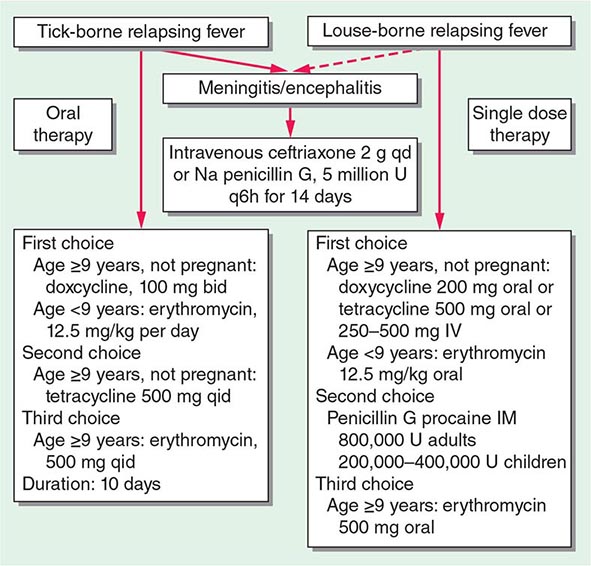
More than 150 species of NTM have been identified. Only a minority of these environmental organisms, which are found in soil and water, are important human pathogens. NTM cause extensive disease, primarily in persons with preexisting pulmonary disease or immunocompromise, but also can cause nodular/bronchiectatic disease in otherwise seemingly healthy hosts. NTM are also important causes of infections in surgical settings. The two major classes of NTM are the slow-growing and rapidly growing species; subcultures of the latter grow within 1 week. The growth characteristics of NTM have diagnostic, therapeutic, and prognostic implications. The rate of growth can provide useful preliminary information within a specific clinical context, in that growth within 2–3 weeks is much more likely to indicate an NTM than M. tuberculosis. When NTM do grow from cultures, colonization should be distinguished from active disease in order to optimize the risk and benefit of prolonged treatment with multiple medications. According to the recommendations of the American Thoracic Society and the Infectious Diseases Society of America, significant clinical manifestations and/or sputum radiographic evidence of progressive disease consistent with NTM infection as well as either reproducible sputum culture results or a single positive culture are required for the diagnosis of NTM pulmonary disease. Isolation of NTM from blood or from an infected-appearing extrapulmonary site, such as soft tissue or bone, is usually indicative of disseminated or local NTM infection (Chap. 204). Treatment of NTM disease is prolonged and requires multiple medications. Side effects of the regimens employed are common, and intermittent therapy is often used to mitigate these adverse events. Treatment regimens depend on the NTM species, the extent or type of disease, and—to some degree—drug susceptibility test results. The nodular bronchiectatic form of MAC infection is generally treated three times per week, whereas fibrocavitary or disseminated MAC infection is treated daily.
THERAPEUTIC CONSIDERATIONS FOR SPECIFIC NTM
M. avium Complex Among the NTM, MAC organisms most commonly cause human disease. In immunocompetent hosts, MAC species are most often found in conjunction with underlying significant lung disease, such as chronic obstructive pulmonary disease or bronchiectasis. For patients with nodular or bronchiectatic MAC lung disease, an initial regimen consisting of clarithromycin or azithromycin, rifampin or rifabutin, and ethambutol is given three times per week. Routine initial testing for macrolide resistance is recommended, as is testing at 6 months with a failing regimen (i.e., with cultures persistently positive for NTM).
In immunocompromised individuals, disseminated MAC infection is generally treated with clarithromycin, ethambutol, and rifabutin. Azithromycin may be substituted in patients unable to tolerate clarithromycin. Amikacin and fluoroquinolones are often used in salvage regimens. Treatment for disseminated MAC infection in AIDS patients may be lifelong in the absence of immune reconstitution. At least 12 months of MAC therapy and 6 months of effective immune reconstitution may be adequate.
M. kansasii M. kansasii is the second most common NTM causing human disease. It is also the second most common cause of NTM pulmonary disease in the United States, where it is most often reported in the southeastern region. M. kansasii infection can be treated with isoniazid, rifampin, and ethambutol; therapy continues for 12 months after culture conversion. Rifampin-resistant M. kansasii has been treated with clarithromycin, trimethoprim-sulfamethoxazole, and streptomycin.
Rapidly Growing Mycobacteria Rapidly growing mycobacteria causing human disease include Mycobacterium abscessus, Mycobacterium fortuitum, and Mycobacterium chelonae. Treatment of these mycobacteria is complex and should be undertaken with input from experienced clinicians. Testing for macrolide resistance is recommended. However, in rapidly growing mycobacteria, an inducible erm gene may confer in vivo macrolide resistance to isolates that are susceptible in vitro.
M. marinum M. marinum is an NTM found in salt water and freshwater, including swimming pools and fish tanks. It is a cause of localized soft-tissue infections, which may require surgical management. Combination regimens include clarithromycin and either ethambutol or rifampin. Other agents with activity against M. marinum include doxycycline, minocycline, and trimethoprim-sulfamethoxazole.
DRUGS FOR THE TREATMENT OF NTM
Clarithromycin
Clarithromycin is a macrolide antibiotic with broad activity against many gram-positive and gram-negative bacteria as well as NTM. This drug is active against MAC organisms and many other NTM species, inhibiting protein synthesis by binding to the 50S mycobacterial ribosomal subunit. NTM resistance to macrolides is probably caused by overexpression of the gene ermB, with consequent methylation of the binding site. Clarithromycin is well absorbed orally and distributes well to tissues. It is cleared both hepatically and renally; the dosage should be reduced in renal insufficiency. Clarithromycin is a substrate for and inhibits cytochrome 3A4 and should not be administered with cisapride, pimozide, or terfenadine because cardiac arrhythmias may occur. Numerous drugs interact with clarithromycin through the CYP3A4 metabolic pathway. Rifampin lowers clarithromycin levels; conversely, rifampin levels are increased by clarithromycin. However, the clinical relevance of this interaction does not appear to be great.
For patients with nodular/bronchiectatic MAC infection, the dosage of clarithromycin is 500 mg, given morning and evening three times a week. For the treatment of fibrocavitary or severe nodular/bronchiectatic MAC infection, a dose of 500–1000 mg is given daily. Disseminated MAC infection is treated with 1000 mg daily. Clarithromycin is used in combination regimens that typically include ethambutol and a rifamycin in order to avoid the development of macrolide resistance. Adverse effects include frequent gastrointestinal intolerance, hepatotoxicity, headache, rash, and rare instances of hypoglycemia. Clarithromycin is contraindicated during pregnancy because of its teratogenicity in animal models.
Azithromycin Azithromycin is a derivative of erythromycin. Although technically an azalide and not a macrolide, it works similarly to macrolides, inhibiting protein synthesis through binding to the 50S ribosomal subunit. Resistance to azithromycin is almost always associated with complete cross-resistance to clarithromycin. Azithromycin is well absorbed orally, with good tissue penetration and a prolonged half-life (~48 h). The usual dosage for treatment of MAC infection is 250 mg/d or 500 mg three times per week. Azithromycin is used in combination with other agents to avoid the development of resistance. For prophylaxis against disseminated MAC infection in immunocompromised individuals, a dose of 1200 mg once per week is given. Because azithromycin is not metabolized by cytochrome P450, it interacts with few drugs. Adjustment of the dosage on the basis of renal function is not necessary.
Cefoxitin Cefoxitin is a second-generation parenteral cephalosporin with activity against rapidly growing NTM, particularly M. abscessus, M. marinum, and M. chelonae. Its mechanism of action against NTM is unknown but may involve inactivation of cell wall synthesis enzymes. High doses are used for treatment of NTM: 200 mg/kg IV three or four times per day, with a maximal daily dose of 12 g. The half-life of cefoxitin is ~1 h, with primarily renal clearance that requires adjustment in renal insufficiency. Adverse effects are uncommon but include gastrointestinal manifestations, rash, eosinophilia, fever, and neutropenia.
CONCLUSION
Treatment of mycobacterial infections requires multiple-drug regimens that often exert significant side effects with the potential to limit tolerability. The prolonged duration of treatment has vastly improved results over those obtained in past decades, but drugs and regimens that will shorten treatment duration and limit adverse drug effects and interactions are needed.
SECTION 9 |
SPIROCHETAL DISEASES |
206 |
Syphilis |
DEFINITION
Syphilis, a chronic systemic infection caused by Treponema pallidum subspecies pallidum, is usually sexually transmitted and is characterized by episodes of active disease interrupted by periods of latency. After an incubation period averaging 2–6 weeks, a primary lesion appears—often associated with regional lymphadenopathy—that resolves without treatment. The secondary stage, associated with generalized mucocutaneous lesions and generalized lymphadenopathy, is followed by a latent period of subclinical infection lasting years or decades. Central nervous system (CNS) involvement may occur early in infection and may be symptomatic or asymptomatic. In the preantibiotic era, about one-third of patients with untreated cases developed the tertiary stage, characterized by progressive destructive mucocutaneous, musculoskeletal, or parenchymal lesions; aortitis; or late CNS manifestations.
ETIOLOGY
The Spirochaetales include four genera that are pathogenic for humans and for a variety of other animals: Leptospira species, which cause leptospirosis (Chap. 208); Borrelia species, which cause relapsing fever and Lyme disease (Chaps. 209 and 210); Brachyspira species, which cause intestinal infections; and Treponema species, which cause the diseases known collectively as treponematoses (see also Chap. 207e). The Treponema species include T. pallidum subspecies pallidum, which causes venereal syphilis; T. pallidum subspecies pertenue, which causes yaws; T. pallidum subspecies endemicum, which causes endemic syphilis or bejel; and T. carateum, which causes pinta. Until recently, the subspecies were distinguished primarily by the clinical syndromes they produce. Researchers have now identified molecular signatures that can differentiate the three subspecies of T. pallidum by culture-independent methods based on polymerase chain reaction (PCR), but other sequence signatures cross subspecies boundaries in certain strains. Other Treponema species found in the human mouth, genital mucosa, and gastrointestinal tract have been associated with disease (e.g., periodontitis), but their role as primary etiologic agents is unclear.
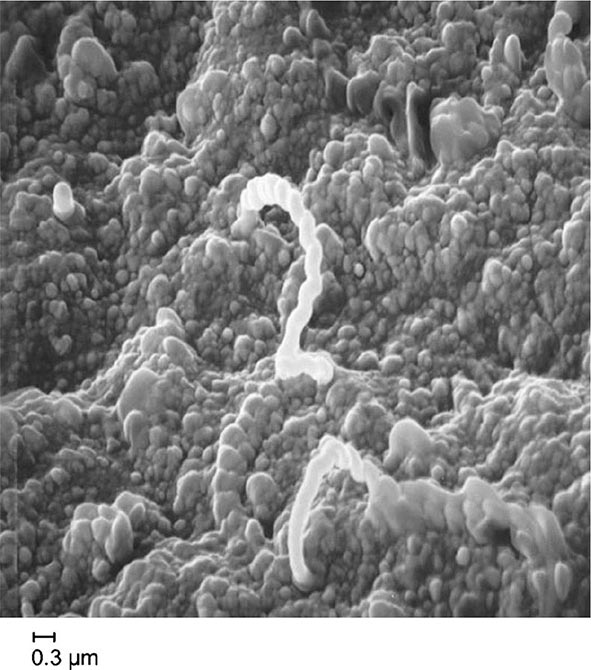
T. pallidum subspecies pallidum (referred to hereafter as T. pallidum), a thin spiral organism, has a cell body surrounded by a trilaminar cytoplasmic membrane, a delicate peptidoglycan layer providing some structural rigidity, and a lipid-rich outer membrane containing relatively few integral membrane proteins. Endoflagella wind around the cell body in the periplasmic space and are responsible for motility.
![]() T. pallidum cannot be cultured in vitro, and little was known about its metabolism until the genome was sequenced in 1998. This spirochete possesses severely limited metabolic capabilities, lacking the genes required for de novo synthesis of most amino acids, nucleotides, and lipids. In addition, T. pallidum lacks genes encoding the enzymes of the Krebs cycle and oxidative phosphorylation. The organism contains numerous compensatory genes predicted to encode transporters of amino acids, carbohydrates, and lipids. In addition, genome analyses and other studies have revealed the existence of a 12-member gene family (tpr) that bears similarities to variable outer-membrane antigens of other spirochetes. One member, TprK, has discrete variable (V) regions that undergo antigenic variation during infection, providing a mechanism for immune evasion.
T. pallidum cannot be cultured in vitro, and little was known about its metabolism until the genome was sequenced in 1998. This spirochete possesses severely limited metabolic capabilities, lacking the genes required for de novo synthesis of most amino acids, nucleotides, and lipids. In addition, T. pallidum lacks genes encoding the enzymes of the Krebs cycle and oxidative phosphorylation. The organism contains numerous compensatory genes predicted to encode transporters of amino acids, carbohydrates, and lipids. In addition, genome analyses and other studies have revealed the existence of a 12-member gene family (tpr) that bears similarities to variable outer-membrane antigens of other spirochetes. One member, TprK, has discrete variable (V) regions that undergo antigenic variation during infection, providing a mechanism for immune evasion.
The only known natural host for T. pallidum is the human. T. pallidum can infect many mammals, but only humans, higher apes, and a few laboratory animals regularly develop syphilitic lesions. Rabbits are used to propagate virulent strains of T. pallidum and serve as the animal model that best reflects human disease and immunopathology.
TRANSMISSION AND EPIDEMIOLOGY
Nearly all cases of syphilis are acquired by sexual contact with infectious lesions (i.e., the chancre, mucous patch, skin rash, or condylomata lata; see Fig. 25e-20). Less common modes of transmission include nonsexual personal contact, infection in utero, blood transfusion, and organ transplantation.
SYPHILIS IN THE UNITED STATES
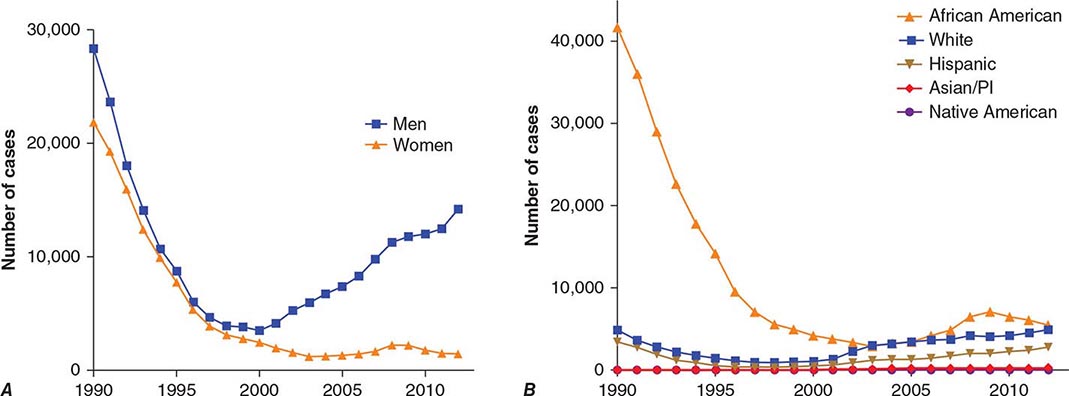
With the advent of penicillin therapy, the total number of cases of syphilis reported annually in the United States declined significantly to a low of 31,575 cases in 2000—a 95% decrease from 1943—with <6000 reported cases of infectious primary and secondary syphilis (the latter being a better indicator of disease activity than total syphilis cases). Since 2000, the number of cases of primary and secondary syphilis has more than doubled, with more than 14,000 cases reported in 2012 (Fig. 206-1). Approximately 70% of these cases were in men who have sex with men (MSM), 20–70% of whom are co-infected with HIV (depending on geographic location). The number of primary and secondary cases among women in the United States increased from 2004 to 2008 but has since been declining in conjunction with a decline in congenital syphilis. Surveillance of the number of new cases of primary and secondary syphilis has revealed multiple 7- to 10-year cycles, which may be attributed to herd immunity in at-risk populations, changing sexual behaviors, and changes in control efforts.
FIGURE 206-1 Primary and secondary syphilis in the United States, 1990–2012, by sex (A) and by race or ethnicity (B). (Data from the Centers for Disease Control and Prevention.)
The populations at highest risk for acquiring syphilis have changed over time, with outbreaks among MSM in the pre-HIV era of the late 1970s and early 1980s as well as at present. It is speculated that recent increases in syphilis and other sexually transmitted infections in MSM may be due to unprotected sex between persons who are HIV concordant and to disinhibition caused by highly effective antiretroviral therapies. The syphilis epidemic that peaked in 1990 predominantly affected African-American heterosexual men and women and occurred largely in urban areas, where infectious syphilis was correlated with the exchange of sex for crack cocaine. The rate of primary and secondary syphilis among African Americans nearly doubled between 2003 and 2009, remains higher than rates for other racial/ethnic groups, but has since declined somewhat (Fig. 206-1).
The incidence of congenital syphilis roughly parallels that of infectious syphilis in women. In 2011, 360 cases in infants <1 year of age were reported, for a decline of 20% since 2008. The case definition for congenital syphilis was broadened in 1989 and now includes all live or stillborn infants delivered to women with untreated or inadequately treated syphilis.
One-third to one-half of individuals named as sexual contacts of persons with infectious syphilis become infected. Many have already developed manifestations of syphilis when they are first seen, and ∼30% of asymptomatic contacts examined within 30 days of exposure actually have incubating infection and will later develop infectious syphilis if not treated. Thus, identification and treatment of all recently exposed sexual contacts continue to be important aspects of syphilis control.
GLOBAL SYPHILIS
![]() Syphilis remains a significant health problem globally; the number of new infections is estimated at 11 million per year. The regions that are most affected include sub-Saharan Africa, South America, China, and Southeast Asia. During the past decade, the incidence rate in China has increased by approximately eightfold, and higher rates of infectious syphilis have been reported among MSM in many European countries. Worldwide, there are estimated to be 1.4 million cases of syphilis among pregnant women, with 500,000 adverse pregnancy outcomes annually (e.g., stillbirth, neonatal and early fetal death, prematurity/low birth weight, and infection in newborns). Congenital syphilis rates in China are ∼150 cases per 100,000 live births.
Syphilis remains a significant health problem globally; the number of new infections is estimated at 11 million per year. The regions that are most affected include sub-Saharan Africa, South America, China, and Southeast Asia. During the past decade, the incidence rate in China has increased by approximately eightfold, and higher rates of infectious syphilis have been reported among MSM in many European countries. Worldwide, there are estimated to be 1.4 million cases of syphilis among pregnant women, with 500,000 adverse pregnancy outcomes annually (e.g., stillbirth, neonatal and early fetal death, prematurity/low birth weight, and infection in newborns). Congenital syphilis rates in China are ∼150 cases per 100,000 live births.
NATURAL COURSE AND PATHOGENESIS OF UNTREATED SYPHILIS
T. pallidum rapidly penetrates intact mucous membranes or microscopic abrasions in skin and, within a few hours, enters the lymphatics and blood to produce systemic infection and metastatic foci long before the appearance of a primary lesion. Blood from a patient with incubating or early syphilis is infectious. The generation time of T. pallidum during early active disease in vivo is estimated to be ∼30 h, and the incubation period of syphilis is inversely proportional to the number of organisms inoculated. The 50% infectious dose for intradermal inoculation in humans has been calculated to be 57 organisms, and the treponeme concentration generally reaches 107/g of tissue before a clinical lesion appears. The median incubation period in humans (∼21 days) suggests an average inoculum of 500–1000 infectious organisms for naturally acquired disease; the incubation period rarely exceeds 6 weeks.
The primary lesion appears at the site of inoculation, usually persists for 4–6 weeks, and then heals spontaneously. Histopathologic examination shows perivascular infiltration, chiefly by CD4+ and CD8+ T lymphocytes, plasma cells, and macrophages, with capillary endothelial proliferation and subsequent obliteration of small blood vessels. The cellular infiltration displays a TH1-type cytokine profile consistent with the activation of macrophages. Phagocytosis of opsonized organisms by activated macrophages ultimately causes their destruction, resulting in spontaneous resolution of the chancre.
The generalized parenchymal, constitutional, and mucocutaneous manifestations of secondary syphilis usually appear ∼6–8 weeks after the chancre heals, although primary and secondary manifestations may overlap. In contrast, some patients may enter the latent stage without ever recognizing secondary lesions. The histopathologic features of secondary maculopapular skin lesions include hyperkeratosis of the epidermis, capillary proliferation with endothelial swelling in the superficial dermis, dermal papillae with transmigration of polymorphonuclear leukocytes, and—in the deeper dermis—perivascular infiltration by CD8+ T lymphocytes, CD4+ T lymphocytes, macrophages, and plasma cells. Treponemes are found in many tissues, including the aqueous humor of the eye and the cerebrospinal fluid (CSF). T. pallidum invades the CNS during the first weeks or months of infection, and CSF abnormalities are detected in as many as 40% of patients during the secondary stage. Clinical hepatitis and immune complex–induced glomerulonephritis are relatively rare but recognized manifestations of secondary syphilis; liver function tests may yield abnormal results in up to one-quarter of patients with early syphilis. Generalized nontender lymphadenopathy is noted in 85% of patients with secondary syphilis. The paradoxical appearance of secondary manifestations despite high titers of antibody (including immobilizing antibody) to T. pallidum may result from immune evasion due to antigenic variation or changes in expression of surface antigens. Secondary lesions generally subside within 2–6 weeks, and the infection enters the latent stage, which is detectable only by serologic testing. In the preantibiotic era, up to 25% of untreated patients experienced at least one generalized or localized mucocutaneous relapse, usually during the first year. Therefore, identification and examination of sexual contacts are most important for patients with syphilis of <1 year’s duration.
As stated earlier, about one-third of patients with untreated latent syphilis developed clinically apparent tertiary disease in the preantibiotic era, when the most common types of tertiary disease were the gumma (a usually benign granulomatous lesion); cardiovascular syphilis (usually involving the vasa vasorum of the ascending aorta and resulting in aneurysm); and late symptomatic neurosyphilis (tabes dorsalis and paresis). In Western countries today, specific treatment for early and latent syphilis and coincidental therapy (i.e., therapy with antibiotics that are given for other conditions but are active against treponemes) have nearly eliminated tertiary syphilis. Asymptomatic CNS involvement, however, is still demonstrable in up to 40% of persons with early syphilis and 25% of patients with late latent syphilis, and cases of general paresis and tabes dorsalis are being reported from China. The factors that contribute to the development and progression of tertiary disease are unknown.
The course of untreated syphilis was studied retrospectively in a group of nearly 2000 patients with primary or secondary disease diagnosed clinically (the Oslo Study, 1891–1951) and was assessed prospectively in 431 African-American men with seropositive latent syphilis of ≥3 years’ duration (the notorious Tuskegee Study, 1932–1972). In the Oslo Study, 24% of patients developed relapsing secondary lesions within 4 years, and 28% eventually developed one or more manifestations of tertiary syphilis. Cardiovascular syphilis, including aortitis, was detected in 10% of patients; 7% of patients developed symptomatic neurosyphilis, and 16% developed benign tertiary gummatous syphilis. Syphilis was the primary cause of death in 15% of men and 8% of women. Cardiovascular syphilis was documented in 35% of men and 22% of women who eventually came to autopsy. In general, serious late complications were nearly twice as common among men as among women.
The Tuskegee Study showed that the death rate among untreated African-American men with syphilis (25–50 years old) was 17% higher than the rate among uninfected subjects and that 30% of all deaths were attributable to cardiovascular or, to a lesser extent, CNS syphilis. Anatomic evidence of aortitis was found in 40–60% of autopsied subjects with syphilis (vs 15% of control subjects), whereas CNS syphilis was found in only 4%. Rates of hypertension were also higher among the infected subjects. The ethical issues eventually raised by this study, begun in the preantibiotic era but continuing into the early 1970s, had a major influence on the development of current guidelines for human medical experimentation, and the history of the study may still contribute to a reluctance of some African Americans to participate as subjects in clinical research.
CLINICAL MANIFESTATIONS
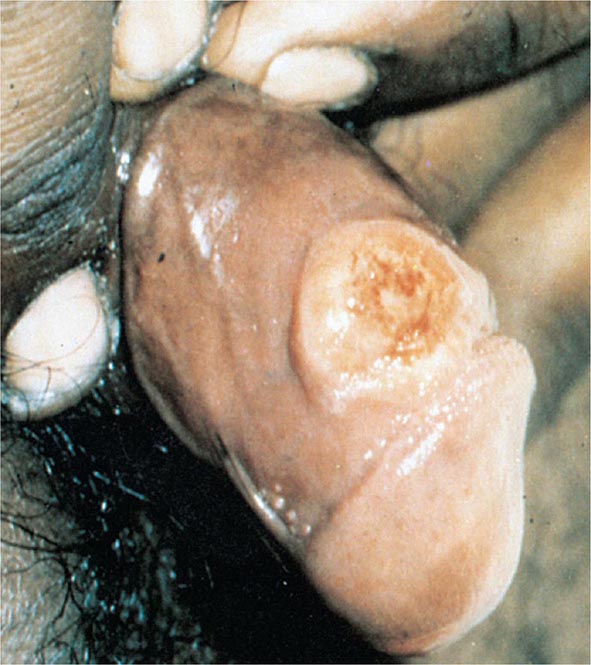
Primary Syphilis The typical primary chancre usually begins as a single painless papule that rapidly becomes eroded and usually becomes indurated, with a characteristic cartilaginous consistency on palpation of the edge and base of the ulcer. Multiple primary lesions are seen in a minority of patients. In heterosexual men the chancre is usually located on the penis (Fig. 206-2; see also Fig. 25e-17), whereas in MSM it may be found in the anal canal or rectum, in the mouth, or on the external genitalia. Oral sex has been identified as the source of infection in some MSM. In women, common primary sites are the cervix and labia. Consequently, primary syphilis goes unrecognized in women and homosexual men more often than in heterosexual men.
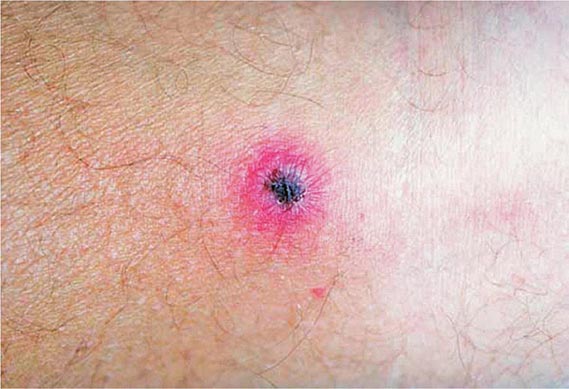
FIGURE 206-2 Primary syphilis with a firm, nontender chancre.
Atypical primary lesions are common. The clinical appearance depends on the number of treponemes inoculated and on the immunologic status of the patient. A large inoculum produces a dark-field-positive ulcerative lesion in nonimmune volunteers but may produce a small dark-field-negative papule, an asymptomatic but seropositive latent infection, or no response at all in some individuals with a history of syphilis. A small inoculum may produce only a papular lesion, even in nonimmune individuals. Therefore, syphilis should be considered even in the evaluation of trivial or atypical dark-field-negative genital lesions. The genital lesions that most commonly must be differentiated from those of primary syphilis include those caused by herpes simplex virus infection (Chap. 216), chancroid (Chap. 182), traumatic injury, and donovanosis (Chap. 198e). Regional (usually inguinal) lymphadenopathy accompanies the primary syphilitic lesion, appearing within 1 week of lesion onset. The nodes are firm, nonsuppurative, and painless. Inguinal lymphadenopathy is bilateral and may occur with anal as well as with external genital chancres. The chancre generally heals within 4–6 weeks (range, 2–12 weeks), but lymphadenopathy may persist for months.
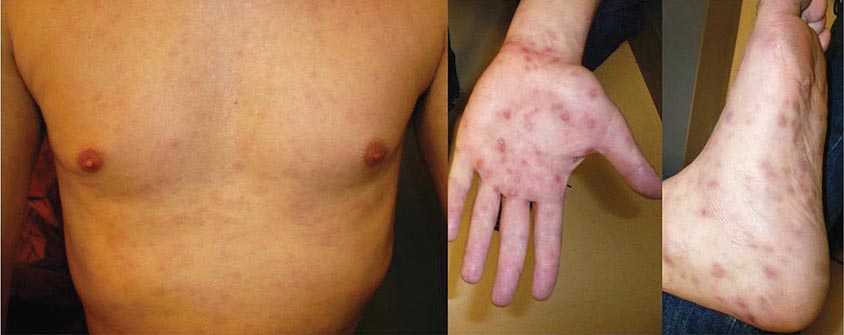
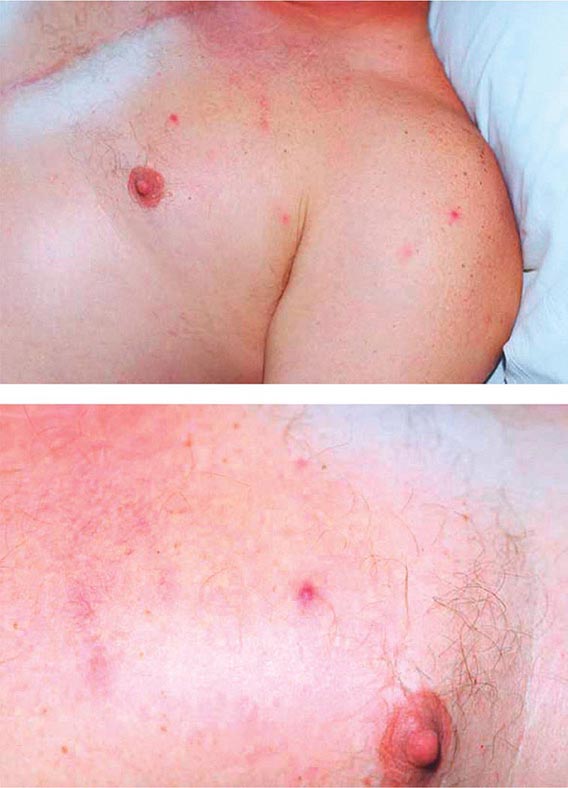
Secondary Syphilis The protean manifestations of the secondary stage usually include mucocutaneous lesions and generalized nontender lymphadenopathy. The healing primary chancre may still be present in ∼15% of cases, and the stages may overlap more frequently in persons with concurrent HIV infection. The skin rash consists of macular, papular, papulosquamous, and occasionally pustular syphilides; often more than one form is present simultaneously. The eruption may be very subtle, and 25% of patients with a discernible rash may be unaware that they have dermatologic manifestations. Initial lesions are pale red or pink, nonpruritic, discrete macules distributed on the trunk and proximal extremities; these macules progress to papular lesions that are distributed widely and that frequently involve the palms and soles (Fig. 206-3; see also Figs. 25e-18 and 25e-19) Rarely, severe necrotic lesions (lues maligna) may appear; they are more commonly reported in HIV-infected individuals. Involvement of the hair follicles may result in patchy alopecia of the scalp hair, eyebrows, or beard in up to 5% of cases.
FIGURE 206-3 Secondary syphilis. Left: Maculopapular truncal eruption. Middle: Papules on the palms. Right: Papules on the soles. (Courtesy of Jill McKenzie and Christina Marra.)
In warm, moist, intertriginous areas (commonly the perianal region, vulva, and scrotum), papules can enlarge to produce broad, moist, pink or gray-white, highly infectious lesions (condylomata lata; see Fig. 25e-20) in 10% of patients with secondary syphilis. Superficial mucosal erosions (mucous patches) occur in 10–15% of patients and commonly involve the oral or genital mucosa (see Fig. 25e-21). The typical mucous patch is a painless silver-gray erosion surrounded by a red periphery.
Constitutional signs and symptoms that may accompany or precede secondary syphilis include sore throat (15–30%), fever (5–8%), weight loss (2–20%), malaise (25%), anorexia (2–10%), headache (10%), and meningismus (5%). Acute meningitis occurs in only 1–2% of cases, but CSF cell and protein concentrations are increased in up to 40% of cases, and viable T. pallidum organisms have been recovered from CSF during primary and secondary syphilis in 30% of cases; the latter finding is often but not always associated with other CSF abnormalities.
Less common complications of secondary syphilis include hepatitis, nephropathy, gastrointestinal involvement (hypertrophic gastritis, patchy proctitis, or a rectosigmoid mass), arthritis, and periostitis. Ocular findings associated with secondary syphilis include pupillary abnormalities and optic neuritis as well as the classic iritis or uveitis. The diagnosis of ocular syphilis is often considered in affected patients only after they fail to respond to steroid therapy. Anterior uveitis has been reported in 5–10% of patients with secondary syphilis, and T. pallidum has been demonstrated in aqueous humor from such patients. Hepatic involvement is common in syphilis; although it is usually asymptomatic, up to 25% of patients may have abnormal liver function tests. Frank syphilitic hepatitis may be seen. Renal involvement usually results from immune complex deposition and produces proteinuria associated with an acute nephrotic syndrome. Like those of primary syphilis, the manifestations of the secondary stage resolve spontaneously, usually within 1–6 months.
Latent Syphilis Positive serologic tests for syphilis, together with a normal CSF examination and the absence of clinical manifestations of syphilis, indicate a diagnosis of latent syphilis in an untreated person. The diagnosis is often suspected on the basis of a history of primary or secondary lesions, a history of exposure to syphilis, or the delivery of an infant with congenital syphilis. A previous negative serologic test or a history of lesions or exposure may help establish the duration of latent infection, which is an important factor in the selection of appropriate therapy. Early latent syphilis is limited to the first year after infection, whereas late latent syphilis is defined as that of ≥1 year’s duration (or of unknown duration). T. pallidum may still seed the bloodstream intermittently during the latent stage, and pregnant women with latent syphilis may infect the fetus in utero. Moreover, syphilis has been transmitted through blood transfusion or organ donation from patients with latent syphilis. It was previously thought that untreated late latent syphilis had three possible outcomes: (1) persistent lifelong infection; (2) development of late syphilis; or (3) spontaneous cure, with reversion of serologic tests to negative. It is now apparent, however, that the more sensitive treponemal antibody tests rarely, if ever, become nonreactive without treatment. Although progression to clinically evident late syphilis is very rare today, the occurrence of spontaneous cure is in doubt.
Involvement of the CNS Traditionally, neurosyphilis has been considered a late manifestation of syphilis, but this view is inaccurate. CNS syphilis represents a continuum encompassing early invasion (usually within the first weeks or months of infection), months to years of asymptomatic involvement, and, in some cases, development of early or late neurologic manifestations.
ASYMPTOMATIC NEUROSYPHILIS The diagnosis of asymptomatic neurosyphilis is made in patients who lack neurologic symptoms and signs but who have CSF abnormalities including mononuclear pleocytosis, increased protein concentrations, or CSF reactivity in the Venereal Disease Research Laboratory (VDRL) test. CSF abnormalities are demonstrated in up to 40% of cases of primary or secondary syphilis and in 25% of cases of latent syphilis. T. pallidum has been recovered by inoculation into rabbits of CSF from up to 30% of patients with primary or secondary syphilis but less frequently by inoculation of CSF from patients with latent syphilis. The presence of T. pallidum in CSF is often associated with other CSF abnormalities, but organisms can be recovered from patients with otherwise normal CSF. Although the prognostic implications of these findings in early syphilis are uncertain, it may be appropriate to conclude that even patients with early syphilis who have such findings do indeed have asymptomatic neurosyphilis and should be treated for neurosyphilis; such treatment is particularly important in patients with concurrent HIV infection. Before the advent of penicillin, the risk of development of clinical neurosyphilis in untreated asymptomatic persons was roughly proportional to the intensity of CSF changes, with the overall cumulative probability of progression to clinical neurosyphilis ∼20% in the first 10 years but increasing with time. Most experts agree that neurosyphilis is more common in HIV-infected persons, while immunocompetent patients with untreated latent syphilis and normal CSF probably run a very low risk of subsequent neurosyphilis. In several recent studies, neurosyphilis was associated with a rapid plasma reagin (RPR) titer of ≥1:32, regardless of clinical stage or HIV infection status.
SYMPTOMATIC NEUROSYPHILIS The major clinical categories of symptomatic neurosyphilis include meningeal, meningovascular, and parenchymatous syphilis. The last category includes general paresis and tabes dorsalis. The onset of symptoms usually occurs <1 year after infection for meningeal syphilis, up to 10 years after infection for meningovascular syphilis, at ∼20 years for general paresis, and at 25–30 years for tabes dorsalis. Neurosyphilis is more frequently symptomatic in patients who are co-infected with HIV, particularly in the setting of a low CD4+ T lymphocyte count. In addition, recent evidence suggests that syphilis infection worsens the cognitive impairment seen in HIV-infected persons and that this effect persists even after treatment for syphilis.
Meningeal syphilis may present as headache, nausea, vomiting, neck stiffness, cranial nerve involvement, seizures, and changes in mental status. This condition may be concurrent with or may follow the secondary stage. Patients presenting with uveitis, iritis, or hearing loss often have meningeal syphilis, but these clinical findings can also be seen in patients with normal CSF.
Meningovascular syphilis reflects meningitis together with inflammatory vasculitis of small, medium, or large vessels. The most common presentation is a stroke syndrome involving the middle cerebral artery of a relatively young adult. However, unlike the usual thrombotic or embolic stroke syndrome of sudden onset, meningovascular syphilis often becomes manifest after a subacute encephalitic prodrome (with headaches, vertigo, insomnia, and psychological abnormalities), which is followed by a gradually progressive vascular syndrome.
The manifestations of general paresis reflect widespread late parenchymal damage and include abnormalities corresponding to the mnemonic paresis: personality, affect, reflexes (hyperactive), eye (e.g., Argyll Robertson pupils), sensorium (illusions, delusions, hallucinations), intellect (a decrease in recent memory and in the capacity for orientation, calculations, judgment, and insight), and speech. Tabes dorsalis is a late manifestation of syphilis that presents as symptoms and signs of demyelination of the posterior columns, dorsal roots, and dorsal root ganglia. Symptoms include ataxic wide-based gait and foot drop; paresthesia; bladder disturbances; impotence; areflexia; and loss of positional, deep-pain, and temperature sensations. Trophic joint degeneration (Charcot’s joints) and perforating ulceration of the feet can result from loss of pain sensation. The small, irregular Argyll Robertson pupil, a feature of both tabes dorsalis and paresis, reacts to accommodation but not to light. Optic atrophy also occurs frequently in association with tabes.
Other Manifestations of Late Syphilis The slowly progressive inflammatory process leading to tertiary disease begins early during infection, although these manifestations may not become clinically apparent for years or decades. Early syphilitic aortitis becomes evident soon after secondary lesions subside, and treponemes that trigger the development of gummas may have seeded the tissue years earlier.
CARDIOVASCULAR SYPHILIS Cardiovascular manifestations, usually appearing 10–40 years after infection, are attributable to endarteritis obliterans of the vasa vasorum, which provide the blood supply to large vessels; T. pallidum DNA has been detected by PCR in aortic tissue. Cardiovascular involvement results in uncomplicated aortitis, aortic regurgitation, saccular aneurysm (usually of the ascending aorta), or coronary ostial stenosis. In the preantibiotic era, symptomatic cardiovascular complications developed in ∼10% of persons with late untreated syphilis. Today, this form of late syphilis is rarely seen in the developed world. Linear calcification of the ascending aorta on chest x-ray films suggests asymptomatic syphilitic aortitis, as arteriosclerosis seldom produces this sign. Only 1 in 10 aortic aneurysms of syphilitic origin involves the abdominal aorta.
LATE BENIGN SYPHILIS (GUMMA) Gummas are usually solitary lesions ranging from microscopic to several centimeters in diameter. Histologic examination shows a granulomatous inflammation, with a central area of necrosis due to endarteritis obliterans. Although rarely demonstrated microscopically, T. pallidum has been detected by PCR or recovered from these lesions, and penicillin treatment results in rapid resolution, confirming the treponemal stimulus for the inflammation. Common sites include the skin and skeletal system; however, any organ (including the brain) may be involved. Gummas of the skin produce indolent, painless, indurated nodular or ulcerative lesions that may resemble other chronic granulomatous conditions, including tuberculosis, sarcoidosis, leprosy, and deep fungal infections. Skeletal gummas most frequently involve the long bones, although any bone may be affected. Upper respiratory gummas can lead to perforation of the nasal septum or palate.
Congenital Syphilis Transmission of T. pallidum across the placenta from a syphilitic woman to her fetus may occur at any stage of pregnancy, but fetal damage generally does not occur until after the fourth month of gestation, when fetal immunologic competence begins to develop. This timing suggests that the pathogenesis of congenital syphilis, like that of adult syphilis, depends on the host immune response rather than on a direct toxic effect of T. pallidum. The risk of fetal infection during untreated early maternal syphilis is ∼75–95%, decreasing to ∼35% for maternal syphilis of >2 years’ duration. Adequate treatment of the woman before the 16th week of pregnancy should prevent fetal damage, and treatment before the third trimester should adequately treat the infected fetus. Untreated maternal infection may result in a rate of fetal loss of up to 40% (with stillbirth more common than abortion because of the late onset of fetal pathology), prematurity, neonatal death, or nonfatal congenital syphilis. Among infants born alive, only fulminant congenital syphilis is clinically apparent at birth, and these babies have a very poor prognosis. The most common clinical problem is the healthy-appearing baby born to a mother with a positive serologic test
![]() Routine serologic testing in early pregnancy is considered cost-effective in virtually all populations, even in areas with a low prenatal prevalence of syphilis. Low-tech point-of-care tests have been developed and are being widely implemented to facilitate antenatal testing in resource-poor settings. A recent study demonstrated the high cost-effectiveness of using these tests for screening (with subsequent treatment) in sub-Saharan Africa. Adverse outcomes were reduced, with 64,000 fewer stillbirths, 25,000 fewer neonatal deaths, and up to 25,000 fewer live births of infants with syphilis. The intervention would remain cost-effective even if the current syphilis seroprevalence among pregnant women declined from its present 3.1% to 0.4%. Where the prevalence of syphilis is high or when the patient is at high risk of reinfection, serologic testing should be repeated in the third trimester and at delivery. Neonatal congenital syphilis must be differentiated from other generalized congenital infections, including rubella, cytomegalovirus or herpes simplex virus infection, and toxoplasmosis, as well as from erythroblastosis fetalis.
Routine serologic testing in early pregnancy is considered cost-effective in virtually all populations, even in areas with a low prenatal prevalence of syphilis. Low-tech point-of-care tests have been developed and are being widely implemented to facilitate antenatal testing in resource-poor settings. A recent study demonstrated the high cost-effectiveness of using these tests for screening (with subsequent treatment) in sub-Saharan Africa. Adverse outcomes were reduced, with 64,000 fewer stillbirths, 25,000 fewer neonatal deaths, and up to 25,000 fewer live births of infants with syphilis. The intervention would remain cost-effective even if the current syphilis seroprevalence among pregnant women declined from its present 3.1% to 0.4%. Where the prevalence of syphilis is high or when the patient is at high risk of reinfection, serologic testing should be repeated in the third trimester and at delivery. Neonatal congenital syphilis must be differentiated from other generalized congenital infections, including rubella, cytomegalovirus or herpes simplex virus infection, and toxoplasmosis, as well as from erythroblastosis fetalis.
The manifestations of congenital syphilis include (1) early manifestations, which appear within the first 2 years of life (often at 2–10 weeks of age), are infectious, and resemble the manifestations of secondary syphilis in the adult; (2) late manifestations, which appear after 2 years and are noninfectious; and (3) residual stigmata. The earliest manifestations of congenital syphilis include rhinitis, or “snuffles” (23%); mucocutaneous lesions (35–41%); bone changes (61%), including osteochondritis, osteitis, and periostitis detectable by x-ray examination of long bones; hepatosplenomegaly (50%); lymphadenopathy (32%); anemia (34%); jaundice (30%); thrombocytopenia; and leukocytosis. CNS invasion by T. pallidum is detectable in 22% of infected neonates. Neonatal death is usually due to pulmonary hemorrhage, secondary bacterial infection, or severe hepatitis.
Late congenital syphilis (untreated after 2 years of age) is subclinical in 60% of cases; the clinical spectrum in the remainder of cases may include interstitial keratitis (which occurs at 5–25 years of age), eighth-nerve deafness, and recurrent arthropathy. Bilateral knee effusions are known as Clutton’s joints. Neurosyphilis was present in about one-quarter of untreated patients with late congenital syphilis in the preantibiotic era. Gummatous periostitis occurs at 5–20 years of age and, as in nonvenereal endemic syphilis, tends to cause destructive lesions of the palate and nasal septum.
Classic stigmata include Hutchinson’s teeth (centrally notched, widely spaced, peg-shaped upper central incisors), “mulberry” molars (sixth-year molars with multiple, poorly developed cusps), saddle nose, and saber shins.
LABORATORY EXAMINATIONS
Demonstration of the Organism T. pallidum cannot be detected by culture. Historically, dark-field microscopy and immunofluorescence antibody staining have been used to identify this spirochete in samples from moist lesions such as chancres or condylomata lata, but these tests are rarely available outside of research laboratories. Sensitive and specific PCR tests have been developed but are not commercially available, although a number of laboratories perform in-house validated PCR testing.
T. pallidum can be found in tissue with appropriate silver stains, but these results should be interpreted with caution because artifacts resembling T. pallidum are often seen. Tissue treponemes can be demonstrated more reliably in research laboratories by PCR or by immunofluorescence or immunohistochemical methods using specific monoclonal or polyclonal antibodies to T. pallidum.
Serologic Tests for Syphilis There are two types of serologic test for syphilis: nontreponemal and treponemal. Both are reactive in persons with any treponemal infection, including yaws, pinta, and endemic syphilis.
The most widely used nontreponemal antibody tests for syphilis are the RPR and VDRL tests, which measure IgG and IgM directed against a cardiolipin-lecithin-cholesterol antigen complex. The RPR test is easier to perform and uses unheated serum or plasma; it is the test of choice for rapid serologic diagnosis in a clinical setting. The VDRL test remains the standard for examining CSF and is superior to the RPR for this purpose. The RPR and VDRL tests are recommended for screening or for quantitation of serum antibody. The titer reflects disease activity, rising during the evolution of early syphilis, often exceeding 1:32 in secondary syphilis, and declining thereafter without therapy. After treatment for early syphilis, a persistent fall by fourfold or more (e.g., a decline from 1:32 to 1:8) is considered an adequate response. VDRL titers do not correspond directly to RPR titers, and sequential quantitative testing (as for response to therapy) must employ a single test. As will be discussed (see “Evaluation for Neurosyphilis,” below), the RPR titer may be useful in determining which patients will benefit from CSF examination.
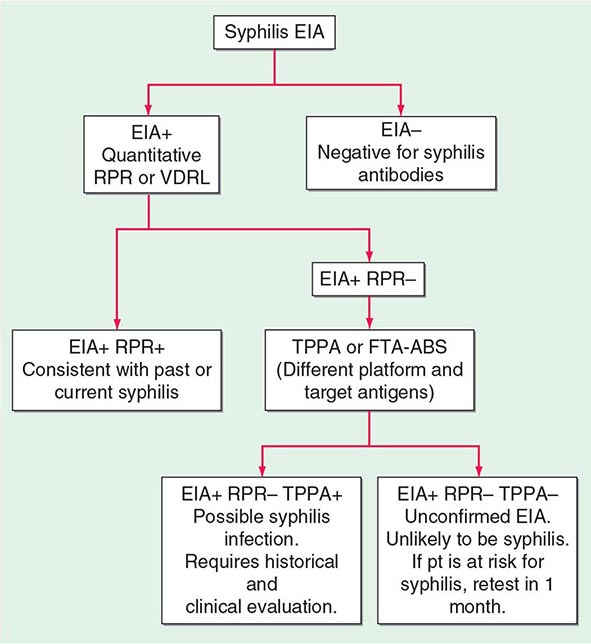
Treponemal tests measure antibodies to native or recombinant T. pallidum antigens and include the fluorescent treponemal antibody–absorbed (FTA-ABS) test and the T. pallidum particle agglutination (TPPA) test, both of which are more sensitive for primary syphilis than the previously used hemagglutination tests. The T. pallidum hemagglutination (TPHA) test is widely used in Europe but is not available in the United States. When used to confirm positive nontreponemal test results, treponemal tests have a very high positive predictive value for diagnosis of syphilis. Treponemal enzyme or chemiluminescence immunoassays (EIAs/CIAs), based largely on reactivity to recombinant antigens, have also been developed and are now widely used as screening tests by large laboratories. In a screening setting, however, treponemal tests give false-positive results at rates as high as 1–2%, and the rate is higher with the EIA/CIA tests. Treponemal tests are likely to remain reactive even after adequate treatment and cannot differentiate past from current T. pallidum infection. Figure 206-4 provides a suggested algorithm for management of such cases.
FIGURE 206-4 Algorithm for interpretation of results from syphilis enzyme immunoassays (EIAs) used for screening. FTA-ABS, fluorescent treponemal antibody–absorbed; RPR, rapid plasma reagin; TPPA, Treponema pallidum particle agglutination; VDRL, Venereal Disease Research Laboratory. (Based on the 2010 Sexually Transmitted Diseases Treatment Guidelines from the Centers for Disease Control and Prevention.)
Both nontreponemal and treponemal tests may be nonreactive in early primary syphilis, although treponemal tests are slightly more sensitive (85–90%) during this stage than nontreponemal tests (∼80%). All tests are reactive during secondary syphilis. (Fewer than 1% of patients with high titers have a nontreponemal test that is nonreactive or weakly reactive with undiluted serum but is reactive with diluted serum—the prozone phenomenon.) VDRL and RPR sensitivity and titers may decline in untreated persons with late latent syphilis, but treponemal tests remain sensitive in these stages. After treatment for early syphilis, nontreponemal test titers will generally decline or the tests will become nonreactive, whereas treponemal tests often remain reactive after therapy and are not helpful in determining the infection status of persons with past syphilis.
For practical purposes, most clinicians need to be familiar with three uses of serologic tests for syphilis recommended by the Centers for Disease Control and Prevention (CDC): (1) screening or diagnosis (RPR or VDRL), (2) quantitative measurement of antibody to assess clinical syphilis activity or to monitor response to therapy (RPR or VDRL), and (3) confirmation of a syphilis diagnosis in a patient with a reactive RPR or VDRL test (FTA-ABS, TPPA, EIA/CIA). Studies have not demonstrated the utility of IgM testing for adult syphilis. Whereas IgM titers appear to decline after therapy, the presence or absence of specific IgM does not strictly correlate with T. pallidum infection. Moreover, no commercially available IgM test is recommended, even for evaluation of infants with suspected congenital syphilis.
False-Positive Serologic Tests for Syphilis The lipid antigens of nontreponemal tests are similar to those found in human tissues, and the tests may be reactive (usually with titers ≤1:8) in persons without treponemal infection. Among patients being screened for syphilis because of risk factors, clinical suspicion, or history of exposure, ∼1% of reactive tests are falsely positive. Modern VDRL and RPR tests are highly specific, and false-positive reactions are largely limited to persons with autoimmune conditions or injection drug use. The prevalence of false-positive results increases with advancing age, approaching 10% among persons >70 years old. In a patient with a false-positive nontreponemal test, syphilis is excluded by a nonreactive treponemal test.
False-positive reactions may also occur with the treponemal tests, particularly the new, very sensitive EIA/CIA tests. When a low-prevalence population for syphilis is screened, the number of false-positive reactions may outnumber true positives, leading to unnecessary treatment. Although the precise reason is not known, it has been shown that sera from patients with periodontal disease react with antigens used in the EIA/CIA tests, presumably as a result of cross-reactive epitopes in the many treponemes that infect the gingival crevices during periodontal disease.
Evaluation for Neurosyphilis Involvement of the CNS is detected by examination of CSF for pleocytosis (>5 white blood cells/μL), increased protein concentration (>45 mg/dL), or VDRL reactivity. Elevated CSF cell counts and protein concentrations are not specific for neurosyphilis and may be confounded by HIV co-infection. Because CSF pleocytosis may also be due to HIV, some studies have suggested using a CSF white-cell cutoff of 20 cells/μL as diagnostic of neurosyphilis in HIV-infected patients with syphilis. The CSF VDRL test is highly specific and, when reactive, is considered diagnostic of neurosyphilis; however, this test is insensitive and may be nonreactive even in cases of symptomatic neurosyphilis. The FTA-ABS test on CSF is reactive far more often than the VDRL test on CSF in all stages of syphilis, but reactivity may reflect passive transfer of serum antibody into the CSF. A nonreactive FTA-ABS test on CSF, however, may be used to rule out asymptomatic neurosyphilis. The utility of measuring CXCL13 in CSF to distinguish between neurosyphilis and HIV-related CSF abnormalities has been demonstrated.
Clearly, all T. pallidum–infected patients who have signs or symptoms consistent with neurologic disease (e.g., meningitis, hearing loss) or ophthalmic disease (e.g., uveitis, iritis) should have a CSF examination, regardless of disease stage. The appropriate management of asymptomatic persons is less clear. Lumbar puncture on all asymptomatic patients with untreated syphilis is impractical and unnecessary. Because standard therapy with penicillin G benzathine fails to result in treponemicidal drug levels in CSF, however, it is important to identify those persons at higher risk for having or developing neurosyphilis so that appropriate therapy may be given. Viable T. pallidum has been isolated from the CSF of several patients (with and without concurrent HIV infection) after penicillin G benzathine therapy for early syphilis. Large-scale prospective studies have now provided evidence-based guidelines for determining which syphilis patients may benefit most from CSF examination for evidence of neurosyphilis. Specifically, patients with RPR titers of ≥1:32 are at higher risk of having neurosyphilis (11-fold and 6-fold higher in HIV-infected and HIV-uninfected persons, respectively), as are HIV-infected patients with CD4+ T cell counts of ≤350/μL. Guidelines for CSF examination are shown in Table 206-1.
|
INDICATIONS FOR CEREBROSPINAL FLUID EXAMINATION IN ADULTS WITH ALL STAGES OF SYPHILIS |
Source: Adapted from the 2010 Sexually Transmitted Diseases Treatment Guidelines from the Centers for Disease Control and Prevention.
Evaluation of HIV-Infected Patients for Syphilis Because persons at highest risk for syphilis are also at increased risk for HIV infection, these two infections frequently coexist. There is evidence that syphilis and other genital ulcer diseases are important risk factors for acquisition and transmission of HIV infection. Some manifestations of syphilis may be altered in patients with concurrent HIV infection, and multiple cases of neurologic relapse after standard therapy have been reported in these patients.
Persons with newly diagnosed HIV infection should be tested for syphilis; conversely, all patients with newly diagnosed syphilis should be tested for HIV infection. Some authorities, persuaded by reports of persistent T. pallidum in CSF of HIV-infected persons after standard therapy for early syphilis, recommend CSF examination for evidence of neurosyphilis for all co-infected patients, regardless of the stage of syphilis, with treatment for neurosyphilis if CSF abnormalities are found. Others, on the basis of their own clinical experience, believe that standard therapy—without CSF examination—is sufficient for all cases of early syphilis in HIV-infected patients without neurologic signs or symptoms. As described above, RPR titer and CD4+ T cell count can be used to identify patients at higher risk of neurosyphilis for lumbar puncture, although some cases of neurosyphilis will be missed, even when these criteria are used. Table 206-1 summarizes guidelines suggested by published studies. Serologic testing after treatment is important for all patients with syphilis, particularly for those also infected with HIV.
|
TREATMENT |
SYPHILIS |
TREATMENT OF ACQUIRED SYPHILIS
![]() The CDC’s 2010 guidelines for the treatment of syphilis are summarized in Table 206-2 and are discussed below. Penicillin G is the drug of choice for all stages of syphilis. T. pallidum is killed by very low concentrations of penicillin G, although a long period of exposure to penicillin is required because of the unusually slow rate of multiplication of the organism. The efficacy of penicillin against syphilis remains undiminished after 60 years of use, and there is no evidence of penicillin resistance in T. pallidum. Other antibiotics effective in syphilis include the tetracyclines and the cephalosporins. Aminoglycosides and spectinomycin inhibit T. pallidum only in very large doses, and the sulfonamides and the quinolones are inactive. Azithromycin has shown significant promise as an effective oral agent against T. pallidum; however, strains harboring 23S rRNA mutations that confer macrolide resistance are widespread; such strains represent >80% of recent isolates from Seattle and San Francisco and have now been identified in multiple North American and European sites. Macrolide resistance mutations have been identified in nearly all samples reported from some regions of China. In contrast, a study based in Madagascar documented the equivalence of benzathine penicillin and azithromycin for treatment of early syphilis, although a sample from one azithromycin clinical failure in that study showed the presence of a 23S rRNA resistance mutation. A more recent survey from South Africa showed a very low (1%) frequency of known 23s rRNA resistance mutations. In short, the prevalence of resistant strains varies widely by geographic location, and routine treatment of syphilis with azithromycin is not recommended. In all cases, careful follow-up of any patient treated for syphilis with azithromycin must be ensured.
The CDC’s 2010 guidelines for the treatment of syphilis are summarized in Table 206-2 and are discussed below. Penicillin G is the drug of choice for all stages of syphilis. T. pallidum is killed by very low concentrations of penicillin G, although a long period of exposure to penicillin is required because of the unusually slow rate of multiplication of the organism. The efficacy of penicillin against syphilis remains undiminished after 60 years of use, and there is no evidence of penicillin resistance in T. pallidum. Other antibiotics effective in syphilis include the tetracyclines and the cephalosporins. Aminoglycosides and spectinomycin inhibit T. pallidum only in very large doses, and the sulfonamides and the quinolones are inactive. Azithromycin has shown significant promise as an effective oral agent against T. pallidum; however, strains harboring 23S rRNA mutations that confer macrolide resistance are widespread; such strains represent >80% of recent isolates from Seattle and San Francisco and have now been identified in multiple North American and European sites. Macrolide resistance mutations have been identified in nearly all samples reported from some regions of China. In contrast, a study based in Madagascar documented the equivalence of benzathine penicillin and azithromycin for treatment of early syphilis, although a sample from one azithromycin clinical failure in that study showed the presence of a 23S rRNA resistance mutation. A more recent survey from South Africa showed a very low (1%) frequency of known 23s rRNA resistance mutations. In short, the prevalence of resistant strains varies widely by geographic location, and routine treatment of syphilis with azithromycin is not recommended. In all cases, careful follow-up of any patient treated for syphilis with azithromycin must be ensured.
|
RECOMMENDATIONS FOR THE TREATMENT OF SYPHILISa |
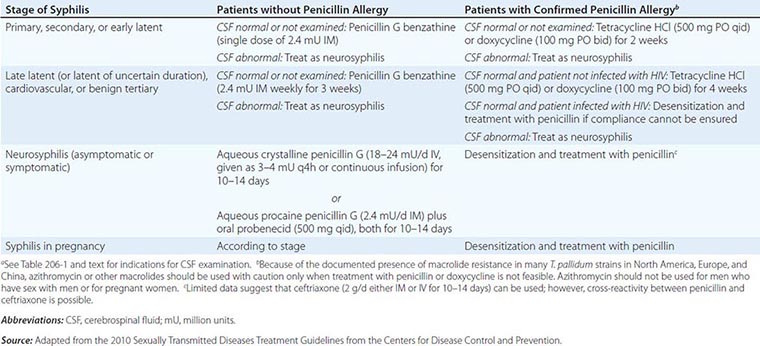
Early Syphilis Patients and Their Contacts Penicillin G benzathine is the most widely used agent for the treatment of early syphilis; a single dose of 2.4 million units is recommended. Preventive treatment is also recommended for individuals who have been exposed to infectious syphilis within the previous 3 months. The regimens recommended for prevention are the same as those recommended for early syphilis. Penicillin G benzathine cures >95% of cases of early syphilis, although clinical relapse can follow treatment, particularly in patients with concurrent HIV infection. Because the risk of neurologic relapse may be higher in HIV-infected patients, CSF examination is recommended in HIV-seropositive individuals with syphilis of any stage, particularly those with a serum RPR titer of ≥1:32 or a CD4+ T cell count of ≤350/μL. Therapy appropriate for neurosyphilis should be given if there is any evidence of CNS infection.
Late Latent Syphilis or Syphilis of Unknown Duration If the CSF is normal or is not examined, the recommended treatment is penicillin G benzathine (7.2 million units total; Table 206-2). If CSF abnormalities are found, the patient should be treated for neurosyphilis.
Tertiary Syphilis CSF examination should be performed. If the CSF is normal, the recommended treatment is penicillin G benzathine (7.2 million units total; Table 206-2). If CSF abnormalities are found, the patient should be treated for neurosyphilis. The clinical response to treatment for benign tertiary syphilis is usually impressive. However, responses to therapy for cardiovascular syphilis are not dramatic because aortic aneurysm and aortic regurgitation cannot be reversed by antibiotics.
Syphilis in Penicillin-Allergic Patients For penicillin-allergic patients with syphilis, a 2-week (early syphilis) or 4-week (late or late latent syphilis) course of therapy with doxycycline or tetracycline is recommended (Table 206-2). These regimens appear to be effective in early syphilis but have not been tested for late or late latent syphilis, and compliance may be problematic. Limited studies suggest that ceftriaxone (1 g/d, given IM or IV for 8–10 days) is effective for early syphilis. These nonpenicillin regimens have not been carefully evaluated in HIV-infected individuals and should be used with caution. If compliance and follow-up cannot be ensured, penicillin-allergic HIV-infected persons with late latent or late syphilis should be desensitized and treated with penicillin.
Neurosyphilis Penicillin G benzathine, given in total doses of up to 7.2 million units, does not produce detectable concentrations of penicillin G in CSF and should not be used for treatment of neurosyphilis. Asymptomatic neurosyphilis may relapse as symptomatic disease after treatment with benzathine penicillin, and the risk of relapse may be higher in HIV-infected patients. Both symptomatic and asymptomatic neurosyphilis should be treated with aqueous penicillin (Table 206-2). Administration either of IV aqueous crystalline penicillin G or of IM aqueous procaine penicillin G plus oral probenecid in recommended doses is thought to ensure treponemicidal concentrations of penicillin G in CSF. The clinical response to penicillin therapy for meningeal syphilis is dramatic, but treatment of neurosyphilis with existing parenchymal damage may only arrest disease progression. No data suggest that additional therapy (e.g., penicillin G benzathine for 3 weeks) is beneficial after treatment for neurosyphilis.
The use of antibiotics other than penicillin G for the treatment of neurosyphilis has not been studied, although very limited data suggest that ceftriaxone may be used. In patients with penicillin allergy demonstrated by skin testing, desensitization and treatment with penicillin are recommended.
Management of Syphilis in Pregnancy Every pregnant woman should undergo a nontreponemal test at her first prenatal visit and, if at high risk of exposure, again in the third trimester and at delivery. In the untreated pregnant patient with presumed syphilis, expeditious treatment appropriate to the stage of the disease is essential. Patients should be warned of the risk of a Jarisch-Herxheimer reaction, which may be associated with mild premature contractions but rarely results in premature delivery.
Penicillin is the only recommended agent for the treatment of syphilis in pregnancy. If the patient has a documented penicillin allergy, desensitization and penicillin therapy should be undertaken according to the CDC’s 2010 guidelines. After treatment, a quantitative nontreponemal test should be repeated monthly throughout pregnancy to assess therapeutic efficacy. Treated women whose antibody titers rise by fourfold or whose titers do not decrease by fourfold over a 3-month period should be re-treated.
EVALUATION AND MANAGEMENT OF CONGENITAL SYPHILIS
Whether or not they are infected, newborn infants of mothers with reactive serologic tests may themselves have reactive tests because of transplacental transfer of maternal IgG antibody. For asymptomatic infants born to women treated adequately with penicillin during the first or second trimester of pregnancy, monthly quantitative nontreponemal tests may be performed to monitor for appropriate reduction in antibody titers. Rising or persistent titers indicate infection, and the infant should be treated. Detection of neonatal IgM antibody may be useful, but no commercially available test is currently recommended.
An infant should be treated at birth if the treatment status of the seropositive mother is unknown; if the mother has received inadequate or nonpenicillin therapy; if the mother received penicillin therapy in the third trimester; or if the infant may be difficult to follow. The CSF should be examined to obtain baseline values before treatment. Penicillin is the only recommended drug for the treatment of syphilis in infants. Specific recommendations for the treatment of infants and older children are included in the CDC’s 2010 treatment guidelines.
JARISCH-HERXHEIMER REACTION
A dramatic although usually mild reaction consisting of fever, chills, myalgias, headache, tachycardia, increased respiratory rate, increased circulating neutrophil count, and vasodilation with mild hypotension may follow the initiation of treatment for syphilis. This reaction is thought to be a response to lipoproteins released by dying T. pallidum organisms. The Jarisch-Herxheimer reaction occurs in ∼50% of patients with primary syphilis, 90% of those with secondary syphilis, and a lower proportion of persons with later-stage disease. Defervescence takes place within 12–24 h. In patients with secondary syphilis, erythema and edema of the mucocutaneous lesions may increase. Patients should be warned to expect such symptoms, which can be managed with symptom-based treatment. Steroid or other anti-inflammatory therapy is not required for this mild transient reaction.
FOLLOW-UP EVALUATION OF RESPONSES TO THERAPY
Efficacy of treatment should be assessed by clinical evaluation and monitoring of the quantitative VDRL or RPR titer for a fourfold decline (e.g., from 1:32 to 1:8). Patients with primary or secondary syphilis should be examined 6 and 12 months after treatment and persons with latent or late syphilis at 6, 12, and 24 months. More frequent clinical and serologic examination (3, 6, 9, 12, and 24 months) is recommended for patients concurrently infected with HIV, regardless of the stage of syphilis.
After successful treatment of seropositive first-episode primary or secondary syphilis, the VDRL or RPR titer progressively declines, becoming negative by 12 months in 40–75% of seropositive primary cases and in 20–40% of secondary cases. Patients with HIV infection or a history of prior syphilis are less likely to become nonreactive in the VDRL or RPR test. Rates of decline of serologic titers appear to be slower, and serologically defined treatment failures more common, among HIV-infected patients than among those without HIV co-infection; however, effective antiretroviral therapy may reduce these differences. Re-treatment should be considered if serologic responses are not adequate or if clinical signs persist or recur. Because it is difficult to differentiate treatment failure from reinfection, the CSF should be examined, with treatment for neurosyphilis if CSF is abnormal and treatment for late latent syphilis if CSF is normal. A minority of patients treated for early syphilis may experience a one-dilution titer increase within 14 days after treatment; however, this early elevation does not significantly affect the serologic outcome at 6 months after treatment. Patients treated for late latent syphilis frequently have low initial VDRL or RPR titers and may not have a fourfold decline after therapy with penicillin. In such patients, re-treatment is not warranted unless the titer rises or signs and symptoms of syphilis appear. Because treponemal tests may remain reactive despite treatment for seropositive syphilis, these tests are not useful in following the response to therapy.
The activity of neurosyphilis (symptomatic or asymptomatic) correlates best with CSF pleocytosis, and this measure provides the most sensitive index of response to treatment. Repeat CSF examinations should be performed every 6 months until the cell count is normal. An elevated CSF cell count falls to normal in 3–12 months in adequately treated HIV-uninfected patients. The persistence of mild pleocytosis in HIV-infected patients may be due to the presence of HIV in CSF; this scenario may be difficult to distinguish from treatment failure. Elevated levels of CSF protein fall more slowly, and the CSF VDRL titer declines gradually over several years. In patients treated for neurosyphilis, a fourfold reduction in serum RPR titer has been positively correlated with normalization of CSF abnormalities; this correlation is stronger in HIV-uninfected patients and in HIV-infected patients receiving effective antiretroviral therapy.
IMMUNITY TO SYPHILIS
The rate of development of acquired resistance to T. pallidum after natural or experimental infection is related to the size of the antigenic stimulus, which depends on both the size of the infecting inoculum and the duration of infection before treatment. Both humoral and cellular responses are considered to be of major importance in immunity and in the healing of early lesions. Cellular infiltration, predominantly by T lymphocytes and macrophages, produces a TH1 cytokine milieu consistent with the clearance of organisms by activated macrophages. Specific antibody enhances phagocytosis and is required for macrophage-mediated killing of T. pallidum. Recent studies demonstrate antigenic variation of the TprK protein, which may lead to persistence of infection and determine susceptibility to reinfection with another strain. Comparative genomic studies have revealed some sequence variations among T. pallidum strains, which can be differentiated by molecular typing methods. Possible correlations between molecular type and clinical manifestations are being examined.
207e |
Endemic Treponematoses |
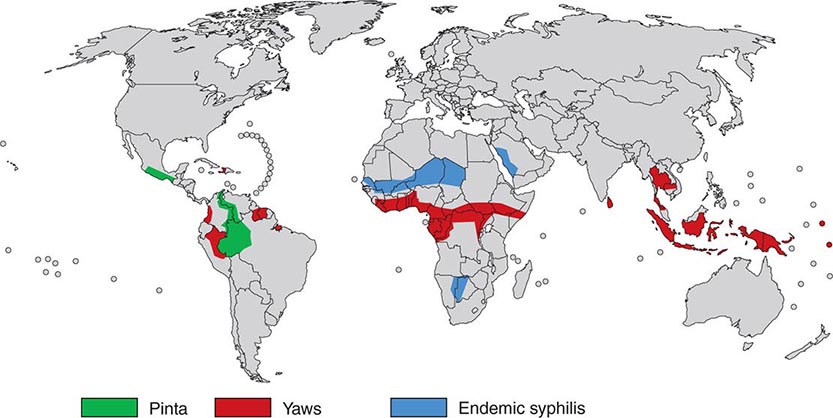
The endemic treponematoses are chronic diseases that are transmitted by direct contact, usually during childhood, and, like syphilis, can cause severe late manifestations years after initial infection. These diseases are caused by very close relatives of Treponema pallidum subspecies pallidum, the etiologic agent of venereal syphilis (Chap. 206). Yaws, pinta, and endemic syphilis are traditionally distinguished from venereal syphilis by mode of transmission, age of acquisition, geographic distribution, and clinical features; however, there is some overlap for each of these factors. Generally, yaws flourishes in moist tropical areas of several regions, endemic syphilis is found primarily in arid climates, and pinta is found in temperate foci in the Americas (Fig. 207e-1). These infections are usually limited to rural areas of developing nations and are seen in developed countries only among recent immigrants from endemic regions. Our “knowledge” about the endemic treponematoses is based on observations by health care workers who have visited endemic areas; virtually no well-designed studies of the natural history, diagnosis, or treatment of these infections have been conducted. The treponemal infections are compared and contrasted in Table 207e-1.
FIGURE 207e-1 Geographic distribution of endemic treponematoses. (Courtesy of the World Health Organization; updated from www.who.int/yaws/epidemiology/Map_yaws_90s.jpg.)
|
COMPARISON OF THE TREPONEMES AND ASSOCIATE DISEASES |
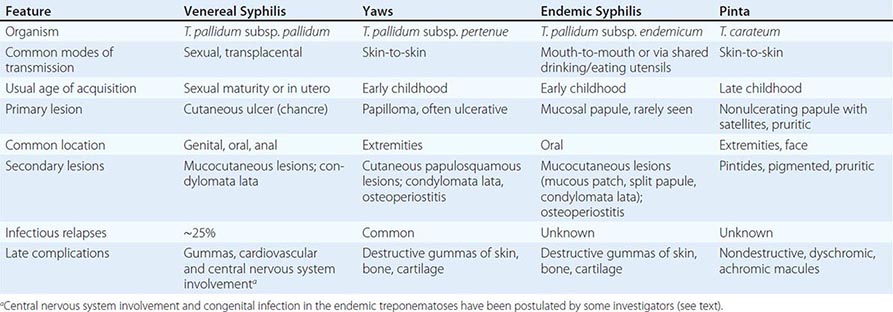
EPIDEMIOLOGY
![]() In a World Health Organization (WHO)–sponsored mass eradication campaign from 1952 to 1969, more than 160 million people in Africa, Asia, and South America were examined for treponemal infections, and more than 50 million cases, contacts, and persons with latent infections were treated. This campaign reduced the prevalence of active yaws from >20% to <1% in many areas. In recent decades, lack of focused surveillance and diversion of resources have resulted in documented resurgence of these infections in some regions. The most recent WHO global estimate (1995) suggested that there are 460,000 new cases per year (mostly yaws) and a prevalence of 2.5 million infected persons; during subsequent years, an increased incidence was documented in some countries. Recent areas of resurgent yaws morbidity include West Africa (Ivory Coast, Ghana, Togo, Benin), the Central African Republic, Nigeria, and rural Democratic Republic of the Congo. The prevalence of endemic syphilis is estimated to be >10% in some regions of northern Ghana, Mali, Niger, Burkina Faso, and Senegal. In Asia and the Pacific Islands, reports suggest active outbreaks of yaws in Indonesia, Papua New Guinea, the Solomon Islands, East Timor, Vanuatu, Laos, and Kampuchea. India actively renewed its focus on yaws control in 1996, achieved zero-case status in 2003, and declared elimination in 2006. In the Americas, foci of yaws are thought to persist in Haiti and other Caribbean islands, Peru, Colombia, Ecuador, Brazil, Guyana, and Surinam, although recent data are lacking. Pinta is limited to Central America and northern South America, where it is found rarely and only in very remote villages. Evidence of yaws-like and venereal diseases, with treponemal seroreactivity, in wild gorillas and baboons in Africa has led to speculation that there may be an animal reservoir for yaws.
In a World Health Organization (WHO)–sponsored mass eradication campaign from 1952 to 1969, more than 160 million people in Africa, Asia, and South America were examined for treponemal infections, and more than 50 million cases, contacts, and persons with latent infections were treated. This campaign reduced the prevalence of active yaws from >20% to <1% in many areas. In recent decades, lack of focused surveillance and diversion of resources have resulted in documented resurgence of these infections in some regions. The most recent WHO global estimate (1995) suggested that there are 460,000 new cases per year (mostly yaws) and a prevalence of 2.5 million infected persons; during subsequent years, an increased incidence was documented in some countries. Recent areas of resurgent yaws morbidity include West Africa (Ivory Coast, Ghana, Togo, Benin), the Central African Republic, Nigeria, and rural Democratic Republic of the Congo. The prevalence of endemic syphilis is estimated to be >10% in some regions of northern Ghana, Mali, Niger, Burkina Faso, and Senegal. In Asia and the Pacific Islands, reports suggest active outbreaks of yaws in Indonesia, Papua New Guinea, the Solomon Islands, East Timor, Vanuatu, Laos, and Kampuchea. India actively renewed its focus on yaws control in 1996, achieved zero-case status in 2003, and declared elimination in 2006. In the Americas, foci of yaws are thought to persist in Haiti and other Caribbean islands, Peru, Colombia, Ecuador, Brazil, Guyana, and Surinam, although recent data are lacking. Pinta is limited to Central America and northern South America, where it is found rarely and only in very remote villages. Evidence of yaws-like and venereal diseases, with treponemal seroreactivity, in wild gorillas and baboons in Africa has led to speculation that there may be an animal reservoir for yaws.
MICROBIOLOGY
![]() The etiologic agents of the endemic treponematoses are listed in Table 207e-1. These little-studied organisms are morphologically identical to T. pallidum subspecies pallidum (the agent of venereal syphilis), and no definitive antigenic differences among them have been identified to date. A controversy has existed about whether the pathogenic treponemes are truly separate organisms, as genome sequencing indicates that yaws and syphilis treponemes are 99.8% identical. Three of the four organisms are classified as subspecies of T. pallidum; the fourth (T. carateum) remains a separate species simply because no organisms have been available for genetic studies. Based on analysis of the small number of strains currently available, molecular signatures—assessed by polymerase chain reaction (PCR) amplification of tpr genes and restriction digestion—have been identified that can differentiate the T. pallidum subspecies. Whether these genetic differences are related to distinct clinical characteristics of these diseases has not been determined. Full genome sequencing of an unclassified strain (Fribourg-Blanc) isolated from a baboon in 1966 shows a very high degree of homology with available strains of T. pallidum subspecies pertenue. This observation is consistent with an earlier report that the Fribourg-Blanc strain can cause experimental infection of humans. Molecular analyses of additional samples from affected baboons suggests that the nonhuman primate samples diverge from the evolutionary tree prior to the clade that contains the human isolates, but uncertainty remains about the importance of the nonhuman primate reservoir for human infection.
The etiologic agents of the endemic treponematoses are listed in Table 207e-1. These little-studied organisms are morphologically identical to T. pallidum subspecies pallidum (the agent of venereal syphilis), and no definitive antigenic differences among them have been identified to date. A controversy has existed about whether the pathogenic treponemes are truly separate organisms, as genome sequencing indicates that yaws and syphilis treponemes are 99.8% identical. Three of the four organisms are classified as subspecies of T. pallidum; the fourth (T. carateum) remains a separate species simply because no organisms have been available for genetic studies. Based on analysis of the small number of strains currently available, molecular signatures—assessed by polymerase chain reaction (PCR) amplification of tpr genes and restriction digestion—have been identified that can differentiate the T. pallidum subspecies. Whether these genetic differences are related to distinct clinical characteristics of these diseases has not been determined. Full genome sequencing of an unclassified strain (Fribourg-Blanc) isolated from a baboon in 1966 shows a very high degree of homology with available strains of T. pallidum subspecies pertenue. This observation is consistent with an earlier report that the Fribourg-Blanc strain can cause experimental infection of humans. Molecular analyses of additional samples from affected baboons suggests that the nonhuman primate samples diverge from the evolutionary tree prior to the clade that contains the human isolates, but uncertainty remains about the importance of the nonhuman primate reservoir for human infection.
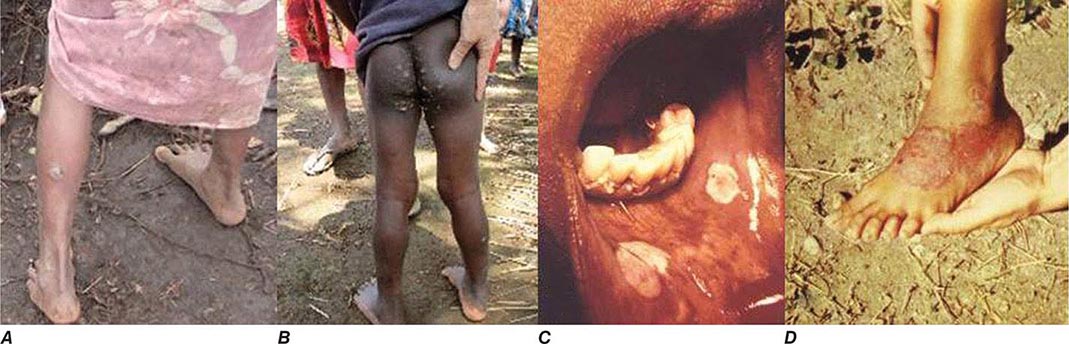
CLINICAL FEATURES
All of the treponemal infections, including syphilis, are chronic and are characterized by defined disease stages, with a localized primary lesion, disseminated secondary lesions, periods of latency, and possible late lesions. Primary and secondary stages are more frequently overlapping in yaws and endemic syphilis than in venereal syphilis, and the late manifestations of pinta are very mild relative to the destructive lesions of the other treponematoses. The current preference is to divide the clinical course of the endemic treponematoses into “early” and “late” stages.
The major clinical distinctions made between venereal syphilis and the nonvenereal infections are the apparent lack of congenital transmission and of central nervous system (CNS) involvement in the nonvenereal infections. It is not known whether these distinctions are entirely accurate. Because of the high degree of genetic relatedness among the organisms, there is little biological reason to think that T. pallidum subspecies endemicum and T. pallidum subspecies pertenue would be unable to cross the blood-brain barrier or to invade the placenta. These organisms are like T. pallidum subspecies pallidum in that they obviously disseminate from the site of initial infection and can persist for decades. The lack of recognized congenital infection may be due to the fact that childhood infections often reach the latent stage (low bacterial load) before girls reach sexual maturity. Neurologic involvement may go unrecognized because of the lack of trained medical personnel in endemic regions, the delay of many years between infection and possible CNS manifestations, or a low rate of symptomatic CNS disease. Some published evidence supports congenital transmission as well as cardiovascular, ophthalmologic, and CNS involvement in yaws and endemic syphilis. Although the reported studies have been small, have failed to control for other causes of CNS abnormalities, and in some instances have not included serologic confirmation, it may be erroneous to accept unquestioningly the frequently repeated belief that these organisms fail to cause such manifestations.
Yaws Also known as pian, framboesia, or bouba, yaws is characterized by the development of one or several primary lesions (“mother yaw”) followed by multiple disseminated skin lesions. All early skin lesions are infectious and may persist for many months; cutaneous relapses are common during the first 5 years. Late manifestations, affecting ~10% of untreated persons, are destructive and can involve skin, bone, and joints.
The infection is transmitted by direct contact with infectious lesions, often during play or group sleeping, and may be enhanced by disruption of the skin by insect bites or abrasions. After an average of 3–4 weeks, the first lesion begins as a papule—usually on an extremity—and then enlarges (particularly during moist warm weather) to become papillomatous or “raspberry-like” (thus the name “framboesia”) (Fig. 207e-2A). Regional lymphadenopathy develops, and the lesion usually heals within 6 months; dissemination is thought to occur during the early weeks of infection. A generalized secondary eruption (Fig. 207e-2B), accompanied by generalized lymphadenopathy, appears either concurrent with or after the primary lesion; may take several forms (macular, papular, or papillomatous); and may become secondarily infected with other bacteria. Painful papillomatous lesions on the soles of the feet result in a crablike gait (“crab yaws”), and periostitis may result in nocturnal bone pain and polydactylitis. Late yaws is manifested by gummas of the skin and long bones, hyperkeratoses of the palms and soles, osteitis and periostitis, and hydrarthrosis. The late gummatous lesions are characteristically extensive. Destruction of the nose, maxilla, palate, and pharynx is termed gangosa and is similar to the destructive lesions seen in leprosy and leishmaniasis.
FIGURE 207e-2 Clinical manifestations of endemic treponematoses. A. Papillomatous initial lesion of early yaws. B. Disseminated lesions of early yaws. C. Mucous patches of endemic syphilis. D. Pigmented macules of pinta. (Photos published with permission from Dr. David Fegan, Brisbane, Australia [A and B]; and from PL Perine et al: Handbook of Endemic Treponematoses, Geneva, World Health Organization, 1984 [C and D].)
Endemic Syphilis The early lesions of endemic syphilis (bejel, siti, dichuchwa, njovera, skerljevo) are localized primarily to mucocutaneous and mucosal surfaces. The infection is reportedly transmitted by direct contact, by kissing, by premastication of food, or by sharing of drinking and eating utensils. A role for insects in transmission has been suggested but is unproven. The initial lesion, usually an intraoral papule, often goes unrecognized and is followed by mucous patches (Fig. 207e-2C) on the oral mucosa and mucocutaneous lesions resembling the condylomata lata of secondary syphilis. This eruption may last for months or even years, and treponemes can readily be demonstrated in early lesions. Periostitis and regional lymphadenopathy are common. After a variable period of latency, late manifestations may appear, including osseous and cutaneous gummas. Destructive gummas, osteitis, and gangosa are more common in endemic syphilis than in yaws.
Pinta Pinta (mal del pinto, carate, azul, purupuru) is the most benign of the treponemal infections. This disease has three stages that are characterized by marked changes in skin color (Fig. 207e-2D), but pinta does not appear to cause destructive lesions or to involve tissues other than the skin. The initial papule is most often located on the extremities or face and is pruritic. After one to many months of infection, numerous disseminated secondary lesions (pintides) appear. These lesions are initially red but become deeply pigmented, ultimately turning a dark slate blue. The secondary lesions are infectious and highly pruritic and may persist for years. Late pigmented lesions are called dyschromic macules and contain treponemes. Over time, most pigmented lesions show varying degrees of depigmentation, becoming brown and eventually white and giving the skin a mottled appearance. White achromic lesions are characteristic of the late stage.
DIAGNOSIS
Diagnosis of the endemic treponematoses is based on clinical manifestations and, when available, dark-field microscopy and serologic testing. The same serologic tests that are used for venereal syphilis (Chap. 206) become reactive during all treponemal infections. Although several targets have been evaluated for specific serodiagnosis, to date there is no antibody test that can discriminate among the different infections. The nonvenereal treponemal infections should be considered in the evaluation of a reactive syphilis serology in any person who has emigrated from an endemic area. Sensitive PCR assays can be used to confirm treponemal infection and to identify the etiologic agent in research laboratories.
|
TREATMENT |
ENDEMIC TREPONEMATOSES |
The WHO-recommended therapy for patients and their contacts is benzathine penicillin G (1.2 million units IM for adults; 600,000 units for children <10 years old). This dose is half of that recommended for early venereal syphilis, and no controlled efficacy studies have been conducted. Definitive evidence of resistance to penicillin is lacking, although relapsing lesions have been reported after penicillin treatment in Papua New Guinea. A recent study in that nation demonstrated equivalence between IM benzathine penicillin G and a single oral dose of azithromycin (30 mg/kg, up to a maximum of 2 g). This finding provided the WHO’s revitalized yaws eradication program with a much easier regimen for use in mass treatment. Although macrolide resistance mutations are common in circulating strains of T. pallidum subspecies pallidum in many parts of the world, analysis of a limited number of yaws samples from Papua New Guinea has yielded no evidence of resistance mutations to date. Limited data suggest the efficacy of tetracycline for treatment of yaws, but no data exist for other endemic treponematoses. Solely on the basis of experience with venereal syphilis, it is thought that doxycycline or tetracycline (at doses appropriate for syphilis; Chap. 206) are alternatives for patients allergic to penicillin. A Jarisch-Herxheimer reaction (Chap. 206) may follow treatment of endemic treponematoses. Nontreponemal serologic titers (in the Venereal Disease Research Laboratory [VDRL] slide test or the rapid plasma reagin [RPR] test) usually decline after effective therapy, but patients may not become seronegative.
CONTROL
Buoyed by the successful elimination of yaws in India in 2006 and the availability of an inexpensive, single-dose oral drug for treatment, in 2012 the WHO renewed its efforts to eradicate yaws globally by 2020. Enthusiasm is high; several planning meetings have been held to develop country-specific plans of action; and resources are being sought. Some caution is warranted: The possible animal reservoir will need to be evaluated. There may be only a window of time during which countries can successfully use azithromycin for yaws eradication before resistance begins to appear in yaws organisms. Given the ongoing lower-dose azithromycin mass treatment campaigns against trachoma, often in populations also at high risk for yaws, development of macrolide resistance is likely at some point. Complete drug coverage and continued careful surveillance by health centers (the weak link in prior control efforts) will be essential for success.
208 |
Leptospirosis |
Leptospirosis is a globally important zoonotic disease whose apparent reemergence is illustrated by recent outbreaks on virtually all continents. The disease is caused by pathogenic Leptospira species and is characterized by a broad spectrum of clinical manifestations, varying from asymptomatic infection to fulminant, fatal disease. In its mild form, leptospirosis may present as nonspecific symptoms such as fever, headache, and myalgia. Severe leptospirosis, characterized by jaundice, renal dysfunction, and hemorrhagic diathesis, is often referred to as Weil’s syndrome. With or without jaundice, severe pulmonary hemorrhage is increasingly recognized as an important presentation of severe disease.
ETIOLOGIC AGENT
Leptospira species are spirochetes belonging to the order Spirochaetales and the family Leptospiraceae. Traditionally, the genus Leptospira comprised two species: the pathogenic L. interrogans and the free-living L. biflexa, now designated L. interrogans sensu lato and L. biflexa sensu lato, respectively. Twenty-two Leptospira species with pathogenic (10 species), intermediate (5 species), and nonpathogenic (7 species) status have now been described on the basis of phylogenetic and virulence analyses (Fig. 208-1). Genome sequences of five Leptospira species (L. biflexa, L. interrogans, L. santarosai, L. borgpetersenii, and L. licerasiae) have been published, and the availability of genome sequences of a wide variety of Leptospira strains will undoubtedly lead to a better understanding of the pathogenesis of leptospirosis. However, classification based on serologic differences better serves clinical, diagnostic, and epidemiologic purposes. Pathogenic Leptospira species are divided into serovars according to their antigenic composition. More than 250 serovars make up the 26 serogroups.
FIGURE 208-1 Differentiation of pathogenic, intermediate, and nonpathogenic (saprophytic) Leptospira species by molecular phylogenetic analysis using the rrs gene and including the potentially new pathogenic species Leptospira borgpetersenii group B and the saprophytic species Leptospira idonii. Scale bar indicates the rate of nucleotide substitutions per base pair. (Figure prepared and provided by Dr. A. Ahmed, KIT Biomedical Research, Amsterdam, The Netherlands.)
Leptospires are coiled, thin, highly motile organisms that have hooked ends and two periplasmic flagella, with polar extrusions from the cytoplasmic membrane that are responsible for motility (Fig. 208-2). These organisms are 6–20 μm long and ~0.1 μm wide; they stain poorly but can be seen microscopically by dark-field examination and after silver impregnation staining of tissues. Leptospires require special media and conditions for growth; it may take weeks to months for cultures to become positive.
FIGURE 208-2 Transmission electron microscopic image of Leptospira interrogans invading equine conjunctival tissue. (Image kindly provided by Dr. JE Nally, National Animal Disease Center, U.S. Department of Agriculture, Ames, IA. This image appears on the homepage of the European Leptospirosis Society website [http://eurolepto.ucd.ie/].)
EPIDEMIOLOGY
![]() Leptospirosis has a worldwide distribution but occurs most commonly in the tropics and subtropics because the climate and occasionally poor hygienic conditions favor the pathogen’s survival and distribution. In most countries, leptospirosis is an underappreciated problem. Most cases occur in men, with a peak incidence during the summer and fall in both the Northern and Southern Hemispheres and during the rainy season in the tropics.
Leptospirosis has a worldwide distribution but occurs most commonly in the tropics and subtropics because the climate and occasionally poor hygienic conditions favor the pathogen’s survival and distribution. In most countries, leptospirosis is an underappreciated problem. Most cases occur in men, with a peak incidence during the summer and fall in both the Northern and Southern Hemispheres and during the rainy season in the tropics.
Reliable data on morbidity and mortality from leptospirosis have gradually started to appear. Current information on global human leptospirosis varies but indicates that approximately 1 million severe cases occur per year, with a mean case–fatality rate of nearly 10%.
As a zoonosis, leptospirosis affects almost all mammalian species and represents a significant veterinary burden. Rodents, especially rats, are the most important reservoir, although other wild mammals as well as domestic and farm animals may also harbor these microorganisms. Leptospires establish a symbiotic relationship with their host and can persist in the urogenital tract for years. Some serovars are generally associated with particular animals—e.g., Icterohaemorrhagiae and Copenhageni with rats, Grippotyphosa with voles, Hardjo with cattle, Canicola with dogs, and Pomona with pigs—but may occur in other animals as well.
Leptospirosis presents as both an endemic and an epidemic disease. Transmission of leptospires may follow direct contact with urine, blood, or tissue from an infected animal or, more commonly, exposure to environmental contamination. The dogma that human-to-human transmission is very rare is challenged by recent findings on household clustering, asymptomatic renal colonization, and prolonged excretion of leptospires. (Both of the latter features imply human infection sources that are not recognized.) Because leptospires can survive in a humid environment for many months, water is an important vehicle in their transmission. Epidemics of leptospirosis are not well understood. Outbreaks may result from exposure to flood waters contaminated by urine from infected animals, as has been reported from several countries. However, it is also true that outbreaks may occur without floods, and floods often occur without outbreaks.
The vast majority of infections with Leptospira cause no or only mild disease in humans. A small percentage of infections (~1%) lead to severe, potentially fatal complications. The proportion of leptospirosis cases that are mild is unknown because patients either do not seek or do not have access to medical care or because the nonspecific symptoms are interpreted as an influenza-like illness. Reported cases surely represent a significant underestimation of the total number. Certain occupational groups are at especially high risk, including veterinarians, agricultural workers, sewage workers, slaughterhouse employees, and workers in the fishing industry. Risk factors include direct or indirect contact with animals, including exposure to water and soil contaminated with animal urine. Leptospirosis has also been recognized in deteriorating inner cities and suburban areas where rat populations are expanding.
Recreational exposure and domestic-animal contact are prominent sources of leptospirosis. Recreational freshwater activities, such as canoeing, windsurfing, swimming, and waterskiing, place persons at risk for infection. Several outbreaks have followed sporting events. For example, an outbreak took place in 1998 among athletes after a triathlon in Springfield, Illinois. Ingestion of one or more swallows of lake water during the swimming leg of the triathlon was a prominent risk factor for illness. Heavy rains that preceded the triathlon, with consequent agricultural runoff, are likely to have increased the level of leptospiral contamination in the lake water. In another outbreak, 42% of participants contracted leptospirosis during the 2000 Eco-Challenge-Sabah multisport endurance race in Malaysian Borneo. Swimming in the Segama River was shown to be an independent risk factor.
In addition, leptospirosis is a traveler’s disease. Large proportions of patients acquire the infection while traveling in tropical countries, usually during adventurous activities such as whitewater rafting, jungle trekking, and caving. Transmission via laboratory accidents has been reported but is rare. New data indicate that leptospirosis may develop after unanticipated immersion in contaminated water (e.g., in an automobile accident) more frequently than has generally been thought and can also result from an animal bite.
PATHOGENESIS
Transmission occurs through cuts, abraded skin, or mucous membranes, especially the conjunctival and oral mucosa. After entry, the organisms proliferate, cross tissue barriers, and disseminate hematogenously to all organs (leptospiremic phase). During this initial incubation period, leptospires can be isolated from the bloodstream (Fig. 208-3). The organisms are able to survive in the nonimmune host: they evade complement-mediated killing by binding factor H, a strong inhibitor of the complement system, on their surface. Moreover, pathogenic leptospires resist ingestion and killing by neutrophils, monocytes, and macrophages. During the immune phase, the appearance of antibodies coincides with the disappearance of leptospires from the blood. However, the bacteria persist in various organs, including liver, lung, kidney, heart, and brain. Autopsy findings illustrate the involvement of multiple organ systems in severe disease. Renal pathology shows both acute tubular damage and interstitial nephritis. Acute tubular lesions progress in time to interstitial edema and acute tubular necrosis. Severe nephritis is observed in patients who survive long enough to develop it and seems to be a secondary response to acute epithelial damage. The reported deregulation of the expression of several transporters along the nephron, including the proximal sodium-hydrogen exchanger 3 (NHE3), aquaporins 1 and 2 (AQP1 and AQP2), Na+-K+ ATPase, and the Na-K-2Cl cotransporter NKCC2, contributes to tubular potassium wasting, hypokalemia, and polyuria. Histopathology of the liver shows focal necrosis, foci of inflammation, and plugging of bile canaliculi. Widespread hepatocellular necrosis is not found. Petechiae and hemorrhages are observed in the heart, lungs (Fig. 208-4), kidneys (and adrenals), pancreas, liver, gastrointestinal tract (including retroperitoneal fat, mesentery, and omentum), muscles, prostate, testis, and brain (subarachnoid bleeding). Several studies show an association between hemorrhage and thrombocytopenia. Although the underlying mechanisms of thrombocytopenia have not been elucidated, it seems likely that platelet consumption plays an important role. A consumptive coagulopathy may occur, with elevated markers of coagulation activation (thrombin–antithrombin complexes, prothrombin fragments 1 and 2, D-dimer), diminished anticoagulant markers (antithrombin, protein C), and deregulated fibrinolytic activity. Overt disseminated intravascular coagulation (DIC) has been documented in patients from Thailand and Indonesia. Elevated plasma levels of soluble E-selectin and von Willebrand factor in patients with leptospirosis reflect endothelial cell activation. Experimental models show that pathogenic leptospires or leptospiral proteins are able to activate endothelial cells in vitro and to disrupt endothelial-cell barrier function, promoting dissemination. Platelets have been shown to aggregate on activated endothelium in the human lung, whereas histology reveals swelling of activated endothelial cells but no evident vasculitis or necrosis. Immunoglobulin and complement deposition have been demonstrated in lung tissue involved in pulmonary hemorrhage.
FIGURE 208-3 Biphasic nature of leptospirosis and relevant investigations at different stages of disease. Note that an incubation period of up to 1 month has now been documented. Specimens 1 and 2 for serology are acute-phase serum samples; specimen 3 is a convalescent-phase serum sample that may facilitate detection of a delayed immune response; and specimens 4 and 5 are follow-up serum samples that can provide epidemiologic information, such as the presumptive infecting serogroup. CSF, cerebrospinal fluid. (Reprinted as adapted by PN Levett: Clin Microbiol Rev 14:296, 2001 [from LH Turner: Leptospirosis. BMJ 1:231, 1969] with permission from the American Society for Microbiology and the BMJ Publishing Group.)
FIGURE 208-4 Severe pulmonary hemorrhage in leptospirosis. Left panel: Chest x-ray. Right panel: Gross appearance of right lower lobes of lung at autopsy. This patient, a 15-year-old from the Peruvian Amazonian city of Iqitos, died several days after presentation with acute illness, jaundice, and hemoptysis. Blood culture yielded Leptospira interrogans serovar Copenhageni/Icterohaemorrhagiae. (Adapted with permission from E Segura et al: Clin Infect Dis 40:343, 2005. © 2005 by the Infectious Diseases Society of America.)
Leptospira species have a typical double-membrane cell wall structure harboring a variety of membrane-associated proteins, including an unusually high number of lipoproteins. The peptidoglycan layer is located close to the cytoplasmic membrane. The lipopolysaccharide (LPS) in the outer membrane has an unusual structure with a relatively low endotoxic potency. Pathogenic leptospires contain a variety of genes coding for proteins involved in motility and in cell and tissue adhesion and invasion that represent potential virulence factors. Many of these are surface-exposed outer-membrane proteins (OMPs). To date, the only leptospiral virulence factor shown to satisfy Koch’s molecular postulates is loa22 encoding a surface-exposed protein with an unknown function. However, the gene is not confined to pathogenic Leptospira species.
Immunity to Leptospira depends on the production of circulating antibodies to serovar-specific LPS. It is unclear whether other antigens play a significant role in protective humoral immunity. Moreover, immunity may not be confined to antibody responses; involvement of the innate-immune Toll-like receptor 2 (TLR2) and TLR4 activation pathways in controlling infection has been demonstrated, whereas in vaccinated cattle a cell-mediated immune response is correlated with protection.
It is likely that several surface-exposed proteins mediate leptospire–host cell interactions, and these proteins may represent candidate vaccine components. Although animal-model studies have shown various degrees of vaccine efficacy for various putative virulence-associated OMPs, it is not yet clear whether such proteins elicit acceptable levels of sterilizing immunity.
CLINICAL MANIFESTATIONS
Although leptospirosis is a potentially fatal disease with bleeding and multiorgan failure as its clinical hallmarks, the majority of cases are thought to be relatively mild, presenting as the sudden onset of a febrile illness. The incubation period is usually 1–2 weeks but ranges from 1 to 30 days. (Figure 208-3 indicates a slightly different range, but an incubation period of up to 1 month has now been documented.) Leptospirosis is classically described as biphasic. The acute leptospiremic phase is characterized by fever of 3–10 days’ duration, during which time the organism can be cultured from blood. During the immune phase, resolution of symptoms may coincide with the appearance of antibodies, and leptospires can be cultured from the urine. The distinction between the first and second phases is not always clear: milder cases do not always include the second phase, and severe disease may be monophasic and fulminant. The idea that distinct clinical syndromes are associated with specific serogroups has been refuted, although some serovars tend to cause more severe disease than others.
Mild Leptospirosis Most patients are asymptomatic or only mildly ill and do not seek medical attention. Serologic evidence of past inapparent infection is frequently found in persons who have been exposed but have not become ill. Mild symptomatic leptospirosis usually presents as a flu-like illness of sudden onset, with fever, chills, headache, nausea, vomiting, abdominal pain, conjunctival suffusion (redness without exudate), and myalgia. Muscle pain is intense and especially affects the calves, back, and abdomen. The headache is intense, localized to the frontal or retroorbital region (resembling that occurring in dengue), and sometimes accompanied by photophobia. Aseptic meningitis may be present and is more common among children than among adults. Although Leptospira can be cultured from the cerebrospinal fluid (CSF) in the early phase, the majority of cases follow a benign course with regard to the central nervous system; symptoms disappear within a few days but may persist for weeks.
Physical examination may include any of the following findings, none of which is pathognomonic for leptospirosis: fever, conjunctival suffusion, pharyngeal injection, muscle tenderness, lymphadenopathy, rash, meningismus, hepatomegaly, and splenomegaly. If present, the rash is often transient; may be macular, maculopapular, erythematous, or hemorrhagic (petechial or ecchymotic); and may be misdiagnosed as due to scrub typhus or viral infection. Lung auscultation may reveal crackles, and mild jaundice may be present.
The natural course of mild leptospirosis usually involves spontaneous resolution within 7–10 days, but persistent symptoms have been documented. In the absence of a clinical diagnosis and antimicrobial therapy, the mortality rate in mild leptospirosis is low.
Severe Leptospirosis Although the onset of severe leptospirosis may be no different from that of mild leptospirosis, severe disease is often rapidly progressive and is associated with a case–fatality rate ranging from 1 to 50%. Higher mortality rates are associated with an age >40, altered mental status, acute renal failure, respiratory insufficiency, hypotension, and arrhythmias. The classic presentation, often referred to as Weil’s syndrome, encompasses the triad of hemorrhage, jaundice, and acute kidney injury.
Patients die of septic shock with multiorgan failure and/or severe bleeding complications that most commonly involve the lungs (pulmonary hemorrhage), gastrointestinal tract (melena, hemoptysis), urogenital tract (hematuria), and skin (petechiae, ecchymosis, and bleeding from venipuncture sites). Pulmonary hemorrhage (with or without jaundice) is now recognized as a widespread public health problem, presenting with cough, chest pain, respiratory distress, and hemoptysis that may not be apparent until patients are intubated.
Jaundice occurs in 5–10% of all patients with leptospirosis; it can be profound and give an orange cast to the skin but usually is not associated with fulminant hepatic necrosis. Physical examination may reveal an enlarged and tender liver.
Acute kidney injury is common in severe disease, presenting after several days of illness, and can be either nonoliguric or oliguric. Typical electrolyte abnormalities include hypokalemia and hyponatremia. Loss of magnesium in the urine is uniquely associated with leptospiral nephropathy. Hypotension is associated with acute tubular necrosis, oliguria, or anuria, requiring fluid resuscitation and sometimes vasopressor therapy. Hemodialysis can be life-saving, with renal function typically returning to normal in survivors.
Other syndromes include (necrotizing) pancreatitis, cholecystitis, skeletal muscle involvement, rhabdomyolysis (with moderately elevated serum creatine kinase levels), and neurologic manifestations including aseptic meningitis. Cardiac involvement is commonly reflected on the electrocardiogram as nonspecific ST- and T-wave changes. Repolarization abnormalities and arrhythmias are considered poor prognostic factors. Myocarditis has been described. Rare hematologic complications include hemolysis, thrombotic thrombocytopenic purpura, and hemolytic-uremic syndrome.
Long-term symptoms following severe leptospirosis include fatigue, myalgia, malaise, and headache and may persist for years. Autoimmune-associated uveitis, a potentially chronic condition, is a recognized sequela of leptospirosis.
DIAGNOSIS
The clinical diagnosis of leptospirosis should be based on an appropriate exposure history combined with any of the protean manifestations of the disease. Returning travelers from endemic areas usually have a history of recreational freshwater activities or other mucosal or percutaneous contact with contaminated surface waters or soil. For nontravelers, recreational water contact and occupational hazards that involve direct or indirect animal contact should be explored (see “Epidemiology,” above).
Although biochemical, hematologic, and urinalysis findings in acute leptospirosis are nonspecific, certain patterns may suggest the diagnosis. Laboratory results usually show signs of a bacterial infection, including leukocytosis with a left shift and elevated markers of inflammation (C-reactive protein level and erythrocyte sedimentation rate). Thrombocytopenia (platelet count ≤100 × 109/L) is common and is associated with bleeding and renal failure. In severe disease, signs of coagulation activation may be present, varying from borderline abnormalities to a serious derangement compatible with DIC as defined by international criteria. The kidneys are invariably involved in leptospirosis. Related findings range from urinary sediment changes (leukocytes, erythrocytes, and hyaline or granular casts) and mild proteinuria in mild disease to renal failure and azotemia in severe leptospirosis. Nonoliguric hypokalemic renal insufficiency (see “Clinical Manifestations,” above) is characteristic of early leptospirosis. Serum bilirubin levels may be high, whereas rises in aminotransferase and alkaline phosphatase levels are usually moderate. Although clinical symptoms of pancreatitis are not a common finding, amylase levels are often elevated. When symptoms of aseptic meningitis develop, examination of the CSF shows pleocytosis that can range from a few cells to >1000 cells/μL, with a polymorphonuclear cell predominance. The protein concentration in the CSF may be elevated; CSF glucose levels are normal.
In severe leptospirosis, pulmonary radiographic abnormalities are more common than would be expected on the basis of physical examination (Fig. 208-4). The most common radiographic finding is a patchy bilateral alveolar pattern that corresponds to scattered alveolar hemorrhage. These abnormalities predominantly affect the lower lobes. Other findings include pleura-based densities (representing areas of hemorrhage) and diffuse ground-glass attenuation typical of acute respiratory distress syndrome (ARDS).
A definitive diagnosis of leptospirosis is based on isolation of the organism from the patient, on a positive result in the polymerase chain reaction (PCR), or on seroconversion or a rise in antibody titer. In cases with strong clinical evidence of infection, a single antibody titer of 1:200–1:800 (depending on whether the case occurs in a low- or high-endemic area) in the microscopic agglutination test (MAT) is required. Preferably, a fourfold or greater rise in titer is detected between acute- and convalescent-phase serum specimens. Antibodies generally do not reach detectable levels until the second week of illness. The antibody response can be affected by early treatment with antibiotics.
The MAT, which uses a battery of live leptospiral strains, and the enzyme-linked immunosorbent assay (ELISA), which uses a broadly reacting antigen, are the standard serologic procedures. The MAT usually is available only in specialized laboratories and is used for determination of the antibody titer and for tentative identification of the involved leptospiral serogroup—and, when epidemiologic background information is available, the putative serovar. This point underscores the importance of testing antigens representative of the serovars prevalent in the particular geographic area. However, cross-reactions occur frequently, and thus definitive identification of the infecting serovar or serogroup is not possible without isolation of the causative organism. Because serologic testing lacks sensitivity in the early acute phase of the disease (up to day 5), it cannot be used as the basis for a timely decision about whether to start treatment.
In addition to the MAT and the ELISA, various rapid tests with diagnostic value have been developed, and some of these are commercially available. These rapid tests mainly apply lateral flow, (latex) agglutination, or ELISA methodology and are reasonably sensitive and specific, although results reported in the literature vary, probably as a consequence of differences in test interpretation, (re)exposure risks, serovar distribution, and the use of biased serum panels. These methods do not require culture or MAT facilities and are useful in settings that lack a strong medical infrastructure. PCR methodologies, notably real-time PCR, have become increasingly widely implemented. Compared with serology, PCR offers a great advantage: the capacity to confirm the diagnosis of leptospirosis with a high degree of accuracy during the first 5 days of illness.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of leptospirosis is broad, reflecting the diverse clinical presentations of the disease. Although leptospirosis transmission is more common in tropical and subtropical regions, the absence of a travel history does not exclude the diagnosis. When fever, headache, and myalgia predominate, influenza and other common and less common (e.g., dengue and chikungunya) viral infections should be considered. Malaria, typhoid fever, ehrlichiosis, viral hepatitis, and acute HIV infection may mimic the early stages of leptospirosis and are important to recognize. Rickettsial diseases, hantavirus infections (hemorrhagic fever with renal syndrome or hantavirus cardiopulmonary syndrome), and dengue share epidemiologic and clinical features with leptospirosis. Dual infections have been reported. In this light, it is advisable to conduct serologic testing for hantavirus, rickettsiae, and dengue virus when leptospirosis is suspected. When bleeding is detected, dengue hemorrhagic fever and other viral hemorrhagic fevers, including hantavirus infection, yellow fever, Rift Valley fever, filovirus infections, and Lassa fever, should be considered.
|
TREATMENT |
LEPTOSPIROSIS |
Severe leptospirosis should be treated with IV penicillin (Table 208-1) as soon as the diagnosis is considered. Leptospires are highly susceptible to a broad range of antibiotics, and early intervention may prevent the development of major organ system failure or lessen its severity. Although studies supporting antibiotic therapy have produced conflicting results, clinical trials are difficult to perform in settings where patients frequently present for medical care with late stages of disease. Antibiotics are less likely to benefit patients in whom organ damage has already occurred. Two open-label randomized studies comparing penicillin with parenteral cefotaxime, parenteral ceftriaxone, and doxycycline showed no significant differences among the antibiotics with regard to complications and mortality risk. Thus ceftriaxone, cefotaxime, or doxycycline is a satisfactory alternative to penicillin for the treatment of severe leptospirosis.
|
TREATMENT AND CHEMOPROPHYLAXIS OF LEPTOSPIROSIS IN ADULTSa |
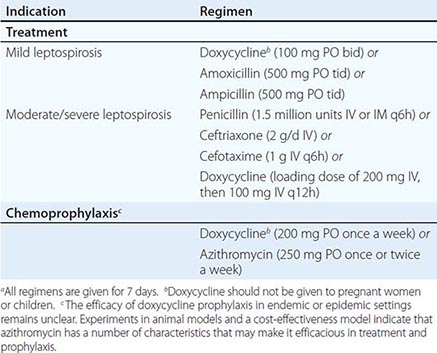
In mild cases, oral treatment with doxycycline, azithromycin, ampicillin, or amoxicillin is recommended. In regions where rickettsial diseases are coendemic, doxycycline or azithromycin is the drug of choice. In rare instances, a Jarisch-Herxheimer reaction develops within hours after the initiation of antimicrobial therapy.
Aggressive supportive care for leptospirosis is essential and can be life-saving. Patients with nonoliguric renal dysfunction require aggressive fluid and electrolyte resuscitation to prevent dehydration and precipitation of oliguric renal failure. Peritoneal dialysis or hemodialysis should be provided to patients with oliguric renal failure. Rapid initiation of hemodialysis has been shown to reduce mortality risk and typically is necessary only for short periods. Patients with pulmonary hemorrhage may have reduced pulmonary compliance (as seen in ARDS) and may benefit from mechanical ventilation with low tidal volumes to avoid high ventilation pressures. Evidence is contradictory for the use of glucocorticoids and desmopressin as adjunct therapy for pulmonary involvement associated with severe leptospirosis.
PROGNOSIS
Most patients with leptospirosis recover. However, post-leptospirosis symptoms, mainly of a depression-like nature, may occur and persist for years after the acute disease. Mortality rates are highest among patients who are elderly and those who have severe disease (pulmonary hemorrhage, Weil’s syndrome). Leptospirosis during pregnancy is associated with high fetal mortality rates. Long-term follow-up of patients with renal failure and hepatic dysfunction has documented good recovery of renal and hepatic function.
PREVENTION
Individuals who may be exposed to Leptospira through their occupations or their involvement in recreational freshwater activities should be informed about the risks. Measures for controlling leptospirosis include avoidance of exposure to urine and tissues from infected animals through proper eyewear, footwear, and other protective equipment. Targeted rodent control strategies could be considered.
![]() Vaccines for agricultural and companion animals are generally available, and their use should be encouraged. The veterinary vaccine used in a given area should contain the serovars known to be present in that area. Unfortunately, some vaccinated animals still excrete leptospires in their urine. Vaccination of humans against a specific serovar prevalent in an area has been undertaken in some European and Asian countries and has proved effective. Although a large-scale trial of vaccine in humans has been reported from Cuba, no conclusions can be drawn about efficacy and adverse reactions because of insufficient details on study design. The efficacy of chemoprophylaxis with doxycycline (200 mg once a week) or azithromycin (in pregnant women and children) is being disputed, but focused pre- and postexposure administration is indicated in instances of well-defined short-term exposure (Table 208-1).
Vaccines for agricultural and companion animals are generally available, and their use should be encouraged. The veterinary vaccine used in a given area should contain the serovars known to be present in that area. Unfortunately, some vaccinated animals still excrete leptospires in their urine. Vaccination of humans against a specific serovar prevalent in an area has been undertaken in some European and Asian countries and has proved effective. Although a large-scale trial of vaccine in humans has been reported from Cuba, no conclusions can be drawn about efficacy and adverse reactions because of insufficient details on study design. The efficacy of chemoprophylaxis with doxycycline (200 mg once a week) or azithromycin (in pregnant women and children) is being disputed, but focused pre- and postexposure administration is indicated in instances of well-defined short-term exposure (Table 208-1).
209 |
Relapsing Fever |
Relapsing fever is caused by infection with any of several species of Borrelia spirochetes. Physicians in ancient Greece distinguished relapsing fever from other febrile disorders by its characteristic clinical presentation: two or more fever episodes separated by varying periods of well-being. In the nineteenth century, relapsing fever was one of the first diseases to be associated with a specific microbe by virtue of its characteristic laboratory finding: the presence of large numbers of spirochetes of the genus Borrelia in the blood.
The host responds with systemic inflammation that results in an illness ranging from a flulike syndrome to sepsis. Other manifestations are the consequences of central nervous system (CNS) involvement and coagulopathy. Antigenic variation of the spirochetes’ surface proteins accounts for the infection’s relapsing course. Acquired immunity follows the serial development of antibodies to each of the several variants appearing during an infection. Treatment with antibiotics results in rapid cure but at the risk of a moderate to severe Jarisch-Herxheimer reaction.
![]() Louse-borne relapsing fever caused large epidemics well into the twentieth century and currently occurs in northeastern Africa. At present, however, most cases of relapsing fever are tick-borne in origin. Sporadic cases and small outbreaks are focally distributed on most continents, with Africa most affected. In North America, the majority of reports of relapsing fever have been from the western United States and Canada. Nevertheless, the recent discovery that another species in the relapsing fever group causes human disease in the same geographic distribution as Lyme disease (Chap. 210) confounds epidemiologic distinctions between the two major types of Borrelia infection.
Louse-borne relapsing fever caused large epidemics well into the twentieth century and currently occurs in northeastern Africa. At present, however, most cases of relapsing fever are tick-borne in origin. Sporadic cases and small outbreaks are focally distributed on most continents, with Africa most affected. In North America, the majority of reports of relapsing fever have been from the western United States and Canada. Nevertheless, the recent discovery that another species in the relapsing fever group causes human disease in the same geographic distribution as Lyme disease (Chap. 210) confounds epidemiologic distinctions between the two major types of Borrelia infection.
ETIOLOGIC AGENT
Coiled, thin microscopic filaments that swim in one direction and then coil up before heading in another were first observed in the blood of patients with relapsing fever in the 1880s (www.youtube.com/watch?v=VxDPV2lBd9U). These microbes were categorized as spirochetes and grouped as several species in the genus Borrelia. It was not until the 1960s that the organisms were isolated in pure culture. The breakthrough cultivation medium and its derivatives are rich in their ingredients, ranging from simple (e.g., amino acids and N-acetylglucosamine) to more complex (e.g., serum and protein hydrolysates). The limited biosynthetic capacity of Borrelia cells is accounted for by a genome content one-quarter that of Escherichia coli.
Like other spirochetes, the helix-shaped Borrelia cells have two membranes, the outer of which is more loosely secured than in other double-membrane bacteria, such as E. coli. As a consequence, fixed organisms with damaged membranes can assume a variety of morphologies in smears and histologic preparations. The flagella of spirochetes run between the two membranes and are not on the cell surface. Although technically gram-negative in their staining properties, the 10- to 20-μm-long Borrelia cells, with a diameter of 0.1–0.2 μm, are too narrow to be seen by bright-field microscopy of Gram-stained specimens.
EPIDEMIOLOGY
![]() The several species of Borrelia that cause relapsing fever have restricted geographic distributions (Table 209-1). The exception is Borrelia recurrentis, which is also the only species transmitted by the louse. Louse-borne relapsing fever (LBRF) is usually acquired from a body louse (Pediculus humanus corporis), with humans serving as the reservoir. Acquisition occurs not from the bite itself but from either rubbing the insect’s feces into the bite site with the fingers in response to irritation or inoculation of feces into the conjunctivae or an open wound. Although LBRF transmission is currently limited to Ethiopia and adjacent countries, the disease has had a global distribution in the past, and that potential remains. Epidemics with thousands of cases of LBRF can occur under circumstances of famine, natural disaster, refugee migration, and war.
The several species of Borrelia that cause relapsing fever have restricted geographic distributions (Table 209-1). The exception is Borrelia recurrentis, which is also the only species transmitted by the louse. Louse-borne relapsing fever (LBRF) is usually acquired from a body louse (Pediculus humanus corporis), with humans serving as the reservoir. Acquisition occurs not from the bite itself but from either rubbing the insect’s feces into the bite site with the fingers in response to irritation or inoculation of feces into the conjunctivae or an open wound. Although LBRF transmission is currently limited to Ethiopia and adjacent countries, the disease has had a global distribution in the past, and that potential remains. Epidemics with thousands of cases of LBRF can occur under circumstances of famine, natural disaster, refugee migration, and war.
|
RELAPSING FEVER BORRELIA SPECIES, BY GEOGRAPHIC REGION, VECTOR, AND PRIMARY RESERVOIR |
All other known species of relapsing fever agents are tick-borne—in most cases, by soft ticks of the genus Ornithodoros (Fig. 209-1). Tick-borne relapsing fever (TBRF) is found on most continents but is absent or rare in tropical, low-desert, arctic, or alpine environments. For most species, the reservoirs of infection are small to medium-sized mammals, usually rodents but sometimes pigs and other domestic animals living in or around human habitats. However, one species, Borrelia duttonii in sub-Saharan Africa, is largely maintained by tick transmission between human hosts. In North America, TBRF occurs as single cases or small case clusters through transient exposure of persons to infested buildings or caves in less populated areas where the rodent reservoirs have nests. The two main Borrelia species involved in North America are Borrelia hermsii (in the mountainous west) and Borrelia turicatae (in the southwestern and south-central regions). The soft tick vectors typically feed for no more than 30 min, usually without being noticed, while the victim is sleeping. Transovarial transmission from one generation of ticks to the next means that infection risk may persist in an area long after incriminated mammalian reservoirs have been eradicated.
FIGURE 209-1 Ornithodoros turicata soft ticks of different ages.
A newly recognized pathogen, Borrelia miyamotoi, belongs to the clade of relapsing fever species but is transmitted to humans from other mammals by hard ticks (e.g., Ixodes scapularis in the eastern United States) that also transmit Lyme disease, babesiosis, anaplasmosis, ehrlichiosis, and arboviral encephalitis. B. miyamotoi is acquired through outdoor activities and through contact with ticks in forested and shrubby areas during recreation, work, or activities around the home. In residents of areas where B. miyamotoi and Borrelia burgdorferi coexist, the prevalence of antibodies to the former is about one-third of that to the latter.
PATHOGENESIS AND IMMUNITY
Unlike LBRF spirochetes, TBRF spirochetes enter the body in the tick’s saliva with the onset of feeding. From an inoculum of a few cells, the spirochetes proliferate in the blood, doubling every 6 h to numbers of 106–107/mL or more. Borrelia species are extracellular pathogens; their presence inside cells likely represents a dead end for the bacteria after phagocytosis. Binding of the spirochetes to erythrocytes leads to aggregation of red blood cells, their sequestration in the spleen and liver, and hepatosplenomegaly and anemia. A bleeding disorder is probably the consequence of thrombocytopenia, impaired hepatic production of clotting factors, and/or blockage of small vessels by aggregates of spirochetes, erythrocytes, and platelets. Some species are neurotropic and frequently enter the brain, where they are comparatively sheltered from host immunity. Relapsing fever spirochetes can cross the maternal-fetal barrier and cause placental damage and inflammation, leading to intrauterine growth retardation and congenital infection.
Although Borrelia species do not have potent exotoxins or a lipopolysaccharide endotoxin, they have abundant membrane-associated lipoproteins whose recognition and binding by Toll-like receptors on host cells can lead to a proinflammatory process similar to that in endotoxemia, with elevations of tumor necrosis factor α, interleukin 6, and interleukin 8 concentrations.
IgM antibodies specific for the serotype-defining surface lipoprotein appear after a few days of infection and soon reach a concentration that causes lysis of bacteria in the blood through either direct bactericidal action or opsonization. The release of large amounts of lipoproteins and other bacterial products from dying bacteria provokes a “crisis,” during which there can be an increase in temperature, hypotension, and other signs of shock. A similar phenomenon occurring in some patients soon after the initiation of antibiotic treatment is characterized by the abrupt worsening of the condition, which is called a Jarisch-Herxheimer reaction (JHR).
CLINICAL MANIFESTATIONS
Relapsing fever presents with the sudden onset of fever. Febrile periods are punctuated by intervening afebrile periods of a few days; this pattern occurs at least twice. The patient’s temperature is ≥39°C and may be as high as 43°C. The first fever episode often ends in a crisis lasting ~15–30 min and consisting of rigors, a further elevation in temperature, and increases in pulse and blood pressure. The crisis phase is followed by profuse diaphoresis, falling temperature, and hypotension, which usually persist for several hours. In LBRF, the first episode of fever is unremitting for 3–6 days; it is usually followed by a single milder episode. In TBRF, multiple febrile periods last 1–3 days each. In both forms, the interval between fevers ranges from 4 to 14 days, sometimes with symptoms of malaise and fatigue.
The symptoms that accompany the fevers are usually nonspecific. Headache, neck stiffness, arthralgia, myalgia, and nausea may accompany the first and subsequent febrile episodes. An enlarging spleen and liver cause abdominal pain. A nonproductive cough is common during LBRF and—in combination with fever and myalgias—may suggest influenza. Acute respiratory distress syndrome may occur during TBRF.
On physical examination, the patient may be delirious or apathetic. There may be body lice in the patient’s clothes or signs of insect bites. In regions with B. miyamotoi infection, a hard tick may be embedded in the skin. Epistaxis, petechiae, and ecchymoses are common during LBRF but not in TBRF. Splenomegaly or spleen tenderness is common in both forms of relapsing fever. The majority of patients with LBRF and ~10% of patients with TBRF have discernible hepatomegaly.
Localizing neurologic findings are more common in TBRF than in LBRF. In North America, B. turicatae infection has neurologic manifestations more often than B. hermsii infection. Meningoencephalitis can result in residual hemiplegia or aphasia. Myelitis and radiculopathy may develop. Unilateral or bilateral Bell’s palsy and deafness from seventh or eighth cranial nerve involvement are the most common forms of cranial neuritis and typically present in the second or third febrile episode, not the first. Visual impairment from unilateral or bilateral iridocyclitis or panophthalmitis may be permanent. In LBRF, neurologic manifestations such as altered mental state or stiff neck are thought to be secondary to spirochetemia and systemic inflammation rather than to direct invasion of the nervous system.
Myocarditis appears to be common in both forms of relapsing fever and accounts for some deaths. Most commonly, myocarditis is evidenced by gallops on cardiac auscultation, a prolonged QTc interval, and cardiomegaly and pulmonary edema on chest radiography.
General laboratory studies are not specific. Mild to moderate normocytic anemia is common, but frank hemolysis and hemoglobinuria do not develop. Leukocyte counts are usually in the normal range or only slightly elevated, and leukopenia can occur during the crisis. Platelet counts can fall below 50,000/µL. Laboratory evidence of hepatitis can be found, with elevated serum concentrations of unconjugated bilirubin and aminotransferases; the prothrombin and partial thromboplastin times may be moderately prolonged.
Analysis of the cerebrospinal fluid (CSF) is indicated in cases of suspected relapsing fever with signs of meningitis or meningoencephalitis. The presence of mononuclear pleocytosis and mildly to moderately elevated protein levels justifies intravenous antibiotic therapy in TBRF.
The manifestations and course of B. miyamotoi infection remain incompletely characterized, but reports to date indicate that the sign most often reported by patients at presentation is fever without respiratory symptoms starting 1–2 weeks after a tick bite and recurring once or twice in some cases. Meningoencephalitis with spirochetes in the CSF was documented in one immunodeficient adult.
DIAGNOSIS
Relapsing fever should be considered in a patient with the characteristic fever pattern and a history of recent exposure—i.e., within 1–2 weeks before illness onset—to body lice or soft-bodied ticks in geographic areas with documented current or past transmission. Because of the longevity of the ticks and the transovarial transmission of the pathogen in the ticks, a case of relapsing fever may be diagnosed many years after the last case reported in that locale.
The bedrock for laboratory diagnosis remains the same as it has been for a century: direct detection of the spirochetes by microscopy of the blood. Manual differential counts of white blood cells by Wright or Giemsa stain usually reveal spirochetes in thin blood smears if their concentration is ≥105/mL and several oil-immersion fields are examined (Fig. 209-2). The preferred time to obtain a blood specimen is between the fever’s onset and its peak. Lower concentrations of spirochetes may be revealed by a thick blood smear that is either directly stained with acridine orange and then examined by fluorescence microscopy or treated with 0.5% acetic acid before Giemsa or Wright staining. An alternative to a fixed blood smear is a wet mount of anticoagulated blood mixed with saline and examined by phase-contrast or dark-field microscopy for motile spirochetes.
FIGURE 209-2 Photomicrograph of tick-borne relapsing fever spirochete (Borrelia turicatae) in a Wright-Giemsa-stained thin blood smear. Included in the figure are a polymorphonuclear leukocyte and two platelets.
Polymerase chain reaction (PCR) and similar DNA amplification procedures are increasingly used for examination of an extract of blood. PCR may reveal spirochetes between febrile episodes, since there are already escape variants in the population when the first wave of bacteria is neutralized.
Culture of blood or CSF in Barbour-Stoenner-Kelly broth medium is an option for isolation of Borrelia species except for B. miyamotoi, which is noncultivable or poorly cultivable. However, few laboratories offer this service. An alternative for tick-borne Borrelia species, including B. miyamotoi, is inoculation of blood or CSF into immunodeficient mice and examination of the animal’s blood after a few days.
Options for serologic confirmation of infection are limited. Most assays that are available commercially or in reference laboratories are based on whole cells of a single Borrelia species. These assays may not detect the major variant antigens to which the patient is mainly responding or may yield false-positive results due to antibodies to cross-reactive antigens of related bacteria, including B. burgdorferi. The most promising new assays under development are based on recombinant antigens such as GlpQ, a protein antigen of all relapsing fever Borrelia species (including B. miyamotoi) but not of any Lyme disease species.
DIFFERENTIAL DIAGNOSIS
Depending on the patient’s history of residential, occupational, travel, and recreational exposures, the differential diagnosis of relapsing fever includes one or more of the following infections that feature either periodicity in the fever pattern or an extended single febrile period with nonspecific constitutional symptoms: Colorado tick fever (which, along with dengue, can have a “saddleback” fever course), Rocky Mountain spotted fever and other rickettsioses, ehrlichiosis, anaplasmosis, tick-borne arbovirus infection, and babesiosis in North America, Europe, Russia, and northeastern Asia. Elsewhere in the Americas and Asia and in most of Africa, malaria, typhoid fever, typhus and other rickettsioses, dengue, brucellosis, and leptospirosis may also be considered. Depending on the geographic area and types of exposure, malaria, louse-borne typhus, typhoid fever, or Lyme disease may complicate relapsing fever.
|
TREATMENT |
RELAPSING FEVER |
Penicillins and tetracyclines have been the antibiotics of choice for relapsing fever for several decades. Erythromycin has been a long-standing second choice. There is no evidence of acquired resistance to these antibiotics. Borrelia species are also susceptible to most cephalosporins and chloramphenicol, but there is less clinical experience with these drugs. Borreliae are relatively resistant to rifampin, sulfonamides, fluoroquinolones, and aminoglycosides. Spirochetes are no longer detectable in the blood within a few hours after the first dose of an effective antibiotic.
A single dose of antibiotic is usually sufficient for the treatment of LBRF (Fig. 209-3). The recurrence rate after antibiotic treatment is ≤5%. For adults, a single dose of oral tetracycline (500 mg), oral doxycycline (200 mg), or intramuscular penicillin G procaine (400,000–800,000 units) is effective. The corresponding doses for children are oral tetracycline at 12.5 mg/kg, oral doxycycline at 5 mg/kg, and intramuscular penicillin G procaine at 200,000–400,000 units. When an adult patient is stuporous or nauseated, the intravenous dose is 250–500 mg. Tetracycline is contraindicated in pregnant and nursing women and in children <9 years old; for individuals in these groups who are allergic to penicillin, oral erythromycin (500 mg for adults and 12.5 mg/kg for children) is an alternative. Tetracycline is marginally superior to penicillin G in terms of time to fever clearance and relapse rate.
FIGURE 209-3 Algorithm for treatment of relapsing fever. If it is not known whether the patient has tick-borne or louse-borne relapsing fever, the patient should be treated for the tick-borne form. The dashed line indicates that central nervous system invasion in louse-borne relapsing fever is uncommon.
The accumulated anecdotal reports on TBRF therapy indicate a recurrence rate of ≥20% after single-dose treatment. This high rate of recurrence plausibly is due to the greater propensity of tick-borne species than of B. recurrentis to invade the CNS, from which they can reinvade the bloodstream after antibiotic levels decline. Accordingly, multiple antibiotic doses are recommended. The preferred treatment for adults is a 10-day course of tetracycline (500 mg or 12.5 mg/kg orally every 6 h) or doxycycline (100 mg twice daily). When tetracyclines are contraindicated, the alternative is erythromycin (500 mg or 12.5 mg/kg orally every 6 h) for 10 days. If a β-lactam antibiotic is given, it should be administered intravenously rather than orally, especially if CNS involvement is confirmed or suspected. For adults, the regimen is penicillin G (5 million units IV every 6 h) or ceftriaxone (2 g IV daily) for 10–14 days.
Experience with the treatment of B. miyamotoi infection is limited, but this organism likely has the same antibiotic susceptibilities as other Borrelia species. Until more is known about treatment efficacy, therapy for B. miyamotoi infection can follow the guidelines for Lyme disease—including parenteral therapy for CNS involvement—because it may be difficult to rule out co-infection.
The JHR during treatment of relapsing fever can be severe and can even end in death if precautions are not in place for close monitoring and provision of cardiovascular and volume support as needed. Rigors, fever, and hypotension occur within 2–3 h of initiation of antibiotic treatment. The incidence of the JHR is ~80% in LBRF and ~50% in TBRF. Both penicillin and tetracycline can elicit the JHR.
PROGNOSIS
The mortality rates for untreated LBRF and TBRF are in the ranges of 10–70% and 4–10%, respectively, and are largely determined by coexisting conditions, such as malnutrition and dehydration. Death from untreated relapsing fever is most common during the first fever episode. With prompt antibiotic treatment, the mortality rate is 2–5% for LBRF and <2% for TBRF. Features associated with a poor prognosis include concurrence with malaria, typhus, or typhoid; pregnancy; stupor or coma on admission; diffuse bleeding; poor liver function; myocarditis; and bronchopneumonia. The mortality rate from the JHR in LBRF, in the absence of adequate monitoring and resuscitation measures, is ~5%. Some patients have survived the crisis or the JHR only to die suddenly either later that day or on the next day. Relapsing fever during pregnancy frequently leads to abortion or stillbirth, but congenital malformations have not been reported. Although it is possible that spirochetes may persist in the CNS or other sequestered sites after bacteremia has resolved, chronic disease or disability from a persistent infection has not been attributed to relapsing fever. Partial immunity against reinfection seems to develop in residents of endemic areas.
PREVENTION
There is no vaccine for either LBRF or TBRF. Reduction of exposure to lice and ticks is the key strategy for prevention. LBRF can be prevented through improved personal hygiene, reduction of crowding, better access to washing facilities, and selected use of pesticides. Infested clothing is an important factor in maintaining body lice. The risk of TBRF can be reduced by construction of houses with concrete or sealed plank floors and without thatched roofs or mud walls. Log cabins pose a particular risk in North America when rodents nest in the roof or beneath the house or porch. Interiors of buildings infested with Ornithodoros ticks can be treated with pesticides. If residing in a high-risk environment, individuals should not sleep on the floor, and beds should be moved away from the wall. With an exposure to TBRF, postexposure treatment with doxycycline (200 mg on day 1 followed by 100 mg/d for 4 days) was efficacious in preventing infection in a placebo-controlled trial.
210 |
Lyme Borreliosis |
DEFINITION
Lyme borreliosis is caused by a spirochete, Borrelia burgdorferi sensu lato, that is transmitted by ticks of the Ixodes ricinus complex. The infection usually begins with a characteristic expanding skin lesion, erythema migrans (EM; stage 1, localized infection). After several days or weeks, the spirochete may spread to many different sites (stage 2, disseminated infection). Possible manifestations of disseminated infection include secondary annular skin lesions, meningitis, cranial neuritis, radiculoneuritis, peripheral neuritis, carditis, atrioventricular nodal block, or migratory musculoskeletal pain. Months or years later (usually after periods of latent infection), intermittent or persistent arthritis, chronic encephalopathy or polyneuropathy, or acrodermatitis may develop (stage 3, persistent infection). Most patients experience early symptoms of the illness during the summer, but the infection may not become symptomatic until it progresses to stage 2 or 3.
Lyme disease was recognized as a separate entity in 1976 because of a geographic cluster of children in Lyme, Connecticut, who were thought to have juvenile rheumatoid arthritis. It became apparent that Lyme disease was a multisystemic illness that affected primarily the skin, nervous system, heart, and joints. Epidemiologic studies of patients with EM implicated certain Ixodes ticks as vectors of the disease. Early in the twentieth century, EM had been described in Europe and attributed to I. ricinus tick bites. In 1982, a previously unrecognized spirochete, now called Borrelia burgdorferi, was recovered from Ixodes scapularis ticks and then from patients with Lyme disease. The entity is now called Lyme disease or Lyme borreliosis.
ETIOLOGIC AGENT
![]() B. burgdorferi, the causative agent of Lyme disease, is a fastidious microaerophilic bacterium. The spirochete’s genome is quite small (~1.5 Mb) and consists of a highly unusual linear chromosome of 950 kb as well as 17–21 linear and circular plasmids. The most remarkable aspect of the B. burgdorferi genome is that there are sequences for more than 100 known or predicted lipoproteins—a larger number than in any other organism. The spirochete has few proteins with biosynthetic activity and depends on its host for most of its nutritional requirements. It has no sequences for recognizable toxins.
B. burgdorferi, the causative agent of Lyme disease, is a fastidious microaerophilic bacterium. The spirochete’s genome is quite small (~1.5 Mb) and consists of a highly unusual linear chromosome of 950 kb as well as 17–21 linear and circular plasmids. The most remarkable aspect of the B. burgdorferi genome is that there are sequences for more than 100 known or predicted lipoproteins—a larger number than in any other organism. The spirochete has few proteins with biosynthetic activity and depends on its host for most of its nutritional requirements. It has no sequences for recognizable toxins.
![]() Currently, 13 closely related borrelial species are collectively referred to as Borrelia burgdorferi sensu lato (i.e., “B. burgdorferi in the general sense”). The human infection Lyme borreliosis is caused primarily by three pathogenic genospecies: B. burgdorferi sensu stricto (“B. burgdorferi in the strict sense,” hereafter referred to simply as B. burgdorferi), Borrelia garinii, and Borrelia afzelii. B. burgdorferi is the sole cause of the infection in the United States; all three genospecies are found in Europe, and the latter two species occur in Asia.
Currently, 13 closely related borrelial species are collectively referred to as Borrelia burgdorferi sensu lato (i.e., “B. burgdorferi in the general sense”). The human infection Lyme borreliosis is caused primarily by three pathogenic genospecies: B. burgdorferi sensu stricto (“B. burgdorferi in the strict sense,” hereafter referred to simply as B. burgdorferi), Borrelia garinii, and Borrelia afzelii. B. burgdorferi is the sole cause of the infection in the United States; all three genospecies are found in Europe, and the latter two species occur in Asia.
Strains of B. burgdorferi have been subdivided according to several typing schemes: one based on sequence variation of outer-surface protein C (OspC), a second based on differences in the 16S–23S rRNA intergenic spacer region (RST or IGS), and a third called multilocus sequence typing. From these typing systems, it is apparent that strains of B. burgdorferi differ in pathogenicity. OspC type A (RST1) strains seem to be particularly virulent and may have played a role in the emergence of Lyme disease in epidemic form in the northeastern United States in the late twentieth century.
EPIDEMIOLOGY
![]() The 13 known genospecies of B. burgdorferi sensu lato live in nature in enzootic cycles involving 14 species of ticks that are part of the I. ricinus complex. I. scapularis (Fig. 475-1) is the principal vector in the eastern United States from Maine to Georgia and in the midwestern states of Wisconsin, Minnesota, and Michigan. I. pacificus is the vector in the western states of California and Oregon. The disease is acquired throughout Europe (from Great Britain to Scandinavia to European Russia), where I. ricinus is the vector, and in Asian Russia, China, and Japan, where I. persulcatus is the vector. These ticks may transmit other agents as well. In the United States, I. scapularis also transmits Babesia microti; Anaplasma phagocytophilum; Ehrlichia species Wisconsin; Borrelia miyamotoi; and, in rare instances, Powassan encephalitis virus (the deer tick virus) (see “Differential Diagnosis,” below). In Europe and Asia, I. ricinus and I. persulcatus also transmit tick-borne encephalitis virus.
The 13 known genospecies of B. burgdorferi sensu lato live in nature in enzootic cycles involving 14 species of ticks that are part of the I. ricinus complex. I. scapularis (Fig. 475-1) is the principal vector in the eastern United States from Maine to Georgia and in the midwestern states of Wisconsin, Minnesota, and Michigan. I. pacificus is the vector in the western states of California and Oregon. The disease is acquired throughout Europe (from Great Britain to Scandinavia to European Russia), where I. ricinus is the vector, and in Asian Russia, China, and Japan, where I. persulcatus is the vector. These ticks may transmit other agents as well. In the United States, I. scapularis also transmits Babesia microti; Anaplasma phagocytophilum; Ehrlichia species Wisconsin; Borrelia miyamotoi; and, in rare instances, Powassan encephalitis virus (the deer tick virus) (see “Differential Diagnosis,” below). In Europe and Asia, I. ricinus and I. persulcatus also transmit tick-borne encephalitis virus.
Ticks of the I. ricinus complex have larval, nymphal, and adult stages. They require a blood meal at each stage. The risk of infection in a given area depends largely on the density of these ticks as well as their feeding habits and animal hosts, which have evolved differently in different locations. For I. scapularis in the northeastern United States, the white-footed mouse and certain other rodents are the preferred hosts of the immature larvae and nymphs. It is critical that both of the tick’s immature stages feed on the same host because the life cycle of the spirochete depends on horizontal transmission: in early summer from infected nymphs to mice and in late summer from infected mice to larvae, which then molt to become the infected nymphs that will begin the cycle again the following year. It is the tiny nymphal tick that is primarily responsible for transmission of the disease to humans during the early summer months. White-tailed deer, which are not involved in the life cycle of the spirochete, are the preferred host for the adult stage of I. scapularis and seem to be critical to the tick’s survival.
Lyme disease is now the most common vector-borne infection in the United States and Europe. Since surveillance was begun by the Centers for Disease Control and Prevention (CDC) in 1982, the number of cases in the United States has increased dramatically. More than 30,000 new cases are now reported each summer, but the actual number of new cases is probably closer to 300,000 annually. In Europe, reported frequencies of the disease are highest in the middle of the continent and in Scandinavia.
PATHOGENESIS AND IMMUNITY
To maintain its complex enzootic cycle, B. burgdorferi must adapt to two markedly different environments: the tick and the mammalian host. The spirochete expresses outer-surface protein A (OspA) in the midgut of the tick, whereas OspC is upregulated as the organism travels to the tick’s salivary gland. There, OspC binds a tick salivary-gland protein (Salp15), which is required for infection of the mammalian host. The tick usually must be attached for at least 24 h for transmission of B. burgdorferi.
After injection into the human skin, the spirochete downregulates OspC and upregulates the VlsE lipoprotein. This protein undergoes extensive antigenic variation, which is necessary for spirochetal survival. After several days to weeks, B. burgdorferi may migrate outward in the skin, producing EM, and may spread hematogenously or in the lymph to other organs. The only known virulence factors of B. burgdorferi are surface proteins that allow the spirochete to attach to mammalian proteins, integrins, glycosaminoglycans, or glycoproteins. For example, spread through the skin and other tissue matrices may be facilitated by the binding of human plasminogen and its activators to the surface of the spirochete. Some Borrelia strains bind complement regulator–acquiring surface proteins (FHL-1/reconectin, or factor H), which help to protect spirochetes from complement-mediated lysis. Dissemination of the organism in the blood is facilitated by binding to the fibrinogen receptor on activated platelets (αIIbβ3) and the vitronectin receptor (αvβ3) on endothelial cells. As the name indicates, spirochetal decorin-binding proteins A and B bind decorin, a glycosaminoglycan on collagen fibrils; this binding may explain why the organism is commonly aligned with collagen fibrils in the extracellular matrix in the heart, nervous system, or joints.
To control and eradicate B. burgdorferi, the host mounts both innate and adaptive immune responses, resulting in macrophage- and antibody-mediated killing of the spirochete. As part of the innate immune response, complement may lyse the spirochete in the skin. Cells at affected sites release potent proinflammatory cytokines, including interleukin 6, tumor necrosis factor α, interleukin 1β, and interferon γ. Patients who are homozygous for a Toll-like receptor 1 polymorphism (1805GG), particularly when infected with highly inflammatory B. burgdorferi RST1 strains, have exceptionally high levels of proinflammatory cytokines. The purpose of the adaptive immune response appears to be the production of specific antibodies, which opsonize the organism—a step necessary for optimal spirochetal killing. Studies with protein arrays expressing ~1200 B. burgdorferi proteins detected antibody responses to a total of 120 spirochetal proteins (particularly outer-surface lipoproteins) in a population of patients with Lyme arthritis. Histologic examination of all affected tissues reveals an infiltration of lymphocytes, macrophages, and plasma cells with some degree of vascular damage, including mild vasculitis or hypervascular occlusion. These findings suggest that the spirochete may have been present in or around blood vessels.
In enzootic infection, B. burgdorferi spirochetes must survive this immune assault only during the summer months before returning to larval ticks to begin the cycle again the following year. In contrast, infection of humans is a dead-end event for the spirochete. Within several weeks or months, innate and adaptive immune mechanisms—even without antibiotic treatment—control widely disseminated infection, and generalized systemic symptoms wane. However, without antibiotic therapy, spirochetes may survive in localized niches for several more years. For example, B. burgdorferi infection in the United States may cause persistent arthritis or, in rare cases, subtle encephalopathy or polyneuropathy. Thus, immune mechanisms seem to succeed eventually in the near or total eradication of B. burgdorferi from selected niches, including the joints or nervous system, and symptoms resolve in most patients.
CLINICAL MANIFESTATIONS
Early Infection: Stage 1 (Localized Infection) Because of the small size of nymphal ixodid ticks, most patients do not remember the preceding tick bite. After an incubation period of 3–32 days, EM usually begins as a red macule or papule at the site of the tick bite that expands slowly to form a large annular lesion (Fig. 210-1). As the lesion increases in size, it often develops a bright red outer border and partial central clearing. The center of the lesion sometimes becomes intensely erythematous and indurated, vesicular, or necrotic. In other instances, the expanding lesion remains an even, intense red; several red rings are found within an outside ring; or the central area turns blue before the lesion clears. Although EM can be located anywhere, the thigh, groin, and axilla are particularly common sites. The lesion is warm but not often painful. Approximately 20% of patients do not exhibit this characteristic skin manifestation.
FIGURE 210-1 A classic erythema migrans lesion (9 cm in diameter) is shown near the right axilla. The lesion has partial central clearing, a bright red outer border, and a target center. (Courtesy of Vijay K. Sikand, MD; with permission.)
Early Infection: Stage 2 (Disseminated Infection) In cases in the United States, B. burgdorferi often spreads hematogenously to many sites within days or weeks after the onset of EM. In these cases, patients may develop secondary annular skin lesions similar in appearance to the initial lesion. Skin involvement is commonly accompanied by severe headache, mild stiffness of the neck, fever, chills, migratory musculoskeletal pain, arthralgias, and profound malaise and fatigue. Less common manifestations include generalized lymphadenopathy or splenomegaly, hepatitis, sore throat, nonproductive cough, conjunctivitis, iritis, or testicular swelling. Except for fatigue and lethargy, which are often constant, the early signs and symptoms of Lyme disease are typically intermittent and changing. Even in untreated patients, the early symptoms usually become less severe or disappear within several weeks. In ~15% of patients, the infection presents with these nonspecific systemic symptoms.
Symptoms suggestive of meningeal irritation may develop early in Lyme disease when EM is present but usually are not associated with cerebrospinal fluid (CSF) pleocytosis or an objective neurologic deficit. After several weeks or months, ~15% of untreated patients develop frank neurologic abnormalities, including meningitis, subtle encephalitic signs, cranial neuritis (including bilateral facial palsy), motor or sensory radiculoneuropathy, peripheral neuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis—alone or in various combinations. In children, the optic nerve may be affected because of inflammation or increased intracranial pressure, and these effects may lead to blindness. In the United States, the usual pattern consists of fluctuating symptoms of meningitis accompanied by facial palsy and peripheral radiculoneuropathy. Lymphocytic pleocytosis (~100 cells/μL) is found in CSF, often along with elevated protein levels and normal or slightly low glucose concentrations. In Europe and Asia, the first neurologic sign is characteristically radicular pain, which is followed by the development of CSF pleocytosis (meningopolyneuritis or Bannwarth’s syndrome); meningeal or encephalitic signs are frequently absent. These early neurologic abnormalities usually resolve completely within months, but in rare cases chronic neurologic disease may occur later.
Within several weeks after the onset of illness, ~8% of patients develop cardiac involvement. The most common abnormality is a fluctuating degree of atrioventricular block (first-degree, Wenckebach, or complete heart block). Some patients have more diffuse cardiac involvement, including electrocardiographic changes indicative of acute myopericarditis, left ventricular dysfunction evident on radionuclide scans, or (in rare cases) cardiomegaly or fatal pancarditis. Cardiac involvement lasts for only a few weeks in most patients but may recur in untreated patients. Chronic cardiomyopathy caused by B. burgdorferi has been reported in Europe.
During this stage, musculoskeletal pain is common. The typical pattern consists of migratory pain in joints, tendons, bursae, muscles, or bones (usually without joint swelling) lasting for hours or days and affecting one or two locations at a time.
Late Infection: Stage 3 (Persistent Infection) Months after the onset of infection, ~60% of patients in the United States who have received no antibiotic treatment develop frank arthritis. The typical pattern comprises intermittent attacks of oligoarticular arthritis in large joints (especially the knees), lasting for weeks or months in a given joint. A few small joints or periarticular sites also may be affected, primarily during early attacks. The number of patients who continue to have recurrent attacks decreases each year. However, in a small percentage of cases, involvement of large joints—usually one or both knees—is persistent and may lead to erosion of cartilage and bone.
White cell counts in joint fluid range from 500 to 110,000/μL (average, 25,000/μL); most of these cells are polymorphonuclear leukocytes. Tests for rheumatoid factor or antinuclear antibodies usually give negative results. Examination of synovial biopsy samples reveals fibrin deposits, villous hypertrophy, vascular proliferation, microangiopathic lesions, and a heavy infiltration of lymphocytes and plasma cells.
Although most patients with Lyme arthritis respond well to antibiotic therapy, a small percentage in the northeastern United States have persistent (antibiotic-refractory) arthritis for months or even for several years after receiving oral and IV antibiotic therapy for 2 or 3 months. Although more often these patients are initially infected with RST1 strains of B. burgdorferi, this complication is not thought to result from persistent infection. Results of culture and polymerase chain reaction (PCR) for B. burgdorferi in synovial tissue obtained in the postantibiotic period have been uniformly negative. Rather, infection-induced autoimmunity, retained spirochetal antigens, or both may play a role in this outcome. Antibiotic-refractory arthritis is associated with a higher frequency of certain class II major histocompatibility complex molecules (particularly HLA-DRBI*0401 or -*0101 molecules); the Toll-like receptor 1 polymorphism 1805GG, which leads to exceptionally high levels of cytokines and chemokines in affected joints; and low frequencies of FoxP3+ T regulatory cells in synovial fluid, which correlate with longer posttreatment durations of arthritis. The recent identification of a novel human autoantigen, endothelial cell growth factor, as a target of T and B cell responses in patients with Lyme disease provided the first direct evidence of autoimmune T and B cell responses in this illness. However, multiple spirochetal or additional yet-to-be identified autoantigens may have a role in antibiotic-refractory arthritis.
Although rare, chronic neurologic involvement also may become apparent from months to several years after the onset of infection, sometimes after long periods of latent infection. The most common form of chronic central nervous system involvement is subtle encephalopathy affecting memory, mood, or sleep, and the most common form of peripheral neuropathy is an axonal polyneuropathy manifested as either distal paresthesia or spinal radicular pain. Patients with encephalopathy frequently have evidence of memory impairment in neuropsychological tests and abnormal results in CSF analyses. In cases of polyneuropathy, electromyography generally shows extensive abnormalities of proximal and distal nerve segments. Encephalomyelitis or leukoencephalitis, a rare manifestation of Lyme borreliosis associated primarily with B. garinii infection in Europe, is a severe neurologic disorder that may include spastic paraparesis, upper motor-neuron bladder dysfunction, and, rarely, lesions in the periventricular white matter.
![]() Acrodermatitis chronica atrophicans, the late skin manifestation of Lyme borreliosis, has been associated primarily with B. afzelii infection in Europe and Asia. It has been observed especially often in elderly women. The skin lesions, which are usually found on the acral surface of an arm or leg, begin insidiously with reddish-violaceous discoloration; they become sclerotic or atrophic over a period of years.
Acrodermatitis chronica atrophicans, the late skin manifestation of Lyme borreliosis, has been associated primarily with B. afzelii infection in Europe and Asia. It has been observed especially often in elderly women. The skin lesions, which are usually found on the acral surface of an arm or leg, begin insidiously with reddish-violaceous discoloration; they become sclerotic or atrophic over a period of years.
The basic patterns of Lyme borreliosis are similar worldwide, but there are regional variations, primarily between the illness found in North America, which is caused exclusively by B. burgdorferi, and that found in Europe, which is caused primarily by B. afzelii and B. garinii. With each of the Borrelia species, the infection usually begins with EM. However, B. burgdorferi strains in the eastern United States often disseminate widely; they are particularly arthritogenic, and they may cause antibiotic-refractory arthritis. B. garinii typically disseminates less widely, but it is especially neurotropic and may cause borrelial encephalomyelitis. B. afzelii often infects only the skin but may persist in that site, where it may cause several different dermatoborrelioses, including acrodermatitis chronica atrophicans.
Post–Lyme Syndrome (Chronic Lyme Disease) Despite resolution of the objective manifestations of the infection with antibiotic therapy, ~10% of patients (although the reported percentages vary widely) continue to have subjective pain, neurocognitive manifestations, or fatigue symptoms. These symptoms usually improve and resolve within months but may last for years. At the far end of the spectrum, the symptoms may be similar to or indistinguishable from chronic fatigue syndrome (Chap. 464e) and fibromyalgia (Chap. 396). Compared with symptoms of active Lyme disease, post-Lyme symptoms tend to be more generalized or disabling. They include marked fatigue, severe headache, diffuse musculoskeletal pain, multiple symmetric tender points in characteristic locations, pain and stiffness in many joints, diffuse paresthesias, difficulty with concentration, and sleep disturbances. Patients with this condition lack evidence of joint inflammation, have normal neurologic test results, and may exhibit anxiety and depression. In contrast, late manifestations of Lyme disease, including arthritis, encephalopathy, and neuropathy, are usually associated with minimal systemic symptoms. Currently, no evidence indicates that persistent subjective symptoms after recommended courses of antibiotic therapy are caused by active infection.
DIAGNOSIS
The culture of B. burgdorferi in Barbour-Stoenner-Kelly (BSK) medium permits definitive diagnosis, but this method has been used primarily in research studies. Moreover, with a few exceptions, positive cultures have been obtained only early in the illness—particularly from biopsy samples of EM skin lesions, less often from plasma samples, and occasionally from CSF samples. Later in the infection, PCR is greatly superior to culture for the detection of B. burgdorferi DNA in joint fluid; this is the major use for PCR testing in Lyme disease. However, because B. burgdorferi DNA may persist for at least weeks after spirochetal killing with antibiotics, detection of spirochetal DNA in joint fluid is not an accurate test of active joint infection in Lyme disease and cannot be used reliably to determine the adequacy of antibiotic therapy. The sensitivity of PCR determinations in CSF from patients with neuroborreliosis has been much lower than that in joint fluid. There seems to be little if any role for PCR in the detection of B. burgdorferi DNA in blood or urine samples. Moreover, this procedure must be carefully controlled to prevent contamination.
Because of the problems associated with direct detection of B. burgdorferi, Lyme disease is usually diagnosed by the recognition of a characteristic clinical picture accompanied by serologic confirmation. Although serologic testing may yield negative results during the first several weeks of infection, almost all patients have a positive antibody response to B. burgdorferi after that time. The limitation of serologic tests is that they do not clearly distinguish between active and inactive infection. Patients with previous Lyme disease—particularly in cases progressing to late stages—often remain seropositive for years, even after adequate antibiotic treatment. In addition, ~10% of patients are seropositive because of asymptomatic infection. If these individuals subsequently develop another illness, the positive serologic test for Lyme disease may cause diagnostic confusion. According to an algorithm published by the American College of Physicians (Table 210-1), serologic testing for Lyme disease is recommended only for patients with at least an intermediate pretest probability of Lyme disease, such as those with oligoarticular arthritis. It should not be used as a screening procedure in patients with pain or fatigue syndromes. In such patients, the probability of a false-positive serologic result is higher than that of a true-positive result.
|
ALGORITHM FOR TESTING FOR AND TREATING LYME DISEASE |

For serologic analysis of Lyme disease in the United States, the CDC recommends a two-step approach in which samples are first tested by enzyme-linked immunosorbent assay (ELISA) and equivocal or positive results are then tested by western blotting. During the first weeks of infection, both IgM and IgG responses to the spirochete should be determined, preferably in both acute- and convalescent-phase serum samples. Approximately 20–30% of patients have a positive response detectable in acute-phase samples, whereas ~70–80% have a positive response during convalescence (2–4 weeks later). After 4–8 weeks of infection (by which time most patients with active Lyme disease have disseminated infection), the sensitivity and specificity of the IgG response to the spirochete are both very high—in the range of 99%—as determined by the two-test approach of ELISA and western blot. At this point and thereafter, a single test (that for IgG) is usually sufficient. In persons with illness of >2 months’ duration, a positive IgM test result alone is likely to be false-positive and therefore should not be used to support the diagnosis.
According to current criteria adopted by the CDC, an IgM western blot is considered positive if two of the following three bands are present: 23, 39, and 41 kDa. However, the combination of two such bands may still represent a false-positive result. Misuse or misinterpretation of IgM blots has been a factor in the incorrect diagnosis of Lyme disease in patients with other illnesses. An IgG blot is considered positive if 5 of the following 10 bands are present: 18, 23, 28, 30, 39, 41, 45, 58, 66, and 93 kDa. In European cases, no single set of criteria for the interpretation of immunoblots results in high levels of sensitivity and specificity in all countries.
The most promising second-generation serologic test is the VlsE C6 peptide IgG ELISA, which employs a 26-mer of the sixth invariant region of the VlsE lipoprotein of B. burgdorferi. The results achieved with this test are similar to those obtained with the standard two-test approach (sonicate IgM and IgG ELISA and western blot). The principal advantage of the C6 peptide ELISA is the early detection of an IgG response, which renders an IgM test unnecessary. However, not all patients with late Lyme disease have a response to the C6 peptide, and this test is not quite as specific as sonicate western blot. Thus, at present, a two-test approach that includes western blot is still recommended. Blotting can also be helpful in assessing the duration of current or past disease.
After successful antibiotic treatment, antibody titers decline slowly but responses (including that to the VlsE C6 peptide) may persist for years. Moreover, not only the IgG but also the IgM response may persist for years after therapy. Therefore, even a positive IgM response cannot be interpreted as confirmation of recent infection or reinfection unless the clinical picture is appropriate.
DIFFERENTIAL DIAGNOSIS
Classic EM is a slowly expanding erythema, often with partial central clearing. If the lesion expands little, it may represent the red papule of an uninfected tick bite. If the lesion expands rapidly, it may represent cellulitis (e.g., streptococcal cellulitis) or an allergic reaction, perhaps to tick saliva. Patients with secondary annular lesions may be thought to have erythema multiforme, but neither the development of blistering mucosal lesions nor the involvement of the palms or soles is a feature of B. burgdorferi infection. In the eastern United States, an EM-like skin lesion, sometimes with mild systemic symptoms, may be associated with Amblyomma americanum tick bites. However, the cause of this Southern tick-associated rash illness (STARI) has not yet been identified. This tick may also transmit Ehrlichia chaffeensis, a rickettsial agent (Chap. 211).
As stated above, I. scapularis ticks in the United States may transmit not only B. burgdorferi but also B. microti, a red blood cell parasite (Chap. 249); A. phagocytophilum, the agent of human granulocytotropic anaplasmosis (Chap. 211); Ehrlichia species Wisconsin; B. miyamotoi, a relapsing fever spirochete (Chap. 209); or (rarely) Powassan encephalitis virus (the deer tick virus, which is closely related to European tick-borne encephalitis virus) (Chap. 233). Although babesiosis and anaplasmosis are most often asymptomatic, infection with any of these agents may cause nonspecific systemic symptoms, particularly in the young or elderly, and co-infected patients may have more severe or persistent symptoms than patients infected with a single agent. Standard blood counts may yield clues regarding the presence of co-infection with Anaplasma or Babesia. Anaplasmosis may cause leukopenia or thrombocytopenia, and babesiosis may cause thrombocytopenia or (in severe cases) hemolytic anemia. IgM serologic responses may confuse the diagnosis. For example, A. phagocytophilum may elicit a positive IgM response to B. burgdorferi. The frequency of co-infection in different studies has been variable. In one prospective study, 4% of patients with EM had evidence of co-infection.
Facial palsy caused by B. burgdorferi, which occurs in the early disseminated phase of the infection (often in July, August, or September), is usually recognized by its association with EM. However, in rare cases, facial palsy without EM may be the presenting manifestation of Lyme disease. In such cases, both the IgM and the IgG responses to the spirochete are usually positive. The most common infectious agents that cause facial palsy are herpes simplex virus type 1 (Bell’s palsy; Chap. 216) and varicella-zoster virus (Ramsay Hunt syndrome; Chap. 217).
Later in the infection, oligoarticular Lyme arthritis most resembles reactive arthritis in an adult or the pauciarticular form of juvenile idiopathic arthritis in a child. Patients with Lyme arthritis usually have the strongest IgG antibody responses seen in Lyme borreliosis, with reactivity to many spirochetal proteins.
The most common problem in diagnosis is to mistake Lyme disease for chronic fatigue syndrome (Chap. 464e) or fibromyalgia (Chap. 396). This difficulty is compounded by the fact that a small percentage of patients do in fact develop these chronic pain or fatigue syndromes in association with or soon after Lyme disease. Moreover, a counterculture has emerged that ascribes pain and fatigue syndromes to chronic Lyme disease when there is little or no evidence of B. burgdorferi infection. In such cases, the term chronic Lyme disease, which is equated with chronic B. burgdorferi infection, is a misnomer, and the use of prolonged, dangerous, and expensive antibiotic treatment is not warranted.
|
TREATMENT |
LYME BORRELIOSIS |
ANTIBIOTIC TREATMENT
As outlined in the algorithm in Fig. 210-2, the various manifestations of Lyme disease can usually be treated successfully with orally administered antibiotics; the exceptions are objective neurologic abnormalities and third-degree atrioventricular heart block, which are generally treated with IV antibiotics, and arthritis that does not respond to therapy. For early Lyme disease, doxycycline is effective and can be administered to men and nonpregnant women. An advantage of this regimen is that it is also effective against A. phagocytophilum, which is transmitted by the same tick that transmits the Lyme disease agent. Amoxicillin, cefuroxime axetil, and erythromycin or its congeners are second-, third-, and fourth-choice alternatives, respectively. In children, amoxicillin is effective (not more than 2 g/d); in cases of penicillin allergy, cefuroxime axetil or erythromycin may be used. In contrast to second- or third-generation cephalosporin antibiotics, first-generation cephalosporins, such as cephalexin, are not effective. For patients with infection localized to the skin, a 14-day course of therapy is generally sufficient; in contrast, for patients with disseminated infection, a 21-day course is recommended. Approximately 15% of patients experience a Jarisch-Herxheimer-like reaction during the first 24 h of therapy. In multicenter studies, more than 90% of patients whose early Lyme disease was treated with these regimens had satisfactory outcomes. Although some patients reported symptoms after treatment, objective evidence of persistent infection or relapse was rare, and re-treatment was usually unnecessary.
FIGURE 210-2 Algorithm for the treatment of the various early or late manifestations of Lyme borreliosis. AV, atrioventricular. *For arthritis, oral therapy should be tried first; if arthritis is unresponsive, IV therapy should be administered. **For Lyme arthritis, IV ceftriaxone (2 g given once a day for 14–28 days) also is effective and is necessary for a small percentage of patients; however, compared with oral treatment, this regimen is less convenient to administer, has more side effects, and is more expensive.
Oral administration of doxycycline or amoxicillin for 30 days is recommended for the initial treatment of Lyme arthritis in patients who do not have concomitant neurologic involvement. Among patients with arthritis who do not respond to oral antibiotics, re-treatment with IV ceftriaxone for 28 days is appropriate. In patients with arthritis in whom joint inflammation persists for months or even several years after both oral and IV antibiotics, treatment with nonsteroidal anti-inflammatory agents, therapy with disease-modifying antirheumatic drugs, or synovectomy may be successful.
In the United States, parenteral antibiotic therapy is usually used for objective neurologic abnormalities (with the exception of facial palsy alone). Patients with neurologic involvement are most commonly treated with IV ceftriaxone for 14–28 days, but IV cefotaxime or IV penicillin G for the same duration also may be effective. In Europe, similar results have been obtained with oral doxycycline and IV antibiotics in the treatment of acute neuroborreliosis. In patients with high-degree atrioventricular block or a PR interval of >0.3 s, IV therapy for at least part of the course and cardiac monitoring are recommended, but the insertion of a permanent pacemaker is not necessary.
It is unclear how and whether asymptomatic infection should be treated, but patients with such infection are often given a course of oral antibiotics. Because maternal-fetal transmission of B. burgdorferi seems to occur rarely (if at all), standard therapy for the manifestations of the illness is recommended for pregnant women. Long-term persistence of B. burgdorferi has not been documented in any large series of patients after treatment with currently recommended regimens. Although an occasional patient requires a second course of antibiotics, there is no indication for multiple, repeated antibiotic courses in the treatment of Lyme disease.
CHRONIC LYME DISEASE
After appropriately treated Lyme disease, a small percentage of patients continue to have subjective symptoms, primarily musculoskeletal pain, neurocognitive difficulties, or fatigue. This chronic Lyme disease or post–Lyme syndrome is sometimes a disabling condition that is similar to chronic fatigue syndrome or fibromyalgia. In a large study, one group of patients with post–Lyme syndrome received IV ceftriaxone for 30 days followed by oral doxycycline for 60 days, while another group received IV and oral placebo preparations for the same durations. No significant differences were found between groups in the numbers of patients reporting that their symptoms had improved, become worse, or stayed the same. Such patients are best treated for the relief of symptoms rather than with prolonged courses of antibiotics.
PROPHYLAXIS AFTER A TICK BITE
The risk of infection with B. burgdorferi after a recognized tick bite is so low that antibiotic prophylaxis is not routinely indicated. However, if an attached, engorged I. scapularis nymph is found or if follow-up is anticipated to be difficult, a single 200-mg dose of doxycycline, which usually prevents Lyme disease when given within 72 h after the tick bite, may be administered.
PROGNOSIS
The response to treatment is best early in the disease. Later treatment of Lyme borreliosis is still effective, but the period of convalescence may be longer. Eventually, most patients recover with minimal or no residual deficits.
REINFECTION
Reinfection may occur after EM when patients are treated with antimicrobial agents. In such cases, the immune response is not adequate to provide protection from subsequent infection. However, patients who develop an expanded immune response to the spirochete over a period of months (e.g., those with Lyme arthritis) have protective immunity for a period of years and rarely, if ever, acquire the infection again.
PREVENTION
Protective measures for the prevention of Lyme disease may include the avoidance of tick-infested areas, the use of repellents and acaricides, tick checks, and modification of landscapes in or near residential areas. Although a vaccine for Lyme disease used to be available, the manufacturer has discontinued its production. Therefore, no vaccine is now commercially available for the prevention of this infection.
SECTION 10 |
DISEASES CAUSED BY RICKETTSIAE, MYCOPLASMAS, AND CHLAMYDIAE |
211 |
Rickettsial Diseases |
The rickettsiae are a heterogeneous group of small, obligately intracellular, gram-negative coccobacilli and short bacilli, most of which are transmitted by a tick, mite, flea, or louse vector. Except in the case of louse-borne typhus, humans are incidental hosts. Among rickettsiae, Coxiella burnetii, Rickettsia prowazekii, and R. typhi have the well-documented ability to survive for an extended period outside the reservoir or vector and to be extremely infectious: inhalation of a single Coxiella microorganism can cause pneumonia. High-level infectivity and severe illness after inhalation make R. prowazekii, R. rickettsii, R. typhi, R. conorii, and C. burnetii bioterrorism threats.
Clinical infections with rickettsiae can be classified according to (1) the taxonomy and diverse microbial characteristics of the agents, which belong to seven genera (Rickettsia, Orientia, Ehrlichia, Anaplasma, Neorickettsia, Candidatus Neoehrlichia, and Coxiella); (2) epidemiology; or (3) clinical manifestations. The clinical manifestations of all the acute presentations are similar during the first 5 days: fever, headache, and myalgias with or without nausea, vomiting, and cough. As the course progresses, clinical manifestations—including occurrence of a macular, maculopapular, or vesicular rash; eschar; pneumonitis; and meningoencephalitis—vary from one disease to another. Given the 15 etiologic agents with varied mechanisms of transmission, geographic distributions, and associated disease manifestations, the consideration of rickettsial diseases as a single entity poses complex challenges (Table 211-1).
|
FEATURES OF SELECTED RICKETTSIAL INFECTIONS |
Establishing the etiologic diagnosis of rickettsioses is very difficult during the acute stage of illness, and definitive diagnosis usually requires the examination of paired serum samples after convalescence. Heightened clinical suspicion is based on epidemiologic data, history of exposure to vectors or reservoir animals, travel to endemic locations, clinical manifestations (sometimes including rash or eschar), and characteristic laboratory findings (including thrombocytopenia, normal or low white blood cell [WBC] counts, elevated hepatic enzyme levels, and hyponatremia). Such suspicion should prompt empirical treatment. Doxycycline is the drug of choice for most of these infections. Only one agent, C. burnetii, has been documented to cause chronic illness. One other species, R. prowazekii, causes recrudescent illness (Brill-Zinsser disease) when latent infection is reactivated years after resolution of the acute illness.
Rickettsial infections dominated by fever may resolve without further clinical evolution. However, after nonspecific early manifestations, the illnesses can also evolve along one or more of several principal clinical lines: (1) development of a macular or maculopapular rash; (2) development of an eschar at the site of tick or mite feeding; (3) development of a vesicular rash (often in rickettsialpox and African tick-bite fever); (4) development of pneumonitis with chest radiographic opacities and/or rales (Q fever and severe cases of Rocky Mountain spotted fever [RMSF], Mediterranean spotted fever [MSF], louse-borne typhus, human monocytotropic ehrlichiosis [HME], human granulocytotropic anaplasmosis [HGA], scrub typhus, and murine typhus); (5) development of meningoencephalitis (louse-borne typhus and severe cases of RMSF, scrub typhus, HME, murine typhus, MSF, and [rarely] Q fever); and (6) progressive hypotension and multiorgan failure as seen with sepsis or toxic shock syndromes (RMSF, MSF, louse-borne typhus, murine typhus, scrub typhus, HME, HGA, and neoehrlichiosis).
Epidemiologic clues to the transmission of a particular pathogen include (1) environmental exposure to ticks, fleas, or mites during the season of activity of the vector species for the disease in the appropriate geographic region (spotted fever and typhus rickettsioses, scrub typhus, ehrlichioses, anaplasmosis); (2) travel to or residence in an endemic geographic region during the incubation period (Table 211-1); (3) exposure to parturient ruminants, cats, and dogs (Q fever); (4) exposure to flying squirrels (R. prowazekii infection); and (5) history of previous louse-borne typhus (recrudescent typhus).
Clinical laboratory findings, such as thrombocytopenia (particularly in spotted fever and typhus rickettsioses, ehrlichioses, anaplasmosis, and scrub typhus), normal or low WBC counts, mild to moderate serum elevations of hepatic aminotransferases, and hyponatremia, suggest some common pathophysiologic mechanisms.
Application of these clinical, epidemiologic, and laboratory principles requires consideration of a rickettsial diagnosis and knowledge of the individual diseases.
TICK-, MITE-, LOUSE-, AND FLEA-BORNE RICKETTSIOSES
These diseases, caused by organisms of the genera Rickettsia and Orientia in the family Rickettsiaceae, result from endothelial cell infection and increased vascular permeability. Pathogenic rickettsial species are very closely related, have small genomes (as a result of reductive evolution, which eliminated many genes for biosynthesis of intracellularly available molecules), and are traditionally separated into typhus and spotted fever groups on the basis of lipopolysaccharide antigens. Some diseases and their agents (e.g., R. africae, R. parkeri, and R. sibirica) are too similar to require separate descriptions. Indeed, the similarities among MSF (R. conorii [all strains] and R. massiliae), North Asian tick typhus (R. sibirica), Japanese spotted fever (R. japonica), and Flinders Island spotted fever (R. honei) far outweigh the minor variations. The Rickettsiaceae that cause life-threatening infections are, in order of decreasing case-fatality rate, R. rickettsii (RMSF); R. prowazekii (louse-borne typhus); Orientia tsutsugamushi (scrub typhus); R. conorii (MSF); R. typhi (murine typhus); and, in rare cases, other spotted fever–group organisms. Some agents (e.g., R. parkeri, R. africae, R. akari, R. slovaca, R. honei, R. felis, R. massiliae, R. helvetica, R. heilongjiangensis, R. aeschlimannii, and R. monacensis) have never been documented to cause a fatal illness.
ROCKY MOUNTAIN SPOTTED FEVER
![]() Epidemiology RMSF occurs in 47 states (with the highest prevalence in the south-central and southeastern states) as well as in Canada, Mexico, and Central and South America. The infection is transmitted by Dermacentor variabilis, the American dog tick, in the eastern two-thirds of the United States and California; by D. andersoni, the Rocky Mountain wood tick, in the western United States; by Rhipicephalus sanguineus in Mexico, Arizona, and probably Brazil; and by Amblyomma cajennense and A. aureolatum in Central and/or South America. Maintained principally by transovarian transmission from one generation of ticks to the next, R. rickettsii can be acquired by uninfected ticks through the ingestion of a blood meal from rickettsemic small mammals.
Epidemiology RMSF occurs in 47 states (with the highest prevalence in the south-central and southeastern states) as well as in Canada, Mexico, and Central and South America. The infection is transmitted by Dermacentor variabilis, the American dog tick, in the eastern two-thirds of the United States and California; by D. andersoni, the Rocky Mountain wood tick, in the western United States; by Rhipicephalus sanguineus in Mexico, Arizona, and probably Brazil; and by Amblyomma cajennense and A. aureolatum in Central and/or South America. Maintained principally by transovarian transmission from one generation of ticks to the next, R. rickettsii can be acquired by uninfected ticks through the ingestion of a blood meal from rickettsemic small mammals.
Humans become infected during tick season (in the Northern Hemisphere, from May to September), although some cases occur in winter. The mortality rate was 20–25% in the preantibiotic era and remains at ~3–5%, principally because of delayed diagnosis and treatment. The case-fatality ratio increases with each decade of life above age 20.
Pathogenesis R. rickettsii organisms are inoculated into the dermis along with secretions of the tick’s salivary glands after ≥6 h of feeding. The rickettsiae spread lymphohematogenously throughout the body and infect numerous foci of contiguous endothelial cells. The dose-dependent incubation period is ~1 week (range, 2–14 days). Occlusive thrombosis and ischemic necrosis are not the fundamental pathologic bases for tissue and organ injury. Instead, increased vascular permeability, with resulting edema, hypovolemia, and ischemia, is responsible. Consumption of platelets results in thrombocytopenia in 32–52% of patients, but disseminated intravascular coagulation with hypofibrinogenemia is rare. Activation of platelets, generation of thrombin, and activation of the fibrinolytic system all appear to be homeostatic physiologic responses to endothelial injury.
Clinical Manifestations Early in the illness, when medical attention usually is first sought, RMSF is difficult to distinguish from many self-limiting viral illnesses. Fever, headache, malaise, myalgia, nausea, vomiting, and anorexia are the most common symptoms during the first 3 days. The patient becomes progressively more ill as vascular infection and injury advance. In one large series, only one-third of patients were diagnosed with presumptive RMSF early in the clinical course and treated appropriately as outpatients. In the tertiary-care setting, RMSF is all too often recognized only when late severe manifestations, developing at the end of the first week or during the second week of illness in patients without appropriate treatment, prompt return to a physician or hospital and admission to an intensive care unit.
The progressive nature of the infection is clearly manifested in the skin. Rash is evident in only 14% of patients on the first day of illness and in only 49% during the first 3 days. Macules (1–5 mm) appear first on the wrists and ankles and then on the remainder of the extremities and the trunk. Later, more severe vascular damage results in frank hemorrhage at the center of the maculopapule, producing a petechia that does not disappear upon compression (Fig. 211-1). This sequence of events is sometimes delayed or aborted by effective treatment. However, the rash is a variable manifestation, appearing on day 6 or later in 20% of cases and not appearing at all in 9–16% of cases. Petechiae occur in 41–59% of cases, appearing on or after day 6 in 74% of cases that manifest a rash. Involvement of the palms and soles, often considered diagnostically important, usually develops relatively late in the course (after day 5 in 43% of cases) and does not develop at all in 18–64% of cases.
FIGURE 211-1 Top: Petechial lesions of Rocky Mountain spotted fever on the lower legs and soles of a young, previously healthy patient. Bottom: Close-up of lesions from the same patient. (Photos courtesy of Dr. Lindsey Baden; with permission.)
Hypovolemia leads to prerenal azotemia and (in 17% of cases) hypotension. Infection of the pulmonary microcirculation leads to noncardiogenic pulmonary edema; 12% of patients have severe respiratory disease, and 8% require mechanical ventilation. Cardiac involvement manifests as dysrhythmia in 7–16% of cases.
Besides respiratory failure, central nervous system (CNS) involvement is the other important determinant of the outcome of RMSF. Encephalitis, presenting as confusion or lethargy, is apparent in 26–28% of cases. Progressively severe encephalitis manifests as stupor or delirium in 21–26% of cases, ataxia in 18%, coma in 10%, and seizures in 8%. Numerous focal neurologic deficits have been reported. Meningoencephalitis results in cerebrospinal fluid (CSF) pleocytosis in 34–38% of cases; usually there are 10–100 cells/μL and a mononuclear predominance, but occasionally there are >100 cells/μL and a polymorphonuclear predominance. The CSF protein concentration is increased in 30–35% of cases, but the CSF glucose concentration is usually normal.
Renal failure, often reversible with rehydration, is caused by acute tubular necrosis in severe cases with shock. Hepatic injury with increased serum aminotransferase concentrations (38% of cases) is due to focal death of individual hepatocytes without hepatic failure. Jaundice is recognized in 9% of cases and an elevated serum bilirubin concentration in 18–30%.
Life-threatening bleeding is rare. Anemia develops in 30% of cases and is severe enough to require transfusions in 11%. Blood is detected in the stool or vomitus of 10% of patients, and death has followed massive upper-gastrointestinal hemorrhage.
Other characteristic clinical laboratory findings include increased plasma levels of proteins of the acute-phase response (C-reactive protein, fibrinogen, ferritin, and others), hypoalbuminemia, and hyponatremia (in 56% of cases) due to the appropriate secretion of antidiuretic hormone in response to the hypovolemic state. Myositis occurs occasionally, with marked elevations in serum creatine kinase levels and multifocal rhabdomyonecrosis. Ocular involvement includes conjunctivitis in 30% of cases and retinal vein engorgement, flame hemorrhages, arterial occlusion, and papilledema with normal CSF pressure in some instances.
In untreated cases, the patient usually dies 8–15 days after onset. A rare presentation, fulminant RMSF, is fatal within 5 days after onset. This fulminant presentation is seen most often in male black patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency and may be related to an undefined effect of hemolysis on the rickettsial infection. Although survivors of RMSF usually return to their previous state of health, permanent sequelae, including neurologic deficits and gangrene necessitating amputation of extremities, may follow severe illness.
Diagnosis The diagnosis of RMSF during the acute stage is more difficult than is generally appreciated. The most important epidemiologic factor is a history of exposure to a potentially tick-infested environment within the 14 days preceding disease onset during a season of possible tick activity. However, only 60% of patients actually recall being bitten by a tick during the incubation period.
The differential diagnosis for early clinical manifestations of RMSF (fever, headache, and myalgia without a rash) includes influenza, enteroviral infection, infectious mononucleosis, viral hepatitis, leptospirosis, typhoid fever, gram-negative or gram-positive bacterial sepsis, HME, HGA, murine typhus, sylvatic flying-squirrel typhus, and rickettsialpox. Enterocolitis may be suggested by nausea, vomiting, and abdominal pain; prominence of abdominal tenderness has resulted in exploratory laparotomy. CNS involvement can masquerade as bacterial or viral meningoencephalitis. Cough, pulmonary signs, and chest radiographic opacities can lead to a diagnostic consideration of bronchitis or pneumonia.
At presentation during the first 3 days of illness, only 3% of patients exhibit the classic triad of fever, rash, and history of tick exposure. When a rash appears, a diagnosis of RMSF should be considered. However, many illnesses considered in the differential diagnosis also can be associated with a rash, including rubeola, rubella, meningococcemia, disseminated gonococcal infection, secondary syphilis, toxic shock syndrome, drug hypersensitivity, idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, Kawasaki syndrome, and immune complex vasculitis. Conversely, any person in an endemic area with a provisional diagnosis of one of the above illnesses could have RMSF. Thus, if a viral infection is suspected during RMSF season in an endemic area, it should always be kept in mind that RMSF can mimic viral infection early in the course; if the illness worsens over the next couple of days after initial presentation, the patient should return for reevaluation.
The most common serologic test for confirmation of the diagnosis is the indirect immunofluorescence assay. Not until 7–10 days after onset is a diagnostic titer of ≥64 usually detectable. The sensitivity and specificity of the indirect immunofluorescence IgG assay are 89–100% and 99–100%, respectively. It is important to understand that serologic tests for RMSF are usually negative at the time of presentation for medical care and that treatment should not be delayed while a positive serologic result is awaited.
The only diagnostic test that has proven useful during the acute illness is immunohistologic examination of a cutaneous biopsy sample from a rash lesion for R. rickettsii. Examination of a 3-mm punch biopsy from such a lesion is 70% sensitive and 100% specific. The sensitivity of polymerase chain reaction (PCR) amplification and detection of R. rickettsii DNA in peripheral blood is improving. However, although rickettsiae are present in large quantities in heavily infected foci of endothelial cells, there are relatively low quantities in the circulation. Cultivation of rickettsiae in cell culture is feasible but is seldom undertaken because of biohazard concerns. The recent dramatic increase in the reported incidence of RMSF correlates with the use of single-titer spotted fever–group cross-reactive enzyme immunoassay serology. Few cases are specifically determined to be caused by R. rickettsii. Currently, many febrile persons who do not have RMSF present with cross-reactive antibodies, possibly because of previous exposure to the highly prevalent spotted fever–group rickettsia R. amblyommii.
|
TREATMENT |
ROCKY MOUNTAIN SPOTTED FEVER |
The drug of choice for the treatment of both children and adults with RMSF is doxycycline, except when the patient is pregnant or allergic to this drug (see below). Because of the severity of RMSF, immediate empirical administration of doxycycline should be strongly considered for any patient with a consistent clinical presentation in the appropriate epidemiologic setting. Doxycycline is administered orally (or, in the presence of coma or vomiting, intravenously) at 200 mg/d in two divided doses. For children with suspected RMSF, up to five courses of doxycycline may be administered with minimal risk of dental staining. Other regimens include oral tetracycline (25–50 mg/kg per day) in four divided doses. Treatment with chloramphenicol, a less effective drug, is advised only for patients who are pregnant or allergic to doxycycline. The antirickettsial drug should be administered until the patient has been afebrile and improving clinically for 2–3 days. β-Lactam antibiotics, erythromycin, and aminoglycosides have no role in the treatment of RMSF, and sulfa-containing drugs are associated with more adverse outcomes than no treatment at all. There is little clinical experience with fluoroquinolones, clarithromycin, and azithromycin, which are not recommended. The most seriously ill patients are managed in intensive care units, with careful administration of fluids to achieve optimal tissue perfusion without precipitating noncardiogenic pulmonary edema. In some severely ill patients, hypoxemia requires intubation and mechanical ventilation; oliguric or anuric acute renal failure requires hemodialysis; seizures necessitate the use of antiseizure medication; anemia or severe hemorrhage necessitates transfusions of packed red blood cells; or bleeding with severe thrombocytopenia requires platelet transfusions. Heparin is not a useful component of treatment, and there is no evidence that glucocorticoids affect outcome.
Prevention Avoidance of tick bites is the only available preventive approach. Use of protective clothing and tick repellents, inspection of the body once or twice a day, and removal of ticks before they inoculate rickettsiae reduce the risk of infection. Prophylactic doxycycline treatment of tick bites has no proven role in preventing RMSF.
MEDITERRANEAN SPOTTED FEVER (BOUTONNEUSE FEVER), AFRICAN TICK-BITE FEVER, AND OTHER TICK-BORNE SPOTTED FEVERS
![]() Epidemiology R. conorii is prevalent in southern Europe, Africa, and southwestern and south-central Asia. Regional names for the disease caused by this organism include Mediterranean spotted fever, Kenya tick typhus, Indian tick typhus, Israeli spotted fever, and Astrakhan spotted fever. The disease is characterized by high fever, rash, and—in most geographic locales—an inoculation eschar (tâche noire) at the site of the tick bite. A severe form of the disease (mortality rate, 50%) occurs in patients with diabetes, alcoholism, or heart failure.
Epidemiology R. conorii is prevalent in southern Europe, Africa, and southwestern and south-central Asia. Regional names for the disease caused by this organism include Mediterranean spotted fever, Kenya tick typhus, Indian tick typhus, Israeli spotted fever, and Astrakhan spotted fever. The disease is characterized by high fever, rash, and—in most geographic locales—an inoculation eschar (tâche noire) at the site of the tick bite. A severe form of the disease (mortality rate, 50%) occurs in patients with diabetes, alcoholism, or heart failure.
African tick-bite fever, caused by R. africae, occurs in rural areas of sub-Saharan Africa and in the Caribbean islands and is transmitted by Amblyomma hebraeum and A. variegatum ticks. The average incubation period is 4–10 days. The mild illness consists of headache, fever, eschar, and regional lymphadenopathy. Amblyomma ticks often feed in groups, with the consequent development of multiple eschars. Rash may be vesicular, sparse, or absent altogether. Because of tourism in sub-Saharan Africa, African tick-bite fever is the rickettsiosis most frequently imported into Europe and North America. A similar disease caused by the closely related species R. parkeri is transmitted by A. maculatum in the United States and by A. triste in South America.
R. japonica causes Japanese spotted fever, which also occurs in Korea. Similar diseases in northern Asia are caused by R. sibirica and R. heilongjiangensis. Queensland tick typhus due to R. australis is transmitted by Ixodes holocyclus ticks. Flinders Island spotted fever, found on the island for which it is named as well as in Tasmania, mainland Australia, and Asia, is caused by R. honei. In Europe, patients infected with R. slovaca after a wintertime Dermacentor tick bite manifest an afebrile illness with an eschar (usually on the scalp) and painful regional lymphadenopathy.
Diagnosis Diagnosis of these tick-borne spotted fevers is based on clinical and epidemiologic findings and is confirmed by serology, immunohistochemical demonstration of rickettsiae in skin biopsy specimens, cell-culture isolation of rickettsiae, or PCR of skin biopsy, eschar, or blood samples. Serologic diagnosis detects antibodies to antigens shared among spotted fever–group rickettsiae, hindering identification of the etiologic species. In an endemic area, a possible diagnosis of rickettsial spotted fevers should be considered when patients present with fever, rash, and/or a skin lesion consisting of a black necrotic area or a crust surrounded by erythema.
|
TREATMENT |
TICK-BORNE SPOTTED FEVERS |
Successful therapeutic agents include doxycycline (100 mg bid orally for 1–5 days) and chloramphenicol (500 mg qid orally for 7–10 days). Pregnant patients may be treated with josamycin (3 g/d orally for 5 days). Data on the efficacy of treatment of mildly ill children with clarithromycin or azithromycin should not be extrapolated to adults or to patients with moderate or severe illness.
RICKETTSIALPOX
R. akari infects mice and their mites (Liponyssoides sanguineus), which maintain the organisms by transovarial transmission.
![]() Epidemiology Rickettsialpox is recognized principally in New York City, but cases have also been reported in other urban and rural locations in the United States and in Ukraine, Croatia, Mexico, and Turkey. Investigation of eschars suspected of representing bioterrorism-associated cutaneous anthrax revealed that rickettsialpox occurs more frequently than previously realized.
Epidemiology Rickettsialpox is recognized principally in New York City, but cases have also been reported in other urban and rural locations in the United States and in Ukraine, Croatia, Mexico, and Turkey. Investigation of eschars suspected of representing bioterrorism-associated cutaneous anthrax revealed that rickettsialpox occurs more frequently than previously realized.
Clinical Manifestations A papule forms at the site of the mite’s feeding, develops a central vesicle, and becomes a 1- to 2.5-cm painless black crusted eschar surrounded by an erythematous halo (Fig. 211-2). Enlargement of the regional lymph nodes draining the eschar suggests initial lymphogenous spread. After an incubation period of 10–17 days, during which the eschar and regional lymphadenopathy frequently go unnoticed, disease onset is marked by malaise, chills, fever, headache, and myalgia. A macular rash appears 2–6 days after onset and usually evolves sequentially into papules, vesicles, and crusts that heal without scarring (Fig. 211-3); in some cases, the rash remains macular or maculopapular. Some patients develop nausea, vomiting, abdominal pain, cough, conjunctivitis, or photophobia. Without treatment, fever lasts 6–10 days.
FIGURE 211-2 Eschar at the site of the mite bite in a patient with rickettsialpox. (Reprinted from A Krusell et al: Emerg Infect Dis 8:727, 2002. Photo obtained by Dr. Kenneth Kaye.)
FIGURE 211-3 Top: Papulovesicular lesions on the trunk of the patient with rickettsialpox shown in Fig. 211-2. Bottom: Close-up of lesions from the same patient. (Reprinted from A Krusell et al: Emerg Infect Dis 8:727, 2002. Photos obtained by Dr. Kenneth Kaye.)
Diagnosis and Treatment Clinical, epidemiologic, and convalescent serologic data establish the diagnosis of a spotted fever–group rickettsiosis that is seldom pursued further. Doxycycline is the drug of choice for treatment.
FLEA-BORNE SPOTTED FEVER
![]() An emerging rickettsiosis caused by R. felis occurs worldwide. Maintained transovarially in the geographically widespread cat flea Ctenocephalides felis, the infection has been described as moderately severe, with fever, rash, and headache as well as CNS, gastrointestinal, and pulmonary symptoms.
An emerging rickettsiosis caused by R. felis occurs worldwide. Maintained transovarially in the geographically widespread cat flea Ctenocephalides felis, the infection has been described as moderately severe, with fever, rash, and headache as well as CNS, gastrointestinal, and pulmonary symptoms.
EPIDEMIC (LOUSE-BORNE) TYPHUS
Epidemiology The human body louse (Pediculus humanus corporis) lives in clothing under poor hygienic conditions and usually in impoverished cold areas. Lice acquire R. prowazekii when they ingest blood from a rickettsemic patient. The rickettsiae multiply in the louse’s midgut epithelial cells and are shed in its feces. The infected louse leaves a febrile person and deposits infected feces on its subsequent host during its blood meal; the patient autoinoculates the organisms by scratching. The louse is killed by the rickettsiae and does not pass R. prowazekii to its offspring.
![]() Epidemic typhus haunts regions afflicted by wars and disasters. An outbreak involved 100,000 people in refugee camps in Burundi in 1997. A small focus was documented in Russia in 1998; sporadic cases were reported from Algeria, and frequent outbreaks occurred in Peru. Eastern flying squirrels (Glaucomys volans) and their lice and fleas maintain R. prowazekii in a zoonotic cycle.
Epidemic typhus haunts regions afflicted by wars and disasters. An outbreak involved 100,000 people in refugee camps in Burundi in 1997. A small focus was documented in Russia in 1998; sporadic cases were reported from Algeria, and frequent outbreaks occurred in Peru. Eastern flying squirrels (Glaucomys volans) and their lice and fleas maintain R. prowazekii in a zoonotic cycle.
Brill-Zinsser disease is a recrudescent illness occurring years after acute epidemic typhus, probably as a result of waning immunity. R. prowazekii remains latent for years; its reactivation results in sporadic cases of disease in louse-free populations or in epidemics in louse-infested populations.
Rickettsiae are potential agents of bioterrorism (Chap. 261e). Infections with R. prowazekii and R. rickettsii have high case–fatality ratios. These organisms cause difficult-to-diagnose diseases and are highly infectious when inhaled as aerosols. Organisms resistant to tetracycline or chloramphenicol have been developed in the laboratory.
Clinical Manifestations After an incubation period of ~1–2 weeks, the onset of illness is abrupt, with prostration, severe headache, and fever rising rapidly to 38.8°–40.0°C (102°–104°F). Cough is prominent, developing in 70% of patients. Myalgias are usually severe. A rash begins on the upper trunk, usually on the fifth day, and then becomes generalized, involving the entire body except the face, palms, and soles. Initially, this rash is macular; without treatment, it becomes maculopapular, petechial, and confluent. The rash often goes undetected in black skin; 60% of African patients have spotless epidemic typhus. Photophobia, with considerable conjunctival injection and eye pain, is common. The tongue may be dry, brown, and furred. Confusion and coma are common. Skin necrosis and gangrene of the digits as well as interstitial pneumonia may occur in severe cases. Untreated disease is fatal in 7–40% of cases, with outcome depending primarily on the condition of the host. Patients with untreated infections develop renal insufficiency and multiorgan involvement in which neurologic manifestations are frequently prominent. Overall, 12% of patients with epidemic typhus have neurologic involvement. Infection associated with North American flying squirrels is a milder illness; whether this milder disease is due to host factors (e.g., better health status) or attenuated virulence is unknown.
Diagnosis and Treatment Epidemic typhus is sometimes misdiagnosed as typhoid fever in tropical countries (Chap. 190). The means even for serologic studies are often unavailable in settings of louse-borne typhus. Epidemics can be recognized by the serologic or immunohistochemical diagnosis of a single case or by detection of R. prowazekii in a louse found on a patient. Doxycycline (200 mg/d, given in two divided doses) is administered orally or—if the patient is comatose or vomiting—intravenously. Although under epidemic conditions a single 200-mg oral dose is effective, treatment is generally continued until 2–3 days after defervescence. Pregnant patients should be evaluated individually and treated with chloramphenicol early in pregnancy or, if necessary, with doxycycline late in pregnancy.
Prevention Prevention of epidemic typhus involves control of body lice. Clothes should be changed regularly, and insecticides should be used every 6 weeks to control the louse population.
ENDEMIC MURINE TYPHUS
Epidemiology R. typhi is maintained in mammalian host/flea cycles, with rats (Rattus rattus and R. norvegicus) and the Oriental rat flea (Xenopsylla cheopis) as the classic zoonotic niche. Fleas acquire R. typhi from rickettsemic rats and carry the organism throughout their life span. Nonimmune rats and humans are infected when rickettsia-laden flea feces contaminate pruritic bite lesions; less frequently, the flea bite transmits the organisms. Transmission can also occur via inhalation of aerosolized rickettsiae from flea feces. Infected rats appear healthy, although they are rickettsemic for ~2 weeks.
![]() Murine typhus occurs mainly in Texas and southern California, where the classic rat/flea cycle is absent and an opossum/cat flea (C. felis) cycle is prominent. Globally, endemic typhus occurs mainly in warm (often coastal) areas throughout the tropics and subtropics, where it is highly prevalent though often unrecognized. The incidence peaks from April through June in southern Texas and during the warm months of summer and early fall in other geographic locations. Patients seldom recall exposure to fleas, although exposure to animals such as cats, opossums, and rats is reported in nearly 40% of cases.
Murine typhus occurs mainly in Texas and southern California, where the classic rat/flea cycle is absent and an opossum/cat flea (C. felis) cycle is prominent. Globally, endemic typhus occurs mainly in warm (often coastal) areas throughout the tropics and subtropics, where it is highly prevalent though often unrecognized. The incidence peaks from April through June in southern Texas and during the warm months of summer and early fall in other geographic locations. Patients seldom recall exposure to fleas, although exposure to animals such as cats, opossums, and rats is reported in nearly 40% of cases.
Clinical Manifestations The incubation period of experimental murine typhus averages 11 days (range, 8–16 days). Headache, myalgia, arthralgia, nausea, and malaise develop 1–3 days before onset of chills and fever. Nearly all patients experience nausea and vomiting early in the illness.
The duration of untreated illness averages 12 days (range, 9–18 days). Rash is present in only 13% of patients at presentation for medical care (usually ~4 days after onset of fever), appearing an average of 2 days later in half of the remaining patients and never appearing in the others. The initial macular rash is often detected by careful inspection of the axilla or the inner surface of the arm. Subsequently, the rash becomes maculopapular, involving the trunk more often than the extremities; it is seldom petechial and rarely involves the face, palms, or soles. A rash is detected in only 20% of patients with darkly pigmented skin.
Pulmonary involvement is frequently prominent; 35% of patients have a hacking, nonproductive cough, and 23% of patients who undergo chest radiography have pulmonary densities due to interstitial pneumonia, pulmonary edema, and pleural effusions. Bibasilar rales are the most common pulmonary sign. Less common clinical manifestations include abdominal pain, confusion, stupor, seizures, ataxia, coma, and jaundice. Clinical laboratory studies frequently reveal anemia and leukopenia early in the course, leukocytosis late in the course, thrombocytopenia, hyponatremia, hypoalbuminemia, mildly increased serum hepatic aminotransferases, and prerenal azotemia. Complications can include respiratory failure, hematemesis, cerebral hemorrhage, and hemolysis. Severe illness necessitates the admission of 10% of hospitalized patients to an intensive care unit. Greater severity is generally associated with old age, underlying disease, and treatment with a sulfonamide; the case-fatality rate is 1%. In a study of children with murine typhus, 50% suffered only nocturnal fevers, feeling well enough for active daytime play.
Diagnosis and Treatment Serologic studies of acute- and convalescent-phase sera can provide a diagnosis, and an immunohistochemical method for identification of typhus group-specific antigens in biopsy samples has been developed. Cultivation and PCR are used only infrequently and are not widely available. Nevertheless, most patients are treated empirically with doxycycline (100 mg bid orally for 7–15 days) on the basis of clinical suspicion. Ciprofloxacin provides an alternative if doxycycline is contraindicated.
SCRUB TYPHUS
Epidemiology O. tsutsugamushi differs substantially from Rickettsia species both genetically and in cell wall composition (i.e., it lacks lipopolysaccharide). O. tsutsugamushi is maintained by transovarial transmission in trombiculid mites. After hatching, infected larval mites (chiggers, the only stage that feeds on a host) inoculate organisms into the skin. Infected chiggers are particularly likely to be found in areas of heavy scrub vegetation during the wet season, when mites lay eggs.
![]() Scrub typhus is endemic and reemerging in eastern and southern Asia, northern Australia, and islands of the western Pacific and Indian Oceans. Infections are prevalent in these regions; in some areas, >3% of the population is infected or reinfected each month. Immunity wanes over 1–3 years, and the organism exhibits remarkable antigenic diversity.
Scrub typhus is endemic and reemerging in eastern and southern Asia, northern Australia, and islands of the western Pacific and Indian Oceans. Infections are prevalent in these regions; in some areas, >3% of the population is infected or reinfected each month. Immunity wanes over 1–3 years, and the organism exhibits remarkable antigenic diversity.
Clinical Manifestations Illness varies from mild and self-limiting to fatal. After an incubation period of 6–21 days, onset is characterized by fever, headache, myalgia, cough, and gastrointestinal symptoms. Some patients recover spontaneously after a few days. The classic case description includes an eschar where the chigger has fed, regional lymphadenopathy, and a maculopapular rash—signs that are seldom seen in indigenous patients. Fewer than 50% of Westerners develop an eschar, and fewer than 40% develop a rash (on day 4–6 of illness). Severe cases typically manifest with encephalitis and interstitial pneumonia due to vascular injury. The case-fatality rate for untreated classic cases is 7% but would probably be lower if all mild cases were diagnosed.
Diagnosis and Treatment Serologic assays (indirect fluorescent antibody, indirect immunoperoxidase, and enzyme immunoassays) are the mainstays of laboratory diagnosis. PCR amplification of Orientia genes from eschars and blood also is effective. Patients are treated with doxycycline (100 mg bid orally for 7–15 days), azithromycin (500 mg orally for 3 days), or chloramphenicol (500 mg qid orally for 7–15 days). Some cases of scrub typhus in Thailand are caused by strains that have high doxycycline or chloramphenicol minimal inhibitory concentrations (MICs) but that are susceptible to azithromycin and rifampin.
EHRLICHIOSES AND ANAPLASMOSIS
Ehrlichioses are acute febrile infections caused by members of the family Anaplasmataceae, which is made up of obligately intracellular organisms of five genera: Ehrlichia, Anaplasma, Wolbachia, Candidatus Neoehrlichia, and Neorickettsia. The bacteria reside in vertebrate reservoirs and target vacuoles of hematopoietic cells (Fig. 211-4). Three Ehrlichia species and one Anaplasma species are transmitted by ticks to humans and cause infection that can be severe and prevalent. E. chaffeensis, the agent of HME, and an E. muris–like agent (EMLA) infect predominantly mononuclear phagocytes; E. ewingii and A. phagocytophilum infect neutrophils. Infection with Candidatus Neoehrlichia mikurensis is less well characterized, but the agent has been identified in human blood neutrophils.
FIGURE 211-4 Peripheral-blood smear from a patient with human granulocytotropic anaplasmosis. A neutrophil contains two morulae (vacuoles filled with A. phagocytophilum). (Photo courtesy of Dr. J. Stephen Dumler.)
Ehrlichia, Candidatus Neoehrlichia, and Anaplasma are maintained by horizontal tick-mammal-tick transmission, and humans are only inadvertently infected. Wolbachiae are associated with human filariasis, since they are important for filarial viability and pathogenicity; antibiotic treatment targeting wolbachiae is a strategy for filariasis control. Neorickettsiae parasitize flukes (trematodes) that in turn parasitize aquatic snails, fish, and insects. Only a single human neo-rickettsiosis has been described: sennetsu fever, an infectious mononucleosis–like illness that was first identified in 1953 and is associated with the ingestion of raw fish containing N. sennetsu–infected flukes.
HUMAN MONOCYTOTROPIC EHRLICHIOSIS
Epidemiology More than 8404 cases of E. chaffeensis infection had been reported to the Centers for Disease Control and Prevention (CDC) as of April 2013. However, active prospective surveillance has documented an incidence as high as 414 cases per 100,000 population in some U.S. regions. Most E. chaffeensis infections are identified in the south-central, southeastern, and mid-Atlantic states, but cases have also been recognized in California and New York. All stages of the Lone Star tick (A. americanum) feed on white-tailed deer—a major reservoir. Dogs and coyotes also serve as reservoirs and often lack clinical signs. Tick bites and exposures are frequently reported by patients in rural areas, especially in May through July. The median age of HME patients is 52 years; however, severe and fatal infections in children also are well recognized. Of patients with HME, 60% are male.
![]() E. chaffeensis has been detected in South America, Africa, and Asia.
E. chaffeensis has been detected in South America, Africa, and Asia.
Clinical Manifestations E. chaffeensis disseminates hematogenously from the dermal blood pool created by the feeding tick. After a median incubation period of 8 days, illness develops. Clinical manifestations are undifferentiated and include fever (96% of cases), headache (72%), myalgia (68%), and malaise (77%). Less frequently observed are nausea, vomiting, and diarrhea (25–57%); cough (28%); rash (26% overall, 6% at presentation); and confusion (20%). HME can be severe: 49% of patients with documented cases are hospitalized, and ~2% die. Severe manifestations include a toxic shock–like or septic shock–like syndrome, adult respiratory distress syndrome, cardiac failure, hepatitis, meningoencephalitis, hemorrhage, and—in immunocompromised patients—overwhelming ehrlichial infection. Laboratory findings are valuable in the differential diagnosis of HME; 61% of patients have leukopenia (initially lymphopenia, later neutropenia), 73% have thrombocytopenia, and 84% have elevated serum levels of hepatic aminotransferases. Despite low blood cell counts, the bone marrow is hypercellular, and noncaseating granulomas can be present. Vasculitis is not a component of HME.
Diagnosis HME can be fatal. Early empirical antibiotic therapy based on clinical diagnosis diminishes adverse outcomes. This diagnosis is suggested by fever with a known tick exposure during the preceding 3 weeks, thrombocytopenia and/or leukopenia, and increased serum aminotransferase levels. Morulae are demonstrated in <10% of peripheral-blood smears. HME can be confirmed during active infection by PCR amplification of E. chaffeensis nucleic acids in blood obtained before the start of doxycycline therapy. Retrospective sero-diagnosis requires a consistent clinical picture and a fourfold increase in E. chaffeensis antibody titer to ≥64 in paired sera obtained ~3 weeks apart. Separate specific diagnostic tests are necessary for HME and HGA.
EWINGII EHRLICHIOSIS AND EHRLICHIA MURIS–LIKE INFECTIONS
Ehrlichia ewingii, originally a neutrophil pathogen causing fever and lameness in dogs, resembles E. chaffeensis in its tick vector (A. americanum) and vertebrate reservoirs (white-tailed deer and dogs). An E. muris–like agent (EMLA) has been discovered and identified as the cause of human infections in Wisconsin and Minnesota. E. ewingii and EMLA illnesses are similar to but less severe than HME. Many cases occur in immunocompromised patients. No specific serologic diagnostic tests for ewingii or EMLA ehrlichiosis are readily available.
CANDIDATUS NEOEHRLICHIA MIKURENSIS INFECTION
![]() Candidatus Neoehrlichia mikurensis, a bacterium in a phylogenetic clade between Ehrlichia and Anaplasma, was originally identified in Ixodes ricinus ticks from the Netherlands and in mice and Ixodes ovatus ticks from Japan. By means of broad-range 16S rRNA gene amplification and sequence analysis, this organism was identified as the cause of severe and sometimes prolonged febrile illnesses in European immunocompromised patients with tick bites or exposures and in Chinese patients with a mild febrile illness after being bitten by Ixodes persulcatus and Haemaphysalis concinna ticks. The clinical presentation is similar to those of HME and HGA. Specific diagnostic methods have been developed but are not widely available.
Candidatus Neoehrlichia mikurensis, a bacterium in a phylogenetic clade between Ehrlichia and Anaplasma, was originally identified in Ixodes ricinus ticks from the Netherlands and in mice and Ixodes ovatus ticks from Japan. By means of broad-range 16S rRNA gene amplification and sequence analysis, this organism was identified as the cause of severe and sometimes prolonged febrile illnesses in European immunocompromised patients with tick bites or exposures and in Chinese patients with a mild febrile illness after being bitten by Ixodes persulcatus and Haemaphysalis concinna ticks. The clinical presentation is similar to those of HME and HGA. Specific diagnostic methods have been developed but are not widely available.
|
TREATMENT |
EHRLICHIOSES |
Doxycycline is effective for HME as well as for ewingii and EMLA ehrlichioses; the use of this drug in Candidatus N. mikurensis infection is associated with disease resolution. Therapy with doxycycline (100 mg given PO or IV twice daily) or tetracycline (250–500 mg given PO every 6 h) lowers hospitalization rates and shortens fever duration. E. chaffeensis is not susceptible to chloramphenicol in vitro, and the use of this drug is controversial. While a few reports document E. chaffeensis persistence in humans, this finding is rare; most infections are cured by short courses of doxycycline (continuing for 3–5 days after defervescence). Although poorly studied, rifampin may be suitable when doxycycline is contraindicated.
PREVENTION
HME, ewingii ehrlichiosis, EMLA infection, and Candidatus N. mikurensis infection can be prevented by the avoidance of ticks in endemic areas. The use of protective clothing and tick repellents, careful postexposure tick searches, and prompt removal of attached ticks probably diminish infection risk.
HUMAN GRANULOCYTOTROPIC ANAPLASMOSIS
![]() Epidemiology As of April 2013, 10,181 cases of HGA had been reported to the CDC, most in the upper midwestern and northeastern United States; the geographic distribution is similar to that for Lyme disease because of the shared I. scapularis tick vector. White-footed mice, squirrels, and white-tailed deer in the United States and red deer in Europe are natural reservoirs for A. phagocytophilum. HGA incidence peaks in May through July, but the disease can occur throughout the year with exposure to Ixodes ticks. HGA often affects males (59%) and older persons (median age, 51 years).
Epidemiology As of April 2013, 10,181 cases of HGA had been reported to the CDC, most in the upper midwestern and northeastern United States; the geographic distribution is similar to that for Lyme disease because of the shared I. scapularis tick vector. White-footed mice, squirrels, and white-tailed deer in the United States and red deer in Europe are natural reservoirs for A. phagocytophilum. HGA incidence peaks in May through July, but the disease can occur throughout the year with exposure to Ixodes ticks. HGA often affects males (59%) and older persons (median age, 51 years).
Clinical Manifestations Seroprevalence rates are high in endemic regions; thus it seems likely that most individuals develop subclinical infections. The incubation period for HGA is 4–8 days, after which the disease manifests as fever (75–100% of cases), myalgia (77%), headache (82%), and malaise (97%). A minority of patients develop nausea, vomiting, or diarrhea (22–39%); cough (27%); or confusion (17%). Rash (6%) is almost invariably concurrent erythema migrans attributable to Lyme disease. Most patients develop thrombocytopenia (75%) and/or leukopenia (55%) with increased serum hepatic aminotransferase levels (83%).
Severe complications occur most often in the elderly and include adult respiratory distress syndrome, a toxic shock–like syndrome, and life-threatening opportunistic infections. Meningoencephalitis is rarely documented with HGA, but brachial plexopathy, cranial nerve involvement, and demyelinating polyneuropathy are reported. For HGA, 7% of patients require intensive care, and the case-fatality rate is 0.6%. Neither vasculitis nor granulomas are components of HGA. While co-infections with Borrelia burgdorferi and Babesia microti (transmitted by the same tick vector[s]) occur, there is little evidence of comorbidity or persistence. HGA is rarely acquired via transfusion.
Diagnosis HGA should be included in the differential diagnosis of influenza-like illnesses during seasons with Ixodes tick activity (May through December), especially with known tick bite or exposure. Concurrent thrombocytopenia, leukopenia, or elevated serum levels of alanine or aspartate aminotransferase further increase the likelihood of HGA. Many HGA patients develop Lyme disease antibodies in the absence of clinical findings consistent with that diagnosis. Thus, HGA should be considered in the differential diagnosis of atypical severe Lyme disease presentations. Peripheral-blood film examination for neutrophil morulae can yield a diagnosis in 20–75% of infections. PCR testing of blood from patients with active disease before doxycycline therapy is sensitive and specific. Serodiagnosis is retrospective, requiring a four-fold increase in A. phagocytophilum antibody titer (to ≥160) in paired serum samples obtained 1 month apart. Since seroprevalence is high in some regions, a single acute-phase titer should not be used for diagnosis.
|
TREATMENT |
HUMAN GRANULOCYTOTROPIC ANAPLASMOSIS |
No prospective studies of therapy for HGA have been conducted. However, doxycycline (100 mg PO twice daily) is effective. Rifampin therapy is associated with improvement of HGA in pregnant women and children. Most treated patients defervesce within 24–48 h.
Prevention HGA prevention requires tick avoidance. Transmission can be documented as few as 4 h after a tick bite.
Q FEVER
The agent of Q fever is Coxiella burnetii, a small intracellular prokaryote that only recently was grown in cell-free medium. C. burnetii, a pleomorphic coccobacillus with a gram-negative cell wall, survives in harsh environments; it escapes intracellular killing in macrophages by inhibiting the final step in phagosome maturation (cathepsin fusion) and has adapted to the acidic phagolysosome by producing superoxide dismutase. Infection with C. burnetii induces a range of immunomodulatory responses, from immunosuppression in chronic Q fever to the production of autoantibodies, particularly those to smooth muscle and cardiac muscle.
Q fever encompasses two broad clinical syndromes: acute and chronic infection. The host’s immune response (rather than the particular strain) most likely determines whether chronic Q fever develops. C. burnetii survives in monocytes from patients with chronic Q fever but not in monocytes from patients with acute Q fever or from uninfected subjects. Impairment of the bactericidal activity of the C. burnetii–infected monocyte is associated with overproduction of interleukin 10. The CD4+/CD8+ ratio is decreased in Q fever endocarditis. Very few organisms and a strong cellular response are observed in patients with acute Q fever, while many organisms and a moderate cellular response occur in chronic Q fever. Immune control of C. burnetii is T cell–dependent, but 80–90% of bone marrow aspirates obtained years after recovery from Q fever contain C. burnetii DNA. C. burnetii’s ready multiplication within trophoblasts accounts for the high concentrations it can reach in the placenta.
Epidemiology Q fever is a zoonosis. The primary sources of human infection are infected cattle, sheep, and goats. However, cats, rabbits, pigeons, and dogs also serve as sources for transmission of C. burnetii to humans. The wildlife reservoir is extensive and includes ticks, coyotes, gray foxes, skunks, raccoons, rabbits, deer, mice, bears, birds, and opossums. In female animals C. burnetii localizes to the uterus and mammary glands. Infection is reactivated during pregnancy and after radiotherapy in mouse models. High concentrations of C. burnetii are found in the placenta. At the time of parturition, the bacteria are released into the air, and infection follows inhalation of aerosolized organisms by a susceptible host. Windstorms can generate C. burnetii aerosols months after soil contamination during parturition. Individuals up to 18 km from the source have been infected. Because it is easily dispersed as an aerosol, C. burnetii is a potential agent of bioterrorism (Chap. 261e), with a high infectivity rate and pneumonia as the major manifestation.
Determining the source of an outbreak of Q fever can be challenging. An outbreak of Q fever at a horse-boarding ranch in Colorado in 2005 was due to spread of infection from two herds of goats that had been acquired by the owners. PCR testing confirmed the presence of C. burnetii in the soil and among the goats. Of 138 persons who lived within 1 mile of the ranch and who were also tested, 11 (8%) had evidence of C. burnetii infection, and 8 of these 11 individuals had no direct contact with the ranch.
Persons at risk for Q fever include abattoir workers, veterinarians, farmers, and other individuals who have contact with infected animals (particularly newborn animals) or products of conception. The organism is shed in milk for weeks to months after parturition. The ingestion of contaminated milk in some geographic areas probably represents a major route of transmission to humans. A recent outbreak of Q fever associated with ingestion of raw milk confirms the oral route of transmission. In rare instances, person-to-person transmission follows labor and childbirth in an infected woman, autopsy of an infected individual, or blood transfusion. Some evidence suggests that C. burnetii can be sexually transmitted among humans. Crushing an infected tick between the fingers has resulted in Q fever; the implication is that percutaneous transmission can occur.
![]() Infections due to C. burnetii occur in most geographic locations except New Zealand and Antarctica. Thus Q fever can be associated with travel. The number of reported cases of Q fever in the United States ranges from 28 to 54 per year. More than 70% of these cases occur in males, and April, May, and June are the most common months for acquisition. Q fever continues to be common in Australia, with 30 cases per 1 million population per year. Cases among abattoir workers in Australia declined dramatically as a result of a vaccination program. An outbreak of Q fever began in the Netherlands in 2007, and by 2010 more than 4000 cases had been reported. Pneumonia was a common manifestation in this outbreak. The outbreak was due to a combination of high-density goat farming in areas abutting large urban populations and environmental factors. Farms where spread did not occur had high vegetation densities and lower groundwater concentrations.
Infections due to C. burnetii occur in most geographic locations except New Zealand and Antarctica. Thus Q fever can be associated with travel. The number of reported cases of Q fever in the United States ranges from 28 to 54 per year. More than 70% of these cases occur in males, and April, May, and June are the most common months for acquisition. Q fever continues to be common in Australia, with 30 cases per 1 million population per year. Cases among abattoir workers in Australia declined dramatically as a result of a vaccination program. An outbreak of Q fever began in the Netherlands in 2007, and by 2010 more than 4000 cases had been reported. Pneumonia was a common manifestation in this outbreak. The outbreak was due to a combination of high-density goat farming in areas abutting large urban populations and environmental factors. Farms where spread did not occur had high vegetation densities and lower groundwater concentrations.
The primary manifestations of acute Q fever differ geographically (e.g., pneumonia in Nova Scotia and granulomatous hepatitis in Marseille). These differences could reflect the route of infection (i.e., ingestion of contaminated milk for hepatitis and inhalation of contaminated aerosols for pneumonia) or strain differences. In the Netherlands outbreak, sequelae of infection in pregnant women were rare; this was not the case among pregnant women elsewhere.
Young age seems to be protective against disease caused by C. burnetii. In a large outbreak in Switzerland, symptomatic infection occurred five times more often among persons >15 years of age than among younger individuals. In many outbreaks, men are affected more commonly than women; the proposed explanation is that female hormones are partially protective.
Clinical Manifestations • ACUTE Q FEVER The symptoms of acute Q fever are nonspecific; common among them are fever, extreme fatigue, photophobia, and severe headache that is frequently retro-orbital. Other symptoms include chills, sweats, nausea, vomiting, and diarrhea, each occurring in 5–20% of cases. Cough develops in about half of patients with Q fever pneumonia. Neurologic manifestations of acute Q fever are uncommon; however, in one outbreak in the United Kingdom, 23% of 102 patients had neurologic signs and symptoms as the major manifestation. A nonspecific rash may be evident in 4–18% of patients. The WBC count is usually normal. Thrombocytopenia occurs in ~25% of patients, and reactive thrombocytosis (with platelet counts exceeding 106/μL) frequently develops during recovery. Chest radiography can show opacities similar to those seen in pneumonia caused by other pathogens, but multiple rounded opacities in patients in endemic areas suggest a diagnosis of Q fever pneumonia.
Acute Q fever occasionally complicates pregnancy. In one series, it resulted in premature birth in 35% of cases and in abortion or neonatal death in 43%. Neonatal death (previous or current) and lower infant birth weight are three times more likely among women seropositive for C. burnetii.
![]() After the usual incubation period of 3–30 days, 1070 patients with acute Q fever in southern France presented with hepatitis (40%), both pneumonia and hepatitis (20%), pneumonia (17%), isolated fever (14%), CNS involvement (2%), and pericarditis or myocarditis (1%). Acalculous cholecystitis, pancreatitis, lymphadenopathy, spontaneous rupture of the spleen, transient hypoplastic anemia, bone marrow necrosis, hemolytic anemia, histiocytic hemophagocytosis, optic neuritis, and erythema nodosum were less common manifestations.
After the usual incubation period of 3–30 days, 1070 patients with acute Q fever in southern France presented with hepatitis (40%), both pneumonia and hepatitis (20%), pneumonia (17%), isolated fever (14%), CNS involvement (2%), and pericarditis or myocarditis (1%). Acalculous cholecystitis, pancreatitis, lymphadenopathy, spontaneous rupture of the spleen, transient hypoplastic anemia, bone marrow necrosis, hemolytic anemia, histiocytic hemophagocytosis, optic neuritis, and erythema nodosum were less common manifestations.
POST–Q FEVER FATIGUE SYNDROME Prolonged fatigue can follow Q fever and can be accompanied by a constellation of symptoms including headaches, sweats, arthralgia, myalgias, blurred vision, muscle fasciculations, and enlarged and painful lymph nodes. Long-term persistence of a noninfective, nonbiodegraded complex of Coxiella cell components, with its antigens and specific lipopolysaccharide, has been detected in the affected persons. Patients who develop this syndrome have a higher frequency of carriage of HLA-DRB1*11 and of the 2/2 genotype of the interferon γ intron 1 microsatellite.
CHRONIC Q FEVER Chronic Q fever almost always implies endocarditis and usually occurs in patients with previous valvular heart disease, immunosuppression, or chronic renal insufficiency. Fever is usually absent or low grade. Valvular vegetations are detected in only 12% of patients by transthoracic echocardiography, but the rate of detection is higher (21–50%) with transesophageal echocardiography. The vegetations in chronic Q fever endocarditis differ from those in bacterial endocarditis, manifesting as endothelium-covered nodules on the valves. A high index of suspicion is necessary for timely diagnosis. Patients with chronic Q fever are often ill for >1 year before the diagnosis is made. The disease should be suspected in all patients with culture-negative endocarditis. In addition, all patients with valvular heart disease and an unexplained purpuric eruption, renal insufficiency, stroke, and/or progressive heart failure should be tested for C. burnetii infection. Patients with chronic Q fever have hepatomegaly and/or splenomegaly, which—in combination with rheumatoid factor, elevated erythrocyte sedimentation rate, high C-reactive protein level, and/or increased γ-globulin concentrations (up to 60–70 g/L)—suggests this diagnosis. Other manifestations of chronic Q fever include infection of vascular prostheses, aneurysms, and bone as well as chronic sternal wound infection. Unusual manifestations include chronic thrombocytopenia, mixed cryoglobulinemia, and livedo reticularis.
Diagnosis Isolation of C. burnetii from buffy-coat blood samples or tissue specimens by a shell-vial technique is easy but requires a biosafety level 3 laboratory. PCR detects C. burnetii DNA in tissue specimens, including paraffin-embedded samples. Serology is the most commonly used diagnostic tool. Indirect immunofluorescence is sensitive and specific and is the method of choice. Rheumatoid factor should be adsorbed from the specimen before testing. With chronic infection, the titer to phase I antigen is usually much higher than that to phase II antigen (i.e., C. burnetii that has truncated lipopolysaccharide associated with gene deletions during laboratory passages), and the diagnosis should not be based on serology alone. Rather, the entire clinical setting must be taken into consideration. An anti–phase I IgG titer of ≥6400 would be considered a major criterion for the diagnosis of chronic Q fever, while a titer of ≥800 but ≤6400 would be a minor criterion. In acute Q fever, a fourfold rise in titer can be demonstrated between acute- and convalescent-phase serum samples.
Fluorodeoxyglucose positron emission tomography combined with CT (FDG-PET/CT) can be useful because it can detect not only valvular infection but also intravascular infection elsewhere as well as osteomyelitis.
|
TREATMENT |
Q FEVER |
ANTIBIOTICS
Treatment of acute Q fever with doxycycline (100 mg twice daily for 14 days) is usually successful. Quinolones also are effective. When Q fever is diagnosed during pregnancy, treatment with trimethoprim-sulfamethoxazole (TMP-SMX) is recommended for the duration of the pregnancy. One study showed no intrauterine fetal deaths and substantial reduction of obstetric complications in a group of Q fever patients treated with TMP-SMX.
The treatment of chronic Q fever is difficult and requires careful follow-up. Addition of hydroxychloroquine (to alkalinize the phagolysosome) renders doxycycline bactericidal against C. burnetii, and this combination is currently the favored regimen. Treatment with doxycycline (100 mg bid) and hydroxychloroquine (200 mg tid; plasma concentration maintained at 0.8–1.2 μg/mL) for 18 months is superior to a regimen of doxycycline and ofloxacin. Among 21 patients who received doxycycline and hydroxychloroquine, 1 died of a surgical complication, 2 were still being treated at the end of the study, 1 was still being evaluated, and 17 were cured. The mean duration of treatment was 31 months. In the ofloxacin and doxycycline group of 14 patients, 1 had died, 1 was still being treated, 7 had relapsed, and 5 had been cured by the end of the study. Optimal management of Q fever endocarditis entails determining the MIC of doxycycline for the patient’s isolate and measuring serum doxycycline levels. A serum level–to–doxycycline MIC ratio of ≥1 is associated with a rapid decline in phase I antibodies with the doxycycline-hydroxychloroquine regimen. Patients treated with this regimen must be advised about photosensitivity and retinal toxicity risks. The doxycycline-hydroxychloroquine regimen was successful in one patient with HIV infection and Q fever endocarditis. The Jarisch-Herxheimer reaction occasionally complicates the treatment of chronic Q fever. Treatment of C. burnetii–infected aortic aneurysms is the same as that for Q fever endocarditis. Surgical intervention is often required.
If doxycycline-hydroxychloroquine cannot be used, the regimen chosen should include at least two antibiotics active against C. burnetii. Rifampin (300 mg once daily) combined with doxycycline (100 mg twice daily) or ciprofloxacin (750 mg twice daily) has been used successfully. The management of patients with Q fever endocarditis is complex and should preferably be undertaken by individuals with experience in managing this illness. Monitoring of antibody titers on a quarterly basis is an essential part of the management of these patients. Thus the laboratory should be contacted and asked to save all serum samples from such patients so that the current sample can be run with the previous one. There is incomplete agreement on the antibody titer at which therapy can be stopped. However, it is reasonable to discontinue treatment if IgG antibody levels have decreased by fourfold at 1 year, if IgM antibody to phase II has disappeared, and if the patient is clinically stable.
Patients with acute Q fever and lesions of native heart valves (e.g., bicuspid aortic valve), prosthetic valves, or prosthetic intravascular material should undergo serologic monitoring every 4 months for 2 years. If the phase I IgG titer is >800, further investigation is warranted. Some authorities recommend that patients with valvulopathy and acute Q fever receive doxycycline and hydroxychloroquine to prevent chronic Q fever. For women who exhibit a serologic profile of chronic Q fever after childbirth, hydroxychloroquine and doxycycline should be given for 1 year.
BIOLOGIC MODIFYING AGENTS
Interferon γ was successful in the treatment of a 3-year-old boy with prolonged fever, abdominal pain, and thrombocytopenia due to C. burnetii that had not been eradicated with conventional antibiotic therapy. Many patients with granulomatous hepatitis due to Q fever have a prolonged febrile illness that is unresponsive to antibiotics. For these individuals, treatment with prednisone (0.5 mg/kg) has resulted in defervescence within 2–15 days. After defervescence, the glucocorticoid dose is tapered over the next month.
Prevention A whole-cell vaccine (Q-Vax) licensed in Australia effectively prevents Q fever in abattoir workers. Before administration of the vaccine, skin testing with intradermal diluted C. burnetii vaccine is performed, serologic testing is undertaken, and a history of possible Q fever is sought. Vaccine is given only to patients with no history of Q fever and negative results in serologic and skin testing.
Good animal-husbandry practices are important in preventing widespread contamination of the environment by C. burnetii. These practices include isolating aborting animals for up to 14 days, raising feed bunks to prevent contamination of feed by excreta, destroying aborted materials (by burning and burying fetal membranes and stillborn animals), and wearing masks and gloves when handling aborted materials. Vaccination of sheep and goats and a culling program were effective in the Netherlands outbreak. Only seronegative pregnant animals should be used in research settings, and only seronegative animals should be permitted in petting zoos.
During an outbreak of Q fever and for 4 weeks after it ceases, blood donations should not be accepted from individuals who live in the affected area.
ACKNOWLEDGMENT
The contributions of Didier Raoult, MD, to this chapter in previous editions are gratefully acknowledged.
212 |
Infections Due to Mycoplasmas |
Mycoplasmas are prokaryotes of the class Mollicutes. Their size (150–350 nm) is closer to that of viruses than to that of bacteria. Unlike viruses, however, mycoplasmas grow in cell-free culture media; in fact, they are the smallest organisms capable of independent replication.
![]() The entire genomes of many Mycoplasma species have been sequenced and have been found to be among the smallest of all prokaryotic genomes. Sequencing information for these genomes has helped define the minimal set of genes necessary for cellular life. The absence of genes related to the synthesis of amino acids, fatty acid metabolism, and cholesterol dictates the mycoplasmas’ parasitic or saprophytic dependence on a host for exogenous nutrients and necessitates the use of complex fastidious media to culture these organisms. Mycoplasmas lack a cell wall and are bound only by a cell membrane. The absence of a cell wall explains the inactivity of β-lactam antibiotics (penicillins and cephalosporins) against infections caused by these organisms.
The entire genomes of many Mycoplasma species have been sequenced and have been found to be among the smallest of all prokaryotic genomes. Sequencing information for these genomes has helped define the minimal set of genes necessary for cellular life. The absence of genes related to the synthesis of amino acids, fatty acid metabolism, and cholesterol dictates the mycoplasmas’ parasitic or saprophytic dependence on a host for exogenous nutrients and necessitates the use of complex fastidious media to culture these organisms. Mycoplasmas lack a cell wall and are bound only by a cell membrane. The absence of a cell wall explains the inactivity of β-lactam antibiotics (penicillins and cephalosporins) against infections caused by these organisms.
At least 13 Mycoplasma species, two Acholeplasma species, and two Ureaplasma species have been isolated from humans. Most of these species are thought to be normal inhabitants of oral and urogenital mucous membranes. Only four species—M. pneumoniae, M. hominis, U. urealyticum, and U. parvum—have been shown conclusively to be pathogenic in immunocompetent humans. M. pneumoniae primarily infects the respiratory tract, while M. hominis, U. urealyticum, and U. parvum are associated with a variety of genitourinary tract disorders and neonatal infections. Some data indicate that M. genitalium may be a cause of disease in humans. Other mycoplasmas may cause disease in immunocompromised persons.
MYCOPLASMA PNEUMONIAE
PATHOGENESIS
M. pneumoniae is generally thought to act as an extracellular pathogen. Although the organism has been shown to exist and replicate within human cells, it is not known whether these intracellular events contribute to the pathogenesis of disease. M. pneumoniae attaches to ciliated respiratory epithelial cells by means of a complex terminal organelle at the tip of one end of the organism. Cytoadherence is mediated by interactive adhesins and accessory proteins clustered on this organelle. After extracellular attachment, M. pneumoniae causes injury to host respiratory tissue. The mechanism of injury is thought to be mediated by the production of hydrogen peroxide and of a recently identified ADP-ribosylating and vacuolating cytotoxin of M. pneumoniae that has many similarities to pertussis toxin. Because mycoplasmas lack a cell wall, they also lack cell wall–derived stimulators of the innate immune system, such as lipopolysaccharide, lipoteichoic acid, and murein (peptidoglycan) fragments. However, lipoproteins from the mycoplasmal cell membrane appear to have inflammatory properties, probably acting through Toll-like receptors (primarily TLR2) on macrophages and other cells. Lung biopsy specimens from patients with M. pneumoniae respiratory tract infection reveal an inflammatory process involving the trachea, bronchioles, and peribronchial tissue, with a monocytic infiltrate coinciding with a luminal exudate of polymorphonuclear leukocytes.