CHAPTER 4 Respiratory disorders
Respiratory assessment: general
Goal of system assessment
Evaluate for ineffective breathing patterns, impaired gas exchange, and airway obstruction.
Continuous pulse oximetry (spo2 monitoring)
• Evaluate for changes over time and/or since the last recorded reading. Results should be correlated with the arterial oxygen saturation (SaO2) readings derived from arterial blood gases.
• Pulse oximetry accuracy is dependent on the presence of an adequate pulse in the area in which the measurement probe has been applied.
• Ensure readings are done using an appropriate probe placed on the anatomical location with the best pulse and least interference. Probes are available for the finger, forehead, or ear lobe.
• Readings must be correlated with physical assessment findings and can remain normal despite signs of impending deterioration. Physical assessment findings such as use of accessory muscles or presence of tachypnea are indicative of respiratory distress but may not be reflected in a change in SpO2. If an increasing amount of oxygen (O2) is needed to maintain SpO2, this is also indicative of impending deterioration of the patient.
Observation
• Evaluate for use of accessory muscles, shortness of breath, and air hunger.
• Ensure the patient is evaluated for the presence of chronic obstructive pulmonary disease (COPD) prior to applying O2 therapy so appropriate liter flow is determined to prevent respiratory impairment.
• Evaluate facial and lip color for pallor or cyanosis indicative of hypoxemia.
Auscultation
• Listen to breath sounds to evaluate for presence of adventitious sounds that reflect factors contributing to respiratory distress, including those related to both airway obstruction and impaired gas exchange.
• Adventitious sounds: crackles (rales) indicative of fluid in alveoli, bubbles (rhonchi) indicative of secretions in bronchioles, wheezing (inflammation), inspiratory stridor (narrowing of airways due to massive inflammation or obstruction by secretions or foreign body), or pleural friction rub (inflammation)
• Lungs must be auscultated anteriorly and posteriorly in all three lobes of the right lung, the two lobes of the left lung, over the right and left main bronchi, and over the trachea.
Screening labwork
• Arterial blood gas analysis can reveal increases or decreases in pH; levels of O2, O2 saturation, CO2, and bicarbonate; base excess or base deficit indicative of impending respiratory failure; hyperpnea/tachypnea; and metabolic derangements affecting breathing patterns. Blood gas analysis may be done using either arterial blood or mixed venous blood samples. Mixed venous blood samples are available only using a pulmonary artery catheter and can be used to calculate efficacy of both O2 delivery and O2 consumption. Arterial blood gases cannot be used to calculate O2 consumption.
CARE PLANS: GENERAL APPROACHES TO RESPIRATORY DISORDERS
Impaired spontaneous ventilation with or without impaired gas exchange
Goals/outcomes
1. Assess for patent airway; if snoring, crowing, stridor, or strained respirations are present, indicative of partial or full airway obstruction, open airway using chin lift or jaw thrust.
2. Insert an oral airway if patient becomes unconscious and cannot maintain patent airway; use a nasopharyngeal airway if patient is conscious to avoid provoking vomiting. If severely distressed, patient may require endotracheal intubation.
3. Position patient to alleviate dyspnea and insure maximal ventilation; generally, sitting in an upright position unless severe hypotension is present.
4. Monitor changes in oxygenation following position change: SpO2, SvO2, ScVO2, end-tidal CO2, A−aDO2 levels and arterial blood gases (ABGs).
5. Clear secretions from airway by having patient cough vigorously, or provide nasotracheal, oropharyngeale, or endotracheal tube suctioning, as needed.
6. Have patient breathe slowly or manually ventilate with manual resuscitator or bag-valve-mask device slowly and deeply between coughing or suctioning attempts.
7. Assist with use of incentive spirometer as appropriate.
8. Turn patient every 2 hours if immobile. Encourage patient to turn self, or get out of bed as much as tolerated if he or she is able.
9. Provide mucolytic and bronchodilating medications orally, intravenously, or by inhaler, aerosol, or nebulizer as ordered to assist with thinning secretions and relaxing muscles in lower airways.
10. Provide chest physical therapy as appropriate, if other methods of secretion removal are ineffective.
1. Ensure humidity is provided when using O2 or bilevel positive airway pressure (BiPAP) device for more than 12 hours to help thin secretions.
2. Administer supplemental O2 using liter flow and device as ordered.
3. Restrict patient and visitors from smoking while O2 is in use.
4. Document pulse oximetry with O2 liter flow in place at time of reading as ordered. Oxygen is a drug; the dose of the drug must be associated with the O2 saturation or the reading is meaningless.
5. Obtain arterial blood gases if patient experiences behavioral changes or respiratory distress to check for hypoxia or hypercapnia.
6. Monitor for hypoventilation, especially in patients with COPD.
7. Monitor for changes indicative of O2 toxicity in patients receiving higher concentrations of O2 (more than FIO2 45%) for longer than 24 hours. Changes will be apparent in chest radiograph and breath sounds. Absorption atelectasis may be present. The higher the O2 concentration, the greater is the chance of toxicity.
8. Monitor for skin breakdown where O2 devices are in contact with the skin, such as nares, around the ears, and around edges of mask devices.
9. Provide O2 therapy during transportation and when patient gets out of bed.
10. If patient is unable to maintain SpO2 reading of more than 88% off O2, consult with the respiratory care practitioner/therapist and the physician about the need for home O2 therapy.
1. Monitor rate, rhythm, and depth of respirations.
2. Note chest movement for symmetry of chest expansion and signs of increased work of breathing such as use of accessory muscles or retraction of intercostal or supraclavicular muscles. Consider use of noninvasive positive pressure ventilation for impending respiratory failure.
3. Monitor for snoring, coughing, and possibly choking-type respirations when patients have a decreased level of consciousness to assess if airway is obstructed by tongue.
4. Monitor for new breathing patterns that impair ventilation, which may need aggressive management in a specialized, highly skilled setting.
5. Note that trachea remains midline, as deviation may indicate patient has a tension pneumothorax.
6. Auscultate breath sounds before and after administration of respiratory medications to assess for improvement.
7. Evaluate changes in O2 saturation (SaO2), pulse oximetry (SpO2), end-tidal CO2 (ETCO2), ScVO2, and ABGs as appropriate.
8. Monitor for dyspnea and note causative activities/events.
9. If increased restlessness or unusual somnolence occur, evaluate patient for hypoxemia and hypercapnia as appropriate.
10. Monitor chest radiograph reports as new images become available.
Acute asthma exacerbation
Pathophysiology
Life-threatening asthma exacerbation results from bronchial smooth muscle contraction (bronchospasm), bronchial inflammation leading to airway edema, and mucus plugging. When an episode of bronchospasm (critical airway narrowing) is not reversed after 24 hours of maximal doses of traditional inhaled short-acting beta2-adrenergic agonists (SABAs) such as albuterol or levalbuterol, injected systemic beta2-agonists such as epinephrine, inhaled anticholinergics such as ipratropium, and systemic steroid therapy with prednisone, prednisolone, or methylprednisolone, the refractory patient may be diagnosed with status asthmaticus (SA). Common triggers for asthma exacerbations include respiratory tract infections, allergens (airborne or ingested), air pollutants, smoke, and physical irritants (e.g., cold air, exercise). Anxiety or “panic” attacks and use of beta-adrenergic blocking agents and nonsteroidal anti-inflammatory drugs (NSAIDs) may predispose patients to development or exacerbation of severe asthma.
Assessment
Goal of system assessment
• Evaluate for ineffective breathing patterns, impaired gas exchange, and airway obstruction.
• Determine patient’s prior treatment regimen; classify which “step” of treatment has been needed to control symptoms; patient may need to move to the next step of treatment to maintain control.
• Classify severity of exacerbation: should be determined following initial assessment and diagnostic testing.
History and risk factors
• Asthma symptoms: Cough (especially if worse at night), wheezing, recurrent difficulty breathing, recurrent chest tightness
• Family history: Patients with either family history or atopic disease are at higher risk of asthma.
• Common triggers: Symptoms worsen with viral respiratory infections, environmental airborne allergens, irritants in the home (mold, mildew, wood-burning stove, cockroaches, dust mites, animal dander, carpeting laid over concrete), recent emotional upset, aggressive exercise, fear, frustration, food, new medications, changes in weather (especially exposure to cold air), occupational chemicals or allergens, and hormonal changes (menstrual cycle).
• Comorbid conditions: Sinusitis, rhinitis, gastroesophageal reflux disease (GERD), obstructive sleep apnea (OSA), allergic bronchopulmonary aspergillosis (ABPA)
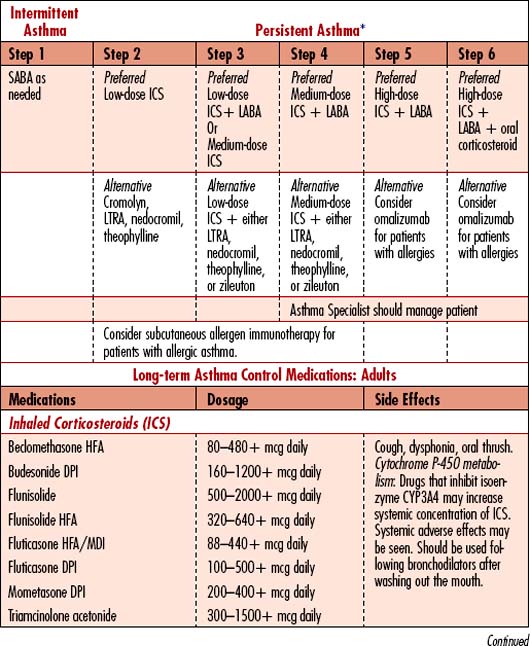
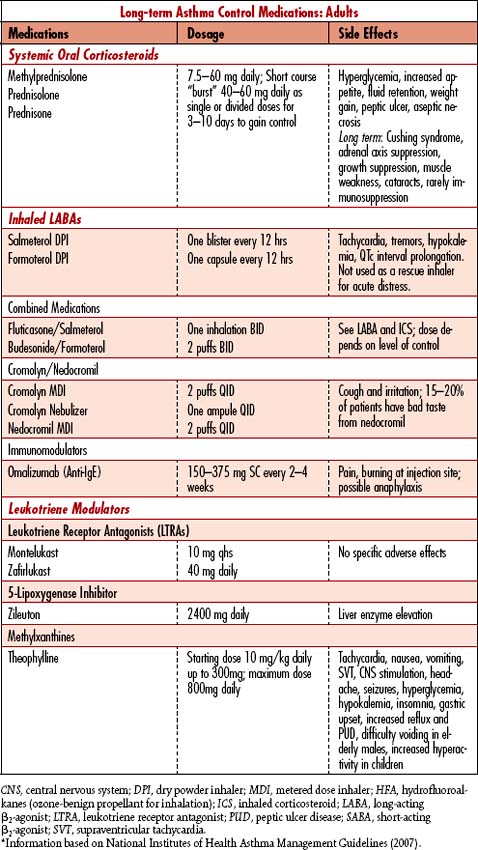
1. Classify asthma severity: Intermittent (step 1 treatment) or persistent: mild, moderate, severe (steps 2, 3, 4, 5, and 6 treatments); steps differ for children under age 5, children between 5 and 12 years old, and adults.
2. Classify severity of exacerbation: Mild to severe or life threatening
3. Assess control: Determine if pattern of previous exacerbations is inherent to the current episode.
4. Compliance/ability to control: Assess the patient’s knowledge and skills for self-management.
5. Identify precipitating factors: Situation: exposure at home, work, daycare, or school to inhalant allergens or irritants; time of day, season or time of year, relationship of symptoms to meals, deterioration in other health conditions or menses
6. Identify comorbid conditions that may impair asthma management (e.g., sinusitis, rhinitis, GERD, OSA, obesity, stress, or depression).
7. Surgery: Asthmatic patients are at high risk for exacerbations following endotracheal intubation, general anesthesia, and ventilation provided during surgical or other invasive procedures. Impaired cough, hypoxemia, and hypercapnia may trigger exacerbation.
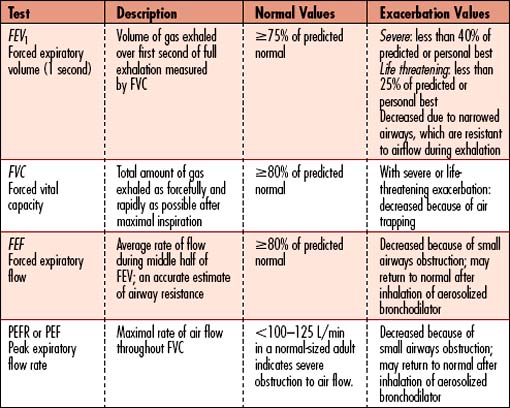
Spirometry or peak expiratory flow
• Peak expiratory flow (PEF): Measurement of rate or force of exhalation; those with easier breathing will have higher values than those in distress. A peak flowmeter is used by patients at home to assess asthma control. Those with more severe asthma may have difficulty discerning worsening of symptoms and may use PEF several times daily to assess for declining rate of exhalation.
• Assesses degree of obstruction and reversibility in patients older than 5 years
• Spirometry is essential for establishing the diagnosis of asthma. Patients’ perceptions of airflow obstruction are highly variable. Spirometry or PEF provides an objective measurement to help classify severity of exacerbation.
• Decreased to less than 40% of predicted value indicates severe exacerbation; less than 25% of predicted value for life threatening
Vital signs (severe to life-threatening asthma exacerbation)
• Presence of fever: Temperature elevation helps discern whether patient’s condition is related to a microbe (fever) versus an allergen (afebrile).
• Pulse oximetry: Oxygen saturation is decreased from patient’s baseline value.
• Tachycardia (HR greater than 140 bpm) and tachypnea (RR greater than 40 breaths/min)
• Hypotension may be present; hypotension is exacerbated by underlying dehydration often present with patients with severe asthma.
Observation
• Severe attacks render patients unable to speak due to breathlessness.
• Use of accessory muscles; fatigued, with or without diaphoresis
• Ashen, pale, or gray/blue facial color, lip color, or nail beds
• Chest expansion may be decreased or restricted.
• Altered level of consciousness (confusion, disorientation, agitation)
• Agitation is more commonly associated with hypoxemia while somnolence is associated with hypercapnia (elevated CO2 level).
• Increased nasal secretions, mucosal swelling, nasal polyps
Screening labwork
• Complete blood count (CBC with WBC differential): Evaluates for elevated white blood cells indicative of chronic inflammation due to allergic response and infection including presence of eosinophils, neutrophils, and mononuclear cells
4-1 RESEARCH BRIEF
From Etminan M, Sadatsafavi M, Jafari S, et al: Acetaminophen use and the risk of asthma in children and adults. Chest 136(5):1316–1323, 2009.
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Arterial blood gas analysis (ABG) | Assess for abnormal gas exchange or compensation for metabolic derangements. Initially PaO2 is normal and then decreases as the ventilation-perfusion mismatch becomes more severe. A normal PCO2 in a distressed asthma patient receiving aggressive treatment may indicate respiratory fatigue, which causes a progressively ineffective breathing pattern, which can also lead to respiratory arrest. Oxygenation assessment differs from acid base balance assessment, wherein the PCO2 value is used as the hallmark sign for respiratory failure induced acidosis. | pH changes: Acidosis may reflect respiratory failure; alkalosis may reflect tachypnea. Carbon dioxide: Elevated CO2 reflects respiratory failure; decreased CO2 reflects tachypnea; rising PCO2 is an ominous, since it signals severe hypoventilation, which can lead to respiratory arrest. Hypoxemia: PaO2 less than 80 mm Hg) Oxygen saturation: SaO2 less than 92% Bicarbonate: HCO3 less than 22 meq/L Base Deficit: less than -2 |
| Complete blood count (CBC) with WBC differential | WBC differential evaluates the strength of the immune system’s response to the trigger of exacerbation and for presence of infection. | Eosinophils: increased in patients not receiving corticosteroids; indicative of magnitude of inflammatory response. Increased WBC count: More than 11,000/mm3 is seen with bacterial pneumonias. WBCs may be increased by asthma in the absence of infection. The Hematocrit (Hct): may be increased from hypovolemia and hemoconcentration. |
| Pulmonary function tests (PFTs)/spirometry | The hallmark sign of asthma is a decreased FEV1 (forced expiratory volume in the first second)/FVC (forced vital capacity.) If PEF rate does not improve with initial aggressive inhaled bronchodilator treatments, morbidity increases. | Forced expiratory volume (FEV): decreased during acute episodes; if less than 0.7, narrowed airways prevent forceful exhalation of inspired volume (Table 4-1). Peak expiratory flow rate (PEF): less than 100–125 L/min in a normal-sized adult indicates severe obstruction to air flow. |
| Pulse oximetry (SpO2) | Noninvasive technology that measures the oxygen saturation of arterial blood intermittently or continuously using a probe placed on the patient’s finger or ear. When using pulse oximetry, it is helpful to obtain ABG values to compare the oxygen saturation and evaluate the PaO2, PaCO2, and pH. | Normal Spo2: more than 95%. Correlation of SpO2 with SaO2 (arterial saturation) is within 2% when SaO2 is more than 50%. Temperature, pH, PaCO2, anemia, and hemodynamic status may reduce the accuracy of pulse oximetry measurements. Presence of other forms of Hgb in the blood (carboxyhemoglobin or methemoglobin) can produce falsely high readings. |
| Serologic studies | Acute and convalescent titers are drawn to diagnose a viral infection. | Increased antibody titers: a positive sign for viral infection. |
| Chest radiograph | Evaluates the severity of air trapping; also useful in ruling out other causes of respiratory failure (e.g., foreign body aspiration, pulmonary edema, pulmonary embolism, pneumonia). | The x-ray usually shows lung hyperinflation caused by air trapping and a flat diaphragm related to increased intrathoracic volume. |
| 12-Lead ECG (electrocardiogram) | Evaluates for dysrhythmias associated with stress response and asthma medications. | Sinus tachycardia: important baseline indicator; use of some bronchodilators (e.g., metaproterenol) may produce cardiac stimulant effects and dysrhythmias. |
| Sputum gram stain, culture and sensitivity | Culture and sensitivity may show microorganisms if infection is the precipitating event. The most reliable specimens are obtained via bronchoalveolar lavage (BAL) during bronchoscopy, or using a protected telescoping catheter (mini or using BAL) to decrease risk of contamination from oral flora. |
Gross examination may show increased viscosity or actual mucous plugs. Gram stain positive: Indicates organism is present. Culture: Identifies organism. Sensitivity: Reflects effectiveness of drugs on identified organism. |
| Diagnostic fiberoptic bronchoscopy using PSB (protected specimen brush) and BAL | Obtains specimens during simple bronchoscopy without contaminating the aspirate; modified technique (mini-BAL) is also effective without the need of full bronchoscopy. | Gram stain positive: Indicates organism is present. Culture: Identifies organism. Sensitivity: Reflects effectiveness of drugs on identified organism. |
| Serum theophylline level | Important baseline indicator for patients who take theophylline regularly; therapeutic level is close to the toxic level. If additional theophylline is given, serial levels should be measured within the first 12–24 hr of treatment and daily thereafter. Patients are monitored for side effects (e.g., nausea, nervousness, dysrhythmias). | Acceptable therapeutic range is 10–20 mcg/ml. There is little evidence to support clinical benefit for adding theophylline to inhaled β-adrenergic blocking agents and steroids for patients with acute, severe asthma who were not already using theophylline regularly. |
Collaborative management
Care priorities
The goal of asthma management is to control the disease using a stepped approach to therapies. Ideal control is attained when patients are free of daytime symptoms, do not awaken breathless or coughing at night, have few or no limitations on activities, do not regularly use rescue medications, have no exacerbations, and maintain a forced expiratory volume in 1 second (FEV1) and/or peak expiratory flow rate (PEFR) greater than 80% of the predicted value. When prevention fails, the potential for life-threatening respiratory failure is high during exacerbations unresponsive to treatment within the first hour. Management is directed toward decreasing bronchospasm and increasing ventilation. Other interventions are directed toward treatment of complications (Table 4-1).
1. Determine severity of asthma exacerbation:
a. Acute severe: PEFR is less than 40% of predicted or personal best in a patient who is unable to speak a complete sentence in one breath, with RR greater than 25 breaths/min and HR greater than 110 bpm.
b. Life threatening: In a patient with severe asthma, the PEFR is less than 25% of predicted or personal best, SpO2 less than 92%, PaO2 less than 80 mm Hg; PCO2 35 to 35 mm Hg, silent chest, weak respiratory effort, exhaustion, cyanosis, bradycardia, hypotension, dysrhythmias, confusion, coma.
c. Near fatal: PCO2 greater than 45 mm Hg and/or requiring mechanical ventilation using increased positive pressure to overcome inspiratory pressures; patient also has other findings of life-threatening exacerbation.
Patients have profound hypoxia and can tolerate high doses of O2 (FIO2) unless they retain CO2 and breathe by hypoxic drive. Most asthmatics are able to tolerate high flow O2, versus those with other obstructive lung disease who cannot. Oxygen dosage must be limited in nonintubated, mechanically ventilated patients who breathe via hypoxic drive to avoid hypoventilation and respiratory arrest. Humidified O2 therapy is begun immediately to correct hypoxemia and thin secretions. PaO2 is kept slightly above normal unless the patient retains CO2, to compensate for the increased O2 demands imposed by the increased work of breathing. The degree of hypoxemia and patient response determine the method of O2 delivery. A high-flow device (e.g., 100% nonrebreather mask) delivers more precise and higher FIO2. Management of anxiety must be considered, especially if the patient will not wear a mask because of feelings of suffocation.
5. Pharmacotherapy to manage acute asthma exacerbation:
Vigorous therapy is initiated to decrease bronchospasms, help reduce airway inflammation, and help remove secretions. Treatment is continued until wheezing is eliminated and pulmonary function tests return to baseline (Table 4-1).
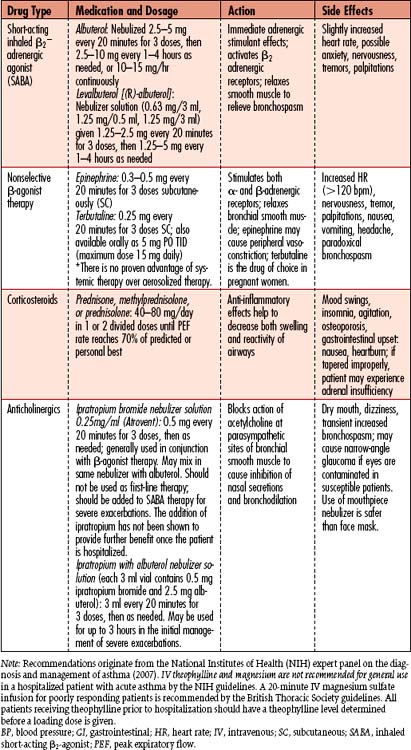
• Bronchodilators: Dilate smooth muscles of the airways to help relieve bronchospasms, resulting in increased diameter of functional airways. SABAs are the mainstay of asthma exacerbation management, while long-acting beta-adrenergic agonists (LABAs) are used for long-term control of asthma. Theophylline and aminophylline are no longer recommended for management of acute bronchospasms.
• Corticosteroids: Given intravenously during the acute phase of the exacerbation to decrease the inflammatory response, which causes edema in upper airways. Administration should decrease reactivity and swelling of the airways. Dosage varies according to severity of episode and whether patient currently is taking steroids. The patient may be converted to inhaled corticosteroids once the acute phase has been resolved. Acute adrenal insufficiency can develop in patients who take steroids routinely at home, if these drugs are not given to the patient during hospitalization.
• Anticholinergics: Inhaled medications used to reduce vagal tone of the airways, thus helping to reduce bronchospasms. Ipratropium (Atrovent) is used in combination with inhaled SABAs for severe, acute asthma.
• Magnesium sulfate: American Thoracic Society asthma management guidelines (2008) recommend consideration of a single dose of magnesium sulfate 1.2 to 2 g over 20 minutes for patients with severe, life-threatening, or fatal exacerbation who have an inadequate or ineffective response to inhaled bronchodilators.
• Sedatives and analgesics: Used in more limited doses in patients who are not intubated or mechanically ventilated, unless the person is extremely agitated and unable to cooperate with therapy. These agents depress the central nervous system (CNS) response to hypoxia and hypercapnia. Once mechanical ventilation is in place, the dosage is titrated until the patient is comfortable and/or hypoxemia or hypercapnia begins to resolve.
• Buffers: Sodium bicarbonate may be given to correct severe acidosis not corrected by intubation and mechanical ventilation. Generally, this is only a temporizing measure to help relieve lactic acidosis. The physiologic response to bronchodilators improves with correction of metabolic acidosis.
• Antibiotics: Given if a respiratory infection is suspected, as evidenced by fever, purulent sputum, or leukocytosis.
Generally contraindicated in acute phases of exacerbation because of acute respiratory decompensation and hyperreactive airways. Once the crisis is over, the patient may benefit from percussion and postural drainage every 2 to 4 hours to help mobilize secretions.
CARE PLANS: ACUTE ASTHMA EXACERBATION
related to ineffective breathing patterns secondary to narrowed airways
1. ![]() Monitor for signs of increasing hypoxia at frequent intervals: Restlessness, agitation, and personality changes are indicative of severe exacerbation. Cyanosis of the lips (central) and of the nail beds (peripheral) are late indicators of hypoxia.
Monitor for signs of increasing hypoxia at frequent intervals: Restlessness, agitation, and personality changes are indicative of severe exacerbation. Cyanosis of the lips (central) and of the nail beds (peripheral) are late indicators of hypoxia.
2. Monitor for signs of hypercapnia at frequent intervals: Confusion, listlessness, and somnolence are indicative of respiratory failure and near-fatal asthma exacerbation.
3. Monitor ABGs when continuous pulse oximetry values or patient assessment reflects progressive hypoxemia or development of hypercapnia. Be alert to decreasing PaO2 and increasing PaCO2 or decreasing O2 saturation levels, indicative of impending respiratory failure.
4. Monitor for decreased breath sounds or changes in wheezing at frequent intervals. Absent breath sounds in a distressed asthma patient may indicate impending respiratory arrest.
5. Position patient for comfort and to promote optimal gas exchange. High-Fowler’s position, with the patient leaning forward and elbows propped on the over-the-bed table to promote maximal chest excursion, may reduce use of accessory muscles and diaphoresis due to work of breathing.
6. Monitor FIO2 to ensure that O2 is within prescribed concentrations. If patient does not retain CO2, 100% nonrebreather mask may be used to provide maximal O2 support. If the patient retains CO2 and is unrelieved by positioning, lower-dose O2, bronchodilators, and steroids, intubation and mechanical ventilation may be necessary sooner than in patients who are able to receive higher doses of O2 by mask.
1. Monitor intubated and mechanically ventilated patients for increased intrathoracic pressure (auto-PEEP) due to “breath stacking,” wherein the next breath is delivered prior to complete emptying of the first breath. Each subsequent breath failing to completely empty increases lung volume and predisposes the patient to volutrauma, pneumothorax, and decreased cardiac output (CO) resulting from the hyperinflated lungs causing pressure increases inside the thorax which impede venous return to the heart.
2. Monitor for hypotension. Decreased venous return can lead to hypotension. Auto-PEEP should be suspected in an intubated asthmatic patient who is hypotensive following intubation and initiation of mechanical ventilation, when there is no other obvious cause (e.g., tension pneumothorax). If auto-PEEP is suspected, consult with the respiratory therapist and the physician to modify ventilator settings.
Respiratory Status: Airway Patency
1. Monitor patient’s ability to clear tracheobronchial secretions frequently. Set up suction equipment at the bedside.
2. Encourage oral fluid intake or administer intravenous (IV) fluids within patient’s prescribed limits to help decrease viscosity of the secretions.
3. Encourage coughing to clear secretions and deep breathing unless patient is already coughing uncontrollably or going into respiratory failure. If the patient can manage to take deep breaths, respiratory failure is manageable.
4. Provide humidified O2 to help liquefy tracheobronchial secretions.
5. Evaluate whether patient may benefit from chest physiotherapy, after crisis phase of exacerbation has been resolved. Discuss with physician. If appropriate, teach significant others to perform chest physiotherapy.
6. Teach patient proper coughing technique for effective management of secretions.
7. Instruct patient to take several deep breaths. Instruct significant others in coaching this technique.
8. After the last inhalation, teach patient to perform a succession of coughs (usually three or four) on the same exhalation until most of the air has been expelled.
9. Explain that patient may need to repeat this technique several times before the cough becomes productive.
1. Determine patient’s previous asthma control status, including which “step” of therapy was implemented (Table 4-3).
2. Compare current status to past exacerbation responses to determine respiratory status.
3. Ensure spirometry measurements (FEV1, FVC, FEV1/FVC ratio) or PEFR readings are obtained before and after use of a short-acting bronchodilator.
4. Educate patient about use of a PEFR meter at home.
5. Determine patient’s compliance with treatments.
6. Note onset, frequency, and duration of coughing and advise patient to avoid triggers of coughing if identified.
7. Coach in breathing or relaxation exercises.
8. Encourage patient to breathe slowly and deeply. Teach pursed-lip breathing technique to assist patient with controlling respirations as appropriate:
9. Teach patient and family how to decrease metabolic demands for O2 by limiting or pacing patient’s activities and procedures.
10. Schedule rest times after meals to avoid competition for O2 supply during digestion.
11. Monitor SpO2 by pulse oximetry during activity to evaluate limits of activity, set future activity goals, and recommend optimal positions for oxygenation.
12. Assess for fever 2 to 4 hours. Consult physician and provide treatment as prescribed to decrease temperature and thus O2 demands.
1. Ascertain and alleviate the cause of restlessness to decrease metabolic demands (e.g., if restlessness is related to anxiety, help reduce anxiety by providing reassurance, enabling family members to stay with patient, and offering distractions such as soft music or television).
2. Be aware that restlessness may be an early sign of hypoxemia.
3. Explain all procedures and offer support to minimize fear and anxiety, which can increase O2 demands.
Acute lung injury and acute respiratory distress syndrome
Pathophysiology
The terms acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are used to describe a continuum of lung dysfunction. There may be a primary (intrapulmonary) or secondary (extrapulmonary) insult to both the lung endothelium and the epithelium. The associated release of mediators, increasing vascular and alveolar permeability (leak), eventually perpetuates alveolar collapse and supports the accumulation of fluids in the pulmonary interstitium. As the capillary permeability and alveolar epithelial damage continue to worsen, surfactant activity is reduced, protein production increases, and therefore gas exchange decreases due to widened diffusion distance and intrapulmonary shunting . The alveoli tend to collapse, communicating the loss of opening pressure to other alveoli in the sac. All resist re-expansion in the absence of surfactant and the presence of significant infiltration and collapsing fluid pressure. Initially, acute hypoxemia develops, worsens, and ultimately progresses into hypercapnic respiratory failure. The shunt fraction (blood flow past de-recruited alveoli rejoins in the pulmonary venous circulation without adequate O2 exposure) as well as alveolar (physiologic) dead space (overventilation of the unaffected alveolar sacs) increases, ultimately progressing to a profoundly noncompliant, de-recruited, and gas dysfunctional state. Current evidence supports that the over distension and force of opening-closing also profoundly affect the healthy lung. ALI and the more severe and exacerbated process, ARDS, are primarily defined once the evolution of damage has required intubation and mechanical ventilation. The progression is measured by a worsening of the patient’s oxygen exchange (Table 4-4). The presence of refractory hypoxemic respiratory failure in conjunction with diffuse pulmonary infiltrates in the absence of left atrial hypertension is considered the primary indicator of the continuum of acute respiratory failure. Despite advances in the treatment of the primary inflammatory process and progress in the method of ventilatory support, the continuum of ALI/ARDS continues to be associated with high morbidity and mortality, reaching greater than 60%. Since 1964, when the continuum was first described, the understanding of etiology, pathophysiology, and epidemiology, as well as the relationship of genetic prodrome and ventilator induced lung injury process, has significantly increased (Table 4-5).
Table 4-4 CRITERIA FOR CLASSIFICATION OF ACUTE LUNG INJURY (ALI)/ ACUTE RESPIRATORY DISTRESS CRITERIA SYNDROME (ARDS)
| Criteria | Indicators |
|---|---|
| ALI | Acute onset |
| PaO2/FIO2 < 250 mm Hg with 0.40 FIO2 Regardless of PEEP | |
| Bilateral infiltrates on frontal chest radiograph | |
| No clinical evidence of left atrial hypertension or left ventricular dysfunction | |
| ARDS | Same as for ALI with the exception of oxygenation issues |
| PaO2/FIO2 < 200 mm Hg with 0.40 FIO2 Regardless of PEEP |
Table 4-5 RISK FACTORS FOR ACUTE LUNG INJURY/ACUTE RESPIRATORY DISTRESS SYNDROME
| Direct Injury | Indirect Injury |
|---|---|
| Pneumonia | Severe sepsis |
| Aspiration | Trauma |
| Lung contusions | Pancreatitis |
| Inhalation/burn injury | Transfusion-related lung injury (TRALI) |
| Severe acute respiratory syndrome (SARS) | Ventilation-associated lung injury (VALI) |
Assessment
Goal of system assessment
Evaluate for decreasing PaO2/FIO2 and increasing requirements for pressure control and PEEP. (See Acid-Base Imbalances, p. 1 )
History and risk factors
Shock: Trauma, hemorrhagic shock, sepsis, massive blood transfusion, and multiple liters of intravascular volume replacement
Respiratory: Inhalation of toxic substances, pneumonia, severe pneumonitis, aspiration of gastric contents, drowning, air or fat embolus, O2 toxicity, ventilator-induced lung injury (VILI)
Other: Acute pancreatitis, post perfusion cardiopulmonary bypass, drug overdose, neurologic injury, immunosuppression, malaria
Observation: oxygenation failure
• Nasal flaring and expiratory grunt may be present.
• Use of accessory muscles indicates respiratory distress.
• May appear fatigued, with or without diaphoresis
• May present with ashen, pale, or gray/blue facial color, lip color, or nail beds
• Chest expansion may be decreased, restricted, or asymmetrical with severe changes in one lung manifesting severe atelectasis or pleuritic pain.
• ![]() Altered level of consciousness (confusion, disorientation, agitation) is more common with older adults but is a very significant sign in any age group.
Altered level of consciousness (confusion, disorientation, agitation) is more common with older adults but is a very significant sign in any age group.
• Agitation is more commonly associated with hypoxemia, while somnolence is associated with hypercarbia (elevated CO2 level).
Hypoxemic hypoxia in acute lung injury
• Initially: Dyspnea, restlessness, hyperventilation, cough, increased work of breathing; chest may appear to be clear on auscultation or there may be late inspiratory crackles. Patient may be significantly agitated and if intubated may appear combative.
• Ventilator pressures: As most of these patients will already be ventilated, increasing peak airway pressure (PawP or PIP) and a validation of increased pressure measured during inspiratory hold (Pplateau) when administering a volume-controlled breath should be evaluated and documented. The rising pressure (Pplateau) indicates a loss of functional alveolar surface, and as the compliance of the lung decreases, the pressure measured when a volume breath is delivered will rise.
• The patient’s proportionate O2 ratio will decrease (P/F). Initially there may be a shift from volume control ventilation to pressure control as well as an increase in PEEP (see Table 4-1).
Hypoxemic hypoxia in acute respiratory distress syndrome
• Initially: Respiratory failure including cyanosis, pallor, grunting respirations, mid to late inspiratory rales, rapid and shallow breathing, intercostal-suprasternal retractions, tachypnea, tachycardia, diaphoresis, mental obtundation
• Ventilator pressures: The increasing peak and plateau pressures will be measured when the patient is given a volume control breath (cannot be measured during a pressure controlled breath).
• The O2 (P/F) ratio will decline further and generally requires a change in ventilation support to a mean airway pressure strategy.
Screening labwork
• CBC: Evaluates for elevated white blood cells indicative of infection. Bandemia (immature neutrophils) of greater than 10% is especially concerning.
• Sputum gram stain, culture and sensitivity: Identifies infecting organism
• Blood culture and sensitivity: If positive, may indicate organism has migrated into the bloodstream to cause a systemic infection
• ABG analysis: Evaluates for hypoxemia and eventually hypercapnia
A−a gradient/a−adO2/p(a−a)O2
• The A−a gradient or Alveolar−arterial O2 tension difference is a clinically useful calculation. The calculation is based on a model as though the lung were one large alveolus and the entire blood flow of the right heart passed around it. Utilizing the rules of partial pressure as well as the laws of CO2 production at the cell and the content of CO2 exerting alveolar pressure, the theoretical alveolar PO2 (PAO2) is calculated. Once the theoretical PAO2 has been calculated, the gradient is achieved by subtracting the measured arterial PaO2. The calculated “gradient” represents the difference between the calculated Alveolar oxygen (PAO2) and the measured arterial oxygen (Pao2 ).
• When the FIO2 is above 0.21, the A−a gradient becomes less accurate in the measurement of proportional gas exchange, although the difference should always be less than 150 mm Hg.
• ![]() Extrapulmonary failure: The A−a gradient generally remains normal or narrow. With shunt or V./Q. mismatch, the gradient is usually wider than normal. The A−a gradient also measures the severity of gas exchange impairment. At any age, an A−a gradient exceeding 20 mm Hg on room air or greater than 100 on increased FIO2 should be considered abnormal and indicative of pulmonary dysfunction.
Extrapulmonary failure: The A−a gradient generally remains normal or narrow. With shunt or V./Q. mismatch, the gradient is usually wider than normal. The A−a gradient also measures the severity of gas exchange impairment. At any age, an A−a gradient exceeding 20 mm Hg on room air or greater than 100 on increased FIO2 should be considered abnormal and indicative of pulmonary dysfunction.
• P/F ratio: The PaO2 divided by the FIO2 (PaO2/FIO2 ratio or, more simply, P/F) can be used to more simply assess the severity of the gas exchange defect. The normal value for the ratio of the partial pressure of arterial blood O2 to FIO2 ( {PaO2/FIO2} FIO2 is expressed as a decimal ranging from 0.21 to 1.00) is 300 to 500. A value of less than 300 indicates gas exchange derangement, and a value below 200 on greater than 40% FIO2 is indicative of severe impairment and is a major component of the diagnostic criteria for ALI and ARDS. The inverse relationships of these measures are important to consider when discussing the level of gas exchange failure.
• QS/QT: The shunt fraction compares the nonoxygenated (shunted: QS) blood exiting the pulmonary bed to the total blood flow (cardiac output: QT). This mathematical calculation, which requires mixed venous blood gas and pulmonary blood gas, evaluates total intrapulmonary shunting. Normal physiologic shunt is 3% to 4% and may increase to 15% to 20% or more in ARDS. The routine measurements of ABGs, chest radiograph, A−a gradient, and P/F ratio as well as the presence of refractory hypoxemia are much more routinely used to diagnose intrapulmonary shunting, a core feature of ARDS.
Diagnostic tests
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Noninvasive Pulmonary Volumes and Pressures | ||
| Pulmonary function studies | Evaluates inspiratory volumes and exhalation volumes as well as capacities of the lung | Persons with ALI/ARDS have decreased inspiratory volume (tidal volume and inspiratory reserve) as well as exhalation volumes (tidal volume and expiratory reserve) because the functional lung surface is significantly reduced. The amount of volume that stays in the lung at the end of a normal exhalation is significantly ↓↓ and promotes continuous alveolar collapse. |
| Pulmonary pressures measured during volume control breath | Measures the relationship of volume delivered and the compliance of the surface, which contains it | Patients presenting with lung injury and distress will have significant increases in Pplateau pressures to more than 25 cm H2O. This increase may or may not manifest as a proportional increase in PIP. |
| Normal PawP or PIP when receiving a 10 ml/kg/IDW breath is <35 cm H2O. | For example, with a 350 ml breath, the patient with ARDS may have a PIP of 48 and a Pplateau of 43. | |
| Normal Pplateau when holding a 10 ml/kg/IDW breath at the end of inspiration is <25 cm H2O. | ||
| Blood Studies | ||
| Arterial blood gas analysis | Evaluates the oxygenation of the arterial blood as well as the presence or absence of acid and the effect on the pH (environment of the cells). See Acid-Base Imbalances, p. 1. |
Although not always predictable when in the disease process changes will occur, generally patients will develop hypoxemia, which may initially be resolved with increasing the FIO2, but eventually will require great increases in FIO2 and ultimately will no longer respond to oxygen therapy. |
| Complete blood count (CBC) Hemoglobin (Hgb) Hematocrit (Hct) RBC count (RBCs) WBC count (WBCs) |
Assesses for anemia, inflammation, and infection | Decreased RBCs, Hgb, or Hct reflects anemia; WBCs and shift to the left may indicate ongoing inflammation. |
| Coagulation profile Prothrombin time (PT) with international normalized ratio (INR) Partial thromboplastin time (PTT) Fibrinogen D-dimer |
Assesses for causes of bleeding, clotting, and disseminated intravascular coagulation (DIC) indicative of the abnormal clotting present in shock or ensuing shock | Decreased PT with low INR promotes clotting; elevation promotes bleeding. In severe sepsis, PT and INR may increase, but in the presence of ALI/ARDS, these measures along with elevated fibrinogen and D-dimer reflect a microcoagulopathy. |
| Radiology | ||
| Chest radiograph (CXR) | Assesses size of lungs, presence of fluids, abnormal gas or fluids in the pleural sac, diaphragmatic margins, the pulmonary hilum, as well as integrity of the rib cage | Presence of fluids in the lung parenchyma initially presents as pulmonary edema. The continuous accumulation differentiates this edema formation to one that is not cardiac. |
| Computed tomography | ||
| Cardiac CT scan | Assesses the three-dimensional lung capacities, fluid load, and primary displacement of the fluid | Normally a large gas-filled surface, the ALI/ARDS lung when seen on CT is frequently whited out, filled ¼ to ¾ with fluid that has extravagated through the endothelial deficits (capillary leak). |
| Invasive Measures | ||
| Tracheal-protein/plasma-protein ratio | A relatively new diagnostic tool used to differentiate between cardiogenic and noncardiogenic pulmonary edema (ARDS). It compares total protein in tracheal aspirate with total protein in plasma. | Ratio in cardiogenic pulmonary edema is <0.5, whereas the ratio in ARDS generally is >0.7. |
Collaborative management
Maintaining adequate arterial oxygenation while protecting the functional lung is the highest priority in both traditional and more recent approaches to ventilator management for ARDS. In addition, the primary goal is to determine and treat the underlying pathophysiologic condition.
Care priorities
2. Facilitate ventilation and gas exchange:
Mechanical ventilation: Provide mechanical ventilation with moderate to high levels of PEEP (to prevent tidal collapse) and low tidal volumes of about 6 ml/kg ideal body weight, to protect the functional lung from overdistention. This lung-protective ventilatory strategy has been shown to ensure adequate gas exchange, decrease the levels of intra-alveolar and systemic mediators, and improve outcomes in patients with ALI and ARDS. Many clinicians have successfully used strategies to treat ARDS by reducing the delivered tidal volume (from 8 to 10 ml/kg ideal body weight [IBW] to 4 to 6 ml/kg IBW) balanced with a RR (12–40) necessary to maintain adequate minute ventilation. This decrease of volume in the noncompliant lung reduces both peak inspiratory and plateau pressures. At the same time, the use of a lower tidal volume protects the functional lung surface from volutrauma and pressure trauma, both of which cause overdistention and stimulation of inflammation. If PEEP trials fail, other strategies designed to open and maintain opening of the alveoli may be considered. These methods such as airway pressure release ventilation (APRV), inverse ratio (I greater than E), and high-frequency oscillation (HFOV) are also mean airway pressure strategies, but the discussion of this type of advanced ventilation is beyond the scope of this book.
Patient positioning: Primary lung edema occurs most aggressively in the dependent areas of the lung. Repositioning the patient at least every 2 hours is indicated in patients with hypoxemia; however, if staffing allows and the patient can tolerate it, more frequent (every 30 minutes) turning could be beneficial. Continuous lateral motion therapy beds may also be used to continuously turn the patient. Motion therapy assists in the redistribution of interstitial edema and may improve oxygenation.
Prone patient positioning: Prone positioning of the patient improves the oxygenation of many patients with ARDS. There are various methods to turn the patient prone: staff generated with pillows, foam wedges, Vollman prone positioned, or mechanically with the Roto-Prone bed.
Patients who are unable to achieve appropriate ventilation due to agitation and dyssynchrony or are hemodynamically unstable may require heavy sedation with agents such as propofol (Diprivan) or, in extreme cases, the diaphragm may need to be paralyzed with a neuromuscular blocking agent such as vecuronium bromide (Norcuron) or cisatracurium (Nimbex). Although very user dependent, train of four should be performed when evaluating level of pharmacologic paralysis. The caregiver must recognize that, although pharmacologically paralyzed patient may appear to be resting quietly or may even be comatose, he or she may be alert and extremely anxious because of the total lack of muscle control. These patients must receive appropriate sedation (e.g., lorazepam [Ativan]) and analgesia (e.g., morphine), and they will require expert psychosocial nursing interventions. See Sedating and Neuromuscular Blockade, p. 158. When patients appear agitated, ventilation should be evaluated first (as long as the patient is not in danger of extubation or self-harm) followed by pain evaluation and analgesia, followed by anxiety-relieving medications. Neuromuscular paralysis should be performed as a last resort and only when necessary to control ventilation.
5. Provide nutritional support:
![]() Energy outlay with respiratory failure is high, in part because of the increased work of breathing. If the patient is unable to consume adequate calories with enteral feedings, total parenteral nutrition (TPN) is added. It is important to perform an occasional evaluation of the patient’s caloric and metabolic needs to make certain that the patient is being adequately nourished but not overfed. All efforts should be made to feed enterally so the gut is used. Newer elemental feedings require no digestion and can be used in the stomach, duodenum, or jejunum. (See Nutritional Support, p. 117.)
Energy outlay with respiratory failure is high, in part because of the increased work of breathing. If the patient is unable to consume adequate calories with enteral feedings, total parenteral nutrition (TPN) is added. It is important to perform an occasional evaluation of the patient’s caloric and metabolic needs to make certain that the patient is being adequately nourished but not overfed. All efforts should be made to feed enterally so the gut is used. Newer elemental feedings require no digestion and can be used in the stomach, duodenum, or jejunum. (See Nutritional Support, p. 117.)
4-2 RESEARCH BRIEF
From The ARDS Clinical Trials Network; National Heart, Lung, and Blood Institute; National Institutes of Health: Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med 31(11):2592–2597, 2003.
4-3 RESEARCH BRIEF
Goldhill DR, Imhoff M, McLean B, et al. performed a meta analysis of the published studies regarding use of rotational turning beds. Twenty prospective randomized controlled trials on rotational therapy were published between 1987 and 2004. Various types of beds were studied, but few details on the rotational parameters were reported. The usual control was manual turning of patients by nurses every 2 hours. One animal investigation and 12 clinical trials addressed the effectiveness of rotational therapy in preventing respiratory complications. Significant benefits to patients were reported in the animal study and 4 of the trials. Significant benefits to patients were reported in two of another four studies focused on treatment of established complications. Little convincing evidence is available regarding the most effective rotation parameters (e.g., degree, pause time, and amount of time per day). Meta-analysis suggests that rotational therapy decreases the incidence of pneumonia but has no effect on duration of mechanical ventilation, number of days in intensive care, or hospital mortality.
From Rotational bed therapy to prevent and treat respiratory complications: a review and meta-analysis. Am J Clin Cardiol 16(1), 2007.
CARE PLANS FOR ALI AND ARDS
1. Assess and document character of respiratory effort: rate, depth, rhythm, and use of accessory muscles of respiration.
2. Assess patient for signs and symptoms of respiratory distress: restlessness, anxiety, confusion, tachypnea (RR greater than 20 breaths/min), and use of accessory muscles.
3. Assess breath sounds with each vital signs check. Adventitious sounds, which usually are present in the later stages of ARDS, are not as likely to occur during the early stage.
4. Monitor serial ABG values, and consult physician for significant changes. Explain need for frequent analysis to patient and significant others.
5. Compare ABG saturation with pulse oximetry saturation for accuracy. Consult physician or mid level practitioner for pulse oximetry values less than 90%.
6. Administer O2 and monitor FIO2 as prescribed.
7. Monitor and record pulmonary function tests as prescribed, especially tidal volume and minute ventilation. Expect decreased tidal volume and increased minute ventilation with respiratory distress.
8. Position patient for comfort and to promote adequate gas exchange. Usually, semi-Fowler’s to high Fowler’s position is therapeutic.
9. Keep oral airway and self-inflating manual ventilating bag at the bedside for emergency use. Keep emergency intubation equipment at the bedside for use should patient’s condition deteriorate.
related to dislodging of life-sustaining equipment during positioning or repositioning
Personal Safety Behavior; Risk Control
Environmental management: safety
1. Secure the ET tube/other devices to prevent accidental movement or dislodging.
2. Provide the appropriate length ventilator tubing to facilitate positioning of the patient without risk of pulling on the ET tube.
3. Facilitate tolerance of rotational therapy by managing anxiety and promoting sleep with medications.
4. Assess oxygenation once patient is prone. Typical responders will demonstrate at least 10 mm Hg increase in PaO2 within 10 minutes of being placed prone.
5. Collaborate with the respiratory care practitioner to decrease the delivered O2 as the patient’s oxygenation status improves.
Acute pneumonia
Pathophysiology
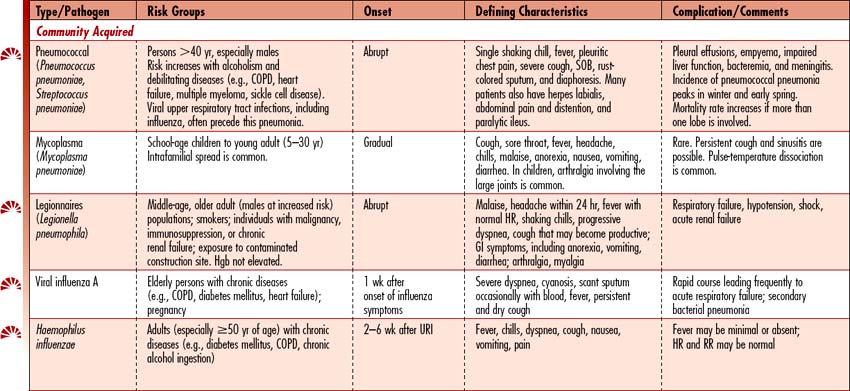
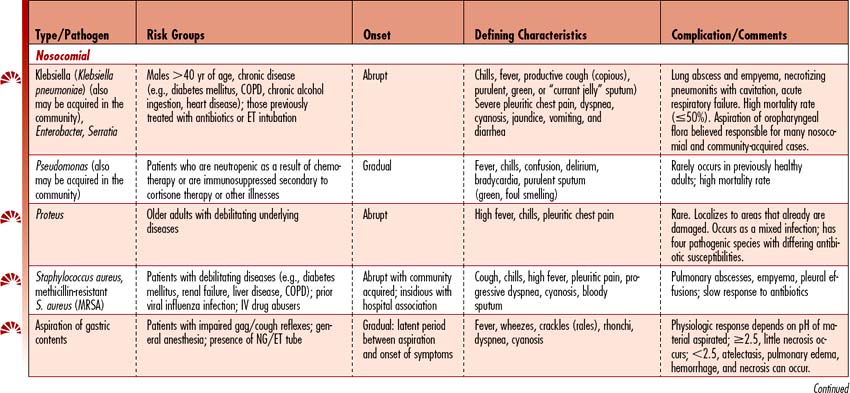
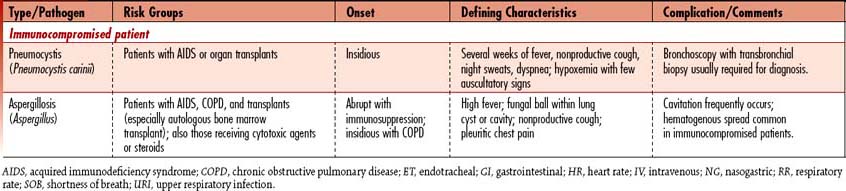
Pneumonia is the sixth leading cause of death in the United States and the leading cause of death due to infectious disease. Pneumonia is an acute infection that causes inflammation of the lung parenchyma (alveolar spaces and interstitial tissue), resulting in the alveoli filling with liquid. Pneumonias can be classified into two groups: community-acquired (CAP) and hospital-associated/nosocomial (HAP). (See Table 4-6 for a detailed discussion by pneumonia type.)
Ventilator-associated pneumonia
![]() A patient who acquires pneumonia more than 48 hours following endotracheal intubation and initiation of mechanical ventilation may be classified as having ventilator-associated pneumonia (VAP), a subgroup of HAP. VAP is the leading cause of death compared with all hospital-acquired infections. Hospital mortality of ventilated patients who develop VAP is 46% compared to 32% for mechanically ventilated patients without VAP. VAP prolongs time on the ventilator and increases length of ICU stay and length of hospital stay following discharge from the ICU, adding an estimated cost of $40,000 to an average hospital admission. The Centers for Medicare and Medicaid (CMS) have recognized VAP as a preventable illness when appropriate patient care is provided. Studies have identified a series of interventions that comprise the “VAP Bundle,” which is considered the standard of care for prevention of VAP (see Collaborative Management, p. 379).
A patient who acquires pneumonia more than 48 hours following endotracheal intubation and initiation of mechanical ventilation may be classified as having ventilator-associated pneumonia (VAP), a subgroup of HAP. VAP is the leading cause of death compared with all hospital-acquired infections. Hospital mortality of ventilated patients who develop VAP is 46% compared to 32% for mechanically ventilated patients without VAP. VAP prolongs time on the ventilator and increases length of ICU stay and length of hospital stay following discharge from the ICU, adding an estimated cost of $40,000 to an average hospital admission. The Centers for Medicare and Medicaid (CMS) have recognized VAP as a preventable illness when appropriate patient care is provided. Studies have identified a series of interventions that comprise the “VAP Bundle,” which is considered the standard of care for prevention of VAP (see Collaborative Management, p. 379).
Assessment
History and risk factors
In addition to the risk factors listed in Table 4-6, any factor that alters the integrity of the lower airways, thereby inhibiting ciliary activity, increases the likelihood of pneumonia. Impairment of the “mucociliary elevator” system impairs the ability of the patient to move secretions from the airways to the oral cavity for expectoration. These factors include hypoventilation, hyperoxia (increased FIO2), hypoxia, airway irritants such as smoke, and the presence of an artificial airway.
Cough
Vital signs
• Fever occurs in response to infection; some patients are not febrile.
• Pulse oximetry: Oxygen saturation is decreased from patient’s normal baseline value.
• P/F ratio (ratio of arterial O2 tension to fractional inspired O2) is decreased when O2 therapy is applied.
• Tachycardia and tachypnea are present if pneumonia is moderate to severe.
• Hypotension may be present if sepsis is ensuing; hypotension is exacerbated by underlying dehydration often present with pneumonia patients.
Observation
• Nasal flaring and expiratory grunt may be present.
• Use of accessory muscles indicates respiratory distress.
• May appear fatigued, with or without diaphoresis if coughing has been relentless
• Ashen, pale, or gray/blue facial color, lip color, or nail beds
• Chest expansion may be decreased, restricted, or asymmetrical with severe pneumonia in one lung manifesting severe atelectasis or pleuritic pain.
• ![]() Altered level of consciousness (confusion, disorientation, agitation) is more common with older adults.
Altered level of consciousness (confusion, disorientation, agitation) is more common with older adults.
• Agitation is more commonly associated with hypoxemia, while somnolence is associated with hypercarbia (elevated CO2 level).
Screening labwork
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Arterial blood gas analysis (ABG) | Oxygenation status and acid/base balance are evaluated with ABGs. | pH changes: Acidosis may reflect respiratory failure; alkalosis may reflect tachypnea. Carbon dioxide: Elevated CO2 reflects respiratory failure; decreased CO2 reflects tachypnea. Hypoxemia: PaO2 <80 mm Hg Oxygen saturation: SaO2 < 92% Bicarbonate: HCO3 <22 mEq/L Base deficit: <−2 |
| Complete blood count (CBC) | Evaluates for presence of infection | Increased WBC count: >11,000/mm3 is seen with bacterial pneumonias. Normal or low WBC count: Seen with viral or mycoplasma pneumonias |
| Sputum gram stain, culture and sensitivity | Identifies infecting organism; A sputum culture should be obtained from the lower respiratory tract before initiation of antimicrobial therapy. The most reliable specimens are obtained via bronchoalveolar lavage (BAL) during bronchoscopy, suctioning with a protected telescoping catheter (mini-BAL), or open-lung biopsy (used occasionally to reduce contamination of specimen with oral flora). |
Gram stain positive: Indicates organism is present Culture: Identifies organism Sensitivity: Reflects effectiveness of drugs on identified organism |
| Blood culture and sensitivity | Identifies whether pneumonia organism has become systemic; blood cultures help to identify the causative organism. | Secondary bacteremia: A frequent finding; patients with bacteremia are at higher risk for developing respiratory failure. |
| Serologic studies | Acute and convalescent titers are drawn to diagnose viral pneumonia. Both serologic and urine tests are available for Legionnaires pneumonia. | Increased antibody titers: A positive sign for viral infection |
| Acid-fast stain | To rule out mycobacterial infection (e.g., tuberculosis) | Positive: Mycobacterial infection is present. |
| Chest radiograph | Identifies anatomic involvement, extent of disease, presence of consolidation, pleural effusions, or cavitation | Lobar: Entire lobe involved Segmental (lobular): Only parts of a lobe involved Bronchopneumonia: Affects alveoli contiguous to the involved bronchi |
| Diagnostic fiberoptic bronchoscopy using PSB (protected specimen brush) and BAL | Obtains specimens during simple bronchoscopy without contaminating the aspirate; modified technique (mini-BAL) is also effective without the need of full bronchoscopy. | Gram stain positive: Indicates organism is present Culture: Identifies organism Sensitivity: Reflects effectiveness of drugs on identified organism |
| Thoracentesis | Removal of pleural effusion fluid from the pleural space using a needle to drain the chest cavity. Pleural effusion fluid may be cultured following thoracentesis to identify the causative organism. | Gram stain positive: Indicates organism is present Culture: Identifies organism Sensitivity: Reflects effectiveness of drugs on identified organism |
Collaborative management
COMMUNITY-ACQUIRED PNEUMONIA (CAP) HOSPITAL QUALITY ALLIANCE (HQA) INDICATORS
| In December 2002, the American Hospital Association (AHA), Federation of American Hospitals (FAH), and Association of American Medical Colleges (AAMC) launched the Hospital Quality Alliance (HQA), an initiative to provide the public with specific reported information about hospital performance. This national public-private collaboration encourages hospitals to voluntarily collect and report quality performance information. The Centers for Medicare and Medicaid Services and The Joint Commission participate in HQA. Hospitals are expected to track and analyze their performance ratings and use the information to improve quality. The table below reflects HQA measures considered essential when caring for patients with community-acquired pneumonia (CAP). All indicators are evidence-based actions that should be included in the plan of care. The measurement describes the details of each indicator. Evidence of performance is derived from review of each patient’s medical record following hospital discharge. | |
| Indicators | Measure |
| Initial antibiotic timing | Initial antibiotic is received within 4 hours of hospital arrival. |
| Appropriate antibiotic selection | Initial antibiotic is appropriate for CAP in immunocompetent patients. |
| Blood cultures drawn | Cultures are performed within 24 hours prior to or after hospital arrival. |
| Blood cultures prior to antibiotics | Blood culture is performed before the first antibiotic is received in the hospital. |
| Oxygenation assessment | Assessed after arriving at the hospital |
| Pneumococcal vaccination | Administered during hospitalization |
| Influenza vaccination | Administered during hospitalization |
| Smoking cessation counseling | Counseling is provided for patients with history of smoking. |
Care priorities
• Oxygen therapy: Administered when patient has an SpO2 less than 92% or becomes symptomatic for air hunger. Special care must be taken not to abolish the hypoxic drive needed for effective breathing if patient has COPD and is known to retain CO2. For patients with chronic CO2 retention, O2 is delivered in low concentrations while O2 saturation (SpO2) is closely monitored. The physician should be consulted for parameters of “acceptable” O2 saturation values in any patient with CO2 retention. Patients in need of higher-level O2 may be considered for noninvasive, positive pressure ventilation to help reduce work of breathing.![]()
• Intubation and mechanical ventilation: Intubation may be necessary if a patient experiences progressive respiratory distress despite treatments. Mechanical ventilation is required if patient is unable to maintain adequate ABG values (PaO2 greater than 60 mm Hg) with supplemental O2. High concentrations of O2 and positive end-expiratory pressure (PEEP) may be necessary in severe cases of pneumonia that lead to acute respiratory failure. (See Acute Respiratory Failure, p. 383.)
2. Determine severity of pneumonia:
• RR greater than 30 breaths/min
• Systolic blood pressure (BP) less than 90 mm Hg
• Diastolic BP less than 60 mm Hg
• Bilateral or multilobar involvement on chest radiograph
• P/F ratio (ratio of PaO2 to FIO2) less than 250
• Urine output less than 20 ml/hr or a total output of less than 80 ml/hr over 4 hours
• A 50% increase in size of the pulmonary infiltrate during the first 48 hours following diagnosis
• Patient requires endotracheal intubation and mechanical ventilation.
• Initiate Early Goal Directed Therapy and Surviving Sepsis Guidelines if patient is septic (http://www.survivingsepsis.com/aboutcampaign).
• Antibiotics or anti-infectives: Prescribed empirically on the basis of presenting signs and symptoms, clinical findings, and chest radiograph results until sputum or blood culture results are available. Pneumococcus is the most common pathogen associated with CAP, whereas enteric gram-negative bacteria are the most common pathogens identified with HAP. Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus are the most common organisms seen in patients on long-term mechanical ventilation. Antimicrobial therapy in critically ill patients usually is parenteral and guided by sensitivity of the causative organism. Many of the organisms responsible for nosocomial pneumonias are resistant to multiple antibiotics or antimicrobials. Proper identification of the organism, determination of sensitivity to the medication, and attainment of therapeutic drug levels are critical for effective therapy.
• Isolation: Some patients with pneumonia may require isolation and transmission-based precautions.
8. Support nutritional status:
Malnutrition is a causative factor in development of infections. In severely ill patients, enteral nutrition may provide the best protection against development of sepsis, owing to probable prevention of bacterial translocation from the gut. A nutritional therapy consultation is warranted for all patients who have developed an infection and those at high risk of infection.
INSTITUTE FOR HEALTHCARE IMPROVEMENT (IHI) VENTILATOR-ASSOCIATED PNEUMONIA (VAP) BUNDLE
| The IHI has composed a group of interventions for all patients on mechanical ventilation that when implemented together, result in better outcomes than when implemented individually. Reducing mortality due to VAP requires an organized approach to early recognition and consistent use of evidence-based practices. | |
| Indicator | Measure |
| Elevation of head of the bed | Head of the bed is elevated ≥30 degrees for the majority of the day (unless medically contraindicated). |
| Daily “sedation vacation” with assessment for readiness to extubate | Sedation is interrupted until the patient is able to follow commands and can be assessed for discontinuation of mechanical ventilation. |
| Peptic ulcer disease (PUD) prophylaxis | Gastric acid–controlling medications are administered to increase gastric pH. H2 blockers are preferred over sucralfate. Proton pump inhibitors have not been fully studied. |
| Deep vein thrombosis (DVT) prophylaxis | Thrombin-inhibiting medications or mechanical devices are used to reduce the risk of clot development in lower extremities. |
CARE PLANS FOR ACUTE PNEUMONIA
related to respiratory compromise present with pneumonia
Infection Severity, Infection Protection
1. Implement standard precautions for infection prevention.
2. Provide additional infection control measures if infecting organism requires isolation.
3. Maintain a closed or inline suction system or an aseptic environment when suctioning the patient.
4. Inform visitors of effective precautions or pertinent isolation procedures.
5. Encourage and help provide turning, coughing, deep breathing, and use of incentive spirometer. Educate significant others to assist with these activities.
6. Encourage and assist with ambulation as soon as possible.
1. ![]() Identify presurgical candidates at increased risk for nosocomial pneumonia (see Table 4-6).
Identify presurgical candidates at increased risk for nosocomial pneumonia (see Table 4-6).
2. Provide presurgical patients and significant others with verbal and written instructions and demonstrations of turning, coughing, and deep-breathing exercises performed after surgery to prevent atelectasis, which may lead to pneumonia.
3. Postoperatively encourage lung expansion: turning and repositioning in bed, deep breathing, coughing at frequent intervals. Mobilization of secretions is facilitated by movement.
4. Encourage and assist with ambulation as soon as possible.
5. Recognize the following ways in which nebulizer reservoirs can contaminate patient: introduction of nonsterile fluids or air; manipulation of nebulizer cup; or backflow of condensation into reservoir or into patient when delivery tubing is manipulated.
6. Use only sterile fluids, and dispense them aseptically.
7. Recognize and manage risk factors for patients with tracheostomy or ET tubes and mechanically ventilated patients:
8. For patients who cannot remove secretions effectively by coughing, perform procedures that stimulate coughing such as chest physiotherapy, which includes breathing exercises, postural drainage, and percussion.
9. If pain interferes with lung expansion, control it by administering as-needed analgesics 0.5 hour before deep-breathing exercises, and provide splint support of wound areas with hands or pillows placed firmly across site of incision.
10. Identify patients at risk for aspiration, such as those with a decreased level of consciousness or dysphagia or who have a nasogastric or gastric tube in place.
11. For patients with decreased level of consciousness (LOC) who are unable to eat normally, consult physician regarding need for a method of feeding in which risk of aspiration is minimal such as postpyloric feeding (e.g., weighted small bore feeding tube that imports enteral feeding to the duodenum or percutaneous endoscopic gastrostomy [PEG tube]).
12. Elevate head of bed (HOB) to at least 30 degrees during feedings and for 1 hour after any feeding or medication to reduce the risk of aspiration.
related to insensible fluid losses associated with pneumonia
Fluid Balance, Electrolyte and Acid-Base Balance
1. Identify patients at risk for dehydration, including those with poor nutritional status, reduced fluid intake, history of severe coughing (may be associated with inability to eat and/or vomiting), increased insensible loss secondary to hyperventilation, fever, and use of supplemental O2.
2. Monitor input and output (I&O) hourly. Initially, intake should exceed output during volume replacement therapy. Consult physician for urine output less than 0.5 ml/kg/hr for 2 consecutive hours.
3. Monitor vital signs and hemodynamic pressures for signs of continued hypovolemia. Be alert to decreased values in BP, CVP, PAP, CO, and MAP, as well as increased HR and SVR.
4. Weigh patient daily, at the same time of day (preferably before breakfast), on a balanced scale, with the patient wearing the same type of clothing.
5. Administer fluids by mouth (PO) and IV as prescribed. Document patient’s response to replacement therapy.
6. Monitor for signs and symptoms of fluid overload or too-rapid fluid administration: crackles (rales), shortness of breath (SOB), tachypnea, tachycardia, increased CVP, increased PAPs, jugular vein distention, and edema.
Additional nursing diagnoses
Also see Drowning (p. 307), and Acute Asthma Exacerbation (p. 354). As appropriate, see nursing diagnoses and interventions in Nutritional Support (p. 117), Acute Respiratory Failure (p. 383), Mechanical Ventilation (p. 99), Prolonged Immobility (p. 149), and Emotional and Spiritual Support of the Patient and Significant Others (p. 200).
4-4 RESEARCH BRIEF
From Rello J, Koulenti D, Blot S, et al: Oral care practices in intensive care units: a survey of 59 European ICUs. Intensive Care Med 33(6):1066–1070, 2007; and Binkley C, Furr LA, Carrico R, et al: Survey of oral care practices in US intensive care units. Am J Infect Control 32(3):161–169, 2004.
Acute respiratory failure
Pathophysiology
The primary goal of the pulmonary system is to promote an appropriate and reasonable gas exchange at the alveolar-capillary surface, generally measured by pulse oximetry and arterial blood gases. Acute respiratory failure is a general term that identifies a primary lung dysfunction. That dysfunction results in failure to remove CO2 (known as hypoventilation), and/or failure to promote appropriate and proportionate O2 uptake at the alveolar-capillary interface. Type I (hypoxemic) is oxygenation failure, whereas type II (hypercapnic) is ventilation failure. Many patients manifest respiratory failure of types I and II simultaneously. Clinically, type I failure exists when PaO2 is less than 50 mm Hg with the patient at rest and breathing room air (FIO2 = 0.21 or 21% of the atmospheric pressure, which is 760 mm Hg at sea level). PaCO2 greater than 50 mm Hg is significant for acute ventilation failure or hypercapnia. A wide variety of disease states create a single or mixed respiratory failure. One of the simplest methods of evaluating patients relates to the understanding of basic gas exchange. Oxygenation occurs primarily during inspiration and the removal of CO2 occurs during exhalation. The basic concepts applied here include compliance and recoil. Lung compliance is the measure of expansion of the alveoli (the gas-exchanging surface), which occurs on inspiration, whereas elasticity refers to the ability of the alveoli to recoil, as they do on exhalation. Restrictive airway diseases general present with significant hypoxemia, whereas obstructive disorders are more likely to develop a persistent and chronic hypercapnia. See Box 4-1 for a description of some of the disease processes that can lead to acute respiratory failure. Careful consideration should be given to evaluate neurologic conditions and OSA as these are commonly overlooked causes of respiratory failure. The evaluation of respiratory failure includes the understanding of the following:
Box 4-1 DISEASE PROCESSES LEADING TO THE DEVELOPMENT OF RESPIRATORY FAILURE
Obstructive disease states, impaired exhalation, impaired minute ventilation, co2 retention
• COPD (emphysema, bronchitis, asthma, cystic fibrosis)
• Neuromuscular defects (Guillain-Barré syndrome, myasthenia gravis, multiple sclerosis, muscular dystrophy, polio, brain/spinal injury)
• Depression of respiratory control centers (drug-induced cerebral infarction, inappropriate use of high-dose oxygen therapy, drug/toxic agents)
V./q. match
Components of an abnormal V./Q. ratio include:
Alveolar dead space ventilation:
This is a primary problem with pulmonary perfusion. Alveoli may be compliant and elastic, but in a condition where the alveoli are normal or hyperventilated, and the perfusion is proportionately lower than the ventilation, there is a primary gas exchange problem. This is measured or evaluated as a high V./Q. mismatch, wherein ventilation is proportionately greater than perfusion. This is frequently seen with low cardiac output states, or pulmonary embolus, and in overventilation of the independent lung surface.
Assessment
Goal of system assessment
To evaluate for poor gas exchange and increased ventilatory support requirements (see Acid-Base Balance, p. 1, and Mechanical Ventilation, p. 99).
History and risk factors
![]() Indicators of acute respiratory failure vary according to the underlying disease process and severity of the failure. Acute respiratory failure is one of the most common causes of impaired LOC and agitation. Respiratory failure is often associated with heart failure, pneumonia, or stroke. Sometimes the onset of acute respiratory failure is so insidious it is missed or misinterpreted. A patient may be somnolent due to hypercapnia (CO2 retention causing elevated CO2) from ventilatory failure or agitated and combative due to hypoxia. Patients with COPD have reduced airway diameter as well as chronic inflammation and airway remodeling, and when the underlying chronic condition is exacerbated, mucus hypersecretion and airway edema compound the initial condition. Increased effort is needed to mobilize gas in and out of the lungs, particularly during exhalation. The failure to exhale leads to air trapping and hyperinflation, which further compromise inspiratory effort and contribute to increasing hypoxia and hypercapnia.
Indicators of acute respiratory failure vary according to the underlying disease process and severity of the failure. Acute respiratory failure is one of the most common causes of impaired LOC and agitation. Respiratory failure is often associated with heart failure, pneumonia, or stroke. Sometimes the onset of acute respiratory failure is so insidious it is missed or misinterpreted. A patient may be somnolent due to hypercapnia (CO2 retention causing elevated CO2) from ventilatory failure or agitated and combative due to hypoxia. Patients with COPD have reduced airway diameter as well as chronic inflammation and airway remodeling, and when the underlying chronic condition is exacerbated, mucus hypersecretion and airway edema compound the initial condition. Increased effort is needed to mobilize gas in and out of the lungs, particularly during exhalation. The failure to exhale leads to air trapping and hyperinflation, which further compromise inspiratory effort and contribute to increasing hypoxia and hypercapnia.
Vital signs
• Early indicators: Dyspnea, restlessness, anxiety, headache, fatigue, cool and dry skin, increased BP, tachycardia, and persistent rapid respiratory rate, which indicate hypoxia. Hypercapnia results in slurred speech and headache.
• Intermediate indicators: Confusion, profound lethargy, tachypnea, hypotension and somnolence (if pH is less than 7.25), and cardiac dysrhythmias
• Late indicators: Cyanosis, diaphoresis, coma, and respiratory arrest
Auscultation
1. Late inspiratory rales (crackles): Anterior, lateral, and posterior: alveolar fluid or late opening
2. Mid inspiratory rales (crackles): Anterior, lateral, and posterior alveolar consolidation
3. Early loud, coarse rales (bubbles or rhonchi): Anterior, lateral, and posterior: conducting airway inflammation and mucus secretion
4. Inspiratory wheezes: Anterior, lateral, and posterior: early airway narrowing
5. Expiratory wheezes: Anterior, lateral, and posterior: late airway narrowing
Diagnostic tests for acute respiratory failure
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Blood Studies | ||
| Arterial blood gas analysis | Assesses adequacy of oxygenation and effectiveness of ventilation. Evaluates the oxygenation of the arterial blood as well as the presence or absence of acid and the effect on the pH (environment of the cells). | Typical results predicting respiratory failure are PaO2 <60 mm Hg, PaCO2 >45 mm Hg, with a pH that may be within normal range consistent with compensation via an increase in HCO3 (bicarbonate), or the pH may be less than 7.35 consistent with acute (uncompensated) respiratory acidosis. Although changes are not always predictable in the disease process, generally patients will develop hypoxemia, which may initially be resolved with increasing the FIO2 but eventually will require great increases in FIO2 and ultimately will no longer respond to oxygen therapy. |
| PaO2/FIO2 ratio | The PaO2 divided by the FIO2 (PaO2/FIO2 ratio, or more simply P/F) can be used to more simply assess the severity of the gas exchange defect. The normal value for the ratio of the partial pressure of oxygen in arterial blood to FIO2 (PaO2/FIO2) (FIO2 is expressed as a decimal ranging from 0.21 to 1.00) is 300–500. | A value of less than 300 indicates gas exchange derangement, and a value below 250 is indicative of severe impairment, and compliance calculations should be performed to encourage alveolar recruitment strategies. |
| Radiology | ||
| Chest radiograph (CXR) | Assesses the size of lungs, presence of fluids, abnormal gas or fluids in the pleural sac, diaphragmatic margins, the pulmonary hilum, and the integrity of the rib cage | Presence of fluids in the lung parenchyma initially presents as pulmonary edema. The continuous accumulation differentiates this edema formation to one that is not cardiac. |
| Computed tomography (CT) lung scan | Assesses the three-dimensional lung capacities, fluid load, and primary displacement of the fluid | Normally a large gas-filled surface, the ALI/ARDS lung when seen on CT is frequently fluffy and white due to fluid that has extravagated through the endothelial deficits (capillary leak). |
Collaborative management
Care priorities
2. Correction of respiratory acidosis (hypercapnia):
• Noninvasive (NPPV): Ventilator support that is given without endotracheal intubation or tracheotomy. Administered via a face or nasal mask. Requires skilled management but not necessarily intensive care admission. NPPV or invasive mechanical support is used as a continuous replacement for normal lung function until the underlying cause of the failure can be corrected and the patient can resume ventilatory efforts independently.
• Invasive: Ventilator support that is given through endotracheal intubation or tracheotomy; requires intensive or high-acuity care. The purposes of intubation and mechanical ventilation are to restore alveolar ventilation and systemic oxygenation, provide compensation in metabolic acidosis/alkalosis, and decrease work of breathing. Early intubation can prevent further airway collapse and tissue injury. In most cases of respiratory failure, the patient will require intubation and mechanical ventilation to support adequate respiratory function and stabilize ABG levels. (See Mechanical Ventilation, p. 99.)
3. Correction of acidotic ph due to hypercapnia (hypoventilation):
![]() Adequate cellular and metabolic functioning are hindered when pH level remains outside the normal range of 7.35 to 7.45. When the pH is less than 7.20, evaluate for signs of systemic compromise such as failure to maintain vascular tone and therefore BP. After efforts to correct ventilation have failed or if the patient presents with clinical symptoms such as hypotension refractory to volume and vasopressors, IV sodium bicarbonate may be used conservatively to return the pH to a level higher than 7.25. The use of sodium bicarbonate is a short-term solution for most acid-base disturbances and its use may actually make the overall situation worse.
Adequate cellular and metabolic functioning are hindered when pH level remains outside the normal range of 7.35 to 7.45. When the pH is less than 7.20, evaluate for signs of systemic compromise such as failure to maintain vascular tone and therefore BP. After efforts to correct ventilation have failed or if the patient presents with clinical symptoms such as hypotension refractory to volume and vasopressors, IV sodium bicarbonate may be used conservatively to return the pH to a level higher than 7.25. The use of sodium bicarbonate is a short-term solution for most acid-base disturbances and its use may actually make the overall situation worse.
4. Correction of alkalotic ph due to hypocapnia (hyperventilation):
4-5 RESEARCH BRIEF
From Scala R, Naldi M, et al: Noninvasive positive pressure ventilation in patients with acute exacerbations of COPD and varying levels of consciousness. Chest 128(3):1657–1666, 2005.
CARE PLANS FOR ACUTE RESPIRATORY FAILURE
related to disease process underlying impending respiratory failure
1. Monitor for signs of increasing hypoxia at frequent intervals: restlessness, agitation, and personality changes are indicative of severe exacerbation. Cyanosis of the nail beds (peripheral) and/or lips (central) are early and late indicators of hypoxia, respectively.
2. Monitor for signs of hypercapnia at frequent intervals: confusion, listlessness, and somnolence may indicate respiratory failure or near-fatal asthma exacerbation.
3. Monitor ABGs when continuous pulse oximetry values or patient assessment reflects progressive hypoxemia or development of hypercapnia. Be alert to decreasing PaO2 and increasing PaCO2 or decreasing O2 saturation levels, indicative of impending respiratory collapse.
4. Monitor for synchronous, bilateral lung expansion, decreased breath sounds, or changes in wheezing at frequent intervals. Absent breath sounds in a distressed asthma patient may indicate impending respiratory arrest.
1. Ascertain and alleviate the cause of restlessness to decrease metabolic demands (e.g., if restlessness is related to anxiety, help reduce anxiety by providing reassurance, enabling family members to stay with patient, and offering distractions such as soft music or television).
2. ![]() Be aware that restlessness may be an early sign of hypoxemia.
Be aware that restlessness may be an early sign of hypoxemia.
3. Explain all procedures and offer support to minimize fear and anxiety, which can increase O2 demands.
1. Provide humidity in O2 if used for more than 12 hours to help thin secretions.
2. Administer supplemental O2 using liter flow device as ordered.
3. Document pulse oximetry with O2 liter flow in place at time of reading as ordered. Oxygen is a drug; the dose of the drug must be associated with the O2 saturation or the reading is meaningless.
4. Monitor for skin breakdown where O2 devices are in contact with the skin, such as nares, and around edges of mask devices.
5. Monitor FIO2 to ensure that O2 is within prescribed concentrations. If patient does not retain CO2, 100% nonrebreather mask may be used to provide maximal O2 support. If the patient retains CO2 and is unrelieved by positioning, lower-dose O2, bronchodilators, and steroids, intubation, and mechanical ventilation may be necessary sooner than in patients who are able to receive higher doses of O2 by mask.
1. Obtain arterial blood gases if patient experiences behavioral changes or respiratory distress to check for hypoxia or hypercapnia.
2. Monitor for induced hypoventilation, especially in patients with COPD.
3. Monitor for changes in chest radiograph and breath sounds indicative of O2 toxicity and absorption atelectasis in patients receiving higher concentrations of O2 (more than FIO2 45%) for longer than 24 hours. The higher the O2 concentration, the greater is the chance of toxicity.
4. Position patient for comfort and to promote optimal gas exchange. High-Fowler’s position, with the patient leaning forward and elbows propped on the over-the-bed table to promote maximal chest excursion, may reduce use of accessory muscles and diaphoresis due to work of breathing.
5. Consider use of NPPV prior to endotracheal intubation and mechanical ventilation.