Safety in the Immunology-Serology Laboratory
At the conclusion of this chapter, the reader should be able to:
• Name the federal or national agencies responsible for safety issues.
• Discuss the occupational transmission of hepatitis B virus (HBV) and human immunodeficiency virus (HIV).
• Describe the practice of Standard Blood and Body Fluid Precautions.
• Explain the proper handling of hazardous material and waste management, including infectious waste, chemicals, and radioactive waste.
• Describe the basic aspects of infection control policies, including the use of personal protective equipment or devices (gowns, gloves, goggles) and the purpose of Standard Precautions.
• Compare preexposure and postexposure prophylactic measures for handling potential occupational transmission of certain pathogens (HBV, HCV, HIV).
• Demonstrate the proper decontamination of a work area at the start and completion of work and after a hazardous spill.
• Explain the process of properly segregating and disposing of various types of waste products generated in the clinical laboratory.
• Analyze a safety case study to identify violations and remediations for the violations.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Identify items essential to safety in the clinical laboratory.
• Correctly answer end of chapter review questions.
Safety Standards and Agencies
• U.S. Department of Labor, Occupational Safety and Health Administration (OSHA)
• Clinical and Laboratory Standards Institute (CLSI), a nonprofit educational organization that provides a forum for the development, promotion, and use of national and international standards
• Centers for Disease Control and Prevention (CDC), part of the U.S. Department of Health and Human Services Public Health Service
1. Staff must wear laboratory coats and be additionally protected from contamination by infectious agents.
2. Food and drinks should not be consumed in work areas or stored in the same area as specimens. Containers, refrigerators, or freezers used for specimens should be marked as containing a biohazard.
3. Specimens needing centrifugation are capped and placed into a centrifuge with a sealed dome.
4. A gauze square is used when opening rubber-stoppered test tubes to minimize aerosol production (introduction of substances into the air).
5. Autodilutors or safety bulbs are used for pipetting. Pipetting of any clinical material by mouth is strictly forbidden.
Prevention of Transmission of Infectious Diseases
• Educate and train all health care workers in Standard Precautions and in preventing bloodborne infections.
• Concentration of HBV or HIV; viral concentration is higher for HBV than HIV
• Presence or skin lesions or abrasions on the hands or exposed skin of the health care worker
• Percutaneous (parenteral) inoculation of blood, plasma, serum, or certain other body fluids from accidental needlesticks
• Contamination of the skin with blood or certain body fluids without overt puncture, caused by scratches, abrasions, burns, weeping, or exudative skin lesions
• Exposure of mucous membranes (oral, nasal, or conjunctival) to blood or certain body fluids, as the direct result of pipetting by mouth, splashes, or spattering
• Centrifuge accidents or the improper removal of rubber stoppers from test tubes, producing droplets. If these aerosol products are infectious and come into direct contact with mucous membranes or nonintact skin, direct transmission of virus can result.
Protective Techniques for Infection Control
Selection and Use of Gloves
General guidelines related to the selection and general use of gloves include the following:
1. Use sterile gloves for procedures involving contact with normally sterile areas of the body or during procedures in which sterility has been established and must be maintained.
2. Use nonsterile examination gloves for procedures that do not require the use of sterile gloves. Gloves must be worn when receiving phlebotomy training. The National Institute of Occupational Safety and Health mandates the use of gloves for phlebotomy.
3. Gloves should be changed between each patient contact.
4. Wear gloves when processing blood specimens, reagents, or blood products, including reagent red blood cells.
5. Gloves should be changed frequently and immediately if they become visibly contaminated with blood or certain body fluids or if physical damage occurs.
6. Do not wash or disinfect latex or vinyl gloves for reuse. Washing with detergents may cause increased penetration of liquids through undetected holes in the gloves. Rubber gloves may be decontaminated and reused, but disinfectants may cause deterioration. Rubber gloves should be discarded if they have punctures, tears, or evidence of deterioration or if they peel, crack, or become discolored.
7. Using items potentially contaminated with human blood or certain body fluids (e.g., specimen containers, laboratory instruments, countertops)
Care must be taken to avoid indirect contamination of work surfaces or objects in the work area. Gloves should be properly removed (Fig. 6-1) or covered with an uncontaminated glove or paper towel before answering the telephone, handling laboratory equipment, or touching doorknobs.
Handwashing
Frequent handwashing is an important safety precaution. It should be performed after contact with patients and laboratory specimens (Box 6-1). Gloves should be used as an adjunct to, not a substitute for, handwashing.
1. After completing laboratory work and before leaving the laboratory
2. After removing gloves. The Association for Professionals in Infection Control and Epidemiology has reported that extreme variability exists in the quality of gloves, with leakage in 4% to 63% of vinyl gloves and in 3% to 52% of latex gloves.
3. Before eating, drinking, applying makeup, and changing contact lenses, and before and after using the bathroom
4. Before all activities that involve hand contact with mucous membranes or breaks in the skin
5. Immediately after accidental skin contact with blood, body fluids, or tissues
a. If the contact occurs through breaks in gloves, the gloves should be removed immediately and the hands thoroughly washed.
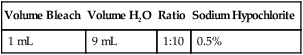
b. If accidental contamination occurs to an exposed area of the skin or because of a break in gloves, wash first with a liquid soap, rinse well with water, and then apply a 1:10 dilution of bleach (Table 6-1) or 50% isopropyl or ethyl alcohol. The bleach or alcohol is left on skin for at least 1 minute before final washing with liquid soap and water.
Table 6-1
Preparation of Diluted Household Bleach
| Volume Bleach | Volume H2O | Ratio | Sodium Hypochlorite |
| 1 mL | 9 mL | 1:10 | 0.5% |
Two important points in the practice of hand hygiene technique follow (see Box 6-1):
• When decontaminating hands with a waterless antiseptic agent (e.g., alcohol-based handrub), apply product to the palm of one hand and rub hands together, covering all surfaces of hands and fingers, until hands are dry. Follow the manufacturer’s recommendations on the volume of product to use. If an adequate volume of an alcohol-based handrub is used, it should take 15 to 25 seconds for hands to dry.
• When washing with a nonantimicrobial or antimicrobial soap, wet hands first with warm water, apply 3 to 5 mL of detergent to hands, and rub hands together vigorously for at least 15 seconds, covering all surfaces of the hands and fingers. Rinse hands with warm water and dry thoroughly with a disposable towel. Use the towel to turn off the faucet.
Decontamination of Work Surfaces, Equipment, and Spills
1. Wear gloves and a laboratory coat.
2. Absorb the blood with disposable towels. Bleach solutions are less effective in the presence of high concentrations of protein. Remove as much liquid blood or serum as possible before decontamination.
3. Using a diluted bleach (1:10) solution, clean the spill site of all visible blood.
4. Wipe down the spill site with paper towels soaked with diluted bleach.
5. Place all disposable materials used for decontamination into a biohazard container.
Disposal of Infectious Laboratory Waste
OSHA has defined infectious waste as blood and blood products, contaminated sharps, pathologic wastes, and microbiological wastes. Infectious waste is packaged for disposal in color-coded containers and labeled as such with the standard symbol for a biohazard (Fig. 6-2).
1. Conspicuously marked “Biohazard” with the universal biohazard symbol
2. Universal color—orange, orange and black, or red
3. Rigid, leakproof, and puncture resistant. (Cardboard boxes lined with leakproof plastic bags are available.)
4. Used for blood and other potentially infectious body fluids, as well as disposable materials contaminated with blood or fluid
Disease Prevention
Immunization
1. Immunizing agents strongly recommended for health care workers
2. Other immunologic agents that are or may be indicated for health care workers
Postexposure Prophylaxis
Although the most important strategy for reducing the risk of occupational HIV transmission is to prevent occupational exposures, plans for postexposure management of health care personnel should be in place. The U.S. Public Health Service has issued guidelines for the management of health care personnel exposure to HIV and recommendations for postexposure prophylaxis (PEP).∗
HBsAg-Positive Exposure Source
• Persons who have written documentation of a complete hepatitis B vaccine series and who did not receive postvaccination testing should receive a single vaccine booster dose.
• Persons who are in the process of being vaccinated but who have not completed the vaccine series should receive the appropriate dose of hepatitis B immune globulin (HBIG) and should complete the vaccine series.
• Unvaccinated persons should receive both HBIG and hepatitis B vaccine as soon as possible after exposure (preferably within 24 hours). Hepatitis B vaccine may be administered simultaneously with HBIG in a separate injection site. The hepatitis B vaccine series should be completed in accordance with the age-appropriate vaccine dose and schedule (see Table 2 and Box 5).∗
Human Immunodeficiency Virus
1. Refrain from donating blood or plasma.
2. Inform potential sex partners of the exposure.
4. Inform health care providers of their potential exposure so they can take necessary precautions.
5. Do not share razors, toothbrushes, or other items that could become contaminated with blood.
6. Clean and disinfect surfaces on which blood or body fluids have spilled.
 Test Your Safety Knowledge
Test Your Safety Knowledge
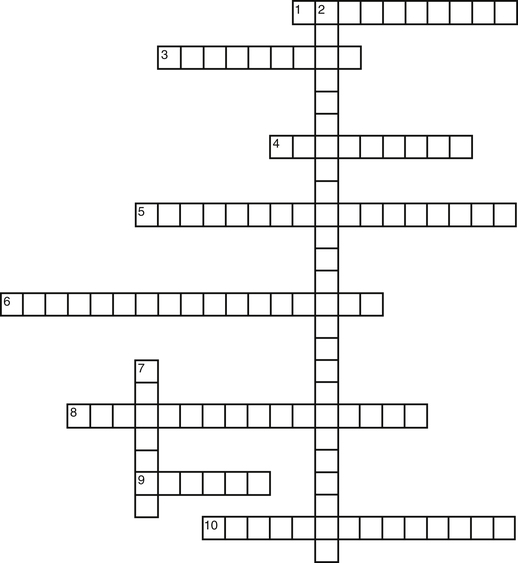
Using the clues provided, fill in the puzzle for objects found in a laboratory.
1. Used to handle concentrated acids
3. Used to clean the surface of a laboratory bench
4. Used for chemical or heat burns on the skin
5. Indicates flammability and is red in color
6. Used to discard sharp objects, such as used needles
8. Can have a rating of a, b, c, or a combination rating
9. Used for emergencies, such as lab coat catching on fire
10. Used for handling specimens that generate infectious aerosols
Chapter Highlights
• Clinical laboratories have instituted Standard Blood and Body Fluid Precautions, or Standard Precautions, to prevent parenteral, mucous membrane, and nonintact skin exposures of health care workers to bloodborne pathogens such as HIV and HBV.
• Although HIV has been isolated from blood, semen, vaginal secretions, saliva, tears, breast milk, cerebrospinal fluid, amniotic fluid, and urine, only blood, semen, vaginal secretions, and possibly breast milk have been implicated in the transmission of HIV to date.
• Medical personnel should be aware that HBV and HIV are different diseases caused by unrelated viruses. The most feared hazard of all, the transmission of HIV through occupational exposure, is among the least likely to occur if proper safety practices are followed.
• The control of infectious, chemical, and radioactive waste is regulated by various governmental agencies (e.g., OSHA, FDA).
∗U.S. Public Health Service: Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis, MMWR Recomm Rep 50(RR-11):1–52, 2001.
∗U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC): MMWR Appendix B PostExposure Guidelines, 55(RR16):30-31, / 2006.
∗Table 2 and Box 5 can be found in U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC): MMWR Appendix B PostExposure Guidelines, 55(RR16):30-31, / 2006.




