11 Right Heart Anomalies
Basic Principles
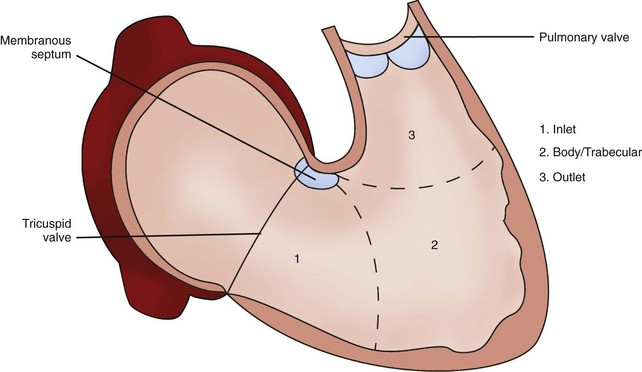
Right Ventricle
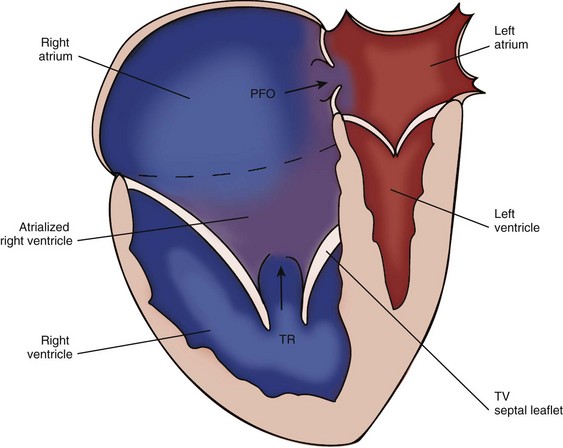
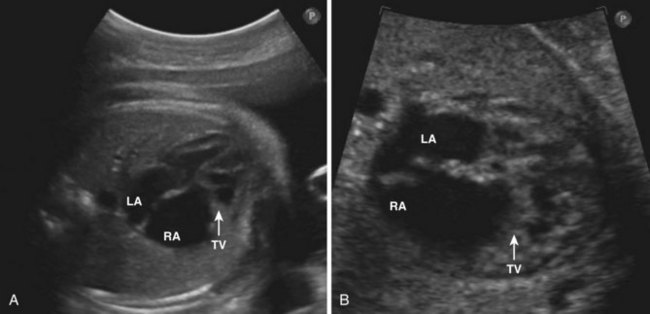
Ebstein’s Anomaly
Background
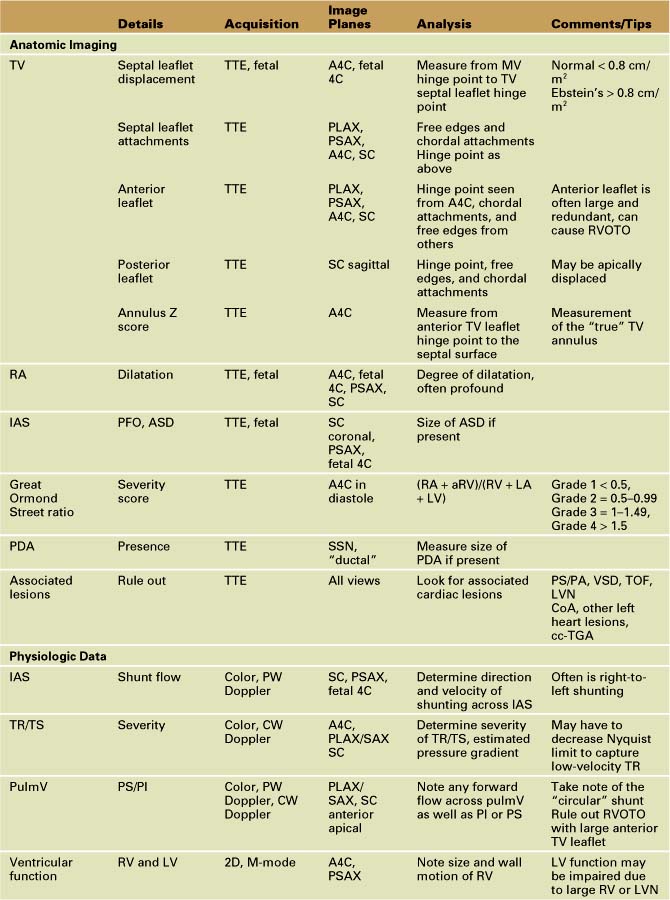
Overview of Echocardiographic Approach
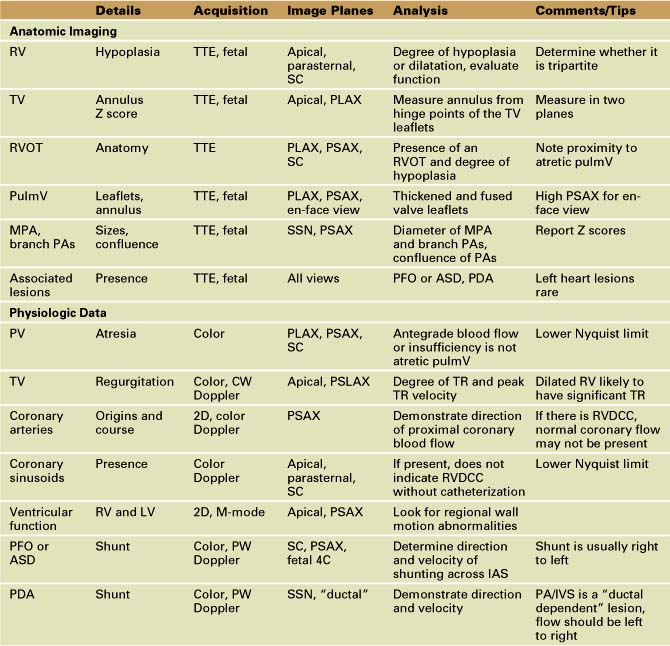
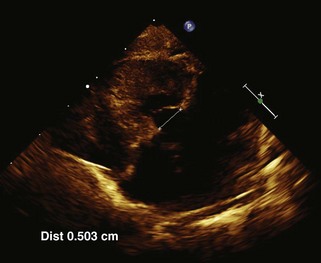
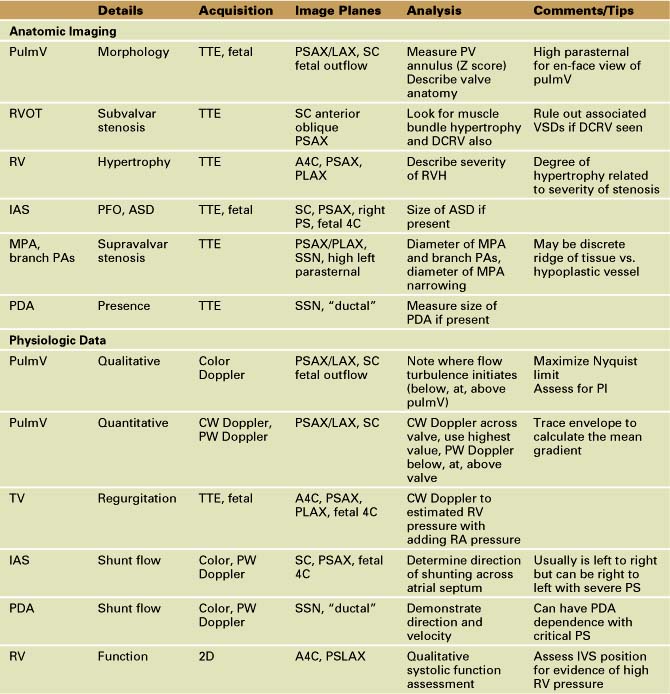
Anatomic Imaging
Acquisition
Analysis
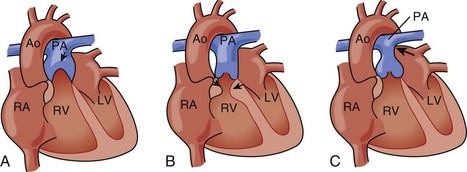
| Type A | Septal and posterior leaflet adherance without functional RV restriction of volume |
| Type B | Atrialized RV with normal anterior leaflet hinge point |
| Type C | Anterior leaflet stenosis |
| Type D | RV entirely atrialized except for a small infundibulum |
Data from Carpentier A, Chauvaud S, Mace L, et al. A new reconstructed operation for Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 2006;132:1285–1290.
Pitfalls
Physiologic Data
Acquisition
Analysis
Alternate Approaches
Key Points
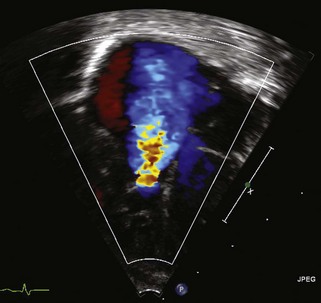
Pulmonary Atresia with Intact Ventricular Septum
Background
Overview of Echocardiographic Approach
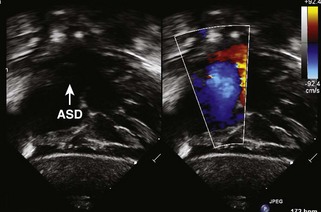
TTE plays an integral role in defining the anatomy and clinical severity of patients with pulmonary atresia with IVS (Table 11-3).
Anatomic Imaging
Acquisition
Analysis
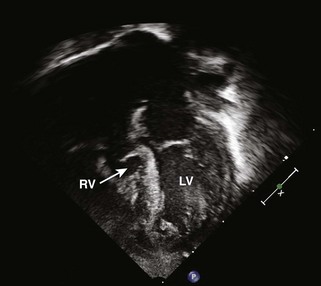
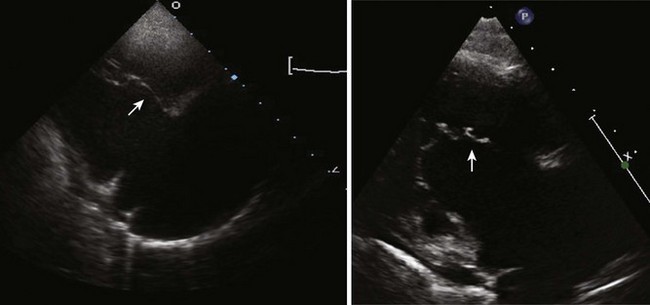
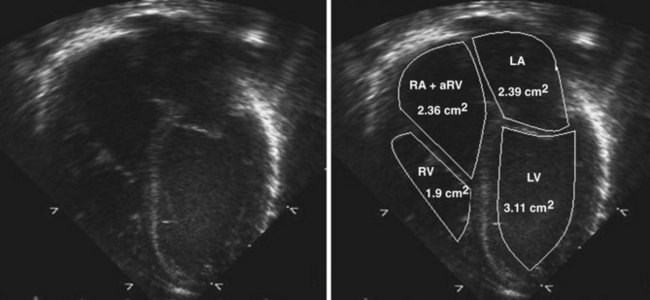
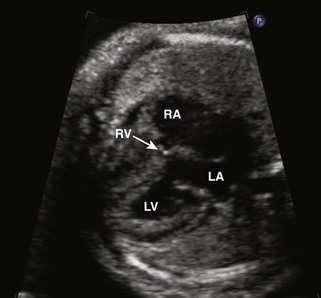
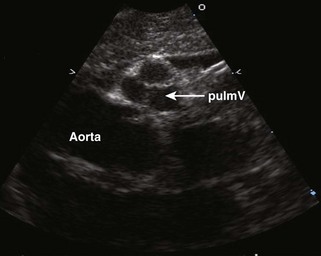
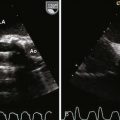
Figure 11-10 Apical view. Note the severely hypoplastic RV. A small inlet portion of the RV is seen in this image.
Physiologic Data
Analysis
Alternate Approaches
Key Points
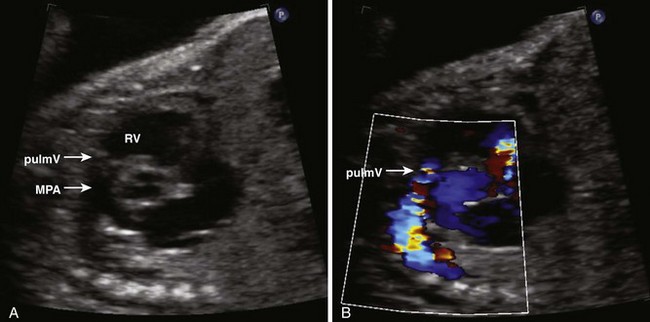
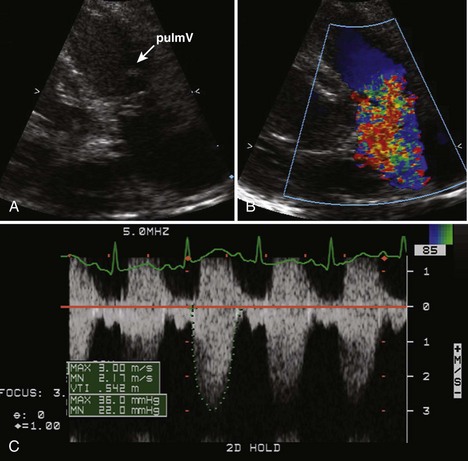
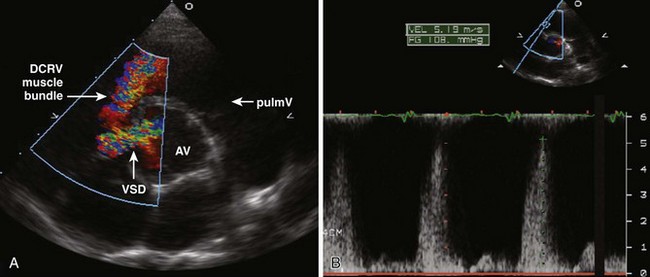
Pulmonary Stenosis
Background
Overview of Echocardiographic Approach
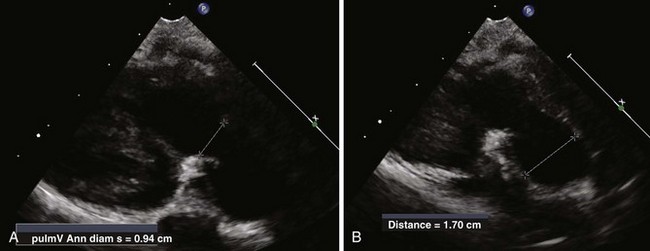
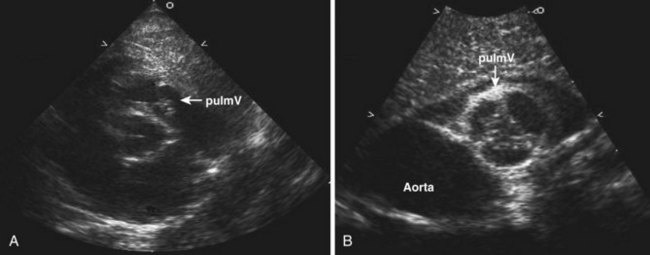
Anatomic Imaging
Acquisition
Analysis
Pitfalls
Physiologic Data
Acquisition
Analysis
Alternate Approaches
Key Points
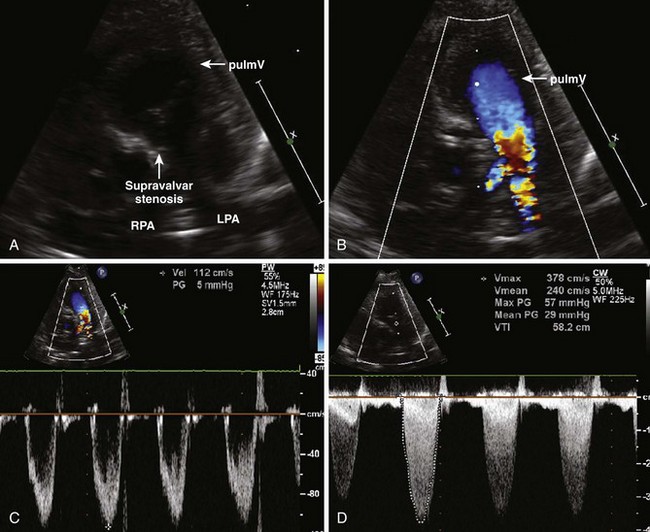
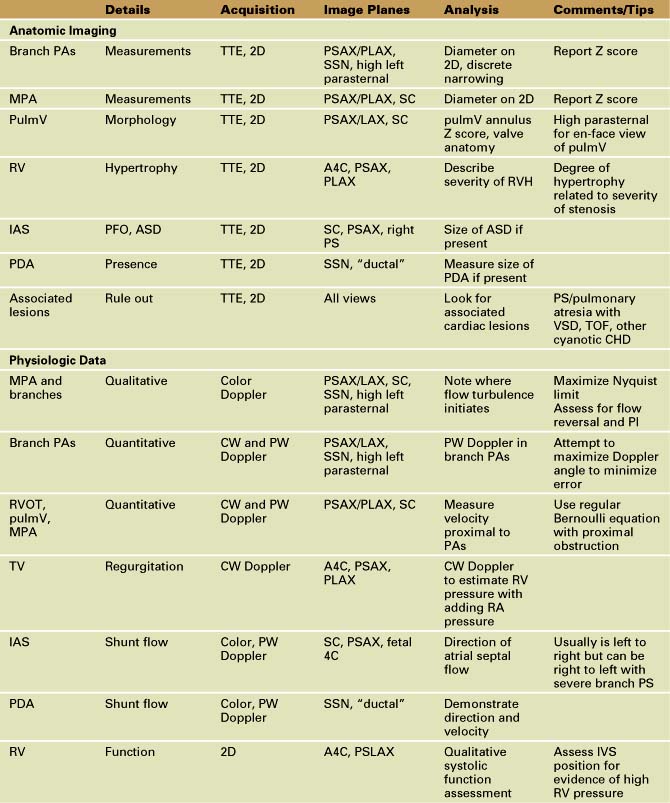
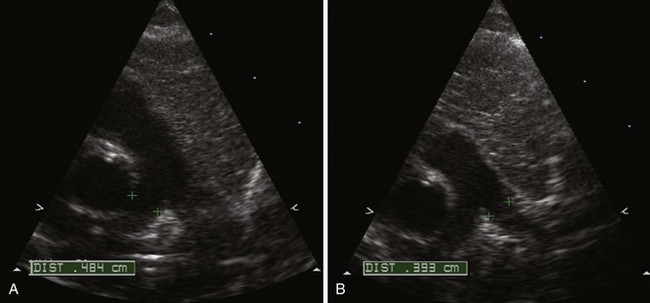
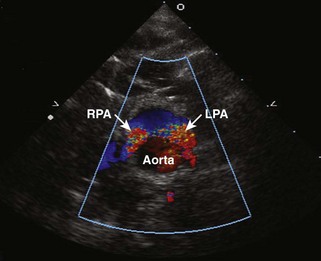
Branch Pulmonary Artery Stenosis
Background
Overview of Echocardiographic Approach
Anatomic imaging
Acquisition
Analysis
Pitfalls
Physiologic Data
Acquisition
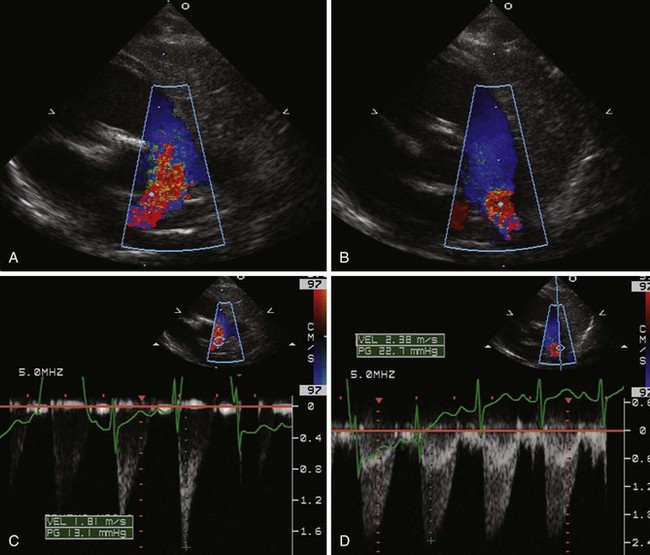
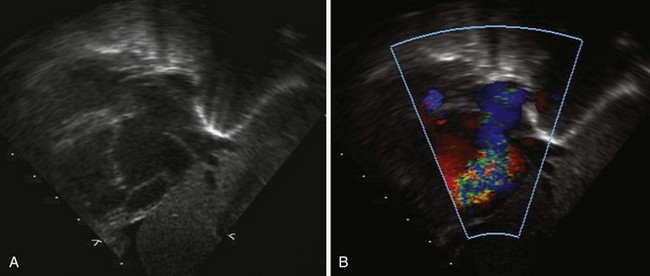
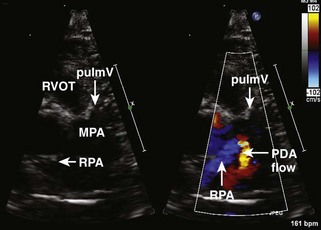
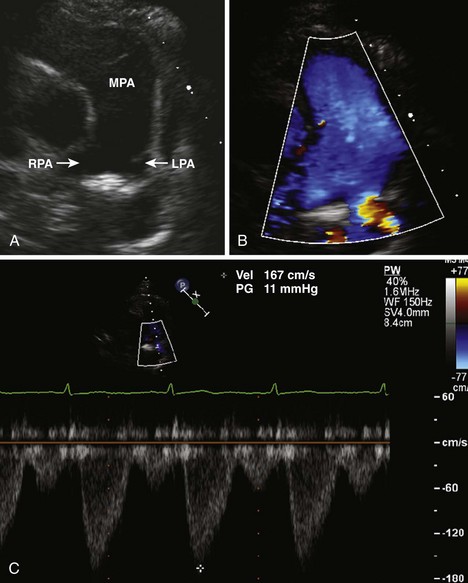
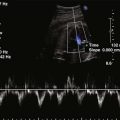
Figure 11-26 PSAX images in an infant with bilateral branch PA stenosis. This is the same patient as in Figure 11-23. A, Color Doppler demonstrating flow turbulence in the RPA. B, Color Doppler demonstrating flow turbulence in the LPA. C, Spectral Doppler of the RPA showing stenosis (peak velocity 1.81 m/s). D, Spectral Doppler of the LPA showing stenosis (peak velocity 2.38 m/s).
Analysis
Pitfalls
Alternate Approaches
Key Points
1 Allen HD, Driscoll DJ, Shaddy RE, Feltes TF, ed. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adults. 2007.
2 Keane JF, Lock JE, Flyer DC. Nadas’ Pediatric Cardiology, 2nd ed. Philadelphia: Saunders/Elsevier; 2006.
3 Attenhofer Jost CH, Connolly HM, Dearani JA, et al. Ebstein’s anomaly. Circulation. 2007;115:277-285.
4 Silverman NH, Gerlis LM, Horowitz ES, et al. Pathologic elucidation of the echocardiographic features of Ebstein’s malformation of the morphologically tricuspid valve in discordant atrioventricular connections. Am J Cardiol. 1995;76:1277-1283.
5 Lai WW, Mertens LL, Cohen MS, Geva T, editors. Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult. Oxford, UK: Wiley-Blackwell, 2009.
6 Paranon S, Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart. 2008;94:237-243.
7 Carpentier A, Chauvaud S, Mace L, et al. A new reconstructive operation for Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 1988;96:92-101.
8 Patel V, Nanda NC, Rajdev S, et al. Live/real time three-dimensional transthoracic echocardiographic assessment of Ebstein’s anomaly. Echocardiography. 2005;22:847-854.
9 Celermajer DS, Cullen S, Sullivan ID, et al. Outcome in neonates with Ebstein’s anomaly. J Am Coll Cardiol. 1992;19:1041-1046.
10 Celermajer DS, Bull C, Till JA, et al. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994;23:170-176.
11 Ammash NM, Warnes CA, Connolly HM, et al. Mimics of Ebstein’s anomaly. Am Heart J. 1997;134:508-513.
12 Steinberger J, Berry JM, Bass JL, et al. Results of a right ventricular outflow patch for pulmonary atresia with intact ventricular septum. Circulation. 1992;86(5 Suppl):167-175.
13 Odim J, Laks H, Plunkett MD, Tung TC. Successful management of patients with pulmonary atresia with intact ventricular septum using a three tier grading system for right ventricular hypoplasia. Ann Thorac Surg. 2006;81:678-684.
14 Hanley FL, Sade RM, Blackstone EH, et al. Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multiinstitutional study. J Thorac Cardiovasc Surg. 1993;105:406-423.
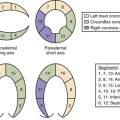
15 Satou GM, Perry SB, Gauvreau K, Geva T. Echocardiographic predictors of coronary artery pathology in pulmonary atresia with intact ventricular septum. Am J Cardiol. 2000;85(11):1319-1324.
16 Park MK. Pediatric Cardiology for Practitioners, 4th ed. St. Louis, MO: Mosby; 2002.
17 Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1-23.
18 Developed in Collaboration With the Society of Cardiovascular Anesthesiologists, Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons, Writing Committee Meers, et al. ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Circulation. 2006;114:e84-e231.
19 Kovalchin JP, Forbes TJ, Nihill MR, Geva T. Echocardiographic determinants of clinical course in infants with critical and severe pulmonary valve stenosis. J Am Coll Cardiol. 1997;29(5):1095-1101.
20 Silvilairat S, Cabalka AK, Cetta F, et al. Echocardiographic assessment of isolated pulmonary valve stenosis: which outpatient Doppler gradient has the most clinical validity? J Am Soc Echocardiogr. 2005;18:1137-1142.
21 King ME, Braun H, Goldblatt A, et al. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: a cross-sectional echocardiographic study. Circulation. 1983;68(1):68-75.
22 Rodriguez RJ, Riggs TW. Physiologic peripheral pulmonic stenosis in infancy. Am J Cardiol. 1990;66:1478-1481.
23 Frank DU, Minich LL, Shaddy RE, Tani LY. Is Doppler an accurate predictor of catheterization gradients for postoperative branch pulmonary stenosis? J Am Soc Echocardiogr. 2002;15(10 Pt 2):1140-1144.
24 Rocchini AP, Kveselis D, Dick M, et al. Use of balloon angioplasty to treat peripheral pulmonary stenosis. Am J Cardiol. 1984;54:1069-1073.
25 Suda K, Matsumura M, Hayashi H, Nishimura K. Comparison of efficacy of medium-sized cutting balloons versus standard balloons for dilation of peripheral pulmonary stenosis. Am J Cardiol. 2006;97:1060-1063.
1 Attenhofer Jost CH, Connolly HM, Dearani JA, et al. Ebstein’s anomaly. Circulation. 2007;115:277-285.
2 Paranon S, Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart. 2008;94:237-243.
3 Reemtsen BL, Fagan BT, Wells WJ, Starnes VA. Current surgical therapy for Ebstein anomaly in neonates. J Thorac Cardiovasc Surg. 2006;132(6):1285-1290.
4 Powell AJ, Mayer JE, Lang P, Lock JE. Outcome in infants with pulmonary atresia, intact ventricular septum, and right ventricle-dependent coronary circulation. Am J Cardiol. 2000;86(11):1272-1274.
5 Odim J, Laks H, Plunkett MD, Tung TC. Successful management of patients with pulmonary atresia with intact ventricular septum using a three tier grading system for right ventricular hypoplasia. Ann Thorac Surg. 2006;81:678-684.
6 ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Circulation. 2006;114:e84-e231.
7 Silvilairat S, Cabalka AK, Cetta F, et al. Echocardiographic assessment of isolated pulmonary valve stenosis: which outpatient Doppler gradient has the most clinical validity? J Am Soc Echocardiogr. 2005;18:1137-1142.
8 Frank DU, Minich LL, Shaddy RE, Tani LY. Is Doppler an accurate predictor of catheterization gradients for postoperative branch pulmonary stenosis? J Am Soc Echocardiogr. 2002;15(10 Pt 2):1140-1144.