Chapter 35 Salivary Gland Malignancies
Etiology and Epidemiology
The causes of salivary gland tumors, both benign and malignant, have not been clearly established. Reports consistently suggest etiologic associations with nutritional deficiencies, exposure to ionizing radiation, ultraviolet light exposure, genetic predisposition, history of previous cancer of the skin of the face, occupational exposure, viral (e.g., Epstein-Barr virus) infection, alcohol use, hair dye use, and higher educational attainment.1,2,3,4,5
Of these associations, exposure to ionizing radiation may be regarded as the one established risk factor for salivary gland tumors, both benign and malignant. Studies have demonstrated not only a strong association6 but also a temporal latency (10 to 25 years) and a dose-response relationship with the exposure, especially for mucoepidermoid carcinomas.4,7,8 The risk is greater for malignant than benign salivary gland tumors.4,8 These observations are based on several independent long-term cohort studies of victims of atomic bomb exposure6,7,9 and patients receiving therapeutic radiation for benign8 and malignant indications.4 Radiation-induced malignant salivary gland tumors may occur with a higher frequency in the minor salivary glands.
Salivary gland tumors are regarded as rare, with a reported overall incidence in the Western world of approximately 2.5 cases to 3.0 cases per 100,000 per year.10 Malignant salivary gland tumors account for more than 0.5% of all malignancies and 3% to 5% of all head and neck cancers.10 They comprise approximately 11% of all oropharyngeal cancers.11 Most patients with malignant salivary gland tumors are in the sixth or seventh decade of life.12
The incidence patterns of salivary gland cancers were recently reported in a Surveillance, Epidemiology, and End Results (SEER) population-based study that offers the advantage of eliminating the inherent biases of clinical series.13 Almost 6,400 major salivary gland carcinomas were estimated to have been diagnosed from 1992 to 2006. The most common nonsquamous salivary gland cancers were mucoepidermoid carcinomas, followed by adenoid cystic and acinic carcinomas with comparable incidence rates. Males had a 51% higher incidence rate compared with females. The parotid gland accounted for 80% of all major salivary gland malignancies,13 but tumors of the parotid are in general less likely to be malignant compared with the other major salivary glands and the palate of the oral cavity.10 Except for adenoid cystic carcinomas, which were equally seen in the parotid and submandibular glands, other histologies occurred mainly in the parotid gland.
Among males, the most common nonsquamous salivary gland cancers were mucoepidermoid carcinoma and adenocarcinoma not otherwise specified (NOS).13 Among females, the most common nonsquamous salivary gland cancers were mucoepidermoid, acinic cell, and adenoid cystic carcinomas. Squamous cell carcinoma, adenocarcinoma NOS, and salivary duct carcinoma occurred with more than a twofold higher incidence among males. In contrast, females had a higher incidence of acinic cell and adenoid cystic carcinomas.
Acinic cell carcinomas were more likely to be diagnosed at younger ages compared with other cell types.13 Incidence ratios of salivary gland cancers were significantly lower among African Americans, Asians, and Pacific Islanders compared with whites. However, incidences of mucoepidermoid carcinoma and adenoid cystic carcinoma occurred equally among all races.
Prevention and Early Detection
The recognition that radiation exposure is a risk factor for benign and malignant salivary gland tumors (especially mucoepidermoid carcinomas) argues that long-term surveillance is likely a prudent recommendation but with unproven benefit. Patients with a known history of accidental, diagnostic, or therapeutic radiation head and neck exposure should be counseled about the potential risk of late manifestation of malignant tumors, especially if their anticipated life span may be 10 to 25 years. It is not clear if younger patients receiving therapeutic radiation are at an increased risk,4,8 necessitating more vigilant observation. Issues of cost-effectiveness of various surveillance investigations and the frequency of follow-up have not been established.
Because a dose-response relationship for developing salivary gland tumors from therapeutic radiation has been described (especially for mucoepidermoid carcinomas), this raises the intriguing question of whether this risk may be mitigated with modern radiation treatment planning techniques. Modern radiotherapy techniques have the advantage that the dose and volume of normal tissue irradiated may be reduced. This includes the use of proton radiation with its favorable Bragg peak physical beam characteristic. Details of this issue are further discussed in the section considering the role of protons in the management of salivary gland tumors. At this time this possibility remains largely speculative because the risk of developing salivary gland tumors has been demonstrated even with low doses (i.e., doses less than 280 cGy to the parotid glands).8 Thus the judicious use of diagnostic and therapeutic radiation and the avoidance of any unnecessary radiation exposure are currently the most effective preventive strategies that can be recommended.
Biologic Characteristics and Molecular Biology
Salivary Gland Genetic and Molecular Biology
Chromosomal Rearrangements
In recent years, investigations have demonstrated recurrent, nonrandom, and hallmark chromosomal rearrangements, especially translocations that characterize both benign and malignant salivary gland tumors.14 These translocations result in fusion oncogenes that affect the apoptotic threshold, cell cycle regulation, tumor angiogenesis, and growth independence. These gene arrangements have been a more common theme among leukemias, lymphomas, sarcomas, and recently with various epithelial malignancies, including thyroid and breast carcinomas. Characterizing these recurring translocations and their clinical significance is an active area of investigation in hopes of identifying future prognostic and therapeutic targets. Several noteworthy observations have been made to date.
In 2003, the recurrent and hallmark t(11;19)(q21;p13) translocation was described by several groups, demonstrating it to be a key pathogenic event that underlies the development of mucoepidermoid carcinomas.15,16 This translocation has been shown to result in the cyclic adenosine monophosphate–responsive element binding protein (CREB) regulated transcription coactivator 1 (CRTC1)/mastermind-like 2 (Drosophila) (MAML2) fusion transcript that results in a transcription factor acting on the Notch and the CREB pathways.17
Okabe and colleagues have demonstrated that this fusion transcript was associated with a longer disease-free (DFS) and overall survival (OS) on univariate and multivariate analysis.18 Others have reported on its favorable and independent prognostic impact on survival.19,20 Despite this translocation correlating with low-grade mucoepidermoid carcinoma, in multivariate analysis for OS this translocation was found to be independent of tumor grading. Thus this translocation can potentially identify a favorable cohort of high-grade mucoepidermoid carcinomas.21 Lower rates of local and distant relapses have been reported in fusion-positive mucoepidermoid carcinomas.20 The prognostic relevance of this translocation offers not only the promise of future risk-stratified treatment approaches but also that insights into the development of novel therapeutic targets may be gained from further characterization of this fusion gene. For example, unfavorable fusion-positive mucoepidermoid carcinomas are believed to have acquired further somatic mutations conferring increased invasiveness, such as deletion in the CDKN2A (formerly p16) gene.22
Persson and colleagues23 have also demonstrated a recurrent and hallmark t(6 : 9) translocation in adenoid cystic carcinomas resulting in the MYB-NFIB fusion oncoprotein. These investigators suggest that this may also be a key oncogenic event in the pathogenesis of adenoid cystic carcinoma.23
Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (ERBB2)
EGFR and ERBB2 (formerly and often referred to as HER2/neu) are members of the epidermal growth factor receptor transmembrane receptor family and when activated transduce mitogenic signals. Several studies have reported high immunohistochemical expression levels and that it may be more dominantly expressed in non–adenoid cystic carcinomas.24–26 However, the clinical significance of EGFR protein overexpression remains controversial, with multivariate analyses demonstrating both an independent effect27 and no independent association with poor survival.26
Similarly, ERBB2 overexpression has been reported for malignant salivary gland tumors. These include strong overexpression in mucoepidermoid carcinomas28 and in salivary duct carcinomas29,30 but rarely seen for adenoid cystic carcinomas.30 Univariate31,32 and multivariate independent prognostic significance has been reported for ERBB2 overexpression.33,34 Agulnik and colleagues24 suggest that greater clinical activity with the dual EGFR and ERBB2 inhibitor lapatinib may come in identifying tumors with both high EGFR and ERBB2 expression and that ERBB2 may be especially important. Identifying subgroups of patients more dependent on ERBB2 signaling may be important in further defining the therapeutic benefit with ERBB2 inhibition.
c-KIT/CD117
The c-KIT protein is a membrane tyrosine kinase receptor that when activated through binding to the ligands stem cell factor or mast cell growth factor provides signals for cell survival, proliferation, and differentiation. Its overexpression has been particularly noted in adenoid cystic carcinomas35 and has been suggested as one way in which adenoid cystic carcinomas may be distinguished from polymorphous low-grade adenocarcinomas,36 although contradictory findings have also been reported.37
Despite its overexpression, it is clear that it is the nature of any point mutations in the protein that impacts on its response to inhibition with imatinib, a small molecule tyrosine kinase inhibitor that competitively inhibits the activation of the c-KIT receptor and several other structurally similar receptor tyrosine kinases. Although several investigators have not demonstrated the presence of mutations, recent studies using more sensitive polymerase chain reaction techniques confirm the presence of multiple mutations in the c-KIT gene in adenoid cystic carcinomas.38 Although both gain of function (activating function of the receptor) and loss of function c-KIT mutations have been described for other malignancies, the therapeutic implications of these recently described mutations in the management of adenoid cystic carcinomas remain to be determined. Although the independent prognostic significance for c-KIT overexpression has not been evaluated, it has remained an attractive therapeutic target owing to the clinical success of imatinib.
Salivary Gland Tumor Microenvironment
Neoneurogenesis
The study of perineural invasion is an important biologic consideration for many cancers, especially for malignant salivary gland tumors such as adenoid cystic carcinomas. The molecular determinants of this mechanism of spread have only begun to receive attention in recent years. Observations in other cancer sites currently suggest that perineural invasion is the result of a mutual neurotropic interaction with cancer cells characterized by the release of various paracrine growth factors.39 As with neoangiogenesis, it is clear that the ingrowth of nerve endings into a tumor may be stimulated by the tumor release of various neurotrophins such as brain-derived neurotrophic factor (BDNF),40 nerve growth factor (NGF), and its receptor tyrosine kinase A (TrkA).41 Neurotropin staining has been demonstrated to be present in adenoid cystic carcinomas. NGF and TrkA both correlated with the presence of perineural invasion.41 These tumor-innervating nerve cells may release neurotransmitters that function as proliferative and promigratory signals for the tumor cells. Furthermore, nerve fibers are used as routes for tumor cell dissemination. For pancreatic carcinoma, detailed studies of serial histologic sections have demonstrated that tumor cells can progress along branching nerve fascicles in a continuous fashion.42
Hypoxia
Preliminary investigations using methodologies such as immunohistochemical staining for the oxygen-dependent binding of 2-nitroimidazoles suggest that salivary gland malignancies may be well-oxygenated tumors.43 This observation would not support the hypothesis that an underlying mechanism for not only the radioresistance but also the high rates of distant metastasis characterizing malignant salivary gland tumors is the presence of tumor hypoxia. In a study of 12 patients receiving surgery, the hypoxia marker pimonidazole was administered preoperatively. Of the 8 patients yielding sufficient tumor specimen for analysis, immunohistochemical staining was not seen with pimonidazole and for the hypoxia-inducible factor (HIF)-1a and the HIF-1a regulated carbonic anhydrase (CA) IX and glucose transporters (GLUT) 1 and 3. Given that salivary gland malignancies are rare and of diverse histologies, further evaluation is needed before more generalized conclusions can be made.
Pathology and Pathways of Spread
Pathology
The classification of salivary gland tumors is primarily based on morphologic patterns, with two classification systems often recognized. This includes the Armed Forces Institute of Pathology (AFIP) system; and, in 2005, the World Health Organization (WHO), which further developed the classification scheme for salivary cancers originally set forth by Foote and Frazell44 (Table 35-1). The WHO classification includes the following categories: adenomas, carcinomas, nonepithelial tumors, malignant lymphomas, secondary tumors, unclassified tumors, and tumor-like lesions. In contrast, the AFIP classification includes benign epithelial neoplasms, malignant epithelial neoplasms, mesenchymal neoplasms, malignant lymphomas, metastatic tumors, and non-neoplastic tumor-like conditions. Speight and Barrett10 have reported a comprehensive summary of salivary gland histopathology. Given the histologic diversity, this chapter will focus on histopathologies that impact on the pattern of spread and its clinical manifestations.
TABLE 35-1 Classification of Malignant Salivary Gland Tumors
NOS, not otherwise specified.
From Barnes L, Eveson J, Reichart PA, Sidransky D, editors: World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Head and Neck. Lyon, 2005, IARC Press.
Important prognostic factors for disease relapse and survival are summarized in Table 35-2. Practically, histologies may be categorized as high-grade at a greater risk of nodal and distant metastases or low-grade with a lower risk of nodal metastasis. The former includes high-grade mucoepidermoid carcinomas, high-grade adenocarcinoma NOS, carcinoma ex-pleomorphic adenoma, salivary duct carcinomas, and squamous cell carcinomas. Low-grade histologies include low-grade mucoepidermoid carcinomas, adenoid cystic carcinomas, acinic cell carcinomas, and polymorphous low-grade adenocarcinomas. An intermediate group has also been described consisting of intermediate mucoepidermoid carcinomas.
TABLE 35-2 Summary of Prognostic Factors
| Endpoint | Prognostic Factors |
|---|---|
| Local relapse | T3 and T4 category, unresectability/skull base invasion |
| Neck metastasis | Male sex, T3 and T4 category, primary site involving the pharynx, high-grade histology, especially mucoepidermoid and adenocarcinoma NOS |
| Distant metastasis | Clinical perineural invasion, skull base invasion, nodal metastasis |
| Survival | Stage IV, nodal metastasis |
NOS, not otherwise specified.
For mucoepidermoid carcinomas, the histologic grading has been shown to be of prognostic significance. Several three-tiered grading schemes have been shown to be reproducible and predictive of the patient’s outcome by defining low-, intermediate-, and high-grade tumors using five histopathologic features yielding a numerical score for differentiation.45–47 High-grade mucoepidermoid carcinoma has a greater proportion of epidermoid cells than mucus-producing cells, with a solid tumor appearance often mistaken for squamous cell carcinoma. High-grade disease also increases the risk of nodal metastasis sufficient to warrant elective management (e.g., a neck dissection). Despite this, the risk of nodal metastasis with low and especially intermediate grades can be as high as 24% to 30% in some series. High-grade mucoepidermoid carcinomas demonstrated nodal metastases in 56% of patients. In the modified Healey grading schema, lymph node involvement was 0% (low grade), 22% (intermediate grade), and 72% (high grade). T category also is associated with an increased risk of nodal metastasis for both major and minor salivary gland mucoepidermoid carcinomas.48 T1 high-grade disease may be at low risk for nodal metastasis in major salivary glands, that is, anatomic sites not involving the mucosa.48
Pathways of Spread
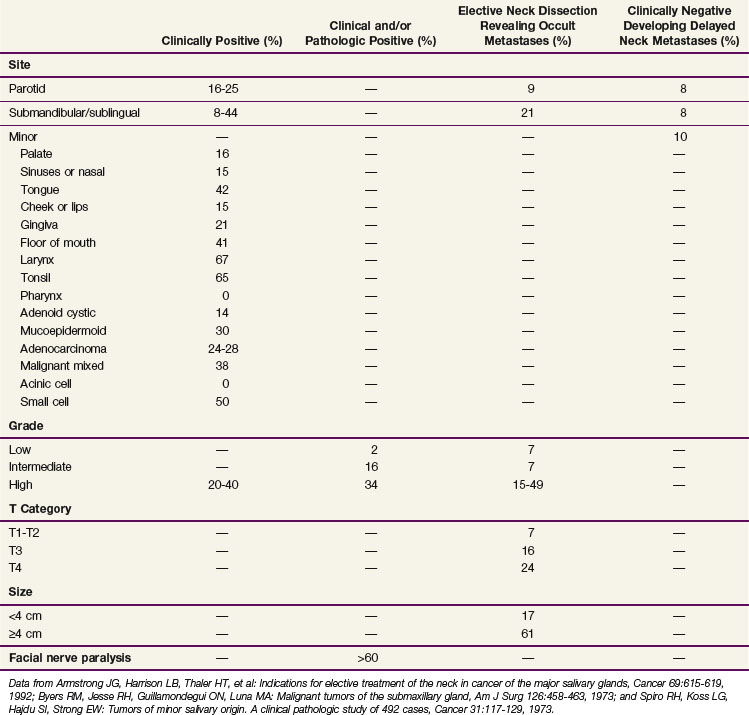
When considering all histologies together, the risk of lymph node metastasis is increased with T3 and T4 disease, involvement of a pharyngeal site, and high-grade mucoepidermoid carcinomas and adenocarcinoma NOS.49 Table 35-3 shows similar observations with T category, anatomic site, and histology.50 For minor salivary glands, the density of the lymphatics in the anatomic site has a significant influence on the risk of nodal metastases. In general, cancers arising within the oropharynx or nasopharynx have about a 60% incidence of lymph node metastasis compared with 5% to 10% for hard palate and paranasal sinus sites. Minor salivary gland cancers arising within the tongue and floor of mouth have an approximately 40% incidence of lymph node metastasis; those arising within the gingiva have a 20% incidence of lymph node metastasis; tumors of the nasal cavity, buccal mucosa, and lip have a 15% incidence or less.
TABLE 35-3 Incidence of Lymph Node Metastases at Initial Diagnosis by Site, Grade, T Category, Size, and Facial Nerve Paralysis
Adenoid cystic carcinomas at the primary site have a predilection for perineural invasion as a unique pattern of local disease extension. Adenoid cystic carcinomas occur most commonly in the palate and frequently spread by perineural invasion. This may be seen in more than 50% of cases. Spread centrally within and peripherally along the nerve are common routes in which this tumor spreads. Growth along the nerve has been shown to have “skip” areas of involvement and noninvolvement, so that a negative nerve margin does not guarantee a final negative margin. However, a recent study of serial sections for pancreatic carcinoma suggests that perineural invasion may, in fact, be contiguous along the branching nerve fascicles and suggest that it may be the branching nature of the nerve and pathologic sampling of the sections evaluated that contribute to the uncertainty in margin assessment.51
The risk of distant metastases is increased with the presence of lymph node metastasis, skull base involvement, and high-grade histology.52 Common distant metastatic sites include lung, liver, and brain and are also associated with the histologic grade.
Clinical Manifestations, Patient Evaluation, and Staging
Clinical Manifestations
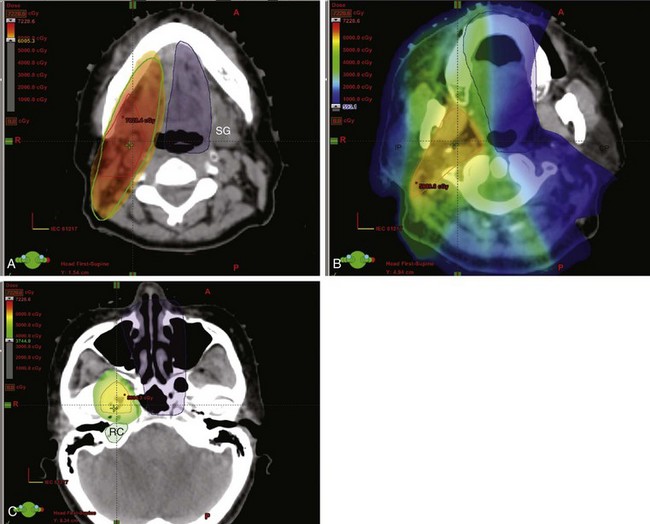
Perineural invasion may result in symptoms of pain and numbness but more commonly presents as an asymptomatic mass with an indolent history of growth. Clinical symptoms are more likely to be associated with radiologic evidence of perineural invasion (Fig. 35-1). Extension through the skull base with intracranial growth can also occur and result in mass effect symptoms. Because adenoid cystic carcinomas commonly occur in the palate, spread along the palatine nerves through the greater and lesser palatine foramen is an important route of local spread.
Patient Evaluation
As for any patient with a suspected or documented malignancy, proper evaluation begins with a detailed history and physical examination (Table 35-4). Presence of a new mass in the face, neck, or mouth should be investigated for associated symptoms based on the surrounding anatomic structures that may be affected, thus helping to localize the disease extension. This should also include symptoms that may relate to perineural spread of the cancer. Physical examination should include a full oral cavity inspection and bimanual palpation of structures that are or may be involved. Minor salivary gland tumors typically appear as a submucosal mass and commonly involve the palate. The proximity of the mass and any changes to the greater and lesser palatine foramen should be noted. Flexible fiberoptic nasopharyngolaryngoscopy should be performed to assess for the location and extent of any mucosal lesions in the pharynx or to assess the function of critical cranial nerves that may be suspected to be involved because of a mass at the skull base.
TABLE 35-4 Diagnostic Algorithm for Malignant Salivary Gland Tumors
| General |
Fine-needle aspiration or ultrasound-guided core needle biopsy of suspicious lesions may be useful to distinguish malignant from benign processes. In one retrospective study of 879 patients, fine-needle aspiration demonstrated a sensitivity of 83% and specificity of 99%, with overall accuracy of 93%.53 However, other studies have demonstrated false-negative rates as high as 33% and 43% for adenoid cystic carcinoma and low-grade mucoepidermoid carcinoma, respectively.54
Both CT and MRI are necessary to assess the extent of disease. Bony destruction and regional lymph node metastases are better visualized with CT, whereas MRI provides information regarding soft tissue infiltration, intracranial spread, and perineural invasion.55 Although the role of PET/CT for salivary gland cancers has yet to be established, early studies have demonstrated an accuracy of more than 90% for detection of the primary tumor and an increased ability in identifying unrecognized local, nodal, and distant metastases.56,57
Staging of Major and Minor Salivary Gland Malignancies
Unlike minor salivary gland tumors, which are staged according to the anatomic site of origin, major salivary gland cancers have their own staging system. In general, tumors of the major salivary glands are staged according to the tumor size and whether there is any extraparenchymal extension (Table 35-5). Extraparenchymal extension is described as either clinical or macroscopic evidence of invasion of the soft tissues. Microscopic extension does not constitute extraparenchymal extension. For parotid tumors the prognostic significance of seventh cranial nerve involvement is also considered. Although tumor grade does affect outcome, the current (7th edition) American Joint Committee on Cancer staging system does not incorporate tumor grade as a component.
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor 2 cm or less in greatest dimension without extraparenchymal extension* |
| T2 | Tumor ≥2 cm but <4 cm in greatest dimension without extraparenchymal extension* |
| T3 | Tumor ≥4 cm and/or tumor having extraparenchymal extension* |
| T4a | Tumor invades skin, mandible, ear canal, and/or facial nerve |
| T4b | Tumor invades base of skull and/or pterygoid plates and/or encases the carotid artery |
* Extraparenchymal extension is defined as the presence of any clinical or macroscopic evidence of invasion of the adjacent soft tissues. Microscopic evidence alone does not constitute extraparenchymal extension for classification purposes.
Nodal category and group staging for major salivary gland tumors follows other head and neck cancer sites and is not described here.58
Primary Therapy
The treatment approach for salivary gland tumors continues to be based on a surgical resectability paradigm. The therapeutic dilemma occurs when there is significant involvement of a functional region in the head and neck involved in speech or swallowing or where unresectable disease occurs. Therapeutic advancements have largely been limited in recent years to the development of novel organ-preserving surgical approaches (Figs. 35-2 and 35-3). Radiotherapy advancements include the development of more conformal radiotherapy techniques and the advent of particle beam therapy. Advancements in systemic agents have included the translation of molecular therapeutics that offer a potentially more favorable therapeutic ratio.
Role of Surgery
The surgical treatment of salivary malignancies can be divided into categories based on the gland involved. The most common site for salivary malignancy is the parotid gland. The submandibular gland and the minor salivary glands have a lower absolute number of cancers; however, the risk of a neoplasm being malignant is higher in submandibular and minor salivary glands than it is in the parotid gland.59
Parotid Gland Malignancy
Surgery involves an incision in the preauricular crease extending postauricularly and then into the upper neck (see web-only Fig. 35-1 on the Expert Consult website![]() ). This incision, known as the modified Blair incision, gives access to the seventh cranial nerve as it comes out of the stylomastoid foramen. One of the keys to the procedure is to first identify the nerve, trace it out along its main branches, and then manage the tumor once its relationship to the nerve has been clarified. In cases in which the nerve is compromised by the tumor, or in which the tumor abuts the stylomastoid foramen, it may be necessary to identify the nerve before it comes out of the mastoid bone. This can be accomplished by drilling out the mastoid bone and identifying the nerve before it comes out of the foramen.
). This incision, known as the modified Blair incision, gives access to the seventh cranial nerve as it comes out of the stylomastoid foramen. One of the keys to the procedure is to first identify the nerve, trace it out along its main branches, and then manage the tumor once its relationship to the nerve has been clarified. In cases in which the nerve is compromised by the tumor, or in which the tumor abuts the stylomastoid foramen, it may be necessary to identify the nerve before it comes out of the mastoid bone. This can be accomplished by drilling out the mastoid bone and identifying the nerve before it comes out of the foramen.
Once the appropriate branches of the nerve have been identified, the tumor is removed. Great care is taken to avoid entry into the tumor, and attempts are made to avoid residual positive margins, because this is a known poor prognostic indicator.60,61 Because most tumors involve only the superficial lobe of the parotid, dissecting the tumor carefully off the seventh cranial nerve is appropriate surgical care. Tumors that involve the deep lobe of the parotid gland or that involve the parapharyngeal space just medial to the parotid gland may require extended procedures to perform complete resections. These procedures are often possible through the same parotid incision but occasionally require extended neck incisions or mandible-splitting transoral procedures. Excision of soft tissue, skin, muscle, and neurovascular structures in the region is appropriate if they are involved with tumor.
Submandibular Gland Malignancy
Although the absolute number of malignancies of the submandibular gland is far less than the parotid gland, the chance of a lesion being malignant is much higher, nearing 50%.62 For this reason, a high level of vigilance must be in place when managing masses in the submandibular region. Most commonly, patients present with painless masses in the region. Less commonly, painful masses or neck adenopathy may occur. Any of these presentations warrants a workup of the area, including possibly CT or MRI and fine-needle aspiration biopsy.
As with parotid cancers, salivary gland tumors of the submandibular gland are treated by surgery. Definitive treatment often includes a combination of therapies but almost always begins with surgical excision. The surgical approach should include, at the minimum, a complete submandibular gland excision with clear margins. There is no role for enucleation of the tumor, because this has been clearly associated with a higher rate of local recurrence.63 In patients who have undergone subtotal surgery, only to be diagnosed with cancer postoperatively, one should strongly consider reoperation to obtain negative margins before considering further therapy.
Sublingual and Minor Salivary Malignancy
Malignancies of the sublingual gland and minor salivary glands are rare. Minor salivary glands are present throughout the upper aerodigestive tract, and salivary gland tumors can occur at any of the sites. The oral cavity and oropharynx are the most common sites of disease, but the nasal cavity, paranasal sinuses, hypopharynx, larynx, nasopharynx, and parapharyngeal space are all at risk for these cancers.64
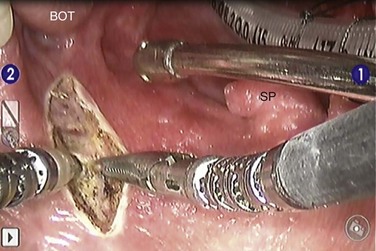
Organ-preserving surgical approaches that do not use transcervical exposure techniques are valuable in limiting the swallowing complications resulting from surgery. These include various transoral approaches, including transoral laser microsurgery (TLM) and transoral robotic surgery (TORS)65,66 (see Figs. 35-2 and 35-3). TORS has several advantages, including the improved visualization of an en bloc tumor resection in three dimensions avoiding sight-line limitations with magnified optics. Its application has included function-preserving resection of salivary gland tumors of the base of the tongue67 and in the parapharyngeal space65 (see Fig. 35-3).
Management of the Neck
Patients who present with clinically palpable or radiologically evident disease in the neck lymph nodes require management of this disease. This generally involves a planned neck dissection at the time of the primary surgery with or without postoperative radiation.68 What remains more controversial is the role of elective neck treatment in patients with no clinicoradiologic evidence of disease in the neck.
Some studies have demonstrated that all high-grade malignancies (regardless of histology) would benefit from elective management of the neck.69 Other studies have suggested elective management of the neck based on histologic type and/or size. This includes elective neck surgery for all squamous cell carcinomas, adenocarcinomas, high-grade mucoepidermoid carcinomas, undifferentiated carcinomas, and all T2 or higher T-category tumors.48,70 Still, other groups have suggested either nodal sampling of the upper neck lymph nodes at the time of the primary surgery or including the neck in the primary radiotherapy field for certain histologies.71,72 What does appear clear is that high-grade, high-stage cancers benefit from aggressive multimodality therapy, including treatment of the primary site and the lymphatic drainage pathway for the tumors.
Role of Radiotherapy
Postoperative Radiotherapy
At present no randomized studies have been conducted to establish the value of postoperative radiotherapy. Tables 35-6, 35-7, and 35-8 summarize the treatment results from modern series comparing surgery with surgery and postoperative, adjuvant irradiation for malignant major salivary gland, submandibular gland, and minor salivary gland cancers, respectively. These demonstrate that the addition of postoperative radiation therapy, when indicated (Table 35-9), appears to be associated with improved local control rates and survival in patients with malignant salivary gland cancers.
TABLE 35-6 Results of Surgery Alone and Combined Surgery and Postoperative External Irradiation for Malignant Salivary Gland Tumors
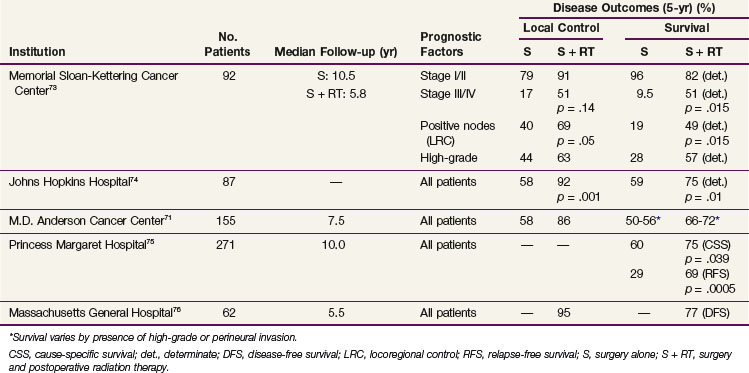
TABLE 35-7 Results of Surgery and Surgery with Postoperative, Adjuvant Radiation Therapy for Malignant Tumors of the Submandibular Gland
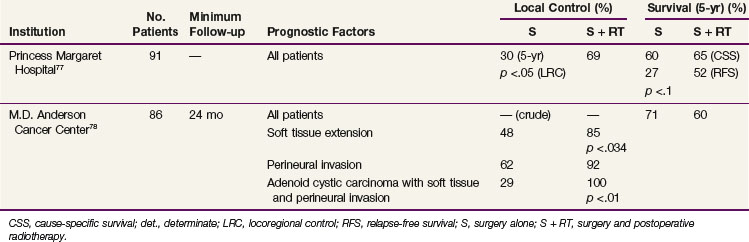
TABLE 35-8 Results of Surgery and Surgery with Postoperative, Adjuvant Radiation Therapy for Malignant Minor Salivary Gland Tumors
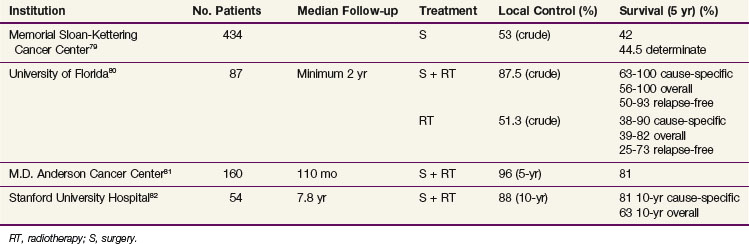
TABLE 35-9 Indications for Postoperative, Adjuvant Radiotherapy for Malignant Salivary Gland Tumors
In general, the use of postoperative radiotherapy doses typically to 60 Gy appears to be sufficient for perineural involvement of unnamed nerves.50 Skull base relapses may be reduced from 15% to 5% with elective skull base irradiation, especially for T4 disease.83 With perineural invasion of a major named nerve, elective skull base irradiation has also been shown to be effective.81 Patients with skull base relapses had a poorer survival when compared with those who did not experience a relapse in a retrospective review of 140 patients.83
Doses between 50 and 60 Gy have also been used for elective irradiation of the neck with relapses less than 5%.84 Terhaard and colleagues50 observed a trend to a lower rate of neck control when elective radiotherapy doses less than 46 Gy were used, compared with 46 Gy or more (p = .07). Again, patients with neck relapses have been associated with a poorer survival compared with those patients without neck relapses. When a higher burden of microscopic disease is present based on the presence of a positive margin or perineural invasion of a major named nerve, doses less than 56 Gy are insufficient and doses more than 60 Gy may be associated with improved local control rates.85
Locally Advanced Disease and Palliation
Definitive Radiotherapy: Conventional Fractionation
With locally unresectable salivary gland malignancies, the use of definitive irradiation on the order of 70 Gy with conventionally fractionated schedules typically results in locoregional control rates of 20% to 30% or less.86,87,88 These results are also limited by the inherent biases seen with retrospective institutional reviews but are significant in their independent verification of a consistently poor outcome for unresectable salivary gland malignancies when treated with photon radiotherapy alone. These results are also consistent with the standard arm in a randomized trial of neutron versus photon radiotherapy for unresectable salivary gland malignancies.89
Altered Fractionation
Although the Radiation Therapy Oncology Group (RTOG) 9003 study demonstrated that altered fractionation improves locoregional control in other sites of the head and neck,90 the role of this modality in salivary gland tumors is unclear. Wang and Goodman91 reported results using high-dose, accelerated, hyperfractionated photon beam therapy in patients with inoperable and unresectable major and minor salivary gland cancers. All patients were treated with 1.6 Gy per fraction, twice a day, combined with various boost techniques to obtain a total dose of 65 to 70 Gy. Local control and survival rates were promising, with a 5-year 100% local control and 65% OS for parotid gland lesions and a 5-year 78% local control and 93% OS for minor salivary gland lesions. Late complications were minimal. However, the follow-up was relatively short and, thus far, no update has been reported. Although this suggests a potential role for altered fractionation, it should be regarded as investigational until further data are reported.
Neutrons/Heavy Charged-Particle Therapy
The only randomized trial comparing neutron radiation to conventional photon irradiation was conducted by the RTOG and the Medical Research Council (MRC) for patients with locally unresectable primary and recurrent malignant salivary gland tumors.89 The study closed early because of ethical concerns resulting from interim analysis performed on 25 patients. At 2 years, this analysis demonstrated a significantly better local control with neutron versus photon irradiation (67% vs. 17%, p <.005) and a trend toward improved OS (62% vs. 25%, p = 0.1). The local control rate of 67% was consistent with prior single-institution reports associated with the use of fast neutrons.92,93,94 A median survival of 2.97 years with neutrons versus 1.23 years with photons was initially reported. With longer follow-up, the OS curves showed no difference. The majority of failures on the neutron study arm were systemic. In contrast, the major relapse pattern in the photon study arm was predominantly locoregional failures. The incidence of severe or life-threatening toxicity was greater in patients treated with neutrons, with nine patients treated with neutrons having at least one “severe or greater” complication, compared with four patients in the photon arm of the study. This finding of a relatively high rate of severe complications from neutrons has been observed in another comparative series.95
The overall lack of survival difference from the randomized trial, and the high rate of severe complications coupled with the lack of institutions with available treatment capabilities, has limited the broad use of fast neutron therapy. However, mature uncontrolled, single-institution studies continue to demonstrate impressive results, with lower rates of toxicity. Douglas and colleagues96 published a retrospective study of 148 patients with major salivary gland tumors treated at the University of Washington with a 5-year actuarial locoregional control rate of 100% for tumors less than 4 cm and only a 6% grade 3 or 4 complication rate. An updated larger series of 279 patients from the same institution demonstrated a 6-year actuarial locoregional control rate of 59% with a grade 3 or 4 toxicity rate of 10%.52 Neutrons have also been used in the postoperative setting for microscopic residual disease with a 100% 5-year actuarial locoregional control rate reported.97
In part, the increased complication rates are the result of the use of neutrons at the skull base and the adjacent central nervous system. The sensitivity of the central nervous system structures to high LET radiation often precludes safe delivery of therapeutic doses of high LET radiation to tumors with skull base involvement. Despite this, it is interesting to observe that neutron radiation does not appear to be associated with an increased risk of facial nerve palsy as a treatment complication.93,98 Complicating the risk of central nervous system injury is the recognition that skull base involvement is associated with a poorer prognosis with lower rates of local control and survival in a group of 159 patients with unresectable adenoid cystic carcinomas.97
To address this technical limitation of neutrons, investigators from the University of Washington have been investigating the role of boosting the skull base disease with stereotactic radiosurgery after reduced dose neutron beam radiotherapy at the superior portion of the tumor. In 34 patients with skull base disease treated with a Gamma Knife boost (see later discussion), the 40-month actuarial local control was 82%, compared with a historical control rate of 39% in patients with skull base disease treated with neutrons alone (p = .04).99
Heavy charged-particle radiation may also hold promise because it combines the biologic qualities of high LET with the rapid Bragg peak dose fall-off. Limited data with carbon ion radiation suggest similar tumor control as neutron beam therapy, with fewer late effects.100
Stereotactic Radiosurgery
The first is the study of SRS at the skull base as a treatment strategy to more safely escalate the effective biologic radiotherapy dose delivered. Douglas and colleagues99 used a Gamma Knife radiosurgery boost as a strategy to reduce the relative biologic neutron dose to the skull base and minimize the risk of central nervous system injury but to improve on the local control rates. The median prescribed Gamma Knife dose was 12 Gy to the 50% isodose line, resulting in a median neutron dose of 19.2 nGy to the isocenter. The median number of isocenters was 17. The median target volume treated was 12.4 cm3 (range, 1.9 to 28.9) with a median total volume treated of 18.3 cm3 (range, 5.9 to 53.9). This resulted in a median dose of 11.98 nGy to the adjacent tip of the temporal lobe. In total, 34 patients were treated, and with a median follow-up of 20.5 months the actuarial 24- and 40-month local control rates were 82% and 82%, compared with 81% and 39% in historical patients not receiving SRS boost. A total of four failures in the SRS group were described with two in-field and two out-of-field relapses. Noteworthy complications included three cases of radionecrosis with one of three patients with symptomatic headaches responsive to corticosteroids.
The other major indication for SRS or SRT has been for palliative indications. The rationale for the use of an SRT approach is twofold. First, the stereotactic approach affords greater confidence in the accuracy of the target lesion to be irradiated, allowing a smaller margin of uncertainty and thus reducing the volume of normal tissues that receive both unnecessary entry and exit radiation. The second is the potential for greater therapeutic benefit that may be achieved with higher doses delivered over a single fraction or with just a few fractions. To date, the reported experiences are limited and largely amount to small case and heterogeneous cohorts of patients with head and neck cancers, including some recurrent salivary gland malignancies.101–103 These observations limit the ability to draw any definitive generalized conclusions about the risks and benefits of SRS and SRT at this time, including the optimal prescribed dose. The range in prescribed dose includes single fractions of 11 to 18 Gy and fractionated schedules including 30 Gy in six fractions. Effective palliation of pain with durable responses including freedom from local tumor progression (ranging from 10 to 35 months) has been reported.103 In general, the major serious but infrequent risk associated with this palliative treatment approach includes the risk of optic, facial, and trigeminal nerve injury. At this time the use of this treatment approach should be limited to treatment on a clinical protocol or institutions with a broad stereotactic experience at the skull base for other malignancies in which the risks are well defined.
Proton Radiotherapy
There is very limited published experience with the use of proton therapy for salivary gland tumors. The Paul Scherrer Institute in Switzerland published a comparative dosimetry study of intensity modulated photon and proton therapy in the treatment of head and neck tumors.104 The primary end point was the risk of secondary cancers based on the risk estimated using the organ equivalent dose model. For proton plans, both the scatter and the neutron dose contributions were accounted for. Their modeling demonstrated that the risk of secondary malignancies may be reduced with the use of intensity modulated proton therapy (IMPT) compared with IMRT with photons. The risk was particularly reduced by reducing the number of proton fields used.
Pommier and colleagues105 at the Francis H. Burr Proton Center in Boston have treated 23 patients with adenoid cystic carcinomas of the skull base with a combination of proton and photon radiotherapy. Only 2 of 23 patients had a complete gross resection, but with residual positive margins as the indications for treatment. The mean total dose to the primary tumor was 75.9 cobalt-gray equivalent. With a median follow-up of 64 months, the 5-year actuarial local control rate was 93%. The 5-year DFS and OS were 56% and 77%, respectively. Multivariate analysis demonstrated that tumor involvement of the sphenoidal sinus and the clivus and the presence of vision change at presentation were significant adverse risk factors for OS. No grade 4 or 5 ocular toxicities were reported. Significant grade 3 neurologic toxicities were observed in 10 patients, and 2 patients developed grade 5 toxicity. These findings suggest that with radiotherapy dose escalation, which may potentially be safer because of the Bragg peak characteristics of the proton beam, local control rates may be improved beyond the historic 20% to 30% local control rates.86
Chemoradiation
The data for concurrent chemoradiation is scant. In the adjuvant context, a single case control study compared 12 patients with a variety of histologies treated with chemoradiation with 12 matched controls treated with radiation alone.106 This analysis showed a significant survival and local control advantage with chemoradiation, with a 3-year OS and DFS of 83% and 77% compared with 44% and 52% for radiotherapy alone. However, in addition to the small number of patients analyzed, several other key caveats must be noted. Performance status and comorbidity of the two groups were not reported, IMRT was used more frequently in the chemoradiation group, and a variety of histologies were treated. Thus, adjuvant chemoradiation must be considered experimental. However, in the absence of level I data to support its efficacy, it is commonly used for patients with aggressive disease, positive margins, extracapsular extension, and other factors associated with a high risk of recurrence. This practice is largely based on the experience of chemoradiation effects on normal tissues when used typically for squamous cell carcinoma of the head and neck. Hence, its use should be limited to the dose ranges and concurrent chemotherapeutics where the toxicity spectrum and risks have been well established if it is being considered.
Much of the available literature on chemoradiation for salivary gland tumors involves small, single-institution series. One case report describes definitive chemoradiotherapy concurrent with intra-arterial cisplatin and docetaxel in two patients.107 A retrospective study of 13 patients treated with cisplatin chemoradiation demonstrated complete response to initial therapy in all patients.108 A single patient developed delayed local failure at 39 months (but achieved successful surgical salvage), and a second patient developed distant metastases; 5-year OS was 83%. Finally, a retrospective analysis of 14 patients reported a 3-year locoregional control rate of 51.6% and a 3-year OS of 35.7% with 5-fluorouracil and hydroxyurea chemoradiation after a median radiation treatment interval of 48 months.109 Although also experimental, definitive chemoradiation is reasonable for patients who are not candidates for surgery, either based on anatomic constraints or medical inoperability.
Palliation: Role of Chemotherapy
Once metastatic, salivary gland malignancies are not curable. Nonetheless, their course can frequently be indolent, especially for adenoid cystic carcinoma with metastatic disease limited to the lung. Median survival with metastatic disease with adenoid cystic carcinoma is approximately 3 years,110 with some patients living substantially longer. Results with systemic agents, both chemotherapeutic and molecular targeted agents, have been disappointing. Therefore careful consideration must be given to administration of supportive care, with systemic therapy reserved for rapid or symptomatic progression.
Chemotherapeutic agents are of limited efficacy in salivary gland malignancies, with particularly poor responses in adenoid cystic histology. A variety of single-agent chemotherapies have been studied in the phase II setting. Limited responses have been observed with cisplatin, vinorelbine, mitoxantrone, epirubicin, methotrexate, and paclitaxel. For example, the Eastern Cancer Oncology Group (ECOG) 1394 trial evaluated paclitaxel, given at 200 mg/m2 every 3 weeks.111 No responses were seen in 14 patients with adenoid cystic carcinoma, whereas a 26% response rate was seen in patients with mucoepidermoid or adenocarcinoma histologies.
The best studied combination regimen is CAP (cyclophosphamide, doxorubicin [Adriamycin], and cisplatin).112 Although the CAP regimen has demonstrated good response rates across histologies, there is no clear evidence for superior efficacy as compared with single-agent chemotherapy. Only a single randomized study compared single-agent therapy with a platinum-based doublet. Airoldi and associates113 compared vinorelbine (given at 30 mg/m2 on days 1 and 8 every 3 weeks) to cisplatin plus vinorelbine (with cisplatin administered at 80 mg/m2 on day 1 and vinorelbine at 25 mg/m2 on days 1 and 8). This study showed a doubling of response rate from 20% to 44% and a strong trend toward improved median survival (8.5 vs. 11 months, p = .058) with use of the doublet. Of particular note was the 44.4% response rate in the typically chemoresistant adenoid cystic carcinoma. Many oncologists consider cisplatin plus vinorelbine as the standard of care when cytotoxic chemotherapy is chosen, with paclitaxel as a reasonable consideration in patients with non–adenoid cystic carcinoma.
Malignant salivary gland tumors commonly overexpress a variety of molecular targets. Given the success of targeted agents in other cancers overexpressing these markers114–117 and the relative tolerability of the agents targeting these markers, investigators have sought to apply these agents to the malignant salivary gland tumors118–131 (see web-only Table 35-1 on the Expert Consult website![]() ).
).
WEB-ONLY TABLE 35-1 Summary Table of Clinical Trials of Molecular Targeted Agents for Malignant Salivary Gland Tumors

Because c-KIT is expressed by most adenoid cystic carcinomas, imatinib mesylate—an inhibitor of the c-KIT, BCR-ABL, and PDGFR tyrosine kinases—was studied. Although responses were noted in three case reports118,119 there were no responses in 30 patients with adenoid cystic carcinoma treated in two phase II studies.120,121 c-KIT staining was assessed only with no mutational analysis performed. Stable disease was common in these studies, including stable disease longer than 6 months. However, the lack of a control group and the indolent behavior of many of these tumors leave unclear whether this stability was because of activity of imatinib or the underlying indolent nature of the tumors. Pending more effective therapies, many clinicians still consider imatinib for c-KIT-expressing adenoid cystic carcinomas because of its lack of toxicity relative to cytotoxic therapies. An ongoing phase II study is evaluating the more potent c-KIT inhibitor dasatinib.110
The anti-ERBB2 (formerly HER2/neu) antibody trastuzumab and the tyrosine kinase inhibitor lapatinib have demonstrated efficacy in ERBB2 overexpressing breast cancer. All histologies of malignant salivary gland tumors have been reported to overexpress ERBB2 at rates from 24% to 56%, depending on the histology, leading to a phase II study of trastuzumab with planned accrual of 50 patients.106 A total of 137 tumors were screened for ERBB2, with 17% found to be overexpressed at 2+ or 3+.30 This result was substantially lower than previously reported in smaller series, and the study was closed early with only 14 patients treated. A single patient with mucoepidermoid carcinoma histology achieved a long-lasting partial response.123 It is relevant to note for future investigations of ERBB2 as a therapeutic target that ERBB2 overexpression in adenoid cystic carcinoma was rare at 4% but common in secretory duct cancers, with 21% in mucoepidermoid carcinoma, 83% in salivary duct tumors, and 60% in squamous histologies.30 A Southwest Oncology group trial of trastuzumab also closed early owing to poor accrual. In this trial, 2 patients were enrolled and treated: one had stable disease for almost a year and the other progressed on therapy.123
Lapatinib is an oral tyrosine kinase inhibitor that targets both ERBB2 and EGFR. A phase II study of this agent enrolled 29 patients with adenoid cystic carcinoma and 28 patients with non–adenoid cystic malignant salivary gland tumors.24 Eighty-eight percent of the patients with adenoid cystic carcinoma and 97% of those patients with non–adenoid cystic carcinoma expressed EGFR and/or ERBB2. There were no responses in either group, but 47% of the patients with adenoid cystic carcinoma and 24% of the non–adenoid cystic carcinoma patients had stable disease lasting at least 6 months. Although the indolent nature of the disease itself likely contributed to some of this stability, biologic activity of lapatinib was likely greater than that represented by the response rate. To meet response criteria, a tumor must shrink 30%. Many patients classified as having stable disease experienced reductions in tumor volumes that failed to meet this threshold but nonetheless are suggestive of activity. Furthermore, in 36% of the patients with stable disease for more than 6 months, the disease was progressing before starting therapy, this suggests that although the therapy may not have been sufficiently active to shrink the cancer, it could at least stop its growth.
Similar results were found with the tyrosine kinase inhibitor gefitinib, which targets primarily the EGFR. At the time of presentation 21 patients were assessable for response, with no responses.124 Thirteen of 14 patients with stable disease had adenoid cystic carcinoma, but the median duration of stable disease was only 13 weeks, again raising concerns that the disease was secondary to the indolent nature of the cancer rather than efficacy of therapy. Similarly, a phase II study of cetuximab failed to achieve any responses. However, 20 of 23 (87%) patients with adenoid cystic carcinoma achieved stable disease, with 12 of these 23 (52%) achieving stability lasting at least 6 months.125 Of note, 11 patients with adenoid cystic carcinoma had disease that was actively progressing at the time of study entry; all of these patients achieved stable disease for at least 6 months.
Bortezomib inhibits the 26S proteosome, indirectly inhibiting NF-κB activity. A phase II study treated 25 patients with adenoid cystic carcinoma.126 Again, no responses were seen. Patients with progressive disease were treated with the combination of bortezomib and doxil, based on preclinical data for synergy. Four patients were treated with this combination; 1 achieved a partial response and 2 had stable disease. A phase II study of the combination of bortezomib and doxil just closed secondary to poor accrual.127
Data for the efficacy of the hormonal agent tamoxifen is limited to three case reports of long-term stable disease.128,129 Case reports also describe responses, including a complete response, to luteinizing hormone–releasing hormone analogues in patients with androgen-receptor positive adenocarcinoma.130
Irradiation Techniques and tolerance
Target Volume Delineation
Submandibular Glands
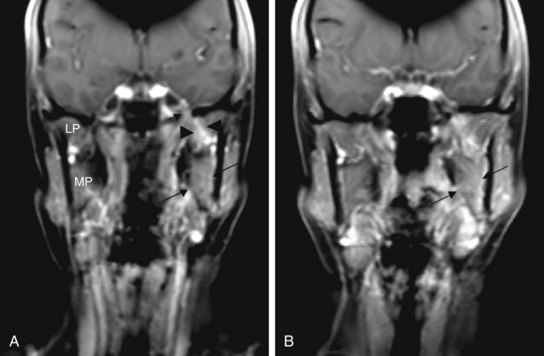
The primary target volume includes the location of the primary tumor or surgical bed for salivary gland tumors meeting indications for radiotherapy. A boost is given to areas of unresected or incompletely resected disease. When perineural invasion is present, but limited to small, unnamed nerves, the target volumes are enlarged in the area of the postoperative bed but the nerve pathways are not treated comprehensively. If named nerves are involved, such as the lingual or hypoglossal nerves, their course should be treated to the base of skull. This involves delineation of the course of the mandibular (V3) branch of cranial nerve V to the foramen ovale. The V3 branch runs from the submandibular space extending into the parapharyngeal and masseter space medial to the course of the medial pterygoid muscle to the foramen ovale (Fig. 35-4). Review of preoperative imaging and intraoperative and pathologic findings are imperative in treatment planning.
Minor Salivary Glands
Elective neck treatment varies with the histology, grade, T category, anatomic site, and nodal category. Lloyd and colleagues49 analyzed the SEER database identifying 2667 cases of minor salivary gland cancers with known lymph node status from 1988 to 2004. Although there are inherent limitations with SEER database analyses, the results are consistent with smaller clinical series that, in turn, lack the size for a multivariate analysis. Analysis demonstrated that male sex, T3 to T4 category, primary site involving the pharynx, and high-grade adenocarcinoma or high-grade mucoepidermoid carcinomas were independent risk factors for the development of nodal metastasis for minor salivary glands.
Although these findings suggest that the biology of salivary gland malignancies do influence the risk of nodal metastases, they also suggest that lymphatic-rich anatomic sites may be relevant for minor salivary gland malignancies as seen with squamous carcinomas. For adenoid cystic carcinomas, Mendenhall and colleagues84 reported that this was a significant consideration in their institutional approach. They reported a 10-year neck control rate of 90% when the neck was observed versus 98% with elective irradiation. Other relevant but unestablished anatomic factors that have been well described for squamous carcinomas can also be considered during treatment planning for malignant salivary gland tumors. These include the presence of mucosal disease at the coronal midline increasing the risk for contralateral nodal metastases132 and the involvement of the posterior pharyngeal mucosa influencing the risk of retropharyngeal lymph node metastases.133
Simulation/Field Arrangements
IMRT may also allow for additional sparing of structures such as the cochlea and temporal lobes. Historically, hearing deficits, soft tissue necrosis, and bone necrosis have been observed in approximately 20% of patients treated with a traditional beam arrangement, especially with wedge-pair fields.134 By using these techniques to treat the tumor bed with or without the ipsilateral neck, mean doses to the contralateral salivary glands can be effectively limited to less than 10% of the prescription dose, thereby preventing any symptoms of xerostomia during and after treatment.
Dose/Fractionation and Pathologic Considerations
For perineural invasion, an increased risk of locoregional relapse has been correlated especially for adenoid cystic carcinomas. Recent detailed histologic studies of serial sections suggest that the mechanism in which perineural invasion may be contributing to relapse is the result of the direct contiguous spread of cancer along the branches of the nerves.51 Therefore a judicious clinical target volume surrounding the primary tumor bed should be created to reflect the potential subclinical extent of cancer. The use of postoperative radiotherapy appears to be effective in reducing the risk of local relapse caused by perineural invasion (10-year local control rates of tumors with perineural invasion treated with surgery vs. surgery and postoperative radiotherapy were 60% vs. 88%, p = .01).50
For more extensive perineural invasion resulting in clinically evident (symptomatic or radiologic) disease there is a further increased risk of locoregional relapse, especially with adenoid cystic carcinomas.79 This observation is consistent with other cancer sites in which the size of the involved nerve appears to be increase the risk of relapse.135 With involvement of major named nerves, the increased risk of relapse can be caused by the spread to the skull base and also with an increased risk of distant relapses. Thus elective irradiation along the course of the nerve is recommended.
Gross, unresectable disease has typically been treated to 70 Gy, again with a conventional daily fractionated schedule. The optimal dose and fractionation schedule has not been clearly defined especially for unresectable gross disease. This is important to consider in light of evidence suggesting that radiotherapy dose escalation with photons99 and protons105 may offer additional improvements in local control rates. Unknown at this time is whether there may be incremental gains in locoregional disease control rates with the practice of a simultaneous in-field boost (“dose painting”) that is now possible with the use of IMRT.
Treatment Algorithm, Conclusions, Controversies, and Future Possibilities
In conclusion, the treatment options for patients with salivary gland tumors have significantly improved in recent years, offering the potential for more effective locoregional therapy that preserves function. The authors’ preferred treatment algorithm is seen in Table 35-10. Major survival gains will likely be realized only with the development of more effective systemic therapy.
TABLE 35-10 Treatment Algorithm for Malignant Major and Minor Salivary Gland Cancers
| Clinical Situation | Standard Therapy | Proposed Clinical Trial |
|---|---|---|
| Complete resection, adjuvant therapy | Postoperative, adjuvant EBRT when indicated (see Table 35-9) | Intergroup trial of surgical resection + postoperative adjuvant EBRT vs. surgical resection + postoperative adjuvant EBRT with concomitant and maintenance chemotherapy |
| Locally advanced (primary or recurrent; unresectable or resected but residual) | 1.High-dose conventional photon irradiation (consider altered fractionation) | 1.Neoadjuvant chemotherapy followed by resection (if feasible) and EBRT with concomitant and maintenance chemotherapy |
| 2.Maximal surgical resection, conventional EBRT with concomitant chemotherapy | 2.High-dose conventional or altered-fractionation EBRT (photons or protons) with concomitant and maintenance chemotherapy | |
| 3.Neutron beam therapy ± SRS boost | 3.Carbon ion therapy | |
| Locally recurrent, prior irradiation | Low- to moderate-dose EBRT, consider SRS or SRT Palliative chemotherapy |
EBRT, external beam irradiation; SRS, stereotactic radiosurgery; SRT stereotactic radiotherapy.
1 Spitz MR, Fueger JJ, Goepfert H, Newell GR. Salivary gland cancer. A case-control investigation of risk factors. Arch Otolaryngol Head Neck Surg. 1990;116:1163-1166.
4 Beal KP, Singh B, Kraus D, et al. Radiation-induced salivary gland tumors. A report of 18 cases and a review of the literature. Cancer J. 2003;9:467-471.
8 Modan B, Chetrit A, Alfandary E, et al. Increased risk of salivary gland tumors after low-dose irradiation. Laryngoscope. 1998;108:1095-1097.
18 Okabe M, Miyabe S, Nagatsuka H, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12:3902-3907.
50 Terhaard CH. Postoperative and primary radiotherapy for salivary gland carcinomas. Indications, techniques, and results. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S52-S55.
52 Douglas JG, Koh WJ, Austin-Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg. 2003;129:944-948.
71 Frankenthaler RA, Luna MA, Lee SS, et al. Prognostic variables in parotid gland cancer. Arch Otolaryngol Head Neck Surg. 1991;117:1251-1256.
73 Armstrong JG, Harrison LB, Spiro RH, et al. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiation therapy. Arch Otorhinolaryngol Head Neck Surg. 1990;116:290-293.
74 North CA, Lee DJ, Piantadosi S, et al. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 1990;18:1319-1326.
75 Theriault C, Fitzpatrick PJ. Malignant parotid tumors. Prognostic factors and optimum treatment. Am J Clin Oncol. 1986;9:510-516.
76 Spiro IJ, Wang CC, Montgomery WW. Carcinoma of the parotid gland. Cancer. 1993;71:2699-2705.
77 Bissett RJ, Fitzpatrick PJ. Malignant submandibular gland tumors. A review of 91 patients. Am J Clin Oncol. 1988;11:41-51.
78 Weber RS, Byers RM, Petit B, et al. Submandibular gland tumors. Adverse histologic factors and therapeutic implications. Arch Otolaryngol Head Neck Surg. 1990;116:1055-1060.
79 Spiro RH, Koss LG, Hajdu SI, Strong EW. Tumors of minor salivary gland origin. A clinical pathologic study of 492 cases. Cancer. 1973;31:117-129.
80 Parsons JT, Mendenhall WM, Stringer SP, et al. Management of minor salivary gland carcinomas. Int J Radiat Oncol Biol Phys. 1996;35:443-454.
81 Garden AS, Weber RS, Ang KK, et al. Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer. 1994;73:2563-2569.
82 Le Q, Birdwell S, Terris DJ, et al. Postoperative irradiation of minor salivary gland malignancies of the head and neck. Radiother Oncol. 1999;52:165-171.
83 Chen AM, Garcia J, Granchi P, et al. Base of skull recurrences after treatment of salivary gland cancer with perineural invasion reduced by postoperative radiotherapy. Clin Otolaryngol. 2009;34:539-545.
84 Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154-162.
85 Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619-626.
86 Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer. 2005;103:2544-2550.
89 Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors. Final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235-240.
92 Battermann JJ, Mijnheer BJ. The Amsterdam fast neutron therapy project. A final report. Int J Radiat Oncol Biol Phys. 1986;12:2093-2099.
96 Douglas JG, Lee S, Laramore GE, et al. Neutron radiotherapy for the treatment of locally advanced major salivary gland tumors. Head Neck. 1999;21:255-263.
97 Douglas JG, Laramore GE, Austin-Seymour M, et al. Treatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:551-557.
99 Douglas JG, Goodkin R, Laramore GE. Gamma knife stereotactic radiosurgery for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 2008;30:492-496.
105 Pommier P, Liebsch NJ, Deschler DG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1242-1249.
106 Tanvetyanon T, Qin D, Padhya T, et al. Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:687-692.
1 Spitz MR, Fueger JJ, Goepfert H, Newell GR. Salivary gland cancer. A case-control investigation of risk factors. Arch Otolaryngol Head Neck Surg. 1990;116:1163-1166.
2 Albeck H, Bentzen J, Ockelmann HH, et al. Familial clusters of nasopharyngeal carcinoma and salivary gland carcinomas in Greenland natives. Cancer. 1993;72:196-200.
3 Gallo O, Santucci M, Calzolari A, Storchi OF. Epstein-Barr virus (EBV) infection and undifferentiated carcinoma of the parotid gland in Caucasian patients. Acta Otolaryngol. 1994;114:572-575.
4 Beal KP, Singh B, Kraus D, et al. Radiation-induced salivary gland tumors. A report of 18 cases and a review of the literature. Cancer J. 2003;9:467-471.
5 Pinkston JA, Cole P. Incidence rates of salivary gland tumors. Results from a population-based study. Otolaryngol Head Neck Surg. 1999;120:834-840.
6 Takeichi N, Hirose F, Yamamoto H. Salivary gland tumors in atomic bomb survivors, Hiroshima, Japan. I. Epidemiologic observations. Cancer. 1976;38:2462-2468.
7 Land CE, Saku T, Hayashi Y, et al. Incidence of salivary gland tumors among atomic bomb survivors, 1950-1987. Evaluation of radiation-related risk. Radiat Res. 1996;146:28-36.
8 Modan B, Chetrit A, Alfandary E, et al. Increased risk of salivary gland tumors after low-dose irradiation. Laryngoscope. 1998;108:1095-1097.
9 Saku T, Hayashi Y, Takahara O, et al. Salivary gland tumors among atomic bomb survivors, 1950-1987. Cancer. 1997;79:1465-1475.
10 Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8(5):229-240.
11 Horner MJ, Ries LAG, Krapcho M, et al. SEER cancer statistics review, 1975-2006. Bethesda, MD: National Cancer Institute; 2009.
12 Wahlberg P, Anderson H, Biörklund A, et al. Carcinoma of the parotid and submandibular glands—a study of survival in 2465 patients. Oral Oncol. 2002;38:706-713.
13 Boukheris H, Curtis RE, Land CE, Dores GM. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006. A population-based study in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:2899-2906.
14 Stenman G. Fusion oncogenes and tumor type specificity—insights from salivary gland tumors. Semin Cancer Biol. 2005;15:224-235.
15 Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208-213.
16 Enlund F, Behboudi A, Andrén Y, et al. Altered Notch signaling resulting from expression of a WAMTP1-MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin’s tumors. Exp Cell Res. 2004;292:21-28.
17 O’Neill ID. t(11;19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinoma. Oral Oncol. 2009;45:2-9.
18 Okabe M, Miyabe S, Nagatsuka H, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12:3902-3907.
19 Miyabe S, Okabe M, Nagatsuka H, et al. Prognostic significance of p27Kip1, Ki-67, and CRTC1-MAML2 fusion transcript in mucoepidermoid carcinoma. A molecular and clinicopathologic study of 101 cases. J Oral Maxillofac Surg. 2009;67:1432-1441.
20 Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas. Prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470-481.
21 Tirado Y, Williams MD, Hanna EY, et al. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors. Implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46:708-715.
22 Anzick SL, Chen WD, Park Y, et al. Unfavorable prognosis of CRTC1-MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes Chromosomes Cancer. 2010;49:59-69.
23 Persson M, Andrén Y, Mark J, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740-18744.
24 Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978-3984.
25 Vered M, Braunstein E, Buchner A. Immunohistochemical study of epidermal growth factor receptor in adenoid cystic carcinoma of salivary gland origin. Head Neck. 2002;24:632-636.
26 Monteiro LS, Bento MJ, Palmeira C, Lopes C. Epidermal growth factor receptor immunoexpression evaluation in malignant salivary gland tumours. J Oral Pathol Med. 2009;38:508-513.
27 Ettl T, Schwarz S, Kleinsasser N, et al. Overexpression of EGFR and absence of c-KIT expression correlate with poor prognosis in salivary gland carcinomas. Histopathology. 2008;53:567-577.
28 Nguyen LH, Black MJ, Hier M, et al. HER2/neu and Ki-67 as prognostic indicators in mucoepidermoid carcinoma of salivary glands. J Otolaryngol. 2003;32:328-331.
29 Nabili V, Tan JW, Bhuta S, et al. Salivary duct carcinoma. A clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29:907-912.
30 Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas. Dependence on histological subtype. Clin Cancer Res. 2004;10:944-946.
31 Nagler RM, Kerner H, Ben-Eliezer S, et al. Prognostic role of apoptotic, Bcl-2, c-erbB-2 and p53 tumor markers in salivary gland malignancies. Oncology. 2003;64:389-398.
32 Stenman G, Sandros J, Nordkvist A, et al. Expression of the ERBB2 protein in benign and malignant salivary gland tumors. Genes Chromosomes Cancer. 1991;3:128-135.
33 Giannoni C, El-Naggar AK, Ordoñez NG, et al. c-erbB-2/neu Oncogene and Ki-67 analysis in the assessment of palatal salivary gland neoplasms. Otolaryngology Head Neck Surg. 1995;112:391-398.
34 Press MF, Pike MC, Hung G, et al. Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland. Correlation with poor prognosis. Cancer Res. 1994;54:5675-5682.
35 Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett. 2000;154:107-111.
36 Beltran D, Faquin WC, Gallagher G, August M. Selective immunohistochemical comparison of polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma. J Oral Maxillofac Surg. 2006;64:415-423.
37 Edwards PC, Bhuiya T, Kelsch RD. C-kit expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and monomorphic adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:586-593.
38 Vila L, Liu H, Al-Quran SZ, et al. Identification of c-kit gene mutations in primary adenoid cystic carcinoma of the salivary gland. Mod Pathol. 2009;22:1296-1302.
39 Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77-82.
40 Kowalski PJ, Paulino AF. Perineural invasion in adenoid cystic carcinoma. Its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33:933-936.
41 Wang L, Sun M, Jiang Y, et al. Nerve growth factor and tyrosine kinase A in human salivary adenoid cystic carcinoma: Expression patterns and effects on in vitro invasive behavior. J Oral Maxillofac Surg. 2006;64:636-641.
42 Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218-223.
43 Wijffels KI, Hoogsteen IJ, Lok J, et al. No detectable hypoxia in malignant salivary gland tumors. Preliminary results. Int J Radiat Oncol Biol Phys. 2009;73:1319-1325.
44 Barnes L, Eveson J, Reichart P, editors. Tumors of the Salivary Gland. Lyon: IARC Press, 2005.
45 Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer. 1992;69:2021-2030.
46 Hicks MJ, Adel KE-N, Catherine MF, et al. Histocytologic grading of mucoepidermoid carcinoma of major salivary glands in prognosis and survival. A clinicopathologic and flow cytometric investigation. Head Neck. 1995;17:89-95.
47 Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma. A clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835-845.
48 Guzzo M, Andreola S, Sirizzotti G, Cantu G. Mucoepidermoid carcinoma of the salivary glands. Clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002;9:688-695.
49 Lloyd S, Yu JB, Ross DA, et al. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Int J Radiat Oncol Biol Phys. 2010;76:169-175.
50 Terhaard CH. Postoperative and primary radiotherapy for salivary gland carcinomas. Indications, techniques, and results. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S52-S55.
51 Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218-223.
52 Douglas JG, Koh WJ, Austin-Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg. 2003;129:944-948.
53 Christensen RK, Bjorndal K, Godballe C, Krogdahl A. Value of fine-needle aspiration biopsy of salivary gland lesions. Head Neck. 2010;32:104-108.
54 Hughes JH, Volk EE, Wilbur DC. Pitfalls in salivary gland fine-needle aspiration cytology: Lessons from the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology. Arch Pathol Lab Med. 2005;129:26-31.
55 Burke CJ, Thomas RH, Howlett D. Imaging the major salivary glands. Br J Oral Maxillofac Surg. 2010 Apr 7. [Epub ahead of print]
56 Jeong HS, Chung MK, Son YI, et al. Role of 18F-FDG PET/CT in management of high-grade salivary gland malignancies. J Nucl Med. 2007;48:1237-1244.
57 Razfar A, Heron DE, Branstetter BF, et al. Positron emission tomography–computed tomography adds to the management of salivary gland malignancies. Laryngoscope. 2010;120:734-738.
58 Edge SB, et al, editors. American joint committee on cancer, cancer staging manual, 7th ed, Chicago: Springer, 2010.
59 Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146:51-58.
60 Tullio A, Marchetti C, Sesenna E, et al. Treatment of carcinoma of the parotid gland. The results of a multicenter study. J Oral Maxillofac Surg. 2001;59:263-270.
61 Gomez DR, Katabi N, Zhung J, et al. Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer. 2009;115:2128-2137.
62 Batsakis JG. Carcinomas of the submandibular and sublingual glands. Ann Otol Rhinol Laryngol. 1986;95:211-212.
63 Camilleri IG, Malata CM, McLean NR, Kelly CG. Malignant tumours of the submandibular salivary gland. A 15-year review. Br J Plast Surg. 1998;51:181-185.
64 Strick MJ, Kelly CG, Soames JV, McLean NR. Malignant tumours of the minor salivary glands—a 20 year review. Br J Plast Surg. 2004;57:624-631.
65 O’Malley BJ, Weinstein G. Robotic skull base surgery. Preclinical investigation to human clinical application. Arch Otolaryngol Head Neck Surg. 2007;133:1215-1219.
66 O’Malley BWJr, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465-1472.
67 Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer. A preliminary experience. Head Neck. 2009;31:283-289.
68 Harish K. Management of primary malignant epithelial parotid tumors. Surg Oncol. 2004;13:7-16.
69 Armstrong JG, Harrison LB, Thaler HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992;69:615-619.
70 McGuirt WF. Management of occult metastatic disease from salivary gland neoplasms. Arch Otolaryngol Head Neck Surg. 1989;115:322-325.
71 Frankenthaler RA, Luna MA, Lee SS, et al. Prognostic variables in parotid gland cancer. Arch Otolaryngol Head Neck Surg. 1991;117:1251-1256.
72 Nagliati M, Bolner A, Vanoni V, et al. Surgery and radiotherapy in the treatment of malignant parotid tumors. A retrospective multicenter study. Tumori. 2009;95:442-448.
73 Armstrong JG, Harrison LB, Spiro RH, et al. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiation therapy. Arch Otorhinolaryngol Head Neck Surg. 1990;116:290-293.
74 North CA, Lee DJ, Piantadosi S, et al. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 1990;18:1319-1326.
75 Theriault C, Fitzpatrick PJ. Malignant parotid tumors. Prognostic factors and optimum treatment. Am J Clin Oncol. 1986;9:510-516.
76 Spiro IJ, Wang CC, Montgomery WW. Carcinoma of the parotid gland. Cancer. 1993;71:2699-2705.
77 Bissett RJ, Fitzpatrick PJ. Malignant submandibular gland tumors. A review of 91 patients. Am J Clin Oncol. 1988;11:41-51.
78 Weber RS, Byers RM, Petit B, et al. Submandibular gland tumors. Adverse histologic factors and therapeutic implications. Arch Otolaryngol Head Neck Surg. 1990;116:1055-1060.
79 Spiro RH, Koss LG, Hajdu SI, Strong EW. Tumors of minor salivary gland origin. A clinical pathologic study of 492 cases. Cancer. 1973;31:117-129.
80 Parsons JT, Mendenhall WM, Stringer SP, et al. Management of minor salivary gland carcinomas. Int J Radiat Oncol Biol Phys. 1996;35:443-454.
81 Garden AS, Weber RS, Ang KK, et al. Postoperative radiation therapy for malignant tumors of minor salivary glands: Outcome and patterns of failure. Cancer. 1994;73:2563-2569.
82 Le Q, Birdwell S, Terris DJ, et al. Postoperative irradiation of minor salivary gland malignancies of the head and neck. Radiother Oncol. 1999;52:165-171.
83 Chen AM, Garcia J, Granchi P, et al. Base of skull recurrences after treatment of salivary gland cancer with perineural invasion reduced by postoperative radiotherapy. Clin Otolaryngol. 2009;34:539-545.
84 Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26(2):154-162.
85 Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619-626.
86 Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer. 2005;103:2544-2550.
87 Bhide SA, Miah A, Barbachano Y, et al. Radical radiotherapy for treatment of malignant parotid tumours. A single centre experience 1995-2005. Br J Oral Maxillofac Surg. 2009;47:284-289.
88 Vikram B, Strong EW, Shah JP, Spiro RH. Radiation therapy in adenoid-cystic carcinoma. Int J Radiat Oncol Biol Phys. 1984;10:221-223.
89 Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors. Final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235-240.
90 Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas. First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7-16.
91 Wang CC, Goodman M. Photon irradiation of unresectable carcinomas of salivary glands. Int J Radiat Oncol Biol Phys. 1991;21:569-576.
92 Battermann JJ, Mijnheer BJ. The Amsterdam fast neutron therapy project. A final report. Int J Radiat Oncol Biol Phys. 1986;12:2093-2099.
93 Catterall M, Errington RD. The implications of improved treatment of malignant salivary gland tumors by fast neutron radiotherapy. Int J Radiat Oncol Biol Phys. 1987;13:1313-1318.
94 Saroja KR, Mansell J, Hendrickson FR, et al. An update on malignant salivary gland tumors treated with neutrons at Fermilab. Int J Radiat Oncol Biol Phys. 1987;13:1319-1325.
95 Huber PE, Debus J, Latz D, et al. Radiotherapy for advanced adenoid cystic carcinoma. Neutrons, photons or mixed beam? Radiother Oncol. 2001;59:161-1617.
96 Douglas JG, Lee S, Laramore GE, et al. Neutron radiotherapy for the treatment of locally advanced major salivary gland tumors. Head Neck. 1999;21:255-263.
97 Douglas JG, Laramore GE, Austin-Seymour M, et al. Treatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:551-557.
98 Buchholz TA, Laramore GE, Griffin BR, et al. The role of fast neutron radiation therapy in the management of advanced salivary gland malignant neoplasms. Cancer. 1992;69:2779-2788.
99 Douglas JG, Goodkin R, Laramore GE. Gamma knife stereotactic radiosurgery for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 2008;30:492-496.
100 Schulz-Ertner D, Nikoghosyan A, Jakel O, et al. Feasibility and toxicity of combined photon and carbon ion radiotherapy for locally advanced adenoid cystic carcinomas. Int J Radiat Oncol Biol Phys. 2003;56:391-398.
101 Ahn YC, Lee KC, Kim DY, et al. Fractionated stereotactic radiation therapy for extracranial head and neck tumors. Int J Radiat Oncol Biol Phys. 2000;48:501-505.
102 Ryu S, Khan M, Yin FF, et al. Image-guided radiosurgery of head and neck cancers. Otolaryngol Head Neck Surg. 2004;130:690-697.
103 Lee N, Millender LE, Larson DA, et al. Gamma knife radiosurgery for recurrent salivary gland malignancies involving the base of skull. Head Neck. 2003;25:210-216.
104 Steneker M, Lomax A, Schneider U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother Oncol. 2006;80:263-267.
105 Pommier P, Liebsch NJ, Deschler DG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1242-1249.
106 Tanvetyanon T, Qin D, Padhya T, et al. Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:687-692.
107 Maruya S, Namba A, Matsubara A, et al. Salivary gland carcinoma treated with concomitant chemoradiation with intraarterial cisplatin and docetaxel. Int J Clin Oncol. 2006;11:403-406.
108 Wan JFVKRRSW: Upfront concurrent chemoradiation for adenoid cystic carcinoma of the head and neck: A 3-institution experience. 7th International Conference on Head and Neck Cancer. San Francisco, 2008.
109 Pederson AW, Haraf DJ, Blair EA, et al. Chemoreirradiation for recurrent salivary gland malignancies. Radiother Oncol. 2010;95:308-311.
110 Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495-498.
111 Gilbert J, Li Y, Pinto HA, et al. Phase II trial of taxol in salivary gland malignancies (E1394). A trial of the Eastern Cooperative Oncology Group. Head Neck. 2006;28:197-204.
112 Licitra L, Cavina R, Grandi C, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients. Ann Oncol. 1996;7:640-642.
113 Airoldi M, Pedani F, Succo G, et al. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer. 2001;91:541-547.
114 Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123-132.
115 Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792.
116 Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-1127.
117 Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib. Randomised trial. Lancet. 2004;364:1127-1134.
118 Alcedo JC, Fabrega JM, Arosemena JR, Urrutia A. Imatinib mesylate as treatment for adenoid cystic carcinoma of the salivary glands. Report of two successfully treated cases. Head Neck. 2004;26:829-831.
119 Faivre S, Raymond E, Casiraghi O, et al. Imatinib mesylate can induce objective response in progressing, highly expressing KIT adenoid cystic carcinoma of the salivary glands. J Clin Oncol. 2005;23:6271-6273. author reply 6273-6274
120 Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit. A Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585-590.
121 Pfeffer MR, Talmi Y, Catane R, et al. A phase II study of imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007;43:33-36.
122 Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39:724-727.
123 Kane MA: Personal communication, April 27, 2010.
124 Glisson BB, Francisco GJr, Erasmus M, et al. Phase II trial of gefitinib in patients with incurable salivary gland cancer. ASCO annual meeting proceedings. J Clin Oncol. 2005;23(16 Suppl):5532.
125 Locati LD, Bossi P, Perrone F, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2009;45:574-578.
126 Argiris MAG, Burtness B, Deconti R, et al. A phase II trial of PS-341 (bortezomib) followed by the addition of doxorubicin at progression in incurable adenoid cystic carcinoma of the head and neck. An Eastern Cooperative Oncology Group study. ASCO annual meeting proceedings. J Clin Oncol. 2006;24(18 Suppl):5573.
127 Argiris AE: Personal communication, April 27, 2010.
128 Elkin AD, Jacobs CD. Tamoxifen for salivary gland adenoid cystic carcinoma. Report of two cases. J Cancer Res Clin Oncol. 2008;134:1151-1153.
129 Shadaba A, Gaze MN, Grant HR. The response of adenoid cystic carcinoma to tamoxifen. J Laryngol Otol. 1997;111:1186-1189.
130 Locati LD, Quattrone P, Bossi P, et al. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14:1327-1328.
131 Lin CH, Yen RF, Jeng YM, et al. Unexpected rapid progression of metastatic adenoid cystic carcinoma during treatment with imatinib mesylate. Head Neck. 2005;27:1022-1027.
132 O’Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys. 2001;51:332-343.
133 Bussels B, Hermans R, Reijnders A, et al. Retropharyngeal nodes in squamous cell carcinoma of oropharynx. Incidence, localization, and implications for target volume. Int J Radiat Oncol Biol Phys. 2006;65:733-738.
134 Garden AS, El-Naggar AK, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys. 1997;37:79-85.
135 Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion. An investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.