Chapter 248 Roseola (Human Herpes Viruses 6 and 7)
Clinical Manifestations
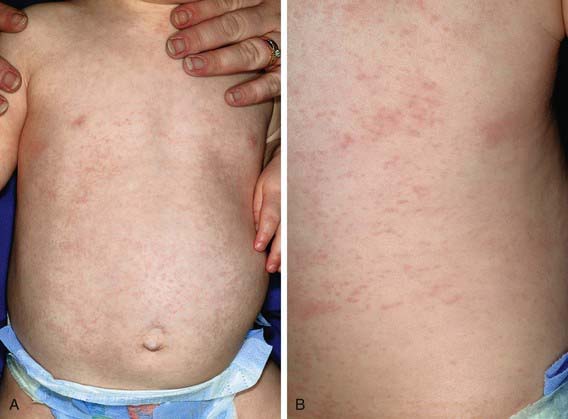
Roseola infantum (exanthem subitum, or sixth disease) is an acute, self-limited disease of infancy and early childhood. It is characterized by the abrupt onset of high fever, which may be accompanied by fussiness. The fever usually resolves acutely after 72 hr (“crisis”) but may gradually fade over a day (“lysis”) coincident with the appearance of a faint pink or rose-colored, nonpruritic, 2- to 3-mm morbilliform rash on the trunk (Fig. 248-1). The rash usually lasts 1-3 days but is often described as evanescent and may be visible only for hours, spreading from the trunk to the face and extremities. Because the rash is variable in appearance, location, and duration, it is not distinctive. Associated signs are sparse but can include mild injection of the pharynx, palpebral conjunctivae, or tympanic membranes and enlarged suboccipital nodes. In Asian countries, ulcers at the uvulopalatoglassal junction (Nagayama spots) are commonly reported in infants with roseola.
Caserta MT, Hall CB, Schnabel K, et al. Primary human herpesvirus 7 infection: a comparison of human herpesvirus 7 and human herpesvirus 6 infections in children. J Pediatr. 1998;133:386-389.
De Bolle LL, Naesens De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
Fotheringham J, Donati D, Akhyani N, et al. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 2007;4:e180.
Hall CB, Caserta MT, Schnabel K, et al. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics. 2008;122:513-520.
Hall CB, Long CE, Schnabel KC, et al. Human herpesvirus-6 infection in children: a prospective study of complications and reactivation. N Engl J Med. 1994;331:432-438.
Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69:156-165.
Ward KN, Andrews NJ, Verity CM, et al. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child. 2005;90:619-623.
Ward KN. Human herpesviruses-6 and -7 infections. Curr Opin Infect Dis. 2005;18:247-252.
Ward KN. The natural history and laboratory diagnosis of human herpesviruses-6 and -7 infections in the immunocompetent. J Clin Virol. 2005;32:183-193.
Yao K, Honarmand S, Espinosa A, et al. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65:257-267.
Zerr DM. Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol. 2006;37:S52-S56.
Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.