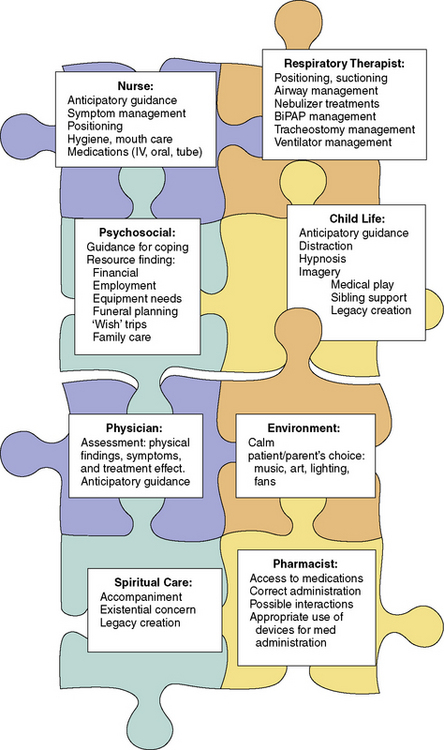
32 Respiratory Symptoms
Of all symptoms that very ill children experience, perhaps the most dreaded and disturbing to patients, parents, and caregivers are pain and difficulty breathing. With regard to the latter, common respiratory difficulties encountered are related to a wide spectrum of conditions. These include illnesses that cause systemic problems such as weakness, anemia, or cystic fibrosis. Other times, localized problems such as airway abnormalities, swallowing problems, aspiration of secretions, or pneumonia may cause breathing difficulties. Metastases from cancer may cause widespread disease within the entire thorax, including multiple tumors, pleural effusions, pneumothorax, or airway compression. This list is by no means exhaustive, but gives some idea as to the variety of causes that can lead to disturbing respiratory symptoms in children, particularly toward the end of life. However, not all respiratory symptoms are necessarily progressive, and are sometimes transient as children recover from other life-threatening illnesses (Fig. 32-1).
Apart from physical or mechanical derangements that lead to breathing problems, anxiety can also compound feelings of shortness of breath and/or difficulty swallowing. There is clearly a role for many specific skills of different health professionals and caregivers to maximize a patient’s comfort. These include numerous psychological strategies,1 careful positioning of the patient, artificial ventilatory supports, and medications. Additionally, children require clear explanations for these distressing symptoms, and reassurance that we will work together to alleviate them.
This interdisciplinary approach to care becomes even more important over time, because as technology continues to develop more children who would have died because of their underlying illness now survive. In this context, some of the therapies used to assist with respiratory symptoms also extend life, but with residual respiratory symptoms or limitations lingering in the background. Examples of these therapies range from treating pneumonia with new generation antibiotics to receiving a lung transplant for cystic fibrosis. In other situations, children who cannot be cured of their disease might have their lives extended by months or years by noninvasive ventilation, such as facemask or nose cradle, or tracheostomy, with or without chronic mechanical ventilation. The number of children who live in the hospital or at home requiring respiratory support by biphasic positive airway pressure (BiPAP), continuous positive air pressure (CPAP) or continuous mechanical ventilation by way of tracheostomy has grown significantly in the past decade.2 Despite this, there are some who believe these procedures are underused in pediatric populations.3 In addition to the array of medical equipment and operative procedures that may alleviate respiratory symptoms, there are also many medical therapies that may be employed, which will be described later in more detail.
Dyspnea
Dyspnea is “a distressful subjective sensation of uncomfortable breathing that may be caused by many disorders, including certain heart and respiratory conditions, strenuous exercise, or anxiety.”4 The differential diagnosis is vast, and the causes can perhaps best be organized by anatomic location. Dyspnea can result from:
In terms of assessing dyspnea, two self-report tools could be located.5,6 One has been tested only in hospitalized patients with asthma, and the other has been limited to small focus groups of children with cystic fibrosis or asthma, compared to normal children. They were found to be reliable in children 6 or 8 years of age, respectively, but not younger. In the absence of any validated tool for dyspnea in other diseases, visual analogue scales (VAS) can also be used for children in these age ranges.
There are many studies evaluating both pharmacologic and non-drug treatment of this distressing problem. Results from meta-analyses in the Cochrane Database of systematic reviews are mixed:7
Many children anecdotally do respond positively to practicing deep, slow breathing, singing, or blowing bubbles or pinwheels when they are feeling anxious and short of breath. The use of self-hypnosis in children to manage dyspnea has also been found to be beneficial.8 This may be directed for younger children by a professional skilled in hypnotherapy. Pediatricians and others can learn to help their patients learn self-hypnosis, which gives the child more immediate and constant access to this form of therapy, through the Society for Developmental and Behavioral Pediatrics. Maintaining a calm and quiet environment is also important, and can be accentuated by use of machines that create light patterns on the ceiling, relaxation carts such a Snoezelen, and favorite music being played quietly.
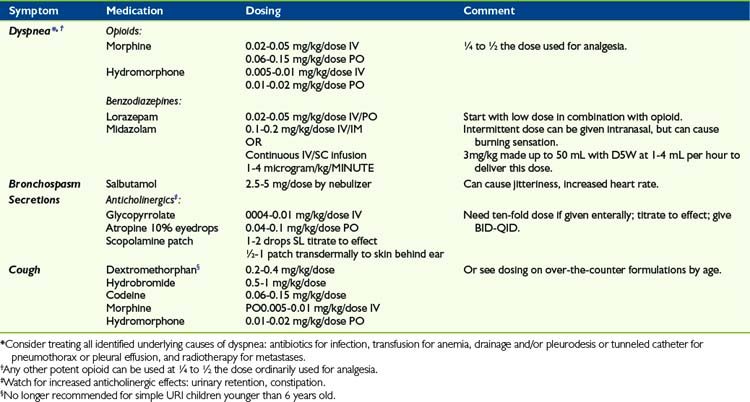
As for drug treatment of dyspnea, the mainstay medications are opioids and benzodiazepines, titrated to minimal effective dose (Table 32-1). There is sometimes an exaggerated concern on the part of health professionals and parents that initiating opioids to treat dyspnea may cause respiratory depression. Very often, the dose of opioid required for the treatment of dyspnea is a quarter to half that required for the treatment of pain. A recent Cochrane review did report benefits of opioid therapy in ameliorating breathlessness in patients due to both malignant and non-malignant disease.9 Although the number of studies was small, the results were significant when these medications were given via the enteral or parenteral route. Educating families and colleagues that judicious use of opioids have been found to be safe and beneficial often allays these fears. Also, opioids can sometimes be used transiently, while other therapies aimed at ameliorating the cause of the dyspnea can be employed and given time to take effect. In addition to systemic administration of opioids, there have been many studies evaluating the possible role of inhaled opioid agonists, the theory being that this might confer a direct benefit by stimulating mu receptors in the lungs themselves, thereby reducing systemic side effects such as pruritis, somnolence, and constipation. A recent meta-analysis reveals that this mode of therapy is not beneficial, even in dose ranges from 1 to 40 mg morphine equivalent,10 despite isolated case reports showing positive effects.
There is no meta-analysis that evaluates the use of benzodiazepines for relief of dyspnea, though the Cochrane Database has a published protocol for such a study. Other published clinical trials in adults have demonstrated that use of midazolam, in addition to morphine, can further diminish the sensation of dyspnea.11 There are no similar trials in children, but the combination of morphine or another opioid in conjunction with benzodiazepines are frequently used to treat this symptom in children despite the paucity of data for both children and adults. Occasionally, suffering from symptoms such as dyspnea becomes intractable, despite multiple combined treatments. In this event, many patients and/or families agree to a course of sedation as a last resort to provide comfort, although conscious awareness may be very diminished as a consequence.12 This treatment might be continued until death occurs, but certainly not always if other treatments such as radiotherapy alleviate the symptom over time in such a way that sedation can be lessened or discontinued. In one study, such sedation was eventually discontinued in a quarter of adult patients.13
Other medications that may provide relief, depending on the underlying disease, are bronchodilators, inhaled steroids, or mucolytics.14 Many patients suffer from orthopnea, and occupational therapists and physiotherapists can often combine their skills to devise modifications to beds and seating such that the patient can maintain a comfortable position even during sleep. Other helpful therapies that may assist with dyspnea include use of a fan for increased air movement in the room. Patients experiencing dyspnea also often feel less symptomatic at an incline of 30 degrees to 90 degrees.
The role of oxygen has been controversial in the management of dyspnea. However, in a 2008 study, patients dying of metastatic lung disease and receiving oxygen therapy were no less dyspneic than those receiving only room air.15 However, oxygen therapy may have a symbolic role for families, particularly if their child has required oxygen frequently throughout his or her life. Therefore, many patients and families do request that oxygen be provided, particularly in home settings. Having oxygen to provide often seems to enhance patient and parental sense of control, and can be important from that point of view.
Cough
A very aggravating symptom is cough. Cough interferes with activities as basic as eating and sleeping, and also as far-reaching as social isolation that prevents one from attending concerts, movies, etc. Moreover, cough leads to fatigue, abdominal or chest pain, and even vomiting and rib fractures. Persistent cough is a serious problem that healthcare professionals sometimes minimize, perhaps because everyone has had a cough at one time or other. Again, medical treatment to this point is somewhat limited, given that mechanical factors such as secretions in the alveoli and bronchi, and irritation of the carina, are potent stimuli of the cough reflex. No meta-analysis looking at treatment of cough in palliative care could be located. Cough may respond to N-methyl-d-aspartate (NMDA) receptor antagonists, such as dextromethorphan. This is often available in low-dose formulations in over-the-counter cough preparations. There are no controlled trials in children evaluating its role in cough due to progressive respiratory illness. However, one study demonstrated more improvement in parent-report of cough compared with placebo when used for viral upper respiratory infection, though not as effective as ingesting honey.16 Some patients get a measure of relief from a relatively small dose of opioid.17 However, for those with a lesion in the bronchi, cough can be fairly intractable without more intense treatments, such as radiotherapy or surgery. With regard to radiotherapy, an area of lung or total lung can be targeted when there is diffuse parenchymal disease, but in the case of bronchial tumors, this can be administered by endobronchial brachytherapy. This is accomplished by delivering a radioactive treatment via an endoscopically placed catheter, which is left in place for a few minutes, then removed.18,19
Secretions
When loss of airway control is caused by imminent death, and causes caregivers distress, it can be treated by a variety of anticholinergic medications, although the evidence for these strategies is quite weak.20 One noninvasive strategy is to use 10% atropine eyedrops sublingually, titrated to effect. Another is to use a scopolamine patch applied transdermally behind the patient’s ear. Systemic medications such as glycopyrrolate can also be used, either enterally or parenterally, and this particular medication has the added advantage of not crossing the blood-brain barrier. These medications need to be carefully titrated such that secretions do not become too thick or tenacious. If this complication arises, secretions may become much harder to move within the airways, leading to the formations of large, solid mucous plugs, which can then worsen the child’s respiratory symptoms. In these situations, families and caregivers also need to be alert to other anticholinergic symptoms, such as urinary retention, which sometimes requires indwelling urinary catheterization, dry mouth, and worsening constipation.
Pleural Effusions and Pneumothorax and/or Hemothorax
A pleural effusion, accumulation of fluid between the lung and chest wall, or pneumothorax, collection of air between the lung and the chest wall, may be asymptomatic when it is small. However, as they enlarge, they often cause increasing dyspnea. Depending on the child’s overall disease trajectory, more conservative attempts to control symptoms might be limited to medications if the child is believed to be very advanced in the course of the underlying disease. On the other hand, if the child is relatively well overall, it often is reasonable to consider other options, including surgery and/or radiotherapy. For malignant pleural effusion, it is not worthwhile to place a chest tube as a sole therapy only transiently, as the effusion will doubtlessly re-accumulate, often within days. Many patients complain of increased pain at the chest tube site for the entire time it is in place, and this discomfort needs to be anticipated and weighed against the benefit that might be conferred by its placement. Surgeries undertaken often include the insertion of pigtail catheters or chest tubes for emergency drainage, and sometimes pleurodesis once the effusion is drained. Pleurodesis is a process by which the pleural surfaces of the chest wall and the lung can be intentionally inflamed by reaction to placement of foreign material in this space, with the goal that the two pleural surfaces permanently adhere to each other, avoiding recurrence of the pneumothorax or pleural effusion. A recent review in adult patients did show that pleurodesis is preferentially done thorascopically rather than by thoracotomy, given the more minimal nature of the former therapy, and that talc is the material of choice to seal the pleural surfaces together.21
A more recent innovation is the insertion of a tunneled catheter for control of malignant pleural effusion. These are increasingly being used for ambulatory adult patients, and can often be inserted by a thoracic surgeon as an outpatient procedure.22 This procedure involves placing a tunneled catheter under the skin and subcutaneous tissue, through the thorax, and into the pleural collection of fluid. The exterior portion of the catheter can then be attached to a sterile collection bottle to perform serial drainages of the effusion as it re-accumulates, to maintain comfort. A 2006 local study23 of 250 insertions in adults suggested that this approach is superior compared with talc pleurodesis, due primarily to the avoidance of a days-long hospital admission and long duration of result, usually until patients’ deaths (Fig. 32-2).
Weakness of respiratory musculature
Children with such diseases are highly symptomatic from a respiratory standpoint. The degree and timing of weakness is variable, depending upon the underlying condition. Symptoms caused by these diseases include dyspnea, swallowing difficulties, resultant excessive oral secretions, and aspiration of feeds and secretions. Recently, more and more families have been advocating for significant respiratory intervention for these children, including long-term CPAP, BiPAP, or tracheostomy and ventilation. With the first two forms of noninvasive ventilation support, children with diseases such as SMA 1 might have life expectancy lengthened from a few months to somewhere into the first decade of life. For many parents, these forms of respiratory supports cannot be emphasized enough in terms of their importance in their child’s care. To that end, many parents correspond through a variety of websites, including www.curesma.org, and have become strong advocates for parents newly adjusting to this serious diagnosis. This has certainly resulted in globalization of medical advice. It is not uncommon for families from around the world to be corresponding with each other, or even with clinicians. This can sometimes be problematic, as parents seek to be involved in clinical trials for their children in other cities, or even foreign countries, but find that practical limitations of their child’s ability to travel, or insurance and other issues, will preclude their participation.24–26 Parents often feel over time that they would be better off in other treatment centers, and this can lead to feelings of frustration and distrust of local care providers, despite the fact that the day-to-day care will include all proven therapies for these incurable diseases.
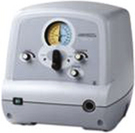
Recent consensus suggests that combination therapy of noninvasive ventilation, gastrostomy feeding and fundoplication, and use of a mechanical insufflation/exsufflation machines that mimic cough, extend life.27 The Cough Assist® machine mimics a cough. When a healthy person coughs, it is preceded by a deep inspiration. With insufflation/exsufflation machines, this is mechanically simulated by applying positive pressure to the airways, followed by rapid switch to a negative pressure through a facemask. This often moves secretions higher into the airway or pharynx to allow for improved suctioning of deeper or inspissated secretions.28 However, despite the advances demonstrated in this most recent consensus statement, the same publication contends that it remains unclear as to which is ethically the more correct path; extension of life through these technologies, or providing care directed at comfort only, but resulting in much earlier death (Fig. 32-3).
With regard to benefit versus burden of these treatments, the proportionality of each is very difficult to ascertain. These children are non-verbal due to their extreme weakness, so one really doesn’t have a way to determine the child’s symptom experience, nor cognitive, emotional, and spiritual dimensions and development in ways adequate for them to inform their own treatment decisions. Some parents have been able to articulate this very well with statements reflecting ambiguity around decision-making. In the words of one mother: “Would we have chosen all this if we could have seen her lying in this bed years later? We did, and I don’t regret it, but I wonder if meeting us would help other families to see what life will be like if they choose this for their babies.” Another related issue that is likely embedded in these choices is the hope for eventual cure of spinal muscular atrophy and other neuromuscular diseases. The advent of stem cell research has been very topical and pervasive in the media, and many families will volunteer that part of their decision making to pursue significant respiratory intervention is the hope that their child will be cured, regardless of the obstacles and time that stretches between their child and this potential therapy. Some parents have been quite pragmatic about banking the cord blood of subsequent babies in the faint hope that a cure will be achieved for their child, however unlikely they know this may be.29,30 Numerous families have requested stem cell transplant in China, and have raised funds in that hope, despite having been counseled that there are no published studies as to outcomes of this therapy, nor any way for their child to be transported that distance. All of these actions suggest their spoken and unspoken desperation in caring for these children.
Children with diseases such as Duchenne (DMD) and other forms of muscular dystrophy present with similar symptoms in the advanced stages of the disease to children with SMA 1. However, the disease trajectory that leads them to this point is often very different. When a child has DMD, he or she becomes gradually weaker, deteriorates from full ambulation in a seemingly normal early childhood, to using a wheelchair, to the point of extreme respiratory muscle weakness that often requires mechanical ventilation by mid-adolescence.31 Child life specialists, and other psychosocial disciplines, are essential in the care of these patients in helping them find a voice, share their feelings, and cultivating their ability to learn strategies to cope with their chronic and progressive illness.
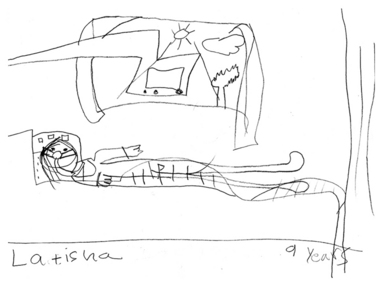
In this situation, the disease trajectory is often well known to the parents, and most often also to the adolescent patient. That said, when patients with DMD present with a sudden severe deterioration in their health, perhaps resulting in ICU admission, it sometimes becomes obvious that these patients and their families have either had little conversation about advance care planning, or have heard mixed messages such that decision making has not occurred prior to the crisis.32 This situation often leads to the sudden addition of BiPAP to the care of these patients. Sometimes, sudden reliance on ventilatory support is the jarring introduction to a more advanced stage of illness as depicted in the drawing by a 9-year-old patient (Fig. 32-4). This can then lead to crisis-oriented decision making, and leave patients and/or families feeling as if they are contributing to the patient’s eventual death if a decision to forgo ventilation is later considered.
Consideration of Tracheostomy
All of the situations in which a child becomes chronically or repeatedly critically ill cause significant angst and distress to children, their families and healthcare professionals. These illnesses often have a high rate of mortality, and therefore the implication is that treatments should not be unduly burdensome, and offer some tangible benefit.33,34 However, because so many of these situations are rare, either the underlying disease itself is rare, or confounding serious complications of a more common primary disease make the constellation of problems rare, there is often virtually no clear evidence on which to base decisions. Many decisions are undertaken with a sense of ambiguity, because of the uncertainty as to whether treatments will lead to a positive outcome. The clinical entities of chronic critical illness and frailty are starting to be explored in the adult intensive care literature, but not yet substantially in the pediatric literature. Therefore, in the case of tracheostomy for the chronically critically ill child, or for those with end-stage neuromuscular disease, it is not always straightforward to ascertain the relative proportionality of burdens and benefits, nor in whose interest decision makers are acting when the child has never been able to speak to his or her own interests. As stated by the Canadian Pediatric Society,35
Our duty, then, as physicians, nurses, and respiratory therapists, is to educate ourselves as to what day-to-day life looks like for these families, in order that parents are fully informed as to the ramifications of taking this decision. Unfortunately, this type of disclosure does not always occur in advance, and sometimes families who have been left to decide about a simple trach come to learn that there is really no such thing. One suggestion for healthcare professionals, and for physicians in particular given their strong role in medical decision making, is mandatory exposure to home care and chronic illness during their training.36 Sometimes, one visit to a family home can suddenly make sense of decisions made by patients or parents that didn’t seem to be clear when they were seen only in a clinical setting. Some European countries, and increasingly North American schools, ensure that all medical students have exposure to home settings, and the role of nursing. The schools require students work as a nurse during their junior clinical training,37 perform interprofessional learning projects,38 and/or by having medical students and residents spend clinical rotations in home care and community care in other sites such as respite centers or hospices.39–41 The first of these cited papers has even advocated short-term hospitalizations of medical students to experience the patient role. The reality that families face after hospital discharge is often overwhelming as they seek to return home and build a new normal life, and this remains foreign to healthcare providers unless we make a concerted effort to educate ourselves as a matter of course about community and home care. There are many assumptions made by us as caregivers, which are communicated to families about the amount and cost of support at home. These discussions often overestimate care provision, such as the amount of available respite, homemaking, night time care, and underestimate costs that families will encounter in caring for their ill child at home.42 Even with the best teaching and preparation that we can offer, families caring for a child with a tracheostomy often leave the hospital after many months, greatly anticipating their return home, only to express despair and fatigue a few months after discharge (personal communication).57 Realistically, disease and treatment management are often very different between a hospital setting and home setting, particularly if the family lives in a rural area.
In recent years, more and more adolescent or young adult patients living with DMD have undergone tracheostomy and continuous ventilation, in order to pursue goals when they feel their quality of life is relatively good. However, even with optimal medical therapy, more than 50% of those aged 15 to 19 years are dependent on ventilation, and the long-term survival is still limited to early adulthood.43 As this decision is often faced by adolescent patients, it is critical that they be active participants in the decision-making process, and be well informed as to what their future care and illness might be like.
Another group of children have systemic weakness caused by chronic critical illness, and this is perhaps a new group in the past decade for whom significant respiratory symptoms are developing.44 Chronic critical illness can be identified by the patient needing to be managed by way of tracheostomy and ongoing mechanical ventilation, because of myriad underlying conditions, often multiorgan dysfunction, that prevent a patient from being weaned from a ventilator in the short term.45 These children often have complex chronic illnesses that lead to multifactorial respiratory symptoms. These factors include months of being bedbound, leading to generalized polyneuropathy and myopathy.46 This weakness is compounded by intrinsic lung and airway disease, caused by factors such as previous pneumonia or adult respiratory distress syndrome (ARDS) and ongoing barotrauma. These children may also be symptomatic from a variety of other organ failures and treatments, as well as from emotional symptoms arising from existential distress, chronic hospitalization, hopelessness, and fear and anxiety.47 The role of psychosocial care providers is essential in reducing some of these psychological symptoms. Child life specialists devise activities that provide a reprieve from the seriousness of the acute care setting in which patients reside for sometimes many months at a time.
Special Considerations for Patients with Cystic Fibrosis
Cystic fibrosis is a common multisystem disease from which children, adolescents, and adults suffer. Over time, progressive respiratory symptoms such as dyspnea, recurrent pneumonias, chronic cough, and hypercarbic headaches are accompanied by other distressing symptoms, such as weight loss, diabetes mellitus, and limited mobility. Aside from being a life-threatening disease, this is a progressive chronic disease that can physically limit the child due to impaired pulmonary function. School absences for appointments, or hospitalization for pneumonia or a so-called CF tune-up are common. This disease also requires compliance to constant daily respiratory treatment regimens, including ingestion of multiple enzymes and medications, inhalation treatment, and chest physiotherapy/postural drainage, all of which are time-consuming, and may be a challenge to maintain. A wonderful example of creativity on the part of a mother to enhance adherence was to devise a marshmallow blower instead of an incentive spirometer, which the child loved to use. Lastly, these children are often of small stature for their age, and therefore they might stand out as different from their healthy peers if they are fairly symptomatic in childhood or adolescence. This combination of factors sometimes leads to social isolation of the child, and more school absences because of psychosocial issues that compound already compromised attendance and involvement. Coupled with these physical symptoms are often fear and anxiety around the possibility of death and dying, fear of symptom progression, and feelings of loss and separation from daily routines and comforts, family and friends. In some situations, children and their families have to make difficult decisions about whether or not to embark on the path to undergo lung transplant. A decision to proceed with transplant often takes them out of their home community to a transplant center for prolonged periods of time, during which they may also have to undergo fairly strenuous pulmonary and general rehabilitation to optimize the chances of successful transplant. To this point, the decision is not an easy one, given the paucity of pediatric data, and controversies about wait-list mortality, etc.48–50
Apnea
Another condition that frequently causes apnea is trisomy 18. Management of these apneas and/or supporting the weak infant with trisomy 18 by modalities such as CPAP or high-flow nasal cannulae are becoming a more common request from parents.51,52 While many centers offer comfort-directed therapy to these babies, other centers are offering increasing supportive therapies such as CPAP or BiPap, and invasive interventions such as open heart surgery for correction of congenital heart disease.53,54 It is true that many conditions would be lethal, were all therapies to be withheld. This is the argument raised by some parents advocating treatment for their babies: that trisomy 18, and even trisomy 13, cause death in infancy and early childhood because nothing has been done therapeutically for these children. On the other hand, most parents still consider the ramifications of trisomies 13 and 18 to be inconsistent with what they believe to be a good life for their child, and the majority choose noninvasive intervention for management of the child’s airway and breathing.
Abnormal breathing patterns
Patients often have erratic breathing patterns toward the end of their lives, sometimes called Cheyne-Stokes respirations. In this situation, the patient may develop very long pauses between breaths, to the extent that caregivers often believe that the patient has died. After the long pause, the patient then compensates with a series of relatively rapid breaths. This is most often described in adult patients with brainstem injury or tumors, and severe congestive heart failure.55 However, this exaggeration of periodic breathing is often seen as pediatric patients approach death from many other types of disease, and the mechanism for it is not fully elucidated. It is helpful for families to know that this breathing irregularity may occur, and that it is a very common finding at the end stages of illness. However, it is important not to make absolute predictions about survival based on this alone, as some patients have had very irregular respirations with apneas for days or weeks before death.
Advance care planning for the child with respiratory symptoms
Most parents want physicians to discuss advance care planning with them and help them make decisions. Effective communication skills are required, including familiarity with developmentally appropriate language if the child is included in the discussions. Rather than asking parents whether they want everything to be provided for their child, it is better to be clear and decide on individual interventions that are feasible within the context of the child’s illness. It is important to be sensitive to emotions that may surface including fear, guilt, anger, denial, and surprise. In anticipation of these reactions, it may help to be explicit about the shift in focus from cure and survival to comfort and well being. The benefits for parents include knowing that they have assured the best care for their child, including preserving quality of life and avoiding unnecessary pain or suffering.35
Another concern is the frequent absence of the child’s wants in these discussions about treatment options and decisions.56 It is hoped that this situation will become less and less common, as more familiarity with the concept and practice of advance care planning evolves. Certainly, some children and adolescents will decline to hear information about, or participate in, decisions related to their care, but it is likely that these are in the minority.
Summary
Although there have been significant advances in the development of medications and technologies to treat respiratory symptoms in children, there is still a relative deficit of high- quality research to evaluate them in pediatric patients. Many treatments are extrapolated from studies of adult patients. Furthermore, medications used for the treatment of dyspnea are largely systemic medications of only two major categories, opioids and benzodiazepines, that work mainly by affecting central nervous system perception, rather than medications that target the lungs directly, leaving further basic science study of dyspnea as an important issue given the high prevalence of this symptom at the end of life. Importantly, even with limitations in current knowledge, in working collaboratively, the interdisciplinary team can ease dyspnea and other distressing symptoms in children with advanced illness (Fig. 32-5).
1 Anbar R.D., Geisler S.C. Identification of children who may benefit from self-hypnosis at a pediatric pulmonary center. BMC Pediatr. 2005;5(1):6.
2 Hadfield P.J., Lloyd-Faulconbridge R.V., Almeyda J., Albert D.M., Bailey C.M. The changing indications for paediatric tracheostomy. Int J Pediatr Otorhinolaryngol. 2003;67(1):7-10.
3 Principi T., Morrison G.C., Matsui D.M., Speechley K.N., Seabrook J.A., Singh R.N., et al. Elective tracheostomy in mechanically ventilated children in Canada. Intensive Care Med. 2008;34(8):1498-1502.
4 Mosby’s Medical Dictionary. 2009. http://medical-dictionary.thefreedictionary.com/Dyspnea.. Accessed December 11, 2009
5 Khan F.I., Reddy R.C., Baptist A.P. Pediatric Dyspnea scale for use in hospitalized patients with asthma. J Allergy Clin Immunol. 2009;123(3):660-664.
6 McGrath P.J., Pianosi P.T., Unruh A.M., Buckley C.P. Dalhousie dyspnea scales: construct and content validity of pictorial scales for measuring dyspnea. BMC Pediatr. 2005;5:33.
7 Bausewein C., Booth S., Gysels M., Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2;2008: 005623.
8 Mize W.L. Clinical training in self-regulation and practical pediatric hypnosis: what pediatricians want pediatricians to know. J Dev Behav Pediatr. 1996;17(5):317-322.
9 Jennings A.L., Davies A.N., Higgins J.P., Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev. 4;2001: 002066.
10 Polosa R., Simidchiev A., Walters E.H. Nebulised morphine for severe interstitial lung disease. Cochrane Database Syst Rev. 2, 2009.
11 Navigante A.H., Cerchietti L.C., Castro M.A., Lutteral M.A., Cabalar M.E. Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. J Pain Symptom Manage. 2006;31(1):38-47.
12 Krakauer E.L., Penson R.T., Truog R.D., King L.A., Chabner B.A., Lynch T.J.Jr. Sedation for intractable distress of a dying patient: acute palliative care and the principle of double effect. Oncologist. 2000;5(1):53-62.
13 Elsayem A., Curry I., Boohene J. Use of palliative sedation for intractable symptoms in the palliative care unit of a comprehensive cancer center. J Pain Symptom Manage. 2006;31(1):38-47.
14 Twycross R., Wilcock A. Respiratory Symptoms. 2009. www.palliativedrugs.com/palliative-care-formulary.html.. Accessed December 11, 2009
15 Cranston J.M., Crockett A., Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev. 3;2008: 004769.
16 Paul I.M., Beiler J., McMonagle A., Shaffer M.L., Duda L., Berlin C.M.Jr. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161(12):1140-1146.
17 Morice A.H., Menon M.S., Mulrennan S.A., Everett C.F., Wright C., Jackson J., et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175(4):312-315.
18 Klopp A.H., Eapen G.A., Komaki R.R. Endobronchial brachytherapy: an effective option for palliation of malignant bronchial obstruction. Clin Lung Cancer. 2006;8(3):203-207.
19 High-dose rate endobronchial brachytherapy. 2009. www.upmccancercenteres.com/radonc/internal/lung_endobronchial.html.. Accessed July 11
20 Wee B., Hillier R. Interventions for noisy breathing in patients near to death. Cochrane Database Syst Rev. 1;2008: 005177.
21 Shaw P., Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev. 1;2004: 002916.
22 Efthymiou C., Masoudi T., Thorpe J., Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of pleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009. July 28 (e-pub)
23 Tremblay A., Michaud G. Single-center experience with 250 tunneled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129(2):362-368.
24 Mercuri E., Bertini E., Messina S., Solari A., D’Amico A., Angelozzi C., et al. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology. 2007;68(1):51-55.
25 Brahe C., Vitali T., Tiziano F.D., Angelozzi C., Pinto A.M., Borgo F., et al. Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. Eur J Hum Genet. 2005;13(2):256-259.
26 Tsai L.K., Yang C.C., Hwu W.L., Li H. Valproic acid treatment in six patients with spinal muscular atrophy. Eur J Neurol. 2007;14(12):e8-e9.
27 Wang C.H., Finkel R.S., Bertini E.S., Schroth M., Simonds A., Wong B., et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027-1049.
28 Fauroux B., Guillemot N., Aubertin G., Nathan N., Labit A., Clement A., et al. Physiologic benefits of mechanical insufflation-exsufflation in children with neuromuscular diseases. Chest. 2008;133(1):161-168.
29 Gross L. Stem cell promise, interrupted: how long do US researchers have to wait? PLoS Biol. 2007;5(1):e32.
30 Nayak M.S., Kim Y.S., Goldman M., Keirstead H.S., Kerr D.A. Cellular therapies in motor neuron diseases. Biochim Biophys Acta. 2006;1762(11–12):1128-1138.
31 Hyde S.A., Steffensen B.F., Floytrup I., Glent S., Kroksmark A.K., Salling B., et al. Longitudinal data analysis: an application to construction of a natural history profile of Duchenne muscular dystrophy. Neuromuscul Disord. 2001;11(2):165-170.
32 Levetown M. American Academy of Pediatrics Committee on, Bioethics. Pediatrics. 2008;121(5):e1441-e1460.
33 Devictor D., Latour J.M., Tissieres P. Forgoing life-sustaining or death-prolonging therapy in the pediatric ICU. Pediatr Clin North Am. 2008;55(3):791-804. xiii
34 Giannini A., Messeri A., Aprile A., Casalone C., Jankovic M., Scarani R., et al. End-of-life decisions in pediatric intensive care. Recommendations of the Italian Society of Neonatal and Pediatric Anesthesia and Intensive Care (SARNePI). Paediatr Anaesth. 2008;18(11):1089-1095.
35 Advance care planning for paediatric patients. J Paediatr Child Health. 2008;13(9):793-796.
36 Kleinman A. Medicine and morality: Health care’s missing care. Globe and Mail. 2009:A13.
37 Hylin U., Nyholm H., Mattiasson A.C., Ponzer S. Interprofessional training in clinical practice on a training ward for healthcare students: a two-year follow-up. J Interprof Care. 2007;21(3):277-288.
38 Sternas K.A., O’Hare P., Lehman K., Milligan R. Nursing and medical student teaming for service learning in partnership with the community: an emerging holistic model for interdisciplinary education and practice. Holist Nurs Pract. 1999;13(2):66-77.
39 Wilkes M., Milgrom E., Hoffman J.R. Towards more empathic medical students: a medical student hospitalization experience. Med Educ. 2002;36(6):528-533.
40 Blasco P.A., Kohen H., Shapland C. Parents-as-teachers: design and establishment of a training programme for paediatric residents. Med Educ. 1999;33(9):695-701.
41 Engelke M.K., Britton B.P., Burhans L., Hall S. Is there a doctor in the house? Integrating medical education and home health care. Home Care Provid. 1998;3(5):260-265. quiz 266-267
42 Carnevale F.A., Alexander E., Davis M., Rennick J., Troini R. Daily living with distress and enrichment: the moral experience of families with ventilator-assisted children at home. Pediatrics. 2006;117(1):e48-e60.
43 Jeppesen J., Green A., Steffensen B.F., Rahbek J. The Duchenne muscular dystrophy population in Denmark, 1977-2001: prevalence, incidence and survival in relation to the introduction of ventilator use. Neuromuscul Disord. 2003;13(10):804-812.
44 Marcin J.P., Slonim A.D., Pollack M.M., Ruttimann U.E. Long-stay patients in the pediatric intensive care unit. Crit Care Med. 2001;29(3):652-657.
45 Camhi S.L., Mercado A.F., Morrison R.S., Du Q., Platt D.M., August G.I., et al. Deciding in the dark: advance directives and continuation of treatment in chronic critical illness. Crit Care Med. 2009;37(3):919-925.
46 Schweickert W.D., Hall J. ICU-acquired weakness,. Chest. 2007;131(5):1541-1549.
47 Melnyk B.M., Alpert-Gillis L., Feinstein N.F., Crean H.F., Johnson J., Fairbanks E., et al. Creating opportunities for parent empowerment: program effects on the mental health/coping outcomes of critically ill young children and their mothers. Pediatrics. 2004;113(6):e597-e607.
48 Huddleston C.B., Bloch J.B., Sweet S.C., de la Morena M., Patterson G.A., Mendeloff E.N. Lung transplantation in children. Ann Surg. 2002;236(3):270-276.
49 Liou T.G., Adler F.R., Cox D.R., Cahill B.C. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med. 2007;357(21):2143-2152.
50 Liou T.G., Adler F.R., Cahill B.C., Cox D.R. Correction: Lung transplantation and survival in children with cystic fibrosis. N Engl J Med. 2008;359(5):536.
51 McGraw M.P., Perlman J.M. Attitudes of neonatologists toward delivery room management of confirmed trisomy 18: potential factors influencing a changing dynamic. Pediatrics. 2008;121(6):1106-1110.
52 Goc B., Walencka Z., Wloch A., Wojciechowska E., Wiecek-Wlodarska D., Krzystolik-Ladzinska J., et al. Trisomy 18 in neonates: prenatal diagnosis, clinical features, therapeutic dilemmas and outcome. J Appl Genet. 2006;47(2):165-170.
53 Graham E.M., Bradley S.M., Shirali G.S., Hills C.B., Atz A.M., Pediatric Cardiac Care C. Effectiveness of cardiac surgery in trisomies 13 and 18 (from the Pediatric Cardiac Care Consortium). Am J Cardiol. 2004;93(6):801-803.
54 Kaneko Y., Kobayashi J., Yamamoto Y., Yoda H., Kanetaka Y., Nakajima Y., et al. Intensive cardiac management in patients with trisomy 13 or trisomy 18. Am J Med Genet A. 2008;146A(11):1372-1380.
55 Cherniack N.S., Longobardo G., Evangelista C.J. Causes of Cheyne-Stokes respiration. Neurocrit Care. 2005;3(3):271-279.
56 Harrison C., Kenny N.P., Sidarous M., Rowell M. Bioethics for clinicians: 9. Involving children in medical decisions. CMAJ. 1997;156(6):825-828.
57 DeVlaming D: Personal communication, professional practice lead, children’s services homecare, Edmonton, Alberta, Canada.