Chapter 28 Rigid Bronchoscopic Intervention for Central Airway Obstruction and Concurrent Superior Vena Cava Syndrome Caused by Small Cell Carcinoma
Case Description
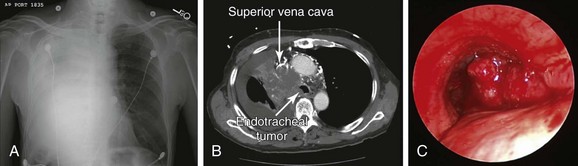
An 85-year-old male with an extensive history of smoking (70 pack-years) developed shortness of breath, which worsened within the week before admission. He had excessive cough, which resulted in hemoptysis (estimated at a few teaspoons/day). Review of systems revealed weight loss (20 kg/6 mo), as well as facial and neck edema for several months. Vital signs showed blood pressure of 150/70 mm Hg, heart rate of 115/min, body temperature of 37.2° C, and respiratory rate of 22/min. On physical examination, the patient had prominent edema of the face, neck, and bilateral upper extremities, with neck vein distention and multiple engorged dilated vessels over the anterior aspect of the chest. Expiratory wheezing was heard on the left hemithorax, and no breath sounds were heard on the right. The rest of the physical examination was normal. Laboratory findings showed WBC count of 19,700 (neutrophils 81.3%, lymphocytes 2%), hemoglobin of 12.8 g/dL, and platelet count of 310,000/mm3. Arterial blood gas analysis showed pH of 7.54, arterial carbon dioxide tension (PaCO2) of 39 mm Hg, and partial pressure of oxygen in arterial blood (PaO2) of 64 mm Hg (O2 = 2 L/min on nasal prong). Electrolytes were within normal limits. Electrocardiography (ECG) showed sinus tachycardia with bifascicular block. Two-dimensional echocardiography showed a small secundum-type atrial septal defect, normal left ventricular function, and no evidence of right ventricular dysfunction. Chest radiograph revealed near complete opacification of the right hemithorax (Figure 28-1). Chest computed tomography showed a 7.3 × 5.7 cm large mediastinal and right hilar mass with near complete occlusion of the superior vena cava (SVC); the mass had eroded into the right mainstem bronchus and lower trachea, causing near complete collapse of the right lung and a right pleural effusion (see Figure 28-1). Bronchoscopic biopsy and washings performed at an outside facility showed small cell carcinoma. The patient was placed on broad-spectrum antibiotics and was transferred to our hospital for consideration for bronchoscopic intervention to restore airway patency. On repeat bronchoscopic examination, the tumor involved the lower trachea above the main carina, completely occluding the entrance to the right main bronchus (see Figure 28-1). The patient was a retired clothing factory owner who lived with his wife. He had a very close family including several children who were actively involved in his care. His family wanted him to receive active and what was hoped would be effective treatment for this tumor. Emergency radiotherapy of first intention had not been recommended by a radiation oncologist because of concerns for worsening tracheal obstruction by radiation-induced edema and ongoing sepsis. Therefore urgent rigid bronchoscopy was scheduled to establish airway patency and to potentially avoid worsening sepsis and respiratory failure.
Discussion Points
1. List two major complications during general anesthesia in patients with large mediastinal masses.
2. Comment on the safety of therapeutic rigid bronchoscopy in people 80 years of age and older.
3. Enumerate seven measures to reduce operative and anesthetic complications in patients with concurrent superior vena cava syndrome and central airway obstruction.
4. Describe three major complications during rigid bronchoscopy in patients with large carinal tumors completely occluding a mainstem bronchus.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient had a new diagnosis of small cell lung cancer (SCLC). The Veterans Affairs Lung Study Group (VALSG)* staging system has been used traditionally to stage SCLC because of its simplicity. Based on this system, limited disease is seen in 30% to 40% at presentation, and extensive disease in 60% to 70% of patients. Accurate staging is clinically relevant because patients with limited-stage disease are treated with combined modality therapy, and those with extensive disease receive chemotherapy alone. Staging by the VALSG system is controversial in patients with locally advanced disease such as contralateral hilar or supraclavicular nodes, pericardial effusions, or malignant pleural effusions, as were seen in this case; for instance, this group is neither precisely defined (as limited or extended disease) nor uniformly managed by different investigators and is frequently excluded from protocols for limited-stage disease. In this regard, the consensus report from the International Association for the Study of Lung Cancer (IASLC) modified the VALSG classification based on the tumor-node-metastasis (TNM) staging system, and only patients with TxNxM1 were considered as having extended disease, so that IASLC criteria include more patients in the prognostically superior limited disease category than are assigned by the VALSG criteria.1 The TNM staging system used for non–small cell lung cancer (NSCLC) has been increasingly advocated by the IASLC to stage SCLC because it describes the extent of disease more accurately than the VALSG system. In fact, the new IASLC M1a descriptors (pleural effusion, pericardial effusion, and contralateral/bilateral intrapulmonary metastasis) adequately prognosticate SCLC patients as having metastatic disease. In fact, the IASLC recommends the use of TNM for all cases of SCLC.2
Similar to our case, patients with advanced-stage lung cancer of any type may present with a variety of loco-regional complications, including central airway obstruction (CAO), superior vena cava (SVC) syndrome, hemoptysis, and post obstructive pneumonia. Patients with CAO usually are not candidates for surgical resection for physiologic or oncologic reasons. Furthermore, chemotherapy and/or radiotherapy in the setting of post obstructive pneumonia may exacerbate the risk for sepsis. The prognosis is guarded, and in the presence of atelectasis, the ability of external beam radiation alone to restore airway patency was shown to be as low as 23%.3 SVC obstruction by lymph node metastasis into the right paratracheal or precarinal station or by direct invasion of lung cancer can cause SVC syndrome* in up to 10% of newly diagnosed cases of SCLC.4 Tumor growth in most cases is gradual, allowing sufficient time for collateral circulation to develop, but many patients eventually present with headache, swelling of the face and neck, and even coma. However, SVC syndrome is no longer considered an emergency, and the use of intravascular stents is recommended only for relapsed or persistent SVC obstruction following chemotherapy or radiation therapy in SCLC.5
Comorbidities
SCLC is the most common malignancy associated with neurologic paraneoplastic syndromes produced by autoantibodies that cross-react with both SCLC cells and the central nervous system or the neuromuscular junction. These antibodies can cause the Lambert-Eaton myasthenic syndrome (LEMS)† in 3% of patients suffering from SCLC.6 Our patient had no obvious neurologic symptoms suggesting LEMS or other paraneoplastic neurologic syndromes seen in SCLC such as limbic encephalitis,‡ paraneoplastic cerebellar degeneration, autonomic neuropathy, or subacute peripheral sensory neuropathy. SCLC cells can also produce a number of polypeptide hormones, including adrenocorticotropic hormone (ACTH) and antidiuretic hormone, resulting in the syndrome of inappropriate antidiuretic hormone and Cushing’s syndrome, respectively. Our patient had no symptoms or laboratory markers suggesting these diagnoses, all of which could affect anesthesia and procedural planning.
Support System
Cancer treatment is emotionally and physically exhausting for patients, so it is important for them to have a good support system during this critical time of their lives. This patient had many family members who were eager to help. Strong family support and faith are noted to have a positive effect on response to cancer treatment.7 If patients, friends, or family members have difficulty coping with the emotional aspects of the illness, experienced professionals in mental health services, social work services, and pastoral services, and local support groups can assist.8
Patient Preferences and Expectations
Although rigorous techniques are frequently used to evaluate survival and response, less rigor is often used when the impact of treatment on quality of life is assessed. Similar to our case, many patients with lung cancer are elderly with complex medical histories and multiple comorbidities. Given limited survival expectations, symptom palliation, quality of life, and convenience of therapy are especially important end points. This patient wished to be minimally involved in treatment decisions, deferring entirely to his family and the cancer care team. He expressed only his wish to not suffer from “chemo” like his brother had a few years previously. Other patients prefer that family members be excluded from treatment decisions and want to take charge themselves. Becoming actively involved in one’s own cancer treatment may actually improve care and recovery after treatment. For instance, when patients are made fully aware of the potential side effects of treatment, they can promptly alert their cancer care team in case of problems.8
Procedural Strategies
Indications
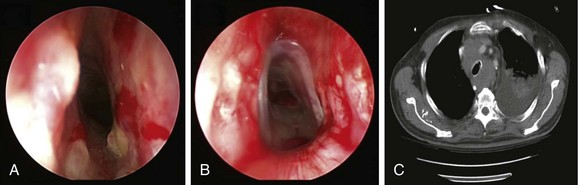
The gold standard treatment for malignant CAO is surgical resection. Similar to this case, however, many patients are poor surgical candidates on the basis of their physiology or oncologic criteria (e.g., inoperable because of advanced tumor stage). Interventional bronchoscopic procedures, when indicated in patients with inoperable NSCLC or SCLC, are expected to restore airway patency and improve lung function, symptoms, and functional status, thus allowing initiation of systemic therapy, which might improve survival.9,10 Resectional techniques are used in cases of intraluminal disease. Airway stent insertion is performed in the setting of malignant CAO caused by severe extrinsic compression (Figure 28-2), or when more than 50% obstruction is present after debulking of the intraluminal component of the disease.11
Contraindications
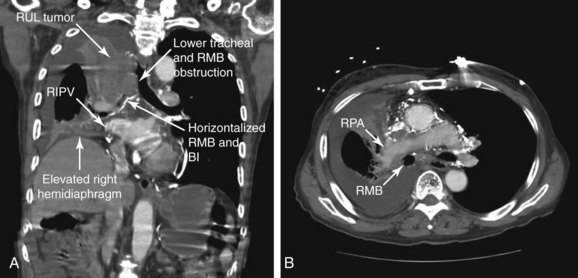
No absolute contraindications to rigid bronchoscopy were noted. Because complete obstruction of the right upper lobe (RUL) bronchus occurred with no identifiable lumen and a potentially nonfunctioning distal lung parenchyma* (Figure 28-3), bronchoscopic attempts to restore RUL bronchial patency were contraindicated. SVC syndrome was a relative contraindication given concerns for hemodynamic instability.
Expected Results
This patient had complete obstruction of the right main bronchus and severe (70%) obstruction in the distal trachea. Palliating CAO was expected to improve dyspnea, lung function, and quality of life.9,12,13 In patients with inoperable or recurrent NSCLC or SCLC occluding a central airway, studies showed no difference in overall survival between those who received both neodymium-doped yttrium aluminum garnet (Nd:YAG) laser treatment and radiation therapy (mean external dose, 53.1 Gy) as compared with historical controls treated with radiation therapy alone. In patients with restored airway lumen,† however, the time interval from treatment to death was prolonged by 4 months compared with those for whom a fully patent airway could not be restored.14 Successful restoration of patency of a major airway occluded by intraluminal tumor using laser resection reduces the likelihood of respiratory failure as a cause of death and does not affect the likelihood of massive fatal hemorrhage, which was a major cause of death in these patients with or without laser treatment.14 Therefore a major issue in these patients is whether they and their families should be warned of the possibility of major bleeding and informed of measures to take should this unfortunate event occur outside the hospital setting.*
Team Experience
Maintaining hemodynamic and respiratory stability during anesthesia requires constant communication between the bronchoscopist and the anesthesiologist. Procedures may be complicated by frequent periods of apnea, compromised airway seals, and the need for special ventilatory techniques such as spontaneous-assisted ventilation or high-frequency jet ventilation. Rigid bronchoscopic procedures involve repeated alternating periods of high and low stimulation requiring rapid titration of intravenous anesthetics to meet fluctuating demands.15
Risk-Benefit Analysis
Because the goal of the procedure is palliation, treatment should have the least possible risk of side effects and discomfort. The risks of intervention warrant careful consideration when patients have significant comorbidities such as a large mediastinal mass, SVC syndrome, or very advanced age.16,17 In our patient, the risks of further physiologic compromise, massive bleeding, and hemodynamic instability were considered to be outweighed by the potential benefit of restoring airway patency to improve functional status and offer systemic therapy. Therapeutic strategies should be elaborated on a case-by-case basis, and advantages and disadvantages of various alternatives discussed with the patient and family if desired, before or during the informed consent process.
Therapeutic Alternatives for Restoring Airway Patency
1. Emergent external beam radiation therapy (EBRT) could have been used for palliating the airway obstruction and the SVC syndrome.18 Initiation of EBRT as primary treatment without attempts at restoration of airway patency in this patient in our opinion would have been of doubtful benefit. EBRT is only variably effective for cancer-induced CAO when the obstruction is severe enough to cause atelectasis, as occurred in our patient. In a study of 330 patients, EBRT palliated hemoptysis in 84% of patients and SVC syndrome in 86% of patients, but atelectasis in only 23%.3 However, EBRT following effective laser treatment could potentially improve survival.19 EBRT had not been recommended as the primary treatment in our patient because of concerns for worsening tracheal obstruction by radiation-induced edema and because of ongoing sepsis in the setting of post obstructive pneumonia. It is noteworthy, from a systemic therapy perspective, that a meta-analysis showed no obvious benefit for combined chemotherapy and radiation therapy over chemotherapy alone in limited-stage SCLC patients older than 70 years. However, more recent trials have revealed a clear-cut benefit for physically “fit elderly”* patients to receive combined modality therapy versus chemotherapy alone, although outcome generally remains inferior to that of younger patients.20
2. Chemotherapy for SCLC involves cisplatin-etoposide regimens and in general is combined with chest radiotherapy for limited disease.21 A systematic review of 29 trials involving 5530 patients found that platinum-based chemotherapy regimens did not offer a statistically significant survival benefit or overall tumor response compared with non–platinum-based regimens. However, platinum-based chemotherapy regimens did increase complete response rates† at the cost of more frequent adverse events, including nausea and vomiting, anemia, and thrombocytopenia.22 CAO is present in as many as 20% to 30% of patients with lung cancer. These patients may develop post obstructive pneumonia.23 Acute infection, as was seen in our patient, is a contraindication to administration of chemotherapy and is an exclusion criterion in most clinical trials.24 We therefore decided to initially proceed with bronchoscopic restoration of airway patency with the patient under general anesthesia.
Techniques and Results
Anesthesia and Perioperative Care
In a patient with severe CAO, premedication with opiates or benzodiazepines in the preoperative period, along with concerns for airway obstruction, anxiety, pain, and periprocedural agitation, should be avoided, or these agents should be administered with caution because they can lead to tachypnea and high airflow velocity during breathing, which increases already turbulent flow in the narrowed airways, exacerbates the pressure drop along the stenosis, and worsens the work of breathing. If possible, patients should be transported to the operating room in a head-up sitting position to avoid worsening preexistent airway and vascular obstruction.25 This simple maneuver can reduce the likelihood of precipitating airway obstruction.26
Careful preoperative assessment for neuromuscular weakness is important in patients with SCLC because approximately 8% of patients with LEMS develop respiratory failure requiring mechanical ventilation. This may develop spontaneously or may be induced by general anesthesia.27 Patients are sensitive to neuromuscular blocking agents and volatile anesthetics, which may cause prolonged paralysis and residual muscle weakness.15 Ideally, these agents should be avoided. Furthermore, the delivery of volatile anesthetics may be problematic when rigid bronchoscopy is performed using an open system with spontaneous-assisted ventilation because the quantity of anesthetic gas reaching the patient is uncertain, and the operating room personnel and operator are at risk for pollution from the escaped anesthetics.15 Total intravenous anesthesia (TIVA) is a preferred technique for rigid bronchoscopy using an open system.*
Securing the airway by rigid bronchoscopic intubation in patients with a large mediastinal mass associated with SVC syndrome and CAO can be challenging. In patients with SVC syndrome, airway management can be complicated by upper airway edema, hemodynamic instability during general anesthesia, and procedure-related bleeding.28 The same degree of edema that is seen externally in the face and neck can be present in the mouth, oropharynx, hypopharynx, and larynx.29 Prompt and atraumatic insertion of the rigid bronchoscope prevents further worsening of preexisting upper airway edema related to severe SVC obstruction. By standing at the head of the patient’s bed during induction, the bronchoscopist is ready and equipped to ensure airway control and, if necessary, to bypass the obstruction, especially in cases of tracheal lesions.30
Anesthesia in the supine position can lead to a decrease in the dimensions of the thoracic cage, a cephalad displacement of the dome of the diaphragm, and a reduction in thoracic volume. Although patients may be asymptomatic while awake, they may develop critical airway obstruction during anesthesia as the result of reduction in the dimensions of the chest wall. This limits the available space for the airways relative to the tumor and mediastinal structures. The decrease in tracheal distention pressure caused by the action of anesthetic agents on chest wall muscle tone also promotes central airway collapse,26 and the supine position prompts an increase in central blood volume, which further increases tumor blood volume and size, worsening both the SVC syndrome and the CAO. Positional changes should certainly be initiated if inadequate ventilation is evident, because airway obstruction sometimes might be ameliorated by placing patients into a lateral decubitus or sitting position.
Although TIVA is used routinely in our institution, loss of airway control has been reported during induction using both intravenous and inhalation anesthesia for rigid bronchoscopy.26,30,31 Muscle relaxants or a dose of propofol that produces apnea can be disastrous in a patient who cannot be ventilated because of CAO.17 Anesthesia induction is the most dangerous period in terms of cardiovascular instability when drugs with a marked tendency for hemodynamic depression are used. Careful hemodynamic monitoring should be provided because patients with SVC syndrome are prone to decreased venous return, reduced cardiac output, and refractory hypotension. Acute worsening of symptoms has been reported as a result of overly generous fluid administration, prompting some authors to recommend diuresis in patients with clinically overt findings of SVC syndrome. It is assumed that diuresis will also decrease tumor volume,25 but diuresis can decrease cardiac preload leading to hypotension, worsening a situation already complicated by compromised venous return.
Spontaneous-assisted ventilation* was shown to have a good cardiac safety profile in patients undergoing rigid bronchoscopy under general anesthesia.31 Although a deeper level of anesthesia is required occasionally to avoid excessive cough and bucking secondary to bronchoscope-induced stimuli, light anesthesia allows spontaneous ventilation. This helps maintain hemodynamic stability and improve oxygenation. Again, we prefer to avoid using neuromuscular blocking agents because they eliminate airway muscular tone that helps maintain airway patency, which may result in prolonged weakness leading to postoperative respiratory failure.30 Positive-pressure ventilation will increase the flow velocity and promote turbulent flow past the region of stenosis. Subsequently, the laminar flow pattern cannot be resumed, potentially resulting in ineffective ventilation of the distal airways and loss of effective gas exchange.29
Ideally, we like to see our patients waking up on the table, avoiding any need for postprocedural endotracheal intubation. During anesthesia maintenance, continuous positive-pressure ventilation and deep anesthesia are also avoided to preserve a normal transpulmonary pressure gradient and to maintain airway patency during spontaneous inspiration.17 This may result in parts of the procedure being performed in a slightly moving or even an occasionally bucking patient. Although precautions are warranted to avoid airway trauma or excessive bronchoscope-related stimulation, short maneuvers are possible, and brief patient movements do not always warrant additional anesthetic to make the patient immobile.
Anatomic Dangers and Other Risks
Because of complete RUL bronchial obstruction and volume loss, normal anatomic relationships in the mediastinum were altered; the right mainstem bronchus (RMB) was more horizontal, the right pulmonary artery was anterior to the proximal RMB, and the right inferior pulmonary vein was medial and posterior to the distal bronchus intermedius (BI) (see Figure 28-3). Therefore laser treatment had to be performed carefully in this area to avoid direct application onto the anterior or posteromedial walls. Because the tumor was hypervascular (see Figure 28-1), superficial absorption of laser energy was expected to be high. This would result in suboptimal deep coagulation and greater risk for bleeding during and after tumor debulking; this risk was probably further increased by the presence of collateral vessels and tumor congestion due to SVC syndrome. In addition, airway obstruction from blood and tumor debris could cause hypoxemia, which in this patient might have been poorly tolerated.
Results and Procedure-Related Complications
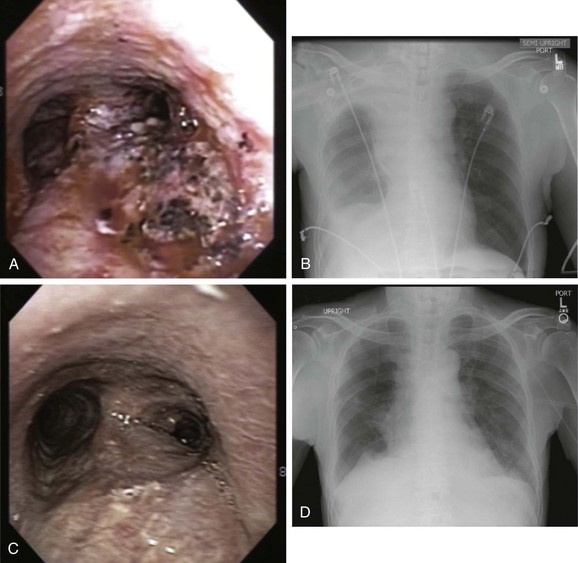
When general anesthesia was induced with intravenous propofol and remifentanil, the patient was difficult to ventilate with the bag mask, probably because of edema in the oropharynx and larynx. Therefore prompt intubation was performed using a 12 mm ventilating rigid bronchoscope. On bronchoscopic examination, the tumor involved the lower third of the trachea, starting 3 cm above the main carina. It completely occluded the entrance to the right main bronchus (see Figure 28-1). The carina was infiltrated, but the left bronchial tree was normal. Nd:YAG laser coagulation and tumor vaporization using a total of 7120 joules were followed by tumor debulking. Abundant thick secretions were suctioned from the previously obstructed right middle and lower lobe bronchi, and bleeding areas were treated using Nd:YAG laser photocoagulation. Flexible bronchoscopy at the end of the procedure confirmed patency of the right lower lobe and right middle lobe bronchi, but the right upper lobe remained completely occluded, and residual tumor was noted along the right lateral wall of the trachea (see video on ExpertConsult.com) (Video VI.28.1![]() ). At extubation, the presence of laryngeal edema prompted us to intubate the patient with a 7.5 mm endotracheal tube, given our concerns for postoperative respiratory failure. Corticosteroids and ventilatory support were provided overnight in the intensive care unit. We explained to the family that this temporary setback would most likely be corrected by the following morning. Indeed, the patient was fully awake with satisfactory weaning parameters and a good leak test, allowing extubation the next day. We did not extubate over a flexible bronchoscope, even though we had noted laryngeal edema at the time of rigid bronchoscopy. His chest radiograph showed improved aeration with re-expansion of a significant portion of the atelectatic right lung (Figure 28-4).
). At extubation, the presence of laryngeal edema prompted us to intubate the patient with a 7.5 mm endotracheal tube, given our concerns for postoperative respiratory failure. Corticosteroids and ventilatory support were provided overnight in the intensive care unit. We explained to the family that this temporary setback would most likely be corrected by the following morning. Indeed, the patient was fully awake with satisfactory weaning parameters and a good leak test, allowing extubation the next day. We did not extubate over a flexible bronchoscope, even though we had noted laryngeal edema at the time of rigid bronchoscopy. His chest radiograph showed improved aeration with re-expansion of a significant portion of the atelectatic right lung (Figure 28-4).
Long-Term Management
Referral
Currently, the standard approach in limited-disease SCLC consists of four to six cycles of platinum-based polychemotherapy combined with radiotherapy of the tumor region and the mediastinum, followed by prophylactic cranial irradiation in case of complete remission. The most widely used chemotherapy regimen in limited-disease SCLC consists of cisplatin and etoposide.32 Both radiation oncology and medical oncology services evaluated this patient, and a decision was made to initially proceed with chemotherapy. Even though it is recommended by American College of Chest Physicians (ACCP) lung cancer guidelines, the survival benefit of concurrent rather than sequential radiation delivery rests largely on findings of a single multicenter trial.33 Emergent radiation therapy was not needed because airway patency had been restored. In fact, a meta-analysis showed no significant reductions in 2-year and 3-year mortality rates despite early thoracic radiotherapy.33
Follow-up Tests and Procedures
Systemic chemotherapy is advocated for both limited- and extensive-stage disease. Once a tissue diagnosis was made and airway patency was restored, the staging workup included chest, liver, and adrenal computed tomography, cranial magnetic resonance imaging (MRI), and a bone scan; in selected cases, unilateral or bilateral bone marrow aspirates and biopsies are also performed because bone marrow can be involved in 15% to 30% of patients at presentation.34
Our elderly patient had an improved but still poor performance status (Eastern Cooperative Oncology Group [ECOG] 2). He was offered combination chemotherapy with etoposide and carboplatin.33 Follow-up bronchoscopy showed patent airways (see Figure 28-4), and chest radiograph showed marked improvement in right lung atelectasis 1 month later (see Figure 28-4). Symptoms, quality of life, and SVC syndrome–related clinical findings improved further after a second cycle of chemotherapy. Unfortunately, our patient expired suddenly from a suspected cardiovascular event while watching television in his home approximately 3 months after the rigid bronchoscopic intervention.
Median survival for SCLC patients without treatment is only 2 to 4 months from the time of diagnosis. For patients undergoing treatment, however, median ranges of survival for limited disease are 15 to 20 months, and for extensive-stage SCLC 8 to 13 months. Approximately 20% to 40% of patients with limited-stage disease and less than 5% of patients with extensive-stage disease survive 2 years.35 The 5-year survival rates are 10% to 13%, and 1% to 2%, respectively.36 For patients older than 75 years of age, median survival time for limited disease SCLC is reportedly 10.3 months. In these patients, 1 year and 2 year survival rates are 47.6% and 11.3%, respectively.37 In addition to the extent of the disease, important adverse prognostic factors in SCLC include male sex (for unknown reasons), poor performance status, weight loss, and continuation of smoking, which may contribute to chemoresistance.38
Quality Improvement
Airway patency was restored, which allowed prompt initiation of chemotherapy. The patient’s need for endotracheal intubation and overnight mechanical ventilatory support was considered a perioperative complication. We also discussed with our anesthesiologist our concerns regarding a transient inability to ventilate during induction. Remifentanil is an ultra-short-acting fentanyl derivative with a rapid onset time of 1 minute and a short duration of action (3 to 10 minutes). This drug is particularly useful for rigid bronchoscopic procedures because it allows active anesthetic management of fluctuating periods of high and low airway stimulation.15 We wondered, however, whether safer alternatives to propofol were available for our elderly patient. Dexmedetomidine, for instance, is an α2-agonist sedative-analgesic that inhibits endogenous norepinephrine release. Some anesthesiologists believe that this drug offers several physiologic benefits, including a reduced sympathetic response to a surgical stimulus, which can have cardioprotective effects in the elderly; other potential benefits of dexmedetomidine include a reduced rise in systemic and pulmonary vascular resistance and a potentially reduced occurrence of postoperative respiratory depression.15 In fact, case reports show that dexmedetomidine, almost without any additional anesthetic drug, maintained spontaneous ventilation in a patient with combined SVC syndrome and severe CAO.39 Alternatively, ketamine, through its profound analgesic, sedative, and amnestic properties, could have been a valuable adjunct drug in our patient because ketamine does not suppress respiration, it reduces the narcotic requirement, and, through its sympathomimetic effects, it could have been useful if blood pressure had needed to be maintained in the presence of volume restriction.15
We realized that our patient’s quality of life should have been objectively evaluated using a validated instrument. In one study, 269 patients undergoing combination chemotherapy and radiotherapy for SCLC of limited extent were asked to complete a Daily Diary Card that enabled an assessment of their quality of life during and after treatment. Results showed that although cytotoxic chemotherapy had an adverse effect on quality of life, the impairment affected only the first 2 or 3 days following each course of treatment. This information could have assisted us in counseling the patient about the likely effects of treatment.40
As an adjunct to general and cancer-specific diagnostic procedures, a comprehensive geriatric assessment (CGA) should be an integral part of cancer treatment in the elderly patient. CGA is based on standardized interviews and covers areas of physical and psychological dysfunction. The routine introduction of CGA in clinical research and daily practice might help clinicians identify those cancer patients for whom the greatest benefit from treatment is to be expected, and to formulate appropriate treatment and management strategies on an individualized basis.32
Discussion Points
1. List two major complications during general anesthesia in patients with large mediastinal masses.
2. Comment on the safety of therapeutic rigid bronchoscopy in people 80 years of age and older.
3. Enumerate seven measures to reduce operative and anesthetic complications in patients with concurrent superior vena cava syndrome and central airway obstruction.29
4. Describe three major complications during rigid bronchoscopy in patients with large carinal tumors completely occluding a mainstem bronchus.
Expert Commentary
This case is far more complicated than most cases of malignant central airway obstruction in that the patient is quite elderly, has an occlusion of the superior vena cava (SVC) with venous engorgement and facial edema, and has a bifascicular cardiac conduction block, as well as an atrial septal defect (ASD). Such comorbid conditions suggest that the patient is at high risk for perioperative complications and even for succumbing during treatment. Two additional clinical constraints influence our management. First, definitive open surgical resection of the malignancy is not an appropriate option; second, avoiding the risks of general anesthesia is impractical. In particular, although the idea of initially treating the patient with radiotherapy is appealing, from clinical experience we know that the severe airway edema that such therapy produces can present a significant danger to the patient in the absence of airway stent insertion.43
Without doubt, airway management is the greatest challenge in this case. Hence, the importance of the bronchoscopist and the anesthesiologist working together to plan and implement a management strategy cannot be overemphasized.44 Airway edema resulting from SVC obstruction can render both intubation and ventilation difficult. One consideration, therefore, is whether general anesthesia should be preceded by awake intubation. A related issue is whether to immediately proceed to rigid bronchoscopy should it be decided to first induce general anesthesia. Yet another issue is whether bronchoscopy should be performed under general anesthesia with the patient breathing spontaneously, albeit with assistance. The bronchoscopy and anesthesia teams will find it necessary to discuss advantages and disadvantages of these various clinical options in light of the patient’s condition, available equipment, and the preferences and training of the two clinical teams. In our experience, in cases such as this one, the bronchoscopy team often prefers to use a rigid bronchoscope with the assistance of muscle relaxants. In the United States, where sugammadex* still is not available, succinylcholine is often used as an initial relaxant. Its short duration of action adds a measure of safety should the patient become impossible to intubate or ventilate after its administration. Many Europeans would prefer to use rocuronium 0.6 mg/kg as a relaxant, followed by a “sugammadex rescue,”45 should ventilation and intubation prove impossible after its administration.
Because of the presence of SVC syndrome, drugs administered via the upper body take longer to enter the central circulation. Consequently, placing an intravenous catheter in a lower body location such as the foot may be advantageous in severe cases of SVC obstruction. In this patient, the additional presence of an ASD makes the use of air trap devices† in the intravenous lines desirable.
In addition to standard monitors (e.g., electrocardiogram, pulse oximeter, capnograph, temperature, spirometry), two special monitors are worth considering. First, an arterial line, sometimes placed before the induction* of general anesthesia in patients with poor ventricular function or other severe cardiac problems, will allow early warning of hemodynamic difficulties. An arterial line will additionally facilitate the drawing of blood for blood gas studies† (useful in respiratory monitoring) and for hemoglobin levels (useful in the event of blood loss and blood transfusions). Second, in cases of TIVA, as in this case, we frequently employ electroencephalographic (EEG) monitoring of the depth of anesthesia, such as the use of bispectral index (BIS) monitoring (Aspect Medical Systems, Norwood, Mass), employing a target BIS score between 40 and 60.
Extubation in these cases can sometimes be challenging, and stridor and even frank respiratory failure sometimes follow. In awakening these patients, we sometimes transition from rigid bronchoscopy to an anesthesia face mask, but more frequently, we transition to a supraglottic airway such as a laryngeal mask airway or even an endotracheal tube before waking the patient, depending on the expected difficulty of maintaining adequate spontaneous respiration. Most cases will benefit from routine administration of intravenous dexamethasone to reduce edema. Stridor is sometimes encountered after extubation; although this may require reintubation, we are often able to avoid this via the use of inhaled racemic epinephrine or the use of heliox, a mixture of helium (typically 70%) and oxygen.46 Extubation over a tube exchanger can be helpful in cases where the need for reintubation is a concern and would be expected to be challenging.47
* Sugammadex (Bridion) is modified cyclodextrin used for reversal of rocuronium-induced neuromuscular blockade. It acts by encapsulating the rocuronium molecule, rendering it unable to bind to the acetylcholine receptor at the neuromuscular junction. The dosage varies between 2 and 16 mg/kg IV, depending on the degree of blockade to be reversed. The most common side effect is dysgeusia (metal or bitter taste); concerns about cardiac rhythm disturbances and hypersensitivity following sugammadex administration have caused the FDA to decline approval of the drug at this time.
† An air trap device captures air bubbles in intravenous lines to prevent them from entering the circulation. Unfortunately, protocol cannot be administered through them (at least in some models) because the traps will clog up. Although one solution might be to avoid the use of propofol (using ketamine, thiopental, or some other drug instead), most clinicians simply inject propofol via a stopcock distal to the filter to avoid this problem.
* The arterial line can also be placed after induction; one should be prepared, however, for hemodynamic instability in patients with poor left ventricular function.
† The frequency of blood draws will depend on how the case proceeds and is very much a judgment issue.
1. Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer. 2002;37:271-276.
2. Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067-1077.
3. Slawson RG, Scott RM. Radiation therapy in bronchogenic carcinoma. Radiology. 1979;132:175-176.
4. Baker GL, Barnes HJ. Superior vena cava syndrome: etiology, diagnosis, and treatment. Am J Crit Care. 1992;1:54-64.
5. Gauden SJ. Superior vena cava syndrome induced by bronchogenic carcinoma: is this an oncological emergency? Australas Radiol. 1993;37:363-366.
6. Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85:838-854.
7. Lissoni P, Messina G, Parolini D, et al. A spiritual approach in the treatment of cancer: relation between faith score and response to chemotherapy in advanced non-small cell lung cancer patients. In Vivo. 2008;22:577-581.
8. NCCN guidelines for patients. http://www.nccn.com/component/content/article/73-life-with-cancer-overview/854-things-to-consider-during-treatment.html. Accessed March 22, 2011
9. Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg. 2003;76:167-174.
10. Morris CD, Budde JM, Godette KD, et al. Palliative management of malignant airway obstruction. Ann Thorac Surg. 2002;74:1928-1933.
11. Bolliger CT. Multimodality treatment of advanced pulmonary malignancies. In: Bolliger CT, Mathur PN. Interventional Bronchoscopy: Progress Respiratory Research. Basel: Karger; 2000:187-196.
12. Mohsenifar Z, Jasper AC, Koerner SK. Physiologic assessment of lung function in patients undergoing laser photoresection of tracheobronchial tumors. Chest. 1988;93:65-69.
13. Vergnon JM, Costes F, Bayon MC, et al. Efficacy of tracheal and bronchial stent placement on respiratory functional tests. Chest. 1995;107:741-746.
14. Macha HN, Becker KO, Kemmer HP. Pattern of failure and survival in endobronchial laser resection: a matched pair study. Chest. 1994;105:1668-1672.
15. Purugganan RV. Intravenous anesthesia for thoracic procedures. Curr Opin Anaesthesiol. 2008;21:1-7.
16. Davoudi M, Shakkottai S, Colt HG. Safety of therapeutic rigid bronchoscopy in people aged 80 and older: a retrospective cohort analysis. J Am Geriatr Soc. 2008;56:943-944.
17. Mackie AM, Watson CB. Anaesthesia and mediastinal masses: a case report and review of the literature. Anaesthesia. 1984;39:899-903.
18. Escalante CP. Causes and management of superior vena cava syndrome. Oncology. 1993;7:61-68. discussion 71-72, 75-77
19. Eichenhorn MS, Kvale PA, Miks VM, et al. Initial combination therapy with YAG laser photoresection and irradiation for inoperable non-small cell carcinoma of the lung: a preliminary report. Chest. 1986;89:782-785.
20. Langer CJ. Elderly patients with lung cancer: biases and evidence. Curr Treat Options Oncol. 2002;3:85-102.
21. Paumier A, Le Péchoux C. Radiotherapy in small-cell lung cancer: where should it go? Lung Cancer. 2010;69:133-140.
22. Amarasena IU, Walters JA, Wood-Baker R, et al. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev. 4, 2008. D006849
23. Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-1297.
24. Thomas P, Robinet G, Gouva S, et al. Randomized multicentric phase II study of carboplatin/gemcitabine and cisplatin/vinorelbine in advanced non-small cell lung cancer GFPC 99-01 study. Lung Cancer. 2006;51:105-114.
25. Pullerits J, Holzman R. Anesthesia for patients with mediastinal masses. Can J Anaesth. 1989;36:681-690.
26. Conacher ID. Anaesthesia and tracheobronchial stenting for central airway obstruction in adults. Br J Anaesth. 2003;90:367-374.
27. O’Neill JH, Murray NM, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome: a review of 50 cases. Brain. 1988;111:577.
28. Narang S, Harte BH, Body SC. Anesthesia for patients with a mediastinal mass. Anesthesiol Clin North Am. 2001;19:559-579.
29. Sibert KS, Biondi JW, Hirsch NP. Spontaneous respiration during thoracotomy in a patient with a mediastinal mass. Anesth Analg. 1987;66:904-907.
30. McMahon CC, Rainey L, Fulton B, et al. Central airway compression: anaesthetic and intensive care consequences. Anaesthesia. 1997;52:158-162.
31. Perrin G, Colt HG, Martin C, et al. Safety of interventional rigid bronchoscopy using intravenous anesthesia and spontaneous assisted ventilation: a prospective study. Chest. 1992;102:1526-1530.
32. Weinmann M, Jeremic B, Bamberg M, et al. Treatment of lung cancer in elderly part II: small cell lung cancer. Lung Cancer. 2003;40:1-16.
33. Samson DJ, Seidenfeld J, Simon GR, et al. Evidence for management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition), American College of Chest Physicians, Chest, 2007;132:314S-323S
34. Tritz DB, Doll DC, Ringenberg QS, et al. Bone marrow involvement in small cell lung cancer: clinical significance and correlation with routine laboratory variables. Cancer. 1989;63:763.
35. Osterlind K, Hansen HH, Hansen M, et al. Long-term disease-free survival in small-cell carcinoma of the lung: a study of clinical determinants. J Clin Oncol. 1986;4:1307.
36. Lassen U, Osterlind K, Hansen M, et al. Long-term survival in small-cell lung cancer: posttreatment characteristics in patients surviving 5 to 18+ years—an analysis of 1,714 consecutive patients. J Clin Oncol. 1995;13:1215.
37. Matsui K, Masuda N, Fukuoka M, et al. Phase II trial of carboplatin plus oral etoposide for elderly patients with small-cell lung cancer. Br J Cancer. 1998;77:1961-1965.
38. Martinez-Garciaz E, Irigoyen M, Gonzalez-Moreno O, et al. Repetitive nicotine exposure leads to a more malignant and metastasis-prone phenotype of SCLC: a molecular insight into the importance of quitting smoking during treatment. Toxicol Sci. 2010;116:467-476.
39. Abdelmalak B, Marcanthony N, Abdelmalak J, et al. Dexmedetomidine for anesthetic management of anterior mediastinal mass. J Anesth. 2010;24:607-610.
40. Fayers PM, Bleehen NM, Girling DJ, et al. Assessment of quality of life in small-cell lung cancer using a Daily Diary Card developed by the Medical Research Council Lung Cancer Working Party. Br J Cancer. 1991;64:299-306.
41. Mohan A, Guleria R, Mohan C, et al. Laser bronchoscopy—current status. J Assoc Physicians India. 2004;52:915-920.
42. Vanderschueren RG, Westermann CJ. Complications of endobronchial neodymium-Yag (Nd:Yag) laser application. Lung. 1990;168:1089-1094.
43. Casal RF. Update in airway stents. Curr Opin Pulm Med. 2010;16:321-328.
44. Abernathy JH3rd, Reeves ST. Airway catastrophes. Curr Opin Anaesthesiol. 2010;23:41-46.
45. Duvaldestin P, Plaud B. Sugammadex in anesthesia practice. Expert Opin Pharmacother. 2010;11:2759-2771.
46. Galway U, Doyle DJ, Gildea T. Anesthesia for endoscopic palliative management of a patient with a large anterior mediastinal mass. J Clin Anesth. 2009;21:150-151.
47. Cooper RM. The use of an endotracheal ventilation catheter in the management of difficult extubations. Can J Anaesth. 1996;43:90-93.
* Limited disease patients are characterized by (1) disease confined to one hemithorax, although local extensions may be present; (2) no extrathoracic metastases, except for possible ipsilateral, supraclavicular nodes if they can be included in the same portal as the primary tumor; and (3) primary tumor and regional nodes, which can be treated adequately and totally encompassed in every portal. Extensive disease patients are inoperable patients who cannot be classified as having limited disease.
* SVC syndrome is characterized by signs and symptoms of central venous obstruction (dyspnea, facial swelling, head fullness). Other symptoms include arm swelling, cough, chest pain, and dysphagia. Patients with cerebral edema may have headaches, confusion, or possibly coma.
† LEMS is an uncommon disorder of neuromuscular junction transmission with the primary clinical manifestation of progressive proximal muscle weakness, autonomic symptoms (dry mouth, blurred vision, constipation), and cranial nerve symptoms (dysarthria, dysphagia, and difficulty chewing). Antibodies directed against the voltage-gated calcium channel (VGCC), a large transmembrane protein with multiple subunits, play a central role in the pathophysiology of LEMS. These antibodies interfere with the normal calcium flux required for the release of acetylcholine.
‡ This disorder is caused by SCLC in 40% to 50% of cases; it develops over days to months and is characterized by mood changes, hallucinations, and memory loss, and less commonly by hypothalamic symptoms such as hyperthermia, somnolence, and endocrine dysfunction.
* No perfect method is available to assess this issue; radionuclide ventilation-perfusion studies can be performed, but reduced perfusion is expected in the lack of ventilation due to bronchial obstruction; in fact, studies show that ventilation and perfusion scores could improve after radiotherapy and/or laser therapy in inoperable patients with malignant bronchial obstruction.
† Complete restoration of airway patency (aka full recanalization) was assessed subjectively by bronchoscopy as restoration of the full shape of the obstructed bronchus and objectively by documenting normalization of peak expiratory flow (PEF); no recanalization was recorded when restoration of bronchial patency failed and distal lobar or segmental bronchi were not visible, and no improvement in PEF was observed.
* Death by massive bleeding is traumatic for all involved. We ourselves have frequently witnessed this event in both hospital and home settings. Advance directives are essential to help guide the behaviors of family members and emergency health care personnel. Specific instructions may be required when patients and families elect to not proceed with resuscitative or life-prolonging measures. Children, if present at the scene, probably should be removed, and everything should be done to comfort the dying patient.
* Patients older than 70 years of age with a good performance status (Karnofsky score >60).
† Tumor response for objective overall response and complete response were defined per World Health Organization (WHO) guidelines for tumor response evaluation. Objective response can be determined clinically, radiologically, or biochemically, or by surgico-pathologic restaging. Complete response represents the disappearance of all known disease, determined by two observations not less than 4 weeks apart. Partial response consists of a 50% or greater decrease in total tumor load of lesions that have been measured to determine the effect of therapy by two observations not less than 4 weeks apart. Overall tumor response refers to partial and complete response rates together.
* Open systems allow the use of jet ventilation to maintain effective gas exchange. Controlled ventilation with a closed system requires capping of the proximal end of the bronchoscope and any small side ports, as well as connecting the large side port to an anesthesia machine. Some method of preventing air leaking through the vocal cords must be provided (e.g., packing the pharynx with damp gauze). A totally closed system allows the use of inhalational anesthetics. Open systems allow the use of jet ventilation to maintain effective gas exchange.
* Spontaneous-assisted ventilation without the use of muscle relaxants requires an Ambu bag with a high-flow oxygen source, which is connected to the side port of the rigid bronchoscope.