Chapter 3. Respiratory medicine
The breathless patient: the general approach 56
Is this patient’s breathlessness due to his heart or his lungs?56
Introduction
This chapter explains the basic principles underlying these two areas and then applies them to the assessment and management of the types of respiratory cases that you will encounter on a day-to-day basis: asthma, COPD and pneumonia.
The Breathless Patient: The General Approach
Is this patient’s breathlessness due to his heart or his lungs?
The two most important and most difficult questions in a breathless patient are always:
• is this cardiac or respiratory breathlessness?
• is this a pulmonary embolus?
Differentiating the causes of breathlessness can be very difficult, and the history and examination will not always provide an answer. Symptoms and signs are shared by many of the common respiratory and cardiac conditions. Thus a patient with LVF and a patient with acute asthma will both have nocturnal breathlessness and severe wheeze. Ankle swelling is characteristic of congestive heart failure, but it can also be a complication of severe COPD. The difficulties are often age related. If a young person presents with a history of intermittent wheeze, breathlessness and cough, the diagnosis is almost certain to be asthma; but what about the obese, wheezy, 70-year-old smoker with previous myocardial infarcts and chronic venous disease in both legs? Why is the patient short of breath? COPD? Pulmonary oedema? Pulmonary embolus?
What tests will be needed to make the right diagnosis?
It can be helpful to remember a few ground rules about the investigation of patients with unexplained breathlessness.
• In breathlessness caused by LVF:
— the chest X-ray will show pulmonary oedema
— the ECG is abnormal, showing ischaemia, strain or an arrhythmia
— a cardiac echogram will confirm poor left ventricular function
• In breathlessness caused by pulmonary emboli:
— the diagnosis is often missed, usually because it is not considered
— risk factors are usually present (recent surgery, phlebitic legs, etc.)
— when in doubt, anticoagulate and scan the lungs to confirm later
Principles of emergency treatment
The principles of emergency treatment are set out in Box 3.1 below. It must be recognised, however, that on occasions in a critically ill patient with unexplained breathlessness it may be appropriate to cover a number of diagnostic options until the picture becomes more clear and the results of further tests become available.
Box 3.1
1. Correct the immediate and life-threatening problems
| — Hypoxia | Oxygen Ventilation |
| — Acidosis | Correct high arterial carbon dioxide |
| — Hypotension | Fluids ± inotropic support |
2. Treat the cause
| — Pulmonary oedema | Diuretics Vasodilators |
| — Bronchoconstriction | Bronchodilators Steroids |
| — Pulmonary embolism | Anticoagulants |
| — Pneumonia | Antibiotics |
| — Tension pneumothorax | Intercostal drain |
3. Prevent further attacks
| — Asthma | Education |
| — Pulmonary oedema | Review previous cardiac therapy |
| — Pulmonary embolism | Warfarin |
Respiratory Failure
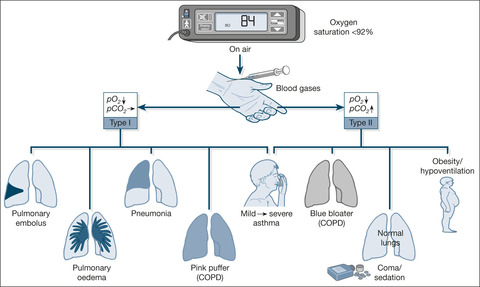
Normal ventilation is a process in which fresh air is brought through the airways to the alveoli, where it is in intimate contact with blood in the pulmonary capillaries. Gas exchange occurs across the alveolar-capillary interface: oxygen passes from the alveoli into the blood and carbon dioxide passes from the blood into the alveoli. We measure blood gas partial pressures to assess the efficiency of this two-way traffic: the arterial pO2 tells us about oxygenation; the arterial pCO2 indicates how well the lungs are venting carbon dioxide.
Type I and Type II Respiratory Failure
The lungs are more efficient at venting carbon dioxide than they are at oxygenating blood. As a result, during the development of respiratory failure, problems with oxygenation occur well before carbon dioxide starts to build up. When things start to go wrong, the patient compensates for lesser degrees of carbon dioxide retention by increasing ventilation and blowing off the build-up of carbon dioxide. Thus, if you look at the blood gases in a case of acute asthma, you will find that, in the early stage of an attack, carbon dioxide levels in the blood are often lower than normal.
Unfortunately, this compensatory mechanism does not work at all well for problems with oxygenation. You will find, for example, that arterial oxygen levels decrease early on in an attack of asthma. Several conditions have a similar pattern of hypoxia without carbon dioxide retention: this pattern is termed ‘Type I’ respiratory failure (→Fig. 3.1 and Box 3.2). (Look at ward blood gas results in some of the conditions mentioned in Box 3.2– in Type I respiratory failure the arterial pO2 is less than 8kPa, with a normal or low carbon dioxide at 4kPa or less. You need to remember that these figures apply to patients whose gases are measured while they are breathing room air; once oxygen is given to patients with Type I respiratory failure, the pO2 increases.)
Box 3.2
• The early part of an attack of asthma
• The thin breathless emphysematous patient
• Pneumonia
• Pulmonary oedema (LVF)
• Pulmonary embolism
With more advanced disease in which the compensatory mechanism of trying to blow off carbon dioxide has ‘worn out’, carbon dioxide retention occurs in addition to hypoxia. This is Type II respiratory failure (pO2 < 8kPa, pCO2 > 6kPa while not receiving oxygen; →Fig. 3.1). Examples are listed in Box 3.3; the patient in Case study 3.1 has Type II respiratory failure.
Box 3.3
• Severe life-threatening asthma (Type I failure progresses to Type II failure)
• The obese oedematous patient with severe COPD
• Respiratory centre depression in a severe drug overdose
• Obesity/hypoventilation syndrome
Case Study 3.1
A 68-year-old non-smoking woman was admitted because of increasing drowsiness following a fall at home.The emergency doctor thought she had probably had a stroke.
She and her husband led ‘separate lives’ at home but he said that she had become increasingly immobile and had taken to sleeping in an armchair over the previous 3 years. Her only medication was a mild diuretic. After a fall in her bedroom she had not been able to get up and became increasingly difficult to rouse.
On admission she was drowsy and uncooperative; she was morbidly obese and deeply cyanosed. Her respiratory rate was five breaths per minute and she was wheezy; her legs, thighs and abdomen were grossly oedematous.There was no evidence of a head injury or any focal neurological signs. Her chest film was normal. Her arterial gases showed a pO2 of 4.5kPa, a pCO2 of 16kPa and a pH of 7.1. A diagnosis of severe Type II respiratory failure due to a combination of obesity/hypoventilation syndrome and asthma was made.
The anaesthetists declined to ventilate her and attempts at NIV (→ p. 78; non-invasive ventilation in COPD, ) were only partially successful. Intravenous doxapram infusion, 24% oxygen and a combination of twice daily high-dose frusemide and aminophylline were started.With considerable difficulty she was nursed in a sitting position. On this regimen she had a marked diuresis with reduction of her oedema, although with a negative deficit of 10L she still weighed 150kg. She became increasingly alert and started to cough up purulent sputum. She slowly mobilised and lost weight over several weeks on the continuing care ward.
Hypoxia and hypercapnia: the blood gases
Carbon dioxide is a central nervous system depressant, its build up (termed hypercapnia) leads to a cycle of confusion, lack of cooperation, increasing drowsiness and hence further carbon dioxide retention. Hypoxia impairs the function of the heart, brain and kidneys. The combination of excessive carbon dioxide and the tissue hypoxia causes an acidosis that produces its own damaging effects on major organ function. (The pH in the blood gas results reflects the degree of tissue acidosis. A pH of less than 7.3 in respiratory failure indicates a major problem and a pH of less than 7.25 requires urgent and aggressive intervention to improve gas exchange.)
Principles of treatment
Before vital organ function is compromised, the blood gases must be corrected, at the simplest level by giving additional oxygen and in more complex cases by assisting or even replacing normal respiratory efforts. The introduction of new forms of ‘non-invasive’ respiratory support (i.e. the patient does not need endotracheal intubation), now means that selected patients with quite severe respiratory failure can be managed on the Acute Medical Unit with a ‘half-way house’ type of ventilation. This approach does not have the implications of full ITU treatment with the problems of endotracheal tubes, weaning, hospital-acquired infection and so forth.
Pulse oximetry
The oximeter measures the oxygen saturation in the arterial blood. The range of values for a normal saturation is small: thus a saturation of 97% is seen in the healthy population, whereas a saturation of 90% can indicate impending respiratory failure. The pulse oximeter is invaluable in recognising early respiratory failure, particularly when readings are taken on admission, before the patient has been given oxygen. You must remember, however, that the oximeter only measures oxygen saturation – it will tell you nothing about the level of carbon dioxide or the degree of acidosis.
Why and when do we do blood gases?
Oxygen saturations will be low in all cases of respiratory failure. Only the blood gases will tell you whether this is Type I or Type II respiratory failure. In acute asthma, for example, there is a world of difference between the patient with hypoxia and a normal pCO2 and the patient who is similarly hypoxic but, having passed from acute asthma to near exhaustion, is now retaining carbon dioxide. If you are in doubt about the severity of the patient’s condition, examine the blood gases. As a general rule, in Acute Medical Unit patients, arterial blood gases must be checked when the oxygen saturations are less than 92% with the patient breathing room air (you will see patients with severe Type II respiratory failure who have been given unlimited oxygen on their way in to hospital: the oxygen saturations look wonderful at 97%, but the gases reveal grossly increased levels of carbon dioxide).
Acute Severe Asthma
Mechanisms
Who gets acute severe asthma and why?
Any patient with asthma may have an acute attack, but those who are at particular risk of dying from acute asthma are:
• chronic severe asthma sufferers
• non-compliant with therapy and follow-up
• those who have previous acute/near-fatal attacks
• poor at recognising the severity of their asthma
• exposed to a high allergic load with a strong history of allergy
• normally requiring regular oral steroids
• those with a history of brittle asthma/frequent AED attendances
• those with serious psychosocial problems
• inappropriately prescribed beta blockers and NSAIDs
The number of acute exacerbations per year in which asthma treatment has to be intensified is a useful marker of the severity and control of the underlying asthma. The patient in Case Study 3.2 had been getting attacks of nocturnal breathlessness for months, but had not appreciated their significance.
Case Study 3.2
A 38-year-old man had had asthma for 30 years. Contrary to medical advice his only treatment was occasional inhaled salbutamol. He described chronic sleep disturbance from early morning wheezing, but even the presence of frequent symptoms did not dissuade him from the view that ‘normal people like him’ should not need to rely on inhalers. His asthma worsened over 2 weeks in spite of oral steroids.After a day of exposure to building dust and grass cuttings he became severely breathless and collapsed. He was admitted after his wife dialled 999.
The patient was too breathless to speak and unable to tolerate a mask.The peak flow could not be recorded. He was clammy with quiet breath sounds and a pulse rate of 160. He was given high-flow oxygen, nebulised bronchodilators and i.v. steroids. Emergency blood gases with the patient breathing oxygen showed a pO2 of 5.6kPa, a pCO2 of 16kPa and a pH of 6.9. A portable chest film showed no sign of a pneumothorax.
The patient was transferred immediately from the Acute Medical Unit to ITU and ventilated for 48h. His peak flows thereafter slowly improved on 40mg of prednisolone and nebulised salbutamol. He continued to have early morning wheezing for the next four mornings which required extra nebulisers before his symptoms slowly improved. He was transferred to the chest unit.
Three major factors narrow the airways in acute asthma:
• airway muscle contraction
• thickening of the airway wall with inflammatory cells
• airway clogging by thickened secretions
Without inhaled steroid treatment, the patient’s airways will have been chronically inflamed (→Case Study 3.2). Uncontrolled airway inflammation in asthma is a potent cause for persistent symptoms and repeated severe attacks.
What goes wrong during an attack?
As an attack develops, breathing becomes progressively more difficult because the patient has to drag and push air through increasingly narrow tubes. Air becomes trapped behind the blocked tubes so that the lungs over-inflate, making the work of breathing even harder. There are two consequences as the asthma attack progresses:
• gas exchange worsens
• the muscles used for breathing start to fatigue
The abnormal gas exchange, if uncorrected, goes through two phases.
1. Early in an attack of asthma, a combination of necessity and fear drives breathing sufficiently hard to blow off carbon dioxide. At this point, the levels of carbon dioxide in the blood are normal or low. In contrast, oxygen uptake is impaired and there is hypoxia, even at this early stage.
The patient in Case Study 3.2 had already passed this stage by the time he was admitted to hospital.
2. Later, as breathing tires, carbon dioxide builds up, oxygen levels continue to decrease and the patient’s condition becomes critical. The increase in carbon dioxide, which can be rapid and dramatic, now causes a sudden and dangerous decrease in the blood pH. If this is unchecked, a respiratory arrest will follow.
The implications for emergency management are to identify the patients who are at risk:
• those who are tiring (history, observation and blood gases)
• those with severe airway narrowing (peak expiratory flow rate)
• those with hypoxaemia (oxygen saturation) and a build-up of carbon dioxide (blood gases)
Assessment of Acute Severe Asthma
History
It is unusual for the patient to overestimate the severity of their attack. Patients like that in Case Study 3.2 who are panicking and uncooperative are far more likely to be doing so because of impending asphyxia than because of over-anxiety.
Examination
The signs of severe asthma must be recognised:
• a pulse rate of more than 110 beats/min
• a respiratory rate of more than 25 breaths/min
• the patient is too breathless to complete a sentence in one breath
• a PFR between a third and a half of their best or their predicted
A common sequence leading to respiratory arrest is of a desperately breathless patient who becomes ashen, clammy and quietly ‘cooperative’. The chest becomes quiet, the pulse slows and respiratory efforts become increasingly feeble. You may have seen patients who are poor at perceiving the severity of their own attacks, and noted how they can pass from the severe to the life-threatening stage with little apparent fuss.
The signs of life threatening asthma are any one of:
• oxygen saturations of less than 92%
• cyanosis
• a silent chest with feeble respiratory effort and exhaustion
• increasing confusion or a reduction in consciousness level/sudden collapse
• a bradycardia or arrhythmia
• hypotension
• PFR less than one-third of their best or their predicted
• PaO2 < 8.0kPa (if the PaCO2 is also > 6.0kPa the asthma is very severe)
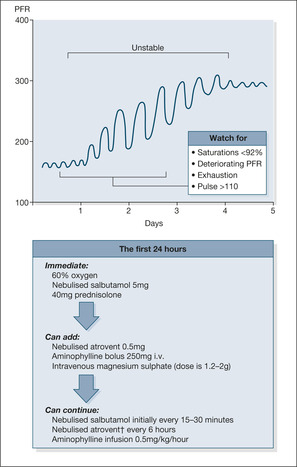
Peak flow rate
The PFR is the simplest and most important objective way to assess acute asthma and to monitor the effectiveness of treatment (→Fig. 3.2). It must be measured in every case; most patients can produce a reading. However, even the observation that the patient is ‘too breathless to do a peak flow’ is a clear indication of the severity of the asthma.
It is important to try and find out how the patient is when at their best. There is a world of difference between an admission peak flow of 250L/min in a steroid-dependent asthmatic patient who never blows better than 275L/min, and a peak flow of 250L/min in a previously ‘mild’ asthmatic individual who is used to peak flows of 550L/min. This information may be available in their hospital case notes, GP records and in home peak-flow diaries. The best ever peak flow is not necessarily the same as the predicted peak flow, but both values are useful to decide the current state of the patient’s asthma. To know the predicted PFR you will need a table giving the normal value for a person of the same height, age and sex. These tables should be freely available on the Acute Medical Unit.
• An initial PFR of less than 50% of the predicted value or, if available, 50% of their best ever PFR reading indicates severe asthma. Peak flows down to one-third of these values indicate life-threatening asthma. As a rough guide, acute severe asthma is associated with PFR readings of less than 300L/min for most males and less than 200L/min for most females.
• The change in PFR with emergency treatment predicts the likely course of events.
• The subsequent PFR chart shows the level of asthma control and stability. Peak flows seesaw as an attack resolves (Fig. 3.2). Patients are particularly vulnerable during the morning dips in PFR, which occur during the first 2–3 days after an acute attack.
Nursing the Patient with Acute Severe Asthma
The balance between resuscitation and communication will depend on your initial assessment. A patient with an unrecordable peak flow, a pulse rate of 140 beats/min and an oxygen saturation of 75% will not welcome a discussion on preventative inhalers. He will, however, be terrified by his suffocating breathlessness and will be desperate for your reassurance that he is not going to stop breathing and die (→Box 3.4). There will be excellent educational opportunities, before the patient is discharged, and while the attack is fresh in the patient’s mind. Your approach will be tailored to the individual patient. There will be an obvious difference between the communication issues in a case of chronic asthma with repeated admissions to hospital and the case of a new asthmatic presenting with their first attack. There will also be sometimes surprising similarities, not least in the most basic of requirements: the understanding and practical use of inhalers.
Box 3.4
• I will measure your PFR; we will treat you and we will see how it improves.
• The hard work of breathing will lessen with treatment.
• You will regain control of your breathlessness soon.
• We will stay with you until the treatment works.
• We will sort things out for you – try and relax if possible.
• Find your most comfortable position.
• Slow your breathing rate.
• Take deep slow breaths through the mask.
• Allow the nebuliser to do its work.
• If the mask is too claustrophobic we can use oxygen tubing to your nostrils.
• The nurses and doctors will be here near you at all times.
• Call us if your breathing seems to be getting more difficult.
• Don’t worry about all the noises from the monitors and drips.
• The oximeter shows that you are getting enough oxygen into your lungs.
• Steroid side-effects will be minimised by the short course that you need.
Critical nursing tasks during the acute attack
Give reassurance and support
Take the patient through the ways in which each aspect of his treatment acts to bring the attack to an end and enables him to regain control of his breathing (→Box 3.4).
Explain
Explain the importance of effective, continuous oxygen therapy. Show the patient how to use the nebuliser and how to perform a peak flow reading. Explain how the patient can get immediate assistance from the staff.
Minimise the work of breathing
Provide effective and timely asthma medication. Sit the patient up in his most comfortable position (this will often involve the patient wanting to sit up, either leaning over a bed table or on the edge of the bed).
▪ Pulse rate
▪ Respiratory rate
▪ PFR
▪ Oxygen saturation (preferably before oxygen is started)
▪ Ability to complete a sentence
▪ Blood pressure (may be increased by anxiety)
▪ Urine dip stick (for steroid-induced diabetes)
▪ Sputum colour (wallpaper glue sputum is common in asthma)
▪ Temperature (a sign of respiratory infection)
▪ Inhaler technique (critical for future management)
Monitor the patient’s progress
This may need to be continuous for the critical first hour or so. Beware of an increasing pulse rate, decreasing peak flow and a tiring patient. Do not be falsely reassured by a normal oxygen saturation if the patient looks awful. Just before a respiratory arrest, many asthmatic patients become quiet, clammy and cooperative.
Plan to prevent this happening again
Identify why this attack occurred. Start a re-evaluation of the patient’s long-term management that can be taken over by appropriate follow-up arrangements.
Management of Acute Severe Asthma
Initial emergency treatment
• Bronchodilators (to increase airway calibre). Nebulised salbutamol 5mg combined, if there are life-threatening features, with nebulised ipratropium 0.5mg.
• Corticosteroids (to reduce airway inflammation). Intravenous (i.v.) hydrocortisone 100mg and/or 40–50mg oral prednisolone.
• Oxygen (to correct gas exchange). High-flow oxygen at 40–60% and oxygen-driven nebulisers. The target oxygen saturations are 94–98%.
Subsequent treatment
If there is no improvement by 15min:
• repeat the salbutamol and ipratropium
• consider a single dose of i.v. magnesium sulphate 1.2–2g over 20min
• continue to give nebulised salbutamol every 15min (alternatively use a special nebuliser to give continuous salbutamol at 10mg/h)
• consider i.v. aminophylline 5mg/kg over 20min. Particular care is needed in choosing the doses, especially if the patient has been taking theophyllines as his usual asthma therapy. Start an infusion of aminophylline 0.5 to 0.7mg/kg/hour over 24h. Check daily theophylline levels
Assessing response
Following the PFR and oximetry. The key to assessment lies in a measurement of PFR. This is done on admission and, while the patient is in the unstable phase, at 15-min intervals. Thereafter, measure the PFR before and after bronchodilator treatment at least four times per day. Oximetry is monitored with the aim of keeping the oxygen saturations above 94%.
Repeating the blood gases. During the first few critical hours, you may need to use the arterial blood gases if there is any doubt about the patient’s progress. The purpose of the blood gas measurement is to identify an increase in the PaCO2 (and a fall in the pH). Thus the blood gases should be repeated 1h after the start of treatment (or earlier) if:
• poor blood gases on admission (pO2 < 8.0kPa, with a normal or high pCO2)
• saturations cannot be kept above 92%
• the patient’s condition deteriorates
Transfer to Intensive Therapy Unit? If the patient is tiring, confused or drowsy, if the PFR is deteriorating or unchanged and the patient feels worse, and particularly if the pCO2 is increasing and the pH is decreasing, you should prepare to move the patient to the ITU (→Case Study 3.2). Respiratory arrest, when it complicates acute asthma, occurs with alarming speed and always poses a major challenge to the resuscitation team. With proper assessment and by monitoring the response to treatment, you can avoid this complication.
Non-invasive ventilation? CPAP/Bi PAP are of unproven value in acute severe asthma and should only be considered in an ITU setting.
A good response to treatment. In most cases you will see a rapid response to treatment. Salbutamol and ipratropium can be reduced to 4–6-hourly. The PFRs increase and the patient will feel better. However, if you examine the PFR charts of patients who are recovering from acute asthma, you will often see that, despite a trend of improvement, the readings continue to fluctuate for several days. These patients remain at risk, particularly in the early morning when their peak flows are at their lowest. You will find that some asthma guidelines use the degree of variation in PFR between the morning and evening as an indication that the patient is fit to be discharged from hospital.
Acute Severe Asthma in Pregnancy
Control deteriorates in about a third of asthmatics during pregnancy, particularly in the second and third trimesters. Acute severe asthma will threaten the life of both the mother and the foetus. Fortunately the drug treatment for severe asthma is the same as for the non-pregnant. Oxygenation is a priority with urgent measures to keep saturations between 94% and 98%. Foetal monitoring is required from the onset and if there is any doubt about foetal well-being the patient will need to be transferred to ITU.
▪ Use of British Thoracic Society guidelines for the treatment of acute asthma
▪ Admission peak flow rates recorded
▪ Blood gases measured if saturations under 92% and appropriate action taken
▪ Serial PFRs documented
▪ The documentation of ‘best ever’ lung function (PFR or spirometry)
▪ Referral for follow-up to a respiratory nurse or chest team
▪ Review of educational needs, compliance and self-management plans for each patient
Answering Relatives’ Questions in Severe Asthma
The patient’s relatives will have found this as frightening as the patient, and will also need reassurance. You may need to address several issues with them.
Should we have acted sooner? Examine their understanding of asthma. Establish if they were aware of a self-management plan. Consider referral to the asthma service or recommend up-to-date written information (e.g. National Asthma Campaign leaflets).
Was there anything else that we could have done? Had the patient been underestimating the severity of his asthma? Had there been over-reliance on the reliever medication? What were the methods used to assess the severity of the attack? What about the use of rescue steroid medication?
The patient has been under stress – is it all nerves? Provide reassurance about the physical nature of asthma. Any ‘nervous’ component is almost certainly an effect rather than a cause of the asthma. Ensure that the patient’s visitors do not have an adverse effect on his management, particularly in the early stage when cooperation with oxygen therapy, nebuliser treatment and so forth is critical.
Could any of his other medication upset the asthma? Examine and list all of the patient’s medication. Some drugs such as aspirin, non-steroidal antiinflammatories and beta-blockers (tablets, or beta-blocker-containing eye drops) can worsen asthma. Assess the patient’s compliance with his asthma medication.
Upper Airway Obstruction: Not to be Confused with Asthma
Upper airway obstruction also produces severe airway noise and breathlessness. Although it can be difficult, a distinction in these cases must be made from acute severe asthma (→Case Studies 3.3 and 3.4).
Case Study 3.3
A 35-year-old man had flu-like symptoms for 5 days, culminating in a severe sore throat. For 24h he could not swallow fluids and had a hoarse voice. On admission he was flushed, pyrexial and breathless. He had a harsh superficial inspiratory and expiratory upper airway noise (stridor), which was easily heard at the mouth. He could open his mouth but could not swallow his saliva. His tonsils were inflamed but not particularly enlarged. His PFR was 250. Based on the characteristic history and examination a diagnosis of acute adult epiglottitis with impending respiratory obstruction was made. Intravenous antibiotics were started and he was transferred, accompanied by a competent anaesthetist, to the ENT unit for close observation and upper airway management.
Case Study 3.4
A 55-year-old male smoker was admitted with increasing breathlessness, panic attacks and noisy breathing. On direct questioning he admitted to occasional haemoptysis. He had a virtually unrecordable peak flow. Bronchodilators were ineffective and it became clear his wheeze was in fact stridor. At bronchoscopy his trachea was narrowed to a pinhole at its mid point by a carcinoma. Urgent laser treatment and chemotherapy produced an excellent symptomatic response.
Chronic Obstructive Pulmonary Disease
Mechanisms
What is COPD?
The term COPD describes patients who suffer from long-standing airflow obstruction but in whom the response to bronchodilator therapy, no matter how intensive, is either absent or incomplete. The airflow obstruction is predominantly fixed, so most of the patients have symptoms that vary little from day to day or from week to week. Acute exacerbations occur when infection temporarily worsens the airflow obstruction.
COPD embraces a spectrum of cases that range from the steroid-dependent chronic asthmatic patient to the heavy-smoking chronic bronchitic individual with advanced emphysema. The ‘mix’ in any one patient with COPD concerns how much is asthma (reversible) and how much is irreversible smoking-related damage to both the alveoli (emphysema) and the small airways (→Case Study 3.5).
Case Study 3.5
A 67-year-old man was admitted with breathlessness, general weakness and a productive cough. He had smoked 20 cigarettes a day since his late teens but had given up 3 months previously. He had been ‘chesty’ for more than 10 years with wheeziness and chest tightness. Colds always went to his chest, leaving him with a prolonged cough with green sputum. He eventually retired in his late 50s because of respiratory problems: a chronic cough, frequent chest infections and increasing breathlessness on exertion. For 2 weeks his cough and wheeze had worsened, he had become weaker and his ankles had started to swell. He had suffered a myocardial infarction 2 years previously and had occasional attacks of angina. He had also been troubled by chronic varicose veins complicated by a venous ulcer. A widower, he lived alone.
On admission the patient was pink, overweight, tremulous and mildly confused. He was receiving oxygen at 6L/min via a bag and reservoir, which had been started by the paramedics. He had a weak, productive-sounding cough but could not bring sputum up. He was wheezy, tachypnoeic at 28 breaths/min and had quite marked ankle oedema.
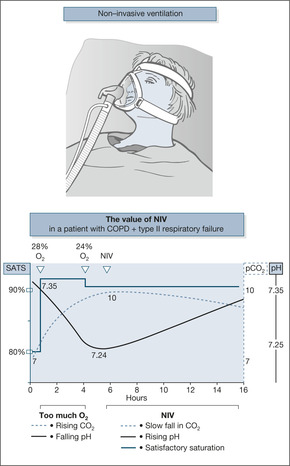
The patient’s temperature was 37.6°C and he had an irregular pulse, with an apical rate of 125 beats/min. His oxygen supply was changed to 24% by face mask, but his saturations on pulse oximetry decreased to 75%. Immediate physiotherapy helped him to cough up moderate amounts of green sputum and, after an air-driven nebuliser of ipratropium 0.5mg, he appeared less drowsy. His arterial blood gases on admission, breathing room air, were pO2 4.5kPa, pCO2 10kPa, pH 7.25.An ECG confirmed atrial fibrillation and a chest film showed overinflation but no evidence of pneumonia.
The inspired oxygen was increased by changing to a 28% mask.This improved the patient’s oxygen saturation to 85% but gases repeated after an hour showed a rising CO2 level, and the patient had become more drowsy. It was decided to try non-invasive ventilatory support by assisting his respirations with a tight-fitting nasal mask attached to a portable ventilator (nasal intermittent positive pressure ventilation).
There are three elements to COPD, all of which are seen in Case Study 3.5.
1. Asthma. This provides the responsive component to the airflow obstruction, which will improve with aggressive bronchodilator treatment. The patient will exhibit wheeze and chest tightness. There will be features of airway irritability: tightness on exposure to cold air and fumes and an early morning wheeze.
2. Chronic bronchitis. A smoking-related condition in which there is a long standing productive cough as a result of the formation of excessive mucus (phlegm) in the lungs. This is usually worse in the winter months. When the mucus becomes infected, the airflow obstruction temporarily worsens.
3. Emphysema and small airway damage. These are fixed changes in the structure of the lungs. In emphysema, the alveoli are destroyed and the lung’s elastic supporting tissue is lost. Airways collapse through loss of support and the lungs over-inflate to try and hold them open. Patients with over-inflated lungs appear barrel-chested and, to prevent airway collapse, breathe out through pursed lips: a common sign in severe emphysema that becomes particularly obvious during acute exacerbations.
The combination of airway damage and alveolar destruction disrupts the primary function of the lungs: the exchange of oxygen and carbon dioxide between the air and the blood. Too much disruption and the patient becomes hypoxic (blue) and loses the battle to blow off carbon dioxide; a combination described previously and termed Type II respiratory failure.
COPD complicated by cor pulmonale
Why did the patient in Case Study 3.5 develop ankle oedema, and why is it of such importance to his future management? Patients who are chronically hypoxic eventually develop fluid retention. A major reason for this is the increased strain on the right side of the heart that occurs because the heart has to force blood through abnormal lungs. The first sign of fluid retention, the development of ankle oedema, often occurs (as in the case study) during an acute exacerbation of COPD. The presence of ankle oedema should act as a warning to take a more aggressive approach to the treatment of the underlying lung disease. Right-sided heart failure that occurs as a result of lung disease is known as cor pulmonale. The most common cause of cor pulmonale is COPD, but it can occur in the setting of any chronic severe lung disease.
Long-term oxygen therapy
Patients with COPD who develop cor pulmonale can be helped by long-term oxygen therapy, which is administered for 15h per day or more. By correcting the underlying problem, hypoxia, the length and quality of the patient’s life can be improved. Once the patient in Case Study 3.5 has recovered from the exacerbation, he should be referred to the respiratory team for possible long-term oxygen therapy.
Assessment of acute exacerbations of COPD
Patients with COPD who are ill enough to be admitted to hospital during an exacerbation do not fare particularly well. The in-hospital mortality is around 10% and only 50% of the patients will still be alive 2 years after the exacerbation. Information from the initial assessment will help discussions on possible ventilation should the need arise, but such decisions are fraught with difficulty. The patients most in need of ventilation are least able to give a good history. Relatives’ information is vital, but may not be objective. There has to be particular caution when a patient such as that in Case Study 3.5 comes in, who is not previously known to the hospital service. Quite often, the patient has been on suboptimal treatment and may do well once the crisis is passed. The prognosis in patients with COPD does not seem to depend on whether they require emergency ventilation, but more on the severity of their pre-existing lung disease.
History in COPD
Important points in the history are:
• exercise tolerance when the patient is well and during exacerbations (use the MRC breathlessness scale; →Table 3.1)
| Grade | Degree of breathlessness related to activities |
|---|---|
| 1 | Not troubled by breathlessness except on strenuous exercise |
| 2 | Short of breath when hurrying or walking up a slight hill |
| 3 | Walks slower than contemporaries on level ground because of breathlessness, or has to stop for breath when walking at own pace |
| 4 | Stops for breath after walking about 100m or after a few minutes on level ground |
| 5 | Too breathless to leave the house, or breathless when dressing or undressing |
• the number of exacerbations per year
• previous hospital admissions and their outcome
• any history of ankle oedema
• co-morbidity, especially heart disease
• is the patient using nebulisers?
• is the patient on long-term oxygen therapy?
• is the patient still smoking?
• is there evidence of an advanced directive or of the wishes of the patient concerning resuscitation?
The initial assessment must take note of the following:
• Is the patient being given oxygen inappropriately (the patient in Case Study 3.5 was receiving a potentially disastrous quantity of oxygen)?
• Is the patient confused (confusion suggests CO2 retention)?
• Is the respiratory rate above 25 breaths/min (the patient is already at their limits of reserve)?
• Is the pulse more than 110 beats/min?
• Does oximetry indicate saturations on room air of less than 92%?
• Is the temperature above 38.5°C (indicates severe infection, particularly pneumonia)?
• How long has this attack lasted (is the patient likely to be tiring)?
• Is the cough effective and are there retained secretions?
• Is the sputum purulent?
• Is breathing hard work (pursed lips, the overuse of accessory muscles, patient sitting forwards with arms fixed)?
• How much wheeze is there (bronchodilators and steroids should help)?
• Is there ankle oedema (cor pulmonale)?
▪ Respiratory rate
▪ Oxygen saturation (preferably before oxygen is started)
▪ Is the patient at all confused (possible CO2 retention)?
▪ Is there an effective cough?
▪ Is there ankle oedema?
Analysis of the blood gases of the patient in Case Study 3.5 indicates that he needs urgent and aggressive intervention. Any of the following results needs to be acted on quickly:
• pO2 less than 6.7kPa
• pCO2 more than 9.3kPa
• pH less than 7.3
Management of acute exacerbations of COPD
In an unfamiliar patient, it can be hard to differentiate a case of end-stage COPD from a case of moderately severe COPD made worse by a reversible factor such as pulmonary embolus, infection or heart failure. If there is any doubt, it is safer to assume there is a treatable component and to manage the patient aggressively. If all else fails, this may have to include ventilation.
Controlled oxygen therapy
Many patients with severe COPD and Type II respiratory failure are in a parlous state with regards to their oxygen needs. During periods of instability there is a fine balance between:
1. the deleterious effects of hypoxia on cardiac, cerebral and kidney function, and
2. the stimulant effects of hypoxia on respiratory drive
If too little oxygen is given, hypoxia damages the function of vital organs. If too much oxygen is given, respiratory drive is switched off, carbon dioxide builds up and as a result the patient becomes acidotic (pH falls), which in itself causes organ damage.
How much oxygen is safe? The aim of controlled oxygen treatment is to maintain oxygen saturations between 88 and 92% without triggering worsening hypercapnia and a decrease in pH (increasing acidosis). Venturi masks giving 24% or 28% oxygen are safe as an initial step until the arterial gas results are known. It should be noted that COPD patients with very high respiratory rates (>30 breaths per minute) may not receive sufficient gas flow with a 24% venturi mask at the specified 2L per minute and in these the flow rate should be increased to 3L per minute. Nasal cannulae may give more than 28% and are probably not safe as first-line treatment in the unstable patient, although occasionally patients will not tolerate a mask. Gases should be rechecked 1h after controlled oxygen is started. A good response sees the pO2 increasing above 7.5kPa, with no fall in pH or increase in CO2. A poor response sees a falling pH and difficulty in keeping oxygen levels above 6.6kPa.
If the pO2 can only be corrected at the expense of a decreasing respiratory effort (as indicated by the falling pH and increasing pCO2), then an additional stimulus or aid to respiratory drive may be needed to drive off CO2. Intervention must not be delayed and if, in spite of controlled oxygen, the pH decreases to 7.35 or less, assisted ventilation should be considered. There are three ways to provide respiratory support:
• non-invasive ventilation (NIV)
• i.v. doxapram – a respiratory stimulant largely replaced by NIV
• formal ventilation on the ITU
Why measure gases when we have an oximeter? Pulse oximetry measures oxygen saturation and as such it can be a useful guide to oxygen requirements. It must be clearly understood, however, that oximetry does not measure pCO2 or pH, both of which are critical factors in Type II (hypercapnic) respiratory failure, particularly during the first 12h of treatment. It is dangerous to rely entirely on pulse oximetry in the unstable patient, in whom blood gases must be measured regularly to evaluate the effect of any change in treatment.
Antibiotics in exacerbations of COPD
Most exacerbations of COPD are primarily viral in origin. Nonetheless, antibiotics are generally given in patients with COPD who exhibit increasing breathlessness, if the quantity and colour of the sputum are changing. Amoxycillin is the first choice unless it has been given unsuccessfully at home. The emergence of the organisms Moraxella catarrhalis and Chlamydia pneumoniae as pathogens in some exacerbations of COPD means that other antibiotics such as co-amoxiclav (Augmentin®) and erythromycin may need to be considered. Although the choice of antibiotics should be determined by the result of sputum cultures, in most cases these are not available until the patient has been in hospital for several days. If the patient remains unwell with persistent breathlessness, a productive cough and sputum that will not clear, it is justified to try a potent i.v. antibiotic such as a third-generation cephalosporin.
If pneumonia is diagnosed from the chest film, the patient is treated in accordance with the appropriate guidelines.
Oral steroids in exacerbations of COPD
Steroids are used in COPD in much the same way that they are given in acute asthma: 30mg of oral prednisolone daily for 7–14 days. There is some evidence that steroids will shorten the duration of an acute exacerbation. The response will be less marked than in asthma – peak flows will show only modest increases as the patient’s symptoms improve. Typical values would be peak flows of around 120L/min on admission and perhaps 150L/min before discharge. The question of whether oral steroids should be followed by inhaled steroids will have to be considered at a later stage. In selected, severely affected cases, inhaled steroids reduce the frequency of exacerbations and slow the decline in quality of life. Overall, about 10% of patients with COPD will benefit from long-term inhaled steroids, and they can be difficult to identify during an acute illness. Inappropriate maintenance oral steroids, a common problem in COPD, can only be withdrawn under careful supervision.
Non-invasive ventilation
Non-invasive ventilation is the best way to treat patients with acute exacerbations of COPD who remain in Type II (hypercapnic) respiratory failure in spite of maximum medical treatment and controlled oxygen therapy (→Case Study 3.5). NIV has largely replaced the use of the respiratory stimulant, intravenous doxapram. Patients receiving NIV are less likely to need tracheal intubation, they stay in hospital for less time and are more likely to survive than patients treated conventionally. Success is not guaranteed, however, and before embarking on NIV there must be an explicit decision as to whether or not the patient should be intubated and ventilated if NIV fails – a decision which will be critically dependent on the patient’s pre-existing level of dependency, which must be documented in the notes.
The clearest indication for NIV is Type II respiratory failure with a pCO2 > 6.0kPa and an arterial pH less than 7.35 in spite of one hour of maximum medical treatment and controlled oxygen administration.
There must be an explicit record of whether NIV is the ceiling in any management plan, or whether the patient can go on to intubation if NIV fails.
Ventilator. The simplest and most popular machine is a Bipap ventilator, which provides two levels (Bi) of positive airways pressure (pap) – a higher pressure in inspiration (IPAP) to drive air into the lungs and a lower pressure during expiration (EPAP) to keep the bases of the lungs expanded, so helping oxygenation. The machine senses the onset of an inspiratory effort and is triggered to assist the patient by pushing in a bigger breath than would otherwise have been taken. If the machine is not triggered, either because the effort is too feeble or the patient’s respiratory rate is too low, the machine takes over and supplies the whole of the breath.
Masks. The full face mask is easier to fit than the nasal mask, and it ventilates more effectively, as many patients can only breathe through their mouth. There are established techniques for sizing and fitting the masks, ensuring that leaks are minimised and that the patient is reassured and comfortable.
Setting IPAP and EPAP levels. The starting level for IPAP is 10cm H20 increasing by 2cm H20 every 10 minutes to a maximum, if tolerated, of 20cm H20. EPAP is set at 4–5cm H20 and is not usually changed.
The first 24h on NIV. Patients who do well settle on the NIV and synchronise with the machine; they struggle less to breathe, use their accessory (neck) muscles less, their respiratory rate falls and, within 1–2h, their blood gases start to improve. Patients who do less well show lots of air leaks, they are confused or restless, their chest does not expand well in time with the machine, their vital signs are unstable and blood gases at 4h are unchanged or worse. The aim of NIV is to allow oxygenation to take place without an increase in CO2. If it is successful, the CO2 will slowly fall and the pH rise (→Fig. 3.3).
 |
| Fig. 3.3 |
Typical signs of success would include:
• respiratory rate falling below 24 breaths/min
• heart rate below 110 beats/min
• saturations more than 90% on oxygen 4L/min
• pH more than 7.35 (blood gases are checked at 0, 1, 4 and 12h)
Failure of NIV is usually due to
• Mask intolerance
• Excessive airway secretions
• Persistent mask leaks
• Nasal bridge erosions
• Patient choice
• The need to escalate treatment (intubation)
The first 24 hours of NIV. Success is determined by improvement in arterial blood gases (ABGs) after NIV has been started. These are taken after one hour (to optimise the NIV settings and oxygen administration), after four hours (a critical result which often determines escalation of care to ITU and ventilation if improvement is not apparent by then) and after twelve hours.
Frequent nursing observations (accompanied by intensive reassurance) are critical during the first four hours: every 15 minutes during the first hour, every 30 minutes for the next three. Thereafter hourly observations are continued until 12 hours, and then reduced further as appropriate.
Observations include
• Mask comfort and compliance
• Oxygen saturations
• Respiratory rate
• Pulse and blood pressure
• Extent of chest movement/use of accessory muscles
• Consciousness level (AVPU) and degree of confusion
A typical five day NIV regime
0–24 hours:
As long as possible but a minimum of six hours. One ten-minute break per hour for nebulisers, oral intake and personal care.
NIV for sixteen hours including six to eight hours overnight
48–72 hours (day three)
NIV for twelve hours including six to eight hours overnight
72–96 hours (day four)
NIV for two hours morning and afternoon and six hours overnight
96–120 hours (day five)
NIV overnight only
Clearly some patients will recover with only two to three days NIV and others will decide for themselves when to discontinue based, often, on intolerance of the whole process. Some patients need longer than five days, indeed some may require long-term overnight NIV. Late failure of NIV (after using it for 48 hours) has a poor outlook – unless the patient is suitable for treatment escalation with ITU care, intubation and ventilation.
The outlook after ventilation for COPD is surprisingly good: mean survival times are around 3 years. The patients who do well have usually been on suboptimal treatment before their admission to hospital. There is a general view that, in the UK, too few COPD patients with respiratory failure are given the chance of ventilation.
Excellence in the provision of acute NIV?
• Acohort of appropriately skilled staff
• Minimum nurse: patient ratios of 1:2 during the first 24 hours of NIV
• Structured training: induction and annual updates
• Written protocols and patient/relative information leaflets
• Individualised NIV ‘prescriptions’ and documentation of compliance and hours of use
• Explicit documentation concerning ceilings of care and plans for escalation
• Annual audit/NIV register or database
Nursing the Patient with COPD
Patients who are admitted with COPD often have complex nursing and medical needs. Most will have several major health problems besides COPD, and there is invariably a background of increasing disability and dependence. For a number, however, their usual treatment will have been less than optimum and there may be some treatable condition that has tipped the balance and brought them into hospital. As a general rule, therefore, your safest course is to assume that there is a major reversible component in all of your COPD patients. Remember, most of your patients will respond to treatment, they will be discharged with a new and better management plan and will have an improved quality of life. For those who do not improve or for those who deteriorate in spite of maximum treatment, your role becomes one of symptom palliation, but this will not be clear to you in the first 24h of the patient’s admission. Your priorities are therefore to address airflow obstruction, improve gas exchange and prevent respiratory failure. The skill for you as a nurse is to combine this with an understanding of the psychological burden that this condition carries and which colours the patient’s physical response to it (→Box 3.5).
Box 3.5
• Your breathlessness and tightness will improve as our therapies start to work.
• You will start to cough up sputum and clear your chest as the treatment takes effect.
• The more you cough up, the better you will feel.
• You can have the nebuliser on frequent occasions during the first few days.
• Because you are getting oxygen through the mask you do not have to fight for breath.
• Try not to panic on the mask, it needs to stay on to boost your oxygen levels.
• The oximeter reading is more than 80% so you are getting plenty of oxygen into your lungs.
• Your ankle swelling is due to fluid retention; it will improve slowly with treatment. It is not a sign of heart weakness.
• Find your most comfortable position even if it means sitting out in the chair.
• You will start to eat again as the infection clears and your oxygen levels improve.
• We will work hard on your chest for the next 24h and you can rest after that.
• You will get some sleep once things improve; a sleeping tablet would be dangerous.
• We will give you your other medications just as you were having them at home.
Critical nursing tasks during the acute attack
Give reassurance and support
Take a positive approach. Most patients with COPD will have experienced very negative attitudes towards their condition. Some patients will be very fearful that they have reached the terminal phase of their illness. Reassure them about the new techniques and approaches to treatment that we are now using.
Minimise the work of breathing
Many of your COPD patients will be bordering on panic. Prop the patient up with pillows or allow them to rest on pillows across a bed table. Obese and oedematous patients will be nursed best sitting out or resting with their legs over the side of the bed. One close relative can be very helpful in ensuring cooperation with masks and so forth: any more than this is counterproductive.
Monitor the patient’s progress
The first 12h are critical. Keep oxygen saturations around 88–92%. Patients who are becoming drowsy or confused need a detailed reassessment, including blood gases. Fluid balance becomes critical if diuretics are being used. Monitor the pulse: your patient is at risk from arrhythmias, particularly if they are hypoxic, are receiving aminophylline or have a cardiac history.
Prepare the patient for more active intervention if there is no improvement
Explain about the possible need for nasal ventilation. Many patients will feel relieved that the work of breathing is going to be taken from them. It is helpful to give some warning about the need for ITU ventilation if this looks like becoming an option.
▪ Documentation of existing exercise tolerance and quality of life
▪ Attempts to identify the patient’s wishes regarding active treatment and resuscitation
▪ Documentation of discussions with the patient’s family
▪ Chest film report in the notes
▪ Evidence that old hospital notes have been reviewed
▪ Appropriate use of blood gases
▪ Safe oxygen prescribing
▪ Documentation of inspired oxygen at the time arterial gases were measured
▪ Statement of treatment aims (e.g.‘To maintain saturations above 88%’)
▪ Availability of NIV
▪ PFR chart or regular spirometry
▪ Close liaison with Early Discharge COPD teams and urgent referral for pulmonary rehabilitation
Answering Relatives’ Questions in COPD
Is this asthma or emphysema? The lay view is that emphysema is untreatable and often fatal. Explain that, in most cases of COPD, there is a mixture of asthma and emphysema, and that our aim is to correct the asthmatic component. Emphasise the role of smoking cessation in preventing further deterioration.
Why is he so depressed and lacking in energy? This may be caused by chronic lack of oxygen, and we can look into this after this episode is over. There may also be a true depressive element or an anxiety state. If you suspect this, note it down for future action.
Why does he keep coming back in so quickly? You may have to accept that your patient has reached a stage where there is no further respiratory reserve. However, readmissions can also be due to social factors: loss of confidence, fear and isolation. These issues should be noted and tackled later. Social work and occupational therapy input is likely to be helpful here.
Is the heart affected? Patients with COPD often have coexisting heart disease, simply because they share the main risk factors: age and smoking. More often than not, however, ankle swelling is due to cor pulmonale rather than to congestive cardiac failure. Reassure the relatives that, as the lungs improve, the ankle oedema should resolve.
Can we get a nebuliser or oxygen for him? There are guidelines for nebulisers and home oxygen in COPD. These are best applied after specialist assessment by the respiratory team.
What can we do when he panics at home? Stay with him, give him a nebuliser followed, if it is available, by oxygen. Wait to see the effect. If he deteriorates, particularly if he appears to be drowsy, or more blue than normal or confused, ask for help.
Respiratory Infections and Pneumonia
Respiratory infections are a common cause for admission to the Acute Medical Unit (→Case Study 3.6). Most occur in a setting of pre-existing lung disease, usually COPD, or some other debilitating condition. An infective exacerbation of COPD is characterised by an increase in cough, an increase in the volume and stickiness of the sputum, and a change in sputum colour to green. There may be a fever and the infection may precipitate respiratory failure or cor pulmonale. In a frail, debilitated patient, a respiratory infection may simply cause a general deterioration with nothing more in the chest than a fruity-sounding cough and an increase in the respiratory rate. Major risk factors for the development of respiratory infection are mouth sepsis, a weak and ineffective cough, and associated conditions such as stroke or coma that increase the risk of aspiration.
Case Study 3.6
A 54-year-old fit man had been unwell for 8 days. His symptoms progressed from generalised flu-like symptoms to severe malaise and confusion. On admission he was pyrexial and unwell with a respiratory rate of 30 breaths/min and a blood pressure of 80/40mmHg. His chest X-ray showed a solid upper lobe in the right lung. His blood count showed a very low white cell count (polymorphs 0.2 × 109), a raised urea of 25mmol/L and a low albumin of 19g/L.The bacterium Streptococcus pneumoniae was grown in both sputum and blood.The pneumococcal antigen was positive in the urine.
The patient was treated with i.v. benzyl penicillin, oxygen and aggressive fluid replacement, combined with careful monitoring of the urine output. His temperature settled over 4 days and he slowly improved.
Pneumonia
Pneumonia is a type of respiratory infection that leads to consolidation of part of the lung. Consolidation impairs gas exchange and is visible on the chest X-ray. Even in a previously fit person who acquires pneumonia outside hospital, it can be a clinical challenge and there are still a substantial number of deaths. The mortality rate in cases of pneumonia admitted to hospital is 10%, but this increases to 50% in patients who are ill enough to need ITU care.
Signs and symptoms of pneumonia
In severe pneumonia, there are signs of systemic upset, including myalgia (muscle pains), diarrhoea and, in extreme cases, restlessness and shock. Even without pre-existing lung disease, pneumonia can cause severe Type I respiratory failure. Any two of the following key features are associated with a mortality rate of 20%:
• respiratory rate more than 30 breaths/min
• diastolic pressure less than 60mmHg
• blood urea more than 7mmol/L
Assessment of pneumonia
The history will identify patients who are particularly vulnerable to the development of pneumonia:
• over 65 years of age
• chronic lung disease, especially in heavy smokers
• underlying ill health or alcohol abuse
• patients who are immunosuppressed (recent chemotherapy, AIDS, myeloma, etc.)
The severity score in pneumonia: CURB-65
CURB-65 (→Table 3.2) is a simple five-point scoring system based on nursing observations and a blood test. It is used to assess the severity of the pneumonia and is a fundamental part of the initial assessment. Severe pneumonia is defined as a score of 3 or more; a score of 4 or 5 carries a high mortality rate of up to 40% (compared to 2% if the CURB-65 score is 1), and these patients should be considered for early transfer to an HDU/ITU bed.
| SCORE 0–2 = mild SCORE 3–5 = severe; SCORE 4–5 = move to HDU/ITU | ||
| CONFUSION | New-onset disorientation for time, place or person/AMTS 8 or less | SCORE 1 |
| UREA | Greater than 7.0mmol/L (as a new finding) | SCORE 1 |
| RESPIRATORY RATE | Greater than 30 breaths/min | SCORE 1 |
| BLOOD PRESSURE | Systolic less than 90mmHg or diastolic less than 60mmHg | SCORE 1 |
| 65 | 65 years of age or over | SCORE 1 |
Finding the cause of pneumonia
In order to identify the likely cause of pneumonia (which is critical in severe cases), and therefore to choose the most appropriate treatment:
• note the history (AIDS, recent travel, etc.)
• culture blood, sputum, urine, pleural fluid (if an effusion is present)
▪ Respiratory rate
▪ Blood pressure
▪ Consciousness level (particularly confusion)
▪ Oxygen saturation
▪ Pulse (rate and rhythm)
• send the blood and urine for immunology (Legionnaire’s, Pneumococcus, atypical pneumonia) and arrange for convalescent samples to be sent for comparison at 10 days. There should be an urgent testing and reporting service for Legionnaire’s urinary antigen that should be used for patients with severe pneumonia. If the urinary antigen is positive, respiratory secretions must be sent for Legionella culture.
• PCR, a molecular diagnostic technique, can accurately type viral and bacterial infection and can be applied to blood, throat swabs and respiratory secretions.
Management of pneumonia
If the assessment indicates features associated with a high risk of death (respiratory failure, shock, acidosis, disseminated intravascular coagulopathy) and particularly if the CURB-65 score is 4 or more, the patient should be managed where ventilation is available at short notice. The key to treatment lies in:
• fluid balance
• oxygenation
• appropriate antibiotics
• early mobilisation and DVT prophylaxis
Basic aims of treatment
Fluids. Suitable fluids are administered:
• to maintain a blood pressure of more than 90/60mmHg
• to maintain a urine output of more than 20ml/h (crystalloids such as normal saline are preferable to colloids such as polygeline (Haemaccel®)). Fluids may need to include inotropic support
Oxygen. Sufficient oxygen is administered to maintain saturations of between 94 and 98%. In the absence of COPD (where the target saturations would be 88 to 92%), patients with pneumonia are not at risk from a worsening of CO2 retention by receiving too much oxygen. A high-concentration mask should be used. NIV or CPAP should be used in pneumonia only in an HDU/ITU setting, as at least half the patients who reach this stage will need tracheal intubation and ventilation of the lungs.
Antibiotics. Appropriate antibiotics are administered to cover the likely causative organisms. These are based on the ‘best guess’ for the likely organism within the context of local antibiotic guidelines. The choice of antibiotics may have to be modified once results of cultures and blood tests become available.
Which antibiotic for which patient?
Examples of suitable initial regimens pending microbiological results include the following:
• Non-severe pneumonia
— oral amoxycillin 500mg tds
plus
— oral erythromycin 500mg qds or clarithromycin 500mg bd
• Non-severe pneumonia, but intolerant or allergic to first-line drugs
— oral levofloxacin 500mg od
• Severe pneumonia (CURB-65 score 3 or more)
— i.v. co-amoxiclav (Augmentin®) 1.2g tds and i.v. clarithromycin 500mg bd
or
— i.v. cefuroxime 1.5g tds and i.v. clarithromycin 500mg bd
• Severe pneumonia with other problems
— i.v. cefotaxime 1g tds and i.v. clarithromycin 0.5g bd
or
— i.v. ceftriaxone 2g od and i.v. clarithromycin 0.5g bd
• Intravenous rifampicin 600mg bd or an oral solution of levofloxacin 500mg od can also be added in very severe cases
Regimens must be reviewed at 24 hours when the initial microbiology results are available. Intravenous antibiotics are a major burden for the Acute Medical Unit in terms of both time and expense, so patients should be transferred to oral antibiotics at the earliest opportunity. The current recommendation is to switch the i.v. regimens to oral co-amoxiclav 625mg tds and clarithromycin. Antibiotics can usually be stopped after 7 days, except in particularly severe cases of pneumonia.
Pleural effusion and pleural empyema
One of the most common complications of pneumonia is the development of a pleural effusion. If the fluid in an effusion becomes infected, an empyema (pus in the pleural cavity) will develop.
Empyemas are seen in the elderly and debilitated, particularly when the treatment of a pneumonia has been delayed (→Case Study 3.7). An empyema should be suspected if there is persistent fever associated with weight loss, pleuritic pain and weakness despite appropriate antibiotics. The treatment of an empyema involves clearing the infected fluid from the pleural cavity by intercostal drainage.
Case Study 3.7
A 17-year-old youth was admitted with severe chest pain and malaise.Three weeks before admission he had become acutely unwell with a cough, shivering and breathlessness. He treated himself with aspirin. On examination he was pyrexial, pale and unwell looking. He had a productive cough, severe right-sided chest pain on inspiration and signs of a right-sided pleural effusion. His chest film showed a solid right lower lobe and fluid in the pleural space.
A diagnosis of unresolved pneumonia with possible empyema was made. His effusion was aspirated to dryness and sent for examination. He was started on ampicillin and erythromycin.
Aspiration pneumonia
Frail patients, especially with swallowing difficulties due to neurological disorders, are at risk from pneumonia through aspirating food or nasophayngeal secretions into the lungs. The usual antibiotic regime for aspiration pneumonia is i.v. co-amoxiclav 1.2g tds. Metronidazole is given with i.v. cefuroxime if the patient is allergic to penicillin. Aspiration pneumonia due to MRSA should be considered if:
• The patient has had previous MRSA
• The patient has come from a nursing or residential home with chronic breaks in the skin
• There is a long-term catheter
• The patient has been in hospital within the previous six months
Nursing the Patient with Pneumonia
The patient with pneumonia combines the challenges of uncontrolled sepsis with the management of threatened or actual respiratory failure (→Case Study 3.7). For many patients, the pneumonia will be complicating pre-existing medical conditions, so your assessment will need to be both comprehensive and searching. For example, has your patient with a CVA developed pneumonia because he cannot swallow and has aspirated? Your most important immediate task is to grade the severity of the pneumonia. This is simple to do and is based on the combination of vital signs (notably respiratory rate and blood pressure) and basic laboratory investigations. As a general rule, the more ‘multisystem’ the symptoms appear (e.g. diarrhoea, muscle pains, headache), the worse the pneumonia. Some organisms attack several systems at once. Has your patient with pneumonia, a splitting headache and photophobia also got meningitis? If you are lucky, the history may help you establish the cause of the pneumonia (for example on our unit a failure to seek out appropriate risk factors led to a lengthy delay in recognising an AIDS-related pneumonia). The history is more likely, however, to highlight other relevant medical conditions that will influence the speed of recovery.
Patients with pneumonia will be in need of reassurance (→Box 3.6).
Box 3.6
• Pneumonia is a treatable infection in the lungs.We are trying to trace its source.
• It would be very unusual for other members of your family to be at risk.
• The drip is to give you fluid and antibiotics.
• For the first few days you will be helped by continuous oxygen through the mask.
• The chest pain is due to inflammation in the lining of the lung – it is not from your heart.
• Your fever and sweats will take several days to subside.
• Your breathlessness is due to temporary lung damage, which is starting to heal. Although you will feel better in a few days we would not expect your X-ray to clear completely for 3 or 4 weeks.
• It is quite normal to have no appetite at all and feel very weak at first.
• You will probably be able to cough up sputum more effectively as you start to improve.
Five critical nursing tasks during the acute attack
Provide timely therapy
Once the diagnosis is suspected, your patient must receive their antibiotics. Hypotension needs urgent attention with fluids. Hypoxia is treated with high concentrations of oxygen.
Explain
Explain the importance of continuous oxygen. Reassure the patient that the i.v. antibiotics are given for the first few doses only.
Monitor the patient’s progress
The hypotensive and ill patient will need a urinary catheter to ensure an adequate urine output (20+ ml/h). Oxygen saturations should be maintained between 94 and 98%. The pulse and temperature should start to decrease within 24–48h of the start of treatment. Persistent confusion in your patient is a worrying sign.
Assess the need for analgesia
Opiates are not advisable because of respiratory depression, but you must try and relieve any pleuritic pain. The pain of pleurisy prevents an adequate cough and impairs deep inspiration.
Watch for complications
• Ankle oedema and extreme lethargy: either cardiac failure or a low albumin due to systemic illness (lung abscess?)
• Low urine output and nausea: renal failure complicating sepsis
Answering Relatives’ Questions in Pneumonia
Pneumonia has a bad reputation with the lay public, who still associate it with death, especially in the elderly. This reputation is not undeserved, particularly when the pneumonia complicates other serious diseases such as cancer, diabetes and stroke. Many patients with pneumonia will have passed through a flu-like stage at home and will have seen their family doctor on two or three occasions, with or without the prescription of antibiotics. Many of you will recognise the words, ‘why wasn’t something done sooner!’, and you may have had to deal with combinations of resentment and anger. You must remember that it is easy to diagnose pneumonia in hindsight, particularly with the aid of on-site chest films. It is probably best to explain to the relatives how the disease can progress rather than to imply any criticism of their initial management. Common questions that you will have to deal with are listed below.
Is this bronchopneumonia, lobar pneumonia or double pneumonia? The term ‘bronchopneumonia’ is commonly used to describe the terminal respiratory distress in a frail and usually elderly patient. In practice it means infection, usually in the lung bases, as a result of retained secretions and an ineffective cough.
‘Bronchopneumonia’ is often stated on the death certificate to be the immediate cause of death in patients in whom it was simply the terminal event after, say, severe stroke, advanced malignant disease or dementia.
‘Lobar pneumonia’ is a more exact term, indicating the extent (a lobe) of the consolidation. ‘Double pneumonia’ is an entirely lay expression, presumably indicating involvement of both lungs.
Can the rest of us catch it? There can be a common source of infection (such as a faulty water-cooling system in an outbreak of Legionnaire’s disease), but person-to-person spread is rare.
Will the confusion clear? Providing the oxygen mask is kept on and the antibiotics work, the confusion will clear. It is, however, an indication of the severity of the case that you are dealing with.
Will there be any permanent lung damage? There should be a complete recovery, but this should be seen as an opportunity to ensure that the patient stops smoking.
What if he gets worse? If your patient has poor prognostic signs, introduce the relatives to the possibility of ITU care and possible ventilation. One way to discuss this is as ‘a stop-gap measure to allow the lungs to rest while the antibiotics get to work’.
Can you feed him up? He has not eaten for days. To start with, the priority is fluid, antibiotics and oxygen. As he improves his appetite will return.
Why don’t you X-ray him again to see how he is progressing? Unless we need to look for complications, the chest X-ray will not improve for at least 2 weeks. The best sign of recovery is when the patient starts to feel better. Special tests such as CT scan are only helpful to sort out any complications.
Spontaneous Pneumothorax
A spontaneous pneumothorax occurs when a defect on the surface of the lung ‘pops’, letting air out under positive pressure into the pleural space. This pressurised air prevents expansion of the lung and can push the mediastinal structures to the opposite side of the chest (a tension pneumothorax implies a large, high-pressure pneumothorax with a major effect on lung function). The symptoms are pain, breathlessness and, in severe cases, cardiorespiratory collapse. As you can see, a pneumothorax can be confused with three major medical conditions:
• pulmonary embolus
• myocardial infarction
• pleurisy
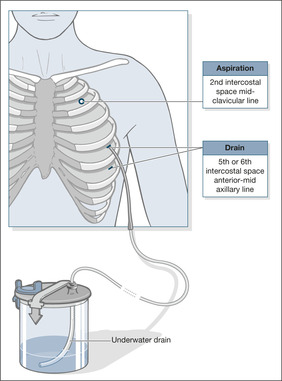
Small pneumothoraces (a rim of air less than 2cm wide on the chest X-ray) produce few symptoms and improve without treatment. A rim of more than 2cm is a ‘large’ pneumothorax; the patient is usually breathless and intervention is necessary. Active treatment of a pneumothorax involves removing the air from the pleural cavity by simple aspiration or, in more difficult cases, by placement of an intercostal drain. Tube drainage is best performed with a small-bore (9–14F) catheter inserted very easily using a Seldinger technique and attached to either an under-water seal or a flutter valve.
Whether a patient is admitted for observation and possible intervention depends primarily on:
• the degree of lung collapse
• the amount of breathlessness
• the presence of pre-existing lung disease (patients with emphysema and asthma are prone to pneumothoraces. Even a small pneumothorax in a patient with severe lung disease or occurring during an asthma attack can be the last straw)
A primary pneumothorax occurs in otherwise normal lungs; a secondary pneumothorax is caused by underlying lung disease (usually emphysema) or trauma.
If aspiration does not lead to re-expansion of the lung, or if there is expansion followed by further collapse within 72h, then an intercostal drain will be needed (→Fig. 3.4). Except when very small and asymptomatic, all secondary pneumothoraces require tube drainage.
Once a drain is in place:
• It should be clamped only under exceptional circumstances (not because the patient is going for a chest film).
• Chest drains that are still bubbling should not be removed unless they are to be replaced.
• If the drain is in place for more than 24h, the patient should be referred at that time to the respiratory team.
The management of a pneumothorax can be troublesome, particularly if there is also chronic lung disease present. A safe course of action is to refer all cases of pneumothorax to the respiratory team.
Safety Issues: Pleural Aspiration and Pleural Drainage
There are increasing concerns about the safety of pleural aspiration and drainage when it is carried out on non-specialist wards, including the AMU, particularly when performed ‘out of hours’. Inexpertly placed drains can penetrate the liver, lung, spleen and heart; inattention to sterile techniques can lead to infection; inadequate pain relief and a clumsy technique can leave the patient (and the staff) traumatised. Current guidelines provide clear advice to minimise the risk and reduce the stress and discomfort to the patient:
• Except where urgent clinical need dictates, pleural procedures should not be done ‘out of hours’
• Unless in an absolute emergency, there must be documented patient consent
• These are sterile procedures (clean space, adequate skin cleansing, gown, gloves and drapes)
• Commercial chest drain packs should be used which contain everything that is needed: gowns, drapes, skin preparations, syringes, needles, scalpel and appropriate dilators, guide wires, drains, 3-way taps and a variety of connections.
• The positioning of the patient and the choice of aspiration/drainage sites are critical to success
• For pleural effusions, bedside ultra-sound will become standard for identifying safe aspiration/drain insertion sites
• Oxygen saturations must be monitored throughout the procedure
• The nursing staff who are responsible for the patient must be familiar with the management of chest drains
• Patients should be encouraged, when appropriate, to take some responsibility for the care of their drains (and keep the drainage bottle below the level of the drain entry site at all times)
Nursing the Patient with a Chest Drain
Air, fluid, blood or pus in the pleural space can result in an acutely ill patient who is severely breathless and requires an urgent chest drain. The small Seldinger drains (10–14F) are easy to use, generally well-tolerated and are increasingly used in preference to large-bore tubes. There are still occasions, however, when a large (24–28F) tube may be required.
Few procedures on the Acute Medical Unit cause as much fear and anxiety to the patient and the staff as the insertion of a large intercostal drain. Under stress they are put in and under darkness they fall out.
Prepare your patient for the procedure
The patients are often young and distressed and will need a lot of reassurance, with an honest explanation of what to expect: ‘There may be initial discomfort and a sensation of pushing, but the local anaesthetic will deaden any pain; if the pain is severe, tell us and we will put in more anaesthetic’. Explain that the breathlessness will go as soon as the tube is in place. It is wise to be non-committal about how long the tube will stay in. A sizeable proportion of pneumothoraces need prolonged drainage and surgical intervention: ‘If things go according to plan, it will be removed in a few days, but if it is less straightforward, the tube will need to stay in longer until the lung goes up and stays up’.
Positioning the patient
The patient needs to be in a position exposing the ‘triangle of safety’ at the base of the axilla – the area chosen for the majority of drains. The patient can sit in bed at 45 degrees with the appropriate arm behind his head. Alternatively the patient can sit in or out of bed and lean forward with his head bent across his folded arms resting either on a bedside table or, if he is in a chair, across the bed.
Alleviate the pain of the intercostal drain
If there is no contraindication, a small dose of i.v. midazolam administered at the time of the skin incision may make the difference between a tense, panicking patient and one who is relaxed and cooperative. Check whether your patient is being given sufficient analgesia. Many doctors underestimate analgesic requirements in medical conditions.
Problems with intercostal drains falling out or falling apart
There should be a local protocol for a standardised technique for placing and securing a chest drain. Common problems that you will see include:
• the weight of the connecting tubing pulling the drain out of the chest
• untaped connections coming apart
• failure to recognise, and act, on a displaced or disconnected tube (clamp an open-ended tube, seal over a sucking chest drain site)
Try to use transparent dressings, so that you can assess the state and site of the tube on at least a twice-daily basis. Simple follow-up measurements of how much tube is visible will warn of imminent displacement. If a tube is on suction and works its way out of the chest cavity, constant bubbling will be seen as soon as the drainage holes are exposed to the air. Important nursing observations are of the amount of fluid drained (effusions), whether the tube is bubbling (pneumothorax) and whether it is swinging (confirming the tube is patent and remains in the pleural cavity).
Three simple tube rules
• bubbling tubes need to stay in and should never be clamped
• swinging tubes can probably be removed if the lung has expanded
• non-swinging tubes are often blocked (hence the failure to re-expand?)
Further Reading
Asthma
Hart, S.R.; Davidson, A.C., Acute adult asthma – assessment of severity and management and comparison with British Thoracic Society guidelines, Respiratory Medicine 93 (1) (1999) 8–10.
COPD
Bardi, G.; Pierotello, R.; Desideri, M.; et al., Nasal ventilation in COPD exacerbations: early and late results of a prospective, controlled study, European Respiratory Journal 15 (1) (2000) 98–104.
Pneumonia
Lim, W.S.; Lewis, S.; Macfarlane, J.T., Severity prediction rules in community acquired pneumonia: a validation study, Thorax 55 (2000) 219–223.
Pneumothorax
Hyde, J.; Sykes, T.; Graham, T., Reducing morbidity from chest drains, British Medical Journal 314 (1997) 914–915.
Currie, G.P.; Alluri, R.; Christie, G.; et al., Pneumothorax an update, Postgraduate Medical Journal 83 (2007) 461–465.
Website
Access to all the British Thoracic Society Guidelines (Asthma, COPD, NIV, Pneumonia, Pneumothorax), http://www.BritThoracic.Org.Uk/Public_Content.Asp?Pageid=7&Catid=36&Old.