CHAPTER 23 Revision Anterior Cruciate Ligament Reconstruction
Reconstruction of the torn anterior cruciate ligament (ACL) has seen a significant increase in volume over the past 3 decades. It is currently one of the most common procedures performed by orthopaedic surgeons in the United States and across the globe. Current estimates cite approximately 250,000 procedures performed annually in the United States. Although the most of these patients have a fairly uneventful recovery and return to sport and/or work, there are patients who present as clinical failures, with three categories represented by recurrent instability, loss of motion, and pain.1 With the ever-increasing numbers of primary surgeries performed, it follows that the numbers of failed primary procedures will subsequently increase. Orthopedic surgeons will be required to tackle these challenging situations.
Although the causes of failure may be multifactorial, these failures may be broadly classified into three categories: loss of motion, recurrent instability, and pain.2 Another potential category may be progressive joint deterioration without the above findings after ACL reconstruction. Any of these factors may play a role in the failure of a reconstruction, but the final goal of revision reconstruction remains constant—provide a stable and functional ACL that most accurately reproduces the normal knee kinematics, allow the individual to return to an active lifestyle, and potentially decrease the development of early osteoarthritis.3,4
ANATOMY
Although commonly overlooked throughout the past 1 to 2 decades, recent anatomic studies have further defined our precise understanding of the anatomy of the native ACL, grossly and arthroscopically.5,6 Recognition of the ACL as a functional two-bundle ligament comprised of distinct anteromedial and posterolateral bundles, as well as their relationship to bony insertion site anatomy, has led to a revolution in discussing ACL reconstruction techniques.7 It is now understood that re-creation of the native anatomy of the ACL may lead to improved kinematic and functional results. Prior nonadherence to these principals may be a major root cause of many primary reconstruction failures.8
Causes of Reconstruction Failure
Loss of Motion
Loss of motion after reconstruction of the ACL may lead to profound functional disability. Depending on the source, the patient may experience loss of extension, flexion, patellar entrapment, and/or global loss of motion as measured in all three planes. The loss of extension often has a more deleterious functional effect on the patient than loss of flexion, because it leads to difficulty with simple ambulation and restoration of the normal gait cycle by feedback loop inhibition of the extensor mechanism.9–11 This may also lead to patellofemoral symptoms, referred or secondary joint arthralgias caused by antalgic gait, and loss of athletic performance. Increased forces are experienced in the patellofemoral compartment, leading to early osteoarthritic changes in these young patients. Loss of minor flexion is often asymptomatic and rarely causes significant disability unless total flexion is limited to less than 120 degrees.10
The causative factors for motion loss are often complex and may be intimately interrelated. Possible factors include poor technique causing graft impingement, infection, immobilization, inadequate or excessive rehabilitation, capsulitis-arthrofibrosis, and complex regional pain syndrome.9,10,12 Nonanatomic technique often creates a situation in which the ACL graft is not anatomically positioned in respect to the femoral or tibial insertion sites, or both. This nonanatomic placement may result in a situation in which the graft impinges on the wall of the lateral femoral condyle, roof of the intercondylar notch, or posterior cruciate ligament (PCL), resulting in mechanical loss of motion in flexion or extension and graft attrition, as well as eventual stretching of the graft because of nonanatomic placement of the graft. Loss of flexion may be related to relative anterior placement of the graft on the femoral and tibial sides; conversely, loss of extension may occur with excessive posterior placement of the graft in relation to the tibial and femoral anatomic positions.1,2,13
Another cause of mechanical motion loss includes the phenomenon known as the cyclops lesion, which is rather uncommon. This represents fibroproliferative nodules that may occur on the anterior surface of the ACL graft that limit extension by a mechanical block in the final few degrees.14 These may develop as a result of failure to remove excess debris from the intercondylar notch produced during reaming, or may be a scarring response to repeated impingement of the graft on the roof of the intercondylar notch. Preventive measures against the cyclops lesions include immediate passive and active extension exercises prior to formation of the nodules.
Some patients may experience a condition known as regional capsulitis, or arthrofibrosis, which may also result in loss of motion.1,15 This may be broken down into primary and secondary conditions, with primary being an idiopathic exaggerated healing response to surgical trauma and secondary representing the results of inappropriate surgical timing and/or rehabilitation. In primary or idiopathic arthrofibrosis, the patient presents with excessive postoperative pain, swelling, warmth, resistance to motion in flexion and extension, and severely limited patellar motion in all planes. Treatment generally focuses on restoration of full motion through gentle rehabilitation modalities while, most importantly, reducing the associated pain and inflammation. Early operative intervention should be avoided, because this often accentuates the process. Eventually, this process may result in permanent or fixed global loss of motion caused by caused by intra-articular adhesions.16 If initial conservative measures are inadequate for restoration of motion, surgical manipulation and lysis of these adhesions may be considered once the period of acute inflammation has defervesced and pain controlled. Although this may result in improved motion, these patients rarely achieve objective full motion and only occasionally experience clinical recurrent instability.
Technical Errors
Nonanatomic surgical technique in the primary ACL reconstruction represents the most common cause of failure of these procedures.4 Technical errors include inadequate clearance for the graft in the intercondylar notch, malpositioned nonanatomic tunnels, poor tensioning of the grafts, and inadequate fixation of the graft. Although the concepts of roofplasty and wallplasty are controversial, the surgeon must confirm that the graft is free of bony impingement throughout the full knee range of motion, which may require some degree of bone and soft tissue removal, particularly in the chronic revision ACL-deficient knee.
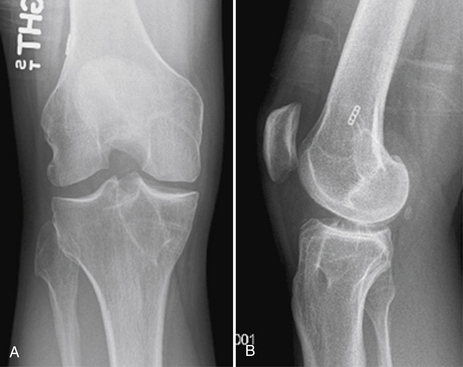
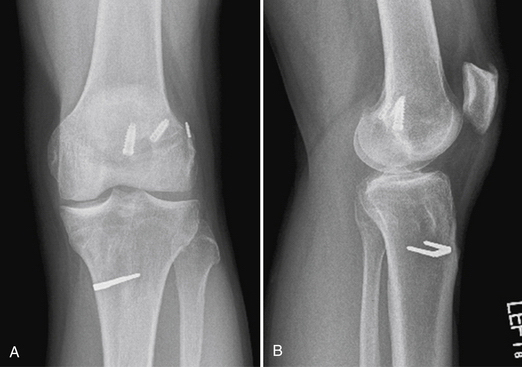
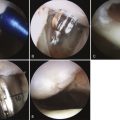
Placement of tibial and femoral tunnels in nonanatomic positions may result in graft attrition and mechanical entrapment or capture of the knee. Additionally, these errors in tunnel management may result in a graft that allows for persistent or recurrent instability, even in the face of an intact graft. Estimates show that approximately 70% to 80% of failures may be attributed to improper tunnel placement, with nonanatomic femoral locations representing the most commonly encountered error in tunnel placement. Cadaveric and arthroscopic studies have confirmed the topographic bony insertion and origin sites of the ACL and their relationship to the reconstructed ligament. Related studies cast doubt that the common endoscopic transtibial approach to femoral tunnel placement may result in an anatomic location of the femoral insertion of the reconstructed ACL.17,18 This transtibial endoscopic technique most commonly leads to common mistakes in graft placement, including excessive posterior placement on the tibial side combined with a femoral site too high and/or anterior on the medial wall of the lateral femoral condyle. In the best case scenario, there is a mismatch in graft placement within the native insertion site, more closely recreating the posterolateral (PL) site on the tibia to the anteromedial (AM) site on the femur. This combination results in poor tensioning in relation to overall knee kinematics, possible impingement of the graft on the PCL, and a vertical graft (Figs. 23-1 and 23-2).19 This situation leaves the graft in a position that may provide some anteroposterior stability, but sacrifices rotational stability and possibly limits range of motion of the knee. In this case, the knee may actually have been made worse by the reconstruction. Revision surgery is almost inevitably required, depending on the patient’s level of activity.
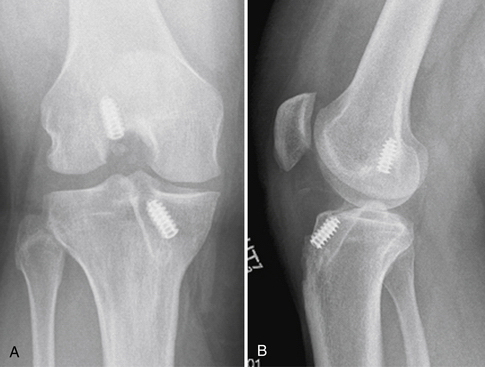
FIGURE 23-1 Failed vertical ACL reconstruction. A, AP view. B, Lateral view.
(From Shaver J, Johnson D. Revision anatomic double-bundle anterior cruciate ligament surgery. Oper Tech Sports Med. 2008;16:157-164.)
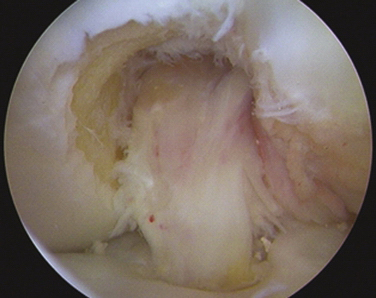
FIGURE 23-2 Failed ACL reconstruction with a vertical intact graft but a grossly positive pivot shift on EUA.
The tibial insertion site may be somewhat more forgiving to mistakes in tunnel placement, but significant deviations from anatomic locations may result in less ideal outcomes. Excessive anterior placement results in superior root impingement in full extension and excessive graft forces in flexion, whereas posterior placement results in flexion laxity and possible impingement on the PCL. Additionally, medial or lateral deviations may result in PCL and/or wall impingement and eventual graft attrition and failure.20–23
Failure of Biologic Incorporation
Failure of the graft to incorporate into the host bone may also represent a source of failure of primary ACL reconstruction. This often presents with recurrent instability in the face of an anatomically well-positioned graft. This may be a result of poor fixation on the femoral or, more commonly, the tibial side, excessive forces experienced by the graft during rehabilitation and biologic incorporation, immune responses of the host versus the graft in the case of allografts, or lack of recognition of certain graft-host mismatches. The tibial metaphyseal bone has been identified as the weak link in graft fixation, and therefore additional backup fixation is often recommended, particularly with all soft tissue ACL reconstruction.24 Excessively aggressive rehabilitation protocols may not allow the proper ligamentization of the graft and may eliminate or delay the reparative process that incorporates the graft with the host bone. Each graft substitute has a biologic timetable of incorporation that must be recognized and rehabilitation tailored to the individual situation.25,26 Immune responses have been known to occur between host bone and allografts and/or certain fixation devices, which may result in tunnel lysis and failure of incorporation of the graft. Recognized graft-host mismatches include soft tissue allografts and autografts in young hyperlax females and high-demand athletes, which may stretch out over time and result in recurrent instability.27–29
Concurrent Laxity
Another cause of failure of primary ACL reconstructions is lack of recognition of concurrent injuries to the important secondary stabilizers of the ACL. The native ACL is the primary restraint to anterior displacement of the tibia, but must work in concert with multiple other structures to function optimally.30 This remains true for the reconstructed ACL, and may be even more important because prior to realization of full strength, the graft must undergo incorporation and ligamentization over an extended period of time. If the secondary stabilizers are absent or nonfunctional, nonphysiologic forces are placed on the graft during the ligamentization process, resulting in a nonfunctional lax graft that does not recreate the stability of the native ACL. The secondary stabilizers are numerous, and include the menisci (medial > lateral), medial collateral ligament (MCL), posteromedial corner (including the posterior oblique ligament [POL]), posterior cruciate ligament (PCL), and the posterolateral corner (PLC).31 These structures may be injured in conjunction with the original ACL injury, or may become lax because of repeated episodes of instability in a chronically ACL-deficient knee.32 A reconstruction of the ACL in the face of concurrent laxity places nonphysiologic stresses on the graft, and often results in premature failure of these reconstructions.33,34 Recognition by thorough examination and proper management strategies for these additional sources of laxity must be taken into account in the primary and revision ACL reconstruction situations.
Traumatic Causes
Finally, the well-placed primary graft may simply fail because of traumatic events inherent to the stresses experienced in grafts of many of this active subset of patients. These failures can be caused by excessive strain placed on the immature graft by excessive rehabilitation, premature return to high-level sports or activities, or shear bad luck during high-level sporting activities. These are generally characterized as early failures (less than 1 year), which occur during graft remodeling and incorporation prior to realization of the final graft strength, or late failures (more than 1 year, with no subjective evidence of prior instability).13 Late failures often present in a similar fashion to their initial traumatic event, often with an acute hemarthrosis and recurrent instability. It is important to evaluate the patient critically who has failed primary surgery and has never returned to high-level sports. These patients often have some component of technical errors that precluded their full return after the index procedure.
PATIENT EVALUATION
The most important aspect of preoperative planning for the revision ACL patient is elucidation of the primary cause of the index surgery’s failure, as noted. Another vital component is the patient’s expectations and desires following the revision reconstruction. It is imperative that the surgeon and patient have realistic goals in the revision situation, as studies have shown a significantly lower rate of return to high-level sports.35–37 Some patients may have a degree of injury that makes this goal unrealistic, and the revision may be considered a salvage procedure to limit any further damage.38 The patient and surgeon must also understand and agree that in the revision situation, a significant investment of time and effort is often required for successful outcomes.
Physical Examination
The physical examination in the revision ACL situation must include a global assessment of the limb. This includes an evaluation of overall limb alignment and gait, because significant variations such as a varus thrust may compromise the longevity of an ACL reconstruction.39 Limitations of motion of not only the knee but also the hip and ankle must be taken into account and possibly addressed prior to revision. The condition and any dysfunction of the supporting thigh musculature need to be addressed, because this may be key to rehabilitation after any revision reconstruction. Residual infectious processes must be ruled out in all cases, because this will doom any revision. The locations of previous incisions are taken into account, and future incisions planned to ensure cutaneous tissue viability.
Diagnostic Imaging
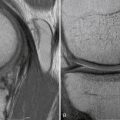
Good-quality radiographic evaluation of the knee aids significantly in preoperative planning and should always be performed. These include standing full extension anteroposterior and lateral views of the knee, as well as 45-degree flexion weight-bearing posteroanterior views. Additional examinations that may be of aid include axial views of the patella, full-length mechanical axis views, varus and valgus stress views, and views of the contralateral knee for comparison. These radiographic studies are evaluated for prior tunnel placement, tunnel expansion, location of prior hardware, presence of degenerative changes, and notch geometry.40 These patients often present with MRI scans, which this may be reviewed for concomitant injury to the secondary stabilizers, condition of the menisci and articular cartilage, and placement and condition of the prior graft.
Arthroscopic Technique
Staging
When faced with the revision patient, one consideration faced by the surgeon is whether the case can be appropriately managed in one surgical session. Situations such as significant limb malalignment, motion loss, and tunnel osteolysis may represent occasions when a staged procedure must be considered. Addressing limb malalignment with osteotomies concurrently with revision ACL surgery is technically feasible; however, this situation may have a propensity for a difficult rehabilitation and portend poor results in all but the most motivated of patients.41 The same concept can be applied to addressing significant motion restriction at the time of revision reconstruction. Most would recommend addressing this with surgical manipulation, lysis of adhesions, and/or aggressive physical therapy, with subsequent revision reconstruction when restoration of motion is achieved. Revision surgery proceeds only if true clinical instability is the problem, not instability caused by lack of rehabilitation strength in the extremity.
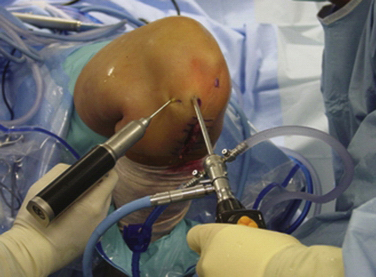
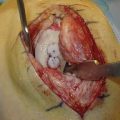
If the degree of tunnel expansion, osteolysis, or previous hardware placement will not allow both anatomic reconstruction and secure postoperative fixation of the grafts for immediate motion postoperatively, the procedure should be staged. Generally, this includes surgical removal of previous hardware and bone grafting of the tunnels, followed by full motion and quadriceps rehabilitation while protecting the knee from recurrent buckling episodes. Revision surgery may then proceed after incorporation of this bone graft material, typically at 4 to 6 months.3,42 When possible, autogenous bone graft should be used in preference to allograft substitutes (Fig. 23-3).
Graft Selection
Thus far, no source of graft in the revision situation has been proven to be the gold standard or clinically superior. The choice of graft must be tailored to the patient’s age, gender, factors such as hyperlaxity, and the physical demands that the reconstructed graft will experience, similar to the recommendations for primary ACL surgery. The full spectrum of grafts may be considered with the revision ACL reconstruction, including numerous autograft and allograft choices. Autograft availability may be limited by their prior use, and scarring from prior procedures may make this technically difficult, if not impossible, although contralateral harvest may be a viable choice in these situations. Cases involving bone loss and tunnel expansion may be more suitable for autografts or allografts that have bone attached, because these are thought to provide better tunnel fill and secure graft fixation characteristics.
Advantages to allograft revision reconstruction are numerous. Many revision surgeons believe that this may represent the optimal graft selection. The lack of donor site morbidity, smaller incisions resulting in less soft tissue trauma, less operative theatre time, and ability to customize the graft size dimensions to the revision situation represent some of the major advantages of allografts in revision ACL reconstructions.43 However, disadvantages such as patient preference, longer incorporation times, and concerns regarding disease transmission remain. Currently, the most common allografts choices include the Achilles tendon-calcaneus and bone-patellar tendon-bone grafts, and all soft tissue grafts such as the anterior and posterior tibialis tendon grafts.
Retained Hardware
Having to plan for and deal with retained hardware or absorbable implants is ubiquitous to revision ACL surgery, and may significantly influence the technical aspects of the procedure. Often, when the primary surgery was performed in a nonanatomic fashion, the hardware may not interfere with anatomic tunnel and graft placement and is therefore retained.44 If it does interfere, the surgeon should have detailed knowledge of the retained implant and an armamentarium of instruments available that are designed for removal of this hardware. The surgeon must also be comfortable with alternative fixation strategies (over the top and two-incision techniques) to deal with the consequences that may occur after removal of offending hardware and technical problems such as short femoral tunnels or posterior wall blowout.
Primary Tunnels
As a result of the primary reconstruction, it is inevitable that the revision surgeon must account for and often incorporate previous tunnels into the surgical plan. These may interfere with the placement of new anatomic tunnels. If they are located in a position significantly away from the planned revision tunnels, surgery may proceed much like a primary reconstruction (Fig. 23-4).
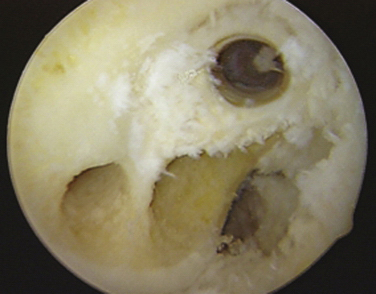
FIGURE 23-4 Vertical tunnel with screw removed and anatomic PL and AM tunnel placement.
(From Shaver J, Johnson D. Revision anatomic double-bundle anterior cruciate ligament surgery. Oper Tech Sports Med. 2008;16:157-164.)
Another strategy involves use of the concept of divergence to allow the surgeon to miss the previous bony tunnel while incorporating the intra-articular entry and exit points of prior tunnels.45 On the femoral side, this usually includes the use of additional accessory portals or varying angles of knee flexion while changing the inclination or position of the tunnel on the tibial metaphyseal side. The surgeon may also choose to use outside-in drilling or the two-incision technique.
Anatomic Single- Versus Double-Bundle Revision
In regard to the type of revision surgery to perform, there are several considerations. Patient size, instability pattern and degree (grade I vs. grade III pivot shift), intraoperative findings, and surgeon comfort influence this choice tremendously. Patients of small stature may not be ideal candidates for the use of anatomic double-bundle techniques, whereas patients with subtle concurrent secondary laxity (i.e., previous significant medial damage or meniscectomies) may be ideal candidates for this technique. Anatomic double-bundle techniques allow for a greater volume of intra-articular graft volume to counteract the secondary laxity often encountered in revision situations. This has been shown to be superior for the restoration of normal knee kinematics versus traditional single-bundle reconstructions.8,46,47 However, the anatomic double-bundle technique remains a technically challenging procedure, with a steep learning curve with an increased risk of intraoperative complications, which further accentuates the difficulty of these already complex revision cases.
Often, previous nonanatomic grafts may lie in a position that adequately controls the anteroposterior motion of the tibia, effectively eliminating the Lachman test and reducing the negative results of the anterior drawer test; however, they experience recurrent rotational instability, clinically expressing the pivot shift test. This may result in significant functional instability in regard to athletic participation. These patients may be candidates for an augmentation procedure to add the second anatomic posterolateral bundle, eliminating the rotational instability.48 This requires a thorough knowledge of the functional and arthroscopic anatomy of the ACL, with expertise in placing this graft while not damaging the prior graft.
Principles of Revision Procedures
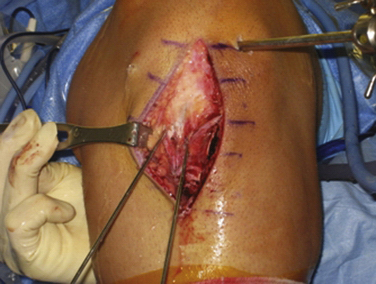
It is our belief that use of the anatomic double-bundle anterior cruciate ligament techniques and principles in revision surgeries is advantageous and the treatment of choice for most of these patients. Adherence to this philosophy will lead to improved restoration of the native kinematics of the knee, thereby improving overall outcomes. The larger volume of tissue counteracts the effects of chronic laxity of the ACL and supporting structures, once again aiding in the restoration of the native environment of the knee. However, certain patient situations may not be conducive to this technique. These include individuals of significantly smaller stature, which will not allow adequate surface area and volume of bone in the lateral femoral condyle that are required to perform a double-bundle reconstruction safely. In these situations, we prefer to revise them to an anatomic single-bundle reconstruction, but still adhere strictly to the principles of tunnel placement previously noted (Fig. 23-5).
Examination Under Anesthesia
A global examination under anesthesia (EUA) should be performed and the condition of not only the degree of ACL laxity but also the supporting structures documented. This should be evaluated in comparison to the contralateral knee to determine the true degree of pathologic laxity. It is our experience that the in-office clinical examination is very inconsistent with the EUA, especially in regard to the degree of anterolateral rotatory instability as noted by the pivot shift test. The office examination often underestimates the degree of pathologic laxity.
Patient Positioning
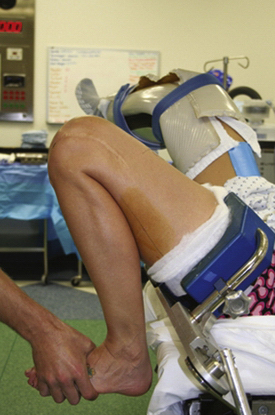
Our preferred setup uses an operating room table that provides full clearance for the lower extremities by a break in the lower half of the bed, with the patient’s buttocks positioned at this break. The nonoperative extremity is held in a padded arthroscopic well-leg holder with the hip flexed to approximately 90 degrees and mildly externally rotated, providing clearance for access to the medial side of the operative extremity. A padded tourniquet is placed high on the thigh and the arthroscopic leg holder is placed on the most distal portion of the bed. The leg holder is then flexed to approximately 30 to 45 degrees, providing mild hip flexion and allowing for deep hyperflexion of the knee (Fig. 23-6). This position makes drilling of the femoral tunnels at 110 and 130 degrees possible from the accessory medial portal, which we recommend for anatomic tunnel placement in primary and revision surgery.49 This also allows full access to the distal half of the thigh, which may be needed to access and remove previous fixation hardware or perform a two-incision technique when required.
Portals and Diagnostic Arthroscopy
Correct portal placement is essential for adequate visualization and allows a significant degree of freedom for alternative visualization during the procedure. The initial anterolateral portal created is high and tight to the patellar tendon, which skirts the inferolateral pole of the patella. After insertion of the arthroscope, a low and tight to patellar tendon anteromedial portal is created under direct visualization of a spinal needle. This should be low enough not to interfere with use of the tip or elbow tibial ACL guide later in the procedure.49
Preparation of the Notch
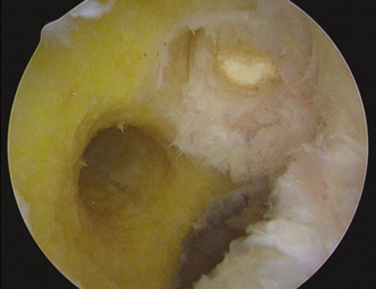
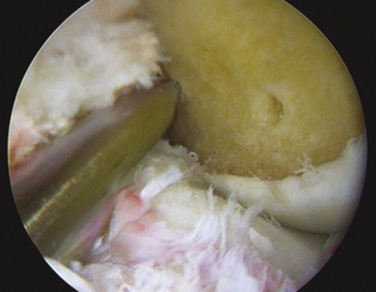
With complete failure of the previous graft or frank nonanatomic prior surgery, the residual graft is removed from the notch to improve visualization. Although the degree of roofplasty and wallplasty performed is controversial, the surgeon must visualize the entire wall and the over the back position, including the lateral intercondylar and bifurcate ridges, if still recognizable. The notchplasty is only done to allow for visualization and restoration of the native anatomy. It has been shown that the bony notch grows back after ACL reconstruction. Therefore, a notchplasty simply for graft placement is not necessary. Previous tunnels and hardware must be cleared of soft tissue and overgrowth of bone by curettes, osteotomes, and picks to allow for easier removal of previous hardware (if required), with a minimum of bony disruption (Fig. 23-7). We have found that at minimum the most inferior surface of the lateral femoral condyle should be contoured away from the notch to limit any potential impingement on this area after graft placement.
Femoral Tunnels
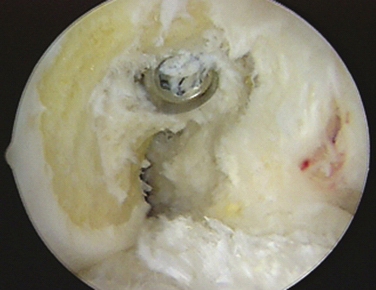
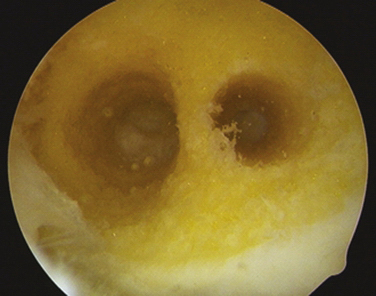
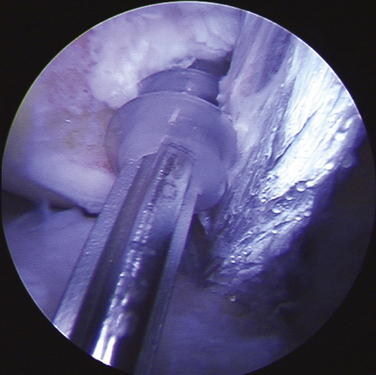
The arthroscope is then inserted in the low tight AM portal for direct visualization of the lateral wall. If the patient’s size and position of previous tunnels allow for two new anatomic femoral tunnels to be created safely, the technique follows as for a standard anatomic double-bundle surgery. Using an arthroscopic awl at 90 degrees of knee flexion, the anatomic positions of the proposed AM and PL tunnels are picked and marked (Fig. 23-8). Guide pins are placed and tunnels reamed at 110 degrees to create first the 5- to 6-mm PL tunnel and next at 130 degrees to create a divergent 7- to 9-mm AM tunnel. Intraoperative fluoroscopy or radiography may be used at this time to assist in placement of tunnels in an anatomic position. These tunnels are both drilled from the accessory medial portal using half-fluted reamers to avoid articular cartilage damage on entry while visualized from the low tight AM portal (Fig. 23-9). Overall length of the PL and AM tunnels are 28 to 34 and 32 to 40 mm, respectively. The integrity of the tunnel walls can then be visualized after removal of bony debris and contouring of the edges using a 5.5-mm shaver to reduce graft impingement (Fig. 23-10).
Tibial Tunnels
If the previous scar allows for anatomic placement of the tibial tunnels, this is used to access the primary tibial tunnel and remove hardware as indicated. A guide pin is placed across this prior tunnel, and the primary graft is removed to the interarticular position. This position can be then checked to determine its relationship to anatomic tibial tunnels. If in an anatomic or near-anatomic position, this tunnel may be overreamed to remove soft tissue and provide healthy bleeding bone or directionally expanded as described for the femoral tunnels to create the tibial AM tunnel. If previous tunnels are far from anatomic, a new 8- to 9-mm AM tunnel can be created with the guide set at 55 to 60 degrees and perpendicular to the joint line, and the previous tunnel bone grafted with excess bone from the Achilles-calcaneus allograft.
The 5- to 6-mm PL tunnel is created by placing the ACL drill guide through the accessory medial portal or low tight anteromedial portal and referencing off the lateral downslope of the medial tibial spine for its interarticular entry site. The angle of inclination for this guide is approximately 45 degrees, with its entry site at the anterior edge of the MCL on the tibial metaphysis (Fig. 23-11). Once again, intraoperative fluoroscopy or radiography may be of assistance in placement of anatomic tunnels. The AM and PL tunnels may often converge at the joint line into a singular intra-articular exit point, but with separate bony tunnels for each graft and later incorporation (Fig. 23-12).
Graft Passage and Fixation
Passing pins are directed through the femoral tunnels in identical trajectories to drilling from the accessory medial portal, and passing sutures are retrieved through the tibial tunnels using a probe or pituitary ronguer. At this time, anatomic placement of the tunnels can be checked using the exit points of the pins. They should exit the skin 2 to 4 cm apart on the lateral distal femur along the iliotibial band, and should be parallel to each other along the long axis of the femur (Fig. 23-13).
The PL bundle is passed first and femoral-sided fixation of this graft is done, usually with a 15-mm EndoButton (Smith & Nephew; Andover, Mass) fixation. Tension is kept on this graft while the AM bundle is passed and femoral-sided fixation is placed. Use of a probe or a 90- degree instrument may aid in passing the AM bundle past the PL graft. The AM bundle fixation is variable, and may be accomplished using an EndoButton or metal–soft tissue interference screw. The grafts are then cycled to evaluate isometry and pretension the grafts. The appropriately placed PL graft should have minimal translation with range of motion, but the AM may have a few millimeters of retraction in full extension (Fig. 23-14).
Final arthroscopic visualization confirms no impingement of the grafts, and postprocedure EUA is compared to the preoperative examination for re-creation of anteroposterior stability and elimination of the pivot shift (Fig. 23-15).
PEARLS& PITFALLS
PEARLS
PITFALLS
CONCLUSIONS
Current published outcomes for revision ACL reconstructions are far from optimal, with only approximately 60% of patients returning to sports participation. Of these who do return, most do so at a level inferior to their previous goals. There are a myriad of causes of a failed primary surgery, but the goal of revision surgery remains the same—to provide a stable and functional ACL that most accurately reproduces the kinematics of the native knee. We believe that adherence to the philosophy, principles, and techniques of anatomic double-bundle anterior cruciate ligament reconstruction in the revision setting will improve overall outcomes, especially in regard to high-level sports participation.
1. Johnson DL, Fu FH. Anterior cruciate ligament reconstruction: why do failures occur?. Instr Course Lect. 1995;44:391-406.
2. Johnson DL, Harner CD, Maday MG, Fu FH. Revision anterior cruciate ligament surgery. In: Fu FH, Harner CD, Vince KG, editors. Knee Surgery. Baltimore: Williams & Wilkins; 1994:877-895.
3. Getelman MH, Friedman MJ. Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg. 1999;7:189-198.
4. Wetzler MJ, Getelman MH, Friedman MJ, et al. Revision anterior cruciate ligament surgery: etiology of failures. Oper Tech Sports Med. 1998;6:64-70.
5. Chhabra A, Starman JS, Ferretti M, et al. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;4(suppl):2-10.
6. Ferretti M, Levicoff EA, Macpherson TA, et al. The fetal anterior cruciate ligament: an anatomic and histologic study. Arthroscopy. 2007;23:278-283.
7. Fu FH, Jordan SS. The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89:2103-2104.
8. Yagi M, Kuroda R, Nagamune K, et al. Double-bundle ACL reconstruction can improve rotational stability. Clin Orthop Relat Res. 2007;(454):100-107.
9. Harner CE, Irrgang JJ, Paul J. Loss of motion after anterior cruciate ligament surgery. Am J Sports Med. 1992;20:499-506.
10. Irrgang JJ, Harner CD. Loss of motion following knee ligament surgery. Sports Med. 1995;19:150-159.
11. Sachs RA, Daniel DM, Stone ML. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17:760-765.
12. Bach BR, Wojtys EM, Lindenfeld TN. Reflex sympathetic dystrophy, patella infera contracture syndrome, and loss of motion following anterior cruciate ligament surgery. Instr Course Lect. 1997;46:251-260.
13. Maday MG, Harner CD, Fu FH. Revision ACL surgery: evaluation and treatment. In: Feagin JA, ed. The Crucial Ligaments. New York: Churchill-Livingstone; 1994; 711-723.
14. Jackson DW, Schaefer RK. Cyclops syndrome: loss of extension following intra-articular anterior cruciate ligament reconstruction. Arthroscopy. 1990;6:171-178.
15. Shelbourne KD, Wilckens JH, Mollasbashy A. Arthrofibrosis in acute anterior cruciate reconstruction: the effects of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19:332-336.
16. Paulos L, Meislin R. Patellar entrapment following anterior cruciate ligament surgery. In: Jackson DW, Arnoczky SP Woo SLY. The Anterior Cruciate Ligament: Current and Future Concepts. New York: Raven Press; 1993:365-372.
17. Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9:194-199.
18. Paessler H, Rossis J, Mastrokalos D, et al. Anteromedial vs. transtibial technique for correct femoral tunnel placement during arthroscopic ACL reconstruction with hamstrings: an in vivo study. J Bone Joint Surg Br. 2004; 86(suppl): 234.
19. Stevenson WW 3rd, Johnson DL. Vertical grafts: a common reason for functional failure after ACL reconstruction. Orthopedics. 2007;30:206-209.
20. Howell SM, Taylor MA. Failure of reconstruction of the anterior cruciate ligament due to impingement on the intercondylar roof. J Bone Joint Surg Am. 1993;21:572-581.
21. Howell SM, Barad SJ. Knee extension and its relationship to the slope of the intercondylar roof: implications for positioning the tibial tunnel in anterior cruciate ligament reconstructions. Am J Sports Med. 1995;23:288-294.
22. Jackson DW, Gasser SI. Tibial tunnel placement in ACL reconstruction. Arthroscopy. 1994;10:124-131.
23. Muneta T, Yamamoto H, Ishibashi T. The effects of tibial tunnel placement and roofplasty on reconstructed anterior cruciate ligament knees. Arthroscopy. 1995;11:57-62.
24. Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:225-229.
25. Schepsis A, Getelman MH, Zimmer J. Revision ACL reconstruction: overall results and a comparison between autograft and allograft, Paper presented at: Book of Abstracts and Instructional Courses of the 14th Annual Meeting of the Arthroscopy Association of North America; May 4-7, Arthroscopy Association of North America. San Francisco: 1995.
26. Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795-1803.
27. Gobbi A, Domzalski M, Pascual J. Comparison of anterior cruciate ligament reconstruction in male and female athletes using the patellar tendon and hamstring autografts. Knee Surg Sports Traumatol Arthrosc. 2004;12:534-539.
28. Barrett GR, Noojin FK, Hartzog CW, et al. Clinical comparison of intra-articular anterior cruciate ligament reconstruction using autogenous semitendinosus and gracilis tendons in men versus women. Am J Sports Med. 2000;28:783-789.
29. Kim SJ, Kim TE, Lee DH, et al. Anterior cruciate ligament reconstruction in patients who have excessive joint laxity. J Bone Joint Surg Am. 2008;90:735-741.
30. Muller W. Muller W. Kinematics. In: The Knee: Form, Function, and Ligament Reconstruction. New York: Springer-Verlag; 1983:8.
31. Noyes FR, Stowers SF, Grood ES. Posterior subluxations of the medial and lateral tibiofemoral compartments: an in vitro ligament sectioning study in cadaveric knees. Am J Sports Med. 1993;21:407-414.
32. Gersoff WK, Clancy WG. Diagnosis of acute and chronic anterior cruciate ligament tears. Clin Sports Med. 1988;7:835-848.
33. Papageorgiau CD, Woo SLY, Fu FH. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sports Med. 2001;29:1-6.
34. Paulos LE, Rosenberg TD, Parker RD. The medial knee ligaments: pathomechanics and surgical repair with emphasis on the external-rotation pivot-shift test. Tech Orthop. 1987;2:37-46.
35. Johnson DL, Swenson TM, Irrgang JJ, et al. Revision anterior cruciate ligament surgery: experience from Pittsburgh. Clin Orthop Relat Res. 1996;(325):100-109.
36. Uribe JW, Hechtman KS, Zvijac JE, Tjin-A-Tsoi EW. Revision anterior cruciate ligament surgery: experience from Miami. Clin Orthop Relat Res. 1996;325:91-99.
37. Grossman MG, El Attrache NS, Shields CL, Glousman RE. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005;21:418-423.
38. Johnson DL, Coen MJ. Revision ACL surgery: etiology, indications, techniques, and results. Am J Knee Surg. 1995;8:155-167.
39. Noyes FR, Schipplein OD, Andriacchi TP. The anterior cruciate ligament deficient knee with varus alignment: an analysis of gait adaptations and dynamic joint loadings. Am J Sports Med. 1992;20:707-716.
40. Gomoll AH, Bach Jr BR. Managing tunnel malposition and widening in revision anterior cruciate ligament surgery. Oper Tech Sports Med. 2006;14:36-44.
41. Gilbart MK, Chhabra A, Harner CD. ACL surgery-the Pittsburgh experience. Sports Med Arthrosc. 2005;13:79-85.
42. Thomas NP, Kankate R, Wandless F, et al. Revision anterior cruciate ligament reconstruction using a two-stage technique with bone grafting of the tibial tunnel. Am J Sports Med. 2005;33:1701-1709.
43. Olsen EJ. Use of soft tissue allografts in sports medicine. Adv Oper Orthop. 1993;1:111-128.
44. Miller M. Revision cruciate ligament surgery with retention of femoral interference screws. Arthroscopy. 1998;14:111-114.
45. Dworsky B, Jewell BF, Bach BRJr. Interference screw divergence in endoscopic anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:45-49.
46. Seon JK, Song EK, Bae BH, Park SJ. Kinematic study following double-bundle anterior cruciate ligament reconstruction. Int Orthop. 2007;31:623-628.
47. Yasuda K, Kondo E, Ichiyama H, et al. Clinical evaluation of anatomic double bundle anterior cruciate ligament reconstruction using hamstring tendon grafts: comparisons among three different procedures. Arthroscopy. 2006;22:240-251.
48. Brophy RH, Selby RM, Altchek DW. Anterior cruciate ligament revision: double bundle augmentation of a primary vertical graft. Arthroscopy. 2006;22:683.
49. Cohen SB, Fu FH. Three-portal technique for anterior cruciate ligament reconstruction: use of a central medial portal. Arthroscopy. 2007;23:325.