Rhombic Flaps
Introduction
Today’s standards for aesthetic and functional results after reconstruction of facial defects are high, requiring meticulous planning and execution to achieve outcomes that are acceptable to both surgeon and patient. Since the advent of Mohs’ micrographic surgery for the treatment of cutaneous malignant neoplasms, complex surgical defects of the head and neck have become increasingly challenging to repair with an acceptable functional and aesthetic result.1 Primary wound closure for many skin defects is impossible and recruitment of adjacent tissue is required for repair. Over the years, careful study and practice have yielded a wealth of techniques and methods to aid the reconstructive surgeon with the repair of facial cutaneous defects. The use of local and regional cutaneous flaps to achieve wound closure with minimal distortion of surrounding facial landmarks is a hallmark of a well-trained plastic surgeon.
Cutaneous defects that are too large for primary wound closure must be addressed with grafts or flaps. In most instances, flaps are preferred to grafts because of their superior color and texture match with the surrounding skin. With proper planning, local flaps usually provide the preferred method of repair with the best aesthetic results and with minimal distortion of surrounding structures.2 A variety of classification schemes for local flaps have been proposed on the basis of blood supply, flap configuration, location, and method of transfer.2 A transposition flap typically has a linear configuration and pivots toward the defect over an incomplete bridge of skin. The base of transposition flaps is always adjacent to the defect. The rhombic flap is one type of transposition flap (Fig. 11-1).
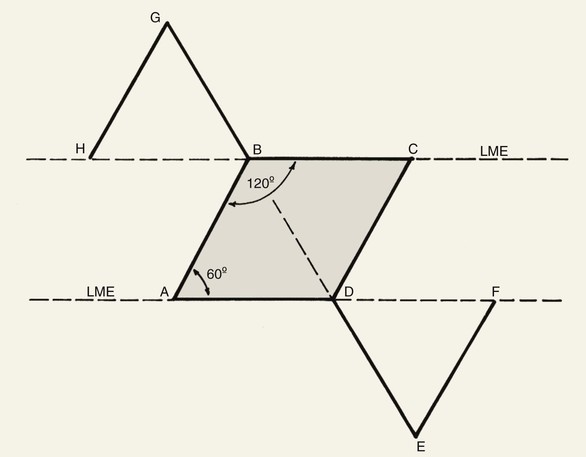
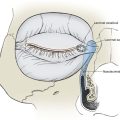
FIGURE 11-1 Proper orientation of two rhombic flaps to take advantage of lines of maximal extensibility (LME) of facial skin. Both sides of defect (B-C and A-D) are parallel to LME. Short diagonal is extended equal to its length, and cutback lines G-H and E-F are drawn parallel to nearest side of defect. Theoretical lines H-B and D-F complete equilateral triangles with bases on LME.
A thorough understanding of vascular anatomy and skin physiology and adherence to the principles of facial aesthetic regions are critical to the successful implementation of any local flap. The microcirculatory system of the superficial, dermal, subdermal, and musculocutaneous vascular plexuses provides a rich, redundant source of nourishment to local flaps.2 Flap survival hinges on two factors: the blood supplied through the base of the flap and the growth of new vascular channels between the flap and the recipient wound bed. Inosculation and neovascularization typically occur 3 to 7 days after flap transfer. Before this time, the flap is supplied by the perfusion pressure provided from the base of the flap and imbibition of nutrients from the wound bed itself.3
The evaluation of a defect begins in a systematic way, which will often aid the surgeon in the appropriate flap selection and design. Four specific factors should be addressed before bringing knife to skin. First, mobile structures such as the eyelid, lip, and melolabial fold must be accounted for in planning the flap and care taken to avoid distortion of these structures due to unfavorable vectors of tension. Second, an area of skin must be identified with adequate tissue laxity that is best suited for construction of a flap. Third, a careful evaluation of relaxed skin tension lines (RSTLs), lines of maximal extensibility (LME), and aesthetic boundaries must be made to plan incisions that will maximize scar camouflage and minimize wound closure tension.1 Fourth, scars and all vectors of tension after transfer of a flap must be anticipated, and these are the determining factors for flap selection and orientation. By use of this systematic approach to the analysis of cutaneous defects, the optimal flap may be chosen for reconstruction that will result in the least functional detriment and facial distortion.1
The first rhombic transposition flap was initially described by Alexander A. Limberg in 1946 and was later published in 1963. On the basis of extensive work with paper models, Limberg described the use of a rhombic transposition flap with internal angles of 60° and 120° to fill a rhombus-shaped surgical defect with similar internal angles.4 If the cutaneous defect did not have the configuration of a 60°-120° rhombus, additional skin was removed to create the rhombus. A rhombus-shaped transposition flap with internal angles of 60° and 120° is often referred to as a Limberg flap. By working with paper models, the geometry of the Limberg flap can be appreciated as having a precise and exacting design.5 Paper lacks the distensibility of normal tissue, and therefore the standing cutaneous deformities produced from Limberg’s paper models are less forgiving than those in clinical practice (Fig. 11-2).
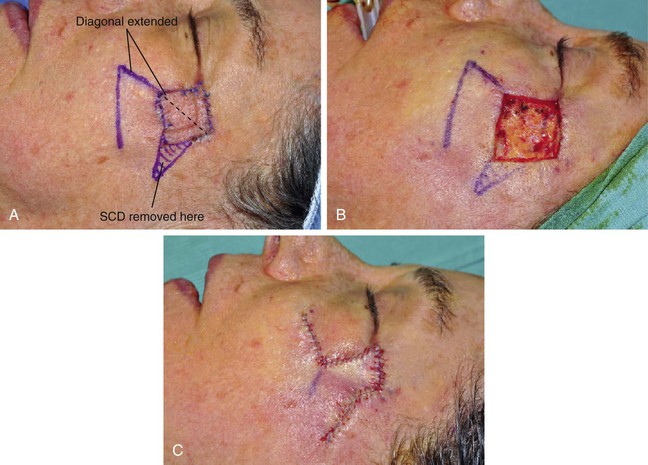
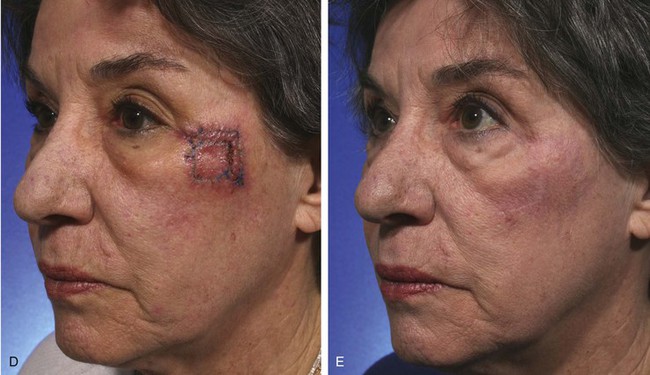
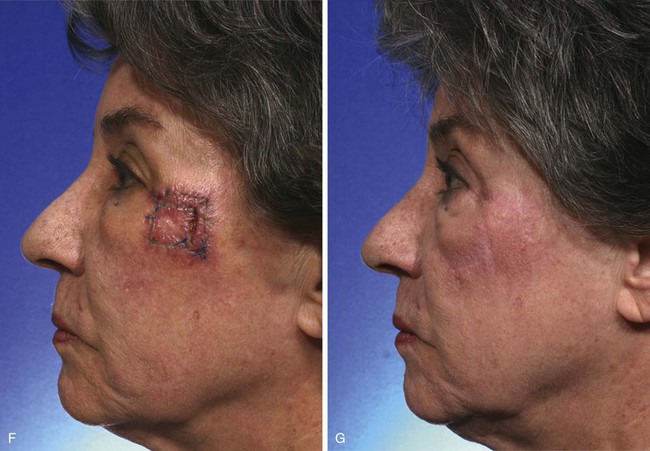
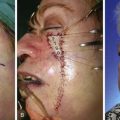
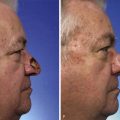
FIGURE 11-2 A, A 2 × 2-cm melanoma in situ marked for excision. Rhombic flap designed for repair after excision. Anticipated standing cutaneous deformity (SCD) marked for excision. B, C, Melanoma excised and wound repaired. D-G, Preoperative and 6-month postoperative views. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
The use of the flap described by Limberg had some limitations, which gave rise to a number of variations, including those designed by Dufourmentel, Becker, and Webster.6–8 These modifications addressed issues of wound closure tension, the need to discard skin to create a rhombus-shaped defect, and standing cutaneous deformities. In 1962, Dufourmentel expanded on Limberg’s model and described a method to close any acute-angled rhombus-shaped defect. In 1977, Webster published another significant modification of the Limberg flap. He used a narrower 30° angled flap coupled with a W-plasty at the flap’s base to facilitate wound closure. This design required less excision of normal tissue to achieve repair of a defect. It also created less wound closure tension on the flap donor site.
The rhombic flap is a cutaneous, random vascular patterned flap that is transferred primarily by transposition with some limited advancement movement. The flap is dependent on its dermal and subdermal plexuses for survival.9 As with any random flap, care must be taken when undermining and transferring the flap to ensure that the dermal and subdermal plexuses are not injured.
Limberg Flap
When a Limberg flap is planned, the defect is modified to create a rhombus-shaped defect with internal angles of 60° and 120°. The 60°-120° rhombus may be thought of as two 60° equilateral triangles aligned base to base. Thus all sides of the defect are of equal lengths, which are in turn equal to the short diagonal of the defect.5 To design a Limberg flap, the line of the short diagonal of the defect is extended, bisecting one of the internal 120° angles. The line is extended a length equal to the short diagonal. This creates the first side of the flap (Fig. 11-1). The second side of the flap is designed by marking a second line of the same length as the first, parallel to the adjacent side of the rhombic defect, creating a 60° angle at the flap’s apex. The designed flap is equal in size to the defect. Four possible rhombic flaps can be designed for any rhombus-shaped defect. After transfer of a Limberg flap, the greatest wound closure tension is at the donor site.10 The tension vector runs roughly parallel to the original border of the defect adjacent to the flap. There is minimal tension across the other portions of the wound (Fig. 11-3).1 This is an important consideration when distortion of surrounding tissues must be avoided. Therefore, it is the axis of the maximal wound closure tension vector in relationship to LME that is the greatest determinant of flap selection. The scar that results from the Limberg flap is highly predictable and consists of straight, precise lines, not all of which run parallel to RSTLs. The surgeon can easily visualize the anticipated scar configuration and approximate vector of maximum wound closure tension by drawing the flap and then covering the two parallel sides of the flap with his or her fingers.
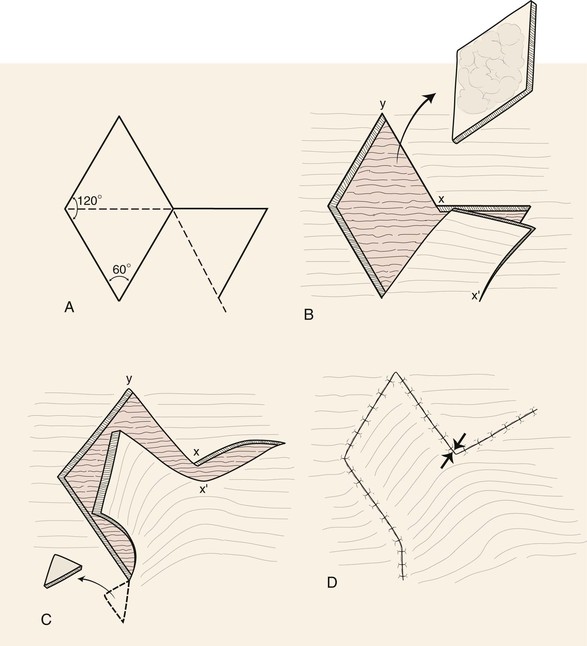
FIGURE 11-3 A, Limberg flap design. B, Defect is modified so configuration is 60°-120° rhombus. C, Flap transposed. Standing cutaneous deformity excised. D, Primary vector of tension is approximately parallel to original border of defect adjacent to flap (opposing arrows). Standing cutaneous deformity excised at base of flap.
The Limberg flap has been used to repair defects of the cheek, temple, eyelids, nose, lip, chin, and neck (Fig. 11-4).9 The horizontal parallel furrows of the forehead make this region of the face particularly unsuitable for use of rhombic flaps. Disadvantages of the Limberg flap include the formation of a standing cutaneous deformity and the need to discard normal skin to convert the defect into a rhombus if the defect does not already have the configuration of a 60°-120° rhombus. In addition, a portion of the scar resulting from the flap does not lie in RSTLs, and thus scar camouflage is not as good as when certain alternative flaps are used.
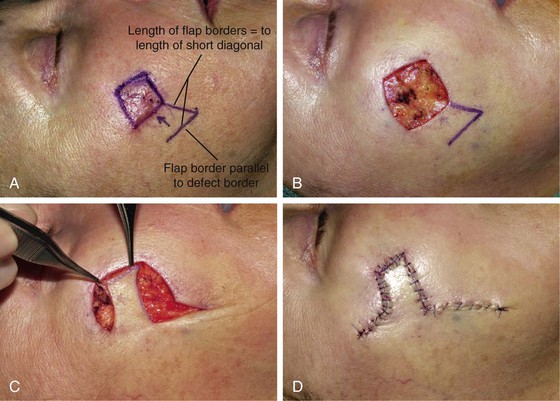
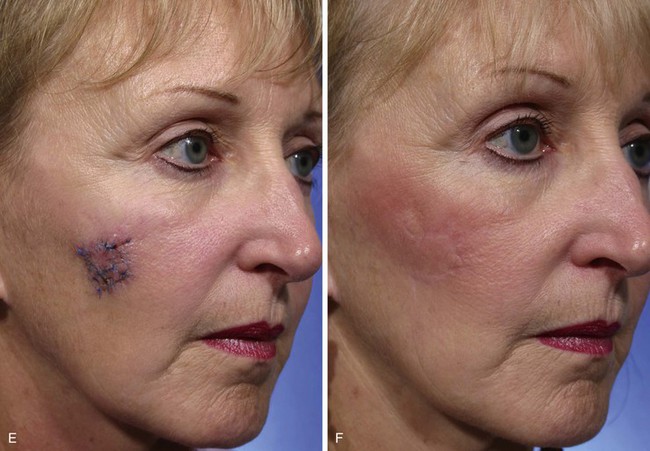
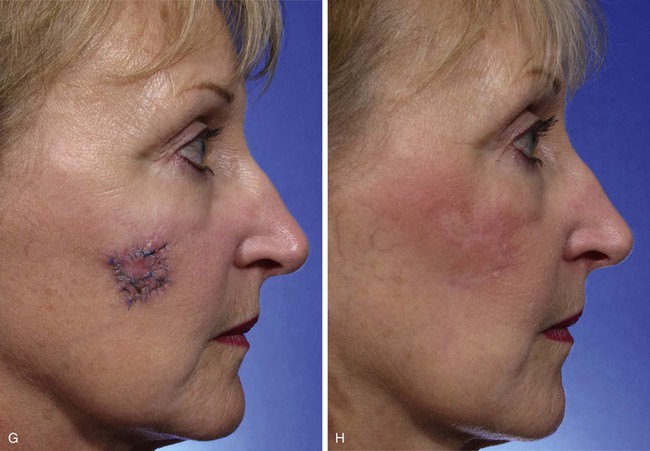
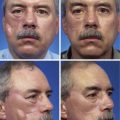
FIGURE 11-4 A, A 1.5 × 1.5-cm melanoma in situ marked for excision. Rhombic flap designed for wound repair after excision. Flap designed by extending short diagonal a length equal to length of diagonal. Second side of equal length drawn parallel to defect border. B-D, Melanoma excised. Wound repaired. E-H, Preoperative and 4-month postoperative views. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Dufourmentel Flap
The Dufourmentel modification of the Limberg flap was introduced as a method of broadening the application of the rhombic flap. Dufourmentel flaps enable the repair of rhombus-shaped defects with any combination of internal angles, in contrast to those with angles limited to 60° and 120°.7 In addition, for defects that are more “square,” less normal tissue is sacrificed when this flap design is used. Like the Limberg flap, the Dufourmentel flap can be used to repair only skin defects in which all sides of the defect are equal. However, the length of the short diagonal will vary on the basis of the acuteness of the internal angles of the defect.5 To design the flap, two lines are created. The first line is an extension of the short diagonal of the defect, and the second is an extension of one side of the defect. The angle created by these two lines is bisected by a third line to create the first side of the flap, which is equal in length to the sides of the defect. The second side of the flap is drawn parallel to the long diagonal of the defect and is also of equal length to the defect’s sides (Fig. 11-5). The Dufourmentel flap has the advantage of a lesser arc of pivot, thus creating a smaller standing cutaneous deformity than is observed with the Limberg flap. Like the Limberg flap, the Dufourmentel flap must be oriented with respect to LME. The flap covers the defect completely, thus minimizing tension and distortion of tissue surrounding the defect. A limited standing cutaneous deformity must be excised, so some normal skin is discarded in the process of reconstructing the defect. As with the Limberg flap, not all lines of the resultant scar will fall within RSTLs (Fig. 11-5).
Webster 30° Flap
The Webster 30° flap uses two modifications in an attempt to decrease wound closure tension at the donor site and to reduce the size of the standing cutaneous deformity that forms from pivoting the flap. First, the excision of the standing cutaneous deformity is performed as a W-plasty to limit the length of the excision of the deformity. Second, the apex of the flap is designed with an angle of 30° (Fig. 11-6). This design creates a narrow-angled donor site that facilitates closure with minimal wound closure tension. The Webster 30° flap, also known as the 30° rhombic flap, is designed by approximately halving an equilateral triangle. The height of the triangle is designed with a height equal to the length of a side of the defect. The angle of the apex of the transposition flap is 30°. The width of the base of the flap is half the greatest width of the defect. The Webster 30° transposition flap allows a more even distribution of wound closure tension but is more likely to distort neighboring structures.8 This is because the surface area of the flap is less than the surface area of the defect that the flap is designed to repair. Thus secondary tissue movement is necessary to assist the flap with closure of the primary defect. Furthermore, care must be taken to ensure that the angle created between the flap and the adjacent side of the defect is at least 110°.5 A lesser angle may result in vascular compromise of the repair. The Webster modification results in an irregular closure line because of the W-plasty but does so at the expense of an additional limb of scar (Fig. 11-6B).
Variations of Flap Design
In 1987, Quaba and Sommerlad described a modification of the rhombic flap that used a flap smaller than the surface area of the defect and without conversion of the defect into a rhombus.11 Their design could be used to repair circular defects with an angular flap. This “square peg into a round hole” technique was introduced in an effort to spare skin wasted in an attempt to create a defect with a rhombic configuration and to minimize the size of the flap. Their modification of the rhombic flap is similar to the note flap described in Chapter 8. The flap is designed by selecting a diameter of the defect based on the area of greatest tissue laxity and the desired location for placement of flap donor site scars. To create the first side of the flap, the line of the diameter is extended beyond the defect for a distance approximately two-thirds of the length of the diameter. The second limb of the flap, of equal length to the first, is drawn from the end of the first line, creating a 60° angle. The line follows the curve of the adjacent border of the defect (Fig. 11-7). Care is taken to avoid narrowing the flap’s base as the line parallels the curved border of the defect. After dissection of the flap and appropriate undermining of adjacent skin, the flap is transferred to the defect and key sutures are placed to distribute wound closure tension. The scar resulting from flap transfer tends to look remarkably like that occurring after use of a Limberg flap (Fig. 11-8). Examples of the use of this modification of the Limberg flap are shown in Figures 11-9 and 11-10. The authors note three distinct advantages of this cutaneous flap design: (1) depending on the location of the defect, the flap may be designed anywhere along the circumference of the circular defect; (2) this enables a relatively unlimited choice of donor sites about the defect, allowing scars to be positioned as inconspicuously as possible; and (3) there is limited sacrifice of normal tissue because the configuration of the defect is not modified and only a standing cutaneous deformity is excised.

FIGURE 11-7 A, Design of Limberg flap for 60°-120° rhombic defect. B, Modified flap design for circular defect. In contrast to Limberg flap, surface area of modified flap is less than surface area of defect.

FIGURE 11-8 Modified rhombic flap used to repair circular defect. First side of flap is two-thirds of length of diameter of circle. Second side is equal in length to first side and forms 60° angle at apex of flap. Standing cutaneous deformity excised at base of flap.

FIGURE 11-9 A, Skin lesion marked for excision. Modified Limberg flap designed for repair. B, Lesion excised. C, Flap in place. (Courtesy of Shan R. Baker, MD.)

FIGURE 11-10 A, A 2.5 × 2-cm skin defect of cheek. Modified Rhombic flap designed for repair. B, Flap transposed. C, Postoperative view at 2 months. (Courtesy of Shan R. Baker, MD.)
There are some disadvantages with the method described by Quaba and Sommerlad.11 The surface area of the flap is less than the defect; therefore, more wound closure tension is on the tissues surrounding the defect when the flap is transferred than when a Limberg flap is used. This may lead to distortion of critical facial landmarks and must be considered before flap design. Furthermore, standing cutaneous deformities were noted in 22% of their series of 175 patients treated by use of this flap.11 By creation of a flap smaller in surface area than the defect, the flap’s donor site wound closure is subjected to less tension and therefore closes more easily than when the Limberg or Dufourmentel flap is used. Thus this modification of flap design allows greater versatility at the expense of additional tension placed on tissues surrounding the defect for which the flap repair is designed.
Bilateral Rhombic Flaps
Johnson and colleagues describe the use of bilateral rhombic flaps to facilitate closure of larger cutaneous defects (Fig. 11-11).12 By visualization of the defect as two adjacent rhombuses, two separate rhombic flaps are designed to fill a single defect. The principles of rhombic flap geometry and design are the same as for a single flap. Use of two rhombic flaps enables the surgeon to use redundant skin at two different locations about the defect. Because two flaps are used, each flap is required to cover only half of the defect, so each flap can be designed smaller than if a single flap were selected for repair. This method of wound closure results in additional scar limbs, albeit in an irregular line, that may be more aesthetically undesirable.

FIGURE 11-11 Bilateral rhombic flaps designed for repair of large defect. Defect divided into two adjacent rhombuses to assist with design of rhombic flaps.
Desciak and Eliezri have proposed the use of bilateral rhombic flaps to close large defects of the nasal dorsum and tip.13 They describe visualizing the defect as two overlapping, equally sized rhombuses, each representing approximately half the total surface area of the defect. The rhombuses overlap in the center of the defect. By design of the flaps superiorly and symmetrically positioned on either side of the nose, the resulting wound closure tension vectors are identical. This prevents asymmetric distortion of the nasal base (Fig. 11-12). Furthermore, the tip is often elevated by the wound closure, which can be functionally advantageous in patients with tip ptosis. Superior and inferior midline Burow triangles are removed to facilitate wound closure. The medial corners of the flaps will overlap in the midline and are trimmed. The resultant scar line is irregular and well suited to the nasal dorsum. Bilateral rhombic nasal flaps allow reconstruction of larger (2 cm) midline nasal defects with local skin that possesses maximum color and texture match for the repair. Bilateral rhombic flaps that are based in opposite directions can also be used to repair skin defects of the nasal dorsum. Each flap is responsible for repair of half of the defect, and each pivots in opposite (clockwise and counterclockwise) directions.



FIGURE 11-12 A-C, Bilateral rhombic flaps designed for repair of circular defect of nasal dorsum and tip. Defect visualized as two overlapping, equally sized rhombuses, each representing half of surface area of defect. Superior and inferior midline Burow triangles removed. Medial corners of flaps trimmed to prevent overlap.
Turan and associates have described the use of four 60° rhombic flaps to close large wounds of the trunk and extremities that would normally require a skin graft.14 Four Limberg flaps are designed an equal distance around a central defect, so that the two opposing flaps pivot in opposite directions, one clockwise and the other counterclockwise.14 Care is taken during flap design to prevent one flap from restricting the base of another. By use of this design, wound closure tension is distributed in four directions rather than in one, and the orientation of the flaps can be designed to use LME and available tissue reserves. The technique produces additional scars, compared with a single flap, but may be a better alternative to skin grafts. This technique may be used with defects of moderate size of the cheek and scalp.
Other examples of the use of multiple rhombic flaps are described in the medical literature. Limberg himself described the use of three rhombic flaps to close defects of the forehead and scalp.4 A similar technique has been used to remove crown alopecia.5 The triple rhombic flap technique is based on the visualization of a circular defect as a hexagon with equal sides. The sides of the flaps are designed so that their length equals the radius of the defect. The flaps are created at alternating corners of the hexagon (Fig. 11-13). This design prevents the flaps from sharing a common side. In this manner, the tension of the wound closure is divided equally by the three flaps. All three flaps are pivoted in the same direction, which may lead to distortion of surrounding tissue when this method of reconstruction is used.

FIGURE 11-13 Circular cutaneous defect conceptualized as hexagon. Sides of hexagon are equal to radius of circle. First side of flap created by direct extension equal in length to radius at alternate corners to prevent sharing of common sides. Second side of flap designed parallel to adjacent side of hexagon.
Some modifications of the Limberg flap aim to extend the clinical applicability of rhombic flaps. An example is the use of Z-plasties to expand the base of the rhombic flap for larger cutaneous defects.15 Johnson and Bennett describe the use of multiple Z-plasties at the base of rhombic flaps (Fig. 11-14).15 This modification allows greater mobility of the flap at the expense of an additional length of the scar. The technique has been shown to be both reliable and predictable when one encounters restriction in tissue movement during initial flap transfer.
Case Reports
Case 1
A 47-year-old man underwent excision of a melanoma in situ located on the right temple. After adequate margins had been resected, the resulting circular defect measured 2 × 2-cm, with the anterior border of the defect 1 cm lateral to the lateral orbital bony rim (Fig. 11-15). Key factors influencing cutaneous flap selection and design are highlighted by this example. The lateral border of the periorbita represents an aesthetic boundary that must be protected from distortion from undue wound closure tension. Any distortion of the eyelid or eyebrow could result in an unsatisfactory functional and aesthetic outcome. Similarly, the anterior hairline should not be retracted inferiorly. With these factors in mind, an inferiorly based rhombic flap was designed to recruit skin from the adjacent cheek while avoiding tension around the primary defect. A Dufourmentel flap was designed for repair to decrease the pivotal arc of the flap. The flap was based posteriorly, enabling the recruitment of cheek skin along LME. The surface area of the Dufourmentel flap is the same as that of the primary defect it is designed to repair; therefore, there is minimal wound closure tension at the recipient site. The immediate postoperative photographs demonstrated preservation of complete eyelid closure and no distortion of the eyebrow. The appropriate orientation of the created rhombus defect and the flap resulted in scar lines that in part paralleled RSTLs, as seen in the scars lateral to the malar eminence and along the lateral bony orbital rim. The multiple scar lines resulting from reconstruction with a rhombic flap typically prevent all the scars from lying in a favorable position. In fact, approximately half of the entire length of the scar that results from wound repair with a rhombic flap is not parallel to and does not lie in RSTLs.
Case 2
A 39-year-old man underwent micrographic surgical excision of a large recurrent basal cell carcinoma located lateral to the left oral commissure. The resulting defect measured 5.5 × 3.5-cm and abutted the commissure (Fig. 11-16). Orbicularis muscle was exposed in the depths of the defect. It was elected to use a Dufourmentel flap for wound closure. The flap was designed with regard to preventing distortion of the oral commissure foremost in mind. It was oriented so that the apex of the flap was transposed toward the medial superior border of the defect, thus minimizing inferior traction on the commissure. The labiomandibular crease, which was resected with the specimen, was partially restored by the border of the flap, thus preserving this aesthetic boundary. The inferior medial cheek provided the source for the flap. This area of the face has the greatest skin redundancy and serves as the preferred location for harvesting of transposition cheek flaps. Postoperatively, the patient demonstrated oral symmetry.
Case 3
A 76-year-old man underwent a square technique (see Chapter 8, Case 5) to establish disease-free margins around a melanoma in situ of the cheek. Once disease-free margins were confirmed, the involved area had a rhombus configuration (Fig. 11-17). In this circumstance, a logical surgical plan to reconstruct the cheek after excision of the melanoma was to use a rhombic flap. An inferiorly based rhombic flap provided an excellent method of reconstruction.


FIGURE 11-17 A, A 4 × 3-cm melanoma in situ marked for excision. Rhombic (Limberg) flap designed for wound repair after excision. Anticipated standing cutaneous deformity (SCD) marked for excision. B, Melanoma excised and wound repaired. C, D, Preoperative and 4-month postoperative views. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Case 4
Similar to the patient discussed in Case 3, a 57-year-old man developed melanoma in situ of the cheek. Tumor-free margins were established by the square technique, resulting in a skin area with a rhombus configuration. The planned excision was located in the preauricular region of the cheek. There is less skin laxity and redundancy in this region of the face compared with the medial cheek or temple. For this reason, a Dufourmentel flap was used to repair the cutaneous defect after excision of the melanoma (Fig. 11-18). Similar to the Limberg flap, the Dufourmentel flap can be used to repair only skin defects in which all sides of the defect are equal. However, the Dufourmentel flap has the advantage of a lesser arc of pivot compared with the Limberg flap, thus creating a smaller flap donor site. A smaller donor site requires less skin advancement to repair the donor site, an important consideration in areas where facial skin is less redundant, as in the preauricular region.

FIGURE 11-18 A, A 2 × 2-cm melanoma in situ marked for excision. Dufourmentel flap designed for wound repair after excision. Lines of defect border and short diagonal extended to create angle that is bisected by third line. Third line equal in length to sides of defect. Third line is first side of flap. Second side of flap equal in length to first side is drawn parallel to long diagonal of defect. B, Melanoma excised and wound repaired. C, D, Postoperative views at 2 months. (Courtesy of Shan R. Baker, MD.)
Case 5
A 27-year-old woman developed a basal cell carcinoma of the malar eminence. Review of the histopathologic features of a biopsy specimen of the lesion showed an aggressive growth pattern. The tumor was treated by micrographic excision, resulting in a 4 × 4-cm circular cutaneous defect. Figure 11-19A, B shows two modified rhombic flaps designed for repair. Their corresponding anticipated standing cutaneous deformities are marked with horizontal lines. One advantage of raising rhombic flaps to repair facial cutaneous defects is the opportunity to often position the flap in variable positions at the periphery of the defect. This increases the number of options available to the surgeon and enables the selection of the preferred location for placement of the secondary defect. In this case, the superiorly based flap was selected for wound repair (Fig. 11-19). The repair was accomplished under moderate wound closure tension because of the large size of the defect relative to paucity of redundant skin for construction of the flap. As a result, some scar hypertrophy developed at the flap donor site.

FIGURE 11-19 A, B, A 4 × 4-cm cutaneous defect of malar eminence. Two modified rhombic flaps designed for repair. Their corresponding anticipated standing cutaneous deformities are marked with horizontal lines. C, D, Superiorly based rhombic flap selected for repair of wound. E, Postoperative view at 1 year. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Summary
Since Limberg’s initial description of the rhombic flap in 1946, many advances in our understanding of wound healing, wound tension vectors, and aesthetic facial boundaries have been made.1 Despite these advances, the rhombic flap and its derivatives remain useful for the plastic surgeon. The geometry and reliability of the rhombic flap allow the surgeon to proceed with an accurate knowledge of the expected scar lines and the effects of wound closure on surrounding structures. When it is planned properly, this technique can provide excellent results with minimal distortion of surrounding facial features and predictable scar lines.
References
1. Park, SS. Local and regional cutaneous flaps. In: Papel ID, ed. Facial plastic and reconstructive surgery. 2nd ed. New York: Thieme; 2002:528.
2. McNay, AT, Ostad, A, Moy, RL. Surgical pearl: modified rhombic flap. J Am Acad Dermatol. 1997; 37(Pt 1):256.
3. Clark, JM, Wang, DW. Skin flaps, design, October 2003. Available at http://www.emedicine.com/, 2004. [Accessed August 19].
4. Limberg, AA. Design of the local flaps. In: Gibson T, ed. Modern trends in plastic surgery. Sevenoaks, England: Butterworth, 1966.
5. Bray, DA. Rhombic flaps. In: Baker S, Swanson NA, eds. Local flaps in facial reconstruction. St. Louis: Mosby; 1995:151.
6. Becker, FF. Rhomboid flap in facial reconstruction. New concept of tension lines. Arch Otolarygol. 1979; 105:10.
7. Dufourmentel, C. An L-shaped flap for lozenge-shaped defects. In: Transactions of the Third International Congress of Plastic Surgery. Amsterdam: Excerpta Medica; 1964.
8. Webster, RC, Davidson, TM, Smith, RC. The 30-degree transposition flap. Laryngoscope. 1978; 88:85.
9. Tollefson, TT, Murakami, CS, Kriet, JD. Cheek repair. Otolaryngol Clin North Am. 2001; 34:627.
10. Larrabee, WF, Jr., Trach, R, Sutton, D. Rhomboid flap dynamics. Arch Otolaryngol. 1983; 107:755.
11. Quaba, AA, Sommerlad, BC. “A square peg into a round hole”: a modified rhomboid flap and its clinical application. Br J Plast Surg. 1987; 40:163.
12. Johnson, TM, Wang, TS, Fader, DJ. The birhombic transposition flap for soft tissue reconstruction. J Am Acad Dermatol. 1999; 41:232.
13. Desciak, EB, Eliezri, YD. Bilateral rhombic flaps for defects on the nasal dorsum and supra-tip. Dermatol Surg. 2003; 29:1163.
14. Turan, T, Kuran, I, Ozcan, H, Bas, L. Geometric limit of multiple local Limberg flaps: a flap design. Plast Reconstr Surg. 1999; 104:1675.
15. Johnson, SC, Bennett, RG. Double Z-plasty to enhance rhombic flap mobility. J Dermatol Surg Oncol. 1994; 20:128.