Chapter 21 Rigid Bronchoscopic Tumor Debulking and Silicone Stent Insertion for Mixed Malignant Tracheal Obstruction Caused by Esophageal Carcinoma
Case Description
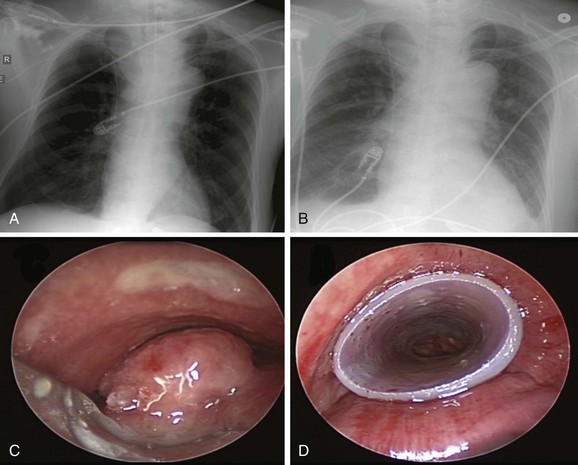
Chest radiograph showed a soft tissue mass density projecting to the right of the trachea. Lung volumes appeared to be enlarged bilaterally (Figure 21-1). Laboratory markers revealed WBC 21.3, Hb 12, and Plt 164. Results from the esophageal mass biopsy showed squamous cell carcinoma. Ventilator settings included assist-control volume-control ventilation with a tidal volume of 500 mL, rate 16, PEEP 5, and FiO2 0.5. At these settings, peak airway pressure was 60 cm H2O and plateau pressure 28 cm H2O. Weaning was attempted but failed. Rigid bronchoscopy was performed, revealing extrinsic compression and an exophytic endoluminal mass protruding from the posterior wall, completely occluding the mid-trachea (see Figure 21-1). The mass was 2 cm in length and was located 5 cm above the carina and 5 cm below the vocal cords. The endoluminal tumor was cored out using Nd:YAG laser for hemostasis. Airway patency was improved, but tracheal obstruction from extrinsic compression (>70%) remained significant. A 16 × 50 mm straight studded silicone stent was therefore placed in the mid-trachea, restoring airway patency satisfactorily. The patient was successfully extubated the next day (see Figure 21-1).
Discussion Points
1. Describe three patient-related factors associated with difficult rigid bronchoscopic intubation.
2. Explain the rationale for stent insertion after tumor debulking in this patient.
3. List at least three strategies to prevent airway perforation and hemorrhage during rigid bronchoscopy.
4. Describe five ways to avoid complications of rigid bronchoscopy.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
Advanced, unresectable esophageal cancer with airway invasion has a very poor prognosis (5 year survival rate <10%).1 Patients with this disease have not only a limited life expectancy but also many potentially debilitating complications from the local effects of their tumors. These include dysphagia, dysphonia, dyspnea from airway involvement, chest pain, and, rarely, massive fatal hemorrhage from aortic erosion.2,3 Airway involvement in the setting of esophageal carcinoma includes tissue invasion, infiltration, or the presence of an esophago-respiratory fistula (ERF).* Airway complications may be caused (1) by extrinsic compression on the airways by the tumor or by an esophageal stent and (2) by direct invasion by the tumor into the airways. These processes can result in severe airway narrowing or ERF. Among a large series of 372 patients seen over the 14 year study period, 74 (20%) were found to have airway involvement: 35 (47%) of these patients had an ERF; in the absence of ERF, airway involvement identified as invasion or infiltration was biopsy proven in 29 of 39 patients (74%).4 Other investigators report airway involvement in 10.9% to 62% of cases.4,5 Because of the proximity of the major airways to the upper two thirds of the esophagus in the thorax, major airway obstruction is also common. In fact, patients with esophageal tumors above the level of the main carina have a worse prognosis than those with lower tumors because of this anatomic relationship with the large airways.6 Hence, up to 25% of patients with advanced esophageal cancer may require airway stent insertion to alleviate airway obstruction.7–10 The mean duration of survival for patients requiring airway stent insertion for obstruction due to esophageal cancer reportedly ranges from only 35 to 121 days, with many patients dying at home or in hospice.9
Severe respiratory symptoms in these patients may occur because of ERF or severe airway narrowing. For tumors extending into the airway lumen, as is seen in our patient, primary goals of therapy include palliative relief of the malignant obstruction of the esophageal lumen and central airway. If present, attempts can be made to close fistulas between the esophagus and the central airway or, at the least, to alleviate or diminish risks of aspiration and inability to swallow. Recurrent aspiration pneumonia may be caused by dysphagia due to esophageal obstruction or by ERF and can lead to respiratory failure. Initial chest radiographs are often normal; in these cases, they are useful for excluding other causes of respiratory symptoms such as pneumonia, pneumothorax, and pleural effusion.11 In our patient, for example, pneumonia was not evident on chest x-ray (CXR) nor was ERF on bronchoscopy. The critical airway obstruction was therefore the likely culprit for respiratory failure in view of notably high peak airway pressures and normal plateau pressures, which point to an airway resistance process. In the absence of evidence for bronchospasm, mucus plugging, or kinking of the endotracheal tube, the most plausible explanation for high peak airway pressures and respiratory failure in this patient was tracheal obstruction by the esophageal mass. This was probably worsened by the moderate sedation used for EGD.
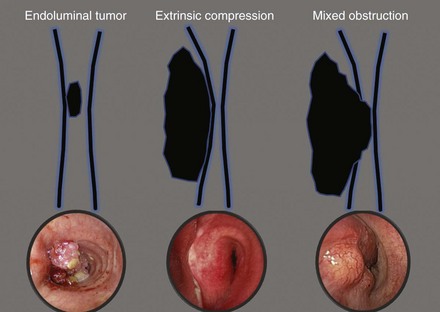
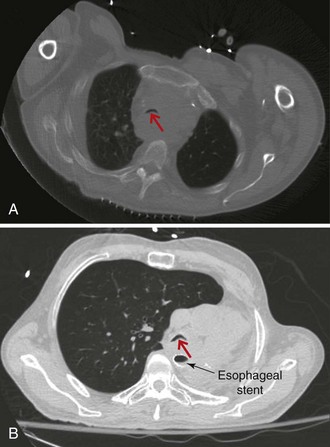
On bronchoscopy, this patient’s central airway obstruction was classified as mixed because it comprised an endoluminal component and extrinsic compression (Figure 21-2).12 In addition to bronchoscopy, computed tomography (CT) provides a noninvasive means of confirming the presence and severity of suspected airway involvement and is valuable in assessing the length of a stenosis, especially when a bronchoscope may not be passable beyond the stenosis. Before bronchoscopy, CT may help to determine whether double stent insertion is necessary (Figure 21-3). In cases of suspected ERF, a barium swallow is usually performed.
Comorbidities
It is possible that our patient had hypoventilation post EGD, caused by sedation-induced reduction in minute ventilation in the setting of critical tracheal obstruction, or possibly by upper airway obstruction unmasked by sedatives. The redundant pharyngeal and laryngeal tissues, as seen in patients with obstructive sleep apnea (OSA), may further lose tonicity, contributing to the hypoventilation and hypoxemia induced by moderate sedation, which potentially leads to respiratory failure and endotracheal intubation (see video on ExpertConsult.com) (Video V.21.1![]() ). Although not documented in his medical history, based on the bronchoscopic findings, we suspected OSA in our patient. This can also contribute to poor outcomes caused by perioperative complications. Indeed, evidence suggests that patients with OSA may have more postoperative hypoxemia, upper airway obstruction, cardiac arrhythmias, and myocardial infarction.13 Results of observational studies show that most patients with OSA who are undergoing surgery have not been diagnosed before surgery. In a study of 2877 elective surgery patients, 24% were found to be at risk for having OSA, and 81% of these had not been diagnosed previously.14 These data raise the question whether all patients undergoing surgery should be screened for OSA.
). Although not documented in his medical history, based on the bronchoscopic findings, we suspected OSA in our patient. This can also contribute to poor outcomes caused by perioperative complications. Indeed, evidence suggests that patients with OSA may have more postoperative hypoxemia, upper airway obstruction, cardiac arrhythmias, and myocardial infarction.13 Results of observational studies show that most patients with OSA who are undergoing surgery have not been diagnosed before surgery. In a study of 2877 elective surgery patients, 24% were found to be at risk for having OSA, and 81% of these had not been diagnosed previously.14 These data raise the question whether all patients undergoing surgery should be screened for OSA.
Patient Preferences and Expectations
The prognosis was discussed with the patient’s wife. By investing in patient-centered conversations with family members, health care providers can better meet the needs of families and patients during a medical crisis such as respiratory failure or admission to the intensive care unit.15 His wife hoped he could be weaned from the ventilator, so she could speak with him about his wishes.
Procedural Strategies
Indications
The primary goal was to restore airway patency to facilitate extubation and change the level of care. Interventional bronchoscopic procedures have been reported to facilitate weaning from mechanical ventilation.16 Relevant to this patient’s condition, in a study of 11 inoperable patients with esophageal carcinoma and airway involvement treated with a combination of rigid bronchoscopy, neodymium-doped yttrium aluminum garnet (Nd:YAG) laser resection, and silicone stent placement, authors noted that 4 patients who required mechanical ventilation for respiratory failure were successfully weaned from mechanical ventilation after the procedure.9 Overall, airway stent insertion is considered a palliative intervention for airway complications in patients with unresectable esophageal cancer.9 Airway stents are effective for treating airway narrowing from both extrinsic compression and direct tumor invasion and have been shown to be useful in the treatment of ERF, if present.4,17,18 The choice of stent depends on the clinician, the place of practice, financial or health care social policy circumstances, institutional biases, and equipment availability, as well as the particular needs of the individual patient. Clinician factors include personal training, familiarity with rigid and flexible bronchoscopy, and personal preference. Institution-related factors include costs (self-expanding metallic stents [SEMS] are generally more expensive), availability of operating theaters and anesthesiologists for emergency rigid bronchoscopy, and stent availability. In terms of patient factors, one might consider the fitness of the patient for general anesthesia, the risk of provoking complete airway occlusion outside of an operating theater while performing flexible bronchoscopy, and any foreseeable chance that a stent may need to be adjusted or replaced subsequently.11
Expected Results
Stent insertion in the involved airway of esophageal cancer has been shown to improve the life quality and outcomes of patients with advanced esophageal cancer.4 In the largest case series to date, 66 airway stents (65 studded silicone stents and 1 Wallstent metal stent) were inserted in 51 patients with airway involvement from esophageal carcinoma. Forty stents were inserted in the trachea, 16 in the left main bronchus, and 10 in the right main bronchus. In 47 patients (92%), improvement in respiratory symptoms was significant. The mean survival was 107.7 days.8
Although some might say that bronchoscopic stent insertion is an unwarranted and potentially costly therapeutic alternative with patients with ultimately and rapidly fatal disease, we contend that stent insertion is a palliative intervention that always warrants consideration, not only because it results in greater comfort, even over the short term, but also because it improves quality of life, might allow withdrawal from mechanical ventilation so that patients can communicate more fully with their loved ones, and may result in a reduction in level of care or more rapid discharge from the intensive care or hospital setting. In the Netherlands, where euthanasia is a legal alternative to certain palliative therapies for patients with advanced cancer, 7 out of 12 patients with esophageal cancer who elected to have airway stents inserted were judged by their family physicians to have received “worthwhile” palliation in terms of quality of life during the terminal phase of their disease, despite their relatively short remaining survival time.19
Team Experience
In high esophageal tumors and in locally invasive tumors with evidence of ERF, a shared interdisciplinary care approach between gastroenterology and pulmonary teams is recommended.20 The type of airway stent selected may depend on the level of team experience and training in the technique of rigid bronchoscopy.21 Bronchoscopic resectional techniques such as laser, electrocautery, cryotherapy, argon plasma coagulation (APC), and photodynamic therapy (PDT), in addition to metal or silicone stent insertion, were available at our institution. Therefore in our case, the choice of treatment was guided by (1) a need for immediate improvement in airway patency to facilitate extubation, (2) a need to maintain airway patency in view of extrinsic compression, and (3) a desire for a stent that could be removed or changed easily in case of changes in clinical illness. Thus we elected to perform our procedure using a resection technique such as Nd:YAG laser for possible hemostasis and photocoagulation of tumor in conjunction with coring out, and to insert a silicone rather than a covered metal stent because of its greater expansile force.22,23
Therapeutic Alternatives for Restoring Airway Patency
Alternative therapeutic modalities include insertion of SEMS, radiation therapy, chemotherapy, surgical intervention (esophageal bypass, diversion, and attempted resection), and comfort measures. This patient was not considered a candidate for surgical intervention given his advanced disease, poor functional status, and respiratory failure. Less invasive palliative interventions considered included other palliative bronchoscopic options such as mechanical debridement, dilation, laser ablation, electrocautery, cryotherapy, photodynamic therapy, and brachytherapy alone.24 Without stent insertion, however, restoration of airway patency may not be immediate or long-lasting. In addition, any remaining extrinsic compression after debulking would require stent placement to maintain patency.
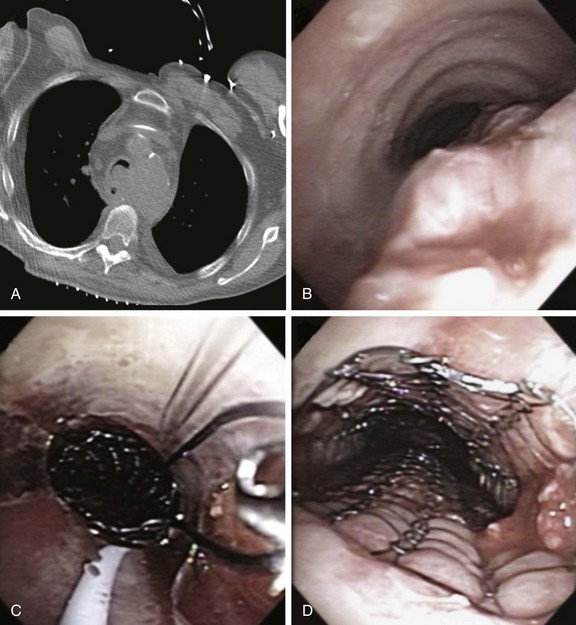
1. Covered self-expandable metallic stents (SEMS) have been used to relieve the airway obstruction and seal ERFs to avoid aspiration symptoms.10,17 Insertion of SEMS in mechanically ventilated patients could be performed via rigid bronchoscopy under general anesthesia (Figure 21-4) or via flexible bronchoscopy under fluoroscopic guidance.25 Some patients are not suitable for rigid bronchoscopy with a general anesthetic because of the severity of their illness, comorbidities, or refusal to undergo intervention. Fluoroscopy requires special facilities that may not be available in every intensive care unit (ICU). In the absence of ready availability of an operating room, and for those operators not versed in the techniques of rigid bronchoscopy,21 this procedure can, however, be performed while the patient is on the ventilator in the ICU. Because a significant part of the obstruction was caused by malignant extrinsic compression in our patient, stent insertion could have restored airway patency with or without minimal flexible bronchoscopic debulking of the mass using electrocautery or APC. A technique for placing these stents without fluoroscopy includes the following steps: The bronchoscope is inserted into the mouth through a bite block alongside the endotracheal tube (ETT) and is advanced into the space between the tracheal wall and the endotracheal tube; the scope is then positioned proximal to the lesion; a guidewire is inserted through the bronchoscope and is passed alongside the lesion, after which the bronchoscope is withdrawn, leaving the guidewire in place. The scope is reinserted into the ETT to confirm guidewire location. A stent delivery catheter is advanced over the guidewire, and the stent is deployed under bronchoscopic visualization. The delivery catheter and the guidewire are withdrawn together, with the stent left in position (Figure 21-5). If necessary, the stent can be repositioned by grasping its proximal loop with a flexible alligator forceps (see video on ExpertConsult.com) (Video V.21.2![]() ).
).
2. Definitive chemo-radiotherapy has been proposed for patients with esophageal cancer and airway invasion with or without ERF. Radiation therapy is effective in palliating dysphagia in 34% to 48% of patients with inoperable esophageal cancer.26 Concurrent chemotherapy with radiotherapy affords even greater benefit in terms of survival and loco-regional control,1 although it is associated with significant treatment-related morbidity and mortality. Anecdotal evidence suggests a clinical complete response with induction chemotherapy followed by consolidation with concurrent chemo-radiotherapy—an approach that may reduce the morbidity of upfront radiation.27 Progression to ERF, however, has been reported with radiotherapy; in one case series, the incidence was approximately 10% (9 of 85 patients).28 This palliative intervention was not considered a first step in our patient given his poor performance status and the presence of respiratory failure.
3. Double stent insertion can effectively palliate both dyspnea and dysphagia. Several reports have described the use of “double” stenting of the esophagus and the airway. Results in terms of immediate relief of respiratory and swallowing symptoms appear to be excellent. Esophageal stents, however, are known to compress the adjacent airway, precipitating or exacerbating airway narrowing (see Figure 21-3).7,29 Acute airway obstruction accompanied by stridor, respiratory failure, and death has been reported after placement of esophageal stents in the proximal one third of the esophagus.7,30 Double stenting of the esophagus and the airway has been described to manage this complication,7,8,17 particularly when malignancy obstructs the lumina of the esophagus and the tracheobronchial tree. The incidence of airway obstruction following esophageal stent insertion ranges from about 1% to 10%.9 Airway stent insertion effectively restores airway patency in this setting.7 Airway stent insertion should always be considered when the esophagus requires stent placement, and certainly respiratory compromise accompanying or following esophageal stent insertion should raise the possibility of airway obstruction by the stent itself. A multidisciplinary approach including an initial airway evaluation for patients being considered for esophageal stent insertion improves prognosis and decreases airway complications related to the esophageal stent.31 The double stenting procedure is not without complications, however. When double stenting is used in combined esophago-airway lesions, massive bleeding is reported in up to 27%.32 Double stenting is associated with ERF in up to 18% to 38% of patients with esophageal cancer,29,32 although it is unclear whether fistulas occur as a direct result of stent-related erosion of altered neoplastic infiltration or airway and esophageal mucosa, or if they are a natural result of disease progression.
Pneumothorax may occur in 3% to 4% of esophageal cancer patients receiving airway stents.8 Patients with previous radiotherapy to the stented area may be especially vulnerable to airway damage.33 In patients with respiratory symptoms, we prefer to place airway and esophageal stents in stages, inserting the airway stent first. In patients without respiratory symptoms, palliation of the esophageal obstruction takes precedence, and an airway stent is inserted only in case of development or suspicion of airway compromise. Of course, in a patient with an esophageal tumor above the level of the tracheal bifurcation, it is advisable to exclude tracheal stenosis before esophageal stent insertion even if the patient is not dyspneic. Although bronchoscopic inspection of the airway before insertion of an esophageal stent is routine practice in many centers, it is not clear from the literature whether this procedure is absolutely cost-effective. An alternative is to insert an airway stent preventively before esophageal stent placement if significant airway compromise is present on CT scan or bronchoscopic evaluation.34 Otherwise, airway compromise after esophageal stent insertion can be effectively palliated by airway stents. Colt et al.7 performed bronchoscopy in 39 patients requiring esophageal stents for esophageal cancer. Airway stents were required in 10 of these patients (26%) because of airway obstruction. Placement of the esophageal prosthesis contributed significantly to airway compromise in 4 of these patients (10% overall). In our patient, we elected to proceed with conversations regarding esophageal stent insertion only after airway patency had been safely established and the patient had been successfully extubated.
4. Supportive care measures include intravenous hydration, feeding tubes, antibiotics, analgesics, and comfort care, including medication for sedation and pain control.
Cost-Effectiveness
No formal cost-effectiveness evaluations of these various modalities have yet been published. Emergent bronchoscopic interventions in the setting of central airway obstruction (CAO) favorably affect health care utilization in patients with acute respiratory distress from CAO who require ICU hospitalization.16 Successful withdrawal from mechanical ventilation and substantial level of care changes from ICU hospitalization to the medical ward in more than 50% of patients, whether for initiation of systemic therapy or for initiation of comfort/supportive care, suggest that consideration should be given to emergent bronchoscopic intervention in cases of CAO resulting in ICU admission. Early intervention probably is justified from both a clinical and an economic standpoint. If mechanical ventilation cannot be discontinued, the long-term prognosis is dismal, and conversations addressing supportive care, including transfer to hospice, should be initiated.
With regard to the placement of metallic stents, bronchoscopic removal, when indicated, has been shown to be associated with significant complications, health care resource utilization, and costs. Their use therefore should be restricted to patients with advanced malignant airway disease with a short life expectancy, in which case long-term complications requiring stent removal are unlikely to occur.35
Techniques and Results
Anesthesia and Perioperative Care
In patients with OSA, the effects of general anesthesia, neuromuscular agents (when used), narcotics, and sedatives may enhance pharyngeal muscle relaxation and depress the arousal response. This results in more frequent and longer apneas postoperatively.13 Nasal continuous positive airway pressure (CPAP) after extubation may improve outcomes, but only limited data suggest possible benefit.13
At our institution, we perform procedures with patients under general anesthesia, usually achieved by intravenous propofol and, when necessary, remifentanil. Neuromuscular blocking drugs are not used because we aim to keep patients breathing spontaneously throughout the procedure. Ideally, the depth of anesthesia should allow spontaneous breathing while preventing excessive body movement in response to rigid bronchoscopy stimulation.36 We routinely use a local anesthetic, such as 1%, 2%, or 4% lidocaine, sprayed directly to the larynx before insertion of the rigid bronchoscope to prevent laryngospasm, to reduce patient discomfort leading to coughing and bucking during the procedure, and to help avoid throat soreness afterward.
Instrumentation
Selection of a particular stent for an individual patient is often based on the operator’s preference and previous experience with a particular stent, evolution in stent technology, and the local availability of various stents. In addition to the geometry (extent, morphology, severity) of the tumor, knowledge of the mechanical properties of the stent is helpful in selecting the appropriate stent.37 We therefore consider the biomechanical properties of various stents before insertion because the expansile force (physical strength) and ability to withhold angulation (buckling) vary widely among different types. The important attribute of a stent in a case of extrinsic compression is its expansile force because this determines whether the stent is likely to expand fully. However, in a distorted, curved airway, the buckling radius becomes important as well because this determines whether the stent can conform to an acutely angulated tumor and yet remain functional. Stents may differ greatly in their elasticity and resistance to angulation. Available information on this topic for airway stents is unfortunately very limited.* For example, Ultraflex stents (Boston Scientific, Natick, Mass) were shown to withstand angulation, but their expansile force is not very great, and a studded silicone stent (Dumon stent, Novatech, Cedex, France) has the opposite behavior.22,23 In this regard, a recent study evaluating the role of therapeutic bronchoscopy for malignant CAO showed that the stent used most commonly in the trachea and the right mainstem bronchi (relatively straight airways) was the Dumon stent, and the one used most commonly in the left mainstem bronchus (curved, tapered airway; often distorted in the setting of malignancy) was the Ultraflex stent, likely because of its better ability to withhold angulation.38 In this study, patients with esophageal carcinoma involving the airway most often required only stent placement without laser-assisted debulking, probably because the main problem was extrinsic compression.38
Results and Procedure-Related Complications
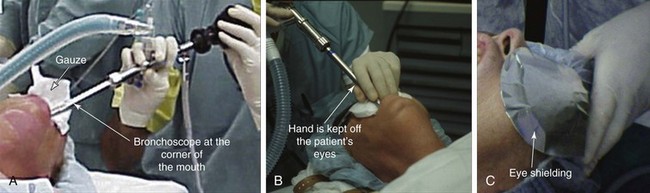
Because of the limited neck mobility, we chose to insert the rigid scope via the corner of the mouth (Figure 21-6). We used a large-diameter tube (13 mm) to facilitate placement of a large stent. The ETT was removed under direct visualization, and the bronchoscope was inserted through the larynx as the endotracheal tube was being removed (see video on ExpertConsult.com) (Video V.21.3![]() ). We avoided laser resection of tumor to avoid necrosis and rupture of the posterior membrane of the trachea, which might precipitate ERF formation. Partial resection and coring out of the tumor within the airway were achieved using the beveled edge of the rigid bronchoscope; once tissues were partially removed, significant extrinsic compression remained and extended for 3 cm (see video on ExpertConsult.com) (Video V.21.4
). We avoided laser resection of tumor to avoid necrosis and rupture of the posterior membrane of the trachea, which might precipitate ERF formation. Partial resection and coring out of the tumor within the airway were achieved using the beveled edge of the rigid bronchoscope; once tissues were partially removed, significant extrinsic compression remained and extended for 3 cm (see video on ExpertConsult.com) (Video V.21.4![]() ). This began at 5 cm below the vocal cords and extended to an area 2.5 cm above the carina. Flexible bronchoscopy was performed through the rigid tube to remove blood and secretions from the airway. Hemostasis was achieved by using an Nd:YAG laser at 30 W, with 1 second pulse photocoagulation, using a total of 935 joules. We chose to insert a Dumon silicone stent (16 mm × 50 mm) because its advantages include ease of adjustment in cases of migration, as well as protection against tumor invasion. Its main disadvantage is the need for rigid bronchoscopy and general anesthesia, which may be poorly tolerated in malnourished patients or in those with concurrent cardiopulmonary disease. Compared with flexible bronchoscopy, however, rigid bronchoscopy affords superior airway control and the ability to safely ventilate patients during the procedure.
). This began at 5 cm below the vocal cords and extended to an area 2.5 cm above the carina. Flexible bronchoscopy was performed through the rigid tube to remove blood and secretions from the airway. Hemostasis was achieved by using an Nd:YAG laser at 30 W, with 1 second pulse photocoagulation, using a total of 935 joules. We chose to insert a Dumon silicone stent (16 mm × 50 mm) because its advantages include ease of adjustment in cases of migration, as well as protection against tumor invasion. Its main disadvantage is the need for rigid bronchoscopy and general anesthesia, which may be poorly tolerated in malnourished patients or in those with concurrent cardiopulmonary disease. Compared with flexible bronchoscopy, however, rigid bronchoscopy affords superior airway control and the ability to safely ventilate patients during the procedure.
The relative thickness of the stent wall tends to lower the maximum achievable luminal diameter. After stent insertion, the rigid scope was removed, and the patient was intubated with a No. 7 endotracheal tube positioned so that its distal aspect was within the stent itself. No intraoperative or postoperative complications were reported. During rigid bronchoscopy with stent insertion, the airway walls can be traumatized or perforated by instrumentation during stent placement and by pressure exerted onto the walls by the stent itself. Complications such as hemorrhage, fistula formation, pneumothorax, pneumomediastinum, or infection cannot always be avoided. Expected delayed adverse events post silicone stent insertion include migration, obstruction by mucus, and granulation. Migration rates for silicone airway stents placed in esophageal cancer patients are reportedly 8% to 12%.11 With migration, not only does the stenotic region again become obstructed, but the solid walls of a displaced silicone stent may potentially block off a bronchial orifice. To prevent mucus plugging after stent insertion, we routinely begin chest physiotherapy as soon as the patient can tolerate it, usually later on the day of stent insertion. Inhaled air is moisturized by means of steam inhalation or nebulized saline, and mucolytics can be given occasionally. Pneumonia, which may result from sputum plugging, has been reported in 4% of esophageal cancer patients after airway stent insertion.8 Granulation tissue overgrowth can be very rapid and can cause significant obstruction of either or both ends of an airway stent within days or weeks. Excessive pressure from too large a stent or friction of a loose stent rubbing against the airway wall might also enhance granulation tissue formation.39
Long–Term Management
Outcome Assessment
The underlying disease, the location of the obstructing lesion, the nature of the lesion (extrinsic, intraluminal, or mixed), and the treatment modality are independent predictors of survival in patients with CAO who undergo therapeutic bronchoscopy.38 In one study, for instance, the median survival for esophageal carcinoma was 2.5 months versus 5.5 months for lung cancer; the median survival for patients with tracheal and bilateral mainstem bronchial stenosis was 1.6 months versus 4.7 and 4.8 months for patients with left- and right-sided obstruction, respectively. Patients with mixed obstruction had a median survival of 2.3 months versus 5.7 months for patients with purely intraluminal disease.38 Patients treated with laser and stent had a median survival of 3 months versus 2.7 months for stent only and 10.7 months for laser only treatment. These data do not necessarily imply that one treatment modality is better than another but may simply reflect the nature and severity of the airway obstruction. For example, patients who needed only laser therapy had less severe airway obstruction (e.g., intrinsic exophytic obstructive lesion) and lesions amenable to this modality alone.38
Follow-up Tests and Procedures
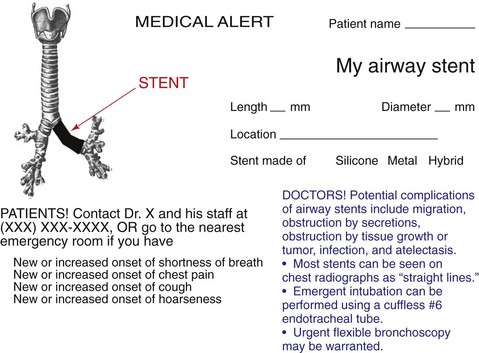
After stent insertion, a follow-up CXR was performed to confirm its location (see Figure 21-1). Surveillance bronchoscopy is warranted in those patients suspected of having stent-related adverse effects. Secretions, migration, tumor progression, fistula formation, and pneumothorax usually are detected by follow-up bronchoscopy or on chest imaging studies. Complications usually are detected by the onset of new respiratory symptoms and do not necessitate systematic flexible bronchoscopy. Preventive measures such as aerosol therapy, respiratory physiotherapy, and clinical visits8 are advocated. If patients undergo radiation treatment, bronchoscopy can reveal radiation-induced changes and can help the clinician determine the need for a prolonged indwelling airway stent. In the vast majority of cases of advanced esophageal carcinoma, airway stents are, in fact, permanent once placed. Although this probably reflects the short survival of this group of patients, given the potential for stent-related complications, arguments for removing airway stents in cases of subsequent tumor-specific therapy are valid here, as well as in cases of remission of the airway stenosis.40 We provided the family with a stent alert card. This document includes the patient’s name; indication for stent insertion; type, location, and size of stent inserted; and contact information, as well as instructions for both patients and physicians in case of stent-related complications (Figure 21-7).
Quality Improvement
We did not believe that an earlier bronchoscopy could have altered the outcome in our patient because, after all, he had no diagnosis before the time of his initial hospitalization. The high incidence of airway involvement by esophageal cancer and the difficulty in predicting it accurately with clinical data or other staging procedures, however, argue for the routine use of bronchoscopy in patients with known tumor located at or above the level of the tracheal bifurcation. Excluding airway invasion is warranted, especially in patients with esophageal tumors longer than 8 cm, or in those for whom CT findings suggest airway invasion. A multivariate logistic regression model indicated, for example, that suspect CT findings (odds ratio, 4.4) and maximal tumor length greater than 8 cm (odds ratio, 3.7) were associated independently with airway invasion.41 Although esophageal ultrasound and CT are considered the best means of assessing the local extent of esophageal cancer, bronchoscopy is best to identify invasion of the cancer into the airways. If patients have previously undergone chemo-radiotherapy, interpreting bronchoscopic findings is more difficult than at baseline; the positive predictive value of macroscopic abnormalities without microscopic proof of cancer is low, and even with extensive sampling for histology and cytology, the false-negative rate is 9.4%.42
Discussion Points
1. Describe three patient-related factors associated with difficult rigid bronchoscopic intubation.
2. Explain the rationale for stent insertion after tumor debulking in this patient.
In patients with CAO, stents are placed for extrinsic compression and combined extrinsic and intrinsic obstruction, and in patients with intrinsic obstruction, they are placed when more than 50% of the lumen is obstructed after debulking or when involvement and instability of the airway wall (malacia) are significant.43
The physiologic rationale for the “50%” cutoff rests on several facts:
3. List at least three strategies to prevent airway perforation and hemorrhage during rigid bronchoscopy.
4. Describe five ways to avoid complications of rigid bronchoscopy.
Expert Commentary
Regarding the issue of operator and team experience and expertise, we need experienced anesthetists to enable us to perform interventions for severe airway stenosis. In patients with severe airway stenosis, obstruction by hemorrhage, secretion, and edema occur easily; therefore certain measures should be taken for the operation, and various precautions are required during the perioperative period. For example, anesthesia usually is maintained with propofol (2.0 to 4.0 mg/kg/hr) and fentanyl citrate (0.1 to 0.2 mg/body weight) for therapeutic rigid bronchoscopy.45 In select cases of severe tracheal stenosis, it is our custom to ensure percutaneous cardiopulmonary support (PCPS) via the femoral artery and vein before induction of anesthesia. Figure 21-3 shows a patient who has had a left pneumonectomy. In such cases, pulmonary reserve is limited, and the shift of the trachea may hinder insertion of rigid bronchoscopy. This is an example in which we find preparation by PCPS extremely helpful, especially if less-experienced operators are performing the procedure.
With regard to risk-benefit analysis and therapeutic alternatives, it is true that a standard method of operation would consist of tumor debulking and silicone stent insertion under rigid bronchoscopy in cases such as that described. This is probably the most effective way to improve the caliber of the severely restricted airway diameter. Patients can receive chemotherapy and radiotherapy after performance status and respiratory condition improve.46 However, we should explain ERF may develop as an effect of the treatment. Fistula formation prompted by the treatment itself will probably adversely affect the patient’s quality of life and survival. Therefore alternatives should be considered and possibly offered to the patient during or before the informed consent process. An expandable metallic stent may be inserted by using a rigid bronchoscope or a flexible bronchoscope. When the flexible bronchoscope is used, intubation with an endotracheal tube is necessary in patients with poor respiratory condition. Advantages of intubation include the ability to maintain sufficient ventilation; the ability to control airway bleeding by compressing the bleeding area or tumor with the inflated cuff of the endotracheal tube; and the possibility for unilateral intubation to protect the contralateral airway in case of massive bleeding. To provide a safeguard against any surprises that might be found during intervention, I insist on performing an inspection flexible bronchoscopy before proceeding with therapeutic procedures, especially if deviation of the proximal airway is suspected on chest x-ray or CT. Doing so allows me to prepare equipment and my team for any otherwise unexpected events, thereby enhancing patient safety and building team confidence.
1. Herskovic A, Martz K, Al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared to radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598.
2. Nelson DB, Axelrad AM, Fleischer DE, et al. Silicone-covered Wallstent prototypes for palliation of malignant esophageal obstruction and digestive-respiratory fistulas. Gastrointest Endosc. 1997;45:31-37.
3. Cheng SL, Wang HC, Lee YC, et al. The role of bronchoscopic assessment in esophageal cancer—clinical and survival analysis in 153 patients. J Formos Med Assoc. 2005;104:168-173.
4. Alexander EP, Trachiotis GD, Lipman TO, et al. Evolving management and outcome of esophageal cancer with airway involvement. Ann Thorac Surg. 2001;71:1640-1644.
5. Altorki NK, Migliore M, Skinner DB. Esophageal carcinoma with airway invasion. Chest. 1994;106:742-745.
6. Kato H, Tachimori Y, Watanabe H, et al. Thoracic esophageal carcinoma above the carina: a more formidable adversary? J Surg Oncol. 1997;65:28-33.
7. Colt HG, Meric B, Dumon JF. Double stents for carcinoma of the esophagus invading the tracheo-bronchial tree. Gastrointest Endosc. 1992;38:485-489.
8. Belleguic C, Lena H, Briens E, et al. Tracheobronchial stenting in patients with esophageal cancer involving the central airways. Endoscopy. 1999;31:232-236.
9. Chan KP, Eng P, Hsu AA, et al. Rigid bronchoscopy and stenting for esophageal cancer causing airway obstruction. Chest. 2002;122:1069-1072.
10. Takamori S, Fujita H, Hayashi A, et al. Expandable metallic stents for tracheobronchial stenoses in esophageal cancer. Ann Thorac Surg. 1996;62:844-847.
11. Sihoe AD, Wan IY, Yim AP. Airway stenting for unresectable esophageal cancer. Surg Oncol. 2004;13:17-25.
12. Murgu SD, Colt HG. Interventional bronchoscopy from bench to bedside: new techniques for central and peripheral airway obstruction. Clin Chest Med. 2010;31:101-115.
13. Gupta RM, Parvizi J, Hanssen AD, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897-905.
14. Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10:753-758.
15. Leon AM, Knapp S. Involving family systems in critical care nursing: challenges and opportunities. Dimens Crit Care Nurs. 2008;27:255-262.
16. Colt HG, Harrell JH. Therapeutic rigid bronchoscopy allows level of care changes in patients with acute respiratory failure from central airways obstruction. Chest. 1997;112:202-206.
17. Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest. 1996;110:1155-1160.
18. Sihoe AD, Wan IY, Yim AP. Airway stenting for unresectable esophageal cancer. Surg Oncol. 2004;13:17-25.
19. Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Tracheobronchial stenting in the terminal care of cancer patients with central airways obstruction. Chest. 2001;120:1811-1814.
20. Gottlieb J, Wedemeyer J. Endoscopic palliation of esophageal and bronchial carcinomas. Internist (Berl). 2010;51:237-245.
21. Colt H, Prakash U, Offord K. Bronchoscopy in North America: survey by the American Association for Bronchology, 1999. J Bronchol. 2000;7:8-25.
22. Freitag L, Eicker K, Donovan TJ, et al. Mechanical properties of airway stents. J Bronchol. 1995;2:270-278.
23. Hautmann H, Rieger J, Huber RM, et al. Elastic deformation properties of implanted endobronchial wire stents in benign and malignant bronchial disease: a radiographic in vivo evaluation. Cardiovasc Intervent Radiol. 1999;22:103-108.
24. Stephens KEJr, Wood DE. Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg. 2000;119:289-295.
25. Lee KE, Shin JH, Song HY, et al. Management of airway involvement of oesophageal cancer using covered retrievable nitinol stents. Clin Radiol. 2009;64:133-141.
26. Petrovich Z, Langholz B, Formenti S, et al. Management of carcinoma of the esophagus: the role of radiotherapy. Am J Clin Oncol. 1991;14:80-86.
27. Ku GY, Goodman KA, Rusch VW, et al. Successful treatment of esophageal cancer with airway invasion with induction chemotherapy and concurrent chemoradiotherapy. J Thorac Oncol. 2009;4:432-434.
28. Lewinsky BS, Annes GP, Mann SG, et al. Carcinoma of the esophagus. Radiol Clin Biol. 1975;44:192-196.
29. Nomori H, Horio H, Imazu Y, et al. Double stenting for esophageal and tracheobronchial stenoses. Ann Thorac Surg. 2000;70:1803-1807.
30. Dasgupta A, Jain P, Sandur S, et al. Airway complications of esophageal self-expandable metallic stent. Gastrointest Endosc. 1998;47:532-535.
31. Paganin F, Schouler L, Cuissard L, et al. Airway and esophageal stenting in patients with advanced esophageal cancer and pulmonary involvement. PLoS One. 2008;3:e3101.
32. Yamamoto R, Tada H, Kishi A, et al. Double stent for malignant combined esophago-airway lesions. Jpn J Thorac Cardiovasc Surg. 2002;50:1-5.
33. Yorozu A, Dokiya T, Ogita M, et al. Complications of esophageal stenting after radiotherapy and brachytherapy. Jpn J Clin Radiol. 1997;42:1579-1585.
34. Neuhaus H. The use of stents in the management of malignant esophageal strictures. Gastrointest Endosc Clin North Am. 1998;8:503-519.
35. Alazemi S, Lunn W, Majid A, et al. Outcomes, health-care resources use, and costs of endoscopic removal of metallic airway stents. Chest. 2010;138:350-356.
36. Wong AYC, Lawmin JC, Irwin MG. Anaesthetic management of a patient with low tracheal obstruction requiring placement of a T-Y stent. Anaesth Intensive Care. 2000;28:196-198.
37. Chan AC, Shin FG, Lam YH, et al. A comparison study on physical properties of self-expandable esophageal metal stents. Gastrointest Endosc. 1999;49:462-465.
38. Chhajed PN, Somandin S, Baty F, et al. Therapeutic bronchoscopy for malignant airway stenoses: choice of modality and survival. J Cancer Res Ther. 2010;6:204-209.
39. Cotran RS, Kumar V, Robbins SL. Robbins Pathologic Basis of Disease, 4th ed. Philadelphia, Pa: WB Saunders; 1989.
40. Witt C, Dinges S, Schmidt B, et al. Temporary tracheobronchial stenting in malignant stenoses. Eur J Cancer. 1997;33:204-208.
41. Riedel M, Stein HJ, Mounyam L, et al. Predictors of tracheobronchial invasion of suprabifurcal oesophageal cancer. Respiration. 2000;67:630-637.
42. Riedel M, Stein HJ, Mounyam L, et al. Influence of simultaneous neoadjuvant radiotherapy and chemotherapy on bronchoscopic findings and lung function in patients with locally advanced proximal esophageal cancer. Am J Respir Crit Care Med. 2000;162:1741-1746.
43. Bolliger CT. Multimodalities treatment of advanced pulmonary malignancies. In: Interventional Bronchoscopy: Progress in Respiratory Research. Basel: Switzerland: S. Karger AG; 2000.
44. Brouns M, Jayaraju ST, Lacor C, et al. Tracheal stenosis: a flow dynamics study. J Appl Physiol. 2007;102:1178-1184.
45. Furukawa K, Ishida J, Yamaguchi G, et al. The role of airway stent placement in the management of tracheobronchial stenosis caused by inoperable advanced lung cancer. Surg Today. 2010;40:315-320.
46. Saji H, Furukawa K, Tsutsui H, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg. 2010;11:425-428.
* Juxtaposition, abutment, bulge, and deviation are terms used to describe extrinsic compression, which is not considered adequate evidence of true airway involvement.
* For esophageal stents, on the other hand, methods of measurement under experimental conditions simulating actual stent implantation have been developed, and the test results for various types of metal stents are available (ASAIO J. 2001 Nov-Dec;47:646-650).
* The Mallampati rule states that there is a relationship between what is seen on direct peroral pharyngeal visualization and what is seen with laryngoscopy. To perform a Mallampati evaluation, with the patient seated, have the patient extend his neck, open his mouth fully, protrude his tongue, and say “ah.” Visualize the airway, looking for the tongue, soft and hard palate, uvula, and tonsillar pillars. In patients with a Mallampati score of 1, the entire posterior pharynx is easily visualized; with a 4, no posterior structures can be seen. Patients with a higher Mallampati grade tend to have poorer visualization during direct laryngoscopy. The examination can be approximated in supine and comatose patients using a tongue blade.