Chapter 14 Rotational and directional atherectomy
 Coronary atherectomy and atheroma ablation devices were introduced to overcome the acute failings of balloon angioplasty and in an attempt to reduce restenosis.
Coronary atherectomy and atheroma ablation devices were introduced to overcome the acute failings of balloon angioplasty and in an attempt to reduce restenosis. Directional coronary atherectomy was introduced before stenting emerged as the dominant technique of PCI.
Directional coronary atherectomy was introduced before stenting emerged as the dominant technique of PCI. In general, stenting is easier and is associated with results as good as or better than those seen with DCA, even when performed optimally.
In general, stenting is easier and is associated with results as good as or better than those seen with DCA, even when performed optimally. There are a number of lesion subsets associated with less than ideal results with stenting (even with DES); directional atherectomy using new catheter designs may still have a role in some of these.
There are a number of lesion subsets associated with less than ideal results with stenting (even with DES); directional atherectomy using new catheter designs may still have a role in some of these.INTRODUCTION
Although stenting has become the default method of PCI, the understanding in the early and mid-1980s of the components of acute failure and restenosis led to two major lines of development – atherectomy and ablative methods as well as methods of scaffolding the vessel. The new technologies that emerged included the directional atherectomy device and two methods of ablation, namely laser techniques and rotablation. Although some of the scenarios when these devices were used can now be treated with stents, certain clinical and anatomic scenarios exist where the use of ablative methods are essential to allow optimal delivery and expansion of stents and in some cases they may be the only means of achieving percutaneous dilatation of a lesion. One of the worst clinical situations to occur in the current era favouring direct stenting is the delivery of a stent to a previously unrecognised undilatable lesion. Only experience, recognition of a number of clinical clues that this situation might be met, and the ability to use ablative technologies can prevent this. This chapter will review the use of atherectomy devices and coronary rotablation.
DIRECTIONAL ATHERECTOMY
Directional coronary atherectomy was developed by John Simpson to enable debulking of lesions. It was argued that excision of atheromatous material would overcome many of the limitations of balloon dilatation alone, allowing for better dilatation of the lesion with less dependence on stretch (and thus barotrauma), more controlled dilatation (and thus fewer dissections), and that there would, as a result, be better acute clinical results with less restenosis. The initial registries appeared favourable, but the results of several randomised trials were disappointing. The major studies of DCA are listed in Table 14.1. In particular, the use of DCA resulted in a relatively high peri-procedural release of cardiac enzymes, and clinical results were either only marginally better or in some cases appeared worse.13–15 There are several reasons why early registry experience is not always translated into clinical benefit in a multi-centre randomised trial. These include the performance of a trial before the technique has been adequately developed, designing a trial that partially reflects the learning curve of operators with only limited experience of the technique and, perhaps most importantly, the fact that early registry experience is obtained in carefully selected patients by operators who have the greatest experience of the technique. Moreover, the original concepts of why DCA might be favourable might have been countered by the stimulus to restenosis caused by cutting into the plaque.
| STUDY | TITLE | REF |
|---|---|---|
| CAVEAT | Coronary Angioplasty Versus Excisional Atherectomy Trial | 1 |
| CCAT | Canadian Coronary Atherectomy Trial | 2 |
| CAVEAT-II | As CAVEAT, but for saphenous vein graft lesions | 3 |
| OARS | Optimal Atherectomy Restenosis Study | 4 |
| BOAT | Balloon vs. Optimal Atherectomy Trial | 5 |
| ABACAS | Adjunctive Balloon Angioplasty following Coronary Atherectomy Study | 6 |
| GUIDE II | Guidance by Ultrasound Imaging for Decision Endpoints | 7 |
| START | STent versus directional coronary Atherectomy Randomized Trial | 8 |
| SOLD | Stenting after Optimal Lesion Debulking Study | 9 |
| Bramucci Registry | DCA before stenting | 10 |
| AtheroLink Registry | DCA before stenting | 11 |
| AMIGO | Atherectomy before Multilink Improves luminal Gain and clinical Outcomes | 12 |
Although the CAVEAT and CCAT trials did not establish a clear benefit from DCA, possibly because of inadequate debulking and a higher complication rate compared to balloon angioplasty alone, the OARS, BOAT, ABACAS and GUIDE II studies suggested that optimal debulking, with attempts to reduce residual plaque volume to a minimum (aided if necessary by IVUS guidance), would result in lower restenosis rates. A residual angiographic percent diameter stenosis after DCA (with or without adjunctive balloon angioplasty) of <10–15% was aimed for. However, the higher CK release during procedures remains an issue and given that DCA is more technically demanding, the emergence of stenting as an easier-to-use technique with equal or better results has resulted in a major reduction in the number of DCA procedures being performed worldwide. However, when only BMS were available, the Japanese START study suggested that DCA could still be considered as an alternative to stenting as it achieved a lower rate of restenosis with no differences in clinical events.8
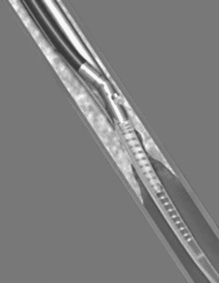
Several generations of devices were produced by Devices for Vascular Intervention (acquired initially by Guidant Ltd and subsequently by FoxHollow). Following the original AtheroCath®, sequential improvements were seen in the SCA-1™, SCA-EX™, Short-Cutter™, the GTO®, the Bantam™ and the Flexi-Cut™ devices, with improved debulking capability and use even in partially calcified lesions. The original devices needing 10F sheaths and guide catheters gave way to 8F-compatible devices. Currently, only the Flexi-Cut™ device is available (Fig. 14.1). The technique requires stiffer than average guide catheters such as the Viking XT™ range (Guidant) with stiff wires such as the Iron Man™ (Guidant).
The device is inserted into the vessel, rotated until the cutting window is lined up with the side of the vessel containing the bulk of the lesion, the cutter is then drawn back and the balloon on the side of the device opposite to the window is then inflated at low pressure. The balloon stabilises the device and pushes the cutting window against the plaque. The device is then activated and the rotating cup-shaped cutter is advanced, slicing off the atheroma and pushing it forward into the collection nosecone. The balloon is then deflated, the device rotated slightly and further excisions made until adequate atherectomy has been performed. Different sized catheters are available for different vessels and lesions. In smaller vessels, the development of ischaemia during the procedure sometimes requires withdrawal of the device back into the guiding catheter between cuts. This is less of a problem with the Flexi-Cut™ device.
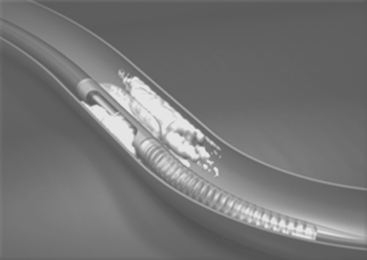
More recently, the FoxHollow SilverHawk™ Plaque Excision System has been introduced (Fig. 14.2), which has the advantage of being 6 F compatible and it does not have a contra-lateral balloon opposite the cutting window but instead a unique hinge design.16 This potentially reduces barotrauma. Three tip lengths are available with different collection chambers, allowing for a number of clinical scenarios in coronary cases, and the device can be used in vessels ranging from 1.5 to 3.5 mm in diameter. Catheters designed for peripheral vascular cases are also available. The carbide blade allows for excision of calcified plaque. Like the earlier DCA devices, the shaved plaque is stored in the nosecone of the device, allowing its retrieval. These atherectomy devices are the only available tools that allow for retrieval of human atheromatous tissue for research purposes. Another device is the Arrow-Fischell Pullback Atherectomy Device (Arrow Medical Devices Co, Reading, Pennsylvania, US).17,18
Although stenting has emerged as the dominant method of PCI, there are still some anatomical situations where stenting is technically difficult or may not achieve optimal results, and stenting itself has produced the problem of how to deal with in-stent restenosis. The latter is due entirely to neointimal proliferation within the stent, but inadequate stent expansion (which thereby reduces the size of the lumen that can accommodate the proliferative tissue) has been recognised to be one of the major determinants of sub-optimal clinical results. DCA enthusiasts have, therefore, continued to use this technique in difficult anatomy, and have also explored the role of debulking prior to stent implantation. Again, early registry evidence was favourable but the randomised multi-centre AMIGO trial could find no benefit from this technique, although benefit was seen in the subset of patients where aggressive debulking (<20% residual stenosis) was achieved prior to stenting. Although certain groups have suggested that the benefits only emerge with aggressive debulking prior to stent implantation, this has become a somewhat academic exercise with the emergence of DES.
The lesions for which some operators continue to use DCA rather than stenting include eccentric lesions, ostial stenoses, bifurcations, in-stent restenoses, and some continue to use the technique of aggressive debulking prior to stenting (especially for left main stem lesions). Its use in these lesions has been reviewed recently.19 Although the use of DES has seen a major reduction in the use of DCA, these lesions remain difficult to treat and results with DES are less favourable than with more straightforward lesions.
ROTATIONAL ATHERECTOMY (ROTABLATION)
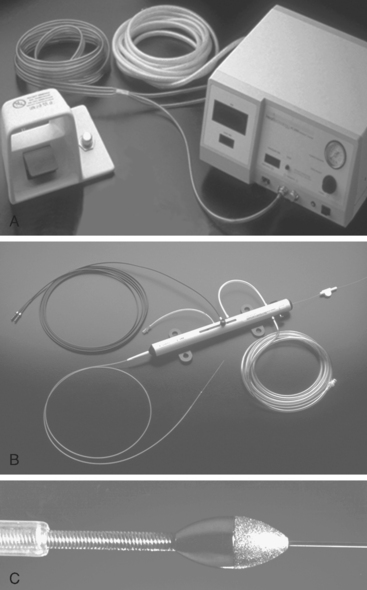
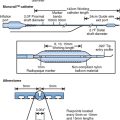
Rotablation is probably a more appropriate name for this technique as it results in virtual emulsification of the atheromatous plaque rather than being a method of excising atheroma. It utilises a high-speed rotating elliptically shaped burr, the front face of which is coated with 2000 to 3000 diamond microchips (30–50 μm in diameter). As the burr meets the plaque, the latter is fragmented into particles 5–10 μm in diameter (smaller than red cells) which are washed downstream, pass through the coronary capillary bed and then enter the venous circulation and are removed from the circulation by the reticulo-endothelial system (Fig. 14.3).
Since its introduction, the technique has changed somewhat. Modifications include the use of a verapamil/nitrate flush solution used down the guide catheter whilst rotablation is performed, a Rotaglide™ lubricant that reduces friction and disperses heat, shorter runs with only gentle advancement into the plaque using a pecking technique that allows for as much coronary perfusion during each run, lower burr speeds, avoidance of drops in speed of >5000 rpm, and up-front use of a glycoprotein IIb/IIIa inhibitor.20,21 Many operators do not use the latter though until after rotablation in particularly difficult, angulated and heavily calcified vessels because of the small risk of perforation of the vessel, but instead consider its use during the remainder of the procedure once the rotablation is completed. For lesions in dominant right or circumflex coronary arteries, significant bradycardia or heart block can occur. The patients should be well hydrated, atropine pre-treatment should be considered and in some cases it is wise to insert a pacing electrode. Right ventricular pacing electrodes (especially if a glycoprotein IIb/IIIa inhibitor is used) can result in cardiac tamponade if an apical electrode perforates the ventricle, and it is probably wiser to select either a septal position for the pacing electrode or use a flotation pacing catheter.
MAJOR STUDIES OF ROTABLATION
The major studies of rotablation are listed in Table 14.2.20–31 The earlier studies compared balloon angioplasty with rotablation (and laser technology in the ERBAC trial) in relatively complex lesion subsets and small vessels. In ERBAC, 685 patients with complex lesions were randomised between rotablation, excimer laser angioplasty or balloon angioplasty. Procedural success was highest in the rotablator arm but there was no difference overall in complications. Further revascularisation at six months was required least frequently with balloon angioplasty. In the COBRA study, procedural success rates were similar in both arms of the study, and restenosis rates were similar although there was more late loss in the rotablator arm. These studies, performed in the pre- and early stenting era, did not provide compelling evidence to use rotablation in the majority of complex lesions (B2 and C category). A number of nonrandomized studies have looked at the role of the rotablator in lesions such as chronic total occlusions, ostial lesions, and bifurcation lesions. In general, there is no evidence to suggest any benefit, although it may be necessary to use the device in individual resistant, fibrotic or calcified lesions. There is no particular advantage of using the device in small vessels (as shown in DART).
| STUDY | TITLE | REF |
|---|---|---|
| SARS | San Antonio Rotablator Study | 20 |
| Rota ReoPro | Rotational atherectomy with ReoPro | 21 |
| ERBAC | Excimer laser, Rotational atherectomy and Balloon Angioplasty Study Comparison | 22 |
| COBRA | Comparison of Balloon vs. Rotational Angioplasty | 23 |
| DART | Dilation vs. Ablation Revascularization Trial | 24 |
| NACI Registry | New Approaches to Coronary Intervention registry | 25 |
| STRATAS | STudy to determine Rotablator And Transluminal Angioplasty Strategy | 26 |
| CARAT | Coronary Angioplasty and Rotablator Atherectomy Trial | 27 |
| BARASTER | Balloon Angioplasty or Rotational Atherectomy in the treatment of in-STEnt Restenosis | 28 |
| ELCA/ROTA, ISR | Excimer Laser Coronary Angioplasty vs. ROTational Atherectomy in treatment of In-Stent Restenosis | 29 |
| ROSTER | ROtational atherectomy verSus balloon angioplasty for diffuse in-stent Restenosis trial | 30 |
| ARTIST | Angioplasty versus Rotational atherectomy for Treatment of diffuse In-Stent Restenosis Trial | 31 |
The issue of the optimal method to use rotablation was the subject of the STRATAS and CARAT studies. Two techniques were considered – a simpler technique utilising sufficient plaque ablation to allow successful dilatation with ballooning at reasonably low pressure (‘facilitated angioplasty’) versus more aggressive debulking using sequential burrs (final burr:artery ratio >0.7) and then finishing with either no ballooning or ballooning at very low pressure (to avoid deep tissue injury and thereby minimise the stimulus to restenosis). These trials did not show the hoped-for lower restenosis rate with the more aggressive technique, which was associated with a higher release of CK and other complications. Nowadays, rotablation using small burrs (burr:artery ratio <0.6) is most often used to remove difficult calcification and to alter vessel compliance prior to ballooning (and nowadays stenting). Use of the larger burrs has reduced significantly and most operators use a single 1.5 or 1.75 mm burr (reserving the 1.25 mm burr for those with a very tight or heavily calcified lesion). There are, however, individual cases that have such deep napkin-ring calcification that sequential burrs are necessary to achieve a satisfactory lumen. If there is any doubt about whether a lesion might be heavily calcified, it is advisable to inspect it first with IVUS. One tip is that if the IVUS catheter cannot cross the lesion, it needs rotablating.
Complications with rotablation are lower with the less aggressive debulking technique but there is an incidence of Q wave infarction (1–1.3%), CK-MB >3 times the upper limit of normal in 4–6%, dissection (10–13%), abrupt closure (1.8–11.2%), the slow flow phenomenon (1.2–7.6%), perforation (0–1.5%) and severe spasm (1.6–6.6%). Many of these can be avoided by correct technique using smaller burrs. If they occur, they can be managed with post-rotablation ballooning and stenting (including the use of covered stents in the case of perforation), and generous administration of intra-coronary nitrates, verapamil or adenosine. If tamponade occurs, emergency pericardiocentesis and sometimes surgical bailout is necessary. If severe slow flow or spasm occurs, it is wise to abandon the rotablator and to use balloon angioplasty and stenting as appropriate, as continued rotablation in these circumstances greatly increases the chances of perforation. Particular care with the rotablator must be used in very angulated or heavily calcified lesions. In these cases it is sensible to start with the smallest 1.25 mm burr and to concentrate on optimal technique. In cases with poor left ventricular function it might be necessary to use an intra-aortic balloon pump prior to starting. The device is not necessary in very soft lesions, it is not designed as a thrombus removal device, and it should not be used in the body of vein grafts with friable disease.
1 Topol EJ, Leya F, Pinkerton CA, et al. A comparison of directional atherectomy with coronary angioplasty in patients with coronary artery disease. The CAVEAT Study Group. N Engl J Med. 1993;329:221-227.
2 Adelman AG, Cohen EA, Kimball BP, et al. A comparison of directional atherectomy with balloon angioplasty for lesions of the left anterior descending artery. N Engl J Med. 1993;329:228-233.
3 Holmes DRJr, Topol EJ, Califf RM, et al. A multicenter, randomised trial of coronary angioplasty versus directional atherectomy for patients with saphenous vein bypass graft lesions. CAVEAT-II Investigators. Circulation. 1995;91:1966-1974.
4 Simonton CA, Leon MB, Baim DS, et al. “Optimal” directional atherectomy: final results of the Optimal Atherectomy Restenosis Study (OARS). Circulation. 1998;97:332-339.
5 Baim DS, Cutlip DE, Sharma SK, et al. Final results of the Balloon vs Optimal Atherectomy Trial (BOAT). Circulation. 1998;97:322-331.
6 Suzuki T, Hosokawa H, Katoh O, et al. Effects of adjunctive balloon angioplasty after intravascular ultrasound-guided optimal directional coronary atherectomy: the result of Adjunctive Balloon Angioplasty after Coronary Atherectomy Study (ABACAS). J Am Coll Cardiol. 1999;34:1028-1035.
7 The GUIDE Trial Investigators. IVUS-determined predictors of restenosis in PTCA and DCA: final report from the GUIDE trial, phase II. (abstr). J Am Coll Cardiol. 1996;29(Suppl.):156A.
8 Tsuchikane E, Sumitsuji S, Awata N, et al. Final results of the Stent versus Directional Coronary Atherectomy Randomized Trial (START). J Am Coll Cardiol. 1999;34:1050-1057.
9 Moussa I, Moses J, Di Mario C, et al. Stenting after optimal lesion debulking (SOLD) registry. Angiographic and clinical outcome. Circulation. 1998;98:1604-1609.
10 Bramucci E, Angoli L, Merlini PA, et al. Adjunctive stent implantation following directional coronary atherectomy in patients with coronary artery disease. J Am Coll Cardiol. 1998;32:1855-1860.
11 Hopp HW, Baer FM, Ozbek C, Kuck KH, Scheller B. A synergistic approach to optimal stenting: directional coronary atherectomy prior to coronary artery stent implantation — the AtheroLink Registry. AtheroLink Study Group. J Am Coll Cardiol. 2000;36:1853-1859.
12 Stankovic G, Colombo A, Bersin R, et al. for the AMIGO Investigators. Comparison of directional coronary atherectomy and stenting versus stenting alone for the treatment of de novo and restenotic coronary artery narrowing. Am J Cardiol. 2004;93:953-958.
13 Harrington RA, Lincoff AM, Califf RM, et al. Characetristics and consequences of myocardial infarction after percutaneous coronary intervention: insights from the Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT). J Am Coll Cardiol. 1995;25:1693-1699.
14 Lefkovits J, Blankenship JC, Anderson KM, et al. Increased risk of non-Q wave myocardial infarction after directional atherectomy is platelet dependent: evidence from the EPIC trial. J Am Coll Cardiol. 1996;28:849-855.
15 Bittl JA, Chew DP, Topol EJ, Kong DF, Califf RM. Meta-analysis of randomised trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherotomy, or laser angioplasty. J. Am. Coll. Cardiol. 2004;43:936-942.
16 Orlic D, Reimers B, Stankovic G, Corvaja N, Chieffo A, Airoldi F, Spanos V, Favero L, Di Mario C, Colombo A. Initial experience with a new 8 French-compatible directional atherectomy catheter: immediate and mid-term results. Catheter Cardiovasc Interv. 2003;60:159-166.
17 Fischell TA, Drexler H. Pullback atherectomy (PAC) for the treatment of complex bifurcation coronary artery disease. Cathet Cardiovasc Diagn. 1996;38:218-221.
18 Veinot JP, Ma X, Jelley J, O’Brien ER. Preliminary clinical experience with the pullback atherectomy catheter and the study of proliferation in coronary plaques. Can J Cardiol. 1998;12:1457-1463.
19 Repetto A, Ferlini M, Ferrario M, Angoli L, Bramucci E. Directional coronary atherectomy in 2005. Ital Heart J. 2005;6:494-497.
20 Kiesz RS, Rozek MM, Ebersole DG, Mego DM, Chang CW, Chilton RL. Novel approach to rotational atherectomy results in low restenosis rates in long, calcified lesions: long-term results of the San Antonio Rotablator Study (SARS). Catheter Cardiovasc Interv. 1999;48:48-53.
21 Kini A, Reich D, Marmur JD, Mitre CA, Sharma SK. Reduction in periprocedural enzyme elevation by abciximab after rotational atherectomy of type B2 lesions: results of the Rota ReoPro randomised trial. Am Heart J. 2001;142:965-969.
22 Reifart N, Vandormael M, Krajcar M, et al. Randomized comparison of angioplasty of complex coronary lesions at a single center. Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison (ERBAC) Study. Circulation. 1997:91-98.
23 Dill T, Dietz U, Hamm CW, et al. A randomised comparison of balloon angioplasty versus rotational atherectomy in complex lesions (COBRA study). Eur Heart J. 2000;21:1759-1766.
24 Mauri L, Reisman M, Buchbinder M, et al. Comparison of rotational atherectomy with conventional balloon angioplasty in the prevention of restenosis of small coronary arteries: results of the Dilatation vs Ablation Revascularization Trial Targeting Restenosis (DART). Am Heart J. 2003;145:847-854.
25 Brown DL, George CJ, Steenkiste AR, et al. High-speed rotational atherectomy of human coronary stenoses: acute and one-year outcomes from the New Approaches to Coronary Intervention (NACI) registry. Am J Cardiol. 1997;80:60K-67K.
26 Whitlow P, Bass T, Kipperman R, et al. Results of the study to determine rotablator and transluminal angioplasty strategy (STRATAS). Am J Cardiol. 2001;87:699-705.
27 Safian R, Feldman T, Muller D, et al. Coronary angioplasty and Rotablator atherectomy trial (CARAT): immediate and late results of a prospective multicentre randomised trial. Catheter Cardiovasc Interv. 2001;53:213-220.
28 Goldberg SL, Berger P, Cohen DJ, et al. Rotational atherectomy or balloon angioplasty in the treatment of intra-stent restenosis: BARASTER multicenter registry. Catheter Cardiovasc Interv. 2000;51:407-413.
29 Mehran R, Dangas G, Mintz GS, et al. Treatment of in-stent restenosis with excimer laser coronary angioplasty versus rotational atherectomy: comparative mechanisms and results. Circulation. 2000;101:2484-2489.
30 Sharma SK, Kini A, Mehran R, Lansky A, Kobayashi Y, Marmur JD. Randomized trial of totational atherectomy versus balloon angioplasty for diffuse in-stent restenosis (ROSTER). Am Heart J. 2004;147:16-22.
31 vom Dahl J, Dietz U, Haager PK, et al. Rotational atherectomy does not reduce recurrent in-stent restenosis results of the angioplasty versus rotational atherectomy for treatment of diffuse in-stent restenosis trial (ARTIST). Circulation. 2002;105:583-588.