CHAPTER 16 Rotator Interval Pathology
The term rotator interval was first attributed to Neer in 1970,1 when describing the capsular structures in the anterior shoulder found between the supraspinatus and subscapularis tendons. Although originally identified as a safe zone from which to access the glenohumeral joint, the role of the rotator interval in shoulder pathology and disease has become more defined as greater knowledge of shoulder kinematics and function has accumulated.
ANATOMY
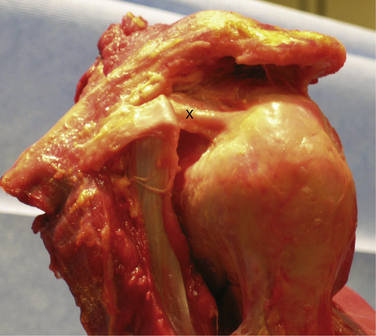
Knowledge of the complex anatomy of the rotator interval is essential to an understanding of the pathologic processes that may lead to shoulder pain and dysfunction. The rotator interval is triangular in shape and consists of the fibers that span the area between the supraspinatus and subscapularis tendons, medially from the coracoid to their insertions onto the greater and lesser tuberosities. The base of the triangle is made up of the base of the coracoid process and its apex is formed by the convergence of the lesser and greater tuberosities and bicipital groove laterally. The superior margin of the subscapularis tendon and inferior margin of the supraspinatus tendon make up the remaining respective sides of the interval. The interval contains the fibers of the coracohumeral ligament (CHL), superior glenohumeral ligament (SGHL), and biceps tendon, with their interdigitations with the supraspinatus, subscapularis, and glenohumeral joint capsule (Fig. 16-1). Distinct structures have been identified within the rotator interval, but histologic analysis has shown poorly organized collagen fibers, and a thinner, less distinct capsular structure in comparison to the well-defined inferior glenohumeral ligaments and capsule.2
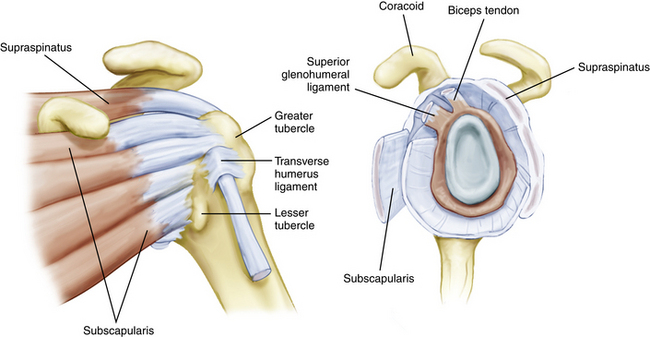
FIGURE 16-1 The rotator interval as viewed extra-articularly and intra-articularly.
(From Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74:53-66.)
The most recent detailed description of the anatomy of the interval was performed by Jost and colleagues.3 In their description, the rotator interval (RI) began as a two-layered structure medially and transitioned to a four-layered structure laterally near the insertions on the tuberosities. Medially, the two layers consisted of the coracohumeral ligament superficially and the superior glenohumeral ligament and glenohumeral joint capsule deeply. As the tissue fans out toward the humerus, two further layers form between the superficial CHL and SGHL. The more superficial layer consists of fibers of the supraspinatus and subscapularis, which interdigitate with those of the superficial CHL, and a deeper layer consisting of fibers of the deep coracohumeral ligament.
The CHL origin is about 2 cm wide and begins at the base of the lateral side of the coracoid process.4 The more superficial fibers cross over the interval and insert onto the medial portion of the greater tuberosity, blending with the fibers of the anterior supraspinatus and forming the lateral portion of the biceps sling (Fig. 16-2). The deeper fibers of the CHL blend with those of the SGHL and the upper subscapularis tendon to form the medial portion of the biceps sling.
The ligamentous structure of the CHL has been debated, and many authors5–7 have described it as an extra-articular fibrous structure that is a thickening or fold of the glenohumeral capsule. Others, such as Neer and associates,8 have recognized it as a distinct identifiable structure starting laterally at the coracoid base, but acknowledged that laterally there are slips that blend into the tendons of the rotator cuff. Microscopic examination has shown that it blends with the glenohumeral capsule with bundles and sheets of collagenous tissue without a discrete orientation, which is not consistent with a true ligamentous structure.9
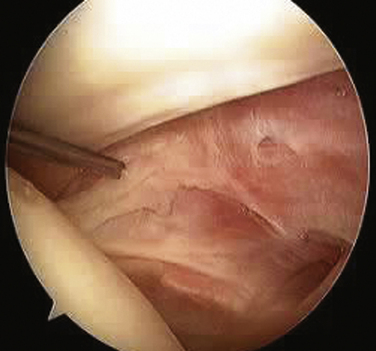
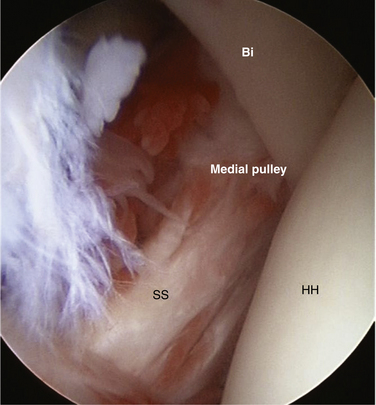
The SGHL originates from the glenoid labrum at roughly the 12 o’clock position.7,9 It crosses the floor of the rotator interval deep to the CHL, blending with the fibers of the glenohumeral capsule. Laterally, it joins the fibers of the deep CHL to form a reflection pulley for the biceps tendon. Similar to the CHL, the SGHL may be recognized as only a capsular thickening.2 Unlike the CHL, which can be seen extra-articularly, the deeper fibers of the SGHL are best seen within the joint as they cross across the RI and insert on the lesser tuberosity (Fig. 16-3).
BIOMECHANICS
Glenohumeral Joint Stability and Motion
Many studies have attempted to clarify the role of the rotator interval structures in passive motion and stability of the glenohumeral joint. Most studies agree that in concert, the RI works to provide resistance to posteroinferior translation of the humeral head and that imbrication or tightening of the RI will lead to restrictions in external rotation. However, disagreement exists about which of the structures may play the more predominant role. Ovesen and Nielsen10 originally identified the CHL as the more important inferior stabilizer, but did not specifically address the SGHL as separate from the CHL or anterior glenohumeral capsule. Boardman and coworkers11 have determined that the CHL can withstand higher tensile loads than the SGHL. Warner and colleagues12 were able to test the SGHL and CHL separately and determined that only the SGHL plays a significant role in preventing inferior translation. Cooper and associates9 have suggested that because the CHL does not consist of a truly ligamentous structure, it is unlikely to play a significant suspensory role as a soft tissue restraint in the shoulder.
Many cadaveric studies have noted that isolated release of the RI structures will affect both passive glenohumeral motion and humeral head translation. Ferrari and coworkers13 have confirmed through visual inspection that the anterior and proximal glenohumeral capsule became taut with external rotation in the adducted position. Similar findings have been noted by others in observational cadaveric studies.6,14
In an oft-cited paper, Harryman and colleagues7 performed sequential sectioning and successive repair of the RI capsule on cadaveric specimens to determine their effect on stability and motion. They found increases in flexion, extension, and external rotation with sectioning of the CHL and rotator interval capsular fibers. Furthermore, obligate translations of the humeral head were coupled with certain motions as a result of the sectioning. RI sectioning increased anterior translation in the abduction–external rotation position and inferior sulcus. However, the most striking finding was that a posteriorly directed force in slight abduction–external rotation resulted in posteroinferior dislocation in 50% of specimens. The authors concluded that the interval capsule acts as a checkrein to flexion and external rotation, and secondarily functions to provide additional posterior stability in the position of abduction–external rotation. In agreement with their findings, most cadaveric studies examining release of the RI and CHL have noted increases in passive external rotation of the shoulder. Neer and associates8 noted an increase of 32 degrees of passive external rotation with CHL sectioning. Tetro and coworkers15 more recently confirmed an increase of external rotation (in adduction) of 36 degrees and increased external rotation of 12 degrees in abduction after arthroscopic release of the rotator interval.
Biceps Stability
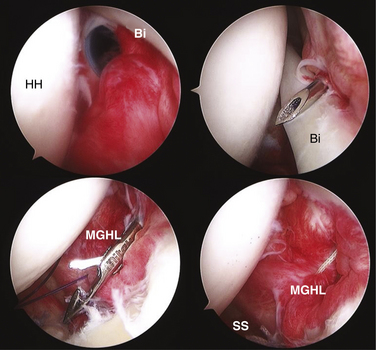
The structures of the RI play a key role in stabilization of the long head of the biceps tendon as it enters the bicipital groove. Werner and colleagues16 have examined the biceps sling anatomically and found the superior glenohumeral ligament and fasciculus obliquus as the primary stabilizers of the tendon at the entrance to the groove. Slatis and Aalto17 have determined through anatomic dissection that the medial coracohumeral ligament is the primary restraint to medial subluxation. Taken together, these and other studies have emphasized the role of the rotator interval capsular structures in biceps stability. Walch and associates18 have described hidden lesions of the anterosuperior cuff and interval capsule that can lead to medial subluxation and instability of the biceps tendon. In their group’s experience, subluxation of the biceps was always associated with subscapularis tendon tears and often with associated tears of the anterior supraspinatus. Gleason and coworkers19 performed an anatomic dissection of cadavers and were unable to find a distinct transverse humeral ligament; they found that the roof of the bicipital groove is made up of continuing fibers from the subscapularis tendon and coracohumeral ligament. Alternatively, Bennett20 has described two separate lateral divisions of the coracohumeral ligament, a lateral and medial division. The medial CHL and SGHL combined with the subscapularis tendon insertion form the medial stabilizers of the biceps sling, and it was noted that involvement of one structure, and not necessarily both, could result in biceps instability. In addition, the lateral CHL and anterior supraspinatus provide stabilization of the biceps tendon on the lateral side of the bicipital groove, and anterosuperior cuff tears involving these structures could also result in subluxation of the biceps tendon (Fig. 16-4).
PATHOPHYSIOLOGY
For the purposes of this chapter, pathology of the rotator interval will be divided into three groups. Taking from the original classification of rotator interval lesions by Nobuhara and Ikeda,21 two classifications of RI pathology will be reviewed: (1) contracture of the rotator interval, resulting in loss of glenohumeral motion; and (2) deficiency of the rotator interval, resulting in loss of normal glenohumeral stability. The third aspect is biceps tendon pathology as it relates to stability in the bicipital groove; this will also be discussed.
Rotator Interval Contracture and Adhesive Capsulitis
Many authors have cited contracture of the rotator interval as the primary involvement in adhesive capsulitis (frozen shoulder) and have focused on the RI (coracohumeral ligament and superior glenohumeral ligament) as the primary reason for loss of external rotation associated with the disorder.6,22,23 Bunker and coworkers22 have performed coracohumeral release for primary frozen shoulder and examined the specimens histologically for changes. They found an active fibroblast population, which was similar to the myofibroblast cell described in Dupuytren disease. Later work confirmed that Dupuytren disease is much more common in patients with idiopathic frozen shoulder, and that similar underlying pathologic processes may be involved.24 These results have been confirmed by others,25 suggesting that an unchecked fibroblast proliferation, combined with inflammation of the anterior interval capsule, leads to contracture of the RI and loss of active and passive motion. Although the underlying biochemical pathways leading to frozen shoulder have not been identified, clear risk factors have been identified, including female gender, diabetes mellitus, and hypothyroidism.
Rotator Interval Laxity and Instability
Glenohumeral instability is known to be a multifactorial process in which alterations in normal rotator cuff function, capsuloligamentous structures, and bony anatomy can lead to altered kinematics, with resultant instability. In their original report, Harryman and colleagues7 found that with sectioning of the RI capsule, posterior-inferior dislocation of the humeral head was possible in the abducted, externally rotated position. Although some have described rotator interval lesions in the settings of open surgery for instability, in the arthroscopic setting it becomes difficult to assess the tissue quality of anterior capsule. Usually, a layer of synovium and capsule will be present anteriorly, without the presence of a clear defect.21,26,27 We believe that with open reconstructive surgery for shoulder instability, friable tissue may be present in the rotator interval, which is easily disrupted and seen as a defect in the capsule. This is especially true with taking down the subscapularis when doing a capsular procedure.
The biomechanical effects of arthroscopic rotator interval closure have been examined in cadaveric models, but published data vary. Harryman and associates7 originally performed imbrication of the rotator interval capsule after cadaveric sectioning and found improved restraint to inferior and posterior translation. The procedure described was essentially a medial to lateral pants over vest imbrication of the coracohumeral ligament of approximately 1 cm. This was thought to be valuable in the setting of multidirectional instabilities, but the open procedure for rotator interval tightening described is very different from current arthroscopic procedures. Most descriptions of RI closure both arthroscopically and open used an inferior to superior closure, leaving limited applicability of this early data. The dramatic losses of external rotation (approximately 35 degrees) seen in their group are not noted in more recent arthroscopic cadaveric studies.
As noted, current arthroscopic techniques use an inferior to superior closure, which does not shorten the anterior capsule as described in the original paper by Harryman and coworkers.7 Using two superior-inferior sutures closing the anterior edge of the SGHL to the upper margin of the subscapularis or the MGHL, Yamamoto and colleagues28 have found decreased anterior translation in the abduction–external rotation (ER) position and decreased posterior translation in the adducted position. At the same time, passive ER and abduction were diminished. Plausinis and associates29 performed an arthroscopic RI closure and found decreased passive external rotation and anterior translations, but no change in posterior translation. However, the closure did significantly decrease anterior translation. Provencher and coworkers30 compared arthroscopic interval closure versus open closure in cadaveric specimens and found no significant benefit to posterior and inferior translation with the arthroscopic technique. They did find improved anterior stability in abduction-ER, but passive external rotation was sacrificed in exchange (approximately 25 degrees). A repeat follow-up study performed by the same group confirmed similar findings.31
Shafer and colleagues32 more recently looked at RI closure in a simulated multidirectional instability model and found that RI closure (although not arthroscopically performed) may help reduce anterior-posterior translations in the abducted positions, but again found similar losses in total rotation. Taken together, it can be inferred from these studies that arthroscopic rotator interval closure is likely to have some effect on anterior translations in the abducted position, but improvement in posterior and inferior translation may be unpredictable, and a clear loss of external rotation can be expected.
Physical Examination
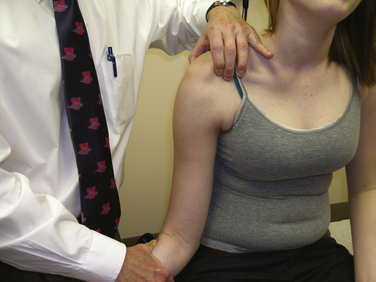
In the setting of instability, the examination should be tailored toward identifying the direction of symptoms. Anterior apprehension and relocation tests should be performed. The jerk test can be performed to determine whether there is posterior instability. Our preference is to perform the load and shift test in the supine position. Often, the patient may guard and prevent painful subluxation of the joint. Even in this setting, the patient may complain of pain or discomfort with translations in a certain direction, which allows the surgeon to assign a primary direction to the instability. A sulcus sign more than 1 cm is suggestive of a lax inferior capsule. As described by Nobuhara and Ikeda,21 a sulcus sign that does not diminish with external rotation of the humerus is suggestive of a deficient rotator interval (Fig. 16-5). Findings of generalized ligamentous laxity should be noted.
Biceps Sling Pathology
History
Pain related to the biceps sling is much more difficult to isolate based on history. Often, patients may complain of only vague soreness in the anterior portion of the shoulder. Elbow flexion maneuvers are rarely painful. In a setting in which suspicion of a rotator cuff tear is high, biceps involvement must be suspected because of the possibility of the anterosuperior cuff tear, as described by Bennett,20 resulting in biceps instability.
Physical Examination
Isolating biceps instability on physical examination is a much less defined process. Cuff strength testing may provide suspicion of a cuff tear that may involve the CHL-SGHL insertion. The abdominal compression test and lift-off examination, although negative, may reproduce pain and suggest an upper subscapularis tear which may result in biceps instability. The Speed and Yergason tests may reproduce pain but are nonspecific. In an arthroscopically confirmed study, Bennett33 confirmed that the Speed test is nonspecific for biceps pathology and does not isolate any specific part of the tendon anatomy. Bennett20 also described a provocative maneuver for reproducing biceps subluxation. The patient’s arm is passively brought from a position of abduction-ER to adduction and internal rotation. A catching or clicking sensation is consistent with biceps instability, but no sensitivity or specificity values for this maneuver are given. Diagnostic injections of the bicipital sheath and glenohumeral joint are useful, but may not fully identify the location of the pathologic process.
IMAGING STUDIES
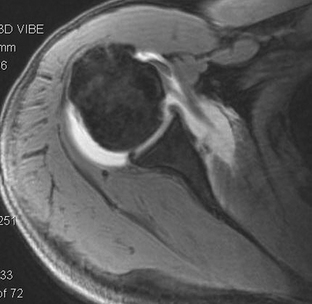
To date, many studies have attempted to quantify the rotator interval in terms of contracture and laxity, but it is thought that many of these do not provide material that is clinically relevant to the surgical approach. When using magnetic resonance arthrography (MRA) for the evaluation of instability pathology, it is important to remember that the interval is the most often used location for the injection of intra-articular contrast material,34 and that small amounts of extravasation may occur and are not indicative of a rotator interval defect or deficiency. Emig and associates35 have determined that with MR imaging (MRI), an anterior capsular thickness more than 4 mm is a highly specific and sensitive threshold for the diagnosis of frozen shoulder, and similar findings were seen in other published reports.36 Provencher and coworkers37 have found no differences in rotator interval dimensions in patients with anterior, posterior, and multidirectional instability, but noted that the biceps tendon assumes a more anterior position in those patients with posterior instability in comparison with other groups.
Given the difficulty of translating the results of published reports into clinical practice, MRI is used primarily as an adjunctive tool to identify concomitant shoulder pathology that may adjust the surgical approach. The diagnosis of adhesive capsulitis in our hands is primarily clinical, but when weakness exists in conjunction with loss of motion, MRI can be used to confirm the presence or absence of rotator cuff tears. In the setting of recurrent instability, MRA may demonstrate discrete labral lesions, which may respond to surgical treatment with repair alone. Biceps instability highly correlates with tears of the upper margin of the subscapularis18 and, when seen, should alert the surgeon to potentially repairable tears of the tendon (Fig. 16-6).
TREATMENT
Rotator Interval Contracture and Adhesive Capsulitis
A variety of nonoperative treatments have been proposed for idiopathic adhesive capsulitis, including benign neglect, chiropractic manipulation, oral or injectable corticosteroids, and physical therapy. Instructive data is unfortunately rather sparse, because numerous early studies lacked objective and subjective outcome measures. More recent studies have used more rigorous study design and data collection. One study38 included 75 patients with idiopathic adhesive capsulitis treated with a formal exercise program consisting of pendulum circumduction, passive forward elevation, external rotation, and internal rotation. Data were collected prospectively and 90% of patients reported satisfactory outcomes, with significant improvements in pain at rest, pain with activity, and active and passive range of motion. Prior physical therapy, a pending or active workers compensation claim or litigation, male gender, and diabetes mellitus were associated with failure of the therapy protocol. Levine and colleagues39 have reported on 105 shoulders treated at a single institution for adhesive capsulitis. Almost 90% of patients’ symptoms resolved with nonoperative management, including oral nonsteroidal anti-inflammatory drugs (NSAIDs) and physical therapy, with varied use of corticosteroids. Gender was not found to play a significant role, and 90% of their diabetic patients were managed successfully without operative intervention. Regardless of operative or nonoperative management, no significant difference was noted in outcome after completion of treatment.
For cases refractory to the aforementioned treatments, operative intervention may be required. Closed manipulation under anesthesia (MUA), arthroscopic capsular release, with or without additional manipulation, and open capsular release have been described, with good results. Typically, operative intervention, including MUA, has been relatively contraindicated in the early inflammatory phase of the disease. Operative intervention is generally considered after the patient’s initial painful symptoms have resolved and he or she is left with a dysfunctional but relatively pain-free shoulder. However, a recent review of 145 patients treated with MUA demonstrated that patients reported better Oxford Shoulder Scores when treated within 9 months of the onset of symptoms.40
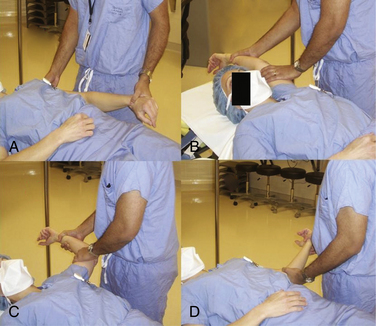
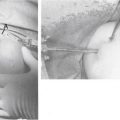
Closed MUA has been the central operative treatment for decades, but it carries the risk of humerus fracture and rotator cuff tear. Significant torsional forces can be applied to the humerus with forceful external rotation of the forearm and should be uniformly avoided. With the patient supine on the gurney, firm pressure should be applied to the anterior thorax to immobilize the scapula between the body and gurney. With the elbow flexed to 90 degrees and the humerus fully internally rotated, controlled but firm superior pressure is applied to the humerus. Once sufficient forward elevation has been achieved, the humerus is then directed from the elevated position through a fully abducted position to return to the patient’s side without altering the position of the elbow. Using Codman’s paradox, external rotation can be regained without applying any large torsional loads to the humeral shaft (Fig. 16-7).41
Arthroscopic Capsular Release
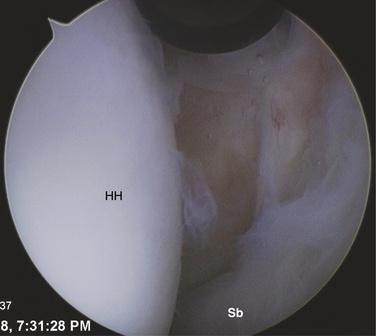
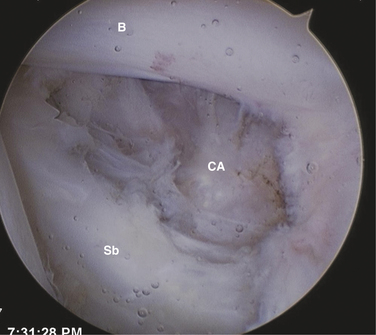
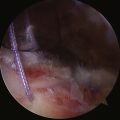
The patient may be positioned laterally or in the beach chair position, according to the surgeon’s preference. A standard posterior portal is established and the arthroscope is introduced into the joint. The inferior joint is typically not easily accessible at this stage because of significant contracture. The diagnosis is verified by the presence of synovial villi and petechial hemorrhaging in the rotator interval and a thickened scarred capsule (Fig. 16-8). An anterior portal is placed after verification of position with an 18-gauge spinal needle. A side-cutting arthroscopic diathermy probe is then inserted via this portal. The sequence begins superiorly with division of the superior glenohumeral ligament and coracohumeral ligament. This dissection may be carried as far medially as the coracoid. if needed. Visualization of the vertically oriented fibers of the coracoacromial ligament and conjoint tendon confirms complete release of the RI tissue (Fig. 16-9).
Arthroscopic Analysis of Bicipital Sheath Lesions
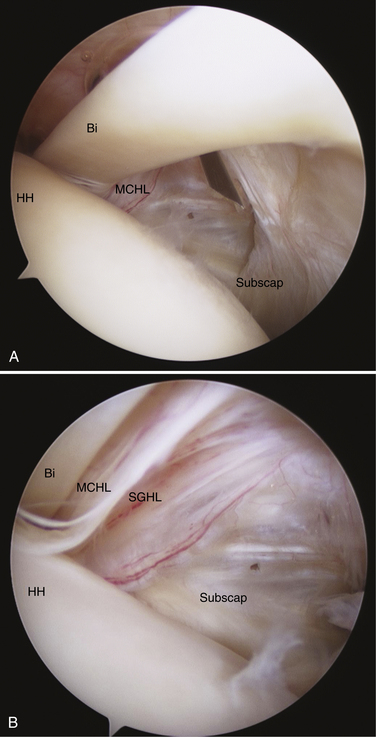
Stepwise diagnostic arthroscopy of the glenohumeral joint is an essential initial phase of any reconstructive procedure. From the typical posterior portal, the lateral rotator interval, including the insertion of the subscapularis tendon, SGHL, medial CHL (MCHL), and biceps sheath can be difficult to visualize. With the addition of a standard anterior portal, the outflow cannula or some other instrument may further obscure these lateral lesions.
A standard 30-degree arthroscope can be advanced anteriorly to the anterior margin of the humeral head. The shoulder should be elevated from 60 to 90 degrees and internally rotated. A probe placed through the anterior portal then can be placed under the subscapularis tendon and SGHL-CHL complex. If a 70-degree arthroscope is used, the examination may be completed with less elevation. In this way, a thorough evaluation of the rotator interval and medial biceps pulley can be safely and reproducibly performed.42 Careful positioning of the arm can also assist the surgeon with improved visualization. Internal rotation combined with adduction of the arm will relax the subscapularis tendon, increasing maneuverability in the rotator interval and bringing the biceps sling more anteriorly for visualization. With use of an arthroscopic probe, subluxation of the biceps tendon can be tested with a medially directed force (Fig. 16-10).
Bennett has described arthroscopic repair of the biceps sheath, but results are inconsistent.43 Despite the success reported and the proof of feasibility offered by this study, it remains somewhat of a diagnostic and treatment dilemma. After prolonged instability, these patients often suffer from substantial fraying of the biceps tendon. It is difficult to repair the medial biceps pulley with confidence if it encloses a frayed and inflamed tendon. Predictable results can be obtained by treating these lesions more definitively with tenotomy or tenodesis, depending on patient age, expectations, and activity level. Arthroscopic or subpectoral tenodesis can be chosen according to surgeon experience and comfort level. These techniques are discussed elsewhere in the text.
Rotator Interval Laxity in Instability Surgery
Surgical Technique for Rotator Interval Closure
Blind Tying Technique.
In this scenario, the interval closure is addressed after all capsular plication and labral repair have been performed. With the arthroscope in the posterior portal, looking at the rotator interval, either of the anterior portals is withdrawn until it has just exited the capsule of the shoulder anteriorly. Often, the surgeon may identify this; the cannula will have increased mobility because it has entered a separate tissue plane. A straight suture delivery device is introduced through the cannula and pierces the superior glenohumeral ligament at the level of the biceps. A monofilament suture is then shuttled into the joint. The suture delivery device is removed, leaving abundant free monofilament within the joint. The cannula is moved inferiorly, and often the surgeon can identify its location extracapsularly by visualizing tenting in the anterior capsule. A sharp suture retriever is then passed through the cannula and pierces the anterior capsule immediately superior to the subscapularis. Fibers of the middle glenohumeral ligament are also pierced prior to retrieving the previously passed monofilament and bringing it back through the anterior cannula. The upper subscapularis can also be included in this suture, if desired. Using this monofilament suture, a braided or nonabsorbable suture can be shuttled through the capsule and cannula. The sutures can be tagged with the use of a small surgical clamp to facilitate suture management. Sutures are passed in a medial to lateral direction in the interval. Typically, one or two sutures are used, depending on the space available in the rotator interval. With the arthroscope in the posterior portal, the surgeon can visualize the amount of volume reduction and suture tension. A blind tying technique must be used through the anterior cannula. Our preference is to use a sliding SMC knot with three alternating half-hitches. A closed-ended suture cutter is then used to prevent injury to the sutures or knot to remove the extra suture material. We recommend tying each suture at the time of individual suture passage, rather than after passage of all the interval closure stitches. Because of the single cannula and blind tying technique used, suture tying after passage of all sutures can become difficult (Fig. 16-11).
Subacromial Technique.
PEARLS& PITFALLS
TECHNICAL PEARLS
1. Neer CS2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52:1077-1089.
2. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop Relat Res. 2001;390:129-137.
3. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9:336-341.
4. Hunt SA, Kwon YW, Zuckerman JD The rotator interval: anatomy, pathology, and strategies for treatment. J Am Acad Orthop Surg, 15; 2007:218-227.
5. Cooper DE, O’Brien SJ, Warren RF Supporting layers of the glenohumeral joint. An anatomic study. Clin Orthop Relat Res (289); 1993:144-155.
6. Edelson JG, Taitz C, Grishkan A. The coracohumeral ligament. Anatomy of a substantial but neglected structure. J Bone Joint Surg Br. 1991;73:150-153.
7. Harryman DT2nd, Sidles JA, Harris SL, Matsen FA3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74:53-66.
8. Neer CS2nd, Satterlee CC, Dalsey RM, Flatow EL The anatomy and potential effects of contracture of the coracohumeral ligament. Clin Orthop Relat Res (280); 1992:182-185.
9. Cooper DE, O’Biren SJ, Arnoczky SP, Warner RF The structure and function of the coracohumeral ligament: an anatomic and microscopic surgery. J Shoulder Elbow Surg, 2; 1993:70-77.
10. Ovesen J, Nielsen S. Experimental distal subluxation in the glenohumeral joint. Arch Orthop Trauma Surg. 1985;104:78-81.
11. Boardman ND, Debski RE, Warner JJ, et al. Tensile properties of the superior glenohumeral and coracohumeral ligaments. J Shoulder Elbow Surg. 1996;5:249-254.
12. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20:675-685.
13. Ferrari DA. Capsular ligaments of the shoulder. Anatomical and functional study of the anterior superior capsule. Am J Sports Med. 1990;18:20-24.
14. Plancher KD, Johnston JC, Peterson RK, Hawkins RJ. The dimensions of the rotator interval. J Shoulder Elbow Surg. 2005;14:620-625.
15. Tetro AM, Bauer G, Hollstien SB, Yamaguchi K Arthroscopic release of the rotator interval and coracohumeral ligament: An anatomic study in cadavers. Arthroscopy, 18; 2002:145-150.
16. Werner A, Mueller T, Boehm D, Gohlke F. The stabilizing sling for the long head of the biceps tendon in the rotator cuff interval. A histoanatomic study. Am J Sports Med. 2000;28:28-31.
17. Slatis P, Aalto K. Medial dislocation of the tendon of the long head of the biceps brachii. Acta Orthop Scand. 1979;50:73-77.
18. Walch G, Nove-Josserand L, Boileau P, Levigne C. Subluxations and dislocations of the tendon of the long head of the biceps. J Shoulder Elbow Surg. 1998;7:100-108.
19. Gleason PD, Beall DP, Sanders TG, et al The transverse humeral ligament: a separate anatomical structure or a continuation of the osseous attachment of the rotator cuff. Am J Sports Med, 34; 2006:72-77.
20. Bennett WF. Subscapularis, medial, and lateral head coracohumeral ligament insertion anatomy. Arthroscopic appearance and incidence of “hidden” rotator interval lesions. Arthroscopy. 2001;17:173-180.
21. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop Relat Res. 1987;223:44-50.
22. Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br. 1995;77:677-683.
23. Ozaki J, Nakagawa Y, Sakurai G, Tamai S. Recalcitrant chronic adhesive capsulitis of the shoulder. Role of contracture of the coracohumeral ligament and rotator interval in pathogenesis and treatment. J Bone Joint Surg Am. 1989;71:1511-1515.
24. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
25. Hand GC, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. 2007;89:928-932.
26. Field LD, Warren RF, O’Brien SJ, et al. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23:557-563.
27. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63:863-872.
28. Yamamoto N, Itoi E, Tuoheti Y, et al Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg, 15; 2006:750-758.
29. Plausinis D, Bravman JT, Heywood C, et al Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med, 34; 2006:1656-1661.
30. Provencher MT, Mologne TS, Hongo M, et al Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy, 23; 2007:583-592.
31. Mologne TS, Zhao K, Hongo M, et al The addition of rotator interval closure after arthroscopic repair of either anterior or posterior shoulder instability: effect on glenohumeral translation and range of motion. Am J Sports Med, 36; 2008:1123-1131.
32. Shafer BL, Mihata T, McGarry MH, et al. Effects of capsular plication and rotator interval closure in simulated multidirectional shoulder instability. J Bone Joint Surg Am. 2008;90:136-144.
33. Bennett WF Specificity of the Speed’s test: arthroscopic technique for evaluating the biceps tendon at the level of the bicipital groove. Arthroscopy, 14; 1998:789-796.
34. Depelteau H, Bureau NJ, Cardinal E, et al Arthrography of the shoulder: a simple fluoroscopically guided approach for targeting the rotator cuff interval. AJR Am J Roentgenol, 182; 2004:329-332.
35. Emig EW, Schweitzer ME, Karasick D, Lubowitz J Adhesive capsulitis of the shoulder: MR diagnosis. AJR Am J Roentgenol, 164; 1995:1457-1459.
36. Sofka CM, Ciavarra GA, Hannafin JA, et al Magnetic resonance imaging of adhesive capsulitis: correlation with clinical staging. HSS J, 4; 2008:164-169.
37. Provencher MT, Dewing CB, Bell SJ, et al. An analysis of the rotator interval in patients with anterior, posterior, and multidirectional shoulder instability. Arthroscopy. 2008;24:921-929.
38. Griggs SM, Ahn A, Green A. Idiopathic adhesive capsulitis. A prospective functional outcome study of nonoperative treatment. J Bone Joint Surg Am. 2000;82:1398-1407.
39. Levine WN, Kashyap CP, Bak SF, et al. Nonoperative management of idiopathic adhesive capsulitis. J Shoulder Elbow Surg. 2007;16:569-573.
40. Flannery O, Mullett H, Colville J Adhesive shoulder capsulitis: does the timing of manipulation influence outcome. Acta Orthop Belg, 73; 2007:21-25.
41. Hollis R, Lahav A, West HSJr. Manipulation of the shoulder using Codman’s paradox. Orthopedics. 2006;29:971-973.
42. Bennett WF. Visualization of the anatomy of the rotator interval and bicipital sheath. Arthroscopy. 2001;17:107-111.
43. Bennett WF Arthroscopic bicipital sheath repair: two-year follow-up with pulley lesions. Arthroscopy, 20; 2004:964-973.