CHAPTER 24 Roux-en-y gastric bypass (linear stapler)
Step 1. Surgical anatomy
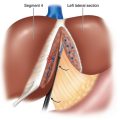
♦ Hepatomegaly—large left lateral segment may require division of falciform ligament for optimal hepatic retraction and visualization of gastric pouch.
♦ Ligament of Treitz—ensure proper identification by careful manipulation and verification that the small bowel is attached to the retroperitoneum and passes through the transverse mesocolon.
♦ Where the circular muscular fibers of the esophagus join the oblique fibers of the stomach. Externally, the gastroesophageal fat pad is a consistent identifier of the GE junction.
Step 2. Preoperative considerations
Patient preparation
♦ Bowel preparation consists of a liquid diet the day before surgery.
♦ Prophylaxis for deep venous thrombosis with sequential compression devices and subcutaneous heparin is started before induction with anesthesia.
♦ Intravenous cefazolin (or alternatively clindamycin) is administered prophylactically.
♦ After induction of anesthesia, an orogastric tube and a urinary catheter are placed to decompress the stomach and bladder, respectively.
Equipment and instrumentation
♦ When operating on a morbidly obese patient, it is an obvious and important consideration to have an operating table that is safe for the patient’s weight. The table base needs to support the patient’s weight plus the weight of foot plates in steep reverse Trendelenburg position without tipping. We prefer a hydraulically operated operating room table that can drop low to the ground, which makes it possible for all surgeons and staff to stand on the ground instead of step stools.
♦ An optical trocar is used to access the abdomen once insufflation is obtained with a Veress needle. We prefer this to the open Hassan technique so that we can avoid an umbilical incision, which decreases the risk of hernia formation.
♦ A long 45-degree, 10-mm endoscope allows for optimal visualization of the operative anatomy despite variations in body shapes.
♦ The expandable liver paddle is easily deployed and offers blunt retraction of the left lobe of the liver, even in very large patients.
Room setup and patient positioning
♦ The patient is placed supine on a well-padded operating table. The arms are extended on padded arm boards and secured with padded straps. Foot plates as well as safety straps on the waist and legs are used to ensure secure positioning, especially during steep reverse Trendelenburg position.
♦ The operative surgeon stands on the patient’s right side while the assistant surgeon and camera operator, if available, are on the patient’s left side.
♦ Monitors are placed at the head of the bed near each shoulder.
Step 3. Operative steps
Access and port placement
♦ The abdomen is insufflated using a Veress needle in the left upper quadrant. After adequate carbon dioxide pneumoperitoneum to 15 mmHg, an 11-mm optical trocar is advanced in the same quadrant under laparoscopic visualization using a zero-degree scope. A 45-degree laparoscope is then advanced through the Visiport and used to visualize the peritoneal cavity.
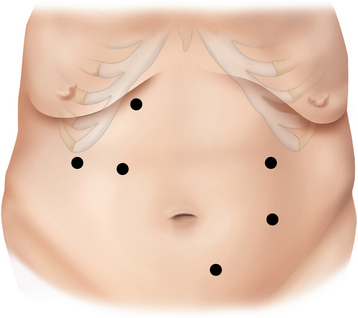
♦ A 10-mm trocar is placed inferolateral to the umbilicus, and the laparoscope is transferred to that position. Two 12-mm and one 5-mm trocars are placed into the right upper quadrant, and a 5-mm trocar is placed lateral to the umbilicus in the left midabdomen (Figure 24-1).
Creation of jejunojejunostomy (J-J)
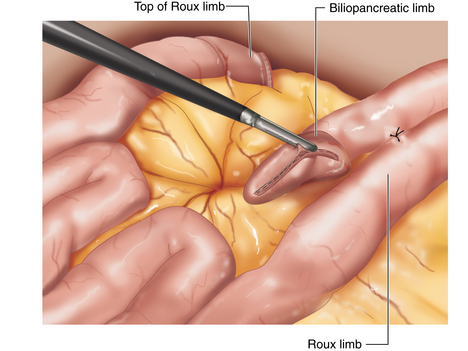
♦ The transverse colon and omentum are retracted superiorly and the ligament of Treitz identified. The proximal jejunum is divided 50 cm from the ligament of Treitz with a linear stapler (2.5 mm). The mesentery between the two limbs of bowel is taken down using the LigaSure device. The proximal jejunum is the pancreaticobiliary limb, containing secretions from remnant stomach as well as liver and pancreas. The distal jejunum will be brought up to the stomach pouch to contain ingested food and is referred to as the Roux limb or alimentary limb.
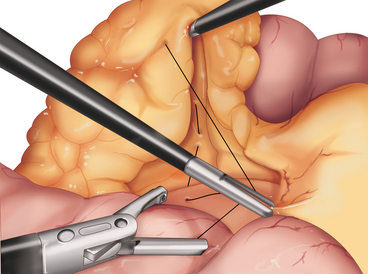
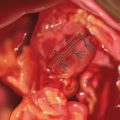
♦ The two limbs are joined by a stapled anastomosis after measuring 100 cm distally on the Roux limb. A tacking suture secures the position in preparation for the anastomosis (Figure 24-2).
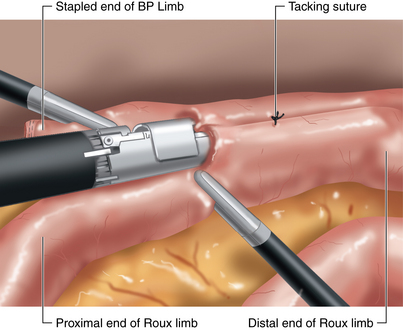
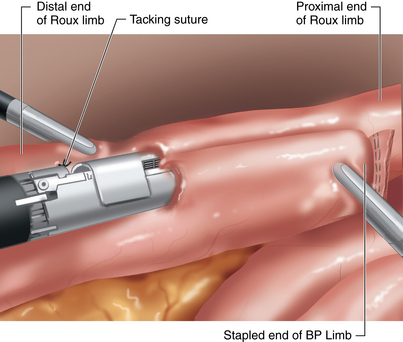
♦ Adjoining enterotomies are created. A linear stapler (2.5 mm) is fired distally first (Figure 24-3), then rotated counterclockwise and fired proximally (Figure 24-4). The remaining enterotomy is tacked with 2-0 silks for retraction into a linear stapler (2.5 mm). The enterotomy is closed in a transverse direction to minimize potential narrowing. The small bowel mesenteric defect is closed with a running 2-0 silk suture.
♦ To prevent obstruction of the biliopancreatic limb (BP) at jejunojejunostomy, we prefer the 180-degree double fire technique.
Mobilization of roux limb to stomach
♦ The Petersen space defect, located between the mesentery of the colon and the mesentery of the Roux limb, is closed with a running 2-0 silk, up to the level of the transverse colon (Figure 24-5). By closing the transverse mesocolon onto the retroperitoneum, shortening of the Roux limb mesenteric length is minimized.
♦ The omentum is inverted over the dome of the liver and then divided from the colon attachment toward the free edge. The fully divided omentum is brought down on either side of the Roux limb.
♦ Care is taken to ensure no twisting of the Roux limb mesentery when retracted superiorly in an antecolic fashion. This limb is tacked to the anterior wall of the fundus of the stomach to facilitate retrieval during creation of the G-J when the patient is placed in steep reverse Trendelenburg.
Creation of gastric pouch
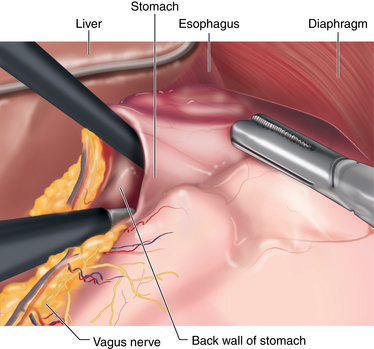
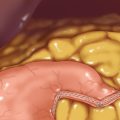
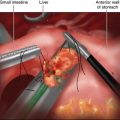
♦ The patient is placed into the reverse Trendelenburg position. Through a lateral right upper quadrant trocar, an expandable liver paddle is used to retract the left lateral segment of the liver anteriorly off of the stomach, and then the paddle is fixed in position. The location of the GE Junction is determined and at a spot 5 cm distal along the lesser curve of the stomach it is dissected away from the lesser omentum. Through this space dissection is carried into the lesser sac posterior to the stomach, while preserving the vagal trunks (Figure 24-6). The NG tube and esophageal temperature probe are removed from the stomach.
♦ The pouch of the gastric bypass is created with a series of firings of the linear stapler (3.5 mm). The first firing is with a short stapler straight across the lesser curve followed by a series of firings with a long stapler toward the angle of His. This long staple line is carried parallel to the inferolateral edge of the gastroesophageal fat pad (approximately 1 to 2 cm toward the greater curve of the stomach). Care is taken to place each stapler into the crotch of the previous stapler firing and to ensure that the pouch is completely separated from the gastric remnant. This creates a pouch of approximately 15 mL in size lying along the lesser curve. The transition zones between stapler firings are oversewn with 2-0 silk figure of eight sutures, both along the pouch as well as along the gastric remnant.
Creation of gastrojejunostomy (G-J)
♦ The posterior wall of the inferior margin of the pouch is cleared of adhesions and any vascular tissue with the LigaSure device. In preparation for a retrogastric anastomosis, a gastrotomy is created in the posterior wall of the pouch and marked with a silk suture. The stitch previously placed to hold the end of the Roux limb to the fundus is cut and an enterotomy is created approximately 2 cm from the stapled end.
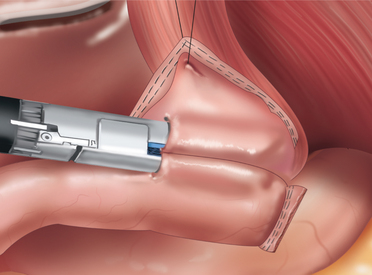
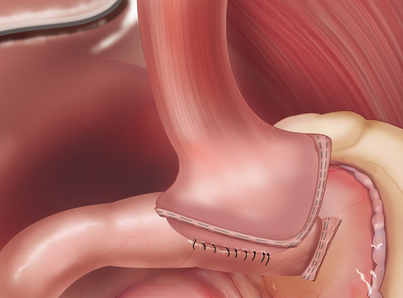
♦ A 30mm long endoscopic gastrointestinal stapler is used to create the gastrojejunostomy by firing it across the common walls of the posterior pouch and distal Roux limb for approximately 2 cm in length (Figure 24-7). Care is taken to ensure that the gastrojejunostomy is at least 1 cm away from the staple line in the lateral wall of the pouch. Following the staple firing, the anesthesia team places an NG tube and advances it under direct vision across the gastrojejunostomy. The gastrojejunostomy is closed over the NG tube with a running 2-0 silk. This layer is carefully inspected to look for any gaps in the suture line. A second layer of 2-0 silk (running Lembert) is placed circumferentially around the entire anastomosis (Figure 24-8).
♦ The pouch and the gastrojejunostomy are tested with a methylene blue by clamping the Roux limb, distending the area with blue dye placed through the NG tube, and wrapping the G-J with sponges. Next, an air test is performed by placing the patient in the Trendelenburg position and filling the left upper quadrant with saline. The pouch is distended with air placed through the NG tube while the Roux limb is clamped. The NG tube is advanced forward, and then pulled back into position by the anesthesia team to ensure that it had not been caught by one of the gastrojejunostomy sutures, and securely taped in place.
♦ The trocars are removed under direct visualization and the pneumoperitoneum is released; 3-0 absorbable suture is used for subcuticular port site closure, and sterile dressings are placed.
Step 4. Postoperative care
♦ DVT prophylaxis consists of subcutaneous heparin, bilateral lower extremity pneumatic compression devices while in bed and early ambulation.
♦ Patients are placed on an aggressive antiemetic regimen.
♦ The nasogastric tube is placed to gravity drainage and used to stent open the gastrojejunal anastomosis.
♦ On postoperative day 1, an upper GI is performed before the patient begins a liquid diet. The contrast is first passed through the NG tube to ensure patency. Then the radiologist removes the NG tube, and oral contrast is administered. Often the oral study shows an obstruction at the G-J anastomosis (secondary to the intentionally small anastomosis); however, patients generally tolerate a liquid diet without difficulty. Over the years, we have found that this anastomotic size is the smallest possible without a significant rate of stenosis.
♦ Oral medication is offered in crushed or elixir form.
♦ A liquid protein diet is tolerated prior to discharge on the second postoperative day.
Step 5. Pearls and pitfalls
♦ Severity of obesity. Super obese patients and patients with a long abdomen may require additional port placement. To facilitate creating the J-J anastomosis, an extra 12-mm trocar can be placed to the right and lateral to the umbilicus. As well, long stem trocars may be needed.
♦ Long abdomen. A second camera port can be placed supraumbilically and an additional working port in the right lower quadrant. (see Figure 24-1.)
♦ Mobilization of the Roux limb. Division of the small bowel mesentery to the base usually includes two crossing vascular arcades to ensure adequate length of the Roux limb. Take care to avoid encroachment on the superior mesenteric vessels.
♦ BP and Roux limb orientation. The BP biliopancreatic limb is oriented on the left and toward the end of the Roux limb when creating J-J to prevent BP limb obstruction and allow for closure of Peterson’s defect. As well, this orientation will allow preservation of the length of the Roux limb mesentery (the critical length) to facilitate reaching the gastric pouch without tension.
♦ All mesenteric defects should be closed completely. Although internal hernias are rare, they can be fatal.
♦ Preserving vagal trunks during the division along the lesser curve when creating the gastric pouch may decrease the incidence of postoperative dysmotility syndrome.
♦ An 18-gauge NG tube is preferred to stent across G-J during creation of this anastomosis. A smaller diameter tube often curls in the stomach and is difficult to pass.
♦ Stapler choice—if the patient has a thicker stomach (more common in males), it may be beneficial to use a 4.8-mm (green) load across the antrum; however, in these cases, the 3.5-mm (blue) load should still be used for creation of the G-J.
♦ Postoperative tachycardia: persistent heart rate greater than 120 bpm for 4 hours mandates further investigation of cause, with a primary focus toward pulmonary embolus or anastomotic leak.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systemic review and meta-analysis. Am J Med. 2009;122(3):248-256.
Kelly JJ, Shikora S, Jones DB, et al. Best practice updates for surgical care in weight loss surgery. Obesity (Silver Spring). 2009;17(5):863-870.
Lehman Center Weight Loss Surgery Expert Panel. Commonwealth of Massachusetts Betsy Lehman Center for Patient Safety and Medical Error Reduction Expert Panel on Weight Loss Surgery: Executive Report update. Obes Res. 2005;13;2:205-226.
. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115;12:956-961.
Sjo’strom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683-2693.