Chapter 6 Respiratory infections and immune insufficiency
OVERVIEW
Respiratory infections are caused by pathogenic invasion and colonisation.1 Upper respiratory tract infections (URTI) involve the nose, sinuses, pharynx or larynx, while lower respiratory tract infections (LRTI) involve the lungs and bronchi, and include pneumonia, lung abscess, acute bronchitis and bronchiectasis.
The term coryza, (the common cold) encompasses for a number of viral URTIs with heterogeneous presentation.1 They may in turn lead to secondary LRTI, or predispose to bacterial URTI. The common cold is most frequently experienced with nasal congestion and drainage, sneezing, sore or scratchy throat, cough and general malaise.1,2 Influenza is regarded as a separate disease entity, although the two often overlap.1 Manifestations of influenza commonly include fever, myalgia, fatigue, malaise, headache, pain behind the eyes, dry cough, runny nose and sore throat (see Table 6.1).3,4
| SYMPTOM | COMMON COLD | INFLUENZA |
|---|---|---|
| Onset | Gradual: 1–3 days | Sudden: within a few hours |
| Site of infection | Upper respiratory tract | Entire respiratory tract |
| Nasal congestion | Frequent | Occasionally |
| Rhinorrhea | Frequent | Occasionally |
| Sneezing | Frequent | Occasionally |
| Sore throat | Frequent | Occasionally |
| Cough | Less common | Can be quite severe |
| Chest discomfort | Mild | Pronounced |
| Fever | Rare | High |
| Myalgia | Occasional or insignificant | Severe |
| Fatigue/malaise | Mild | Long lasting |
| Headache | Rare, more mild | Prominent and severe |
| Exhaustion | Rare | Early and prominent |
Source: Adapted from Meissner 20053 and Roxas & Jurenka 20075
Respiratory tract infections (RTIs) are among the most commonly experienced illnesses, and place large economic costs on the community in terms of absences from work and school, and also in visits to medical professionals.1,2 While most are self-limiting and resolve within time, they can be quite serious. Influenza kills approximately 36,000 in the United States of America every year,3 with higher mortality rates in the elderly, who have weaker immune systems.6 Many more Americans (exceeding 200,000) experience complications that require hospital admission.3
URTIs are more commonly experienced in the cooler months of the year, and in the rainy periods of tropical areas.1 Children contract a greater number of infections throughout the year than adults, possible due to their close socialisation in day care and at school.3,7
Transmission occurs by one of three main methods:1–37
Most acute respiratory illnesses are viral in origin.8 There are several viruses which cause the common cold, with rhinoviruses (HRV) playing a prominent aetiological role.1,2,9 In autumn, the peak time for colds, the HRV accounts for up to 80% of URTIs.10 Other predominant viral agents include respiratory syncytial virus (RSV), influenza virus (INF) and parainfluenza viruses (PIV).1,3,8
Genetics play a large role in immune function, and innate resistance to infection is principally inherited rather than acquired.11 The set of host genes controlling this is termed the ‘resistome’, and this varies from person to person.11 Defective genes in the resistome present most strikingly in congenital immunodeficiency syndromes, but variance in the genetic set may also lead to variability in the individual capacity of resistance to infections.12,13
Epidemiological data and the evidence from viral-challenge studies suggest that psychological stress is a risk factor for the development of URTI.14
Diet also has a key role in immune capacity. Malnourished children in developing countries, particularly those with vitamin A deficiency, are at far higher risk of contracting respiratory infection and also experience more severe morbidity.15 Many nutrients play a role in responding to microbial and viral challenge, and deficiencies in zinc, vitamin A, C, B6, iron, copper, selenium, protein intake and omega 3 fatty acids have all been shown to negatively affect immune capacity.6,16–21
Environmental chemicals are known to be immunotoxic and children exposed to these chemicals at an early age (pre- and postnatally) experience higher rates of respiratory infection than comparative populations with low levels of exposure.15,22
An inverse relationship has been demonstrated between the level of physical activity and the number of URTIs experienced by adult males.23 However, while moderate exercise enhances immune function and lowers the risk of URTI, intensive exertion actually produces a temporary suppressive effect on both the adaptive and the innate systems.24
Traditional knowledge links the development of URTI with a drop in body temperature due to cold exposure. This connection was examined in healthy subjects, who each received a 20-minute foot chill at 10°C. Approximately 29% of those receiving a foot chill were diagnosed with a cold 4 days after the chill experience, compared to 9% of control subjects (a significant difference).25 In another study, a clear connection was demonstrated between the incidence of URTI and LRTI and decreases in average ambient temperature.26
PATHOPHYSIOLOGY
The pathogenesis of URTI differs depending on the organism, but generally involves interaction between viral replication and the host immune inflammatory response.1 Viral infection stimulates a local inflammatory response, which causes the classic symptoms of the common cold—rhinorrhea and nasal obstruction.1,9 Sneezing and excess mucus production are precipitated by increased cholinergic stimulation in the area.1 Inflammatory mediators interleukin (IL) 6 and IL-8 appear to be particularly involved, with some studies correlating their concentration in nasal secretions directly with the severity of cold symptoms.27,28
Contrastingly, influenza viruses inhabit and replicate in the epithelium of the tracheobronchial area.4 Here they generate a similar inflammatory response, but also cause overt epithelial toxicity, which contributes to the severity of symptoms.4,9
CONVENTIONAL TREATMENT
As the common cold is caused by a multitude of different virus types with varying pathogenic mechanisms, a universal medical treatment remains elusive. Until more effective antiviral treatments are available, the treatment of choice for URTIs remains rest, increased fluids and symptomatic relief (usually with over-the-counter medications).29
Nasal congestion and rhinorrhea are the two most vexing symptoms of URTI, and are often addressed with intranasal or oral decongestants (see Table 6.2).30 Sneezing and rhinorrhoea may be treated with first-generation (but not second-generation) antihistamines though these drugs have no role in shortening the duration of the virus.30,31 Non-steroidal anti-inflammatory drugs are effective at reducing soreness of the throat, cough and systemic effects of URTI such as fever and malaise.32 Cough medications, both antitussives and mucolytic agents, are also frequently used, although their efficacy seems variable and limited.33
| URTI SYMPTOM | TREATMENT GOAL | PHARMACOLOGICAL TREATMENT |
|---|---|---|
| Nasal congestion | Reduce inflammation and size of nasal turbinates | Decongestants |
| Rhinorrhoea | Reduce seromucous gland secretion |
Source: Adapted from Gwaltney 200232
Antibiotic use is contentious in the treatment of URTI. The general consensus is that there is no benefit in treating the common cold with antibiotics.34 Most studies show no difference in symptom improvement between those treated with antibiotics immediately and those with delayed prescriptions.35 However, in patients who are more predisposed to complications, such as those with underlying lung disease, evidence does exist to support the use of these drugs in the treatment of URTIs.34,36
At present, specific antiviral treatments for respiratory viruses are commercially available only for influenza viruses.37 Because of the leading role of rhinoviruses in the common cold, effective antivirals against these viruses could be expected to have the greatest effect in the treatment of this disease. Although these agents exist, they are still in the developmental stages, and often are not as clinically efficacious as in vitro studies would suggest; they must be taken early and frequently in order to have an effect, and it is often too late by the time a patient presents to their general practitioner.2
KEY TREATMENT PROTOCOLS
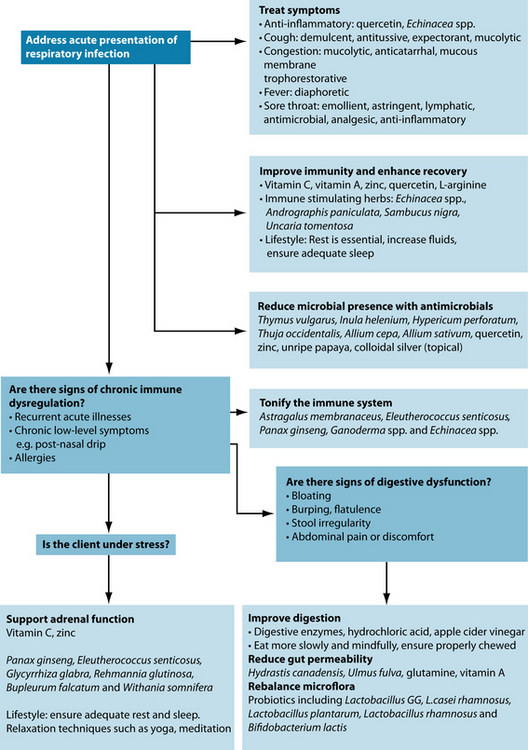
From a clinical perspective, it is important to address the acute presentation first. Amelioration of symptoms, limitation of causative pathogens, and strengthening immune defences and clearance will address the patient’s immediate concerns and help them to feel better. Once this is resolved, the practitioner should endeavour to restore the health and wellbeing of the patient, and address causes of immune insufficiency that may predispose to future infections.
Addressing the symptoms
While it is important to take a holistic view of the disease process and underlying causes, symptomatic treatment of URTI is also essential (see Table 6.3). Clearing the infection and ameliorating the symptoms can be accomplished concurrently. Overall, many symptoms are the result of an inflammatory response, and thus reducing this will address the symptom cluster as a whole.
Quercetin demonstrates a potent ability to inhibit inflammatory cytokine and chemokine production in acute and chronic inflammation, including the cytokines IL-6 and IL-8, which are both implicated in the aetiology of cold symptoms.38,39 Echinacea spp. may also be of use in this regard, as it is known to modulate levels of these two cytokines.40 (See ‘Immune modulation—humoral and cellular immune system’ below.)
For the protocols for addressing symptoms such as phlegm, cough and nasal congestion, please refer to Chapter 8 on congestive respiratory illness.
Fever management
Overview
Fever is a state of elevated core temperature, which is part of the defensive response to the invasion of live or inanimate matter recognised as pathogenic or alien.46 This response is a complex physiological reaction involving a cytokine-mediated rise in core temperature, generation of acute phase reactants, and activation of numerous physiological, endocrinological, and immunological systems (see Figure 6.1).46
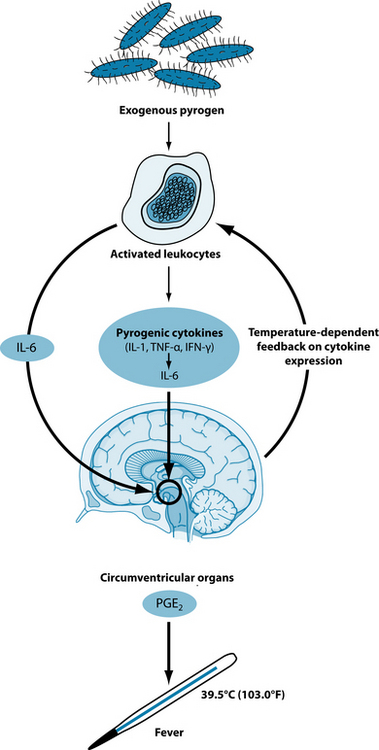
Figure 6.1 Hypothetical model for the febrile response. IL indicates interleukin; TNF, tumour necrosis factor; IFN, interferon; and PGE2, prostaglandin E2.46
Accompanying the development in antipyretic therapies such as external cooling and specific pharmacological antipyretic agents has come a change in medical philosophy: from fever as a beneficial host defence mechanism to fever as a symptom indicating the need for aggressive therapy.47
Treating fever presentation with antipyretic medication is commonly considered by the medical community to do no harm, nor to slow the resolution of common viral and bacterial infections.48 However, other data suggest a beneficial effect from fever and, correspondingly, adverse effects from antipyretics on infection outcomes. A positive correlation was found between maximum temperature on the day of bacteraemia and survival in a retrospective analysis of 218 patients with bacteraemia.49 In an examination of factors influencing the prognosis of spontaneous bacterial peritonitis, a positive correlation was identified between a temperature greater than 38°C and likelihood of survival.50 Paracetamol may potentially prolong chicken pox, as treated subjects experience a longer time to total crusting of lesions than placebo-treated controls,51 possibly allowing for a longer period of viral spread. Adults infected with rhinovirus exhibit more nasal viral shedding when they receive aspirin than when administered placebo.52 A trend towards longer duration of rhinoviral shedding was found in association with antipyretic therapy, showing that the use of aspirin or paracetamol is associated with suppression of the serum neutralising antibody response, and increased nasal signs and symptoms.53
An open, randomised, prospective clinical trial compared an aggressive fever treatment strategy (650 mg paracetamol every 6 hours for fever > 38.5°C and cooling blanket added if > 39.5°C) with a permissive strategy (treatment reserved for fever > 40°C only) in stable but critically ill patients. The aggressive treatment group had a higher number of infections compared with the permissive treatment group and a slightly higher rate of antibiotic use. No significant difference in the length of ICU stay was observed. However, the study was ceased prematurely due to safety concerns, after interim analysis revealed an excess mortality rate of 16% (seven of 44) in the aggressive group compared with 3% (one of 38) in the permissive group.47
Naturopathic management of fever
This has led some researchers to suggest that fever suppression may be potentially harmful, and that treating moderate fever with antipyretic or direct cooling therapies may be counterproductive.54 Key pyrogenic cytokines have also demonstrated immune-potentiating capabilities that may in theory enhance resistance to infection, lending credence to the idea of fever as beneficial.55
However, the potentially life-threatening nature of fever also needs to be emphasised and respected. Full use should be made of current medical understanding and diagnostic techniques to ensure the fever is not related to a serious condition. Close monitoring of the fever should also be employed, to ensure it stays within an acceptable range (up to 38.9oC),55 although this can vary in significance depending on whether the fever is deemed to be continuing to rise (the patient feels chilled, indicating they have not reached the new temperature set point) or has reached a peak and has started to fall (the patient feels hot).55
According to traditional herbal principles, first line treatments for fever have a ‘normalising’ effect, being regarded as mildly heating diaphoretics. Herbal examples of these include Achillea millefolium, Verbena officinalis, Hyssopus officinalis, Tilia europaea, Sambucus nigra, Eupatorium perfoliatum, Thymus vulgaris, Nepeta cataria, Tanacetum vulgare, Melissa officinalis, Oxalis acetosella and Polygonum bistorta.41,43
It is possible that a fever may require enhancement if it is considered that it is not adequately materialising, and sufficient effort has also been made to ensure there is no underlying pathology or life-threatening infection.41,43 Here moderately warming/circulation-stimulating remedies such as Cinnamomum zeylanicum, Allium sativum and Elettaria cardamomum can be used readily. Stronger heating remedies like Capsicum spp., Zingiber officinale and Armoracia rusticana require additional care as they can prove more stimulating to the fever process.41
Just as there may be reason to enhance a fever, it is possible that it may need to be controlled. In this instance cooling bitters are recommended, for example Taraxacum officinale, Cinnamomum cassia, Gentiana lutea and Andrographis paniculata.41,43
One trial found that zinc supplementation resolved fever 3.1 times more rapidly in 2- to 24-month-old boys.56 It is suggested that this nutrient is most beneficial in treating fever when the patient has sub-optimum zinc serum levels (see Table 6.6 for a review of the evidence).
Address pathogens
For a discussion of the role of antimicrobials, antivirals and antifungal agents in respiratory illness, refer to Chapter 8 on congestive respiratory illness.
Immune modulation—humoral and cellular immune system
Any factor which depletes immune function may predispose a person to develop a RTI, and those who suffer immune insufficiency, such as the elderly, those with co-existent disease, and the immunocompromised are particularly at risk of recurrent or severe infection.57
The causes of lowered innate and adaptive immune resistance are multiple and dynamic in interaction, and immune resistance varies throughout life58 (see ‘Immunity’). The innate homeostasis of individual immune response is influenced by genes, stress, diet, environmental influences, age and prior infection or inflammatory events.58
Adequate host defence mechanisms play a role in the symptom severity and clinical outcome of URTIs. While both specific and humoral immunity are important in host response to rhinoviral infection, it seems that the innate response is dominant early after infection, and modulates the symptomatic presentation.9 This is also the division that is responsible for immunosurveillance of pathogens, and for preventing initial entry.59 Thus, it is important to strengthen this general defence mechanism.
Antigen-specific humoral and cellular immune responses to URTI are elicited, but are generally not detectable until after symptoms have abated.9 Thus, enhancement of these systems is more relevant with regard to prevention of re-infection.
Vitamin C has been shown to reduce the incidence and improve the outcome of a number of infections, including RTI.19 A Cochrane review suggests that vitamin C supplementation is consistently associated with a modest reduction in the duration and severity of colds.60 The results were most positive in those trials using high doses of 8000 mg daily or more. This intervention works on a number of pathways, although not all of the mechanisms are completely understood.61,62 Vitamin C is known to be a regulator of redox and metabolic checkpoints that organise the activation and continued survival of immune cells.19 Ingestion of buffered vitamin C has been shown to significantly enhance both natural killer cells’ numbers and activity,63 and may also enhance T- and B-cell function, suggesting benefits for adaptive immunity and prophylaxis.63
Vitamin C may also ameliorate symptoms via its influences on cytokine production, as it inhibits the expression of IL-6 in particular.64 Other studies show effects on phagocyte function, production of interferon and gene expression of monocyte adhesion molecules, thereby enhancing immune function.16,61,65
Nitric oxide (NO) production by epithelial cells has shown a clear role in the body’s antiviral responses9 and reduces epithelial cell release of cytokines and chemokines induced by viral infections.66 Vitamin C has demonstrated the ability in cell lines to increase NO production, and thus may aid in viral clearance and symptom reduction via this mechanism.67
Arginine, the physiological precursor for NO, may also be a novel supplement consideration in this area.68
One of the most widely used immune modulating nutrients is zinc. Low levels of this mineral affect almost all aspects of innate and adaptive immunity. It is crucial for the development and function of natural killer cells, phagocytes, macrophages and neutrophils and lack predisposes a person to lymphocytopenia, reduced type 1 T helper (Th1) cells, and decreased thymic function.16,69–71 Prolonged states of deficiency effectively ‘reprogram’ the immune system, by increasing glucocorticoid secretion, which accelerates pre-T-cell and pre-B-cell apoptosis.72 Zinc is also essential for cytokine production and secretion.16,69–71
Several studies have demonstrated that zinc administration (up to 30 mg) is effective to ameliorate symptoms, shorten duration and decrease incidence of respiratory infections.19,73 In children, preventative zinc supplementation (at varying doses) may decrease episodes of RTI by approximately 15%, increasing to 25% in a zinc-deficient population.74,75 The most bioavailable forms of zinc supplementation appear to be glycinates, gluconates and zinc-enriched yeast.76–78
Reviews conclude that the effectiveness of zinc lozenges remains to be established. About half of studies seem to indicate beneficial effects, but a number of these fail to meet rigorous design criteria.79–81 Zinc nasal gel, however, has shown efficacy in reducing the duration of cold symptoms.82
Quercetin may be a useful additional supplement in acute respiratory illness. It seems to exert antiviral effects via Th1:Th2 modulation, encouraging the production of Th1-derived cytokine INF-γ, which eliminates or blocks viral replication in infected cells.83 Studies suggest that doses of up to 1000 mg/day may protect athletes from URTI in the period of immune depletion following heavy exertion.84,85 The mechanism seems to be directly antiviral, rather than correction of immune dysregulation.59 There is some evidence that quercetin supplementation may decrease expression of IL-886—possibly reducing nasal inflammation in the common cold. In influenza, it may protect the lung from the damaging free radicals generated in the disease process.87
Low levels of vitamin A cause a wide range of immunological defects, and may predispose a person to develop respiratory (and other) infections.16,88 Theoretically, vitamin A should be of benefit in LRTI due to its ability to up-regulate Th1 and down-regulate Th2-mediated immune responses.89 The nutrient also enhances innate defences by supporting healthy mucosal barriers and the function of macrophages, neutrophils and natural killer cells.90 However, although a Cochrane review in 2008 found that supplementation of this nutrient could prevent LRTI in retinol-deficient children or those with a poor nutritional status, evidence did not that it could beneficially affect other LRTI symptoms.89 One factor which may be responsible for this puzzling result is that many of these studies were mega-dose studies, and vitamin A supplementation seems more efficacious when administered via frequent low-dosing.89,90
The immunological activity of different herbal medicines is being increasingly documented, with labels such as ‘immuno-stimulant’ and ‘immune-enhancer’ being ascribed to herbs such as Echinacea spp.91 While ‘stimulation’ of certain immunological systems does occur, the term ‘immuno-modulation’ is perhaps more apt, as the modulation of the immune system by phytoconstituents is more complex than a purely ‘stimulating’ effect. Various plant constituents modulate the humoral and/or the cellular components of the immune system.92 While up-regulating the humoral immune system (increasing macrophage activity and phagocytosis) is often beneficial in cases of pathogenic challenge or in a deficient immune system, the workings of the cellular immune system are more complex. Some studies show that herbal medicines may ‘up’ or ‘down’ regulate varying aspects of the cellular immune system, and in isolation some constituents may modulate cytokines that individually are anti-inflammatory and/or inflammatory.∗
Herbal medicines modulate a unique combination of immune pathways, with different medicinal plants often used for similar therapeutic outcomes (e.g. acute infection). They express varying biological activities to achieve this effect. Herbal medicines may enhance the activity of immune components that are beneficial in fighting pathogens via increased humoral activity; natural killer cell, leukocyte, selective cytokine and immunoglobulin production; modulation of Th1 and Th2 cells; and/or down-regulation of NF-κB and TNF-α91 (see Table 6.4).
It should be noted that some herbals, for example Hemidesmus indicus and Tylphora indica, may actually ‘suppress’ many components of the immune system.107 While it is advised that these plants be avoided in acute conditions,40 they have a place in treating disease processes where an ‘over-activated’ cellular immune system is apparent (see Chapter 28 on autoimmunity). The clinician may find the complexities of the modulation of individual immune components by herbal medicines to be complex and confusing.
ECHINACEA QUALITY ISSUES
Alkylamides have been found to be most active immune-enhancing constituents in Echinacea spp., with a combination of E. purpurea and E. angustifolia108–110 enhancing the bioavailability of these compounds. Combining the two extracts provides a protective effect on the alkylamides from E. angustifolia, as alkyamides from E. purpurea protect them from liver metabolism via the P450 pathway.109 In order to obtain the highest amounts of these active constituents, not only is the species important, but also the plant part. Research has identified the following differences in the alkylamide content (mg/g) of the various parts of E. purpurea: flower 2.7, leaf 0.2, rhizome 5.7 and root 1.7.111 This would suggest that the rhizome is the preferable plant part to use, though a synergy has been shown between the alkylamides from the aerial parts and the root of E. purpurea.112 Thus, a combination of root and herb tinctures may also provide enhanced immunomodulatory and anti-inflammatory effects.
However, while in vitro and in vivo studies assist in the understanding of mechanisms of action, the key clinical principle is always to prescribe in relation to traditional usage and evidence from human clinical trials.
Three main species of Echinacea are used medicinally: E. palladia, E. purpurea and E. angustifolia. There is a long history of Echinacea spp. use in infectious conditions, and both E. purpurea and E. angustifolia were used by the eclectics for respiratory illness such as catarrh and chronic bronchitis.113 Given the quality issues noted in the side-bar, the only relevant studies are those which use the correct plant part at reasonable doses.
Echinacea spp. exert a strong inhibitory effect in vitro on the expression of IL-6 and IL-8 expression by bronchial cells infected with a number of common respiratory pathogens.40,114 This helps ameliorate URTI symptoms, and is effective both prophylactically and symptomatically.40
Meta-analyses and reviews of Echinacea spp. efficacy have shown differing results, as, in general, they have not screened studies for use of a particular plant part. One recent meta-analysis included a subgroup of standardised products, and found that these generated reductions in the incidence of infection by 58%, and symptom duration 1.4 days.115 Patients who were given a mix of aerial part and root tincture in addition to vitamin C and propolis showed an 86% decrease in incidence of common cold.115,116
Andrographis paniculata is used in Ayurvedic medicine to treat a range of illnesses including influenza, pneumonia and bronchitis.117 Studies demonstrate reasonably strong evidence that doses of up to 6 g per day may reduce symptom severity and duration when administration is begun within 36 to 48 hours of the onset of URTI.118,119 Some trials indicate that the effect may be dose-dependent, increasing in effectiveness with higher quantities of andrographolides.119,120 There is also preliminary evidence indicating a protective effect against infection.121 In one study, very low doses (200 mg/day) produced a relative risk of URTI contraction 2.1 times lower in the active group than in placebo by the third month.118 Adverse effects appear minimal, although the herb should be used with caution in pregnancy, bleeding disorders, and with patients taking hypoglycaemic or antiplatelet medication.118,121 It should be noted that high doses can occasionally lead to gastric discomfort, anorexia and, in extreme cases, vomiting.42 One way to mitigate this when prescribing high doses is to give smaller doses more frequently.
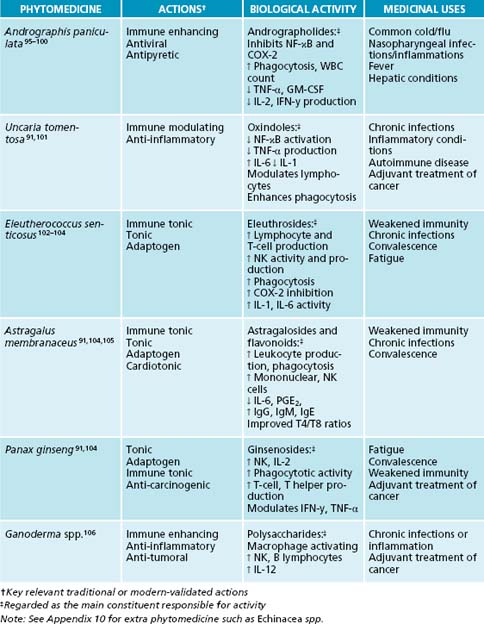
In comparison to A. paniculata, Astragalus membranaceus is considered more specific for chronic or recurrent infection, and in instances of immune depletion. In traditional Chinese medicine, it is used for ‘all diseases caused by “insufficient Qi”, typically those manifesting in weakness, fatigue, and vulnerability to infection’.104,122 Clinically, A. membranaceus improves lymphocyte function in both immune-depressed and healthy patients.104 While no clinical studies examining the use of this botanical agent in respiratory illness could be found thus far, centuries of traditional use and demonstrated immune stimulating activity suggest that it would be of benefit in treating and preventing URTI.
Sambucus nigra has strong traditional indications for respiratory tract diseases.123 Constituents in the berries neutralise the spikes on a number of enveloped viruses, including Influenza types A and B, rendering them unable to enter cells and replicate.5,124 They also have an ability to modulate cytokine expression in vitro, and thus potentially enhance phagocytic activity and chemotaxis for clearance of infection.125,5 This immunomodulary action has also been demonstrated in a number of human trials. Fifteen millilitres of syrup containing S. nigra (38% standardised S. nigra extract, with other non-active ingredients) administered twice daily produces effective inhibition of the influenza viruses,124,126 causing symptom duration and severity to decline significantly, with nearly 90% of the S. nigra group experiencing ‘complete cure’ within 2 or 3 days (compared to 6 days in the other group).126
For further information and other herbs with immune-stimulating activity refer to Table 6.3. Any one of these may be of use in treating RTI presentations, depending upon the patient. Their use should be guided by the indications suggested.
Table 6.3 Suggested actions and agents for symptomatic relief41,42–45
| SYMPTOM | ACTION REQUIRED | HERBAL AND NUTRITIONAL REMEDIES |
|---|---|---|
| Nasal congestion/sinusitis | Mucolytic |
Preventing respiratory infections and the role of herbal ‘tonics’ and adaptogens
All traditional healing systems, and variants of complementary and alternative medicine (CAM) recognise a concept of ‘vitalism’, or a vital force that sustains a person.127,128 This is at once greater than, and in a different form to, the physical and chemical interactions of the human being.129,130 This is the concept referred to variously as prana, Qi, vital force, life force and universal intelligence, among other names.129 In its naturopathic form, the concept of vis medicatrix naturae (‘the healing power of nature’) positions the practitioner as facilitator of this energy.130,131 When it is allowed to flow unhindered, the being exists in a state of optimal functioning, wellbeing or ‘vitality’.
Many traditional medical systems incorporate use of treatments in order to maintain the vitality of the body, or keep it ‘in tune’.127 In the Malaysian and Indonesian systems, these are called jamu, in Ayurvedia, they are rasayanas (for rejuvenation), and in Russia the term toniziruyuzhie sredstva literally translates to ‘tonic substances’.127 In the Western tradition, they are referred to as adaptogens or tonics. The general understanding of these remedies is that they are plants containing ‘biologically active substances which … induce a state of … increase[d] resistance to … aversive assaults which threaten internal homeostasis’.128
Traditionally, immune tonics were used to fortify the immune system in infection or immune challenge from pathogens, or to ‘tonify’ and restore the immune system if deemed deficient.41 A state of dysregulation was perceived to exist in people with recurrent infections, or presenting with symptoms such as a lingering cough, or unresolved infection. In traditional Chinese medicine this is seen as Qi (vital force) deficiency, and the patient often appears with a pale face and tongue, a weak pulse, fatigue and shortness of breath.135
The concept of an immune tonic implies a degree of bidirectionality. While immune stimulants/modulators may, for example, encourage the up-regulation of bodily processes (such as an increase in natural killer cell function), they do not possess the ability to down-regulate this function in cases of overactivity. Thus, they are unidirectional. A true immune tonic expresses an ability to restore the immune system to balance no matter which way it departs from homeostasis.136 A good example is Echinacea spp., which exhibits the ability to regulate and balance T helper cell function, with differing actions depending upon the existing state (basal or stimulated) of the immune response.137 Thus, an immune tonic is a true balancer of dysregulated immune function. Beneficial immune tonic herbs include Astragalus membranaceus, Eleutherococcus senticosus, Panax ginseng, Ganoderma spp. and Echinacea spp.
There is sometimes controversy over the use of certain herbs for either ‘acute’ or ‘chronic’ conditions. A core naturopathic principle is to first use immune enhancers/modulators to assist in the elimination of the pathogen, and follow this with a tonic to strengthen immunity. For example, the use of Echinacea spp. and Andrographis paniculata for an acute cold/flu would initially be employed, followed by Astragalus membranaceus and Eleutherococcus senticosus to tonify. However, it is not always so clear cut. Evidence does not preclude the long-term use of immune modulators in chronic conditions as they may be beneficial in preventing infection and RTIs.138 Also, the use of tonics is contraindicated in most acute cases, as a strong stimulating effect can
KEEPING IT SIMPLE
lead to more florid immune/inflammatory responses and promote a short-term worsening of the condition/symptoms. However, as an exception to the rule, these may traditionally be prescribed in cases of acute cold/flu where the person’s energy is so low they cannot fight the pathogen (presenting with marked fatigue, shortness of breath and daytime sweating).138
Psychoneuroimmunology, adrenal and nervine tonics
Psychoneuroimmunology is the study of the interactions between the central nervous system, the autonomic and endrocrine systems, and the immune system.139,144 Research demonstrates that stress, depression, anxiety, insomnia and other nervous conditions common in today’s society may all have detrimental effects on immune function.134,145,146 Epidemiological data have long shown a link between life stress and higher likelihood of infectious and inflammatory disease,148 but it is only relatively recently that the mechanisms behind this connection have begun to be elucidated. It is likely that the effect is mediated by hormones of the hypothalamus-pituitary-adrenal (HPA) axis (see Chapter 15 on adrenal fatigue).140,133 Generally, these hormones are immune suppressing, and even the transient cortisol spike after intensive exertion may increase susceptibility to URTI.146 Animal studies illustrate that early-life stress is likely to promote lifelong immune dysregulation.147 Prolonged psychological stress results in chronic exposure to these hormones, and may predispose a person to lowered immunity and repeated infection.
Additionally, viral illnesses and infections are, in themselves, physiologically stressful, as they induce concomitant activation of the HPA axis, and may favour the evolution of psychological illnesses.148 Thus, there is a clear role for adrenal support where patients suffer from both repeated infection/poor immunity and chronic or high levels of stress.
Useful nutrients include vitamin C and zinc. Human and animal studies suggest that vitamin C supplementation may be beneficial in reducing circulating cortisol levels both post-exercise and in situations of psychosocial stress.145,148 It is also an important cofactor for both adrenal medulla and adrenal cortex hormone synthesis, and will be rapidly depleted in situations of over-activity and secretion.149 Zinc deficiency, rather than impairing adrenal function, directly leads to HPA activation and chronically high circulating levels of glucocorticoids.73 In populations at risk
EFFECTS OF HORMONES ON IMMUNITY
High levels of corticotropin-releasing hormone (CRH) may lead to vigorous declines in innate and cellular immune responses.140
Of the botanical adrenal tonics available, Eleutherococcus senticosus, Glycyrrhiza glabra, Rehmannia glutinosa, Bupleurum falcatum, Rhodiola rosea, Panax ginseng and Withania somnifera all demonstrate activity on the adrenal glands or hormones. Eleutherococcus senticosus is native to Russia and northern Asia and has been used in traditional Chinese medicine use since 190 AD and, more recently, in Russia as an adaptogen.103,151 Clinical trials demonstrate that Eleutherococcus senticosus elicits a biphasic stress response from the adrenal glands, raising cortisol levels when they are too low, and reducing them in elevated states.152 This may be due to an increased binding of the natural ligands to receptors involved in the feedback systems of stress hormones.152 It is suggested to inhibit catechol-O-methyl transferase, an enzyme which normally catalyses the degradation of stress hormones and, in doing so, prolongs their action in the body. This activates the negative feedback loop, lowering glucocorticoid secretion, and thus potentially diminishing the immune suppressive effects of stress.
Glycyrrhiza glabra and Rehmannia glutinosa may have similar action on cortisol, delaying its breakdown and hence, eventually, lowering the levels expressed.153,154 The Ayurvedic rasayana Withania somnifera also demonstrates activity on the adrenal glands, and an ability to reduce adrenal hypertrophy and circulating levels of glucocorticoids.155–157 In doing so, it improves the chronic stress-induced decrease of the T-lymphocyte population (CD3+, CD4+/CD8+ T-cells).155–157 Finally, early experimental evidence suggests that Rhodiola rosea also may reduce secretion of CRF, and prevent depletion of adrenal hormones in chronic stress.158,159
Panax ginseng has renowned adaptogenic and tonic activity, which is suspected to be due to activity on the adrenal glands.160–162 In chronic stress, the herb has demonstrated the ability to normalise adrenal gland weight and serum corticosteroid levels via restoration of regular HPA feedback cycles.163 Restoration of regular feedback loops will decrease abnormally high levels of cortisol that may depress immune function. Panax ginseng also increases adrenal capacity, thus toning the organs and promoting normal functioning.164 Caution needs to be applied when using Panax ginseng as there is potential for overstimulation, and concomitant use with caffeine, nicotine or other stimulants should be avoided.165
Traditionally, nervine tonics such as Avena sativa, Scutellaria lateriflora and Hypericum perforatum are employed to nourish and strengthen the nervous system in cases of stress and nervous debility.41 Avena sativa may be given green or as seed—each has slightly different actions, but both are nervine tonics.42 The fresh green juice has demonstrated efficacy in reducing nicotine cravings and assisting in smoking cessation,166 possibly through a stress-relieving, anxiolytic activity. Scutellaria lateriflora also has a history of use, and demonstrated clinical efficacy as a nervine tonic.167–169 Finally, Hypericum perforatum, best known for its antidepressant activity, has traditionally been suggested to be of ‘value in nervous disorders’.167 Doses between 300 and 1200 mg have shown clinical efficacy in reducing symptoms of many stress-related disorders including depression,170 premenstrual syndrome171 and anxiety (see Section 3 on the nervous system).
Correction of digestive function
The gastrointestinal tract plays a key role in immune function (see Section 1 on the gastrointestinal system). Not only can hypochlorhydria and malabsorption lead to key nutrient deficiencies, but they can also predispose a patient to increased intestinal permeability (leaky gut), which then contributes to systemic immune dysregulation via gut associated lymphatic tissue (GALT) dysfunction. This can then disrupt the mucosal immunity of extra-gastrointestinal tissues including the respiratory tract.172,173
Altered intestinal microflora may also contribute to immune deficiency, and may be a further underlying cause of recurrent URTIs. Probiotic supplementation with Lactobacillus GG, L. casei rhamnosus, Lactobacillus plantarum, Lactobacillus rhamnosus and Bifidobacterium lactis has been shown to be useful in controlling respiratory infection.174–176 A recent meta-analysis of 14 trials has also reported that supplementation of a number of different strains of probiotics may reduce the symptoms and duration of respiratory tract infection.177
INTEGRATIVE MEDICAL CONSIDERATIONS
Homoeopathy
Homoeopathic treatment has been shown to be more cost-effective than conventional drug therapy in recurrent respiratory infections.178 Individualised treatment may also be more efficacious at preventing URTI in the paediatric population.179
The homoeopathic remedy Oscillococcinum has evidence of mild effectiveness in reducing duration and severity of URTI, though no evidence of a preventive role.180,181 Individualised homoeopathy also has evidence of effectiveness in treatment of URTI,179 though this appeared to be equally effective when using self-administered acute remedies as opposed to classical homoeopathic consultations (see Table 6.5).182
Table 6.5 The most common homoeopathic treatments in URTI7–9
| REMEDY | INDICATION |
|---|---|
| Aconite | Used at first sign of cold/flu (first 12 hours). Accompanied by restlessness, fear, thirst. Dry, hoarse cough. |
| Allium Cepa | Colds with clear, runny mucous. Burning discharge. Acute catarrhal inflammation of mucous membranes. |
| Arsenicum Album | Flu with tossing and turning, restlessness, anxiety and desire for sips of water. Difficulty breathing. |
| Belladonna |
Acupuncture
Most of the studies using acupuncture as a treatment method have been undertaken in China—and to a lesser extent Japan—and therefore many published results are unavailable in English.183 Additionally, acupuncture trials are complicated by the difficulty of practising sham acupuncture as placebo treatment.184
In traditional Chinese medicine, URTI may be viewed as pathogenic invasion of wind (wind-cold or wind-heat) into the lung.185 The studies available do show benefit in the use of this treatment in respiratory infections, but not as prophylaxis.186 Acupuncture manipulation on the neck produces a significant decrease in the symptoms of the common cold, potentially via immune system activation.184,186–188
Example treatment
The patient presents with discomfort due to a range of acute symptoms. Therefore the initial treatment focus needs to be on symptomatic relief. Not only is this the patient’s priority, but, in meeting her needs, the practitioner will engender trust. Building upon
Sambucus nigra is included for its antiviral properties, as well anti-catarrhal and diaphoretic properties.
Dietary and lifestyle modification (traditional wisdom)
TANNINS AND ALKALOIDS—TROUBLE BREWING?
If tannins and alkaloids are mixed in a liquid extract prescription, a muddy precipitate is observed to form. It is considered that this renders the alkaloids less available for absorption and consequently it is recommended that these not be mixed in a formula.41
The main alkaloid-containing herb of concern is Hydrastis canadensis,190 as berberine and hydrastine (the alkaloid components) are considered important actives. If these precipitate and are not bioavailable, then the herb may be inactive.
Tannin-rich herbs to be aware of are Agrimonia eupatoria, Camellia sinensis, Filipendula ulmaria, Geranium maculatum, Rubus idaeus,190 Albizzia lebbeck, Cinnamomum zeylanicum, Matricaria recutita, Rhodiola rosea, Mentha piperita, Rosmarinus officinalis, Hypericum perforatum, Sambucus nigra, Thymus vulgaris and Prunus serotina.
Long-term dietary suggestions for supporting healthy immune function
Cunningham-Rundles S., et al. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115(6):1119-1128.
Murray M.T. A comprehensive review of vitamin C. American Journal of Natural Medicine. 1996;3:8-21.
Spelman K., et al. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev.. Jun 2006;11(2):128-150.
1. Heikkinen T., Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
2. Mackay I.M. Human rhinoviruses: the cold wars resume. J Clin Virol. 2008;42(4):297-320.
3. Meissner H.C. Reducing the impact of viral respiratory infections in children. Pediatr Clin North Am. 2005;52(3):695-710. v
4. Kuiken T., Taubenberger J.K. Pathology of human influenza revisited. Vaccine. 2008;26(Suppl. 4):D59-D66.
5. Roxas M., Jurenka J. Colds and influenza: a review of diagnosis and conventional, botanical, and nutritional considerations. Altern Med Rev. 2007;12(1):25-48.
6. Wardwell L., et al. Nutrient intake and immune function of elderly subjects. J Am Diet Assoc. 2008;108(12):2005-2012.
7. Hemming V.G. Viral respiratory diseases in children: classification, etiology, epidemiology, and risk factors. J Pediatr. 124(5 Pt 2), 1994. S13–S16
8. Sloots T.P., et al. Emerging respiratory agents: new viruses for old diseases? J Clin Virol. 2008;42(3):233-243.
9. Proud D. Upper airway viral infections. Pulm Pharmacol Ther. 2008;21(3):468-473.
10. Arruda E., et al. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35(11):2864-2868.
11. Beutler B., et al. Genetic dissection of innate immunity to infection: the mouse cytomegalovirus model. Curr Opin Immunol. 2005;17(1):36-43.
12. Leonard W.J. Genetic effects on immunity: editorial overview. Current Opinion in Immunology. 2000;12(4):465-467.
13. Mouton D., et al. Genetic factors of immunity against infection. Ann Inst Pasteur Immunol. 1985;136D(2):131-141.
14. Cohen S. Psychological stress and susceptibility to upper respiratory infections. Am J Respir Crit Care Med. 1995;152(4 Pt 2):S53-S58.
15. Cashat-Cruz M., . Azpiri M. Respiratory tract infections in children in developing countries. Semin Pediatr Infect Dis. 2005;16(2):84-92.
16. Cunningham-Rundles S. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115(6):1119-1128. quiz 1129
17. Villamor E., Fawzi W.W. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18(3):446-464.
18. Schaible U.E., Kaufmann S.H. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4(5):e115.
19. Wintergerst E.S., et al. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85-94.
20. Trakatellis A., et al. Pyridoxine deficiency: new approaches in immunosuppression and chemotherapy. Postgrad Med J. 1997;73(864):617-622.
21. Arredondo M., Nunez M.T. Iron and copper metabolism. Mol Aspects Med. 2005;26(4–5):313-327.
22. Glynn A., et al. Immune cell counts and risks of respiratory infections among infants exposed pre- and postnatally to organochlorine compounds: a prospective study. Environ Health. 2008;7:62.
23. Kostka T., et al. Physical activity and upper respiratory tract infections. Int J Sports Med. 2008;29(2):158-162.
24. Neiman D. Exercise and immunity: clinical studies. Psychoneuroimmunology. 4th edn.. 2007:661-673.
25. Johnson C., Eccles R. Acute cooling of the feet and the onset of common cold symptoms. Fam Pract. 2005;22(6):608-613.
26. Falagas M.E., et al. Effect of meteorological variables on the incidence of respiratory tract infections. Respir Med. 2008;102(5):733-737.
27. Zhu Z., et al. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest. 1996;97(2):421-430.
28. Turner R.B., et al. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis. 1998;26(4):840-846.
29. Mayhew M. Treatment of the common cold: the evidence. Journal for Nurse Practitioners. 2008;4(1):61-63.
30. Woo T. Pharmacology of cough and cold medicines. J Pediatr Health Care. 2008;22(2):73-79. quiz 80–82
31. Arroll B. Common cold. Clin Evid 2005;(13):1853-1861.
32. Gwaltney J.M. Viral respiratory infection therapy: historical perspectives and current trials. Am J Med. 2002;112(Suppl 6A):33S-41S.
33. Arroll B. Non-antibiotic treatments for upper-respiratory tract infections (common cold). Respir Med. 2005;99(12):1477-1484.
34. Arroll B. Antibiotics for upper respiratory tract infections: an overview of Cochrane reviews. Respir Med. 2005;99(3):255-261.
35. Spurling G., et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev. (3):2007;. CD004417
36. Ram F., et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2):2006;. CD004403
37. Nicholson K.G., et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355(9218):1845-1850.
38. Kim B.H., et al. Quercetin 3-O-beta-(2’-galloyl)-glucopyranoside inhibits endotoxin LPS-induced IL-6 expression and NF-kappa B activation in macrophages. Cytokine. 2007;39(3):207-215.
39. Liu J., et al. The inhibitory effect of quercetin on IL-6 production by LPS-stimulated neutrophils. Cell Mol Immunol. 2005;2(6):455-460.
40. Sharma M., et al. Echinacea as an antiinflammatory agent: the influence of physiologically relevant parameters. Phytother Res. 2009;23(6):863-867.
41. Mills S., Bone K. Principles and practice of phytotherapy. Edinburgh: Churchill Livingstone; 2000.
42. Bone K.M. A clinical guide to blending liquid herbs. Philadelphia: Churchill Livingstone; 2003.
43. Scientific Committee of the British Herbal Medical Association. British herbal pharmacopoeia. Bournemouth: British Herbal Medicine Association, 1983.
44. Fisher C.P. Materia medica of western herbs for the southern hemisphere. Auckland: CP Fisher; 1996.
45. Osiecki H. The nutrient bible, 6th edn. Eagle Farm: Bio Concepts Publishing; 2004.
46. Mackowiak P.A. Concepts of fever. Arch Intern Med. 1998;158(17):1870-1881.
47. Schulman C.I., et al. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized prospective study. Surg Infect (Larchmt). 2005;6(4):369-375.
48. Dinarello C., Porat R. Fever and rash. In: Fauci A.S., et al, editors. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill Companies, Inc, 2008.
49. Bryant R.E., et al. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971;127(1):120-128.
50. Weinstein M.I., et al. Spontaneous bacterial peritonitis: a review of 28 cases with emphasis on improved survival and factors influencing prognosis. American Journal of Medicine. 1978;64(4):592-598.
51. Doran T., et al. Acetaminophen: more harm than good for chicken pox? Journal of Pediatrics. 1989;114(6):1045-1048.
52. Stanley E.D., et al. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231(12):1248-1251.
53. Graham N.M., et al. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162(6):1277-1282.
54. Laupland K.B. Fever in the critically ill medical patient. Crit Care Med. 2009;37(7 Suppl):S273-S278.
55. Dinarello C. Endogenous pyrogens: the role of cytokines in the pathogenesis of fever. In: Mackowiak P.A., editor. Fever: basic mechanisms and management. New York: Raven Press; 1997:23-47.
56. Mahalanabis D., et al. Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J Clin Nutr. 2004;79(3):430-436.
57. Canton R., et al. Respiratory tract infections: at-risk patients who are they? Implications for their management with levofloxacin. Int J Antimicrob Agents. 2006;28(Suppl 2):S115-S127.
58. Wissinger E., et al. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol. 2009;21(3):147-155.
59. Nieman D.C. Immunonutrition support for athletes. Nutr Rev. 2008;66(6):310-320.
60. Douglas R., et al. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 18(3), 2007. CD000980
61. Hemilä H. Vitamin C respiratory infections and the immune system. Trends Immunol. 2003;24(11):579-580.
62. Hemilä H., Douglas R.M. Vitamin C and acute respiratory infections. Int J Tuberc Lung Dis. 1999;3(9):756-761.
63. Heuser G., Vojdani A. Enhancement of natural killer cell activity and T and B cell function by buffered vitamin C in patients exposed to toxic chemicals: the role of protein kinase-C. Immunopharmacol Immunotoxicol. 1997;19(3):291-312.
64. Hartel C., et al. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27(4–5):101-106.
65. Rayment S.J., et al. Vitamin C supplementation in normal subjects reduces constitutive ICAM-1 expression. Biochem Biophys Res Commun. 2003;308(2):339-345.
66. Proud D. Nitric oxide and the common cold. Curr Opin Allergy Clin Immunol. 2005;5(1):37-42.
67. Mizutani A., et al. Ascorbate-dependent enhancement of nitric oxide formation in activated macrophages. Nitric Oxide. 1998;2(4):235-241.
68. Coman D. New indications and controversies in arginine therapy. Clin Nutr. 2008;27(4):489-496.
69. Fraker P.J. Roles for cell death in zinc deficiency. J Nutr. 2005;135(3):359-362.
70. Fraker P.J., et al. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130(5S Suppl):1399S-1406S.
71. Prasad A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43(5):370-377.
72. Fraker P.J., King L.E. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277-298.
73. Sazawal S., et al. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics. 1998;102(1 Pt 1):1-5.
74. Brown K.H., et al. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull. 2009;30(1 Suppl):S12-S40.
75. Roth D.E., et al. Acute lower respiratory infections in childhood: opportunities for reducing the global burden through nutritional interventions. Bull World Health Organ. 2008;86(5):356-364.
76. Gandia P., et al. A bioavailability study comparing two oral formulations containing zinc (Zn bis-glycinate vs. Zn gluconate) after a single administration to twelve healthy female volunteers. Int J Vitam Nutr Res. 2007;77(4):243-248.
77. Siepmann M., et al. The pharmacokinetics of zinc from zinc gluconate: a comparison with zinc oxide in healthy men. Int J Clin Pharmacol Ther. 2005;43(12):562-565.
78. Tompkins T.A., et al. Clinical evaluation of the bioavailability of zinc-enriched yeast and zinc gluconate in healthy volunteers. Biol Trace Elem Res. 2007;120(1–3):28-35.
79. Caruso T.J., et al. Treatment of naturally acquired common colds with zinc: a structured review. Clin Infect Dis. 2007;45(5):569-574.
80. Marshall I. WITHDRAWN: Zinc for the common cold. Cochrane Database Syst Rev. (3):2006;. CD001364
81. Marshall I. Zinc for the common cold. Cochrane Database Syst Rev. (2):2000;. CD001364
82. Hirt M., et al. Zinc nasal gel for the treatment of common cold symptoms: a double-blind, placebo-controlled trial. Ear Nose Throat J. 2000;79(10):778-782.
83. Nair M.P., et al. The flavonoid, quercetin, differentially regulates Th-1 (IFNgamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim Biophys Acta. 2002;1593(1):29-36.
84. Nieman D.C., et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exerc. 2007;39(9):1561-1569.
85. Davis J.M., et al. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R505-R509.
86. Nieman D.C., et al. Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007;103(5):1728-1735.
87. Kumar P., et al. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp Lung Res. 2005;31(5):449-459.
88. Cameron C., et al. Neonatal vitamin A deficiency and its impact on acute respiratory infections among preschool Inuit children. Can J Public Health. 2008;99(2):102-106.
89. Chen H., et al. Vitamin A for preventing acute lower respiratory tract infections in children up to seven years of age. Cochrane Database Syst Rev. (1):2008;. CD006090
90. Stephensen C.B. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167-192.
91. Spelman K., et al. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006;11(2):128-150.
92. Huang C.F., et al. The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol Immunol. 2008;5(1):23-31.
93. Barrett B. Medicinal properties of echinacea: a critical review. Phytomedicine. 2003;10(1):66-86.
94. Mishima S., et al. Antioxidant and immuno-enhancing effects of Echinacea purpurea. Biol Pharm Bull. 2004;27(7):1004-1009.
95. Abu-Ghefreh A.A. In vitro and in vivo anti-inflammatory effects of andrographolide. Int Immunopharmacol. 2009;9(3):313-318.
96. Burgos R.A., et al. Andrographolide inhibits IFN-gamma and IL-2 cytokine production and protects against cell apoptosis. Planta Med. 2005;71(5):429-434.
97. Shen Y.C., et al. Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. Br J Pharmacol. 2002;135(2):399-406.
98. Hidalgo M.A., et al. Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br J Pharmacol. 2005;144(5):680-686.
99. Naik S.R., Hule A. Evaluation of immunomodulatory activity of an extract of andrographolides from Andographis paniculata. Planta Med. 2009;75(8):785-791.
100. Qin L.H., et al. Andrographolide inhibits the production of TNF-alpha and interleukin-12 in lipopolysaccharide-stimulated macrophages: role of mitogen-activated protein kinases. Biol Pharm Bull. 2006;29(2):220-224.
101. Laus G. Advances in chemistry and bioactivity of the genus Uncaria. Phytother Res. 2004;18(4):259-274.
102. Drozd J., et al. Estimation of humoral activity of Eleutherococcus senticosus. Acta Pol Pharm. 2002;59(5):395-401.
103. Bleakney T.L. Deconstructing an adaptogen: Eleutherococcus senticosus. Holist Nurs Pract. 2008;22(4):220-224.
104. Block K.I., Mead M.N. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003;2(3):247-267.
105. Prieto J.M., et al. Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions. J Pharm Pharmacol. 2003;55(9):1275-1282.
106. Zhou X., et al. Ganodermataceae: natural products and their related pharmacological functions. Am J Chin Med. 2007;35(4):559-574.
107. Bone K. Clinical applications of Ayurvedic and Chinese herbs. Warwick: Phytotherapy Press; 1996.
108. Matthias A., et al. Permeability studies of alkylamides and caffeic acid conjugates from echinacea using a Caco-2 cell monolayer model. J Clin Pharm Ther. 2004;29(1):7-13.
109. Matthias A., et al. Echinacea alkylamide disposition and pharmacokinetics in humans after tablet ingestion. Life Sci. 2005;77(16):2018-2029.
110. Agnew L.L., et al. Echinacea intake induces an immune response through altered expression of leucocyte hsp70, increased white cell counts and improved erythrocyte antioxidant defences. J Clin Pharm Ther. 2005;30(4):363-369.
111. Perry N.B., et al. Alkylamide levels in Echinacea purpurea: a rapid analytical method revealing differences among roots, rhizomes, stems, leaves and flowers. Planta Med. 1997;63(1):58-62.
112. Chicca A., et al. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int Immunopharmacol. 2009;9(7–8):850-858.
113. Felter HW, Lloyd JU. King’s American dispensatory. Online. Available: http://www.henriettesherbal.com/eclectic/kings/extracta
114. Sharma M., et al. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized echinacea, a potent antiviral herbal extract. Antiviral Res. 2009;83:165-170.
115. Shah S.A., et al. Evaluation of echinacea for the prevention and treatment of the common cold: a meta-analysis. Lancet Infect Dis. 2007;7(7):473-480.
116. Cohen H.A., et al. Effectiveness of an herbal preparation containing echinacea propolis and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled multicenter study. Arch Pediatr Adolesc Med. 2004;158(3):217-221.
117. Williamson E., editor. Major herbs of Ayurveda. London: Churchill Livingstone, 2002.
118. Kligler B., et al. Andrographis paniculata for the treatment of upper respiratory infection: a systematic review by the Natural Standard Research Collaboration. Explore (NY). 2006;2(1):25-29.
119. Poolsup N., et al. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharm Ther. 2004;29(1):37-45.
120. Thamlikitkul V., et al. Efficacy of Andrographis paniculata Nees for pharyngotonsillitis in adults. J Med Assoc Thai. 1991;74(10):437-442.
121. Coon J.T., Ernst E. Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy. Planta Med. 2004;70(4):293-298.
122. Brush J., et al. The effect of Echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD69 expression and immune cell activation in humans. Phytother Res. 2006;20(8):687-695.
123. Boericke W. Boericke’s Materia Medica. 1901.
124. Zakay-Rones Z., et al. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J Altern Complement Med. 1995;1(4):361-369.
125. Barak V., et al. The effect of Sambucol, a black elderberry-based natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur Cytokine Netw. 2001;12(2):290-296.
126. Zakay-Rones Z., et al. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J Int Med Res. 2004;32(2):132-140.
127. Davydov M., Krikorian A.D. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: a closer look. J Ethnopharmacol. 2000;72(3):345-393.
128. Kannur D.M., et al. Screening of anti-stress properties of herbal extracts and adaptogenic agents. Pharmacognosy Reviews. 2008;2(3):95-101.
129. Coulter I.D., Willis E.M. The rise and rise of complementary and alternative medicine: a sociological perspective. MJA. 2004;180:587-589.
130. Evans S. Changing the knowledge base in western herbal medicine. Soc Sci Med. 2008;67(12):2098-2106.
131. Whorton J.C. Nature cures: the history of alternative medicine in America. New York: Oxford University Press; 2004.
132. Rakel D.P., et al. Practitioner empathy and the duration of the common cold. Fam Med. 2009;41(7):494-501.
133. Kemeny M.E., Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21(8):1009-1018.
134. Maciocia G. The foundations of Chinese medicine. Singapore: Churchill Livingstone; 1989.
135. Mowery D.B. Herbal tonic therapies. New York: Wings Books; 1993.
136. Matthias A., et al. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79(1):53-58.
137. Shah S., et al. Evaluation of echinacea for the prevention and treatment of the common cold: a meta-analysis. Lancet Infect Dis. 2007;7(7):347-348.
138. Maciocia G. The practice of Chinese medicine: the treatment of diseases with acupuncture and Chinese herbs. London: Churchill Livingstone; 1994.
139. Strausbaugh H., Irwin M. Central corticotropin-releasing hormone reduces cellular immunity. Brain Behav Immun. 1992;6(1):11-17.
140. DeRijk R., et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6. J Clin Endocrinol Metab. 1997;82(7):2182-2191.
141. Singh A., et al. Lymphocyte subset responses to exercise and glucocorticoid suppression in healthy men. Med Sci Sports Exerc. 1996;28(7):822-828.
142. Moser M., et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25(10):2818-2824.
143. Irwin M.R. Human psychoneuroimmunology: 20 years of discovery. Brain Behav Immun. 2008;22(2):129-139.
144. Kainuma E., et al. Association of glucocorticoid with stress-induced modulation of body temperature, blood glucose and innate immunity. Psychoneuroendocrinology. 2009. In press
145. Kemeny M. Emotions and the immune system. In: Ader R., editor. Psychoneuroimmunology. San Diego: Elsevier Academic Press, 2007.
146. Carrillo A.E., et al. Vitamin C supplementation and salivary immune function following exercise-heat stress. Int J Sports Physiol Perform. 2008;3(4):516-530.
147. Avitsur R., et al. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20(4):339-348.
148. Gibb J., et al. Neurochemical and behavioral responses to inflammatory immune stressors. Front Biosci (Schol Ed). 2009;1:275-295.
149. Satterlee D.G., et al. Vitamin C amelioration of the adrenal stress response in broiler chickens being prepared for slaughter. Comp Biochem Physiol A Comp Physiol. 1989;94(4):569-574.
150. Patak P., et al. Vitamin C is an important cofactor for both adrenal cortex and adrenal medulla. Endocr Res. 2004;30(4):871-875.
151. Grieve M. A modern herbal. New York: Dover; 1931.
152. Gaffney B.T., et al. Panax ginseng and Eleutherococcus senticosus may exaggerate an already existing biphasic response to stress via inhibition of enzymes which limit the binding of stress hormones to their receptors. Med Hypotheses. 2001;56(5):567-572.
153. Kato H., et al. 3-Monoglucuronyl-glycyrrhetinic acid is a major metabolite that causes licorice-induced pseudoaldosteronism. J Clin Endocrinol Metab. 1995;80(6):1929-1933.
154. Zhang R.X., et al. Rehmannia glutinosa: review of botany, chemistry and pharmacology. J Ethnopharmacol. 2008;117(2):199-214.
155. Bhattacharya S.K., Muruganandam A.V. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav. 2003;75(3):547-555.
156. Kaur P., et al. Effect of 1-oxo-5beta 6beta-epoxy-witha-2-ene-27-ethoxy-olide isolated from the roots of Withania somnifera on stress indices in Wistar rats. J Altern Complement Med. 2003;9(6):897-907.
157. Kour K., et al. Restoration of stress-induced altered T cell function and corresponding cytokines patterns by Withanolide A. Int Immunopharmacol Int Immunopharmacol. 2009;9(10):1137-1144.
158. Perfumi M., Mattioli L. Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother Res. 2007;21(1):37-43.
159. Kelly G.S. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293-302.
161. Blumenthal M., et al, editors. Herbal medicine: expanded Commission E monographs (English translation). Austin: Integrative Medicine Communications, 2000.
160. Tachikawa E., Kudo K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: suppression of adrenal medullary function in vitro by ginseng. J Pharmacol Sci. 2004;95(2):140-144.
162. Nocerino E., et al. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71(Suppl 1):S1-A5.
163. Rai D., et al. Anti-stress effects of Ginkgo biloba and Panax ginseng: a comparative study. J Pharmacol Sci. 2003;93(4):458-464.
164. Fulder S.J. Ginseng and the hypothalamic-pituitary control of stress. Am J Chin Med. 1981;9(2):112-118.
165. Braun L., Cohen M. Herbs and natural supplements: an evidence-based guide, 2nd edn. Marrickville: Elsevier; 2007.
166. Anand C.L. Effect of Avena sativa on cigarette smoking. Nature. 1971;233(5320):496.
167. Felter H. The eclectic materia medica pharmacology and therapeutics. 1922. Online. Available: http://www.swsbm.com/FelterMM/Felters.html
168. Cook W. The Physiomedical Dispensatory. 1896. Online. Available: http://medherb.com/cook/home.htm
169. Wolfson P., Hoffmann D.L. An investigation into the efficacy of Scutellaria lateriflora in healthy volunteers. Altern Ther Health Med. 2003;9(2):74-78.
170. Kasper S., et al. Superior efficacy of St John’s wort extract WS 5570 compared to placebo in patients with major depression: a randomized, double-blind, placebo-controlled, multi-center trial [ISRCTN77277298]. BMC Med. 2006;4:14.
171. Stevinson C., Ernst E. A pilot study of Hypericum perforatum for the treatment of premenstrual syndrome. BJOG. 2000;107(7):870-876.
172. Fasano A. Physiological pathological and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol. 2008;173(5):1243-1252.
173. Lamblin C., et al. [The common mucosal immune system in respiratory disease]. Rev Mal Respir. 2000;17(5):941-946.
174. Hatakka K., et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind randomised trial. BMJ. 2001;322(7298):1327.
175. Lin J.S., et al. Different effects of probiotic species/strains on infections in preschool children: A double-blind randomized controlled study. Vaccine. 2009;27(7):1073-1079.
176. Pregliasco F., et al. A new chance of preventing winter diseases by the administration of synbiotic formulations. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S224-S233.
177. Vouloumanou E.K., et al. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34(3):197. .e1–197.e10
178. Rossi E., et al. Cost-benefit evaluation of homeopathic versus conventional therapy in respiratory diseases. Homoeopathy. 2009;98(1):2-10.
179. Steinsbekk A., et al. Homeopathic care for the prevention of upper respiratory tract infections in children: a pragmatic, randomised, controlled trial comparing individualised homeopathic care and waiting-list controls. Complement Ther Med. 2005;13(4):231-238.
180. Vickers A., Smith C. Homoeopathic Oscillococcinum for preventing and treating influenza and influenza-like syndromes. Cochrane Database Syst Rev. (3):2006;. CD001957
181. Jaber R. Respiratory and allergic diseases: from upper respiratory tract infections to asthma. Prim Care. 2002;29(2):231-261.
182. Steinsbekk A., et al. An exploratory study of the contextual effect of homeopathic care. A randomised controlled trial of homeopathic care vs. self-prescribed homeopathic medicine in the prevention of upper respiratory tract infections in children. Prev Med. 2007;45(4):274-279.
183. Suzuki M., et al. Research into acupuncture for respiratory disease in Japan: a systematic review. Acupunct Med. 2009;27(2):54-60.
184. Kawakita K., et al. Preventive and curative effects of acupuncture on the common cold: a multicentre randomized controlled trial in Japan. Complement Ther Med. 2004;12(4):181-188.
185. Zhou Z.Y., Jin H.D., et al. Clinical manual of Chinese herbal medicine and acupuncture. Oxford: Churchill Livingstone; 1997.
186. Kawakita K., et al. Do Japanese style acupuncture and moxibustion reduce symptoms of the common cold? Evid Based Complement Alternat Med. 2008;5(4):481-489.
187. Sato T., et al. Acupuncture stimulation enhances splenic natural killer cell cytotoxicity in rats. Jpn J Physiol. 1996;46(2):131-136.
188. Ye F., et al. Effects of electro-acupuncture on T cell subpopulations, NK activity, humoral immunity and leukocyte count in patients undergoing chemotherapy. J Tradit Chin Med. 2007;27(1):19-21.
189. Pengelly A. The constituents of medicinal plants, 2nd edn. Crows Nest: Allen & Unwin; 2004.
190. Amin M., Kapadnis B.P. Heat stable antimicrobial activity of Allium ascalonicum against bacteria and fungi. Indian J Exp Biol. 2005;43(8):751-754.
191. Douglas R.M., et al. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. (2):2000. CD000980
192. Douglas R.M., et al. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. (4):2004;. CD000980
193. Hemilä H. Vitamin C supplementation and respiratory infections: a systematic review. Mil Med. 2004;169(11):920-925.
194. Hemilä H., Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. (1):2007. CD005532
195. Anderson T.W., Suranyi G., Beaton G.H. The effect on winter illness of large doses of vitamin C. Can Med Assoc J. 1974;111(1):31-36.
196. Anderson T.W., et al. Winter illness and vitamin C: the effect of relatively low doses. Can Med Assoc J. 1975;112(7):823-826.
197. Anderson R., et al. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr. 1980;33(1):71-76.
198. Schoop R., et al. Echinacea in the prevention of induced rhinovirus colds: a meta-analysis. Clin Ther. 2006;28(2):174-183.
199. O’Neil J.H. Effects of echinacea on the frequency of upper respiratory tract symptoms: a randomized, double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2008;100(4):384-388.