CHAPTER 12 RESPIRATORY PATHOLOGY
DEALING WITH THE BIOPSY OR RESECTION SPECIMEN
INDICATIONS FOR REMOVAL OF LUNG TISSUE (Table 12.1)
Table 12.1 Indications for submission of pediatric lung tissue for histopathological examination
| Identification or assessment of primary or secondary neoplasm |
| Assessment of diffuse interstitial lung disease |
SPECIMEN TYPES
Bronchoalveolar lavage (Columbo et al 1987, ERSTask force 2000)
Wedge biopsy (Langston et al 2006)
ARTEFACTS, INCIDENTAL FINDINGS AND VARIANTS OF NORMAL
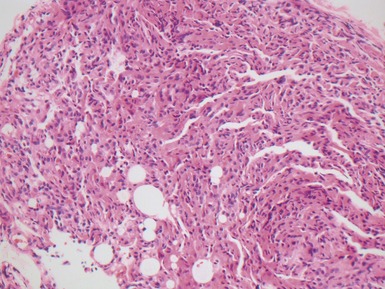
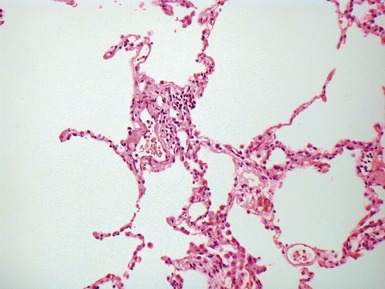
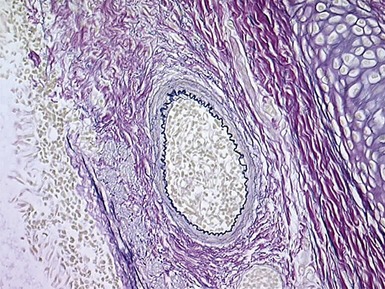
Fig 12.4 Transbronchial biopsy of lung. The tissue is collapsed and also partly distorted. Assessment of architecture and cellularity is almost impossible on such a specimen.
MALFORMATIONS AND CYSTIC LUNG LESIONS
Introduction
| Bronchopulmonary malformations (see Table 12.3) |
(data from Langston 2003)
Table 12.3 Bronchopulmonary malformations
|
Bronchial atresia
|
(data from Langston 2003)
Classification
| Type 0 | acinar dysgenesis/dysplasia |
| Type 1 | large cyst type |
| Type 2 | small cyst type |
| Type 3 | adenomatoid type |
| Type 4 | peripheral type |
(data from Stocker 2002)
CONGENITAL PULMONARY AIRWAY MALFORMATION
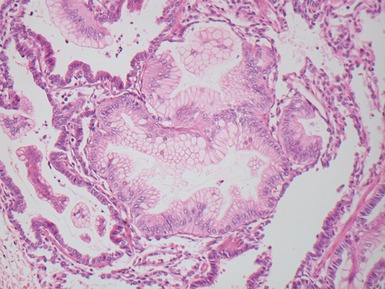
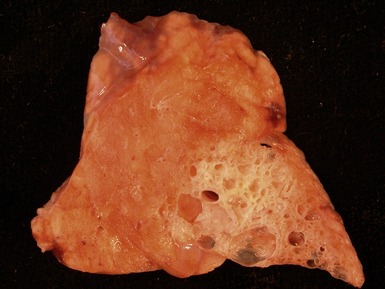
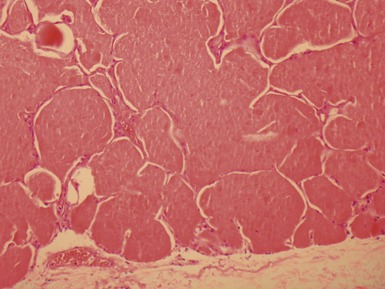
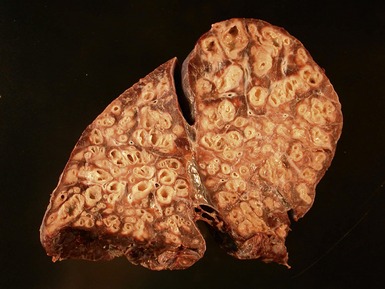
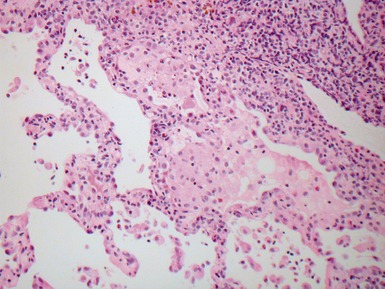
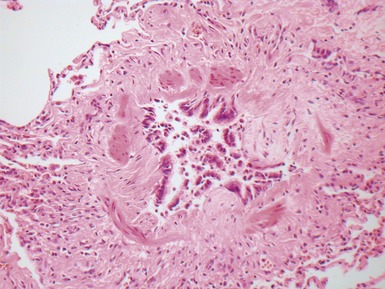
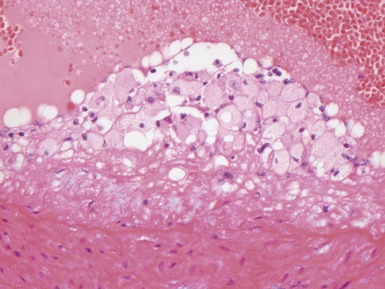
Fig 12.14 CPAM type 1. One-month-old baby girl with cystic lesion in left lower lobe of lung. The excised lobe showed obvious cysts, the largest 1.8cm in diameter. The lining of the cysts resembles that in Fig 12.13 but, in addition, there are areas of mucin-secreting epithelium, with a resemblance to gastric epithelium. Some smooth muscle is evident in the cyst walls.
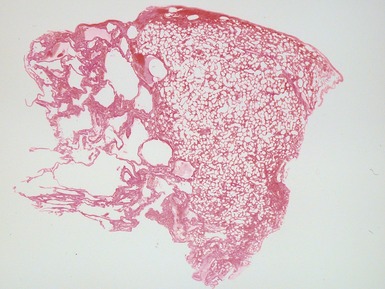
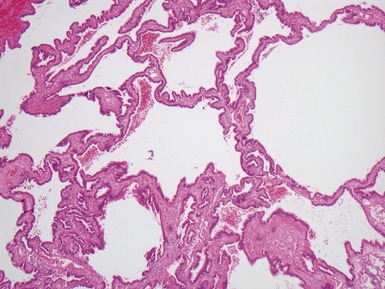
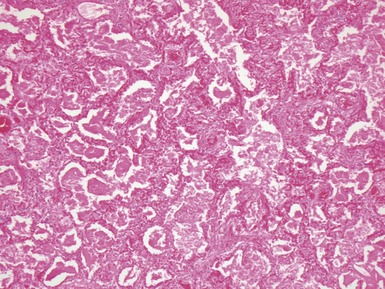
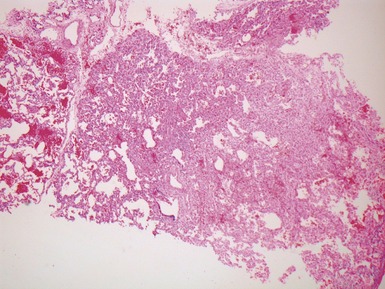
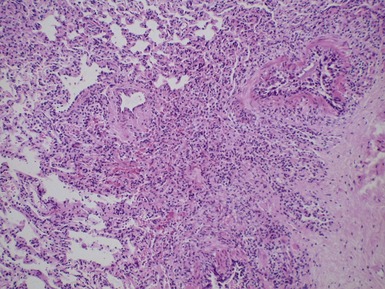
Fig 12.16 CPAM type 2. Same case as Fig 12.15. A low power view of a section from junction of normal and cystic lung. The multiple small cysts with thin walls are well appreciated as is the relatively well-defined border of the lesion. (H&E)
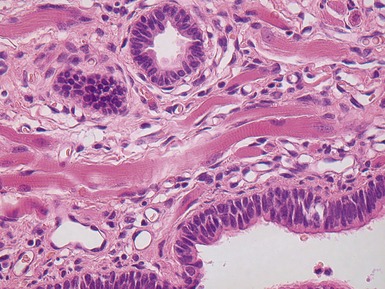
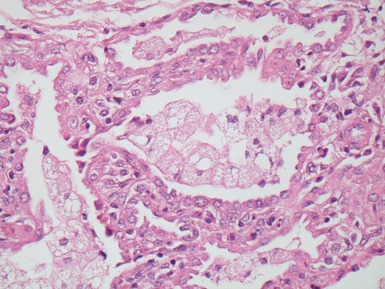
Fig 12.18 CPAM type 2. Striated muscle fibers run through the stroma of the cyst walls; so-called rhadomyomatous dysplasia. Rhabdomyomatous dysplasia may also be seen in other abnormal lungs such as in pulmonary hypoplasia with or without diaphragmatic hernia and may be focal or diffuse throughout the lung parenchyma. The origin of the skeletal muscle is not known. It may represent ingrowth from adjacent structures such as esophagus, but equally it may represent differentiation of pulmonary mesenchyme.
BRONCHIAL ATRESIA
EXTRALOBAR SEQUESTRATION
Histopathological features
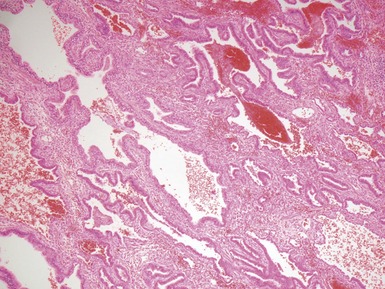
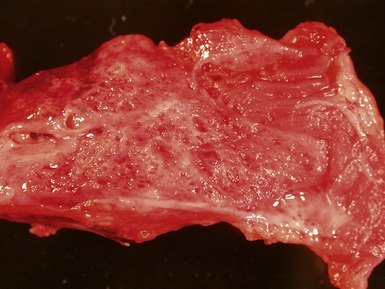
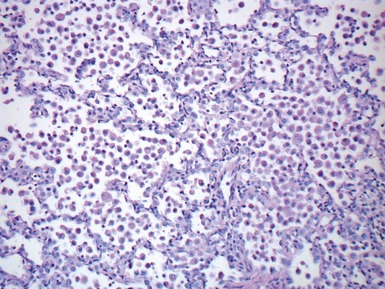
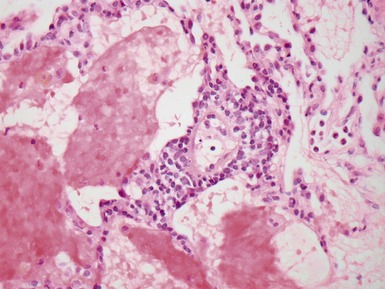
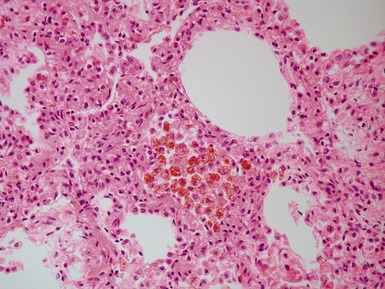
Fig 12.21 Extralobar sequestration. Photomicrograph of the same specimen as Fig 12.20. The lung shows cystic change resembling CPAM type 2. There is some fresh traumatic hemorrhage. There was also chronic inflammation. It contained striated muscle fibers. Cartilage-containing bronchi were also present.
INTRALOBAR SEQUESTRATION
Histopathological features
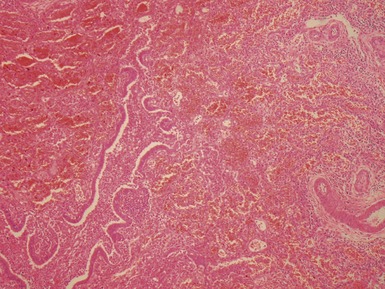
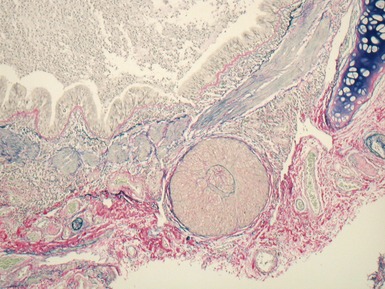
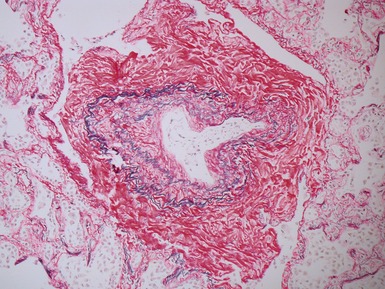
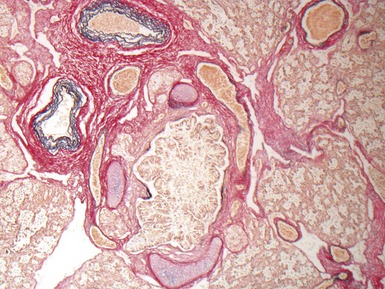
Fig 12.23 Intralobar sequestration. Photomicrograph of specimen Fig 12.22. There is extensive fresh hemorrhage and there is active inflammation. The bronchi and bronchioles are distended by purulent material. The arteries appear thick-walled.
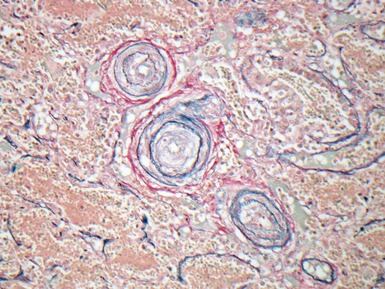
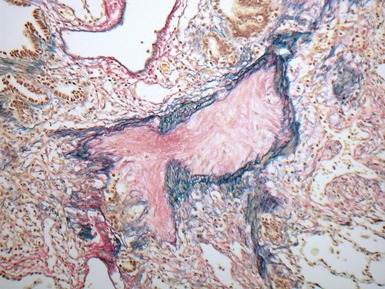
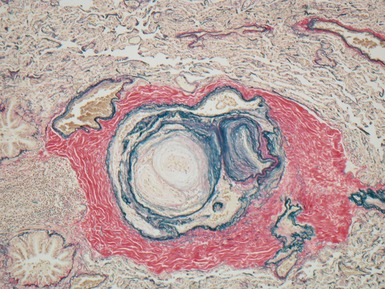
Fig 12.24 Intralobar sequestration. Photomicrograph of the peripheral vasculature in the same case as Figs 12.22 and 12.23. There is muscularization of the intra-acinar arteries and there is also intimal cellular proliferation, both features indicating pulmonary arterial hypertension, presumably from exposure to the systemic arterial pressure of the anomalous systemic arterial supply. (EVG)
LOBAR EMPHYSEMA
Congenital lobar overinflation
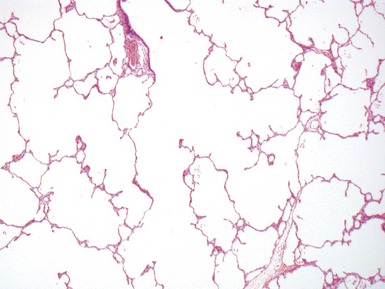
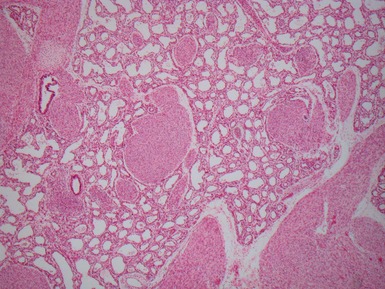
Fig 12.26 Congenital lobar overinflation. Photomicrograph from specimen in Fig 12.25. There is overdistension of alveoli and alveolar ducts (to four to five times the normal diameter). The magnitude of the distension may not be evident until compared with an age-matched control section of lung. Interstitial emphysema was also evident but no other abnormality. No bronchial obstruction could be demonstrated. (H&E)
BRONCHOGENIC CYST
HYDATID CYST
INTERSTITIAL EMPHYSEMA
INTERSTITIAL LUNG DISEASE
Classification
Table 12.5 North American classification of diffuse lung disease in children under 2 years
(data from Deutsch et al 2007)
CONGENITAL SURFACTANT DEFICIENCY
Genetics
SP-B gene deficiency
SP-C deficiency
PULMONARY ALVEOLAR PROTEINOSIS
Clinical features
PEDIATRIC INTERSTITIAL PNEUMONITIS (IP)
DESQUAMATIVE INTERSTITIAL PNEUMONITIS (DIP)
NEUROENDOCRINE CELL HYPERPLASIA OF INFANCY
LUNG TRANSPLANTATION PATHOLOGY
CYSTIC FIBROSIS
Histopathological features
PATHOLOGY OF THE TRANSPLANTED LUNG (DISHOP, MALLORY ET AL 2008)
Acute cellular rejection
Classification
Table 12.6 Revised working formulation for classification and grading of pulmonary allograft rejection
(data from Stewart et al 2007)
Infectious complications
Chronic lung allograft dysfunction
PULMONARY VASCULAR DISEASE
NORMAL PULMONARY VASCULAR ANATOMY
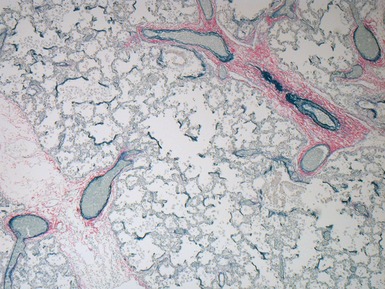
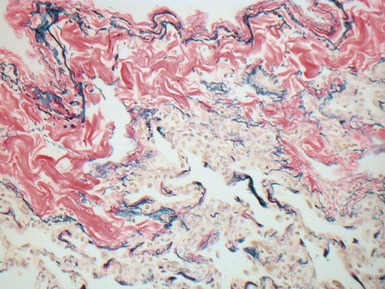
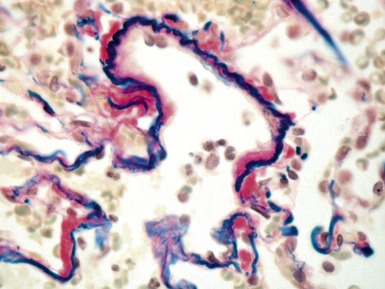
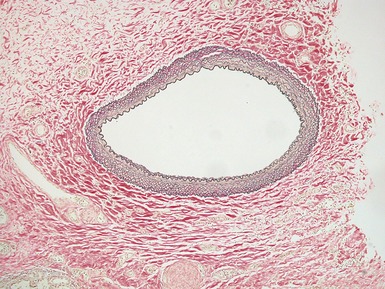
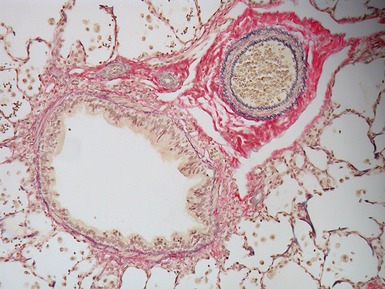
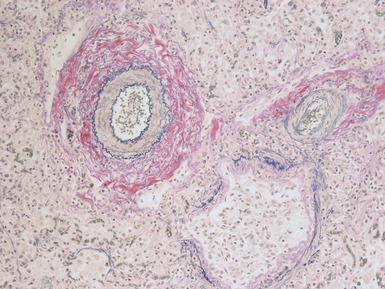
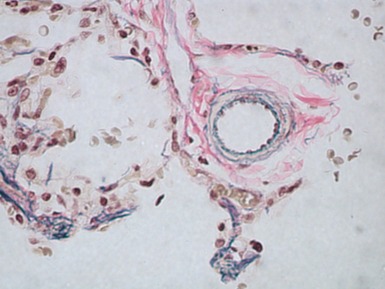
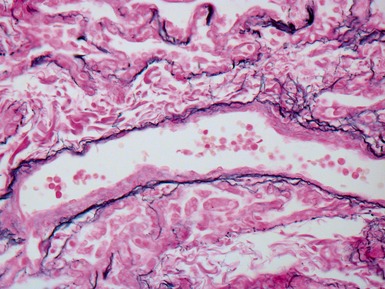
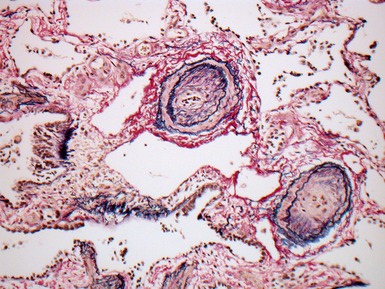
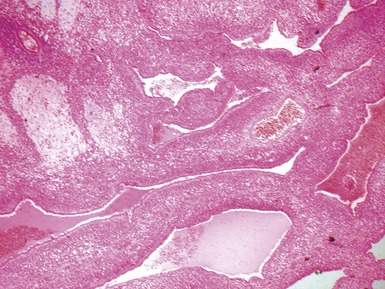
Fig 12.61 Normal vascular anatomy in the lobule. Photomicrograph demonstrating, in the center of the lobule, a bronchiole with accompanying muscular pulmonary artery. An interlobular septum is present at the bottom left and contains two pulmonary veins. Note the wide separation of veins and arteries at this level. (EVG)
|
Muscular pulmonary arteries
|
PULMONARY HYPERTENSION
Classification
Table 12.8 Clinical classification of pulmonary hypertension
|
Pulmonary arterial hypertension
|
Diagnosis of pulmonary hypertension on lung biopsy
Histopathological features
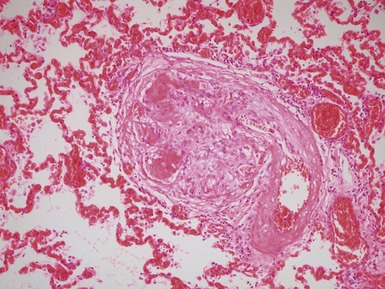
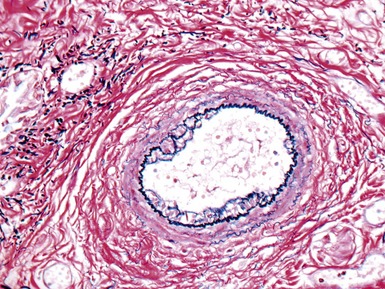
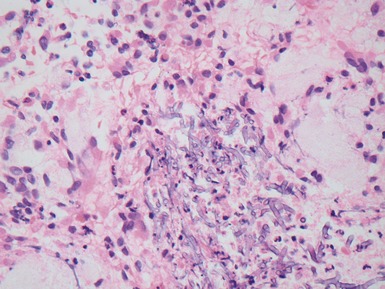
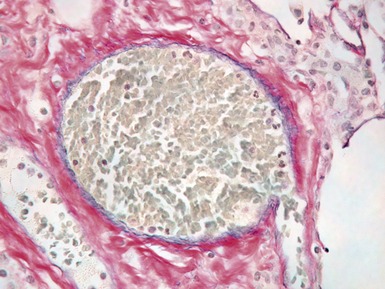
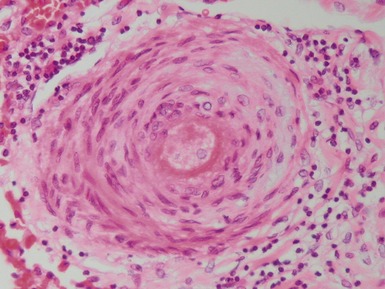
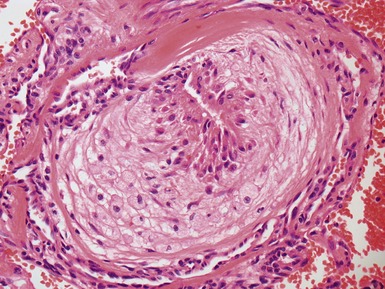
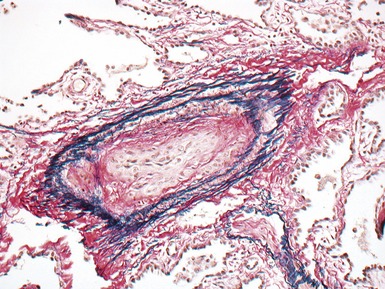
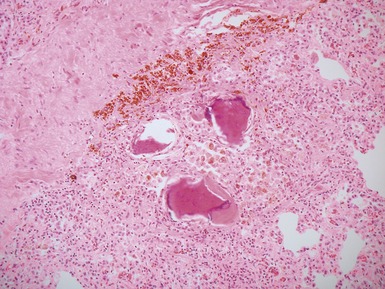
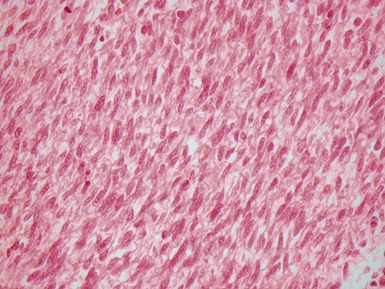
Fig 12.72 Pulmonary arterial hypertension: plexiform lesion. Photomicrograph demonstrating a muscular pulmonary artery with polypoid expansion with proliferation of spindle cells in the lumen to form tiny channels, some of which contain fibrin. Plexiform lesions tend to occur in supernumerary branches of large muscular pulmonary arteries.
Grading
Table 12.9 Heath–Edwards grading system for pulmonary hypertension in congenital heart disease
| Grade 1 | Medial hypertrophy of small muscular pulmonary arteries |
| Grade 2 | Cellular intimal proliferation in muscular arteries |
| Grade 3 | Concentric intimal fibrosis in muscular arteries |
| Grade 4 | Plexiform lesions |
| Grade 5 | Dilatation lesions and angiomatoid lesions |
| Grade 6 | Fibrinoid necrosis |
(data from Heath & Edwards 1958)
PULMONARY ARTERIAL HYPERTENSION
Congenital heart disease and pulmonary vascular disease
PULMONARY VENOUS HYPERTENSION
Veno-occlusive disease
ALVEOLAR CAPILLARY DYSPLASIA
Histopathological features
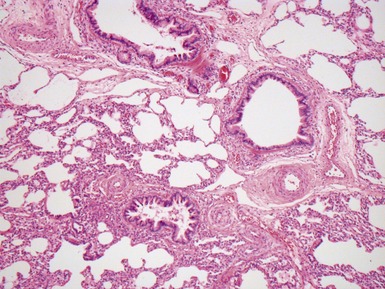
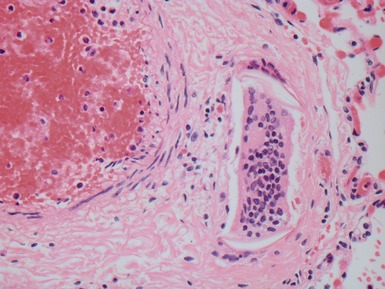
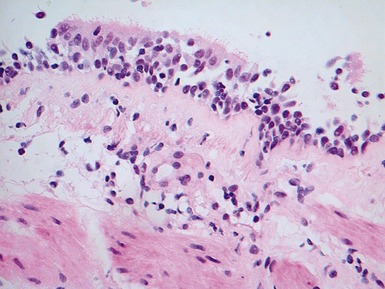
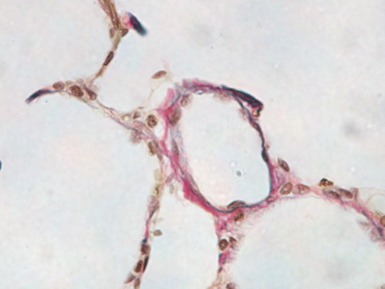
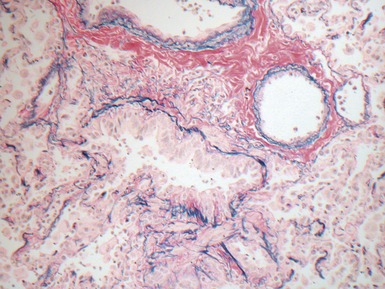
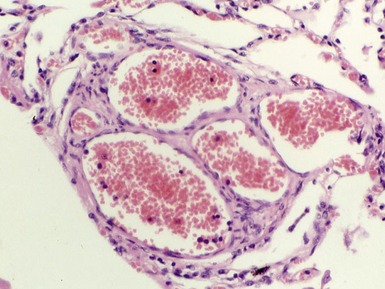
Fig 12.83 Alveolar capillary dysplasia with misalignment of the pulmonary veins. Photomicrograph demonstrating misalignment of the pulmonary veins. The interlobular septa were empty and the pulmonary veins ran with the muscular pulmonary arteries. The veins are the thin walled vessels accompanying the arteries in their collagenous sheaths. Note also the characteristic interstitial thickening and lymphangiectasia.
PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN
CAPILLARY HEMANGIOMATOSIS
PULMONARY LYMPHATIC DISORDERS
Histopathological features
Lymphangiectasia
PEDIATRIC PULMONARY / THORACIC TUMORS
INFLAMMATORY MYOFIBROBLASTIC TUMOR
PLEUROPULMONARY BLASTOMA
Type I
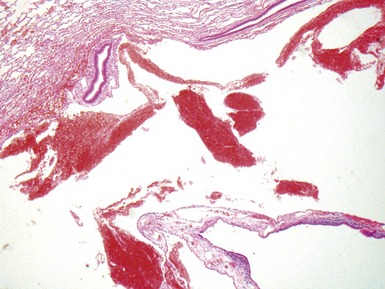
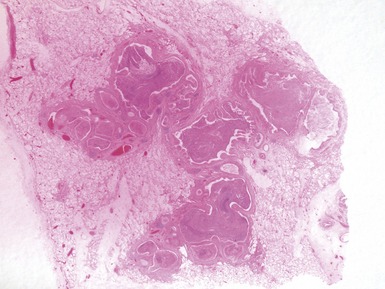
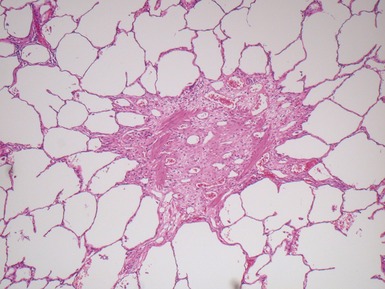
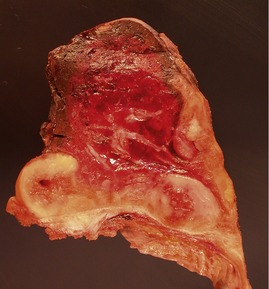
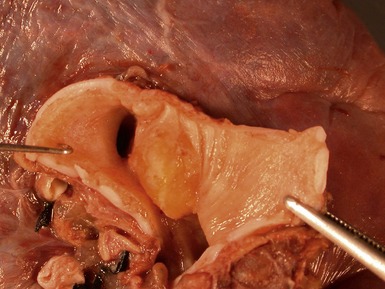
Fig 12.91 Pleuropulmonary blastoma type 1. A one-year-old boy with a cyst in the right middle lobe. Subsequently he developed cysts in the left lung with similar morphology. Photomicrograph demonstrating a cyst with thin septa. Compressed normal lung is visible on the upper part of the picture. There is some fresh hemorrhage. The septa are covered by epithelium and, focally, have a cellular stroma.
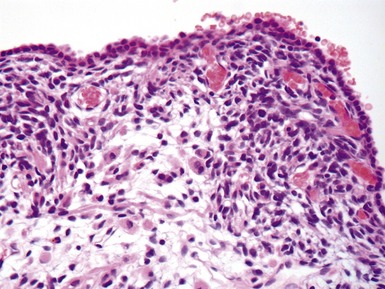
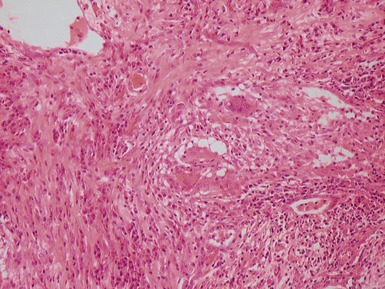
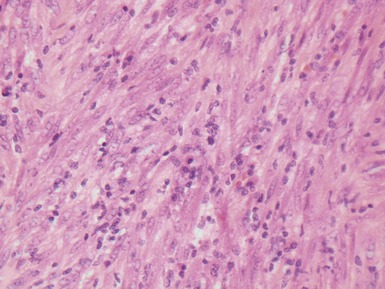
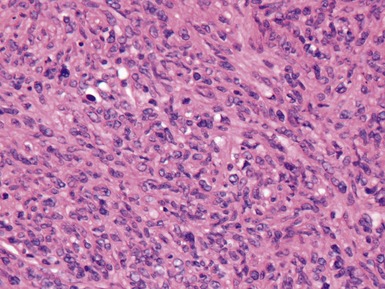
Fig 12.92 Pleuropulmonary blastoma type 1. Photomicrograph demonstrating higher power view of a septum from Fig 12.91. There is a bland epithelial covering. The stroma is cellular. The constituent cells have hyperchromatic nuclei. There are numerous cells with obvious rhabdomyoblastic differentiation.
Type II
PRIMARY PULMONARY CARCINOID
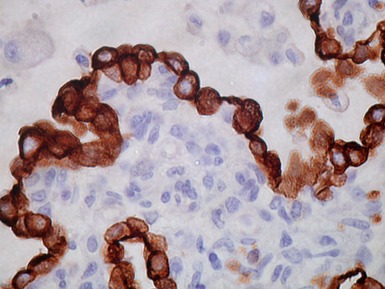
Histopathological features
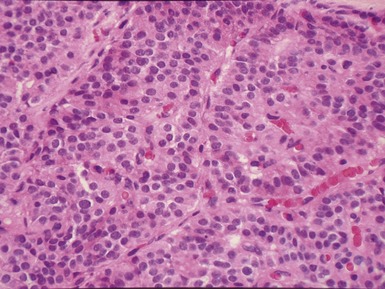
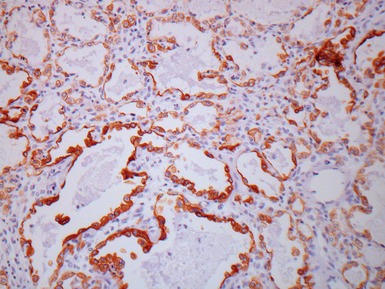
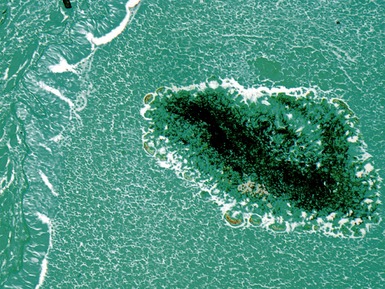
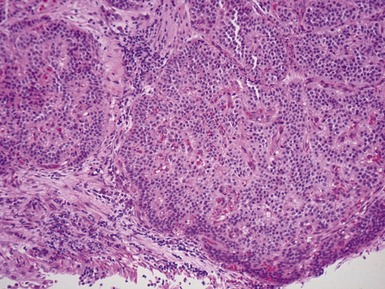
Fig 12.96 Bronchial carcinoid. Photomicrograph demonstrating higher power view of Fig 12.95. The cells have very regular nuclei. There are no mitotic figures and there is no necrosis at the centers of the cell nests. These features, representative of the lesion as a whole, indicate that this is a typical carcinoid.
MUCOEPIDERMOID TUMOR
Histopathological features
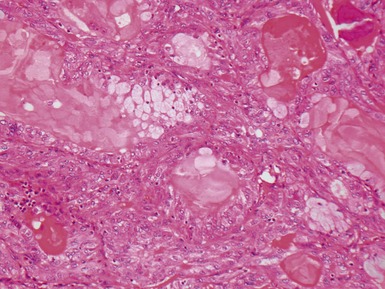
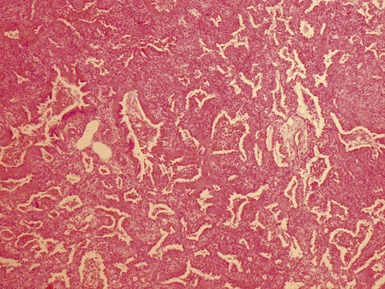
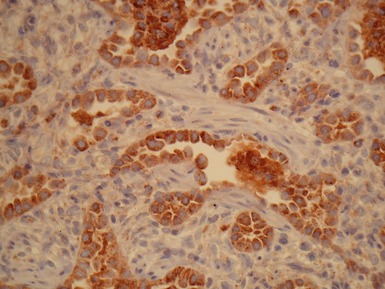
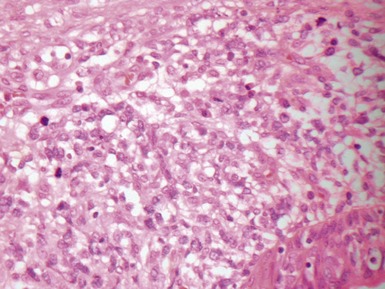
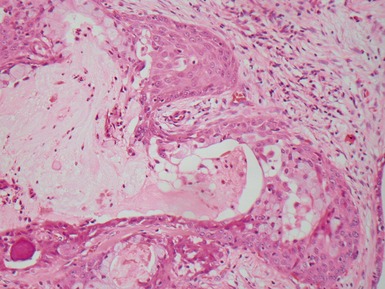
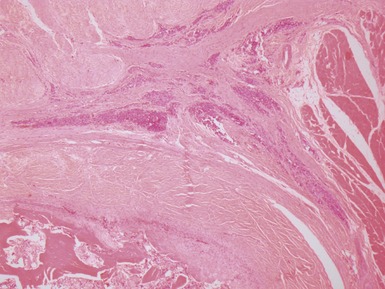
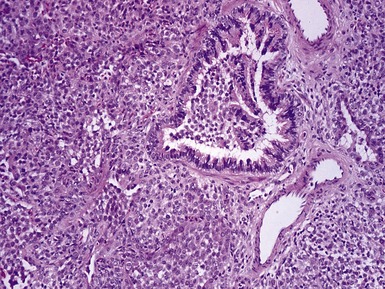
Fig 12.98 Mucoepidermoid carcinoma. Photomicrograph of specimen in Fig 12.97. There are multiple dilated tumor glands containing eosinophilic mucus. Goblet cells are also evident. Smaller glandular structures without much mucus are also present. There are also intermediate cells in the background. The tumor ulcerated the mucosa and extended beneath intact mucosa. There was extensive calcification of the mucus.
PULMONARY LANGERHANS CELL HISTIOCYTOSIS (LCH)
CONGENITAL PERIBRONCHIAL MYOFIBROBLASTIC TUMOR
INFANTILE CHEST WALL HAMARTOMA
PULMONARY METASTATIC DISEASE
(Kayton et al 2006; Dishop, Kuruvilla et al 2008)
Adatia I. Recent advances in pulmonary vascular disease. Curr Opin Pediatr. 2002;14:292.
Becker AE, Becker MJ, Edwards JE. Dilated bronchial veins within pulmonary parenchyma. Arch Pathol. 1971;21:256-260.
Bullard JE, Wert SE, Nogee LM. ABCA3 deficiency: neonatal respiratory failure and interstitial lung disease. Semin Perinatol. 2006;30:327-334.
Columbo JL, Hallberg TK. Recurrent aspiration in children. Lipid-laden alveolar macrophage quantitation. Pediatr Pulmonol. 1987;3:86-89.
Deyrup AT, Lee VK, Hill CE, et al. Epstein–Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients. Am J Surg Pathol. 2006;30:75-82.
Deutsch GH, Young LR, Deterding RR, et al. Diffuse lung disease in children: application of a novel classification scheme. Am J Resp. Crit Care Med. 2007;176:1120-1128.
Dishop MK, Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: a review and 25 year experience at a large children’s hospital. Arch Pathol Lab Med. 2008;132(7):1079-1103.
Dishop MK, Mallory GB, White FV. Pediatric lung transplantation: perspectives for the pathologist. Pediatr Development Pathol. 2008;11:85-105.
Doan ML, Guillerman RP, Dishop MK, et al. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366-373.
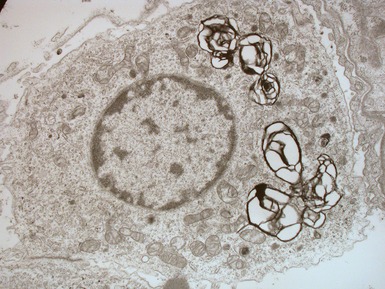
Edwards V, Cutz E, Viero S, Moore AM, Nogee L. Ultrastructure of lamellar bodies in congenital surfactant deficiency. Ultrastruct Pathol. 2005;29:503-509.
ERS Task force on bronchoalveolar lavage in children. Bronchoalveolar lavage in children. Eur Respir J. 2000;15:217-231.
France NE, Brown RJK. Congenital pulmonary lymphangiectasis: report of 11 examples with special reference to cardiovascular findings. Arch Dis Child. 1971;46:528.
Gonzalez OR, Gomez IG, Recalde AL, Landing BH. Postnatal development of the cystic lung lesion of Down’s syndrome: suggestion that the cause is reduced formation of peripheral air spaces. Pediatr Pathol. 1991;11:623-633.
Gorenflo M, Nelle M, Schnabe PA, Ullmann MV. Pulmonary hypertension in infancy and childhood. Cardiol Young. 2003;13:219.
Guillemot N, Troadec C, de Villemeur TB, et al. Lung disease in Niemann–Pick disease. Pediatr Pulmonol. 2007;42:1207-1214.
Hamvas A. Inherited surfactant protein-B deficiency and surfactant protein-C associated disease: clinical features and evaluation. Semin Perinatol. 2006;30:316-326.
Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease: a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18:533-547.
Hill DA, Jarzembowski JA, Priest JR, et al. Type 1 pleuropulmonary blastoma: pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol. 2008;32:282-295.
Kayton ML. Pulmonary metastasectomy in pediatric patients. Thorac Surg Clin. 2006;16:167-183.
Langston C, Patterson K, Dishop MK, et al. A protocol for the handling of tissue obtained by operative lung biopsy: recommendations of the child Pathology Co operative Group. Pediatr Devel Pathol. 2006;9:173-180.
Langston C. New concepts in the pathology of congenital lung malformations. Sem Pediatr Surg. 2003;12:17-37.
Lantuejoul S, Sheppard MN, Corrin B, Burke MM, Nicholson AG. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis: a clinicopathological study of 35 cases. Am J Surg Pathol. 2006;30:850-857.
Lantuejoul S, Nicholson AG, Sartori G, et al. Mucinous cells in Type 1 pulmonary congenital cystic adenomatoid malformation as mucinous bronchioalveolar carcinoma precursors. Am J Surg Pathol. 2007;31:961-969.
Murphy JD, Rabinovitch M, Goldstein JD, Reid LM. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981;98:962.
Nichols WC, Koller DL, Slovis B, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31-32. Nature Genet. 1997;15:277.
Parto K, Kallajoki M, Aho H, Simell O. Pulmonary alveolar proteinosis and glomerulonephritis in lysinuric protein intolerance: case reports and autopsy findings of four patients. Hum Pathol. 1994;25:400-407.
Riedlinger WFJ, Vargas SO, Jennings RJ, et al. Bronchial atresia is common to extralobar sequestration, intralobar sequestration, congenital cystic adenomatoid malformation and lobar emphysema. Ped Dev Pathol. 2006;9:361-373.
Rosen R, Fritz J, Nurko A, Simon D, Nurko S. Lipid-laden macrophage index is not an indicator of gastroesophageal reflux-related respiratory disease in children. Pediatrics. 2008;121:e879-e884.
Simmonneau G, Galie N, Rubin NJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S-12S.
Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229-1242.
Stocker JT, McGill CL, Orsini EN. Post-infarction peripheral cysts of the lung in pediatric patients: a possible cause of idiopathic spontaneous pneumothorax. Ped Pulmonol. 1985;1:7-18.
Stocker TJ. Congenital pulmonary airway malformation – a new name for and an expanded classification of congenital cystic adenomatoid malformation of the lung. Histopathol. 2002;41:424-431.
Veeraraghavan S, Koss MN, Sharma OP. Pulmonary veno-occlusive disease. Curr Opin Pulm Med. 1999;5:310.
Widlitz A, Barst RJ. Pulmonary arterial hypertension in children. Eur Respir J. 2003;21:155.