CHAPTER 99 Revision Spine Surgery
The number of elective spinal surgeries performed yearly continues to increase at an accelerating pace.1 Although advancements in diagnostics, implant design, and biologic adjuncts have made these operations more predictable in outcome, a significant number of patients report persistent or recurrent symptoms. These patients represent a major challenge to spine care providers. In general, the odds for success continue to fall with each additional surgical undertaking. From a statistical standpoint, the second operation has a 50% chance of success, and after the second operation, patients are more likely to be made worse than better.2 The risk of reoperation holds true independent of the index diagnosis, procedure performed, and comorbidities. As such, in the classic words of Waddell and colleagues, “caution and restraint are required when contemplating repeat back surgery.”3
Patient Evaluation
The patient’s main symptoms before the index surgery should be elicited, as well their index diagnosis and details of their operative procedure. It is helpful to obtain the medical records and imaging studies that were performed before the previous surgical procedure to compare with the current situation. In some cases, after such careful analysis an incorrect initial diagnosis may be identified.4
The time frame over which the new or recurrent symptoms developed may suggest their underlying etiology. Failure to achieve any pain-free interval after the primary surgery has been associated with incorrect preoperative diagnosis, wrong-level surgery, and inadequate decompression, particularly in the lateral recess or foraminal region.5–7 Early onset of new radicular pain after surgery may suggest nerve root injury. If pedicle screws or interbody fusion devices have been placed, their position should be evaluated with computed tomography (CT).4 In the intermediate postoperative period (1 to 6 months), mild new or recurrent pain may develop as patients progress through rehabilitation. Symptoms that develop suddenly after a specific inciting event may be from recurrent disc herniation or hardware failure. Recurrent symptoms of gradual onset in the intermediate time frame are often related to the development of scar tissue such as epidural fibrosis or adhesive arachnoiditis.2 Symptomatic pseudarthroses may also begin to manifest themselves toward the end of the 6-month postoperative period. Other causes of late postoperative pain include recurrent disc herniations or stenosis and the development of adjacent level pathology. For the surgeon who may evaluate patients initially treated elsewhere, this timeline regarding the recurrence of symptoms is crucial to establishing the proper diagnosis.
A frequent cause of failed spine surgery is poor patient selection, which unfortunately is often more apparent in retrospect. Several authors have reported that psychologic factors documented on the Minnesota Multiphasic Personality Inventory (MMPI) can be related to poor outcomes after spinal surgery.8–10 Elevation of hypochondriasis, hysteria, and depression scales are most predictive. Poor results are also more likely in patients who exhibit abnormal pain behavior, receive worker’s compensation, or are involved in litigation.11,12 The influence of these factors on outcome, however, is poorly understood. The combination of physical, psychosocial, and economic problems with which patients with FBSS present make individualized treatment plans necessary.
Another regrettably common cause of persistent symptoms is performance of the wrong surgical procedure. Wrong-level discectomy is the most common avoidable error in spine surgery. The North American Spine Society has advocated intraoperative radiographic localization in almost all cases to help prevent this misfortune.7 Another example of an improper procedure is to decompress only what appears to be the most stenotic level and leave other areas, particularly in the lateral recess or foraminal region, unaddressed. Likewise, failure to identify the pain generator before performing an interbody fusion for discogenic pain can lead to disappointing results. Many patients will have one or two significantly abnormal discs on magnetic resonance evaluation but other areas with comparably mild changes, which may be surprisingly concordant at discography. In our experience, such patients are advised against surgery.
Leg Pain Versus Back Pain
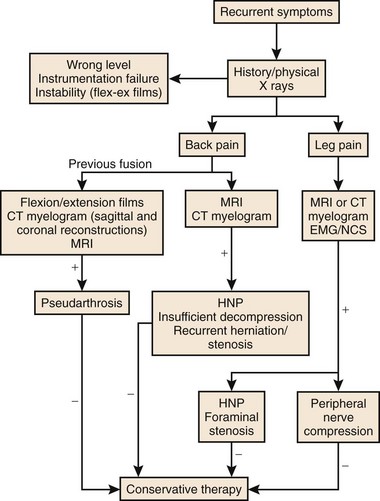
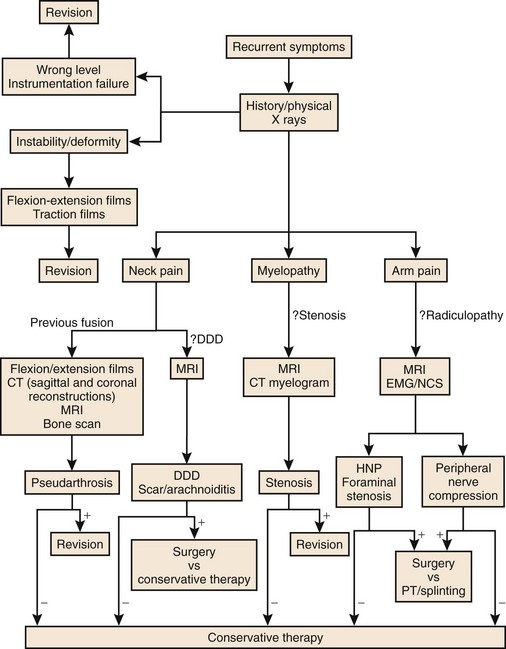
As with primary lumbar degenerative syndromes, differentiating the degree of axial back pain from radicular or neurogenic claudicatory symptoms is paramount to formulating an effective treatment plan. Although the diagnostic tests that follow will help identify the pathoanatomy, all of the findings should be kept in context with the patient’s main complaint. An algorithm based on the patient’s main complaint is presented in Figure 99–1.
Physical Examination
The physical examination of patients with FBSS is similar to the initial examination for nonoperated patients. Posture, alignment, and gait should be observed. Range of motion, tenderness, and nerve root tension signs should be elicited and a thorough neurologic examination should be performed. Although the physical examination findings of this condition may be nonspecific, a thorough examination may be helpful to exclude other conditions that may have similar presentations. In patients with leg pain, thorough examination of the hip and knee and assessment of distal pulses may reveal other potential causes of their symptoms. Nonorganic physical findings should be recorded, as described by Waddell.13
Diagnostic Testing
Plain Radiography
Plain radiographs of the lumbar spine should include biplanar standing films. A coned-down lateral of the lumbosacral junction and an anteroposterior Ferguson view may be particularly helpful in evaluating patients who have had previous surgery at the L5-S1 level. Patients with pain after a discectomy should be evaluated for iatrogenic pars fracture. Endplate rarefaction suggests a postoperative discitis. Previously placed spinal implants should be scrutinized for malposition, loosening, subsidence, or breakage. In addition to evaluation of the disc height, endplate characteristics, and alignment, anterolisthesis or retrolisthesis at the adjacent levels should be noted (Figs. 99–2A and 99–2B). Flexion-extension radiographs should be scrutinized for motion involving segments where fusion was attempted because gross motion detectable in this manner typically indicates pseudarthrosis. These films should also be evaluated for dynamic instability at levels adjacent to the previous surgery. The overall sagittal balance should be assessed on standing 36-inch radiographs. A flatback deformity from poorly contoured constructs or adjacent segment degeneration (ASD) is best evaluated with this technique.
Advanced Imaging
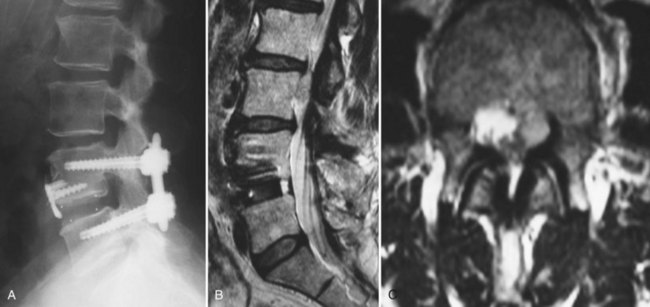
CT with fine-cuts and sagittal/coronal reconstructions can help delineate fusion status.14 The adjacent levels should be carefully evaluated for hypertrophic facet arthropathy, spondylolysis, and pedicle stress fractures. Magnetic resonance imaging (MRI) (Fig. 99–3) with and without gadolinium enhancement is the most sensitive test for evaluating neurologic compression at both the previously operated site and the adjacent levels.15 Comparison of the enhanced and nonenhanced sequences can help differentiate epidural scar (enhancing) from recurrent disc herniations and hypertrophic ligamentum flavum (nonenhancing). With the advent of MRI, CT myelography is less commonly required but is still helpful in certain situations. Patients with contraindications to MRI, pronounced scoliosis, known stainless steel implants, or patients with substantial imaging artifact degrading magnetic resonance images can be better evaluated with CT myelography.
Diagnostic Injections
Selective nerve root injections have been shown to have both therapeutic and diagnostic efficacy in the management of patients with lumbar radicular pain.16,17 Controlled studies documenting their predictive value in patients with failed back surgery syndromes are lacking. The authors have found them useful in situations where MRI findings are equivocal and in situations where multilevel pathology is present. The use of provocative discography is controversial, and its role in the evaluation of previously operated discs is not universally accepted.18 Some reports indicate that discography may predict surgical outcome for patients undergoing anterior lumbar interbody fusion for persistent pain after successful posterior fusion.19,20 The role of discography in evaluating degenerated adjacent discs as pain generators is also unclear. Although this has been suggested as a useful test in determining whether a degenerated segment below a long thoracolumbar fusion is a significant pain generator,21 the role of provocative discography in the more common setting of adjacent segment disease cranial to more limited lumbar procedures continues to be poorly defined.
Nonoperative Treatment
Nonoperative treatment will typically be involved to varying degrees in many patients with FBSS because measures such as physical therapy and pain medications are part of the usual postoperative regimen for most index lumbar procedures. When persistent complaints have triggered the workup outlined earlier, a diagnosis can be rendered in many cases. Several conditions may be amenable to nonoperative treatment (Box 99–1). The degree to which a patient’s symptoms can be attributed to epidural scar formation is unclear. Epidural scar is an incidental finding in many patients who do not develop symptoms, and most feel that the scarring will inevitably return to some degree after revision, making the indication for repeat surgical intervention unclear. As new agents to help prevent proliferative epidural scarring are developed, the overall management of such patients may continue to evolve.
Nonoperative treatment may have a potential role in several other conditions. Fractures involving noninstrumented fusion masses, pedicles within the fused segments, or compression fractures cephalad to a fusion may be treated with bracing in the absence of frank instability. Postoperative discitis may be treated with biopsy, immobilization, and directed antibiotic therapy, but infection after instrumented fusions generally merits surgical intervention (see Chapter 98). Sacroiliac symptoms are rarely treated with arthrodesis, and medical/physical therapy plus fluoroscopically guided injections are favored. Leg symptoms due to peripheral neuropathy should be managed medically, and leg symptoms due to peripheral nerve compression may be amenable to bracing or specialized local care. Leg symptoms due to recurrent disc herniation or stenosis can be treated initially with physical therapy and nonsteroidals. Epidural injections may be offered as a treatment option; however, their effectiveness in the postoperative patient is less predictable. Transforaminal and caudal injections have some reported therapeutic success in uncontrolled studies.22,23 Some have theorized that the presence of postoperative scar tissue interferes with dispersion of the injectate and leads to diminished effectiveness. Patients with diffuse degenerative disease and axial symptoms are typically advised to pursue intensive multidisciplinary rehabilitation combining physical training with cognitive behavioral interventions, vocational planning, and consultation with a specialist in chronic pain medical management (covered separately in Chapters 104–107).
Spinal cord stimulation has been used for more than 30 years to treat chronic pain including FBSS. A thorough review is conducted in the chapter on interventional procedures for chronic pain. As one can imagine, it has been difficult to conduct well-designed trials to compare spinal cord stimulation to revision surgery. In a review published in 2004 of 583 studies of spinal cord stimulation, the only class I randomized controlled trial did not include patients with FBSS.24 A subsequent randomized controlled trial compared reoperation and spinal cord stimulation for patients with FBSS. Using at least 50% pain relief as a criterion for success, the authors reported that spinal cord stimulation was significantly more successful than reoperation.25 The authors typically consider implantable spinal cord stimulators an option for patients with back and leg pain after spinal fusion when solid arthrodesis is apparent by imaging studies and all other potential causes of persistent pain have been excluded. A trial stimulator is typically done first and converted to a permanent device if the patient demonstrates a favorable response.
Surgical Treatment—Back Pain Predominant
Pseudarthroses and Failed Instrumentation
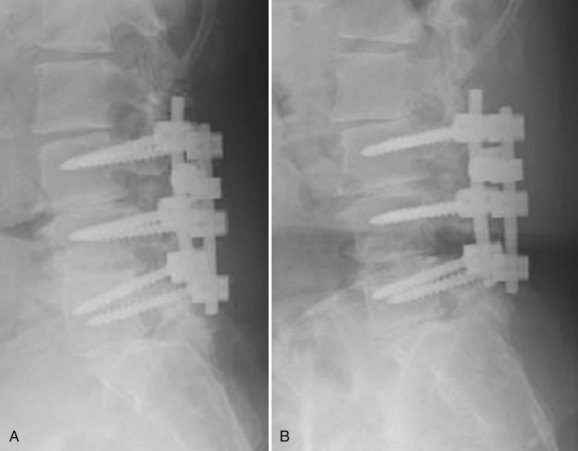
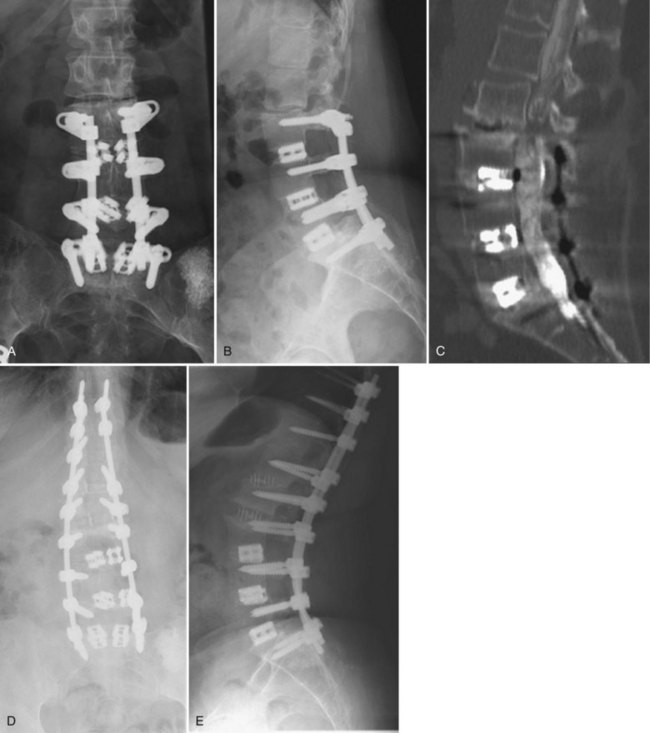
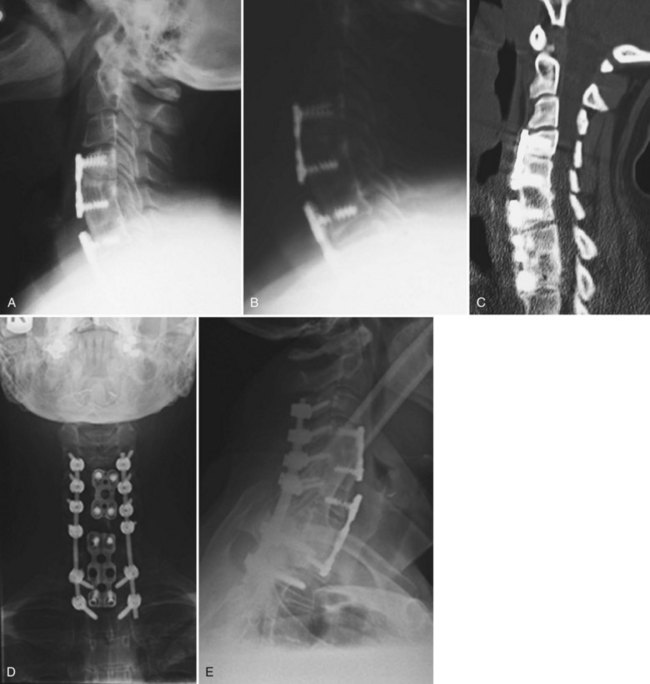
Although pseudarthroses are not always symptomatic, they do account for a considerable proportion of cases of FBSS. Likewise, instrumentation failure typically occurs in the setting of a pseudarthrosis; however, broken implants have been reported in cases of solid fusion. When either of these conditions is felt to be the cause of a patient’s FBSS, any revision surgery should be carefully planned and executed. There is no commonly accepted treatment algorithm for surgical management of pseudarthrosis. The surgical approach must be individualized on the basis of the location and etiology of the nonunion, the absence or presence of failing implants requiring revision (Fig. 99–4A to E), the medical status of the patient (including any previous abdominal surgeries), and the surgeon’s comfort level with specific techniques.
Nonunions after a posterior fusion that have adequate bone stock can often be managed by a revision posterior approach.26 This should involve decortication of the pseudarthrosis and posterolateral fusion with instrumentation. At exploration of the fusion, the rods and screws are typically removed first. If the screw lengths and diameters are not known from the previous operative note, these should be determined from direct inspection and measurement. The posterolateral fusion masses are then exposed, and any fibrous tissue associated with pseudarthrosis is débrided. If any question remains regarding the gross stability of the fused segments, we typically cannulate the previous pedicle screw holes with blunt probes or appropriate-sized curettes and attempt to elicit segmental motion by cantilevering the instruments. Use of a gouge to decorticate the previous fusion masses helps preserve any remaining bone stock. Because removing and replacing a pedicle screw results in a 34% decrease in pullout strength, some attempts must be made to improve purchase. If the anatomy permits, increasing the diameter of the screw by at least 1 mm or increasing the length by 5 to 10 mm can help improve fixation. Using a different pedicle screw system is beneficial because the screws will have a different thread pitch. In severely osteoporotic bone, polymethylmethacrylate (PMMA) can augment fixation strength. Achieving fusion can be challenging in such revision scenarios. As such, local bone is preferentially augmented by morsellized autograft harvested from the iliac crest. Allograft and demineralized bone matrix are used as extenders rather than graft substitutes. If both iliac crests have been previously used, other strategies for enhancing fusion should be considered. Options include electrical stimulation, bone marrow concentrates, or bone morphogenetic protein, although few studies have been performed to delineate their role in this circumstance.
When breakage of a pedicle screw is encountered, spanning that level with the addition of a crosslink is an option. If the affected level is at the end of the construct, extension of the fusion can be considered. If it is felt that reinstrumentation of the level with the broken screw is necessary, then screw removal is necessary. Commercially available screw removal sets are available, but most techniques involve removal of additional bone, so augmentation may be necessary. Careful patient selection is necessary to identify those patients who will benefit from a posterior or posterolateral strategy rather than a more involved procedure. West and colleagues26 reported a 35% pseudarthrosis rate and 47% clinical failure rate in patients with preexisting pseudarthrosis in whom posterior fusion and pedicle screw fixation were performed.
In cases of failed posterolateral fusion with inadequate bone stock (such as after a wide decompression), the interbody region may provide the best area of vascularized host surface for revision grafting. Excessive epidural scarring makes entry into the interbody space through virgin areas more desirable. Anterior lumbar interbody fusion (ALIF) permits placement of structural allografts or cages with large footprints. Using an anterior-posterior approach in this situation has been associated with the best fusion rates.27 For salvage of a single-level pseudarthrosis where decompression is not necessary, anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation has recently been described, with radiographic fusion reported in 52 of 54 cases.28
In cases where an anterior approach is problematic, a transforaminal interbody fusion (TLIF) is a good option29,30 because the entry site to the interbody space may be less involved by postoperative scar. Studies examining this technique specifically for the management of pseudarthrosis, however, are lacking. Another recently described technique, the extreme lateral interbody fusion (XLIF), offers an additional approach to the interbody space without a formal anterior exposure (Fig. 99–5A to E). This involves development of the plane between the posterolateral margin of the paraspinals and the peritoneal sac, followed by splitting the fibers of the psoas muscle. This technique requires high-quality intraoperative neurophysiologic monitoring of the nerve roots of the lumbar plexus31 and intraoperative fluoroscopy. Although early reports of the technique have demonstrated encouraging results with low complication rates,32 data on this technique for revision arthrodeses are currently lacking.
Revision of pseudarthroses after attempted posterior lumbar interbody fusion (PLIF) or TLIF can be challenging. The authors prefer to remove the cage from an anterior approach to the disc space (Fig. 99–6A to D). Osteotomes are used to create planes adjacent to the cage and assist removal. The choice of implant to then use for the ALIF is based on the defect left after removal of the cage. Whether a revision posterior fusion is required is then based on the integrity of the previously placed posterior implants and the overall segmental stability.
Anterior nonunions and failed lumbar disc arthroplasty present unique challenges. If the failed ALIF or arthroplasty device is not grossly unstable, a posterolateral fusion with instrumentation may suffice.33, 34 A recent article reported that removal of the prosthesis and circumferential fusion yielded slightly better results than posterior fusion at 1-year follow-up.35 The authors, however, acknowledged that significant risks are inherent with removal of the arthroplasty device, so further studies are necessary to clarify management of this scenario. If the patient had a 360-degree fusion and the posterior fusion is solid, an isolated revision ALIF can be performed. The entire construct should be revised in cases of failed 360-degree fusions with both anterior and posterior pseudarthroses and unstable implants. Revision anterior lumbar procedures are technically demanding. Vascular injuries are common, and an experienced approach or vascular surgeon should be considered.36 Strategies to help navigate the hazards of the revision anterior approach have been recommended. A previous left retroperitoneal approach to L5-S1 may be best revised through a right retroperitoneal or transperitoneal approach. A previous left retroperitoneal approach to L4-5 may be best revised through a transperitoneal or transpsoas approach.37 Because retroperitoneal scarring may obscure the anatomy, other measures such as preoperative ureteral stenting and intraoperative cannulation of the iliac veins with balloon catheters can be considered.
Painful Hardware
A small subgroup of patients may report recurrent pain despite intact implants, a solid fusion, and no other obvious pain generators. Implant removal in such cases remains controversial, particularly because the mechanism as to how the implants generate pain is not well understood. Some authors have recommended injection of local anesthetic around the hardware as a diagnostic step.38 For patients who report temporary relief of their pain with the injection, removal of the implants can be considered, provided that the patient clearly understands that such an intervention may not have a predictable outcome.
Surgical Treatment—Leg Pain Predominant
Revision Discectomy
The first step in a revision discectomy is to carefully remove the pseudomembrane and scar tissue to identify the borders of the previous laminotomy.39 The level of the dura in relation to the edge of the laminotomy should be determined from the preoperative imaging studies. If the spinous processes about the laminotomy site are preserved, these are convenient starting points to start elevating the pseudomembrane. Once the margins of the previous laminotomy have been cleared, a curved curette is used to carefully detach the scar from the edges and then the undersurfaces of the lamina. Kerrison rongeurs are used to enlarge the laminotomy and identify normal dura. The pedicle can be palpated with a Penfield or Woodson and the traversing nerve root passing medial and exiting below can be identified. Mobilizing the nerve root from epidural scar should be done carefully. A reverse angle curette firmly anchored on the surface of the disc just adjacent to the pedicle can be rotated toward the root to tease it free from adhesions and visualize the recurrent herniation. Once mobilization of the nerve root and visualization of the herniation is accomplished, the revision discectomy can be performed in a more standard manner.
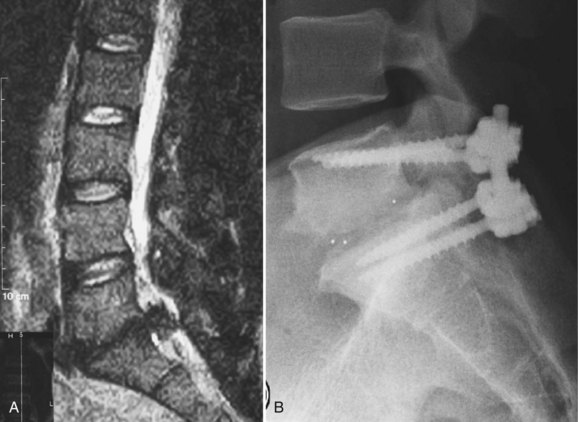
Patients who develop a third-time herniation or those who report a significant component of back pain (in addition to leg pain) may require interbody fusion at the time of revision discectomy. A transforaminal interbody approach (Fig. 99–7A and B) is favored by some because removing the facet and working more lateral to the traversing root requires less mobilization of the epidural scar.40 Even with this strategy, dural tears are not infrequent and should be anticipated. An anterior-posterior approach is favored by some in this situation, but the authors rarely advocate a stand-alone ALIF if leg pain is a significant part of the symptom complex. Although some have reported successful removal of extruded disc fragments at the time of ALIF, direct posterior neurolysis and decompression allow better intraoperative confirmation that the leg pain issue is effectively addressed.
Revision Laminectomy and Treatment of Adjacent-Level Stenosis
Preoperative Planning
The presence of a spondylolisthesis combined with stenosis at the adjacent segment should prompt strong consideration of extending the fusion. Retrolisthesis, rotatory listhesis, or substantial hypermobility on flexion-extension films may also warrant extension of the fusion. The present authors routinely extend the fusion at the time of decompression for junctional stenosis even in the absence of evidence of instability because as motion can be considered one of the root causes of ASD.41,42 Others, however, have reported that decompression alone in this setting can be effective.43–45 Patients are typically advised of an increased incidence of durotomy, as well as all other complications, compared with the risk attendant with the index procedure.
Technical Aspects of Surgery
The cranial margin of the previous scar is generally where the stenosis is most severe. Even in cases in which an extensive revision of the previous decompression is unnecessary, the scar must be mobilized in this region. Working laterally, the pseudomembrane is carefully detached from the bony rim with a sharp, curved microcurette. After the initial release with curettes, the transition zone between scar and virgin dura can be progressively mobilized. In our practice, the cupped end of a Penfield-1 elevator is useful for this maneuver. The edge is placed directly along the bony rim, and the wide surface helps to gradually develop the plane. Once the epidural scar and dura have been separated from the bone, the decompression can be performed. Kerrison rongeurs are usually effective; however, if the bone edge is broad and steep, efficient resection may be difficult. Using an osteotome or chisel to undercut the wall or create a thinner shelf of bone, a more controlled and effective use of the Kerrison is permitted. We have found a beveled-edge chisel to be helpful in that the bevel can help direct the initial pass laterally and can then redirect the cut downward by inverting the chisel edge. This can detach a long strip of bone as one piece, making it easier to remove.46 Because many decompressions are performed, “pedicle-to-pedicle” palpation of the pedicle of the proximal segment from the previous decompression is helpful for orientation and to verify adequate decompression of the neural elements.
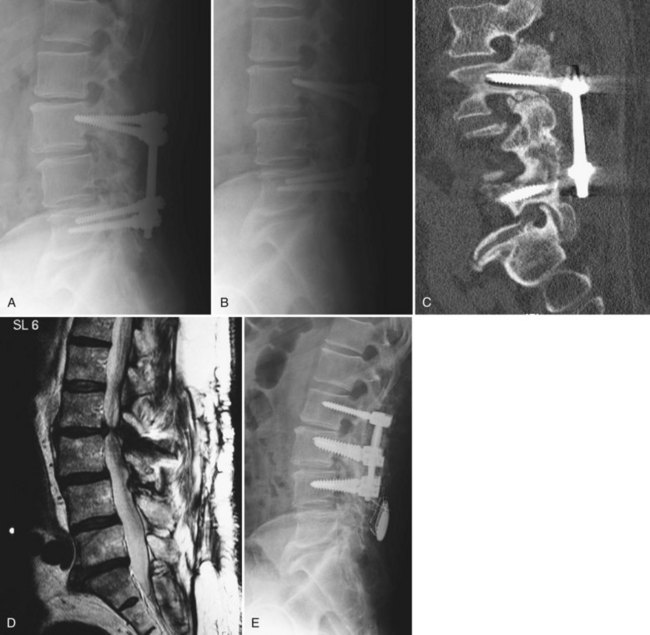
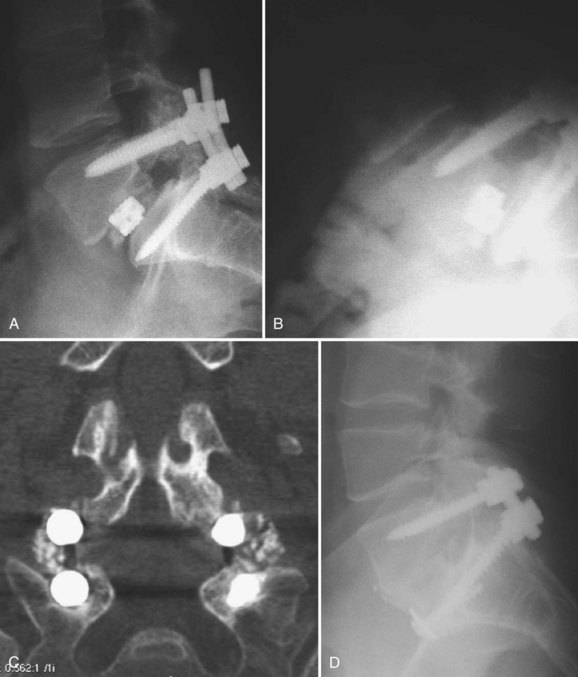
Pedicle screws are then placed in the adjacent level using standard technique (Fig. 99–8A to E). If the previous screws have been removed, they are replaced with larger-diameter screws or same-size screws with different thread pitch if this fits snugly in the pedicle. If the previous fusion involved multiple levels and appeared solid at exploration, the more distant levels may not require reinstrumentation. Achieving a solid fusion can be challenging in these cases. Whitecloud and colleagues47 reported the results of patients undergoing decompression and fusion for ASD. Eighty percent of cases where noninstrumented fusion was attempted developed pseudarthroses compared with 17% of instrumented fusions. As such, the authors recommend segmental instrumentation and use of iliac crest autograft or other strategies for enhancing fusion, as discussed earlier.
The clinical outcomes for patients undergoing decompression and fusion for adjacent segment stenosis are mixed. Phillips and colleagues reported the results of 26 patients who underwent decompression or decompression and fusion for stenosis at the adjacent level.45 Eleven of the 26 patients were either neutral or dissatisfied with their surgery. Other studies have reported more encouraging results. Schlegel reported the results of 37 patients who were treated surgically for ASD.44 Twenty-three patients were treated with decompression alone, and 14 with decompression and fusion. Twenty-six of the 37 patients reported good-to-excellent improvement of back and leg pain; however, the length of follow-up was limited to 2 years. Chen and colleagues42 reported the results of 39 patients undergoing surgery for ASD. All of these patients were treated with a wide decompression and extension of their fusion using instrumentation. At an average follow-up of approximately 5 years, 77% of patients had excellent or good results and their fusion rate was 95%. A sobering note was that five of these patients subsequently developed new disease at the neighboring segment, typically leading to a poor clinical result.
Revision Cervical Spine Surgery
Patient Evaluation
As discussed previously in the lumbar section, a thorough history must be obtained. Essential historical facts include the nature and duration of the preoperative symptoms and the type of operative procedure that was performed. Obtaining the patient’s old imaging studies and medical records can be helpful in fully assessing the original problem leading to the index surgery. Recollection of the perioperative period after the initial surgery should then be pursued. Note should be made of any complications and the initial results of the surgery. Clarifying whether there was an initial period of improved pain or neurologic function versus unresolved or worsened symptoms can help establish if the appropriate procedure was performed. As with lumbar procedures, poor initial patient selection is one of the primary causes of failed surgery. Establishing the principal residual/recurrent problem as primarily axial neck pain, radiculopathy, or myelopathy is essential for formulating a treatment strategy. An algorithm for such patients is presented in Figure 99–9.
Imaging
In addition to reviewing previous diagnostic studies, additional imaging is warranted to evaluate failed procedures. Upright plain radiographs should be obtained, and the position and integrity of previously placed implants and bone grafts should be scrutinized. The overall alignment should be noted. The levels adjacent to a previous fusion should be evaluated for degeneration. Flexion and extension lateral films should be performed to evaluate potential pseudarthrosis. Radiographic criteria suggesting a pseudarthrosis include lack of bridging bony trabeculae between the graft and adjacent vertebral body and segmental motion on flexion-extension films.48–50 Cannada and colleagues51 conducted a study comparing measurement of Cobb angles and change in distance between the spinous processes for identifying pseudarthrosis after anterior cervical discectomy and fusion. They found that measurement of the change in distance between the spinous processes is more reproducible and accurate for diagnosing pseudarthrosis than the Cobb method. A change in distance of 2 mm or more was highly suggestive of pseudarthrosis.
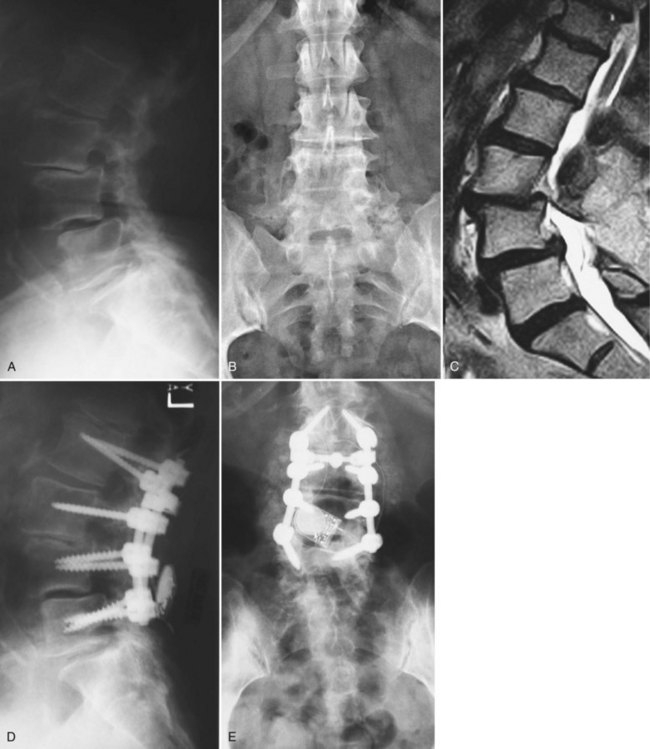
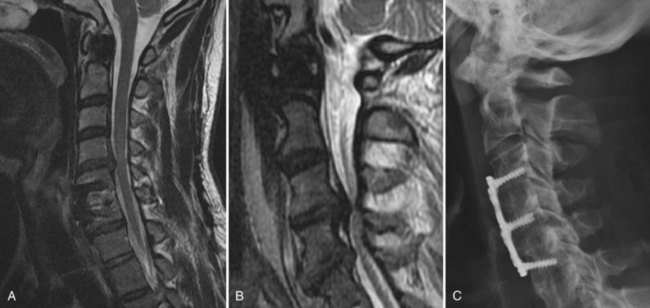
CT is an excellent modality for evaluation of failed cervical surgeries. Position of lateral mass or anterior vertebral body screws can be readily assessed. Sagittal and coronal reconstructions are helpful for evaluating the position of interbody or strut grafts. Residual spondylotic bony compression or ossification of the posterior longitudinal ligament can also be readily assessed. Findings on CT that are suggestive of a pseudarthrosis include absence of bridging trabeculae, bony lucency at the graft-vertebral body junction, or fracture and graft resorption. Buchowski and colleagues52 conducted a study assessing the reliability of plain radiographs, CT, and MRI to detect a pseudarthrosis after an anterior cervical fusion compared with intraoperative exploration. The study involved 14 patients, 8 of which were found to have pseudarthrosis at repeat surgery. Their results suggested that CT most closely agreed with intraoperative findings (Fig. 99–10A to E).
Nonspinal etiologies should be considered in cases where the spinal imaging workup is equivocal. Reports of pain in the shoulder with overhead activities or impingement signs should prompt imaging of the shoulder.53 Numbness in the hands, particularly at night, or positive findings on provocative tests of the carpal or cubital tunnel should raise suspicion of peripheral nerve compressive syndromes. These conditions, as well as peripheral neuropathies, may be further assessed by electromyography/nerve conduction studies.54 Intrinsic conditions of the spinal cord can also mimic compressive myelopathies, and consultation with a neurologist may be helpful in these cases.
Late Postoperative Failure
Adjacent Segment Degeneration
Degeneration of segments adjacent to a prior fusion has been the subject of intense interest (Fig. 99–11A to C). Concern about degeneration of neighboring motion segments has been one of the primary driving forces behind the development of nonfusion technologies. The classic study of this phenomenon was published by Hilibrand and colleagues in 1999.55 In their series of 374 patients with extended follow-up, symptomatic adjacent segment degenerative disease occurred at a rate of 2.9% per year over a 10-year period. In this series, more than two thirds of all patients in whom the new disease developed failed nonoperative management. Despite these odds, nonoperative measures are initially tried in the absence of a progressive neurologic condition. Nonoperative measures include physical therapy, nonsteroidal anti-inflammatories, and injection therapy.56,57
Patients who fail nonoperative treatment should be considered for surgical intervention. Patients with radiculopathy due to a lateral disc herniation may be considered for a laminoforaminotomy, provided there is no substantial axial pain or instability. This procedure has demonstrated success as an index operation for patients with this pathology.58 Its role for disease adjacent to an anteriorly fused segment is unknown, and theoretic concerns exist that the abnormal stress transfer may lead to recurrent problems.
For patients with symptomatic adjacent segment disease and substantial axial pain, bilateral radiculopathy, or myelopathy, anterior surgery should be considered. Although the authors preferentially use a left-sided approach for most primary cases, the decision to attempt re-exposure through the same incision must be considered carefully. Obliteration of tissue planes leaves the trachea, esophagus, and vascular structures vulnerable. This issue may outweigh the risk of injury to the recurrent laryngeal nerve through a contralateral right-sided approach. Some have suggested otolaryngologic evaluation of vocal cord motility, if a contralateral approach is entertained.59 If a vocal cord is noted to be paretic from the previous approach, then the repeat approach should be same sided to avoid the possibility of injuring the contralateral intact recurrent laryngeal nerve. Hilibrand and colleagues60 reported a fusion rate of 63% for nonplated interbody fusions for adjacent segment disease using iliac crest autograft. The authors of that study suggested using a strut graft in hopes of increasing the likelihood of fusion. It is our strategy, however, to perform a plated interbody fusion for such adjacent-level procedures in hopes of improving the odds of fusion. If the previous fusion also used an anterior plate, removal may be required to allow proper position of the plate for the adjacent level. The specific design of the previously placed plate should be identified preoperatively so that appropriate instruments can be made available. Newer devices such as threaded cages, staples, or cage-staple hybrids have been introduced as alternatives that would obviate the need for plate removal/replating, but further study is necessary to clarify their role.
Pseudarthrosis
The incidence of pseudarthrosis after anterior cervical interbody fusion ranges from 0% to 20% after single-level fusion.61–65 The incidence has been reported as high as 50% after multilevel fusions.66–68 The presence of a pseudarthrosis does not necessarily result in a failed operation,69 but recent studies have suggested that successful bony fusion increases the likelihood of a favorable result.70,71 In a retrospective study of 48 patients who developed pseudarthrosis after anterior cervical discectomy and fusion (ACDF), 67% were symptomatic at latest follow-up or at the time of further surgery.72 A younger age at the time of the ACDF increased the likelihood of the pseudarthrosis becoming symptomatic. In multiple-level ACDFs, the caudal-most operated level accounted for 82% of the pseudarthroses. Symptoms associated with pseudarthrosis include neck pain and a “radiculitis” or recurrent radiculopathy similar to the patient’s preoperative symptoms.70,71
When nonoperative modalities fail, surgical management may be considered. Modifiable underlying factors that contribute to the risk of nonunion include metabolic abnormalities, smoking, and patient noncompliance with postoperative restrictions.73,74 Once these issues have been addressed, the surgical approach must be selected. Both repeat anterior fusion and posterior laminoforaminotomy plus fusion have been described. The typical observation has been that the anterior pseudarthrosis eventually fuses as a posterior arthrodesis is achieved.48 Published studies have reported success in 59% to 80% of patients treated with anterior repair of cervical nonunions.71,74,75 As discussed in the previous section, a repeat anterior approach involves dissection through scar tissue with the associated risk of injuring the recurrent laryngeal nerve, carotid vessels, esophagus, and trachea.76,77 Postoperative hoarseness and dysphagia are also not uncommon.65,78,79
Several studies have retrospectively compared posterior fusion versus anterior pseudarthrosis repair. Brodsky and colleagues74 studied 34 patients being surgically treated for pseudarthrosis after anterior cervical fusion. Patients treated with a posterior fusion using wiring techniques had a 94% fusion rate, compared with 57% in patients who had revision anterior surgery. Lowery and colleagues75 also reported a 94% fusion rate with posterior fusion in patients with anterior cervical nonunion compared with 57% fusion rate for revision anterior surgery. More recently, Carreon and colleagues80 published a retrospective study of 120 patients undergoing repeat surgery for anterior cervical pseudarthrosis. Twenty-seven had repeat anterior procedures and 93 had posterior procedures. Patients in the anterior group had less blood loss and shorter average hospital stays. The anterior group had a 4% complication rate, while the posterior group had an 8% complication rate. All of the complications in both groups were wound or bone graft harvest site infections. Forty-four percent of the anterior revision group required an additional surgery for persistent nonunion, however, compared with only 2% in the posterior fusion group. The authors concluded that despite the higher blood loss, longer hospital stay, and higher complication rate, posterior fusion was preferable to anterior revision due to the higher fusion rate and much lower incidence of repeat revision surgery.
Given these considerations, we typically use the posterior approach as our preferred method of treatment for anterior cervical pseudarthroses. This approach should have three components: decompression of the affected nerve root, initial stabilization with internal fixation, and achieving solid arthrodesis. Decompression can be achieved through a standard laminoforaminotomy. We typically focus on dorsally decompressing the nerve root rather than manipulating it and trying to remove ventral osteophytes or fibrous material. Lateral mass screws can be augmented by spinous process wiring if there is concern of the purchase after laminoforaminotomy. Posterior iliac crest autograft is usually preferred in order to maximize chances of a solid arthrodesis. In our practice, the main indication that warrants a repeat anterior approach is an anterior nonunion associated with unstable or prominently migrating anterior implants. There have been several reports of implants eroding through the esophagus in such cases left untreated.81–84 At the time of such revisions, careful evaluation of the esophagus is necessary to detect perforation. Placement of dilute indigo Carmen dye directly into the esophagus through a nasogastric or orogastric tube can aid in the detection of occult injuries. If dye is noted in the wound, then a defect is present and intraoperative consultation by a general or head and neck surgeon may be necessary.
Failed Multilevel Decompression for Cervical Myelopathy
The treatment goal in surgical management of patients with cervical myelopathy is to prevent further neurologic deterioration or promote recovery.85 Barring the previously mentioned conditions, failure to improve after decompression may be due to advanced, irreversible neurologic changes, missed confounding diagnoses, or inadequate surgical decompression.86 In these cases, further imaging studies should be performed to evaluate for any continued thecal sac compression. The study of choice is a gadolinium-enhanced MRI. In symptomatic patients, MRI may reveal continued anterior thecal sac compression in up to 40% of patients.87 Myelography and postmyelography CT is also helpful when MRI findings are equivocal or for those patients who cannot undergo MRI.88 Unfortunately, in the absence of focal dramatic findings, there are no good guidelines to select patients for repeat decompression.87,89
Two relatively common scenarios that can lead to disappointing results after multilevel surgery for myelopathy are progressive ossification of the posterior longitudinal ligament90,91 and untreated or progressive kyphosis.92–94 Both conditions are covered in separate chapters elsewhere. In their absence, for selected cases where further surgery is contemplated, we individualize the approach on the basis of the patient’s previous operation. If the previous procedure was an anterior decompression, the posterior approach is safer and enables adequate visualization of each nerve root. A laminoplasty may be appropriate for residual myelopathy in the absence of neck pain or instability adjacent to the previously fused levels. Laminectomy and fusion may be more appropriate in patients who had previous multilevel corpectomies, instability above or below previous fusions, or those with significant axial pain. In patients who had previous posterior decompressions, an anterior approach may be indicated. Posterior cervical revision surgery is potentially dangerous because the anatomy is obscured by scar tissue and the potential for dural injury is high.95 In some of these cases, limited decompression and anterior fusion of segments with large disc osteophyte complexes may be adequate to address residual symptoms.
Pearls
Key Points
1 Finnegan WJ, Tenlen JM, Marvel JP, et al. Results of surgical intervention in the symptomatic multiply-operated back patient. J Bone Joint Surg Am. 1979;61:1077-1082.
Classic article detailing the declining chances for success with additional spinal procedures.
2 Waddell G, McCulloch JA, Kummel E, Venner RM. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
Discusses nonorganic physical signs that are associated with failure to improve postoperatively.
3 Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion. Spine. 2000;25:123-130.
4 Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;4:519-528.
5 Carreon L, Glassman SD, Campbell MJ. Treatment of anterior cervical pseudarthrosis: posterior fusion versus anterior revision. Spine J. 2006;6:154-156.
1 Weinstein JN, Lurie JD, Olson PR, et al. United States trends and regional variations in lumbar spinal surgery:1992-2003. Spine. 2006;31:2707-2714.
2 Finnegan WJ, Tenlen JM, Marvel JP, et al. Results of surgical intervention in the symptomatic multiply-operated back patient. J Bone Joint Surg Am. 1979;61:1077-1082.
3 Waddell G, Kummel EG, Lotto WN, et al. Failed lumbar disk surgery and repeat surgery following industrial injuries. J Bone Joint Surg Am. 1979;61:201-207.
4 Guyer RD, Patterson M, Ohnmeiss DD. Failed back surgery syndrome: Diagnostic evaluation. J Am Acad Orthop Surg. 2006;14:534-543.
5 Crock HV. Observations on the management of failed spinal operations. J Bone Joint Surg Br. 1976;58:193-199.
6 Maistrelli GL, Vaughan PA, Evans DC, et al. Lumbar disc herniations in the elderly. Spine. 1987;12:63-66.
7 Wong DA. Present initiatives and future directions: How to best serve our patients and members North American Spine Society Presidential Address, San Diego, CA. Spine J, 4. 2004:8-14.
8 Block AR, Gatchel RJ, Deardorff WW, Guyer RD. The Psychology of Spine Surgery. Washington DC: American Psychological Association; 2003.
9 Spengler DM, Freeman C, Westbrook R, Miller JW. Low-back pain following multiple lumbar spine procedures: Failure of initial selection? Spine. 1980;5:356-360.
10 Wiltse LL, Rocchio PD. Preoperative psychological tests as predictors of success of chemonucleolysis in the treatment of the low-back syndrome. J Bone Joint Surg Am. 1975;57:478-483.
11 Klekamp J, McCarty E, Spengler DM. results of elective lumbar discectomy for patients involved in the workers’ compensation system. J Spinal Disord. 1998;11:277-282.
12 Taylor VM, Deyo RA, Ciol M, et al. Patient-oriented outcomes from low back surgery: A community-based study. Spine. 2000;25:2445-2452.
13 Waddell G, McCulloch JA, Kummel E, Venner RM. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
14 Lang P, Genant HK, Chafetz N. Three dimensional computed tomography and multiplanar reformations in the assessment of pseudarthrosis in posterior lumbar fusion patients. Spine. 1988;13:69-75.
15 Ross JS, Masaryk TJ, Schrader M. MR imaging of the postoperative lumbar spine: assessment with gadopentetate dimeglumine. Am J Roentgenol. 1990;155:867-872.
16 Riew KD, Park JB, Cho YS, et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am. 2006;88:1722-1725.
17 Sasso RC, Macadaeg K, Nordmann D, et al. Selective nerve root injetions can predict surgical outcome for lumbar and cervical radiculopathy. J Spinal Disord Tech. 2005;18:471-478.
18 Carragee EJ, Chen Y, Tanner CM, et al. Provocative discography in patients after limited lumbar discectomy: a controlled, randomized study of pain response in symptomatic and asymptomatic subjects. Spine. 2000;25:3065-3071.
19 Weatherley CR, Prickett CF, O’Brien JP. Discogenic pain persisting despite solid posterior fusion. J Bone Joint Surg Br. 1986;68:142-143.
20 Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels or prior posterolateral fusion. Spine. 2000;25:853-857.
21 Kostuik JP. Decision making in adult scoliosis. Spine. 1979;4:521-525.
22 Mavrocordatos P, Cahana A. Minimally invasive procedures for the treatment of failed back surgery syndrome. Adv Tech Stand Neurosurg. 2006;31:221-252.
23 Barre L, Lutz G, Southern D, et al. Fluoroscopically guided caudal epidural injections for lumbar spinal stenosis: a retrospective evaluation of long term efficacy. Pain Physician. 2004;7(2):187-193.
24 Turner J, Loeser J, Deyo R, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-147.
25 North RB, Kidd DH, Farrikhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2006;56:98-107.
26 West JL3rd, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg Am. 1991;73:1179-1184.
27 Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion. Spine. 2000;25:123-130.
28 Lee SH, Kang BU, Jeon SH, et al. Revision surgery of the lumbar spine: anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation. J Neurosurg Spine. 2006;5:228-233.
29 Potter BK, Freedman BA, Verwiebe EG, et al. Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech. 2005;18:337-346.
30 Lowe TG, Tahernia AD, O’Brien MF, et al. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2 year results. J Spinal Disord Tech. 2002;15:31-38.
31 Bengalis DMJr, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139-144.
32 Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF); a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
33 Patel AA, Brodke DS, Pimenta L, et al. Revision strategies in lumbar total disc arthroplasty. Spine. 2008;33:1276-1283.
34 Bertagnoli R, Funk JF, Schneider SV. Complications and strategies for revision surgery in total disc replacement. Orthop Clin North Am. 2005;36:389-395.
35 Punt IM, Visser VM, Lodewijk WVJ, et al. Complications and reoperations of the SB CHARITÉ lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17:36-43.
36 Nguyen HV, Akbarnia BA, van Dam BE, et al. Anterior exposure of the spine for removal of lumbar interbody devices and implants. Spine. 2006;31:2449-2453.
37 Leary SP, Regan JJ, Lanman TH. Revision and explantation strategies involving the CHARITÉ lumbar artificial disc replacement. Spine. 2007;32(9):1001-1011.
38 Alanay A, Vyas R, Shamie A, et al. Safety and efficacy of implant removal for patients with recurrent back pain after a failed degenerative lumbar spine surgery. J Spinal Disord Tech. 2007;20:271-277.
39 LaRocca H, Macnab I. The laminectomy membrane. J Bone Joint Surg Br. 1974;56:545-550.
40 Chen Z, Zhao J, AiGang L. Surgical treatment of recurrent lumbar disc herniation by transforaminal lumbar interbody fusion. Int Orthop. 2009;33:197-201.
41 Hansraj K, O’Leary P, Cammisa F, et al. Decompression, fusion, and instrumentation surgery for complex lumbar spinal stenosis. Clin Orthop Rel Res. 2001;384:18-25.
42 Chen WJ, Lai PL, Chi-Chien N, et al. Surgical treatment of adjacent instability after lumbar spine fusion. Spine. 2001;26:E519-E524.
43 Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86:1497-1503.
44 Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970-981.
45 Phillips FM, Carlson GD, Bohlman HH, et al. Results of surgery for spinal stenosis adjacent to previous lumbar fusion. J Spinal Disord. 2000;13:432-437.
46 Vives MJ, Bono CM, Garfin SR. Management of adjacent segment degeneration and spinal stenosis (scar). Semin Spine Surg. 2008;20:284-292.
47 Whitecloud TS, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine. 1994;19:531-536.
48 Farey ID, McAfee PC, Davis RF, et al. Pseudarthrosis of the cervical spine after anterior arthrodesis. J Bone Joint Surg Am. 1990;72:1171-1177.
49 Hilibrand AS, Dina TS. The use of diagnostic imaging to assess spinal arthrodesis. Orthop Clin North Am. 1998;29:591-601.
50 Martin GJ, Haid RW, MacMillan M, et al. Anterior cervical discectomy with freeze-dried fibula allograft: Overview of 317 cases and literature review. Spine. 1999;24:852-859.
51 Cannada LK, Scherping SC, Yoo JU, et al. Pseudarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine. 2003;28:46-51.
52 Buchowski JM, Liu G, Bunmaprasert T, et al. Anterior cervical fusion assessment: surgical exploration versus radiographic evaluation. Spine. 2008;33:1185-1191.
53 Manifold SG, McCann PD. Cervical radiculitis and shoulder disorders. Clin Orthop Relat Res. 1999;368:105-113.
54 Epstein NE, Epstein JA, Carras R. Coexisting cervical spondylotic myelopathy and bilateral carpal tunnel syndromes. J Spinal Disord. 1989;2:36-42.
55 Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;4:519-528.
56 Weinstein SM, Herring SA, Derby R. Contemporary concepts in spine care. Epidural steroid injections. Spine. 1995;20:1842-1846.
57 Ellenger MR, Honet JC, Treanor WJ. Cervical radiculopathy. Arch Phys Med Rehabil. 1994;75:342-352.
58 Herkowitz HN, Kurz LT, overholt DP. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine. 1990;15:1026-1030.
59 Winslow CP, Meyers AD. Otolaryngologic complications of the anterior approach to the cervical spine. Am J Otolaryngol. 1999;20:16-27.
60 Hilibrand AS, Yoo JU, Carlson GD, et al. The success of anterior cervical arthrodesis adjacent to a previous fusion. Spine. 1997;22:1574-1579.
61 Aronson N, Filtzer DL, Bagan M. Anterior cervical fusion by the Smith-Robinson approach. J Neurosurg. 1968;29:397-404.
62 Riley LH, Robinson RA, Johnson KA, et al. The results of anterior interbody fusion of the cervical spine. J Neurosurg. 1969;30:127-133.
63 Robinson RA, Walker AE, Ferlic DC, et al. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg Am. 1962;44:1569-1587.
64 Rogers WA. Fractures and dislocations of the cervical spine. An end-result study. J Bone Joint Surg Am. 1957;39:341-376.
65 Simmons EH, Bhalla SK. Anterior cervical discectomy and fusion. J Bone Joint Surg Br. 1969;51:225-237.
66 Conolly ES, Seymour RJ, Adams JE. Clinical evaluation of anterior cervical fusion for degenerative cervical disc disease. J Neurosurg. 1965;23:431-437.
67 Emery SE, Bolesta MJ, Banks MA, et al. Robinson anterior cervical fusion. Comparison of standard and modified techniques. Spine. 1994;19:660-663.
68 White AA, Southwick WO, Duponte R, et al. Relief of pain by anterior cervical spine fusion for spondylosis. J Bone Joint Surg Am. 1968;55:525-534.
69 Fernyhough JC, White JI, La Rocca H. Fusion rates in multi-level cervical spondylosis comparing allograft fibula and auto graft fibula in 126 patients. Spine. 1994;16:s561-s564.
70 Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical diskectomy and arthrodesis for cervical radiculopathy. J Bone Joint Surg Am. 1993;75:1298-1307.
71 Newman M. The outcome of pseudarthrosis after cervical anterior fusion. Spine. 1993;18:22380-22382.
72 Phillips FM, Carlson G, Emery SE. Anterior cervical pseudarthrosis: natural history and treatment. Spine. 1997;22:1585-1589.
73 Steinman JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80-90.
74 Brodsky AE, Khalil MA, Sassard WR, et al. Repair of symptomatic pseudarthrosis of anterior cervical fusion: posterior versus anterior repair. Spine. 1992;17:1137-1143.
75 Lowery GL, Swank ML, McDonough RF. Surgical revision for failed anterior cervical fusion: articular pillar plating or anterior revision? Spine. 1995;20:2336-2341.
76 Beutler WJ, Sweeney CA, Connolly PT. Recurrent laryngeal nerve injury with anterior cervical spine surgery: risk with laterality of surgical approach. Spine. 2001;26:1337-1342.
77 Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien). 1989;99:41-50.
78 Baron EM, Soliman AM, Gaughan JP, et al. Dysphagia, hoarseness, and unilateral true vocal fold motion impairment following anterior cervical discectomy and fusion. Ann Otol Rhinol Laryngol. 2003;112:921-926.
79 Winslow CP, Winslow TJ, Wax MK. Dysphonia and dysphagia following the anterior approach to the cervical spine. Arch Otolaryngol Head Neck Surg. 2001;127:51-55.
80 Carreon L, Glassman SD, Campbell MJ. Treatment of anterior cervical pseudarthrosis: posterior fusion versus anterior revision. Spine J. 2006;6:154-156.
81 Pompili A, Canitano S, Caroli F, et al. Asymptomatic esophageal perforation caused by late screw migration after anterior cervical plating: report of a case and review of relevant literature. Spine. 2002;27:E499-E502.
82 Fountas KN, Kapsalaki EZ, Machinis T, et al. Extrusion of a screw into the gastrointestinal tract after anterior cervical spine plating. J Spinal Disord Tech. 2006;19:199-203.
83 Gazzeri R, Tamorri M, Faiola A, et al. Delayed migration of a screw into the gastrointestinal tract after anterior cervical spine plating. Spine. 2008;33:E268-E271,.
84 Lu DC, Theodore P, Korn WM, et al. Esophageal erosion 9 years after anterior cervical plate implantation. Surg Neurol. 2008;69:310-312.
85 Bernard TNJr, Whitecloud TSIII. Cervical spondylotic myelopathy and myeloradiculopathy. Anterior decompression and stabilization with autogenous fibula strut graft. Clin Orthop. 1987;221:149-160.
86 Emery SE, Bohlman HH, Bolesta MJ, et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80:941-951.
87 Okamoto A, Shinomiya K, Furuya K, et al. Post-operative magnetic resonance imaging in patients with cervical myelopathy. Spine. 1991;16:s530-s533.
88 Fager CA. Evaluation of cervical spine surgery by postoperative myelography. Neurosurgery. 1983;12:416-421.
89 Martins AN. Anterior cervical discectomy with and without interbody graft. J Neurosurg. 1976;44:290-295.
90 Chiba K, Ogawa Y, Ishii K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy-average 14-year follow-up study. Spine. 2006;31:2998-3005.
91 Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7-13.
92 Guigui P, Benoist M, Deburge A. Spinal deformity and instability after multilevel cervical laminectomy for spondylotic myelopathy. Spine. 1998;23:440-447.
93 Kaptain GJ, Simmons NE, Replogle RE, et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93:199-204.
94 Albert TJ, Vaccaro AR. Postlaminectomy kyphosis. Spine. 1998;23:2738-2745.
95 Farcy JP, Weidenbaum M, Sola C. Surgical management of severe cervical kyphosis following extensive laminectomy. Spine. 1990;15:41-45.