13 RESPIRATORY SYSTEM
NASAL CAVITIES AND PARANASAL SINUSES
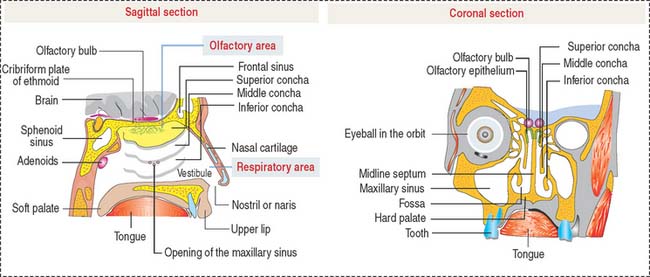
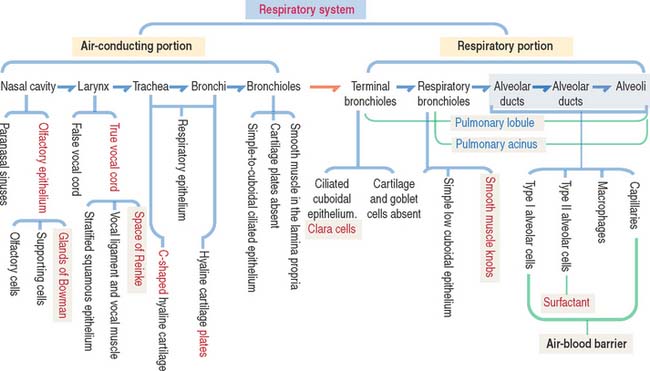
Each nasal cavity, separated from the other by the septum, consists of the vestibule, the respiratory portion, and the olfactory area (Figure 13-1).
The respiratory portion is lined by a pseudostratified ciliated epithelium with goblet cells supported by the lamina propria, which consists of connective tissue with seromucous glands. The lamina propria has a rich superficial venous plexus, known as cavernous or erectile tissue. The lamina propria is continuous with the periosteum or perichondrium of bone or cartilage, respectively, forming the wall of the nasal cavities.
Nasopharynx
The auditory tubes (eustachian tubes), extending from the middle ear, open into the lateral walls of the nasopharynx.
Olfactory epithelium
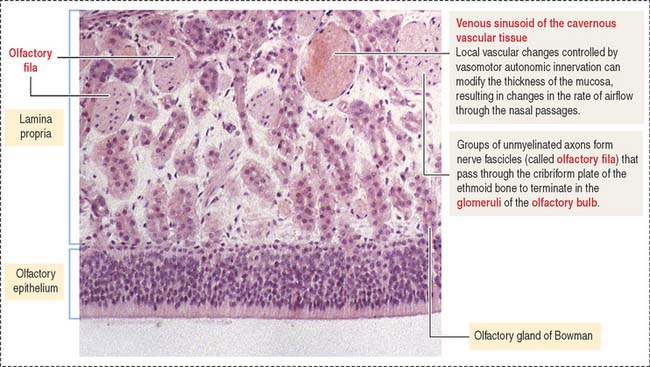
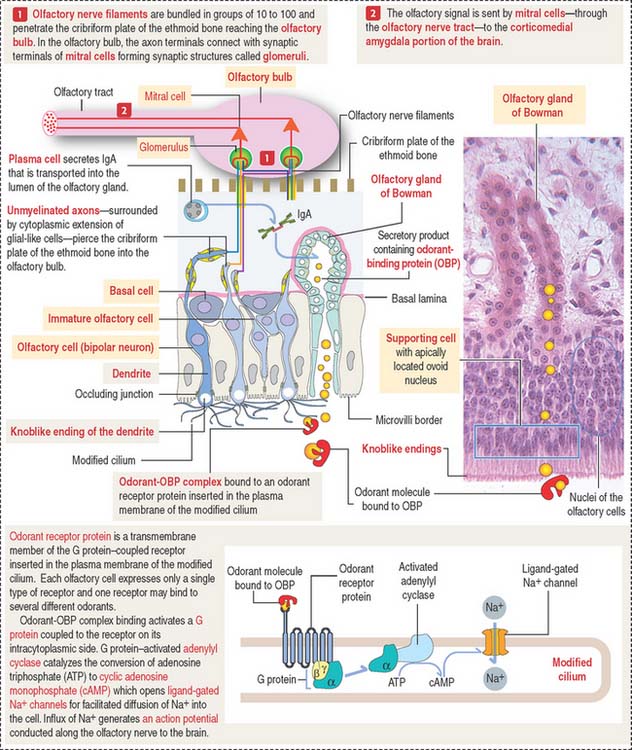
The olfactory epithelium contains three major types of cells (Figures 13-2 and 13-3): (1) basal cells, (2) olfactory cells (bipolar neurons), and (3) supporting or sustentacular cells.
The olfactory cell is highly polarized (see Figure 13-3). The apical region, facing the surface of the mucosa, forms a knoblike ending (called olfactory vesicle or olfactory knob) with 10 to 20 modified cilia. The basal region gives rise to an axon. Several axons, projecting from the olfactory cells, form small unmyelinated bundles (called olfactory fila; from Latin filum, thread) surounded by glial-like cells. Nerve bundles cross the cribriform plate of the ethmoid bone and contact in the glomerulus dendrites of mitral cells, neurons of the olfactory bulb, to establish appropriate synaptic connections (see Box 13-A).
Box 13-A Olfactory epithelium
LARYNX
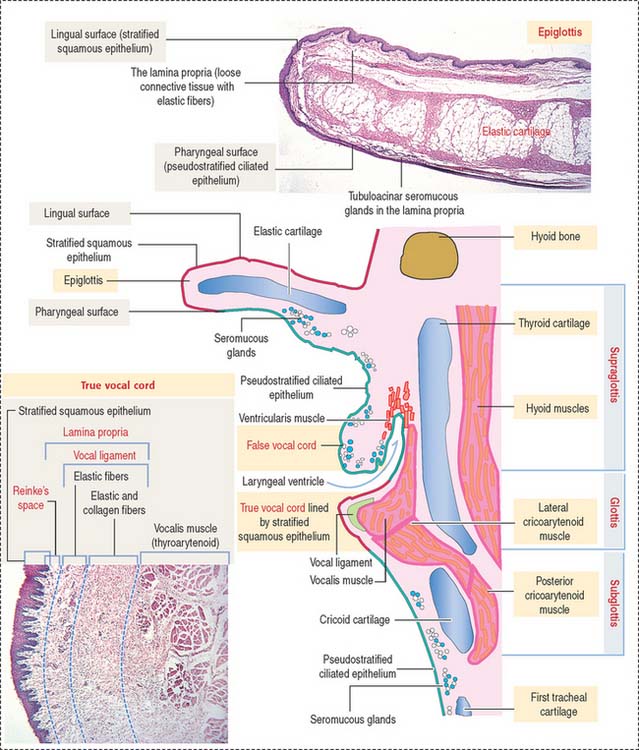
The wall of the larynx is made up of the thyroid and cricoid hyaline cartilage and the elastic cartilage core of the epiglottis extending over the lumen (Figure 13-4).
The larynx can be subdivided into three regions:
During forced inspiration, vocal cords are abducted, and the space between the vocal cords widens.
During phonation, the vocal cords are adducted and the space between the vocal cords changes into a linear slit. The vibration of the free edges of the cords (a cover consisting of both the stratified squamous epithelial covering and the superficial layer of the lamina propria, known as Reinke’s space) during passage of air between them produces sound. The contraction of the intrinsic muscles of the larynx, forming the body of the cords, increases tension on the vocal cords, changing the pitch of the produced sound (see Box 13-B).
Box 13-B True vocal cords or folds
Laryngeal seromucous glands are found throughout the lamina propria, except at the level of the true vocal cords. The lamina propria of the true vocal cords consists of three layers (see Figure 13-4): (1) a superficial layer containing extracellular matrix and few elastic fibers. This layer is known as Reinke’s space; (2) an intermediate layer with an increased content of elastic fibers; and (3) a deep layer with abundant elastic and collagen fibers.
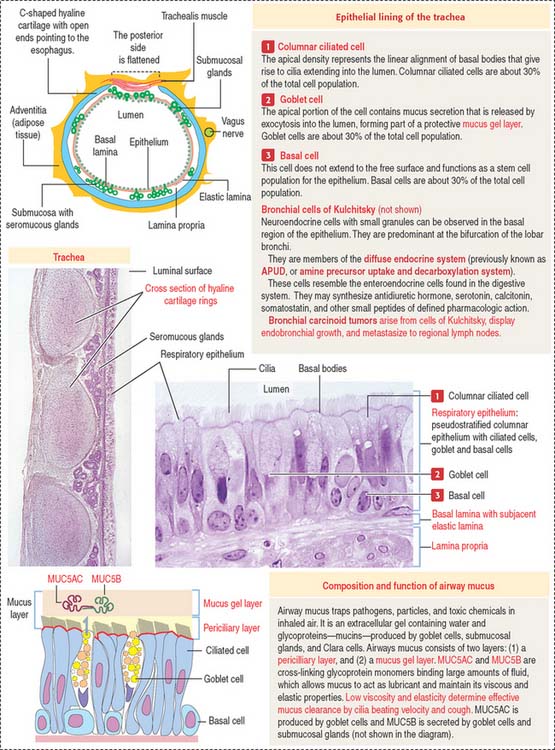
TRACHEA
The right lung has three lobes, whereas the left lung has two lobes.
The trachea and main bronchi are lined by pseudostratified columnar ciliated epithelium resting on a distinct basal lamina. Several types of cells can be identified (Figure 13-5):
The lamina propria contains elastic fibers. The submucosa displays mucous and serous glands that, together with goblet cells, produce components of the airways mucus (see Box 13-C).
Box 13-C Airway mucus
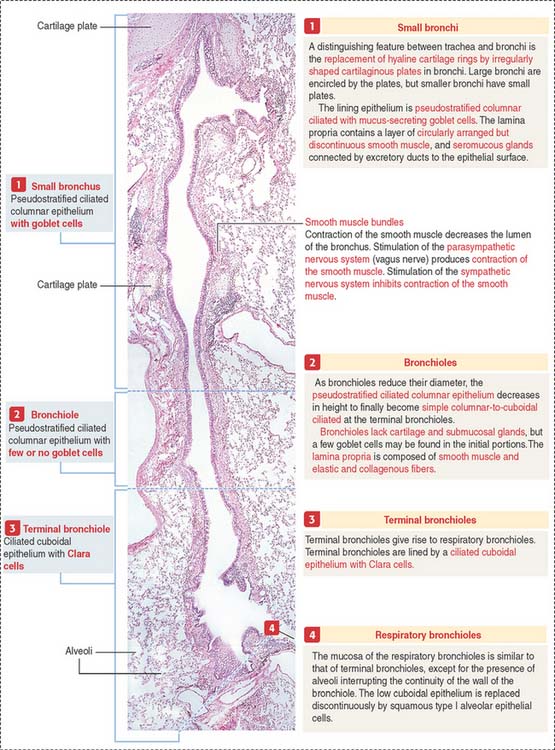
The framework of the trachea and extrapulmonary bronchi consists of a stack of C-shaped hyaline cartilages, each surrounded by a fibroelastic layer blending with the perichondrium. In the trachea and primary bronchi, the open ends of the cartilage rings point posteriorly to the esophagus. The lowest tracheal cartilage is the carinal cartilage. Transverse fibers of the trachealis muscle attach to the inner ends of the cartilage. In branching bronchi, cartilage rings (see Figure 13-5) are replaced by irregularly shaped cartilage plates (Figure 13-6), surrounded by smooth muscle bundles in a spiral arrangement.
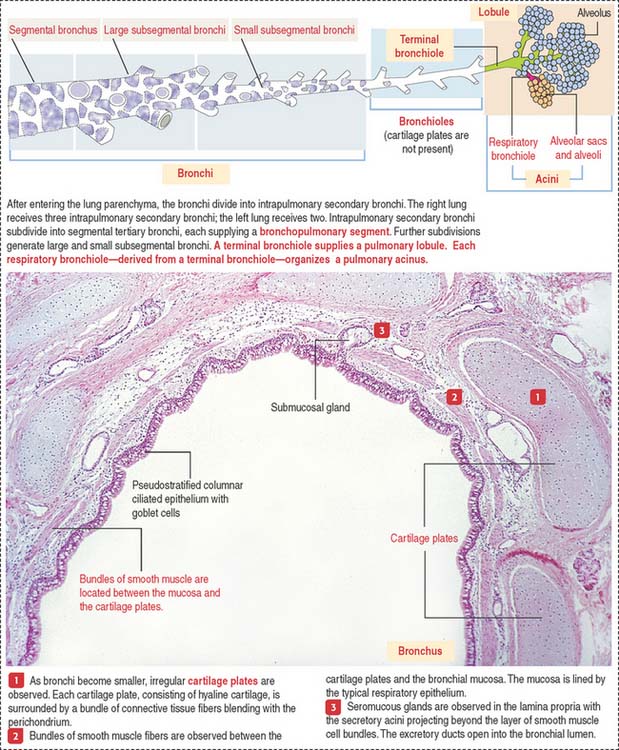
INTRAPULMONARY SEGMENTATION OF THE BRONCHIAL TREE
Within the pulmonary parenchyma, a segmental bronchus gives rise to large and small subsegmental bronchi. A small subsegmental bronchus is continuous with a bronchiole. This transition involves the loss of cartilage plates in the bronchiole and a progressive increase in the number of elastic fibers.
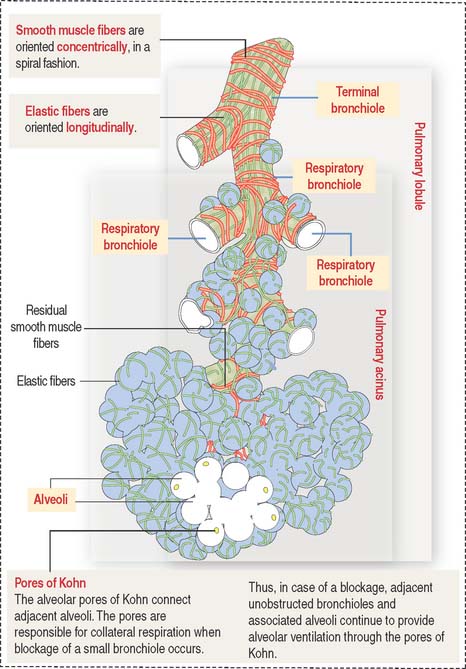
The intrapulmonary segmentation results in the organization of a pulmonary lobule and a pulmonary acinus (Figure 13-7; see also Figure 13-6).
Pulmonary lobule and pulmonary acinus
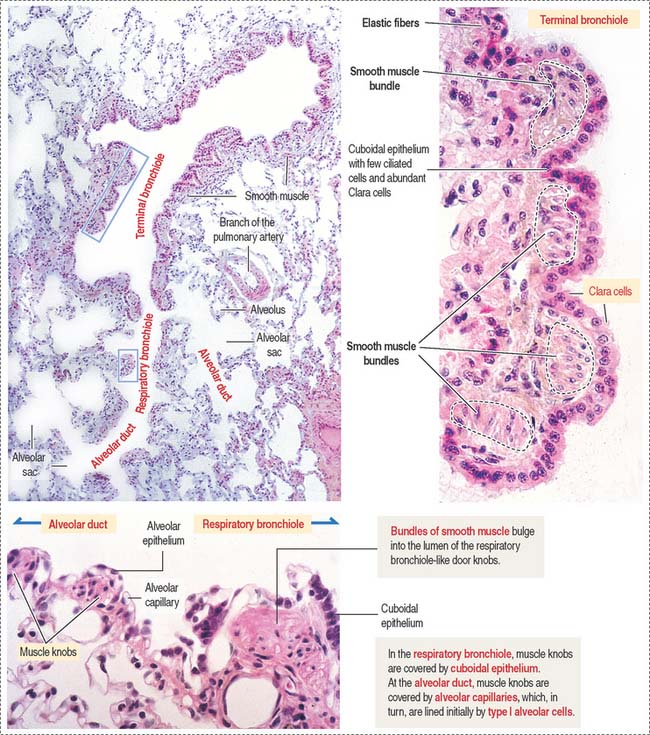
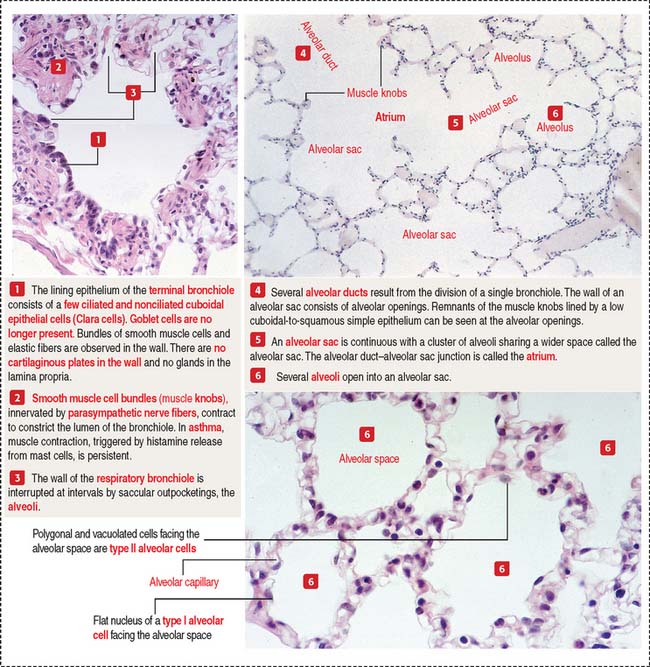
A terminal bronchiole and the associated region of pulmonary tissues that it supplies constitute a pulmonary lobule (Figure 13-8). A pulmonary lobule includes the respiratory bronchioles, alveolar ducts, alveolar sacs, and alveoli.
Distal to the respiratory bronchiole is the alveolar duct. The alveolar duct is characterized by an interrupted wall with typical smooth muscle knobs bulging into the lumen (Figure 13-9).
Clinical significance: Emphysema
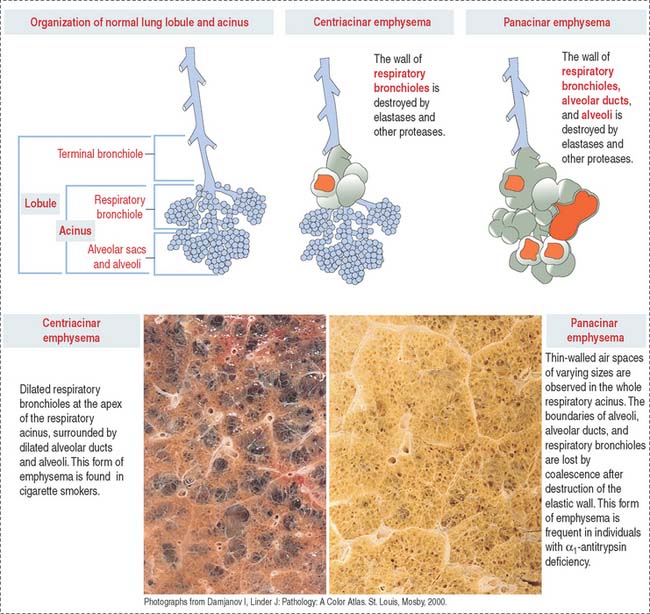
COPD occurs in the peripheral airways—the bronchioles—and lung parenchyma. Elastic fibers are important components of bronchioles and alveolar walls. A loss of elasticity and breakdown of elastic fibers give rise to emphysema, characterized by chronic airflow obstruction. As a result, adjacent alveoli become confluent, creating large air spaces, or blebs (Figure 13-10).
Let us review the concepts of the pulmonary lobule and the acinus to understand the types of emphysema. Figures 13-6 and 13-8 show that a pulmonary lobule includes the terminal bronchiole and the first to third generations of derived respiratory bronchioles. Each respiratory bronchiole gives rise to alveolar ducts and alveoli, an arrangement known as the acinus—so called because aggregates of alveoli cluster like acini in connection with the ductlike respiratory bronchiole. Because a pulmonary lobule generates several respiratory bronchioles, each resolved into an acinus, a pulmonary lobule is made up of several acini.
In panacinar (or panlobular) emphysema, blebs are observed from the respiratory bronchiole down to the alveolar sacs. This type of emphysema is more common in patients with a deficiency in the α1–antitrypsin gene encoding a serum protein.
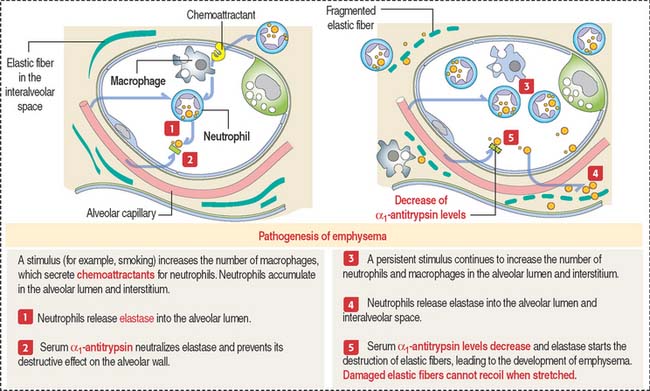
Protein α1-antitrypsin is a major inhibitor of proteases, in particular elastase, secreted by neutrophils during inflammation (Figure 13-11). Under the influence of a stimulus, such as cigarette smoke, macrophages in the alveolar wall and alveolar lumen secrete proteases and chemoattractants (mainly leukotriene B4) to recruit neutrophils.
Clinical significance: Asthma
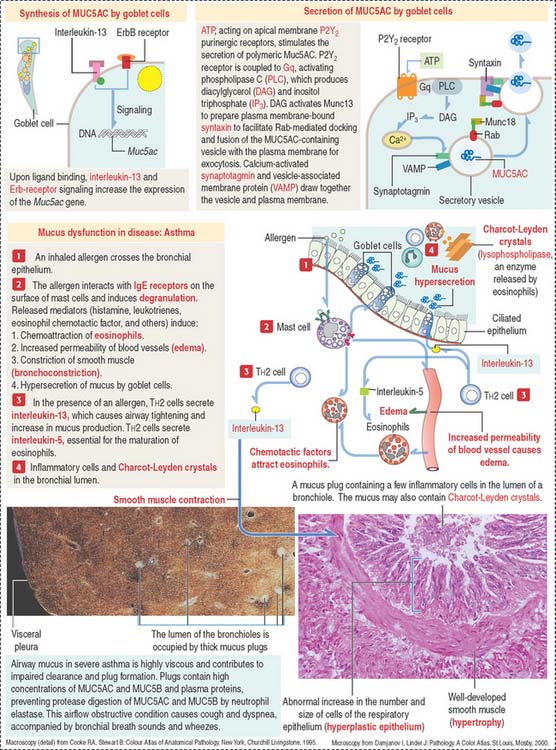
Asthma is characterized by airway hyperresponsiveness, defined by three salient features (Figure 13-12): (1) airway wall inflammation involving neutrophils, T cells (CD8+), and macrophages. Asthma is characterized by the recruitment of T cells (CD4+) and eosinophils (see Figure 13-12); (2) luminal obstruction of airways by mucus, caused by hypersecretion of bronchial mucous glands, along with infiltration by inflammatory cells; and (3) vasodilation of the bronchial microvasculature with increased vascular permeability and edema.
Asthma can be triggered by repeated antigen exposure (allergic asthma) or by an abnormal autonomic neural regulation of airway function (nonallergic asthma).
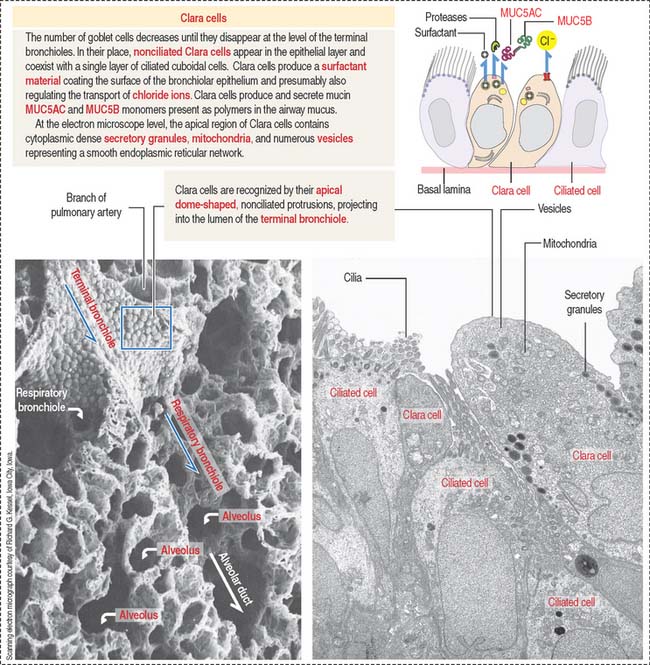
Nonciliated Clara cells
Clara cells are epithelial cells with a dome-shaped apical domain lacking cilia. They represent 80% of the epithelial cell population of the terminal bronchiole (Figure 13-13). Clara cells secrete surfactant that differs from that produced by type II alveolar cells. After airway injury, Clara cells can proliferate and migrate to replenish alveolar epithelial cells. This process is known as alveolar bronchiolization More recently, Clara cells have been associated with Cl− release mediated by a chloride channel regulated by a cyclic guanosine monophosphate (cGMP)– guanylyl cyclase C mechanism.
Clinical significance: Cystic fibrosis
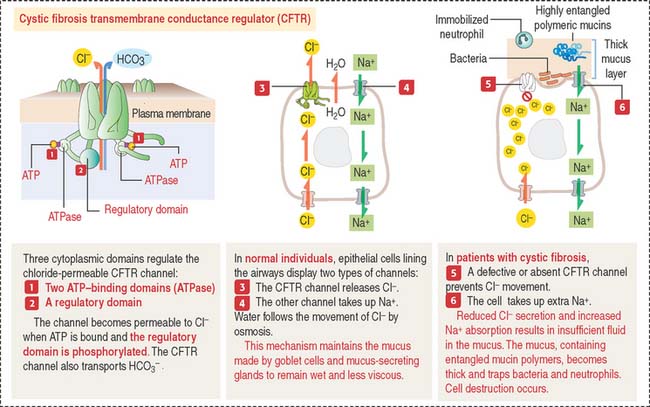
Cystic fibrosis is a recessive genetic disease affecting children and young adults. Cystic fibrosis is caused by mutations in the gene encoding cystic fibrosis transmembrane conductance regulator (CFTR), which results in reduced chloride secretion, increased sodium absorption, and insufficient airway luminal fluid (Figure 13-14).
In most patients, the blockage of pancreatic ducts by mucus causes pancreatic dysfunction. Pancreatic ductules release a bicarbonate-rich fluid under regulation of secretin. Secretin is produced by enteroendocrine cells in response to acidic gastric contents entering the duodenum (see Chapter 17, Digestive Glands).
In the skin, the excessive presence of salt secretion by sweat glands is diagnostic of cystic fibrosis (see Chapter 11, Integumentary System).
The cystic fibrosis gene encodes the protein CFTR, belonging to the ABC transporter family—so called because it contains adenosine triphosphate (ATP)– binding domains, or ATP-binding cassettes, and requires ATP hydrolysis to transport ions, sugars, and amino acids. In 70% of patients with cystic fibrosis, the amino acid 508—of a total of 1480 amino acids in CFTR protein—is missing.
RESPIRATORY PORTION OF THE LUNG
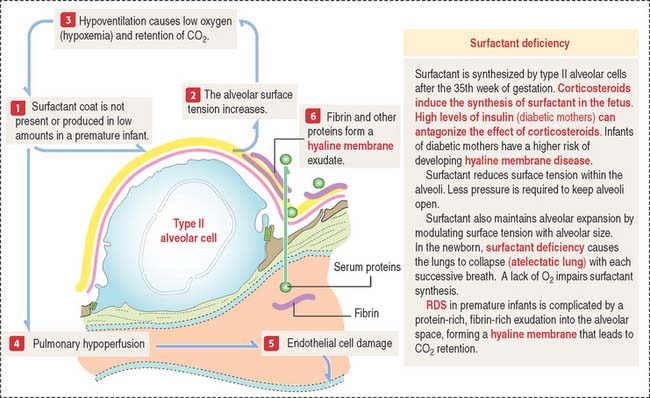
Respiratory bronchioles represent the transition from the conducting to the respiratory portion of the lung (Figure 13-15). They are lined initially by simple cuboidal epithelial cells, some of which are ciliated. The epithelium becomes low cuboidal and nonciliated in subsequent branches. The respiratory bronchiole subdivides to give rise to an alveolar duct (see Figure 13-15). The alveolar duct is continuous with the alveolar sac. Several alveoli open into an alveolar sac.
The alveolus is the functional unit of the pulmonary acinus
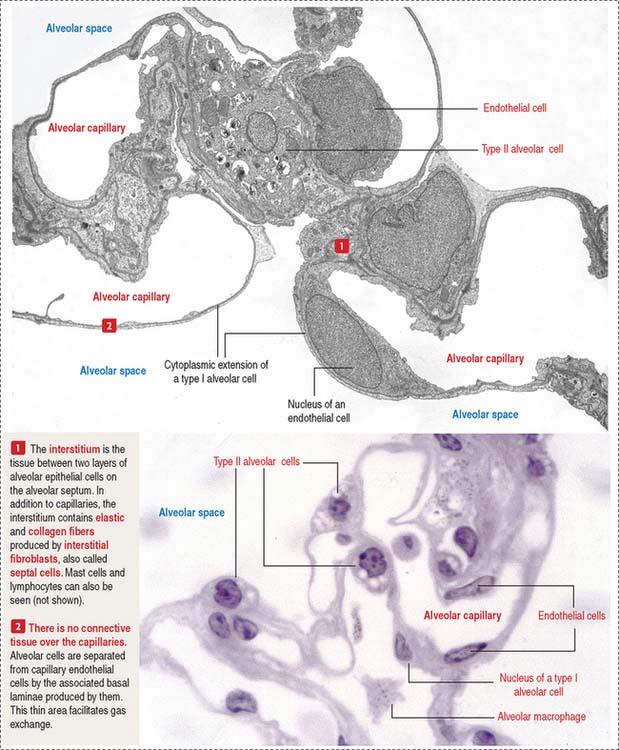
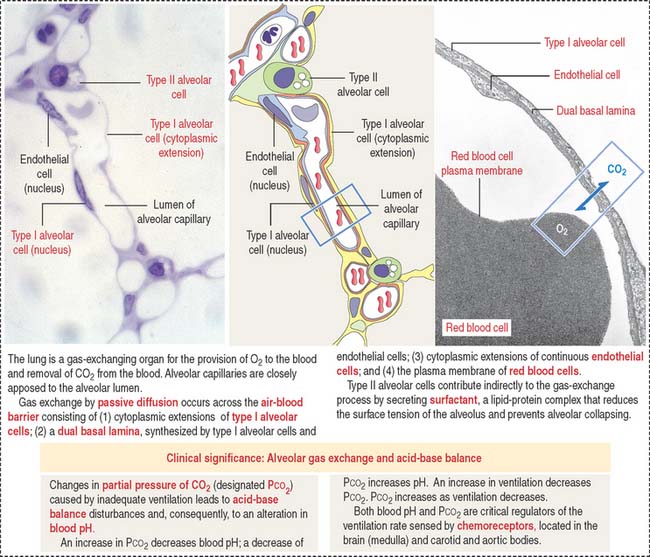
About 300 million air sacs, or alveoli, in each lung provide a total surface area of 75 m2 for oxygen and carbon dioxide exchange. Each alveolus has a thin wall with capillaries lined by simple squamous epithelial cells (Figure 13-16) forming part of the air-blood barrier (Figure 13-17).
The alveolar epithelium consists of two cell types (see Figures 13-16 and 13-17): (1) type I alveolar cells, representing about 40% of the epithelial cell population but lining 90% of the alveolar surface; and (2) type II alveolar cells, approximately 60% of the cells, covering only 10% of the alveolar surface area.
Each alveolus opens into an alveolar sac. However, a few of them open directly into the respiratory bronchiole (see Figure 13-15). This particular feature distinguishes the respiratory bronchiole from the terminal bronchiole, whose wall is not associated with alveoli.
The low cuboidal epithelium of the respiratory bronchiole is continuous with the squamous type I alveolar cells of the alveolus (see Figure 13-9).
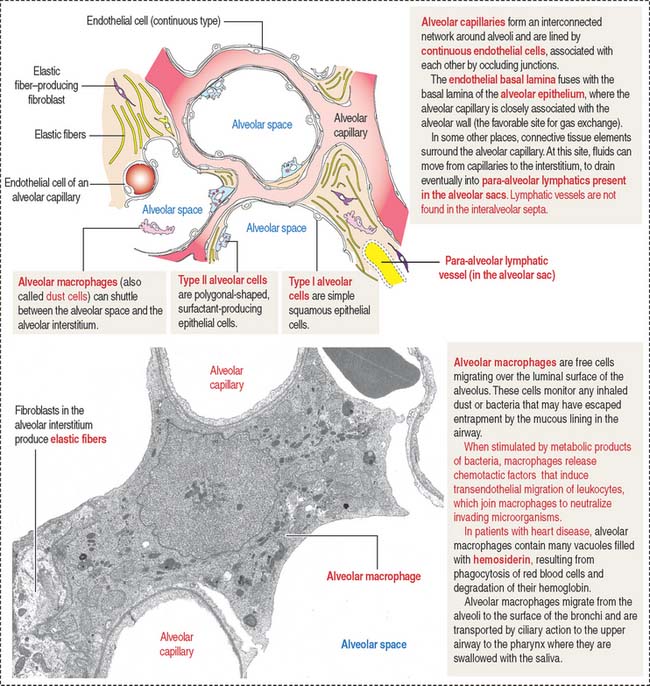
Additional cells of the alveolar septa are the alveolar macrophages (Figure 13-18) (also called dust cells; they are derived from bone-marrow monocytes and frequently seen in the alveolar lumen), fibroblasts (producing elastic fibers), and mast cells.
Alveolar capillaries are lined by continuous endothelial cells juxtaposed to type I alveolar cells through a dual basal lamina produced by these two cells. Alveolar endothelial cells contain angiotensin-converting enzyme for the conversion of angiotensin I to angiotensin II (see Figure 14-18 in Chapter 14, Urinary System).
Type II alveolar cells
Type II alveolar cells are predominantly located at the angles formed by adjacent alveolar septa. Contrasting with the more squamous type I alveolar cells, type II alveolar cells are polygonal-shaped, vacuolated and extend beyond the level of the surrounding epithelium.
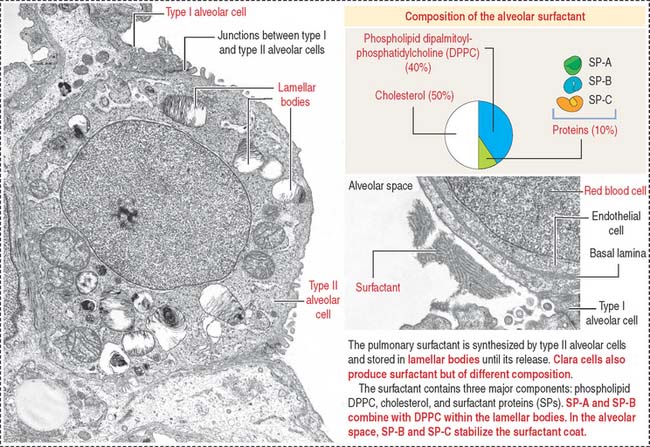
The free surface of type II alveolar cells is covered by short microvilli. The cytoplasm displays dense membrane-bound lamellar bodies, representing secretory granules containing pulmonary surfactant (Figure 13-19).
The pulmonary surfactant contains (1) phospholipids, (2) cholesterol, and (3) proteins (see Figure 13-19).
In the alveolar space, SP-B and SP-C stabilize the phospholipid layer and enhance the surfactant action of the phospholipid DPPC–protein complex (Figure 13-20).
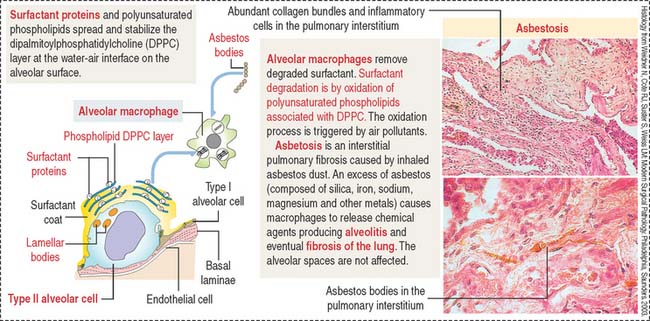
Surfactant turnover is facilitated by the phagocytic function of alveolar macrophages (see Figures 13-18 and 13-20). Macrophages can also take up inhaled asbestos and trigger interstitial pulmonary fibrosis, asbestosis, characterized by extensive deposition of collagen and asbestos bodies (asbestos fibers coated by iron particles, see Figure 13-20). The alveolar spaces are generally uninvolved but type II alveolar cells increase in number (hyperplasia),
An additional function of type II alveolar cells is the maintenance and repair of the alveolar epithelium when injury occurs. When type I alveolar cells are damaged, type II alveolar cells increase in number and differentiate into type I alveolar-like cells (see Figure 13-20). As already discussed, Clara cells also have a reparative function during injury of the alveolar epithelium (alveolar bronchiolization).
Clinical significance: Acute respiratory distress syndrome
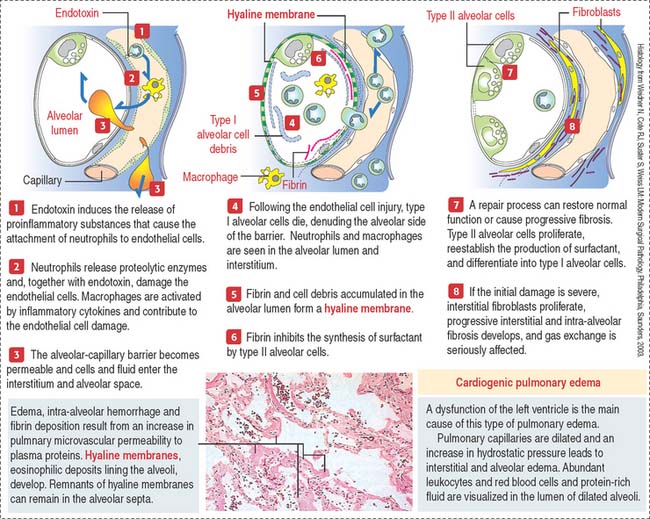
A common pathologic pattern of diffuse alveolar damage (Figure 13-21) can be observed in cardiogenic and noncardiogenic ARDS. The first phase of ARDS is an acute exudative process defined by interstitial and alveolar edema, neutrophil infiltration, hemorrhage, and deposits of fibrin. Cellular debris, resulting from dead type I alveolar cells, and fibrin deposited in the alveolar space form hyaline membranes (Figure 13-22).
The second phase is a proliferative process in which alveolar cells proliferate and differentiate to restore the epithelial alveolar lining, returning gas exchange to normal in most cases. In other cases, the interstitium displays inflammatory cells and fibroblasts. Fibroblasts proliferate and invade the alveolar spaces through gaps of the basal lamina. The hyaline membranes either are removed by phagocytosis by macrophages or are invaded by fibroblasts.
Pleura
The pleura consists of two layers: (1) a visceral layer and (2) a parietal layer.
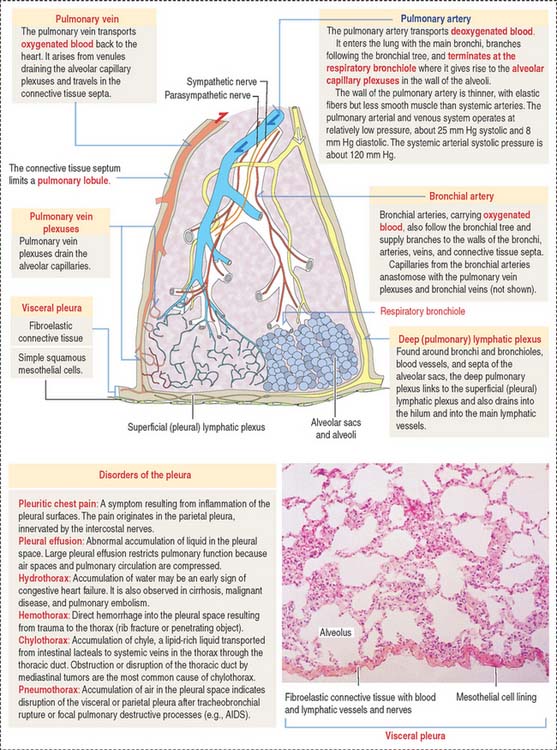
The visceral layer is closely attached to the lung. It is lined by a simple squamous epithelium, called mesothelium, and consists of cells with apical microvilli resting on a basal lamina applied to a connective tissue rich in elastic fibers (Figure 13-23). This connective tissue is continuous with the interlobular and interlobar septa of the lung. The parietal layer is also lined by the mesothelium.
Blood vessels to the visceral pleura derive from pulmonary and bronchial blood vessels (see Figure 13-23). The vascular supply to the parietal pleura derives from the systemic blood vessels. Branches of the phrenic and intercostal nerves are found in the parietal pleura; the visceral pleura receives branches of the vagus and sympathetic nerves supplying the bronchi.
Clinical significance: Disorders of the pleura
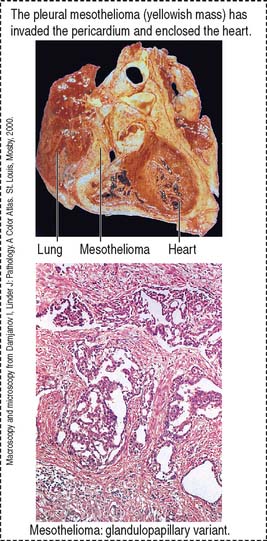
Mesothelioma is a tumor that originates in the mesothelial cell lining of the pleura, the peritoneum, and the pericardium. Mesothelioma is associated with previous long exposure (15 to 40 years) to asbestos. Pleural mesothelioma spreads within the thoracic cavity (pericardium or diaphragm, Figure 13-24) and metastasis can involve any organ, including the brain. Symptoms include pleural effusion, chest pain, or dyspnea. Organ imaging studies of the thorax can detect thickening of the pleura (asbestos plaques) and fluid containing tumoral cells.
Respiratory System
Anosmia refers to deprivation of the sense of smell by disease or injury. Olfactory cells have a life span of about 1 to 2 months and are replaced throughout life by undifferentiated basal cells. Sensory endings of the trigeminal nerve, found in the olfactory epithelium, are responsible for the harmful sensation caused by irritants such as ammonia.
Chronic obstructive pulmonary disease (COPD) includes emphysema and asthma.
Additional components of the alveolus include endothelial cells (lining the alveolar capillaries), macrophages (alveolar phagocytes or dust cells), fibroblasts in the interalveolar septum (producing elastic fibers), and mast cells.