Chapter 35 Risk Stratification after Acute ST-Segment Elevation and Non-ST-Segment Elevation Myocardial Infarction
INTRODUCTION
Each year in the United States alone, over 400,000 patients suffer an acute ST-segment elevation myocardial infarction (STEMI).1 Initial treatment of these patients has rapidly evolved over the past decade, particularly with the introduction of thrombolytic agents to achieve early coronary artery patency and the more selective use of primary percutaneous coronary intervention (PCI) for those admitted to hospitals with cardiac catheterization facilities. Along with these therapeutic advancements, there has been a complementary refinement in risk-stratification algorithms based on specific clinical criteria and the results of noninvasive imaging modalities. Nuclear cardiac imaging has retained an important role in the initial evaluation and risk stratification of patients surviving acute myocardial infarction (AMI), in guiding subsequent therapeutic decision making, and in monitoring the benefits of these therapeutic measures.
CLINICAL PREDICTORS OF RISK
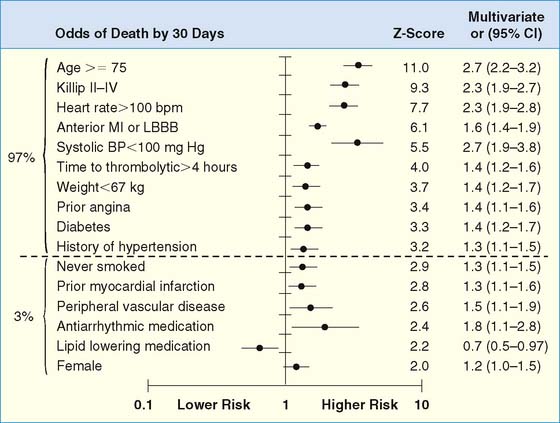
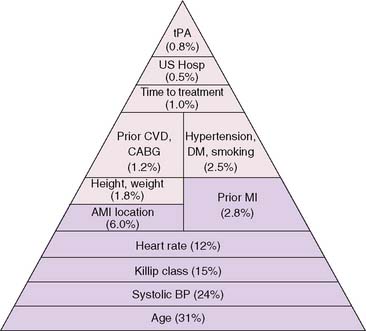
The development of clinical models from large patient cohorts has greatly enhanced risk stratification following STEMI. Data from the Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries (GUSTO)-I study, on 41,021 patients, identified age as the single most important predictor of 30-day mortality, followed by markers of myocardial dysfunction (i.e., lower systolic blood pressure, higher Killip class, elevated heart rate, and presence of anterior infarction).2 These five variables contained over 90% of the prognostic information derived from all recorded baseline clinical information (Fig. 35-1). Patients younger than age 60 years had a 2.4% 30-day mortality compared to a 20.5% mortality in those older than age 75. Mortality increased dramatically with increasing Killip class from I (5.1%) to IV (57.8%).
(From Ryan TJ, Antman EM, Brooks NH, et al: ACC/AHA guidelines for the management of patients with acute myocardial infarction: 1999 update: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Committee on Management of Acute Myocardial Infarction]. Available at www.acc.org. Adapted from ACC/AHA guidelines for the perioperative cardiovascular evaluation for noncardiac surgery, J Am Coll Cardiol 27: 910–948, 1996, with permission. Copyright © 1996 The American College of Cardiology and American Heart Association, Inc.)
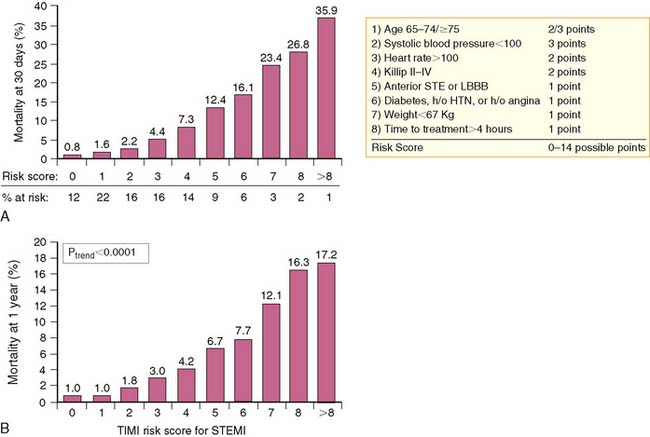
A simpler “bedside” prognostic tool was derived from the 14,114 patients enrolled in the Intravenous nPA for Treatment of Infarcting Myocardium Early (INTIME)-II trial.3 As observed from GUSTO-I, patient age older than 75 was the most important predictor of early (30-day) mortality, followed by hemodynamic instability, defined as increasing Killip class, heart rate over 100 beats/min, anterior AMI, and systolic hypotension (<100 mm Hg).3 Ten clinical variables accounted for 97% of all prognostic risk derived from the multivariate model and were selected for inclusion in the thrombolysis in myocardial infarction (TIMI) risk score (Fig. 35-2). The TIMI score predicted both 30-day and 1-year mortality (Fig. 35-3) and offered similar prognostic capability as the more complex multivariate model derived from GUSTO-I. The TIMI risk score was recently validated from 84,029 patients comprising the National Registry of Myocardial Infarction (NRMI) 3.4 The predictive behavior of the risk score was similar to the INTIME-II results among patients undergoing either coronary reperfusion with thrombolytic therapy or primary PCI. Current American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend coronary angiography in clinically high-risk, unstable patients.5
IMAGING PREDICTORS OF RISK
The most important predictors of subsequent patient outcome after AMI are infarct size,6–9 left ventricular (LV) ejection fraction (LVEF),6,10–12 LV volumes,13,14 and the presence and extent of residual myocardial ischemia.15–18 All of these variables can be directly determined through scintigraphic approaches.
Infarct Size
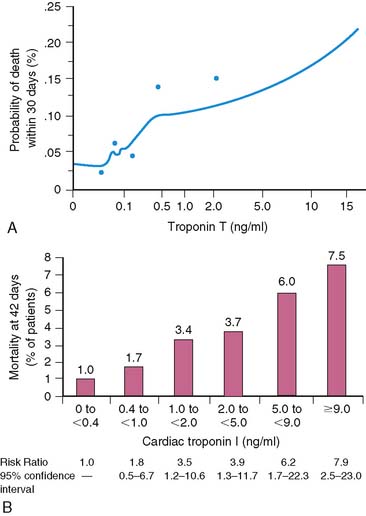
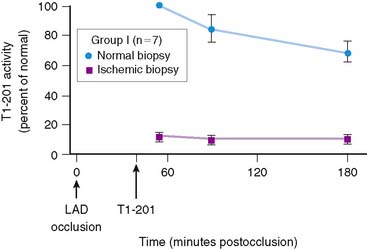
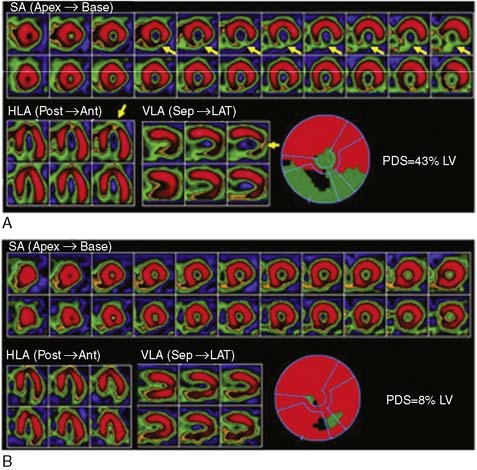
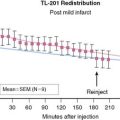
Serum levels of enzymatic markers accurately quantify infarct size and predict mortality (Fig. 35-4).8,9 Likewise, resting myocardial perfusion scintigraphy can accurately assess infarct size, since the uptake of thallium 201Tl and technetium 99mTc-based radiopharmaceuticals is primarily dependent on coronary blood flow and the presence of myocardial viability (see Chapter 28).19–21 In animal models of permanent coronary occlusion, initial 201Tl activity within the infarct zone correlates very well with coronary blood flow as measured by radiolabeled microspheres.22 However, the relative gradient in 201Tl activity between normal and infarcted myocardium decreases over time, as this isotope washes out from normal regions (Fig. 35-5). Due to the kinetics of 201Tl, the relative count activity within the infarct zone might appear to improve over time, simulating an artifactual reduction in scintigraphic infarct size.
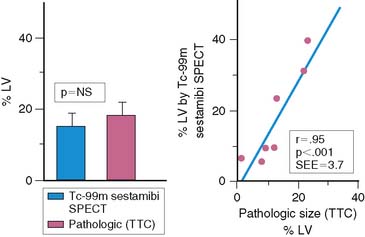
An alternative radiopharmaceutical for assessing infarct size is 99mTc sestamibi, since this isotope minimally redistributes once it is taken up by the myocardium.21,23–25 Unlike 201Tl, which can bidirectionally cross the cell membrane, sestamibi is actively taken up by mitochondria and trapped within the nucleus. Thus imaging can be performed even several hours after the initial resting injection. Animal investigations with sestamibi demonstrate close correlations between its initial uptake and occluded flows by microspheres, and the gradient in count activity between normally perfused and infarcted zones remains relatively constant over time.23,25 In animal models of coronary occlusion, there is a close correlation between scintigraphic and pathologic infarct size (Fig. 35-6) that is not influenced by the early reactive hyperemia observed following rapid reperfusion.23,25,26 These studies indicate that sestamibi imaging during AMI can estimate infarct size following coronary reperfusion independent of temporal restraints.
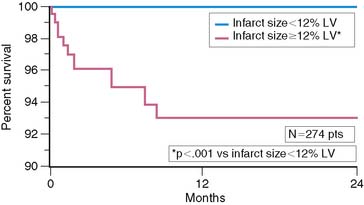
Scintigraphic infarct size predicts subsequent survival following AMI. In the Western Washington Study, resting 201Tl single-photon emission computed tomography (SPECT) was performed in 307 survivors of AMI a mean of 8.1 ± 6.4 weeks after enrollment.6 Patients with a quantified LV infarct size of 20% or greater had a significantly higher mortality rate than those with smaller infarcts (Fig. 35-7). Miller and colleagues prospectively followed 274 patients who received thrombolytic therapy for STEMI and had resting 99mTc sestamibi imaging a mean of 7 ± 8 days after hospital admission.7 The median LV infarct size for the entire cohort was 12% (range, 0% to 68%) with a 2-year mortality rate of 3%. Patients with an LV infarct size above the median had a significantly higher mortality rate compared to those with a smaller infarction (7% versus 0%, respectively; Fig. 35-8). This result is not unexpected, since infarct size and LVEF are inversely related.27 Infarct size as determined by resting 99mTc sestamibi SPECT is now commonly used as a surrogate marker for mortality in clinical trials assessing therapies during AMI.28–34
Left Ventricular Ejection Fraction
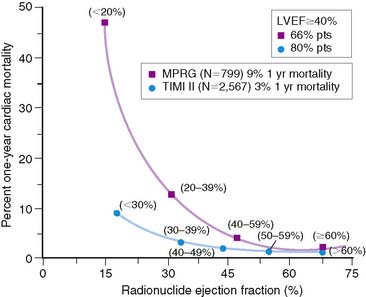
The LVEF remains the single best long-term predictor of mortality in survivors of AMI.6,10–12 Mortality rates are low in patients with an LVEF greater than 40% but rapidly increase with more severe LV dysfunction. In the Multicenter Post Infarction Research Group (MPRG)10 study, 799 survivors of AMI had gated radionuclide angiography following admission. The 1-year mortality rate for all patients was 9%, but 60% of all deaths occurred in the 33% with an LVEF less than 40%. A very high mortality rate of 47% was observed in the 3% of patients with an LVEF less than 20% (Fig. 35-9).
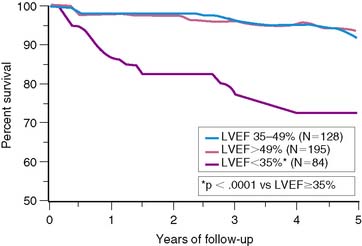
The important prognostic information obtained from LVEF has been confirmed in patients receiving reperfusion therapy during STEMI.6,11,35–37 The resultant LVEF and subsequent mortality rate are clearly dependent on the degree to which early coronary artery patency is achieved. In the GUSTO-I trial, LVEF and 30-day survival were both significantly higher in patients who achieved TIMI grade 3 flow (61% ± 14% [95.6%]) versus TIMI grade 0/1 (55% ± 14% [91.1%]) or TIMI grade 2 (56% ± 14% [92.6%]) flows, respectively.38 In the Survival and Ventricular Enlargement (SAVE) study, gated radionuclide angiography was performed within the first 2 weeks of AMI, and patients with an LVEF less than 40% were randomized to receive either placebo or captopril therapy.39 One-third of all patients had thrombolytic therapy during AMI. The mean LVEF in patients randomized to placebo was 31%, and the associated 1-year mortality approximately 12%—similar to the 15% mortality reported by the MPRG in patients with an LVEF less than 40%.10 Likewise, in the Western Washington Streptokinase Trials, LVEF measured 8.7 ± 6 weeks after enrollment was the best univariate and multivariate predictor of survival.6 In the 20% of patients with an LVEF less than 35%, the 1-year mortality was 15%, which increased to 22% by 3 years—virtually identical to the 22% placebo mortality reported in SAVE (Fig. 35-10).
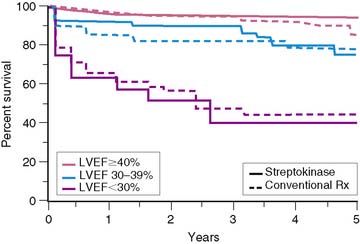
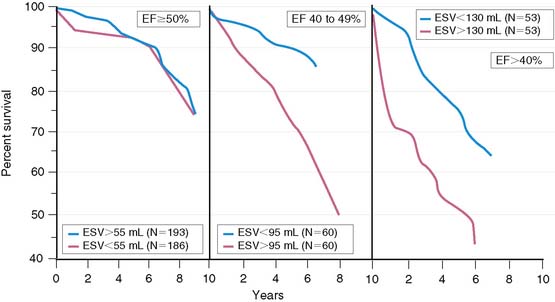
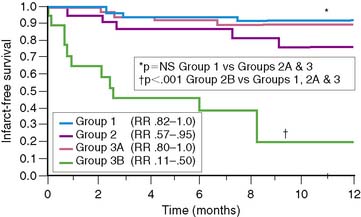
Similar results are reported by Simoons and coworkers in 422 patients randomized to intracoronary streptokinase versus placebo, where the LVEF was measured 10 to 40 days after AMI.11 In patients with LVEF greater than 40%, the 3-year mortality rate remained low (4.3%). Conversely, in patients with an LVEF less than 40%, the 1- and 3-year mortality rates increased with worsening LVEF, but irrespective of initial therapy (Fig. 35-11). Mortality was strongly influenced by LVEF and the extent of angiographic coronary artery disease (CAD) (Fig. 35-12), the latter presumably an indicator of the extent of jeopardized myocardium. In the series by Dakik and colleagues, the LVEF was the only significant predictor of infarct-free survival. The relative risk of death or nonfatal MI doubled for every 10% decrease in LVEF (RR = 2.06, 95% CI, 1.17–3.64; P = 0.01) (Fig. 35-13).35
The TIMI-II36 and Grupo Italiano por lo Studio della Streptochinase Nell’Infarcto Miocardico (GISSI)-237 trials indicate that survival at any given LVEF is better in patients who receive thrombolytic therapy compared to historical controls in the prethrombolytic era (see Fig. 35-9).10 This may be due to differing patient characteristics, refinements in risk stratification, therapeutic improvements, or the use of thrombolytics, which may prevent long-term remodeling by maintaining arterial patency. In addition, patients who achieve early coronary reperfusion during AMI frequently exhibit regional myocardial stunning that can persist for weeks.27,30 Patients with minimal LV dysfunction (i.e., LVEF > 40%) are expected to have a low subsequent mortality rate. The TIMI-II36 and GISSI-237 data confirm a similar and comparably low mortality rate in patients with normal LV function (LVEF > 50%; 1.2%), as reported earlier by the MPRG (see Fig. 35-9).10 However, if the LVEF is reduced due to extensive myocardial stunning that later resolves, cardiac risk could be spuriously overestimated. This may partially explain the lower 1-year mortality rate in patients with LV dysfunction in the TIMI-II and GISSI-2 studies compared to the MPRG. Measuring LVEF 1 to 2 months after infarction rather than within the first 2 weeks would reduce the confounding influence of stunning and offer a more reliable estimate of risk. Despite these caveats, the final LVEF remains an important predictor of long-term survival irrespective of initial therapy during STEMI.
Left Ventricular Volumes
Left ventricular enlargement increases mortality in patients with AMI and particularly when coexisting myocardial dysfunction is present. White and colleagues showed that survival decreased with progressive LV dilation and a decrease in LVEF.13 However, LV dilation influenced survival only in patients with LVEF less than 50% (Fig. 35-14). Likewise, the SAVE investigators demonstrated in 512 patients with LVEF less than 40% that 1-year survivors had a significantly smaller increase in LV dimensions, compared to patients who died.14 LV enlargement is also known to increase mortality in patients with chronic CAD who have a depressed LVEF less than 45%.40
Left ventricular dilation develops early in patients after AMI, presumably as a compensatory mechanism for maintaining stroke volume.41 This is particularly true in patients with anterior infarction, who generally have the greatest degree of initial LV dysfunction and are therefore most likely to develop early infarct zone expansion.41,42 Over the ensuing months, structural and geometric changes occur that entail scar formation and thinning of the infarct zone as well as hypertrophy and dilation of noninfarcted regions.43–45 The initial loss of myocardium, if large enough, leads to progressive LV dilation, increasing wall stress, further LV dysfunction, and ultimately end-stage heart failure. One study in survivors of AMI reported a progressive increase in both LV end-diastolic and end-systolic volumetric indices over a 6-month period, but this occurred almost exclusively in those with an initial LVEF less than 40% (Fig. 35-15).46 Various therapies that limit LV dilation can improve survival in patients with LV dysfunction.14,46 LV volumes can be accurately measured with both gated radionuclide angiography47 and gated SPECT48 techniques.
Myocardial Ischemia
The presence and extent of myocardial ischemia are strong predictors of both fatal and nonfatal cardiac events and improve risk stratification beyond the information gleaned from clinical variables15–18 or the extent of CAD.17,35,49,50 The detection of myocardial ischemia using exercise electrocardiography51–59 has been largely supplanted by more sensitive techniques, including stress echocardiography,60,61 exercise radionuclide angiography,62,63 and most important, stress myocardial perfusion scintigraphy.15–18
RISK STRATIFICATION FOLLOWING ACUTE MYOCARDIAL INFARCTION
Exercise Stress Testing
Exercise stress testing has been extensively studied for identifying high- and low-risk survivors of AMI.51–59 Predictors of high risk include a poor exercise effort (<4 METs) and exercise-induced angina, ischemic (>1 mm) ST-segment depression, hypotension, and ventricular arrhythmias. Inability to perform a predischarge exercise test is, in itself, a poor prognostic finding.37,64 In the TIMI-II trial, the mortality rate at 1 year was 7.7% in those who did not perform an exercise test, compared to 1.8% in those who did (P < 0.001).64 In GISSI-2, the mortality rate at 6 months increased from 1.3% to 9.8% based on whether patients could perform an exercise test.37
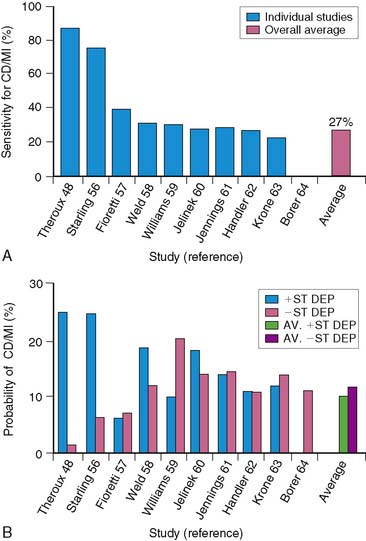
Electrocardiographic (ECG) ischemia during submaximal exercise predicts subsequent cardiac death.51 In an early study from the Montreal Heart Institute, the 1-year mortality rate among all patients was 9.5%, but death occurred almost exclusively in the 30% of patients with ECG ischemia. Patients without ischemia had only a 2.1% mortality, compared to a 27% mortality in those with ST-segment depression.51
Low-level exercise ECG testing predicts mortality in seemingly low-risk groups after AMI, but it is of limited value in predicting other morbid events. The stress ECG is insensitive for detecting significant CAD,52,65 particularly in patients who perform only submaximal exercise.66 The exercise ECG is much less accurate in risk stratification than myocardial perfusion scintigraphy.16,18,49 Furthermore, an ischemic ECG response during treadmill exercise currently occurs less frequently than previously reported. In the prethrombolytic era, approximately 31% of patients with uncomplicated AMI exhibited ECG ischemia on predischarge exercise testing.15,16,67,68 However, this has decreased to approximately 15% among patients evaluated in the thrombolytic era.35,50,64,69–71 All of these factors limit the ability of the treadmill test to accurately predict which stable patients after AMI are at increased risk for subsequent events (Fig. 35-16).
Gated Radionuclide Angiography
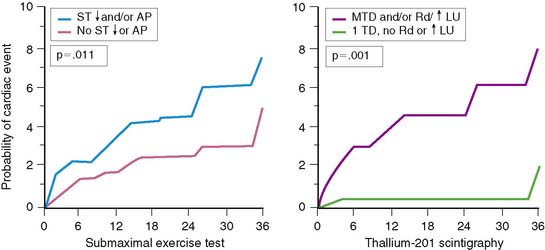
Gated radionuclide angiography allows assessment of LVEF at rest and during dynamic bicycle exercise (see Chapter 11). The resting LVEF identifies patients at high risk for death,6,10–12,35–37 and the presence of exercise-induced ischemia may further improve risk stratification, depending on the population studied and the types of subsequent events considered (Table 35-1).62,63,72–77 Morris and coworkers studied 106 patients, of whom 24 died and an additional 38 had either recurrent AMI, readmission for unstable angina, or refractory angina necessitating coronary revascularization.77 The resting and exercise LVEF both predicted mortality but no other cardiac event. The lack of change in LVEF from resting to exercise identified patients at high risk for developing refractory angina who then underwent coronary revascularization. Abraham and colleagues reported a 58% event rate at 2 years in patients with an exercise LVEF less than 50%, compared to a 17% event rate among those with normal LV function.72 A more than 5% increase in the LVEF during exercise identified a very low-risk group for subsequent cardiac events, particularly if the resting function was greater than 40%.
Table 35-1 Gated Exercise Radionuclide Angiography for Risk Assessment after Acute Myocardial Infarction
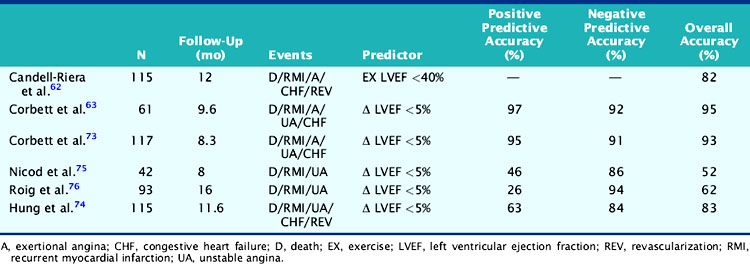
In a study by Hung and colleagues, 115 patients had resting/exercise radionuclide angiography 3 weeks after AMI.74 Twenty-two patients subsequently died (n = 3) or had recurrent infarction (n = 5), readmission for unstable angina (n = 4), congestive heart failure (n = 1), or need for bypass surgery (n = 9), primarily due to refractory angina. The change in LVEF during exercise was a significant predictor of both hard (i.e., death, recurrent infarction) and all cardiac events. Corbett and coworkers showed that 97% of patients who failed to increase their LVEF during exercise returned with a cardiac event by 6 months; however, most of these events were ischemic (i.e., angina or recurrent infarction).63 Conversely, in the series by Nicod and coworkers, where the majority of events were death or recurrent infarction (65%), the exercise LVEF was less discriminating (positive predictive accuracy = 46%).75 Knowing the extent of resting and inducible LV dysfunction appears to identify patients who might best be further evaluated with coronary angiography.
Patients who receive thrombolytic therapy frequently have exercise-induced ischemic LV dysfunction. In the TIMI-II trial, 59% of 2143 patients who had resting and exercise radionuclide angiography prior to hospital discharge had an ischemic response, defined as either a less than 5% increase (48%) or a greater than 5% decrease (11%) in exercise LVEF.36 The 1-year mortality rate in the total TIMI cohort of 3197 patients was 3%, which decreased to 2.2% in the 2567 who underwent gated radionuclide angiography and 1.7% in those who had both resting and exercise radionuclide angiography. Although the resting (see Fig. 35-9), peak exercise (Fig. 35-17), and change in LVEF with exercise all predicted survival, the exercise variables did not improve predictive accuracy over the resting LVEF alone. This result may partially be explained by the exclusion of 1045 patients who could not exercise and had a high mortality rate of 5.8% (see Fig. 35-17). The exercise LVEF variables may also better predict nonfatal ischemic cardiac events, which were not evaluated in this trial. Although important from an historical perspective, exercise gated radionuclide angiography has been largely supplanted by gated SPECT perfusion imaging for risk stratification.
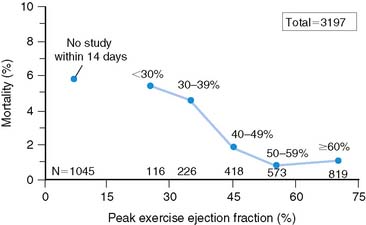
Figure 35-17 Relation of all-cause mortality to peak exercise ejection fraction.
(From Zaret BL, Wackers FJT, Terrin ML, et al, for the TIMI Study Group: Value of radionuclide rest and exercise left ventricular ejection fraction in assessing survival of patients after thrombolytic therapy for acute myocardial infarction: Results of Thrombolysis in Myocardial Infarction (TIMI) Phase II Study, J Am Coll Cardiol 26:73, 1995.)
Exercise Myocardial Perfusion Scintigraphy
Exercise myocardial perfusion scintigraphy can accurately define risk in stable survivors of AMI (Table 35-2).15,16,50,68,78 Patients without scintigraphic ischemia have a very low cardiac event rate (<5%), whereas 40% to 50% of patients with ischemia will develop subsequent cardiac events. Early reports demonstrated increased lung uptake of 201Tl and ischemia in multiple vascular territories as additional high-risk predictors.16 With the advent of quantitative SPECT analysis, the size of the stress-induced perfusion defect, in relation to the presence and quantified extent of scintigraphic ischemia, has added a new dimension to risk stratification.17 Furthermore, with gated SPECT, the important prognostic variables of LVEF and LV volumes can be calculated directly from the perfusion images (see Chapter 12).
Table 35-2 Comparison of Exercise Versus Pharmacologic Coronary Vasodilators for Risk Assessment after Acute Myocardial Infarction
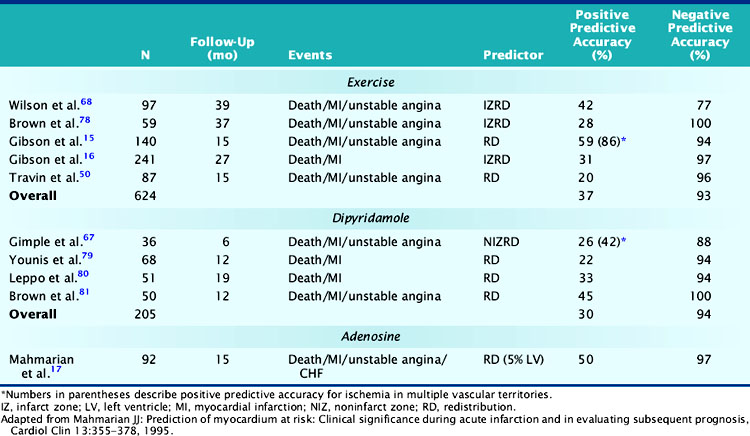
In a landmark study from Gibson and colleagues, 140 seemingly low-risk patients were evaluated with submaximal exercise 201Tl scintigraphy and coronary angiography.16 Over 15 ± 12 months of follow-up, 36% of patients either died (n = 7), had recurrent AMI (n = 9), or had readmission for unstable angina (n = 34). The presence of scintigraphic ischemia, particularly when involving multiple vascular territories, was the most powerful prognosticator. The scintigraphic variables were superior to the treadmill exercise variables in defining high- and low-risk individuals. Fifty-nine percent of patients with evidence of 201Tl redistribution and 86% of those with redistribution in multiple vascular beds (an indicator of multivessel CAD) had a subsequent cardiac event (Fig. 35-18), compared to only 49% of those with ECG ischemia. Moreover, only 6% of patients without scintigraphic ischemia had a cardiac event versus 26% of those with a low-risk exercise test (see Fig. 35-18).
More recently, Travin and colleagues demonstrated the value of exercise 99mTc sestamibi SPECT in stratifying risk after AMI.50 Submaximal exercise SPECT was performed in 134 stable patients within 14 days (mean, 7.5 ± 2 days) of AMI. Ischemic ECG changes were observed in only 23% of patients, whereas 70% had scintigraphic ischemia. Thirty-three patients who had early coronary revascularization were excluded from analysis, and most (79%) of these patients had ischemia by SPECT. Cardiac events occurred in 13 patients over 15 ± 10 months of follow-up. Patients without scintigraphic ischemia had a very low 7% event rate. In patients with ischemia, both the presence and extent of this variable predicted outcome. Overall, 19% of patients with ischemia had a subsequent cardiac event, but this increased from 12% in those with one or two ischemic defects to 38% in patients with more than three ischemic defects. By Cox regression analysis of clinical, exercise treadmill, and scintigraphic variables, only the number of ischemic defects on SPECT predicted outcome.
Pharmacologic Stress Perfusion Scintigraphy
Dipyridamole and adenosine stress are effective and preferable to exercise SPECT as methods for assessing risk in stable patients after AMI.17,67,79–81 Pharmacologic vasodilators maximize heterogeneity in coronary blood flow and can thereby accurately identify the extent of LV hypoperfusion and residual ischemia.82,83 Adenosine induces a similar perfusion defect size as observed with maximal exercise stress.84 Since these tests can be safely performed even within 1 to 2 days after AMI, patients can be rapidly triaged to early coronary angiography or hospital discharge.85–88 The scintigraphic risk variables identified with exercise stress have been confirmed in studies using dipyridamole and adenosine (see Table 35-2).
Dipyridamole SPECT
In the initial series by Leppo and colleagues, dipyridamole 201Tl scintigraphy was performed in 51 patients 1 to 2 weeks after uncomplicated AMI.80 The presence of 201Tl redistribution was the only significant predictor of cardiac death or recurrent AMI. Brown and coworkers safely performed dipyridamole imaging in 50 stable patients very early (mean, 62 ± 121 hours) after hospitalization.81 The only significant predictor of in-hospital and late cardiac events among clinical, scintigraphic, and angiographic variables was the presence of infarct zone 201Tl redistribution. Conversely, none of the patients without redistribution had a subsequent cardiac event over the ensuing year. Other investigators have confirmed the prognostic importance of 201Tl redistribution on dipyridamole imaging after AMI.89,90
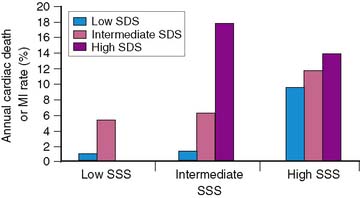
Brown and associates reported the results of a large multicenter trial evaluating dipyridamole SPECT for predicting early and late cardiac events.18 Stable patients postinfarction underwent 3:1 randomization to either early (2 to 4 days) dipyridamole 99mTc sestamibi SPECT followed by exercise SPECT (at 6 to 12 days) (n = 284) or submaximal exercise SPECT alone (n = 309). Twenty-nine patients who had in-hospital cardiac events and 24 who had early coronary revascularization were excluded from long-term follow-up. Following hospital discharge, death or recurrent AMI occurred in 37 patients assigned to dipyridamole testing and in 31 who had submaximal exercise SPECT. A semiquantitative summed stress score (SSS) and summed difference score (SDS) were generated to assess the size of the stress-induced perfusion defect and the extent of scintigraphic ischemia, respectively. Multivariate predictors of in-hospital events among clinical, dipyridamole stress ECG, and scintigraphic variables were only the SSS, SDS, and peak creatine kinase. Multivariate predictors of death or MI following hospital discharge were the dipyridamole SPECT-derived SSS and SDS, as well as anterior infarction location. The extent of scintigraphic ischemia (i.e., SDS) further improved risk stratification—particularly in patients with intermediate-size perfusion defects (Fig. 35-19). Risk stratification was significantly better with dipyridamole than with submaximal exercise SPECT (Fig. 35-20). This study emphasizes that pharmacologic stress testing after infarction is safe and effective at identifying low- and high-risk groups based on the extent of stress-induced hypoperfusion and scintigraphic ischemia. This is the first study to demonstrate improved risk stratification with dipyridamole stress, compared to submaximal treadmill exercise in the same patients.
Adenosine SPECT
Adenosine is a potent direct coronary artery vasodilator that predictably induces maximal hyperemia82,83 and thereby produces a similar perfusion defect extent as observed with maximal exercise stress in patients who have CAD.84 Based on these considerations, its high safety profile,87,88 and exceedingly short half-life,82 this agent is well suited for evaluating patients after AMI for residual myocardial ischemia.
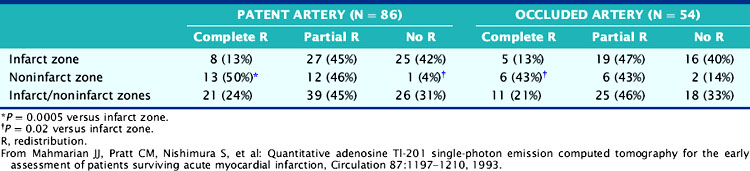
The role of adenosine 201Tl SPECT for detecting residual ischemia and predicting in-hospital cardiac events was first reported in 120 stable survivors of AMI who were imaged early (5 ± 3 days) after infarction.86 The overall sensitivity for detecting significant (>50%) CAD was 87%. Sixty-three percent of patients with double-vessel and 91% of patients with triple-vessel CAD were accurately predicted to have multivessel involvement. Scintigraphic ischemia was common within the infarct zone (59%) but also in noninfarct-zone (63%) territories in patients with multivessel CAD. Neither angiographic patency of the infarct and noninfarct-related arteries (Table 35-3) nor the presence of collaterals (Table 35-4) predicted the presence of scintigraphic ischemia.
Table 35-4 Prevalence of Redistribution Associated with Occluded Infarct and Noninfarct Arteries: Relation to Coronary Collaterals
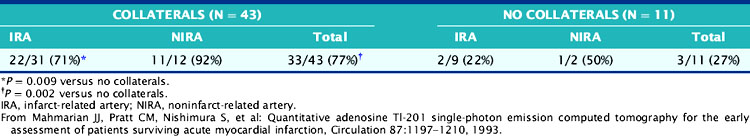
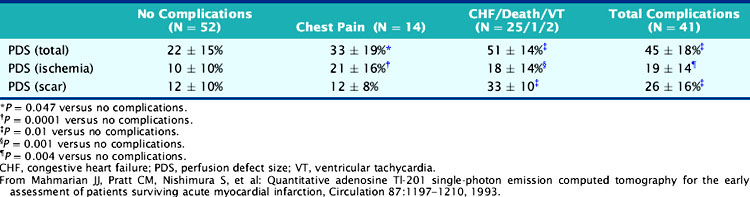
The adenosine-induced LV perfusion defect size was significantly larger in the 41 patients with in-hospital complications (45 ± 15%) compared to those without such complications (22 ± 15%) (Table 35-5). No patient with a small (<10%) LV perfusion defect size had an in-hospital cardiac event, compared to 51% of those with larger defects. A positive predictive value of 43% and a negative predictive value of 91% were observed when the ischemic perfusion defect size was dichotomized at 12%. This study emphasized that (1) adenosine SPECT can readily identify patients with multivessel CAD, (2) the scintigraphic total and ischemic perfusion defect size can identify patients at high and low risk for in-hospital events, and (3) the angiographic information alone is a poor predictor of myocardial viability and may be misleading when trying to decide the appropriateness of coronary revascularization.
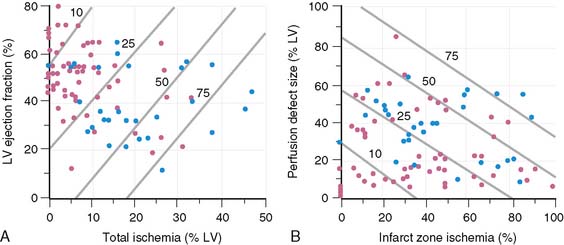
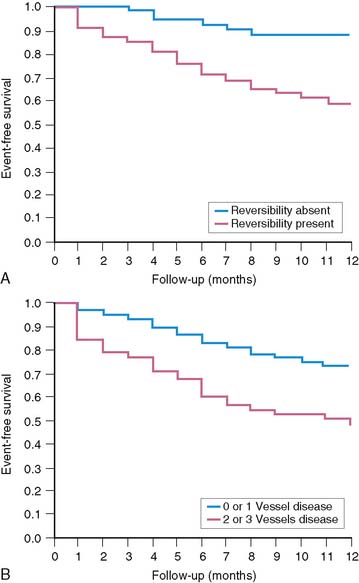
A subsequent trial17 from the same group studied 92 stable patients with adenosine 201Tl SPECT 5 ± 3 days after AMI. Cardiac events occurred in 30 (33%) patients over 15.7 ± 4.9 months of follow-up (8 deaths, 12 recurrent infarctions, 7 admissions for unstable angina, and 3 for congestive heart failure). Clinical predictors of risk were patient age, gender, prior history of AMI, and prior coronary revascularization. Scintigraphic risk predictors were the LVEF (P < 0.0001), the quantified LV perfusion defect size (P < 0.0001), and absolute extent of scintigraphic ischemia (P < 0.000001) (Fig. 35-21).
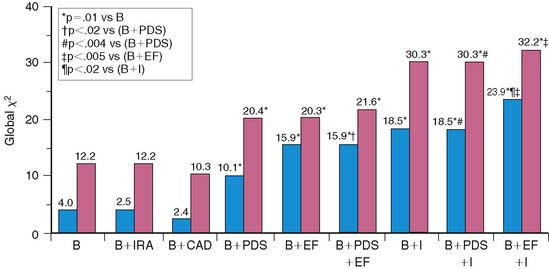
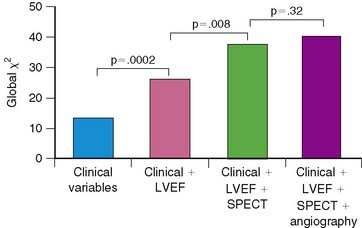
Multivariate analysis incorporating clinical, angiographic, and scintigraphic variables identified several models for predicting risk. The most powerful model was based on the absolute extent of scintigraphic ischemia and LVEF, where 10% increments in these variables increased risk by 82% or decreased risk by 24%, respectively. Only female gender added to the model, at a P value of 0.03. At any given LVEF, risk increased dramatically according to the extent of LV ischemia (Fig. 35-22). A second risk model was based solely on the scintigraphic perfusion variables of total LV perfusion defect size and infarct-zone ischemia (see Fig. 35-22). Chi-square analysis using a baseline model of clinical variables demonstrated improved risk stratification when LVEF as well as total and ischemic perfusion defect size were added in an incremental fashion. The addition of coronary angiographic findings did not improve the clinical model (Fig. 35-23).
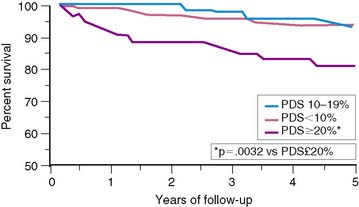
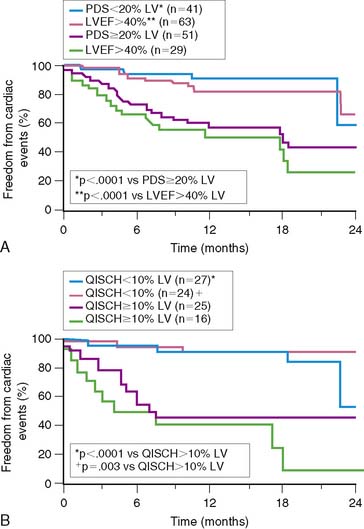
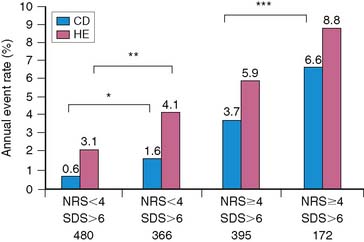
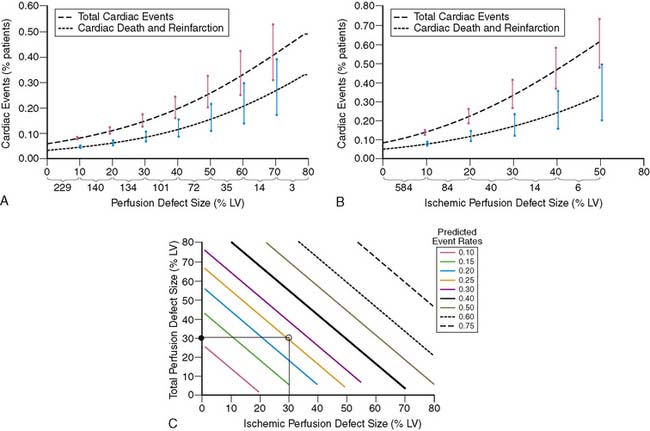
To further validate these retrospective results, 133 stable patients after AMI were prospectively risk stratified according to their initial perfusion defect size and the extent of scintigraphic ischemia (Fig. 35-24).91 Patients with a small (<20%) adenosine-induced LV perfusion defect size were classified as low risk, those with a large (>20%) but predominantly nonischemic (<10%) perfusion defect size as intermediate risk, and patients with a large (>20%) and predominantly ischemic (>10%) LV perfusion defect size as high risk. High-risk patients who were considered good revascularization candidates following coronary angiography underwent randomization to receive either intensive antiischemic medical therapy or coronary revascularization, whereas the remainder received medical therapy as tolerated.
Patients classified as low risk had a relatively low overall cardiac event rate (17%) with no deaths and few reinfarctions (7%) over 11 ± 5 months; patients classified as intermediate risk had an overall higher event rate (29%). The patients with large ischemic defects who were not randomized to intensive antiischemic therapy had a significantly higher event rate than those with scintigraphic scar (78% versus 29%, P < 0.001, respectively), despite a comparable LVEF of 36%. This was also true when events were limited to death and nonfatal reinfarction (see Fig. 35-24). These data imply that stable patients after AMI who have a small or nonischemic perfusion defect size can generally be managed conservatively, with aggressive antiischemic therapy reserved for those with large reversible defects who are at high risk for subsequent cardiac events. This paradigm is further supported by a study that focused primarily on patients with a remote (>6 months) history of AMI. In this study of 1413 patients, the defect size and extent of ischemia again predicted outcome, with the lowest annual event rate in patients who had a small MI and no inducible ischemia (0.6%), and the greatest prognostic impact of ischemia observed among those with an intermediate-size perfusion defect (Fig. 35-25).92
RISK STRATIFICATION IN THE THROMBOLYTIC ERA
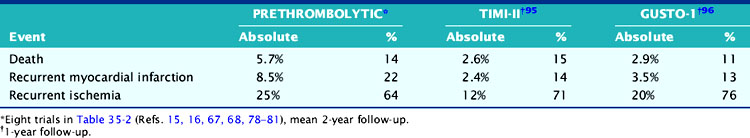
The low cardiac mortality in patients who undergo acute coronary reperfusion appears to be due in part to a reduction in infarct size,28,29 with subsequent preservation of LV function.38,93 In the TIMI-II trial, over 50% of all deaths occurred within the first 3 weeks, with only an additional 4.5% mortality by 2 years.94 This low late mortality rate has also been confirmed by the GUSTO-I investigators.95 However, most cardiac events after hospital discharge reported in current studies94,95 and in those from the prethrombolytic era15,16,67,68,78–81 were attributable to recurrent AMI and particularly, readmission for unstable angina rather than death (Table 35-6). This is because prognostic studies from the prethrombolytic era appropriately excluded clinically unstable patients from study participation (i.e., those at highest risk for death).
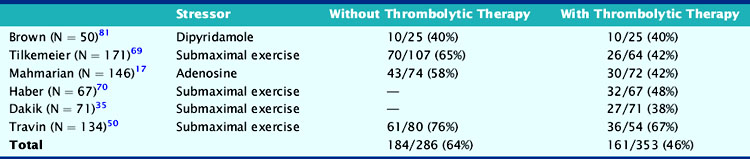
Another issue is that the prevalence of ischemia in patients receiving thrombolytic therapy may be significantly lower than previously reported. This is apparently true for exercise-induced ECG ischemia, which has decreased from a prevalence of approximately 31% to 15%.15,16,35,50,64,68–71 The prevalence of scintigraphic ischemia has also decreased but to a much lesser degree than observed with treadmill testing, with approximately 46% of patients still demonstrating ischemia on perfusion scintigraphy (Table 35-7).17,35,50,69,70,81 Patients who undergo PCI of the infarct-related artery also commonly demonstrate residual ischemia.93
Two early trials, owing to study design issues, predictably demonstrated little benefit of scintigraphy in risk-stratifying patients who received thrombolytic therapy.96,97 In the study by Krone and colleagues,96 67% of patients had coronary angiography, and 39% had angioplasty prior to scintigraphy. Patients who underwent bypass surgery or had resting ST-segment/T-wave changes on the baseline ECG were excluded from study entry. Since scintigraphy was performed relatively late after AMI (2.7 months), many patients already had events prior to noninvasive testing. In the study by Miller and colleagues,97 73% of patients had coronary angiography, with subsequent coronary angioplasty prior to (35%) or early after (17%) scintigraphy. In both of these studies, most of the anticipated high-risk patients were effectively excluded or treated prior to noninvasive testing, thereby limiting the ability of scintigraphic results to predict outcome.
Both exercise and pharmacologic stress myocardial perfusion imaging (MPI) have been shown to predict subsequent outcome in patients receiving thrombolytic therapy. Tilkemeier and coworkers compared submaximal planar 201Tl scintigraphy in 171 patients who did or did not have interventions (i.e., primary angioplasty or thrombolytic therapy) during AMI.69 The positive predictive value of exercise-induced scintigraphic ischemia for predicting subsequent events was similar in both groups (36% versus 33%, respectively). The presence of scintigraphic ischemia identified 80% (4 out of 5) of intervened patients who died or had recurrent infarction. Travin and colleagues followed 87 patients after submaximal exercise SPECT, of whom 34 received thrombolytic therapy.50 The number of ischemic segments predicted subsequent cardiac events equally well, irrespective of initial therapy.
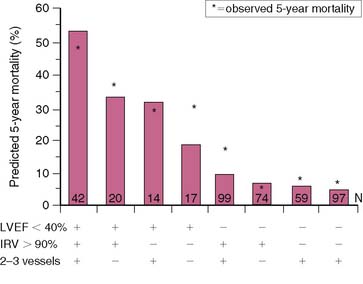
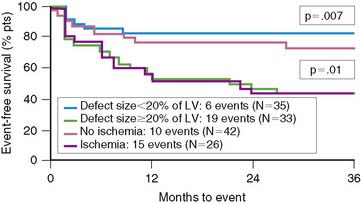
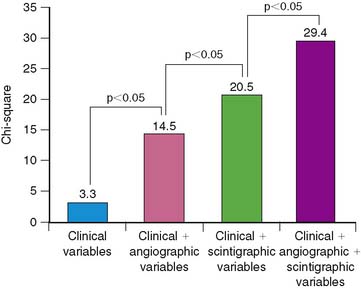
Dakik and coworkers studied 71 patients who received thrombolytic therapy during AMI and had exercise 201Tl SPECT and coronary angiography prior to hospital discharge.35 Twenty-five (37%) patients either died (n = 2), had recurrent MI (n = 5), or were rehospitalized due to unstable angina (n = 11) or heart failure (n = 7). The LVEF (P < 0.005) (see Fig. 35-13) as well as the exercise-induced total (P = 0.002) and ischemic (P < 0.0005) SPECT perfusion defect size (Fig. 35-26) were all strong univariate predictors of subsequent cardiac events over 26 ± 18 months of follow-up. This is despite the fact that 45% of patients had coronary revascularization prior to SPECT imaging. None of the treadmill exercise variables predicted subsequent outcome. By multivariate analysis, the best predictors of risk were the LVEF (RR 1.85 for a 10% decrease) and quantified ischemic perfusion defect size (RR 1.38 for a 5% increase). The LVEF and scintigraphic variables significantly contributed to predicting risk beyond the clinical variables alone, with no additional information gained from the angiographic results (Fig. 35-27).
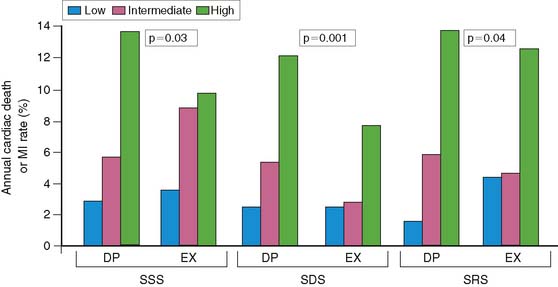
These results with submaximal exercise testing have been confirmed in patients studied with pharmacologic vasodilators. Brown and colleagues18 reported better separation of high and low risk with dipyridamole sestamibi SPECT in patients who received thrombolytic therapy (P = 0.02). Mahmarian and colleagues likewise showed that adenosine 201Tl SPECT imaging could predict events comparably well in patients who did or did not receive thrombolytic therapy during AMI, based on the quantified ischemic perfusion defect size (see Fig. 35-21B).17
The prognostic value of SPECT is evident even in seemingly very low-risk patients.49 In one study, 203 clinically stable enrolled patients (of whom 62% received thrombolytic therapy) had a normal submaximal exercise treadmill test prior to dipyridamole SPECT. Most patients, by coronary angiography, had either no significant CAD (23%) or only single-vessel involvement (52%). Over a mean follow-up of 15 ± 3 months, cardiac events occurred in 69 patients (34%), with 1 cardiac death, 7 recurrent infarctions, 26 admissions for unstable angina, and 35 subsequent revascularization procedures. Multivariate predictors of all events were the angiographic extent of CAD and presence of scintigraphic ischemia (Fig. 35-28). The scintigraphic data provided greater prognostic value than the angiographic results (Fig. 35-29). These data all indicate that the resultant extent of residual scintigraphic ischemia, rather than the initial thrombolytic strategy per se, best predicts future cardiac risk. Since patients postthrombolysis who lack ischemia by noninvasive testing have an excellent prognosis, it seems unlikely that coronary revascularization in this population would further improve outcome.98
RISK STRATIFICATION WITH NUCLEAR CARDIAC IMAGING IN THE ERA OF INTERVENTIONAL CARDIOLOGY
The cornerstone of risk stratification in stable survivors of STEMI is to identify (1) high-risk individuals who might benefit from coronary revascularization and (2) low-risk individuals in whom medical therapy and early hospital discharge is warranted. It is generally accepted that patients admitted with AMI who have signs of clinical instability are a high-risk group who should undergo coronary angiography with the intent to revascularize. These patients generally have ongoing or recurrent ischemic chest pain despite medical therapy, congestive heart failure, or hemodynamic instability and thereby incorporate many of the variables contained within the TIMI risk score.3 Patients with a high TIMI risk score (>5) have a reported in-hospital mortality of approximately 21% but fortunately represent only a relatively small (<20%) percentage of patients with STEMI. Importantly, these patients have been typically, and appropriately, excluded from trials assessing the role of nuclear cardiac imaging in risk stratification.
A further level of complexity exists in patients who receive acute PCI as the initial treatment of STEMI, where the coronary anatomy is, by default, revealed. Primary PCI is increasingly recognized as the treatment of choice for restoring coronary blood flow, preventing reinfarction, and improving survival.99–101 The Danish Multicenter Randomized Study on Fibrinolytic Therapy versus Acute Coronary Angioplasty in Acute Myocardial Infarction (DANAMI-2) study showed a significant reduction in the 30-day composite end point of death, recurrent MI, and disabling stroke among the 1572 patients who were randomized to PCI (8.0%) versus alteplase (13.7%).99 However, despite growing support for performing PCI over thrombolytic therapy in STEMI, for various reasons, only 18% of patients currently undergo primary PCI in the United States, with most either receiving thrombolytic agents (52%) or no reperfusion therapy (30%).102 Patients not treated with primary PCI would be good candidates for noninvasive risk stratification using nuclear cardiac imaging techniques. In those who do have primary PCI, nuclear cardiac imaging could still be selectively used to identify viable myocardium within the infarct zone, to assess for ischemia outside the infarct zone in patients with multivessel CAD, and to predict recovery in LV function in patients with myocardial stunning.
Rationale for an Invasive Approach
Beyond the benefits of coronary reperfusion during the early stages of STEMI, the rationale behind a routine invasive approach is to identify patients with a high-risk coronary anatomy where appropriate revascularization can be readily performed. Implicit to this approach is that coronary revascularization following AMI will improve survival and reduce morbidity from CAD, and do so more effectively than intensive medical therapy. If so, this should result in a more cost-effective strategy by limiting initial hospital stay and preventing subsequent admissions due to recurrence of clinical instability. Currently, only one trial has directly compared the relative efficacy of medical therapy versus coronary revascularization in patients surviving AMI who had stress-induced ischemia by noninvasive testing.103 Although mortality rates were similar over 2.4 years, infarct-free survival was significantly better in patients randomized to revascularization (90.5%) than in those who received medical therapy (85.1%).103 However, this difference may have occurred because of suboptimal use of medical therapy rather than a significant beneficial effect from revascularization per se.
Rationale for a Conservative Approach
The rationale behind a noninvasive conservative approach is that (1) most patients with AMI do not have subsequent cardiac events, (2) the subset of high-risk patients who are likely to benefit from revascularization can be accurately identified by noninvasive testing, and (3) intensive medical therapy can prevent anginal symptoms, and do so as well as routine revascularization. For a conservative approach to be effective, patients must undergo early risk stratification and with an accurate technique such as stress MPI. Exercise treadmill testing alone is insensitive for detecting high-risk patients with residual myocardial ischemia, but when combined with perfusion scintigraphy, it readily identifies ischemic patients and those with multivessel CAD.86 This is a critical issue, since patients who have preserved LV function and no residual ischemia are at very low risk, which is not further reduced with coronary revascularization.98 Likewise, coronary angioplasty and stenting of an occluded infarct-related artery does not improve outcome over medical therapy in patients who have depressed LV function and minimal or no residual ischemia.104 Based on the total and ischemic PDS and LVEF by gated SPECT, individual patient risk can be determined, followed by appropriate selection of patients for either invasive procedures or medical therapy and early hospital discharge.88,105 With the introduction of pharmacologic vasodilators, in lieu of exercise stress, perfusion imaging can be performed safely within 1 to 2 days of admission, thereby providing rapid risk stratification to guide patient-management decisions.17,18,85,87,88 Imaging early after admission is of paramount importance, since cardiac instability in susceptible patients tends to recur within days of the initial event.106,107 The TIMI investigators reported a significant reduction in overall survival among the approximately 4% of patients who received thrombolytic therapy for acute ST-segment elevation and had subsequent in-hospital reinfarction.106 This has also been shown in patients admitted with an ACS where prolonged antithrombotic therapy prior to a diagnostic procedure to assess risk leads to a higher event rate.107
Clinical Trial Support for Either Strategy
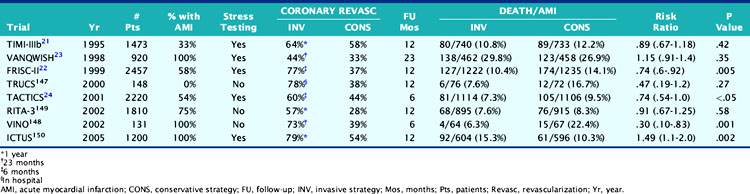
The widespread acceptance of an invasive strategy as the community standard of care for patients following AMI has largely been fueled by favorable results reported from trials in acute coronary syndromes (Table 35-8).108–115 Trials demonstrating an outcome advantage with a routine invasive approach have generally compared “state-of-the-art” interventional strategies to one of submaximal treadmill testing.110,112 Other trials have not even included routine noninvasive risk stratification in their study design (see Table 35-8).111,113,114 Furthermore, in many of these trials, such as the Fast Revascularization during Instability in Coronary artery disease (FRISC) and Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy (TACTICS)-TIMI 18 trials, it is unclear the degree to which medical therapy was administered in patients with residual ischemia.110,112 This is particularly relevant, since patients in the conservative strategy only crossed over to coronary angiography when ischemia was considered severe by treadmill testing. Since exercise treadmill testing was the stressor used in both of these trials, many patients randomized to the conservative strategy suffered a recurrent cardiac event during the “watchful waiting” period—before the index stress test could be safely performed.
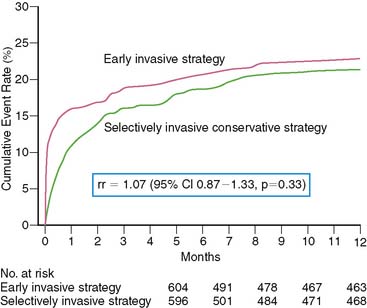
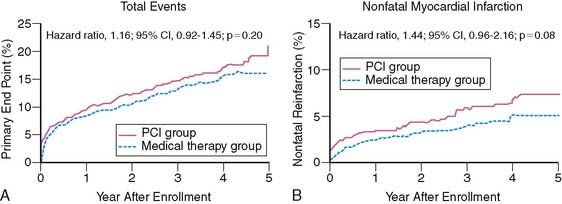
A more recent study disputes the finding from TACTICS112 and FRISC-II110 in ACS patients who all had enzymatic evidence of non-ST-elevation AMI. The Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) trial randomized 1200 patients to either a routine invasive strategy or a conservative strategy where coronary angiography was selectively performed based on clinical criteria and predischarge exercise stress test results.115 Similar to other trials, the primary end point was a composite of death, reinfarction, or rehospitalization for angina at 1 year. Coronary revascularization was performed by 1 year in 79% of patients assigned to the early invasive strategy and in 54% randomized to the selectively invasive group. Most PCI procedures were performed with stents (88%) and abciximab. The study was powered at 80% to detect a 25% risk reduction between the two groups at an alpha level of 0.05. The 1-year cumulative event rate was similar in the two groups, balanced by the higher reinfarction rate in the invasive (15%) versus the conservative (10%) group (RR 1.50, 95% CI 1.10–2.04, P = 0.005), but the higher rehospitalization rate in the conservative versus invasive limb (10.9% versus 7.4%, P = 0.04) (Fig. 35-30). Subgroup analysis based on age, gender, diabetes status, ST-segment deviation, and baseline troponin T showed no advantage of the early invasive over the conservative strategy. The results did not change when the more strict definitions of reinfarction used in FRISC-II and TACTICS were applied to the ICTUS population.
Beyond these trials in acute coronary syndromes, several studies in patients with STEMI showed no significant prognostic advantage with an invasive versus a conservative strategy. In the TIMI-IIB study, 3339 patients with acute STEMI received thrombolytic therapy and were then randomized to either early coronary angiography or a conservative strategy where coronary angiography was performed only in patients who had spontaneous ischemia or inducible ischemia on treadmill testing.116 Although twice as many patients had either angioplasty or coronary artery bypass surgery by 1 year in the invasive (72%) versus the conservative limb (35%), 1-year71 and 3-year94 infarct-free survival were virtually identical in both groups.
Similar results were reported in the Should We Intervene Following Thrombolysis (SWIFT) study, where 800 patients with STEMI received anistreplase and were then randomized to either a conservative or invasive strategy.117 Coronary revascularization was performed in 57% of patients randomized to the invasive strategy, which increased to 61% by 1 year. In the conservative strategy group, 5% of patients had coronary revascularization at hospital discharge and only 15% by 1 year. As in TIMI-IIB, the conservative therapy group did as well as those assigned to an invasive strategy, with a 1-year infarct-free survival of 83.4% versus 80.9% (P = NS), respectively.
In the Medicine versus Angiography in Thrombolytic Exclusion (MATE) trial, 201 patients with STEMI who were ineligible for thrombolysis were randomized.118 In the invasive strategy, 58% of patients were revascularized, compared to 37% in the conservative medical therapy limb (P = 0.004). Of note, 27 of 54 patients who underwent coronary angiography in the conservative limb did so as a result of physician preference and not because of clinical instability. After 21 months of follow-up, no significant differences in death (11% versus 10%) or infarct-free survival (14% versus 12%) were observed between the two groups.
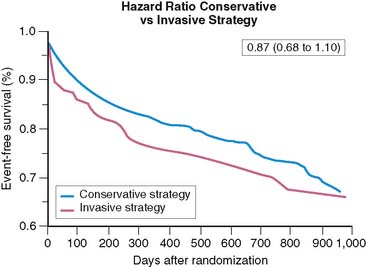
The Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) trial is the only one that selected myocardial perfusion scintigraphy as the noninvasive testing modality in 920 patients randomized to either an invasive or conservative strategy following non-Q-wave AMI.109 In this study, 30% of randomized patients had STE on the initial ECG.119 During the trial, significantly higher rates of coronary angiography (94% versus 48%) and revascularization (44% versus 33%) were observed in the invasive versus conservative limbs, despite a comparable 1-year infarct-free survival (76% versus 81%; Fig. 35-31). A recent analysis from the VANQWISH investigators demonstrated that the conservative strategy was more cost-effective than the routine invasive approach.120
These large randomized trials are further supported by subgroup analyses from the SAVE121 and GUSTO-I trials,122 which showed comparable infarct-free survival among patients treated in the United States and Canada, despite a two- to threefold higher rate of coronary revascularization in the former country. In SAVE, only effort-related angina was less frequent in the United States than Canada (27% versus 33%).122 Data from the Organization to Assess Strategies for Ischemic Syndromes (OASIS) registry also show no difference in 6-month outcome among patients admitted to hospitals in countries where coronary angiography and revascularization are more frequently performed.123 In addition, a recent substudy from the OASIS-5 trial in 184 women with non-STE AMI showed no added benefit to an early invasive versus a selective-invasive approach.124 In fact in this study, as in ICTUS, there was a strong trend for a higher event rate (death, AMI, stroke) in women randomized to the invasive versus the conservative limb (HR 1.46, 95% CI 0.73–2.94). Consistent with current clinical practice, most patients in the conservative limb were administered aspirin (98.9%), an angiotensin receptor blocker (80.4%), a β-blocker (93.5%), and lipid-lowering therapy (85.9%). The sum of these trials indicate that a routine invasive approach may be most appropriate in the clinically highest-risk patients and particularly when timely noninvasive risk stratification is not available and/or appropriate intensive medical therapy is not administered.
THE INSPIRE TRIAL—IMPLICATIONS FOR RISK STRATIFICATION
The Adenosine Sestamibi SPECT Post-Infarction Evaluation (INSPIRE) trial was a large prospective multicenter randomized study that enrolled 728 stabilized patients with ST-elevation (60%) or non-ST-elevation (40%) AMI.88,105,125 Adenosine 99mTc sestamibi SPECT was used as an initial noninvasive method to assess risk and guide subsequent therapeutic decision making early after AMI. INSPIRE is the only prospective trial other than VANQWISH109 to use myocardial perfusion scintigraphy for risk stratification. Unlike previous studies, all patients in INSPIRE were assigned to a conservative strategy of stress SPECT, with subsequent triage to invasive procedures based on the imaging results. Imaging was performed early after AMI, with online study interpretation by a central core laboratory within 24 hours. This was a critical study design feature, recognizing that cardiac events tend to recur early after both ST-elevation106 and non-ST-elevation AMI.107 In TACTICS112 and FRISC,110 exercise stress testing in the conservative limb led to an unavoidable delay in identifying ischemia. In INSPIRE, over half the patients at U.S. sites were safely imaged with adenosine within 2 days of hospital admission so as to facilitate subsequent patient care and avoid the pitfalls of these earlier trials. No cardiac events occurred in INSPIRE patients prior to noninvasive testing, due in part to the expedited imaging protocol.
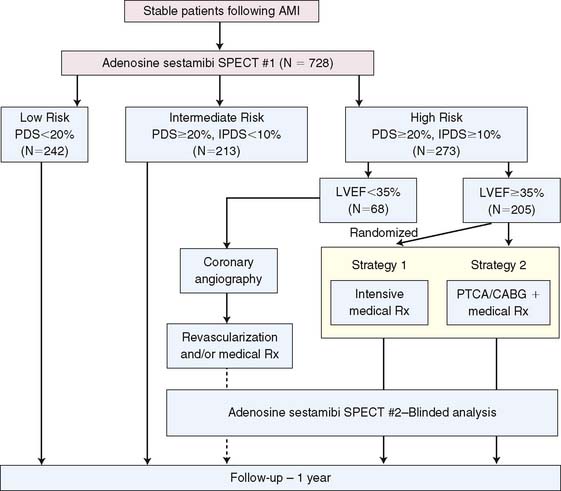
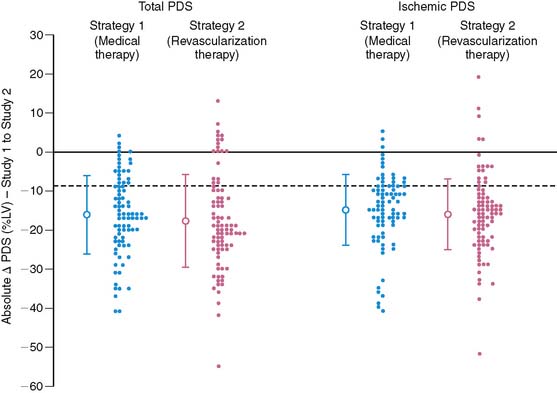
Based on previous preliminary studies,17,91,126 patient risk and management decisions were prospectively defined by the scintigraphic findings (Fig. 35-32).125 Patients with a small quantified PDS (<20%) were considered low risk with an anticipated greater than 95% infarct-free survival at 1 year. These patients were medically managed and targeted for early hospital discharge. Patients with large (>20%) primarily nonischemic (<10%) defects were recognized to be at higher (intermediate) risk but thought unlikely to benefit from coronary revascularization. Patients with large (>20%) and ischemic (>10%) perfusion defects who had an LVEF less than 35% were defined as high risk and encouraged to have coronary angiography with the intent to revascularize, whereas those with an LVEF greater than 35% were randomized to receive either intensive medical therapy or coronary revascularization.125 Following optimization of therapy, SPECT was repeated at 6 to 8 weeks to assess the relative effect of therapy on total and ischemic PDS and subsequent patient outcome. Follow-up was complete at 1 year in 98% of patients.
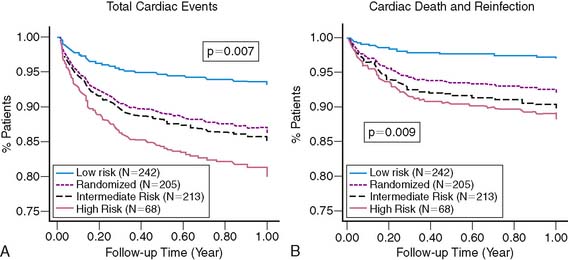
As anticipated, overall cardiac and death/reinfarction event rates significantly increased across INSPIRE risk groups (Fig. 35-33).88 However, a very low 1.8% death and re-infarction rate was observed at 1 year in the prospectively defined low-risk group, even though 38% were at intermediate or high clinical risk based on their TIMI score, and their mean troponin T was elevated at 1.1 ± 1 μg/mL. Adenosine SPECT identified this low-risk population irrespective of age, gender, site of infarction, or clinical risk. The total and ischemic defect sizes were the best multivariate risk predictors and allowed estimation of risk in individual patients (Fig. 35-34). This was true both in patients with STEMI and in those with non-ST-elevation MI.
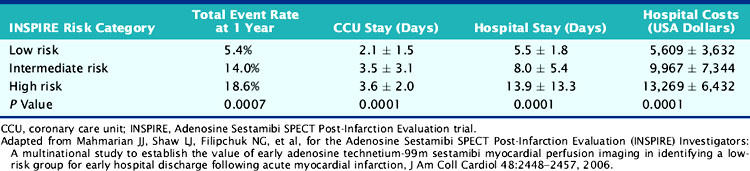
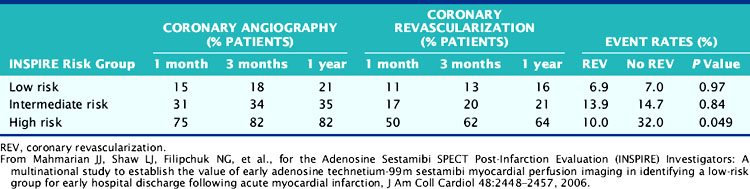
The results from INSPIRE,88 ICTUS,115 and the Occluded Artery Trial (OAT)104 question the recommendations from TACTICS112,127 that all patients with an elevated troponin should undergo routine coronary angiography, since it is unlikely that coronary revascularization will further benefit scintigraphically low-risk patients or those with minimal or no residual ischemia. The prospectively defined low-risk group in INSPIRE, which represented one-third of all enrolled patients, had the lowest rate of coronary revascularization, the shortest hospital stay, the lowest hospital-related costs, and yet the lowest overall event rate (Table 35-9). Coronary revascularization did not improve outcome in this group (Table 35-10). Based on the cost-analysis approach used in TACTICS,128 the cost savings in this group would have been approximately 65% compared to a routine invasive approach.
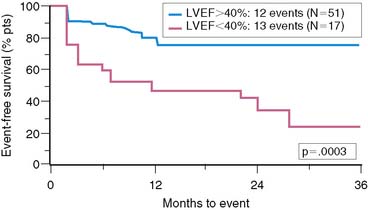
It must be emphasized that the purpose of risk stratification is not only to identify high-risk patients but also to determine in whom risk can be reduced. The INSPIRE intermediate-risk group represented an additional 29% of patients who were at higher clinical risk for events but unlikely to benefit from coronary revascularization, since they had minimal residual ischemia.104,129,130 The recently published OAT trial demonstrated no prognostic advantage to dilating and stenting occluded infarct-related arteries over medical therapy alone in patients with minimal or no residual ischemia (Fig. 35-35).104 In fact, a disturbing trend toward a higher reinfarction rate was observed in those randomized to PCI. The intermediate-risk group in INSPIRE also showed similar event rates among those who were or were not revascularized (see Table 35-10). The high-risk INSPIRE group who had extensive residual ischemia and significantly depressed LV function were generally selected for coronary revascularization but represented only 9% of all enrolled patients. As supported by retrospective analyses,129–130 those who were revascularized had a significantly lower overall 1-year event rate than the remainder who received medical therapy alone (10% versus 32%, P = 0.049) (see Table 35-10). The Surgical Treatment for Ischemic Heart Failure (STICH) trial is a prospective randomized trial comparing medical therapy to cardiac surgery in similar patients but who have chronic ischemic cardiomyopathy and an LVEF less than 35%.131
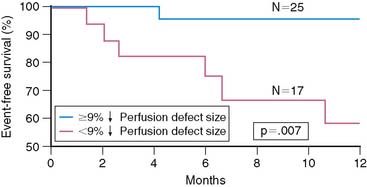
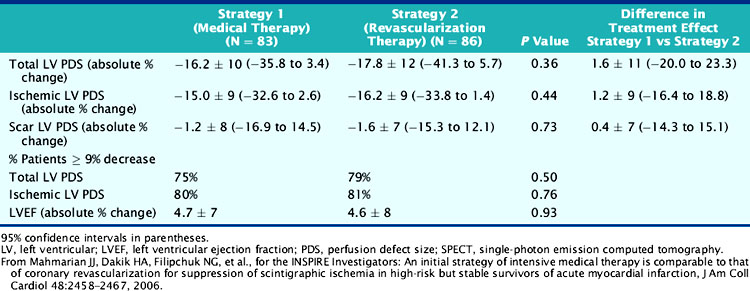
Among the randomized patients with preserved LV function, intensive medical therapy was as effective as coronary revascularization in reducing total and ischemic PDS, as determined by serial SPECT imaging pre- and post-therapy (Table 35-11) (Fig. 35-36).105 Patients with ischemia in the conservative limbs of other trials have generally been referred for coronary angiography with the intent to revascularize. Similar to ICTUS,115 FRISC-II,110 and TACTICS,112 most patients randomized to the interventional arm had coronary revascularization (81%) with over 80% of significantly (>70%) stenosed arteries revascularized. Stents were placed in 94% of arteries undergoing PCI. However, unlike most trials, patients randomized to medical therapy had prospectively defined intensive treatment with antiplatelet (100%), β-blocker (93%), ACE inhibitor (77%), and statin (84%) medications so as to ensure a fair comparison to coronary revascularization. The suppression of scintigraphic ischemia with medical therapy is consistent with earlier small reports in patients with chronic CAD132 and similar to the findings from the pilot study to INSPIRE.126 The comparable event rates in the two groups support the scintigraphic findings and indicate that currently available intensive medical therapy may be a reasonable alternative in stable AMI patients who are not optimal revascularization candidates (Fig. 35-37). The pilot study to INSPIRE also indicated improved event-free survival in patients who had a significant (>9%) reduction in PDS following either medical therapy or PCI (Fig. 35-38).126 These data suggest that MPI may be used not only to assess initial risk but also to track subsequent risk by evaluating the efficacy of various therapies on myocardial ischemia. A larger adequately powered prospective randomized event trial in ischemic patients after AMI is warranted.
Table 35-11 Changes in Gated SPECT Results in the Medical Versus Revascularization Strategies in INSPIRE
The INSPIRE results are consistent with over 2 decades of clinical studies evaluating a broad spectrum of patients with ACS in whom stress myocardial perfusion scintigraphy provided accurate risk stratification.15–18,35,49–50,80–81,91 The implications from INSPIRE are that “state-of-the-art” adenosine SPECT imaging performed very early after AMI can safely and reliably identify a large patient cohort unlikely to benefit from an invasive evaluation, either because of their low-risk scintigraphic profile or lack of inducible ischemia. Conversely, adenosine SPECT can also identify the subset of high-risk patients with extensive ischemia where intensive medical and/or interventional therapies are appropriate. The INSPIRE results complement the findings from earlier randomized clinical trials comparing invasive approaches to conservative approaches, which have incorporated either routine noninvasive risk stratification as a method for triaging patients and/or used current medical therapy approaches.
CURRENT GUIDELINES FOR STRATIFYING RISK IN ST-ELEVATION AMI
Exercise MPI is recommended in stable patients with ST-elevation AMI who have not yet undergone in-hospital coronary angiography and who have an uninterpretable ECG for assessing ischemia.5 This would include patients who receive thrombolytics or patients who have not had reperfusion therapy (Table 35-12). Treadmill testing alone is still recommended in patients with an interpretable ECG. This may change in subsequent guidelines, since treadmill testing is inferior to exercise SPECT for detecting residual ischemia18 and predicting subsequent outcome,16,18,35,49,50 and cannot localize ischemia to the culprit coronary artery.
Table 35-12 Current Practice Guidelines: Risk Stratification Following ST-Elevation AMI5
AMI, acute myocardial infarction; CHF, congestive heart failure; ECG, electrocardiogram.
From Antman EM: 2004 Update: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). Available at www.acc.org.
Pharmacologic vasodilators are currently recommended with SPECT only in patients who cannot exercise, even though submaximal exercise SPECT is less sensitive for detecting ischemia18 and cannot be performed earlier than 3 to 4 days after admission. This latter issue significantly limits the value of a noninvasive approach, since events recur early after AMI.106–107 INSPIRE corroborates earlier trial data attesting to the safety of adenosine in assessing risk even when administered within 24 hours of admission.88 Future guidelines may liberalize the use of pharmacologic vasodilators to patients regardless of their exercise capability so as to expedite risk stratification.
Clinically unstable patients with postinfarction angina, decompensated CHF, life-threatening cardiac dysrhythmias, or hemodynamic instability are recommended for coronary angiography.5 Such patients are appropriately excluded from a noninvasive assessment but represent a small minority (15% to 20%) of those with AMI. However, the guidelines also categorize patients with an LVEF less than 40% as a uniformly high-risk group who will benefit from routine catheterization. This recommendation may also change based on the recent OAT results, where PCI did not improve outcome in patients who had significant LV dysfunction104 but minimal or no ischemia. Gated adenosine SPECT can readily identify which patients have depressed LV function and whether there is ischemic or nonviable myocardium present so as to guide management.88
CURRENT GUIDELINES FOR STRATIFYING RISK IN NON-ST-ELEVATION AMI
The most recent practice guidelines addressing management of patients with unstable angina and non-ST-elevation AMI have been strongly influenced by the FRISC-II110 and TACTICS-TIMI 18112 results. Prior to these trials, a routine conservative approach was considered an acceptable alternative to an invasive strategy except in the clinically highest-risk unstable patients. Since then, coronary angiography has been recommended as a frontline approach in all but the most stable lowest-risk patients and in all who are troponin positive. However, the most recent ACC/AHA 2007 guideline revision once again lends stronger support for a noninvasive conservative strategy (Table 35-13).133 The INSPIRE,88 ICTUS,115 and OAT104 trial results should further promote this philosophy and emphasize the role of perfusion imaging in patient evaluation.
Table 35-13 Current Practice Guidelines: Risk Stratification Following Non-ST-Elevation AMI and Unstable Angina
AMI, acute myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; UA, unstable angina.
From Anderson JL, Adams CD, Antman EM, et al: ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction), developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons, endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine, J Am Coll Cardiol 50:e1–e157, 2007.
1. Detailed diagnoses and procedures. National Hospital Discharge Survey, 1996. National Center for Health Statistics. Vital Health Stat. 1998;13:138.
2. Lee K.L., Woodlief L.H., Topol E.J., for the GUSTO-I Investigators. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. Circulation. 1995;91:1659-1668.
3. Morrow D.A., Antman E.M., Charlesworth A., et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation. An Intravenous nPA for Treatment of Infarcting Myocardium Early II trial substudy. Circulation. 2000;102:2031-2037.
4. Morrow D.A., Antman E.M., Parsons L., et al. Application of the TIMI risk score for ST-elevation MI in the National Registry of Myocardial Infarction 3. JAMA. 2001;286:1356-1359.
5. Antman E.M. 2004: update: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction), Available at www.acc.org.
6. Cerqueira M.D., Maynard C., Ritchie J.L., et al. Long-term survival in 618 patients from the Western Washington Streptokinase in Myocardial Infarction trials. J Am Coll Cardiol. 1992;20:1452-1459.
7. Miller T.D., Christian T.F., Hopfenspirger M.R., et al. Infarct size after acute myocardial infarction measured by quantitative tomography Tc-99m sestamibi imaging predicts subsequent mortality. Circulation. 1995;92:334-341.
8. Ohman E.M., Armstrong P.W., Christenson R.H., et alfor the GUSTO-IIa Investigators. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. N Engl J Med. 1996;335:1333-1341.
9. Antman E.M., Tanasijevic M.J., Thompson B., et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342-1349.
10. The Multicenter Postinfarction Research Group. Risk stratification and survival after myocardial infarction. N Engl J Med. 1983;309:331-336.
11. Simoons M.L., Vos J., Tijssen J.G., et al. Long-term benefit of early thrombolytic therapy in patients with acute myocardial infarction: 5-year follow-up of a trial conducted by the Interuniversity Cardiology Institute of the Netherlands. J Am Coll Cardiol. 1989;14:1609-1615.
12. Sanz G., Castaner A., Betriu A., et al. Determinants of prognosis in survivors of myocardial infarction. A prospective clinical angiographic study. N Engl J Med. 1987;306:1065-1070.
13. White H.D., Norris R.M., Brown M.A., et al. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44-51.
14. St John Sutton M., Pfeffer M.A., Plappert T., et alfor the SAVE investigators. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68-75.
15. Gibson R.S., Beller G.A., Gheorghiade M., et al. The prevalence and clinical significance of residual myocardial ischemia 2 weeks after uncomplicated non-Q wave infarction: A prospective natural history study. Circulation. 1986;73:1186-1198.
16. Gibson R.S., Watson D.D., Craddock G.B., et al. Prediction of cardiac events after uncomplicated myocardial infarction: A prospective study comparing predischarge exercise thallium-201 scintigraphy and coronary angiography. Circulation. 1983;68:321-336.
17. Mahmarian J.J., Mahmarian A.C., Marks G.F., Pratt C.M., Verani M.S. Role of adenosine thallium-201 tomography for defining long-term risk in patients after acute myocardial infarction. J Am Coll Cardiol. 1995;25:1333-1340.
18. Brown K.A., Heller G.V., Landin R.S., et al. Early dipyridamole 99mTc-sestamibi single photon emission computed tomographic imaging 2 to 4 days after acute myocardial infarction predicts in-hospital and postdischarge cardiac events: Comparison with submaximal exercise imaging. Circulation. 1999;100:2060-2066.
19. Leppo J.A., Meerdink D.A. Comparison of the myocardial uptake of a technetium-labeled isonitrile analogue and thallium. Circ Res. 1989;65:632-639.
20. Nishiyama H., Adolph R.J., Gabel M., et al. Effect of coronary blood flow on thallium-201 uptake and washout. Circulation. 1982;65:534-542.
21. Okada R.D., Glover D., Gaffney T., et al. Myocardial kinetics of technetium-99m-hexakis-2-methoxy-2-methylpropyl-isonitrile. Circulation. 1988;77:491-498.
22. Granato J.E., Watson D.D., Flanagan T.L., et al. Myocardial thallium-201 kinetics during coronary occlusion and reperfusion: Influence of method of reflow and timing of thallium-201 administration. Circulation. 1986;73:150-160.
23. DeCoster P.M., Wijns W., Cauwe F., et al. Area-at-risk determination by technetium-99m-hexakis-2-methoxyisobutyl isonitrile in experimental reperfused myocardial infarction. Circulation. 1990;82:2152-2162.
24. Liu P., Houle S., Mills L., et al. Kinetics of Tc-99m MIBI in clearance in ischemia-reperfusion: Comparison with Tl-201 (abstr). Circulation. 1987;76(Suppl IV):216.
25. Sinusas A.J., Trautman K.A., Bergin J.D., et al. Quantification of area at risk during coronary occlusion and degree of myocardial salvage after reperfusion with technetium-99m methoxyisobutyl isonitrile. Circulation. 1990;82:1424-1437.
26. Verani M.S., Jeroudi M.O., Mahmarian J.J., et al. Quantification of myocardial infarction during coronary occlusion and myocardial salvage after reperfusion using cardiac imaging with technetium-99m hexakis 2-methoxyisobutyl isonitrile. J Am Coll Cardiol. 1988;12:1573-1581.
27. Christian T.F., Behrenbeck T., Pellikka P.A., et al. Mismatch of left ventricular function and infarct size demonstrated by technetium-99m isonitrile imaging after reperfusion therapy for acute myocardial infarction: Identification of myocardial stunning and hyperkinesia. J Am Coll Cardiol. 1990;16:1632-1638.
28. Gibbons R.J., Verani M.S., Behrenbeck T., et al. Feasibility of tomographic 99mTc-hexakis-2-methoxy-2-methylpropyl-isonitrile imaging for the assessment of myocardial area at risk and the effect of treatment in acute myocardial infarction. Circulation. 1989;80:1277-1286.
29. Gibbons R.J., Holmes D.R., Reeder G.S., et al. Immediate angioplasty compared with the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction. N Engl J Med. 1993;328:685-691.
30. Santoro G.M., Bisi G., Sciagra R., et al. Single photon emission computed tomography with technetium-99m hexakis 2-methoxyisobutyl isonitrile in acute myocardial infarction before and after thrombolytic treatment: Assessment of salvaged myocardium and prediction of late functional recovery. J Am Coll Cardiol. 1990;15:301-314.
31. Dixon S.R., Whitbourn R.J., Dae M.W., et al. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40:1928-1934.
32. Kopecky S.L., Aviles R.J., et alfor the AmP579 Delivery for Myocardial Infarction REduction Study. A randomized, double-blinded, placebo-controlled, dose-ranging study measuring the effect of an adenosine agonist on infarct size reduction in patients undergoing primary percutaneous transluminal coronary angioplasty: The ADMIRE (AmP579 Delivery for Myocardial Infarction REduction) study. Am Heart J. 2003;146:146-152.
33. Angeja B.G., Gunda M., Murphy S.A., et al. TIMI myocardial perfusion grade and ST segment resolution: Association with infarct size as assessed by single photon emission computed tomography imaging. Circulation. 2002;105:282-285.
34. Faxon D.P., Gibbons R.J., Chronos N.A., et alfor the HALT-MI Investigators. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: The results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199-1204.
35. Dakik H.A., Mahmarian J.J., Kimball K.T., et al. Prognostic value of exercise thallium-201 tomography in patients treated with thrombolytic therapy during acute myocardial infarction. Circulation. 1996;94:2735-2742.
36. Zaret B.L., Wackers F.J.T., Terrin M.L., et alfor the TIMI Study Group. Value of radionuclide rest and exercise left ventricular ejection fraction in assessing survival of patients after thrombolytic therapy for acute myocardial infarction: Results of Thrombolysis in Myocardial Infarction (TIMI) Phase II Study. J Am Coll Cardiol. 1995;26:73-79.
37. Volpi A., DeVita C., Franzosi M.G., et althe Ad hoc Working Group of the Gruppo Italiano per lo Studio della Sopravivienza nell’Infarto Miocardico (GISSI)-2 Data Base. Determinants of 6-month mortality in survivors of myocardial infarction after thrombolysis: Results of the GISSI-2 Data Base. Circulation. 1993;88:416-429.
38. The GUSTO: Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med. 1993;329:1615-1622.
39. Pfeffer M.A., Braunwald E., Moye L.A., et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement Trial. N Engl J Med. 1992;327:669-677.
40. Sharir T., Germano G., Kavanagh P.B., et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999;100:1035-1042.
41. Seals A.A., Pratt C.M., Mahmarian J.J., et al. Relation of left ventricular dilation during acute myocardial infarction to systolic performance, diastolic dysfunction, infarct size and location. Am J Cardiol. 1988;61:224-229.
42. Pfeffer M.A., Lamas G.A., Vaughan D.E., et al. Effect of captopril on progressive left ventricular dilation after anterior myocardial infarction. N Engl J Med. 1988;319:80-86.
43. Pfeffer M.A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161-1172.
44. Erlebacher J.A., Weiss J.L., Weisfeldt M.L., et al. Early dilation of the infarcted segment in acute transmural myocardial infarction: Role of infarct expansion in acute left ventricular enlargement. J Am Coll Cardiol. 1984;4:201-208.
45. Erlebacher J.A., Weiss J.L., Eaton L.W., et al. Late effects of acute infarct dilation on heart size: A two-dimensional echocardiographic study. Am J Cardiol. 1982;49:1120-1126.
46. Mahmarian J.J., Moye L.A., Chinoy D.A., et al. Transdermal nitroglycerin patch therapy improves left ventricular function and prevents remodeling after acute myocardial infarction: Results of a multicenter prospective randomized double-blind placebo controlled trial. Circulation. 1998;97:2017-2024.
47. Mahmarian J.J., Moye L., Verani M.S., et al. Criteria for the accurate interpretation of changes in left ventricular ejection fraction and cardiac volumes as assessed by rest and exercise gated radionuclide angiography. J Am Coll Cardiol. 1991;18:112-119.
48. Hyun I.Y., Kwan J., Park K.S., et al. Reproducibility of Tl-201 and Tc-99m sestamibi gated myocardial perfusion SPECT measurement of myocardial function. J Nucl Cardiol. 2001;8:182-187.
49. Chiamvimonvat V., Goodman S.G., Langer A., et al. Prognostic value of dipyridamole SPECT imaging in low-risk patients after myocardial infarction. J Nucl Cardiol. 2001;8:136-143.
50. Travin M.I., Dessouki A., Cameron T., et al. Use of exercise technetium-99m sestamibi SPECT imaging to detect residual ischemia and for risk stratification after acute myocardial infarction. Am J Cardiol. 1995;75:665-669.
51. Theroux P., Waters D.D., Halphen C., et al. Prognostic value of exercise testing soon after myocardial infarction. N Engl J Med. 1979;301:341-345.
52. Starling M.R., Crawford M.H., Kennedy G.T., et al. Exercise testing early after myocardial infarction: Predictive value of subsequent unstable angina and death. Am J Cardiol. 1980;46:909-914.
53. Fioretti P., Brower R.W., Simoons M.L., et al. Prediction of mortality during the first year after acute myocardial infarction from clinical variables and stress test at hospital discharge. Am J Cardiol. 1984;55:1313-1318.
54. Weld F.M., Chu K.-L., Bigger J.T., et al. Risk stratification with low-level exercise testing 2 weeks after acute myocardial infarction. Circulation. 1981;64:306-314.
55. Williams W.L., Nair R.C., Higginson L.A., et al. Comparison of clinical and treadmill variables for the prediction of outcome after myocardial infarction. J Am Coll Cardiol. 1984;4:477-486.
56. Jelinek V.M., McDonald I.G., Ryan W.F., et al. Assessment of cardiac risk 10 days after uncomplicated myocardial infarction. BMJ. 1982;284:227-230.
57. Jennings K., Reid D.S., Hawkins T., et al. Role of exercise testing early after myocardial infarction in identifying candidates for coronary surgery. BMJ. 1984;288:185-187.
58. Handler C.E. Submaximal predischarge exercise testing after myocardial infarction: Prognostic value and limitations. Eur Heart J. 1985;6:510-517.
59. Krone R.J., Gillespie J.A., Weld F.M., et al. Low-level exercise testing after myocardial infarction: Usefulness in enhancing clinical risk stratification. Circulation. 1985;71:80-89.
60. Applegate R.J., Dell’Italia L.J., Crawford M.H. Usefulness of two-dimensional echocardiography during low-level exercise testing early after uncomplicated acute myocardial infarction. Am J Cardiol. 1987;60:10-14.
61. Picano E., Landi P., Bolognese L., et al. Prognostic value of dipyridamole echocardiography early after uncomplicated myocardial infarction: A large-scale, multicenter trial. Am J Med. 1993;95:608-618.
62. Candell-Riera J., Permanyer-Miralda G., Castell J., et al. Uncomplicated first myocardial infarction: Strategy for comprehensive prognostic studies. J Am Coll Cardiol. 1991;18:1207-1219.
63. Corbett J.R., Nicod P., Lewis S.E., et al. Prognostic value of submaximal exercise radionuclide ventriculography after myocardial infarction. Am J Cardiol. 1983;52:82A-91A.
64. Chaitman B.R., McMahon R.P., Terrin M., et al. Impact of treatment strategy on predischarge exercise test in the Thrombolysis in Myocardial Infarction (TIMI) II Trial. Am J Cardiol. 1993;71:131-138.
65. Gianrossie R., Detrano R., Mulvihill D., et al. Exercise induced ST depression in the diagnosis of coronary artery disease: A meta-analysis. Circulation. 1989;80:87-98.
66. Heller G.V., Ahmed I., Tilkemeier P.L., et al. Influence of exercise intensity on the presence, distribution, and size of thallium-201 defects. Am Heart J. 1992;123:909-916.
67. Gimple L.W., Hutter Am, Guiney T.E., et al. Prognostic utility of predischarge dipyridamole-thallium imaging after uncomplicated acute myocardial infarction. Am J Cardiol. 1989;64:1243-1248.
68. Wilson W.W., Gibson R.S., Nygaard T.W., et al. Acute myocardial infarction associated with single vessel coronary artery disease. An analysis of clinical outcome and the prognostic importance of vessel patency and residual ischemic myocardium. J Am Coll Cardiol. 1988;11:223-234.
69. Tilkemeier P.L., Guiney T.E., LaRaia P.J., et al. Prognostic value of predischarge low-level exercise thallium testing after thrombolytic treatment of acute myocardial infarction. Am J Cardiol. 1990;66:1203-1207.
70. Haber H.L., Beller G.A., Watson D.D., et al. Exercise thallium-201 scintigraphy after thrombolytic therapy with or without angioplasty for acute myocardial infarction. Am J Cardiol. 1993;71:1257-1261.
71. Williams D.O., Braunwald E., Knatterud G., et al. One-year results of the Thrombolysis in Myocardial Infarction Investigation (TIMI) Phase II trial. Circulation. 1992;85:533-542.
72. Abraham R.D., Harris P.J., Roubin G.S., et al. Usefulness of ejection fraction response to exercise one month after acute myocardial infarction in predicting coronary anatomy and prognosis. Am J Cardiol. 1987;60:225-230.
73. Corbett J.R., Dehmer G.J., Lewis S.E., et al. The prognostic value of submaximal exercise testing with radionuclide ventriculography before hospital discharge in patients with recent myocardial infarction. Circulation. 1981;64:535-544.
74. Hung J., Goris M.L., Nash E., et al. Comparative value of maximal treadmill testing, exercise thallium myocardial perfusion scintigraphy and exercise radionuclide ventriculography for distinguishing high- and low-risk patients soon after acute myocardial infarction. Am J Cardiol. 1984;53:1221-1227.
75. Nicod P., Corbett J.R., Firth B.G., et al. Prognostic value of resting and submaximal exercise radionuclide ventriculography after acute myocardial infarction in high-risk patients with single and multivessel disease. Am J Cardiol. 1983;52:30-36.
76. Roig E., Magrina J., Armengol X., et al. Prognostic value of exercise radionuclide angiography in low-risk acute myocardial infarction survivors. Eur Heart J. 1993;14:213-218.
77. Morris K.G., Palmeri S.T., Califf R.M., et al. Value of radionuclide angiography for predicting specific cardiac events after acute myocardial infarction. Am J Cardiol. 1985;55:318-324.
78. Brown K.A., Weiss R.M., Clements J.P., et al. Usefulness of residual ischemic myocardium within prior infarct zone for identifying patients at high risk late after acute myocardial infarction. Am J Cardiol. 1987;60:15-19.
79. Younis L.T., Byers S., Shaw L., et al. Prognostic value of intravenous dipyridamole-thallium scintigraphy after acute myocardial ischemic events. Am J Cardiol. 1989;64:161-166.
80. Leppo J.A., O’Brien J., Rothendler J.A., et al. Dipyridamole-thallium-201 scintigraphy in the prediction of future cardiac events after acute myocardial infarction. N Engl J Med. 1984;310:1014-1018.
81. Brown K.A., O’Meara J., Chambers C.E., et al. Ability of dipyridamole-thallium-201 imaging 1 to 4 days after acute myocardial infarction to predict in-hospital and late recurrent myocardial ischemic events. Am J Cardiol. 1990;65:160-167.
82. Wilson R.F., Wyche K., Christensen B.V., Zimmer S., Laxson D.D. Effects of adenosine on human coronary arterial circulation. Circulation. 1990;82:1595-1606.
83. Rossen J.D., Quillen J.E., Lopez A.G., Stenberg R.G., Talman C.L., Winniford M.D. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18:485-491.
84. Nishimura S., Mahmarian J.J., Verani M.S. Effect of exercise level on equivalence between adenosine and exercise thallium-201 myocardial tomography in coronary artery disease (abstr). J Nucl Med. 1993;34:95P.
85. Heller G.V., Brown K.A., Landin R.J., et aland the Early Post MI IV Dipyridamole Study (EPIDS). Safety of early intravenous dipyridamole technetium 99m sestamibi SPECT myocardial perfusion imaging after uncomplicated first myocardial infarction. Am Heart J. 1997;134:105-111.
86. Mahmarian J.J., Pratt C.M., Nishimura S., et al. Quantitative adenosine Tl-201 single-photon emission computed tomography for the early assessment of patients surviving acute myocardial infarction. Circulation. 1993;87:1197-1210.
87. Abreu A., Mahmarian J.J., Nishimura S., et al. Tolerance and safety of pharmacologic coronary vasodilation with adenosine in association with thallium-201 scintigraphy in patients with suspected coronary artery disease. J Am Coll Cardiol. 1991;18:730-735.
88. Mahmarian J.J., Shaw L.J., Filipchuk N.G., et alfor the Adenosine Sestamibi SPECT Post-Infarction Evaluation (INSPIRE) Investigators. A multinational study to establish the value of early adenosine technetium-99m sestamibi myocardial perfusion imaging in identifying a low-risk group for early hospital discharge following acute myocardial infarction. J Am Coll Cardiol. 2006;48:2448-2457.
89. Bosch X., Magrina J., March R., et al. Prediction of in-hospital cardiac events using dipyridamole-thallium scintigraphy performed very early after acute myocardial infarction. Clin Cardiol. 1996;19:189-196.
90. Pirelli S., Inglese E., Suppa M., et al. Dipyridamole-thallium-201 in the early post-infarction period: Safety and accuracy in predicting the extent of coronary disease and future recurrence of angina in patients suffering from their first myocardial infarction. Eur Heart J. 1988;9:1324-1331.
91. Dakik H.A., Wendt J.A., Kimball K., Pratt C.M., Mahmarian J.J. Prognostic value of adenosine Tl-201 myocardial perfusion imaging after acute myocardial infarction: results of a prospective clinical trial. J Nucl Cardiol. 2005;12:276-283.
92. Zellweger M.J., Dubois E.A., Lai S., et al. Risk stratification in patients with remote prior myocardial infarction using rest-stress myocardial perfusion SPECT: Prognostic value and impact on referral to early catheterization. J Nucl Cardiol. 2002;9:23-32.
93. Grines C.L., Browne K.F., Marco J., et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med. 1993;328:673-679.
94. Terrin M.L., Williams D.O., Kleiman N.S., et al. Two- and three-year results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II clinical trial. J Am Coll Cardiol. 1993;22:1763-1772.
95. Califf R.M., White H.D., VandeWerf F., et alfor the GUSTO-I Investigators. One-year results from the Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries (GUSTO-I) Trial. Circulation. 1996;94:1233-1238.
96. Krone R.J., Gregory J.J., Freedland K.E., et al. Limited usefulness of exercise testing and thallium scintigraphy in evaluation of ambulatory patients several months after recovery from an acute coronary event: Implications for management of stable coronary heart disease. Multicenter Myocardial Ischemia Research Group. J Am Coll Cardiol. 1994;24:1274-1281.
97. Miller T.D., Gersh B.J., Christian T.F., et al. Limited prognostic value of thallium-201 exercise treadmill testing early after myocardial infarction in patients treated with thrombolysis. Am Heart J. 1995;130:259-266.
98. Ellis S.G., Mooney M.R., George B.S., et al. Randomized trial of late elective angioplasty versus conservative management for patients with residual stenoses after thrombolytic treatment of myocardial infarction. Treatment of Post Thrombolytic Stenoses (TOPS) Study Group. Circulation. 1992;86:1400-1406.
99. Andersen H.R., Nielsen T.T., Rasmussen K., et alfor the DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733-742.
100. Zijlstra F., de Boer M.J., Hoorntje J.C., et al. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680-684.
101. Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 2003;361(9351):3-20.
102. Rogers W.J., Canto J.G., Lambrew C.T., et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: The National Registry of Myocardial Infarction 1, 2 and 3. J Am Coll Cardiol. 2000;36:2056-2063.
103. Madsen J.K., Grande P., Saunamaki K., et al. Danish multicenter randomized study of invasive versus conservative treatment in patients with inducible ischemia after thrombolysis in acute myocardial infarction (DANAMI). DANish trial in Acute Myocardial Infarction. Circulation. 1997;96:748-755.
104. Hochman J.S., Lamas G.A., Buller C.E., Dzavik V., Reynolds H.R., Abramsky S.J., Forman S., Ruzyllo W., Maggioni A.P., White H., Sadowski Z., Carvalho A.C., Rankin J.M., Renkin J.P., Steg P.G., Mascette A.M., Sopko G., Pfisterer M.E., Leor J., Fridrich V., Mark D.B., Knatterud G.L. Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395-2407.
105. Mahmarian J.J., Dakik H.A., Filipchuk N.G., Shaw L.J., Iskander S.S., Ruddy T.D., Keng F., Henzlova M.J., Allam A., Moye L.A., Pratt C.M., INSPIRE. Investigators. An initial strategy of intensive medical therapy is comparable to that of coronary revascularization for suppression of scintigraphic ischemia in high-risk but stable survivors of acute myocardial infarction. J Am Coll Cardiol. 2006;48:2458-2467.
106. Gibson C.M., Karha J., Murphy S.A., James D., Morrow D.A., Cannon C.P., Giugliano R.P., Antman E.M., Braunwald E. TIMI Study Group. Early and long-term clinical outcomes associated with reinfarction following fibrinolytic administration in the Thrombolysis in Myocardial Infarction trials. J Am Coll Cardiol. 2003;42:7-16.
107. Neumann F.J., Kastrati A., Pogatsa-Murray G., Mehilli J., Bollwein H., Bestehorn H.P., Schmitt C., Seyfarth M., Dirschinger J., Schomig A. Evaluation of prolonged antithrombotic pretreatment (“cooling-off” strategy) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA. 2003;290:1593-1599.
108. The TIMI IIIB Investigators. Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Circulation. 1994;89:1545-1556.
109. Boden W.E., O’Rourke R.A., Crawford M.H., et al. Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators. N Engl J Med. 1998;338:1785-1792.
110. FRagmin and Fast Revascularisation during InStability in Coronary artery disease (FRISC II) Investigators. Invasive compared with noninvasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999;354:708-715.
111. Michalis L.K., Stroumbis C.S., Pappas K., Sourla E., Niokou D., Goudevenos J.A., Siogas C., Sideris D.A. Treatment of refractory unstable angina in geographically isolated areas without cardiac surgery. Invasive versus conservative strategy (TRUCS study). Eur Heart J. 2000;21:1954-1959.
112. Cannon C.P., Weintraub W.S., Demopoulos L.A., et alfor the TACTICS-Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879-1887.
113. Fox K.A., Poole-Wilson P.A., Henderson R.A., Clayton T.C., Chamberlain D.A., Shaw T.R., Wheatley D.J., Pocock S.J. Randomized Intervention Trial of unstable Angina Investigators. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743-751.
114. Spacek R., Widimsky P., Straka Z., Jiresova E., Dvorak J., Polasek R., Karel I., Jirmar R., Lisa L., Budesinsky T., Malek F., Stanka P. Value of first day angiography/angioplasty in evolving Non-ST segment elevation myocardial infarction: an open multicenter randomized trial. The VINO Study. Eur Heart J. 2002;23:230-238.
115. de Winter R.J., Windhausen F., Cornel J.H., Dunselman P.H., Janus C.L., Bendermacher P.E., Michels H.R., Sanders G.T., Tijssen J.G., Verheugt F.W. Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) Investigators. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med. 2005;353:1095-1104.
116. TIMI Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction: Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. N Engl J Med. 1989;320:618-627.
117. SWIFT (Should We Intervene Following Thrombolysis?) Trial Study Group. SWIFT trial of delayed elective intervention v conservative treatment after thrombolysis with anistreplase in acute myocardial infarction. BMJ. 1991;302:555-560.
118. McCullough P.A., O’Neill W.W., Graham M., et al. A prospective randomized trial of triage angiography in acute coronary syndromes ineligible for thrombolytic therapy. Results of the Medicine Versus Angiography in Thrombolytic Exclusion (MATE) Trial. J Am Coll Cardiol. 1998;32:596-605.
119. Ferry D.R., O’Rourke R.A., Blaustein A.S., et al. Design and baseline characteristics of the Veterans Affairs Non-Q-Wave Infarction Strategies In-Hospital (VANQWISH) trial. VANQWISH Trial Research Investigators. J Am Coll Cardiol. 1998;31:312-320.
120. Barnett P.G., Chen S., Boden W.E., et al. Cost-effectiveness of a conservative, ischemia-guided management strategy after non-Q-wave myocardial infarction: Results of a randomized trial. Circulation. 2002;105:680-684.
121. Rouleau J.L., Moyé L.A., Pfeffer M.A., et alfor the SAVE Investigators. A comparison of management patterns after acute myocardial infarction in Canada and the United States. N Engl J Med. 1993;328:779-784.
122. Mark D.B., Naylor C.D., Hlatky M.A., et al. Use of medical resources and quality of life after acute myocardial infarction in Canada and the United States. N Engl J Med. 1994;331:1130-1135.
123. Piegas L.S., Flather M., Pogue J., et alfor the OASIS Registry Investigators. The Organization to Assess Strategies for Ischemic Syndromes (OASIS) Registry in patients with unstable angina. Am J Cardiol. 1999;84:7M-12M.
124. Swahn E, Alfredsson J, Afzal R, Budaj A, Chrolavicius S, Fox K, Jolly S, Mehta S.R, de Winter R, Yusuf S: Early invasive compared with a selective invasive strategy in women with non-ST-elevation acute coronary syndromes: A substudy of the OASIS 5 trial and a meta-analysis of previous randomized trials [Epub ahead of print], Eur Heart J doi:10.1093/eurheartj/ehp009
125. Mahmarian J.J., Shaw L.J., Olszewski G.H., Pounds B.K., Frias M.E., Pratt C.M., INSPIRE Investigators. Adenosine sestamibi SPECT post-infarction evaluation (INSPIRE) trial: A randomized, prospective multicenter trial evaluating the role of adenosine Tc-99m sestamibi SPECT for assessing risk and therapeutic outcomes in survivors of acute myocardial infarction. J Nucl Cardiol. 2004;11:458-469.
126. Dakik H.A., Kleiman N.S., Farmer J.A., et al. Intensive medical therapy versus coronary angioplasty for suppression of myocardial ischemia in survivors of acute myocardial infarction. A prospective, randomized pilot study. Circulation. 1998;98:2017-2023.
127. Morrow D.A., Cannon C.P., Rifai N., Frey M.J., Vicari R., Lakkis N., Robertson D.H., Hille D.A., DeLucca P.T., DiBattiste P.M., Demopoulos L.A., Weintraub W.S., Braunwald E., TACTICS-TIMI: 18 Investigators. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA. 2001;286:2405-2412.
128. Mahoney E.M., Jurkovitz C.T., Chu H., Becker E.R., Culler S., Kosinski A.S., Robertson D.H., Alexander C., Nag S., Cook J.R., Demopoulos L.A., DiBattiste P.M., Cannon C.P., Weintraub W.S., TACTICS-TIMI: 18 Investigators. Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial Infarction. Cost and cost-effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non-ST-segment elevation myocardial infarction. JAMA. 2002;288:1851-1858.
129. Hachamovitch R., Hayes S.W., Friedman J.D., Cohen I., Berman D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900-2907.
130. Allman K.C., Shaw L.J., Hachamovitch R., Udelson J.E. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151-1158.
131. Joyce D., Loebe M., Noon G.P., McRee S., Southard R., Thompson L., Skrabal C., Youker K., Torre-Amione G. Revascularization and ventricular restoration in patients with ischemic heart failure: the STICH trial. Curr Opin Cardiol. 2003;18:454-457.
132. Mahmarian J.J. Monitoring medical therapy: The role of noninvasive imaging. In: Dilsizian V., Narula J., Braunwald E., editors. Atlas of Nuclear Cardiology. ed 2. Philadelphia: Current Medicine Group; 2006:191-210.
133. Anderson J.L., Adams C.D., Antman E.M., et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1-e157.