Chapter 30 Screening for Esophageal Squamous Cell Carcinoma and Its Precursor Lesions
![]() Video related to this chapter’s topics: Endoscopic Screening for Esophageal Squamous Cell Carcinoma
Video related to this chapter’s topics: Endoscopic Screening for Esophageal Squamous Cell Carcinoma
Introduction
Esophageal cancer is the sixth leading cause of cancer death worldwide.1 In 2002, it was estimated that there were 462,000 new esophageal cancer cases and 386,000 deaths caused by esophageal cancer, only 25,000 fewer deaths than were caused by breast cancer.1 About 80% of esophageal cancer cases occur in developing countries.1 In the United States, esophageal cancer is the ninth leading cause of cancer death; an estimated 16,470 new cases and 14,530 deaths occurred as a result of esophageal cancer in 2009.2
One striking characteristic of esophageal cancer throughout the world is its great geographic variation in incidence, with 10-fold differences reported over distances of a few hundred kilometers.3 Worldwide, the highest risk populations are found in two geographic belts: one in central Asia from the Caspian Sea to north central China and one from eastern to southern Africa (Fig. 30.1).4 Age-adjusted incidence rates over 100 cases per 100,000 inhabitants per year have been reported from some areas in these regions.3,5,6 Populations with intermediate risk are found in southern South America (in southern Brazil, Uruguay, Paraguay, and northern Argentina) and in northwestern France.5 People throughout most of the world are considered to be at low risk, with incidence rates less than 10 per 100,000 inhabitants per year.5 In the Surveillance Epidemiology and End Results (SEER) cancer registries in the United States, the age-adjusted incidence of EC per 100,000 population per year in 2002–2006 was 7.9 in white men, 1.9 in white women, 9.3 in black men, and 3.0 in black women.7 In low-risk countries such as the United States, the male-to-female ratio of cases is usually about 3 : 1 to 4 : 1,7 but in the highest risk populations, this ratio approaches or even falls below 1 : 1.3,6
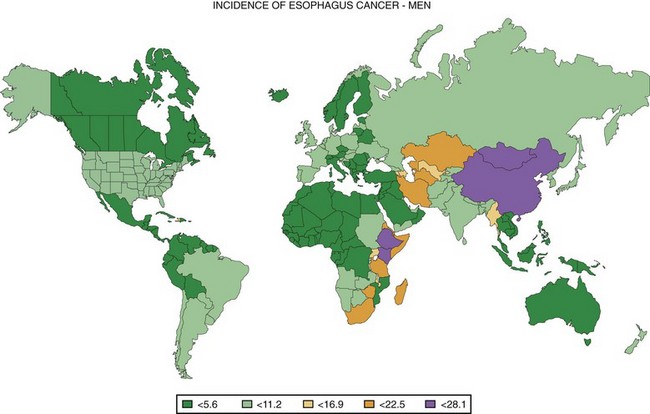
Fig. 30.1 Worldwide incidence of esophageal cancer in men, 2002.
(Data from Ferlay J, Bray F, Pisani P, et al: GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide: IARC cancer base No. 5. version 2.0, Lyon, 2004, IARC Press.)
Throughout most of the world, most esophageal cancer cases are esophageal squamous cell carcinomas (ESCCs).5 In Western countries, the incidence of ESCC has been gradually declining, and the incidence of esophageal adenocarcinoma has been rapidly increasing over the last 30 years,5,8 so that now greater than 50% of esophageal cancer cases in the United States are esophageal adenocarcinoma.5,7
In most low-risk countries, cigarette smoking and alcohol consumption are the dominant risk factors for ESCC.9–11 In the United States, greater than 90% of ESCC cases can be attributed to these two exposures alone.12 Additional contributing risk factors include a low dietary intake of fruits and vegetables and factors related to low socioeconomic status.13–16 A few host medical conditions have also been associated with increased risk of ESCC in low-risk populations, including previous or concurrent squamous cell carcinoma of the head and neck region, achalasia, tylosis, caustic esophageal strictures, and Plummer-Vinson syndrome.17
In most high-risk populations, tobacco and alcohol are not major risk factors for ESCC. Tobacco consumption in these groups is typically low, both in terms of the prevalence of smoking and in the amount of tobacco consumed by smokers, and alcohol consumption is even lower.18–20 In addition, in the highest risk areas, where there are nearly as many ESCC cases in women as in men, virtually none of the women smoke or drink.18–20 These high-risk groups may be exposed to some of the major tobacco carcinogens, however, such as polycyclic aromatic hydrocarbons (PAHs), nitrosamines, and acetaldehyde, in other ways. Studies have documented high levels of PAH exposure in Linxian, a county in the high-risk region in north central China21; in northeastern Iran22; and in southern Brazil.23 The source of these exposures is unknown; in Linxian, it may be related to ingestion of ambient soot particles released from heating and cooking with soft coal in unvented stoves,24,25 and in Brazil, it may be caused by drinking the beverage maté.23,26 Nontobacco exposure to nitrosamines and acetaldehyde has also been suggested in Linxian27,28 and in northeastern Iran29 possibly secondary to changes in oral bacteria that accompany poor oral hygiene and tooth loss. Other risk factors reported in high-risk areas include diets low in fruits and vegetables20,30,31; low levels of certain micronutrients, especially selenium and zinc32,33; low socioeconomic status34; exposure to fungal toxins such as fumonisins35; and drinking hot liquids.30,36,37
One of the most consistent risk factors for ESCC in high-risk populations is family history,20,38,39 and preliminary molecular studies support a role for genetic susceptibility in the etiology of ESCC in these areas. Studies have shown high frequencies of loss of heterozygosity (LOH),40 characteristic patterns of gene expression,41 and significant differences in both LOH and gene expression by family history41,42 in tumors from north central China, but no major susceptibility gene for ESCC has yet been identified.
Both cell types of esophageal cancer have a very poor prognosis. In SEER data for 1999–2005, the overall 5-year relative (disease-specific) survival for EC patients was 16.4%.7 This survival rate is improved from 4.7% in 1975–1979,43 but it is still the third lowest survival rate (after pancreas and liver) among major cancers. In developing countries, the 5-year survival rates are usually less than 10%.5
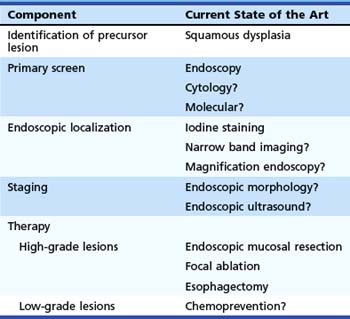
Table 30.1 summarizes these components for ESCC and our understanding of the current techniques available for each component. Techniques that are possible but are not yet established are followed by a question mark. Because the subject of this chapter is screening, we discuss only the first three of these components. Staging and endoscopic therapy are discussed in other chapters of this text.
Table 30.1 Components Needed for Successful Early Detection and Treatment Program for Esophageal Squamous Cell Carcinoma
| Component | Current State of the Art |
|---|---|
| Identification of precursor lesion | Squamous dysplasia |
| Primary screen | Endoscopy |
| Cytology? | |
| Molecular? | |
| Endoscopic localization | Iodine staining |
| Narrow band imaging? | |
| Magnification endoscopy? | |
| Staging | Endoscopic morphology? |
| Endoscopic ultrasound? | |
| Therapy | |
| High-grade lesions | Endoscopic mucosal resection |
| Focal ablation | |
| Esophagectomy | |
| Low-grade lesions | Chemoprevention? |
Precursor Lesions of Esophageal Squamous Cell Carcinoma
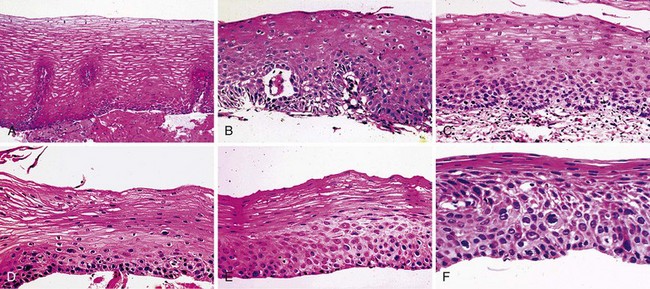
In low-risk countries, squamous dysplasia (including carcinoma in situ) is accepted as the histologic precursor of ESCC because it is the established precursor in other organs lined by squamous epithelium, such as the cervix, and because it is often found adjacent to invasive cancer in esophagectomy specimens.44 In high-risk populations, squamous dysplasia is also accepted as a precursor of ESCC,45–49 but other histologic lesions, such as chronic esophagitis, atrophy, and basal cell hyperplasia, have also been proposed as precursors, based primarily on differences in the prevalence of these lesions in high-risk and low-risk groups.46,49,50 These ecologic comparisons have not always been consistent, however.51 In the only two prospective studies, in which patients who underwent biopsy were followed over time, only squamous dysplasia was significantly associated with the later development of ESCC,45,47,48 and increasing grades of dysplasia were associated with increasing risk.45,48 In the larger of these prospective studies, members of our group followed 682 adults from Linxian who underwent endoscopy without initial evidence of invasive cancer for up to 13.5 years and compared the cumulative incidence and relative risk of developing ESCC among patients with different initial biopsy diagnoses (Fig. 30.2 and Table 30.2).
Table 30.2 Incidence and Relative Risk of Esophageal Squamous Cell Carcinoma (ESCC) during 13.5 Years of Follow-up, by Initial Histology, in an Endoscopy Cohort from Linxian, China
We believe that squamous dysplasia is the only confirmed, clinically relevant precursor lesion of ESCC in high-risk and low-risk populations. More recent consensus conferences and World Health Organization guidelines prefer to use the term intraepithelial neoplasia rather than dysplasia throughout the gastrointestinal (GI) tract, and they prefer to subdivide these lesions into two grades: low-grade intraepithelial neoplasia and high-grade intraepithelial neoplasia.52,53 In the squamous lesions of the esophagus, the guidelines suggest that high-grade intraepithelial neoplasia should include severe dysplasia and carcinoma in situ.52 The above-described data suggest, however, that there are three quite different levels of risk that are identifiable by histologic examination, and both moderate and severe dysplasia need to be targeted for screening and treatment.
Nonendoscopic Screening Techniques
Cytologic Techniques
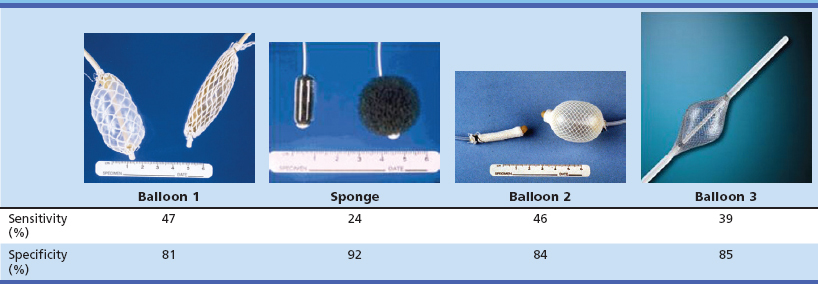
The most common nonendoscopic screening technique for early detection of esophageal cancer is esophageal balloon cytology screening in high-risk populations. Two principal types of cytologic samplers have been used in these screenings: an inflatable balloon sampler first developed in China54–57 and an encapsulated sponge sampler first developed in Japan (Fig. 30.3).58–61 In the balloon technique, a deflated balloon covered by a cloth net or rubber ribbing is swallowed into the stomach, inflated, and withdrawn, collecting exfoliated cells and scraping the mucosal surface of the esophagus. At the upper esophageal sphincter, the balloon is deflated and removed. In the sponge technique, a polyurethane mesh is compressed inside a gelatin capsule and attached to a string or a thin plastic stylet. The capsule is swallowed into the stomach, where the gelatin dissolves, and the mesh expands. The mesh is pulled up the esophagus by the string, collecting exfoliated and scraped mucosal cells. In both methods, the collected cells are processed and stained for cytology and read for cellular abnormalities. Several studies of both of these methods have reported high sensitivities for detecting ESCC in symptomatic patients, but there are few data on their accuracy for detecting squamous dysplasia or for detecting ESCC in asymptomatic individuals, who would be the target group for any population screening effort.
To investigate further the potential of nonendoscopic esophageal cytology for screening asymptomatic high-risk individuals, we performed two studies in Linxian to evaluate the screening characteristics of two commonly used Chinese balloons, a new American balloon, and an American-made encapsulated sponge. In this study, asymptomatic adults were examined by the cytology samplers, followed by Lugol’s iodine chromoendoscopy of all participants. The cytology slides were read using the Bethesda System, the standard Western cytologic criteria for diagnosing cervical and vaginal smears, and the cytologic diagnoses were compared with the “gold standard” biopsy diagnoses. In the first study,62 439 patients were examined by both a Chinese balloon and the encapsulated sponge, in random order, and in the second study,63 359 patients were examined by the second Chinese balloon, and 381 patients were examined by the American balloon. The results of these studies were not encouraging: None of the samplers achieved a sensitivity of 50% for identifying biopsy-proven squamous dysplasia or cancer (Table 30.3). The most important problem with this screening technique appears to be two kinds of sampling error: blind sampling of a large organ and missing small lesions and morphologic evaluation of only a small percentage of the collected cells.
Table 30.3 Screening Characteristics of Esophageal Balloon Cytology Samplers for Identifying Patients with Biopsy-Proven Esophageal Squamous Dysplasia in Linxian, China
Molecular Techniques
Blood or one of its components would be an ideal clinical sample for primary screening purposes. Possible serologic approaches to screening include looking for tumor-specific DNA, RNA, or proteins that are secreted into the circulation or are released during tumor cell death and looking for autoantibodies produced by the host in response to tumor antigens. A few authors have looked for tumor-specific hypermethylated genes in the serum or plasma of patients with ESCC and have found them to be present in a few cases.64,65 Hibi and colleagues64 found hypermethylated p16 in tumor tissue in 31 (82%) of 38 ESCCs and found this same marker in the serum of 7 (23%) of the 31 patients with positive tumors. Kawakami and associates65 found hypermethylated APC in tumor tissue from 16 (50%) of 32 ESCCs and in the corresponding serum from 2 (12%) of the 16 tumor-positive patients. In the latter study, detection of hypermethylated APC in the plasma of esophageal adenocarcinomas was significantly associated with tumor stage (1 of 26 [4%] positive in stage I-II tumors; 12 of 26 [46%] positive in stage III-IV tumors); this should probably also be true of ESCCs. Although the proportion of tumors showing some hypermethylation increases when multiple genes are evaluated,66 it still seems unlikely that many intraepithelial precursor lesions or stage I ESCCs would shed altered DNA into the serum or plasma that can be detected by such evaluations. Preliminary serologic studies looking for tumor-associated RNA67 and proteins68 have also been performed. Another possible serologic screening approach that has shown promise for detection of early squamous cell carcinomas of the lung and the head and neck region is identification of host autoantibodies generated against tumor-specific antigens.69–71
Stool is another clinical sample that could potentially contain information about the esophagus and could be collected noninvasively. Several authors have shown that neoplasia-specific DNA mutations can be detected in stool from patients with colorectal adenomas and adenocarcinomas,72,73 and the sensitivity of these assays can be increased by testing panels of markers73a and by employing more sensitive new technologies.74 There are also two reports of detection of other, more proximal aerodigestive malignancies, including esophageal carcinomas, by measuring high-molecular-weight or “long” DNA (DNA from nonapoptotic cells, which are more commonly sloughed from neoplasms)75 or tumor-specific DNA mutations76 in stool. Detection of esophageal precursor lesions or early invasive ESCCs by these methods may be unlikely, but it should still be evaluated.
At least for the near future, nonendoscopic molecular screening for early ESCC and its precursor lesions may still need to depend on evaluation of esophageal cell samples. As discussed previously, current morphology-based cytologic techniques are not sufficiently sensitive to find patients with focal squamous dysplasia, but molecular techniques may improve this sensitivity, especially DNA changes that can be amplified. One study reported promoter methylation findings for eight genes in esophageal balloon cytology samples from 147 patients with endoscopic biopsy diagnoses ranging from normal to severe squamous dysplasia and showed a 50% sensitivity and 68% specificity for identifying patients with high-grade dysplasia.77 The most promising possibility may be the detection of molecular changes that have undergone clonal expansion and affect large areas of the squamous mucosa, similar to the “field effects” previously documented for p16 and p53 lesions in Barrett’s esophagus.78,79 If such field-wide molecular abnormalities are identified that reliably precede or accompany squamous dysplasia, a simple, imperfect sampler that is acceptable to patients, such as an encapsulated sponge, may be able to identify accurately or rule out the presence of an abnormal field, which may be sufficient to triage patients appropriately to endoscopy.
Endoscopic Screening Techniques
Various different endoscopes have been used to screen for early ESCC and its precursor lesions. These endoscopes may differ in size, resolution, and magnification characteristics. Ultrathin endoscopes measuring less than 6 mm (with a biopsy channel) may be used. A trial with a 3.1-mm stand-alone battery-powered fiberoptic esophagoscope (which did not have a biopsy channel) showed that it was feasible and accurate in detecting esophageal pathology.80 Typically, instruments with diameters of 9 mm are employed. Standard resolution and magnification instruments suffice, but advantages of higher resolution and magnification have been described.81 Conventional videoendoscopes are equipped with CCD chips of 100k to 300k pixels, meaning that each image is built up from 100,000 to 300,000 individual pixels. This technical feature, pixel density, determines the resolution. Newer instruments with CCDs having 600,000 to 1 million pixels are now commercially available and are referred to as high-resolution instruments. The feature of high resolution is distinct from high magnification, which can be accomplished by either optical magnification or electronic magnification. With optical magnification, a mechanical system that can move a lens at the tip of the endoscope along the longitudinal axis of the endoscope creates the ability for the endoscopist to adjust the focal distance of the device to allow for close viewing of mucosal details. Depending on which endoscope and which optical magnification system is used, the image can be enlarged 75 to 115 times. With optical magnification, image resolution can be preserved during the magnification process. With an electronic magnification technique, the actual magnification is performed by the videoprocessor. An area in the center of the endoscopic images magnifies only the pixels in the target area, which are used to generate the enlarged image. It is often more difficult to preserve image resolution in this method. Features of high resolution and high magnification can be combined. Most of the published literature regarding endoscopic screening describes the use of standard endoscopes.
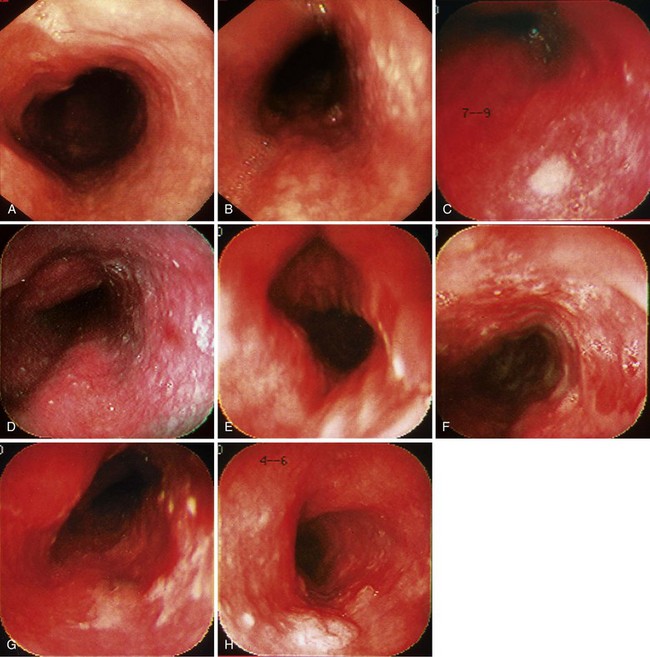
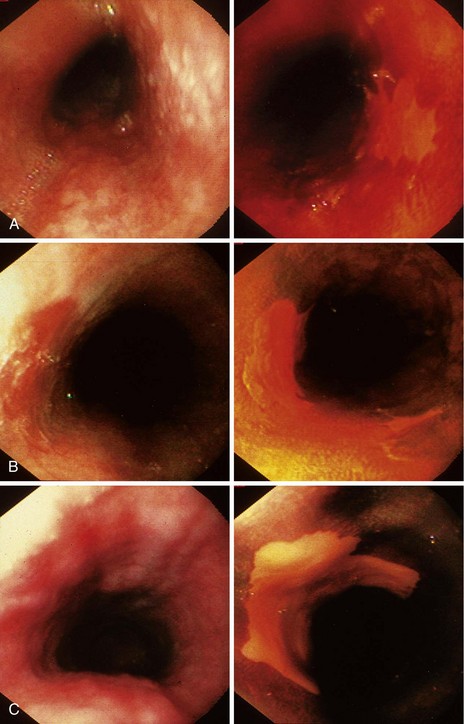
When screening is performed endoscopically, visual inspection to identify pathology is the first step. In the absence of staining, dysplasia may appear as normal mucosa, irregular mucosa, a small white patch, a focal red area, an erosion, or a plaque. Early ESCC is usually seen as an erosion, a plaque, or a nodule (Fig. 30.4).82
Chromoendoscopy
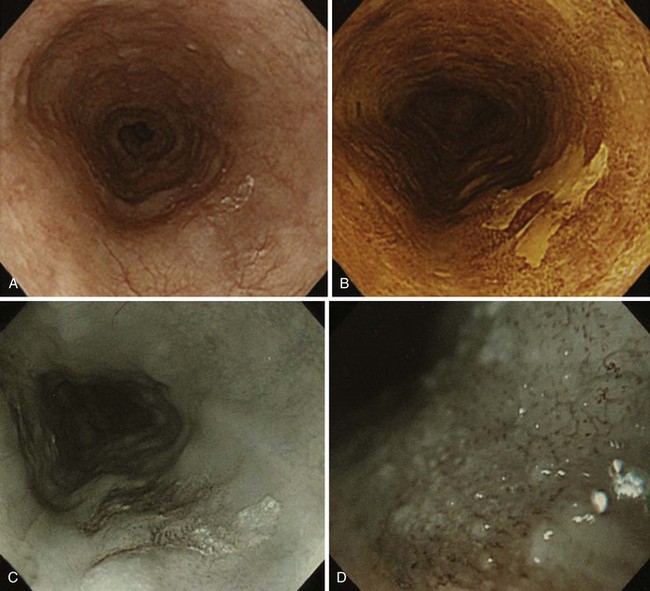
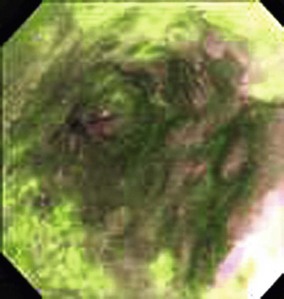
Chromoendoscopy has been shown to assist with definition of early precancerous and cancerous lesions in numerous patients. When chromoendoscopy is used, Lugol’s iodine is generally employed for staining. The first description of iodine staining was by Schiller in 1933,83 and it was used in the uterine cervix. The first reports using iodine as a supplement to esophagoscopy were by Brodmerkel,84 Nothmann and colleagues,85 and Voegeli.86 Iodine reversibly binds to glycogen, which is abundant in normal nonkeratinized squamous epithelial cells. Iodine staining causes a brown color change in normal esophageal mucosa. Inflamed, dysplastic, or cancerous cells are relatively depleted of glycogen and do not take up as much of the dye. Foci of glycogenic acanthosis have more than the usual amount of glycogen and appear overstained. Lugol’s iodine can be used to identify abnormal mucosal areas (unstained lesions), including esophagitis, dysplasia, and ESCC (Fig. 30.5). It also can be valuable in delineating the margins of these lesions, which is particularly relevant if endoscopic mucosal resection or an ablative treatment is contemplated.
In a typical procedure, the patient is placed in the left lateral decubitus position. Sedation may or may not be used. Often a “Cetacaine-type” medication is sprayed onto the throat, or a “Dicaine-type” slurry is drunk. If sedation is used, typically a benzodiazepine with or without a narcotic is given. The esophageal mucosa must be assessed in its entirety, so evaluation must begin as soon as the instrument passes the upper esophageal sphincter. The endoscopist searches for mucosal abnormalities as described previously (see Fig. 30.4).
If the initial endoscopy without staining is to be followed by chromoendoscopy, biopsies are deferred until after the staining. Various concentrations of Lugol’s iodine have been employed, but in most cases the concentration is between 1% and 2%. The formula of the iodine stain that we use (12 g iodine + 24 g potassium iodide dissolved in 1000 mL of water) was taken from the work of Endo and Ide.87 This formula is slightly stronger than Lugol’s original solution (1 g iodine + 2 g potassium iodide in water to 100 g). The formula we use has been called 1.2% Lugol’s by some authors, referring to its elemental iodine content, and 3% Lugol’s by other authors, referring to the total (iodine + potassium iodide) iodine content. For this reason, it is important to specify the formula that is used.
Some investigators advocate pretreatment with a water rinse or a mucolytic agent to remove mucus from esophageal mucosa.88 It has not been our practice to do so.
Our group evaluated the utility of mucosal iodine staining to improve endoscopic visualization of dysplasia and ESCC in the high-risk population of Linxian, China.89 In our mucosal staining study, 225 patients with evidence of dysplasia or carcinoma found on previous balloon cytology were evaluated with endoscopy before and after staining with 1.2% Lugol’s iodine solution. In these patients, 253 unstained lesions, 94 foci of high-grade (moderate or severe) dysplasia and 20 invasive ESCCs were found. Before staining, the sensitivity of visible lesions for identifying high-grade dysplasia or ESCC was 62%, and the specificity was 79%. After staining, the sensitivity of unstained lesions for identifying high-grade dysplasia or ESCC was 96%, and the specificity was 63%. Mucosal iodine staining significantly improved endoscopic detection of high-grade dysplasia and ESCC.
In this study, Lugol’s staining also greatly improved delineation of the significant mucosal abnormalities. Examples of dysplastic lesions before and after iodine staining are shown in Fig. 30.6. The outlines of the lesions are much clearer after staining. Clarity of the lesion outlines is very important when endoscopic treatment is delivered (Fig. 30.7).90
Misumi and colleagues88 evaluated the role of Lugol’s dye endoscopy in diagnosing ESCC in 17 lesions in 10 patients. In 14 instances, there was an abnormality before staining—either a color change (redness) or elevation or depression. The lesions ranged in size from 0.7 to 4.0 cm. There was good correlation in all cases between the endoscopic detection of the unstained area and the lesion margins in the final surgical specimen. The investigators concluded that Lugol’s staining was useful in diagnosing ESCC because it found some abnormalities not detected by routine endoscopy, and it provided more accurate information about the extent of the cancer.
Freitag and coworkers91 studied patients at risk for squamous cell carcinoma in southern Brazil, where esophageal cancer is the fourth most frequent neoplasm in men and the eighth most common neoplasm in women. When Lugol’s solution was applied in this high-risk group, if unstained lesions were found, there was an 80% sensitivity and 63% specificity for the detection of dysplasia.
Shiozaki and associates92 used Lugol’s iodine to screen asymptomatic patients with head and neck cancer for esophageal cancer. Of 178 screened patients, 9 (5%) had one or more esophageal cancers identified. Of the 13 cancers found, 8 were early stage disease, with no lymph node metastases. Only 4 of the 13 lesions were seen at routine endoscopy. The investigators concluded that Lugol’s chromoendoscopy should be considered in asymptomatic patients with a history of head and neck cancer and in any other patient populations at high risk for ESCC.
Ina and colleagues93 also evaluated the utility of Lugol’s staining in male patients with oral or oropharyngeal cancer who had no symptoms of esophageal disease. Lugol’s dye was used to screen 101 patients with oral cancer and 26 patients with oropharyngeal cancer. Of these 127 patients, 8 (6%) had esophageal cancer; in 5 of the 8 patients, no abnormality was seen on routine endoscopy or barium swallow.
Muto and associates94 performed chromoendoscopy with Lugol’s solution in 389 patients with head and neck cancer and found 54 (14%) who had a synchronous primary ESCC. They characterized the staining pattern in the 389 patients into four groups. Type I patients had normal brown staining; type II patients had 10 or fewer unstained areas; type III patients had more than 10 unstained areas; and type IV patients had irregular-shaped, multiform unstained lesions. In the latter group, 55% of the patients had ESCC. Muto and associates94 also followed their patients for more than 1 year, and patients who had the type IV pattern were more likely to develop a metachronous lesion during that time. They concluded that the irregular-shaped, multiform lesions likely represented a “field cancerization.”
Using iodine staining, Hashimoto and coworkers95 found 8 cases of dysplasia (7%) and 10 cases of ESCC (8%) in 118 Brazilian patients with head and neck cancer. Because most reports using Lugol’s staining in high-risk patients are from Asia, this Brazilian study lends support to its utility in any patient population at risk throughout the world.
In addition to patients with head and neck cancer, alcoholics are another group at high risk for developing esophageal squamous cancer. Yokoyama and colleagues96 studied a cohort of 629 male alcoholics by endoscopy with iodine staining. Unstained lesions were observed in 162 patients (26%), and 36 unstained lesions in 21 patients (3%) were superficial ESCC. Because these lesions were superficial, endoscopic mucosal resection could be performed in 17 patients. In a similar group of 255 alcoholic Japanese patients, Ban and associates97 found unstained lesions in 22% and cancer in 4%.
In southern Brazil, Fagundes and colleagues98 studied 190 patients who were at risk for esophageal cancer because of increased consumption of alcohol or the beverage maté (a hot infusion of herbs). Unstained lesions were present in 23 of these asymptomatic patients, of which 6 showed dysplasia. In some patients with a normally stained midesophagus, there was also evidence of dysplasia, but the likelihood of finding dysplasia was eightfold higher in areas that were unstained. The investigators concluded that iodine chromoendoscopy improves the detection of dysplasia and should be added to conventional upper GI endoscopies in patients in selected populations at high risk for squamous cell carcinoma.
Strader and colleagues (D. Strader, personal communication, January 1, 2004)99 screened 98 alcoholic American veterans with endoscopy and iodine staining; 28 (29%) had unstained lesions, but no foci of dysplasia or cancer were found. Screening in this study began at age 40, which may have been too young in this population. Meyer and associates100 prospectively compared the diagnostic accuracy of videoendoscopy with and without Lugol’s staining for the detection of esophageal cancer in 158 alcoholic and smoking patients in France. Before staining, 12 patients had 14 endoscopically identifiable dysplastic or cancerous lesions. After staining, these numbers increased to 13 patients with 17 lesions. The unstained areas were significantly larger than the mucosal abnormalities seen before staining.
In an attempt to understand staining patterns with Lugol’s iodine better, Mori and colleagues101 applied iodine staining to 24 ESCC specimens resected by subtotal esophagectomy. They divided the staining patterns into four groups: type I, hyperstaining; type II, normal brown staining; type III, less intense staining; and type IV, unstained. Most type IV lesions were invasive carcinomas, carcinomas in situ, or severe dysplasia. Moderate to mild dysplasia or atrophy appeared as type III staining. The investigators also showed that there was good correlation between the histologic tumor margin and the absence of staining.
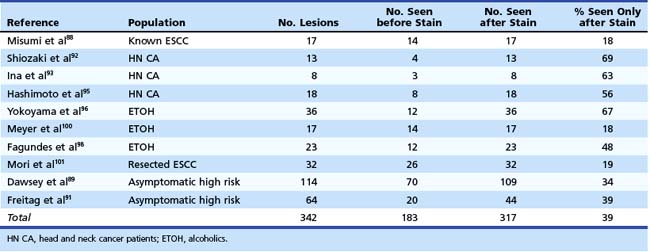
Table 30.4 summarizes the detection of significant lesions before and after iodine staining in the above-described studies. In these patients, who had various degrees of risk for developing ESCC, 39% of the lesions were detected only after staining with iodine. This finding underscores our belief that mucosal iodine staining improves endoscopic detection and delineation of high-grade squamous dysplasia and ESCC.
Table 30.4 Detection of Squamous Dysplasia and Esophageal Squamous Cell Carcinoma (ESCC) in Studies Using Iodine Chromoendoscopy
Although Lugol’s iodine is generally regarded as safe when sprayed onto esophageal mucosa to facilitate early detection of squamous cell carcinoma, some side effects have been reported.102,103 Mucosal irritation can be manifested by retrosternal pain, discomfort, or nausea. Free iodine, one of the components of Lugol’s, causes these side effects, and the risk of complication is greater with increasing concentrations of iodine.
Sodium thiosulfate (STS), a water-soluble neutral chemical, can be used as an intravenous antidote for cyanide, iodine, arsenic, or heavy metal intoxication. STS spray was first reported as being effective for reducing symptoms caused by staining with iodine solutions in 1993.103,104 Kondo and coworkers105 compared the utility of 5% STS with no treatment and with aluminum magnesium hydroxide gel (Maalox) in 120 patients after spraying the esophagus with 3% Lugol’s iodine. STS was most effective in reducing adverse symptoms, and the authors recommended its routine use after staining with iodine.
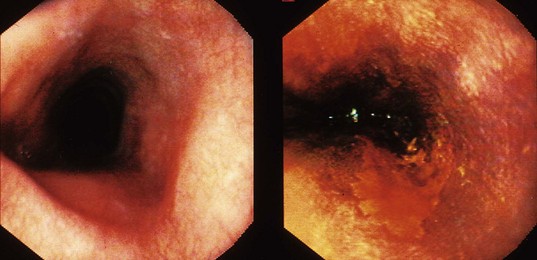
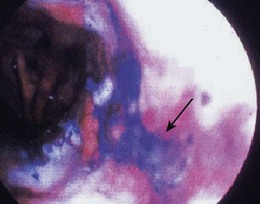
Although the largest experience using dyes to assist with screening for ESCC and its precursor lesions is with Lugol’s iodine, there are also some reports using toluidine blue (also called tolonium chloride). Toluidine blue stains cellular nuclei, and this property makes it useful for identifying malignant tissue, which has an increased DNA content and a higher nuclear-to-cytoplasmic ratio. The mucosa of a normal esophagus does not stain with toluidine blue (Fig. 30.8).
When toluidine blue is used, a proteolytic enzyme (e.g., proteinase) in water is frequently used.87 It may be ingested by mouth or sprayed on the mucosal surface. Toluidine blue is delivered as a 2% solution through a spray catheter in a manner identical to Lugol’s iodine.
Seitz and colleagues106 screened 100 patients with a history of alcohol and tobacco abuse using toluidine blue. Two carcinomas and 15 cases of dysplasia were detected. Two studies have looked at the use of toluidine blue dye spraying in patients with head and neck cancers. Hix and Wilson107 found a 17% prevalence of ESCC in 18 asymptomatic patients with a history of head and neck malignancy. Contini and colleagues108 found three esophageal neoplasms, two of which were not seen on routine endoscopy. Additional cases of leukoplakia and esophagitis were also identified. To date, no complications have been reported with toluidine blue. Because the dye is absorbed through the GI tract and excreted in the urine, caution should be observed in cases with decreased renal function.
New Techniques for Screening without Chromoendoscopy
Various new adjunct techniques have been combined with standard endoscopy to determine if they can improve the diagnosis of neoplastic esophageal lesions and assist with staging and therapy. These “high-tech” approaches have attempted to improve on the “low-tech” method of chromoendoscopy, which has been extremely valuable and has withstood the test of time. Some more promising newer methods are (1) narrow band imaging (NBI) and multiband imaging, (2) autofluorescence, (3) endoscopic ultrasound (EUS), (4) optical coherence tomography, (5) endocytoscopy, and (6) confocal laser microscopy.109
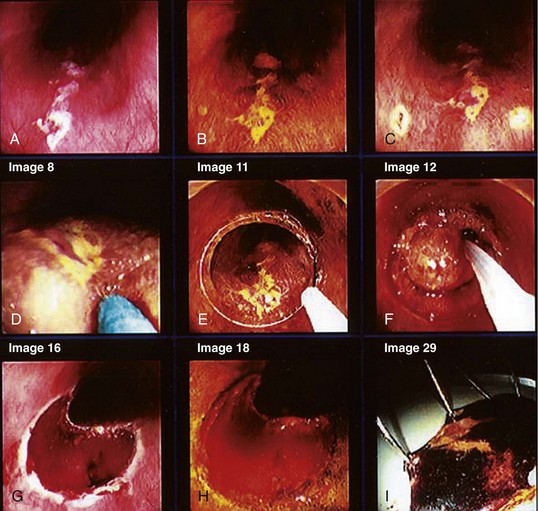
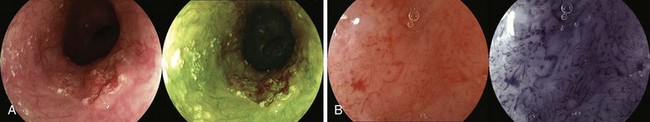
NBI is a technique that uses optical filters instead of dyes. In NBI, the depth of light penetration in tissue depends on its wavelength. The shorter the wavelength, the more superficial the penetration. In the visible light spectrum, blue penetrates most superficially and is the most valuable for mucosal imaging. Red light penetrates more deeply and is more appropriate for submucosal imaging. With NBI, the intensity of the blue light improves, which gives better definition to mucosal patterns and to blood vessels, particularly because blue light is preferentially absorbed by hemoglobin. Several endoscopic images with NBI are shown in Fig. 30.9. There has been considerable investigation with the use of NBI for Barrett’s esophagus. Hamamoto and associates110 were the first to use NBI for Barrett’s esophagus. Kuraoka and colleagues111 reported on the use of NBI and screening for early esophageal cancer in a group of patients who were at high risk because of heavy alcohol intake and previous head and neck cancers. They found a sensitivity, specificity, and positive predictive value of 100%, 59%, and 10%. Katada and coworkers112 studied NBI for detecting metachronous superficial pharyngeal squamous cell carcinomas.
Multiband imaging, initially known as Fujinon Intelligent Chromoendoscopy (FICE), uses certain dedicated wavelengths similar to NBI. However, in contrast to NBI, which uses optical filters to narrow the bandwidth, FICE takes an ordinary endoscopic image from the videoprocessor and processes the photons in a way that allows virtual images to be constructed with a choice of different wavelengths (Fig. 30.10). Pasha and coworkers113 reported on the use of FICE for studying the colonic mucosa. Many exquisite photographs showing high-resolution endoscopy plus NBI are contained in a text edited by Cohen.114
In standard endoscopy, incident light reacts with the tissue, and the reflected light is captured by the endoscope and processor. In addition to the standard imaging that occurs, there are other tissue interactions, one of which is autofluorescence. With autofluorescence, an incoherent violet-blue light is used as the source of excitation. The optical signals that result depend on the structure and molecular composition of the tissues being excited. Because neoplasia changes the composition of the tissue, it is theoretically possible to distinguish between normal tissue, dysplasia, and neoplasia (Fig. 30.11). The Erlangen group has contributed considerably in this area. Mayinger and colleagues115 used 129 endogenous fluorescence spectra in evaluating nine patients with squamous cell carcinoma and four patients with adenocarcinoma. Malignant and benign spectra were differentiated with the aid of a mathematical algorithm; by using this algorithm, the investigators had a sensitivity of 97% and a specificity of 95% for the diagnosis of esophageal carcinoma. A commercially available endoscope by Olympus allows for the detection of autofluorescence.
EUS has made a significant impact on staging cancers of the digestive tract including esophageal cancer. To date, however, it has had limited utility for detecting dysplasia or defining the exact level of mucosal or submucosal penetration by early esophageal cancer. A study by May and colleagues116 looked at the accuracy of staging early esophageal cancer using high-resolution endoscopy and high-resolution EUS. The study was carried out in 100 patients using a 20-MHz miniprobe. Overall, rates of accuracy were 79% using EUS staging for mucosal lesions, but for submucosal tumors the overall accuracy for EUS was only 48%.
The “Holy Grail” for endoscopy has been defined as an imaging system that would allow for an optical biopsy, which is a real-time image that would be equivalent to histopathologic sections. Advances have been made in this area using the principles of light contact microscopy. Endocytoscopy (Olympus, Tokyo, Japan) uses a system by which a 3.2-mm probe is passed through the accessory channel of an endoscope. Kumagai and coworkers117 studied 12 patients with superficial esophageal cancer. They sprayed the esophageal mucosal surface with 1% methylene blue and studied the targeted mucosa. In normal esophageal mucosa, the nuclei showed regular staining and a low nucleus-to-cytoplasm ratio. In contrast, in esophageal cancer, there were irregularities in cell distribution and a high nucleus-to-cytoplasm ratio. Inoue and associates118 studied endocytoscopy in patients who had already undergone chromoendoscopy and NBI. They reported an overall accuracy for differentiating nonmalignant from malignant pathology in 82% of patients.
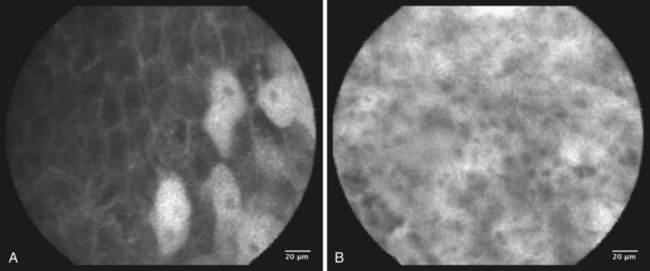
Several authors have reported on their experience using confocal laser and endomicroscopy for imaging of early squamous cell cancer.119–121 The authors were able to show differences in both the cellular structure and the vascular structure, including a demonstration of intrapapillary capillary loops (IPCLs). The confocal images were compared with standard histology. The cancer tissues exhibited more irregular cellular arrangements, increased diameter of IPCLs, and irregularly shaped IPCLs. This technology is promising but is still in its early stages of development. Confocal images are shown in Fig. 30.12. It is hoped that these “high-tech” developments will contribute to meaningful advances in the management of a large number of patients.
Endoscopic Morphology
Several classification systems have been used for defining the morphologic appearance of early GI cancer. The lack of standardization has led to confusion, and two separate literatures have evolved in the “East” and the “West” concerning the macroscopic appearance of superficial neoplastic lesions. This development discouraged international collaboration. A meeting was held in Paris in 2002 with leaders from the East and the West and terms were agreed on; this is referred to as the Paris classification of superficial neoplastic lesions of the GI tract.122 A follow-up meeting was held in Osaka in 2003. A description of this classification follows.
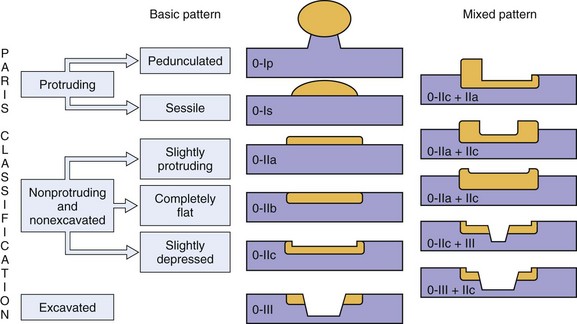
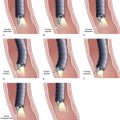
Neoplastic lesions of the esophagus are called superficial when their endoscopic appearance suggests that invasion is limited to the mucosa or submucosa, and these types of lesions are considered to be type 0 (for increasingly deeper lesions, the classification goes to higher numbers I, II, III, and IV). Type 0 is divided into three categories: 0-I, protruding; 0-II, nonprotruding and nonexcavated; and 0-III, excavated. Type 0-II is subdivided further into slightly elevated (IIa), completely flat (IIb), and slightly depressed (IIc). Table 30.5 and Fig. 30.13 summarize this classification.
Table 30.5 Morphologic Classification of Type 0 Esophageal Lesions with a Superficial Appearance at Endoscopy
| Morphologic Appearance | Type |
|---|---|
| Protruding | |
| Pedunculated | 0-Ip |
| Sessile | 0-Is |
| Nonprotruding and nonexcavated | |
| Slightly elevated | 0-IIa |
| Completely flat | 0-IIb |
| Depressed | 0-IIc |
| Excavated | 0-III |
Clinical Application of Screening and Treatment for Early Esophageal Squamous Cell Carcinoma and Its Precursor Lesions
In the United States and Europe, where adenocarcinoma is common and Barrett’s esophagus has been identified as the precursor lesion, screening for Barrett’s esophagus and surveillance for progression in patients who have Barrett’s esophagus have led to the application of several new therapies. When localized elevated endoscopic abnormalities are found in patients with Barrett’s esophagus, endoscopic mucosal resection is a valuable treatment. If the lesion is superficial and fully removed, consideration can be given to other endoscopic therapies that eradicate larger segments of Barrett’s tissues. A study by Shaheen and colleagues123 showed in a randomized sham-controlled trial that radiofrequency ablation was beneficial for patients with low-grade or high-grade glandular dysplasia. There has also been interest in pursuing similar endoscopic therapy for patients with early esophageal cancer.
Pouw and colleagues124 more recently described successful radiofrequency ablation for widespread early squamous cell carcinoma in a single case. A collaborative international effort has begun in China to study radiofrequency ablation for high-grade squamous intraepithelial neoplasia and early ESCC (G. Wang, personal communication, January 15, 2009). Chinese investigators from the Cancer Institute, Chinese Academy of Medical Sciences and Feicheng Medical Center, are collaborating with American and Dutch physicians to evaluate this treatment technique in patients with moderate or severe dysplasia or invasive squamous cell carcinoma limited to the lamina propria (T1m2). To be eligible, the esophageal mucosa must be flat, without nodules or ulceration (type 0-IIb), and patients can have no signs of metastasis on EUS or CT. The primary endpoint will be the percentage of subjects who are completely free of dysplasia or cancer at 12 months after the first ablation procedure. If such endoscopic techniques are shown to be effective for treatment of early neoplastic lesions in such high-risk populations, primary screening for early ESCC and its precursor lesions would be even more valuable.
1 Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
2 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
3 Mahboubi E, Kmet J, Cook PJ, et al. Oesophageal cancer studies in the Caspian Littoral of Iran: The Caspian cancer registry. Br J Cancer. 1973;28:197-214.
4 Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide IARC cancer base No. 5. version 2.0. Lyon: IARC Press; 2004.
5 Blot WJ, McLaughlin JK, Fraumeni JFJr. Esophageal cancer. In: Schottenfeld D, Fraumeni JFJr, editors. Cancer epidemiology and prevention. ed 3. Oxford: Oxford University Press; 2006:697-706.
6 Lu JB, Yang WX, Liu JM, et al. Trends in morbidity and mortality for oesophageal cancer in Linxian County, 1959–1983. Int J Cancer. 1985;36:643-645.
7 SEER Cancer Statistics Review, 1975–2006. National Cancer Institute. http://seer.cancer.gov/csr/1975_2006, 2009. Available at
8 Devesa SS, Blot WJ, Fraumeni JFJr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053.
9 Brown LM, Hoover RN, Greenberg RS, et al. Are racial differences in squamous cell esophageal cancer explained by alcohol and tobacco use? J Natl Cancer Inst. 1994;86:1340-1345.
10 Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424-1433.
11 Lagergren J, Bergstrom R, Lindgren A, et al. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340-346.
12 Brown LM, Hoover R, Silverman D, et al. Excess incidence of squamous cell esophageal cancer among US black men: Role of social class and other risk factors. Am J Epidemiol. 2001;153:114-122.
13 Brown LM, Swanson CA, Gridley G, et al. Dietary factors and the risk of squamous cell esophageal cancer among black and white men in the United States. Cancer Causes Control. 1998;9:467-474.
14 Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer. 2007;121:2753-2760.
15 Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control. 1991;2:325-357.
16 Terry P, Lagergren J, Hansen H, et al. Fruit and vegetable consumption in the prevention of oesophageal and cardia cancers. Eur J Cancer Prev. 2001;10:365-369.
17 Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252.
18 Nasrollahzadeh D, Kamangar F, Aghcheli K, et al. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. Br J Cancer. 2008;98:1857-1863.
19 Pourshams A, Khademi H, Malekshah A, et al. Cohort Profile: The Golestan Cohort Study—a prospective study of esophageal cancer in northern Iran. Int J Epidemiol. 2010;39:52-59.
20 Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456-463.
21 Roth M, Qiao Y, Rothman N, et al. High urine 1-hydroxypyrene glucuronide concentration in Linxian, China, an area of high risk for squamous oesophageal cancer. Biomarkers. 2001;6:381-386.
22 Kamangar F, Strickland PT, Pourshams A, et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res. 2005;25:425-428.
23 Fagundes RB, Abnet CC, Strickland PT, et al. Higher urine 1-hydroxy pyrene glucuronide (1-OHPG) is associated with tobacco smoke exposure and drinking mate in healthy subjects from Rio Grande do Sul, Brazil. BMC Cancer. 2006;6:139.
24 Roth MJ, Strickland KL, Wang GQ, et al. High levels of carcinogenic polycyclic aromatic hydrocarbons present within food from Linxian, China may contribute to that region’s high incidence of oesophageal cancer. Eur J Cancer. 1998;34:757-758.
25 Wei WQ, Abnet CC, Lu N, et al. Risk factors for oesophageal squamous dysplasia in adult inhabitants of a high risk region of China. Gut. 2005;54:759-763.
26 Kamangar F, Schantz MM, Abnet CC, et al. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol Biomarkers Prev. 2008;17:1262-1268.
27 Abnet CC, Qiao YL, Mark SD, et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12:847-854.
28 Yang CS. Research on esophageal cancer in China: A review. Cancer Res. 1980;40(8 Pt 1):2633-2644.
29 Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3062-3068.
30 Castellsague X, Munoz N, De Stefani E, et al. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2000;88:658-664.
31 Cook-Mozaffari PJ, Azordegan F, Day NE, et al. Oesophageal cancer studies in the Caspian Littoral of Iran: Results of a case-control study. Br J Cancer. 1979;39:293-309.
32 Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97:301-306.
33 Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753-1763.
34 Islami F, Kamangar F, Nasrollahzadeh D, et al. Socioeconomic status and esophageal cancer: Results from a population-based case-control study in a high-risk area. Am J Epidemiol. 2009;38:978-988.
35 Turner PC, Nikiema P, Wild CP. Fumonisin contamination of food: Progress in development of biomarkers to better assess human health risks. Mutat Res. 1999;443:81-93.
36 Islami F, Pourshams A, Nasrollahzadeh D, et al. Tea drinking habits and esophageal cancer in a high risk area in northern Iran. BMJ. 2009;338:b929.
37 Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk—a systematic review. Int J Cancer. 2009;125:491-524.
38 Akbari MR, Malekzadeh R, Nasrollahzadeh D, et al. Familial risks of esophageal cancer among the Turkmen population of the Caspian littoral of Iran. Int J Cancer. 2006;119:1047-1051.
39 Hu N, Dawsey SM, Wu M, et al. Family history of oesophageal cancer in Shanxi Province, China. Eur J Cancer. 1991;27:1336.
40 Hu N, Roth MJ, Polymeropolous M, et al. Identification of novel regions of allelic loss from a genomewide scan of esophageal squamous-cell carcinoma in a high-risk Chinese population. Genes Chromosomes Cancer. 2000;27:217-228.
41 Su H, Hu N, Shih J, et al. Gene expression analysis of esophageal squamous cell carcinoma reveals consistent molecular profiles related to a family history of upper gastrointestinal cancer. Cancer Res. 2003;63:3872-3876.
42 Hu N, Goldstein AM, Albert PS, et al. Evidence for a familial esophageal cancer susceptibility gene on chromosome 13. Cancer Epidemiol Biomarkers Prev. 2003;12:1112-1115.
43 Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98-100.
44 Ohta H, Nakazawa S, Segawa K, et al. Distribution of epithelial dysplasia in the cancerous esophagus. Scand J Gastroenterol. 1986;21:392-398.
45 Dawsey SM, Lewin KJ, Wang GQ, et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus: A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686-1692.
46 Munoz N, Crespi M, Grassi A, et al. Precursor lesions of oesophageal cancer in high-risk populations in Iran and China. Lancet. 1982;1:876-879.
47 Qiu SL, Yang GR. Precursor lesions of esophageal cancer in high-risk populations in Henan Province, China. Cancer. 1988;62:551-557.
48 Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: Results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187-192.
49 Wang LD, Qiu SL, Yang GR, et al. A randomized double-blind intervention study on the effect of calcium supplementation on esophageal precancerous lesions in a high-risk population in China. Cancer Epidemiol Biomarkers Prev. 1993;2:71-78.
50 Crespi M, Munoz N, Grassi A, et al. Precursor lesions of oesophageal cancer in a low-risk population in China: Comparison with high-risk populations. Int J Cancer. 1984;34:599-602.
51 Dawsey SM, Lewin KJ. Histologic precursors of squamous esophageal cancer. Pathol Annu. 1995;30(Pt 1):209-226.
52 Gabbert HE, Shimoda T, Hainaut P, et al. Squamous cell carcinoma of the esophagus. In: Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours. Pathology and genetics. Tumours of the digestive system. Lyon: IARC Press; 2000:8-19.
53 Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255.
54 Dawsey SM, Shen Q, Nieberg RK, et al. Studies of esophageal balloon cytology in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1997;6:121-130.
55 Shen O, Liu SF, Dawsey SM, et al. Cytologic screening for esophageal cancer: Results from 12,877 subjects from a high-risk population in China. Int J Cancer. 1993;54:185-188.
56 Shu YJ. Cytopathology of the esophagus: An overview of esophageal cytopathology in China. Acta Cytol. 1983;27:7-16.
57 Shu Y. The cytopathology of esophageal carcinoma. New York: Masson; 1985.
58 Jaskiewicz K, Venter FS, Marasas WF. Cytopathology of the esophagus in Transkei. J Natl Cancer Inst. 1987;79:961-967.
59 Leoni-Parvex S, Mihaescu A, Pellanda A, et al. Esophageal cytology in the follow-up of patients with treated upper aerodigestive tract malignancies. Cancer. 2000;90:10-16.
60 Nabeya K, Onozawa K, Ri S. Brushing cytology with capsule for esophageal cancer. Chir Gastroenterol. 1979;13:101-107.
61 Nabeya K. Markers of cancer risk in the esophagus and surveillance of high-risk groups. In: Sherlock P, Morson B, Barbara L, et al, editors. Precancerous lesions of the gastrointestinal tract. New York: Raven Press; 1983:71-86.
62 Roth MJ, Liu SF, Dawsey SM, et al. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer. 1997;80:2047-2059.
63 Pan QJ, Roth MJ, Guo HQ, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Linxian, China. Acta Cytol. 2008;52:14-23.
64 Hibi K, Taguchi M, Nakayama H, et al. Molecular detection of p16 promoter methylation in the serum of patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2001;7:3135-3138.
65 Kawakami K, Brabender J, Lord RV, et al. Hypermethylated APC DNA in plasma and prognosis of patients with esophageal adenocarcinoma. J Natl Cancer Inst. 2000;92:1805-1811.
66 Brock MV, Gou M, Akiyama Y, et al. Prognostic importance of promoter hypermethylation of multiple genes in esophageal adenocarcinoma. Clin Cancer Res. 2003;9:2912-2919.
67 Koike M, Hibi K, Kasai Y, et al. Molecular detection of circulating esophageal squamous cell cancer cells in the peripheral blood. Clin Cancer Res. 2002;8:2879-2882.
68 Kilic A, Schuchert MJ, Luketich JD, et al. Use of novel autoantibody and cancer-related protein arrays for the detection of esophageal adenocarcinoma in serum. J Thorac Cardiovasc Surg. 2008;136:199-204.
69 Lin HS, Talwar HS, Tarca AL, et al. Autoantibody approach for serum-based detection of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2396-2405.
70 Zhong L, Hidalgo GE, Stromberg AJ, et al. Using protein microarray as a diagnostic assay for non-small cell lung cancer. Am J Respir Crit Care Med. 2005;172:1308-1314.
71 Zhong L, Coe SP, Stromberg AJ, et al. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2006;1:513-519.
72 Sidransky D, Tokino T, Hamilton SR, et al. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science. 1992;256:102-105.
73 Traverso G, Shuber A, Levin B, et al. Detection of APC mutations in fecal DNA from patients with colorectal tumors. N Engl J Med. 2002;346:311-320.
73a Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: Molecular approaches. Gastroenterology. 2005;128:192-206.
74 Zou H, Taylor WR, Harrington JJ, et al. High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology. 2009;136:459-470.
75 Ahlquist D, Cameron A, Jett J, et al. Universal detection of aerodigestive cancers by assays of non-apoptotic human DNA in stool. Gastroenterology. 2000;119:1219-1227.
76 Ahlquist DA. Next-generation stool DNA testing: Expanding the scope. Gastroenterology. 2009;136:2068-2073.
77 Adams L, Roth MJ, Abnet CC, et al. Promoter methylation in cytology specimens as an early detection marker for esophageal squamous dysplasia and early esophageal squamous cell carcinoma. Cancer Prev Res (Phila Pa). 2008;1:357-361.
78 Prevo LJ, Sanchez CA, Galipeau PC, et al. p53-mutant clones and field effects in Barrett’s esophagus. Cancer Res. 1999;59:4784-4787.
79 Wong DJ, Paulson TG, Prevo LJ, et al. p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 2001;61:8284-8289.
80 Mokhashi MS, Wildi SM, Glenn TF, et al. A prospective, blinded study of diagnostic esophagoscopy with a superthin, stand-alone, battery-powered esophagoscope. Am J Gastroenterol. 2003;98:2383-2389.
81 Kiesslich R, Jung M, DiSario JA, et al. Perspectives of chromo and magnifying endoscopy: How, how much, when, and whom should we stain? J Clin Gastroenterol. 2004;38:7-13.
82 Dawsey SM, Wang GQ, Weinstein WM, et al. Squamous dysplasia and early esophageal cancer in the Linxian region of China: Distinctive endoscopic lesions. Gastroenterology. 1993;105:1333-1340.
83 Schiller W. Early diagnosis of carcinoma of the cervix. Surg Gynecol Obstet. 1933;59:210-222.
84 Brodmerkel G. Schiller’s test: An aid in esophagoscopic diagnosis. Gastroenterology. 1971;60:813.
85 Nothmann BJ, Wright JR, Schuster MM. In vivo vital staining as an aid to identification of esophagogastric mucosal junction in man. Am J Dig Dis. 1972;17:919-924.
86 Voegeli R. Die schillersche jodprobe im rahmen der osophagusdiagnostik. Pract Otorhinolaryngol. 1966;28:230-239.
87 Endo M, Ide H. Endoscopic staining in early diagnosis of esophageal cancer. Tokyo: Japan Scientific Societies Press; 1991.
88 Misumi A, Harada K, Murakami A, et al. Role of Lugol dye endoscopy in the diagnosis of early esophageal cancer. Endoscopy. 1990;22:12-16.
89 Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220-231.
90 Fleischer DE, Wang GQ, Dawsey SM, et al. Endoscopic therapy for esophageal dysplasia and early esophageal cancer in Linxian, China. Gastrointest Endosc. 1997;45:AB68.
91 Freitag CP, Barros SG, Kruel CD, et al. Esophageal dysplasias are detected by endoscopy with Lugol in patients at risk for squamous cell carcinoma in southern Brazil. Dis Esophagus. 1999;12:191-195.
92 Shiozaki H, Tahara H, Kobayashi K, et al. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990;66:2068-2071.
93 Ina H, Shibuya H, Ohashi I, et al. The frequency of a concomitant early esophageal cancer in male patients with oral and oropharyngeal cancer: Screening results using Lugol dye endoscopy. Cancer. 1994;73:2038-2041.
94 Muto M, Hironaka S, Nakane M, et al. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517-521.
95 Hashimoto C, Moraes-Filho J, Eisig J. High incidence of esophageal cancer in patients of primary head and neck cancer: The role of Lugol’s staining method in the establishment of early diagnosis of esophageal cancer. Am J Gastroenterol. 1999;94:2586.
96 Yokoyama A, Ohmori T, Makuuchi H, et al. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928-934.
97 Ban S, Toyonaga A, Harada H, et al. Iodine staining for early endoscopic detection of esophageal cancer in alcoholics. Endoscopy. 1998;30:253-257.
98 Fagundes RB, de Barros SG, Putten AC, et al. Occult dysplasia is disclosed by Lugol chromoendoscopy in alcoholics at high risk for squamous cell carcinoma of the esophagus. Endoscopy. 1999;31:281-285.
99 Strader D, Dawsey S, Fleischer DE, et al. Early detection of esophageal cancer/dysplasia in a high-risk population via chromoendoscopy. Gastrointest Endosc. 1997;45:AB84.
100 Meyer V, Burtin P, Bour B, et al. Endoscopic detection of early esophageal cancer in a high-risk population: Does Lugol staining improve videoendoscopy? Gastrointest Endosc. 1997;45:480-484.
101 Mori M, Adachi Y, Matsushima T, et al. Lugol staining pattern and histology of esophageal lesions. Am J Gastroenterol. 1993;88:701-705.
102 Aoyama N, Aruike S, Koizumu H, et al. Investigations of questionnaire about side effects of Lugol’s staining. Jpn J Gastroenterol Soc. 1983;16:939-940.
103 Kameyama H, Murakami M, Shimuzu Y, et al. The efficacy and diagnostic significance of sodium thiosulfate spraying after iodine dyeing of the esophagus. Dig Endosc. 1994;6:181-186.
104 Yonekawa H, Shima S, Yoshizumi Y. The effect of sodium thiosulfate on endoscopic Lugol staining of the esophagus. Endosc Dig. 1993;5:681-685.
105 Kondo H, Fukuda H, Ono H, et al. Sodium thiosulfate solution spray for relief of irritation caused by Lugol’s stain in chromoendoscopy. Gastrointest Endosc. 2001;53:199-202.
106 Seitz JF, Monges G, Navarro P, et al. [Endoscopic detection of dysplasia and subclinical cancer of the esophagus: Results of a prospective study using toluidine blue vital staining in 100 patients with alcoholism and smoking]. Gastroenterol Clin Biol. 1990;14:15-21.
107 Hix WR, Wilson WR. Toluidine blue staining of the esophagus: A useful adjunct in the panendoscopic evaluation of patients with squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1987;113:864-865.
108 Contini S, Consigli GF, Di Lecce F, et al. Vital staining of oesophagus in patients with head and neck cancer: Still a worthwhile procedure. Ital J Gastroenterol. 1991;23:5-8.
109 Curvers WL, Kiesslich R, Bergman JJ. Novel imaging modalities in the detection of oesophageal neoplasia. Best Pract Res Clin Gastroenterol. 2008;22:687-720.
110 Hamamoto Y, Endo T, Nosho K, et al. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J Gastroenterol. 2004;39:14-20.
111 Kuraoka K, Hoshino E, Tsuchida T, et al. Early esophageal cancer can be detected by screening endoscopy assisted with narrow-band imaging (NBI). Hepatogastroenterology. 2009;56:63-66.
112 Katada C, Nakayama M, Tanabe S, et al. Narrow band imaging for detecting metachronous superficial oropharyngeal and hypopharyngeal squamous cell carcinomas after chemoradiotherapy for head and neck cancers. Laryngoscope. 2008;118:1787-1790.
113 Pasha S, Fleischer DE, Foster AM, et al. Role of multiband imaging (Fujinon Intelligent Chromoendoscopy [FICE]) in the diagnosis and characterization of colorectal lesions. Am J Gastroenterol. 104, 2009. in press
114 Cohen J, editor. Advanced digestive endoscopy: Comprehensive atlas of high resolution endoscopy and narrow band imaging. Malden, MA: Blackwell Publishing, 2007.
115 Mayinger B, Horner P, Jordan M, et al. Light-induced autofluorescence spectroscopy for the endoscopic detection of esophageal cancer. Gastrointest Endosc. 2001;54:195-201.
116 May A, Gunter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: A comparative, prospective, and blinded trial. Gut. 2004;53:634-640.
117 Kumagai Y, Inoue H, Nagai K, et al. Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial esophageal carcinoma. Endoscopy. 2002;34:369-375.
118 Inoue H, Sasajima K, Kaga M, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: A pilot trial. Endoscopy. 2006;38:891-895.
119 Deinert K, Kiesslich R, Vieth M, et al. In-vivo microvascular imaging of early squamous-cell cancer of the esophagus by confocal laser endomicroscopy. Endoscopy. 2007;39:366-368.
120 Liu H, Li YQ, Yu T, et al. Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy. 2009;41:99-106.
121 Pech O, Rabenstein T, Manner H, et al. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. Clin Gastroenterol Hepatol. 2008;6:89-94.
122 Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578.
123 Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288.
124 Pouw RE, Gondrie JJ, Curvers WL, et al. Successful balloon-based radiofrequency ablation of a widespread early squamous cell carcinoma and high-grade dysplasia of the esophagus: A case report. Gastrointest Endosc. 2008;68:537-541.