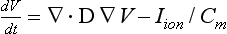Rotors in Human Atrial Fibrillation
In seminal work, Haïssaguerre1 reported that localized ectopy from the pulmonary veins (PVs) may trigger AF. This discovery launched the field of potentially curative AF ablation, with PV isolation as its cornerstone.2 Nevertheless, the mechanisms that perpetuate AF, once triggered, remained undefined.3,4 The multiwavelet hypothesis proposed that multiple spatially meandering electrical waves cause AF.5 However, this did not explain consistent patterns of propagation observed in patients with AF,6,7 the termination of AF after localized ablation,2,8 or the opposite finding—that extensive ablation to constrain wavelets may have little acute impact.2,9 The alternative localized source hypothesis is based on animal and in silico experiments in which rapid localized spiral waves (rotors)4,10 or focal sources7 directly cause AF. Although rotors in human AF have been disputed,5 and direct evidence has been lacking,4 rotors have been indirectly suggested at sites of high dominant frequency where ablation terminates paroxysmal AF11,12 and, if interatrial rate gradients are abolished, improves outcome,11 and by AF mapping.13
Recent data from our14 and other laboratories15 show that human AF is indeed perpetuated by a small number of stable rotors or focal sources14,15 in individuals with paroxysmal, persistent, and long-standing persistent AF. The mechanistic role of stable AF source rotors has been supported by the acute termination and subsequent non-reinducibility of AF by brief targeted ablation (focal impulse and rotor modulation [FIRM]) before any other intervention. Clinically, this approach has been shown to substantially improve the long-term elimination of AF compared with conventional ablation alone.16
Triggers Interact With Sustaining Mechanisms to Cause Human Atrial Fibrillation
The fact that AF may be triggered by ectopic beats1 draws an intuitively attractive parallel with the mechanisms for “simple” supraventricular (SVT) and ventricular (VT) tachycardias, which are also triggered by ectopic beats. In those arrhythmias, triggers engage specific mechanisms of dual atrioventricular (AV) nodal physiology to maintain AV node reentry,17 an accessory pathway for maintaining AV reciprocating tachycardia,18 or a slowly conducting isthmus to maintain ventricular tachycardia.19
We hypothesized that AF may be caused when triggers engage specific AF-maintaining mechanisms that may be created dynamically via conduction block, leading to localized reentry, or by triggering repetitive focal beats. To address this hypothesis, we studied the rate dependence of monophasic action potential duration (APD) in the left and right atria, and bi-atrial patterns of rate-dependent bi-atrial conduction slowing in the left and right atria.20,21 and propagation en route to AF using multipolar basket catheters.
In a series of studies, we recently demonstrated that human AF onset is consistently preceded by alternans and complex oscillations in APD, which create a milieu of heightened repolarization dispersion immediately before AF onset. In patients with persistent and paroxysmal AF, we have found that sustained rapid rates produce marked alternans and complex oscillations in APD22 preceding AF,23 independently of APD restitution. In parallel, bi-atrial conduction velocity slows dynamically (restitution) just before AF onset at the location where AF is initiated.24
Mapping Rotors During Human Atrial Fibrillation
Prior Mapping Studies of Human AF
Several mechanisms for the maintenance of human AF have been proposed, including disorganized multiwavelet reentry,5,25 spatially localized reentrant26 or focal7 sources, and mixed patterns.13 Divergence in these hypotheses in large part may reflect mapping that has not always met “classical” requirements: to broadly map chambers of interest, at sufficient spatiotemporal resolution to identify varying patterns, over long enough periods to capture variability, then to use interventions to demonstrate that proposed mechanisms are causal and are not bystanders. Failure to apply these criteria even to simple supraventricular17,18,27 and ventricular19 arrhythmias is a well-recognized cause of incorrect diagnosis and potentially undesirable therapy.
Many clinical studies over the past decade show that human AF is spatially non-uniform. For instance, human AF exhibits consistent activation patterns,28 consistent rate, or dominant frequency gradients within and between atria,6,29 as well as electrocardiographic (ECG) spectra suggesting conserved global spatiotemporal organization for at least days30 within and between patients. These data have long supported the notion that human AF is maintained by spatially localized mechanisms, further supported by the fact that human AF may terminate with ablation at defined triggers, drivers,1 and other localized regions.12,31 Such regions may arise in either atrium32 and are difficult to identify a priori, but can be ablated in both atria by a systematic stepwise approach.8
Higher-resolution mapping, on the other hand, has produced surprisingly inconsistent results. In seminal intraoperative mapping studies in AF patients, Cox, Schuessler, et al33,34 found stable reentry within disordered AF that were interrupted by lesions that formed the basis for the Maze procedure35 and that still underpin many current ablation lesion sets.2 Conversely, in separate human AF studies,36 Allessie et al found no consistency within disordered AF and concluded that AF was attributed to multiple reentrant waves (as in early computer models37) with “focal” events reflective of transmural breakthrough. However, these studies mapped <20% of the dilated atria in these patients, and did not apply interventions to prove causality of disordered activation. More recent epicardial mapping has revealed localized high-frequency regions in AF patients consistent with sources.7,38 Schilling et al39 and more recently Cuculich et al13 used mathematical inverse solutions to map the atria via noncontact approaches (Ensite 3000™, St Jude Medical, Minneapolis, Minnesota, and EcVue™, Cardioinsight, Cleveland, Ohio, respectively) in AF.13 However, such studies have yet to use focused ablation to establish causality of each proposed mechanism and exclude bystanders.
Requirements to Map Human AF
Detection Design Requirements
We reasoned that the factor most relevant to mapping spatial resolution is the length scale of the mapped event. Figure 43-1 illustrates a rotor with limited movement of the rotor tip (in a locus of migration) and wavebreak to fibrillation, generated in a computer simulation. Electrophysiological model parameters were chosen such that the spiral breaks down far from the migration locus.3,4,23 The rotor controls activation in a “coherent domain” in 1 : 1 fashion with length scale Rrotor, beyond which activation breaks down into complex spatiotemporal patterns. A coherent rotor domain surrounded by incoherent activity can also be generated in simulations of heterogeneous tissue.40,41 To map the rotor core, the required spatial resolution is comparable with the length scale of the reentrant path Rlocus. It is important to note, however, that to simply detect rotational activity around the core (the rotor), the required spatial resolution is coarser and is comparable with its wavelength, λ, which is much larger than Rlocus.

Figure 43-1 Snapshot of a Computer Simulation in Homogeneous Tissue
A rotor, the locus of migration of its tip, and its coherent domain of rotational activity (i.e., tissue controlled in a 1 : 1 fashion by the rotor). The model parameters were chosen such that beyond this region of tissue, the rotor breaks down into other spatiotemporal structures as the result of discordant alternans. The relevant length scales in the problem are the size of the domain of locus migration of the rotor tip, Rlocus, the coherent domain (of detectable rotational activation), Rrotor, and the wavelength of the rotor, λ.
Figure 43-1 also illustrates the importance of mapping a sufficiently large field-of-view. Attempting to map a rotor with a field-of-view smaller than its trajectory of migration (using a small mapping plaque) may lead to results that are difficult to interpret. Hence, if the locations of putative AF sources are unknown, then as much of the atrial surface as possible should be mapped. Finally, the temporal resolution must be able to distinguish activation between neighboring recording sites and thus can be found by dividing the spatial resolution by the dynamic conduction velocity. For example, for a spatial resolution of 5 mm and a range of conduction velocity of 50 to 150 cm/s,42 the required temporal resolution is 3.3 to 10 ms.
We estimated the length scales λ, Rlocus, and Rrotor, and thus the required spatial resolution, to map potential localized sources of human AF, based on animal studies and observations of human AF. Animal models of AF show varying mechanisms, including localized spiral waves (rotors),43,44 focal sources,45 or nonlocalized waves.10 In experiments showing rotors, the length scale for the reentrant path (Rlocus) ranges from 1 cm to >3 cm,10,46,47 requiring a minimum resolution of 0.5 cm for mapping. Spatial organization in some models controlled tissue areas >5 cm2,44 corresponding to a rotor length scale and wavelength >2 cm. Thus, once the location of a rotor is identified, reentry requires mapping a field-of-view of at least 2.5 × 2.5 cm, with a resolution of ≈1 cm.
In humans, the length scale of the reentrant path may be estimated from the concept of tissue wavelength48 as the product of minimum conduction velocity and the shortest refractory period. In AF patients, minimum (dynamic) conduction velocity in left and right atria is ≈40 cm/s42 and the minimum atrial refractory period ≈100 to 110 ms,20,23 resulting in a minimum wavelength of ≈4 to 5 cm, diameter of ≈1.5 cm, and minimum required spatial resolution of ≈1 cm.
Numeric Validation of Design Requirements for AF Mapping
where V is the membrane voltage, Cm represents the membrane capacitance, D is the diffusion tensor, and Iion represents the membrane currents. For the purposes of illustrating propagation in silico, we computed membrane currents using the 3- and 4-variable Fenton-Karma (FK) model.49,50
Figure 43-2 illustrates spiral wave reentry in a 200 × 200-node simulation area with a physical size of 5 × 5 cm, corresponding to a spatial resolution Δx of 0.25 mm (Figure 43-2, A). The spiral is single-armed with a wavelength larger than Lrotor/2 and a period of 90 ms. The computed locus of migration of the rotor tip is illustrated in red in Figure 43-2, A, and consists of a complex meandering trajectory with a length scale of ≈1 cm. Thus, this simulated rotor has Llocus ≈1 cm, Lrotor >5 cm, and λ >2.5 cm.
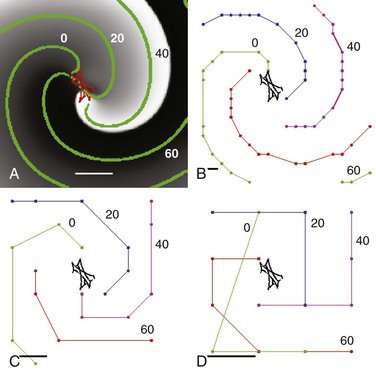
Figure 43-2 Effect of Mapping Resolution on a Simulated Clockwise Rotor
Simulation on a 200 × 200 grid. A, Spatial resolution Δx = 0.25 mm, corresponding to a 5 × 5-cm domain. The activation is plotted using a gray scale, with white corresponding to depolarized and black corresponding to repolarized tissue. The tip of the rotor meanders as shown in red. The green symbols are isochrones superimposed onto the snapshots. These isochrones are 20 ms apart and are computed as all grid points activated within 1 ms. Scalebar = 1 cm. B, Isochrones computed on a 20 × 20 grid obtained by coarsening the original grid. Isochrones are again 20 ms apart with the order green, blue, purple, and red; scalebar represents spatial resolution (Δx = 2.5 mm). C, Isochrones computed on an 8 × 8 grid providing spatial resolution Δx = 6.25 mm. D, Isochrones computed on a 4 × 4 grid providing spatial resolution Δx = 12.5 mm. Note that rotational activity (organized domain) of the rotor remains detectable at all resolutions.
Activation times for each node were determined using a voltage threshold (10% maximal) and were stored at temporal resolution Δt = 1 ms. Activation times were used to compute isochrones separated by 20 ms (Figure 43-2, B, green). To simulate coarser recording resolution, we coarsened stored activation time intervals but did not rerun simulations. Figure 43-2, B-D illustrates isochrones for the same rotor at resulting spatial resolutions of Δx = 2.5 mm (20 × 20 grid), Δx = 6.25 mm (8 × 8 grid), and Δx = 12.5 mm (4 × 4 grid), corresponding to isochronal intervals of 3 ms, 7 ms, and 18 ms, respectively. Notably, all values of Δx preserved rotational activity of the organized domain of the rotor.
Summary of Design Requirements for Human AF Mapping
These estimates and numeric simulations suggest that a spatial resolution of ≈0.5 cm may be able to resolve rotor migration, and a spatial resolution of ≈1 cm may capture rotational activity of a rotor in human AF. The corresponding temporal resolution should be at least 6 ms. These considerations laid the foundation for our direct contact focal impulse and rotor mapping (FIRM) approach,14 which we recently described to identify patient-specific sustaining rotors and focal sources for human AF. Localized AF sources were identified at electrophysiological study and their causal role verified by AF termination by patient-specific targeted ablation alone.
Sustaining Rotors and Focal Impulses for Human Atrial Fibrillation
Recently, our group16 and others15 showed that human paroxysmal, persistent, and long-standing persistent AF are predominantly sustained by localized rotors or focal sources. In a multicenter experience that currently includes more than 200 patients, stable rotor or focal sources were identified in >98% of patients, in diverse bi-atrial locations that were stable for prolonged periods in each individual. Direct targeted ablation was applied to each mapped source (FIRM) before any other intervention, which led acutely to the termination or substantial organization of AF in the vast majority of cases. Patients who received FIRM together with conventional ablation had substantially greater freedom from AF on rigorous long-term follow-up compared with those receiving conventional ablation alone.
Identification of Rotors and Focal Impulses in Human AF: Focal Impulse and Rotor Mapping
We designed FIRM mapping on the basis of the above considerations including the requirement to apply ablation to prove proposed AF mechanisms.14 Accordingly, FIRM mapping is performed during clinical electrophysiology study by advancing 64-pole basket catheters to the right atrium and, after transseptal puncture, to the left atrium. Contact electrodes provide spatial resolution of 4 to 6 mm along each spline, and ≈4 to 10 mm between splines (higher resolution at the poles than at the equator). Heparin is infused to maintain activated clotting time >350 ms. In early work, we mapped both atria simultaneously (Figure 43-3), but we now use one basket: first in the right atrium while performing transseptal puncture, then in the left atrium. Multi-site electrograms are recorded with a temporal resolution of 1 ms as unipoles or as overlapping bipoles to reduce far-field artifact, and are filtered at 0.05 to 500 Hz. AF data are exported digitally as multiple 1-minute epochs over a period of tens of minutes.
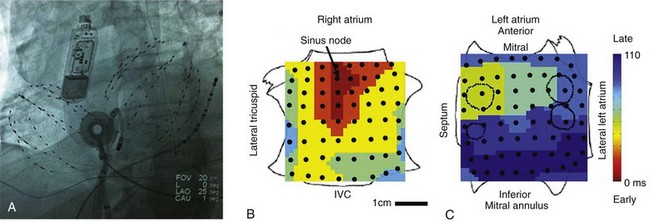
Figure 43-3 Anatomical Reference and Nomenclature for FIRM Mapping
A, Fluoroscopic view of 64-pole basket catheters in the right and left atria. An ablation catheter and a coronary sinus catheter are shown, along with an esophageal temperature probe. The image shows a subcutaneous continuous ECG monitor (Reveal XT™, Medtronic, Minneapolis, Minnesota) used to categorically establish elimination of AF after ablation in clinical series. B, Sinus rhythm map on bi-atrial schematic. Activation at basket electrodes, shown as dots, is displayed as a color-coded map from the sinus node to the lateral inferior left atrium. The right atrium is opened between its poles, with the tricuspid annulus opened laterally and medially; the left atrium is opened along its equator, with the mitral annulus opened superiorly and inferiorly. The pulmonary vein ostia are indicated by dashed lines.
A novel system (RhythmView™, Topera Inc., Palo Alto, California) is then used to analyze multipolar AF signals.14,16,51 Electrograms are filtered to exclude noise and far-field signals guided by the reported rate dynamics of human right and left atrial APD20,22,23,52 to estimate minimum activation time, and conduction velocity20,24 to map propagation paths. The system produces isopotential movies of AF over successive cycles, as the primary display modality used to guide ablation, as discussed later. However, for illustration, single-cycle isochronal snapshots are provided throughout the chapter.
Demonstration of AF Rotors and Focal Sources
Propagation can be mapped in various rhythms. For orientation, Figure 43-3 illustrates bi-atrial propagation in sinus rhythm. The atria are projected onto grids, with the right atrium cut vertically through the tricuspid valve, opening its lateral and medial halves. The left atrium is cut horizontally through the mitral valve, opening its superior and inferior halves. On these projections, sinus activation is represented by color-coded isochrones emanating from the sinoatrial node (in red), crossing Bachmann’s bundle to the left atrium (in blue).
In AF, isopotential movies were analyzed to identify rotors as rotational activity around a center, or focal impulses as centrifugal activation from a point of origin, or laminar activation or disorganized patterns that fell outside the three categories previously described. Rotors and focal sources were diagnosed only if stable and sustained throughout several recording epochs (>100 cycles), to exclude transient rotational and/or focal activations that represent fibrillatory conduction.5
We observed sustained rotors and focal sources in nearly all patients (98/101, or 98%) with paroxysmal, persistent, and long-standing persistent AF.16 Subjects exhibited 2.1 ± 1.0 sources concurrently that were more prevalent in individuals with persistent than paroxysmal AF (2.2 ± 1.0 vs. 1.7 ± 0.9; P = .03). AF sources were sustained for hundreds of cycles analyzed in “time lapse” fashion, representing tens of minutes (i.e., thousands of cycles). Sources lay in diverse atrial locations, and it was surprising to note that 24% were found in the right atrium. The prevalence of rotors was greater than the prevalence of focal beats.14 In patients in whom both spontaneous AF and induced AF were observed, AF propagation movies converged once AF had been sustained for >10 minutes in either case. No complications were noted during mapping.16
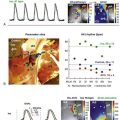
Figure 43-4 illustrates rotors in the (A) right and (B) left atria in patients during AF, showing head-to-tail (red-to-blue) activation in the coherent domain, and peripheral disorganization and/or collision. In each case, electrograms in the coherent domain exhibit sequential 1 : 1 activation.
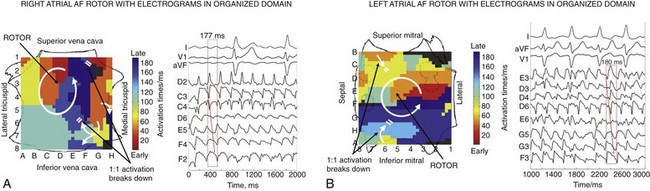
Figure 43-4 Right Atrial and Left Atrial Spiral Waves (Rotors) During Human AF on FIRM Mapping
A, Counterclockwise rotor in posterolateral right atrium, with collision (white double lines) beyond the coherent domain of the spiral arm. Electrograms around the right atrium rotor site indicate sequential activation of counterclockwise rotation with variability at cycle length ≈177 ms. B, Counterclockwise rotor in midposterior left atrium, with collision beyond the spiral arm (double lines). Pulmonary vein ostia indicated by dashed lines. ABL, Ablation electrogram; AF, atrial fibrillation; CS, coronary sinus.
Figure 43-5, A illustrates a right atrial AF rotor with disorganization peripherally and in the left atrium. Figure 43-5, B illustrates, for comparison, right atrial clockwise reentry in reverse typical atrial flutter, showing 1 : 1 propagation in both atria. Figure 43-5, C shows a repetitive focal source driving AF with peripheral disorganization. For comparison, Figure 43-5, D illustrates the distinct bi-atrial propagation maps of a “simple” focal atrial tachycardia in a patient without AF, in which propagation is 1 : 1 throughout ipsilateral and contralateral atria.
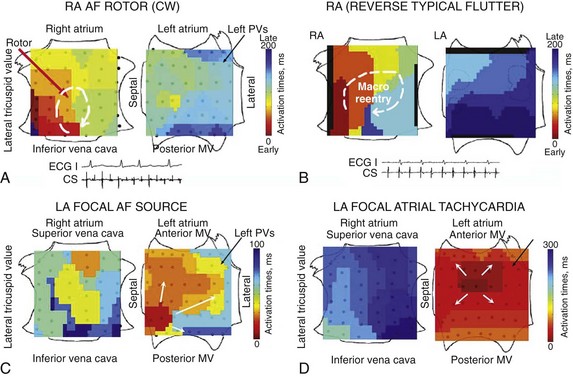
Figure 43-5 AF Sources Are Distinct from Simple Rhythms on FIRM Mapping
A, Clockwise right atrial rotor in AF, showing noncoherent (non-1 : 1) propagation in left atrium. Conversely, (B) clockwise reverse typical flutter with 1 : 1 propagation to the left atrium. C, Repetitive focal beat during paroxysmal AF (in low septal left atrium), with activation to remaining left atrium and fibrillatory conduction to right atrium (CL ≈100 ms). In contrast, (D) focal atrial tachycardia (nonfibrillatory) from the high posterior left atrium differs from AF by showing 1 : 1 activation centrifugally to the ipsilateral then the contralateral atria (CL, 300 ms).
Electrophysiological Evidence That Rotors and Focal Sources Are Primary Sustaining Mechanisms for AF
Mapping Activation Sequence
Rotors exhibited 1 : 1 activation within their coherent domains, and focal sources showed 1 : 1 activation from their origins, with distal disorganization and/or collision from isochronal analyses (see Figures 43-4 through 43-7) and isopotential movies. Directionality was analyzed to further establish that propagation emanated from rotors or focal sources during AF and not was directed to them. Directionality53 was computed by analyzing isochronal AF snapshots in 10-ms bins, then calculating vectors between successive isochronal contours at the spatial boundaries of each bin. Figure 43-6 shows (A) a left atrial AF rotor with (B) activation emanating to remaining atria, and (C) a left atrial focal AF source with (D) activation emanating to remaining atria. Analysis of consecutive cycles revealed that directionality was conserved close to the center of each source but not farther away. These data support the notion that sustained sources control and drive fibrillatory activity over multiple cycles.
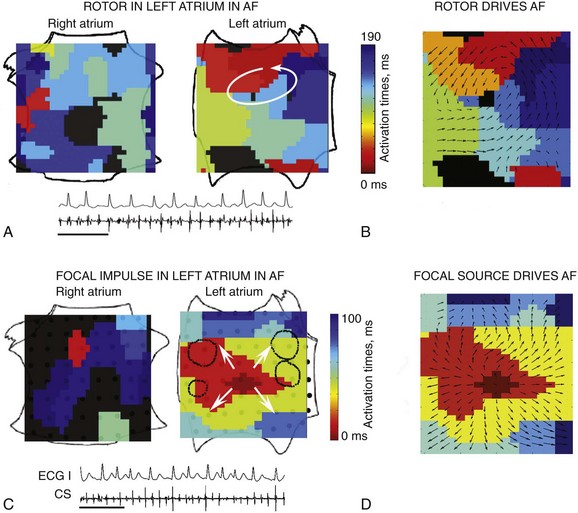
Figure 43-6 Propagation Emanates from Localized Sources to Remaining Atria During Human AF
A, Left atrial AF rotor. B, Directionality shows propagation emanating from rotor to remaining atria. C, Left atrial AF focal source. D, Directionality shows propagation emanating from focal source to remaining atria. The arrows indicate the activation direction53 between isochrones (color bar).
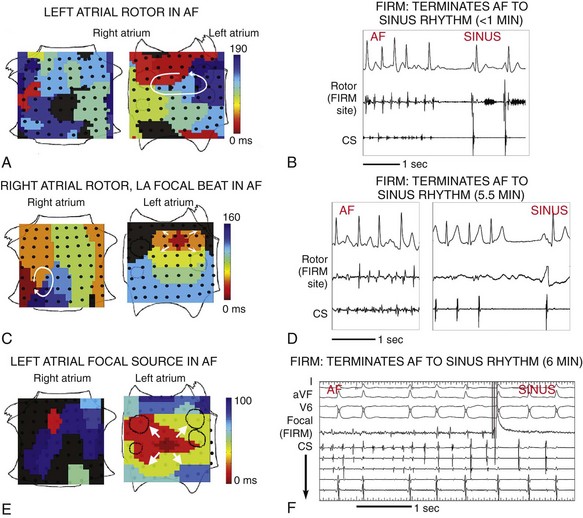
Figure 43-7 Acute Termination of AF to Sinus Rhythm by Focal Impulse and Rotor Modulation (FIRM) Ablation
A, Left atrial rotor with counterclockwise activation (red to blue) and disorganized right atrium in AF. B, FIRM ablation at left atrium rotor terminated AF to sinus rhythm in <1 min. C, Right atrium rotor (clockwise) and simultaneous left atrium focal source during persistent AF. D, FIRM ablation at right atrium rotor terminated AF to sinus rhythm in 5.5 min. E, Left atrium focal source in AF. F, FIRM ablation at left atrium focal source terminated AF to sinus rhythm. All patients are AF-free on implanted cardiac monitors at >1 y. Scalebar = 1 s. CS, coronary sinus electrogram. Atrial orientations as in Figure 43-3. (Adapted from Narayan et al,16 with permission.)
Targeted Ablation Limited to Rotors and Focal Sources Acutely Terminates AF
Recent computational advances enabled us to perform FIRM mapping for human AF in near-real time at electrophysiological study. This provided the opportunity to target these regions for ablation prospectively,16 as illustrated in a recent video case report.51
Radiofrequency energy was targeted directly at identified rotors and focal sources before PV isolation. Radiofrequency energy was delivered using a 3.5-mm-tip irrigated catheter (Thermocool, Biosense-Webster, Diamond Bar, California) at 25 to 35 W, or, in heart failure subjects, an 8-mm-tip nonirrigated catheter (Blazer, Boston Scientific, Natick, Massachusetts) at 40 to 50 W, target 52° C. Other centers have subsequently employed a wide variety of energy sources for FIRM ablation cases, including cryoenergy. In each case, the catheter is maneuvered near basket electrodes overlying each source using fluoroscopy, then ablation is applied for ≈30 s at each site. The catheter is moved within the area representing the center of rotation or focal origin, for the acute end point of AF termination or ≤10 minutes, whichever comes first. AF termination is followed by attempted non-reinducibility of AF. Only if AF is subsequently non-reinducible do we consider the end point of AF termination achieved, unlike prior reports in which AF reinduction is rarely attempted. If AF did not terminate, we assessed for abruptly increased AF cycle length >10%. In our validation studies, an AF cycle length increase >10% indicates elimination of a secondary AF source, as suggested in computer simulations by others (using a 3% to 4% cutpoint).54,55 Our acute end point was thus the composite of AF termination or ≥10% slowing.
The acute effects of targeted ablation limited to rotor or focal sources were reported in the CONFIRM trial,16 in patients aged 63 ± 9 years, of whom 81% had persistent AF, and in an initial report from first cases at other centers,15 in patients aged 58 ± 12 years, of whom 92% had persistent AF.
FIRM ablation achieved the acute end point in 86% (31/36) of patients receiving FIRM-guided ablation in the CONFIRM trial16 and in 100% (12/12) of patients in the first external series.15 Figure 43-7 shows AF termination by FIRM ablation at (A) a posterior left atrial rotor, (B) a right atrial rotor, and (C) a left atrial focal beat. Preliminary findings from a larger multicenter FIRM-guided ablation experience show similar results (manuscript in preparation).
The AF termination end point alone was observed in 56%16 and 67%15 of patients before PV isolation after a median of <5 min FIRM ablation at the primary source. In the CONFIRM trial, this was achieved with a median of 2.5 min ablation at the primary source (interquartile range [IQR], 1 to 3 min).16 Moreover, AF termination by FIRM was predominantly to sinus rhythm (23/28 terminations in pooled studies; 82%15,16), unlike conventional (nonguided) ablation, in which AF terminates typically to an atrial tachycardia (<13% to sinus rhythm55). Notably, AF sources were stable over time in each patient. AF sources were persistent over the time scale required for mapping and ablation (mean, 115 ± 57 minutes). AF sources were conserved for months in a subset of subjects who failed conventional ablation in the control limb of CONFIRM, then re-presented for FIRM-guided ablation.16
Discussion
Our finding that human AF is sustained by a small number (1 to 3) of rotors and focal sources that are spatially stable over time10 was initially surprising and differed from previous studies. A detailed recent epicardial mapping study was interpreted to show that AF is maintained by numerous complex nonrepeating activation fronts,36 yet that study used limited field-of-view (<20% of dilated atrial surfaces) that may miss AF sources outside the mapped field, and did not prove that the disordered waves were causal rather than bystanders from unmapped and unidentified mechanisms.
Our results extend elegant prior studies suggesting rotors in human AF. Atienza et al reported regions of high spectral dominant frequency near the pulmonary veins in patients with paroxysmal AF whose frequency increased after adenosine administration,26 consistent with a reentrant mechanism. Using noninvasive body surface mapping (ECG imaging [ECGI]), Cuculich et al13 detected rotational activity in AF patients, but in a minority of patients, typically of only 1 rotation, and with very short cycle length (<75 ms).13 These differences, which may reflect the respective mapping approaches, require further definition.
Clinical Implications
Ablation of Localized Sources—the CONFIRM Trial
The CONFIRM trial (CONventional ablation with or without Focal Impulse and Rotor Modulation) was a prospective case cohort study16 that enrolled pateints at 107 AF ablation procedures who had failed at least one antiarrhythmic medication with standard indications. Of this population, 36 underwent FIRM-guided ablation then conventional ablation, and 71 underwent conventional ablation alone (1 : 2 allocation). The only exclusion criterion was an unwillingness to consent. Patients had paroxysmal AF (episodes that self-terminate in <7 days), persistent AF (episodes that last >7 days and require electrical cardioversion), and long-standing persistent AF (persistent AF with >1 year of continuous AF).2 Overall, three-quarters of patients had persistent or long-standing persistent AF (conventional, 66%; FIRM-guided, 81%), and subjects had a wide range of ages (20 to 81 years), left ventricular ejection fractions (20% to 75%), and comorbidities.
Conventional ablation was then performed: in FIRM-blinded subjects, immediately after basket data were acquired, and in FIRM-guided subjects, after FIRM-guided ablation was completed. Conventional ablation2 comprised wide area circumferential ablation to isolate the left and right pulmonary veins in pairs, with verification of pulmonary vein isolation using a circular mapping catheter.
CONFIRM Trial Results
The single-procedure freedom from AF was 82.4% in FIRM-guided patients compared with 44.9% in FIRM-blinded patients (P < .001) after 273 days (median IQR, 132 to 681). Notably, no FIRM-guided case recurred after ≈7 months. FIRM-guided therapy maintained its benefit over FIRM-blinded therapy for first-time ablation cases (P < .001; Figure 43-8 for patients off antiarrhythmic medications) and for all prespecified subgroups.
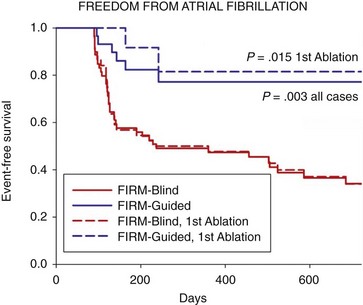
Figure 43-8 Cumulative Freedom from Atrial Fibrillation in Patients Off Antiarrhythmic Medications
For all cases (bold lines), and for those at first ablation (dashed lines). Intention-to-treat analysis and P values reflect the complete follow-up period. (Adapted from Narayan et al,16 with permission.)
Discussion of CONFIRM Trial
The CONFIRM trial is notable for many reasons. First, it reports among the highest single procedure success rate of any AF ablation trial to date, verified using implanted ECG monitors that are far more sensitive than symptoms or the intermittent ECG monitoring used in most prior ablation trials. Second, the CONFIRM trial is one of the few AF trials to date9,56,57 to compare a novel ablation strategy with a control strategy of conventional ablation58 (not to antiarrhythmic medications). Third, CONFIRM is the first trial to identify and directly target demonstrated AF mechanisms, proven by achievement of the acute end point in a patient-tailored fashion. Fourth, FIRM-guided ablation was more effective than conventional ablation in all patient subgroups, including those undergoing their first procedure and those with AF despite prior conventional ablation (see Figure 43-8).
Results of the CONFIRM trial may explain many observations from conventional AF ablation. First, most sources lay in the left atrium. Nevertheless, the presence of right atrial sources in one-quarter of patients may explain the 70% to 80% success ceiling2 of conventional (mostly left atrial) ablation for AF, and the need for right atrial ablation in some patients.32 Second, diverse source locations are consistent with reports that widespread ablation may be required.8 Third, the higher number of sources in persistent than in paroxysmal AF is consistent with lower success rates and more difficult procedures in persistent AF patients. Fourth, how widespread conventional ablation can miss localized sources may be explained by AF source locations often remote from typical lesion sets, and the fact that conventional ablation may dwell briefly at each location (≈30-60 s) before the catheter is moved, but FIRM targets sources for >≈2.5 min of ablation (similar to ablation for AV node reentry, atrial tachycardias, or other “simple” arrhythmias17–19).
References
1. Haïssaguerre, M, Jais, P, Shah, DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339:659–666.
2. Calkins, H, Kuck, KH, Cappato, R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, end points, and research trial design: A report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012; 9:632–696. e621.
3. Nattel, S. New ideas about atrial fibrillation 50 years on. Nature. 2002; 415:219–226.
4. Vaquero, M, Calvo, D, Jalife, J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm. 2008; 5:872–879.
5. Allessie, MA, de Groot, NM, Houben, RP, et al. The electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010; 3:606–615.
6. Lazar, S, Dixit, S, Marchlinski, FE, et al. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004; 110:3181–3186.
7. Sahadevan, J, Ryu, K, Peltz, L, et al. Epicardial mapping of chronic atrial fibrillation in patients: Preliminary observations. Circulation. 2004; 110:3293–3299.
8. Haissaguerre, M, Sanders, P, Hocini, M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: Critical structures for termination. J Cardiovasc Electrophysiol. 2005; 16:1125–1137.
9. Oral, H, Pappone, C, Chugh, A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006; 354:934–941.
10. Skanes, AC, Mandapati, R, Berenfeld, O, et al. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998; 98:1236–1248.
11. Atienza, F, Almendral, J, Jalife, J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009; 6:33–40.
12. Sanders, P, Berenfeld, O, Hocini, M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005; 112:789–797.
13. Cuculich, PS, Wang, Y, Lindsay, BD, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010; 122:1364–1372.
14. Narayan, SM, Krummen, DE, Rappel, W-J. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation (cover article). J Cardiovasc Electrophysiol. 2012; 23:447–454.
15. Shivkumar, K, Ellenbogen, Kenneth, A, et al. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: First multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012; 23(12):1277–1285.
16. Narayan, SM, Krummen, DE, Shivkumar, K, et al. Treatment of atrial fibrillation by the ablation of localized sources: The conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation (CONFIRM) trial. J Am Coll Cardiol. 2012; 60:628–636.
17. Jackman, WM, Beckman, KJ, McClelland, JH, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992; 327:313–318.
18. Jackman, WM, Wang, XZ, Friday, KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991; 324:1605–1611.
19. Stevenson, WG, Khan, H, Sager, P, et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993; 88:1647–1670.
20. Narayan, SM, Kazi, D, Krummen, DE, et al. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: A mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008; 52:1222–1230.
21. Gong, Y, Xie, F, Stein, K, et al. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: A simulation study. Circulation. 2007; 115:2094–2102.
22. Narayan, SM, Bode, F, Karasik, PL, et al. Alternans of atrial action potentials as a precursor of atrial fibrillation. Circulation. 2002; 106:1968–1973.
23. Narayan, SM, Franz, MR, Clopton, P, et al. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011; 123:2922–2930.
24. Lalani, G, Schricker, A, Gibson, M, et al. Dynamic conduction slowing precedes human atrial fibrillation initiation: Insights from bi-atrial basket mapping on transitions to atrial fibrillation. J Am Coll Cardiol. 2012; 59:595–606.
25. Konings, K, Kirchhof, C, Smeets, J, et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994; 89:1665–1680.
26. Atienza, F, Almendral, J, Moreno, J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation. 2006; 114:2434–2442.
27. Feld, GK, Fleck, RP, Chen, PS, et al. Radiofrequency catheter ablation for the treatment of human type 1 atrial flutter: Identification of a critical zone in the re-entrant circuit by endocardial mapping techniques. Circulation. 1992; 86:1233–1240.
28. Gerstenfeld, E, Sahakian, A, Swiryn, S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992; 86:375–382.
29. Dibs, SR, Ng, J, Arora, R, et al. Spatiotemporal characterization of atrial activation in persistent human atrial fibrillation: Multisite electrogram analysis and surface electrocardiographic correlations—a pilot study. Heart Rhythm. 2008; 5:686–693.
30. Xi, Q, Sahakian, AV, Ng, J, et al. Atrial fibrillatory wave characteristics on surface electrogram: ECG to ECG repeatability over twenty-four hours in clinically stable patients. J Cardiovasc Electrophysiol. 2004; 15:911–917.
31. Nademanee, K, McKenzie, J, Kosar, E, et al. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004; 43:2044–2053.
32. Hocini, M, Nault, I, Wright, M, et al. Disparate evolution of right and left atrial rate during ablation of long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2010; 55:1007–1016.
33. Schuessler, RB, Grayson, TM, et al. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992; 71(5):1254–1267.
34. Cox, JL, Schuessler, RB, Boineau, JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991; 101:402–405.
35. Weimar, T, Bailey, MS, Watanabe, Y, et al. The Cox-Maze IV procedure for lone atrial fibrillation: A single center experience in 100 consecutive patients. J Interv Card Electrophysiol. 2011; 31:47–54.
36. Allessie, MA, de Groot, NM, Houben, RP, et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: Longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010; 3:606–615.
37. Moe, GK, Rheinboldt, W, Abildskov, J. A computer model of atrial fibrillation. Am Heart J. 1994; 67:200–220.
38. Wu, T-J, Doshi, RN, Huang, H-LA, et al. Simultaneous biatrial computerized mapping during permanent atrial fibrillation in patients with organic heart disease. J Cardiovasc Electrophysiol. 2002; 13:571–577.
39. Schilling, RJ, Kadish, AH, Peters, NS, et al. Endocardial mapping of atrial fibrillation in the human right atrium using a non-contact catheter. Eur Heart J. 2000; 21:550–564.
40. Samie, FH, Berenfeld, O, Anumonwo, J, et al. Rectification of the background potassium current: A determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001; 89:1216–1223.
41. Baher, A, Qu, Z, Hayatdavoudi, A, et al. Short-term cardiac memory and mother rotor fibrillation. Am J Physiol Heart Circ Physiol. 2007; 292:H180–H189.
42. Lalani, GG, Schricker, A, Gibson, M, et al. Atrial conduction slows immediately before the onset of human atrial fibrillation: A bi-atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol. 2012; 59:595–606.
43. Chou, CC, Chang, PC, Wen, MS, et al. Epicardial ablation of rotors suppresses inducibility of acetylcholine-induced atrial fibrillation in left pulmonary vein-left atrium preparations in a beagle heart failure model. J Am Coll Cardiol. 2011; 58:158–166.
44. Cherry, EM, Fenton, FH. Visualization of spiral and scroll waves in simulated and experimental cardiac tissue. New J Phys. 2008; 10:125016.
45. Ryu, K, Shroff, SC, Sahadevan, J, et al. Mapping of atrial activation during sustained atrial fibrillation in dogs with rapid ventricular pacing induced heart failure: Evidence for a role of driver regions. J Cardiovasc Electrophysiol. 2005; 16:1348–1358.
46. Hill, BC, Courtney, KR. Design of a multi-point laser scanned optical monitor of cardiac action potential propagation: Application to microreentry in guinea pig atrium. Ann Biomed Eng. 1987; 15:567–577.
47. Kirchhof, C, Chorro, F, Scheffer, GJ, et al. Regional entrainment of atrial fibrillation studied by high-resolution mapping in open-chest dogs. Circulation. 1993; 88:736–749.
48. Rensma, P, Allessie, M, Lammers, W, et al. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988; 62:395–410.
49. Fenton, F, Karma, A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: Filament instability and fibrillation. Chaos. 1998; 8:20–47.
50. Fenton, FH, Cherry, EM, Hastings, HM, et al. Multiple mechanisms of spiral wave breakup in a model of cardiac electrical activity. Chaos. 2002; 12:852–892.
51. Narayan, SM, Patel, J, Mulpuru, S, et al. Focal impulse and rotor modulation (FIRM) ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on followup. Heart Rhythm. 2012; 9:1436–1439.
52. Narayan, SM, Franz, MR. Quantifying fractionation and rate in human atrial fibrillation using monophasic action potentials: Implications for substrate mapping. Europace. 2007; 9:vi89–vi95.
53. Kalifa, J, Tanaka, K, Zaitsev, AV, et al. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006; 113:626–633.
54. Haissaguerre, M, Lim, KT, Jacquemet, V, et al. Atrial fibrillatory cycle length: Computer simulation and potential clinical importance. Europace. 2007; 9(Suppl 6):vi64–vi70.
55. Takahashi, Y, O’Neill, MD, Hocini, M, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008; 51:1003–1010.
56. Wazni, OM, Marrouche, NF, Martin, DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA. 2005; 293:2634–2640.
57. Wilber, DJ, Pappone, C, Neuzil, P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA. 2010; 303:333–340.
58. Oral, H, Chugh, A, Yoshida, K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009; 53:782–789.