Resuscitation
INTRODUCTION
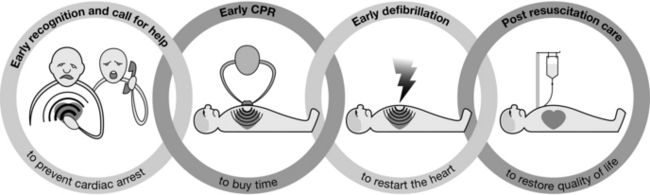
Without intervention, cardiac arrest may lead to permanent neurological injury after just three minutes. The interventions that contribute to a successful outcome after a cardiac arrest can be conceptualized as a chain – the Chain of Survival (Fig. 47.1).
PREVENTION
Guidelines for Prevention of In-Hospital Cardiac Arrest (Resuscitation Council (UK))
1. Place critically ill patients, or those at risk of clinical deterioration, in areas where the level of care is matched to the level of patient sickness.
2. Monitor such patients regularly using simple vital sign observations (e.g. pulse, blood pressure, respiratory rate, conscious level, temperature and SpO2). Match the frequency and type of observations to the severity of illness of the patient.
3. Use an early warning score (EWS) system or ‘calling criteria’ to identify patients who are critically ill, at risk of clinical deterioration or cardiopulmonary arrest, or both.
4. Use a patient vital signs chart that encourages and permits the regular measurement and recording of vital signs and, where used, early warning scores.
5. Ensure that the hospital has a clear policy that requires a timely, appropriate, clinical response to deterioration in the patient’s clinical condition.
6. Introduce into each hospital a clearly identified response to critical illness. This will vary between sites, but may include an outreach service or resuscitation team (e.g. medical emergency team (MET)) capable of responding to acute clinical crises. This team should be alerted, using an early warning system, and the service must be available 24 hours a day.
7. Ensure that all clinical staff are trained in the recognition, monitoring, and management of the critically ill patient, and that they know their role in the rapid response system.
8. Empower staff to call for help when they identify a patient at risk of deterioration or cardiac arrest. Use a structured communication tool to ensure effective handover of information between staff (e.g. SBAR – Situation-Background-Assessment-Recommendation).
9. Agree a hospital do-not-attempt-resuscitation (DNAR) policy, based on current national guidance. Identify patients who do not wish to receive CPR and those for whom cardiopulmonary arrest is an anticipated terminal event for whom CPR would be inappropriate.
10. Audit all cardiac arrests, ‘false arrests’, unexpected deaths, and unanticipated intensive care unit admissions, using a common dataset. Audit the antecedents and clinical responses to these events. All hospitals should consider joining NCAA (http://www.resus.org.uk/pages/NCAA.htm).
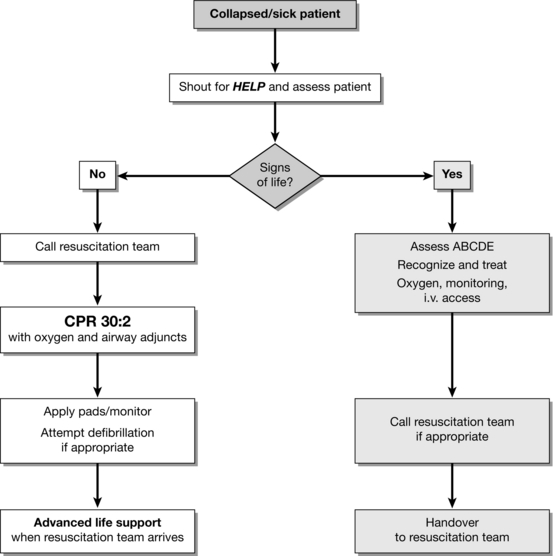
CARDIOPULMONARY RESUSCITATION
The sequence of actions and outcome depends on:
 Location – out-of-hospital versus in-hospital; witnessed versus unwitnessed; monitored versus unmonitored.
Location – out-of-hospital versus in-hospital; witnessed versus unwitnessed; monitored versus unmonitored.
 Skills of the responders – in some public places staff may be trained in CPR and defibrillation. All healthcare professionals should be able to recognize cardiac arrest, call for help, and start resuscitation.
Skills of the responders – in some public places staff may be trained in CPR and defibrillation. All healthcare professionals should be able to recognize cardiac arrest, call for help, and start resuscitation.
 Number of responders – single responders must ensure that help is coming. If other staff are nearby, several actions can be undertaken simultaneously.
Number of responders – single responders must ensure that help is coming. If other staff are nearby, several actions can be undertaken simultaneously.
 Equipment available – AEDs are available in some public places. In hospital, ideally, the equipment used for CPR (including defibrillators) and the layout of equipment and drugs should be standardized throughout the hospital. AEDs should be considered for clinical and non-clinical areas where staff do not have rhythm recognition skills or rarely need to use a defibrillator.
Equipment available – AEDs are available in some public places. In hospital, ideally, the equipment used for CPR (including defibrillators) and the layout of equipment and drugs should be standardized throughout the hospital. AEDs should be considered for clinical and non-clinical areas where staff do not have rhythm recognition skills or rarely need to use a defibrillator.
 Response system to cardiac arrest and medical emergencies – outside hospital the EMS should be summoned. In hospital, the resuscitation team can be a traditional cardiac arrest team (called when cardiac arrest is recognized) or a MET.
Response system to cardiac arrest and medical emergencies – outside hospital the EMS should be summoned. In hospital, the resuscitation team can be a traditional cardiac arrest team (called when cardiac arrest is recognized) or a MET.
ADVANCED LIFE SUPPORT
Arrhythmias associated with cardiac arrest are divided into two groups: shockable rhythms (VF/VT) and non-shockable rhythms (asystole and PEA). The principle difference in management is the need for attempted defibrillation in patients with VF/VT. Subsequent actions, including chest compression, airway management and ventilation, vascular access, injection of adrenaline, and the identification and correction of reversible factors, are common to both groups. The ALS algorithm (Fig. 47.3) provides a standardized approach to the management of adult patients in cardiac arrest.
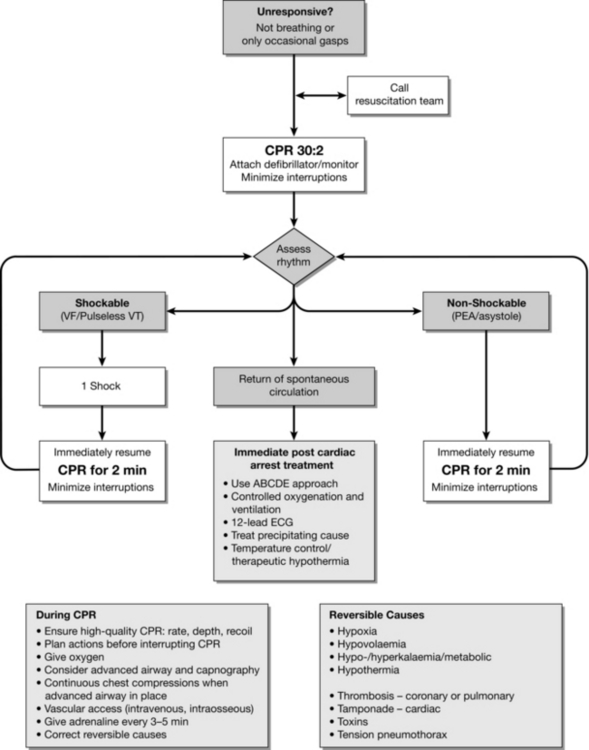
FIGURE 47.3 The advanced life support algorithm (adapted from 2010 Resuscitation Guidelines (Resuscitation Council UK)).
Shockable Rhythms (VF/VT)
Sequence of Actions
 If VF/VT is confirmed, charge the defibrillator while another rescuer continues chest compressions.
If VF/VT is confirmed, charge the defibrillator while another rescuer continues chest compressions.
 Once the defibrillator is charged, pause the chest compressions, quickly ensure that all rescuers are clear of the patient and then give one shock. The person doing compressions, or another rescuer may deliver the shock. This sequence should be planned before stopping compressions.
Once the defibrillator is charged, pause the chest compressions, quickly ensure that all rescuers are clear of the patient and then give one shock. The person doing compressions, or another rescuer may deliver the shock. This sequence should be planned before stopping compressions.
 Resume chest compressions immediately (30:2) without reassessing the rhythm or feeling for a pulse.
Resume chest compressions immediately (30:2) without reassessing the rhythm or feeling for a pulse.
 Continue CPR for 2 min, then pause briefly to check the monitor:
Continue CPR for 2 min, then pause briefly to check the monitor:
 On completion of CPR for 2 min, pause briefly to check the monitor:
On completion of CPR for 2 min, pause briefly to check the monitor:
 If intravascular (i.v./intraosseous) access has been obtained, give adrenaline 1 mg and amiodarone 300 mg once compressions have resumed. On completion of CPR for 2 min, pause briefly to check the monitor:
If intravascular (i.v./intraosseous) access has been obtained, give adrenaline 1 mg and amiodarone 300 mg once compressions have resumed. On completion of CPR for 2 min, pause briefly to check the monitor:
 Give a further (4th) shock; resume CPR immediately and continue for 2 min.
Give a further (4th) shock; resume CPR immediately and continue for 2 min.
 Give adrenaline 1 mg with alternate cycles of CPR (i.e. approximately every 3–5 min).
Give adrenaline 1 mg with alternate cycles of CPR (i.e. approximately every 3–5 min).
 If organized electrical activity is seen during this brief pause in compressions, check for a pulse.
If organized electrical activity is seen during this brief pause in compressions, check for a pulse.
 If a pulse is present, start post-resuscitation care.
If a pulse is present, start post-resuscitation care.
 If no pulse is present, continue CPR and switch to the non-shockable algorithm.
If no pulse is present, continue CPR and switch to the non-shockable algorithm.
 If asystole is seen, continue CPR and switch to the non-shockable algorithm.
If asystole is seen, continue CPR and switch to the non-shockable algorithm.
Defibrillation Strategy
Pads Versus Paddles: Self-adhesive defibrillation pads have practical benefits over hand-held paddles for routine monitoring and defibrillation and are much preferred to standard defibrillation paddles. Use of self-adhesive pads enables the operator to defibrillate the patient from a safe distance and to deliver a shock more rapidly than with paddles.
Safe Use of Oxygen: In an oxygen-enriched atmosphere, sparks from defibrillator paddles applied poorly can cause a fire. The use of self-adhesive defibrillation pads instead of manual paddles reduces the risk of sparks occurring.
 Remove any oxygen mask or nasal cannulae and place them at least 1 m away from the patient’s chest during defibrillation.
Remove any oxygen mask or nasal cannulae and place them at least 1 m away from the patient’s chest during defibrillation.
 Leave the ventilation bag connected to the tracheal tube or other airway adjunct. Alternatively, disconnect the ventilation bag from the tracheal tube and move it at least 1 m from the patient’s chest during defibrillation.
Leave the ventilation bag connected to the tracheal tube or other airway adjunct. Alternatively, disconnect the ventilation bag from the tracheal tube and move it at least 1 m from the patient’s chest during defibrillation.
Single Versus Three-Shock Strategy: If defibrillation is attempted immediately after the onset of VF, it is unlikely that chest compressions will improve the already very high chance of ROSC associated with second or third shocks (i.e. myocardial levels of oxygen and adenosine triphosphate are likely to be adequate for the first minute or so). Thus, if VF/VT occurs during cardiac catheterization or in the early post-operative period after cardiac surgery (when chest compressions could disrupt vascular sutures), consider delivering up to three-stacked shocks before starting chest compressions. This three-shock strategy may also be considered for an initial, witnessed VF/VT cardiac arrest if the patient is already connected to a manual defibrillator – this situation will exist perioperatively if defibrillation pads were applied before induction of anaesthesia.
Defibrillation Energy: All modern defibrillators deliver shocks with a biphasic waveform. The initial biphasic shock should be at least 150 J. Because of the lower efficacy of monophasic defibrillators for terminating VF/VT, the recommended initial energy level for the first shock using a monophasic defibrillator is 360 J. If an initial shock has been unsuccessful, deliver the second and subsequent shocks with a higher energy level if the defibrillator is capable of delivering a higher energy. Manufacturers should display the effective waveform energy range on the face of the biphasic device. If you are unaware of the effective energy range of the device, use 200 J for the first shock.
Non-Shockable Rhythms (PEA and Asystole)
Sequence of Actions for PEA
 Start CPR 30:2 and inject adrenaline 1 mg as soon as intravascular access is achieved.
Start CPR 30:2 and inject adrenaline 1 mg as soon as intravascular access is achieved.
 Continue CPR 30:2 until the airway is secured, then continue chest compressions without pausing during ventilation.
Continue CPR 30:2 until the airway is secured, then continue chest compressions without pausing during ventilation.
 Identify and correct any reversible causes of PEA.
Identify and correct any reversible causes of PEA.
Sequence of Actions for Asystole
 Without stopping CPR, check that the leads are attached correctly.
Without stopping CPR, check that the leads are attached correctly.
 Give adrenaline 1 mg as soon as intravascular access is achieved.
Give adrenaline 1 mg as soon as intravascular access is achieved.
 Continue CPR 30:2 until the airway is secured, then continue chest compression without pausing during ventilation.
Continue CPR 30:2 until the airway is secured, then continue chest compression without pausing during ventilation.
 Identify and correct any reversible causes of asystole.
Identify and correct any reversible causes of asystole.
 Recheck the rhythm after 2 min and proceed accordingly.
Recheck the rhythm after 2 min and proceed accordingly.
During CPR
Potentially Reversible Causes
Potential causes or aggravating factors for which specific treatment exists must be sought during any cardiac arrest (Table 47.1).
TABLE 47.1
Suspect hypothermia in any drowning incident; use a low-reading thermometer.
Use of Ultrasound Imaging During Advanced Life Support: Several studies have examined the use of ultrasound during cardiac arrest to detect potentially reversible causes. This imaging provides information that may help to identify reversible causes of cardiac arrest (e.g. cardiac tamponade, pulmonary embolism, ischaemia (regional wall motion abnormality), aortic dissection, hypovolaemia, pneumothorax). When ultrasound imaging and appropriately trained clinicians are available, use them to assist with assessment and treatment of potentially reversible causes of cardiac arrest. The integration of ultrasound into advanced life support requires considerable training to ensure that interruptions to chest compressions are minimized. A sub-xiphoid probe position has been recommended. Placement of the probe just before chest compressions are paused for a planned rhythm assessment enables a well-trained operator to obtain views within 10 seconds.
Resuscitation in the Operating Room
Cardiac Arrest Caused by Local Anaesthetic
Systemic toxicity of local anaesthetics involves the central nervous system and the cardiovascular system and occurs when a bolus of local anaesthetic inadvertently enters the circulation, usually during regional anaesthesia. Severe agitation, loss of consciousness, with or without convulsions, sinus bradycardia, conduction blocks, asystole and ventricular tachyarrhythmias can all occur. Patients with cardiovascular collapse or cardiac arrest attributable to local anaesthetic toxicity should be treated with i.v. 20% lipid emulsion in addition to standard ALS. Guidelines for treatment with lipid emulsion have been produced by the Association of Anaesthetists of Great Britain and Ireland (http://www.aagbi.org): give an initial i.v. bolus of 20% lipid emulsion followed by an infusion at 15 mL kg− 1 h− 1; give up to three bolus doses of lipid at 5-minute intervals and continue the infusion until the patient is stable or has received up to a maximum of 12 mL kg− 1 of lipid emulsion.
Assisting the Circulation
Drugs
Adrenaline: Despite the widespread use of adrenaline during resuscitation, and several studies involving vasopressin, there is no placebo-controlled study that shows that the routine use of any vasopressor at any stage during human cardiac arrest increases neurologically-intact survival to hospital discharge. A recent prospective, randomized trial of adrenaline versus placebo for out-of-hospital cardiac arrest has documented higher rates of ROSC with adrenaline for both shockable and non-shockable rhythms. The optimal dose of adrenaline is not known, and there are no data supporting the use of repeated doses. There are few data on the pharmacokinetics of adrenaline during CPR. The optimal duration of CPR and number of shocks that should be given before giving drugs is unknown. On the basis of expert consensus, for VF/VT, adrenaline is given after the third shock once chest compressions have resumed, and then repeated every 3–5 min during cardiac arrest (alternate cycles).
Atropine: Several recent studies have failed to demonstrate any benefit from atropine in out-of-hospital or in-hospital cardiac arrests and its routine use for asystole or PEA is no longer recommended.
Anti-Arrhythmic Drugs: No anti-arrhythmic drug given during human cardiac arrest has been shown to increase survival to hospital discharge, although amiodarone has been shown to increase survival to hospital admission after shock-refractory VF/VT. Based on expert consensus, give amiodarone 300 mg by bolus injection (flushed with 20 mL of 0.9% sodium chloride or 5% dextrose) after the third shock. A further dose of 150 mg may be given for recurrent or refractory VF/VT, followed by an infusion of 900 mg over 24 h. Lidocaine 1 mg kg− 1 may be used as an alternative if amiodarone is not available, but do not give lidocaine if amiodarone has been given already.
Bicarbonate: Cardiac arrest causes a combined respiratory and metabolic acidosis because pulmonary gas exchange ceases and cellular metabolism becomes anaerobic. The best treatment of acidaemia in cardiac arrest is chest compression; some additional benefit is gained by ventilation. During cardiac arrest, arterial blood gas values may be misleading and bear little relationship to the tissue acid–base state – analysis of central venous blood may provide a better estimation of tissue pH. Giving sodium bicarbonate routinely during cardiac arrest and CPR, or after ROSC, is not recommended. Give sodium bicarbonate 50 mmol if cardiac arrest is associated with hyperkalaemia or tricyclic antidepressant overdose. Repeat the dose according to the clinical condition of the patient and the results of repeated blood gas analysis.
Calcium: There are no data supporting any beneficial action for calcium after most cases of cardiac arrest. High plasma concentrations achieved after injection may be harmful to the ischaemic myocardium and may impair cerebral recovery. Give calcium during resuscitation only when indicated specifically (cardiac arrest caused by hyperkalaemia, hypocalcaemia, or overdose of calcium channel blocking drugs).
Mechanical CPR
Impedance Threshold Device (ITD): The impedance threshold device (ITD) is a valve that limits air entry into the lungs as the chest recoils during the compression release phase; this decreases intrathoracic pressure and increases venous return to the heart. Recent prospective randomized trials indicate no difference in long term outcome when the ITD is used with standard CPR but one study has documented higher long-term survival rates compared with standard CPR when the ITD is combined with active compression–decompression (ACD) CPR.
Lund University Cardiac Arrest System (LUCAS) CPR: The Lund University cardiac arrest system (LUCAS) is a gas-driven sternal compression device that incorporates a suction cup for ACD-CPR. There are no published randomized human studies comparing LUCAS-CPR with standard CPR although there are two large ongoing prehospital studies.
Load-Distributing Band CPR (AutoPulse): The load-distributing band (LDB) is a circumferential chest compression device comprising a pneumatically actuated constricting band and backboard. A recent large prehospital randomized trial has shown that LDB-CPR produces similar survival rates to that of high-quality manual CPR.
PERI-ARREST ARRHYTHMIAS
Cardiac arrhythmias are common in the peri-arrest period and treatment algorithms for both tachycardia (Fig. 47.4) and bradycardia (Fig. 47.5) have been developed to enable the non-specialist to initiate treatment safely. In all cases, oxygen is given, an i.v. cannula is inserted and the patient is assessed for adverse signs. Whenever possible, record a 12-lead ECG; this will help determine the precise rhythm, either before treatment or retrospectively, if necessary with the help of an expert. Correct any electrolyte abnormalities.
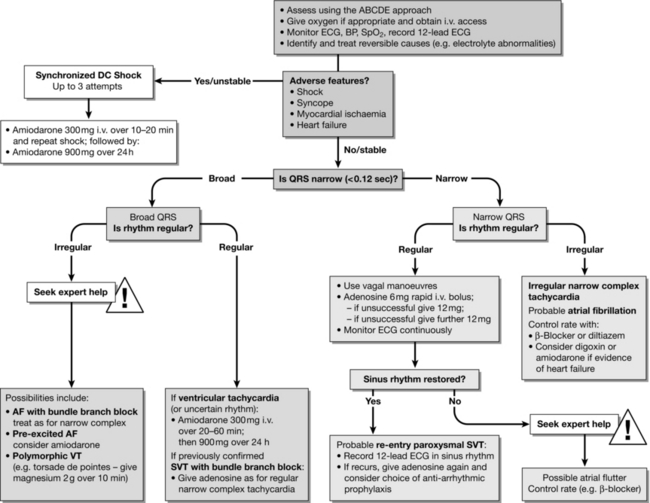
FIGURE 47.4 Tachycardia algorithm (adapted from 2010 Resuscitation Guidelines (Resuscitation Council UK)).
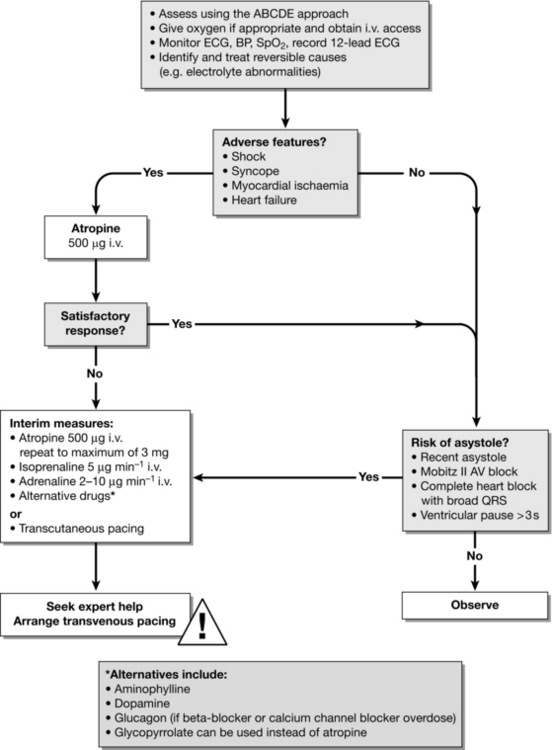
FIGURE 47.5 Bradycardia algorithm (adapted from 2010 Resuscitation Guidelines (Resuscitation Council UK)).
 Shock – pallor, sweating, cold, clammy extremities, impaired consciousness and hypotension (e.g. systolic blood pressure < 90 mmHg).
Shock – pallor, sweating, cold, clammy extremities, impaired consciousness and hypotension (e.g. systolic blood pressure < 90 mmHg).
 Syncope – transient loss of consciousness.
Syncope – transient loss of consciousness.
 Heart failure – pulmonary oedema; 3rd heart sound; raised JVP; hepatic congestion; peripheral oedema.
Heart failure – pulmonary oedema; 3rd heart sound; raised JVP; hepatic congestion; peripheral oedema.
 Myocardial ischaemia – chest pain or evidence of ischaemia on 12-lead ECG.
Myocardial ischaemia – chest pain or evidence of ischaemia on 12-lead ECG.
Tachycardias
The treatment for stable tachycardias is outlined in Figure 47.4. A regular broad-complex tachycardia is likely to be VT or a supraventricular rhythm with bundle branch block. In a stable patient, if there is uncertainty about the source of the arrhythmia, give adenosine.
Regular narrow-complex tachycardias include:
 AV nodal re-entry tachycardia (AVNRT) – the commonest type of regular narrow-complex tachyarrhythmia;
AV nodal re-entry tachycardia (AVNRT) – the commonest type of regular narrow-complex tachyarrhythmia;
 AV re-entry tachycardia (AVRT) – due to Wolff–Parkinson–White (WPW) syndrome;
AV re-entry tachycardia (AVRT) – due to Wolff–Parkinson–White (WPW) syndrome;
POST-RESUSCITATION CARE
Disability (Optimizing Neurological Recovery)
Temperature Control
Treatment of Hyperpyrexia: A period of hyperthermia (hyperpyrexia) is common in the first 48 h after cardiac arrest and this is associated with worse neurological outcome. Treat hyperthermia occurring after cardiac arrest with antipyretics or active cooling.
Therapeutic Hypothermia: Animal and human data indicate that mild hypothermia is neuroprotective and improves outcome after a period of global cerebral hypoxia-ischaemia. Cooling suppresses many of the pathways leading to delayed cell death, including apoptosis (programmed cell death). Hypothermia decreases the cerebral metabolic rate for oxygen by about 6% for each 1 °C reduction in temperature and this may reduce the release of excitatory amino acids and free radicals.
DECISIONS RELATING TO CARDIOPULMONARY RESUSCITATION
 Decisions about CPR must be made on the basis of an individual assessment of each patient’s case.
Decisions about CPR must be made on the basis of an individual assessment of each patient’s case.
 Advance care planning, including making decisions about CPR, is an important part of good clinical care for those at risk of cardiorespiratory arrest.
Advance care planning, including making decisions about CPR, is an important part of good clinical care for those at risk of cardiorespiratory arrest.
 Communication and the provision of information are essential parts of good quality care.
Communication and the provision of information are essential parts of good quality care.
 It is not necessary to initiate discussion about CPR with a patient if there is no reason to believe that the patient is likely to suffer a cardiorespiratory arrest.
It is not necessary to initiate discussion about CPR with a patient if there is no reason to believe that the patient is likely to suffer a cardiorespiratory arrest.
 Where no explicit decision has been made in advance there should be an initial presumption in favour of CPR.
Where no explicit decision has been made in advance there should be an initial presumption in favour of CPR.
 If CPR would not restart the heart and breathing, it should not be attempted.
If CPR would not restart the heart and breathing, it should not be attempted.
 Where the expected benefit of attempted CPR may be outweighed by the burdens, the patient’s informed views are of paramount importance. If the patient lacks capacity, those close to the patient should be involved in discussions to explore the patient’s wishes, feelings, beliefs and values.
Where the expected benefit of attempted CPR may be outweighed by the burdens, the patient’s informed views are of paramount importance. If the patient lacks capacity, those close to the patient should be involved in discussions to explore the patient’s wishes, feelings, beliefs and values.
 If a patient with capacity refuses CPR, or a patient lacking capacity has a valid and applicable advance decision refusing CPR, this should be respected.
If a patient with capacity refuses CPR, or a patient lacking capacity has a valid and applicable advance decision refusing CPR, this should be respected.
 A DNAR decision does not override clinical judgement in the unlikely event of a reversible cause of the patient’s respiratory or cardiac arrest that does not match the circumstances envisaged.
A DNAR decision does not override clinical judgement in the unlikely event of a reversible cause of the patient’s respiratory or cardiac arrest that does not match the circumstances envisaged.
 DNAR decisions apply only to CPR and not to any other aspects of treatment.
DNAR decisions apply only to CPR and not to any other aspects of treatment.
ACKNOWLEDGEMENT
Much of this chapter has been adapted, with permission, from the 2010 Resuscitation Council (UK) Guidelines (www.resus.org.uk).
Association of Anaesthetists of Great Britain & Ireland, Do not attempt resuscitation (DNAR) decisions in the perioperative period. Available at http://www.aagbi.org/sites/default/files/dnar_09_0.pdf
British Medical Association, Resuscitation Council (UK) and Royal College of Nursing. Decisions relating to cardiopulmonary resuscitation, 2007. www.resus.org.uk
Deakin, C.D., Morrison, L.J., Morley, P.T., et al. Advanced life support chapter collaborators. Part 8. Advanced life support. International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(Suppl. 1):e93–e174.
Deakin, C.D., Nolan, J.P., Soar, J., et al. European resuscitation council guidelines for resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81:1305–1352.
General Medical Council Treatment and care towards the end of life: good practice in decision making. General Medical Council: London, 2010. isbn:978-0-901458-46-9, www.gmc-uk.org.
Nolan, J., Soar, J., Lockey, A., et al, Advanced life support, sixth ed. Resuscitation Council UK: London, 2011. isbn:978-1-903812-22-8.
Nolan, J.P. Resuscitation guidelines, 2010. Resuscitation Council (UK): London, 2010. isbn:978-1-903812-21-1, www.resus.org.uk.
Nolan, J.P., Hazinski, M.F., Billi, J.E., et al. Part 1. Executive summary. International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(Suppl. 1):e1–e25.
Nolan, J.P., Neumar, R.W., Adrie, C., et al. Post–cardiac arrest syndrome. Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Resuscitation. 2008;79:350–379.
Nolan, J.P., Soar, J., Zideman, D.A., et al. Guidelines writing group. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81:1219–1276.