Chapter 50 Retrosigmoid Approach to Tumors of the Cerebellopontine Angle
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
SURGICAL ANATOMY
Historically, the earliest approach to the posterior fossa was undertaken through the suboccipital convexity. Krause1 first employed this technique during the latter portion of the 19th century. Until the 1970s, the technique in widespread use was the so-called suboccipital approach. In this procedure, a large bone window is removed, and the anterior limit of the craniectomy is the first mastoid air cell encountered. Curtailment of the anterior opening at the first contact with pneumatization was predicated on the assumption that the mastoid was bacterially contaminated, and that opening its air cell tracts created an increased risk of meningitis. Because of its more posterior angle of view, the suboccipital approach required a greater degree of cerebellar retraction, and sometimes necessitated a partial cerebellar resection.
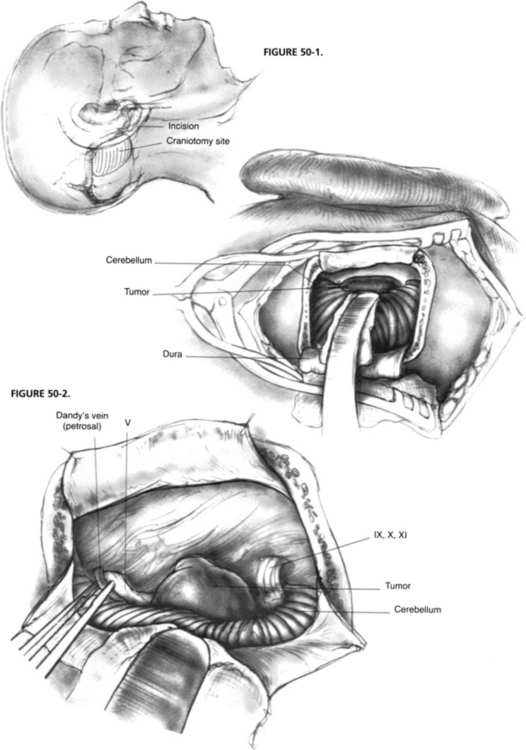
In recent years, as a result of increased experience with CPA surgery, the classic suboccipital approach has been modified to become the retrosigmoid approach, which is now the preferred method for exposing the CPA behind the sigmoid sinus. In this technique, bone is removed anteriorly up to the level of the posterior border of the sigmoid sinus and superiorly to the inferior margin of the transverse sinus (Fig. 50-1). Although mastoid air cells are frequently transected during this maneuver, experience has not shown an increased incidence of postoperative infection. The slightly higher risk of CSF leak associated with this more anterior exposure is more than offset by its more favorable angle of view into the CPA and the markedly reduced need for cerebellar retraction with this approach.
The anatomic exposure of the posterior fossa provided by the retrosigmoid approach is bounded superiorly by the tentorium cerebelli and inferiorly by the jugular foramen and foramen magnum (Fig. 50-2).2–5 Access to the central nervous system includes the lateral cerebellar hemisphere and the lateral surface of the pons and upper medulla. CN V through XI are visible at their root entry zones and over their cisternal courses. Although the theoretical anterior limit of exposure is the clivus and the apical portion of the petrous pyramid, in practice, access to these ventral structures is usually limited by CN VII and VIII superiorly and CN IX through XI inferiorly, which bridge across the CPA, restricting ventral access to narrow intervals. Exposure of the prepontine cistern is largely obstructed by the lateral aspect of the pons, which does not tolerate medial retraction well.
Anatomic variations may affect the CPA exposure provided by the retrosigmoid approach. A posteriorly placed sigmoid sinus course results in the anterior edge of the craniectomy being placed more posteriorly. This placement creates a deeper field of action and a less favorable angle of view with the consequent need for more cerebellar retraction. This disadvantageous exposure may be compromised further by a low transverse sinus course, particularly if the patient also has a short neck and a prominent shoulder. This problem of restricted exposure may be overcome by combining the retrosigmoid approach with an anterosigmoid, retrolabyrinthine decompression to allow anterior retraction of the sigmoid sinus.6 A highly placed jugular bulb restricts access to the internal auditory canal (IAC), and can make the dissection of the inferior bony trough between the canal and the bulb difficult. Occasionally, the bulb may extend superiorly to overlap the IAC, partially obscuring access to the medial aspect of the canal.7
PREOPERATIVE EVALUATION AND PATIENT COUNSELING
The minimal preoperative evaluation for a patient with a CPA tumor comprises a clinical history, a physical examination, pure tone and speech audiometry, and an imaging study (preferably, gadolinium-enhanced magnetic resonance imaging [MRI]). In nonacoustic tumors, computed tomography (CT) scanning for evaluation of the osseous characteristics of the cranial base and angiography to address vascular anatomy and possibly to perform embolization are occasionally indicated. Neither vestibular diagnostic testing nor auditory evoked responses are routinely obtained in patients already diagnosed with an acoustic neuroma.8
Numerous factors affect the selection of posterior fossa craniotomy for tumors of the CPA.7,9,10 As advocates of selective management of these lesions according to the unique attributes of each tumor and the potential surgical options, we involve the patient in the discussion of the relative advantages and disadvantages of each technique. In most cases, an obvious choice can be made, whereas in others, patient preference is important. Our customary preoperative counseling includes the anticipated and potential risks to hearing, balance, and facial motor function. Less common complications that are discussed include CSF leak, meningitis, cerebrovascular accident, and death.11 Although blood transfusion is seldom required, we encourage the patient to donate 1 U of autologous blood.
PATIENT SELECTION
Common Indications in Neurotology
Hearing Preservation
The primary aim of acoustic neuroma management is removing the threat of progressive tumor growth, while avoiding injury to the central nervous system. Preservation of cranial nerve function (facial movement, facial sensation, and hearing), which has become the primary focus of acoustic neuroma surgery in recent years, is a secondary goal. Patients with acoustic tumors can be classified into three groups in terms of potential for hearing preservation. Patients for whom hearing preservation is highly improbable generally undergo translabyrinthine removal. Criteria that place a patient into this group include poor hearing (<30% speech discrimination, >70 dB speech reception threshold), large CPA component (>3 cm), and deep penetration of the IAC. Conversely, patients with good hearing (>70% speech discrimination, <30 dB speech reception threshold), small CPA component (<1 cm), and shallow IAC involvement are considered excellent candidates for a hearing conservation approach.7 It is difficult to codify a set of rules concerning selection of a hearing conservation approach for the numerous patients who lie between these parameters. Each surgical team must rely on its own criteria, based on experience, together with the patient’s wishes in coming to a selection of surgical approach. Neurotologists would always favor undertaking a hearing conservation approach, even when the chances of success were remote, were there not potential adverse consequences from the endeavor. The lower morbidity of the translabyrinthine approach, especially in terms of persistent headache and CSF leak, leads the clinician away from the retrosigmoid hearing conservation approach when the chances of success are limited.
The concept of useful hearing is context dependent. In a patient with a normal contralateral ear, imperfect residual hearing in the tumor ear is often of little practical benefit. When hearing in the contralateral ear is impaired or threatened, such as in cases of bilateral acoustic neuromas associated with neurofibromatosis type 2, a conservative approach to hearing conservation is prudent, occasionally even at the expense of complete tumor excision.12
Hearing preservation is seldom achieved when tumors with a CPA component exceeding 2 cm in diameter are removed.13,14 This rule should not be applied in nonacoustic CPA tumors (e.g., meningiomas), however, because hearing preservation is frequently achieved even with large tumors.15
The retrosigmoid approach exposes a variable amount of the IAC without violating the inner ear while the canal is being drilled open. Two factors should be considered in the decision of whether hearing conservation via the retrosigmoid approach is feasible: the depth to which the tumor penetrates the IAC, and the degree of IAC exposable in that patient. The relationship between the inner ear and the lateralmost extension of the tumor into the IAC may be predicated by preoperative gadolinium-enhanced MRI.16
Use of Retrosigmoid Approach in Combined Therapy of Acoustic Neuroma
Numerous studies have shown that functional outcome after conventional microsurgery is substantially poorer in patients with acoustic neuroma larger than approximately 3 cm. In these patients, the incidence of persistent facial dysfunction is high. There is also an increased risk of persistent balance dysfunction because of infarction of the middle cerebellar peduncle.17 In an effort to improve functional outcome, some centers have begun approaching larger tumors with subtotal resection leaving a rind of tumor on the pons and along the course of the facial nerve. When the patient has serviceable hearing, the retrosigmoid approach is typically used. To reduce the risk of recurrence, it is essential to remove the IAC component. Such surgical remnants resume growth in approximately one third of cases.18 If the remnant grows on serial imaging, it may be treated with stereotactic radiation with a greater than 90% probability of halting its growth.
Acoustic Neuroma in a Patient with Chronic Otitis Media
Although patients with acoustic neuromas rarely have concomitant chronic middle ear infection, in patients who do the translabyrinthine approach for acoustic neuroma resection is contraindicated. The retrosigmoid approach may also open into potentially contaminated mastoid air cells lying behind the sigmoid sinus, and into air cells that may surround the IAC. To avoid potential intracranial infection, chronic middle ear infection should be controlled with tympanoplasty, antibiotics, or both, before tumor surgery whenever possible.7
Tumors with Limited Extension into Meckel’s Cave
The retrosigmoid approach is also useful in approaching extra-axial posterior fossa tumors that possess minor extensions into Meckel’s cave (cavum trigeminale). Most such tumors are trigeminal schwannomas and petroclival meningiomas. Added exposure is obtained by removing the apical petrous bone between the IAC and the tentorium. This maneuver provides access to approximately 1 to 2 cm of the posterior aspect of Meckel’s cave for tumor removal.19–21
Relative Contraindications
Deep Extension into Internal Auditory Canal
Generally, tumors extending into the lateral one third of the IAC are not resectable by the retrosigmoid approach without destroying hearing. In such cases, the translabyrinthine approach ensures complete resection and reduces operative morbidity.7
Large Tumors
Although large CPA tumors (>3 cm) may be approached through either the retrosigmoid or the translabyrinthine technique, when we intend radical removal we prefer to use the latter because it provides ample exposure and minimizes the need for cerebellar retraction. In addition, when the pons and the cerebellar peduncle are substantially displaced medially and posteriorly, the translabyrinthine approach, by virtue of its more anterior placement, provides a more favorable angle of view posteriorly toward the brainstem interface. In cases of facial nerve disruption, which is more common in large tumors, the translabyrinthine approach affords more reconstructive options through mastoid meatal rerouting.7
PATIENT PREPARATION AND POSITIONING
At most centers, the operation is performed by a multidisciplinary team consisting of a neurotologist, neurosurgeon, neuroanesthesiologist, neurophysiologist, and specialized operating room nurses. The operation is done with the patient under general anesthesia. A short-duration muscle relaxant is used to facilitate endotracheal intubation. Thereafter, anesthesia is maintained with inhalational agents alone, avoiding the use of muscle relaxants, which would prevent effective intraoperative cranial nerve electrophysiologic monitoring. In addition to the routine neuroanesthesia monitoring equipment, antithrombotic stockings and a urinary catheter are used. The retrosigmoid approach may be carried out in one of three surgical positions: supine, lateral supine (park bench position), and sitting.10 Supine is the favored position because it affords excellent exposure and carries the lowest risk of complication (discussed later).
The patient is secured in the optimal operating position by means of a head holder attached to the bed frame (e.g., Mayfield). This apparatus facilitates exposure of the suboccipital region while the patient is in the supine position. Optimal surgical field exposure is obtained by a combination of head rotation, neck flexion, and ipsilateral shoulder elevation. Excessive neck torsion should be avoided to prevent cervical injury and to reduce the risk of cerebellar swelling secondary to compromised flow through the vertebral venous system. The cranial nerve–monitoring electromyographic electrodes are placed into the muscles supplied by CN V, VII, and XI. When intraoperative auditory brainstem monitoring is indicated, scalp electrodes are placed, and an earphone is inserted into the ipsilateral external auditory canal.22
SPECIAL INSTRUMENTS
Operating room electric circuitry and neuroanesthesia electric monitoring equipment should be grounded and electronically quiet to minimize 60 Hz noise production, which interferes with the cranial nerve electrophysiologic monitoring setup. The specialized equipment for intraoperative cranial nerve monitoring used in our institution has been described elsewhere in detail.22
SURGICAL TECHNIQUE
Exposure of Internal Auditory Canal
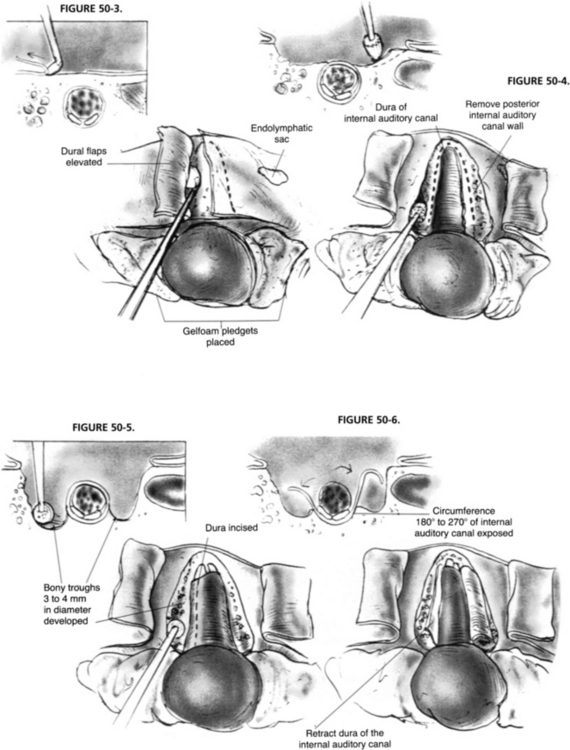
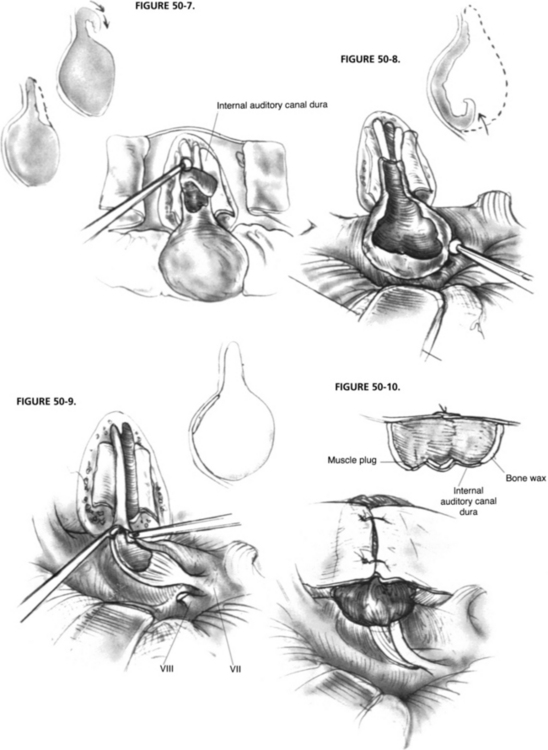
Exposure of the IAC and its contents involves removal of the bone surrounding the posterior, superior, and inferior aspects (Figs. 50-3 to 50-6). Optimal canal visualization may be obtained through a combination of rotation of the operating table away from the side of the surgeon and microscope positioning. These maneuvers bring the posterior petrous face into view centered over the region of the IAC. To locate the canal, the opening of the meatus is gently probed with a blunt, right angle hook. Before IAC opening begins, the operative field is set up to contain as much bone debris as possible and to prevent its dissemination into the subarachnoid space. Absorbable gelatin sponge (Gelfoam) pledgets are placed into the superior and inferior portions of the CPA. A rectangular-shaped rubber dam is fashioned from a surgical glove, placed over the occluding pledgets, and held in place with the cerebellar retractor.
In hearing conservation attempts, the lateral extent of the IAC opening should be restricted to approximately the medial two thirds of the IAC because opening of the lateral one third to expose the fundus may result in a breach of the vestibule or crus commune, militating against hearing conservation. The decision as to how far laterally the IAC is opened depends on the lateral intracanalicular extent of the tumor, which may be accurately predicted from the preoperative gadolinium-enhanced MRI.16,23 Alternatively, the lateral opening can be limited on the premise that an indirect inspection and clearance of the tumor from the lateral IAC can be satisfactorily achieved. This method has the attendant risk of leaving residual tumor in the lateral IAC, however. To avoid this problem, some surgeons have advocated blind curettage using special right angle curettes followed by inspection of the fundus with a small mirror or endoscope to validate the extent of tumor resection.24,25
With these methods, distinguishing residual tumor from the transected vestibular nerves and traumatized dura is sometimes difficult. Dissection of tumor from the fundus without direct visualization risks leaving well-vascularized residual tumor with the potential for clinically significant recurrence.26,27 We advocate exposure of the IAC laterally to a point beyond the tumor interface, where the naked CN VII and residual CN VIII may be visualized. This process sometimes may require opening the canal to the fundus, with resultant entry into labyrinthine structures and sacrifice of residual hearing. It has been proposed that enhanced visualization of the fundus can be achieved by skeletonization of the posterior and superior semicircular canals.28 Similarly, it has been shown that partial resection of the posterior semicircular canal may be helpful in augmenting fundal exposure.29
Acoustic Neuroma Resection
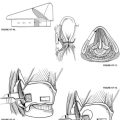
Attention is now turned to planning the actual resection of the tumor; the size of the tumor largely dictates the actual sequence and pattern of removal (Figs. 50-7 to 50-9). We prefer to dissect the IAC initially because this step helps ascertain the probable course of the facial nerve outside of the porus into the CPA, and allows early identification of the facial nerve. A test run of the neural monitoring system can be performed in which positive identification of the nerve by its anatomic relationships is possible. In many cases, ascertaining whether the tumor has arisen from the superior or inferior vestibular nerve is possible. When only one of these nerves is visible on the posterior surface of the tumor, it may be assumed that the other was the nerve of origin.
Alternatively, the anterior tumor capsule with attached nerve may be lifted and rotated to bring the facial nerve course into the surgeon’s view. This action is quite traumatic to the facial nerve, however, and risks disruption of its attenuated fibers. We prefer to dissect the tumor from the facial nerve in situ without mobilizing it from its bed, where it lies supported by an arachnoidal mesh. When the facial nerve is splayed and tightly adherent to the tumor capsule, removing the last remnant of capsule may be impossible without disruption of the nerve.30 In such cases, we prefer to perform a near-total removal, leaving a thin velum of capsule, only 1 to 2 mm thick, attached to the nerve. We believe that this minuscule residual capsule, hanging free in the CPA, is unlikely to generate a recurrent tumor.26 By contrast, tumor left in the distal IAC or in contact with brainstem possesses a vascular supply, and the possibility of regrowth is greater.
Internal Auditory Canal Closure
At the time of IAC closure (Fig. 50-10), the bony troughs developed for the IAC exposure are inspected for opened air cells by palpation with a ball hook. Inspection of the cut bony edge may also be carried out through use of a 90 degree angled rigid endoscope. Bone wax is applied to a small Cottonoid and smeared over the exposed bony trough surfaces to seal overtly and covertly opened air cells to prevent CSF leakage. A small fat graft is harvested from the abdomen and is used to seal the IAC. We prefer fat to muscle as an IAC sealant because, with fat-suppressed MRI, fat creates less obscuration of the tumor bed on follow-up imaging. A 7-0 monofilament nylon suture may be placed through the dural flaps of the posterior petrous face, although this is not always needed. The fat is positioned in the IAC, and the suture is tied. Auditory and facial nerve monitoring are maintained until the fat is secured in place so that any possible neural irritation induced by its placement can be identified.
RESULTS
Historically, the primary issue in acoustic neuroma surgery was the survival of the patient. With the evolution of microsurgical techniques, mortality from acoustic neuroma surgery has become very low—less than 2% in most recent series. Contemporary emphasis includes tumor control and, particularly, functional preservation. Before the data from our own experience and the data published in the literature are addressed, however, it is important to appreciate that limited international standardization exists in the criteria used for reporting results on degree of resection,31 facial nerve function,32 and hearing preservation.33
There are numerous articles in the literature on the subject of facial nerve preservation in acoustic neuroma surgery citing varying degrees of success. Because these results are difficult to compare and draw conclusions from, we confine our commentary to our own series. In our experience, facial nerve outcome from the retrosigmoid approach is similar to that from the other methods of removing acoustic neuromas for tumors of similar size.34 In our acoustic neuroma patients, anatomic continuity of the facial nerve was maintained in 99.2% of cases. Anatomic continuity does not imply functional integrity. The probability of a grade I or II facial function at 1 year after surgery in the context of tumor size was 100% for tumors less than 1 cm; 90% for tumors 1 to 3 cm; and 82% for tumors greater than 3 cm.
With regard to hearing preservation, most published series address residual “measurable” hearing in contrast to the much more relevant concept of “useful” hearing.35 For a patient with a unilateral acoustic neuroma, it could be argued that unless the conserved hearing maintains an interaural difference of less than 30 dB hearing loss with good speech discrimination (>50%), it would be likely to be beneficial. Preservation of useful hearing has been reported to be achieved in 25% to 58% of hearing conservation candidates.13 Very little information is available on the long-term follow-up of patients with preserved hearing. In two published series, significant late decline occurred in 22% to 56% of ears with successful hearing conservation.36,37
Factors relevant to success in hearing conservation approaches to acoustic neuroma include tumor size in the CPA, the depth to which the tumor penetrates the IAC, pure tone hearing level, and auditory brainstem response results. A full discussion of these criteria is beyond the scope of this chapter. It is not yet well established whether intraoperative auditory monitoring materially improves hearing conservation results. In one study using auditory brainstem response monitoring, it was found to be of marginal benefit overall, with the possible exception of tumors less than 1 cm in diameter.38
Several retrospective studies have compared hearing preservation rates after the retrosigmoid and middle fossa approaches.39–41 In each study, the middle fossa approach yielded significantly better hearing results. Although that choice has not yet been universally accepted, the trend among centers undertaking a large volume of acoustic neuroma surgical procedures is to use the middle fossa approach as the preferred means of attempting hearing conservation. In our institution, the upper limit on the use of the extended middle fossa approach is a tumor in the 15 mm range of extracurricular diameter. The retrosigmoid approach is reserved for acoustic neuromas when three conditions are met: (1) excellent hearing, (2) a cisternal component 15 to 20 mm, and (3) no tumor involvement of the distal one third of the IAC. The retrosigmoid approach is still used for selected nonacoustic tumors of the CPA (e.g., meningiomas and epidermoids). Incomplete resection also has a potential role in hearing preservation, particularly in patients with neurofibromatosis type 2 or in patients with a tumor in an only hearing ear.12,42
COMPLICATIONS
The common complications of the retrosigmoid approach to the CPA are persistent headache and CSF leakage.11,43,44 Less common complications include (aseptic or bacterial) meningitis, hydrocephalus, cerebellar dysfunction, vascular compromise (thrombosis and hemorrhage), and problems associated with patient malpositioning during surgery. Medical complications, such as pulmonary thromboembolism and pneumonia, may also occur, but are not specific to surgery of this region. Although the potential complications of acoustic neuroma surgery are similar among the various operative approaches, their relative incidence varies considerably. In the retrosigmoid approach, persistent headache and CSF leakage occur more frequently than with the other technique used in approaching CPA tumors.
Vascular Complications
Hemorrhage
Vascular complications may be extra-axial or intra-axial. The main extra-axial problem is bleeding into the CPA. CPA hematomas may cause brainstem compression and acute obstructive hydrocephalus. The incidence of acute CPA hematomas has been reported to be 0.5% to 2%; however, with modern hemostatic techniques, the incidence is probably considerably less frequent.11 This diagnosis should be suspected when a patient does not promptly awaken after surgery or has a delayed deterioration in the level of consciousness. The diagnosis may be made by noncontrast CT scan, in which fresh blood appears as a hyperdense mass in the CPA and extrinsic pontine compression is noted. If serious neurologic sequelae and death are to be avoided, prompt surgical evacuation of the hemorrhage is essential.
Intra-axial pontine hemorrhage may occur, particularly after removal of very large tumors that have greatly deflected the brainstem. Although major parenchymal hemorrhage is rare, minor amounts of intrinsic pontine bleeding are often evident radiographically after extirpation of giant tumors. Presumably, these bleeds result form the sudden re-expansion of the deeply compressed parenchyma. Supratentorial intra-axial hemorrhages have been reported after retrosigmoid approaches performed with the patient in the sitting position. These hemorrhages were associated with hypertension and may have resulted from subcortical venous tearing resulting from mechanical stress induced by the sitting position.45–47 Extradural hematoma formation, a concern in the middle fossa approach, is uncommon after the retrosigmoid approach.
Anterior Inferior Cerebellar Artery Syndrome
Brainstem infarction may occur after damage to the AICA, the vascular supply to the pons and cerebellar peduncle. Mechanisms of injury include disruption, cauterization, and arteriospasm with thrombosis. A full-fledged AICA syndrome is extremely serious and is often fatal because it results in the loss of respiratory center control.48 Partial interruption of flow in the AICA system, avulsion of one or more of its branches, or obstruction of a nondominant AICA may result in an incomplete AICA syndrome.17 More recently, we have recognized several patients operated on for acoustic neuromas greater than 3 cm in diameter in whom gadolinium-enhanced MRI detected an infarction in the region of the middle cerebellar peduncle. These patients had unilaterally impaired cerebellar function and required prolonged physical therapy rehabilitation.49
Nonvascular Complications
Complications from Patient Positioning
As with any craniotomy, air embolism through breach of the major venous sinuses is a potential hazard. This risk is minimal, however, when a supine or lateral patient position is used.50 Air embolism is the main complication of the sitting position and has been reported in 30% of cases.51 When the sitting position is used, intraoperative monitoring with precordial Doppler ultrasonography alerts the anesthesiologist to venous air entry. The initial maneuvers to perform when air embolism has been detected are to flood the field with fluid and lower the head of the bed.
Quadriplegia (in four cases) and paraplegia have also been reported after acoustic neuroma resection in the sitting position. The degree of cervical flexion in the absence of protective spinal reflexes during anesthesia was thought to have caused spinal cord compression and infarction.52,53 In the supine and lateral supine positions, the unconscious patient must be handled carefully, especially when the head holder is positioned. Excessive head rotation risks cervical injury and may obstruct vertebral venous drainage and contribute to cerebellar swelling. Excessive downward displacement of the shoulder risks traction injury on the brachial plexus.
Cerebrospinal Fluid Leakage
CSF leakage is the most common postoperative complication, occurring in approximately 15% of patients who undergo retrosigmoid approaches for acoustic neuroma.54–56 The patient must be counseled to recognize and report CSF leakage so that steps can be taken to control it rapidly to prevent infectious meningitis. CSF leak occurs either directly through the wound or indirectly through the ear and auditory tube to the nasopharynx, where it manifests as a watery rhinorrhea or salty postnasal discharge. CSF escape into the ear may occur through opened and unsealed mastoid air cells in the region of the craniectomy or through air cells opened and unsealed in the bony IAC dissection.57 CSF drainage often stops spontaneously with simple fluid restriction and avoidance of straining. The use of acetazolamide, a carbonic anhydrase–inhibiting diuretic, may also be beneficial. Alternatively, the early use of a lumbar CSF drain for 48 to 72 hours may halt the drainage.
Some authors have advocated (1) wound re-exploration with rewaxing of the bone to close covert open air cells, (2) replacement of the muscle graft plug to close CSF leakage, and (3) continued lumbar drainage.50 We prefer to address persistent, intractable CSF otorhinorrhea transtemporally. When useful residual hearing is present, a canal wall up mastoidectomy is performed, perilabyrinthine cells are copiously waxed, the fossa incudis is occluded with a fascia graft, and fat is used to obliterate the cavity. When the operated ear is deaf, a canal wall down mastoidectomy is performed. The external auditory canal is sutured closed, and the auditory tube is sealed under direct vision with bone wax and muscle. The mastoid air cells are also waxed, and the cavity is obliterated with fat. Lumbar CSF drainage is maintained for approximately 72 hours after surgery.
Aseptic and Bacterial Meningitis
Entry of blood and bone dust into the subarachnoid space can result in aseptic meningitis. Care is taken during the drilling of the posterior petrous face during the IAC exposure to prevent contamination of the subarachnoid space with bone dust. Gelfoam is placed in the CPA superior and inferior to the tumor and CN VII-VIII complex, and a rubber dam is placed over the cerebellum. After completion of the bone work, the wound is thoroughly irrigated, and the bone debris is removed. Similarly, throughout the tumor dissection and at its completion, a combination of suction and irrigation is used to prevent the buildup of blood and clots because blood and bone debris produce an irritative or chemical aseptic meningitis.11
Hydrocephalus
Hydrocephalus can occur as a result of blood and bone debris contamination of the posterior fossa subarachnoid space. Particulate and proteinaceous debris becomes ingested by arachnoid granulations, impairing their absorptive capabilities, which results in raised intracranial pressure. Cerebellar retraction with subsequent swelling at release may also result in the development of hydrocephalus.11
Persistent Headache
Headache is encountered more frequently after the retrosigmoid approach than after other types of posterior fossa craniotomy.58 In our experience, nearly all retrosigmoid patients have substantial headache during the first postoperative month. By 3 months after surgery, approximately one third continue to complain of this symptom. By 1 year, approximately 15% of patients continue to have chronic moderate to severe headaches compared with very few headaches for patients who underwent the translabyrinthine procedure. Some individuals are unable to return to work or resume other life activities because of this symptom. The highest incidence of persistent headache in our series has been in patients with small tumors who underwent the retrosigmoid approach in an effort to preserve hearing.
Although the headache may have myriad presentations, it is most commonly either frontal or referred to the area of surgery and is often triggered by cough. Numerous potential underlying causes exist for chronic headache after retrosigmoid craniotomy, including aseptic meningitis, coupling of the suboccipital dura to the nuchal musculature, occipital neuralgia, and exacerbation of an underlying headache tendency, such as migraine. Although numerous mechanisms are possible, we believe that most headaches are a result of chronic arachnoiditis incited by contamination with bone dust and blood at the time of surgery. One apparent risk factor for the development of chronic headaches is retrosigmoid craniectomy, in which the calvarial bony defect is left unreconstructed.59,60 Replacement of the retrosigmoidal bone with a flap, bone chips, or even alloplastic material diminishes the incidence of persistent headache.61,62
Acknowledgment
Figures 51-1 to 1-10 were adapted from artwork produced by the authors from Jackler RK: Atlas of Skull Base Surgery and Neurotology, 2nd ed. New York, Thieme. 2009. The original drawings in this chapter were produced by Christine Gralapp, MA.
1. Krause F. Zur Freilegung der hinteren Felsenbeinflache und des Kleinhirns. Beitr Klin Chir. 1903;37:728-764.
2. Rhoton A.L.Jr., Tedeschi H. Microsurgical anatomy of acoustic neuroma. Otolaryngol Clin North Am. 1992;25:257-294.
3. Lang J. Clinical Anatomy of the Posterior Cranial Fossa and its Foramina. New York: Thieme; 1991.
4. Lang J.Jr., Samii A. Retrosigmoidal approach to the posterior cranial fossa: An anatomical study. Acta Neurochir (Wien). 1991;111:147-153.
5. Camins M.B., Oppenheim J.S. Anatomy and surgical techniques in the suboccipital transmeatal approach to acoustic neuromas. Clin Neurosurg. 1992;38:567-588.
6. Silverstein H., Morrell H., Smouha E., Jones R. Combined retrolabretrosigmoid vestibular neurectomy: An evolution in approach. Am J Otol. 1989;10:166-169.
7. Jackler R.K., Pitts L.H. Selection of surgical approach to acoustic neuroma. Otolaryngol Clin North Am. 1992;25:361-387.
8. Selesnick S.H., Jackler R.K. Clinical manifestations and audiologic diagnosis of acoustic neuromas. Otolaryngol Clin North Am. 1992;25:521-551.
9. Cohen N.L., Hammerschlag P., Berg H., Ransohoff J. Acoustic neuroma surgery: An eclectic approach with an emphasis on hearing preservation. Ann Otol Rhinol Laryngol. 1986;95:21-27.
10. Cohen N.L. Retrosigmoid approach for acoustic tumor removal. Otolaryngol Clin North Am. 1992;25:295-310.
11. Wiet R.J., Teixido M., Liang J.G. Complications in acoustic neuroma surgery. Otolaryngol Clin North Am. 1992;25:389-412.
12. Glasscock M.E.III, Hart M.J., Vrabec J.T. Management of bilateral acoustic neuroma. Otolaryngol Clin North Am. 1992;5:449-469.
13. Shelton C. Hearing preservation in acoustic tumor surgery. Otolaryngol Clin North Am. 1992;25:609-621.
14. Yates P.D., Jackler R.K., Satar B., et al. Is it worthwhile to attempt hearing preservation in larger acoustic neuromas? Otol Neurotol. 2003;24:460-464.
15. Nassif P.S., Shelton C., Arriaga M.M. Hearing preservation following surgical removal of meningiomas affecting the temporal bone. Laryngoscope. 1992;102:1357-1362.
16. Blevins N., Jackler R.K. Exposure of the lateral extremity of the internal auditory canal via the retrosigmoid approach: A radioanatomic study. Otolaryngol Head Neck Surg. 1994;11:81-90.
17. Hegarty J.L., Jackler R.K., Rigby P.L., et al. Distal AICA syndrome following acoustic neuroma surgery. Otol Neurotol. 2002;23:560-571.
18. Bloch D., Oghalai J.S., Jackler R.K., Pitts L.H. The role of less-than-complete resection of acoustic neuroma. Otolaryngol Head Neck Surg. 2004;130:104-112.
19. Cheung S.W., Jackler R.K., Pitts L.P., Gutin P.H. Interconnecting the posterior and middle fossa for tumors which traverse Meckel’s cave. Am J Otol. 1995;16:200-208.
20. Seoane E., Rhoton A.L.Jr. Suprameatal extension of the retrosigmoid approach: Microsurgical anatomy. Neurosurgery. 1999;44:553-560.
21. Samii M., Tatagiba M., Carvalho G.A. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: Surgical technique and outcome. J Neurosurg. 2000;92:235-241.
22. Yingling C.D., Gardi J.N. Intraoperative monitoring of facial and cochlear nerves during acoustic neuroma surgery. Otolaryngol Clin North Am. 1992;25:413-448.
23. MacDonald C.B., Hirsch B.E., Kamerer D.B., Sekhar L. Acoustic neuroma surgery: Predictive criteria for hearing preservation. Otolaryngol Head Neck Surg. 1991;104:128.
24. Goksu N., Bayazit Y., Kemaloglu Y. Endoscopy of the posterior fossa and dissection of acoustic neuroma. J Neurosurg. 1999;91:776-780.
25. Wackym P.A., King W.A., Poe D.S., et al. Adjunctive use of endoscopy during acoustic neuroma surgery. Laryngoscope. 1999;109:1193-1201.
26. Lye R.H., Pace-Balzan A., Ramsden R.T., et al. The fate of tumour rests following removal of acoustic neuromas: An MRI Gd-DTPA study. Br J Neurosurg. 1992;6:195-201.
27. Thedinger B.S., Whittaker C.K., Luetje C.M. Recurrent acoustic tumor after a suboccipital removal. Neurosurgery. 1991;29:681-687.
28. Mazzoni A., Calabrese V., Danesi G. A modified retrosigmoid approach for direct exposure of the fundus of the internal auditory canal for hearing preservation in acoustic neuroma surgery. Am J Otol. 2000;21:98-109.
29. Arriaga M., Gorum M. Enhanced retrosigmoid exposure with posterior semicircular canal resection. Otolaryngol Head Neck Surg. 1996;115:46-48.
30. Kemink J.L., Langman A.W., Niparko J.K., Graham M.D. Operative management of acoustic neuromas: The priority of neurologic function over complete resection. Otolaryngol Head Neck Surg. 1991;104:96-99.
31. Moffat DA: Synopsis on near-total, subtotal, or partial removal: Acoustic neuroma. In Tos M, Thomsen J (eds): Proceedings of the First International Conference on Acoustic Neuroma, Copenhagen, August 25-29, 1991. New York, Kugler Publications, 1992, pp 983-984.
32. Baer S, Tos M, Thomsen J, Hughes G: Synopsis on grading of facial nerve function after acoustic neuroma treatment: Acoustic neuroma. In Tos M, Thomsen J (eds): Proceedings of the First International Conference on Acoustic Neuroma, Copenhagen, August 25-29, 1991. New York, Kugler Publications, 1992, pp 993-995.
33. Sanna M, Gamoletti J, Tos M, Thomsen J: Synopsis on hearing preservation following acoustic neuroma surgery: Acoustic neuroma. In Tos M, Thomsen J (eds): Proceedings of the First International Conference on Acoustic Neuroma, Copenhagen, August 25-29, 1991. New York, Kugler Publications, 1992, pp 985-987.
34. Lalwani A., Butt F.Y., Jackler R.K., et al. Facial nerve outcome after acoustic neuroma surgery: A study from the era of cranial nerve monitoring. Otolaryngol Head Neck Surg. 1994;111:561-570.
35. Hinton A.E., Ramsden R.T., Lye R.H., Dutton J.E. Criteria for hearing preservation in acoustic schwannoma surgery: The concept of useful hearing. J Laryngol Otol. 1992;106:500-503.
36. Shelton C, Hitselberger WE, House WF, Brackmann DE: Long-term results of hearing after acoustic tumor removal: Acoustic neuroma. In Tos M, Thomsen J (eds): Proceedings of the First International Conference on Acoustic Neuroma, Copenhagen, August 25-29, 1991. New York, Kugler Publications, 1992, pp 661-664.
37. McKenna M.J., Halpin C., Ojemann R.G., et al. Long-term hearing results in patients after surgical removal of acoustic tumors with hearing preservation. Am J Otol. 1992;13:134-136.
38. Slavit D.H., Harner S.G., Harper C.M.Jr., Beatty C.W. Auditory monitoring during acoustic neuroma removal. Arch Otolaryngol Head Neck Surg. 1991;17:1153-1157.
39. Staecker H., Nadol J.B., Ojeman R., et al. Hearing preservation in acoustic neuroma surgery: Middle fossa versus retrosigmoid approach. Am J Otol. 2000;21:399-404.
40. Irving R.M., Jackler R.K., Pitts L.H. Hearing preservation in patients undergoing vestibular schwannoma surgery: Comparison of middle fossa and retrosigmoid approaches. J Neurosurg. 1998;88:840-845.
41. Arriaga M.A., Chen D.A., Fukushima T. Individualizing hearing preservation in acoustic neuroma surgery. Laryngoscope. 1997;107:1043-1047.
42. Wigand M.E., Haid T., Goertzen W., Wolf S. Preservation of hearing in bilateral acoustic neurinomas by deliberate partial resection. Acta Otolaryngol (Stockh). 1992;112:237-241.
43. Mangham C.A. Complications of translabyrinthine versus suboccipital approach for acoustic tumor surgery. Otolaryngol Head Neck Surg. 1988;99:396-400.
44. Ebersold M.J., Harner S.G., Beatty C.W., et al. Current results of the retrosigmoid approach to acoustic neurinoma. J Neurosurg. 1991;76:901-909.
45. Haines J.H., Maroon J.C., Janetta P.J. Supratentorial intracerebral hemorrhage following posterior fossa surgery. J Neurosurg. 1978;49:881.
46. Harders A., Gilbach J., Weigel K. Supratentorial space-occupying lesions following infratentorial surgery: Early diagnosis and treatment. Acta Neurochir (Wien). 1985;74:57.
47. Seiler R.W., Zurbrugg H.R. Supratentorial intracerebral hemorrhage after posterior fossa operation. Neurosurgery. 1986;18:472.
48. Atkinson J. The anterior cerebellar artery: Its variations, pontine distribution, and significance in the surgery of cerebellopontine angle tumours. J Neurol Neurosurg Psychiatry. 1949;12:137-151.
49. Hegarty J.L., Jackler R.K., Rigby P.L., Pitts L.P., Cheung S.C. Distal AICA syndrome following acoustic neuroma surgery. Otol Neurotol. 2002;23:560-571.
50. Harner S.G., Beatty C.W., Ebersold M.J. Retrosigmoid removal of acoustic neuroma: Experience 1978-1988. Otolaryngol Head Neck Surg. 1990;103:40-45.
51. Duke D.A., Lynch J.J., Harner S.G., et al. Venous air embolism in sitting and supine patients undergoing vestibular schwannoma resection. Neurosurgery. 1998;42:1282-1286.
52. Hitselberger W.E., House W.F. A warning regarding the sitting position for acoustic tumor surgery. Arch Otolaryngol Head Neck Surg. 1980;106:69.
53. Samii M., Turel K.E., Penker G. Management of seventh and eighth nerve involvement by cerebellopontine angle tumors. Clin Neurosurg. 1985;32:242.
54. Becker S.S., Jackler R.K., Pitts L.P. CSF Leak after acoustic neuroma surgery: A comparison of the translabyrinthine, middle fossa, and retrosigmoid approaches. Otol Neurotol. 2003;24:107-112.
55. Baird C.J., Hdeib A., Suk I., et al. Reduction of cerebrospinal fluid rhinorrhea after vestibular schwannoma surgery by reconstruction of the drilled porus acusticus with hydroxyapatite bone cement. J Neurosurg. 2007;107:347-351.
56. Falcioni M., Romano G., Aggarwal N., Sanna M. Cerebrospinal fluid leak after retrosigmoid excision of vestibular schwannomas. Otol Neurotol. 2008;29:384-386.
57. Smith P.G., Leonetti J.P., Grubb R.L. Management of cerebrospinal fluid otorhinorrhea complicating the retrosigmoid approach to the cerebellopontine angle. Am J Otol. 1990;11:178-180.
58. Schessel DA, Nedzelski JM, Rowed Feghali JG: Headache and local discomfort following surgery of the cerebellopontine angle: Acoustic neuroma. In Tos M, Thomsen J (eds): Proceedings of the First International Conference on Acoustic Neuroma, Copenhagen, August 25-29, 1991. New York, Kugler Publications, 1992, pp 899-904.
59. Koperer H., Deinsberger W., Jodicke A., Boker D.K. Postoperative headache after the lateral suboccipital approach: Craniotomy versus craniectomy. Minim Invasive Neurosurg. 1999;42:175-178.
60. Feghali J.G., Elowitz E.H. Split calvarial graft cranioplasty for the prevention of headache after retrosigmoid resection of acoustic neuromas. Laryngoscope. 1998;108:1450-1452.
61. Silverman D.A., Hughes G.B., Kinney S.E., Lee J.H. Technical modifications of suboccipital craniectomy for prevention of postoperative headache. Skull Base. 2004;14:77-84.
62. Schaller B., Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: A long-term follow-up-study. Otolaryngol Head Neck Surg. 2003;128:387-395.
63. Beatty C.W., Ebersold M.J., Harner S.G. Residual and recurrent acoustic neuromas. Laryngoscope. 1987;97:1168-1171.