CHAPTER 42 Results and Complications
The results of revision shoulder arthroplasty are as variable as the indications for which it is performed. In general, outcomes after revision shoulder arthroplasty are less satisfactory than those after primary shoulder arthroplasty. Because of the paucity of results of revision shoulder arthroplasty reported in the literature, this chapter reports the results of revision shoulder arthroplasty by drawing from our own experience. Additionally, the most frequent complications and their treatment are outlined.
RESULTS
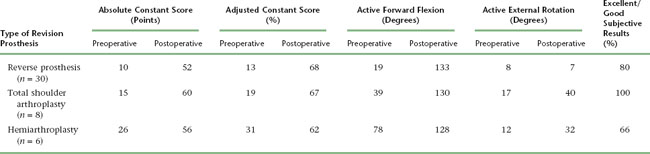
The results of revision shoulder arthroplasty are hard to examine because of the diversity of indications for which revision is performed. A simple indication such as converting a hemiarthroplasty to a total shoulder arthroplasty for symptomatic glenoid erosion would logically yield a better outcome than would implantation of a revision shoulder arthroplasty for a chronic infection after multiple irrigation and débridement sessions. Unfortunately, the relative rarity of revision shoulder arthroplasty prevents definitive conclusions regarding outcomes. Table 42-1 details the results of revision shoulder arthroplasty from our prospective database initiated in 2003. This table expresses the results in terms of active mobility; patient satisfaction; the Constant score, a shoulder-specific outcomes device incorporating pain, mobility, activity, and strength; and the age- and gender-adjusted Constant score.1,2
INTRAOPERATIVE COMPLICATIONS
Humerus
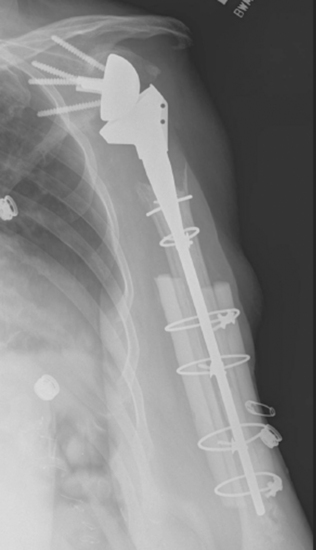
Intraoperative complications involving the humerus are common. The most frequent humeral complication is iatrogenic fracture, which usually occurs during an overly aggressive dislocation maneuver without previous adequate soft tissue release or during extraction of a well-fixed humeral stem. Patients with osteopenia and those with severe preoperative stiffness are most at risk for this complication. These fractures may occur at the humeral diaphysis or proximally and involve the tuberosities. Fractures involving the diaphysis should be reduced and a long-stem humeral implant placed. Allograft struts and cerclage cables may be added in patients with severe osteopenia (Fig. 42-1).
Neurovascular Structures
Catastrophic injury to the neurovascular structures around the shoulder is rare during revision shoulder arthroplasty. The neural structures most at risk during revision shoulder arthroplasty are the axillary and musculocutaneous nerves. If a humeral osteotomy is performed or if revision surgery is performed for a periprosthetic fracture, the radial nerve is also at risk. Nerve injury during revision shoulder arthroplasty can occur as a neuropraxic stretch injury or as a transection injury. Neuropraxic injury caused by stretch most commonly involves the axillary nerve but can involve any nerves within the brachial plexus. Care should be taken when positioning the patient to maintain the cervical spine in neutral alignment to avoid a stretch injury to the brachial plexus. When treating a periprosthetic fracture with revision arthroplasty or when a humeral osteotomy is anticipated for extraction of the humeral stem, the radial nerve should be carefully exposed to ensure its protection. Careful exposure of the radial nerve often results in transient neuropraxia. Patient education preoperatively is of paramount importance in dealing with neuropraxia inasmuch as patients are much more accepting if they have heard about the possibility of this complication before surgery. Axillary and radial nerve neuropraxia is treated by observation, with most patients recovering by 3 to 4 months postoperatively.
POSTOPERATIVE COMPLICATIONS
Glenoid Problems
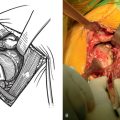
Glenoid component failure after revision to unconstrained total shoulder arthroplasty can occur as a result of loosening of the glenoid component from the host bone or mechanical breakage of the glenoid implant (Fig. 42-2). Glenoid component problems, when symptomatic, generally require further revision surgery.
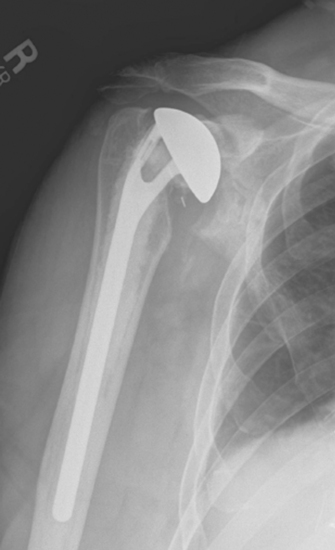
Figure 42-2 Unconstrained glenoid component loosening occurring 3 years after revision shoulder arthroplasty.
Glenoid component failure after revision to reverse shoulder arthroplasty, as with primary reverse shoulder arthroplasty, has been associated in all cases of which we are aware with initial placement of the glenoid component in a superiorly oriented direction, implantation of the prosthesis in the presence of an intraoperative glenoid fracture, or insertion of the glenoid component with the central post anchored only in grafted bone. These complications are best avoided. As with primary reverse shoulder arthroplasty, we implant the reverse prosthesis for revision arthroplasty through a deltopectoral approach to avoid inadvertent placement of the glenoid component in a superiorly oriented position, which can occur with use of the superior lateral approach. If an intraoperative glenoid fracture occurs, we treat it as described previously in this chapter. When glenoid failure occurs, further revision surgery consisting of glenoid reconstruction and conversion to a hemiarthroplasty is required.
Humeral Problems
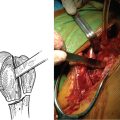
Loosening of a reverse humeral stem after revision surgery is uncommon. The predominant risk factor for aseptic loosening of a revision reverse humeral stem is proximal humeral bone loss (Fig. 42-3). Whenever loosening of a revision reverse humeral stem occurs, infection must be ruled out, as with loosening of an unconstrained revision humeral stem (see later). In the rare instance of symptomatic aseptic loosening of a revision reverse humeral component, treatment is further revision of the humeral stem, usually combined with allograft reconstruction of the proximal humerus to provide osseous support of the proximal portion of the revision stem.
Mechanical problems of a revision reverse humeral component are exceedingly rare and related to the polyethylene liner. Incomplete seating of the polyethylene component at the revision arthroplasty can be responsible for dissociation of the polyethylene liner from the humeral stem. In this scenario, further revision surgery with replacement of the polyethylene liner is indicated. Polyethylene wear occurs medially on the rim of the polyethylene liner in many patients, as seen at the time of retrieval during revision surgery. It occurs as a result of scapular notching, as discussed later.
Periprosthetic humeral fractures after revision arthroplasty are more common than loosening of the humeral component and are almost always the result of a fall or similar low-energy trauma. The majority of these fractures occur just distal to the tip of the humeral stem, and most can be treated nonoperatively. Nonoperative treatment consists of fracture bracing, activity modification, pain medication, and frequent radiographic monitoring. If the fracture has not healed within 3 months, we will incorporate the use of an external bone stimulator (OL 1000 Bone Growth Stimulator, Donjoy Orthopedics, Vista, CA). Despite these measures, periprosthetic humeral fractures treated nonoperatively may take longer than 9 months to heal.3 Our criteria for recommending operative treatment of periprosthetic fractures include complete displacement, angulation greater than 30 degrees, loosening of the humeral component, or failure of nonoperative treatment.
Instability
Instability after Revision Surgery with a Reverse Shoulder Prosthesis
Instability after revision surgery with a reverse shoulder prosthesis is twice as common as that observed in the primary reverse arthroplasty scenario. Dislocations after revision arthroplasty with a reverse prosthesis occur early (within 6 weeks of revision surgery). Instability of a reverse prosthesis can be related to various factors. In revision cases, proximal humeral bone loss seems to be the greatest risk factor for dislocation of a reverse prosthesis. In this scenario, deltoid muscle tension is often solely responsible for the stability of the implant because no rotator cuff or joint capsule exists to provide stability. Even if the deltoid is properly tensioned initially, it can gradually lose its tension and result in dislocation. A second major risk factor for dislocation of a reverse prosthesis is subscapularis insufficiency, a common condition encountered when performing revision shoulder arthroplasty with a reverse prosthesis. A less common factor contributing to dislocation of a reverse prosthesis is mechanical impingement causing the prosthetic socket to be levered away from the glenoid component. This impingement usually occurs inferiorly as the arm is adducted and is often related to too superior positioning of the glenoid component on the glenoid face, as detailed schematically in Chapter 32. Finally, in the revision situation, the integrity and function of the axillary nerve should be ensured before performing revision arthroplasty with a reverse prosthesis because this neural deficit can result in prosthetic dislocation.
In most cases, as with dislocation of a primary reverse prosthesis, treatment initially consists of closed reduction and a period of bracing. Closed reduction is performed in the operating room with the patient either heavily sedated or under general anesthesia. An attempt is made to reduce the dislocation under fluoroscopic guidance. If the prosthesis is successfully reduced, fluoroscopic examination is performed to ensure that mechanical impingement is not responsible for the instability. If the problem is not related to mechanical impingement and the prosthesis is successfully reduced, a brace is applied to maintain the arm with the humeral component centered on the glenoid component, usually in about 90 degrees of abduction and 30 degrees of forward flexion (Fig. 42-4). The patient maintains this brace at all times for 6 weeks, and radiographs are taken in the brace every 7 to 10 days to confirm that the prosthesis has remained located (Fig. 42-5). After 6 weeks the brace is discontinued and a normal rehabilitation regimen ensues.
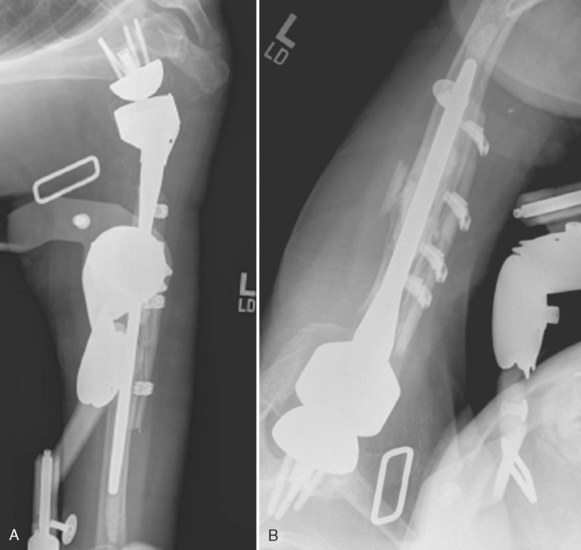
Figure 42-5 A and B, Radiographs obtained in the brace confirming maintenance of prosthetic reduction.
If the prosthesis is not reducible by closed means, mechanical impingement causing dislocation exists, or closed reduction plus bracing has failed, open reduction with insertion of a thicker polyethylene spacer or metallic augment (or both) is performed (Fig. 42-6). Any mechanical impingement can simultaneously be addressed by careful removal of bone at the lateral aspect of the scapula just inferior to the glenoid component, if necessary. Postoperatively, the patient is treated with the same bracing protocol used after closed reduction of a dislocated reverse prosthesis.
Stiffness
Glenohumeral stiffness after revision shoulder arthroplasty is related to capsular contracture or the prosthesis, or both. It is much more commonly observed when using an unconstrained revision implant than when using a reverse revision implant. Prosthetic problems resulting in stiffness are generally the result of implantation of too large an unconstrained humeral component or malpositioning of the revision component (Fig. 42-7). Rehabilitation with capsular stretching can be attempted in an effort to improve mobility. If this fails (no improvement over a 6-month period), revision surgery is indicated and consists of downsizing of the humeral head with open release of any capsular contractures that are present.
Stiffness related to capsular contracture almost always responds to nonoperative management involving aquatic-based rehabilitation (see Chapter 43). If the patient shows no improvement in mobility over a 6-month course of rehabilitation and has no obvious prosthetic problem, we will consider the patient a candidate for arthroscopic capsular contracture release if an unconstrained revision implant was used. We have no experience dealing with capsular contracture in a patient who has undergone revision shoulder arthroplasty with a reverse prosthesis.
Acromial Problems
Occasionally, acromial stress fractures are seen after revision reverse shoulder arthroplasty, just as with primary reverse shoulder arthroplasty. These fractures result from deltoid tension applied to osteopenic bone. Frequently, these fractures exist preoperatively as a result of chronic superior migration of the humeral head with persistent acromiohumeral articulation (Fig. 42-8). Postoperatively, deltoid tension may cause the fracture fragment to tilt inferiorly. Despite these radiographic findings, no specific treatment is necessary for this complication. Additionally, patients obtain satisfactory results even with this radiographic finding.
Scapular Notching
Though debatable whether it should be considered a complication, notching of the scapula occurs within 2 years of surgery in half the patients who have undergone reverse shoulder arthroplasty, both in the primary and in the revision situation (Fig. 42-9). This radiographic finding most likely occurs as a result of mechanical impingement of the medial aspect of the humeral component and the lateral aspect of the scapula just inferior to the glenoid. As the patient internally rotates the shoulder, the impingement is exacerbated. This mechanical impingement theory is further supported by the observation that patients’ internal rotation seems to improve as the scapular notch progresses, thus suggesting that the prosthesis must “carve out” a portion of the scapula to maximize postoperative internal rotation. Another theory of the cause of scapular notching is polyethylene wear causing osteolysis, although this theory currently has less support than the mechanical impingement theory.
Scapular notching is commonly accompanied by a scapular osteophyte just medial to the notch. The degree of scapular notching has also been graded by severity.4 The best way to avoid scapular notching seems to be by initially placing the glenoid component inferiorly on the glenoid face and by introducing slight inferior tilt during glenoid reaming, as discussed in Chapter 29. Despite the concerning appearance of this scapular notch, its clinical implications are unclear, with most of the evidence suggesting no adverse consequence. As long as the glenoid component remains stable, no treatment of an asymptomatic scapular notch is indicated.
1 Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160-164.
2 Constant CR. Assessment of shoulder function. In: Gazielly D, Gleyze P, Thomas T, editors. The Cuff. New York: Elsevier; 1997:39-44.
3 Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86:680-689.
4 Valenti P, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: Long term results (>5 years). In: Walch G, Boileau P, Molé D, editors. 2000 Prosthèses d’Epaule … Recul de 2 à 10 Ans. Paris: Sauramps Medical; 2001:253-259.