Chapter 3 Role of Intact Biological Models for Evaluation of Radiotracers
INTRODUCTION
The development of radiolabeled tracers for clinical application in humans has been critically dependent on evaluation and testing in biological models. Although in vitro models have been important in the evaluation of radiotracers, this chapter will focus only on the use of intact models. Initial evaluation of a radiotracer generally focuses on assessment of in vivo organ selectivity as determined by biodistribution and pharmacokinetics.1 Often, radiolabeled compounds are evaluated in various species to ensure that behavior of a compound does not demonstrate important species differences. More important, radiotracers must be evaluated for specific organ pharmacokinetics in different disease processes.2
This chapter will define the role of intact biological models in the evaluation of radiotracers for use in the diagnosis and management of cardiovascular disease. Important species differences will be defined as they relate to cardiovascular disease. The selection of short-term versus long-term animal models will be discussed, with particular attention to the need for conscious or sedated models. Approaches for determination of myocardial radiotracer kinetics will be reviewed, including dynamic imaging (planar imaging, single-photon emission computed tomography [SPECT],3 or positron emission tomography [PET]), miniature detectors, serial biopsies, and postmortem tissue well counting or autoradiography. Special consideration will be given to imaging in small animals and the utilization of transgenic models. Approaches for evaluation of radiotracer biodistribution and myocardial radiotracer uptake and clearance will be reviewed briefly, with emphasis on the effect of cellular viability, flow, metabolic inhibitors, and pharmacologic stress on radiotracer kinetics.
SELECTION OF ANIMAL MODEL
Use of Small Mammals for Definition of Biodistribution
The first step in the evaluation of any radiopharmaceutical involves determination of the normal biodistribution of a radiotracer over time. These initial screening studies are usually performed in mice, rats, or rabbits to minimize cost. A measured dose is injected intravenously, and the percentage of uptake per gram of tissue is determined for critical organs.4–7 Uptake in the heart, the target organ, is compared with adjacent background structures at multiple time points after injection. Blood clearance can be easily derived by serial sampling of blood for gamma well counting. Tissue clearance curves can be derived either by sacrificing animals at different time points or by performing serial imaging. Segregation of tissue into cell fractions can provide information on tracer localization in specific cellular fractions.8 This information may offer insight into the mechanism of uptake and retention. The smaller hearts in these animals lend themselves to microautoradiography.
Use of Small Mammals for Evaluation of Specific Disease Processes (See Chapter 11)
A limited number of natural biological models are currently available in smaller mammals for evaluation of specific cardiovascular disease processes. The Syrian hamster model is an example of a small model for evaluation of radiotracer kinetics under cardiomyopathic conditions. Other researchers have developed rabbit models of congestive heart failure.9,10 Investigators have also created a chronic volume-overloaded condition by mechanically disrupting the aortic valve, instigating aortic insufficiency.11 It is more difficult to create a small-animal model of regional ischemia or infarction. However, this has been accomplished by placing a ligature or small clamp around a proximal coronary artery. In the mouse and rat, this occlusion can be done blindly with a small needle and suture through a thoracotomy.12,13 Unfortunately, this approach generally necessitates disruption of both venous drainage and arterial inflow. Regarding rat models of infarction, the use of Lewis rats may be preferable over other strains because of the improved survival and greater uniformity of myocardial injury.14 Several investigators have used a rabbit model of regional myocardial ischemia.15,16 In the rabbit, it is possible to selectively isolate and occlude a proximal coronary artery. Fujita et al. recently established a chronic rabbit model of myocardial infarction without the need for endotracheal intubation, which significantly improves survival.16 Production of graded ischemia in smaller animals is nearly impossible. The use of radiolabeled microspheres for independent assessment of regional myocardial blood flow is more difficult in these preparations. In addition, these preparations do not permit regional arterial-venous balance measurement for the independent assessment of regional metabolism. However, with the advent of dedicated small-animal imaging systems, quantitative evaluation of radiotracers in these physiologic small-animal models will become more feasible.17,18
Use of Genetically Engineered Small Mammals for Evaluation of Specific Biological Processes and Radiotracers (See Chapter 45)
The ability to selectively alter gene expression permits the evaluation of the significance of certain gene products for structure-function studies of cardiac proteins and their role in cardiovascular disease. These transgenic models facilitate the evaluation of targeted radiotracers in relevant biological models. Hundreds of mutant mouse strains and also a few mutant rat and rabbit strains have been generated. The number of genetically engineered mouse lines for cardiovascular research is continuously growing. Recent reviews have provided a summary of important new genetically modified animals with cardiovascular disease.19–27 Transgenic mice are most frequently used for this purpose, since mice breed rapidly, the maintenance costs are lower, and our knowledge of mouse genetics is far more advanced. Although the small size of these animals leads to significant challenges in the evaluation of radiotracers, there have been significant advances in small-animal SPECT and PET instrumentation that make imaging of mice feasible.17,18
Approaches for creation of transgenic animals are nicely reviewed by Williams and Wagner in their application for evaluation of integrative biology.28 However, the use of transgenic animals will be equally useful for the development and testing of biologically targeted radiotracers. Once targeted radiotracers, are established, they could also be used to evaluate new transgenic strains in conjunction with radiotracers which provide information about physiologic processes (i.e., perfusion, metabolism). Thus, the use of radiotracer imaging would also permit phenotyping of transgenic animals.
There are two basic approaches to mouse genomic manipulation: random chromosomal integration, which can be used for addition of an exogenous transgene, and homologous recombination of foreign DNA, which leads to targeted mutation of an endogenous gene.28
Random chromosomal integration is based on addition of DNA into fertilized oocytes in order to generate “gain-of-function” mutations, in which the transgene is overexpressed.28 Another common application of random chromosomal integration of transgenes is to identify transcriptional control elements that respond to physiologic stimuli. In this application, the coding region of a so-called reporter gene is linked to the regulatory elements of interest. By definition, reporter genes encode biologically innocuous proteins that are easily detected. The goal of this approach is to avoid perturbation of the cell in which the reporter is expressed while assessing the function of transcriptional regulatory sequences that are attached to the reporter gene. For application with radiotracer imaging, these reporter genes are linked with a radiolabeled “reporter probe” that can be detected in vivo in mice with microPET or microSPECT imaging systems.
Gambhir et al. reported methods for monitoring reporter gene expression by using herpes simplex virus type I mutant thymidine kinase (HSV1-sr39tk) as a PET reporter gene and 9-(4-[18F]-fluoro-3 hydroxymethylbutyl) guanine ([18F]-FHBG) as a PET reporter probe in animal models.29 Wu et al. applied this technology using microPET imaging to noninvasively monitor cardiac reporter gene expression in rats.30 More recently, Wu et al. applied this approach for the noninvasive assessment of myocardial response to cell therapy using embryonic cardiomyoblasts expressing HSV1-sr39tk and/or firefly luciferase.31 The location, magnitude, and survival duration of the transplanted cells were monitored noninvasively using PET and bioluminescence optical imaging.
Gene targeting via homologous recombination in embryonic stem cells is frequently used to create “loss-of-function” mutations, known as knockouts. Targeted inactivation can be accomplished by introducing a positive selection marker, which will disrupt gene structure. A conditional gene targeting approach is preferred, since this approach provides cell-type-specific and/or inducible gene targeting. One conditional gene targeting approach involves Cre/loxP-mediated targeted mutagenesis.32 This approach is based on the ability of Cre recombinase to recognize a unique nucleotide sequence (loxP site), allow the introduction of mutations in the gene of interest, and by the controlled expression of Cre also control the expression during different time points and avoid germline mutations that may be lethal. Alternatively, temporal or cell-type-specific transcriptional control can be accomplished using a tetracycline-responsive promoter.33
Use of Large Mammals for Evaluation of Specific Disease Processes
Dogs, pigs, and sheep are the large mammals most commonly used for the evaluation of cardiovascular physiology. These models have been used for many years, and the methodologies of their use are well established. The higher cost of large-animal models almost precludes their use for simple biodistribution studies; rather, they are mostly used to evaluate radiotracer kinetics in specific disease processes. In the evaluation of radiotracers, dogs and pigs have been most commonly used. Important interspecies differences in radiotracer uptake have been observed over the years. The most notable example is the uptake of technetium-99m (99mTc) 1,2-bis(dimethylphosphino)ethane. This 99mTc-labeled perfusion tracer showed favorable uptake characteristics in rats, dogs, rabbits, and monkeys; however, in phase I trials, no appreciable uptake was noted in the hearts of pigs or humans. Although there was transient uptake of this radiotracer by human heart, trapping was not observed because of a reductive mechanism that is prominent in human myocardium.34
The dog has a dominant left coronary system. In almost all cases, the left circumflex coronary leads to the posterior descending coronary artery; very rarely does the right coronary artery provide any of the blood supply to the left ventricle. The coronary vascular tree in the dog is also highly collateralized. Therefore, some investigators claim that the dog is the ideal model of chronic coronary artery disease in humans, because patients with longstanding critical coronary disease frequently are highly collateralized. In addition, the dog is very tolerant of ischemic injury. In contrast, pigs and sheep have limited native collaterals and tolerate ischemic insults less reliably. When conducting radioisotope experiments in the dog, it may sometimes be necessary to tie off superficial epicardial collaterals to minimize the nearly instantaneous opening of epicardial collateral circuits. Dogs can also be trained to walk on a treadmill, providing an effective model for evaluation of pharmacologic stress agents.35
The pig has a coronary circulation that is more similar than that of the dog to human coronary circulation in that both the right and left coronary artery supply the left ventricle with blood. Pig coronary circulation systems have less native coronary collaterals, a characteristic akin to normal coronaries in humans. Therefore, the pig may be a better model of acute coronary occlusion in humans, because under conditions of acute occlusion, coronary collaterals may not have yet developed. In recent years, porcine models of ischemia, infarction, stunning, and hibernation have been established. However, sheep may provide the best model for studying chronic infarction and left ventricular remodeling.36
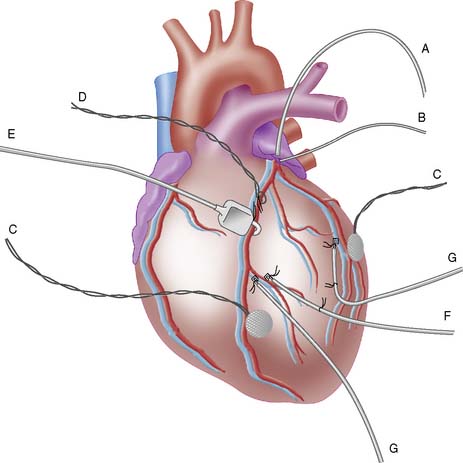
In all of these large-animal models, acute coronary occlusion may be created surgically37 or with a balloon angioplasty catheter under fluoroscopic guidance.38,39 In acute and chronic models, partial occlusions can be created with the aid of a hydraulic occluder or partially occluding ligature. A better way to achieve chronic partial or total occlusion may be placement of an ameroid occluder.40,41 An ameroid occluder is a small ring that is surgically placed around a vessel. This device slowly swells over 3 to 4 weeks. A complete or partial coronary occlusion is thus created very gradually, allowing for the development of coronary collaterals.
Large-animal models offer important advantages over studies in smaller animals. First, studies in larger animals allow for more complete instrumentation and regional interventions.42–47 Second, the distribution of a radiotracer can be easily evaluated with standard radionuclide imaging equipment.48,49 Finally, independent regional measures of flow, function, and metabolism are much easier to obtain in larger animals.50
Many physiologic issues can be addressed by using acute animal models. However, to evaluate changes over an extended period of time, chronic models must be used. Chronic models also offer the ability to evaluate radiotracer behavior under conscious conditions. Animals are usually surgically instrumented and allowed to recover for at least 1 week after surgery. After full surgical recovery, an ischemic insult is implemented or stress is applied while the animal is conscious.35,51
SELECTION OF ANESTHESIA
The cardiovascular effects of anesthesia in experimental animals have been previously reviewed.52 These effects vary with different species and with dosages. Opioids tend to decrease preload, contractility, afterload, and heart rate.52 Benzodiazepines induce taming effects in animals, facilitating imaging of chronically instrumented animals. These drugs may affect the cardiovascular system as a result of their central nervous system effects. Narcotic analgesics tend to have less cardiovascular effects than do general anesthetics. However, these agents require additional use of neuromuscular blocking agents. Inhalant anesthetics tend to decrease contractility, aortic pressure, and cardiac output and cause a compensatory increase in heart rate. Halothane is the most commonly used inhaled anesthetic for research purposes. Halothane at low end-tidal concentrations (1%) has little effect on the coronary circulation.
METHODS OF MEASUREMENT
Dynamic Imaging
Planar Imaging
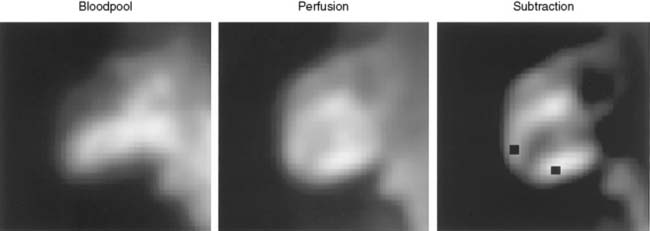
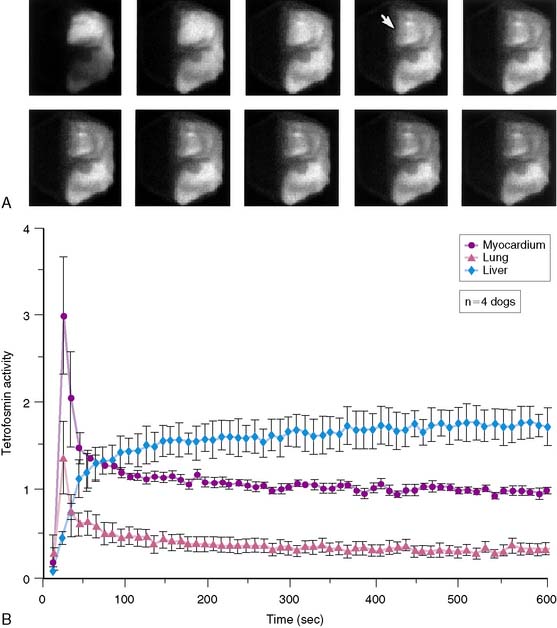
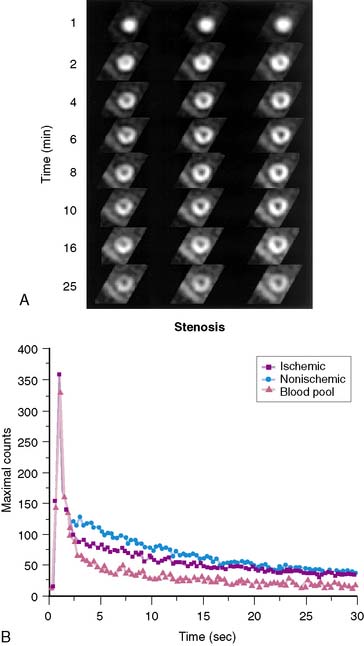
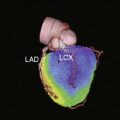
Planar imaging offers some advantages for evaluation of radiotracer pharmacodynamics, including high temporal and spatial resolution. Planar imaging of the heart is complicated by superimposition of activity in adjacent extracardiac structures. This can be obviated in open-chest models by isolation of the heart from other structures by using a flexible lead sheet. It is critical that the shielding material is placed directly under the heart, thereby completely isolating the beating heart from extracardiac structures. Time-activity curves can be derived for determination of blood and regional myocardial pharmacodynamics. In this type of kinetic analysis, placement of the regions of interest is critical. We have found that moving the myocardial region of interest one pixel toward or away from the blood pool can greatly alter the myocardial clearance curves. To facilitate optimal placement of the myocardial region of interest, we generate a reference image by subtracting an early blood-pool image from a later image (Fig. 3-1). An initial left ventricular blood-pool image can be derived by reconstruction of the left ventricular phase of the first-pass list mode acquisition. A myocardial perfusion image can be derived by summing the last 1 minute of the dynamic image sequence. The initial blood-pool image is then subtracted from the final perfusion image, providing an index myocardial image that is minimally contaminated by blood pool. The myocardial clearance curves generated by using this planar imaging approach can provide useful information on myocardial uptake and clearance of radiotracer in carefully controlled experimental models of regional ischemia or infarction. This model allows for selective arterial and venous sampling for simultaneous determination of regional metabolism or radiotracer extraction. Figure 3-2 shows a dynamic image series and corresponding clearance curves that can be derived from it.
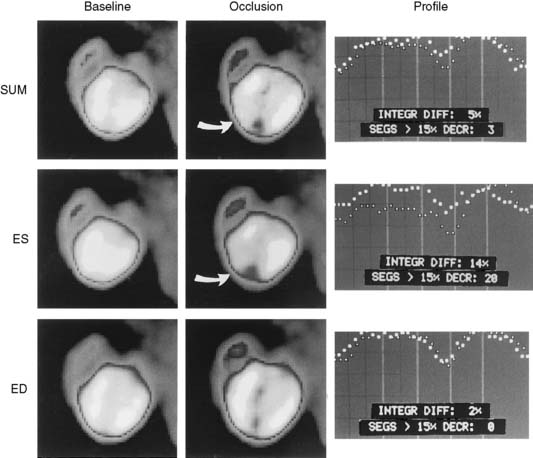
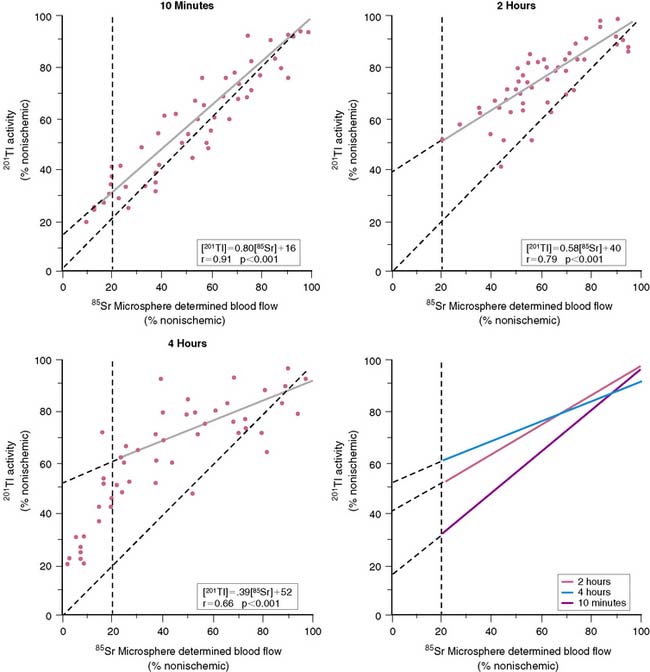
In 1979, it was recognized that distortion of global and regional left ventricular geometry could cause artifact defects in perfusion images obtained with thallium-201 (201Tl) or intracoronary administration of 99mTc-labeled albumin microspheres.53 Sinusas et al. subsequently demonstrated that changes in regional function may confound analysis of planar 99mTc-sestamibi images.44 Thus, changes in regional myocardial thickening may lead to misinterpretation of myocardial tracer uptake or clearance because of partial volume effects.44 Partial volume effects refers to the underestimation of count density from a structure that is thinner than twice the resolution of the imaging system (usually 12 to 20 mm for a gamma camera).54 In models of regional ischemia, systolic thinning may occur in ischemic or postischemic stunned regions. Differences in wall thickness would be most prominent at end systole. The potential impact of these partial volume errors are illustrated in Figure 3-3. We found that analysis of end-diastolic images may compensate somewhat for the partial volume effect associated with regional dyskinesis.
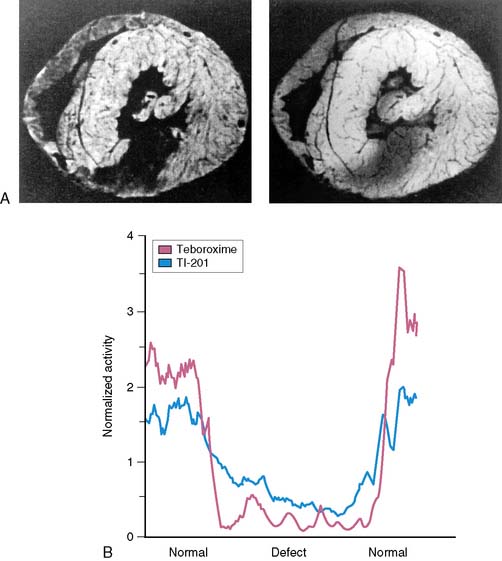
Dynamic planar imaging has been used by several investigators to establish the clearance kinetics of 201Tl,55 99mTc-sestamibi,56 99mTc-tetrofosmin,45 and 99mTc-teboroxime.57 Serial planar imaging permits nontraumatic serial assessment of changes in regional activity. However, dynamic planar imaging is limited by camera resolution, partial volume effects, and potential motion artifacts and may be influenced by background scatter and tissue cross-talk.
Dynamic planar images can also be obtained in animals as small as a mouse using high-resolution pinhole collimators.58 Figure 3-4 illustrates a series of planar pinhole images in a mouse injected with a 99mTc-labeled compound targeted at the αvβ3 integrin, and corresponding tissue clearance curves. Using an external radioactive reference source (~10 μCi), the regional counts within selected organs can be converted to μCi and expressed as a percentage of injected dose.
Single-Photon Emission Computed Tomography
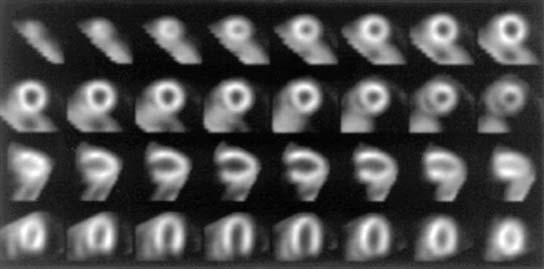
High-quality tomographic images can be acquired to evaluate the biodistribution of a radiotracer. Imaging by SPECT provides better separation of organs than planar imaging. Dynamic SPECT imaging has only recently become feasible with the development of multidetector cameras. Several investigators have used dynamic SPECT imaging for kinetic modeling.59–61 Stewart et al. performed dynamic tomographic imaging with 99mTc-teboroxime in closed-chest dogs by using a single-photon ring tomographic system.61 These investigators demonstrated monoexponential clearance of 99mTc-teboroxime with this approach. By using a triple-detector SPECT system, Smith et al. demonstrated the feasibility of acquiring dynamic 10-second tomographic data with a continuous acquisition protocol.60 Analysis of wash-in of 99mTc-teboroxime in myocardial tissue measured with this dynamic SPECT approach, in conjunction with compartmental modeling, provided an index of regional myocardial blood flow. Smith et al. also demonstrated that the use of attenuation correction in conjunction with dynamic SPECT was critical for compartmental analysis of 99mTc-teboroxime. Figure 3-5 provides an example of a series of high-quality images and corresponding clearance curves derived from dynamic SPECT in a dog. The potential advantage of kinetic modeling with SPECT over PET is the greater availability of SPECT equipment and reduced cost. However, the accuracy of the dynamic SPECT approach is not established.
Pinhole SPECT systems have been developed to evaluate regional properties of radiopharmaceuticals in small animals.62,63 Unfortunately, the ten-fold gain in resolution of these systems is at the expense of a 100-fold loss in sensitivity.64 This limitation has been overcome by using multidetector SPECT systems.62 Optimal reconstruction of the pinhole images will probably require more computer-intensive, three-dimensional, maximum-likelihood expectation-maximization (ML EM) algorithms.64 Although these systems cannot compete with the high resolution of microautoradiography, they may provide an alternative to macroautoradiography or well counting. Hirai et al. demonstrated the feasibility of serial SPECT with pinhole collimators for estimation of flow and metabolism in vivo in rat hearts.65 Wu et al. performed myocardial perfusion imaging of mice using 99mTc-sestamibi and demonstrated the feasibility for measurement of perfusion defect size from pinhole SPECT images (Fig. 3-6).31 Other investigators recently demonstrated the feasibility of electrocardiographic (ECG)-gated pinhole SPECT imaging in mice66 and rats67 for the evaluation of regional left ventricular function by applying standard clinical image analysis software to reconstructed ECG-gated microSPECT images.
High-speed and high-sensitivity SPECT cameras have recently become available that employ cadmium zinc telluride (CZT) detectors and novel schemes for reconstruction and collimation.68,69 The novel design of these new systems significantly increases sensitivity and reduces imaging time while providing higher spatial resolution than the conventional Anger camera approach.70,71 These systems will permit dynamic SPECT imaging, facilitating evaluation of the biodistribution and uptake and the clearance kinetics of new radiotracers under evaluation. They also offer the potential for absolute quantification of radiotracers. The increased sensitivity is at the cost of the size of the field of view, but for experimental imaging, these systems may be ideally suited for dynamic imaging of rabbits or focused imaging of larger animals. These systems should expand the horizons of SPECT imaging in experimental animal models.
Positron Emission Tomography (See Chapter 11)
Dynamic PET imaging in large-animal models plays an important role in evaluation of the kinetics of positron-emitting radiopharmaceuticals. However, this chapter will not review this topic. Technetium-94m (94mTc), a positron emitter with a 53-minute half-life, can be used to evaluate the biodistribution and pharmacokinetics of 99mTc radiopharmaceuticals for SPECT.72,73 Performing PET in animal models with Tc-labeled compounds allows in vivo evaluation of regional myocardial uptake and clearance kinetics. Figure 3-7 provides an example of 94mTc-sestamibi PET images. Imaging with PET offers great advantages in the evaluation of SPECT tracers: It provides high temporal and spatial resolution and reliable attenuation correction. Labeling of SPECT tracers with 94mTc allows the direct comparison of SPECT radiotracers with PET compounds.
Recently, several microPET imaging systems have become commercially available and are capable of performing dynamic PET imaging in mice and rats.74 However, accurate kinetic modeling with microPET requires a reliable arterial input function, which is difficult to obtain in mice. Some investigators have attempted to derive an input function using high-sensitivity detectors placed over an extracorporeal circuit, while others are designing precision devices for serial blood sampling.
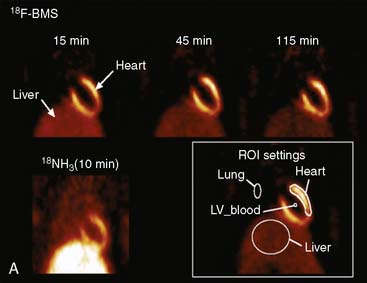
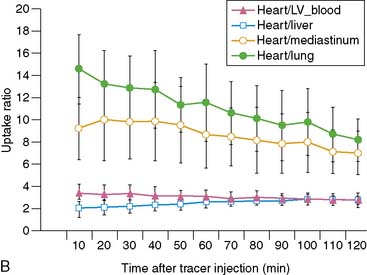
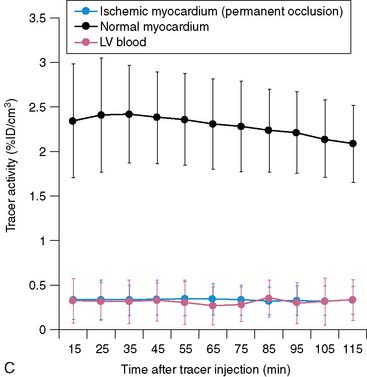
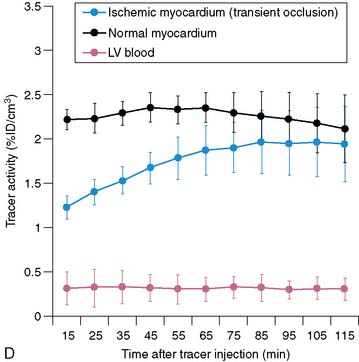
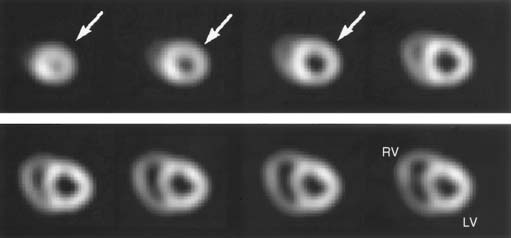
Serial microPET imaging was recently applied in rats following myocardial infarction and transient ischemia, as well as in control rats, to (1) evaluate the biodistribution and uptake and the clearance kinetics of a new 18F-labeled pyridazinone analog (18F-BMS-747158-02) under a range of physiologic conditions, and (2) define the changes in the uptake ratio of heart to adjacent organs (Fig. 3-8A-B). Serial microPET imaging was also used to define the regional uptake and clearance of normal myocardium and myocardium following permanent (see Fig. 3-8C) and transient (see Fig. 3-8D) coronary occlusion.
Miniature Detectors
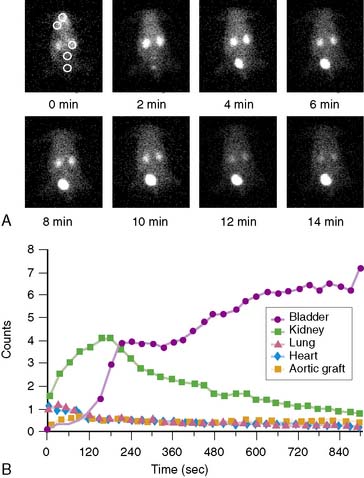
Myocardial radiotracer kinetics can also be derived in large open-chest animal preparations with the use of miniature radiation detectors. In many studies by Okada et al., miniature cadmium telluride radiation detectors were used to evaluate myocardial uptake and clearance of several radiopharmaceuticals, including 201Tl- and 99mTc-sestamibi.56,75,76 These probes allow continuous monitoring and display of regional myocardial activity.77 High-quality myocardial clearance curves derived by using a cadmium telluride detector are shown in Figure 3-9. The use of these probes can be complicated by changes in myocardial thickness and underlying blood pool beneath the probe and in the stability of the instrumentation. In some studies, probes have been placed on the myocardium in combination with a device to measure wall thickening. This system allows online correction of changes in counts associated with cyclic wall thickening. More recently, Stewart et al. measured myocardial clearance of 99mTc-teboroxime with a 1-inch collimated sodium-iodine (Tl) probe.57 The first component of 99mTc-teboroxime washout derived by using this probe was related to microsphere blood flow. This miniature probe approach allows continuous monitoring of regional myocardial activity under different ischemic conditions or during pharmacologic stress. Despite the potential limitations of these miniature probes, much insight into the in vivo behavior of 201Tl-labeled and newer 99mTc-labeled compounds has been derived from probe studies.
Serial Myocardial Biopsy
Investigators have obtained serial myocardial biopsy specimens after injection of a radiotracer to evaluate changes in absolute myocardial activity over time.78–80 This approach is the most accurate method to serially determine changes in absolute myocardial counts per gram. However, serial myocardial biopsy results in myocardial injury, necessitates the analysis of very small pieces of tissue, and has a potential sampling error.
Postmortem Imaging
Imaging of excised organs or tissues can be very useful to establish the final distribution of a radiotracer that has been injected in vivo under a specific physiologic condition. Some investigators have acquired high-resolution, tomography-like images by placing slices of the heart on the surface of a gamma camera (Fig. 3-10).43 Images acquired by using this method are similar to those obtained using macroautoradiography. The gamma camera approach enables the simultaneous acquisition of high-resolution images by using multiple energy windows. Investigators have used this method to compare the distribution of perfusion and metabolic tracers labeled with different radioisotopes. Effective application of this method necessitates sectioning the heart in slices of uniform thickness, which requires an automated slicing device. Alternatively, a complete three-dimensional set of short-axis images of the intact heart can be acquired ex vivo with a SPECT camera (Fig. 3-11). This eliminates the need for precision slicing of the heart and avoids the problems of attenuation and motion. To simulate the in vivo geometry of the heart, hearts are generally stuffed with gauze, frozen in a distended state, or filled with inert dental molding material. All methods of postmortem imaging eliminate partial volume errors associated with cardiac deformation. Images derived using this method can be easily quantified and readily compared with postmortem histochemical stains.
Autoradiography
Microautoradiology and macroautoradiography have been critical in the development and evaluation of radiopharmaceuticals.11,43,81,82 One of the major limitations of this approach is that each animal provides data for only a single experimental time point, possibly two if dual-isotope autoradiographs are acquired. However, these approaches also offer several advantages.
Microautoradiography
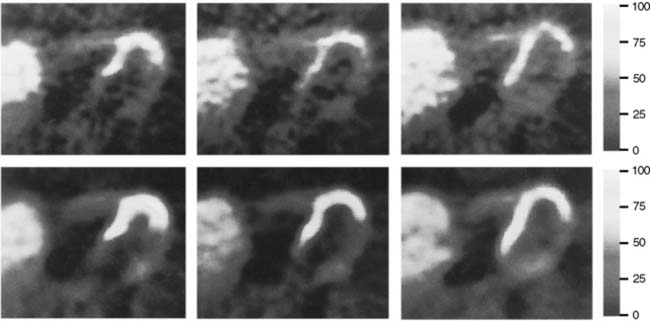
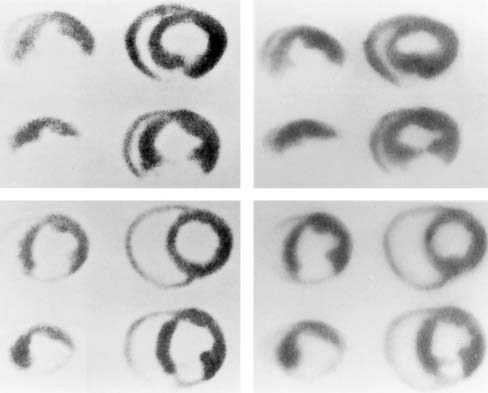
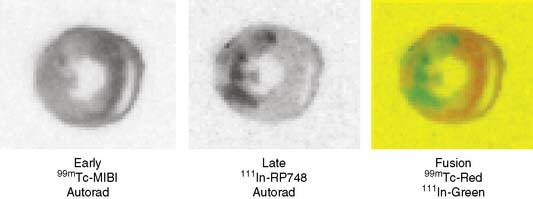
Microautoradiography permits high-resolution assessment of the distribution of radioactivity within an organ. This approach allows several isotopes to be compared directly if they can be separated on the basis of differences in half-life. The appropriate use of this technique requires preparation of tissue standards for each isotope in exponentially increasing activity concentrations.82,83 Tissue standards are necessary to convert autoradiographic intensity into tracer activity. Digitized autoradiographs can be quantitatively analyzed if the appropriate standards are used; the details of this approach have been reported elsewhere.81 Figure 3-12 provides an example of digitized dual-isotope microautoradiographs and quantitative profiles comparing the myocardial distribution of 201Tl- and 99mTc- teboroxime in a rabbit model of acute coronary occlusion. Figure 3-13 provides another example of dual-isotope microautoradiography using 99mTc-sestamibi and an 111In-labeled agent (111In-RP748) targeted at the αvβ3 integrin in a rat following occlusion and reperfusion resulting in nontransmural infarction. Uptake of 111In-RP748 demonstrates increased expression and activation of the αvβ3 integrin in angiogenic vessels within the infarct region as defined by 99mTc-sestamibi.
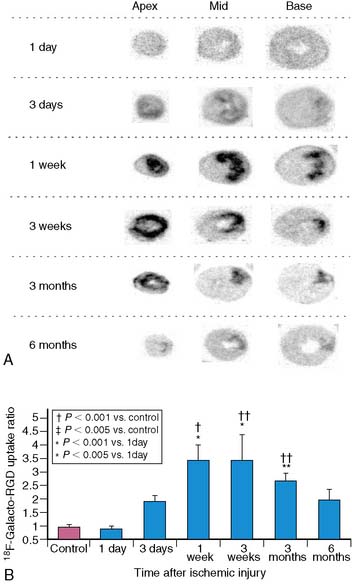
Digital microautoradiography can also be performed with 18F-labeled PET radiotracers using 20-μm thick frozen sections placed in a specimen imaging system capable of detecting either beta or gamma radiation.7,84,85 Higuchi et al. used a specimen imaging system to obtain digital microautoradiographs in rat hearts to determine the time course of an [18F]galacto-RGD compound for targeted imaging of the αvβ3 integrin following myocardial infarction (Fig. 3-14).7
The resolution of autoradiographs depends on the energy level of the tracers and the thickness of the sections.86 Hearts are typically cut into sections that are 10 to 50 μm thick. For quantitative analysis, a high-precision cryotome is required, and the thickness of the specimen must be uniform. Spatial resolution is improved if the specimen is firmly pressed onto the film. This technique is relatively economical because a gamma well counter is not required.
Macroautoradiography
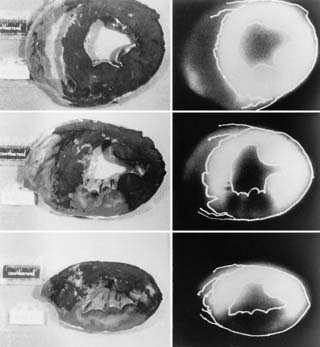
Lower-resolution macroautoradiographs can be more easily obtained in larger animal hearts.43 This approach does not even require a cryotome. Thin slices (2 to 5 mm) can be obtained with an inexpensive meat slicer. These myocardial slices are laid flat on cardboard, covered in plastic wrap, and inverted on x-ray film. Macroautoradiographs obtained in this manner can provide information on the spatial distribution of a radiotracer under experimental conditions of low-flow ischemia, myocardial stunning, or infarction. As shown in Figure 3-15, macroautoradiographs can be directly compared with postmortem stains to relate radiotracer uptake to myocardial viability.
Postmortem Tissue Well Counting
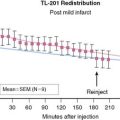
Tissue is counted in a gamma well counter, which has excellent energy discrimination and permits simultaneous evaluation of as many as six radiotracers if each one has gamma emissions at distinguishable energy ranges. Activity spillover from the energy window of one radiotracer to another can be corrected with a matrix derived from counting pure specimens of each radiotracer. This approach has been described in detail by Heymann et al.87 and has been applied by many other investigators.88 Identification of 201Tl redistribution was accomplished using this approach (Fig. 3-16).89
Gamma well counting in postmortem tissue has obvious advantages and disadvantages. A major disadvantage is that the myocardial distribution of a radiotracer can only be determined at discrete time points. In addition, a given animal can be examined only once. Finally, the resolution of this approach is dependent on how finely the heart is sectioned, usually in 0.3-gm samples. The tissue counting approach is very reproducible and avoids interference of background activity. To facilitate comparisons of results between animals, myocardial radiotracer activity is usually normalized. One way to normalize activity is to express activity in each segment as a percentage of a predefined normal region.42 This method enables evaluation of the myocardial activity within an ischemic or infarcted region relative to a normal region in a manner analogous to the interpretation of clinical images. Alternatively, regional myocardial activity can be normalized by the computed average activity for each heart, as proposed by Yipintsoi et al.90 A less desirable approach normalizes activity in each myocardial segment according to the segment with the highest activity.91
PHYSIOLOGIC MODELS
Myocardial Infarction
It has been well established in canine models of coronary occlusion that myocardial necrosis progresses from the subendocardium to the subepicardium in a wavefront manner.37,92 Advancing myocardial necrosis can be halted by coronary reperfusion. In canine models, permanent cellular injury begins after approximately 40 minutes of occlusion. The extent of necrosis depends on the area at risk and the duration of occlusion. In dogs, the maximum degree of infarction occurs after about 6 hours of occlusion. However, the extent of infarction may be affected by heart rate and left ventricular loading conditions. Thus, in anesthetized models, the method of anesthesia may affect infarct size. Myocardial flow following reperfusion varies and is critically dependent on timing after reperfusion, because a period of hyperemic flow is often seen following release of an occlusion.
Connelly et al. reported that complete coronary occlusion in the rabbit produced an expanding necrotic wavefront from the subendocardium.93 This effect is similar to the phenomenon first described by Reimer and Jennings in the canine model of occlusion.92 In the rabbit, mild subendocardial necrosis can be detected by nitroblue tetrazolium staining as early as 15 minutes after occlusion; 1 hour of coronary occlusion consistently produces completed transmural infarction.
Coronary occlusion can be produced by various noninvasive and invasive methods.52 Noninvasive closed-chest approaches include sustained coronary occlusion with angioplasty catheters38,39; selective coronary embolization94; and deployment of intracoronary copper coils,95 which promote thrombosis. Some of these approaches permit subsequent reperfusion. More invasive surgical approaches involve acute occlusion with a surgical ligature37,92 or more gradual occlusion after implantation of an ameroid occluder.96
Small-rodent models of surgical myocardial infarction are now widely used in the evaluation of the pathophysiology of infarction and postinfarction remodeling97–101 and have only recently been used for evaluation of targeted radiotracers following infarction.12,13,18,102 These small-animal imaging studies will be facilitated by the availability of high-resolution microSPECT and microPET imaging systems. Larger experimental models of coronary occlusion and reperfusion are more frequently used to assess the behavior of radiolabeled perfusion tracers under conditions of reduced flow and in the presence of myocardial necrosis. The myocardial uptake and retention of a perfusion tracer is critically dependent on the timing of injection relative to reperfusion.79,103 Appropriate use of experimental occlusion-reperfusion models allows analysis of the uptake and clearance characteristics of a radiotracer in relation to cellular viability independent of flow.43 These models have also been used to evaluate sympathetic innervation of the heart after coronary occlusion and reperfusion with iodine-123 (123I) meta-iodobenzyl guanidine39 or to examine myocardial metabolism with radiolabeled tracers of aerobic or anaerobic metabolism.38,104–106
A model of chronic congestive heart failure can be produced by repeated coronary embolization94,107 or by surgery.108 Animals with extensive infarction producing extensive myocardial necrosis can be created by repetitive transmyocardial direct current shocks.109
Myocardial Stunning
Myocardial stunning is characterized by a condition of postischemic mechanical dysfunction that persists after reperfusion despite the absence of irreversible injury. In the dog, a coronary occlusion lasting less than 20 minutes does not result in any myocardial necrosis; however, it produces regional dysfunction that may persist for hours.110,111 Myocardial stunning can also be produced by repeated brief (5- or 10-minute) coronary occlusions.112 In other species with fewer coronary collaterals, much briefer periods of coronary occlusion result in myocellular necrosis. Myocardial stunning can also occur after a prolonged partial coronary occlusion,113 in association with nontransmural infarction,114 or with exercise-induced ischemia.115
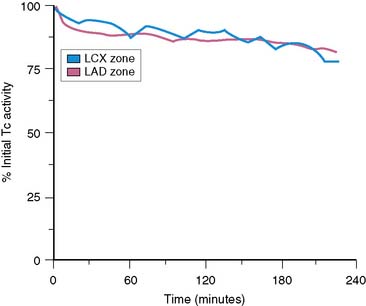
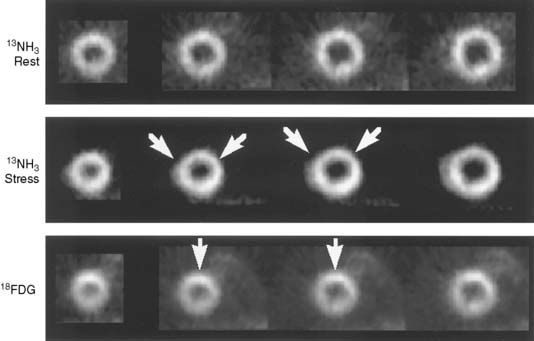
We recently developed a long-term canine model of ameroid-induced gradual subtotal coronary occlusion resulting in regional myocardial dysfunction (Fig. 3-17). Although resting flow was normal in these animals, on PET imaging with nitrogen-13 ammonia, coronary flow reserve was markedly impaired in response to adenosine. This model matches the clinical scenario reported by Vanoverschelde et al. in which reversible left ventricular dysfunction was identified in patients with viable collateralized occluded coronary arteries with preserved resting flow.116 In our canine model, dysfunctional regions with impaired coronary flow reserve also demonstrate increased fluorine-18 deoxyglucose uptake, similar to the clinical condition reported by Camici et al.117 These observations support the contention that the chronic regional dysfunction may be caused by repetitive stress-induced ischemia in the setting of normal resting flows and impaired flow reserve. This chronic model should prove useful in the evaluation of radiolabeled fatty acids such as [123I]β-methyl-iodophenyl pentadecanoic acid (BMIPP), which are reportedly “memory” markers of reversible ischemic injury.106
Myocardial Hibernation (See Chapter 36)
The term myocardial hibernation has generally been applied to patients with coronary artery disease who have chronically depressed left ventricular function in the absence of myocardial infarction that improves after coronary revascularization. It is unclear whether chronic left ventricular dysfunction in humans represents an adaptive response to chronic reduction in coronary blood flow.118 Impairment of left ventricular function under these conditions has been regarded as a protective mechanism by which the heart down-regulates its energy requirements, thereby preventing irreversible myocardial injury.
Short-term canine and porcine studies have successfully demonstrated sustained (1- to 5-hour) matching of reduced flow and function.113,119,120 We also developed a short-term canine model of sustained low-flow ischemia (Fig. 3-18).42,47,121 A hydraulic occluder is placed around a proximal coronary artery, allowing for the creation of a variable stenosis. The distal coronary artery is cannulated with a non–flow-obstructing catheter, which permits accurate regulation of the stenosis severity on the basis of measurement of distal coronary artery pressure and transstenotic pressure gradient. The degree of ischemic dysfunction can be assessed by measuring regional myocardial thickening with epicardial Doppler thickening probes.
Changes in regional metabolism can also be assessed in this model by selective venous sampling for arterial-venous balance measurements. Additional application of atrial pacing or intravenous administration of inotropic agents can provide a model of stress-induced ischemia as it may occur in patients with critical coronary artery disease.47 Many investigators have used this type of low-flow model to evaluate radiolabeled tracers of perfusion, myocardial metabolism, and tissue hypoxia.42,47,89,122 Using this experimental model of sustained low flow, the perfusion tracer 99mTc-NOET (bis[N-ethoxy, N-ethyl dithiocarbamato]-nitrido 99mTc) demonstrates substantial redistribution,122 as was previously observed for 201Tl89– and 99mTc-sestamibi.46
The condition of chronic low-flow perfusion-contraction matching has been more difficult to model experimentally. Recently, investigators succeeded in producing regional dysfunction over extended periods in pigs and dogs.123–125 In each of these studies, the severity of regional dysfunction was out of proportion to the reduction in regional myocardial blood flow. In a clinical study, Vanoverschelde et al. suggested that chronic regional myocardial dysfunction in the presence of relatively preserved resting coronary flow may be attributed to repetitive stunning associated with intermittent stress-induced ischemia.116 The presence of 201Tl rest redistribution in patients with reversible left ventricular dysfunction supports the idea that true myocardial hibernation with chronic resting reduction in flow can and does occur.126 Whereas a resting defect may be attributed to partial volume errors, the presence of 201Tl redistribution suggests differential myocardial clearance that is most likely secondary to reduced resting myocardial flow. Additional imaging studies in animal models of chronic hibernation are needed to establish the pathophysiology of reversible ischemic dysfunction in the presence of coronary artery disease. In an excellent recent review, Canty and Fallavollita suggest that chronic stunning and chronic hibernation represent the extremes of a continuum.127 They present the recent experimental studies that demonstrated that there is a progression from chronic stunning with normal flow to hibernating myocardium with reduced resting flow. The application and evaluation of new metabolic radiotracers in these models will be critical for advancing our understanding of this important clinical condition. These low-flow models also will be invaluable in assessing new radiolabeled perfusion tracers.
Dilated Cardiomyopathy
The value of animal models in the evaluation of cardiomyopathies was recently reviewed by Yarbrough and Spinale.128 Although several landmark discoveries regarding the pathophysiology of cardiomyopathies have arisen from small-animal models, the proof of concept must ultimately be reproduced in animal species that more closely mimic human physiology, function, and anatomy.128 While small-animal models allow the identification of potential novel imaging targets, larger-animal models facilitate the direct translation of radiotracer imaging approaches to applications in patients with cardiomyopathies.
Many canine species are known to have idiopathic dilated cardiomyopathies.52 This is seen almost exclusively in the larger breeds of dogs and is characterized by biventricular cardiac dilation. Dilated cardiomyopathies have also been seen in domestic cats, and there is a cardiomyopathic strain of Syrian hamsters. Several investigators have developed experimental models of congestive heart failure associated with ventricular dilation. Chronic rapid atrial or right ventricular pacing in the dog or pig produces dilated cardiomyopathy.129–131 Chronic mitral insufficiency also results in dilated cardiomyopathy. This can be accomplished surgically or noninvasively by cutting chordae tendineae of the mitral valve with a urologic forceps.88,132 Other investigators have created a volume-overloaded state by creating aortic insufficiency.
Magid et al. developed a rabbit model of chronic aortic insufficiency.133 The regurgitant fraction and resulting left ventricular dilation and hypertrophy produced by this rabbit model are similar to those reported in severe aortic insufficiency in human beings. Lu et al. used this model to evaluate the value of 111In-labeled antimyosin antibody Fab fragment imaging for identification of myocyte necrosis associated with chronic aortic insufficiency.11 These types of experimental studies help to generate hypotheses about the potential role of radionuclide imaging in the evaluation and management of patients with acute or chronic aortic insufficiency.
Atherosclerosis (See Chapter 44)
Several recent reviews have summarized the many models of atherosclerosis that are available for testing of radiotracers targeted at critical components of the atherosclerotic process.134,135 Animal models of atherosclerosis generally involve use of a high-fat diet in combination with endothelial injury. Vascular injury can be produced by direct physical injury, chemical injury, or immunologic injury. Some animals develop atherosclerosis as a naturally occurring disease: The Watanabe heritable hyperlipidemic rabbit, for example, has a natural LDL-receptor deficiency and provides a natural model for homozygous familial hypercholesterolemia.136 The St. Thomas’ Hospital (STH) strain of rabbits resembles human triglyceridemia and combined hyperlipidemia and also develops advanced atherosclerotic lesions.137 The most commonly used animal model of atherosclerosis is the high-cholesterol-diet-fed rabbit.52 The pig is also commonly used as a model of atherosclerosis. Vallabhajosula et al. developed a novel porcine model of complex coronary atherosclerosis that employs balloon injury followed by an injection of cholesterol esters into the coronary arteries of healthy pigs, creating coronary lesions (15 mm in length).138 These investigators demonstrated that the uptake of [18F]fluorodeoxyglucose (FDG) in the coronary lesions was dependent on the progression of the lesion. Within the first 2 to 3 months after the induction of lesion, FDG uptake was seen in some of the lesions. But after 4 months, most of the lesions were clearly identified by FDG. To reduce the problems associated with use of this large species, miniaturized breeds of swine have been developed. Nonhuman primates are considered to provide a model of atherosclerosis that is most similar to the atherosclerosis seen in humans. Limitations of the nonhuman primate include expense and the rapid development of atherosclerotic lesions.
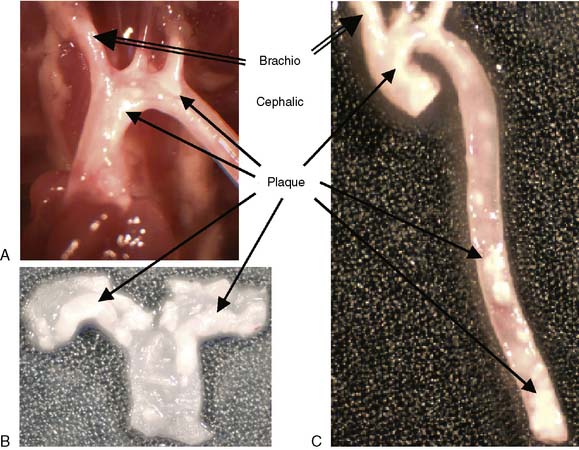
The transgenic/knockout animal models have greatly enhanced our understanding of atherosclerosis. The most widely used mouse models of atherosclerosis are apolipoprotein E–deficient (ApoE−/−), LDL receptor–deficient (LDLr−/−), and ApoE*3Leiden (E3L).26 These models may differ strikingly from one another in their response to specific experimental manipulations. The ApoE−/−, mouse has become an established model of hypercholesterolemia and spontaneous atherosclerotic lesion development.139,140 ApoE mice demonstrate lesions of all phases of atherosclerosis throughout the arterial tree.141 Williams et al. more recently demonstrated that the brachiocephalic lesion that develops in ApoE-deficient mice is characteristic of the unstable plaque seen in human patients.142 The brachiocephalic lesions that develop in the ApoE-deficient mice are characterized by large plaques with a thin fibrous cap and increased lipid core. These are all features associated with plaque rupture. Lesions in this region are also characterized by multiple buried caps, suggesting repeated rupture, and support a higher rupture rate of the brachiocephalic region compared with other vascular segments in these mice. The ApoE mouse fed a high-fat diet develops lesions similar to those in humans. Therefore, the ApoE model provides an excellent approach for evaluation of radiotracers targeted at unstable components of the atherosclerotic plaque. Figure 3-19 illustrates the gross atherosclerotic lesions that develop in ApoE-deficient mice.
The LDLr−/− mouse model develops atherosclerosis, particularly following feeding with a lipid-rich diet. E3L mice have a mutant form of the human apoE3 gene and develop atherosclerosis when fed a high-cholesterol diet. These E3L mice are more sensitive to lipid-lowering drugs then either the ApoE−/− or LDLr−/− mice.26
Mouse models have proved useful in the study of atherosclerosis, but differences in anatomy, lipid metabolism, and gene expression complicate translation of experimental results obtained in mice to humans.26
Models of atherosclerosis have been used to evaluate a number radiotracers targeted at the vulnerable plaque.143,144 As outlined earlier, plaques vulnerable to rupture typically have a large, necrotic lipid core and an attenuated fibrous cap that are significantly infiltrated by macrophages and lymphocytes. There is emerging evidence that apoptosis contributes to the instability of the atherosclerotic lesion. Apoptosis of macrophages contributes substantially to the size of the necrotic core,145 whereas apoptosis of smooth muscle cells results in thinning of the fibrous cap.146 Annexin V can be radiolabeled with 99mTc and has been previously used for the noninvasive detection of apoptosis in myocardial infarction and inflammatory myocardial diseases. Kolodgie et al. applied annexin V planar in vivo and ex vivo imaging in experimental atherosclerotic lesions in hypercholesterolemic rabbits for noninvasive detection of atherosclerosis.143 They demonstrated that annexin V targeted apoptotic macrophages within the atherosclerotic plaque and may provide an attractive imaging agent for the noninvasive detection of unstable atherosclerotic plaques. Narula et al. also demonstrated the feasibility of noninvasive visualization of experimental atherosclerotic lesions with a mouse/human chimeric antibody Z2D3 F(ab′)2 directed against proliferating smooth-muscle cells.147 Narula et al. subsequently demonstrated that 111In-Z2D3 antibody imaging could be used for noninvasive assessment of the rate of smooth-muscle cell proliferation after coronary angioplasty.148 Other investigators using a rabbit model of atherosclerosis demonstrated that radiolabeled MCP-1 may also be a useful tracer for imaging monocyte/macrophage-rich experimental atherosclerotic lesions.144
MODELS OF PLAQUE RUPTURE
There is no single experimental model that perfectly replicates plaque rupture in man.149 Some of the available small- and large-animal models of plaque rupture are summarized in Table 3-1. The large-animal models are essentially models of atherosclerosis. The small-animal models of induced or spontaneous plaque rupture have the limitation that the lesions formed are usually in non-coronary vessels. Despite these limitations, genetically modified mice—particularly apolipoprotein E knockout mice—are routinely used to develop and evaluate radiotracers for identification of vulnerable plaque.
| Species | Manipulation | Refs |
|---|---|---|
| Mouse | ApoE−/−, mechanical injury ApoE−/−, non-constrictive carotid collar, p53 adenovirus ApoE−/−, constrictive carotid collar ApoE−/−, high-fat diet plus cholesterol ApoE−/−, SR-BI−/− |
Reddick159 von der Thusen160 Cheng161 Johnson & Jackson162 Williams163 Johnson164 Braun165 |
| Rat | Heritable inducible hypertension | Herrera166 |
| Rabbit | Cholesterol diet, viper venom, histamine Cholesterol diet, implanted balloon catheter |
Conastantinides167 |
| Pig | Injection into arterial tunica media Heritable hyperlipidemia |
Granada170 Prescott171 |
Modified from Jackson CL, Benbow U, Galley DJ, Karanam S: Models of plaque rupture. Drug Discov Today Dis Models 4:171–175, 2007 (reprinted with permission).
MODELS OF AORTIC ANEURYSMS
Many mouse models of aortic aneurysms have been developed that use a diverse array of methods for producing the disease, including genetic manipulation and chemical induction.150 A common chemical-induced model involves the periaortic application of calcium chloride, which results in aortic dilation over a 2- to 4-week period. These models could provide insight into potential mechanisms in the development of this disease and in the process of vascular remodeling.151 These models have been used to evaluate radiotracers targeted at investigation of vascular remodeling.152
There are also large-animal models of aortic aneurysms. Hynecek et al. have developed a novel porcine model of abdominal aortic aneurysm.153 This porcine model has been shown to replicate the gene expression patterns that have been observed in human studies as well as in rodent models of aneurysms.154 These large-animal models may offer advantages for investigating the interrelationship of vascular remodeling, aneurysm expansion, and regional shear and vascular mechanics.
EVALUATION OF PHARMACOLOGIC STRESSORS
Radiotracers are often used in conjunction with pharmacologic stressors, particularly for the detection of coronary artery disease. Recent evidence suggests that radiotracer pharmacokinetics may be influenced by the type of pharmacologic stress applied.155–158 Therefore, biological models have an important role in evaluation of pharmacologic stressors.
1. Lambrecht R., Eckelman W. Animal models in radiotracer design. New York: Springer-Verlag, 1983.
2. Lambrecht R. Biological models in radiopharmaceutical development. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1996.
3. Bader M., Ganten D. Transgenic rats: tools to study the function of the renin-angiotensin system. Clin Exp Pharmacol Physiol Suppl. 1996;3:S81-87.
4. Pasqualini R., Duatti A., Bellande E., et al. Bis(dithiocarbamato)-nitrido technetium-99m radiopharmaceuticals: A class of neutral myocardial imaging agents. J Nucl Med. 1994;35:334-341.
5. Hatada K., Riou L.M., Ruiz M., et al. 99mTc-N-DBODC5, a new myocardial perfusion imaging agent with rapid liver clearance: Comparison with 99mTc-sestamibi and 99mTc-tetrofosmin in rats. J Nucl Med. 2004;45(12):2095-2101.
6. Higuchi T., Nekolla S.G., Huisman M.M., et al. A new 18F-labeled myocardial PET tracer: Myocardial uptake after permanent and transient coronary occlusion in rats. J Nucl Med. 2008;49(10):1715-1722.
7. Higuchi T., Bengel F.M., Seidl S., et al. Assessment of {alpha}v{beta}3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res. 2008;78(2):395-403.
8. Morishita S., Kusuoka H., Yamamichi Y., Suzuki N., Kurami M., Nishimura T. Kinetics of radioiodinated species in subcellular fractions from rat hearts following administration of iodine-123-labeled 15-(p-iodophenyl)-3-(R,S)-methylpentadecanoic acid (123I-BMIPP). Eur J Nucl Med. 1995;23:383-389.
9. Kubota K., Som P., Oster Z., Brill A., Goodman M., Knapp F.J., et al. Detection of cardiomyopathy in animal model using quantitative autoradiography. J Nucl Med. 1988;29:1697-1703.
10. Takatsu H., Uno Y., Fujiwara H. Modulation of left ventricular iodine-125-MIBG accumulation in cardiomyopathic Syrian hamster using the renin-angiotensin system. J Nucl Med. 1995;36:1055-1061.
11. Lu P., Zanzonico P., Goldfine S., et al. Antimyosin antibody imaging in experimental aortic regurgitation. J Nucl Cardiol. 1997;4:25-32.
12. Lindsey M.L., Escobar G.P., Dobrucki L.W., et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290(1):H232-239.
13. Su H., Spinale F.G., Dobrucki L.W., et al. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005;112(20):3157-3167.
14. Liu Y.-H., Yang X.-P., Nass O., Sabbah H., Peterson E., Carretero O. Chronic heart failure induced by coronary ligation in Lewis inbred rats. Am J Physiol Heart Circ Physiol. 1997;272:H722-H727.
15. Reinhardt C., Weinstein H., Marcel R., Leppo J. Comparison of iodine-125-BMIPP and thallium-201 in myocardial hypoperfusion. J Nucl Med. 1995;36:1645-1653.
16. Fujita M., Morimoto Y., Ishihara M., et al. A new rabbit model of myocardial infarction without endotracheal intubation. J Surg Res. 2004;116(1):124.
17. Sinusas A., Bengel F., Nahrendorf M., et al. Multimodality cardiovascular molecular imaging, Part I. Circ Cardiovasc Imaging. 2008;1:244-256.
18. Nahrendorf M., Sosnovik D., French B., et al. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2:56-70.
19. Bader M., Bohnemeier H., Zollmann F., Lockley-Jones O., Ganten D. Transgenic animals in cardiovascular disease research. Exp Physiol. 2000;85:713-731.
20. Carmeliet P., Collen D. Transgenic mouse models in angiogenesis and cardiovascular disease. J Pathol. 2000;190(3):387-405.
21. Fazio S., Linton M.F. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515-525.
22. Olgin J.E., Verheule S. Transgenic and knockout mouse models of atrial arrhythmias. Cardiovasc Res. 2002;54(2):280-286.
23. de Winther M.P., Hofker M.H. New mouse models for lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol. 2002;13(2):191-197.
24. Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323(1):3-10.
25. Xu Q. A handbook of mouse models of cardiovascular disease. Chichester, England; Hoboken, NJ: John Wiley & Sons, 2006.
26. Zadelaar S., Kleemann R., Verschuren L., et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27(8):1706-1721.
27. Moon A., Robert S.K. Chapter 4 Mouse models of congenital cardiovascular disease. Curr Top Dev Biol. 2008;84:171.
28. Williams R.S., Wagner P.D. Transgenic animals in integrative biology: approaches and interpretations of outcome. J Appl Physiol. 2000;88(3):1119-1126.
29. Gambhir S., Bauer E., Black M., et al. A mutant herpes simplex virus type I thymidine kinase reporter gene shows improved sensitivity for imaging of reporter gene expression with positron emission tomography. Proc Natl Acad Sci USA. 2000;97:2785-2790.
30. Wu J., Inubushi M., Sundaresan G., Schelbert H., Gambhir S. Positron emission tomography imaging of cardiac reporter gene expression in living rats. Circulation. 2002;106:180-183.
31. Wu J.C., Chen I.Y., Sundaresan G., et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108(11):1302-1305.
32. Rajewsky K., Gu H., Kuhn R., et al. Conditional gene targeting. J Clin Invest. 1996;98(3):600-603.
33. Furth P., Onge L., Boger H., et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci USA. 1994;91(20):9302-9306.
34. Deutsch E., Ketring A., Libson K., Vanderheyden J., Hirth W. The Noah’s Ark experiment: Species dependent biodistributions of cationic 99mTc complexes. Int J Nucl Med Biol. 1989;16:191-232.
35. Ball R.M., Bache R.J., Cobb F.R., Greenfield J.C. Regional myocardial blood flow during graded treadmill exercise in the dog. J Clin Invest. 1975;55:43-49.
36. Gupta K.B., Ratcliffe M.B., Fallert M.A., Edmunds L.H.Jr, Bogen D.K. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89(5):2315-2326.
37. Reimer K., Lowe J., Rasmussen M., Jennings R. The wavefront phenomenon of ischemic cell death. I. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786-794.
38. Buxton D., Schelbert H. Measurement of regional glucose metabolic rates in reperfused myocardium. Am J Physiol. 1991;261:H2058-H2068.
39. Dae M., O’Connell J., Botvinick E., Chin M. Acute and chronic effects of transient myocardial ischemia on sympathetic nerve activity, density and norepinephrine content. Cardiovasc Res. 1995;30:270-280.
40. Sinusas A.J. Targeted imaging offers advantages over physiological imaging for evaluation of angiogenic therapy. JACC Cardiovasc Imaging. 2008;1(4):511-514.
41. Johnson L.L., Schofield L., Donahay T., Bouchard M., Poppas A., Haubner R. Radiolabeled arginine-glycine-aspartic acid peptides to image angiogenesis in swine model of hibernating myocardium. JACC Cardiovasc Imaging. 2008;1(4):500-510.
42. Sinusas A., Watson D., Cannon J., Beller G. Effect of ischemia and postischemic dysfunction on myocardial uptake of technetium-99m-labeled methoxyisobutyl isonitrile and thallium-201. J Am Coll Cardiol. 1989;14(7):1785-1793.
43. Sinusas A., Trautman K., Bergin J., et al. Quantification of area at risk during coronary occlusion and degree of myocardial salvage after reperfusion with technetium-99m methoxyisobutyl isonitrile. Circulation. 1990;82:1424-1437.
44. Sinusas A., Shi Q., Vitols P., et al. Impact of regional ventricular function, geometry, and dobutamine stress on quantitative 99mTcSestamibi defect size. Circulation. 1993;88:2224-2234.
45. Sinusas A., Shi Q., Saltzberg M., et al. Technetium-99m-tetrofosmin to assess myocardial blood flow: Experimental validation in an intact canine model of ischemia. J Nucl Med. 1994;35:664-671.
46. Sinusas A., Bergin J., Edwards N., et al. Redistribution of 99mTc-sestamibi and 201Tl in the presence of a severe coronary artery stenosis. Circulation. 1994;89:2332-2341.
47. Shi C., Sinusas A., Dione D., et al. Technetium-99m-nitroimidazole (BMS181321): A positive imaging agen for detecting myocardial ischemia. J Nucl Med. 1995;36:1078-1086.
48. Meoli D.F., Sadeghi M.M., Krassilnikova S., et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113(12):1684-1691.
49. Kalinowski L., Dobrucki L.W., Meoli D.F., et al. Targeted imaging of hypoxia-induced integrin activation in myocardium early after infarction. J Appl Physiol. 2008;104(5):1504-1512.
50. Shi C.Q., Young L.H., Daher E., et al. Correlation of myocardial p-(123)I-iodophenylpentadecanoic acid retention with (18)F-FDG accumulation during experimental low-flow ischemia. J Nucl Med. 2002;43(3):421-431.
51. Mays A., Cobb F. Relationship between regional myocardial blood flow and thallium-201 distribution in the presence of coronary artery stenosis and dipyridamole-induced vasodilation. J Clin Invest. 1984;73:1359-1366.
52. Gross D. Animal models in cardiovascular research, ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1994.
53. Gewirtz H., Grote G., Strauss H., et al. The influence of left ventricular volume and wall motion on myocardial images. Circulation. 1979;59:1172-1177.
54. Kojima A., Matsumoto M., Takahashi M., Hirota Y., Yoshida H. Effect of spatial resolution on SPECT quantification values. J Nucl Med. 1989;30:508-514.
55. Okada R., Pohost G. The use of preintervention and postintervention thallium imaging for assessing the early and late effects of experimental coronary arterial reperfusion in dogs. Circulation. 1984;69:1153-1160.
56. Okada R.D., Glover D., Gaffney T., Williams S. Myocardial kinetics of technetium-99m-hexakis-2-methoxy-2-methylpropyl-isonitrile. Circulation. 1988;77(2):491-498.
57. Stewart R., Heyl B., O’Rourke R., Blumhardt R., Miller D. Demonstration of differential post-stenotic myocardial technetium-99m-teboroxime clearance kinetics after experimental ischemia and hyperemic stress. J Nucl Med. 1991;32:2000-2008.
58. Sadeghi M.M., Krassilnikova S., Zhang J., et al. Detection of injury-induced vascular remodeling by targeting activated alphavbeta3 integrin in vivo. Circulation. 2004;110(1):84-90.
59. Nakajima K., Taki J., Bunko H., et al. Dynamic acquisition with a three-headed SPECT system: application to technitium-99m-SQ30217 myocardial imaging. J Nucl Med. 1991;1991:1273-1277.
60. Smith A., Gullberg G., Christain P., FL D. Kinetic modeling of teboroxime using dynamic SPECT imaging of a canine model. J Nucl Med. 1994;35:484-495.
61. Stewart R., Schwaiger M., Hutchins G., et al. Myocardial clearance kinetics of technetium-99m-SQ30217: A marker of regional myocardial blood flow. J Nucl Med. 1990;31:1183-1190.
62. Ishizu K., Mukai T., Yonekura Y., et al. Ultra-high resolution SPECT system using four pinhole collimators for small animal studies. J Nucl Med. 1995;36:2282-2287.
63. Weber D., Ivanovic M., Franceschi D., et al. An approach to in vivo high-resolution SPECT imaging in small laboratory animals. J Nucl Med. 1994;35:342-348.
64. Weber D., Ivanovic M. Pinhole SPECT: Ultra-high resolution imaging in small animal studies [editorial]. J Nucl Med. 1995;36:2287-2289.
65. Hirai T., Nohara R., Hosokawa R., et al. Evaluation of myocardial infarct size in rat heart by pinhole SPECT. J Nucl Cardiol. 2000;7(2):107.
66. Constantinesco A., Choquet P., Monassier L., Israel-Jost V., Mertz L. Assessment of left ventricular perfusion, volumes, and motion in mice using pinhole gated SPECT. J Nucl Med. 2005;46(6):1005-1011.
67. Vanhove C., Lahoutte T., Defrise M., Bossuyt A., Franken P.R. Reproducibility of left ventricular volume and ejection fraction measurements in rat using pinhole gated SPECT. Eur J Nucl Med Mol Imaging. 2005;32(2):211.
68. Sharir T., Ben-Haim S., Merzon K., et al. High-speed myocardial perfusion imaging: Initial clinical comparison with conventional dual-detector Anger camera imaging. JACC Cardiovasc Imaging. 2008;1(2):156-163.
69. Berman D.S., Kang X., Tamarappoo B., et al. Stress thallium-201/rest technetium-99m sequential dual isotope high-speed myocardial perfusion imaging. JACC Cardiovasc Imaging. 2009;2(3):273-282.
70. Wagenaar D., Parnham K., Sundal B., et al: Advantages of semiconductor CZT for medical imaging, Paper presented at: Penetrating Radiation Systems and Applications VIII, 2007.
71. Montemont G., Bordy T., Rebuffel V., Robert C., Verger L.: CZT pixel detectors for improved SPECT imaging, Paper presented at: Nuclear Science Symposium Conference Record, 2008. NSS ’08. IEEE, 2008.
72. Nickles R., Nunn A., Stone C., Christian B. Technetium-94m-teboroxime: Synthesis, dosimetry and initial PET imaging studies. J Nucl Med. 1993;34:1058-1066.
73. Stone C., Christian B., Nickles R., Perlman S. Technetium 94m-labeled methoxyisobutyl isonitrile: Dosimetry and resting cardiac imaging with positron emission tomography. J Nucl Cardiol. 1994;1:425-433.
74. Berger F., Yu-Po L., Loening A., et al. Whole-body skeletal imaging in mice utilizing microPET: optimization of reproducibility and applications in animal models of bone disease. Eur J Nucl Med. 2002;29:1225-1236.
75. Okada R. Kinetics of thallium-201 in reperfused canine myocardium after coronary artery occlusion. J Am Coll Cardiol. 1984;3:1245-1251.
76. Okada R., Jacobs M., Daggett W., et al. Thallium-201 kinetics in nonischemic canine myocardium. Circulation. 1982;65:70-77.
77. Jacobs M., Okada R., Daggett W., Fowler B., Strauss H., Pohost G. Regional myocardial radiotracer kinetics in dogs using miniature detectors. Am J Physiol. 1982;242:H849-854.
78. Gerson M., Millard R., Roszell N., et al. Kinetic properties of 99mTc-Q12 in canine myocardium. Circulation. 1994;89:1291-1300.
79. Granato J., Watson D., Flanagan T., GAscho J., Beller G. Myocardial thallium-201 kinetics during coronary occlusion and reperfusion: Influence of method of reflow and timing of thallium-201 administration. Circulation. 1986;73:150-160.
80. Li Q.S., Solot G., Frank T.L., Wagner H.J., Becker L.C. Myocardial redistribution of technetium-99m-methoxyisobutyl isonitrile (SESTAMIBI). J Nucl Med. 1990;31(6):1069-1076.
81. Weinstein H., Reinhardt C.P., Leppo J.A. Teboroxime, sestamibi and thallium-201 as markers of myocardial hypoperfusion: Comparison by quantitative dual-isotope autoradiography in rabbits. J Nucl Med. 1993;34:1510-1517.
82. Weinstein H., Reinhardt C.P., Wironen J.F., Leppo J.A. Myocardial uptake of thallium-201 and technetium 99m-labeled sestamibi after ischemia and reperfusion: Comparison by quantitative dual-tracer autoradiography in rabbits. J Nucl Cardiol. 1994;1:351-364.
83. Ito T., Brill A. Validity of tissue paste standards for quantitative whole-body autoradiography using short-lived radionuclides. Appl Radiat Isot. 1990;41:661-667.
84. Crumeyrolle-Arias M., Jafarian-Tehrani M., Cardona A., et al. Radioimagers as an alternative to film autoradiography forin situ quantitative analysis of125I-ligand receptor binding and pharmacological studies. Histochem J. 1996;28(11):801.
85. Barthe N., Chatti K., Coulon P., Maîtrejean S., Basse-Cathalinat B. Recent technologic developments on high-resolution beta imaging systems for quantitative autoradiography and double labeling applications. Nucl Instrum Methods Phys Res A. 2004;527(1–2):41.
86. Tomoike H., Ogata I., Maruoka Y., Sakai K., Kurozumi T., Nakamura M. Differential registration of two types of radionuclides on macroautoradiograms for studying coronary circulation: Concise communication. J Nucl Med. 1983;24:693-699.
87. Heymann M., Payne B., Hoffman J., Rudolph A. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977;20:55-79.
88. Morais D., Richart T., Fritz A., Acree P., Davila J., Glover R. The production of chronic experimental mitral insufficiency. Ann Surg. 1957;145:500-508.
89. Pohost G., Okada R., O’Keefe D., Gewirtz H., Beller G., Strauss H., Chaffin J., Leppo J., Daggett W. Thallium redistribution in dogs with severe coronary stenosis of fixed caliber. Circ Res. 1981;48:439-446.
90. Yipintsoi T., Dobbs W., Scalon P., Knopp T., Bassingthwaighte J. Regional distribution of diffusable tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ Res. 1973;33:573-587.
91. Meleca M., McGoron A., Gerson M., et al. Flow versus uptake comparisons of thallium-201 with technetium-99m perfusion tracers in a canine model of myocardial ischemia. J Nucl Med. 1997;38:1847-1856.
92. Reimer K., Jennings R. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633-644.
93. Connelly C., Vogel W., Hernandez Y., Apstein C. Movement of necrotic wavefront after coronary occlusion in rabbit. Am J Physiol. 1982;243:H682-H690.
94. Stone H., Bishop V., Guyton A. Cardiac function after embolization of coronaries with microspheres. Am J Physiol. 1963;204:16-27.
95. Kordenat R., Kezdi P., Stanley F. A new catheter technique for producing experimental coronary thrombosis and selective coronary visualization. Am Heart J. 1972;83:360-367.
96. Roth D., Maruoka Y., Rogers J., White F., Longhurst J., Bloor C. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol. 1987;253:H1279-H1288.
97. Ducharme A., Frantz S., Aikawa M., et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106(1):55-62.
98. Creemers E.E.J.M., Davis J.N., Parkhurst A.M., et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284(1):H364-H371.
99. Hayashidani S., Tsutsui H., Ikeuchi M., et al. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;285(3):H1229-H1235.
100. Chen J., Song S.-K., Liu W., et al. Remodeling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. Am J Physiol Heart Circ Physiol. 2003;285(3):H946-H954.
101. Chung G., Sinusas A.J. Imaging of matrix metalloproteinase activation and left ventricular remodeling. Curr Cardiol Rep. 2007;9(2):136-142.
102. Morrison A.R., Sinusas A.J. New molecular imaging targets to characterize myocardial biology. Cardiol Clin. 2009;27(2):329.
103. Beller G., Glover D., Edwards N., Ruiz M., Simanis J., Watson D. 99mTc-sestamibi uptake and retention during myocardial ischemia and reperfusion. Circulation. 1993;87:2033-2042.
104. Buxton D., Vaghaiwalla Mody F., Krivokapich J., Phelps M., Schelbert H. Quantitative assessment of prolonged metabolic abnormalities in reperfused canine myocardium. Circulation. 1992;85:1842-1856.
105. Miller D., Gill J., Livni E., et al. Fatty acid analogue accumulation: A marker of myocyte viability in ischemic-reperfused myocardium. Circ Res. 1988;63:681-692.
106. Nishimura T., Sago M., Kihara K., et al. Fatty acid myocardial imaging using 123I-B-methyl-iodophenyl pentadecanoic acid (BMIPP): Comparison of myocardial perfusion and fatty acid utilization in canine myocardial infarction (occlusion and reperfusion model). Eur J Nucl Med. 1989;15:341-345.
107. Young D., Cholrin N., Roth A. Pressure drop across artificially induced stenosis in the femoral arteries in dogs. Circ Res. 1975;36:735-743.
108. Anversa P., Loud A., Leviscky V., Guideri G. Left ventricular failure induced by myocardial infarction. I. Myocyte hypertrophy. Am J Physiol. 1985;248:H876-H882.
109. McDonald K., Francis G., Carlyle P., et al. Hemodynamic, left ventricular structural and hormonal changes after discrete myocardial damage in the dog. J Am Coll Cardiol. 1992;19:460-467.
110. Bolli R., Zhu W., Thornby J., O’Neill P., Roberts R. Time-course and determinants of recovery of function after reversible ischemia in conscious dogs. Am J Physiol. 1988;254:H102-H114.
111. Heyndrickx G., Millard R., McRitchie R., Maroko P., Vatner S. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975;56:978-985.
112. Nicklas J., Becker L., Bulkley B. Effects of repeated brief coronary occlusion on regional left ventricular function and dimension in dogs. Am J Cardiol. 1985;56:473-478.
113. Matsuzaki M., Gallagher K., Kemper W., White F., Ross J.J. Sustained regional dysfunction produced by prolonged coronary stenosis: Gradual recovery after reperfusion. Circulation. 1983;68:170-182.
114. Lavallee M., Cox D., Patrick T., Vatner S. Salvage of myocardial function by coronary artery reperfusion 1,2, and 3 hours after occlusion in conscious dogs. Circ Res. 1983;53:235-247.
115. Homans D., Sublett E., Dai X., Bache R. Persistence of regional left ventricular dysfunction after exercise-induced myocardial ischemia. J Clin Invest. 1986;77:66-73.
116. Vanoverschelde J.-L., Wijns W., Depre C., et al. Mechanism of chronic regional postischemic dysfunction in humans: New insights from the study of noninfarcted collateral dependent myocardium. Circulation. 1993;87:1513-1523.
117. Camici P., Araujo L., Spinks T., et al. Increased uptake of 18F-fluorodeoxyglucose in postischemic myocardium of patients with exercise-induced angina. Circulation. 1986;74:81-88.
118. Vanoverschelde J., Wijns W., Borgers M., et al. Chronic myocardial hibernation in humans: From bedside to bench. Circulation. 1997;95:1961-1971.
119. Arai A., Pantely G., Anselone C., Brislow J., Brislow J. Acive down regulation of myocardial energy requirements during moderate ischemia in swine. Circ Res. 1991;69:1458-1469.
120. Fedele F., Gewirtz H., Capone R., Sharaf B., Most A. Metabolic response to prolonged reduction of myocardial blood flow distal to a severe coronary artery stenosis. Circulation. 1988;78:729-735.
121. Edwards N., Sinusas A., Bergin J., Watson D., Ruiz M., Beller G. Influence of subendocardial ischemia on transmural myocardial function. Am J Physiol. 1992;31:H568-H576.
122. Vanzetto G., Calnon D.A., Ruiz M., et al. Myocardial uptake and redistribution of 99mTc-N-NOET in dogs with either sustained coronary low flow or transient coronary occlusion: Comparison with 201Tl and myocardial blood flow. Circulation. 1997;96(7):2325-2331.
123. Fallavollita J.A. Spatial heterogeneity in fasting and insulin-stimulated 18F-2-deoxyglucose uptake in pigs with hibernating myocardium. Circulation. 2000;102(8):908-914.
124. Gerber B., Laycock S., Melin J., Flameng W., Vanoverschelde J. Perfusion-contraction matching, inotropic reserve and vasodilatory capacity in a canine model of dysfunctional collateral-dependent myocardium. Circulation. 1995;92(suppl):I-314.
125. Shen Y., Vatner S. Mechanism of impaired myocardial function during progressive coronary stenosis in conscious pigs: Hibernation versus stunning? Circ Res. 1995;76:479-488.
126. Berger B., Watson D., Burwell L., et al. Redistribution of thallium at rest in patients with stable and unstable angina and the effects of coronary artery bypass graft surgery. Circulation. 1979;60:1114-1125.
127. Canty J., Fallavollita J. Chronic hibernation and chronic stunning: A continuum. J Nucl Cardiol. 2000;7:509-527.
128. Yarbrough W., Spinale F. Large animal models of congestive heart failure: A critical step in translating basic observations into clinical applications. J Nucl Cardiol. 2003;10:77-86.
129. Packer D., Bardy G., Worley S., et al. Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol. 1986;57:563-570.
130. Spinale F., Fulbright B., Mukherjee R., et al. Relations between ventricular and myocyte function with tachycardia-induced cardiomyopathy. Circ Res. 1992;71:174-187.
131. Spinale F., Zellner J., Tomita M., Tempel G., Crawford F., Zile M. Tachycardia induced cardiomyopathy: Effects on blood flow and capillary structure. Am J Physiol. 1991;261:H140-H148.
132. Zile M., Tomita M., Nakano K., et al. Effects of left ventricular volume overload produced by mitral regurgitation on diastolic function. Am J Physiol. 1991;261:H1471-H1480.
133. Magid N., Opio G., Wallerson D., Young M., Borer J. Heart failure due to chronic experimental aortic regurgitation. Am J Physiol. 1994;267:H226-H233.
134. Brousseau M.E., Hoeg J.M. Transgenic rabbits as models for atherosclerosis research. J Lipid Res. 1999;40(3):365-375.
135. Moghadasian M.H. Experimental atherosclerosis: A historical overview. Life Sci. 2002;70(8):855-865.
136. Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit): Incidence and development of atherosclerosis and xanthoma. Atherosclerosis. 1980;36:261-268.
137. Nordestgaard B., Lewis B. Intermediate density of lipoprotein levels are strong predictors of the extent of aortic atherosclerosis in the St. Thomas’s Hospital rabbit strain. Atherosclerosis. 1992;87:39-46.
138. Vallabhajosula S., Granada J., Kothari P., et al. Porcine model of complex coronary atherosclerosis (CCA) or vulnerable plaque: Role of FDG-PET to study the disease progression and assess lesion macrophage activity. J Nucl Med. 2007;48(Suppl 2):104.
139. Piedrahita J., Zhang S., Hagaman J., Oliver P., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. 1992;89:4471-4475.
140. Zhang S., Reddick R., Burkey B., Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest. 1994;94:937-945.
141. Nakashima Y., Plump A., Raines E., Breslow J., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133-140.
142. Williams H., Johnson J., Carson K., Jackson C. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:788-792.
143. Kolodgie F.D., Petrov A., Virmani R., et al. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: A technique with potential for noninvasive imaging of vulnerable plaque. Circulation. 2003;108(25):3134-3139.
144. Ohtsuki K., Hayase M., Akashi K., Kopiwoda S., Strauss H.W. Detection of monocyte chemoattractant protein-1 receptor expression in experimental atherosclerotic lesions: An autoradiographic study. Circulation. 2001;104(2):203-208.
145. Bjorkerud S., Bjorkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol. 1996;149(2):367-380.
146. Geng Y.-J., Henderson L.E., Levesque E.B., Muszynski M., Libby P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17(10):2200-2208.
147. Narula J., Petrov A., Bianchi C., et al. Noninvasive localization of experimental atherosclerotic lesions with mouse/human chimeric Z2D3 F(ab′)2 specific for the proliferating smooth muscle cells of human atheroma: Imaging with conventional and negative charge–modified antibody fragments. Circulation. 1995;92(3):474-484.
148. Narula J., Petrov A., Pak K.-Y., Ditlow C., Chen F., Khaw B.-A. Noninvasive detection of atherosclerotic lesions by 99mTc-based immunoscintigraphic targeting of proliferating smooth muscle cells. Chest. 1997;111:1684-1690.
149. Jackson C.L., Benbow U., Galley D.J., Karanam S. Models of plaque rupture. Drug Discov Today Dis Models. 2007;4(4):171.
150. Daugherty A., Cassis L.A. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24(3):429-434.
151. Kuivaniemi H., Platsoucas C.D., Tilson M.D.III. Aortic aneurysms: An immune disease with a strong genetic component. Circulation. 2008;117(2):242-252.
152. Nie L., Razavian M., Zhang J., et al. Imaging matrix metalloproteinase activation to predict aneurysm expansion in vivo. J Nucl Med. 2009. (in press)
153. Hynecek R.L., DeRubertis B.G., Trocciola S.M., et al. The creation of an infrarenal aneurysm within the native abdominal aorta of swine. Surgery. 2007;142(2):143.
154. Sadek M., Hynecek R.L., Goldenberg S., Kent K.C., Marin M.L., Faries P.L. Gene expression analysis of a porcine native abdominal aortic aneurysm model. Surgery. 2008;144(2):252.
155. Calnon D.A., Glover D.K., Beller G.A., et al. Effects of dobutamine stress on myocardial blood flow, 99mTc sestamibi uptake, and systolic wall thickening in the presence of coronary artery stenoses: Implications for dobutamine stress testing. Circulation. 1997;96(7):2353-2360.
156. Wu J.C., Yun J.J., Heller E.N., et al. Limitations of dobutamine for enhancing flow heterogeneity in the presence of single coronary stenosis: implications for technetium-99m-sestamibi imaging. J Nucl Med. 1998;39(3):417-425.
157. Calnon D.A., Ruiz M., Vanzetto G., Watson D.D., Beller G.A., Glover D.K. Myocardial uptake of 99mTc-N-NOET and 201Tl during dobutamine infusion: Comparison with adenosine stress. Circulation. 1999;100(15):1653-1659.
158. He Z.-X., Cwajg E., Hwang W., et al. Myocardial blood flow and myocardial uptake of 201Tl and 99mTc-sestamibi during coronary vasodilation induced by CGS-21680, a selective adenosine A2A receptor agonist. Circulation. 2000;102(4):438-444.
159. Reddick R.L., Zhang S.H., Maeda N. Aortic atherosclerotic plaque injury in apolipoprotein E deficient mice. Atherosclerosis. 1998;140(2):297.
160. von der Thusen J.H., van Vlijmen B.J.M., Hoeben R.C., et al. Induction of atherosclerotic plaque rupture in apolipoprotein E-/- mice after adenovirus-mediated transfer of p53. Circulation. 2002;105(17):2064-2070.
161. Cheng C., Tempel D., van Haperen R., et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113(23):2744-2753.
162. Johnson J.L., Jackson C.L. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis. 2001;154(2):399.
163. Williams H., Johnson J.L., Carson K.G.S., Jackson C.L. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22(5):788-792.
164. Johnson J., Carson K., Williams H., et al. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: Model characterization and effects of pravastatin treatment. Circulation. 2005;111(11):1422-1430.
165. Braun A., Trigatti B.L., Post M.J., et al. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90(3):270-276.
166. Herrera V., Didishvili T., Lopez L., et al. Hypertension exacerbates coronary artery disease in transgenic hyperlipidemic Dahl salt-sensitive hypertensive rats. Mol Med. 2001;7:831-844.
167. Constantinides P., Chakravarti R. Rabbit arterial thrombosis production by systemic procedures. Arch Pathol. 1961;72:197-208.
168. Abela G.S., Picon P.D., Friedl S.E., et al. Triggering of plaque disruption and arterial thrombosis in an atherosclerotic rabbit model. Circulation. 1995;91(3):776-784.
169. Rekhter M.D., Hicks G.W., Brammer D.W., et al. Animal model that mimics atherosclerotic plaque rupture. Circ Res. 1998;83(7):705-713.
170. Granada J., Moreno P., Burke A., Schulz D., Raizner A., Kaluza G. Endovascular needle injection of cholesteryl linoleate into the arterial wall produces complex vascular lesions identifiable by intravascular ultrasound: early development in a porcine model of vulnerable plaque. Coron Artery Dis. 2005;16:217-224.
171. Prescott M., McBride C., Hasler-Rapacz J., Von Linden J., Rapacz J. Development of complex atherosclerotic lesions in pigs with inherited hyper-LDL cholesterolemia bearing mutant alleles for apolipoprotein B. Am J Pathol. 1991;139:139-147.