Chapter 62 Seasonal and Acute Allergic Reactions
Allergic rhinitis is the most common atopic disease. Although the prevalence varies by region, it is estimated that nearly 25% of the population may be affected.8 It is the second most prevalent chronic condition in the United States, outranked only by hypertension.72 Allergic rhinitis may be classified as seasonal (commonly referred to as hay fever) or perennial. The seasonal form is caused by aeroallergens released from the pollens of wind-pollinated plants, which include trees, grasses, and weeds. Perennial symptoms are commonly the result of exposure to house dust mites, domestic pets, cockroaches, and mold. In temperate climates, sufferers of perennial allergic rhinitis with a seasonal component often experience worsening symptoms during the warmer months because of the additional burden of seasonal allergens.
Although anaphylaxis is not as common as allergic rhinitis, it represents the most severe form of allergic reaction and can lead to a serious, including fatal, outcome if not treated promptly. The exact prevalence of anaphylaxis is not known. Based on extrapolation from data, the estimated risk for anaphylaxis per person in the United States is 1% to 3%.79 Anaphylactic reactions are probably underrecognized and underreported, particularly in cases of unexplained death. In one study, elevated tryptase levels, which are often found during acute anaphylaxis, were found in 13% of 68 patients postmortem.62 Foods and medications are the most common causes of anaphylaxis. Each year, approximately 40 deaths in the United States are attributed to anaphylaxis resulting from bee stings.2
Allergic Rhinitis
Allergic rhinitis is a chronic immunoglobulin E–mediated (IgE-mediated) inflammatory disease of the nasal mucosa. It is the most common atopic disease, and for reasons not yet clear, its prevalence is increasing worldwide. The economic impact of allergic rhinitis is impressive. In 2002, the total direct and indirect costs were $7.3 billion and $4.2 billion, respectively.61 Indirect costs were mainly attributed to work or school absenteeism. In the United States, allergic rhinitis is responsible for 3.5 million lost work days and 2 million lost school days.61 The disease usually manifests in early childhood or adolescence and peaks in the second or third decade of life. The disease does not appear to favor any gender, ethnic group, or race. The most important risk factor for development of allergic rhinitis is a family history of atopy, especially with early onset of disease. In comparison with the general population, the risk is 30% greater if one parent or sibling is atopic, and 50% greater if both parents are affected.47 On the other hand, lack of complete concordance for atopy in identical twins emphasizes the importance of environmental factors in disease development.4 Allergic rhinitis, like other atopic conditions such as asthma and atopic dermatitis, tends to cluster in families.
Pathophysiology
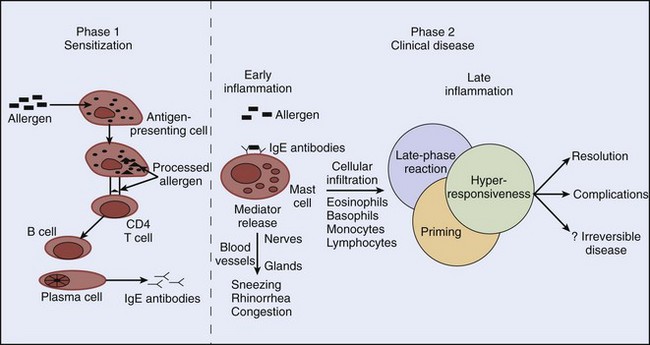
The two important components in the pathogenesis of allergic rhinitis are the acute allergic reaction and late inflammatory events. Type I immediate hypersensitivity reaction accounts for most of the acute clinical manifestations of allergic rhinitis, and cells such as eosinophils, basophils, and T lymphocytes play important roles in late inflammatory events. Production of allergen-specific IgE (sensitization) antibodies forms the underlying basis of immediate hypersensitivity; atopy is defined as the genetic predisposition to develop allergen-specific IgE antibodies. The sensitization process requires a cooperative effort between CD4 T lymphocytes and B lymphocytes (Figure 62-1). It begins with presentation of an allergen to CD4 T lymphocytes by antigen-presenting cells (e.g., macrophages) in the context of a major histocompatibility complex. Cytokines released from CD4 T lymphocytes as a result of this interaction cause differentiation of B lymphocytes into immunoglobulin-secreting plasma cells. This differentiation leads to isotype switching (production of specific antibody types) within the plasma cells. For example, release of cytokine interleukin IL-4 or IL-13 from T lymphocytes promotes IgE switching.14 Once allergen-specific IgE antibody is produced, subsequent exposure and allergen binding to the IgE molecule on the surface of mast cells result in cross-linking of the IgE molecule. Consequently, mast cells or basophils degranulate and release preformed and newly synthesized mediators. The prototype preformed mediator is histamine, and the newly synthesized mediators include those of the arachidonic acid pathway (leukotrienes, prostaglandins, and platelet-activating factor), neuropeptides (e.g., substance P), and cytokines (e.g., IL-4, IL-5).
Release of chemical mediators has various pathologic and clinical consequences. Sneezing and itching result from histamine stimulation of its receptors on sensory nerve endings. Rhinorrhea results from increased vascular permeability produced by all the mediators, and leukotrienes and prostaglandins are believed to play major roles in nasal congestion.74 Our knowledge of the pathophysiology of allergic rhinitis has been greatly enhanced by nasal challenge studies.26 It is now known that the allergic reaction consists of an early phase, characterized by mast-cell or basophil degranulation, and a late phase, which occurs 4 to 6 hours after the early phase (see Figure 62-1). The hallmark of the late-phase reaction is an influx of inflammatory cells, such as eosinophils, basophils, and T lymphocytes.3 For example, basophils cause further histamine release, and T lymphocytes release additional cytokines that enhance IgE production (via IL-4) and eosinophil activation (via IL-5). As a result of further inflammatory activity by these cells, there is recrudescence of symptoms many hours after the initial allergen exposure. Leukotrienes, prostaglandins, and cytokines released in the early-phase reaction play an important role in recruiting the late-phase cellular components to the inflammatory site. Although inhaled corticosteroids block both the early- and late-phase reactions, systemic corticosteroids block only the late-phase reaction.
Allergens
Pollens
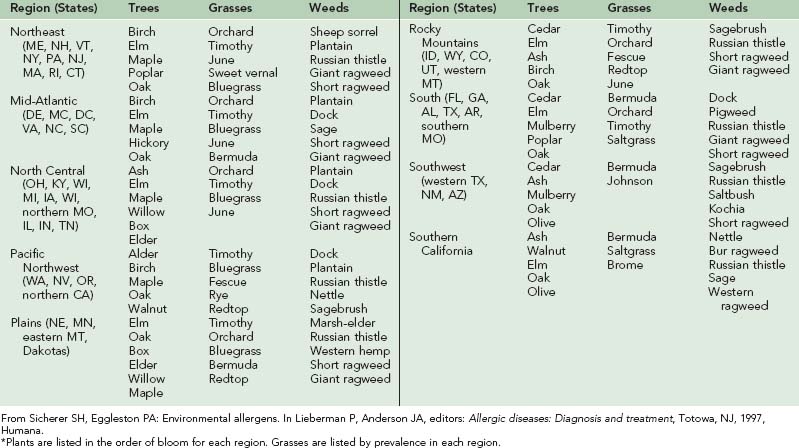
Pollination in higher-order plants consists of transfer of the male gametophyte to the female gametophyte. In this process, pollen grains serve as vectors for male gametophytes. Of the different types of pollen-producing plants, flowering plants (including trees, grasses, and weeds) are the most important from an allergic standpoint. Flowering plants may be divided into those that rely on animal vectors (e.g., insects) for pollination (termed entomophilous) and those that depend on the wind (termed anemophilous). In general, only anemophilous plants (including trees, grasses, and weeds) cause allergic symptoms.67 Pollens of entomophilous plants do not achieve high airborne concentrations. In temperate climates, pollination of trees, grasses, and weeds occurs in predictable time intervals in a given region. Therefore it is important to know the relevant local botany and respective pollination seasons (Table 62-1 and Figure 62-2).
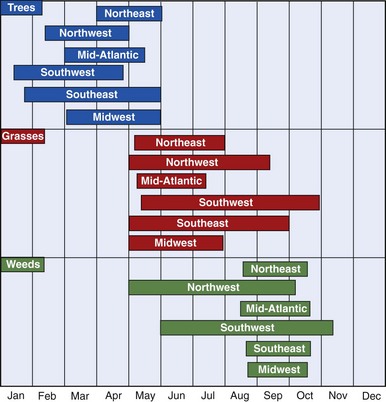
FIGURE 62-2 Pollen seasons by region in the continental United States.
(From Sicherer SH, Eggleston PA: Environmental allergens. In Lieberman P, Anderson JA, editors: Allergic diseases: Diagnosis and treatment, Totowa, NJ, 1997, Humana.)
As shown in Figure 62-2, tree pollination marks the onset of allergy season in most parts of North America. It begins as early as mid-January in the Southwest or early April in the Northeast, and it terminates between early and late May. Trees of allergenic significance in various regions are listed in Table 62-1. Grass pollen, with its worldwide distribution, is a significant source of allergen exposure and a major cause of allergic rhinitis in sensitive individuals. In frost-free areas of North America, grass pollen may be present year-round, whereas in temperate climates, grass pollination peaks between mid-May and mid-July. Important grass types are rye, timothy, Kentucky bluegrass, orchard, Johnson, and Bermuda. Unlike ragweed and tree pollen allergens, grass pollen allergens show extensive cross-reactivity among different species, and allergic individuals are generally sensitive to many of these species. Weeds represent the final pollen producers of the season, typically generating pollen from mid-August through mid-October. Although a variety of weeds cause regionally significant allergies, ragweed is found in most parts of North America.
Dust Mites
Dust mites are microscopic arachnids that are close relatives of ticks and spiders. They feed on human epidermal scales and, like fungi, prefer a warm, humid environment. Dust mites are the most common indoor allergens and can cause significant perennial symptoms in sensitive individuals. Although there is no good correlation between exposure level and degree of rhinitis symptoms, a level of 10 µg/g of dust is considered a risk factor for development of acute asthma symptoms.53 Common indoor sources of dust mite exposure include mattresses, pillows, blankets, upholstery, and stuffed toys. Dust mites may proliferate in sleeping bags stored in humid environments (e.g., damp basements).
Animals
Animals, especially cats, can be highly allergenic and in sensitized individuals can cause significant symptoms. Cats and dogs are the most common pets in the United States, with more than one-third to one-half of all homes housing at least one cat or dog.64 Birds, rabbits, hamsters, guinea pigs, rats, and mice are some other potentially allergenic pets. The major allergens of the cat and the dog are found in their saliva and sebaceous glands. The cat allergen can remain a potential source of allergy months after the removal of the cat, because of its light, sticky qualities.
Outdoors, inhalation of the emanations of moths, locusts, beetles, and flies may cause allergic symptoms. Residents of the area around western Lake Erie may experience allergic symptoms caused by the mayfly.76 Finally, the cockroach is potentially allergenic and is frequently encountered in heavily infested, crowded, or multifamily dwellings.
Functions of the Nose
In most people, cyclic swelling and shrinking of the turbinates between the two sides of the nose is inherent. This nasal cycle, which is approximately 1 to 4 hours in length, results from alternating sympathetic discharge and is responsible for the sensation of alternating unilateral nasal blockage experienced by allergic rhinitis sufferers.22
Clinical Evaluation
A thorough, careful history is crucial in evaluation of allergic rhinitis and is often helpful in determining whether or not an individual is allergic. Allergy testing is used to confirm the underlying clinical suspicion. Symptoms of allergic rhinitis can range from intermittently mild to incapacitating, such as during peak pollen season. The hallmark of allergic rhinitis is temporal correlation of symptoms with allergen exposure.46 The most common symptoms of allergic rhinitis—sneezing, nasal congestion, rhinorrhea, and pruritus of the nose and eyes—are nonspecific. Itching and sneezing are the most distinctive complaints associated with allergic rhinitis,47 whereas nasal congestion seems to be more prominent in perennial rhinitis than in seasonal rhinitis. In some individuals, ocular symptoms predominate over nasal symptoms. Allergic rhinitis sufferers often experience the priming effect (an increase in sensitivity to allergens after repeated exposure) and hyperresponsiveness to nonallergenic environmental stimuli, such as tobacco smoke, strong odors, pollutants, and weather changes.11
Since the time intervals of pollination are predictable in a given season, the timing of symptoms is often helpful in identifying the responsible pollen(s). For example, in the Midwest, ragweed sufferers characteristically start experiencing symptoms in mid-August. Conversely, because any number of allergens may cause symptoms in individuals with perennial rhinitis, determining the specific responsible allergen(s) can be difficult. Other conditions of the upper respiratory tract may mimic allergic rhinitis and must be considered. Complications of allergic rhinitis include sinusitis, otitis media with effusion, and asthma. Additionally, numerous studies have shown that allergic rhinitis may significantly affect quality of life, with effects such as sleep loss, fatigue, poor concentration, reduced productivity, and irritability.28 Although a decline in their quality of life may seem like an obvious clue to the severity of the disease, patients may not be aware of this decline if they have been living with their condition for years and have become accustomed to it.71
As is true for the symptoms described by the patient, many of the signs noted on physical examination are not exclusive to allergic rhinitis. Classically, the nasal mucosa is pale blue and edematous, but this color change is noted in only 60% of sufferers, and many individuals have erythematous mucosa.15 In children, the “allergic salute” (upward nasal rubbing) and “allergic shiners” (dark, puffy circles under the eyes) may be present. Other notable signs include septal deviation, polyps, and an obvious foreign body. If congestion is profound, use of a topical decongestant such as oxymetazoline (Afrin) may improve visualization.
Allergy Testing
Because of the temporal correlation of symptoms with a particular season, diagnosis of seasonal allergic rhinitis can often be made clinically without the need for testing. However, given the ambiguity of various allergen exposures in perennial rhinitis, allergy testing is generally recommended. Allergy testing may be performed either by skin testing or by the radioallergosorbent test (RAST). In skin testing, which is widely accepted and more sensitive,30 minute quantities of specific allergens are introduced under the skin, and a positive response depends on allergen-specific release of histamine. In the RAST, evidence of allergen-specific IgE is determined using the patient’s serum. Given its lesser sensitivity, greater cost, and longer turnaround time for results, RAST is typically used in special circumstances only, such as for patients with severe eczema or dermatographism, in whom skin testing might be difficult to interpret.
Differential Diagnosis
Given the significant and overlapping symptoms of various rhinitis conditions, the differential diagnosis of rhinitis must be considered during the initial evaluation (Box 62-1). Rhinitis can be classified as acute or chronic. The most common cause of acute rhinitis is a viral infection, and allergic rhinitis may be confused with viral rhinitis. However, unlike the symptoms of allergic rhinitis, those of viral rhinitis usually do not persist longer than 2 weeks unless superseding sinusitis develops. A foreign body or trauma also can cause acute rhinitis. Unilateral symptoms are the hallmark of foreign body obstruction.
BOX 62-1 Differential Diagnosis of Rhinitis
The differential diagnosis of chronic nonallergic rhinitis is broad. Nonallergic rhinitis with eosinophilia syndrome (NARES) is characterized by (1) symptoms similar to allergic rhinitis, (2) eosinophilia on nasal smear, and (3) a negative skin test.27 Chronic sinusitis, which can occur as a complication of allergic rhinitis, commonly causes nasal congestion, sinus pressure, postnasal drip, cough, and diminished senses of smell and taste. Symptoms of chronic sinusitis, unlike those of acute sinusitis, are subtle and may require radiologic evaluation, preferably computed tomography. Systemic diseases, such as vasculitis and cystic fibrosis, may cause chronic rhinitis. Rhinitis medicamentosa, which usually results from overuse of topical α-adrenergic vasoconstrictors (e.g., oxymetazoline), is associated with profound nasal congestion caused by rebound effects on withdrawal of the decongestant, usually after 5 to 7 days of topical use. Medications that have been implicated in chronic rhinitis include nonsteroidal antiinflammatory drugs (NSAIDs), angiotensin-converting enzyme inhibitors, and β-blockers.
Mechanical and anatomic abnormalities should always be sought on physical examination. Nasal polyps have a pearly, smooth, peeled grape-like appearance and are often bilateral. Nasal polyps may be associated with chronic sinusitis, asthma, and aspirin sensitivity.63 Unilateral symptoms should always heighten suspicion for a foreign body or tumor. Some degree of septal deviation may be found in most people. Therefore, unless symptoms are severe, treatment is unnecessary. In children, adenoid hypertrophy must be considered.
Symptoms of gustatory rhinitis are caused by a cholinergic response that occurs after such stimuli as eating, running, and exposure to cold air. Atrophic rhinitis is a rare condition that manifests in older adults as a thick, bilateral, odorous discharge with little nasal congestion.21 Rhinitis of pregnancy should be considered in the pregnant woman with congestion, especially if there is no previous history of rhinitis. Although rare, cerebrospinal fluid (CSF) leak must be considered in anyone with clear rhinorrhea and a history of central nervous system trauma. Testing for the presence of glucose in the nasal mucus is helpful in making the diagnosis. The diagnostic test of choice to detect CSF leak is an assay for a β-2 transferrin level in the nasal mucus.65
Often a diagnosis of exclusion, vasomotor (idiopathic) rhinitis is a common cause of nonallergic rhinitis in adults. Although the term implies a cause relating to vascular or neurologic dysfunction, the mechanism of vasomotor rhinitis is poorly understood. The symptoms of vasomotor rhinitis are frequently obstructive rather than secretory. Therefore nasal congestion and sinus pressure predominate over sneezing and rhinorrhea.39 Individuals with vasomotor rhinitis typically experience perennial symptoms that may be triggered by changes in weather, spicy foods, and irritants, such as smoke, strong scents, and chemicals.
Treatment
Avoidance of the offending allergens is the first step in the treatment of allergic rhinitis. However, avoidance is often difficult because of the ubiquitous presence of the allergens in the environment or because of an inability to part from a beloved pet. Therefore pharmacologic intervention is often necessary. The optimal treatment plan should be individualized on the basis of symptoms, seasonal patterns, and presence of possible comorbid conditions. The ideal medication is efficacious, cost effective, and compliance friendly, with minimal side effects. Furthermore, based on the pathophysiology of allergic rhinitis, the best therapeutic agent is one that effectively addresses both early- and late-phase reactions.68 Currently, the main classes of medications available for treatment of allergic rhinitis are antihistamines, decongestants, topical or oral glucocorticoids, anticholinergics, cromolyn sodium, and leukotriene-receptor antagonists. In addition, immunotherapy should be considered in moderately to highly sensitive individuals.
Avoidance
Although it may be difficult to avoid pollen because of its widespread distribution, certain common-sense measures can be taken to decrease exposure. Outdoor activities should be limited or completely avoided on days when pollen counts are high. On any given day, pollen counts typically peak in the late morning to mid-afternoon.77 Limiting activities such as leaf raking, lawn mowing, and farming can reduce exposure to outdoor fungi. Because fungi thrive in moist, humid environments, important indoor control measures include dehumidification, proper ventilation, fungicide use in contaminated areas, and removal of sources of fungal growth. Dust mite exposure can be reduced by several methods, including covering mattresses and pillows with allergen-proof encasings, frequently washing of bedding in hot water, and removing carpeting or treating it with denaturing chemicals. Complete removal of the offending animal from the environment is the best treatment for animal allergy. However, this option is often not exercised. If the pet stays in the home environment, then bathing the pet at regular intervals and keeping it out of the bedroom may be helpful. Use of high-efficiency particulate air (HEPA) filters has been shown to be beneficial in removing animal allergens from the environment.12
Antihistamines and Decongestants
Antihistamines have been used for 50 years and are considered by many to be the first line of therapy in the treatment of allergic rhinitis.54 They are most effective against seasonal rhinitis in which sneezing, itching, rhinorrhea, and watery eyes are the most prominent symptoms. However, only 33% to 50% of seasonal allergic rhinitis sufferers obtain complete relief with antihistamine therapy alone.7 Antihistamines have little or no effect on nasal congestion because of the minor role of histamine in the pathophysiology of congestion. For this reason, antihistamines are usually inadequate for treatment of perennial rhinitis, in which nasal congestion is often a predominant symptom.
Antihistamines act by occupying H1 receptors on cells and thereby blocking histamine binding. Older- and newer-generation antihistamines are equally effective. However, the newer-generation antihistamines have gained tremendous popularity because their use is associated with a much lower incidence of sedation and fewer anticholinergic side effects. Commonly used older-generation antihistamines, most of which are available over the counter, are chlorpheniramine, diphenhydramine, brompheniramine, clemastine, and hydroxyzine. The duration of action of brompheniramine and diphenhydramine is 6 to 10 hours, whereas those of chlorpheniramine and hydroxyzine are 24 and 36 hours, respectively. Although sedation is a well-known side effect of the older-generation antihistamines, many patients are unaware of its effects on cognitive functions44 and impairment in driving performance.50
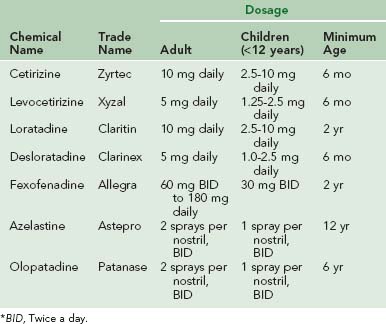
Newer-generation antihistamines are used widely and have been shown to be safe, effective, and well tolerated.29 Moreover, newer-generation antihistamines are more specific in their actions and do not have anticholinergic or anti–α-adrenergic activity. Seven newer-generation antihistamines, including five oral and two intranasal, are currently available (Table 62-2). With the exception of azelastine, olopatadine, and certain formulations of fexofenadine, these medications are given once daily. All seven are approved for use in children; cetirizine, levocetirizine, and desloratadine are approved for those as young as 6 months of age. Azelastine and olopatadine are the only H1 receptor antagonists available in a nasal spray formulation. Studies have shown that both azelastine and olopatadine are safe and effective in relieving most allergic rhinitis symptoms, including nasal congestion.5,34 Although all antihistamines have a relatively rapid onset of action and may be used as needed, they are most effective if either taken before exposure (e.g., before pollen season begins) or used on a regular basis.
Nasal Corticosteroids
Nasal corticosteroids are the gold standard of treatment for allergic rhinitis. Numerous well-designed studies have shown that nasal corticosteroids are superior to antihistamines and cromolyn sodium.6,9 With the exception of ocular symptoms, nasal corticosteroids are effective in controlling all symptoms of allergic rhinitis. Nasal corticosteroids are often the first line of therapy for patients with perennial rhinitis. Corticosteroids control the rate of protein synthesis by either inducing or suppressing gene transcription within the cell.33 The inhibitory effect of all corticosteroids on the late-phase reaction is well established, but studies have shown that nasal corticosteroids, unlike their oral counterparts, also inhibit the early-phase reaction.1
Several preparations of nasal corticosteroids are currently available (Table 62-3). All are equally effective; hence selection is based on convenience, cost, and delivery system. All nasal corticosteroids are in aqueous form and administered either once or twice a day. Because of their relatively slow onset of action, the maximal benefit of nasal corticosteroids may not be realized until 1 to 3 weeks after the initiation of therapy. For individuals with seasonal rhinitis, it is best to start nasal corticosteroid therapy shortly before the onset of pollen season. There is no role for regular use of oral corticosteroids in the treatment of allergic rhinitis. However, a short course (7 to 10 days) of oral corticosteroids may be appropriate when symptoms are severe, such as during peak pollen season. In addition to providing quicker relief, oral corticosteroids may facilitate improved delivery of nasal corticosteroids to the nasal mucosa.
The most common side effects of nasal corticosteroids are local irritation and bleeding, reported in approximately 10% and 2% of patients, respectively.43 The incidence of side effects is higher during winter months because of drier conditions. Although rare, nasal septal perforation has been reported with use of nasal corticosteroids.60 If significant septal ulceration and crust formation are noted, therapy should be discontinued for a few days and nasal hygiene with saline spray should be instituted. Long-term studies have shown no evidence of mucosal atrophy associated with the use of nasal corticosteroids.45 Because of the rapid metabolism of nasal steroids, hypothalamic-pituitary-adrenal axis suppression is generally not a problem. Therefore risk for systemic side effects is low.
Leukotriene-Receptor Antagonists
Leukotriene-receptor antagonists (LTRAs), which were previously indicated only for asthma, are now approved for use in allergic rhinitis. Cysteinyl leukotrienes, which are products of the arachidonic acid pathway, are important mediators of the allergic inflammatory effects. The leukotrienes cause nasal congestion that results from vasodilation. Because leukotrienes play a major role in the pathophysiology of nasal congestion, the LTRAs may significantly reduce nasal congestion.32 Additionally, they cause mucus production and therefore rhinorrhea. Various studies have demonstrated that leukotriene levels are increased in nasal lavage in both seasonal and perennial allergic rhinitis.66 LTRAs block binding of cysteinyl leukotrienes to the CYS-LT1 receptor in the respiratory tract.
Several studies have compared the effects of LTRAs with those of antihistamines and nasal corticosteroids. In one study comparing the LTRA montelukast with loratadine, both were found equally effective in alleviating nasal symptoms.52 However, in a different study comparing montelukast with the nasal corticosteroid fluticasone propionate, montelukast was significantly less effective than fluticasone propionate in relieving nasal symptoms.40 The effectiveness of combining LTRAs and antihistamines have also been studied. One study showed that the combination of montelukast with loratadine was more effective than either agent alone.43 However, other studies have refuted this finding.48 In conclusion, the studies of LTRAs in allergic rhinitis show that they perform as well as antihistamines, but not as well as nasal corticosteroids.
Montelukast, zafirlukast, and zileuton are three currently available oral LTRAs. However, only montelukast is approved for use in both allergic rhinitis and asthma. The other two LTRAs are approved only for use in asthma. The recommended dosage of montelukast for adults and adolescents 15 years of age and older is 10 mg at bedtime. Pediatric dosing is 5 mg and 4 mg at bedtime for ages 6 to 14 years and 6 months to 5 years, respectively. In general, LTRAs appear to be well tolerated. Occasional liver toxicity has been reported with zafirlukast55 and zileuton, but not with montelukast.
Immunotherapy
Allergen immunotherapy is a form of immunomodulation in which a state of tolerance is achieved by injecting gradually increasing amounts of allergen extracts into a sensitized person. Although the precise mechanisms of immunotherapy are unknown, several immunologic changes have been observed, including induction of allergen-specific IgG “blocking antibodies,” decreases in allergen-specific IgE, modulation of mast cell or basophil function, and increases in suppressor T cells (CD8).19
The efficacy of immunotherapy in patients with allergic rhinitis has been well established in numerous studies.16,42 Most individuals who receive immunotherapy for allergic rhinitis obtain a variable degree of relief, enabling better symptom control with less medication. Immunotherapy should be considered in individuals who respond poorly to medical therapy and in individuals who experience significant side effects to medications. Additionally, consideration may be given if allergen avoidance is not possible. In immunotherapy, weekly injections of allergens at escalating dosages are administered until a maintenance dosage is reached, generally within 4 to 6 months. Thereafter, one or more injections are given monthly, depending on the number of allergen sensitivities. Although there are no data regarding optimal duration of immunotherapy, the general recommendations are from 3 to 5 years.75
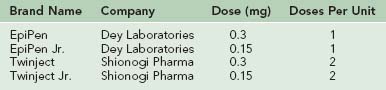
The most common adverse reactions of immunotherapy are local, consisting of erythema, edema, and pruritus at the site of injection. The risk for systemic reaction is approximately 0.1% to 1% per injection.23 Manifestations of a systemic reaction are variable and may include urticaria, flushing, rhinitis, bronchospasm, laryngospasm, abdominal cramping, and hypotension. The risk for systemic reactions is much greater in individuals with poorly controlled asthma. Therefore immunotherapy is generally not recommended in asthmatics with a forced expiratory volume in 1 second (FEV1) of less than 70% of predicted. Because most systemic reactions occur within 20 to 30 minutes after injection, recipients should be observed in the physician’s office for this time period. Some practitioners require patients to carry epinephrine (e.g., EpiPen, Twinject) to self-treat reactions that may occur after leaving the physician’s office.
Anaphylaxis
Anaphylaxis is a systemic life-threatening allergic reaction resulting from IgE-mediated mast-cell and basophil degranulation. It is the most severe form of type I hypersensitivity reaction, and clinical manifestations may occur in various end organs, including the skin, upper respiratory tract, lower respiratory tract, gastrointestinal tract, and cardiovascular system. An anaphylactoid reaction is non-IgE antibody mediated but may be clinically indistinguishable from anaphylaxis (Box 62-2). Mechanisms of anaphylactoid reactions include direct release of mediators from mast cells and basophils (e.g., caused by opiates, radiocontrast material), disturbances in arachidonic acid metabolism (e.g., caused by NSAIDs),17 and complement activation (e.g., caused by transfusion reactions).
BOX 62-2 Classification of Anaphylactic and Anaphylactoid Reactions
Etiology
The various causes of anaphylaxis can be categorized into those that are IgE antibody mediated and those that are non-IgE antibody mediated (see Box 62-2). Foods were the most frequent causative agents of anaphylaxis in one series of 179 subjects spanning 3.5 years, accounting for 36% of episodes.78 Egg, cow’s milk, wheat, soybean, peanut, tree nuts (e.g., hazelnut, walnut, cashew, almond), fish, and shellfish (e.g., shrimp, lobster, crab) account for more than 90% of all food-related anaphylactic reactions. In children, peanuts are probably the most frequent cause of food-induced anaphylaxis.59 Individuals may accidentally ingest the allergenic food when it is disguised in cooking preparations or hidden by misleading labeling or contamination during the preparation process.
Drugs, especially antibiotics, may cause an anaphylactic or an anaphylactoid reaction. Penicillin and its derivatives are most often implicated, with reactions occurring in between 1 and 5 of every 10,000 courses of treatment.25 Opiates and vancomycin cause drug reactions by directly degranulating mast cells and basophils, so prior sensitization is not required.
Aspirin and other NSAIDs can provoke allergic reactions that are either IgE or non-IgE antibody mediated, depending on the presence or absence of underlying asthma or cutaneous diseases (e.g., chronic urticaria). In the presence of asthma and chronic urticaria, disturbance in arachidonic acid metabolism is the predominant mechanism. Aspirin and other NSAIDs inhibit the cyclooxygenase pathway, leading to rapid synthesis of the lipoxygenase pathway products,36 especially leukotrienes, which are important mediators of inflammation.
Important outdoor-related causes of anaphylaxis are envenomation (e.g., bee sting), contact with aquatic proteins, and antivenom therapy (e.g., for snakebite). The estimated incidence of insect sting anaphylaxis in the general population is 0.3% to 3%.56 Bee sting anaphylactic reactions are especially common in individuals younger than 20 years but are more likely to be fatal in older adults. In general, children’s reactions are milder (e.g., urticaria only) than are those of adults. Individuals who experience a sting-related anaphylactic reaction have a 50% to 60% risk for suffering anaphylaxis after subsequent insect stings.56 The offending insects vary depending on geographic location. In the United States, yellow jackets cause the most allergic reactions, whereas in Europe, honey bees and wasps cause the majority of insect sting–related reactions. Although rare, anaphylaxis can result from bites inflicted by certain insects (e.g., the kissing bug Triatoma protracta, the deer fly Chrysops discalis,24 and ticks).10
Physical stimuli may provoke anaphylactoid reactions. In exercise-induced reactions, symptoms typically arise after 5 minutes of moderate to heavy exercise, resolving within 30 minutes to 4 hours after exercise cessation.73 About 50% of affected individuals are atopic, and most engage in regular vigorous exercise. A coinciding factor, such as ingestion of an allergenic food (e.g., shellfish)41,51 or NSAID, may be necessary to induce this type of reaction—that is, exercise only or ingestion of the food only does not cause the reaction. In a cholinergic reaction, symptoms occur after exercise and after passive exposure to heat (e.g., hot showers, sweating, and anxiety). In cold-induced reactions, symptoms occur after exposure to a cold stimulus, such as being outside on a cold day, holding a cold object, or eating a cold food.
Pathophysiology
Nitric oxide (NO), a by-product of inflammation, has been found to be increased during anaphylaxis.57 NO has the potential to be both protective and damaging in anaphylaxis. It relaxes bronchial smooth muscle while dilating vascular smooth muscle. Therefore its effects on smooth muscle can improve bronchospasm while worsening hypotension. The overall effects of NO are adversarial. In both animals and humans, NO synthesis inhibitor exerts a beneficial effect in shock.37 The role of NO in the production of anaphylactic reaction has been questioned. In a study of 77 patients with acute allergic reactions, no correlation was found between NO levels and plasma histamine or serum tryptase.38 In addition, NO levels were no higher in patients with hypotension.
Clinical Features and Diagnosis
Clinical manifestations of anaphylaxis may occur in various end organs, including the skin (urticaria, angioedema, and flushing), upper respiratory tract (rhinitis, stridor, and hoarseness), lower respiratory tract (wheezing, bronchospasm, and cough), gastrointestinal tract (abdominal pain, diarrhea, and vomiting), and cardiovascular system (tachycardia, hypotension, and shock). Urticaria and angioedema are by far the most common manifestations, occurring in 83% to 90% of individuals with anaphylaxis.31 The second most common manifestations are respiratory tract symptoms, followed by dizziness or syncope and gastrointestinal symptoms. Cardiovascular collapse with shock can occur rapidly, without any other antecedent symptoms.
Most anaphylactic reactions occur soon (within 5 minutes to 2 hours) after exposure to an inciting agent, but other patterns are possible. In bimodal anaphylaxis, symptoms begin within minutes of exposure; after transient clinical improvement, the allergic reaction returns 1 to 8 hours later.70 Protracted anaphylaxis can begin suddenly or gradually, but the clinical manifestations are prolonged, sometimes requiring hours or even days of intense resuscitation. Generally, the more rapid the onset, the more severe the episode.37 Fatalities usually result from airway obstruction or cardiovascular collapse, or both.
Plasma histamine and serum tryptase can be helpful in making the diagnosis of anaphylaxis, because both may be elevated during an acute episode. The best time to measure plasma histamine is between 10 minutes and 1 hour after the onset of symptoms, whereas the best time to measure serum tryptase is between 1 and 2 hours after the onset of symptoms.35
Treatment
Short-term management of anaphylaxis is detailed in Box 62-3.69 Because most fatalities occur as a result of delayed treatment,18 the importance of prompt administration of epinephrine cannot be overemphasized. Intravenous epinephrine may be administered if the reaction is life threatening and if the patient does not respond to subcutaneous epinephrine. However, intravenous epinephrine should be used with caution in persons older than 35 years. Aerosolized aqueous epinephrine can prevent upper airway edema but is inadequate to abort systemic anaphylaxis.13 Use of over-the-counter epinephrine inhalation aerosol bronchodilators (e.g., Primatene Mist) is generally not recommended because of its lack of adrenergic specificity (i.e., it is non-β2 selective) and its extremely short half-life. These agents should also be used with caution in individuals with a history of coronary artery disease and arrhythmias.
BOX 62-3 Treatment of Anaphylaxis
General Measures
Epinephrine Use and Treatment of Hypotension
Treatment of Bronchospasm
Antihistamines and Corticosteroids
Repletion of intravascular volume is a mainstay of treatment for hypotension. A large volume of fluid may be necessary and should be given rapidly in either colloid or crystalloid form. Vasopressor drugs may be needed to treat hypotension if the response to subcutaneous epinephrine and fluids is inadequate. Dopamine is often the initial drug of choice. Norepinephrine, a potent vasopressor, can be used to treat severe hypotension that is unresponsive to epinephrine, dopamine, and fluids. Glucagon may be necessary to treat refractory hypotension (e.g., patients on β-blocker medications) that is resistant to standard therapeutic regimens.80 Glucagon is the drug of choice for patients on β-blocker medications.20 Medical antishock trousers have been used successfully to treat refractory hypotension associated with anaphylaxis.49
For prominent wheezing, intermittent or continuous use (depending on severity) of an aerosolized β2-agonist is recommended. Although antihistamines are only adjunctive therapy to epinephrine, they can relieve symptoms dramatically. Combining an H1 antihistamine and an H2 antihistamine may be more effective than administering either alone.58 Most authorities recommend the use of glucocorticoids to decrease the likelihood of a late-phase reaction.
Because the symptoms can recur after the initial anaphylactic event, patients should be observed for 2 to 24 hours after being stabilized, depending on the severity of the episode. Any individual with severe respiratory or cardiac compromise should be hospitalized. Persons at risk for anaphylaxis should carry a device allowing self-injection of epinephrine (Table 62-4) at all times and should wear a medical information bracelet or carry a medical information card. Finally, it is important to identify the precipitating agent for anaphylaxis so that preventive measures can be taken to reduce the risk for future reactions.
1 Andersson M, Andersson P, Pipkorn U. Topical glucocorticoids and allergen-induced increase in nasal reactivity: Relationship between treatment time and inhibitory effect. J Allergy Clin Immunol. 1988;82:1019.
2 Barnard JH. Studies of 400 Hymenoptera sting deaths in the United States. J Allergy Clin Immunol. 1973;52:259.
3 Bascom R, Pipkorn U, Lichtenstein LM, et al. The influx of inflammatory cells into nasal washings during the late response to antigen challenge: Effect of systemic steroid pretreatment. Am Rev Respir Dis. 1988;138:406.
4 Bazaral M, Orgel HA, Hamburger RN. Genetics of IgE and allergy. J Allergy Clin Immunol. 1974;54:288.
5 Berger W, Ratner P, Casale T, et al. Safety and efficacy of olopatadine hydrochloride nasal spray 0.6% in pediatric subjects with allergic rhinitis. Allergy Asthma Proc. 2009;30:612.
6 Bernstein DI, Creticos PS, Busse WW, et al. Comparison of triamcinolone acetonide nasal inhaler with astemizole in the treatment of ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1996;97:749.
7 Bousquet J, Chanez P, Michel FB. Pathophysiology and treatment of seasonal allergic rhinitis. Respir Med. 1990;84:11.
8 Bousquet J, van Cauwenberge P, Khaltaev N, et al. Workshop Expert Panel. Allergic Rhinitis and its Impact on Asthma (ARIA) in collaboration with the World Health Organization. Executive summary of the Workshop Report. Allergy. 2002;57:841.
9 Bronsky EA, Dockhorn RJ, Meltzer EO, et al. Fluticasone propionate aqueous nasal spray compared with terfenadine tablets in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1996;97:915.
10 Brown AF, Hamilton DL. Tick bite anaphylaxis in Australia. J Accid Emerg Med. 1998;15:111.
11 Connell JT. Quantitative intranasal pollen challenges: III. The priming effect in allergic rhinitis. Allergy. 1969;43:33.
12 de Blay F, Chapman MD, Platts-Mills TA. Airborne cat allergen (Fel d I): Environmental control with the cat in situ. Am Rev Respir Dis. 1991;143:1334.
13 deLage C, Irey N. Anaphylactic deaths: A clinicopathologic study of 43 cases. J Forensic Sci. 1972;17:525.
14 de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol. 1998;102:165.
15 Druce HM. Allergic and nonallergic rhinitis. In: Middleton EJr, editor. Allergy: Principles and practice. ed 5. St Louis: Mosby; 1998:1005-1016.
16 Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass pollen immunotherapy. N Engl J Med. 1999;341:468.
17 Fischer AR, Rosenberg MA, Lilly CM, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046.
18 Frazier CA, Wynn SR, Munoz-Furlong A, et al. Anaphylaxis at school: Etiologic factors, prevalence, and treatment [letter]. Pediatrics. 1993;91:516.
19 Gleich GJ, Zimmermann EM, Henderson LL, et al. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: A six-year prospective study. J Allergy Clin Immunol. 1982;70:261.
20 Glick G, Parmley WW, Wechsler AS, et al. Glucagon: Its enhancement of cardiac performance in the cat and dog and persistence of its inotropic action despite (beta-receptor blockade with propranolol. Circ Res. 1968;22:789.
21 Goodman WS, deSouza FM. Atrophic rhinitis. In: English GM, editor. Otolaryngology, vol 2. Philadelphia: Lippincott; 1987:1-11.
22 Hasegawa M, Kern EB. The human nasal cycle. Mayo Clin Proc. 1977;52:28.
23 Hejjaoui A, Ferrando R, Dhivert H, et al. Systemic reactions occurring during immunotherapy with standardized pollen extracts. J Allergy Clin Immunol. 1992;89:925.
24 Hoffman DF. Allergy to biting insects. Clin Rev Allergy Immunol. 1987;5:177.
25 Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159.
26 Iliopoulos O. Histamine-containing cells obtained from the nose hours after antigen challenge have functional and phenotypic characteristics of basophils. J Immunol. 1992;148:223.
27 Jacobs RL, Freedman PM, Boswell RN. Non-allergic rhinitis with eosinophilia (NARES syndrome): Clinical and immunological presentation. J Allergy Clin Immunol. 1981;67:253.
28 Juniper EF. Measuring health-related quality of life in rhinitis. J Allergy Clin Immunol. 1997;99:S742.
29 Kaliner MA. Non-sedating antihistamines: Pharmacology, clinical efficacy and adverse effects. Am Fam Physician. 1992;45:1337.
30 Kelso JM, Sodhi N, Gosselin VA, Yunginger JW. Diagnostic performance characteristics of the standard Phadebas RAST, modified RAST, and Pharmacia CAP system versus skin testing. Ann Allergy. 1991;67:511.
31 Kemp SF, Lockey RF, Wolf BL, Lieberman P. Anaphylaxis: A review of 266 cases. Arch Intern Med. 1995;155:1749.
32 Knapp HR. Reduced allergen-induced nasal congestion and leukotriene synthesis with an orally active 5-lipoxygenase inhibitor. N Engl J Med. 1990;323:1745.
33 LaForce C. Use of nasal steroids in managing allergic rhinitis. J Allergy Clin Immunol. 1999;103:S388.
34 LaForce C, Dockhorn RJ, Prenner BM, et al. Safety and efficacy of azelastine nasal spray (Astelin NS) for seasonal allergic rhinitis: A 4-week comparative multicenter trial. Ann Allergy Asthma Immunol. 1996;76:181.
35 LaRoche D, Vergnaud MC, Sillard B, et al. Biochemical markers of anaphylactoid reactions to drugs: Comparison of plasma histamine and tryptase. Anesthesiology. 1991;75:945.
36 Lee TH. Mechanism of bronchospasm in aspirin-sensitive asthma [editorial]. Am Rev Respir Dis. 1993;148:1442.
37 Lieberman P. Anaphylaxis and anaphylactoid reactions. In: Middleton EJr, editor. Allergy: Principles and practice. ed 5. St Louis: Mosby; 1998:1079-1092.
38 Lin RY, Gupta A. Nitric oxide levels in patients with acute allergic reactions [abstract]. J Allergy Clin Immunol. 2003;111:S100.
39 Lindberg S, Malm L. Comparison of allergic rhinitis and vasomotor rhinitis patients on the basis of a computer questionnaire. Allergy. 1993;48:602.
40 Llanes SJ, Sur S, Grant JA, Alam R. Comparison of the effects of fluticasone and montelukast on early- and late-phase nasal allergic reactions. J Allergy Clin Immunol. 2001;107:S312.
41 Maulitz RM, Pratt DS, Schocket AL. Exercise-induced anaphylactic reactions to shellfish. J Allergy Clin Immunol. 1979;63:433.
42 McHugh SM, Lavelle B, Kemeny DM, et al. A placebo-controlled trial of immunotherapy with two extracts of Dermatophagoides pteronyssinus in allergic rhinitis, comparing clinical outcomes with changes in antigen-specific IgE, IgG, and IgG subclasses. J Allergy Clin Immunol. 1990;86:521.
43 Meltzer EO, Malmstrom K, Lu S, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: A randomized, placebo-controlled clinical trial. J Allergy Clin Immunol. 2000;105:917.
44 Meltzer EO, Welsh MJ. Adverse effects of H1-receptor antagonists in the central nervous system. In: Simons FER, editor. Histamine and H1-receptor antagonists in allergic disease. New York: Marcel Dekker; 1996:357-381.
45 Morrow Brown H, Storey G, Jackson FA. Beclomethasone dipropionate aerosol in treatment of perennial and seasonal rhinitis: A review of five years’ experience. Br J Clin Pharmacol. 1977;4:2835.
46 Naclerio R. Allergic rhinitis. N Engl J Med. 1991;325:860.
47 Naclerio R, Solomon W. Rhinitis and inhalant allergens. JAMA. 1997;278:1842.
48 Nayak AS, Philip G, Lu S, et al. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: A multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002;88:592.
49 Oertel T, Loehr MM. Bee-sting anaphylaxis: The use of medical antishock trousers. Ann Emerg Med. 1984;13:459.
50 O’Hanlon JF. Antihistamines and driving performance: The Netherlands. J Resp Dis. 1988;9:S12.
51 Okazaki M, Kitani H, Mifune T, et al. Food-dependent exercise-induced anaphylaxis. Intern Med. 1992;31:1052.
52 Philip G, Malmstrom K, Hampel FC, et al. Montelukast for treating seasonal allergic rhinitis: A randomized, double-blind, placebo-controlled trial performed in the spring. Clin Exp Allergy. 2002;32:1020.
53 Pollart SM, Chapman MD, Fiocco GP, et al. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J Allergy Clin Immunol. 1989;83:875.
54 Rachelefsky GS. Pharmacologic management of allergic rhinitis. J Allergy Clin Immunol. 1998;101:S367.
55 Reinus JF, Persky S, Burkiewicz JS, et al. Severe liver injury after treatment with the leukotriene receptor antagonist zafirlukast. Ann Intern Med. 2000;133:964.
56 Reisman R. Insect stings. N Engl J Med. 1994;331:523.
57 Rolla G, Nebiolo F, Guida G, et al. Level of exhaled nitric oxide during anaphylaxis. Ann Allergy Asthma Immunol. 2006;97:264.
58 Runge JW, Martinez JC, Caravati EM, et al. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med. 1992;21:237.
59 Sachs M, Yunginger JW. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 1991;11:743.
60 Schoelzel EP. Nasal sprays and perforation of the nasal septum. JAMA. 1985;253:2046.
61 Schoenwetter WF, Dupclay LJr, Appajosyula S, et al. Economic impact and quality-of-life burden of allergic rhinitis. Curr Med Res Opin. 2004;20:305.
62 Schwartz HJ, Yunginger JW, Schwartz LB. Is unrecognized anaphylaxis a cause of sudden unexpected death? Clin Exp Allergy. 1995;25:866.
63 Settipane GA. Nasal polyps. In Rhinitis, ed 2, Providence, RI: OceanSide; 1991:173-183.
64 Sicherer SH, Eggleston PA. Environmental allergens. In: Lieberman P, Anderson JA, editors. Allergic diseases: Diagnosis and treatment. Totowa, NJ: Humana; 1997:37-46.
65 Skedros DG, Cass SP, Hirsch BE, et al. β-2 transferrin assay in clinical management of cerebral spinal fluid and perilymphatic fluid leaks. J Otolaryngol. 1993;22:341.
66 Skoner DP, Lee L, Doyle WJ, et al. Nasal physiology and inflammatory mediators during natural pollen exposure. Ann Allergy. 1990;65:206.
67 Solomon WR, Weber RW, Dolen WK. Common allergenic pollen and fungi. In: Bierman CW, et al, editors. Allergy, asthma, and immunology from infancy to adulthood. ed 3. Philadelphia: WB Saunders; 1996:93-114.
68 Spector S. Ideal pharmacotherapy for allergic rhinitis. J Allergy Clin Immunol. 1999;103:S386.
69 Spector S, et al. Algorithm for the treatment of acute anaphylaxis. Joint Task Force on Practice Parameters]. J Allergy Clin Immunol. 1998;101:S469.
70 Stark BJ, Sullivan TJ. Biphasic and protracted anaphylaxis. J Allergy Clin Immunol. 1986;78:76.
71 Storms W, Meltzer EO, Nathan RA, et al. Allergic rhinitis: The patient’s perspective. J Allergy Clin Immunol. 1997;99:S825.
72 U.S. Department of Health and Human Services, National Center for Health Statistics. Current estimates from the National Health Interview Survey (DHHS Publication no. (PHS) 96–1521). Vital and Health Statistics. 1994. series 10, no. 193
73 Wade JP, Liang MH, Sheffer AL. Exercise-induced anaphylaxis: Epidemiological observations. Prog Clin Biol Res. 1989;297:175.
74 White MV, Kaliner MA. Mediators of allergic rhinitis. J Allergy Clin Immunol. 1992;90:699.
75 WHO/IUIS Working Group report. Current status of allergen immunotherapy. Lancet. 1989;1:259.
76 Wilbur RD, Evans R. An immunologic evaluation of deer fly hypersensitivity. J Allergy Clin Immunol. 1975;55:72.
77 Wood R. Allergens. In: Mygind N, Naclerio R, editors. Allergic and non-allergic rhinitis. Copenhagen: Munksgaard, 1993.
78 Yocum MW. Anaphylaxis [abstract]. J Allergy Clin Immunol. 1993;91:153.
79 Yocum MW, Butterfield JH, Klein JS, et al. Epidemiology of anaphylaxis in Olmsted County: A population-based study. J Allergy Clin Immunol. 1999;104:452.
80 Zaloga GP, DeLacey W, Holmboe E, et al. Glucagon reversal of hypotension in a case of anaphylactoid shock. Ann Intern Med. 1986;105:65.