Chapter 73 Seafood Allergies
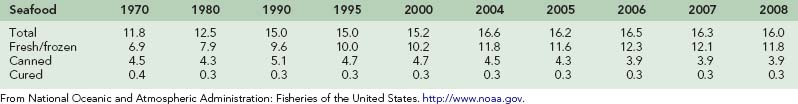
Seafood, including all edible fish and shellfish, has been a mainstay of diets throughout the world for centuries, playing a key role in the nutrition and economy of nations around the globe. In the United States, fish and shellfish consumption has increased in recent decades, perhaps because of its increasingly recognized health benefits. In 2007, the United States was ranked the third largest consumer of seafood in the world, behind China and Japan, consuming a total of 4.908 billion pounds of fish and shellfish.146 This equated to 16.0 lb (7.25 kg) of fish and shellfish per person in 2008, up from 11.8 lb (5.35 kg) in 1970. Table 73-1 illustrates trends in per capita seafood consumption in the United States since 1970. Since 2001, shrimp has continued to rank as the top consumed seafood in the United States, with 4.1 lb (1.86 kg) of shrimp consumed per person in 2008, down from a record of 4.4 lb (1.99 kg) in 2006.145 Table 73-2 lists the most frequently consumed seafood in the United States in 2008.
TABLE 73-2 Top Ten Most Frequently Consumed Seafoods in the United States in 2008
| Seafood | Weight (lb [kg]/capita) |
|---|---|
| 1. Shrimp | 4.1 (1.86) |
| 2. Canned tuna | 2.8 (1.27) |
| 3. Salmon | 1.84 (0.83) |
| 4. Pollock | 1.34 (0.60) |
| 5. Tilapia | 1.19 (0.54) |
| 6. Catfish | 0.92 (0.42) |
| 7. Crab | 0.61 (0.28) |
| 8. Cod | 0.44 (0.20) |
| 9. Flatfish | 0.43 (0.19) |
| 10. Clams | 0.42 (0.19) |
| Total | 16.0 (7.26) |
Data from National Marine Fisheries Service: Top 10 U.S. consumption by species chart, calculated by Howard Johnson, H.M. Johnson & Associates for NFI. http://www.aboutseafood.com/about/about-seafood/Top-10-Consumed-Seafoods.
Epidemiology
Food allergies pose a significant threat to human health. Bock and co-workers estimate that food allergies are the leading identifiable cause of anaphylactic reactions presenting to emergency departments in the United States. There are approximately 29,000 anaphylactic reactions per year, resulting in 150 deaths annually.20 Hughes and Mills estimate that approximately 33% of anaphylactic reactions are caused by foods.80 The actual incidence of anaphylaxis due to food allergies depends on the diagnostic criteria used. One study reported a 13% incidence among a sample of patients presenting with food-related allergic reactions,170 whereas another reported an incidence of 51%.38 In a study of patients presenting with food allergies to an allergy center in Singapore, 66% had a history of anaphylactic reaction to a food allergen.197
Food allergies are common. It is estimated that approximately 4% of adults and 6% of children younger than age 3 years in the United States have a food allergy, with 2.2% of the population having an isolated seafood allergy.184,185 Unlike many food allergies, seafood allergies appear to be more common in adults than in children. Similar to peanut allergy, patients with fish and shellfish allergies generally remain clinically reactive lifelong. A 2002 telephone survey conducted in the United States determined that fish allergies afflicted 0.1% and 0.4% of U.S. children and adults, respectively, whereas shellfish afflicted 0.1% of children and 2% of adults. Shellfish rank as the leading cause of IgE-mediated food allergies in the U.S. adult population.184 Another analysis of persons with food allergies presenting to emergency departments in the United States estimated that shellfish are the number one cause of food allergies among individuals older than age 6 years.170 Overall, seafood allergies have been identified in 2.3% of the U.S. population, with 5.9% of U.S. households reporting at least one seafood allergy.184
Although the specific etiologies of food allergies vary in different countries according to regional dietary patterns, seafood allergies appear to be one of the leading causes of food allergies worldwide. In a study of patients with food allergies in Singapore, crustacea accounted for 34%, mollusks 19%, and fish 4% of the food allergies.197 In a case series of children in Spain, fish and shellfish allergies accounted for 30% and 6.8% of reported food allergies, respectively.41 In several studies of food-induced anaphylaxis, fish allergy was implicated in 30% of cases in a series involving children in Italy,152 and 29% of cases in a study of children in Philadelphia.51
Biologic Classification of Seafood
Seafood can generally be classified into four categories of organisms, including fish, crustacea, mollusks, and echinoderms, with each belonging to a different phylum. Because most individuals with a seafood allergy are not allergic to all types of seafood, a basic understanding of the biologic classification of fish and shellfish can be helpful in guiding patients on selective avoidance diets. Table 73-3 provides an overview of the taxonomic relationships among seafoods.
| Phylum | Class | Common Name Representatives |
|---|---|---|
| Chordata | Actinopterygii | Bony, ray-finned fish (see Table 73-4) |
| Chondrichthyes | Cartilaginous fish (sharks, rays, skates) | |
| Arthropoda | Crustacea | Shrimp, crab, lobster, barnacles, crayfish, krill |
| Mollusca | Gastropoda | Snails, abalone, whelk |
| Bivalvia | Mussels, oysters, scallops, clams, cockles | |
| Cephalopoda | Squid, octopus, cuttlefish | |
| Echinodermata | Echinoidea | Sea urchin |
| Holothuroidea | Sea cucumber |
Data from Myers P, Espinosa R, Parr CS, et al: The animal diversity web, 2008. http://www.animaldiversity.org.
Fish belong to the phylum Chordata, with most edible fish belonging to the class of bony, ray-finned fish, Actinopterygii (super-class Osteichthyes). Sharks (including dogfish), rays, and skates are the exception, belonging to the class of cartilaginous fish Chondrichthyes. The most frequently consumed fish in the United States fall into several orders: Salmoniformes (salmon, trout, whitefish), Siluriformes (catfish), Pleuronectiformes (flounder, halibut, sole, flatfish), Perciformes (bass, perch, snapper, tuna, mackerel, tilapia, swordfish), Gadiformes (codfish, pollock), and Clupeiformes (herring, sardines, anchovies).141 Table 73-4 describes the taxonomic relationships among edible fish species.
TABLE 73-4 Taxonomic Relationships Among the Edible Fishes
| Class | Order (Suborder) | Common Name |
|---|---|---|
| Chondrichthyes | Elasmobranchii | Sharks |
| Actinopterygii | Acipenseriformes | Sturgeons, paddlefish |
| Anguilliformes | Common eels, morays | |
| Atheriniformes | Silversides, jacksmelts, grunions | |
| Beloniformes | Sauries, needlefish, flying fish | |
| Clupeiformes | Herring, sardines, alewives, shad, menhaden, anchovies | |
| Cypriniformes | Minnows, carp, suckers | |
| Elopiformes | Tarpons, ten-pounders | |
| Esociformes | Pike, pickerel, muskellunge | |
| Gadiformes | Codfish, ling cod, pollock, haddock, tomcod, hake, codling, whiting | |
| Gonorynchiformes | Awa, milkfish | |
| Lampridiformes | Opah | |
| Lophiiformes | Monkfish, goosefish | |
| Mugiliformes | Mullets | |
| Osmeriformes | Smelts, eulachon, capelin | |
| Perciformes (Ammodytoidei) | Sand lances | |
| Perciformes (Labroidei) | Cichlids (tilapia), tautogs, wrasss, surf perch | |
| Perciformes (Percodei) | Bass, crappies, bluegills, sea bass, sunfish, perch, bluefish, jacks, pompanos, dolphin fish, snapper, groupers, scups, grunts, porgies, pomfrets, sheepsheads, snooks, robalos, bigeyes, catalufas, croakers, butterfly fish, goatfish, mojarras, rudderfish, weakfish, drums, sauger, threadfins, walleye | |
| Perciformes (Scombroidei) | Mackerel, tuna, cutlassfish, albacore, bonitos, kingfish, swordfish, sailfish, barracuda, billfish, marlin, spearfish, tengirris | |
| Perciformes (Stromateoidei) | Butterfish | |
| Perciformes (Zoarcoidei) | Wolffish | |
| Percopsiformes | Trout-perch, sand rollers | |
| Pleuronectiformes | Flounders, halibut, sole, dabs, turbots, flatfish | |
| Salmoniformes | Trout, salmon, whitefish, graylings, lake herring | |
| Scorpaeniformes | Rockfish, scorpionfish, greenlings | |
| Siluriformes | Catfish | |
| Tetraodontiformes | Pufferfish, boxfish, trunkfish |
From Myers P, Espinosa R, Parr CS, et al: The animal diversity web, 2008. http://www.animaldiversity.org.
Shellfish can be broken down into two distinct phyla. Crustacea, which include shrimp, prawns, crab, lobster, barnacles, krill, and crayfish, are classified as arthropods, sharing the Arthropoda phylum with spiders, centipedes, and insects. The Mollusca phylum includes eight classes, three of which are important for human consumption: Gastropoda (snails, abalone), Bivalvia (mussels, oysters, scallops, clams), and Cephalopoda (squid, octopus, cuttlefish).141,195
Sea cucumbers and sea urchins and their products, including uni, or sea urchin coral, and roe, or sea urchin ovaries, comprise a very small category of marine organisms consumed by humans. Sea urchins and sea cucumbers belong to the phylum Echinodermata, with sea urchins belonging to the class Echinoidea, and sea cucumbers belonging to the class Holothuroidea.141
Immunologic Mechanisms
Although nonimmunologic reactions to fish and shellfish occur, true seafood allergies are immunoglobulin E (IgE)–mediated reactions that represent a failure of the body’s oral tolerance mechanisms. Oral tolerance can be defined as “an active non-response to antigens delivered via the oral route.”124 It involves both prevention of uptake of allergenic proteins from the gut into the bloodstream and suppression of the immune system’s allergenic response to such proteins that enter the system.
Under physiologic conditions, luminal barriers within the gastrointestinal (GI) tract prevent the uptake of the majority of potential food allergens that enter the gut. Potentially allergenic proteins are degraded into nonimmunogenic forms by gastric acid and digestive enzymes, whereas IgA antibodies secreted by B cells in the gut bind foreign proteins and prevent their uptake. However, even under physiologic conditions, approximately 2% of ingested proteins cross the protective epithelium of the GI tract intact and are absorbed into the bloodstream as immunologically active antigens.81,208 Usually these antigens do not elicit allergic reactions because of the body’s innate mechanisms that suppress the immune response to food allergens. This process of immune suppression begins when an intact antigen escapes the protective barriers of the gut and is then taken up and presented by antigen-presenting cells (APCs), including B cells, dendritic cells, and macrophages. APCs then activate regulatory and suppressor T cells, which secrete the suppressive cytokines, transforming growth factor β (TGF-β) and interleukin (IL)-10. Through this series of steps, a state of oral tolerance is achieved whereby the immune system essentially “ignores” the food antigen. In the case of high-dose oral antigen exposure, tolerance is mediated by a different mechanism, specifically, lymphocyte clonal anergy and/or deletion.25,32
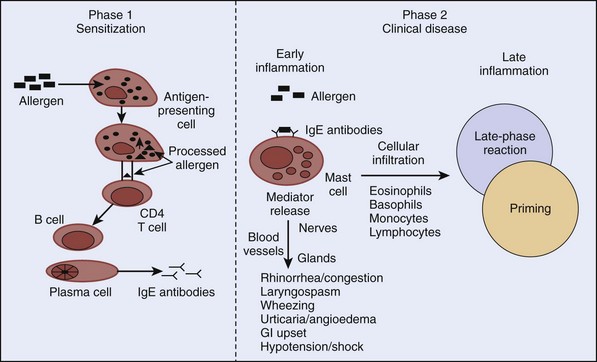
When oral tolerance mechanisms fail to inhibit the body’s immune response to ingested food antigens, food allergies can develop. True seafood allergies are type I immediate hypersensitivity IgE-mediated reactions that result from a chain of molecular and cellular interactions involving APCs, T cells, and B cells (Figure 73-1). Production of allergen-specific IgE (sensitization) antibodies forms the underlying basis of immediate hypersensitivity; atopy is defined as the genetic predisposition to developing allergen-specific IgE antibodies. The sensitization process requires a cooperative effort between CD4 T lymphocytes and B lymphocytes. It begins with presentation of an allergen to CD4 T lymphocytes by APCs in the context of a major histocompatibility complex. Cytokines released from CD4 T lymphocytes as a result of this interaction cause differentiation of B lymphocytes into immunoglobulin-secreting plasma cells. This differentiation leads to isotype switching (production of specific antibody types) within the plasma cells. For example, release of cytokines IL-4 or IL-13 from T lymphocytes promotes IgE switching.49 Once allergen-specific IgE antibodies are produced, subsequent exposure and binding of the allergens to IgE molecules on the surface of mast cells results in cross-linking of the IgE molecules. Consequently, mast cells or basophils degranulate and release preformed and newly synthesized mediators. The prototype preformed mediator is histamine, and the newly synthesized mediators include those of the arachidonic acid pathway (leukotrienes, prostaglandins, and platelet-activating factor), neuropeptides (e.g., substance P), and cytokines (e.g., IL-4, IL-5).
It is now known that allergic reactions consist of an early phase characterized by mast cell or basophil degranulation, and a late phase, which occurs 4 to 6 hours after the early phase. The hallmark of the late-phase reaction is an influx of inflammatory cells, such as eosinophils, basophils, and T lymphocytes.15 For example, basophils cause further histamine release, and T lymphocytes release additional cytokines that enhance IgE production (via IL-4) and eosinophil activation (via IL-5). As a result of further inflammatory activity by these cells, there is recrudescence of symptoms many hours after the initial allergen exposure. Leukotrienes, prostaglandins, and cytokines released in the early-phase reaction play an important role in recruiting the late-phase cellular components to the inflammatory site.
In theory, prior exposure and sensitization to a food allergen must occur before development of a clinically significant allergic reaction. The exposure may occur through cutaneous or inhalational routes, cross-sensitization via similar antigens, placental transfer, or as a result of hidden ingredients or contaminants in other foods. Risk factors for the development of food allergies include early age of antigen exposure, family history of atopy, presence of asthma or other atopic disease, and medications (e.g., antacids) or medical conditions that reduce the acidity of the gut and allow more potential allergens to escape the natural protective barriers of the GI tract.25,201 In one study, more than half of patients with food allergy had concomitant allergic rhinitis, asthma, and/or atopic dermatitis.197 In another study, codfish-allergic individuals were orally challenged with fish digested with gastric enzymes at pH 2.0 and 3.0. Subjects experienced allergic symptoms sooner or at a lower dose when the codfish was predigested at pH 3.0 compared to pH 2.0, underscoring the role of gastric digestion in the process of food allergen tolerance.201
Clinical Manifestations
Clinical manifestations of fish and shellfish allergies are similar to other IgE-mediated food allergy reactions, ranging from mild urticaria to life-threatening anaphylaxis. In the U.S. telephone survey discussed earlier, 55% of finfish reactions and 40% of shellfish reactions were severe enough that evaluation by a physician was sought.184 IgE-mediated reactions are generally rapid in onset, with allergic symptoms developing within minutes to an hour of exposure and most reactions occurring within 30 minutes.1,27,28,46,74 However, delayed onset of symptoms may occur (3 to 24 hours after exposure) and have been noted with, among other seafood, dogfish,136,168 cuttlefish,181 abalone,119 and limpets.136 In 25% to 30% of cases, a biphasic reaction occurs whereby the patient will appear to recover and then experience a late-phase reaction with a recrudescence of symptoms after an asymptomatic period of 1 to 72 hours.99,176
Symptoms of seafood allergy are often, but not always, related to the method of exposure and can occur after ingestion, cutaneous contact, and inhalation. Following ingestion of an offending seafood, the most commonly reported signs and symptoms include: generalized itching and urticaria; angioedema—particularly swelling of the lips and tongue; pulmonary manifestations including dyspnea, wheezing, and chest tightness; gastrointestinal complaints such as nausea, vomiting, diarrhea, and abdominal cramping; and shock.101,184 It is the direct contact of the allergenic food with the oral mucosa that causes pruritus and angioedema of the lips, tongue, throat, and palate—a constellation of symptoms known as oral allergy syndrome (OAS).36,53 In patients with underlying atopic disease, exposure to fish and shellfish allergens can cause exacerbations of eczema173 and, less commonly, asthma symptoms.85 Because ongoing exposure to a food allergen may cause chronic urticaria, the presence of an undiagnosed food allergy should be sought in patients with chronic urticaria.53
In general, ingestion of the allergic seafood leads to gastrointestinal symptoms, urticaria, and possible vascular compromise, whereas skin contact results in mainly dermatologic symptoms, and inhalational exposure typically causes respiratory symptoms. For example, there are documented cases of patients allergic to fish presenting with skin reactions after handling raw fish138,162 as well as symptoms of asthma in fish-allergic children after inhalation of aerosolized fish.166 However, this is by no means the rule, and systemic reactions after cutaneous and inhalational exposure may occur. In one case study, a 2-year-old fish-allergic child experienced facial urticaria and angioedema after her grandfather, who had eaten fish 2 hours earlier, kissed her.132 In another report, a shellfish-allergic patient experienced anaphylaxis after kissing her boyfriend who had recently ingested shrimp.189 In one survey, in 8.6% of fish and 10% of shellfish allergic individuals, more severe reactions occurred following inhalational or dermal exposure rather than ingestion. These allergic individuals were able to consume the offending antigen without significant sequelae.184
Vascular involvement is not uncommon in patients with seafood allergies. In one review of patients with seafood allergies, 8% of subjects with fish allergy and 13% of subjects with shrimp allergy developed anaphylactic shock after seafood challenge.101 Manifestations of vascular involvement may include hypotension, a subjective “sense of doom,” respiratory distress progressing to asphyxia, dysrhythmias, and myocardial infarction. Near-fatal and fatal reactions may start with only mild symptoms, such as OAS, before rapidly progressing to cardiovascular collapse. Risk factors for severe anaphylactic reactions are the presence of other atopic disease(s), inadvertent ingestion of the offending food, rapid onset of symptoms, failure to promptly treat with epinephrine, and history of prior anaphylaxis to the causative food.53
One form of anaphylaxis that occurs in the setting of seafood allergy is food-associated, exercise-induced anaphylaxis. Affected patients develop anaphylaxis if they exercise within 2 to 6 hours of ingesting an allergic food, but remain asymptomatic if the same food is ingested without exercise. Although the mechanism is poorly understood, shellfish and wheat flour are the most common causes of food-associated, exercise-induced anaphylaxis.17,216 Shrimp and mollusks are among the most commonly implicated seafoods.90,123
Occupational Seafood Allergy
Just as is the case on the consumer side, hypersensitivity reactions secondary to occupational exposure to fish and shellfish in the seafood processing industry are increasingly recognized. Rather than ingestion, most reactions are associated with direct contact or inhalational exposure during cutting, cleaning, cooking, or drying of seafood.100 Occupational reactions have been reported in a variety of seafood workers, including fishermen, seafood processing workers, canners, restaurant cooks, delivery persons, and other workers associated with the seafood industry.29,30,50,105,179 Occupational seafood allergy can manifest as rhinitis, conjunctivitis, asthma, urticaria, contact dermatitis, or OAS.3,87 Studies performed on snow crab workers demonstrated a 33% incidence of asthma, 24% incidence of skin rash, and 18% rate of rhinitis or conjunctivitis related to inhalational exposure or skin contact with snow crab meat or by-products.29 In a survey of occupational allergies in seafood workers in Australia and South Africa, skin reactions accounted for 78% to 81% of reported problems, followed by asthmatic symptoms (7% to 10%) and nonspecific allergic symptoms (9% to 15%).117 Although rare, vascular involvement related to occupational seafood exposure has been reported.179
In most studies, occupational asthma appears to be the most prominent clinical presentation of seafood allergy, with a reported prevalence of 7% to 36%.87 Seafood implicated in occupational asthma include all the major seafood groupings: oysters,143 clams,50 shrimp,50,105 prawns,62 fish,42,52 snow and king crabs,29,155 lobsters,105,159 sea squirts,91 abalone,39 powdered marine sponges,14 cuttlefish,198 and clam liver extract.93 In one case study, shark cartilage powder caused a fatal occupational asthma attack.158 Hypersensitivity pneumonitis may also result from occupational exposure to seafood allergens and has been documented secondary to mollusk shell dust inhalation.156,157 Clinical manifestations of hypersensitivity pneumonitis include dyspnea, fever, chills, cough, and malaise. With chronic low-level allergen exposure, fever and chills may be absent, with symptoms of exertional dyspnea, fatigue, and weight loss predominating.104
Dermatologic occupational seafood allergy has been less well studied but generally takes the forms of contact urticaria and a chronic recurrent dermatitis known as protein contact dermatitis (PCD).3,78,140 The estimated prevalence of occupational PCD ranges from 3% to 11%.87 The most frequent clinical presentation is chronic or recurrent eczema that may be limited to the fingertips or extend to the wrists and arms. Initial manifestations include itchy, erythematous, and vesicular lesions, which usually progress to chronic eczema, with episodic acute exacerbations after repeated contact with the culprit allergen.3,78 Some cases of chronic paronychia (after handling the allergic food) may also be a variant of PCD, with redness and swelling of the proximal nail fold.200 In some cases, percutaneous sensitization to seafood allergens may occur via direct skin contact in the workplace, as may occur with seafood packers or delivery persons. If ongoing exposure occurs, the individual may develop allergic symptoms and even anaphylaxis following ingestion of the offending seafood.179 Risk factors for sensitization and development of clinical allergy in seafood workers include the presence of atopy, as well as duration and intensity of exposure to the potential allergen.92,186
Differential Diagnosis
Many non-seafood products contain fish and shellfish, often unbeknownst to the consumer. For example, imitation crab meat is usually made of pollock or monkfish. Surimi, which is processed fish meat usually derived from Alaskan pollock in the United States, is commonly used for seafood-flavored snacks, sauces, flavors, “meatless” hot dogs, sausages, pepperoni sticks, imitation crab, and pizza toppings.210 Anchovies are a routine ingredient in Caesar salad dressing and Worcestershire sauce. Many pills and medications contain chitin, a component of the outer skeleton of crustacea and other arthropods. Additionally, many products, although not purposefully containing seafood products, may be contaminated with seafood because they are processed in a facility that also handles seafood. Although the allergenic potential of some of these products has not been well studied, it is important to consider them as a potential allergenic source in patients presenting with allergic symptoms. A thorough history may help to identify these accidental ingestions, especially in patients with a known seafood allergy who present with an allergic reaction of unknown etiology.
Apparent seafood allergies can also be due to seafood parasites, rather than to the particular fish or shellfish that is consumed. The parasite Anisakis simplex, for example, is gaining increasing recognition as a cause of allergic reactions in individuals after consuming parasitized seafood.9,10,94 A. simplex is a nematode that infects fish worldwide and can cause health issues in humans, either via transient infection after consuming raw or undercooked flesh of infected fish or via Anisakis allergy. The allergic reaction is a typical IgE-mediated reaction, presenting as acute urticaria, angioedema, or anaphylaxis following ingestion of infected fish.10 To date, nine different Anisakis allergens have been characterized.118 One of the responsible allergens, Ani s 3, is the invertebrate pan-allergen, tropomyosin, which is capable of cross-reacting with shellfish tropomyosins, adding to the diagnostic dilemma when faced with a patient with a potential seafood allergy.8,10,68,122,209 In one study, Anisakis allergy was demonstrated in 23 individuals who experienced allergic reactions following ingestion of seafood. All of the subjects had negative skin tests to the actual seafood consumed, and all of them demonstrated sensitization to Anisakis by either positive skin-prick test (SPT) or serum-specific IgE.133 In another study of patients with a history of food allergy, Kimura and colleagues found IgE-reactivity against A. simplex to be higher than that against any specific fish tested, suggesting that sensitization to Anisakis allergen is more common than sensitization to any fish allergens.96
Evidence also suggests that Anisakis allergy contributes to occupational respiratory and skin allergies in seafood workers. Armentia and colleagues found A. simplex to be the cause of occupational asthma in two seafood workers by in vitro and in vivo studies.4 In a case report by Scala and co-workers, Anisakis was found to be the allergen responsible for contact urticaria and inhalational asthma in a seafood factory worker.178 In one study looking at the prevalence of Anisakis sensitization and related symptoms in fish-processing factories, the prevalence of sensitization to Anisakis was found to be higher than that for the fish being processed and was associated with a higher risk of allergic reactions.148 These findings underscore the importance of considering Anisakis allergy in patients presenting with first-time allergic symptoms following consumption of seafood, especially if that seafood has been tolerated in the past. Unfortunately, studies suggest that ingestion of frozen or cooked seafood, which is recommended for anisakiasis prophylaxis, does not prevent IgE-mediated allergic reactions to Anisakis. Given the prevalence of parasitism of fish and shellfish by Anisakis, for patients diagnosed with Anisakis allergy, a seafood-free diet is recommended.133
A common mimicker of IgE-mediated seafood allergy is scombroid poisoning (see Chapter 72). Scombroid intoxication results from the ingestion of some dark-meat fish (tuna, salmon, marlin, mahi-mahi, bluefish, mackerel, and others) that contain high levels of free histamine produced by bacteria in the fish flesh during spoilage.31 Usually within 10 to 30 minutes of ingestion, the histamine produces symptoms that mimic IgE-mediated allergy, including perioral tingling and burning sensations, flushing, urticaria, gastrointestinal complaints, and possibly progressing to bronchospasm, tachycardia, and hypotension. Symptoms that suggest scombroid intoxication include headache, dizziness, perioral tingling and burning, as well as a history of consuming fish that tasted peppery or bitter.31
Other types of seafood poisoning (ciguatoxin, diarrheic shellfish poisoning, and others) may result in a variety of physical complaints, but these are usually clinically distinct from IgE-mediated allergic reactions. Similarly, seafood-associated illness may occur secondary to bacterial and viral etiologies, such as poisoning due to toxins (botulism, Staphylococcus) or gastroenteritis from bacterial or viral infection.31 These illnesses also tend to be clinically distinct from IgE-mediated reactions.
Diagnosis
A critical step in diagnosing seafood allergy, or any other food allergy, is obtaining a thorough and accurate history, including specific symptoms, food(s) ingested around the time of symptom onset, timing of the reaction, prior history of similar reactions, presence of known food allergies, and any exacerbating factors such as exercise. Patients should also be questioned about possible contaminants or hidden allergenic ingredients in ingested food(s), particularly if the inciting allergen is unknown. Although taking a good history is of utmost importance, research suggests that medical history alone is insufficient in diagnosing food allergy. In one study of children with a self-reported food allergy, the allergic reaction was reproducible in only 40% by double-blind, placebo-controlled food challenge.19
In patients with suspected seafood allergy, SPTs are a relatively safe, inexpensive, and useful screening tool. Commercial extracts are not available for every seafood species; therefore, mixed extracts are often used. Additionally, actual raw or cooked food itself can be used for skin testing. SPT may be contraindicated in patients with a history of severe anaphylactic reaction to the seafood being tested or in patients with significant skin disease. Given the fact that SPTs measure sensitization to a particular allergen, and sensitization is not equivalent to allergic disease, caution must be taken in interpreting SPT results. Multiple studies comparing SPTs with double-blind, placebo-controlled food challenges have found that a positive SPT does not always correlate with symptomatic fish allergy.18,19,73 Thus, SPTs have a high sensitivity and excellent negative predictive value, but a low specificity and poor positive predictive value.53 Specifically, patients with positive results on SPT may not necessarily have clinically significant allergic disease. However, a study using mean wheal diameters to predict positive food challenges with shrimp suggested that skin testing for seafood allergy may not be as problematic as was once thought.89 A mean wheal diameter of 30 mm (1.2 inches) after SPT provided an 80% and 95% predictive probability for positive food challenge in subjects with allergies to black tiger prawn and giant freshwater prawn, respectively. This study suggested that the predictive probability of SPT can be helpful in cases where food challenge cannot be performed.89
In vitro diagnostic methods, such as measurement of serum food-specific IgE by radioallergosorbent test, such as the Pharmacia CAP-RAST FEIA, can also be useful screening tools, particularly for patients in whom skin testing is contraindicated. RAST testing is fraught with the same diagnostic dilemmas as is skin testing, in that in vitro reactivity, like cutaneous sensitization, does not necessarily correlate with clinical allergy.131 Thus, many patients with positive RAST testing may not have allergic disease when exposed to the allergen in question.18,73 Studies by Sampson and colleagues, however, suggest that quantitative measurement of food-specific IgE antibodies may be a useful predictive tool in identifying patients with clinical reactivity.174,175 In one study, the CAP System FEIA was used to establish diagnostic levels of IgE—called “decision points”—that could predict clinical reactivity with greater than 95% certainty to a variety of allergic foods, including fish, egg, peanut, and milk. Diagnostic IgE levels were identified at 20 kU (A)/L or greater for fish allergy.175 The predictive value of using diagnostic IgE levels to substantiate clinical reactivity was confirmed in a prospective study in which greater than 95% of clinical food allergies, including fish allergy, were correctly identified using quantitative serum food-specific IgE concentrations.174 These findings suggest that the CAP-RAST FEIA may be a safe alternative to oral challenge in patients suspected of having IgE-mediated food allergy. Of note, the diagnostic levels of IgE for predicting crustacean allergy have not yet been determined.
Atopy patch tests (APTs) have also been evaluated as useful tools in the diagnosis of food allergy. In the classic patch test, the suspected allergen is applied to a piece of cloth or paper, which is then placed on intact skin and covered with an impermeable barrier for 24 to 48 hours. The patch is then removed and the skin examined.104 However, recent studies have found that APTs add little predictive value to the standard SPT and IgE measurements in the diagnostic workup of suspected food allergies and thus cannot be routinely recommended.126
The gold standard test in verifying a particular food allergen is the double-blind, placebo-controlled food challenge (DBPCFC).21 This should not be performed in persons who have experienced life-threatening reactions and should be undertaken only under close physician supervision. Dried or freeze-dried foods are encapsulated in opaque, dye-free capsules; alternatively, the food of interest can be hidden in a food vehicle. Appropriate identical placebo-controls are prepared. Although such testing is time consuming and labor intensive, it permits precise diagnosis.
Although the methods described previously are useful in diagnosing food allergies, diagnosis of occupational allergies often requires a different approach, especially in the case of occupational asthma due to inhalation of a seafood allergen. If the allergic individual notes the onset of asthma symptoms related to work exposure, and there is improvement during weekends or vacation, occupational asthma should be suspected. Asthma is verified by appropriate pulmonary function tests, such as spirometry with and without bronchodilators. If the history of asthma is suspected but not corroborated by physical examination or spirometry, it may be necessary to perform a provocation test with inhaled methacholine or histamine to document airway hyperreactivity. The diagnosis depends ultimately on the provocation of symptoms by a bronchial inhalation challenge with the suspected allergen to simulate industrial exposure.104 Such evaluation can be performed at the workplace or in a controlled laboratory environment. If a workplace challenge is performed, the subject’s lung function is monitored during the workday with the idea that lung function will decline during the work period because of workplace exposure to the offending allergen. Laboratory challenge is the diagnostic method of choice for diagnosis of occupational asthma, as it allows for identification of a specific etiologic agent (unlike a workplace challenge, where many different allergens may confound the test).104 Because of inherent dangers of exposing an allergic individual to high doses of allergen, laboratory challenge should occur under close observation in a hospital setting.
Occupational allergic contact dermatitis may also require a specialized approach. Approximately 90% of occupational dermatitis involves the hands, usually the palm and back of the wrist104; therefore, dermatitis in a different distribution should raise doubt about the diagnosis. Additionally, location of the dermatitis and location of exposure to the allergen must be matched. Although routine atopy patch testing is not recommended as part of the diagnostic workup of food allergy, in the case of suspected occupational skin disease, patch testing may be useful in demonstrating allergic contact dermatitis to a suspected allergen.104
Aftercare
Avoidance is the only treatment for seafood allergy. Because the allergens present in fish and shellfish are molecularly different (see Molecular Biology, later), patients with an allergy to fish generally do not need to avoid shellfish, and vice versa. Allergists generally recommend removing all edible fish from the diet when the patient has a demonstrated history of allergic reaction to any fish and/or if there is a positive skin test or RAST test to a fish extract. In patients with a history of severe anaphylactic reaction or allergic reactions to many types of seafood, avoidance of all fish and shellfish may be the safest strategy. However, in patients with a history of less severe allergic reaction to a particular seafood, research suggests that selective avoidance diets may be reasonable. Studies using double-blind, placebo-controlled fish challenges18,73 and other tests48 in fish-allergic children have shown that individuals are not uniformly sensitive to all fish species; hence, sensitivity to one species does not automatically warrant dietary elimination of all seafood. Studies of fish challenges are often negative in children with negative skin tests.18 Therefore, it seems reasonable to recommend dietary elimination of any seafood species for which there has been a demonstrated allergic reaction or a positive SPT or in vitro test. If a patient tests positive by SPT or in vitro testing to a particular seafood item, but has no history of clinical allergy to that seafood species, DBPCFC can be performed to determine whether true clinical allergy exists. Given the high negative predictive value of SPT, seafood species for which patients have no history of allergic reaction and have tested negative by SPT could be permitted in the diet after oral challenge. In the setting of a newly diagnosed seafood allergy to a particular fish or shellfish species, it is reasonable to allow patients to consume other seafood items that have not caused allergic symptoms before and that they have consumed previously.
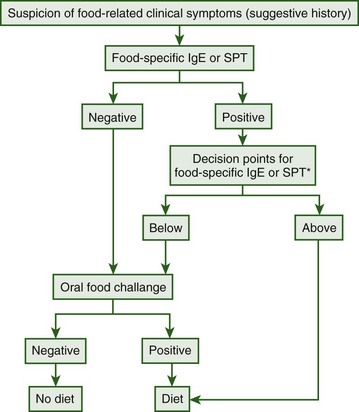
Niggemann and colleagues have proposed an algorithmic approach to diagnosing food allergies, including seafood allergies (Figure 73-2).149 In this algorithm, all patients with a suspected food allergy should undergo food-specific IgE or SPT. If negative, oral challenge can be conducted. If initial IgE or SPT is positive, previously established food-specific IgE or SPT “decision points” should be evaluated. If the patient’s quantitative IgE or SPT wheal diameter is above the previously established decision point, the food should be eliminated from the diet, but if it falls below the decision point, oral challenge can be conducted.149
Education is also crucial following an allergic reaction to seafood. Patients should be counseled to read all food labels for the possibility of hidden or unexpected allergenic ingredients as well as potential allergic contaminants. Since the passage of the United States Food Allergen Labeling and Consumer Protection Act of 2004, food labels have been required to clearly state the presence of eight specified food allergens, including fish and crustacean shellfish. Importantly, mollusks are not included in this labeling mandate.61 Patients should also be informed about the potential for allergic reaction after aerosolization of the offending allergen, such as may occur during cooking of seafood, either at home or in fish markets. Finally, patients should be cautioned about the potential for exposure to seafood allergens via inadvertent cross-contact, such as in restaurants where equipment is shared for seafood and nonseafood cooking, or during contact with contaminated saliva with kissing or utensil sharing.132,189
Molecular Biology of Seafood Allergies
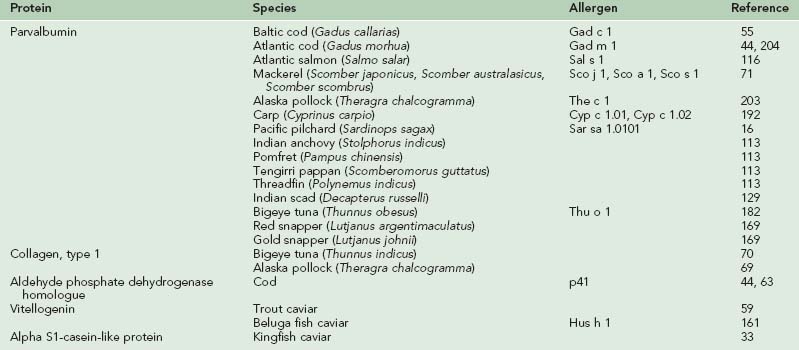
The major allergens responsible for IgE-mediated allergic reactions due to fish and shellfish are the parvalbumin and tropomyosin proteins, respectively.47,54,55,111,180 Since the original characterization of these allergens in codfish and shrimp models, researchers have continued to characterize these proteins and confirm their allergenicity in a wide variety of species of fish and shellfish, as well as identify new classes of proteins also implicated in the development of seafood allergies. Tables 73-5 and 73-6 list the allergens characterized in fish and shellfish to date.
Fish Allergens
Of the seafood allergens that have been isolated and purified, the best characterized is the major allergen of the codfish, Gad c 1, which belongs to the group of muscle tissue proteins, parvalbumins, and was first identified in the Baltic cod (Gadus callarias).55 Parvalbumins are small (12-kD) calcium-binding proteins responsible for mediating the concentration of calcium in white muscle of lower vertebrates and skeletal muscle of higher vertebrates. Parvalbumins exist in two different isoforms, alpha and beta, and in fish, parvalbumin beta appears to be a cross-reactive pan-allergen. Parvalbumins are resistant to heat and enzymatic degradation,210 making them ideally suited food allergens capable of withstanding extreme temperatures during cooking and proteolytic breakdown in the digestive tract.
Since the original characterization of parvalbumin in codfish, parvalbumins have been identified as allergens in numerous other fish species. Lindstrom and co-workers identified a parvalbumin, designated Sal s 1, as the major allergen in Atlantic salmon (Salmo salar).116 A second cod parvalbumin, Gad m 1, has been characterized in the Atlantic cod (Gadus morhua) and was found to have greater homology with Sal s 1 than with Gad c 1 (75% with Sal s 1 compared to 62.3% with Gad c 1).45,204 Parvalbumin antigens have also recently been identified as major allergens in three species of mackerel (Sco j 1, Sco a 1, Sco s 1), carp (Cyp c 1.01, Cyp c 1.02), Alaska pollock (The c 1), pilchard (Sar sa 1.0101), threadfin, Indian anchovy, pomfret, tengirri, and Indian scad.* Studies performed on red and golden snapper revealed a 51-kD protein as a major allergen that is hypothesized to be a parvalbumin tetramer.169 Interestingly, the 12-kD protein isolate believed to be fish parvalbumin was only found to be a minor allergen in both species of snapper.169 Studies on tuna have produced inconclusive results. Bugajska-Schretter and associates demonstrated IgE reactivity to tuna parvalbumins in sera from fish-allergic patients,24 and another study reported the identification of parvalbumin as a major allergen in bigeye tuna (Thu o 1).182 Other studies have failed to detect allergenicity to tuna parvalbumins, suggesting that tuna fish allergy may be caused by an allergen other than parvalbumin.202,214
In addition to parvalbumin proteins, other antigens are also emerging as major fish allergens. A second codfish allergen, p41, has been identified that is a 41-kD IgE-reactive protein homologous to an aldehyde phosphate dehydrogenase (APDH).44,63 The purified p41 protein binds specifically to reaginic IgE from cod-allergic individuals. The p41 protein was also found to bind to monoclonal antibodies specific for the first calcium-binding site of parvalbumins, suggesting that p41 may have a calcium-binding site corresponding to an IgE epitope similar to that of Gad c 1.63
Type 1 collagen, a component of muscle and skin in several fish species, was also recently identified as a potential major allergen. Hamada and colleagues identified a high-molecular-weight allergen recognized by one fish-allergic serum sample in surimi made from walleye pollock.69 IgE immunoblotting and amino acid analysis identified the allergen as collagen. In another study, Sakaguchi and co-workers demonstrated IgE antibodies to fish gelatin (type 1 collagen) in fish-allergic children.172 In a study by Hamada and colleagues, five of eight serum samples obtained from fish-allergic individuals reacted to bigeye tuna collagen.70 Furthermore, studies of allergens in red and golden snapper revealed a heat-stable high-molecular-weight protein believed to be collagen as a minor allergen in both snapper species.169
Fish allergy may also result from allergy to protamine sulfate, which is a protein found in the sperm of salmon, trout, herring, and other species belonging to the families Salmonidae and Clupeidae. Protamine sulfate is a low-molecular-weight protein used as a heparin antagonist. Because of case reports of fish-allergic patients experiencing anaphylaxis after administration of protamine sulfate, extreme caution or use of alternative therapies in fish-allergic patients is advised by some experts.40,97,163
Allergic reactions, including anaphylaxis, have also been reported after ingestion of fish roe and caviar. In several case reports, the allergic individuals experienced a reaction after eating Beluga caviar, trout roe, or whitefish roe but had no allergy to fish or other types of roe.59,120,161 In another case, a woman experienced an allergic reaction after consuming rainbow trout roe, and serum analysis demonstrated cross-reactivity with other types of fish roe.120 A 118-kD protein, Hus h 1, has been identified as the culprit allergen in Beluga caviar allergy.161 This is the hormone, vitellogenin, found in fish eggs, and has also been proposed as the causative allergen in the circumstance of trout roe allergies.59,161 A 33-kD alpha S1-casein-like allergen—a well-known major allergen in cow’s milk—was identified as the culprit allergen in a subject who experienced anaphylactic shock after consuming kingfish caviar.33 Although sea urchins fall into a different phylum from fish, it is worth mentioning sea urchin roe allergy, as several case studies have reported anaphylactic reactions following consumption of sea urchin roe.75,167
Crustacean Allergens
As discussed previously, the crustacea family includes shrimp, prawns, crabs, lobsters, crayfish, krill, and barnacles and is a common reported cause of food allergy. The major allergen in crustacea has been identified as tropomyosin, an essential protein for muscle contraction found in vertebrates and invertebrates. Tropomyosin was originally identified as the major allergen in shrimp,47,111,180 and subsequent studies have identified tropomyosin as the major allergen in other crustacean species, as well as in mollusk species. Although tropomyosins are major allergens in shellfish, and also in arachnids (mites) and insects (cockroaches, midges), the tropomyosins of vertebrates such as cattle and chicken are considered nonallergenic, possibly because of their greater susceptibility to breakdown by digestive enzymes compared to shellfish, arachnid, and insect tropomyosins.127 Invertebrate tropomyosins share high (up to 100%) amino acid sequence homology with different invertebrates and much lesser (50% to 60%) homology with vertebrate tropomyosins, supporting their role as the pan-allergen responsible for cross-reactivity across crustacea, insects, arachnids, and mollusks. This also helps explain the lack of allergenicity of vertebrate tropomyosins (see Cross-Reactivity section).12,67,109
Convincing evidence for the role of tropomyosin as a major shrimp allergen originated with studies by Daul and co-workers.47 Using the brown shrimp (Penaeus aztecus) model, a 36-kD tropomyosin protein named Pen a 1 was identified and shown to react with the sera of 82% of shrimp-allergic individuals.47 Similar tropomyosins were also identified as the major allergens in other shrimp species, including Pen i 1 in Indian white shrimp (Penaeus indicus), Met e 1 in sand shrimp (Metapenaeus ensis), and Par f 1 in Neptune rose shrimp (Parapenaeus fissurus).111,115,180 Immunoblotting analyses revealed two allergens in white shrimp (Penaeus setifecus) that both demonstrated reactivity with greater than 84% of shrimp-allergic individuals. The first, a 36-kD protein designated Pen s Bd36k, was hypothesized to be tropomyosin, and the second, a 29-kD protein named Pen s Bd29k, remains unidentified.134 Studies of other crustacea by Leung and colleagues have identified tropomyosin as the major allergen in American and Chinese spiny lobsters (Homarus americanus and Panulirus stimpsoni), designated Hom a 1 and Pan s 1, respectively, and Red crab (Charybdis feriatus), named Cha f 1.107,108 Two major IgE-binding proteins of 35- to 37-kD and 97-kD were demonstrated in extracts of lobster in pooled sera from subjects with respiratory symptoms to Norwegian lobster (Nephrops norvegicus).211 The 35- to 37-kD allergen likely represented tropomyosin, but only made up 0.02% to 1% of the total protein. The 97-kD allergen made up 7% to 15% of the total protein, suggesting the presence of another major allergen in addition to tropomyosin in Norwegian lobster.
Tropomyosin proteins have also been identified as the major allergens in two species of krill, designated Eup s 1 in Euphausia suerba and Eup p 1 in Euphausia pacifica, with the krill tropomyosins showing high IgE-binding epitope sequence homology to shrimp tropomyosin Pen a 1.142 Tropomyosins were found to be the major allergens in gammaridean and caprellid amphipods.137 Amphipods can be accidentally collected with seaweed during seaweed harvest and therefore become part of nori (dried laver) sheets used in sushi-making and as condiments in other foods, raising concerns about the safety of nori sheets in individuals with shellfish allergies. Finally, tropomyosins have been identified as major allergens in two species of barnacle, Bal r 1 in the Acorn barnacle (Balanus rostratus) and Cap m 1 in the Goose barnacle (Capitulum mitella).190 Interestingly, these tropomyosins shared higher sequence identity with mollusk tropomyosins compared to other crustacean tropomyosins, suggesting that the barnacle tropomyosin is evolutionally more closely related to the molluscan tropomyosin family.190
In addition to tropomyosins, other major allergens have been identified and characterized in crustacea, particularly shrimp. A 356–amino acid protein designated Pen m 2 has been found in tiger shrimp (Penaeus monodon). This protein showed homology to arginine kinase from other crustacea and was found to react with serum IgE from shrimp-allergic individuals.215 A similar 40-kD protein was isolated from white pacific shrimp (Litopenaeus vannamei) and identified as arginine kinase.64 Designated Lit v 2, this new protein was recognized by IgE in serum from shrimp-allergic individuals and had a 96% identity to Pen m 2.64 Recently, another new shrimp allergen, a myosin light chain protein in white pacific shrimp named Lit v 3 has been identified.11 Lit v 3 demonstrated IgE binding in 55% of white pacific shrimp-allergic individuals.11 In another recent study, a new 20-kD allergen was purified from the abdominal muscle of black tiger shrimp and identified as a sarcoplasmic calcium-binding protein (SCP).183 Of sera from 16 crustacea-allergic individuals, eight reacted to SCP, whereas 13 reacted to tropomyosin, supporting SCP as a new crustacean allergen.183
Mollusk Allergens
Molluscan shellfish allergy has been described in the medical literature to nearly all of the commonly consumed types of mollusks, including terrestrial and marine snails, whelk, limpet, and abalone among the gastropods; oyster, clam, scallop, mussel, and cockle among the bivalves; and squid, octopus, and cuttlefish among the cephalopods. Tropomyosins appear to be the major allergens in mollusks, and specific tropomyosin allergens have been characterized in all classes of mollusks.195 However, amino acid sequence homology to crustacean tropomyosins ranges from 56% to 66%, which is only slightly higher than compared with vertebrate tropomyosins, suggesting that other allergens beside tropomyosins may be responsible for allergic reactions to molluscan shellfish.195
In the cephalopod class, a tropomyosin, named Tod p 1, was found to be the major allergen in the Pacific flying squid (Todarodes pacificus).130 In studies on the common octopus (Octopus vulgaris), the tropomyosin protein, Oct v 1, was designated as the major octopus allergen, including identification of several IgE-binding epitopes with sequence similarities to IgE-binding epitopes of other molluscan shellfish and crustacea.84 Amino acid sequence analysis demonstrates 64% homology between Oct v 1 and shrimp tropomyosin Pen a 1, and 63% homology between Tod p 1 and Pen a 1.195
In the bivalve class of mollusks, tropomyosin allergens have been characterized in the Pacific oyster (Cassostrea gigas), razor clam (Ensis macha), mussel (Perna viridis), and scallop (Chlamys nobilis).37,83,88 Cra g 1 and Cra g 2 were isolated from oyster, with Cra g 1 having 76% sequence homology with mussel tropomyosin, 74% with abalone tropomyosin, and 58% with M. ensis (shrimp) tropomyosin.83 Studies on razor clam allergens isolated three major allergens between 30 and 45 kD in size that demonstrated IgE-binding with serum from a razor clam-allergic patient. One allergen, designated Ens m 1, is likely clam tropomyosin, while the others remain unidentified.88 Other studies identified tropomyosin as the major allergen in the scallop, designated Chl n 1, and the mussel, named Per v 1, and confirmed their reactivity to IgE-antibodies from shellfish-allergic subjects.37
Among the gastropods, tropomyosins have been demonstrated to be major allergens in abalone (Hal m 2, Hal d 1, Hal r 1), turban shell (Tur c 1), common whelk (Buc u 1), and fan shell (Pin a 1).* There are at least two major allergens in the abalone, Haliotis midae.119 The first, a 38-kD IgE-binding protein, designated Hal m 2, is likely tropomyosin. The second, a unique 49-kD major IgE-binding protein, named Hal m 1, remains to be identified.119 Another study has identified tropomyosin as the major allergen of Turbo cornutus, a horned turban mollusk and a popular food item in Japan that has been implicated in a case of exercise-induced anaphylaxis.90 The major allergen, named Tur c 1, was found to be 35 kD in size and identified as tropomyosin, but it was found to have an IgE-binding epitope dissimilar to those in oyster and shrimp tropomyosins.82 Studies identifying the major allergens in common whelk revealed three IgE-binding proteins. One, with a molecular weight of 40 kD, was presumed to be tropomyosin (Buc u 1), whereas the other two, with molecular weights of 71 and 82 kD, remain unknown.98 A study of snail tropomyosin found that brown garden snail (Helix aspersa) tropomyosin, named Hel as 1, shared high homology with other edible mollusk tropomyosins (69% to 84% identity). However, tropomyosin reacted with only 18% of the sera from snail-allergic patients, suggesting that tropomyosin may be only a minor allergen in snails.7
In addition to tropomyosins, many studies have identified nontropomyosin allergens in numerous mollusk species, including snails, whelk, pen shell, fan shell, abalone, and limpet in the gastropod family; oyster, scallop, and razor clam in the bivalves; and squid, octopus, and cuttlefish in the cephalopod family.195 Most of these nontropomyosin allergens remain to be identified, although research suggests that some of them may be hemocyanin, myosin heavy chain, and amylase.195
Cross-Reactivity
Cross-reactivity may be defined as “the recognition of distinct antigens by the same IgE antibody, demonstrable by in vivo and in vitro tests, which clinically manifests as reactions caused by antigens that are homologous to different species.”210 Individuals may also demonstrate sensitization by positive allergy testing to multiple species of fish and/or shellfish without demonstrating overt symptoms after consumption of that particular seafood, although the clinical significance of this observation is unclear. As discussed previously, the major allergens responsible for allergies due to fish and shellfish are parvalbumins and tropomyosins, respectively. It is the homology of the epitopes of these proteins across different types of seafood that is thought to produce cross-reactivity.
When looking at cross-reactivity within the class of bony fish, it is estimated that approximately 50% of individuals allergic to a particular fish species will be allergic to another fish species.199 Among crustacea, cross-reactivity appears to be even higher, with approximately 75% of individuals allergic to a crustacean species being allergic to another type of crustacea, likely because of the greater degree of similarity among tropomyosins compared to parvalbumins.199 Both clinical and serologic cross-reactivity among fish and shellfish have been well documented in the literature. However, some studies have produced conflicting results, suggesting that the mechanisms of cross-reactivity and the responsible allergens have not been completely elucidated. Furthermore, most studies have only looked at in vitro and serologic cross-reactivity, with few studies testing for actual clinical cross-reactivity.
Although some degree of cross-reactivity is common, species-specific allergies also occur and have been reported to sole, swordfish, tuna, and shrimp.5,86,95,135 In studies on monospecific fish allergies, subjects with allergies to multiple fish species showed IgE binding to 12- to 13-kD bands (parvalbumins), whereas the monosensitive subjects showed IgE binding to unique bands at 40 kD in tuna,86 25 kD in swordfish,95 and 6 to 7 kD and 40 kD in tropical sole.5 Such monospecific reactions are thought to be secondary to IgE antibodies to minor, species-specific antigens rather than to the major allergenic proteins, parvalbumins, and tropomyosins.
It is also noteworthy that because different antigens are responsible for causing allergic reactions to fish and shellfish, cross-reactivity between fish and shellfish does not occur. Allergy to both fish and shellfish in a single individual may occur, but it is not due to cross-reactivity. Nonetheless, in the American telephone survey previously discussed, it was estimated that 10% of individuals with a seafood allergy have an allergy to both fish and shellfish,184 perhaps reflecting an atopic predisposition in this population.
Fish Cross-Reactivity
Both serologic and clinical cross-reactivity across different fish species has been demonstrated and is hypothesized to be secondary to the major fish allergen parvalbumin. It has been noted that in adults with clinical sensitivity to cod, positive skin prick reactions were reported to mackerel, herring, and plaice, and that sera from the same individuals also demonstrated IgE binding to a protein in the 11- to 14-kD region of mackerel, herring, and plaice extracts, likely representing parvalbumin.72 Additionally, mackerel, herring, and plaice inhibited codfish RAST, demonstrating at least the presence of serologic cross-reactivity to different fish species.72 Cross-reactivity among IgE epitopes for six different fish species, including cod, tuna, salmon, perch, carp, and eel, was demonstrated by IgE-immunoblot inhibition experiments.24 In another study, when sera from fish-allergic individuals were incubated with recombinant carp parvalbumin, IgE-reactivity to cod, tuna, and salmon was lost, suggesting the presence of common epitopes across these fish species.193 In a study of children with codfish allergy, skin testing was most frequently positive with eel (85%), bass, dentex, sole, and tuna (55%), whereas it was least frequently seen with dogfish (10%).48 Again, this suggests the presence of common epitopes, but it also supports the presence of significant variation within these common epitopes. More recently, cross-reactivity across nine commonly consumed fish in Norway was studied, and it was found that cod, salmon, pollock, herring, and wolffish had the most potent cross-reactive allergens, whereas halibut, tuna, flounder, and mackerel were the least allergenic, suggesting that cross-reactivity among IgE epitopes is highest in the setting of close phylogenetic relationships between fish species.202
Other studies have demonstrated similar variable cross-reactivity among different fish species. In one case study, a 4-year-old boy experienced anaphylactic reactions on contact with many different types of fish, including cod, tuna, salmon, trout, and eel, among others.114 In other studies of individuals with fish allergy confirmed by skin test and RAST reactivity, the majority of subjects reacted to only one type of fish, whereas a much smaller proportion of individuals reacted to two or more species of fish on oral challenge.18,73 These studies demonstrated that while clinically significant cross-reactivity exists, it varies across allergic individuals, and that sensitization as indicated by positive allergy testing cannot always predict clinically significant allergic reactions. Similarly, up to 40% of patients sensitized to fish (positive allergy testing) do not present with symptoms on consumption of other fish species,199 supporting the observation that subclinical sensitization is not always predictive of clinical hypersensitivity.
As a whole, the fish parvalbumins share amino acid homologies ranging from 60% to 80%, which both supports the role of parvalbumin as a major fish allergen and helps to account for the variable clinical cross-reactivity seen in fish-allergic patients.118 Variable clinical cross-reactivity and monospecific fish allergies can also be explained by the presence of non-parvalbumin and species-specific fish allergens. Thus, it seems that parvalbumin is a pan-allergen in most or all fish species, whereas some species contain additional species-specific allergens.196
Cross-reactivity with nonfish parvalbumins may also exist. One case report documented a patient who experienced anaphylaxis following consumption of frog legs, with subsequent protein microsequencing implicating the alpha isoform of frog parvalbumin as the causative allergen.76 Subsequent studies have demonstrated in vitro cross-reactivity between frog and fish beta-parvalbumins, suggesting that parvalbumins may be a new family of cross-reactive allergens.77
Shellfish Cross-Reactivity
Cross-reactivity among shellfish is even more extensive compared to fish cross-reactivity and is due to the pan-allergen tropomyosin, which has significant sequence homology throughout crustacea and mollusks, as well as in other invertebrates such as arachnids and insects.165 In the American telephone survey discussed previously, 38% of individuals reported an allergy to more than one type of crustacea, 49% had an allergy to more than one type of mollusk, and 14% reported an allergy to both crustacea and mollusks.184 Studies have demonstrated marked homology between shrimp, crab, and lobster tropomyosins, as well as likely cross-reactivity between shrimp and crab, and shrimp and lobster as evidenced by IgE inhibition assays.107,108 In addition, studies on krill have used immunoblotting to demonstrate in vitro cross-reactivity between krill, shrimp, lobster, and crab tropomyosin.142 Another study found that 81% of atopic shrimp-allergic individuals demonstrated cross-reactivity to crab, crayfish, and lobster by SPT.46 Similarly, cross-reactivity has been demonstrated among shrimp, crab, crayfish, and lobster by positive skin testing.207
Studies of cross-reactivity among mollusks have demonstrated cross-reactivity among abalone, snail, white mussel, black mussel, oyster, and squid using laboratory analysis and SPT.119 Laboratory methods have also been used to demonstrate cross-reactivity of abalone, scallop, and mussel tropomyosins.37 These studies establish subclinical cross-reactivity, but as oral challenges were not performed, the clinical significance of these observations remains to be investigated.
In addition to cross-reactivity within the crustacea and mollusk phyla, cross-reactivity between crustacea and mollusks have also been widely reported in the literature. In one study, inhibition experiments were used to demonstrate cross-reactivity between oyster and crustacea and between squid and shrimp.27,103 One group of researchers was able to demonstrate in vitro cross-reactivity between squid and shrimp tropomyosin allergens, but not between squid and octopus or squid and other mollusks.130 In a study of patients with a history of shrimp anaphylaxis, 100% of patient’s sera reacted with tropomyosins from 13 different crustacea and mollusks, although again, because oral challenges were not conducted, the clinical importance is uncertain.109
Shellfish cross-reactivity has also been reported in circumstances of occupational seafood allergy and food-dependent, exercise-induced anaphylaxis. In one case, a seafood restaurant worker presented with occupational asthma and urticaria after contact with shrimp and scallops, with laboratory analysis confirming the cross-reactivity between shrimp and scallops.66 In another case report, a 14-year-old girl with a recurrent history of oral swelling and discomfort after ingesting shrimp, crab, squid, and octopus presented with similar symptoms after scallop ingestion followed by intensive exercise.216 Laboratory investigation demonstrated that her serum IgE reacted to multiple types of crustacean and mollusk tropomyosins, with the level of IgE-reactivity and species-specific IgE scores correlating directly with the degree of sequence homology between each seafood tropomyosin and shrimp tropomyosin. In the case of scallops, the patient’s scallop-specific IgE score was not as high as for shrimp and other shellfish, consistent with the lesser homology in the amino acid sequence of scallop tropomyosin with shrimp tropomyosin and consistent with the observation that other immunologic mechanisms, specifically food-dependent, exercise-induced anaphylaxis, was necessary for clinical reactivity.216
Shellfish Cross-Reactivity with Insects and Arachnids
Invertebrate tropomyosin is also found in nonmarine allergenic organisms, including cockroaches, dust mites, and other insects and arachnids, and has been demonstrated to be a major allergen in dust mites and cockroaches via inhalational exposure.2,6,177 Between shrimp and fruitfly, shrimp and cockroach, and shrimp and house-dust mite, tropomyosin sequence identities share 87%, 90%, and 89% homologies, respectively, supporting the role of tropomyosin as an invertebrate pan-allergen.165 A growing body of evidence suggests that this highly conserved tropomyosin protein is responsible for causing cross-reactivity between shellfish and nonedible arthropoda and insects.43,58,109,122,212 For example, inhibition experiments demonstrated cross-reactivity between shrimp and nonbiting midges (chironomids).58 In other studies, cross-reactivity was demonstrated between crustacean, chironomid, and cockroach tropomyosins.212 Immunoblotting and inhibition studies have demonstrated in vitro cross-reactivity between Atlantic shrimp and German cockroaches.43 Sera from shrimp-allergic subjects demonstrated IgE reactivity against grasshopper, cockroach, and fruitfly tropomyosins.109 In another study, tropomyosin IgE from shrimp-allergic individuals demonstrated cross-reactivity to mite, cockroach, and lobster tropomyosins.12 In a study of five patients with barnacle allergy, two patients demonstrated in vitro cross-reactivity to house-dust mites, although the responsible cross-reactive allergen was not identified.121
Skin prick studies have also demonstrated cross-reactivity between shellfish and other arachnids and insects. For example, there are significant correlations between positive SPT with chironomid extract and various crustacea.58 In a study of patients attending an allergy clinic in Hong Kong, Wu and colleagues found that 90% of patients with shellfish allergy demonstrated house-dust mite cross-reactivity by SPT.213 In one unique study, Orthodox Jews with dust mite/cockroach hypersensitivity were found to have positive SPTs and IgE reactivity to shrimp. Because they had never been exposed to shellfish due to religious dietary prohibitions, it is hypothesized that sensitization to shrimp tropomyosin occurred via cross-reactivity to house-dust mite or cockroach tropomyosin.60
The previously discussed studies support the presence of in vitro and serologic cross-reactivity between shellfish and nonmarine allergenic organisms. Accumulating studies suggest that this cross-reactivity also has important clinical implications. A series of individuals developed both laboratory and clinical evidence of shrimp allergy over the course of immunotherapy for house-dust mite allergy, suggesting that dust mite allergen served as the sensitizing agent in causing the shrimp allergy.206 In another study, in a series of patients with asthma induced by snail consumption, house-dust mite sensitization was likely the causal event, although tropomyosin was thought to play only a minor role as a cross-reactive allergen.205 Clinical cross-reactivity was also demonstrated in a study in which asthmatic subjects sensitized to house-dust mite showed laboratory and clinical allergy to limpets.13
Future Directions
The current standard of care for managing seafood allergies is avoidance diets and provision of a self-injectable epinephrine device. However, much research is underway to develop new strategies for treating and preventing seafood allergies. Some of the therapeutic modalities currently under investigation include sublingual and oral immunotherapy, anti-IgE therapy, peptide immunotherapy, traditional Chinese medicine, DNA immunization, and development of hypoallergenic seafood for human consumption.26,56
Although traditional allergen-specific immunotherapy (SIT) was discovered nearly a century ago and has been used successfully in the treatment of peanut allergy, it is currently not recommended because of unacceptably high incidence of dangerous systemic allergic reactions during the treatment course.147,154 Additionally, there appears to be a potential for developing hypersensitivity to cross-reacting food allergens, such as shrimp, as described in subjects undergoing house-dust mite immunotherapy.206 Given the high incidence of adverse reactions using traditional immunotherapy, alternatives are currently under investigation and promising new methods are being developed. For example, sublingual immunotherapy (SLIT), originally developed to treat allergic rhinoconjunctivitis and asthma, was used successfully and safely to treat hazelnut food allergy in hazelnut-allergic patients.57 Along similar lines, studies looking at the efficacy of specific oral tolerance induction (SOTI) or oral immunotherapy (OIT) in inducing desensitization to food allergens have yielded some promising results, although the long-term effects of such therapy have not been rigorously investigated.23,26,125,150,160 In one study including patients with fish allergy, a standardized OIT protocol induced desensitization, as evidenced by conversion from skin test positive to skin test negative, following treatment in 78% of subjects who completed the OIT protocol.160
Another promising modality currently in clinical trials is recombinant humanized monoclonal anti-IgE antibodies. These IgG antibodies directed against the IgE molecule bind to freely circulating IgE, creating antigen–antibody complexes that are then cleared from the circulation. Use of anti-IgE appears to decrease levels of circulating free IgE, inhibit early- and late-phase responses to allergens, suppress inflammation, and improve the control of allergic diseases.56,128 Although not yet tested for use in seafood allergies, a recent clinical trial using anti-IgE in the treatment of peanut allergy found that a significant number of patients had a significant decrease in clinical symptoms in response to peanut challenge following treatment.106
Peptide immunotherapy is another novel therapy currently under investigation that uses peptide fragments containing reactive epitopes rather than the complete protein allergen, with the idea that these peptide fragments are immunogenic but are theoretically unable to cross-link IgE molecules, activate mast cells, and cause clinical allergic symptoms.22,26 Use of these peptide fragments for immunotherapy would thus render T cells unresponsive to subsequent allergen exposure without causing dangerous systemic allergic reactions during the course of therapy. Thus far, clinical studies using peptide immunotherapy for bee venom sensitivity and cat allergy have demonstrated promising results, with subjects experiencing a significant decrease in allergic symptoms after allergen exposure following therapy.22,139,151,153,194 Although peptide immunotherapy for food allergy has not yet reached clinical trials, studies underway using peanut allergen peptides suggest that peptide immunotherapy may have a future role in the treatment of food allergies, including seafood allergy.65,79
Traditional Chinese medicine and the use of herbal remedies have recently gained attention as potential modalities for treating allergic diseases, including food allergies. In studies on peanut allergy using murine models, the Food Allergy Herbal Formula-1 (FAHF-1) and the simplified FAHF-2 significantly reduced IgE levels and blocked anaphylactic reactions to peanut for up to 5 weeks following therapy.112,188 Although Chinese herbal remedies hold some promise, only studies with murine models have been conducted and the active ingredients and mechanism of action of these remedies remains to be delineated.
Another new approach in the treatment of food allergy is DNA immunization.187 With this strategy, a plasmid DNA (pDNA) vector encoding a specific food allergenic protein would be injected subcutaneously or delivered orally. The pDNA sequence would then be taken up by APCs, the DNA would be transcribed and translated, and the allergenic protein would then be presented on the surface of the APC as an endogenously produced protein. This endogenous protein would induce a Th1 response (rather than Th2 as occurs in allergic disease) with suppression of allergen-specific IgE production, thus producing desensitization to the specific food allergen.26,171 Although promising, allergen DNA immunization is likely years away from practical use.
Genetic alteration of epitopes on food allergens to suppress their allergenicity is also currently under investigation as a means of producing safer allergens for immunotherapy. Hypoallergenic foods could also be developed for consumption by individuals with food allergies. For example, studies on shrimp tropomyosin (Pen a 1) have demonstrated that substitution of critical amino acids in Pen a 1 epitopes results in significant reduction of IgE binding while still preserving immunogenicity.102 Such a mutated molecule could be used safely and effectively for immunotherapy without the risk of allergic reaction during treatment, or the mutant could be incorporated into the genome to create a hypoallergenic organism.102 A recombinant hypoallergenic carp parvalbumin mutant has been constructed that has 95% reduced IgE reactivity and diminished allergenicity as demonstrated by in vitro assays and in vivo SPT, but has preserved immunogenicity, making it a candidate for immunotherapy.191 Studies using genetic transformation technology to modify the allergic structure in shrimp are also underway,36,164 with the eventual goal of producing nonallergenic transgenic seafood that is safe for consumption by individuals with seafood allergy.
1 Aas K. Studies of hypersensitivity to fish: A clinical study. Int Arch Appl Imunol. 1966;29:346.
2 Aki T, Kodama T, Fujikawa A, et al. Immunochemical characterization of recombinant and native tropomyosins as a new allergen from the house dust mite, Dermatophagoides farinae. J Allergy Clin Immunol. 1995;96:74.
3 Amaro C, Goossens A. Immunological occupational contact urticaria and contact dermatitis from proteins: A review. Contact Dermatitis. 2008;58:67.
4 Armentia A, Lombardero M, Callejo A, et al. Occupational asthma by Anisakis simplex. J Allergy Clin Immunol. 1998;102:831.
5 Asero R, Mistrello G, Roncarolo D, et al. True monosensitivity to a tropical sole. Allergy. 1999;54:1228.
6 Asturias JA, Gómez-Bayón N, Arilla MC, et al. Molecular characterization of American cockroach tropomyosin (Periplaneta americana allergen 7), a cross-reactive allergen. J Immunol. 1999;162:4342.
7 Asturias JA, Eraso E, Arilla MC, et al. Cloning, isolation, and IgE-binding properties of Helix aspersa (brown garden snail) tropomyosin. Int Arch Allergy Immunol. 2002;128:90.
8 Asturias JA, Eraso E, Martínez A. Cloning and high level expression in Escherichia coli of an Anisakis simplex tropomyosin isoform. Mol Biochem Parasitol. 2000;108:263.
9 Audicana MT, Fernández del Corres L, Muñoz D, et al. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J Allergy Clin Immunol. 1995;96:558.
10 Audicana MT, Kennedy MW. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008;21:360.
11 Ayuso R, Grishina G, Bardina L, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol. 2008;122:795.
12 Ayuso R, Reese G, Leong-Kee S, et al. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129:38.
13 Azofra J, Lombardero M. Limpet anaphylaxis: Cross-reactivity between limpet and house-dust mite Dermatophagoides pteronyssinus. Allergy. 2003;58:146.
14 Baldo BA, Krilis S, Taylor KM. IgE-mediated acute asthma following inhalation of a powdered marine sponge. Clin Allergy. 1982;12:179.
15 Bascom R, Pipkorn U, Lichtenstein LM, et al. The influx of inflammatory cells into nasal washings during the late response to antigen challenge: Effect of systemic steroid pretreatment. Am Rev Respir Dis. 1988;138:406.
16 Beale J, Jeebhay MF, Lopata AL. Characterization of purified parvalbumin from five fish species and nucleotide sequencing of the major allergen from Pacific pilchard, Sardinops sagax. Mol Immunol. 2009;46:2985.
17 Beaudouin E, Renaudin JM, Morisset M, et al. Food-dependent exercise-induced anaphylaxis: Update and current data. Allerg Immunol. 2006;38:45.
18 Bernhisel-Broadbent J, Scanlon SM, Sampson HA. I. Fish hypersensitivity: In vitro and oral challenge results in fish-allergic patients. J Allergy Clin Immunol. 1992;89:730.
19 Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr. 1990;117:561.
20 Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191.
21 Bock SA, Sampson HA, Atkins FM, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. J Allergy Clin Immunol. 1988;82:986.
22 Briner TJ, Kuo MC, Keating KM, et al. Peripheral T-cell tolerance induced in naïve and primed mice by subcutaneous injection of peptides from the major cat allergen Fel d 1. Proc Natl Acad Sci USA. 1993;90:7608.
23 Buchanon AD, Green TD, Jones SM, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immnol. 2007;119:199.
24 Bugajska-Schretter A, Elfman L, Fuchs T, et al. Parvalbumin, a cross-reactive fish allergen, contains epitopes sensitive to periodate treatment and Ca2+ depletion. J Allergy Clin Immunol. 1998;101:67.
25 Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy, and immunotherapy: Implications for future treatment. J Allergy Clin Immunol. 2008;121:1344.
26 Burks W, Kulis M, Pons L. Food allergies and hypersensitivity: A review of pharmacotherapy and therapeutic strategies. Expert Opin Pharmacother. 2008;9:1145.
27 Carrillo T, Castillo R, Caminero J, et al. Squid hypersensitivity: A clinical and immunologic study. Ann Allergy. 1992;68:483.
28 Carrillo T, de Castro FR, Cuevas M, et al. Allergy to limpet. Allergy. 1991;46:515.
29 Cartier A, Malo JL, Forest F, et al. Occupational asthma in snow crab-processing workers. J Allergy Clin Immunol. 1984;74:261.
30 Cartier A, Malo JL, Ghezzo H, et al. IgE sensitization in snow crab-processing workers. J Allergy Clin Immunol. 1986;78:344.
31 Chegini S, Metcalfe DD. Contemporary issues in food allergy: Seafood toxin-induced disease in the differential diagnosis of allergic reactions. Allergy Asthma Proc. 2005;26:183.
32 Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3.
33 Chen YH, Wu HJ, Tsai JJ, et al. Anaphylactic shock caused by a 33-kDa alpha S1-casein-like allergen in kingfish caviar. J Investig Allergol Clin Immunol. 2009;19:245.
34 Choi JH, Yoon SH, Suh YJ, et al. Measurement of specific IgE to abalone (Haliotis discus hannai) and identification of IgE-binding components. Korean J Asthma Allergy Clin Immunol. 2003;23:349.
35 Chu RS, Targoni OS, Krieg AM, et al. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623.
36 Chu KH, Tang CY, Wu A. Seafood allergy: Lessons from clinical symptoms, immunological mechanisms and molecular biology. Adv Biochem Engin/Biotechnol. 2005;97:205.
37 Chu KH, Wong SH, Leung PS. Tropomyosin is the major mollusk allergen: Reverse transcriptase polymerase chain reaction, expression and IgE reactivity. Mar Biotechnol. 2000;2:499.
38 Clark S, Bock SA, Gaeta TJ, et al. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004;113:347.
39 Clarks PS. Immediate respiratory hypersensitivity to abalone. Med J Aust. 1979;1:623.
40 Collins C, O’Donnel A. Does an allergy to fish pre-empt an adverse protamine reaction? A case report and a literature review. Perfusion. 2008;23:369.
41 Crespo JF, Pascual C, Burks AW, et al. Frequency of food allergy in a pediatric population from Spain. Pediatr Allergy Immunol. 1995;6:39.
42 Crespo JF, Pascual C, Dominguez C, et al. Allergic reactions associated with airborne fish particles in IgE-mediated fish hypersensitive patients. Allergy. 1995;50:257.
43 Crespo JF, Pascual C, Helm R, et al. Cross-reactivity of IgE-binding components between boiled Atlantic shrimp and German cockroach. Allergy. 1995;50:918.
44 Das DS, Chopin C, Romano A, et al. IgE-binding and cross-reactivity of a new 41 kDa allergen of codfish. Allergy. 2002;57:84.
45 Das DS, Chopin C, Villaume C, et al. A new oligomeric parvalbumin allergen of Atlantic cod (Gad m 1) encoded by a gene distinct from that of Gad c 1. Allergy. 2002;57:79.
46 Daul CB, Morgan JE, Waring NP, et al. Immunologic evaluation of shrimp-allergic individuals. J Allergy Clin Immunol. 1987;80:716.
47 Daul CB, Slattery M, Reese G, et al. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol. 1994;105:49.
48 de Martino M, Novembre E, Galli L, et al. Allergy to different fish species in cod-allergic children: In vivo and in vitro studies. J Allergy Clin Immunol. 1990;86:909.
49 de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol. 1998;102:165.
50 Desjardins A, Malo JL, L’Archevêque J, et al. Occupational IgE-mediated sensitization and asthma caused by clam and shrimp. J Allergy Clin Immunol. 1995;96:608.
51 Dibs SD, Baker MD. Anaphylaxis in children: A 5-year experience. Pediatrics. 1997;99:E7.
52 Douglas JD, McSharry C, Blaikie L, et al. Occupational asthma caused by automated salmon processing. Lancet. 1995;346:737.
53 Ebo DG, Stevens WJ. IgE-mediated food allergy: Extensive review of the literature. Acta Clinica Belgica. 2001;56:234.
54 Elsayed S, Aas K. Characterization of a major allergen (cod): Observation on effect of denaturation on the allergenic activity. J Allergy. 1971;47:283.
55 Elsayed S, Bennich H. The primary structure of allergen M from cod. Scand J Immunol. 1975;4:203.
56 Enrique E, Cisteró-Bahíma A. Specific immunotherapy for food allergy: Basic principles and clinical aspects. Curr Opin Allergy Clin Immunol. 2006;6:466.
57 Enrique E, Pineda F, Malek T, et al. Sublingual immunotherapy for hazelnut food allergy: A randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116:1073.
58 Eriksson NE, Ryden B, Jonsson P. Hypersensitivity to larvae of chironomids (non-biting midges): Cross-sensitization with crustaceans. Allergy. 1989;44:305.
59 Escudero R, Gamboa PM, Anton J, et al. Food allergy due to trout roe. J Investig Allergol Clin Immunol. 2007;17:346.
60 Fernandes J, Reshef A, Patton L, et al. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33:956.
61 . Food allergen labeling and consumer protection act of 2004 (public law 108-282, title II). Available at http://www.fda.gov Accessed on August 30, 2009
62 Gaddie J, Friend JA. Pulmonary hypersensitivity in prawn workers. Lancet. 1980;316:1350.
63 Galland AV, Dory D, Pons L, et al. Purification of a 41 kDa cod-allergenic protein. J Chromatogr B Biomed Sci Appl. 1998;706:63.
64 Garcia-Orozco KD, Aispuro-Hernandez E, Yepiz-Plascencia G, et al. Molecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus vannamei. Int Arch Allergy Immunol. 2007;144:23.
65 Glaspole IN, De Leon MP, Rolland JM, et al. Characterization of the T-cell epitopes of a major peanut allergen, Ara h 2. Allergy. 2005;60:35.
66 Goetz DW, Whisman BA. Occupational asthma in a seafood restaurant worker: Cross-reactivity of shrimp and scallops. Ann Allergy Asthma Immunol. 2000;85:461.
67 Goodman RE, Silvanovich A, Hileman RE, et al. Bioinformatic methods for identifying known or potential allergens in the safety assessment of genetically modified crops. Comments Toxicol. 2002;8:251.
68 Guarneri F, Guarneri C, Benvenga S. Cross-reactivity of Anisakis simplex: Possible role of Ani s 2 and Ani s 3. Int J Dermatol. 2007;46:146.
69 Hamada E, Genka M, Ohira Y, et al. Allergenicity of fish meat paste products and surimi of walleye pollock. J Food Hyg Soc Jpn. 2000;41:38.
70 Hamada Y, Nagashima Y, Shiomi K. Identification of collagen as a new fish allergen. Biosci Biotechnol Biochem. 2001;65:285.
71 Hamada Y, Tanaka H, Ishizaki S, et al. Purification, reactivity with IgE and cDNA cloning of parvalbumin as the major allergen of mackerels. Food Chem Toxicol. 2003;41:1149.
72 Hansen TK, Bindslev-Jensen C, Skov PS, et al. Codfish allergy in adults: IgE cross-reactivity among fish species. Ann Allergy Asthma Immunol. 1997;78:187.
73 Helbling A, Haydel RJr, McCants ML, et al. Fish allergy: Is cross-reactivity among fish species relevant? Double-blind placebo-controlled food challenge studies of fish allergic adults. Ann Allergy Asthma Immunol. 1999;83:517.
74 Helbling A, McCants ML, Musmand JJ, et al. Immunopathogenesis of fish allergy: Identification of fish-allergic adults by skin test and radioallergosorbent test. Ann Allergy Asthma Immunol. 1996;77:48.
75 Hickey RW. Sea urchin roe (uni) anaphylaxis. Ann Allergy Asthma Immunol. 2007;98:493.
76 Hilger C, Grigioni F, Thill L, et al. Severe IgE-mediated anaphylaxis following consumption of fried frog legs: Definition of alpha-parvalbumin as the allergen in cause. Allergy. 2002;57:1053.
77 Hilger C, Thill L, Grigioni F, et al. IgE antibodies of fish allergic patients cross-react with frog parvalbumin. Allergy. 2004;59:653.
78 Hjorth N, Roed-Petersen J. Occupational protein contact dermatitis in food handlers. Contact Dermatitis. 1976;2:28.
79 Hong SJ, Michael JG, Fehringer A, et al. Pepsin-digested peanut contains T-cell epitopes but no IgE epitopes. J Allergy Clin Immunol. 1999;104:473.
80 Hughes DA, Mills C. Food allergy: A problem on the increase. Biologist. 2001;48:201.
81 Husby S, Jensenius JC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults: Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand J Immunol. 1985;22:83.
82 Ishikawa M, Ishida M, Shimakura K, et al. Purification and IgE-binding properties of a major allergen in the gatropod Turbo cornutus. Biosci Biotechnol Biochem. 1998;62:1337.
83 Ishikawa M, Ishida M, Shimakura K, et al. Tropomyosin, the major oyster Crassostrea gigas allergen and its IgE-binding epitopes. J Food Sci. 1998;63:44.
84 Ishikawa M, Suzuki F, Ishida M, et al. Identification of tropomyosin as a major allergen in the octopus Octopus vulgaris and elucidation of its IgE-binding epitopes. Fisheries Science. 2001;67:934.
85 James JM, Bernhisel-Broadbent J, Sampson HA. Respiratory reactions provoked by double-blind food challenges in children. Am J Respir Crit Care Med. 1994;149:59.
86 James JM, Helm RM, Burks AW, et al. Comparison of pediatric and adult IgE antibody binding to fish proteins. Ann Allergy Asthma Immunol. 1997;79:131.
87 Jeebhay MF, Robins TG, Lehrer SB, et al. Occupational seafood allergy: A review. Occup Environ Med. 2001;58:553.
88 Jimenez M, Pineda F, Sanchez I, et al. Allergy due to Enis macha. Allergy. 2005;60:1090.
89 Jirapongsananuruk O, Sripramong C, Pacharn P, et al. Specific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic children. Clin Exp Allergy. 2008;38:1038.
90 Juji F, Takashima H, Suko M, et al. A case of food-dependent exercise-induced anaphylaxis possibly induced by shellfish (Sulculus supertexta and Turbo cornutus). Arerugi. 1990;39:1515.
91 Jyo T, Kohmoto K, Tsubai S, et al. Sea squirt asthma: Occupational asthma induced by inhalation of antigenic substances contained in sea squirt body fluid. Allergy Immunol. 1974;20:435.
92 Kalogeromitros D, Makris M, Gregoriou S, et al. IgE-mediated sensitization in seafood processing workers. Allergy Asthma Proc. 2006;27:399.
93 Karlin JM. Occupational asthma to clam’s liver extract. J Allergy Clin Immunol. 1979;63:197.
94 Kasuya S, Hamano H, Izumi S. Mackerel-induced urticaria and Anisakis. Lancet. 1990;335:665.
95 Kelso JM, Jones RT, Yunginger JW. Monospecific allergy to swordfish. Ann Allergy Asthma Immunol. 1996;77:227.
96 Kimura S, Takagi Y, Gomi K. IgE response to Anisakis compared to seafood. Allergy. 1999;54:1225.
97 Knape JT, Schuller JL, de Haan P, et al. An anaphylactic reaction to protamine in a patient allergic to fish. Anesthesiology. 1981;55:324.
98 Lee BJ, Park HS. Common whelk (Buccinum undatum) allergy: Identification of IgE-binding components and effects of heating and digestive enzymes. J Korean Med Sci. 2004;19:793.
99 Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics. 2000;106:762.
100 Lehrer SB. Hypersensitivity reactions in seafood workers. Allergy Proc. 1990;11:67.
101 Lehrer SB, Ayuso R, Reese G. Seafood allergy and allergens: A review. Mar Biotechnol. 2003;5:339.
102 Lehrer SB, Bannon GA. Risks of allergic reactions to biotech proteins in foods: Perception and reality. Allergy. 2005;60:559.
103 Lehrer SB, McCants ML. Reactivity of IgE antibodies with crustacea and oyster allergens: Evidence for common antigenic structures. J Allergy Clin Immunol. 1987;80:133.
104 Lehrer SB, O’Neil CE. Occupational reactions in the food industry. Food Technology. 1992;May:153.
105 Lemière C, Desjardins A, Lehrer S, et al. Occupational asthma to lobster and shrimp. Allergy. 1996;51:272.
106 Leung DY, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986.
107 Leung PS, Chen YC, Gershwin ME, et al. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J Allergy Clin Immunol. 1998;102:847.
108 Leung PS, Chen YC, Mykles DL, et al. Molecular identification of the lobster muscle protein tropomyosin as a seafood allergen. Mol Mar Biol Biotechnol. 1998;7:12.
109 Leung PS, Chow WK, Duffey S, et al. IgE reactivity against a cross-reactive allergen in crustacea and Mollusca: Evidence for tropomyosin as the common allergen. J Allergy Clin Immunol. 1996;98:954.
110 Leung PS, Chu KH. Molecular and immunological characterization of shellfish allergens. Front Biosci. 1998;3:306.
111 Leung PS, Chu KH, Chow WK, et al. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J Allergy Clin Immunol. 1994;92:837.
112 Li XM, Zhang TF, Huang CK, et al. Food allergy herbal formula-1 (FAHF-1) blocks peanut induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108:639.
113 Lim DL, Neo KH, Yi FC, et al. Parvalbumin: The major tropical fish allergen. Pediatr Allergy Immunol. 2008;19:399.
114 Lin HY, Shyur SD, Fu JL, et al. Fish induced anaphylactic reaction: Report of one case. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1998;39:200.
115 Lin RY, Shen HD, Han SH. Identification and characterization of a 30-kD major allergen from Parapenaeus fissurus. J Allergy Clin Immunol. 1993;92:837.
116 Lindstrom CD, van Do T, Hordvik I, et al. Cloning of two distinct cDNAs encoding parvalbumin, the major allergen of Atlantic salmon (Salmo salar). Scand J Immunol. 1996;44:335.
117 Lopata AL, Baatjies R, Thrower SJ, et al. Occupational allergies in the seafood industry: A comparative study of Australian and South African workplaces. Internat Marit Health. 2004;55:61.
118 Lopata AL, Lehrer SB. New insights into seafood allergy. Curr Opin Allergy Clin Immunol. 2009;9:270.
119 Lopata AL, Zinn C, Potter PC. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J Allergy Clin Immunol. 1997;100:642.
120 Mäkinen-Kiljunen S, Kiistala R, Varjonen E. Severe reactions from roe without concomitant fish allergy. Ann Allergy Asthma Immunol. 2003;91:413.
121 Marinho S, Morais-Almeida M, Gaspar A, et al. Barnacle allergy: Allergen characterization and cross-reactivity with mites. J Investig Allergol Clin Immunol. 2006;16:117.
122 Martínez A, Martínez J, Palacios R, et al. Importance of tropomyosin in the allergy to household arthropods: Cross-reactivity with other invertebrate extracts. Allergol Immunopathol. 1997;25:118.
123 Maulitz RM, Pratt DS, Schocket AL. Exercise-induced anaphylactic reaction to shellfish. J Allergy Clin Immunol. 1979;63:433.
124 Mayer L, Sperber K, Chan L, et al. Oral tolerance to protein antigens. Allergy. 2001;56:12.
125 Meglio P, Bartone E, Plantamura M, et al. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy. 2004;59:980.
126 Mehl A, Rolinck-Werninghaus C, Staden U, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118:923.
127 Mikita CP, Padlan EA. Why is there a greater incidence of allergy to the tropomyosin of certain animals than to that of others? Med Hypotheses. 2007;69:1070.
128 Milgrom H. Anti-IgE therapy in allergic disease. Curr Opin Pediatr. 2004;16:642.
129 Misnan R, Murad S, Jones M, et al. Identification of the major allergens of Indian scad (Decapterus russelli). Asian Pac J Allergy Immunol. 2008;26:191.
130 Miyazawa H, Fukamachi H, Inagaki Y, et al. Identification of the first major allergen of a squid (Todarodes pacificus). J Allergy Clin Immunol. 1996;98:948.
131 Moneret-Vautrin DA, Kanny G, Frémont S. Laboratory tests for diagnosis of food allergy: Advantages, disadvantages and future perspectives. Eur Ann Allergy Clin Immunol. 2003;35:113.
132 Monti G, Bonfante G, Muratore MC, et al. Kiss-induced facial urticaria and angioedema in a child allergic to fish. Allergy. 2003;58:684.
133 Moreno-Ancillo A, Caballero MT, Cabañas R, et al. Allergic reactions to Anisakis simplex parasitizing seafood. Ann Allergy Asthma Immunol. 1997;79:246.
134 Morgan JE, Daul CB, Lehrer SB. Characterization of important shrimp allergens by immunoblot analysis. J Allergy Clin Immunol. 1990;85:170.
135 Morgan JE, O’Neil CE, Daul CB, et al. Species-specific shrimp allergens: RAST and RAST-inhibition studies. J Allergy Clin Immunol. 1989;83:1112.
136 Morikawa A, Kato M, Tokuyama K, et al. Anaphylaxis to grand keyhole limpet (abalone-like shellfish) and abalone. Ann Allergy. 1990;65:415.
137 Motoyama K, Hamada Y, Nagashima Y, et al. Allergenicity and allergens of amphipods found in nori (dried laver). Food Addit Contam. 2007;24:917.
138 Múgica MV, Añíbarro B, Seoane FJ. Contact urticaria by angler fish. Allergy. 2003;58:682.
139 Müller U, Akdis CA, Fricker M, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747.
140 Musmand JJ, Daul CB, Lehrer SB. Crustacea allergy. Clin Exp Allergy. 1993;23:722.
141 Myers P, Espinosa R, Parr CS, et al. The Animal Diversity Web (online). http://www.animaldiversity.org, 2008. Available at Accessed August 27
142 Nakano S, Yoshinuma T, Yamada T. Reactivity of shrimp allergy-related IgE antibodies to krill tropomyosin. Int Arch Allergy Immunol. 2008;145:175.
143 Nakashima T. Studies on bronchial asthma observed in cultured oyster workers. Hiroshima J Med Sci. 1969;18:141.
144 National Marine Fisheries Service. Top 10 U.S. consumption by species chart, calculated by Howard Johnson, H.M. Johnson & Associates for NFI. Available at http://www.aboutseafood.com/about/about-seafood/Top-10-Consumed-Seafoods Accessed August 28, 2009
145 National Oceanic and Atmospheric Administration. Fisheries of the United States. Available at www.noaa.gov Accessed August 27, 2009
146 National Oceanic and Atmospheric Administration. Seafood consumption declines slightly in 2007. Available at www.noaa.gov Accessed August 31, 2009
147 Nelson HS, Lahr J, Rule R, et al. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744.
148 Nieuwenhuizen N, Lopata AL, Jeebhay MF, et al. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. J Allergy Clin Immunol. 2006;117:1098.
149 Niggemann B, Beyer K. Diagnosis of food allergy in children: Toward a standardization of food challenge. J Pediatr Gastroenterol Nutr. 2007;45:399.
150 Niggemann B, Staden U, Rolinck WC, et al. Specific oral tolerance induction in food allergy. Allergy. 2006;61:808.
151 Norman PS, Ohman JLJr, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623.
152 Novembre E, Cianferoni A, Bernardini R, et al. Anaphylaxis in children: Clinical and allergologic features. Pediatrics. 1998;101:E8.
153 Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: A randomized controlled trial. Lancet. 2002;360:47.
154 Oppenheimer JJ, Nelson HS, Bock SA, et al. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90:256.
155 Orford RR, Wilson JT. Epidemiologic and immunologic studies in processors of the king crab. Am J Ind Med. 1985;7:155.
156 Orriols R, Aliaga JL, Antó JM, et al. High prevalence of mollusc shell hypersensitivity pneumonitis in nacre factory workers. Eur Respir J. 1997;10:780.
157 Orriols R, Manresa JM, Aliaga JL, et al. Mollusk shell hypersensitivity pneumonitis. Ann Intern Med. 1990;113:80.
158 Ortega HG, Kreiss K, Schill DP, et al. Fatal asthma from powdering shark cartilage and review of fatal occupational asthma literature. Am J Ind Med. 2002;42:50.
159 Patel PC, Cockcroft DW. Occupational asthma caused by exposure to cooking lobster in the work environment: A case report. Ann Allergy. 1992;68:360.
160 Patriarca G, Nucera E, Roncallo C, et al. Oral desensitizing treatment in food allergy: Clinical and immunological results. Aliment Pharmacol Ther. 2003;17:459.
161 Perez-Gordo M, Sanchez-Garcia S, Cases B, et al. Identification of vitellogenin as an allergen in Beluga caviar allergy. Allergy. 2008;63:479.
162 Porcel S, León F, Cumplido J, et al. Contact urticaria caused by heat-sensitive raw fish allergens. Contact Dermatitis. 2001;45:139.
163 Porsche R, Brenner ZR. Allergy to protamine sulfate. Heart Lung. 1999;28:418.
164 Preston NP, Baule RL, Henderling J, et al. Delivery of DNA to early embryos of the Kuruma prawn, Penaeus japonicus. Aquaculture. 2000;181:225.
165 Reese G, Ayuso R, Lehrer SB. Tropomyosin: An invertebrate pan-allergen. Int Arch Allergy Immunol. 1999;119:247.
166 Roberts G, Golder N, Lack G. Bronchial challenges with aerosolized food in asthmatic, food-allergic children. Allergy. 2002;57:659.
167 Rodriguez V, Bartolome B, Armisen M, et al. Food allergy to Paracentrotus lividus (sea urchin roe). Ann Allergy Asthma Immuol. 2007;98:393.
168 Rodriguez V, Gracia MT, Iriarte P, et al. Allergy to dogfish. Allergy. 2003;58:1315.
169 Rosmilah M, Shahnaz M, Masita A, et al. Identification of major allergens of two species of local snappers: Lutjanus argentimaculatus (merah/red snapper) and Lutjanus johnii (jenahak/golden snapper). Trop Biomed. 2005;22:171.
170 Ross MP, Ferguson M, Street D, et al. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. 2008;121:166.
171 Roy K, Mao HQ, Huang SK, et al. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387.
172 Sakaguchi M, Toda M, Ebihara T, et al. IgE antibody to fish gelatin (type I collagen) in patients with fish allergy. J Allergy Clin Immunol. 2000;106:579.
173 Sampson HA. Role of immediate food hypersensitivity in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1983;71:473.
174 Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891.
175 Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444.
176 Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380.
177 Santos AB, Chapman MD, Aalberse RC, et al. Cockroach allergens and asthma in Brazil: Identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104:329.
178 Scala E, Giani M, Pirrotta, et al. Occupational generalized urticaria and allergic airborne asthma due to Anisakis simplex. Eur J Dermatol. 2001;11:249.
179 Seitz CS, Bröcker EB, Trautmann A. Occupational allergy due to seafood delivery: Case report. J Occup Med Toxicol [serial online]. 2008;3:11, http://www.occup-med.com/. Accessed August 29, 2009
180 Shanti KN, Martin BM, Nagpal S, et al. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE binding epitopes. J Immunol. 1993;151:5354.
181 Shibasaki M, Ehara T, Takita H. Late anaphylactic reaction to cuttlefish. Ann Allergy. 1989;63:421.
182 Shiomi K, Hamada Y, Sekiguchi K, et al. Two classes of allergens, parvalbumins and higher molecular weight substances, in Japanese eel and bigeye tuna. Fish Sci. 1999;65:943.
183 Shiomi K, Sato Y, Hamamoto S, et al. Sarcoplasmic calcium-binding protein: Identification as a new allergen of the black tiger shrimp Penaeus monodon. Int Arch Allergy Immunol. 2008;146:91.
184 Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159.
185 Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2006;117:S470.
186 Siracusa A, Marcucci F, Spinozzi F, et al. Prevalence of occupational allergy due to live fish bait. Clin Exp Allergy. 2003;33:507.
187 Spiegelberg HL, Orozco EM, Roman M, et al. DNA immunization: A novel approach to allergen-specific immunotherapy. Allergy. 1997;52:964.
188 Srivastava KM, Kattan JD, Zou ZM, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171.
189 Steensma DP. The kiss of death: A severe allergic reaction to a shellfish induced by a good-night kiss. Mayo Clin Proc. 2003;78:221.
190 Suma Y, Ishizaki S, Nagashima Y, et al. Comparative analysis of barnacle tropomyosin: Divergence from decapod tropomyosins and role as a potential allergen. Comp Biochem Physiol B Biochem Mol Biol. 2007;147:230.
191 Swoboda I, Bugajska-Schretter A, Linhart B, et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J Immunol. 2007;178:6290.
192 Swoboda I, Bugajska-Schretter A, Valenta R, et al. Recombinant fish parvalbumins: Candidates for diagnosis and treatment of fish allergy. Allergy. 2002;57:94.
193 Swoboda I, Bugajska-Scretter A, Verdino P, et al. Recombinant carp parvalbumin, the major cross-reactive fish allergen: A tool for diagnosis and therapy of fish allergy. J Immunol. 2002;168:4576.
194 Tanabe S. Epitope peptides and immunotherapy. Curr Protein Pept Sci. 2007;8:109.
195 Taylor SL. Molluscan shellfish allergy. Adv Food Nutr Res. 2008;54:139.
196 Taylor SL, Kabourek JL, Hefle SL. Fish allergy: Fish and products thereof. J Food Sci. 2004;69:R175.
197 Thong BY, Cheng YK, Leong KP. Immediate food hypersensitivity among adults attending a clinical immunology/allergy centre in Singapore. Singapore Med J. 2007;48:236.
198 Tomaszunas S, Weclawik Z, Lewinski M. Allergic reactions to cuttlefish in deep-sea fishermen. Lancet. 1988;331:1116.
199 Torres Borrego J, Martínez Cuevas JF, Tejero García J. Cross reactivity between fish and shellfish. Allergol Immunopathol. 2003;31:146.
200 Tosti A, Guerra L, Morelli R, et al. Role of foods in the pathogenesis of chronic paronychia. J Am Acad Dermatol. 1992;27:706.
201 Untersmayr E, Vestergaard H, Malling HF, et al. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J Allergy Clin Immunol. 2007;119:711.
202 Van Do T, Elsayed S, Florvaag E, et al. Allergy to fish parvalbumins: Studies on the cross-reactivity of allergens from 9 commonly consumed fish. J Allergy Clin Immunol. 2005;116:1314.
203 Van Do T, Hordvik I, Endresen C, et al. Characterization of parvalbumin, the major allergen in Alaska pollock, and comparison with codfish allergen M. Mol Immunol. 2005;42:345.
204 Van Do T, Hordvik I, Endresen C, et al. The major allergen (parvalbumin) of codfish is encoded by at least two isotypic genes: cDNA cloning, expression and antibody binding of the recombinant allergens. Mol Immunol. 2003;39:595.
205 van Ree R, Antonicelli L, Akkerdaas JH, et al. Asthma after consumption of snails in house-dust-mite-allergic patients: A case of IgE cross-reactivity. Allergy. 1996;51:387.
206 van Ree R, Antonicelli L, Akkerdaas JH, et al. Possible induction of food allergy during mite immunotherapy. Allergy. 1996;51:108.
207 Waring NP, Daul CB, deShazo RD, et al. Hypersensitivity reactions to ingested crustacea: Clinical evaluation and diagnostic studies in shrimp-sensitive individuals. J Allergy Clin Immunol. 1985;76:440.
208 Warshaw AL, Walker WA, Isselbacher KJ. Protein uptake by the intestine: Evidence for absorption of intact macromolecules. Gastroenterology. 1974;66:987.
209 Weiler CR. Anisakis simplex and cross-reacting antigens. Int J Dermatol. 2007;46:224.
210 Wild LG, Lehrer SB. Fish and shellfish allergy. Curr Allergy Asthma Rep. 2005;5:74.
211 Wiley K, Griffin P. Characterization and purification of allergens from Norwegian lobster. Clin Exp Allergy. 1994;24:175.
212 Witteman AM, Akkerdaas JH, van Leeuwen J, et al. Identification of a cross-reactive allergen (presumably tropomyosin) in shrimp, mite and insects. Int Arch Allergy Immunol. 1994;105:56.
213 Wu AY, Williams GA. Clinical characteristics and pattern of skin test reactivities in shellfish allergy patients in Hong Kong. Allergy Asthma Proc. 2004;25:237.
214 Yamada S, Nolte H, Zychlinsky E. Identification and characterization of allergens in two species of tuna fish. Ann Allergy Asthma Immunol. 1999;82:395.
215 Yu CJ, Lin YF, Chiang BL, et al. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol. 2003;170:445.
216 Zhang Y, Matsuo H, Morita E. Cross-reactivity among shrimp, crab and scallops in a patient with a seafood allergy. J Dermatol. 2006;33:174.