Chapter 13 Restenosis and in-stent restenosis
 Percutaneous coronary intervention (PCI) has become the dominant revascularization modality for obstructive coronary artery disease while restenosis is the ‘Achilles heel’ of balloon angioplasty.
Percutaneous coronary intervention (PCI) has become the dominant revascularization modality for obstructive coronary artery disease while restenosis is the ‘Achilles heel’ of balloon angioplasty. Restenosis occurs at a rate of 40–50% angiographically and 20–30% clinically following balloon angioplasty.
Restenosis occurs at a rate of 40–50% angiographically and 20–30% clinically following balloon angioplasty. The use of bare metal stents (BMS) reduced the incidence of acute vessel closure and restenosis substantially.
The use of bare metal stents (BMS) reduced the incidence of acute vessel closure and restenosis substantially.PATHOPHYSIOLOGY
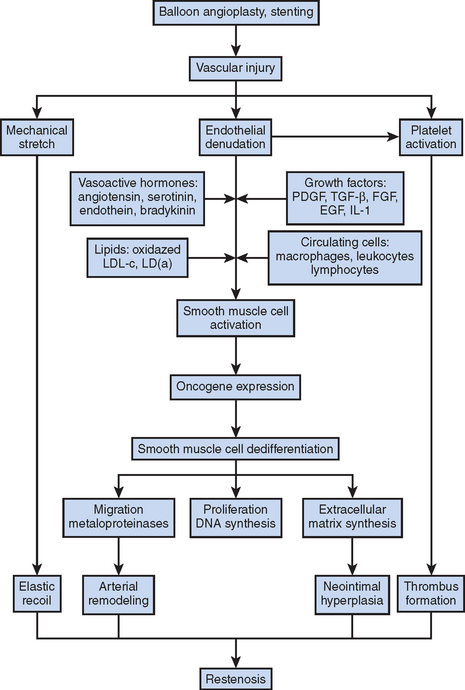
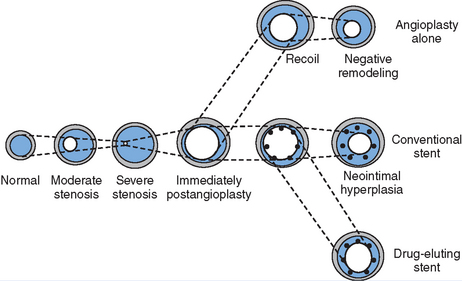
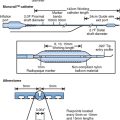
Figure 13.1 Mechanisms of restenosis following PCI.
Source: Chan AW and Moliterno DJ, Clinical Evaluation of Restenosis. In Atherothrombosis and Coronary Artery Disease, edited by V. Fuster, EJ Topol, and EG Nabel, p. 1416 (Figure 93.2).
DEFINITIONS OF RESTENOSIS
Angiographic restenosis
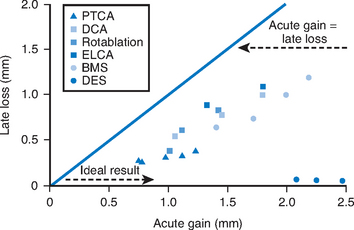
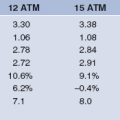
Various definitions for angiographic restenosis have been used. 50% or greater dichotomous diameter stenosis at follow-up angiography is a widely used criterion in clinical trials. However, reporting of the minimum luminal diameter (MLD) may avoid variation of restenosis rates reported in clinical trials due to inconsistent definitions of angiographic restenosis. The difference between pre-procedural MLD and immediate post-procedural MLD is defined as acute gain, and the difference between post-procedural MLD and MLD at follow-up is termed to late loss. Acute gain is lowest with PTCA, followed by atherectomy, and stenting (highest acute gain). Late loss is usually proportional to acute gain, such that the late loss index, defined as late loss divided by the acute gain, is similar after revascularization with PTCA, atherectomy, or stents (Fig. 13.3).
Clinical restenosis
Since angiographic definitions for restenosis are somewhat arbitrary, the importance of restenosis becomes clinically relevant only when it is linked to symptom(s) or functional parameter(s). Because the process of restenosis is usually gradual, and the newly formed lesion is ‘stabilized’ with fibrous tissue and smooth muscle cells, recurrent angina is the most common presenting feature, whereas myocardial infarction (MI) and sudden death are less frequent. Chest pain is reportedly present in 25–93% of patients six months following balloon angioplasty, with an average of approximately 50%.2 The proportion of patients presenting with recurrent chest pain who have restenosis demonstrable at angiography ranges from 48–92%. Hence, the positive predictive value (PPV) of symptoms alone is only modest.
Clinical restenosis is roughly correlated with the need for repeat revascularization (repeat PCI or bypass surgery). Traditionally, target lesion revascularization (TLR) has been used in many of the interventional trials designed to test various pharmacology, while target vessel revascularization (TVR) has been used in device trials in which the effect of the device on the vessel beyond the target lesions also needs to be considered. More recently, the term target vessel failure (TVF) has also become popular, and it refers to TVR plus death or myocardial infarction due to restenosis or reocclusion of the target vessel.
PREDICTORS FOR RESTENOSIS
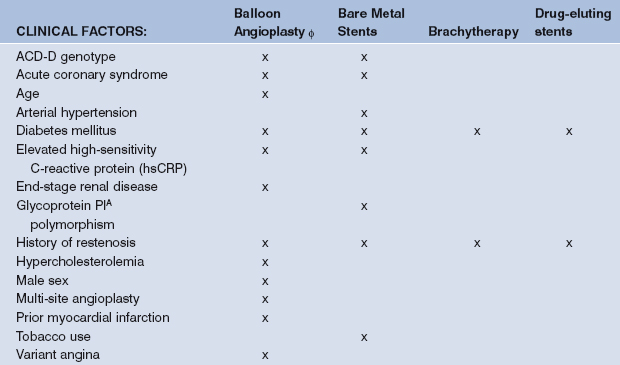
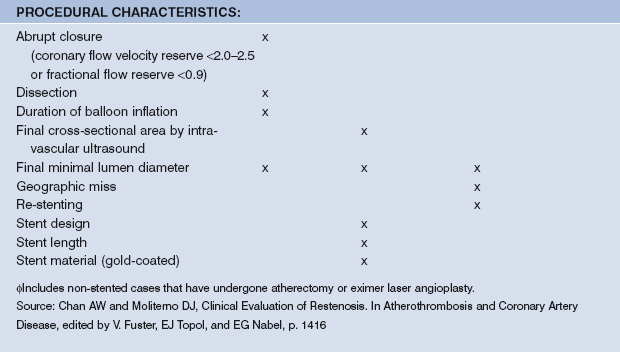
Many identifiable restenosis predictors were derived from large pharmacological trials related to PCI. Predictors for restenosis after balloon angioplasty, stenting, and brachytherapy modestly differ. Risk factors can be categorized into those related to the patient, the lesion, and the procedure, as summarized in Table 13.1.
PRESENTATION AND EVALUATION OF RESTENOSIS
Regardless of the PCI device used, most patients who have clinical restenosis experience return of angina. As mentioned, myocardial infarction or death due to restenosis per se is unusual.3 Atypical chest pain or pain different from the patient’s angina is unlikely to be secondary to restenosis. Similarly, early angina (that is, within the first couple months after PCI) is usually related to incomplete revascularization, whereas angina presenting much beyond nine months is usually due to lesions other than the index lesion.3 The incidence of silent restenosis is variable since the definition of angiographic restenosis is somewhat arbitrary. Absence of any symptom may be related to less severe coronary obstruction, diabetes, non-viable myocardium, or abundant collateralization. The importance of silent restenosis in clinical practice remains questionable, as the available data are limited and inconsistent.
Recurrent angina within nine months of PCI is an ACC/AHA Class I indication for repeat coronary angiography.4 In asymptomatic patients or those with atypical symptoms following PCI, use of non-invasive tests is the recommended evaluation method. A stress test (exercise or pharmacological) with nuclear scintigraphy or echocardiography carries relatively less risk, cost, and time (compared with routine coronary angiography), and it provides physiologic assessment of the restenotic lesion.5 When stress testing is believed to be unreliable and the myocardium at risk is large, angiography is preferable.
PREVENTION OF RESTENOSIS
While pharmacological agents failed to reduce restenosis following PTCA, the use of coronary BMS resulted in significant reductions in both acute vessel closure and restenosis (when compared to PTCA), and the use of DES has further reduced the incidence of angiographic and clinical restenosis to less than 10%.
TREATMENT OF RESTENOSIS
PTCA
Balloon angioplasty as a treatment for restenosis is associated with a particularly high rate (40–50%) of recurrent restenosis. In a multicenter randomized restenosis stent study, 383 patients with clinical and angiographic restenosis following prior balloon angioplasty were randomized to repeat PTCA versus Palmaz-Schatz stent placement. In this study of non-complete lesions, repeat PTCA was associated with a 32% rate of angiographic restenosis at six months.6 With ISR, repeat PTCA is effective when used for management of focal lesions with angiographic restenosis rates as low as 20% at six month.7–8 For more diffuse restenotic lesions, however, repeat PTCA is associated with a six-month angiographic restenosis rate >50%.9
Atherectomy
Several small case series documented the safety of rotational atherectomy to treat restenosis. In a non-randomized registry of 304 patients, the combined use of atherectomy and balloon angioplasty was associated with a reduced rate of one-year clinical events (death, MI, TLR) when compared with balloon angioplasty or atherectomy alone (38% vs. 52% vs. 60%, respectively).11 However, in the multicenter ARTIST trial, 298 patients with ISR were randomly assigned to PTCA or rotational atherectomy; atherectomy was associated with a higher rate of angiographic restenosis (65% vs. 51%) and incidence of composite clinical events (death, MI, TLR) (20% vs. 9%), primarily due to a greater rate of repeat revascularization at six months.12 Similarly, the use of excimer laser and rotational atherectomy in treating in-stent restenotic lesions has been disappointing (one-year TLR rates of 26% and 28%, respectively). These debulking devices cause a greater late lumen loss, so-called device taxing, implying that remodeling or neointimal proliferation are not favorably reduced.13
Stenting
Bare metal stents
By reducing elastic recoil and vascular remodeling, and by compressing the plaque and scaffolding the vessel lumen, BMS has resulted in a significant reduction in acute vessel closure and restenosis. ISR secondary to neointimal hyperplasia remains a significant problem with BMS, however, with 20–30% angiographic and 10–15% clinical restenosis.14,15
Drug-eluting stents
Sirolimus (rapamune) is a macrocyclic lactone with antibiotic, immunosuppressive, and anti-proliferative actions. Biologically it induces cell-cycle arrest and affects proliferation and migration of smooth muscle cells. In the RAVEL trial, which was the first randomized controlled trial of sirolimus-eluting stents (SES) versus BMS, SES reduced ISR at six months by more than 50% when compared to BMS (0 vs. 27%)16, and at one-year follow up, the overall rate of cardiac events (mostly TVR) was lower with SES (6% vs. 29%).
Paclitaxel interferes with function of the microtubules responsible for proper chromosome segregation during cell division. In the TAXUS II trial, 536 low-risk patients were randomly assigned to a BMS or a paclitaxel-eluting stent (PES).17 At six months, PES were associated with significant reductions in ISR (3.5% vs. 19.1%) and TLR (3.9% vs. 13.3%). In a meta-analysis of all randomized clinical trials of sirolimus-versus paclitaxel-eluting stents the rate of angiographic restenosis in patients with SES was 9.3% and the rate of TLR was 5.1%. The rate of angiographic restenosis in patients with PES was 13.1% and the rate of TLR was 7.8%.18
TREATMENT OF ISR
Brachytherapy
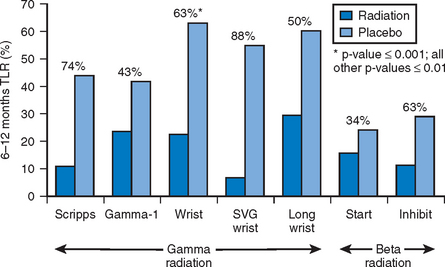
Gamma and beta radiotherapy have been studied for treatment of ISR. During spontaneous decay of nuclei of certain elements, radiation is emitted in the form of either gamma radiation (electromagnetic energy carried by photons which may penetrate more than 10 mm of human tissue) or beta particles (electrons carrying a wide range of energy and traveling within 2–3 mm of human tissue). By affecting DNA in the actively dividing cells, brachytherapy inhibits smooth muscle proliferation and neointima formation. Use of brachytherapy in management of restenosis and ISR has been evaluated in multiple clinical trials and registries, with both gamma (SCRIPPS, GAMMA-1, WRIST, SVG WRIST, and LONG WRIST) and beta (BETA WRIST, START, and INHIBIT) radiation. The effect of brachytherapy is not always consistent or curative. While infrequent, both thrombosis (perhaps due to inadequate re-endothelialization) and restenosis (possibly from paradoxical neointimal stimulation of an adjacent vessel segment) have been reported following brachytherapy. Overall, brachytherapy has reduced the rate of recurrent restenosis by about 40–50%. Figure 13.4 summarizes the results of the noted effects of beta and gamma radiation on TLR.
DES for ISR
Use of DES in the management of ISR was evaluated in small observational studies that suggested benefit. These were soon followed by the ISAR-DESIRE trial, which randomized 300 patients with ISR to treatment with SES, PES or PTCA.19 DES resulted in a significant reduction (≥50%) in the primary end point of angiographic restenosis at six months (14.3% SES, 21.7% PES, and 44.6% PTCA). TVR rate was reduced significantly as well (8% SES, 19% PES, and 33% PTCA).
When restenosis occurs with DES, it may often be a consequence of local vessel injury during the procedure, polymer peeling, non-homogeneous drug elution, gaps in stent coverage, or inadequate stent expansion. In addition to suboptimal stent placement technique, Table 13.2 lists the variables that have been associated with ISR in sirolimus-eluting stents.20
TABLE 13.2 TVR PREDICTORS AMONG 1,726 PATIENTS FROM THE GERMAN CYPHER REGISTRY
| VARIABLES | ADJUSTED OR (95% CI) | P-VALUE |
|---|---|---|
| Target vessel coronary bypass graft | 2.43 (1.41–4.18) | 0.001 |
| 2- or 3-vessel disease | 1.69 (1.05–2.72) | 0.030 |
| Hypertension | 1.66 (0.99–2.79) | 0.056 |
| Additional implantation of BMS | 1.49 (0.71–3.13) | 0.230 |
| Renal insufficiency | 1.41 (0.80–2.48) | 0.230 |
| Indication of in-stent restenosis | 1.40 (0.94–2.07) | 0.095 |
| Chronic total occlusion | 1.39 (0.73–2.68) | 0.320 |
| Ostial lesion | 1.28 (0.79–2.07) | 0.320 |
| Diabetes mellitus | 1.14 (0.76–1.71) | 0.530 |
| Total length of SES (per 10 mm) | 1.07 (0.90–1.26) | 0.450 |
Source: Zahn R, et al. American Journal of Cardiology 2005; 95:1302-8.
CABG for ISR
Limited data exist regarding the use of CABG in the treatment of ISR. In a retrospective analysis of 510 patients with documented first occurrence of ISR, patients who were managed with CABG (n = 117) had a reduced rate of TVR (8%) and major adverse cardiovascular events (23%) at a mean follow up of 19 months, when compared to PCI.21 This study, however, was done before the widespread use of DES. Further investigations are needed to compare the use of CABG versus multivessel PCI with DES in the management of ISR.
1 Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of the human atherosclerotic coronary arteries. NEJM. 1987;316:1371-1375.
2 Simonton CA, Mark DB, Hinohara T, et al. Late restenosis after emergent coronary angioplasty for acute myocardial infarction: comparison with elective coronary angioplasty. J AM Coll Cardiol. 1988;11:698-705.
3 Holmes DR, Vlietstra RE, Smith HC, et al. Restenosis after percutaneous transluminal coronary angioplasty: a report from the PTCA registry of the national Heart, Lung and Blood institute. Am J Cardiol. 1984;53:77c-81c.
4 Ryan TJ, Antman EM, Brooks NH, et al. 1999 Update: ACC/AHA guidelines for the management of patients with acute myocardial infarction: executive summary and recommendations. Circulation. 1999;100:1016-1030.
5 Takeuchi M, Miura Y, Toyokawa T, et al. The comparative diagnostic value of dobutamine stress echocardiography and thallium stress tomography for detecting restenosis after coronary angioplasty. J Am Soc Echocardiography. 1995;8:696-702.
6 Erbel R, Haude M, Hopp HW, et al. Coronary-artery stenting compared with balloon angioplasty for restenosis after initial balloon angioplasty. Restenosis Stent Study Group. N Engl J Med. 1998 Dec 3;339(23):1672-1678.
7 Bauters C, Banos JL, Van Belle E, et al. Six-month angiographic outcome after successful repeat percutaneous intervention for in-stent restenosis. Circulation. 1998;97:318-321.
8 Reimers B, Moussa I, Akiyama T, et al. Long term clinical follow up after successful repeat percutaneous intervention for stent restenosis. J Am Coll Cardiol. 1997;30:186-192.
9 Eltchaninoff H, Koning R, Tron C, et al. Balloon angioplasty for the treatment of coronary in-stent restenosis: Immediate results and 6-month angiographic recurrent restenosis rate. J Am Coll Cardiol. 1998;32:980-984.
10 Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002 Jun 6;346(23):1773-1780.
11 Goldberg SL, Berger P, Cohen DJ, et al. Rotational atherectomy or balloon angioplasty in the treatment of intra-stent restenosis: BARASTER multicenter registry. Cathet Cardiovasc Intervent. 2000;51:407-413.
12 Vom Dahl J, Dietz V, Silber S, et al. Rotational atherectomy does no reduce recurrent in-stent restenosis: results of the angioplasty versus rotational atherectomy for the treatment of diffuse in-stent restenosis trail (ARTIST). Circulation. 2002;105:583-588.
13 Mehran R, Dangas G, Mintz G, et al. Treatment of in-stent restenosis with excimer laser coronary angioplasty versus rotational atherectomy: Comparative mechanisms and results. Circulation. 2000;101:2484-2489.
14 Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994 Aug 25;331(8):496-501.
15 Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994 Aug 25;331(8):489-495.
16 Serruys PW, Degertekin M, Tanabe K, et al. Intravascular ultrasound findings in the multicenter, randomized, double-blind RAVEL (RAndomized study with the sirolimus-eluting VElocity balloon-expandable stent in the treatment of patients with de novo native coronary artery Lesions) trial. Circulation. 2002 Aug 13;106(7):798-803.
17 Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003 Aug 19;108(7):788-794.
18 Kastrati A, Dibra A, Dipl Stat S, et al. Sirolimus-eluting stents vs. Paclitaxel-eluting stents in patients with coronary artery disease. A meta-analysis of randomized trials. JAMA. 2005 Aug 17;294(7):819-825.
19 Kastrati A, Mehilli J, von Beckerath N, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005 Jan 12;293(2):165-171.
20 Zahn R, Hamm CW, Scheinder S. Incidence and predictors of target vessel revascularization and clinical event rates of the sirolimus-eluting coronary stent (results from the prospective multicenter German Cypher Stent Registry). Am J of Cardiology. 2005 Jun 1;95(11):1302-1308.
21 Moustapha A, Assali AR, Sdringola S, et al. Percutaneous and surgical interventions for in-stent restenosis: long-term outcomes and effect of diabetes mellitus. J Am Coll Cardiol. 2001 Jun 1;37(7):1877-1882.