Scuba Diving and Dysbarism
Perspective
Underwater free diving to salvage wrecks and to harvest seafood, sponges, coral, and mother-of-pearl has been practiced for more than 5000 years.1 Historically, divers also used breathing tubes, such as hollow reeds; however, it is nearly impossible to use these at depths of more than 3 feet because of the restriction of inspiration by underwater pressure. Subsequent inventions from the 16th to the 19th centuries, including diving dresses, allowed divers to remain underwater for prolonged periods at depths of up to 12 fathoms (72 feet).2 The first diving dress (1715) was a reinforced, leather-covered barrel with watertight armholes and a viewing porthole.2
With the advent of these technologies, the symptoms of diving-related illness began to be recognized. Colonel William Pasley, the officer in charge of a unit of the British Royal Engineers that salvaged the sunken warship HMS Royal George in 1840, observed symptoms in his divers.3 At approximately the same time, similar symptoms and even fatalities were observed among caisson workers.* The ailment became known as caisson disease, but the construction workers on the Brooklyn Bridge (built from 1870 to 1883) attached the name “the bends,” characterizing the symptoms that often caused the victim to bend forward in pain.3 The first clinical description of caisson disease was by Paul Bert in 1878.3 He correctly attributed the disease to nitrogen gas coming out of solution in the tissues during decompression. This led to the recommendation of slow ascents for pressurized workers and the development of the first recompression chambers.
The significant breakthrough allowing diving at depth was the invention of the aqua-lung by Jacques-Yves Cousteau and Emile Gagnan in 1943.2 The lighter and less expensive equipment, widely known by its acronym SCUBA (self-contained underwater breathing apparatus), does not require a surface supply of air or the support personnel that are necessary for helmet diving. This innovation allowed widespread deep-sea diving, and millions of divers have become certified to date.
Most amateur divers use compressed air, open-circuit scuba equipment at depths of less than 130 feet of seawater (fsw). Systems with artificial mixtures of various gases, however, are used to extend the depths to which divers can descend. Some of these are used in sport diving, but their use is uncommon and is primarily limited to commercial applications (Table 143-1).
Table 143-1
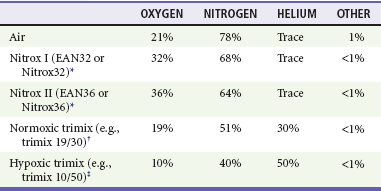
*Enhanced air nitrox, oxygen enriched air, nitrox, EANx, SafeAir, “devil gas,” “voodoo gas.”
†A normoxic mix, such as 19/30, is used in the depth range of 30 m (100 feet) to 60 m (200 feet).
‡A hypoxic mix, such as 10/50, is used for deeper diving, as a “bottom” gas only; it cannot safely be breathed at shallow depths where the oxygen partial pressure is less than 0.18.
The total number of diving-related injuries is unknown, but the absolute numbers of patients with decompression-related illnesses, one of the most serious dive-related injuries, continue to climb as the number of divers has increased despite the rate per 10,000 dives remaining relatively constant since 2001.4 This rate varies somewhat on the basis of the type of diver: 0.015% for scientific divers, 0.01 to 0.019% for recreational divers, 0.030% for U.S. Navy divers, and 0.095% for commercial divers.5 The rate of mortality in diving varies between 1.5 and 9 per 100,000 dives. With the popularity of diving and the relative ease of rapid travel from distant destinations, it behooves even the land-locked emergency physician to be aware of diving-related illnesses.
Principles of Disease
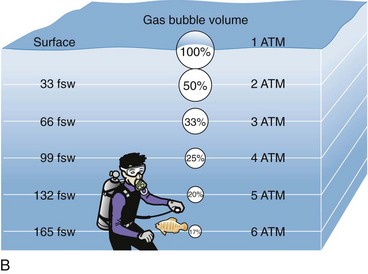
To understand the pathophysiologic processes of dysbarisms and barotrauma, one should be familiar with several of the laws of physics that define the behavior of liquids and gases (Table 143-2; Figs. 143-1 to 143-5). The human body is composed mostly of water and behaves like a liquid subject to Pascal’s law, which states that a pressure applied to any part of a liquid is transmitted equally throughout. Pressure changes, however, do alter the volume within the air-filled spaces of the body, including the lungs, bowel, sinuses, and middle ear, according to Boyle’s law. This law states that at constant temperature, the absolute pressure and the volume of gas are inversely proportional (PV = k). In other words, as pressure increases (with descent), the gas volume is reduced; as the pressure is reduced (with ascent), the gas volume increases.
Table 143-2
| GAS LAW | FORMULA | SIGNIFICANCE |
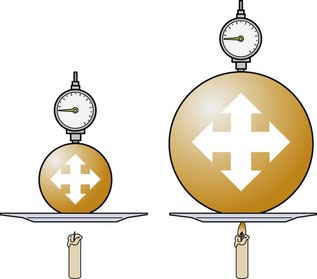
| Pascal’s law: A pressure applied to any part of a liquid is transmitted equally throughout. | ΔP = ρg (Δh) ΔP is the hydrostatic pressure. ρ is the fluid density. g is acceleration due to gravity. Δh is the height of fluid. |
Pressure increases in a contained space are transmitted throughout; significant for inner ear barotrauma and middle ear barotrauma (see Fig. 143-1) |
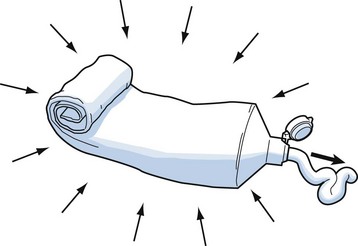
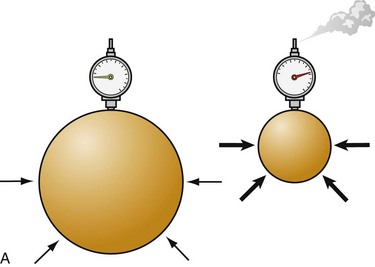
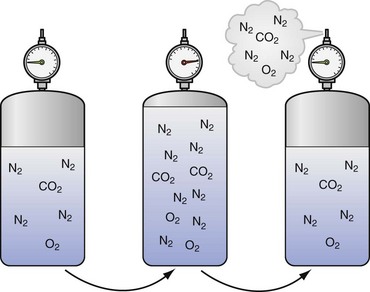
| Boyle’s law: At a constant temperature, the absolute pressure and the volume of gas are inversely proportional. As pressure increases, the gas volume is reduced; as the pressure is reduced, the gas volume increases. | P1 • V1 = P2 • V2 | Relates to change in the volume of a gas caused by the change in pressure due to depth, which defines the relationship of pressure and volume in breathing gas supplies (see Fig. 143-2) |
| Charles’ law: At a constant pressure, the volume of a gas is directly proportional to the change in the absolute temperature. | V1/T1 = V2/T2 | Increasing pressure (filling a scuba tank) causes heat; cooling a tank decreases the pressure (see Fig. 143-3) |
| The general gas law combines these concepts to predict the behavior of a gas when the factors change. | P1 • V1/T1 = P2 • V2/T2 P1 is the initial pressure. V1 is the initial volume. T1 is the initial temperature. P2 is the final pressure. V2 is the final volume. T2 is the final temperature. |
A means of relating pressure, volume, and temperature together in one equation when variables are not constant |
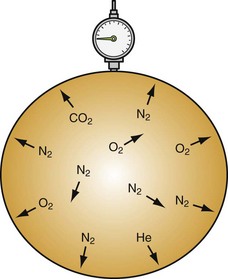
| Dalton’s law: The total pressure exerted by a mixture of gases is equal to the sum of the pressures (partial pressures) of each of the different gases making up the mixture, with each gas acting as if it alone is present and occupies the total volume. | PTotal 3 P1 + P2 + P3 + … + Pn | Nitrogen under pressure acts as if other gases are not present (see Fig. 143-4) |
| Henry’s law: The amount of a gas that will dissolve in a liquid at a given temperature is directly proportional to the partial pressure of that gas. | ep = ekc e is approximately 2.7182818 (the base of the natural logarithm). p is the partial pressure of the solute above the solution. c is the concentration of the solute in the solution. k is the Henry’s law constant. |
More nitrogen is taken into solution (e.g., serum) at high pressures than comes out of solution at lower pressures (see Fig. 143-5) |
Clinical Features
Middle Ear Barotrauma
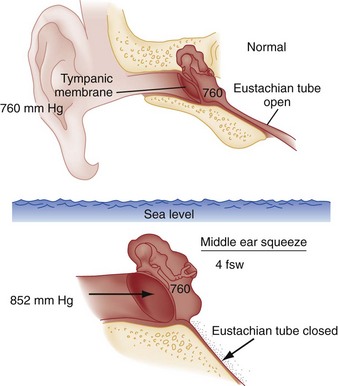
Middle ear barotrauma (MEBT), also known as barotitis or “ear squeeze,” is the most common complaint of scuba divers. It is experienced by 30% of novice scuba divers and 10% of experienced divers.6,7 The middle ear is an air-filled space with solid bone walls except for the tympanic membrane (Fig. 143-6). The eustachian tube is the only anatomic passage to the external environment.
External Ear Barotrauma
External ear barotrauma is less common than MEBT and results from the outward bulging of the tympanic membrane during descent. Normally, the external auditory canal is filled with water during descent. Obstruction of the external auditory canal because of cerumen, stenosis, earplugs, or a tight-fitting wet suit hood can trap air, causing a relative negative pressure. This may lead to localized pain. Patients may have hemorrhages in the wall of the external auditory canal on examination, but the symptoms are typically self-limited.8
Inner Ear Barotrauma
Inner ear barotrauma (IEBT) results in damage to the cochleovestibular apparatus. It is much less common than MEBT but is associated with greater morbidity. A large negative pressure gradient develops in the middle ear if the diver is unable to equalize pressure during descent, similar to MEBT. Inward deflection of the tympanic membrane is transmitted to the oval window of the cochlea through the ossicles. Movement of the oval window creates a pressure wave within the perilymph of the cochlea, which causes an outward distention of the round window into the middle ear. Sudden equilibration of pressure in the middle ear or a vigorous Valsalva maneuver may rupture the round window, lead to hemorrhage into the inner ear, or tear the labyrinthine (Reissner’s) membrane.9–11
Barosinusitis
Obstruction of the sinus ostia by mucosal thickening, polyps, pus, or deviated septum predisposes to sinus barotrauma, the second most common complaint among divers.8,9 The air-filled maxillary, frontal, and ethmoidal sinuses are susceptible to volume-pressure changes on ascent or descent; the most commonly affected is the maxillary sinus, followed by the frontal.11 The most common symptom is facial pain; epistaxis is common.
Facial Barotrauma
As water pressure increases during descent, a negative pressure develops within the dive mask over the eyes and nose, which must be equalized by forced exhalation through the nose. When this is not adequately performed, the large negative pressure gradient may lead to facial and conjunctival edema, diffuse petechial hemorrhages on the face, and subconjunctival hemorrhages.8 Very rarely, optic nerve damage can result from severe facial barotrauma.
Disorders Arising at Depth
Nitrogen narcosis, known as rapture of the deep, results from the intoxicating effects of increased tissue nitrogen concentration at depth. Symptoms include euphoria, false feeling of well-being, confusion, loss of judgment or skill, disorientation, inappropriate laughter, diminished motor control, and tingling and vague numbness of the lips, gums, and legs.3 With breathing of compressed air, symptoms typically begin to occur at approximately 100 feet and often become profound at depths of more than 150 feet.3 Because of these dangers, the use of compressed air is not recommended for sport diving to depths of more than 120 feet. Although the effects of nitrogen narcosis resolve with ascent to shallower depths and are variable between individuals, the diver may drown because of poor judgment or seriously impaired motor skills in the presence of a dive emergency.
Contaminated Air
Rarely, other gases, such as carbon monoxide and carbon dioxide, can contaminate the air that is compressed into a tank. This can happen, for example, if the compressor intake is placed too close to the compressor’s engine exhaust. As in the case of oxygen and nitrogen, the partial pressure of these contaminants in the tissues increases dramatically with depth, potentiating their clinical effects. The symptoms of hypercarbia or carbon monoxide poisoning are more severe at elevated partial pressures. Hypercarbia increases a diver’s susceptibility to CNS oxygen toxicity.3
Rebreathers release microscopic calcium hydroxide or “soda lime” dust particles into the apparatus.12 These particles are small enough and have geometric characteristics that allow them to be deposited in the alveoli. When soda lime comes into contact with water, it forms a caustic liquid. In the event of a hose rupture allowing seawater contamination of the circuit, caustic burns to the mouth, throat, and airways may result. Chronic exposure to soda lime dust may contribute to long-term effects on respiratory function.
Disorders Arising on Ascent
Gastrointestinal Barotrauma
Serious gastrointestinal barotrauma is a rare condition in scuba divers. It results from the expansion of bowel gas in the small intestine and colon on ascent after diving. Predisposing factors include consumption of carbonated beverages, large meals, or gas-producing foods before diving as well as performance of the Valsalva maneuver in the head-down position. Symptoms include eructation, flatulence, bloating, and crampy abdominal pain. In divers with inguinal or other hernias, the potential for expansion of trapped gas within the hernia exists, and expansion may result in incarceration or strangulation.13,14 Gastric rupture is a rare complication.14 Although gastrointestinal barotrauma is a rare entity, it should be suspected in the diver-patient with a provocative history and abdominal pain.
Pulmonary Barotrauma
Asthmatics have a twofold increased risk for pulmonary barotrauma compared with the general diver population.15 There are six mechanisms that contribute to the increased risk in asthmatics:
1. Bronchospasm and mucus plugging predispose local regions of lung to injury.
2. When air is compressed, it becomes denser. This may contribute to greater turbulent flow through narrow airways.
3. During scuba diving, there is a reduction in breathing capacity related to the effects of immersion. At 33 feet underwater, the maximum breathing capacity of a normal scuba diver is only 70% of the surface value. At 100 feet underwater, the reduction is approximately 50%.
4. When compressed air (from the scuba tank) expands in the regulator before delivery to the lungs, it cools (Charles’ law). Breathing of chilled air may trigger bronchospasm in asthmatics who have a cold-induced component of their disease.
5. Scuba diving takes some effort; asthmatics who have an exercise-induced component of their disease may experience bronchospasm.
6. Compressed air may be contaminated by pollen and other allergens.
Traditionally, asthmatics were advised not to dive. Several experts, however, propose more liberal guidelines.16–18 The risk of diving is probably acceptable if the diving candidate with asthma demonstrates normal pulmonary function at rest (forced vital capacity [FVC], midexpiratory flow, forced expiratory volume in 1 second, and forced expiratory flow between 25 and 75% of FVC) and after strenuous exercise. Asthma severity can wax and wane. Because symptoms may worsen for 4 to 6 weeks after an upper respiratory infection or during certain seasons, asthmatics should refrain from diving until they are completely free of symptoms despite the pulmonary function criteria.
Pulmonary barotrauma can also cause alveolar hemorrhage.19,20 Patients may present with hemoptysis coincident with chest pain and dyspnea. Chest radiography may reveal an interstitial infiltrate.
Decompression Sickness
The term decompression sickness (DCS) refers to a spectrum of clinical illnesses resulting from the formation of small bubbles of nitrogen gas in the blood and tissues on ascent.21,22 The clinical expression of DCS depends on the location, destination, and degree of nitrogen bubble formation in blood and tissues. Small, asymptomatic venous gas emboli are common in the ascending diver and are filtered by the lungs without apparent permanent damage.23,24 Persistent intravascular bubbles, however, elicit inflammatory cascades, cytokines, the complement system, platelet aggregation, and thrombosis.25 Furthermore, the bubbles can cause mechanical obstruction, ischemia, and tissue hypoxia. Nitrogen is highly fat soluble, and the heavily myelinized white matter of the CNS is at particular risk for DCS.
The incidence of DCS is estimated to be 2.8 cases per 10,000 dives.4 The potential for development of DCS increases with the length and depth of a dive. Other risk factors include age, obesity, fatigue, heavy exertion, dehydration, fever, cold ambient temperatures after diving, diving at high altitude, and flying after diving. Tobacco and ethanol use may also increase susceptibility to DCS. The risk of DCS is 2.6 times greater for men than for women.26 This difference is possibly due to risk-taking behaviors. There appears to be no increase in DCS in women who are taking oral contraceptive agents or menstruating during diving.
A patent foramen ovale (PFO) is a risk factor for increased susceptibility to DCS.27,28 Sixty-five percent of divers who present with serious DCS have a PFO.27,29 Brain lesions occur in 27% of sport divers.30 This percentage is roughly the same as the prevalence of PFO or other right-to-left shunts in the general population. These multiple brain lesions may be caused by bubbles in the venous circulation that are not filtered by the vasculature of the lungs but enter the arterial circulation, even in the absence of other DCS symptoms.31 Most sport divers do not undergo screening for PFO with echocardiographic bubble studies; some PFOs may open only at increased ambient pressures so that bubble studies conducted at 1 ATA would be normal.32
The U.S. Navy dive tables estimate the amount of nitrogen that accumulates in the body during a dive to a particular depth and duration.3 The tables calculate a maximal dive time, called the no-decompression limit. If the no-decompression limits are exceeded, underwater decompression stops are recommended. Many sport scuba divers use submersible dive computers to calculate maximum dive times in lieu of the tables. These computers use mathematical algorithms to model nitrogen saturation in human tissues. Although they remove human calculation errors, such computers also tend to extend no-decompression times to their maximum limits.
These tables and computers are meant to reduce the likelihood of exceeding the solubility of nitrogen at sea level to produce DCS. The diver still must ascend in a slow, controlled manner to allow the gradual release of nitrogen. Off-gassing continues after the diver has surfaced. Repetitive dives within several hours result in accumulation of tissue nitrogen and shorter no-decompression limits. Because dive tables are based on several assumptions about nitrogen elimination, even strict adherence to these tables does not ensure that DCS will not occur.3 The risk of DCS increases as a diver approaches the no-decompression time limits and almost never occurs after dives to less than 6 meters and rarely at less than 10 meters.33 It is critical for the evaluating physician to realize that divers can develop DCS even when they are within any calculated no-decompression limits.
DCS typically is manifested within hours after surfacing. The U.S. Navy Diving Manual notes that 42% of symptoms occur within 1 hour after diving, 60% within 3 hours, 83% within 8 hours, and 98% within 24 hours.34 Flying shortly after diving or ascending to altitude, however, may cause symptoms in patients later than expected, and some patients present days after diving with DCS.34,35
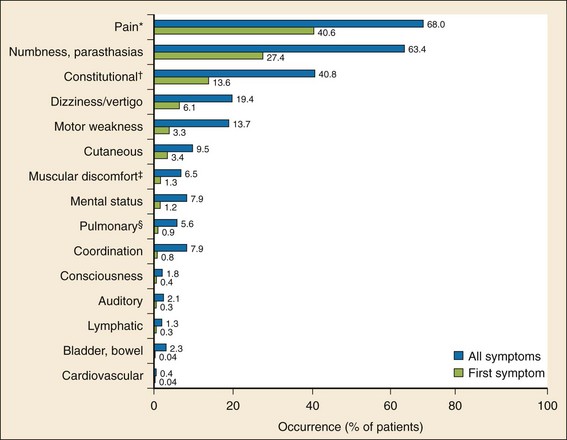
Traditionally, DCS has been divided into two categories, type I and type II. Type I DCS affects the musculoskeletal system, skin, and lymphatic vessels. Type II DCS involves any other organ system. The more inclusive “decompression-related illness” is now also used to encompass DCS I, DCS II, and arterial gas embolism (AGE). This terminology is adapted to aid clinicians in remembering that the distinction is irrelevant in the selection of treatment. All types of decompression illness require recompression.35 DCS I, DCS II, and AGE can coexist. The symptoms of DCS may be subtle and resolve by the time of evaluation. A distribution of the most common symptoms and the first symptoms is presented in Figure 143-7.
Type I DCS can be experienced as variable and periarticular pain in the arms and legs. The elbow and shoulder joints are most commonly affected. Local tenderness and erythema are uncommon. The placement of a blood pressure cuff inflated to 150 to 200 mm Hg on an affected joint produces relief of pain and confirms the diagnosis; however, the sensitivity of this maneuver was as low as 61% in one study.30 Skin marbling, known as cutis marmorata (patchy cyanotic marbling of the skin), results from venous stasis. It may begin as severe pruritus and progress into an erythematous rash and then skin mottling. Cutis marmorata does not follow a dermatomal distribution and commonly involves the trunk and torso. Lymphatic obstruction by air bubbles can also occur, causing extremity edema.
Spinal DCS can occur alone or in combination with cerebral, inner ear, or pulmonary symptoms. Cerebral symptoms include mild to moderate headache, blurred vision, diplopia, dysarthria, unusual fatigue, inappropriate behavior, and a sense of detachment. Loss of consciousness in CNS DCS is rare (in marked contrast to AGE).36 Magnetic resonance imaging, computed tomography (CT), and single-photon emission CT with technetium (Tc-99m)–labeled hexamethylpropyleneamine can identify the bubbles of CNS DCS. No imaging studies are sensitive enough to exclude DCS, however, and normal imaging results should not delay transfer for definitive therapy.
The physical examination of patients suffering from pulmonary DCS may reveal cyanosis and hypotension in association with increased central venous pressure and pulmonary arterial pressure, right-sided strain on an electrocardiogram, and decreased end-tidal carbon dioxide level. The condition may progress to respiratory arrest. Ancillary tests for pulmonary DCS not only are insensitive but also lead to unnecessary treatment delays. Even after ascent from very shallow saturation dives, microbubbles in the venous circulation can be routinely detected by M-mode ultrasonography; however, their presence does not necessarily correlate with symptoms.37–40
DCS may be particularly dangerous to a developing fetus of a scuba diving mother because the majority of the fetal circulation bypasses the pulmonary bed through the foramen ovale and the ductus arteriosus. This bypass prevents the fetal lungs from acting as a filter for microbubbles. In addition, venous gas emboli may appear in the fetal circulation before they are apparent in the maternal circulation.41,42 Data on the effects of diving on pregnant women suggest a higher incidence of low-birth-weight infants, prematurity, congenital malformations, stillbirths, and spontaneous abortions.43,44 There are no safe-diving tables that would protect a fetus from DCS; therefore, pregnant women should be advised to refrain from scuba diving.
Arterial Gas Embolism
AGE results when air bubbles are forced across the alveolar-capillary membrane, escape into the pulmonary venous circulation, and proceed through the left atrium and ventricle into the arterial circulation. It is one of the most common causes of death in divers.4,20,35 Clinical symptoms and signs are in part the result of mechanical obstruction by gas bubbles. AGE can also result from a right-to-left shunt of venous bubbles, such as in a diver with a PFO. AGE may occur alone or in conjunction with DCS.35
Although air bubbles may embolize to any organ, the coronary and cerebral arteries are associated with the most serious consequences. Emboli to the coronary arteries may cause cardiac ischemia, myocardial infarction, dysrhythmias, or cardiac arrest. Dysrhythmias are also indirectly caused by centrally mediated autonomic dysfunction from cerebral emboli.45,46 Mechanical occlusion of the cerebral vasculature from emboli, most commonly to the anterior and middle cerebral arteries, causes a variety of symptoms and signs similar in appearance to an acute stroke.
The clinical manifestations of AGE may be sudden, dramatic, and life-threatening. Divers who have supposedly “drowned” may actually have lost consciousness during ascent as a result of cerebral gas emboli. Any diver breathing compressed air at any depth underwater and who surfaces unconscious or who loses consciousness within 10 minutes of reaching the surface should be assumed to be suffering from AGE. The most common presentation of AGE includes a global alteration of consciousness, headache, dizziness, convulsions, and visual changes. Other common presenting symptoms and signs include cranial nerve symptoms, unilateral weakness, unilateral or bilateral sensory loss, ataxia, and speech changes.20 Pulmonary symptoms, including dyspnea, pleuritic chest pain, and hemoptysis, occur in 25 to 50% of cases.47
Diagnostic Strategies
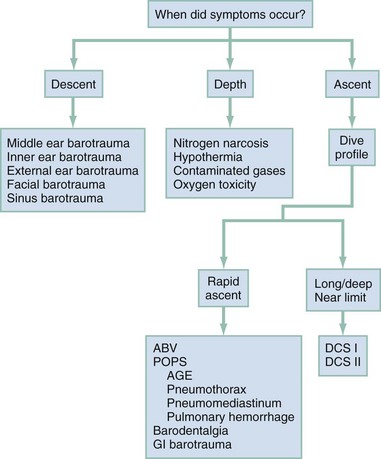
Focused questions concerning the dive profile, including the depth and length of the dive and a careful assessment of when the symptoms first occurred, may provide important diagnostic clues (Box 143-1). A physician unfamiliar with the specifics of scuba diving may rely on the assessment of members of the dive group to determine whether maximum dive limits were approached. In making the diagnosis of a dive injury, it is helpful to think of the injuries in terms of occurring during descent, while at depth, or during ascent (Fig. 143-8). Because recompression therapy is time sensitive, it is important to concentrate on treatment decisions rather than on securing an absolute diagnosis. The primary question to address is whether this dive injury is likely to be helped by recompression. Early consultation with a hyperbaric specialist is recommended.
Differential Considerations
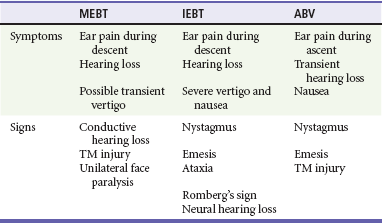
Most diving injuries have limited differential diagnoses that include medical disorders and trauma unrelated to dysbarism. The differential diagnosis of IEBT includes inner ear DCS, ABV, and isolated MEBT with a rupture of the tympanic membrane. It is relatively easy to distinguish IEBT from MEBT and ABV because the vestibular symptoms associated with the last two entities are transient and self-limited. When IEBT occurs simultaneously with MEBT, the presence of both may be documented by an audiogram, which demonstrates both a conductive and a sensorineural hearing loss (Table 143-3).
The differentiation of IEBT from inner ear neurologic DCS is crucial because the treatments differ. An IEBT is more likely when symptoms begin during descent or the diver relates a history of difficulty equilibrating or performing a vigorous Valsalva maneuver. If the dive profile is examined and the no-decompression limits are approached and symptoms began soon after surfacing, inner ear DCS is more likely.49 A history of difficulty in equalizing the ears on descent, or the onset of symptoms early in the dive, suggests barotrauma. A history of a dive that approaches decompression limits, or the onset of symptoms during or soon after ascent, and the presence of other neurologic findings suggest DCS. A trial of recompression therapy is prudent if concerns for DCS exist.
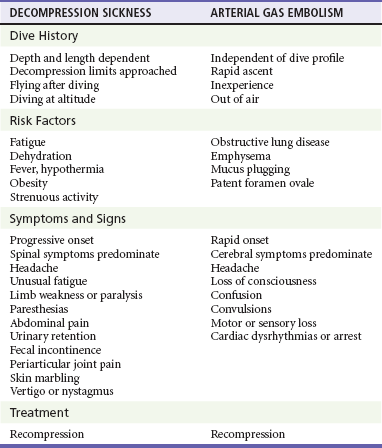
The differential diagnosis of pulmonary DCS includes AGE (Table 143-4). Although the treatment of both requires recompression therapy, they may be differentiated by the timing of the onset of symptoms. Almost all cases of AGE present within the first 10 minutes of surfacing, whereas DCS presents more typically after 10 minutes: 42% of pulmonary DCS symptoms begin within 1 hour of surfacing, 60% within 3 hours, 83% within 8 hours, and 98% within 24 hours.3
Management
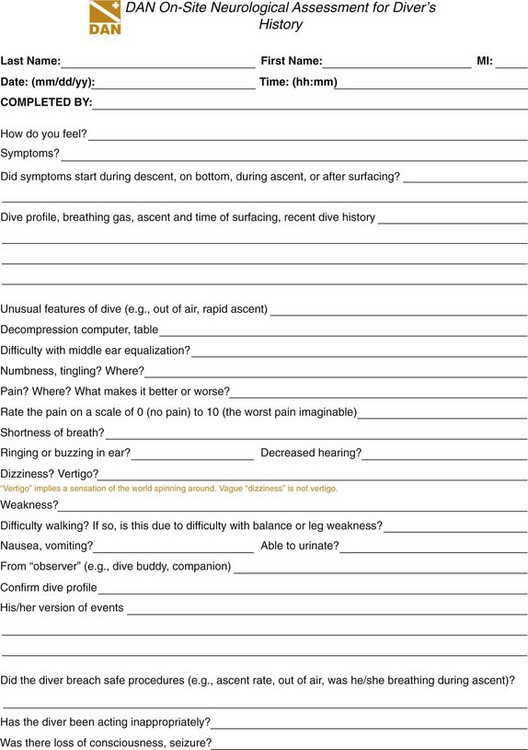
Patients with stable vital signs and suspected dive injuries should receive 100% oxygen until the clinician can exclude pulmonary barotrauma or decompression illness. Patients with unstable vital signs or in cardiac arrest should be treated according to advanced cardiac life support (ACLS) guidelines. The clinician should take a thorough history, including elements in Box 143-1, and then perform a thorough physical examination. Diving-related illnesses are diagnosed and treated on the basis of the history and physical examination, and several invaluable resources are available for advice. The Divers Alert Network (DAN), located at Duke University in Durham, North Carolina, is a membership association that provides courses on diving-related emergencies and publishes data on diving accidents and fatalities. Clinicians may obtain treatment advice by calling DAN. DAN provides a 24-hour medical emergency hotline at 1-919-684-9111 (collect calls are accepted) and a nonemergency advisory line Monday through Friday, 8:30 AM to 5 PM Eastern time, at 1-919-684-2948 or 1-800-446-2671. In addition, DAN international contacts are in Europe, Brazil, Japan, Asia Pacific, and southern Africa. DAN maintains a website with links to key information at www.diversalertnetwork.org. DAN uses a telephone intake form, the DAN On-Site Neurological Assessment for Diver’s History (Fig. 143-9); familiarity with this form may facilitate the emergency physician’s communications with DAN.
The U.S. Navy has its current diving manual (revision 6) available at its website, www.usu.edu/scuba/navy_manual6.pdf. This manual, updated in 2008, contains a wealth of information about diving principles, equipment, operations, and recompression.
Diving Disorders Requiring Recompression Therapy
Diving disorders that require recompression therapy are listed in Box 143-2.
Treatment with 100% oxygen replaces inert gases in the lungs with oxygen. By establishment of a large gradient from the tissues to the alveoli, removal of the inert gases is enhanced and bubble size is reduced.50 In addition, oxygen administration treats the tissue hypoxia caused by gas bubbles. Normobaric oxygen treatment during preparation for recompression therapy is associated with a need for fewer recompressions.51
Dehydration can increase the seriousness of DCS.52 Hydration decreases bubble formation after decompression.53 It also decreases the endothelial inflammation and damage associated with hemoconcentration and hemodynamic instability seen in dehydrated DCS patients.54,55 Therefore, intravenous fluids should be administered to divers suspected of having nonpulmonary (“chokes”) DCS. Overly aggressive hydration in divers with pulmonary DCS may worsen pulmonary edema. Similarly, fluid overload should be avoided in patients with cerebral or spinal cord edema. There is no clear consensus that the best fluid is crystalloid (normal saline or lactated Ringer’s solution) or colloid; many experts, however, advise against dextrose in water without electrolytes (e.g., D5W). Fluids should be given to ensure a urinary output of 0.5 mL/kg/hr.
The intravenous fluid treatment recommendations differ in AGE. Divers with AGE are less likely to be dehydrated than are divers with DCS. This may be because of a shorter period of immersion or because they experience less bubble-induced endothelial damage.3 Further, CNS injuries in AGE may be worsened by cerebral edema. Therefore, large-volume fluid resuscitation is not recommended in divers with AGE after a short, shallow dive.56
The goals of recompression therapy are to reduce the mechanical obstruction of air bubbles, to facilitate the washout of nitrogen by increasing the tissue-blood nitrogen gradient, and to increase oxygen delivery to ischemic tissue. Recompression is the only definitive treatment of DCS and AGE. Treatment of DCS or AGE should not be withheld even if a significant time delay in transfer to a hyperbaric chamber is unavoidable. Although delayed recompression is less effective than immediate recompression in serious cases, the time beyond which recompression offers no benefit is unknown.57
Hyperbaric therapy for AGE should be initiated as soon as possible, although there are cases of significant improvement even in the face of long delays until recompression.58 Patients with AGE who are recompressed within 5 minutes of surfacing have a mortality rate of 5%, and there is an extremely low risk of morbidity among the survivors.32 If recompression is delayed by 5 hours or more, the mortality rate increases to 10%, with 50% morbidity.32 Although spontaneous resolution of symptoms may occur in patients with AGE, all patients should be recompressed. The rationale is that although microbubbles may clear from the cerebral circulation, secondary capillary edema and swelling may result in a delayed recurrence of symptoms. Furthermore, more subtle symptoms may be appreciated only after the resolution of more prominent ones. Finally, minor symptoms may progress, and the relapse rate is high in untreated cases.
Similarly, the prognosis for DCS when it is treated with recompression is generally good but depends on the severity of symptoms at onset and the delay to recompression. A delay to definitive recompression treatment is associated with a worse outcome in cases of severe DCS. Patients can obtain some benefit from recompression, however, even if treatment is initiated more than 24 hours after the dive. Recompression therapy for DCS may be initiated as late as 10 to 14 days after exposure if necessary.59
Recompression for DCS and AGE is most often performed in a multiplace chamber with one or more in-chamber attendants. DCS and AGE have also been successfully treated in monoplace chambers.60 Monoplace chambers are compact, lightweight, and more widely available than multiplace chambers. Unfortunately, because of their design, most cannot be pressurized beyond 3 ATA (100 fsw) or deliver air-oxygen mixtures. The most common recompression schedule is U.S. Navy treatment (or an equivalent procedure); the diver is compressed to 2.8 bar (60 fsw pressure) while breathing 100% oxygen. The time to complete treatment is 4 hours 45 minutes, not including descent time.
In addition to recompression therapy, several adjunctive treatments are proposed in the treatment of DCS and AGE. The treatment or prevention of hypothermia may increase tissue perfusion and off-gassing. The nonsteroidal anti-inflammatory drug tenoxicam (Mobiflex) decreases the number of recompressions required for symptom resolution in DCS but does not change the ultimate outcome.61 Aspirin therapy (as a platelet aggregation inhibitor) has previously been recommended for DCS and AGE. Because of the lack of studies demonstrating safety and efficacy for DCS or AGE treatment, however, it cannot be recommended.3,35 Steroids have previously been advocated clinically for neurologic DCS and AGE. No significant improvements in neurologic sequelae are now demonstrated; however, the elevated blood glucose levels associated with steroid treatment may worsen the outcome of CNS injury.56
Anticoagulants should not be used routinely in the treatment of DCS. The one exception is in divers with lower extremity paralysis caused by neurologic DCS or AGE. Low-molecular-weight heparin (e.g., enoxaparin, 30 mg, subcutaneously every 12 hours; dose adjustment should be made for patients with renal impairment) after recompression therapy may be used in patients with inability to walk to prevent venous embolic disease. Intermittent pneumatic compression devices are alternatives.3,35
Lidocaine is not effective in DCS but may have neuroprotective effects in patients with AGE and serious neurologic symptoms. It is administered as an intravenous bolus of 1 mg/kg, followed by a drip infusion of 2 to 4 mg/min.56,62
Cardiac dysrhythmias may be refractory to standard treatments until the diver is recompressed. Defibrillation in a hyperbaric environment may be a fire hazard and is not recommended. Seizures may be managed with benzodiazepines; however, mannitol should be avoided. Spinal DCS patients often develop urinary retention requiring bladder catheterization. Of note, endotracheal tube and urinary catheter balloons should be inflated with sterile saline (not air) before recompression therapy is initiated. Nitrous oxide should be avoided because it can increase the size of tissue bubbles by inward diffusion.63
Although the head-down position (Trendelenburg) has previously been advocated to prevent migration of intra-arterial bubbles to the brain, it is not effective and may result in worsening cerebral edema and intracranial pressure.64 Transport of the patient with AGE in a flat supine position is recommended to maximize arterial-venous flow.
Diving Disorders Not Requiring Recompression Therapy
Diving disorders that do not require recompression therapy are listed in Box 143-3.
Prevention of MEBT requires that the diver equalize the pressure in both middle ears. Any diver who cannot clear both ears on the surface should not dive. The diver should never perform a forceful Valsalva maneuver during descent or ascent to clear the ears because of the risk of ABV, round or oval window rupture (descent), or pulmonary barotrauma (ascent). The prophylactic use of pseudoephedrine (60 mg taken 30 minutes before diving) or oxymetazoline nasal spray may reduce the incidence and severity of MEBT.7 The use of these medications to facilitate diving with symptoms of an upper respiratory infection, however, is not recommended. Antihistamines should be avoided before diving. Sinusitis and upper respiratory infections increase the likelihood of suffering barotitis. Diving should be avoided for 2 weeks after the resolution of an upper respiratory infection.65
The victim of facial barotrauma may have a dramatic appearance, but the condition is usually benign and requires no specific treatment. The patient should be advised not to resume diving until facial edema resolves.66
The evaluation and management of pneumomediastinum include serial chest radiographs to ensure that no coexisting pneumothorax develops. One hundred percent oxygen therapy may hasten the resolution of symptoms. In the rare case of respiratory compromise, tracheal intubation may be required. Most important, the presence of interstitial pulmonary emphysema should alert the physician to the possible coexistence of more severe forms of pulmonary barotrauma. The need for a surgical decompressive treatment of subcutaneous emphysema alone is extremely unlikely. Supportive therapy to correct hypoxia is indicated in the treatment of alveolar hemorrhage.19
Careful equalization during a slow ascent can prevent the occurrence of ABV. Oral and intranasal decongestants may be indicated if symptoms persist. Myringotomy is occasionally required.67
Disposition
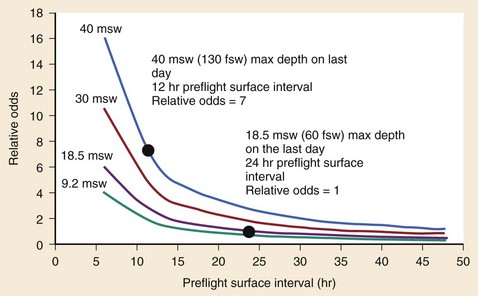
The recommended postdive preflight surface interval (PFSI) before ascending to higher altitudes (or flying) depends on the diver’s repetitive group designator (residual nitrogen time). The relative risk for development of DCS increases with greater residual nitrogen times and shorter PFSI (Fig. 143-10).34 For example, the risk for development of DCS is seven times greater for PFSIs of 12 hours after a dive to 130 fsw than for PFSIs of 24 hours after a dive to 60 fsw.34 Long PFSIs (up to 48 hours) may be necessary to reduce the risk of DCS after deep, multiday repetitive diving, particularly if the dives required decompression stops (exceeding the no-decompression limits).68 Some dive computers calculate time-to-flight intervals, which tend to be somewhat shorter than most guidelines based on residual nitrogen timetables. Flying should be delayed for at least 12 hours after diving if less than 2 hours of total dive time was accumulated in the preceding 48 hours. For multiple-day, unlimited diving, flying should be delayed for at least 24 hours. It is prudent to admit all recompressed patients or to advise them to remain within 60 minutes of the hyperbaric facility for 24 hours. Patients recompressed after DCS or AGE should not fly for 72 hours.
The U.S. Navy’s guidelines recommend that the patient not return to diving for 7 days after recompression for type I DCS and for 4 weeks after type II DCS.3 The sport diver who experiences DCS type II symptoms or AGE should probably never dive again.
After treatment for pulmonary barotrauma, evaluate the diver with a spiral volumetric chest CT scan to determine whether there are any preexisting pathologic conditions (e.g., bullae) that could put the diver at risk for recurrence before further diving.69 Chest CT is not recommended in routine medical screening before participation in scuba diving without a history of pulmonary barotrauma.
Echocardiography to assess for a PFO is often recommended for divers with severe or recurrent neurologic DCS.70,71 A substantial amount of venous gas must embolize through the PFO for significant symptoms to occur; that is unlikely to happen in conservative depth-time profiles. A diver with a diagnosed PFO should weigh the risks of continuing to dive and the risks of surgical repair.
References
1. Brylske, A. A brief history of diving: Part I. Dive Training. 1994.
2. Brylske, A. A brief history of diving: Part II. Dive Training. 1994.
3. U.S. Navy Diving Manual Revision 6. Washington, DC: U.S. Department of the Navy, 2008.
4. Pollock NW, ed. Diver’s Alert Network: Annual Diving Report. Durham, NC: Diver’s Alert Network, 2008.
5. Bove, AA, Davis, JC. Bove and Davis’ Diving Medicine, 4th ed. Philadelphia: WB Saunders; 2004.
6. Green, SM, Rothrock, SG, Green, EA. Tympanometric evaluation of middle ear barotrauma during recreational scuba diving. Int J Sports Med. 1993;14:411–415.
7. Brown, M, Jones, J, Krohmer, J. Pseudoephedrine for the prevention of barotitis media: A controlled clinical trial in underwater divers. Ann Emerg Med. 1992;21:849–852.
8. DeGorordo, A, Vallejo-Manzur, F, Chanin, K, Varon, J. Diving emergencies. Resuscitation. 2003;59:171–180.
9. Melamed, Y, Shupak, A, Bitterman, H. Medical problems associated with underwater diving. N Engl J Med. 1992;326:30–35.
10. Moon, RE. Treatment of diving emergencies. Crit Care Clin. 1999;15:429–456.
11. Cheshire, WP, Jr., Ott, MC. Headache in divers. Headache. 2001;41:235–247.
12. Neubauer, B, Mutzbauer, TS, Tetzlaff, K. Exposure to soda-lime dust in closed and semi-closed diving apparatus. Aviat Space Environ Med. 2000;71:1248–1251.
13. Landon, CW. Gastrointestinal barotrauma in scuba divers. West J Med. 1981;135:242.
14. Cramer, FS, Heimbach, RD. Stomach rupture as a result of gastrointestinal barotrauma in a SCUBA diver. J Trauma. 1982;22:238–240.
15. Neuman, TS, Bove, AA, O’Connor, RD, Kelsen, SG. Asthma and diving. Ann Allergy. 1994;73:344–350.
16. Coetmeur, D, Briens, E, Dassonville, J, et al. [Asthma and scuba diving: Absolute contraindication? in all asthma patients?]. Rev Mal Respir. 2001;18(Pt 1):381–386.
17. Harrison, D, Lloyd-Smith, R, Khazei, A, Hunte, G, Lepawsky, M. Controversies in the medical clearance of recreational scuba divers: Updates on asthma, diabetes mellitus, coronary artery disease, and patent foramen ovale. Curr Sports Med Rep. 2005;4:275–281.
18. British Thoracic Society Fitness to Dive Group, Subgroup of the British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines on respiratory aspects of fitness for diving. Thorax. 2003;58:3–13.
19. Balk, M, Goldman, JM. Alveolar hemorrhage as a manifestation of pulmonary barotrauma after scuba diving. Ann Emerg Med. 1990;19:930–934.
20. Kizer, KW. Dysbaric cerebral air embolism in Hawaii. Ann Emerg Med. 1987;16:535–541.
21. Masurel, G. [The value of ultrasonic detection of circulating bubbles in animal and man—the contribution to physiopathogenesis of a decompression accident]. Schweiz Z Sportmed. 1989;37:41–44.
22. Baj, Z, Olszanski, R, Majewska, E, Konarski, M. The effect of air and nitrox divings on platelet activation tested by flow cytometry. Aviat Space Environ Med. 2000;71:925–928.
23. Spencer, MP, Clarke, HF. Precordial monitoring of pulmonary gas embolism and decompression bubbles. Aerospace Med. 1972;43:762–767.
24. Butler, BD, Hills, BA. The lung as a filter for microbubbles. J Appl Physiol. 1979;47:537–543.
25. Brubakk, AO, Neuman, TS. Bennett and Elliott’s Physiology and Medicine of Diving, 5th ed. Philadelphia: WB Saunders; 2003.
26. St Leger Dowse, M, Bryson, P, Gunby, A, Fife, W. Comparative data from 2250 male and female sports divers: Diving patterns and decompression sickness. Aviat Space Environ Med. 2002;73:743–749.
27. Lairez, O, et al. Risk of neurological decompression sickness in the diver with a right-to-left shunt: Literature review and meta-analysis. Clin J Sport Med. 2009;19:231–235.
28. Gempp, E, Blatteau, JE. Decompression sickness with a right-to-left shunt. Clin J Sport Med. 2009;19:512–513.
29. Moon, RE, Camporesi, EM, Kisslo, JA. Patent foramen ovale and decompression sickness in divers. Lancet. 1989;1:513–514.
30. Reul, J, Weis, J, Jung, A, Willmes, K, Thron, A. Central nervous system lesions and cervical disc herniations in amateur divers. Lancet. 1995;345:1403–1405.
31. Knauth, M, et al. Cohort study of multiple brain lesions in sport divers: Role of a patent foramen ovale. BMJ. 1997;314:701–705.
32. Tomassoni, AJ. Cardiac problems associated with dysbarism. Cardiol Clin. 1995;13:266–271.
33. Van Liew, HD, Flynn, ET. Direct ascent from air and N2-O2 saturation dives in humans: DCS risk and evidence of a threshold. Undersea Hyperb Med. 2005;32:409–419.
34. Freiberger, JJ, et al. The relative risk of decompression sickness during and after air travel following diving. Aviat Space Environ Med. 2002;73:980–984.
35. Vann, RD, Butler, FK, Mitchell, SJ, Moon, RE. Decompression illness. Lancet. 2011;377:153–164.
36. Dick, AP, Massey, EW. Neurologic presentation of decompression sickness and air embolism in sport divers. Neurology. 1985;35:667–671.
37. Bayne, CG, Hunt, WS, Johanson, DC, Flynn, ET, Weathersby, PK. Doppler bubble detection and decompression sickness: A prospective clinical trial. Undersea Biomed Res. 1985;12:327–332.
38. Eckenhoff, RG, Olstad, CS, Carrod, G. Human dose-response relationship for decompression and endogenous bubble formation. J Appl Physiol. 1990;69:914–918.
39. Ikeda, T, Okamoto, Y, Hashimoto, A. Bubble formation and decompression sickness on direct ascent from shallow air saturation diving. Aviat Space Environ Med. 1993;64:121–125.
40. Ikeda, T, Suzuki, S, Shimizu, K, Okamoto, Y, Llewellyn, ME. M-mode ultrasonic detection of microbubbles following saturation diving: A case report and proposal for a new grading system. Aviat Space Environ Med. 1989;60:166–169.
41. Fife, WP, Simmang, C, Kitzman, JV. Susceptibility of fetal sheep to acute decompression sickness. Undersea Biomed Res. 1978;5:287–292.
42. Stock, MK, et al. Responses of fetal sheep to simulated no-decompression dives. J Appl Physiol. 1980;48:776–780.
43. Bolton, ME. Scuba diving and fetal well-being: A survey of 208 women. Undersea Biomed Res. 1980;7:183–189.
44. Turner, G, Unsworth, I. Intrauterine bends? Lancet. 1982;1:905.
45. Cales, RH, Humphreys, N, Pilmanis, AA, Heilig, RW. Cardiac arrest from gas embolism in scuba diving. Ann Emerg Med. 1981;10:589–592.
46. Evans, DE, Kobrine, AI, Weathersby, PK, Bradley, ME. Cardiovascular effects of cerebral air embolism. Stroke. 1981;12:338–344A.
47. Ball, R. Effect of severity, time to recompression with oxygen, and re-treatment on outcome in forty-nine cases of spinal cord decompression sickness. Undersea Hyperb Med. 1993;20:133–145.
48. Wilmshurst, PT, Nuri, M, Crowther, A, Webb-Peploe, MM. Cold-induced pulmonary oedema in scuba divers and swimmers and subsequent development of hypertension. Lancet. 1989;1:62–65.
49. Adkisson, GH, Meredith, AP. Inner ear decompression sickness combined with a fistula of the round window. Case report. Ann Otol Rhinol Laryngol. 1990;99(Pt 1):733–737.
50. Hyldegaard, O, Moller, M, Madsen, J. Effect of He-O2, O2, and N2O-O2 breathing on injected bubbles in spinal white matter. Undersea Biomed Res. 1991;18:361–371.
51. Longphre, JM, Denoble, PJ, Moon, RE, Vann, RD, Freiberger, JJ. First aid normobaric oxygen for the treatment of recreational diving injuries. Undersea Hyperb Med. 2007;34:43–49.
52. Fahlman, A, Dromsky, DM. Dehydration effects on the risk of severe decompression sickness in a swine model. Aviat Space Environ Med. 2006;77:102–106.
53. Gempp, E, Blatteau, JE, Pontier, JM, Balestra, C, Louge, P. Preventive effect of pre-dive hydration on bubble formation in divers. Br J Sports Med. 2009;43:224–228.
54. Brunner, FP, Frick, PG, Buehlmann, AA. Post-decompression shock due to extravasation of plasma. Lancet. 1964;1:1071–1073.
55. Trytko, B, Mitchell, S. Extreme survival: A deep technical diving accident. SPUMS J. 2005;35:23–27.
56. Moon, R. Adjunctive Therapy for Decompression Illness. Kensington, Md: UHMS Research Foundation; 2003.
57. Gempp, E, Blatteau, JE. Risk factors and treatment outcome in scuba divers with spinal cord decompression sickness. J Crit Care. 2010;25:236–242.
58. Tirpitz, D, Schipke, JD. Delayed recompression after SCUBA diving–induced barotrauma: A case report. Aviat Space Environ Med. 1996;67:266–267.
59. Myers, RA, Bray, P. Delayed treatment of serious decompression sickness. Ann Emerg Med. 1985;14:254–257.
60. Cianci, P, Slade, JB, Jr. Delayed treatment of decompression sickness with short, no-air-break tables: Review of 140 cases. Aviat Space Environ Med. 2006;77:1003–1008.
61. Bennett, M, Mitchell, S, Dominguez, A. Adjunctive treatment of decompression illness with a non-steroidal anti-inflammatory drug (tenoxicam) reduces compression requirement. Undersea Hyperb Med. 2003;30:195–205.
62. Mitchell, SJ. Lidocaine in the treatment of decompression illness: A review of the literature. Undersea Hyperb Med. 2001;28:165–174.
63. Acott, CJ, Gorman, DF. Decompression illness and nitrous oxide anaesthesia in a sports diver. Anaesth Intensive Care. 1992;20:249–250.
64. Moon, RE, Sheffield, PJ. Guidelines for treatment of decompression illness. Aviat Space Environ Med. 1997;68:234–243.
65. Parell, GJ, Becker, GD. Conservative management of inner ear barotrauma resulting from scuba diving. Otolaryngol Head Neck Surg. 1985;93:393–397.
66. Rudge, FW. Ocular barotrauma caused by mask squeeze during a scuba dive. South Med J. 1994;87:749–750.
67. Becker, GD. Recurrent alternobaric facial paralysis resulting from scuba diving. Laryngoscope. 1983;93:596–598.
68. Sheffield, PJ. Flying after diving guidelines: A review. Aviat Space Environ Med. 1990;61:1130–1138.
69. Tetzlaff, K, Neubauer, B, Reuter, M, Warninghoff, V. Pulmonary barotrauma of a diver using an oxygen rebreathing diving apparatus. Aviat Space Environ Med. 1996;67:1198–1200.
70. Germonpre, P, Dendale, P, Unger, P, Balestra, C. Patent foramen ovale and decompression sickness in sports divers. J Appl Physiol. 1998;84:1622–1626.
71. Harrah, JD, O’Boyle, PS, Piantadosi, CA. Underutilization of echocardiography for patent foramen ovale in divers with serious decompression sickness. Undersea Hyperb Med. 2008;35:207–211.