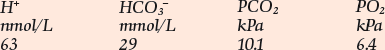Respiratory and mixed acid–base disorders
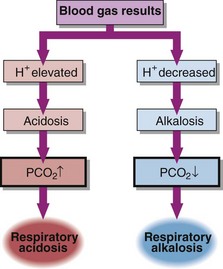
In respiratory acid–base disorders the primary disturbance is caused by changes in arterial blood PCO2 (Fig 22.1). Respiratory disorders are related to changes either in the amount of air moving in or moving out of the lungs (ventilation), or in the ability of gases to diffuse across the alveolar membrane (gas exchange). In both cases PCO2 changes and the carbonic acid concentration rises or falls.
Respiratory acidosis
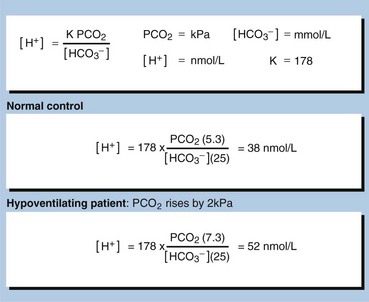
Respiratory acidosis may be acute or chronic. Acute conditions occur within minutes or hours. They are uncompensated. Renal compensation has no time to develop as the mechanisms that adjust bicarbonate reabsorption take 48–72 hours to become fully effective. The primary problem in acute respiratory acidosis is alveolar hypoventilation. If airflow is completely or partially reduced, the PCO2 in the blood will rise immediately and the [H+] will rise quickly (Fig 22.2). A resulting low PO2 and high PCO2 causes coma. If this is not relieved rapidly, death results.
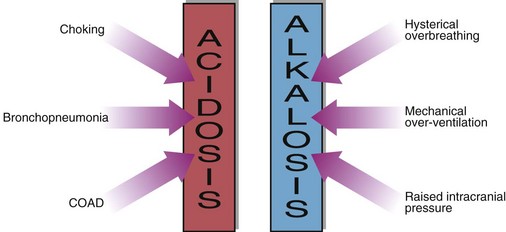
Examples of acute, and hence uncompensated, respiratory acidosis are:
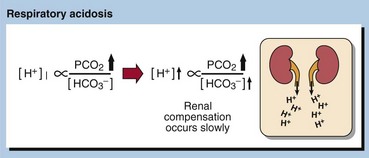
Chronic respiratory acidosis usually results from chronic obstructive airways disease (COAD) and is usually a long-standing condition, accompanied by maximal renal compensation. In a chronic respiratory acidosis the primary problem again is usually impaired alveolar ventilation, but renal compensation contributes markedly to the acid–base picture. Compensation may be partial or complete. The kidney increases hydrogen ion excretion and ECF bicarbonate levels rise. Blood [H+] tends back towards normal (Fig 22.3).
It takes some time for the kidneys to respond to a high PCO2 and a high [H+], and therefore compensation will only be maximal some days after the onset of the clinical problem. In many patients with chronic respiratory conditions, extensive renal compensation will keep the blood [H+] near normal, despite grossly impaired ventilation. In stable chronic bronchitis the [H+] may be within the reference interval despite a very high PCO2. This is achieved only by maintaining a plasma bicarbonate concentration twice that of normal. The PO2 is usually depressed, and becomes more so as lung damage increases with time (pp. 46–47). Examples of chronic respiratory disorders are:
The causes of respiratory acidosis are summarized in Figure 22.4.
Respiratory alkalosis
Respiratory alkalosis is much less common than acidosis but can occur when respiration is stimulated or is no longer subject to feedback control (Fig 22.4). Usually these are acute conditions, and there is no renal compensation. The treatment is to inhibit or remove the cause of the hyperventilation, and the acid–base balance should return to normal. Examples are:
Mixed acid–base disorders
Other examples of mixed acid–base disorders commonly encountered are:
 a patient with chronic obstructive airways disease, causing a respiratory acidosis, with thiazide-induced potassium depletion and consequent metabolic alkalosis
a patient with chronic obstructive airways disease, causing a respiratory acidosis, with thiazide-induced potassium depletion and consequent metabolic alkalosis
 hyperventilation causing a respiratory alkalosis, with prolonged nasogastric suction that causes a metabolic alkalosis
hyperventilation causing a respiratory alkalosis, with prolonged nasogastric suction that causes a metabolic alkalosis
 salicylate poisoning in which respiratory alkalosis occurs due to stimulation of the respiratory centre, together with metabolic acidosis due to the effects of the drug on metabolism.
salicylate poisoning in which respiratory alkalosis occurs due to stimulation of the respiratory centre, together with metabolic acidosis due to the effects of the drug on metabolism.
Care must be taken in the interpretation of the blood gas results in these patients. Knowledge of the clinical picture is essential. Theoretically, the limits of the compensatory responses in simple primary acid–base disorders are known (Fig 22.5). When compensation apparently falls outside of these expected limits, it is likely that a second acid–base disorder is present.
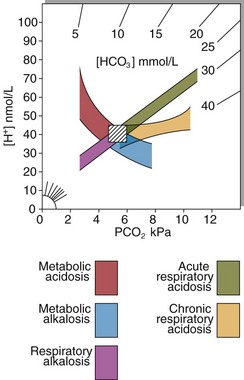
Fig 22.5 The 95% confidence intervals for arterial blood gases in primary acid–base disorders. Arterial [H+] is plotted against PCO2 with lines of equal [HCO3−] radiating from origin. The hatched box shows normal values. Graphs such as these may be used to chart the progress of a patient under treatment to correct an acid–base disorder.
There is further discussion on the interpretation of blood gas results on pages 48–49.