Chapter 11 Rheumatology and bone disease
Rheumatological and musculoskeletal disorders
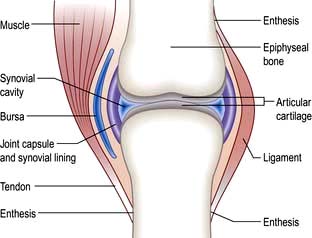
The normal joint
There are three types of joints: fibrous, fibrocartilaginous and synovial.
Synovial joints
These (Fig. 11.1) include the ball-and-socket joints (e.g. hip) and the hinge joints (e.g. interphalangeal).
Juxta-articular bone
The bone which abuts a joint (epiphyseal bone) differs structurally from the shaft (metaphysis) (see Fig. 11.32). It is highly vascular and comprises a light framework of mineralized collagen enclosed in a thin coating of tougher, cortical bone. The ability of this structure to withstand pressure is low and it collapses and fractures when the normal intra-articular covering of hyaline cartilage is worn away as in osteoarthritis (OA; see p. 512). Loss of surface cartilage also leads to the abnormalities of bone growth and remodelling typical of OA (see p. 512).
Ligaments and tendons
These structures stabilize joints. Ligaments are variably elastic and this contributes to the stiffness or laxity of joints (see p. 559). Tendons are inelastic and transmit muscle power to bones. The joint capsule is formed by intermeshing tendons and ligaments. The point where a tendon or ligament joins a bone is called an enthesis and may be the site of inflammation.
Components of extracellular matrix
Collagens. Collagens consist of three polypeptide (α) chains wound into a triple helix. These alpha chains contain repeating sequences of Gly-x-y triplets, where x and y are often prolyl and hydroxypropyl residues. Collagen fibres show genetic heterogeneity, with genes on at least 12 chromosomes. Hyaline cartilage is 90% type II (COL2A1). There are several classes of collagen genes, based on their protein structures, and abnormalities of these may lead to specific diseases (see p. 560).
Skeletal muscle
This consists of bundles of myocytes containing actin and myosin molecules. These molecules interdigitate and form myofibrils which cause muscle contraction in a similar way to myocardial muscle (p. 671). Bundles of myofibrils (fasciculi) are covered by connective tissue, the perimysium, which merges with the epimysium (covering the muscle) and forms the tendon which attaches to the bone surface (enthesis).
Clinical approach to the patient
Taking a musculoskeletal history
 Where is it? Is it localized or generalized? The pattern of joint involvement is a useful clue to the diagnosis (e.g. distal interphalangeal joints in nodal osteoarthritis).
Where is it? Is it localized or generalized? The pattern of joint involvement is a useful clue to the diagnosis (e.g. distal interphalangeal joints in nodal osteoarthritis).
 Is it arising from joints, the spine, muscles or bone, with local tenderness? Soft tissue lesions and inflamed joints are locally tender.
Is it arising from joints, the spine, muscles or bone, with local tenderness? Soft tissue lesions and inflamed joints are locally tender.
 Could it be referred from another site? Joint pain is localized but may radiate distally – shoulder to upper arm; hip to thigh and knee.
Could it be referred from another site? Joint pain is localized but may radiate distally – shoulder to upper arm; hip to thigh and knee.
 Is it constant, intermittent or episodic? How severe is it – aching or agonizing?
Is it constant, intermittent or episodic? How severe is it – aching or agonizing?
 Are there aggravating or precipitating factors? Is it made worse by activity and eased by rest (mechanical) or worse after rest (inflammatory).
Are there aggravating or precipitating factors? Is it made worse by activity and eased by rest (mechanical) or worse after rest (inflammatory).
 Are there any associated neurological features? Numbness, pins and needles and/or loss of power suggest ‘nerve’ involvement. The distribution of symptoms is a useful clue to the nerve or nerve root affected.
Are there any associated neurological features? Numbness, pins and needles and/or loss of power suggest ‘nerve’ involvement. The distribution of symptoms is a useful clue to the nerve or nerve root affected.
Gout (see p. 530), reactive arthritis (p. 529) and ankylosing spondylitis (p. 527) are more common in men. Rheumatoid arthritis and other autoimmune rheumatic diseases are more common in women.
 Is the person young, middle-aged or older?
Is the person young, middle-aged or older?
 How old was the patient when the problem first started? Osteoarthritis (see p. 512) and polymyalgia rheumatica (p. 542) rarely affect the under-50s. Rheumatoid arthritis starts most commonly in women aged 30–50 years.
How old was the patient when the problem first started? Osteoarthritis (see p. 512) and polymyalgia rheumatica (p. 542) rarely affect the under-50s. Rheumatoid arthritis starts most commonly in women aged 30–50 years.
 Is there any associated ill-health or other worrying feature, such as weight loss or fever?
Is there any associated ill-health or other worrying feature, such as weight loss or fever?
 Are there other associated medical conditions that may be relevant? Psoriasis (see p. 1207) or inflammatory bowel disease is associated with spondyloarthritis (see p. 1004). Charcot’s joints (p. 547) are seen in diabetics.
Are there other associated medical conditions that may be relevant? Psoriasis (see p. 1207) or inflammatory bowel disease is associated with spondyloarthritis (see p. 1004). Charcot’s joints (p. 547) are seen in diabetics.
Could a drug be a cause? Diuretics may precipitate gout in men and older women. Hormone replacement therapy or the oral contraceptive pill may precipitate systemic lupus erythematosus (SLE) (p. 535). Steroids can cause avascular necrosis. Some drugs cause a lupus-like syndrome (p. 535).
Is this relevant? Sickle cell disease causes joint pain in young black Africans, but osteoporosis (see p. 552) is uncommon in older black Africans.
Have there been any similar episodes or is this the first? Are there any clues from previous medical conditions? Gout is recurrent; the episodes settle without treatment in 7–10 days. Acute episodes of palindromic rheumatism may predate the onset of rheumatoid arthritis (see p. 519).
The biopsychosocial model of disease is highly relevant to many rheumatic disorders:
 Has there been any recent major stress in family or working life? Could this be relevant? Stress rarely causes rheumatic disease but may precipitate a flare-up of inflammatory arthritis. It reduces a person’s ability to cope with pain or disability.
Has there been any recent major stress in family or working life? Could this be relevant? Stress rarely causes rheumatic disease but may precipitate a flare-up of inflammatory arthritis. It reduces a person’s ability to cope with pain or disability.
 Has there been an injury for which a legal case for compensation is pending?
Has there been an injury for which a legal case for compensation is pending?
The World Health Organization describes the impact of disease on an individual in terms of:
 Impairment: any loss or abnormality of psychological or anatomical structure or function
Impairment: any loss or abnormality of psychological or anatomical structure or function
 Disability (activity limitation): any restriction or lack of ability to perform an activity in the manner or within the range considered normal for a human being
Disability (activity limitation): any restriction or lack of ability to perform an activity in the manner or within the range considered normal for a human being
 Handicap (participation restriction): a disadvantage for an individual resulting from an impairment or disability that limits or prevents the fulfilment of a role that is normal for that individual.
Handicap (participation restriction): a disadvantage for an individual resulting from an impairment or disability that limits or prevents the fulfilment of a role that is normal for that individual.
Examination of the joints
Always observe a patient, looking for disabilities, as he or she walks into the room and sits down. General and neurological examinations are often necessary. Guidelines for rapid examinations of the limbs and spine are shown in Practical Box 11.1.
![]() Practical Box 11.1
Practical Box 11.1
Rapid examinations of the limb and spine
Rapid examination of the upper limbs
 Raise arms sideways to the ears (abduction). Reach behind neck and back. Difficulties with these movements indicate a shoulder or rotator cuff problem.
Raise arms sideways to the ears (abduction). Reach behind neck and back. Difficulties with these movements indicate a shoulder or rotator cuff problem.
 Hold the arms forward, with elbows straight and fingers apart, palm up and palm down. Fixed flexion at the elbow indicates an elbow problem. Examine the hands for swelling, wasting and deformity.
Hold the arms forward, with elbows straight and fingers apart, palm up and palm down. Fixed flexion at the elbow indicates an elbow problem. Examine the hands for swelling, wasting and deformity.
 Place the hands in the ‘prayer’ position with the elbows apart. Flexion deformities of the fingers may be due to arthritis, flexor tenosynovitis or skin disease. Painful restriction of the wrist limits the person’s ability to move the elbows out with the hands held together.
Place the hands in the ‘prayer’ position with the elbows apart. Flexion deformities of the fingers may be due to arthritis, flexor tenosynovitis or skin disease. Painful restriction of the wrist limits the person’s ability to move the elbows out with the hands held together.
 Make a tight fist. Difficulty with this indicates a loss of flexion or grip. Grip strength can be measured.
Make a tight fist. Difficulty with this indicates a loss of flexion or grip. Grip strength can be measured.
Rapid examination of the lower limbs
 Ask the patient to walk a short distance away from and towards you, and to stand still. Look for abnormal posture or stance.
Ask the patient to walk a short distance away from and towards you, and to stand still. Look for abnormal posture or stance.
 Ask the patient to stand on each leg. Severe hip disease causes the pelvis on the non-weight-bearing side to sag (positive Trendelenburg test).
Ask the patient to stand on each leg. Severe hip disease causes the pelvis on the non-weight-bearing side to sag (positive Trendelenburg test).
 Watch the patient stand and sit, looking for hip and/or knee problems.
Watch the patient stand and sit, looking for hip and/or knee problems.
 Ask the patient to straighten and flex each knee.
Ask the patient to straighten and flex each knee.
 Ask the patient to place each foot in turn on the opposite knee with the hip externally rotated. This tests for painful restriction of hip or knee. Abnormal hips or knees must be examined lying.
Ask the patient to place each foot in turn on the opposite knee with the hip externally rotated. This tests for painful restriction of hip or knee. Abnormal hips or knees must be examined lying.
 Move each ankle up and down. Examine the ankle joint and tendons, medial arch and toes whilst standing.
Move each ankle up and down. Examine the ankle joint and tendons, medial arch and toes whilst standing.
Rapid examination of the spine
 Ask the patient to (a) bend forwards to touch the toes with straight knees, (b) extend backwards, (c) flex sideways, and (d) look over each shoulder, flexing and extending and sideflexing the neck. Observe abnormal spinal curves – scoliosis (lateral curve), kyphosis (forward bending) or lordosis (backward bending). A cervical and lumbar lordosis and a thoracic kyphosis are normal. Muscle spasm is worse whilst standing and bending. Leg length inequality leads to a scoliosis which decreases on sitting or lying (the lengths are measured lying).
Ask the patient to (a) bend forwards to touch the toes with straight knees, (b) extend backwards, (c) flex sideways, and (d) look over each shoulder, flexing and extending and sideflexing the neck. Observe abnormal spinal curves – scoliosis (lateral curve), kyphosis (forward bending) or lordosis (backward bending). A cervical and lumbar lordosis and a thoracic kyphosis are normal. Muscle spasm is worse whilst standing and bending. Leg length inequality leads to a scoliosis which decreases on sitting or lying (the lengths are measured lying).
 Ask the patient to lie supine. Examine any restriction of straight-leg raising (see disc prolapse, below).
Ask the patient to lie supine. Examine any restriction of straight-leg raising (see disc prolapse, below).
 Ask the patient to lie prone. Examine for anterior thigh pain during a femoral stretch test (flexing knee whilst prone), which indicates a high lumbar disc problem.
Ask the patient to lie prone. Examine for anterior thigh pain during a femoral stretch test (flexing knee whilst prone), which indicates a high lumbar disc problem.
Examining an individual joint involves three stages: looking, feeling and moving (Table 11.1). A screening examination of the locomotor system, known by the acronym GALS (Global Assessment of the Locomotor System) has been devised. X-ray or ultrasound of the joint often forms an integral part of the examination.
|
LOOK at the appearance of the joint |
Swelling – could be bony, fluid or synovial |
|
Deformity – valgus, where the distal bone is deviated laterally (e.g. knock-knees or genu valgum) |
|
|
Varus where the distal bone is deviated medially (bow-legs or genu varum) |
|
|
Fixed flexion or hyperextension |
|
|
Rash – especially psoriasis |
|
|
Muscle wasting – easier to see in large muscles like the quadriceps |
|
|
Scars – from surgery or trauma |
|
|
Signs of inflammationSymmetry – are the right and left joints (e.g. hips, knees, any other paired joint) the same? If not which do you think is abnormal? |
|
|
FEEL |
Swelling – fluid swelling (effusion) usually represents increased synovial fluid in inflammatory arthritis, but can be due to blood or pus |
|
Synovial swelling is rubbery or boggy and usually occurs in inflammatory arthritis |
|
|
Bony swelling, such as Heberden’s nodes in the fingers is usually seen in osteoarthritis |
|
|
Warmth – a warm joint may be inflamed or infected |
|
|
Tenderness – may represent joint inflammation, but many people have chronic tenderness all over the body (e.g. in fibromyalgia) |
|
|
MOVE |
Active movement – is the range full and pain-free? Is the movement fluid? In the hands – can the patient perform fine movements? In the legs – can the patient walk properly? |
|
Compare movements on the right and left side – are they symmetrical? |
|
|
Is there crepitus when the joint is moved? |
|
|
If active movement is limited try passive movement. In a joint problem both will usually be affected. If it is a muscle or nerve problem passive movement may remain full. |
Investigations
Useful blood screening tests
 Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). An increase of these reflects inflammation. Plasma viscosity is also raised in inflammatory disease.
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). An increase of these reflects inflammation. Plasma viscosity is also raised in inflammatory disease.
 Bone and liver biochemistry. A raised serum alkaline phosphatase may indicate liver or bone disease. A rise in liver enzymes is seen with drug-induced toxicity. For other investigations of bone, see page 550.
Bone and liver biochemistry. A raised serum alkaline phosphatase may indicate liver or bone disease. A rise in liver enzymes is seen with drug-induced toxicity. For other investigations of bone, see page 550.
Serum autoantibody studies
 Rheumatoid factors (RFs) (see also p. 518). Rheumatoid factors are detected by enzyme linked immunoabsorbent assay (ELISA). RFs are antibodies (usually IgM, but also IgG or IgA) against the Fc portion of IgG. They are detected in 70% of people with rheumatoid arthritis (RA), but are not diagnostic. RFs are detected in many autoimmune rheumatic disorders (e.g. SLE), in chronic infections, and in asymptomatic older people (Table 11.2).
Rheumatoid factors (RFs) (see also p. 518). Rheumatoid factors are detected by enzyme linked immunoabsorbent assay (ELISA). RFs are antibodies (usually IgM, but also IgG or IgA) against the Fc portion of IgG. They are detected in 70% of people with rheumatoid arthritis (RA), but are not diagnostic. RFs are detected in many autoimmune rheumatic disorders (e.g. SLE), in chronic infections, and in asymptomatic older people (Table 11.2).
 Anti-citrullinated peptide antibodies (ACPA). These antibodies are directed against citrullinated antigens, vimentin, fibrinogen, alpha enolase and type II collagen. They are measured by an ELISA technique and are present in up to 80% of people with RA. They have a high specificity for RA (90% with a sensitivity of 60%). They are helpful in early disease when the RF is negative to distinguish it from acute transient synovitis (see Box 11.6, p. 519). Positivity for RF and/or ACPA is associated with a worse prognosis and an increase in the likelihood of bony erosions in people with RA.
Anti-citrullinated peptide antibodies (ACPA). These antibodies are directed against citrullinated antigens, vimentin, fibrinogen, alpha enolase and type II collagen. They are measured by an ELISA technique and are present in up to 80% of people with RA. They have a high specificity for RA (90% with a sensitivity of 60%). They are helpful in early disease when the RF is negative to distinguish it from acute transient synovitis (see Box 11.6, p. 519). Positivity for RF and/or ACPA is associated with a worse prognosis and an increase in the likelihood of bony erosions in people with RA.
 Antinuclear antibodies (ANAs). These are detected by indirect immunofluorescent staining of fresh-frozen sections of rat liver or kidney or Hep-2 cell lines. Different patterns reflect a variety of antigenic specificities that occur with different clinical pictures (see Box 11.16, p. 537). ANA is used as a screening test for systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) – a negative ANA makes either condition highly unlikely – but low titres occur in RA and chronic infections and in normal individuals, especially the elderly (Table 11.3).
Antinuclear antibodies (ANAs). These are detected by indirect immunofluorescent staining of fresh-frozen sections of rat liver or kidney or Hep-2 cell lines. Different patterns reflect a variety of antigenic specificities that occur with different clinical pictures (see Box 11.16, p. 537). ANA is used as a screening test for systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) – a negative ANA makes either condition highly unlikely – but low titres occur in RA and chronic infections and in normal individuals, especially the elderly (Table 11.3).
 Anti-double-stranded DNA (dsDNA) antibodies. These are usually detected by a precipitation test (Farr assay), by ELISA, or by an immunofluorescent test using Crithidia luciliae (which contains double-stranded DNA). Raised anti-dsDNA is highly specific for SLE and the levels usually rise and fall in parallel with disease activity so can be used to monitor the level of treatment required.
Anti-double-stranded DNA (dsDNA) antibodies. These are usually detected by a precipitation test (Farr assay), by ELISA, or by an immunofluorescent test using Crithidia luciliae (which contains double-stranded DNA). Raised anti-dsDNA is highly specific for SLE and the levels usually rise and fall in parallel with disease activity so can be used to monitor the level of treatment required.
 Anti-extractable nuclear antigen (ENA) antibodies (see Box 11.16, p. 537). These produce a speckled ANA fluorescent pattern, and can be identified by ELISA. The most commonly measured ENAs are:
Anti-extractable nuclear antigen (ENA) antibodies (see Box 11.16, p. 537). These produce a speckled ANA fluorescent pattern, and can be identified by ELISA. The most commonly measured ENAs are:
 Anti-neutrophil cytoplasmic antibodies (ANCAs) (see p. 544). These are predominantly IgG autoantibodies directed against the primary granules of neutrophil and macrophage lysosomes. They are strongly associated with small-vessel vasculitis. Two major clinically relevant ANCA patterns are recognized on immunofluorescence:
Anti-neutrophil cytoplasmic antibodies (ANCAs) (see p. 544). These are predominantly IgG autoantibodies directed against the primary granules of neutrophil and macrophage lysosomes. They are strongly associated with small-vessel vasculitis. Two major clinically relevant ANCA patterns are recognized on immunofluorescence:
 Antiphospholipid antibodies (see p. 538). These are detected in the antiphospholipid syndrome (see p. 538).
Antiphospholipid antibodies (see p. 538). These are detected in the antiphospholipid syndrome (see p. 538).
 Immune complexes. Immune complexes are infrequently measured, largely because of variability between assays and difficulty in interpreting their meaning. Assays based on the polyethylene glycol precipitation method (PEG) or C1q binding are available commercially.
Immune complexes. Immune complexes are infrequently measured, largely because of variability between assays and difficulty in interpreting their meaning. Assays based on the polyethylene glycol precipitation method (PEG) or C1q binding are available commercially.
 Complement. Low complement levels indicate consumption and suggest an active disease process in SLE.
Complement. Low complement levels indicate consumption and suggest an active disease process in SLE.
Table 11.2 Conditions in which rheumatoid factor is found in the serum
|
Autoimmune rheumatic diseases |
RF (IgM) % |
|
Rheumatoid arthritis |
70 |
|
Systemic lupus erythematosus |
25 |
|
Sjögren’s syndrome |
90 |
|
Systemic sclerosis |
30 |
|
Polymyositis/dermatomyositis |
50 |
|
Juvenile idiopathic arthritis |
Variable |
|
Viral infections |
Hyperglobulinaemias |
|
Hepatitis |
Chronic liver disease |
|
Infectious mononucleosis |
Sarcoidosis |
|
Cryoglobulinaemia |
|
|
Chronic infections |
Normal population |
|
Tuberculosis |
Elderly |
|
Leprosy |
Relatives of people with RA |
|
Syphilis |
|
Table 11.3 Conditions in which serum antinuclear antibodies are found
| (%) | |
|---|---|
|
Systemic lupus erythematosus |
95 |
|
Systemic sclerosis |
70 |
|
Sjögren’s syndrome |
80 |
|
Polymyositis and dermatomyositis |
40 |
|
Rheumatoid arthritis |
30 |
|
Juvenile idiopathic arthritis |
Variable |
|
Other diseases |
|
|
Autoimmune hepatitis |
100 |
|
Drug-induced lupus |
>95 |
|
Myasthenia gravis |
50 |
|
Idiopathic pulmonary fibrosis |
30 |
|
Diabetes mellitus |
25 |
|
Infectious mononucleosis |
5–10 |
|
Normal population |
8 |
Joint aspiration
Examination of joint (or bursa) fluid is used mainly to diagnose septic, reactive or crystal arthritis. The appearance of the fluid is an indicator of the level of inflammation. The procedure is often undertaken in combination with injection of a corticosteroid. Aspiration alone is therapeutic in crystal arthritis (see Practical Box 11.2, p. 508).
Examination of synovial fluid
Diagnostic imaging and visualization
 X-rays can be diagnostic in certain conditions (e.g. established rheumatoid arthritis) and are the first investigation in many cases of trauma. X-rays can detect joint space narrowing, erosions in rheumatoid arthritis, calcification in soft tissue, new bone formation, e.g. osteophytes and decreased bone density (osteopenia) or increased bone density (osteosclerosis):
X-rays can be diagnostic in certain conditions (e.g. established rheumatoid arthritis) and are the first investigation in many cases of trauma. X-rays can detect joint space narrowing, erosions in rheumatoid arthritis, calcification in soft tissue, new bone formation, e.g. osteophytes and decreased bone density (osteopenia) or increased bone density (osteosclerosis):
 Ultrasound (US) is particularly useful for periarticular structures, soft tissue swellings and tendons and for detecting active synovitis in inflammatory arthritis. It is increasingly used to examine the shoulder and other structures during movement, e.g. shoulder impingement syndrome (see p. 500). Doppler US measures blood flow and hence inflammation. US is used to guide local injections.
Ultrasound (US) is particularly useful for periarticular structures, soft tissue swellings and tendons and for detecting active synovitis in inflammatory arthritis. It is increasingly used to examine the shoulder and other structures during movement, e.g. shoulder impingement syndrome (see p. 500). Doppler US measures blood flow and hence inflammation. US is used to guide local injections.
 Magnetic resonance imaging (MRI) shows bone changes and intra-articular structures in striking detail. Visualization of particular structures can be enhanced with different resonance sequences. T1-weighted is used for anatomical detail, T2-weighted for fluid detection and short tau inversion recovery (STIR) for the presence of bone marrow oedema. It is more sensitive than X-rays in the early detection of articular and periarticular disease. It is the investigation of choice for most spinal disorders but is inappropriate in uncomplicated mechanical low back pain. Gadolinium injection enhances inflamed tissue. MRI can also detect muscle changes, e.g. myositis.
Magnetic resonance imaging (MRI) shows bone changes and intra-articular structures in striking detail. Visualization of particular structures can be enhanced with different resonance sequences. T1-weighted is used for anatomical detail, T2-weighted for fluid detection and short tau inversion recovery (STIR) for the presence of bone marrow oedema. It is more sensitive than X-rays in the early detection of articular and periarticular disease. It is the investigation of choice for most spinal disorders but is inappropriate in uncomplicated mechanical low back pain. Gadolinium injection enhances inflamed tissue. MRI can also detect muscle changes, e.g. myositis.
 Computerized axial tomography (CT) is useful for detecting changes in calcified structures but dose of irradiation is high.
Computerized axial tomography (CT) is useful for detecting changes in calcified structures but dose of irradiation is high.
 Bone scintigraphy utilizes radionuclides, usually 99mTc, and detects abnormal bone turnover and blood circulation and, although nonspecific, helps in detecting areas of inflammation, infection or malignancy. It is best used in combination with other anatomical imaging techniques.
Bone scintigraphy utilizes radionuclides, usually 99mTc, and detects abnormal bone turnover and blood circulation and, although nonspecific, helps in detecting areas of inflammation, infection or malignancy. It is best used in combination with other anatomical imaging techniques.
 DXA scanning uses very low doses of X-irradiation to measure bone density and is used in the screening and monitoring of osteoporosis.
DXA scanning uses very low doses of X-irradiation to measure bone density and is used in the screening and monitoring of osteoporosis.
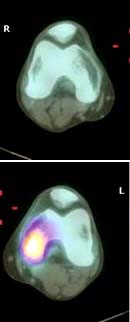
 Positron emission tomography (PET) scanning uses radionuclides, which decay by emission of positrons. 18F-Fluorodeoxyglucose uptake indicates areas of increased glucose metabolism. It is used to locate tumours and demonstrate large vessel vasculitis, e.g. Takayasu’s arteritis (see p. 789). PET scans are combined with CT to improve anatomical details.
Positron emission tomography (PET) scanning uses radionuclides, which decay by emission of positrons. 18F-Fluorodeoxyglucose uptake indicates areas of increased glucose metabolism. It is used to locate tumours and demonstrate large vessel vasculitis, e.g. Takayasu’s arteritis (see p. 789). PET scans are combined with CT to improve anatomical details.
 Arthroscopy is a direct means of visualizing a joint, particularly the knee or shoulder. Biopsies can be taken, surgery performed in certain conditions (e.g. repair or trimming of meniscal tears), and loose bodies removed.
Arthroscopy is a direct means of visualizing a joint, particularly the knee or shoulder. Biopsies can be taken, surgery performed in certain conditions (e.g. repair or trimming of meniscal tears), and loose bodies removed.
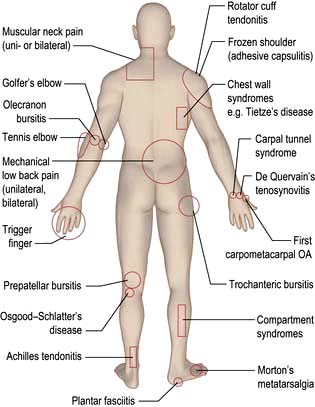
Common regional musculoskeletal problems (fig. 11.2)
Pain in the neck and shoulder (Table 11.4)
Mechanical or muscular neck pain (shoulder girdle pain)
Spondylosis seen on X-ray increases after the age of 40 years, but it is not always causal. Spondylosis can, however, cause stiffness and increases the risk of mechanical or muscular neck pain. Muscle spasm is palpable and tender and may lead to abnormal neck posture (e.g. acute torticollis). Muscular-pattern neck pain is not localized but affects the trapezius muscle, the C7 spinous process and the paracervical musculature (shoulder girdle pain). Pain often radiates upwards to the occiput and is commonly associated with tension headaches. These features are also seen in chronic widespread pain (see p. 509).
Treatment
Patients are given short courses of analgesic therapy along with reassurance and explanation. Physiotherapists can help to relieve spasm and pain, teach exercises and relaxation techniques, and improve posture. An occupational therapist can advise about the ergonomics of the workplace if the problem is work-related (see p. 510).
Nerve root entrapment
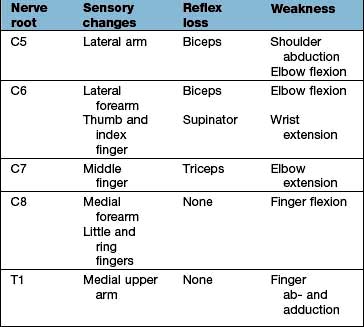
Acute cervical disc prolapse presents with unilateral pain in the neck, radiating to the interscapular and shoulder regions. This diffuse, aching dural pain is followed by sharp, electric shock-like pain down the arm, in a nerve root distribution, often with pins and needles, numbness, weakness and loss of reflexes (Table 11.5).
Cervical spondylosis occurs in the older patient with posterolateral osteophytes compressing the nerve root and causing root pain (see Fig. 22.58, p. 1148), commonly at C5/C6 or C6/C7; it is seen on oblique radiographs of the neck. An MRI scan clearly distinguishes facet joint OA, root canal narrowing and disc prolapse.
Treatment
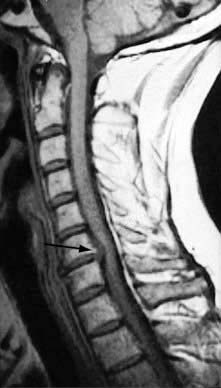
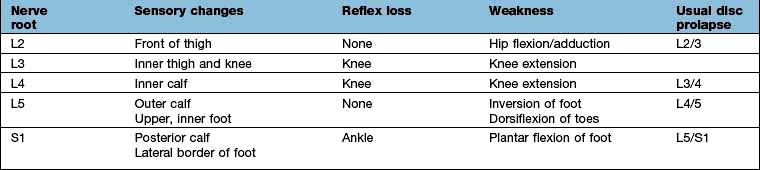
A support collar, rest, analgesia and sedation are used initially as necessary. Patients should be advised not to carry heavy items. It usually recovers in 6–12 weeks. MRI is the investigation of choice if surgery is being considered or the diagnosis is uncertain (Fig. 11.3). A cervical root block administered under direct vision by an experienced pain specialist may relieve pain while the disc recovers. Neurosurgical referral is essential if the pain persists or if the neurological signs of weakness or numbness are severe or bilateral. Bilateral root pain with or without long track symptoms or signs is a neurosurgical emergency because a central disc prolapse may compress the cervical spinal cord. Posterior osteophytes may cause spinal claudication and cervical myelopathy.
Pain in the shoulder
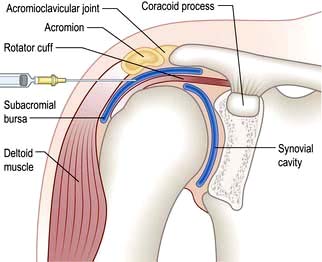
The shoulder is a shallow joint with a large range of movement. The humeral head is held in place by the rotator cuff (Fig. 11.4) which is part of the joint capsule. It comprises the tendons of infraspinatus and teres minor posteriorly, supraspinatus superiorly and teres major and subscapularis anteriorly. The rotator cuff (particularly supraspinatus) prevents the humeral head blocking against the acromion during abduction; the deltoid pulls up and the supraspinatus pulls in to produce a turning movement and the greater tuberosity glides under the acromion without impingement. Shoulder pathology restricts or is made worse by shoulder movement. Specific diagnoses are difficult to make clinically but this may not matter for pain management.
Pain in the shoulder can sometimes be due to problems in the neck. The differential diagnosis of this is shown in Box 11.1. Adhesive capsulitis (true frozen shoulder) is uncommon (see below). Early inflammatory arthritis and polymyalgia rheumatica in the elderly may present with shoulder pain. Shoulder pain is more common in diabetic patients than in the general population.
![]() Box 11.1
Box 11.1
Differential diagnosis of ‘shoulder’ pain
 Rotator cuff tendonitis pain is worse at night and radiates to the upper arm.
Rotator cuff tendonitis pain is worse at night and radiates to the upper arm.
 Painful shoulders produce secondary muscular neck pain.
Painful shoulders produce secondary muscular neck pain.
 Muscular neck pain (also known as shoulder girdle pain) does not radiate to the upper arm.
Muscular neck pain (also known as shoulder girdle pain) does not radiate to the upper arm.
 Cervical nerve root pain is usually associated with pins and needles or neurological signs in the arm.
Cervical nerve root pain is usually associated with pins and needles or neurological signs in the arm.
Rotator cuff (supraspinatus) tendonosis
Treatment
Analgesics, NSAIDs and/or physiotherapy may suffice, but severe pain responds to an injection of corticosteroid into the subacromial bursa (Fig. 11.4). Patients should be warned that 10% will develop worse pain for 24–48 hours after injection. Some 70% improve over 5–20 days and mobilize the joint themselves. Physiotherapy helps persistent stiffness. Further ultrasound-guided corticosteroid injections may be needed but the long-term benefit is unclear.
Pain in the elbow
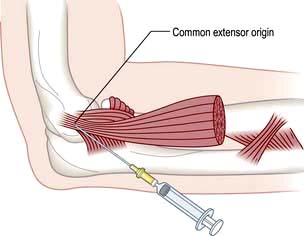
Epicondylitis
Treatment
Advise rest and arrange review by a physiotherapist. A local injection of corticosteroid at the point of maximum tenderness is helpful when the pain is severe but needs physiotherapy follow-up to prevent recurrences (Fig. 11.5). Avoid the ulnar nerve when injecting golfer’s elbow. Both conditions settle spontaneously eventually, but occasionally persist and require surgical release.
Pain in the hand and wrist (table 11.6)
| All ages | Older patients |
|---|---|
|
Trauma/fractures |
Nodal OA: |
|
Tenosynovitis: |
DIPs (Heberden’s nodes) |
|
Flexor with/without triggering |
PIPs (Bouchard’s nodes) |
|
Dorsal |
|
|
De Quervain’s |
Trauma – scaphoid fracture |
|
Pseudogout |
|
|
Gout: |
|
|
Acute |
|
|
Tophaceous |
|
|
|
DIPs, PIPs, distal and proximal interphalangeal joints.
Carpal tunnel syndrome
This is due to median nerve compression in the limited space of the carpal tunnel. Thickened ligaments, tendon sheaths or bone enlargement can cause it, but it is usually idiopathic. (Causes are discussed on p. 1144.) The history is usually typical and diagnostic with the patient waking with numbness, tingling and pain in a median nerve distribution. The pain radiates to the forearm. The fingers feel swollen but usually are not. Wasting of the abductor pollicis brevis develops with sensory loss in the radial three and a half fingers. The pain may be produced by tapping the nerve in the carpal tunnel (Tinel’s sign) or by holding the wrist in flexion (Phalen’s test).
Other conditions causing pain
Nodal osteoarthritis. This affects the DIP and less commonly PIP joints, which are initially swollen and red. The inflammation and pain settle but bony swellings remain (p. 514).
Pain in the lower back
Low back pain is a common symptom. It is often traumatic and work-related, although lifting apparatus and other mechanical devices and improved office seating help to avoid it. Episodes are generally short-lived and self-limiting, and patients attend a physiotherapist or osteopath more often than a doctor. Chronic back pain is the cause of 14% of long-term disability in the UK. The causes are listed in Table 11.7, and the management of back pain is summarized in Box 11.2.
|
Mechanical |
|
Inflammatory |
|
Metabolic |
|
Neoplastic (see p. 589) |
Referred pain
![]() Box 11.2
Box 11.2
Management of back pain
 Most back pain presenting to a primary care physician needs no investigation.
Most back pain presenting to a primary care physician needs no investigation.
 Pain between the ages of 20 and 55 years is likely to be mechanical and is managed with analgesia, brief rest if necessary and physiotherapy.
Pain between the ages of 20 and 55 years is likely to be mechanical and is managed with analgesia, brief rest if necessary and physiotherapy.
 Patients should stay active within the limits of their pain.
Patients should stay active within the limits of their pain.
 Early treatment of the acute episode, advice and exercise programmes reduce long-term problems and prevent chronic pain syndromes.
Early treatment of the acute episode, advice and exercise programmes reduce long-term problems and prevent chronic pain syndromes.
 Physical manipulation of uncomplicated back pain produces short-term relief and enjoys high patient satisfaction ratings.
Physical manipulation of uncomplicated back pain produces short-term relief and enjoys high patient satisfaction ratings.
 Psychological and social factors may influence the time of presentation.
Psychological and social factors may influence the time of presentation.
Investigations
 Spinal X-rays are required only if the pain is associated with certain ‘red flag’ symptoms or signs, which indicate a high risk of more serious underlying problems:
Spinal X-rays are required only if the pain is associated with certain ‘red flag’ symptoms or signs, which indicate a high risk of more serious underlying problems:
 MRI is preferable to CT scanning when neurological signs and symptoms are present. CT scans demonstrate bony pathology better. Interpretation of the relevance of the findings may require a specialist opinion.
MRI is preferable to CT scanning when neurological signs and symptoms are present. CT scans demonstrate bony pathology better. Interpretation of the relevance of the findings may require a specialist opinion.
 Bone scans are useful in infective and malignant lesions but are also positive in degenerative lesions.
Bone scans are useful in infective and malignant lesions but are also positive in degenerative lesions.
 Full blood count, ESR and biochemical tests are required only when the pain is likely to be due to malignancy, infection or a metabolic cause. Normal ESR and CRP distinguish mechanical back pain from polymyalgia rheumatica, a likely differential in the elderly.
Full blood count, ESR and biochemical tests are required only when the pain is likely to be due to malignancy, infection or a metabolic cause. Normal ESR and CRP distinguish mechanical back pain from polymyalgia rheumatica, a likely differential in the elderly.
Mechanical low back pain
Examination and management
 pre-existing chronic widespread pain (fibromyalgia)
pre-existing chronic widespread pain (fibromyalgia)
 psychosocial factors such as high levels of psychological distress, poor self-rated health and dissatisfaction with employment.
psychosocial factors such as high levels of psychological distress, poor self-rated health and dissatisfaction with employment.
Spinal movement occurs at the disc and the posterior facet joints, and stability is normally achieved by a complex mechanism of spinal ligaments and muscles. Any of these structures may be a source of pain. An exact anatomical diagnosis is difficult, but some typical syndromes are recognized (see below). They are often associated with but not necessarily caused by radiological spondylosis (see p.1148).
Postural back pain develops in individuals who sit in poorly designed, unsupportive chairs.
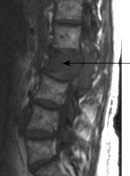
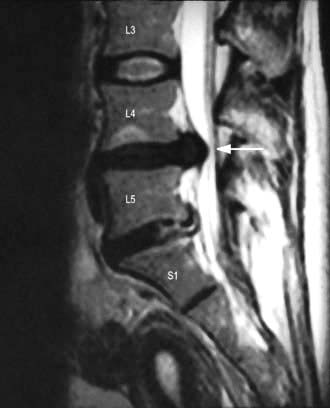
Reactive changes develop in adjacent vertebrae; the bone becomes sclerotic and osteophytes form around the rim of the vertebra (Fig. 11.6). The most common sites of lumbar spondylosis are L5/S1 and L4/L5.
Acute lumbar disc prolapse
The central disc gel may extrude into a fissure in the surrounding fibrous zone and cause acute pain and muscle spasm. These events are often self-limiting. A disc prolapse occurs when the extrusion extends beyond the limits of the fibrous zone (Fig. 11.6). The weakest point is posterolateral, where the disc may impinge on emerging spinal nerve roots in the root canal.
The episode often starts dramatically during lifting, twisting or bending and produces a typical combination of low back pain and muscle spasm, and severe, lancinating pains, paraesthesia, numbness and neurological signs in one leg (rarely both). The back pain is diffuse, usually unilateral and radiates into the buttock. The muscle spasm leads to a scoliosis that reduces when lying down. The nerve root pain develops with, or soon after, the onset. The site of the pain and other symptoms is determined by the root affected (Table 11.8). A central high lumbar disc prolapse may cause spinal cord compression and long tract signs (i.e. upper motor neurone). Below L2/L3 it produces lower motor neurone lesions.
On examination, the back often shows a marked scoliosis and muscle spasm. The straight-leg-raising test, whilst lying, is positive in a lower lumbar disc prolapse – raising the straight leg beyond 30° produces pain in the leg. Slight limitation or pain in the back limiting this movement is seen with mechanical back pain. Pain in the affected leg produced by a straight raise of the other leg suggests a large or central disc prolapse. Look for perianal sensory loss and urinary retention, which indicate a cauda equina lesion – a neurosurgical emergency (see p. 1135). An upper lumbar disc prolapse produces a positive femoral stretch test; pain in the anterior thigh when the knee is flexed in the prone position.
Diffuse idiopathic skeletal hyperostosis (DISH)
DISH (Forestier’s disease) affects the spine and extraspinal locations. It causes bony overgrowths and ligamentous ossification and is characterized by flowing calcification over the anterolateral aspects of the vertebrae. The spine is stiff but not always painful, despite the dramatic X-ray changes. Ossification at muscle insertions around the pelvis produces radiological ‘whiskering’. Similar changes occur at the patella and in the feet. It is commoner in people with metabolic syndrome (high BMI, diabetes mellitus, hypertension and dyslipidaemia; see p. 1006).
Pain in the hip (table 11.9)
| Hip region problems | Main sites of pain |
|---|---|
|
Osteoarthritis of hip |
Groin, buttock, front of thigh to knee |
|
Trochanteric bursitis (or gluteus medius tendonopathy) |
Lateral thigh to knee |
|
Meralgia paraesthetica |
Anterolateral thigh to knee |
|
Referred from back |
Buttock |
|
Facet joint pain |
Buttock and posterior thigh |
|
Fracture of neck of femur |
Groin and buttock |
|
Inflammatory arthritis |
Groin, buttock, front of thigh to knee |
|
Sacroiliitis (AS) |
Buttock(s) |
|
Avascular necrosis |
Groin and buttocks |
|
Polymyalgia rheumatica |
Lumbar spine, buttocks and thighs |
AS, ankylosing spondylitis.
Osteoarthritis (OA)
OA (see p. 512) is the most common cause of hip joint pain in a person over the age of 50 years. It causes pain in the buttock and groin on standing and walking. Stiff hip movements cause difficulty in putting on a sock and may produce a limp. Sudden onset pain may be associated with an effusion on MRI and can be treated by an ultrasound guided steroid injection.
Fracture of the femoral neck
This usually occurs after a fall, occasionally spontaneously. There is pain in the groin and thigh, weight-bearing is painful or impossible, and the leg is shortened and externally rotated. Occasionally, a fracture is not displaced and remains undetected. X-rays are diagnostic. Anyone with a hip fracture, especially after minimal trauma, should be reviewed for osteoporosis (see p. 553).
Avascular necrosis (osteonecrosis) of the femoral head
This is uncommon but occurs at any age. (Risk factors are discussed on p. 556.) There is severe hip pain. X-rays are diagnostic after a few weeks, when a well-demarcated area of increased bone density is visible at the upper pole of the femoral head. The affected bone may collapse. Early, the X-ray is normal but bone scintigraphy or MRI demonstrates the lesion and shows bone marrow oedema.
Pain in the knee (table 11.10)
|
Trauma and overuse |
|
Periarticular problems |
Osteoarthritis/Inflammatory arthritis
Other
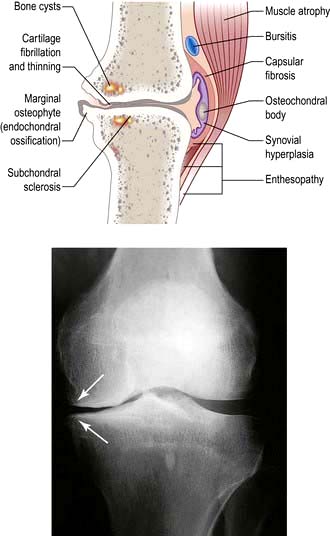
The knee is also a common site of inflammatory arthritis and osteoarthritis. Minor radiographic changes of osteoarthritis (see Fig. 11.11) are common in the over-50s and often coincidental, the cause of the pain being periarticular. Symptomatic osteoarthritis of the knee correlates poorly with the severity of the radiological changes.
Common periarticular knee lesions
Anterior knee pain is common in adolescence. In many cases, no specific cause is found, despite investigation. This is called ‘anterior knee pain syndrome’ and settles with time. Isometric quadriceps exercises and avoidance of high heels both help the condition. Patient and parents often need firm reassurance. Abnormal patellar tracking may be a cause and need surgical treatment. Hypermobility of joints causes joint pain, maltracking and rarely recurrent patellar dislocation (see also p. 546).
Osgood–Schlatter disease (p. 546) causes pain and swelling over the tibial tubercle. It is a traction apophysitis of the patellar tendon and occurs in enthusiastic teenage sports players.
Enthesitis may occur at the patellar end of the tendon (jumper’s knee).
Common intra-articular traumatic lesions of the knee
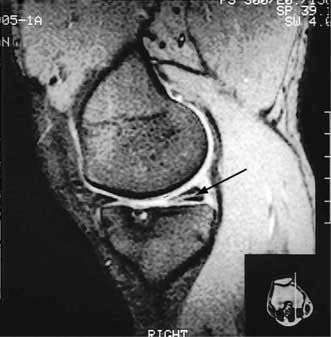
The menisci are partially attached fibrocartilages that stabilize the rounded femoral condyles on the flat tibial plateaux. In the young they are resilient but this decreases with age. They can be torn by an injury, commonly in sports that involve twisting and bending. The history is usually diagnostic. There is immediate medial or lateral knee pain and swelling within a few hours. The affected side is tender. If the tear is large the knee may lock flexed. The immediate treatment is to apply ice. MRI demonstrates the tear (Fig. 11.7). In most circumstances, especially in active sportsmen, early arthroscopic repair or trimming of the torn meniscus is essential. Surgical intervention reduces recurrent pain, swelling and locking but not the risk of secondary osteoarthritis. The long-term benefit of early repair of tears is not yet known. Post-surgical quadriceps exercises aid a return to sport and other activities.
Knee joint effusions
Monoarthritis of the knee, associated with severe pain and marked redness, may be due to septic arthritis, or gout in the middle-aged male, or to gout or pseudogout in an older male or female. A cool, clear, viscous effusion is seen in elderly people with moderate or severe symptomatic OA (see p. 512).
Investigations
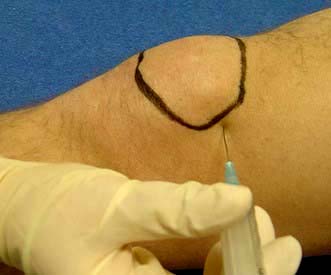
These are (a) blood tests, and (b) aspiration (Fig. 11.8) and examination of the knee effusion. The basic technique of aspiration is described in Practical Box 11.2.
![]() Practical Box 11.2 Joint aspiration
Practical Box 11.2 Joint aspiration
This is a sterile procedure which should be carried out in a clean environment
Explain the procedure to the patient; obtain consent.
1. Decide on the site to insert the needle and mark it.
2. Clean the skin and your hands scrupulously; remove rings and wristwatch. Put on gloves.
3. Draw up local anaesthetic (and corticosteroid if it is being used) and then use a new needle.
4. Warn the patient, insert the needle, injecting local anaesthetic as it advances and, if a joint effusion is suspected, attempt to aspirate as you advance it.
5. If fluid is obtained, change syringes and aspirate fully.
6. Examine the fluid in the syringe and decide whether or not to proceed with a corticosteroid injection (if fluid clear or slightly cloudy) or send for microbiological tests.
7. Cover the injection site and advise the patient to rest the affected area for a few days. Warn the patient that the pain may increase initially but to report urgently if this persists beyond a few days, if the swelling worsens, or if they become febrile, since this might indicate an infected joint.
 Trauma: meniscal, cruciate or synovial lining tear
Trauma: meniscal, cruciate or synovial lining tear
 Clotting or bleeding disorders: such as haemophilia, sickle cell disease or von Willebrand’s disease.
Clotting or bleeding disorders: such as haemophilia, sickle cell disease or von Willebrand’s disease.
A history of previous knee problems and the sudden onset of pain and tenderness high in the calf suggest a ruptured cyst rather than a deep vein thrombosis (DVT). However, the diagnosis is often missed and treated inappropriately with anticoagulants. A diagnostic ultrasound examination distinguishes a ruptured cyst from a DVT (see p. 789). Analgesics or NSAIDs, rest with the leg elevated, and aspiration and injection with corticosteroids into the knee joint are required.
Pain in the foot and heel (table 11.11)
|
Structural (flat (pronated) or high arched (supinated)) |
|
|
Hallux valgus/rigidus (±OA) |
|
|
Metatarsalgia |
|
|
Morton’s neuroma |
|
|
Stress fracture |
|
|
Inflammatory arthritis |
|
|
Acute, monoarticular – gout |
|
|
Chronic, polyarticular – RA |
|
|
Chronic, pauciarticular – spondyloarthritis |
|
|
Tarsal tunnel syndrome |
|
|
Heel pain |
|
|
Plantar fasciitis |
Below heel |
|
Plantar spur |
Below heel |
|
Achilles tendonitis/bursitis |
Behind heel |
|
Sever’s disease |
Behind heel |
|
Arthritis of ankle/subtaloid joints |
|
There are two common types of foot deformity:
 Flat feet: stress the ankle and throw the hindfoot into a valgus (everted) position. A flat foot is rigid and inflexible.
Flat feet: stress the ankle and throw the hindfoot into a valgus (everted) position. A flat foot is rigid and inflexible.
 High-arched feet: place pressure on the lateral border and ball of the foot.
High-arched feet: place pressure on the lateral border and ball of the foot.
The foot is affected by a variety of inflammatory arthritic conditions. After the hand, the foot joints are the most commonly affected by rheumatoid arthritis. The diagnosis depends upon careful assessment of the distribution of the joints affected, the pattern of other joint problems or by finding the associated condition (e.g. psoriasis, see p. 1207).
Pain in the chest
Musculoskeletal conditions are sometimes a cause of chest pain. An example is Tietze’s disease. In this condition, pain arises from the costosternal junctions. It is usually unilateral and affects one, two or three ribs. There is local tenderness, which helps to make the diagnosis. The condition is benign and self-limiting. It often responds well to anti-inflammatory drugs. Other causes of chest wall pain include rib fractures due to trauma or osteoporosis or a malignant deposit. Costochondral pain occurs in ankylosing spondylitis (see p. 527). In people with heart disease, costochondral pain may cause severe anxiety but it is not like angina and the patient should be reassured.
Chronic pain syndromes
Chronic pain syndromes (see p. 1163) are difficult to manage. Psychological factors are at least as relevant as inflammation or damage in determining the patient’s perception of pain. It is essential to be objective and non-judgemental when discussing physical, psychological and social factors without assuming which is primary. Chronic pain syndromes are difficult to explain scientifically. It is all too easy for a doctor to respond to this lack of a clear scientific cause by seeming to ‘blame’ the patient for the symptoms. Many chronic pain states are post-traumatic and some may be exacerbated partly by the process of litigation that may follow an injury.
Chronic widespread pain (fibromyalgia)
Chronic widespread pain is defined as pain for more than three months both above and below the waist (p. 1163). It is a diagnosis of exclusion although it is still not universally accepted as a diagnosis. Multiple trigger points are reported by people with fibromyalgia (see p. 1163; Fig. 11.9). The pain is widespread, with unremitting, aching discomfort. Many patients have sleep disturbances, so they awake unrefreshed and have poor concentration. Multiple other symptoms, e.g. irritable bowel syndrome (IBS), tension headaches, dysmenorrhoea, atypical facial or chest pain, often co-exist. It occurs at any age and affects women more than men (7:1).
Chronic fatigue syndrome
Diffuse muscular pain and stiffness is common in this condition, which is described on page 1162.
Hypermobility and hypermobility syndrome
Many people in the adult population have hypermobile joints (see p. 546). A small proportion are more prone to joint pains, joint instability and autonomic disturbances. This sometimes causes extreme anxiety and manifests as a chronic pain syndrome. Specific exercises to stabilize the joints, recognition of the problem and, sometimes, cognitive behavioral therapy all help. Surgery is best avoided because of problems with healing.
Analgesic and anti-inflammatory drugs for musculoskeletal problems
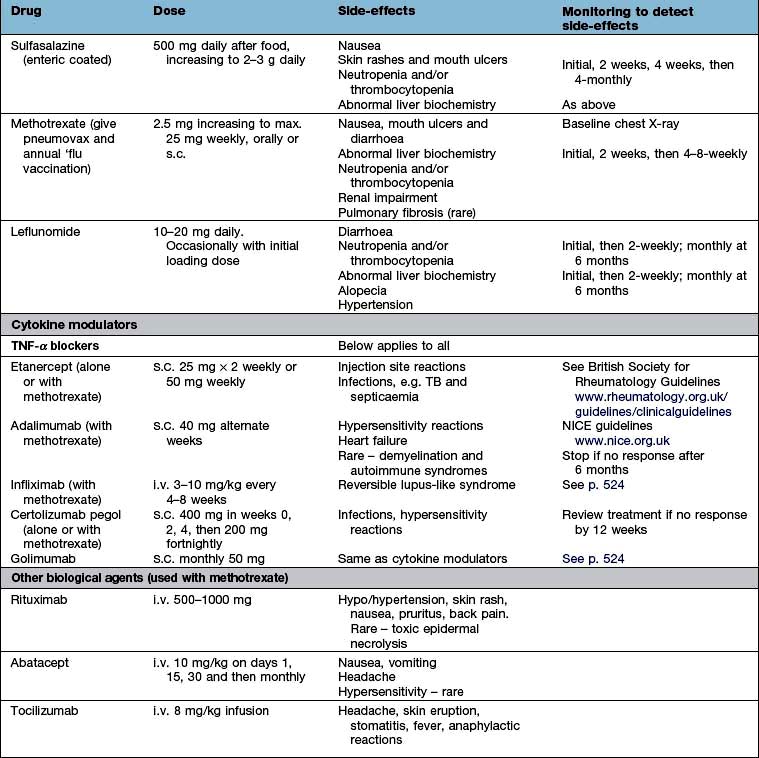
The key to using drugs, particularly in chronic disorders and the elderly, is to balance risk and benefit and constantly to review their appropriateness. Box 11.3 shows the main drugs available.
![]() Box 11.3
Box 11.3
Analgesics and NSAIDs
|
Analgesics (in order of potency) Advise that they be taken only if needed. Maximum doses are indicated here: |
||
|
Paracetamol |
500–1000 mg |
6-hourly |
|
Paracetamol (500 mg) and codeine (8–30 mg) |
1–2 tablets |
6-hourly |
|
Dihydrocodeine |
30–60 mg |
Every 6–8 h |
|
Paracetamol with dihydrocodeine |
1–2 tablets |
Every 6–8 h |
|
Non-steroidal anti-inflammatory drugs (NSAIDs) |
||
|
Always to be taken with food. Use slow-release preparations in inflammatory conditions or if more regular pain control is needed. Examples are: |
||
|
Ibuprofen |
200–400 mg |
Every 6–8 h |
|
Ibuprofen slow release |
600–800 mg |
12-hourly |
|
Diclofenac |
25–50 mg |
8-hourly |
|
Diclofenac slow release |
75–100 mg |
× 1–2 daily |
|
Naproxen |
250 mg |
× 3–4 daily |
|
Naproxen slow release |
550 mg |
× 2 daily |
|
Celecoxiba |
100–200 mg |
× 2 daily |
Non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs have anti-inflammatory and centrally acting analgesic properties. They inhibit cyclo-oxygenase (COX), a key enzyme in the formation of prostaglandins, prostacyclins and thromboxanes (see Fig. 15.30). There are two specific cyclo-oxygenase enzymes:
 COX-1 is the constitutive form present in many normal tissues.
COX-1 is the constitutive form present in many normal tissues.
 COX-2 is the form mainly induced in response to pro-inflammatory cytokines and not found in most normal tissues (except the kidney). It is associated with oedema and the nociceptive and pyretic effects of inflammation.
COX-2 is the form mainly induced in response to pro-inflammatory cytokines and not found in most normal tissues (except the kidney). It is associated with oedema and the nociceptive and pyretic effects of inflammation.
Coxibs and NSAIDs may reduce renal function, especially in the elderly (see Box 12.3, p. 608) and rarely cause cardiovascular events.
 Short courses of NSAIDs or coxibs are used in musculoskeletal pain and in osteoarthritis and spondylosis but simple analgesia is often more appropriate.
Short courses of NSAIDs or coxibs are used in musculoskeletal pain and in osteoarthritis and spondylosis but simple analgesia is often more appropriate.
 In crystal synovitis, NSAIDs and coxibs have a true anti-inflammatory effect (see p. 511).
In crystal synovitis, NSAIDs and coxibs have a true anti-inflammatory effect (see p. 511).
 In chronic inflammatory synovitis, NSAIDs and coxibs do not alter the chronic inflammatory process, or decrease the risk of joint damage, but they do reduce pain and stiffness.
In chronic inflammatory synovitis, NSAIDs and coxibs do not alter the chronic inflammatory process, or decrease the risk of joint damage, but they do reduce pain and stiffness.
 Slow-release preparations are useful for inflammatory arthritis and when more constant pain control is needed.
Slow-release preparations are useful for inflammatory arthritis and when more constant pain control is needed.
 Be aware of the patient’s gastrointestinal and cardiac risks before prescribing NSAIDs or coxibs.
Be aware of the patient’s gastrointestinal and cardiac risks before prescribing NSAIDs or coxibs.
Osteoarthritis (OA)
Epidemiology
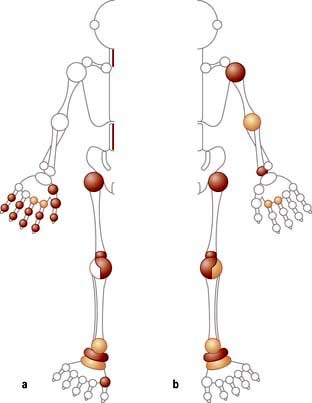
The prevalence of OA increases with age; it is uncommon below the age of 50 years and most people over 60 years will have some radiological evidence of it, although only a quarter of these will be symptomatic. It occurs worldwide, but with a variable distribution, e.g. in Asians, hip OA is less common and knee OA is more common than in Europeans. Women over 55 years are affected more commonly than men of a similar age. There is a familial pattern of inheritance in nodal OA and in primary generalized OA. OA has a variable distribution (Fig. 11.10). The resulting disabilities have major socioeconomic resource implications, particularly in the developed world. OA is the most common cause of disability in the Western world in older adults.
Aetiology (Box 11.4)
![]() Box 11.4
Box 11.4
Factors predisposing to osteoarthritis
 Obesity: Predicts later risk of radiological and symptomatic OA of the hip and hand in population studies
Obesity: Predicts later risk of radiological and symptomatic OA of the hip and hand in population studies
 Heredity: Familial tendency to develop nodal and generalized OA
Heredity: Familial tendency to develop nodal and generalized OA
 Gender: Polyarticular OA is more common in women; a higher prevalence after the menopause suggests a role for sex hormones
Gender: Polyarticular OA is more common in women; a higher prevalence after the menopause suggests a role for sex hormones
 Hypermobility (see p. 546): Increased range of joint motion and reduced stability lead to OA
Hypermobility (see p. 546): Increased range of joint motion and reduced stability lead to OA
 Osteoporosis: There is a reduced risk of OA
Osteoporosis: There is a reduced risk of OA
 Trauma: A fracture through any joint. Meniscal and cruciate ligament tears cause OA of the knee
Trauma: A fracture through any joint. Meniscal and cruciate ligament tears cause OA of the knee
 Congenital joint dysplasia: Alters joint biomechanics and leads to OA. Mild acetabular dysplasia is common and leads to earlier onset of hip OA
Congenital joint dysplasia: Alters joint biomechanics and leads to OA. Mild acetabular dysplasia is common and leads to earlier onset of hip OA
 Joint congruity: Congenital dislocation of the hip or a slipped femoral epiphysis or Perthes’ disease; osteonecrosis of the femoral head (see p. 556) in children and adolescents causes early-onset OA
Joint congruity: Congenital dislocation of the hip or a slipped femoral epiphysis or Perthes’ disease; osteonecrosis of the femoral head (see p. 556) in children and adolescents causes early-onset OA
 Occupation: Miners develop OA of the hip, knee and shoulder, cotton workers OA of the hand, and farmers OA of the hip
Occupation: Miners develop OA of the hip, knee and shoulder, cotton workers OA of the hand, and farmers OA of the hip
 Sport: Repetitive use and injury in some sports causes a high incidence of lower-limb OA.
Sport: Repetitive use and injury in some sports causes a high incidence of lower-limb OA.
Cartilage is a matrix of collagen fibres, which enclose a mixture of proteoglycans and water (see p. 494). The gene for human aggrecan has been cloned, and polymorphisms of the gene have been correlated with OA of the hand in older men.
Cartilage is smooth-surfaced and shock-absorbing. Under normal circumstances, there is a dynamic balance between cartilage degradation by wear and its production by chondrocytes. Early in the development of OA, this balance is lost and, despite increased synthesis of extracellular matrix, the cartilage becomes oedematous. Focal erosion of cartilage develops. Chondrocytes die and, although repair is attempted from adjacent cartilage, the process is disordered. Eventually the synthesis of extracellular matrix fails and the surface becomes fibrillated and fissured. Cartilage ulceration exposes underlying bone to increased stress, producing microfractures and cysts. The bone attempts repair but produces abnormal sclerotic subchondral bone and overgrowths at the joint margins, called osteophytes (Fig. 11.11). There is some secondary inflammation.
Pathogenesis
Several mechanisms have been suggested:
 Abnormal stress and loading leading to mechanical cartilage damage play a role in secondary OA.
Abnormal stress and loading leading to mechanical cartilage damage play a role in secondary OA.
 Obesity is a risk factor for developing OA of the hand and knee, but not the hip in later life. Increased skeletal mass increases cartilage volume.
Obesity is a risk factor for developing OA of the hand and knee, but not the hip in later life. Increased skeletal mass increases cartilage volume.
 Collagenases (MMP-1 and MMP-13) cleave collagen, and other metalloproteinases such as stromelysin (MMP-3) and gelatinases (MMP-2 and MMP-9) are also present in the extracellular matrix. MMPs are secreted by chondrocytes in an inactive form. Extracellular activation then leads to the degradation of both collagen and proteoglycans around chondrocytes.
Collagenases (MMP-1 and MMP-13) cleave collagen, and other metalloproteinases such as stromelysin (MMP-3) and gelatinases (MMP-2 and MMP-9) are also present in the extracellular matrix. MMPs are secreted by chondrocytes in an inactive form. Extracellular activation then leads to the degradation of both collagen and proteoglycans around chondrocytes.
 Tissue inhibitors of metalloproteinases (TIMPs) regulate the MMPs. Disturbance of this regulation may lead to an increase in cartilage degradation over synthesis and contribute to the development of OA. TIMPs have not yet proven to be of therapeutic value.
Tissue inhibitors of metalloproteinases (TIMPs) regulate the MMPs. Disturbance of this regulation may lead to an increase in cartilage degradation over synthesis and contribute to the development of OA. TIMPs have not yet proven to be of therapeutic value.
 Osteoprotegerin (OPG), RANK and RANK ligand (RANKL) control the remodelling of subchondral bone remodelling. Their levels are significantly different in OA chondrocytes. Inhibiting RANKL may prove a new therapeutic approach in OA.
Osteoprotegerin (OPG), RANK and RANK ligand (RANKL) control the remodelling of subchondral bone remodelling. Their levels are significantly different in OA chondrocytes. Inhibiting RANKL may prove a new therapeutic approach in OA.
 Aggrecanase production is stimulated by pro-inflammatory cytokines and aggrecan (the major proteoglycan) levels fall.
Aggrecanase production is stimulated by pro-inflammatory cytokines and aggrecan (the major proteoglycan) levels fall.
 Synovial inflammation is present in OA, and CRP in the serum may be raised. Interleukin-1 (IL-1) and tumour necrosis factor (TNF-α) release stimulates metalloproteinase production and IL-1 inhibits type II collagen production. IL-6 and IL-8 may also be involved. Anticytokine therapy has not yet been tested in OA. The production of cytokines by macrophages and of MMPs by chondrocytes in OA are dependent on the transcription factor NF-κB. Inhibition of NF-κB may have a therapeutic role in OA.
Synovial inflammation is present in OA, and CRP in the serum may be raised. Interleukin-1 (IL-1) and tumour necrosis factor (TNF-α) release stimulates metalloproteinase production and IL-1 inhibits type II collagen production. IL-6 and IL-8 may also be involved. Anticytokine therapy has not yet been tested in OA. The production of cytokines by macrophages and of MMPs by chondrocytes in OA are dependent on the transcription factor NF-κB. Inhibition of NF-κB may have a therapeutic role in OA.
 IL-1 receptor antagonist genes are associated with radiographic severity of knee OA.
IL-1 receptor antagonist genes are associated with radiographic severity of knee OA.
 Growth factors, including insulin-like growth factor (IGF-1) and transforming growth factor (TGF-β), are involved in collagen synthesis, and their deficiency may play a role in impairing matrix repair. However, increased TGF-β may cause increased subchondral bone density.
Growth factors, including insulin-like growth factor (IGF-1) and transforming growth factor (TGF-β), are involved in collagen synthesis, and their deficiency may play a role in impairing matrix repair. However, increased TGF-β may cause increased subchondral bone density.
 Cartilage breakdown products lead to macrophage infiltration and vascular hyperplasia and IL1-β and TNF-α may contribute to further cartilage degradation.
Cartilage breakdown products lead to macrophage infiltration and vascular hyperplasia and IL1-β and TNF-α may contribute to further cartilage degradation.
 Vascular endothelial growth factor (VEGF) from macrophages is a potent stimulator of angiogenesis and may contribute to inflammation and neovascularization in OA. Innervation can accompany vascularization of the articular cartilage.
Vascular endothelial growth factor (VEGF) from macrophages is a potent stimulator of angiogenesis and may contribute to inflammation and neovascularization in OA. Innervation can accompany vascularization of the articular cartilage.
 Mutations in the gene for type II collagen (COL2A1) have been associated with early polyarticular OA.
Mutations in the gene for type II collagen (COL2A1) have been associated with early polyarticular OA.
 A strong hereditary element underlying OA is suggested by twin studies. Further studies may reveal genetic markers for the disease. The influence of genetic factors is estimated at 35–65%.
A strong hereditary element underlying OA is suggested by twin studies. Further studies may reveal genetic markers for the disease. The influence of genetic factors is estimated at 35–65%.
 In the Caucasian population there is an inverse relationship between the risk of developing OA and osteoporosis.
In the Caucasian population there is an inverse relationship between the risk of developing OA and osteoporosis.
 Gender. In women, weight-bearing sports produce a two- to three-fold increase in risk of OA of the hip and knee. In men, there is an association between hip OA and certain occupations: farming and labouring. OA may flare after the female menopause or after stopping hormone replacement therapy.
Gender. In women, weight-bearing sports produce a two- to three-fold increase in risk of OA of the hip and knee. In men, there is an association between hip OA and certain occupations: farming and labouring. OA may flare after the female menopause or after stopping hormone replacement therapy.
 Periarticular enthesitis has been proposed as a factor in the pathogenesis of nodal generalized OA (NGOA; p. 515) and is the subject of investigation.
Periarticular enthesitis has been proposed as a factor in the pathogenesis of nodal generalized OA (NGOA; p. 515) and is the subject of investigation.
The term primary OA is sometimes used when there is no obvious known predisposing factor.
Box 11.4 shows some of the predisposing factors for the development of OA, and Table 11.12 shows other conditions that sometimes cause secondary arthritis.
|
Primary OA |
No known cause |
|
Secondary OA |
Pre-existing joint damage: |
|
Rheumatoid arthritis |
|
|
Gout |
|
|
Spondyloarthritis |
|
|
Septic arthritis |
|
|
Paget’s disease |
|
|
Avascular necrosis, e.g. corticosteroid therapy |
|
|
Metabolic disease: |
|
|
Chondrocalcinosis |
|
|
Hereditary haemochromatosis |
|
|
Acromegaly |
|
|
Systemic diseases: |
|
|
Haemophilia – recurrent haemarthrosis |
|
|
Haemoglobinopathies, e.g. sickle cell disease |
|
|
Neuropathies |
Clinical subsets
Localized OA
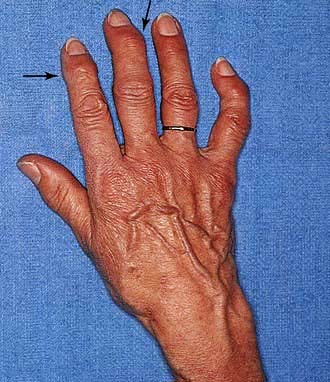
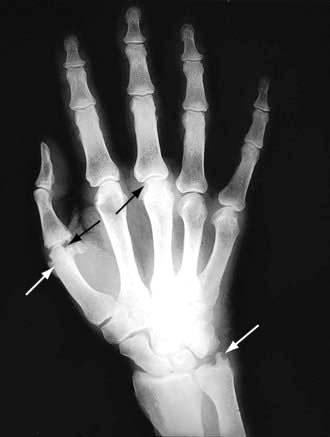
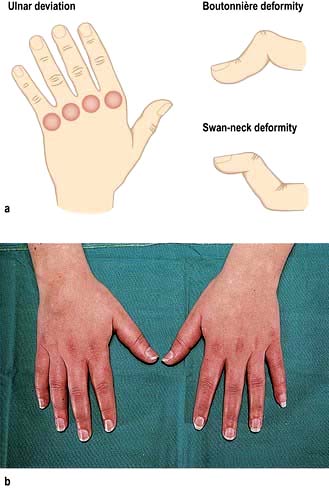
Joints of the hand are usually affected one at a time over several years, with the distal interphalangeal joints (DIPs) being more often involved than the proximal interphalangeal joints (PIPs). Nodal OA often starts around the female menopause. The onset may be painful and associated with tenderness, swelling and inflammation and impairment of hand function. At this stage, enthesitis can be seen on MRI. An intra-articular corticosteroid injection can be used at this stage, if deemed necessary. The inflammatory phase settles after some months or years, leaving painless bony swellings posterolaterally: Heberden’s nodes (DIPs) and Bouchard’s nodes (PIPs), along with stiffness and deformity (Fig. 11.12). Functional impairment is slight for most, although PIP osteoarthritis restricts gripping more than DIP involvement. On X-ray, the nodes are marginal osteophytes and there is joint space loss.
Thumb base OA co-exists with nodal OA and causes pain and disability, which decrease as the joint stiffens. The ‘squared’ hand in OA (Fig. 11.12) is caused by bony swelling of the carpometacarpal joint and fixed adduction of the thumb. Function is rarely severely compromised.
Polyarticular hand OA is associated with a slightly increased frequency of OA at other sites.
Hip OA (see p. 494) affects 7–25% of adult Caucasians but is significantly less common in black African and Asian populations. There are two major subgroups defined by the radiological appearance. The most common is superior-pole hip OA, where joint space narrowing and sclerosis predominantly affect the weight-bearing upper surface of the femoral head and adjacent acetabulum. This is most common in men and unilateral at presentation, although both hips may become involved because the disease is progressive. Early onset of hip OA is associated with acetabular dysplasia or labral tears. Less commonly, medial cartilage loss occurs. This is most common in women and associated with hand involvement (nodal generalized OA – NGOA), and is usually bilateral. It is more rapidly disabling.
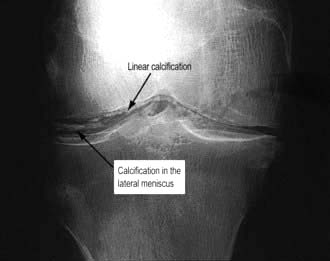
This is most commonly seen with calcium pyrophosphate deposition in the cartilage (chondrocalcinosis). Chondrocalcinosis increases in frequency with age and is seen on over 40% of knee X-rays in the over-80s, but is usually asymptomatic. The joints most commonly affected are the knees (hyaline cartilage and fibrocartilage) and wrists (triangular fibrocartilage, see Fig. 11.10). There is patchy linear calcification on X-ray (Fig. 11.13).
A chronic arthropathy (pseudo-OA) occurs, predominantly in elderly women with severe chondrocalcinosis. There is a florid inflammatory component and marked osteophyte and cyst formation visible on X-rays. The joints affected differ from NGOA, being predominantly the knees, then wrists and shoulders. Chondrocalcinosis is associated with pseudogout, an acute crystal-induced arthritis (see p. 532).
Investigations in OA
 Blood tests. There is no specific test; the ESR is normal although high sensitivity CRP may be slightly raised. Rheumatoid factor and antinuclear antibodies are negative.
Blood tests. There is no specific test; the ESR is normal although high sensitivity CRP may be slightly raised. Rheumatoid factor and antinuclear antibodies are negative.
 X-rays are abnormal only when the damage is advanced. They are useful in preoperative assessments. For knees, a standing X-ray (stressed) is used to assess cartilage loss and ‘skyline’ views in flexion for patello-femoral OA.
X-rays are abnormal only when the damage is advanced. They are useful in preoperative assessments. For knees, a standing X-ray (stressed) is used to assess cartilage loss and ‘skyline’ views in flexion for patello-femoral OA.
 MRI demonstrates meniscal tears, early cartilage injury and subchondral bone marrow changes (osteochondral lesions).
MRI demonstrates meniscal tears, early cartilage injury and subchondral bone marrow changes (osteochondral lesions).
 Arthroscopy reveals early fissuring and surface erosion of the cartilage.
Arthroscopy reveals early fissuring and surface erosion of the cartilage.
 Aspiration of synovial fluid (if there is a painful effusion) shows a viscous fluid with few leucocytes (p. 498).
Aspiration of synovial fluid (if there is a painful effusion) shows a viscous fluid with few leucocytes (p. 498).
Inflammatory arthritis
Inflammatory arthritis (Table 11.14) includes a large number of arthritic conditions in which the predominant feature is synovial inflammation (Box 11.5). This disparate group includes post-viral arthritis, rheumatoid arthritis, spondyloarthritis, crystal arthritis, and Lyme arthritis. The diagnosis of these conditions is helped by the pattern of joint involvement (symmetrical or asymmetrical; large or small) (Table 11.14), along with any non-articular disease; a past and family history is helpful. The periodicity of the arthritis (single acute, relapsing, chronic and progressive) also helps in the diagnosis.
Table 11.14 Pattern of joint involvement in inflammatory arthritis
|
Diseases presenting as an inflammatory monoarthritis |
Diseases presenting as an inflammatory polyarthritis
There is a distinct genetic separation of rheumatoid-pattern synovitis and spondyloarthritis; RA (see below) is associated with a genetic marker in the class II major histocompatibility genes, whilst spondyloarthritis shares certain alleles in the B locus of class I MHC genes, usually B27 (see p. 526).
Early inflammatory polyarthritis
Undifferentiated polyarthritis requires urgent referral to a rheumatologist for diagnosis and treatment, including the early introduction of disease-modifying agents when indicated (see p. 523). In persistent inflammatory arthritis sustained remission depends on rapid diagnosis and intensive treatment. Poor prognostic features for undifferentiated polyarthritis are:
Rheumatoid arthritis (ra)
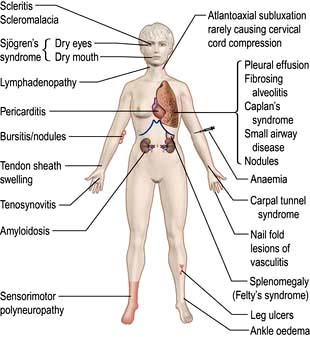
Aetiology and pathogenesis
The cause is multifactorial and genetic and environmental factors play a part.
 Gender. Women before the menopause are affected three times more often than men. After the menopause, the frequency of onset is similar between the sexes, suggesting an aetiological role for sex hormones. A meta-analysis of the use of the oral contraceptive pill has shown no effect on RA overall, but it may delay the onset of disease.
Gender. Women before the menopause are affected three times more often than men. After the menopause, the frequency of onset is similar between the sexes, suggesting an aetiological role for sex hormones. A meta-analysis of the use of the oral contraceptive pill has shown no effect on RA overall, but it may delay the onset of disease.
 Familial. The disease is familial with an increased incidence in first-degree relatives and a high concordance amongst monozygotic twins (up to 15%) and dizygotic twins (3.5%). In occasional families it affects several generations.
Familial. The disease is familial with an increased incidence in first-degree relatives and a high concordance amongst monozygotic twins (up to 15%) and dizygotic twins (3.5%). In occasional families it affects several generations.
 Genetic factors account for about 60% of disease susceptibility. There is a strong association between susceptibility to RA and certain HLA haplotypes: HLA-DR4, which occurs in 50–75% of patients and correlates with a poor prognosis, as does possession of certain shared alleles of HLA-DRB1*04. The possession of these shared epitope alleles in HLA-DRB1 (S2 and S3P) increases susceptibility to RA and may predispose to anti-citrullinated peptide antibodies (ACPA). Citrullination is a process which modifies antigens, allowing them to fit into the shared epitope on HLA alleles. In a genome-wide association study in ACPA-positive RA, an association was found with loci near HLA-DRBI and PTPN22 in people of European descent. These genes affect the presentation of autoantigens (HLA-DRBI), T cell receptor signal transduction (PTPN22) and targets of ACPA (PAD14).
Genetic factors account for about 60% of disease susceptibility. There is a strong association between susceptibility to RA and certain HLA haplotypes: HLA-DR4, which occurs in 50–75% of patients and correlates with a poor prognosis, as does possession of certain shared alleles of HLA-DRB1*04. The possession of these shared epitope alleles in HLA-DRB1 (S2 and S3P) increases susceptibility to RA and may predispose to anti-citrullinated peptide antibodies (ACPA). Citrullination is a process which modifies antigens, allowing them to fit into the shared epitope on HLA alleles. In a genome-wide association study in ACPA-positive RA, an association was found with loci near HLA-DRBI and PTPN22 in people of European descent. These genes affect the presentation of autoantigens (HLA-DRBI), T cell receptor signal transduction (PTPN22) and targets of ACPA (PAD14).
 Smoking is an environmental risk factor for seropositive RA, possibly by activation of the innate immune system.
Smoking is an environmental risk factor for seropositive RA, possibly by activation of the innate immune system.
RA is primarily a synovial disease which invades local tissues and rheumatoid synovitis results when chemoattractants produced in the joint recruit circulating inflammatory cells. Overproduction and overexpression of tumour necrosis factors (TNF) is a key inflammatory element in RA, leading to synovitis and joint destruction. Interaction of macrophages and T and B lymphocytes drives this overproduction. TNF-α stimulates overproduction of interleukin-6 and other cytokines. Antibodies to TNF-α and IL-6 or specific blocking agents produce marked short-term improvement in synovitis, indicating the pivotal role of these cytokines in the chronic synovitis (see p. 523). These antibodies also reduce the malaise and tiredness felt in active RA. Interleukin-1 plays a bigger role in certain subsets, such as systemic juvenile idiopathic arthritis (see p. 545) and adult-onset Still’s disease (see p. 545).
 Synovial cells in chronic rheumatoid synovitis are predominantly abnormally behaving fibroblast-like synoviocytes, and macrophage-like synoviocytes which produce pro-inflammatory cytokines.
Synovial cells in chronic rheumatoid synovitis are predominantly abnormally behaving fibroblast-like synoviocytes, and macrophage-like synoviocytes which produce pro-inflammatory cytokines.
 Abnormal fibroblast-like synoviocytes appear to circulate between joints and may be the trigger for the polyarthritis.
Abnormal fibroblast-like synoviocytes appear to circulate between joints and may be the trigger for the polyarthritis.
 Osteoclasts play an active role in bone and cartilage destruction.
Osteoclasts play an active role in bone and cartilage destruction.
 B cells in the synovium, activated by cytokine-activated macrophages and T cells produce autoantibodies of which IgM and IgA RF is the most typical in RA. As RFs have the Fc portion of IgG as their antigen they have the potential for self aggregation and immune complex formation in the synovium. These may then trigger macrophages via IgGFc receptors to produce even more cytokines including IL-1, IL-8, TNF-α and granulocyte-macrophage colony-stimulating factor, and fibroblasts to produce IL-6.
B cells in the synovium, activated by cytokine-activated macrophages and T cells produce autoantibodies of which IgM and IgA RF is the most typical in RA. As RFs have the Fc portion of IgG as their antigen they have the potential for self aggregation and immune complex formation in the synovium. These may then trigger macrophages via IgGFc receptors to produce even more cytokines including IL-1, IL-8, TNF-α and granulocyte-macrophage colony-stimulating factor, and fibroblasts to produce IL-6.
 CD20 positive B cell ablation (a technique used for treating B cell lymphomas) induces temporary remission, reinforcing the central role of B cells in the chronic inflammation of RA. As the B cells return, the CRP rises and the disease flares again.
CD20 positive B cell ablation (a technique used for treating B cell lymphomas) induces temporary remission, reinforcing the central role of B cells in the chronic inflammation of RA. As the B cells return, the CRP rises and the disease flares again.
 Synovial fibroblasts have high levels of the adhesion molecule, vascular cell adhesion molecule (VCAM-1: a molecule which supports B lymphocyte survival and differentiation), decay accelerating factor (DAF: a factor that prevents complement-induced cell lysis) and cadherin-II (which mediates cell to cell interactions). These molecules may facilitate the formation of ectopic lymphoid tissue in synovium. Recent studies have shown that mice deficient in cadherin-II are resistant to a form of inflammatory arthritis. High-affinity antibodies are not a feature of RA, unlike other autoimmune diseases.
Synovial fibroblasts have high levels of the adhesion molecule, vascular cell adhesion molecule (VCAM-1: a molecule which supports B lymphocyte survival and differentiation), decay accelerating factor (DAF: a factor that prevents complement-induced cell lysis) and cadherin-II (which mediates cell to cell interactions). These molecules may facilitate the formation of ectopic lymphoid tissue in synovium. Recent studies have shown that mice deficient in cadherin-II are resistant to a form of inflammatory arthritis. High-affinity antibodies are not a feature of RA, unlike other autoimmune diseases.
 T cells can be a part of the destructive process but other subsets reduce its severity. T cell-associated cytokines such as IL-2 and IL-4 are not present in high amounts and, when CD4-specific antibodies were used therapeutically to produce a specific helper T cell lymphopenia, they did not significantly alter the disease, suggesting that T cells play a lesser role. This treatment is no longer used. T17 helper cells (see p. 61), which produce IL-17A, 17F, 21 and 22 and TNF-α, may cause inflammation. The normal regulatory T cells are suppressed by TGP-β and interleukins (produced by macrophages and dendritic cells), allowing the T17 helper cells to increase.
T cells can be a part of the destructive process but other subsets reduce its severity. T cell-associated cytokines such as IL-2 and IL-4 are not present in high amounts and, when CD4-specific antibodies were used therapeutically to produce a specific helper T cell lymphopenia, they did not significantly alter the disease, suggesting that T cells play a lesser role. This treatment is no longer used. T17 helper cells (see p. 61), which produce IL-17A, 17F, 21 and 22 and TNF-α, may cause inflammation. The normal regulatory T cells are suppressed by TGP-β and interleukins (produced by macrophages and dendritic cells), allowing the T17 helper cells to increase.
 The role of innate immunity in RA pathogenesis and in predisposing the joint to inflammation are the subjects of increasing interest (see p. 51).
The role of innate immunity in RA pathogenesis and in predisposing the joint to inflammation are the subjects of increasing interest (see p. 51).
Pathology
Rheumatoid arthritis is typified by widespread persistent synovitis (inflammation of the synovial lining of joints, tendon sheaths or bursae). The normal synovium is thin and comprises a lining layer a few cells thick containing fibroblast-like synoviocytes and macrophages overlying loose connective tissue. The synoviocytes play a central role in synovial inflammation. In RA the synovium becomes greatly thickened and causes ‘boggy’ swelling around the joints and tendons. There is proliferation of the synovium into folds and fronds, and it is infiltrated by a variety of inflammatory cells, including polymorphs, which transit through the tissue into the joint fluid, and lymphocytes and plasma cells. There are disorganized lymphoid follicles. The normally sparse surface layer of lining cells becomes hyperplastic and thickened (Fig. 11.14). There is marked vascular proliferation. Increased permeability of blood vessels and the synovial lining layer leads to joint effusions that contain lymphocytes and dying polymorphs.
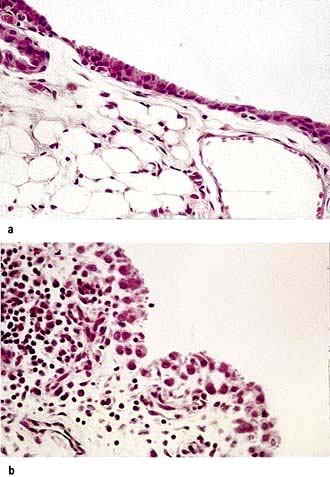
(From Shipley M. Colour Atlas of Rheumatology, 3rd edn. London: Mosby-Wolfe; 1993, with permission.)
Fibroblasts from the proliferating synovium also grow along the course of blood vessels between the synovial margins and the epiphyseal bone cavity and damage the bone. This is shown by MRI to occur in the first 3–6 months following onset of the arthritis, and before the diagnostic, ill-defined juxta-articular bony ‘erosions’ appear on X-ray (Fig. 11.15). This early damage justifies the use of DMARDs (see p. 523) within 3–6 months of onset of the arthritis. Low-dose steroids delay and anti-TNF-α agents halt and occasionally reverse erosion formation. Erosions lead to a variety of deformities and contribute to long-term disability.
Rheumatoid factors (RFs) and anti-citrullinated peptide antibodies (ACPAs)
RFs (see p. 496) are circulating autoantibodies that have the Fc portion of IgG as their antigen. Transient production of RF is an essential part of the body’s normal mechanism for removing immune complexes, but in RA they show a much higher affinity and their production is persistent and occurs in the joints. They are of any immunoglobulin class (IgM, IgG or IgA), but the most common tests employed clinically detect IgM rheumatoid factor. Around 70% of people with polyarticular RA have IgM rheumatoid factor in the serum. Positive titres can predate the onset of RA.
Anti-CCP antibodies (ACPA) (p. 497) are usually present with RF in RA. They are better predictors of a transition from early transient inflammatory arthritis to persistent synovitis and early RA. RF and the ACPA together are even more specific.
Clinical features of ra
Typical presentation
The most typical presentation of rheumatoid arthritis (approximately 70% of cases) begins as a slowly progressive, symmetrical, peripheral polyarthritis, evolving over a period of a few weeks or months. The patient is usually between 30 and 50 years of age, but the disease can occur at any age. Less commonly (15%) a rapid onset can occur over a few days (or explosively overnight) with a severe symmetrical polyarticular involvement, especially in the elderly. Factors which indicate a poor prognosis are listed in Box 11.6. The differential diagnosis of early RA is shown in Box 11.7.
Older classification criteria used to distinguish RA from other forms of arthritis (American College of Rheumatology, ACR criteria 1987) are now mainly used to ensure matched groups for research. The newer criteria that have replaced them are more suitable for assessing early arthritis because they do not rely on later changes such as erosions and extra-articular disease (Box 11.8).
![]() Box 11.8
Box 11.8
ACR/EULAR 2010 criteria for RA
| Criteria | Points |
|---|---|
|
1. Joint involvement |
0–5 |
|
1 medium to large joint |
0 |
|
2–10 medium to large joints |
1 |
|
1–3 small joints (large joints not counted) |
2 |
|
4–10 small joints (large joints not counted) |
3 |
|
>10 joints at least one small joint |
5 |
|
2. Serology |
0–3 |
|
Negative RF and negative ACPA |
0 |
|
Low positive RF or low positive ACPA |
2 |
|
High positive RF or high positive ACPA |
3 |
|
3. Acute-phase reactants |
0–1 |
|
Normal CRP and normal ESR |
0 |
|
Abnormal CRP or abnormal ESR |
1 |
|
4. Duration of symptoms |
0–1 |
|
<6 weeks |
0 |
|
≥6 weeks |
1 |
In early RA, the combination of at least one swollen joint for more than 6 weeks with no prior injury and no associated history or family history of spondyloarthritis or associated conditions such as psoriasis (see p. 1207) and a positive ACPA test is the best way to select patients for earlier treatment to avoid joint damage. This earlier treatment is evidence based and has been shown to reduce the risk of the development of damage and thus reduce permanent joint deformities.
Other presentations
The presentation and progression of RA is variable. Presentations are shown in Box 11.9. Relapses and remissions occur either spontaneously or on drug therapy. In some patients, the disease remains active, producing progressive joint damage. Rarely, the process may cease (‘burnt-out RA’).
![]() Box 11.9
Box 11.9
Presentations of rheumatoid arthritis
 Palindromic: Palindromic monoarticular attacks lasting 24–48 hours; 50% progress to other types of RA.
Palindromic: Palindromic monoarticular attacks lasting 24–48 hours; 50% progress to other types of RA.
 Transient: A self-limiting disease, lasting less than 12 months and leaving no permanent joint damage. Usually seronegative for IgM rheumatoid factor and ACPA. Some of these may be undetected post-viral arthritis.
Transient: A self-limiting disease, lasting less than 12 months and leaving no permanent joint damage. Usually seronegative for IgM rheumatoid factor and ACPA. Some of these may be undetected post-viral arthritis.
 Remitting: There is a period of several years during which the arthritis is active but then remits, leaving minimal damage.
Remitting: There is a period of several years during which the arthritis is active but then remits, leaving minimal damage.
 Chronic, persistent: The most typical form, it may be seropositive or seronegative for IgM rheumatoid factor. The disease follows a relapsing and remitting course over many years. Seropositive (plus ACPA) patients tend to develop greater joint damage and long-term disability. They warrant earlier and more aggressive treatment with disease-modifying agents.
Chronic, persistent: The most typical form, it may be seropositive or seronegative for IgM rheumatoid factor. The disease follows a relapsing and remitting course over many years. Seropositive (plus ACPA) patients tend to develop greater joint damage and long-term disability. They warrant earlier and more aggressive treatment with disease-modifying agents.
 Rapidly progressive: The disease progresses remorselessly over a few years and leads rapidly to severe joint damage and disability. It is usually seropositive (plus ACPA), has a high incidence of systemic complications and is difficult to treat.
Rapidly progressive: The disease progresses remorselessly over a few years and leads rapidly to severe joint damage and disability. It is usually seropositive (plus ACPA), has a high incidence of systemic complications and is difficult to treat.
Seronegative RA initially affects the wrists more often than the fingers and has a less symmetrical joint involvement. It has a better long-term prognosis, but some cases progress to severe disability. This form can be confused with psoriatic arthropathy, which has a similar distribution (p. 528).
Complications (Table 11.15)
This is a serious complication with significant morbidity and mortality. In immunosuppressed patients, the affected joints may not be hot and inflamed with accompanying fever. There is usually a neutrophil leucocytosis. Any effusion, particularly of sudden onset, should be aspirated. Staphylococcus aureus is the most common organism. Blood cultures are often positive. Treatment is with systemic antibiotics (see p. 533) and drainage.
Side-effects of therapy
Amyloidosis (see p. 1042) is found in a very small number of people with uncontrolled rheumatoid arthritis. RA is the most common cause of secondary AA amyloidosis. AL amyloidosis causes a polyarthritis that resembles RA in distribution and is also often associated with carpal tunnel syndrome and subcutaneous nodules.
Joint involvement in RA
 A combination of ulnar drift and palmar subluxation of the MCPs (Fig. 11.16). This leads to unsightly deformity, but function may be remarkably good once the patient has learned to adapt, and pain is controlled
A combination of ulnar drift and palmar subluxation of the MCPs (Fig. 11.16). This leads to unsightly deformity, but function may be remarkably good once the patient has learned to adapt, and pain is controlled
 Fixed flexion (buttonhole or boutonnière deformity) or fixed hyperextension (swan-neck deformity) of the PIP joints, which impairs hand function
Fixed flexion (buttonhole or boutonnière deformity) or fixed hyperextension (swan-neck deformity) of the PIP joints, which impairs hand function
 Swelling and dorsal subluxation of the ulnar styloid, which leads to wrist pain and may cause rupture of the finger extensor tendons, leading in turn to a sudden onset of finger drop of the little and ring fingers predominantly, which needs urgent surgical repair.
Swelling and dorsal subluxation of the ulnar styloid, which leads to wrist pain and may cause rupture of the finger extensor tendons, leading in turn to a sudden onset of finger drop of the little and ring fingers predominantly, which needs urgent surgical repair.
RA commonly affects the shoulders. Initially, the symptoms mimic rotator cuff tendonosis (see p. 500) with a painful arc syndrome and pain in the upper arms at night. As the joints become more damaged, global stiffening occurs. Late in the disease rotator cuff tears are common (see p. 501) and interfere with dressing, feeding and personal toilet.
One of the earliest manifestations of RA is painful swelling of the MTP joints.
 The foot becomes broader and a hammer-toe deformity develops.
The foot becomes broader and a hammer-toe deformity develops.
 Exposure of the metatarsal heads to pressure by the forward migration of the protective fibrofatty pad (Fig. 11.17) causes pain.
Exposure of the metatarsal heads to pressure by the forward migration of the protective fibrofatty pad (Fig. 11.17) causes pain.
 Ulcers or calluses may develop under the metatarsal heads and over the dorsum of the toes.
Ulcers or calluses may develop under the metatarsal heads and over the dorsum of the toes.
 Mid- and hindfoot RA causes a flat medial arch and loss of flexibility of the foot.
Mid- and hindfoot RA causes a flat medial arch and loss of flexibility of the foot.
Massive synovitis and knee effusions occur, but respond well to aspiration and steroid injection (see p. 507). A persistent effusion increases the risk of popliteal cyst formation and rupture (see p. 508). In later disease, erosion of cartilage and bone causes loss of joint space on X-ray and damage to the medial and/or lateral and/or retropatellar compartments of the knees. Depending on the pattern of involvement, the knees may develop a varus or valgus deformity. Secondary OA follows. Total knee replacement is often the only way to restore mobility and relieve pain.
Non-articular manifestations (Fig. 11.18)
Less common non-articular manifestations
 Airways disease: a spectrum from predominant bronchiectasis (cough and daily sputum) to predominant obliterative bronchiolitis (progressive breathlessness)
Airways disease: a spectrum from predominant bronchiectasis (cough and daily sputum) to predominant obliterative bronchiolitis (progressive breathlessness)
 Disease of the pleura: pleural effusion (asymptomatic to mildly breathless) and thickening
Disease of the pleura: pleural effusion (asymptomatic to mildly breathless) and thickening
 Fibrosing alveolitis: a combination of inflammation and basal lung fibrosis
Fibrosing alveolitis: a combination of inflammation and basal lung fibrosis
 Peripheral, intrapulmonary nodules: these are asymptomatic but may cavitate, especially with pneumoconiosis (Caplan’s syndrome)
Peripheral, intrapulmonary nodules: these are asymptomatic but may cavitate, especially with pneumoconiosis (Caplan’s syndrome)
 Infective lesions, e.g. TB in patients on biological DMARDs.
Infective lesions, e.g. TB in patients on biological DMARDs.
Vasculitis (see p. 542) is caused by immune complex deposition in arterial walls. It is uncommon. Smoking is a risk factor. Findings are:
Diagnosis and investigations
The diagnosis relies on the clinical features described above. The predictors of poor prognosis arthritis are listed in Box 11.6. Initial investigations include:
 Blood count. Normochromic, normocytic anaemia may be present.
Blood count. Normochromic, normocytic anaemia may be present.
 The ESR and/or CRP are raised in proportion to the activity of the inflammatory process and are useful in monitoring treatment.
The ESR and/or CRP are raised in proportion to the activity of the inflammatory process and are useful in monitoring treatment.
 Serology. ACPA (see p. 497) is present earlier in the disease (and may predate it by many years), and in early inflammatory arthritis indicates the likelihood of progressing to RA. Rheumatoid factor is present in approximately 70% of cases and ANA at low titre in 30%.
Serology. ACPA (see p. 497) is present earlier in the disease (and may predate it by many years), and in early inflammatory arthritis indicates the likelihood of progressing to RA. Rheumatoid factor is present in approximately 70% of cases and ANA at low titre in 30%.
 X-rays show soft tissue swelling in early disease but MRI demonstrates synovitis and early erosions.
X-rays show soft tissue swelling in early disease but MRI demonstrates synovitis and early erosions.
 Aspiration of the joint if an effusion is present. The aspirate looks cloudy owing to white cells. In a suddenly painful joint septic arthritis should be suspected (see p. 532).
Aspiration of the joint if an effusion is present. The aspirate looks cloudy owing to white cells. In a suddenly painful joint septic arthritis should be suspected (see p. 532).
 Doppler ultrasound is a very effective way of demonstrating persistent synovitis when deciding on the need for DMARDs or assessing their efficacy.
Doppler ultrasound is a very effective way of demonstrating persistent synovitis when deciding on the need for DMARDs or assessing their efficacy.
Management of ra (box 11.10)
![]() Box 11.10
Box 11.10
Management of rheumatoid arthritis
 Establish the diagnosis clinically.
Establish the diagnosis clinically.
 Use NSAIDs and analgesics to control symptoms.
Use NSAIDs and analgesics to control symptoms.
 Try to induce remission with i.m. depot methylprednisolone 80–120 mg if synovitis persists beyond 6 weeks.
Try to induce remission with i.m. depot methylprednisolone 80–120 mg if synovitis persists beyond 6 weeks.
 If synovitis recurs, refer to a rheumatologist to start DMARDs and consider combinations of sulfasalazine, methotrexate and hydroxychloroquine. Give a second dose of i.m. depot methylprednisolone or oral steroids.
If synovitis recurs, refer to a rheumatologist to start DMARDs and consider combinations of sulfasalazine, methotrexate and hydroxychloroquine. Give a second dose of i.m. depot methylprednisolone or oral steroids.
 Refer for physiotherapy and general advice through a specialist team.
Refer for physiotherapy and general advice through a specialist team.
 As improvement occurs, as measured by less pain, less morning stiffness and reduced acute-phase response, tail-off steroids and possibly reduce drugs.
As improvement occurs, as measured by less pain, less morning stiffness and reduced acute-phase response, tail-off steroids and possibly reduce drugs.
Drug therapy
Non-steroidal anti-inflammatory drugs (NSAIDs) and coxibs
Most people with RA are unable to cope without an NSAID to relieve night pain and morning stiffness. NSAIDs do not reduce the underlying inflammatory process. They all act on the cyclo-oxygenase (COX) pathway (see Fig. 15.30). The individual response to NSAIDs varies greatly. It is desirable therefore to try several different drugs for a particular patient in order to find the best (Box 11.13). Each compound should be given for at least a week. Start with an inexpensive NSAID with few side-effects and with which you are familiar. Regular doses are needed to be effective. The major side-effects of NSAIDs and the use of coxibs are discussed on page 511. If gastrointestinal side-effects are prominent, or the patient is over 65 years of age, add a proton pump inhibitor. Slow-release preparations (e.g. slow-release diclofenac, 75 mg, taken after supper), or a suppository at bedtime, usually work well. For additional relief, a simple analgesic is taken as required (e.g. paracetamol or a combination of codeine or dihydrocodeine and paracetamol). Many patients need night sedation.
Corticosteroids
Oral corticosteroids have a number of problems (Boxes 11.11 and 19.11). They are powerful disease-controlling drugs, but are avoided in the long term because side-effects are inevitable. Early intensive short-term regimens are often used. Doses of 5–7.5 mg daily as maintenance therapy are used in some centres. Corticosteroids are invaluable to people with severe disease with extra-articular manifestations such as vasculitis.
![]() Box 11.11
Box 11.11
Problems associated with the use of corticosteroids
 Patients are increasingly anxious about the use of corticosteroids because of adverse publicity about their potential side-effects. This must be discussed frankly and the risks of not using corticosteroids in treatment should be described and balanced against the risks of the drugs themselves.
Patients are increasingly anxious about the use of corticosteroids because of adverse publicity about their potential side-effects. This must be discussed frankly and the risks of not using corticosteroids in treatment should be described and balanced against the risks of the drugs themselves.
 Patients must be warned to avoid sugars and saturated fats and to eat less because of the risk of weight gain.
Patients must be warned to avoid sugars and saturated fats and to eat less because of the risk of weight gain.
 The skin becomes thin and easily damaged.
The skin becomes thin and easily damaged.
 Monitor for diabetes and hypertension.
Monitor for diabetes and hypertension.
 Cataract formation may be accelerated.
Cataract formation may be accelerated.
 Osteoporosis develops within 6 months on doses above 7.5 mg daily. Monitor with DXA scan and treat with calcium and vitamin D and bisphosphonate (see this chapter).
Osteoporosis develops within 6 months on doses above 7.5 mg daily. Monitor with DXA scan and treat with calcium and vitamin D and bisphosphonate (see this chapter).
Disease-modifying anti-rheumatic drugs (DMARDs)
DMARDs, prescribed by a rheumatologist, are listed in Table 11.16 (see also Fig. 11.19).
 Traditional DMARDs, which mainly act through cytokine inhibition, reduce inflammation, with a reduction of joint swelling, a fall in the plasma acute-phase reactants and slowing of the development of joint erosions and irreversible damage. Their beneficial effect is not immediate (hence ‘slow-acting agents’) and may be partial or transient.
Traditional DMARDs, which mainly act through cytokine inhibition, reduce inflammation, with a reduction of joint swelling, a fall in the plasma acute-phase reactants and slowing of the development of joint erosions and irreversible damage. Their beneficial effect is not immediate (hence ‘slow-acting agents’) and may be partial or transient.
 DMARDs often only have a partial effect, achieving between 20% and 50% improvement by ACR criteria for disease remission (Box 11.12).
DMARDs often only have a partial effect, achieving between 20% and 50% improvement by ACR criteria for disease remission (Box 11.12).
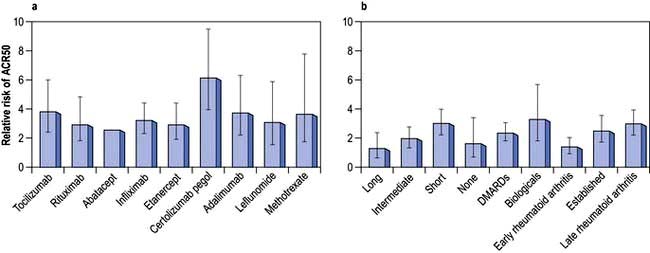
Figure 11.19 American College of Radiology ACR50 responses in trials of DMARDs and biological agents (50% improvement in five of seven measures in the ACR 1987 criteria).a,b (a) ACR50 response in trials of DMARDs and biological agents. (b) Impact of treatment duration, disease stage and prior treatment on ARC50 response. The difference between patients treated with active drug and placebo is greatest in people with late rheumatoid arthritis who have failed biological treatment and whose disease is managed for short periods. The difference is smallest in individuals with early arthritis who have not previously received DMARDs and are treated for a long time. American College of Rheumatology 1987 criteria for diagnosis of RA (revised 1988) have been superseded (see Box 11.8) but are used in trials, with ≥4 criteria necessary for diagnosis: morning stiffness >1 hours for ≥6 weeks; arthritis of ≥3 joints for ≥6 weeks; arthritis of hand joints and wrists for ≥6 weeks; symmetrical arthritis, subcutaneous nodules; positive serum rheumatoid factor; typical radiological changes (erosions and/or periarticular osteopenia). Error bars 95% CIs.
(From Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010; 376:1094–1108, with permission.)
DMARDs are used as early as possible once RA has been diagnosed. Studies suggest that early intervention with DMARDs at 6 weeks to 6 months improves the outcome. Combinations of three or four drugs (steroids, sulfasalazine, methotrexate and hydroxychloroquine) in early RA are increasingly common, reducing the number of agents once remission has been achieved. Most DMARDs are contraindicated in pregnancy (Box 11.13). Effective treatment with DMARDs reduces the increased cardiovascular risk seen in people with RA.
![]() Box 11.13 Drug use during pregnancy in treatment of rheumatoid arthritis
Box 11.13 Drug use during pregnancy in treatment of rheumatoid arthritis
paracetamol – the oral analgesic of choice
 Oral NSAIDs and selective COX-2 inhibitors: can be used after implantation up until the last trimester if symptoms justify their use.
Oral NSAIDs and selective COX-2 inhibitors: can be used after implantation up until the last trimester if symptoms justify their use.
 Corticosteroids: may be used to control disease flares (main maternal risks are hypertension, glucose intolerance and osteoporosis).
Corticosteroids: may be used to control disease flares (main maternal risks are hypertension, glucose intolerance and osteoporosis).
 Cytokine modulators: safety during pregnancy is currently unclear.
Cytokine modulators: safety during pregnancy is currently unclear.
 Etanercept is a fully humanized p75 TNF-α receptor IgG1 fusion protein given by self-administered subcutaneous injection. Around 65% of patients respond well. Some develop an injection reaction.
Etanercept is a fully humanized p75 TNF-α receptor IgG1 fusion protein given by self-administered subcutaneous injection. Around 65% of patients respond well. Some develop an injection reaction.
 Adalimumab is a fully human monoclonal antibody against TNF-α, given along with methotrexate.
Adalimumab is a fully human monoclonal antibody against TNF-α, given along with methotrexate.
 Infliximab is a monoclonal antibody against TNF-α, given intravenously and co-prescribed with methotrexate to prevent loss of efficacy because of antibody formation.
Infliximab is a monoclonal antibody against TNF-α, given intravenously and co-prescribed with methotrexate to prevent loss of efficacy because of antibody formation.
 Certolizumab pegol is an Fab fragment of a humanized TNF-α inhibitor monoclonal antibody approved in the UK and other countries. It is PEGylated, i.e. polyethylene glycol groups are added to reduce its immunogenicity and prolong its half-life. The lack of an Fc portion on the antibody aims to reduce the risk of complement-dependent and antibody dependent cytotoxicity. It is useful with or without methotrexate for severe active RA.
Certolizumab pegol is an Fab fragment of a humanized TNF-α inhibitor monoclonal antibody approved in the UK and other countries. It is PEGylated, i.e. polyethylene glycol groups are added to reduce its immunogenicity and prolong its half-life. The lack of an Fc portion on the antibody aims to reduce the risk of complement-dependent and antibody dependent cytotoxicity. It is useful with or without methotrexate for severe active RA.
 Golimumab is a human IgG1-κ monoclonal antibody against TNF which is awaiting approval in some countries. It is given by subcutaneous injection once monthly for severe RA.
Golimumab is a human IgG1-κ monoclonal antibody against TNF which is awaiting approval in some countries. It is given by subcutaneous injection once monthly for severe RA.
Rituximab is a genetically engineered chimeric monoclonal antibody (p. 72) that causes lysis of CD20-positive B cells. CD20 is a pan-B cell surface antigenic phosphoprotein. Its expression is restricted to pre-B and mature B cells but it is not present on stem cells and is lost before differentiation into plasma cells. Rituximab produces significant improvement in RF-factor positive RA for 8 months to several years when used alone or in combination with corticosteroids and/or methotrexate. This is associated with a 6–9-month B cell lymphopenia with little change in circulating immunoglobulins. A re-flare is often accompanied by a return of peripheral lymphocytes and a rise in CRP. Rituximab can be reused as the disease flares. Repeated courses over up to 5 years are acceptable and well tolerated and around 80% of RF-positive patients respond with 50–60% showing persistent disease control. It is worth trying in patients who have failed to respond to anti-TNF agents. There may be an increased risk of chest infections, and immunoglobulin levels may fall progressively and need to be monitored.
Spleen tyrosine kinase (SYK) inhibitor given orally has been effective in RA in phase 2 studies.
Prognosis
A poor prognosis is indicated by:
 A clinical picture of an insidious rather than an explosive onset of RA, female sex, increasing number of peripheral joints involved and the level of disability at the onset
A clinical picture of an insidious rather than an explosive onset of RA, female sex, increasing number of peripheral joints involved and the level of disability at the onset
 Blood tests showing a high CRP/ESR, normochromic normocytic anaemia and high titres of ACPA antibodies and of rheumatoid factor
Blood tests showing a high CRP/ESR, normochromic normocytic anaemia and high titres of ACPA antibodies and of rheumatoid factor
 X-rays with early erosive damage (note: ultrasound and MRI can show cartilage and bone damage prior to conventional X-rays).
X-rays with early erosive damage (note: ultrasound and MRI can show cartilage and bone damage prior to conventional X-rays).
Prognosis can be altered dramatically with early DMARD therapy under expert supervision.