24 Resuscitation
After reading this chapter, you should be able to:
• identify the clinical assessment used to identify sudden cardiac arrest (SCA).
• outline the role of the chain of survival in the management of SCA.
• outline the management of common arrhythmias associated with SCA.
• describe the use of advanced airway adjuncts and indications for use in SCA.
• discuss indications, actions and routes of administration of medications used in advanced life support.
• describe the appropriate care of persons experiencing SCA including specific circumstances such as the pregnant woman, electrical injuries and drowning.
Introduction
The continuum of critical illness for an individual can span the period before and beyond hospital admission. Resuscitation is often required outside the critical care environment, and the ‘cardiac arrest’ team has evolved to use a more proactive, early-intervention approach, utilising a range of systems and instruments to detect deterioration in patients’ clinical status (see Chapter 3). It is well recognised that improved outcomes from cardiac arrest are dependent on early recognition and initiation of the ‘chain of survival’. This chapter introduces the resuscitation systems and processes in both the prehospital and the in-hospital settings. The chain of survival provides a framework for the management of the person experiencing cardiac arrest and resuscitation in specific circumstances. The chapter expands on the final link in the chain, advanced life support, to outline advanced airway management, rhythm recognition, administration of medications and post resuscitation care. Resuscitation involves many moral and ethical issues, such as family presence during resuscitation, deciding when to cease or initiate resuscitation, and near-death experiences.
Background
Coronary heart disease (CHD) is the leading cause of death in most industrialised countries, with over half of these being due to sudden cardiac arrest (SCA).1–3 Despite advances in CHD management, survival outcome figures from SCA remain poor.4–6 Survival after SCA is dependent on the presenting rhythm, early defibrillation, effective cardiopulmonary resuscitation and advanced life support.6 Because the presenting rhythm with the majority of witnessed SCAs is ventricular fibrillation, bystander cardiopulmonary resuscitation and early defibrillation are the major interventions influencing outcome after SCA.2,6–7 It is possible that the number of ventricular fibrillation/ventricular tachycardia (VF/VT) arrests is actually higher than reported, as often by the time the cardiac arrest team arrives the patient’s rhythm has deteriorated to asystole.8
Incidence/Aetiology of Cardiac Arrests
The prevalence of CHD varies worldwide, thus estimates of the incidence of SCA are difficult to obtain. In Australia, CHD is the leading cause of disease burden (9%) and accounts for 16.5% of all deaths.9,10 There are many factors that contribute to cardiac arrest. In adults, the most common cause of cardiac arrest is a primary cardiac event,11 with coronary artery disease accounting for up to 90% of all victims.12,13 CHD is the most likely cause of death in those over 35 years of age, compared to non-cardiac causes such as drowning, acute airway obstruction or trauma for people less than 35 years of age.13
While causes of cardiac arrest are numerous, most often it is associated with ventricular fibrillation triggered by an acutely ischaemic or infarcted myocardium or primary electrical disturbance.3 Causes of cardiac arrest may be separated into two categories, primary and secondary, as displayed in Table 24.1.
| Primary causes | Secondary causes |
|---|---|
Acute myocardial infarction (AMI) is the most common precursor to cardiac arrest. In victims of trauma, drug overdose and drowning, the predominant cause of cardiac arrest is asphyxia. Cardiac arrest in children is rare and even more rarely sudden,14,15 with the common causes being trauma, congenital heart disease, long QT syndrome, drug overdose, hypoxia and hypothermia. The most common arrhythmia in infants is bradycardia, and the prognosis is especially poor if asystole is present.14,16
Pathophysiology
In sudden cardiac arrest with cardiac origin, it is believed that myocardial ischaemia leads to ventricular irritability and the progression from ventricular tachycardia to ventricular fibrillation (VF) and ultimately asystole.17 After the onset of VF (in animal studies), carotid arterial blood flow continues for approximately 4 minutes even in the absence of cardiac compressions, as coronary perfusion pressure (the pressure gradient between the aorta and the right atrium) falls over this period.17 This initial phase is characterised by minimal ischaemic injury, and it is during this time that defibrillation is most likely to result in the restoration of a perfusing rhythm, while initiation of effective cardiac compressions will increase the coronary perfusion pressure.17
Progression of the cardiac arrest beyond 4 minutes results in accumulation of toxic metabolites, depletion of high-energy phosphate stores, and the initiation of ischaemic cascades.17 A high probability of irreversible cellular injury exists where a cardiac arrest extends for longer than 10 minutes, and the return of a spontaneous circulation during this period may initiate a reperfusion injury17 (see Chapter 11 for further discussion).
Resuscitation Systems and Processes
Since the rediscovery of the effectiveness of closed-chest cardiopulmonary resuscitation (CPR) in 1960 and its subsequent widespread adoption, CPR has saved the lives of many, potentially ensuring years of productive life.18 As CPR quickly became one of the most widely-used and researched procedures, voluntary coordinating bodies developed throughout the world.13 Organisations such as the European Resuscitation Council (ERC), the American Heart Association (AHA), the New Zealand Resuscitation Council (NZRC), the Heart and Stroke Foundation of Canada, and the Southern African and Australian Resuscitation Councils (ARCs) established practice guidelines to improve standards in resuscitation, and coordinated resuscitation activities on a national basis.19,20 However, as standardised recording of outcome data did not exist, resuscitation endeavours could not be compared meaningfully between countries.19Consequently, the International Liaison Committee on Resuscitation (ILCOR) was formed in 1992 to promote global discussion and consistency of guidelines between these international resuscitation councils.19 The AHA, ARC, NZRC, ERC and ILCOR guidelines are subject to constant review and modification based on emerging scientific data. Guidelines and recommendations are classified according to scientific evidence. The most recent substantive guidelines from ILCOR were published in October 2010,20 with the ARC and NZRC guidelines published in January 2011. While it is recognised there are differences between the various councils, this chapter primarily reports on the ARC and NZRC recommendations.
Survival of OUT-Of-Hospital Arrests
Despite recent advances in resuscitation and technology, the survival rate for out-of-hospital cardiac arrest (OHCA) remains poor.6 Factors associated with higher rates of mortality for adults are: age over 80 years, unwitnessed arrest, delays before commencing CPR, defibrillation response times longer than 8 minutes, and non-ventricular tachycardia/fibrillation rhythm.21 The outcome statistics for children after OHCA are similarly poor.14 Marked differences in the inclusion criteria and outcome definitions may, however, also explain the wide variations in survival rates from cardiac arrests.21 In recognition of these variations, the Utstein guidelines were developed and implemented to consistently document, monitor and compare out-of-hospital cardiac arrests. These guidelines:
• establish uniform terms and definitions for out-of-hospital resuscitation
• establish a reporting template for resuscitation studies to ensure comparability
• define time points and time intervals relating to cardiac resuscitation
• define clinical items and outcomes that emergency medical systems should gather
Survival from in-Hospital Arrests
In-hospital resuscitation, as with OHCA, have survival rates of around 20%.22,23 Many factors such as age, presence or absence of morbidity before or during the hospital admission, absence of ‘not-for-resuscitation’ orders, asystole and non-ICU location contribute to the low in-hospital survival rates.24,25
Management
The overall aim of managing a patient in arrest is the prompt restoration of a spontaneous perfusing rhythm with minimal neurological dysfunction. It is well recognised that successful outcome from cardiac arrest is dependent on several key factors: (a) early recognition of cardiac arrest; (b) immediate effective CPR, (c) optimising response times, and (d) early defibrillation.26,27 The probability of an unsuccessful outcome grows with the length of time taken to restore spontaneous circulation.
Chain of Survival
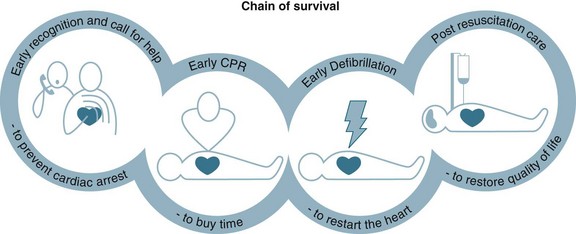
To optimise a person’s chance of survival, the ‘chain of survival’ strategy has been developed,27 that represents the sequence of four events that must occur as quickly as possible: early recognition, early CPR, early defibrillation and postresuscitation care (see Figure 24.1). These time-sensitive, sequential actions must occur to optimise a cardiac arrest victim’s chances of survival. Communities with integrated links along this chain have demonstrated higher survival rates after OHCA than those with deficiencies in these links.2
Early Recognition of Cardiac Arrest
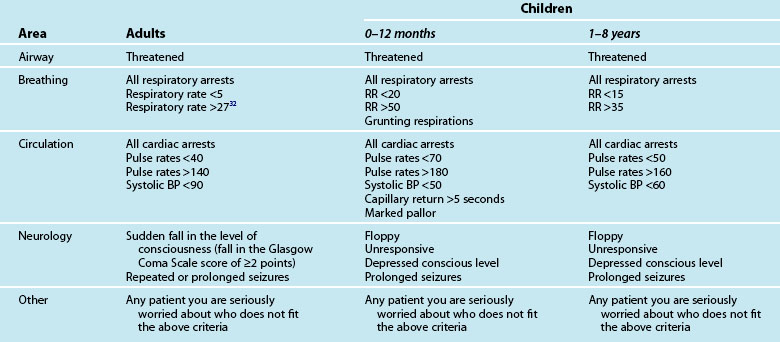
The chain of survival begins with early recognition of a medical emergency and the activation of the medical calling system.2,28 However, the chain of survival has not always been adequate when a cardiac arrest occurs in the hospital, from the point of view of early recognition, timeliness or availability of equipment or staff.24,25 The traditional cardiac arrest team responded to the seriously ill, but the patient was often not salvageable by the time the cardiac arrest team arrived. Two-thirds of in-hospital cardiac arrests are potentially avoidable, with up to 84% of all in-hospital cardiac arrests demonstrating evidence of deterioration in the 6 to 8 hours preceding the arrest.29,30 Consequently, in recent years there has been a move to implement rapid response teams (RRT) that facilitate the early recognition and rapid management of critically ill patients, for example the medical emergency team (MET), the patient-at-risk team (PART) and physiological track and trigger systems (TTS) such as the medical early-warning system (MEWS)31–33 (see Chapter 3 for further discussion). These teams replace the traditional cardiac arrest team by responding to a calling criteria based primarily on abnormal vital signs (see Table 24.2).
Early warning system calling criteria are widely displayed around the hospital and the RRT is activated in the same manner as the cardiac arrest team, ultimately resuscitating patients earlier.34 Recent reviews of the literature and meta-analyses show that in clinically unstable patients, early access – including early recognition and intervention by a MET/rapid response system – can reduce the incidence of cardiac arrests outside ICUs, however there are inconsistent findings regarding their impact on intensive care admission rates and lowering hospital mortality rates.35–37 To further facilitate earlier activation of the RRTs family and patients have been provided with a means to activate the team on a patient’s behalf.38
Basic Life Support
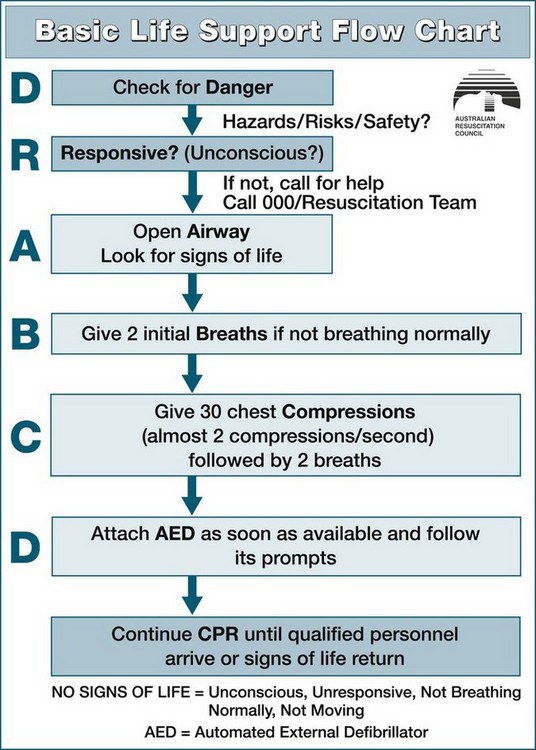
When a patient is identified as in potential or actual arrest, a primary and secondary survey should be conducted in the DRSABCD sequence:39
• Danger. Check for danger (hazards or risks or safety)
• Responsive. Check for response (if responsive/unconscious)
• Airway. Open the airway. Airway assessment is undertaken to establish a patent airway while maintaining cervical spine support (if injury is suspected)
• Breathing. Check breathing. Breathing includes the assessment and establishment of breathing, noting rate, pattern, chest movement and tissue oxygenation
• CPR. Start CPR. Give 30 chest compressions (almost two compressions/second) followed by two breaths.
• Defibrillation. Attach an automated external defibrillator as soon as available and follow its prompts.
Continue CPR until responsiveness and normal breathing return. Ideally, these interventions are performed simultaneously or in rapid sequence and will take no longer than 60–90 seconds to complete. This systematic approach correlates closely with the principles of basic life support (BLS), in that where a life-threatening abnormality is detected, immediate intervention is required before further assessment (see Figure 24.2).
Airway
Recognition of airway obstruction includes listening for inspiratory (stridor), expiratory or grunting noises. The work of breathing can be assessed by the respiratory rate, intercostals, subcostal or sternal recession, use of accessory muscles, tracheal tug or flaring of the alae nasi. Nasal flaring is especially evident in infants with respiratory distress. Noisy breathing is obstructed breathing, but the volume of the noise is not an indicator of the severity of respiratory failure. Should obstruction to air flow be detected, then the airway should be opened using three manoeuvres: the head-tilt, chin-lift and jaw thrust. The ARC recommends assessing a person’s airway without turning them onto the side unless the airway is obstructed with fluid (vomit or blood) or submersion injuries.39
The airway of the infant differs from that of the older child or adult in that the infant has a large head and tongue, small mouth, and the larynx is narrower, shorter, more anterior and acutely angled.17 The airway of an infant is also more cartilaginous and can be easily occluded when the neck is hyperextended; in addition, the large tongue can easily fall back to obstruct the pharynx.40 Hence, the head of an infant should be maintained in the neutral position, whereas a child aged 1–8 will require the ‘sniffing position’ with varying degrees according to age. The chin-lift and head-tilt manoeuvres may be used in children to obtain the appropriate amount of positioning for age. Jaw thrust may be used if head-tilt/chin-lift is contraindicated.40 Do not use the finger sweep to clear the airway of an infant, as this may result in damage to the delicate palatal tissues and cause bleeding, which can worsen the situation. Use of finger sweep can force foreign bodies further down into the airway.40 Suction is more useful for removing vomitus and secretions.
Breathing
To assess for the presence of breathing, look, listen and feel for breath sounds for no more than 10 seconds. If the person is unresponsive with absent or abnormal breathing, call for help and compressions should be commenced immediately. Agonal gasps are not to be considered as normal breathing. Typically, the arterial blood will remain saturated with oxygen for several minutes following the cardiac arrest and as cerebral and myocardial cell oxygenation is limited more by the absence of cardiac output as opposed to the reduced PaO2, effective compressions are more important than rescue breaths.27
CPR
Individuals should commence cardiac compressions if the victim is unconscious, unresponsive, not moving and not breathing normally. Where possible, change the person delivering the compressions every two minutes. Pulse check by lay rescuers and health professionals in BLS is not recommended. Assessment of effective chest compression by healthcare professionals may be made by continuous end tidal CO2 (ETCO2) monitoring. For CPR to be effective the patient should be flat, supine and on a firm surface. The chest should be compressed in the midline over the lower half of the sternum, which equates to the ‘centre of the chest’, at a depth of more than 5 cm (in adults) and at a rate of 100 compressions per minute for adults, infants and children, with the rate rising to 120/min for the newborn.27 CPR should be initiated when the heart rate is 60 beats/min for the neonate, infant and the small child and 40 beats/min for the large child. Performed correctly, external cardiac compressions (ECC) can produce a systolic blood pressure peak of 60–80 mmHg (in adults) and a cardiac output of 20–30% of normal.27,41 With external chest compressions it takes time to reach optimal levels of coronary perfusion pressure and, ultimately, bloodflow. Any interruption to chest compressions therefore decreases the coronary perfusion pressure and resultant blood flow, ultimately reducing survival.42 After 30 compressions open the airway and give two breaths.43
Survival potentially improves when an individual receives a higher number of chest compressions during CPR, even if the person receives fewer ventilations. Because of this, it is recommended that a 30 : 2 compression-to-ventilation ratio is used in adults, children and infants regardless of the number of rescuers, and 3 : 1 for neonates. Having noted this, in the advanced life support paediatric setting, the compression ratio changes to 15 : 2 and a ratio of 3 : 1 for the newborn with any number of rescuers (see Table 24.3). Studies note that the average person may not only be reluctant to initiate mouth-to-mouth resuscitation44 but will also take eight seconds to deliver one breath.45 When a rescuer is reluctant to perform rescue breaths, external cardiac compression (ECC) without expired air resuscitation (EAR) should be encouraged, as the generally held belief is that ECC alone is better than no CPR at all.46–48
Devices to augment compression
As ECC supplies only 30% of normal cardiac output49 and 15% of normal cerebral blood flow, there is a great need to find ways to improve ECC. While no circulatory adjunct is currently recommended, several are being routinely used in the preadmittance and in-hospital settings.20 A few of the recent devices are outlined in Table 24.4.
| Device | Description |
|---|---|
| Active compression–decompression (ACD-CPR) |
• utilises a small portable device to compress and decompress the chest (‘plunger method’) • enhances ventilation and venous return by raising the negative intrathoracic pressure139 which facilitates venous return, thus priming the heart for subsequent compressions. |
| Interposed abdominal compression combined (IAC) with CPR (IAC-CPR) | |
| Non-invasive automated chest compression device (AutoPulse) |
Given the limited available information on the outcome of any of these devices and the absence of evidence to demonstrate these devices are superior to conventional manual CPR, no device is currently recommended as a routine substitute for manual CPR.20
Defibrillation
While CPR has been associated with improved survival to discharge from hospital, it cannot be substituted for the definitive treatment of early defibrillation. It is thought that CPR will supply sufficient oxygen to the brain and heart until defibrillation is available. Ultimately, despite the most effective CPR, the single-most important cause of decreased prognosis in pulseless VT/VF cardiac arrests is a delay in electrical defibrillation.3
Praecordial thump
A praecordial thump is a single, sharp blow delivered with a clenched fist to the midsternum of a victim’s chest from a height of 25–30 cm above the sternum.7 The mechanical energy generated by the praecordial thump may generate a few joules, and therefore if applied within the first few seconds of onset of a shockable rhythm, but it has a very low success rate at converting VF/VT to a perfusing rhythm.50,51 Because of the very low success rate and the brief period for application, delivery of the thump must not delay accessing help or a defibrillator. Only situations where the VF arrest is witnessed and monitored and a defibrillator is not immediately on hand (i.e. critical care environments) would the delivery of the praecordial thump be appropriate.20
Electrical defibrillation
Defibrillation is the passage of a current of electricity through a fibrillating heart to simultaneously depolarise the mass of myocardial cells and allow them to repolarise uniformly to an organised electrical activity.52 There are two defibrillator modes for delivery of electrical energy: monophasic and biphasic waveforms. Monophasic defibrillators are no longer manufactured, however they are still available in clinical settings. Monophasic defibrillators operate by the current travelling in one direction from one paddle through the heart to the opposite paddle.52,53 In comparison, the biphasic defibrillator’s current travels in one direction through the heart for a predetermined time, then reverses.
There are two types of external defibrillators: the manual external defibrillator (MED), and the automatic external defibrillator (AED). The AED can be either fully automatic (FAED) or semiautomatic (SAED). The MED requires the user to be able to immediately and accurately recognise arrhythmias and make the decision whether to initiate defibrillation or not. In comparison, the AED automatically detects and interprets the rhythm without relying on the user’s recognition of arrhythmias. AEDs can be operated in both manual and semiautomatic mode. When using an AED, the user determines whether the person is unresponsive, not breathing and pulseless.54 After checking for a pulse, the AED requires only four steps to operate: turn power on, place self-adhesive electrodes on a victim’s chest, rhythm analysis follows (hands-off period), then (if advised by the machine) press the shock button. The AED will automatically interpret the cardiac rhythm and if VF/VT is present, will advise the operator to provide a shock. This ‘hands-off’ period may result in significant interruptions to chest compressions and adversely impact patient survival.55 The combined preshock and the postshock pause ideally should be less than 5 seconds.53 This can be achieved by continuing compressions while the defibrillator is charging and resuming chest compressions immediately after the delivery of the shock. Biphasic AEDs are safe, easy to use and are effective for detecting and classifying arrhythmias (sensitivity 100%, specificity 97%). FAEDs are programmed to assess the rhythm, charge the defibrillator and deliver shocks without user intervention.
Successful defibrillation and survival to discharge is inversely related to the time from onset of ventricular fibrillation to defibrillation. For every minute that passes, the probability of survival decreases 5–10%,56 so resuscitation bodies place great emphasis on early defibrillation. To facilitate early defibrillation, ILCOR endorses the concept of non-medical individuals being authorised, educated and encouraged to use defibrillators.53 This public access to early defibrillation has seen the placement of defibrillators on aircraft, in casinos and cricket grounds, with non-medical personnel such as police, flight attendants, security guards, family members and even children successfully initiating early defibrillation.57,58 The effectiveness of training non-traditional out-of-hospital first responders to use the AED has improved survival to discharge rates.20 Similarly, in-hospital cardiac arrests also occur in any area, and all healthcare workers should be capable of initiating early defibrillation.53 The ARC notes that while BLS does not have to include the use of adjunctive equipment, the use of AEDs by persons with education in their use is supported and should be taught. Figure 24.3 outlines the integration of defibrillation with BLS.
Practice tip
Remember, when using a monophasic defibrillator for AF cardioversion, the use of hand-held paddles is preferable to the use of self adhesive pads.59
For 90% of people in VF, return of a perfusing rhythm will occur after a single shock. However it is rare that a pulse will be palpable with the perfusing rhythm, hence the immediate resumption of chest compressions in the postshock period is supported.53 Failure to successfully convert VF after the single-shock strategy may indicate the need for a period of effective CPR (30 : 2) for 2 min and rhythm reanalysis, then shock if indicated.53 A single shock strategy is now recommended for all patients in cardiac arrest requiring defibrillation for VF or pulseless VT.39 Not all the electrical energy delivered during defibrillation will traverse the myocardium. Table 24.5 outlines some of the common factors contributing to the success or failure of defibrillation. Studies have demonstrated that lower-energy biphasic defibrillators are associated with greater first-shock efficacy, require lower joules, cause less myocardial dysfunction and increase return of spontaneous circulation when compared with the monophasic defibrillator.60,61 The optimum defibrillation energy level is that which sufficiently abolishes the arrhythmia to enable the return of an organised rhythm, with minimal myocardial damage.53 The recommended first shock for a monophasic defibrillator is 360 J and 200 J for biphasic defibrillators. Other biphasic energy levels may be used providing there is relevant clinical data for a specific defibrillator that suggests that an alternative energy level provides adequate shock success (ARC & NZRC Guideline 11.4).62 If the initial shock is unsuccessful, subsequent shocks should be delivered at the above doses or higher energy levels may be selected.61 In children, it is recommended 4J/kg for the initial and subsequent shocks for both biphasic and monophasic defibrillators.53 Standard adult AEDs and pads are suitable for use in children older than 8 years. Ideally, for children between 1 and 8 years paediatric pads and an AED with paediatric capability should be used.63 These pads also are placed as per the adult methodology. If the AED does not have a paediatric mode or paediatric pads then the standard adult AED and pads can be used.24 Defibrillation of infants less than one year of age is not recommended.53
TABLE 24.5 Factors contributing to the success or failure of defibrillation
| Success | Failure | Precautions |
|---|---|---|
• Inadequate contact with the chest (Excessive chest hair)
• Faulty positioning of the paddles
• Synchronise button in the on position, flat battery or fractures lead
• Positioning over bone/fat or breast tissue
• Drying out of gel conduction pads
• Patient factors: acidosis, hypoxia, electrolyte imbalance, drug toxicity, hypothermia
• Time of respiration (best delivered at end-expiration)
• PEEP and auto-PEEP (air-trapping) should be minimised
• Paddles/electrodes too small (8–12 cm electrodes for adults)
• Place defibrillation electrodes at least 8 cm away from ECG electrodes, or implantable medical devices pacemakers, vascular access devices
• Remove medication patches, wipe the area before applying defibrillation electrodes
• Do not defibrillate unless all clear of the bed/patient
• Do not charge/discharge paddles in the air
• Do not have the patient in contact with metal
• Do not allow oxygen to flow onto the patient during delivery of the shock (at least 1 m from the patient)
• Do not use electrode gels and pastes as these can spread between the paddles and potentially spark.
The importance of early, uninterrupted chest compressions and early defibrillation are well promulgated in the ILCOR guidelines.12 As determining the length of time from collapse is difficult to accurately estimate, it is imperative rescuers perform chest compressions until the defibrillator is both available and charged.64,65
Advanced Life Support
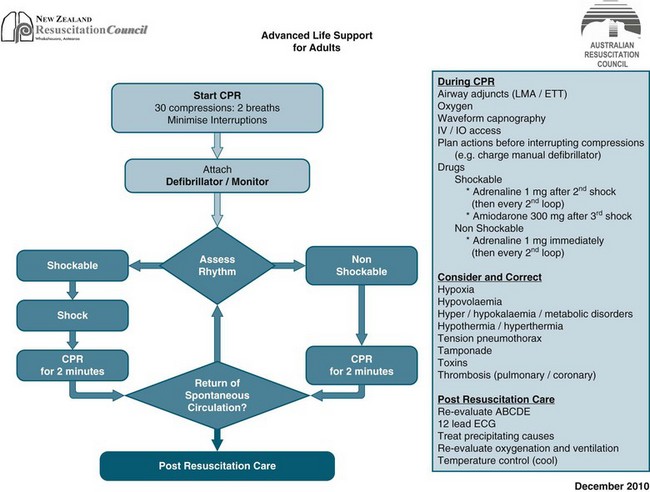
Basic life support can provide around 20–30% of normal cardiac output and a fraction of inspired oxygen (FiO2) of 0.1–0.16. Consequently, a significant number of patients rely on the provision of advanced life support (ALS) for survival. ALS extends BLS to provide the knowledge and skills essential for the initiation of early treatment and stabilisation of people post-cardiac arrest. Advanced skills traditionally include defibrillation, advanced airway management and the administration of resuscitation drugs. While BLS is generally initiated prior to ALS, where a defibrillator and a person trained in its use are available, defibrillation takes precedence over BLS and ALS. The ARC and NZRC algorithm for management of cardiopulmonary arrest (see Figures 24.3 and 24.4) outlines the two decision paths of therapy in ALS: (a) defibrillation and CPR for pulseless VT/VF (shockable); and (b) identifying and treating the underlying cause for non-VT/VF (non shockable).
Advanced Airway Management
A person with signs of acute respiratory distress should be administered oxygen at the highest possible concentration. Initially during CPR, whenever possible, administer the highest possible oxygen concentration.43 Oxygen should never be withheld for fear of adverse effects, as rescue breaths provide an inspired oxygen concentration of only 15–18%. The administration of oxygen alone does not result in adequate ventilation, and as such the establishment of an effective airway is paramount. Airway management is essential in the performance of CPR, and may be administered using a variety of techniques. The choice of advanced airway adjunct is determined by the availability of equipment and experienced personnel (see Table 24.6 and Chapter 15):
| Airway type | Description | Practice considerations |
|---|---|---|
| Oropharyngeal (Guedel’s) airway |
Use with caution in patients with head injuries.
With the exception of infant’s head-tilt, jaw support or jaw thrust is still necessary when using either the oropharyngeal or the nasopharyngeal.
Remember, children under 1 year of age are nose-breathers, and anything that blocks their nose is going to severely compromise their breathing.40
BVMs are often inappropriately used and offer no protection to the airway.
Two-person technique is preferable.12
Single-person BVM ventilation may result in a poor seal around the patient’s mouth and the delivery of less than optimal tidal volumes.142 When using a BVM it is best performed using two rescuers, although not always possible.
As the airway is not protected, smaller tidal volumes with supplementary oxygen can provide adequate oxygenation and reduce the risk of gastric inflation, regurgitation and aspiration.
The mask should be used right-way-up with children and upside-down with infants.
The soft circular mask is preferred for infants, as it provides an excellent seal with low dead space.14
The LMA is inserted orally using a blind technique so that the distal end of the mask abuts against the base of the hypopharynx, behind the cricoid cartilage, and the cuff is inflated to form an airtight seal around the larynx.146
The LMA is used as a first-line adjunct when endotracheal intubation is not available.
The LMA is more rapidly inserted and requires less equipment than the endotracheal tube.142,144
When used as a first-line airway device, the LMA provides a clear airway with a significantly lower risk of gastric overinflation and regurgitation than the BVM.12,144
As with adults, the LMA can be used safely and effectively in infants.14
size 6 >100 kg. ARC & NZRC guideline 12.661
Complications of LMA include gastric aspiration, partial airway obstruction, coughing or gastric insufflation.
Contraindications include patients unable to open their mouths adequately; pharyngeal pathology; airway obstruction at or below level of the larynx; low pulmonary compliance or high airway resistance; or increased risk of aspiration.144
Endotracheal intubation is a difficult skill to acquire and maintain.
In addition to routine clinical methods, ETT placement can be confirmed by either measurement of ETCO2 or oesophageal detector; the latter is more reliable in a non-perfusing rhythm (Class IIb).
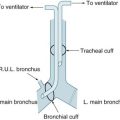
Immediate complications associated with intubation include oesophageal intubation; right main bronchi intubation; or ETT occlusion (kinking, sputum, cuff, blood).
While endotracheal tube (ETT) is considered the ‘gold standard’ for airway management in a cardiac arrest, as it protects the airway, assists effective ventilation, ensures delivery of high concentrations of oxygen and eases suctioning, no studies have found that ETT use during a cardiac arrest increases survival.20 It is vital that CPR not be interrupted for more than 10 seconds during attempts at endotracheal intubation.20 Waveform capnography should be applied to confirm the ETT placement.12 The ETCO2 may also be used to monitor the quality of the CPR. Given the limitations noted in Table 24.6, a variety of adjunct airway/ventilation management devices, such as bag–mask ventilation (BMV) and supraglottic airway devices (SADs) such as laryngeal mask airway (LMA), the classic laryngeal mask airway (cLMA), the oesophageal–tracheal Combitube (ETC) and the I-gel are available. When an LMA-Fasttrach is in place, it can be used to guide the passage of bougies, introducers, a bronchoscope or an ETT into the trachea. The benefit of the SADs is that they are easily inserted without interruption to chest compressions.66 Currently, there is no evidence to support the routine use of any particular advanced adjunct airway devices. Healthcare professionals trained to use supraglottic airway devices (e.g. LMA) may consider their use for airway management during cardiac arrest and as a backup or rescue airway in a difficult or failed tracheal intubation.
If available, automated ventilators can be used. These may be set to deliver a tidal volume of 6–7 mL/kg at a rate of 10 breaths/min. The automated ventilator may be used with either the face mask or other adjunct airway devices such as LMA, Combitube or ETT.16 Having noted this, there is currently no evidence to suggest that the use of automated ventilators during cardiac arrest are more beneficial than bag–valve–mask devices.16
Rhythm
1. ventricular fibrillation (VF) and pulseless ventricular tachycardia (VT)
2. non-VF/VT incorporating asystole and pulseless electrical activity (PEA).
The most common arrhythmias observed in SCA are pulseless VT and VF, with 60–85% of all patients initially presenting with these lethal arrhythmias.6 PEA occurs as the initial rhythm in approximately 13–22% of cases;67 when witnessed by emergency personnel in the prehospital setting, it has been documented as high as 50%.68 Asystole is the most common arrest arrhythmia in children, because their hearts respond to prolonged severe hypoxia and acidosis by progressive bradycardia leading to asystole.53
Ventricular fibrillation and pulseless ventricular tachycardia
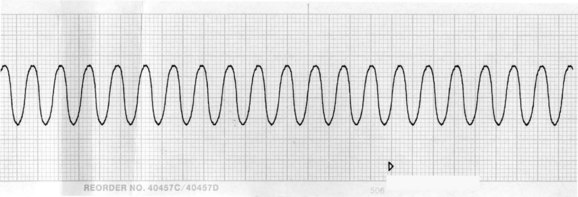
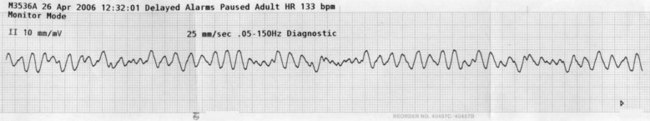
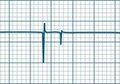
As previously noted, the only intervention shown to unequivocally improve long-term survival after a VF or pulseless VT arrest is prompt and effective BLS, uninterrupted chest compressions and early defibrillation.12 VT and VF rhythms are displayed in Figures 24.5 and 24.6. Energy levels and subsequent shocks are equivalent for both VF and pulseless VT.
Non-VF/VT
Non-VF/VT arrhythmias include pulseless electrical activity and asystole. Pulseless electrical activity (PEA) or electromechanical dissociation (EMD) reflects a dissociation between the heart’s electrical and mechanical activities, and the two terms are used interchangeably. It is important to note that PEA/EMD may present as any rhythm normally compatible with a pulse (e.g. sinus rhythm, sinus tachycardia/bradycardia). PEA is characterised by a stroke volume insufficient to produce a palpable pulse, despite adequate electrical activity.69 PEA often follows defibrillation of VF and has a survival rate of 0–6%.68 Management of PEA includes identifying and correcting reversible causes, summarised as the 4 Hs and 4 Ts in Table 24.7.
| The four Hs | The four Ts |
|---|---|
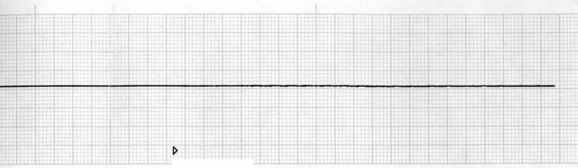
Careful confirmation of asystole (see Figure 24.7) on two leads and the absence of a palpable pulse are essential when making the decision to manage asystole. When an out-of-hospital arrest has an initial rhythm of asystole, survival to discharge is as low as 2%.20
Medications Administered During Cardiac Arrest
Resuscitation drugs can be administered during a cardiac arrest using a variety of routes including peripheral and central veins, or intraosseous (IO). Administration by the central venous route remains the optimal method, but the decision to access peripheral versus central cannulation will depend on the skill of the operator. Peripheral venous cannulation is the quickest and easiest method, however, the patient in cardiac arrest may have inaccessible peripheral veins.20 Should a decision be made to insert a central line during a cardiac arrest, this must not take precedence over defibrillation attempts, CPR or airway maintenance. Medications inserted into a peripheral line should be flushed with at least 20 mL (adults) of an isotonic solution followed by at least 1 minute of continuous external cardiac compressions. Where there is difficulty accessing a peripheral vein, selected medications may be administered via an IO route.20 Tracheal administration of medication is no longer recommended as the dose delivered is unpredictable and the optimal dose is unknown.20
Intraosseous infusion involves the insertion of a metal needle with trocar (usually utilising a drill) into the bone marrow and provides a rapid, safe and reliable access to the circulation.70 The marrow sinusoids of long bones are a non-collapsible venous system in direct connection with the systemic circulation, allowing drugs to reach the central circulation as quickly as medications injected into central veins.71 Intraosseal access is safe and effective for use in patients of all age groups.72,73 General blood specimens such as biochemistry values, blood cultures, haemoglobin and crossmatch studies can also be taken from the marrow at cannulation.17
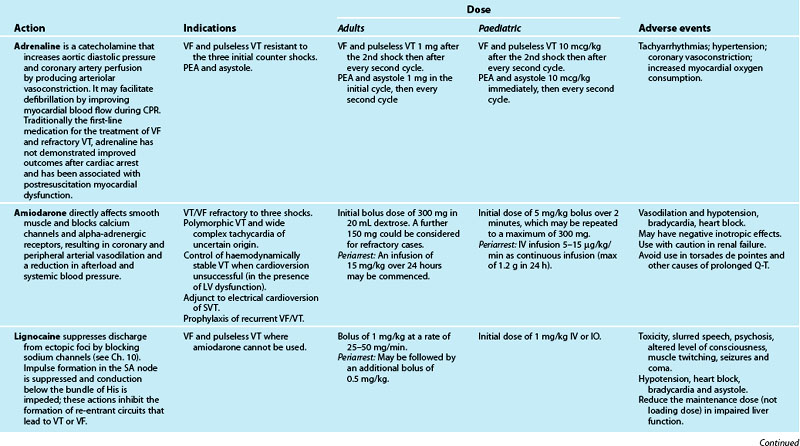
Vasopressors such as adrenaline and vasopressin have been used as adjuncts in cardiac arrests to improve the success of CPR. While there is no evidence that shows that the routine use of any vasopressor during a cardiac arrest will increase survival to discharge from hospital adrenaline is still recommended.12 Adrenaline has been demonstrated to increase the return of spontaneous circulation but not survival to hospital discharge.12 The optimal dose of adrenaline in the prehospital and in-hospital setting remains unclear. Current recommendations propose that adrenaline 1 mg should be administered for VT/VF following the second shock and then every second loop thereafter. For asystole and electromechanical dissociation (EMD) administer 1 mg of adrenaline in the initial loop then every second loop (ARC & NZRC guideline 11.5) (see Table 24.8).62 Studies have reported that vasopressin produced no overall change in survival after cardiac arrest when compared with adrenaline.74–76 Currently there is no evidence to support or refute the use of vasopressin as an alternative to or in combination with adrenaline.
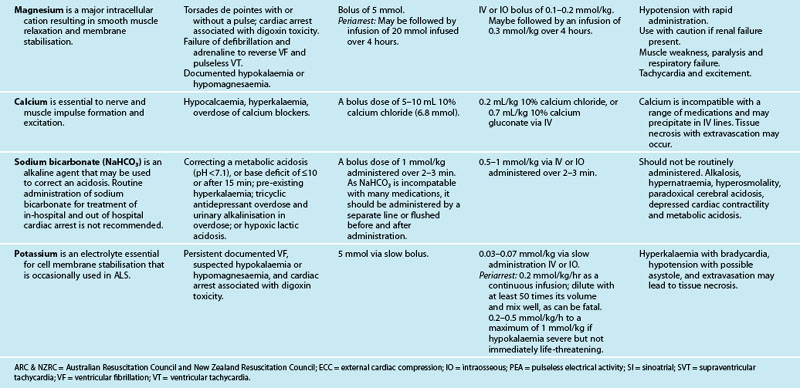
The optimal role and exact benefit of antiarrhythmic medications in cardiac resuscitation is yet to be fully elucidated, but they have very little, if any, role to play in the treatment of cardiac arrests.12 The common antiarrhythmic drugs include amiodarone, lignocaine, magnesium, atropine and calcium (see Table 24.8). While no antiarrhythmic has been shown to improve survival to discharge, recent trials have demonstrated that amiodarone is superior to lignocaine and placebo in improving survival to hospital admission for people with refractory VF in out-of-hospital cardiac arrests.77 The efficacy of IV amiodarone in the setting of VT and VF is 51–100%.78 If after the third shock, the VT/VF has not reverted then a bolus injection of 300 mg of amiodarone is recommended and 150 mg for recurrent or refractory VT/VF.12 Lignocaine (1 mg/kg) may be used as an alternative if amiodarone is not available or cannot be used, but the two should not be used together.12 There is no evidence of improved survival with the use of atropine in a cardiac arrest with asystole or PEA.20 Calcium chloride has little use in the management of arrhythmias unless caused by hyperkalaemia, hypocalcaemia or hypermagnesaemia, or an overdose of calcium channel-blocking drugs. Sodium bicarbonate is no longer administered routinely, as it may cause hypernatraemia, hyperosmolality and intracellular acidosis from the rapid ingress of CO2 generated from its dissociation. Bicarbonate is recommended if the cardiac arrest is associated with hyperkalaemia or tricyclic antidepressant overdose.12
There is insufficient data for the routine use of magnesium in cardiac arrests,79 except if torsades de pointes is suspected.12 Thrombolytics should not be routinely administrated in a cardiac arrest. However, it could be considered in adult patients with proven or suspected pulmonary embolism or acute thrombotic aetiology.12 Effective CPR should be continued for at least 60–90 minutes following the administration of the fibrinolytic medication as there is evidence in these situations of good neurological outcome and survival after following extended periods of CPR.80
During the arrest, strategies should be initiated to prevent the development of serious periarrest arrhythmias. Whenever possible, arterial blood gases, serum electrolytes and a 12-lead ECG should be obtained to assist with determining the precise rhythm and appropriate medical interventions.16 The presence or absence of adverse signs and symptoms will dictate interventions. Adverse factors may include clinical evidence of:
• low cardiac output (unconscious, unresponsive, systolic BP <90 mmHg, increased sympathetic activity)
• reduced diastolic filling time (excessive tachycardia, e.g. heart rates of >150/min, wide complex tachycardia and supraventricular tachycardia)
• excessive bradycardia (heart rates of <40/min)
• raised end-diastolic filling pressure (presence of pulmonary oedema or raised jugular venous pressures)
Interventions can broadly be divided into three options for immediate treatment:
Common periarrest arrhythmias and interventions are covered in Chapter 11. Antiarrhythmic interventions such as medications, physical manoeuvres and electrical therapies may be proarrhythmic.16
Fluid Resuscitation
Fluid resuscitation may be considered if hypovolaemia is suspected as a possible cause of the cardiac arrest. 0.9% sodium chloride or Hartmann’s solution are recommended as a rapid infusion in the initial stages of resuscitation (at least 20 mL/kg). There is no evidence to support the routine administration of fluids during a cardiac arrest in the absence of hypovolaemia.20
Ultrasound Imaging
Ultrasound imaging has shown to have some benefit on the detection and diagnosis of reversible causes of arrest including cardiac tamponade, pulmonary embolism, pneumothorax, aortic dissection or hypovolaemia. Placement of the probe at the sub-xiphoid position prior to stopping for planned rhythm assessment will facilitate views within 10 sec and minimise chest compression interruptions.20 While the use of imaging has not been shown to improve outcome, absence of heart motion on sonography during resuscitation is highly predictive of death.20
Special Considerations
Pregnancy
In 2008, there was an estimated 342,900 maternal deaths worldwide.81 Precipitants included pulmonary embolism, trauma, peripartum haemorrhage, amniotic fluid embolism, eclamptic seizure, congenital and aquired cardiac disease, myocardial infarction, subarachnoid haemorrhage and cerebral aneurysm.82 Regardless of the aetiology, resuscitation following cardiac arrest in late pregnancy is often unsuccessful. Hence, timely delivery by caesarean section in the setting of maternal cardiac arrest may save both infant and mother.
The principles of airway, breathing and circulation remain the same, but modifications must be made because of the physiological changes that occur with normal pregnancy.83 A number of factors may need to be considered when resuscitating a pregnant woman. Any situation that affects haemodynamic status will be exacerbated in a supine position, as autocaval compression may result in a fall in cardiac output of up to 25%.84 The mother may be placed in the left lateral tilt (15 degrees) or supine with a pillow under the right buttock, to displace the uterus from the inferior vena cava, facilitating venous return and cardiac output.83 Often the angle of the tilt is overestimated potentially reducing the quality of the chest compressions.85 The uterus may also be manually and gently displaced to the left to remove caval compression.83
While ventilation : compression ratios remain the same for a pregnant woman, chest compression may be complicated by flaring of the ribs, raised diaphragm, obesity and breast hypertrophy.83
The superior displacement of stomach contents by the gravid uterus and a relaxed cardiac sphincter contribute to an increased risk of gastric aspiration in the pregnant woman.83,86 Because of this increased risk, cricoid pressure should be applied until after the airway is protected by a cuffed tracheal tube.87 Tracheal intubation should be attended to early, utilising a short-handled laryngoscope86 or with a blade mounted at more than 90 degrees,87 as airway anatomy is altered with the larynx more anterior and superior, while pharyngeal mucosa is slightly oedematous and friable.86 A tracheal tube a size smaller than one normally chosen for a similar size non-pregnant woman may be chosen because of potential narrower airways secondary to oedema or swelling.83 Defibrillation energy, drug doses and administration are in accordance with ALS guidelines.87
If maternal cardiac arrest occurs in the labour ward, operating room or emergency department and BLS and ALS measures are unsuccessful, the uterus should be emptied by surgical (scalpel) intervention within 4–5 minutes.87 Maternal resuscitation may not be possible until the fetus is removed. Successful resuscitations have occurred after prompt surgical intervention.87 Refer to Chapter 26 for additional information about critical illness and pregnancy.
Electrical injuries
Electrical burn injuries (EBIs) and lightning injuries are similar in that they occur infrequently, commonly cause widespread acute and delayed tissue damage, and can arrest the heart and respiratory centre. Burn injuries are discussed in Chapter 23. This section focuses on the cardiac arrest situation. High-voltage electrocution is associated with a high incidence of cardiac abnormalities, including arrhythmias, prolongation of the QT interval, ST and T wave changes, and myocardial infarction.83 The most common cause of death with lightning injury is cardiac arrest due to VF or asystole or respiratory arrest.88 Because of the potential for cardiac injuries, all patients should be admitted for cardiac monitoring.
A lightning strike may result in asystole followed by spontaneous return of circulation. If ventilation is initiated early and severe hypoxia does not ensue, a patient’s chance of recovery should be better.88 Initial response of BLS should always begin with D (danger), that is, avoidance of injury to the rescuer. Ensure that the environment is safe for rescuers by disconnecting the electrical supply, where possible, without touching the patient. Where high-voltage lines (power lines) are in contact with the person or the vehicle, no attempt should be made to extricate the person from the vehicle until the situation is deemed safe by an authorised electricity supply person. Once the environment is safe, commence BLS resuscitation. The neck and spine should be protected, as there may be trauma.
In lightning victims, emphasis is on the immediate resuscitation of those who appear unresponsive. Respiratory arrest may be prolonged due to paralysis of the medullary respiratory centre; if not corrected, cardiac arrest secondary to hypoxia ensues. Fixed, dilated pupils should not be used as a poor prognosis of outcome, as victims can benefit from prolonged resuscitation without major sequelae.88
Drowning
General issues in managing drowning presentations are discussed in Chapter 22. This section focuses on resuscitation of a cardiorespiratory arrest. Hypoxia and acute lung injury (ALI) from drowning results in respiratory arrest which, if not corrected may proceed to a cardiac arrest.89,90 A patient’s emotional state, associated diseases, previous hypoxia and water temperature all influence this progression.83
The primary goal of initial intervention is the relief of hypoxaemia83 and restoration of cardiovascular stability.89,90 Resuscitation of drowning victims follows BLS guidelines, with commencement as soon as practical. Rescue breathing may commence while the victim is still in the water, provided it is safe for the rescuer.83 As drowning victims may have swallowed considerable amounts of water, vomiting and aspiration of gastric contents can be a major problem during resuscitation. To minimise the risks of inhalation, abdominal compression, the Heimlich manoeuvre and attempts to drain water from the lungs are not recommended. Instead the victim should be placed on the side for the initial assessment of airway and breathing.63 Cardiac arrest in these victims is secondary to hypoxia, so compression-only CPR is likely to be less effective and should be avoided.83 Once experienced personnel arrive, ALS and administration of oxygen should be initiated. The principles of respiratory support and ventilation are discussed in Chapter 11, and treatment of the sequelae of a drowning victim is discussed in Chapter 16.
Evaluation During Resuscitation
The use of capnometry as a non-invasive technique for monitoring CPR’s effectiveness is recommended.12 As partial pressure of end-tidal carbon dioxide (PetCO2) concentration correlates with pulmonary bloodflow during CPR, the adequacy of resuscitation efforts is evaluated by measuring this parameter. PetCO2 also correlates with cardiac output, return of spontaneous circulation (ROSC) and outcomes in cardiac arrest.91 A mean PetCO2 of 17 mmHg or above has been associated with survival from cardiac arrest, while a mean PetCO2 <10 mmHg is associated with poor outcomes. A rise in PetCO2 during CPR may indicate the return of spontaneous circulation.80 Conversely, experimental studies have demonstrated that cardiac arrest from massive pulmonary embolism is associated with an extremely low PetCO2 readings during CPR.92 Having noted this, hyperventilation during CPR is not recommended and may be harmful. Similarly, animal studies indicate that hyperventilation is associated with raised thoracic pressure, decreased coronary and cerebral perfusion and reduced return of spontaneous circulation. Clinical studies show that rescuers consistently hyperventilate patients during a cardiac arrest.93
Roles During Cardiac Arrest
Resuscitation teams should be organised to ensure that the individual skills of each member are used effectively and efficiently.94 The exact composition of the resuscitation team will vary between organisations, but generally the team should possess the following skills:94
• advanced airway management and intubation skills
• intravenous access skills including central venous access
• defibrillation and external pacing abilities
As members of a resuscitation team in the hospital generally do not work together but come from all areas of the hospital, the team should have a designated leader. The team leader gives direction and guidance, assigns tasks and makes clinical decisions without directly performing specific procedures.16,94 The leader should engender the team’s trust. Where leaders initiate structure within the arrest team, members not only work together better, they also perform the tasks of resuscitation more quickly and more effectively.94 The leader nominates the roles of arrest team members. Roles of team members include airway management, chest compression, medication administration (including IV access), documentation of events and care of family members. The team leader should be responsible for postresuscitation transfer, documentation, communicating with family members and healthcare professionals and debriefing of the team.94
The resuscitation scenario is both complex and stressful for all participants. Often, participants express feelings that too many people are involved, with no one person in control. Unfortunately, the concept of the multidisciplinary team, where all members’ contributions are equally respected, is often not evident in the literature.95 In addition, while nurses already present at a cardiac arrest in the hospital setting may be willing and competent to perform CPR, they may be prevented from doing so because of the arrival of the cardiac arrest team.96,97
Family Presence During an Arrest
The practice of family members witnessing resuscitation has over time become more evident, both in practice and in the literature. This shift in practice has been attributed to increasing patient autonomy and the presence of family at a cardiac arrest in popular television shows.98 This has contributed to public support, family members requesting – and expecting – to be present.99,100 However, the issue of whether the family should be present during a cardiac arrest remains controversial. Proponents argue the importance of family being with loved ones during their last moments, as this shortens the period of grieving and provides closure.98 Indeed, professional resuscitation bodies recommend that family should be afforded the opportunity to be present. However, translating these recommendations into practice varies within health care personnel. Commonly cited is concern that the family may interrupt the work of the resuscitation team, the ethical and medico–legal implications, or concern about offending the family.101–103 Contrary to these beliefs, there is limited evidence that family interfere with the performance of the resuscitation team.101,104,105
Conflicting evidence exists as to the psychological effects of such an event on the family. Effects have been documented as ranging from no adverse effects98 through to expressions of distress, haunting consequences and trauma.106 Where families are provided the option of being present, a staff member should be identified to have sole responsibility of supporting the family.
Ceasing Cpr
The decision to cease CPR is often difficult; continuing CPR beyond 30 minutes without return of spontaneous circulation (ROSC) is usually futile unless the arrest was compounded by hypothermia, submersion in cold water, lightning strike, drug overdose or other identified and treatable conditions such as intermittent VF/VT.16 Prolonged resuscitation of greater than 60 minutes may be made for a severely hypothermic, child victim of near-drowning. Pupillary signs should not be used as a predictor of outcome in infants and children, as 11–33% of children with non-reactive pupils have survived long-term after CPR.17 It is important to have eliminated all causes as far as possible.
Termination of resuscitation is a multifactorial process, influenced by provider comfort and experience, patient prognosis, desires previously expressed, wishes and values, the culture of the hospital, the EMS or emergency department, protocols and resource issues, and national and international guidelines that reflect changing standards of care, resource availability, global interpretations of utility and emerging science.107 With scientific advances and evidence-based protocols becoming more widely implemented, current impressions of termination decisions will change over time.107 It is appropriate to invite suggestions from team members, to ensure that all members are comfortable with a decision to stop the resuscitation attempt.16 Ultimately, terminating CPR is equivalent to a determination of death, and must be made by a physician. In some out-of-hospital circumstances it may be the paramedical staff that make this decision regarding stopping CPR. Prospectively validated termination of resuscitation rules such as the ‘basic life support termination of resuscitation rule’ are recommended to guide termination of prehospital CPR in adults.24
Postresuscitation Phase
The aim of postresuscitation care is the maintenance of cerebral and myocardial perfusion and the return of a patient to a state of best possible health. Resuscitation does not cease with the return of spontaneous circulation. However, the ROSC after cardiac arrest does not always equate to a positive outcome for the patient. Mortality rates following in-hospital cardiac arrests vary between 67 and 71%.108,109 This high mortality rate has been attributed to multiple organs that are involved with whole of body ischaemia during cardiac arrest.109 The reperfusion responses that occur following successful resuscitation is termed postcardiac arrest syndrome.110 Coordinated care and specific interventions initiated in the postarrest phase can influence outcomes.111 Control of body temperature, identification and treatment of acute coronary syndromes and optimisation of mechanical ventilation are a few of the targeted objectives of care (ARC & NZRC Guideline 11.8).62
Role of Hypothermia in Adults after Cardiac Arrest
During cardiac arrest, prolonged global ischaemia coupled with inadequate reperfusion during the immediate postresuscitation period can lead to severe cerebral hypoxic injury.112 Induced moderate hypothermia (28–32°C) has been used in open-heart cardiac surgery since the 1950s to protect the brain against global ischaemia.113 One randomised control trial and other studies have shown that cooling patients postcardiac arrest provides significant survival benefit as well as improved cardiac and neurological function.113–115 Prospective randomised studies have demonstrated that mild hypothermia (32–34°C) increases the rate of favourable neurological outcome in comatosed adult patients resuscitated after out-of-hospital cardiac arrest (OHCA) due to VF.114,115 A variety of cooling techniques are described in Box 24.1.
Therapeutic cooling consists of the induction, maintenance and rewarming phases.116 ILCOR recommends that unconscious adult patients with spontaneous circulation after OHCA should be cooled to 32–34°C for 12–24 hours if the initial rhythm was VF. This cooling may also be beneficial for other rhythms or in-hospital cardiac arrest.113 It is important to note that shivering must be prevented during this phase (ARC & NZRC Guideline 11.8).62
Persistent hyperglycaemia following cardiac arrest has been associated with poor neurological outcome. Monitoring of blood sugar levels and treatment of hyperglycaemia (>10 mmol/L) with insulin is recommended in the post cardiac arrest period.117
Near-Death Experiences
With the rise in survival rates after a critical illness, there are increasing numbers of documented near-death (NDEs) and out-of-body (OBEs) experiences.118,119 Near-death has been described as unusual experiences during a close brush with death.118 Experiences have typically included memories of bright tunnels of light, deceased relatives, out-of-body sensations, feelings of presence of deity and peace.120,121 These experiences may vary between cultures: Euro-Americans may report a golden colour light where as Tibetans may report a clear light.122 People report the experiences as pleasant, and they have resulted in positive life changes for the individual. After-effects of an NDE include absence of fear of death, more spiritual view of life, less regard for material wealth and/or a heightened chemical sensitivity.119,120 The incidence of NDEs after cardiac arrest is reported at 6–18%,118,123 with the frequency generally being higher in people under 60 years of age.119 Hence, an awareness of the incidence of NDEs, the cultural differences and needs of the person with a reported NDE are essential postcardiac arrest.120,124
Legal and Ethical Considerations
Burgeoning technology in the 1960s enabled the support of oxygenation and circulation for people whose illnesses would have been lethal just a few years before. Enthusiasm for restoration of life led healthcare workers to routinely initiate CPR for all patients who died in hospital.125 Unfortunately, this led to inappropriate resuscitation attempts and the realisation of the economic, medical and ethical burden to society when survivors had a resultant poor quality of life.126 In the 1970s, growing concern about inappropriate application of CPR and patient’s rights led authors to suggest means of forgoing resuscitation and involving patients in decision making.127 Traditionally, the decision to initiate or withhold CPR was often made by the treating medical team in the absence of the patient or family.128
Hospitals responded by developing procedures for withholding CPR through the documentation of ‘do not attempt to resuscitate’ (DNAR) orders, physician orders for life-sustaining treatment (POLST), advance directives or living wills128 (see Chapter 5). For patients or their surrogates to meaningfully participate in decision making about CPR, they must have some understanding of survival rates and adverse effects associated with CPR.129 Consequently, much debate has ensued over the right of a person to forgo treatment.125
Research proposes that while patients want to be involved in CPR decision making and want some form of advance directive, their knowledge is limited and often derived from television dramas.128,130 Understanding of morbidity and outcomes after CPR strongly influences their preferences.129 Most patients, and indeed healthcare workers, commonly hold unrealistic expectations of CPR success,131 and will often reverse their preference for commencing CPR once they are informed of the true probability of survival and functional status after resuscitation.129 Regardless of this, healthcare workers continue to demonstrate a reluctance to discuss CPR options with patients. Despite open discussion, poor documentation and communication can result in CPR being inappropriately commenced.132 Approximately one-third of patients successfully resuscitated have subsequently stated that they did not want to be resuscitated.133 Conversely, and contrary to medical and nursing opinions, some people choose CPR even when they have a terminal illness, coma or serious disability.129
Standardised orders for limitations on life-sustaining treatments (e.g. DNAR, POLST) should be considered to decrease the incidence of futile resuscitation attempts and to ensure that adult patient’s wishes are honoured. These orders should be specific, detailed, transferable across healthcare settings, and easily understood. Processes, protocols and systems should be developed that fit within local cultural norms and legal limitations to allow providers to honour patient’s wishes about resuscitation efforts.24 With the exception of a zero survival rate there remains no formal consensus on DNAR decision-making practices or the termination of resuscittion. While researchers have attempted to develop prognostic indicators for cardiac arrest outcome, moralists would argue that the use of such prognostic tools alone reflect utilitarianism,133 and should never be used in isolation of the input of the patient and healthcare team.134
Summary
Research vignette
Critique
The use of therapeutic hypothermia as a modality to improve mortality and morbidity in out of hospital cardiac arrest has been well recognised in the literature since 2002.147 The International Liaison Committee on Resuscitation (ILCOR) published an advisory statement in 2003 recommending the implementation of therapeutic hypothermia.148 The American Heart Association (AHA) and the European Resuscitation Council (ERC) also published therapeutic hypothermia guidelines following the ILCOR 2005 consensus on science and treatment recommendations,149 and these were updated and republished in 2010.150 However, as pointed out in this paper, the uptake of therapeutic hypothermia across the world has been slow and reasons for this have included the cost of equipment used for cooling.151–156 When referring to these statements of utilisation, the reader should be aware that the Australian experience has not been discussed and therefore generalisation of the Australian context cannot be made. Further, as the authors note, the use of a single study site limits the generalisability of the findings.
The ILCOR statement on therapeutic hypothermia states that intravenous ice cold fluids (30 mL/kg) can safely initiate therapeutic hypothermia and the use of ice packs and/or cooling blankets and pads can maintain temperature control.150 In this study, therapeutic hypothermia was initiated at 40 mL/kg; the authors do not state why they used a higher fluid volume than recommended by ILCOR. Similarly, the use of ice-water gastric lavage in the study has not been recommended by ILCOR. Other reported methods of non-invasive cooling not recommended by ILCOR, but evident in the literature, includes the trans-nasal insertion of an evaporative coolant into the nasopharynx.157–159
Various methods have been documented for recording and monitoring the core temperature, including involving the bladder, rectum, pulmonary artery and oesophagus.160 While pulmonary artery catheters are considered to be ‘gold standard’, the use of minimally-invasive monitoring such as oesophageal temperature monitoring is considered to be optimal.159 Temperature monitoring using the bladder and the rectum should be interim measures only as there is typically is a ‘temperature lag time’ behind the core temperature. In addition, variability of measurements occurs with the flow of urine presence and faeces around the catheter. Consistent with ILCOR recommendations, re-warming commenced after 24 hours, however the authors state that the recommended rate is no more than 0.5 °C/h. ILCOR makes no mention of the rate of rewarming and the researchers reference this rate to Scandinavian Clinical Practice.44 The researchers in the study achieved a re-warming rate of 0.18 °C/h.
Cognitive preservation was measured as an outcome measure in the current study using the Glasgow-Pittsburg Cerebral Performance Category (CPC). This scale rates patients from 1 (normal) through to 5 (certified brain-dead) and has been used as a comparable outcome measure in similar recent studies.159 This study noted that 31 percent of participants had a CPC score of 1 or 2. While the Australian experience has not been discussed, generalisation to the Australian context should be made with caution.
Whilst the researchers claim that their modality of therapeutic hypothermia is rapid, safe and low cost, they highlight that the major barrier inhibiting the uptake of this treatment is technical difficulties. The researchers attribute these difficulties to the cost of commercial equipment required to rapidly and effectively implement therapeutic hypothermia. This cost is underexplored in this study; it is eluded that all devices are expensive and therefore unattainable by many hospitals. The researchers then state that their method is labour -intensive, however the cost comparison of the labour as opposed to use of the various devices is not explored. The insertion and confirmation of nasograstric tube (NGT) occurred by auscultation and aspiration of gastric fluid. Evidence cautions against the use of litmus paper, auscultation and bubbling to confirm NGT placement, with pH testing and X-ray confirmation preferred.161 Thus, the reader should be aware that the use of ice-water gastric lavage is not supported by ILCOR and that there are inherent risks of NGT misplacement. Replication of the study without the ice-water gastric lavage cooling technique will likely be beneficial.
The benefits of initiating mild therapeutic hypothermia following an OHCA or IHCA are well documented in the literature. The authors rightly note that transferring this evidence into practice has not been seen and cite the ease of cooling processes as its potential barrier. In the study, the researchers report a reduction in the ROSC to initiation of hypothermia time (257 to 132 minutes) with targeted education, raising clinical awareness through lectures and wide distribution of cooling protocols. Other studies have also found an increase in the therapeutic hypothermia following the implementation of a standardised protocol.162,163
The mix of patients in this study also needs consideration. Definitive data on benefit has been primarily based on out-of-hospital cardiac arrest (OHCA) with ILCOR only highlighting two studies that included both OHCA and in-hospital cardiac arrests (IHCA).150 The researchers in this study had predominately IHCA patients (n = 40) whereas OHCA patients were of lower numbers (n = 25). This study is important as it adds weight to the supportive evidence for therapeutic hypothermia for all patients suffering cardiac arrest who remain comatose post return of spontaneous circulation. Interestingly the IHCA group had better neurological outcomes overall when compared with the OHCA group.
Learning activities
All learning activities relate to the case study.
1. Discuss the management of this patient in relation to the ALS flowchart.
2. Discuss the ethical issues of consent and limitations of treatment as related to the case study.
3. Identify potential causes of PEA.
4. Discuss the pathophysiology of PEA in relation to the case study.
5. Outline the role of therapeutic hypothermia in post arrest care.
6. Outline the postresuscitation management that is related to this case study.
American Heart Association (AHA). http://www.americanheart.org.
Australian Resuscitation Council (ARC). http://www.resus.org.au.
Center for Pediatric Emergency Medicine (CPEM). http://www.med.nyu.edu/peder/cpem.
European Resuscitation Council (ERC). http://www.erc.edu.
International Liaison Committee on Resuscitation (ILCOR). http://www.ilcor.org/en/home.
New Zealand Resuscitation Council (NZRC). http://www.nzrc.org.nz.
The Regional Emergency Medical Services Council of New York City. http://www.nycremsco.org/default.asp.
1 Rubart M, Zipes D. Mechanisms of sudden cardiac death. J Clin Investigation. 2005;115(9):2305–2315.
2 Meyer A, Cameron P, Smith K, McNeil J. Out-of-hospital cardiac arrest. eMJA. 2000;172:73–76.
3 Ali S, Antezano S. Sudden Cardiac Death. South Med J. 2006;99(5):502–510.
4 Rea T, Eisenberg M, Sinibaldi G. Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation. 2004;63(1):17–24.
5 Atwood C, Eisenberg M, Herlitz J. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation. 2005;67(1):75–80.
6 Holmgren C, Bergfeldt L, Edvardsson N, et al. Analysis of initial rhythm, witnessed status and delay to treatment among survivors of out-of-hospital cardiac arrest in Sweden. Heart and Education in Heart. 2010;96(22):1826–1830.
7 Rea T, Pearce R, Raghunathan T. Incidence of out-of-hospital cardiac arrest. Am J of Cardiol. 2004;93:1455–1460.
8 Waalewijn R, Nijpels MA, Tijssen JG, Koster RW. Prevention of deterioration of ventricular fibrillation by basic life support during out-of-hospital cardiac arrest. Resuscitation. 2002;54(1):31–36.
9 Herlitz J, Engdahl J, Svensson L, Young M, Angquist K-A, Holmberg S. Can we define patients with no chance of survival after out-of-hospital cardiac arrest? Heart Lung. 2004;90:1114–1118.
10 Australian Institute of Health and Welfare (AIHW). Australia’s health 2010. Australia’s health series no. 12. Canberra: AIHW; 2010.
11 Muller D, Agrawal R, Arntz H. How sudden is sudden cardiac death? Circulation. 2006;114:1146–1150.
12 Deakin C, Nolan J, Soar J. European Resuscitation Council Guidelines for Resuscitation 2010. Section 4. Adult advanced life support. Resuscitation. 2010;81(10):1305–1352.
13 Herlitz J, Svensson L, Engdahl J. Characteristics of cardiac arrest and resuscitation by age group: an analysis from the Swedish Cardiac Arrest Registry. Am J Emerg Med. 2007;25(9):1025–1031.
14 Dickson E, Anders N. Infant resuscitation. Curr Anaesthetics Crit Care. 2004;15:53–60.
15 Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry – Cardiac Arrest. Circulation. 2009;119(11):1484–1491.
16 Rosen K, Sinz E, Casto J. Basic and advanced life support, acute resuscitation, and cardiac resuscitation. Curr Opin Anaesthesiol. 2001;14(2):177–178.
17 Frenneaux M. Cardiopulmonary resuscitation: some physiological considerations. Resuscitation. 2003;58(3):259–265.
18 Ballew K. Cardiopulmonary resuscitation: recent advances. BMJ. 1997;314(7092):1462–1466.
19 Cummins R, Chamberlain D, Hazinski M, Nadkarni V, Kloeck W, Kramer E. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital ‘Utstein style. Circulation. 1997;95(8):2213–2239.
20 Nolan J, Soar J, Zideman D, et al. European Resuscitation Council Guidelines for Resuscitation 2010. Section 1. Executive summary. Resuscitation. 2010;81(10):1219–1276.
21 Petrie D, De Maio V, Stiell I. Factors affecting survival after prehospital asystolic cardiac arrest in basic life support-defibrillation system OPALS Study Group. Canadian J of Emerg Med. 2001;3(3):113–118.
22 Meaney P, Nadkarni V, Kern K, Indik J, Halperin H, Berg R. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38(1):101–108.
23 Genardi M, Cronin S, Thomas L. Revitalizing an established rapid response team [Evaluation study]. DCCN. 2008;27(3):104–109.
24 Soar J, Mancini M, Bhanji F, et al. On behalf of the Education, Implementation, and Teams Chapter Collaborators. Part 12: Education, implementation, and teams: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81:e288–e330.
25 Smith G. In-hospital cardiac arrest: Is it time for an in-hospital ‘chain of prevention. Resuscitation. 2010;81(9):1209–1211.
26 Hajbaghery M, Mousavi G, Akbar H. Factors influencing survival after in-hospital cardiopulmonary resuscitation. Resuscitation. 2005;66(3):317–321.
27 Koster R, Baubin M, Bossaert L, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2010;81(10):1277–1292.
28 Brindley P, Simmonds M, Gibney R. Medical emergency teams: Is there M.E.R.I.T. Can J Anaesthes. 2007;54(5):389–391.
29 Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hilman K. A comparison of antecedents to cardias arrests, deaths and emergency intensive admissions in Australia and New Zealand, and the United Kingdom – The ACADEMIA study. Resuscitation. 2004;62(2):275–282.
30 Considine J, Botti M. Who, when and where? Identification of patients at risk of an in-hospital adverse event: implications for nursing practice. Int J Nurs Res. 2004;10(1):21–31.
31 DeVita M, Smith G, Adam S, et al. Identifying the hospitalised patient in crisis: A consensus conference on the afferent limb of Rapid Response Systems. Resuscitation. 2010;81(4):375–382.
32 Lee A, Bishop G, Hillman K, Daffurn K. The medical emergency team. Anaesth Intens Care. 1995;23(2):183–186.
33 Prytherch D, Smith G, Schmidt P, Featherstone P. ViEWS – Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932–937.
34 Hillman K. Critical care without walls. Curr Opinion Crit Care. 2002;8(6):594–599.
35 Massey D, Aitken L, Chaboyer W. Literature review: do rapid response systems reduce the incidence of major adverse events in the deteriorating ward patient? J Clin Nurs. 2010;19(23–24):3260–3273.
36 Chan P, Jain R, Nallmothu B, Berg R, Sasson C. Rapid response teams: a systematic review and meta-analysis. Archives of Internal Med. 2010;170(1):18–26.
37 Laurens N, Dwyer T. The effect of Medical Emergency Teams on patient outcome: A review of the literature. Int J Nurs Prac. 2010;16(6):533–544.
38 Ray EM, Smith R, Massie S, et al. Family alert: Implementing direct family activation of a pediatric rapid response team. Joint Comm J on Quality & Patient Safety. 2009;35(11):575–580.
39 Australian Resuscitation Council and New Zealand Resuscitation Council (ARC NRC). Airway: Guideline 4. Available at http://www.resus.org.au/
40 Mackway-Jones K, Molyneux E, Phillips B, Wieteska K. Advanced paediatric life support: the practical approach, 4th edn. Blackwell Publishing; 2005.
41 Wyllie J, Perlman J, Kattwinkel J, et al. On behalf of the Neonatal Resuscitation Chapter Collaborators. Part 11: Neonatal resuscitation: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;10(81):e260–e287.
42 Berg R, Saunders A, Kern K, Hilwig R, Heidenreich J, Porter M. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular defibrillation cardiac arrest. Circulation. 2001;104(20):2465–2470.
43 Nolan J, Hazinski M, Billi J, et al. Part 1: Executive summary: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(1 Suppl1):e1–25.
44 Dwyer T. Psychological factors inhibiting family member’s confidence to initiate CPR. Prehospital Emerg Care. 2008;12(2):157–161. April
45 Assar D, Chamberlain D, Colquhoun M, Donnelly P, Handley A, et al. Randomised controlled trial of staged teaching for basic life support: skill acquisition at bronze stage. Resuscitation. 2000;45(1):7–15.
46 Koster R, Sayre M, Botha M, et al. Part 5: Adult basic life support: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(10):e48–e70.
47 Iwami T, Kawamura T, Hiraide A, et al. Effectiveness of bystander-initiated cardiac-only resuscitation for patients with out-of-hospital cardiac arrest. Circulation. 2007;116(2):900–907.
48 Bobrow B, Spaite D, Berg R, et al. Chest compression-only CPR by lay rescuers and survival from out-of-hospital cardiac arrest. JAMA. 2010;304(3):1447–1454.
49 Delguercio L, Feins N, Cohn J, Coomaraswamy R, Wollman S, State D. Comparison of blood flow during external and internal cardiac massage in man. Circulation. 1965;31(Suppl1):171–180.
50 Pellis T, Kette F, Lovisa D, et al. Utility of pre-cordial thump for treatment of out of hospital cardiac arrest: a prospective study. Resuscitation. 2009;80(1):17–23.
51 Haman L, Parizek P, Vojacek J. Precordial thump efficacy in termination of induced ventricular arrhythmias. Resuscitation. 2009;80(1):14–16.
52 Wilson R, Gwinnutt C. Recent advances in cardiopulmonary resuscitation. Curr Anaesth Crit Care. 2004;15(6):418–426.
53 Deakin C, Nolan J, Sunde K, Koster R. European Resuscitation Council Guidelines for Resuscitation 2010 Section 3. Electrical therapies: Automated external defibrillators, defibrillation, cardioversion and pacing. Resuscitation. 2010;81(10):1293–1304.
54 Dwyer T, Mosel Williams L, Jacobs I. Benefits and use of shock advisory defibrillators in-hospitals. Int J Nurs Prac. 2004;10(2):86–92.
55 vanAlem AP, Sanou BT, Koster RW. Interruption of cardiopulmonary resuscitation with the use of the automated external defibrillator in out-of-hospital cardiac arrest. Annals of Emerg Med. 2003;42(4):449–457.
56 Cummins R, Sanders A, Mancini E, Hazinski M. In-Hospital Resuscitation – A statement for healthcare professionals from the American Heart Association Emergency Cardiac Care Committee and the Advanced Cardiac Life Support, Basic Life Support, Pediatric Resuscitation, and Program Administration subcommitties. Circulation. 1997;95(8):2211–2212.
57 Valenzuela T, Roe D, Nichol Clark L. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. New England J of Med. 2000;343(17):1206–1209.
58 Grundry J, Comess K, De Rook F, Jorgenson D, Brady G. Comparison of naive sixth-grade children with trained professionals in the use of an automatic external defibrillator. Circulation. 1999;100(16):1703–1707.
59 Sunde K, Jacobs I, Deakin C, Hazinski M, Kerber R, et al. on behalf of Defibrillation Chapter Collaborators. Part 6: Paediatric 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81s:e71–e85.
60 Hess EP, Atkinson EJ, White RD. Increased prevalence of sustained return of spontaneous circulation following transition to biphasic waveform defibrillation. Resuscitation. 2008;77(1):39–45.
61 Morrison L, Dorian P, Long J, et al. Out-of-hospital cardiac arrest rectilinear biphasic to monophasic damped sine defibrillation waveforms with advanced life support intervention trial (ORBIT). Resuscitation. 2005;66(2):149–157.
62 Australian Resuscitation Council and New Zealand Resuscitation Council (ARC & NZRC). The Australian Resuscitation Council Online. Available from http://www.resus.org.au/
63 de Caen A, Kleinman M, Chameides L, et al. On behalf of the Paediatric Basic and Advanced Life Support Chapter Collaborators. Part 10: Paediatric basic and advanced life support: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(2):e213–e259.
64 Christenson J, Andrusiek D, Everson-Stewart S. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120(13):1241–1247.
65 Chan P, Krumholz H, Spertus J, et al. Automated external defibrillators and survival after in-hospital cardiac arrest. JAMA. 2010;304(19):2129–2136.
66 Gatward M, Thomas J, Nolan J, Cook T. Effect of chest compressions on the time taken to insert airway devices in a manikin. Brit J Anaest. 2008;100:351–356.
67 Herlitz J, Bang A, Alsen B, Aune S. Characteristics and outcome among patients suffering from in hospital cardiac arrest in relation to whether the arrest took place during office hours. Resuscitation. 2002;53(1):127–133.
68 De Maio V, Stiell J, Wells G, Spaite D. Cardiac arrest witnessed by emergency medical services personnel: descriptive epidemiology, prodromal symptoms, and predictors of survival. Annals of Emerg Med. 2000;35(2):138–146.
69 Rosborough J, Deno D. Electrical therapy for post defibrillatory pulseless electrical activity. Resuscitation. 2004;63(1):65–72.
70 Wenzel V, Lindner KH, Augenstein S, et al. Intraosseous vasopressin improves coronary perfusion pressure rapidly during cardiopulmonary resuscitation in pigs. Crit Care Med. 1999;27(8):1565–1569.
71 Duncan A. The critically ill child. In: Bersten A, Soni N, Oh T. Oh’s intensive care manual. Oxford: Butterworth-Heinemann, 2003.
72 Shavit Y, Hoffmann R, Galbraith A, Waisman Y. Comparison of two mechanical intraosseous infusion devices: a pilot, randomized crossover trial. Resuscitation. 2009;80(9):1029–1033.
73 Leidel B, Kirchhoff C, Braunstein V, Bogner V, Biberthaler P, Kanz K. Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: A prospective, randomized study. Resuscitation. 2010;81(8):994–999.
74 Wenzel V, Krismer A, Arntz H, Sitter H, Stadlbauer K, Lindner K. European Resuscitation Council Vasopressor during Cardiopulmonary Resuscitation Study Group: a comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. New England J Med. 2004;350:105–113.
75 Stiell G, Hebert P, Wells G. Vasopressin versus epinephrine for in hospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358:105–109.
76 Aung K, Htay T. Vasopressin for cardiac arrest: a systematic review and meta-analysis. Archives of Internal Med. 2005;165:17–24.
77 Kudenchuk P, Cobb L, Copass M, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. New England J of Med. 1999;341:871–878.
78 Lunxian T, Hu X, Qing H. Intravenous amiodarine for the treatment of ventricular tachycardia and ventricular fibrillation. Cochrane Database of Systematic Reviews 2003(2). CD004195.
79 Allegra J, Lavery R, Cody R. Magnesium sulfate in the treatment of refractory ventricular fibrillation in the prehospital setting. Resuscitation. 2001;49(3):245–249.
80 Sehra R, Underwood K, Checchia P. End tidal CO2 is a quantitative measure of cardiac arrest. Pacing Clin Electrophysiolgy. 2003;26(1):515–517.
81 Hogan M, Foreman K, Naghavi M. Maternal mortality for 181 countries, 1980–2008: a systemic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375(9726):1609–1623.
82 Lewis G, The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer, 2003–005. The seventh report of the confidential enquiries into maternal deaths in the United Kingdom. London: CEMACH; 2007.
83 Soar J, Perkins G, Alfonzo A, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 8. Cardiac arrest in special circumstances: Electrolyte abnormalities, poisoning, drowning, accidental hypothermia, hyperthermia, asthma, anaphylaxis, cardiac surgery, trauma, pregnancy, electrocution. Resuscitation. 2010;81(10):1400–1433.
84 Cardosi R, Porter K. Cesarean delivery of twins during maternal cardiopulmonary arrest. Obstet Gynecol. 1998;94(4):695–699.
85 Jones SJ, Kinsella SM, Donald FA. Comparison of measured and estimated angles of table tilt at Caesarean section. Brit J Anaesthetics. 2003;90(1):86–87.
86 Luppi C. Cardiopulmonary resuscitation: pregnant women are different. AACN Clin Iss Adv Pract Acute Crit Care. 1997;8(4):574–585.
87 Morris S, Stacey M. Resuscitation in pregnancy. BMJ. 2003;327(7426):1277–1280.
88 Zafrena K, Durrerc B, Herryd J, Bruggere H. Lightning injuries: prevention and on-site treatment in mountains and remote areas: official guidelines of the International Commission for Mountain Emergency Medicine and the Medical Commission of the International Mountaineering and Climbing Federation (ICAR and UIAA MEDCOM). Resuscitation. 2005;65(3):369–372.
89 Layon A, Modell J. Drowning update. Anesthesiology. 2009;110:1390–1401.
90 Salomez F, Vincent J. Drowning: a review of epidemiology, pathology, treatment and prevention. Resuscitation. 2004;63(3):261–268.
91 Frakes M. Measuring end-tidal carbon dioxide: clinical applications and usefulness. Crit Care Nurse. 2001;21(5):23–37.
92 Cortney D, Watts J, Kline J. End tidal CO2 is reduced during hypotension and cardiac arrest in a rat model of massive pulmonary embolism. Resuscitation. 2002;53(1):83–91.
93 Aufderheide T, Sigurdsson G. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–1965.
94 Cooper S, Wakelam A. Leadership of resuscitation teams: ‘lighthouse leadership. Resuscitation. 1999;42(1):27–45.
95 Meerabeau L, Page S. I’m sorry if I panicked you: Nurses’ accounts of teamwork in cardiopulmonary resuscitation. J Interprofessional Care. 1999;13(1):29–35.
96 Dwyer T, Mosel Williams L. Nurses’ behaviour regarding CPR and the theories of reasoned action and planned behaviour. Resuscitation. 2002;52(1):85–90.
97 Dwyer T, Mosel Williams L, Mummery K. Defibrillation beliefs of rural nurses: Focus group discussions guided by the theory of planned behaviour. Int Electronic J Rural Remote Health Research, Education, Practice and Policy. 5(322), 2005. Available from http://www.rrh.org.au/articles/showarticlenew.asp?ArticleID=322
98 MacLean D, Guzzetta C, White C, Fontaine D, Eichhorn D, Meyers T. Family presence during cardiopulmonary resuscitation and invasive procedures: Practices of critical care and emergency nurses. J of Emerg Nursing. 2003;29(3):208–221.
99 Mazer M, Cox L, Capon J. The public’s attitude and perception concerning witnessed cardiopulmonary resuscitation. Crit Care Med. 2006;34(12):2925–2928.
100 Ong M, Chung W, Mei J. Comparing attitudes of the public and medical staff towards witnessed resuscitation in an Asian population. Resuscitation. 2007;73(1):103–108.
101 Miller H, Stiles A. Family Presence During Resuscitation and Invasive Procedures: The Nurse Experience. Qualitative Health Research. 2009;19(10):1431–1442.
102 Newton A. Witnessed resuscitation in critical care: The case against. Intens Crit Care Nursing. 2002;18(3):146–150.
103 Boyd R, White S. Does witnessed cardiopulmonary resuscitation alter perceived stress in accident and emergency staff? Euro J of Emerg Med. 2000;7(1):51–53.
104 Weslien M, Nilstun T, Lundqvist A, Fridlund B. Narratives about resuscitation: Family members differ about presence. Euro J of Cardiovasc Nurs. 2006;5(1):68–74.
105 Schmidt B. Review of Three Qualitative Studies of Family Presence During Resuscitation. The Qualitative Report. 2010;15(3):731–736.
106 Fulbrook P, Albarran J, Latour J. An European survey of critical care nurses’ attitudes and experiences of having family members present during cardiopulmonary resuscitation. Int J Nurs Studies. 2005;45(5):557–567.
107 Larkin G. Termination of resuscitation: the art of clinical decision making. Curr Opin Crit Care. 2002;8(3):224–229.
108 Nadkarni V, Larkin G, Peberdy M. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57.
109 Nolan J, Laver S, Welch A, Harrison D, Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC Case Mix Programme Database. Gupta U. Anaesthesia. 2007;62(12):1207–1216.
110 Nolan J, Neumara R, Adriea C, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication: A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part 1). Int Emerg Nurs. 2009;17(4):203–225.
111 Binks A, Nolan J. Post-cardiac arrest syndrome. Minerva Anestesiol. 2010;76:362–368.
112 Negovsky V. Postresuscitation disease. Crit Care Med. 1988;16(10):942–946.
113 Nolan J, Morley P, Hoek T, Hickey R. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Resuscitation. 2003;57(3):231–235.
114 Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. New England J Med. 2002;346(8):557–563.
115 Bernard S, Gray T, Buist M, Jones B, Silvester W, Gutteridge G. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New Eng J Med. 2002;346(8):557–563.
116 Poldermann K, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–1120.
117 Australian Resuscitation Council and New Zealand Resuscitation Council (ARC NRC). Post-resuscitaion therapy in adult advanced life support: Guideline 11.7. Available from http://www.resus.org.au/
118 Greystone B. Incidence and correlates of near-death experiences in a cardiac care unit. Gen Hosp Psychiat. 2003;25(4):269–276.
119 Van Lommel P, van Wees R, Meyers V, Elfferich I. Near-death experience in survivors of cardiac arrest: a prospective study in the Netherlands. Lancet. 2001;358(15):2039–2045.
120 James D. What emergency department staff need to know about near-death experiences. Top Emerg Med. 2004;26(1):29–34.
121 Parnia S, Fenwick P. Near death experiences in cardiac arrest: vision of a dying brain or versions of a new science of consciousness. Resuscitation. 2002;52(1):5–11.
122 Belanti J, Perera M, Jagadheesan K. Phenomenology of Near-death Experiences: A Cross-cultural Perspective. Transcultural Psychiatry. 2008;45(1):121–133.
123 Parina S, Waller D, Yeates R, Fenwick P. A qualitative and quantitative study of the incidence, features and aetiol-ogy of near death experiences on cardiac arrest survivors. Resuscitation. 2001;48(2):149–156.
124 Daffin C. Near death experiences ‘must be taken seriously. Nursing Standard. 2002;16(17):9–13.
125 Lynn J. Regulating hearts and minds: the mismatch of law, custom and resuscitation decisions. J Am Geriatric Society. 2003;51(10):45–52.
126 Landry F, Parker J, Phillips Y. Outcome of cardiopulmonary resuscitation in the intensive care setting. Archives of Internal Med. 1992;152(11):2305–2308.
127 Rabkin M, Gillerman G, Rice N. Orders not to resuscitate. New Engl J of Med. 1976;295(12):364–372.
128 Kerridge I, Pearson S, Rolfe I, Lowe M. Decision making in CPR: attitudes of hospital patients and health care professionals. Med J Aust. 1998;169:128–131.
129 Kerridge I, Pearson S, Rolfe I, Lowe M, McPhee J. Impact of written information on knowledge and preferences for cardiopulmonary resuscitation. 2. eMJA. 1999;171:239–244.
130 Harris D, Willoughby H. Resuscitation on television: Realistic or ridiculous? A quantitative observational analysis of the portrayal of cardiopulmonary resuscitation in television medical drama. Resuscitation. 2009;80(11):1275–1279.
131 Heyland D, Frank C, Groll D. Understanding cardiopulmonary resuscitation decision making: perspectives of seriously ill hospitalised patients and family members. Chest. 2006;130(2):419–428.
132 Giles H, Moule P. Do not attempt resuscitation’ decision-making: a study exploring the attitudes and experiences of nurses. Nursing Crit Care. 2004;9(3):115–122.
133 Hayward M. Cardiopulmonary resuscitation: are practitioners being realistic? Brit J Nurs. 1999;8(12):810–814.
134 Fritz Z, Fu J. Ethical issues surrounding do not attempt resuscitation orders: decisions, discussions and deleterious effects. J Med Ethics. 2010;36(10):593–597.
135 Smith R, Hickey B, Santamaria J. Automated external defibrillators and survival after in-hospital cardiac arrest: early experience at an Australian teaching hospital. Crit Care Resusc. 2009;11(4):261–265.
136 Forcina M, Farhat A, O’Neil W, Haines D. Cardiac arrest survival after implementation of automated external defibrillator technology in the in-hospital setting. Crit Care Med. 2009;37(4):1229–1236.
137 Zafari A, Zarter S, Heggen V, et al. A program encouraging early defibrillation results in improved in-hospital resuscitation efficacy. J Am Coll Cardiol. 2004;44(4):846–852.
138 Haines D. Automated external defibrillators and the law of unintended consequences. JAMA. 2010;304(19):2178–2179.
139 Kern K, Morley P, Babbs C. Use of adjunctive devices in cardiopulmonary resuscitation. Annals of Emerg Med. 2001;37(4 Suppl):S568–S577.
140 Hickey R. Recent developments and emerging concepts in cardiopulmonary cerebral resuscitation. Clinic Pediatr Emerg Med. 2004;5:217–223.
141 Timerman S, Cardoso L, Ramires J, Halperin H. Improved hemodynamic performance with a novel chest compression device during treatment of in-hospital cardiac arrest. Resuscitation. 2004;61(3):273–280.
142 Hand H. Cardiopulmonary resuscitation: the laryngeal mask airway. Emerg Nurse. 2002;10(104):31–37.
143 Cook T, Strube P, Lees M, Millar J, Baskett P. Randomised crossover comparison of the proseal with the classic laryngeal mask airway in unparalysed anaesthetised patients. Br J Anaesth. 2002;88(4):527–532.
144 Kette F, Reffo I, Giordani G, Buzzi F, Borean V, et al. The use of laryngeal tube by nurses in out-of-hospital emergencies: preliminary experience. Resuscitation. 2005;66(1):21–25.
145 Tiah L, Wong E, Chen M, Sadarangani S. Should there be a change in teaching of airway management in the medical school curriculum? Resuscitation. 2005;64(1):87–91.
146 Kette F, Reffo I, Giordani G, et al. The use of laryngeal tube by nurses in out-of-hospital emergencies: Preliminary experience. Resuscitation. 2005;66(1):21–22.
147 Bernard S, Gray T, Buist M, Jones B, Silvester W, Gutteridge G. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New Eng J Med. 2002;346(8):557–563.
148 Nolan J, Morley P, Hoek T, Hickey R. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Resuscitation. 2003;57(3):231–235.
149 American Heart Association. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 7.5: Postresuscitation Support Care. Circulation. 2005;112(5):84–88.
150 Morrison L, Deakin C, Morley P, Callaway C, Kerber R, Kronick S. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation. 2010;122:S345–S421.
151 Abella B, Rhee J, Huang K, Vanden Hoek T, Becker L. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64:181–186.
152 Merchant R, Soar J, Skrifvars M. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34:1935–1940.
153 Kennedy J, Green R, Stenstrom R. The use of induced hypothermia after cardiac arrest: a survey of Canadian emergency physicians. CJEM. 2008;10:125–130.
154 Oksanen T, Pettila V, Hynyen M, Varpula T. Therapeutic hypothermia after cardiac arrest: implementation and outcome in Finnish intensive care units. Acta Aneaesth Scan. 2007;51:866–871.
155 Wolfrum S, Radke P, Pischon T, Willich S, Schunkert H, et al. Mild therapeutic hypothermia after cardiac arrest-a nationwide survey on the implementation of ILCOR guidelines in German intensive care units. Resuscitation. 2007;72:207–213.
156 Laver S, Padkin A, Atalla A, Nolan J. Therapeutic hypothermia after cardiac arrest: a survey of practice in intensive care units in the United Kingdom. Anesthesia. 2006;61:873–877.
157 Busch H, Janata A, Eichwede F, Födisch M, Wöbker G, et al. Safety and feasibility of a new innovative cooling approach for immediate induction of therapeutic hypothermia in patients after successful resuscitation. trans-nasal cooling after cardiac arrest. Circulation. 2010;118(S):1459.
158 Castren M, Nordberg P, Svensson L, Taccone F, Vincent J, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation. 2010;122(7):729–736.
159 Oommen S, Menon V. Hypothermia after cardiac arrest: beneficial, but slow to be adopted. Cleveland Clin J Med. 2011;78(7):441–449.
160 Insler S, Sessler D. Perioperative thermoregulation and temperature monitoring. Anesthesiology Clinics. 2006;24:823–837.
161 Tho P, Mordiffi S, Ang E, Chen H. Implementation of the evidence review on best practice for confirming the correct placement of nasogastric tube in patients in an acute care hospital. Int J Evidence-Based Healthcare. 2011;9:51–60.
162 Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen L, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39.
163 Dainty K, Scales D, Brooks S, Needham D, Dorian P, et al. A knowledge translation collaborative to improve the use of therapeutic hypothermia in post-cardiac arrest patients: protocol for a stepped wedge randomized trial. Implementation Science. 2011;14(6):4.