14 Respiratory Alterations and Management
After reading this chapter, you should be able to:
• describe the pathophysiological mechanisms of acute respiratory failure (ARF) and key principles of patient management
• differentiate between hypoxaemic (type I) and hypercapnoeic (type II) respiratory failure
• outline the incidence of respiratory alterations in the Australasian critical care context
• discuss the aetiology, pathophysiology, clinical manifestations and management of common respiratory disorders managed in intensive care, specifically pneumonia, respiratory epidemics, asthma, chronic obstructive pulmonary disease (COPD), acute lung injury (ALI) and pneumothorax
• describe the evidence base for key components of nursing and collaborative practice involved in the management of patients with ARF in ICU
• outline the principles and immediate postoperative management for lung transplant recipients.
acute respiratory distress syndrome
hypoxaemic respiratory failure
Introduction
The most common reason that patients require admission to an intensive care unit (ICU) is for support of their respiratory system. Over the last decade, almost half of all patients admitted to ICU in Australia and New Zealand required mechanical ventilation;1 a statistic of 41% in 2008.2 Failure or inadequate function of the respiratory system occurs as a result of direct or indirect pathophysio-logical conditions. The process of mechanical ventilation may also injure a patient’s lungs, further impacting functioning of the respiratory system. Preventing or minimising ventilator-associated lung injury is therefore also a primary goal of patient care. Chapter 13 described the relevant anatomy and physiology and assessment and monitoring practices for a patient with life-threatening respiratory dysfunctions. This chapter describes the incidence, pathophysiology, clinical manifestations and management of common respiratory disorders that result in acute respiratory failure, specifically pneumonia (including discussion of respiratory epidemics), asthma, chronic obstructive pulmonary disease (COPD), acute lung injury (ALI), pneumothorax and lung transplantation. Discussion of oxygenation and ventilation strategies to support respiratory function during a critical illness is presented in Chapter 15.
Incidence of Respiratory Alterations
Respiratory diseases are common and affect significant numbers of the population in Australia, accounting for almost half of all hospital admissions.3 These diseases are also the most common illness responsible for emergency admission to hospital, the most common reason to visit a general practitioner and represent the most commonly reported long-term illnesses in children.4 Despite these findings, the incidence of respiratory alterations is difficult to quantify as the number of patients who require admission to hospital as a result of respiratory disease represent a small proportion of the total number affected. Further, patients who require admission to ICU as a result of respiratory disease represent only a fraction of all hospital admissions.5,6
Data presented in Table 14.17 illustrates the total number of patients (adults and children) admitted to hospital as a result of a range of respiratory diseases. While it is difficult to determine the number of patients in each diagnostic group who required admission to ICU as part of their management, ICU admissions account for around 4% of all overnight hospital admissions.5 Infective processes (influenza and pneumonia), COPD and asthma represent the three largest groups of hospital admissions. Conditions such as adult respiratory distress syndrome (ARDS), pneumothorax, pulmonary embolus and pulmonary oedema are relatively small. It should be noted, however, that these conditions often evolve throughout the course of an illness6 and may not therefore be included as the reason for admission. Common respiratory-related ICU presentations are discussed in the following sections.
TABLE 14.1 Incidence of respiratory alterations in Australia 2007–20087
| Disorder | Hospital admissions | |
|---|---|---|
| n | % | |
| Adult Respiratory Distress Syndrome | 202 | 0.06 |
| Asthma | 37,641 | 10.40 |
| COPD (acute exacerbation) | 56,249 | 15.54 |
| Influenza and pneumonia | 70,232 | 19.41 |
| Lung transplantation | 91 | 0.03 |
| Pneumothorax | 3,177 | 0.88 |
| Pulmonary embolus | 9,234 | 2.55 |
| Pulmonary oedema | 902 | 0.25 |
| Total | 177,728 | 49.11 |
Respiratory Failure
Respiratory failure occurs when there is a reduction in the body’s ability to maintain either oxygenation or ventilation, or both. It may occur acutely, as observed in pneumonia and ARDS or it may exist in chronic form, as observed in asthma and COPD. Respiratory failure, and the disorders that cause it, are responsible for a high proportion of death and disability throughout the world.6
Aetiology of Respiratory Failure
• decreased respiratory drive may be caused by brain trauma, drug overdose or anaesthesia/sedation
• decreased respiratory muscle strength may be caused by Guillain–Barré syndrome, poliomyelitis, myasthenia gravis or spinal cord injury
• decreased chest wall expansion may be caused by postoperative pain, rib fractures or a pneumothorax
• increased airway resistance may be caused by asthma or COPD
• increased metabolic oxygen requirements may be caused by severe sepsis
• decreased capacity for gas exchange may be caused by impairment in either ventilation (e.g. pulmonary oedema, pneumonia, acute lung injury, COPD) or pulmonary perfusion (e.g. pulmonary embolism), or a combination of the two.
Importantly, respiratory failure can be an acute or chronic condition. While acute respiratory failure (ARF) is characterised by life-threatening alterations in function, the manifestations of chronic respiratory failure are more subtle and potentially more difficult to diagnose. Patients with chronic respiratory failure often experience acute exacerbations of their disease, also resulting in the need for intensive respiratory support.6
Pathophysiology
Respiratory failure occurs when the respiratory system fails to achieve one or both of its essential gas exchange functions: oxygenation or elimination of carbon dioxide, and can be described either as type I (primarily a failure of oxygenation) or type II (primarily a failure of ventilation).6
Type I Respiratory Failure
A patient with type I (‘hypoxaemic’) respiratory failure presents with a low PaO2 and a normal or low PaCO2. Hypoxaemic respiratory failure may be caused by a reduction in inspired oxygen pressure (e.g. such as extreme altitude), hypoventilation, impaired diffusion or ventilation-perfusion mismatch. Most major respiratory alterations cause this type of failure, usually as a result of hypoventilation due to alveolar collapse or consolidation, or a perfusion abnormality.6
When there is mismatch between ventilation and perfusion in the lungs, exchange of gases is impaired and hypoxaemia ensues (see Figure 14.1):6
• In some cases, there may be reduced ventilation to a certain area of lung tissue (e.g. pulmonary oedema, pneumonia, atelectasis, ARDS). A severe form of mismatch known as intrapulmonary shunting occurs when adequate perfusion exists but there are sections of lung tissue that are not ventilated. In these alveoli, the oxygen content is similar to that of the mixed venous blood and the CO2 is elevated.
• In other instances, ventilation may be adequate but perfusion is impaired (e.g. pulmonary embolus). In its severe form, this is known as dead space ventilation as the lungs continue to be ventilated but there is no perfusion, and therefore no gas exchange. In this situation, the alveolar oxygen content is similar to that of the inspired gas mixture and the CO2 is minimal6 (see Chapter 13 for further discussion).
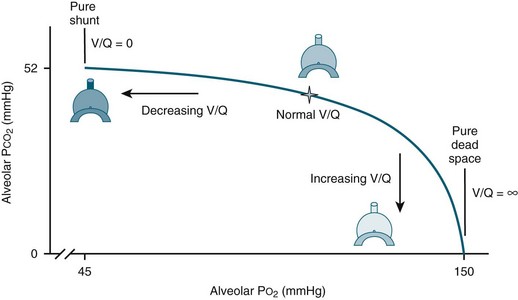
FIGURE 14.1 Ventilation-perfusion mismatches.6 Ventilation-perfusion (V/Q) ratio displays the normal balance (star) between alveolar ventilation and vascular perfusion allowing for proper oxygenation. When ventilation is reduced, the V/Q ratio decreases, in the most extreme case resulting in pure shunt, where V/Q = 0. When perfusion is reduced, the V/Q ratio increases, in the most extreme case resulting in pure dead space, where V/Q = infinite (∞). (published with permission)
Clinical Manifestations
Patient presentations in acute respiratory failure can be quite diverse and are dependent on the underlying pathophysiological mechanism (e.g. hypercapnoea and/or hypoxaemia), the specific aetiology and any comorbidities that may exist.6 Specific clinical manifestations for the clinical disorders discussed in this chapter are provided in each section. Dyspnoea is the most common symptom associated with ARF; this is often accompanied by an increased rate and reduced depth of breathing and the use of accessory muscles. Patients may also present with cyanosis, anxiety, confusion and/or sleepiness.4
A systematic approach to clinical assessment and management of patients with ARF is crucial, given the large number of possible causes. Clinical investigations to assess the cause of respiratory failure vary depending on the suspected underlying aetiology and the progression of disease. Continuous monitoring of oxygen saturation using pulse oximetry, arterial blood gas (ABG) analysis and chest radiograph assessment are used in almost all cases of respiratory failure.8 Other more specialised tests such as computed tomography (CT) of the chest and microbiological cultures may be used in specific circumstances.9 With ABG analysis, the measurement of PaO2, PaCO2, Alveolar–arterial (A–a) PO2 difference and the patient response to supplemental oxygen are key elements in determining the cause of ARF (see Chapter 13).
Independent Nursing Practice
The primary survey (airway, breathing and circulation) and immediate management form initial routine practice.10 Frequent assessment and monitoring of respiratory function, including a patient’s response to supplemental oxygen and/or ventilatory support, is the focus. Patient comfort and compliance with the ventilation mode, ABG analysis and pulse oximetry guide any titration of ventilation. The key goals of management are to treat the primary cause of respiratory failure, maintain adequate oxygenation and ventilation and prevent or minimise the potential complications of positive pressure mechanical ventilation.
Maintaining Oxygenation and Ventilation
The therapeutic aim is to titrate the fraction/percentage of inspired oxygen (FiO2) to achieve a PaO2 of 65–70 mmHg and to maintain minute ventilation to achieve PaCO2 within normal limits where possible.6 Oxygen is not a drug, therefore it does not require prescription for use. Nursing staff in ICU are therefore commonly responsible for titration of oxygen therapy to maintain a specific PaO2 or SpO2, and the alteration of respiratory rate and/or tidal volume to maintain a specified PaCO2. One concern that often arises, particularly with patients who require high concentrations of oxygen, is the risk of oxygen toxicity. The link between prolonged periods of oxygen concentrations approaching 100% and oxidant injuries in airways and lung parenchyma has been established, although mostly from animal research. Although it remains unclear how these data apply to human populations, most consensus groups have argued that FiO2 values less than 0.4 are safe for prolonged periods of time and that FiO2 values of greater than 0.8 should be avoided if possible6 (see Chapter 15 for further discussion of oxygenation).
Ventilator-associated lung injury is also a concern when managing patients with acute respiratory failure. A lung can be injured when it is stretched excessively as a result of tidal volume settings that generate high pressures, often referred to as barotrauma or volutrauma. The most common injury is that of alveolar rupture and/or air in the pleural space (pneumothorax).6 An approach known as ‘lung protective ventilation’ aims to minimise overdistension of the alveoli through careful monitoring of tidal volumes and airway pressures. This method should be considered for all ventilated patients. The approach may result in tolerance of higher PaCO2 than normal in patients presenting with acute lung injury or ARDS (see Chapter 15 for further discussion).
Development of ventilator-associated respiratory muscle weakness has been reported as a significant issue when the respiratory muscles are rendered inactive through adjustment of ventilator settings and administration of pharmacotherapy. While it is not yet possible to provide precise recommendations for interventions to avoid this, clinicians are advised to select ventilator settings that provide for some respiratory muscle use.11
Prevention or minimisation of complications associated with positive pressure mechanical ventilation remains a major focus of nursing practice. These complications may relate to the patient–ventilator interface (artificial airway and ventilator circuitry), infectious complications such as ventilator-associated pneumonia (VAP) or complications associated with sedation and/or immobility. Some common complications and the appropriate management strategies are briefly outlined in Table 14.26,12–14 and discussed further in Chapter 15.
TABLE 14.2 Complications of mechanical ventilation and associated management strategies
| Patient–ventilator interface complications | |
| Airway dislodgement/disconnection | Endotracheal tube (ETT) or tracheostomy tube is secured to optimise ventilation and prevent airway dislodgement or accidental extubation. |
| Circuit leaks | Cuff pressure assessmentCircuit checksExhaled tidal volume measurement |
| Airway injury from inadequate heat/humidity | Maintain humidification of the airway using either a heat-moisture exchanger or a water-bath humidifier. |
| Obstructions from secretions | Assess the need for suctioning regularly and suction as required. |
| Tracheal injury from the artificial airway | Assessment of airway placement and cuff pressure (minimal occlusion method) |
| Infectious complications | |
| Ventilator-associated pneumonia (VAP) | |
Collaborative Practice
A patient with ARF requires extensive multidisciplinary collaboration between nurses, physiotherapists, specialist medical staff, speech and occupational therapists, dietitians, social workers, radiologists and radiographers. Patients may require additional oxygen delivery through an adequate haemoglobin level for oxygen transportation and a cardiac output sufficient to supply oxygenated blood to the tissues.6 At times this may require blood transfusion and/or the use of vasoactive medications (see Chapters 11 and 20).
Chest physiotherapy is a routine activity for managing patients with ARF. This involves positioning, manual hyperinflation, percussion and vibration and suctioning. The evidence base for these techniques is limited, however, with a systematic review not demonstrating an improvement in mortality.12 Guidelines for physiotherapy assessment have enabled identification of patient characteristics for treatments to be prescribed and modified on an individual basis.13 Table 14.36,13,15 outlines a number of collaborative practice issues for patients with respiratory failure, particularly those who may require prolonged mechanical ventilation.
TABLE 14.3 Collaborative practices for patients with respiratory failure
| Long-term patient management | Best practice |
|---|---|
| Timing of tracheostomy insertion | Where mechanical ventilation is expected to be 10 days or more, tracheostomy should be performed as soon as identified. Early tracheostomy is associated with less nosocomial pneumonia, reduced ventilation time and shorter ICU stay. |
| Weaning protocols | Specific plan is patient dependent; better outcomes are achieved when there is an agreed and well communicated weaning plan (see Chapter 15) |
| Nutrition | Consider adequate nutrition for physiological needs – important to not overfeed as this increases CO2 production and need to have balance of vitamins and minerals |
| Swallow assessment | Assess for dysphagia |
| Mobilisation | Sitting out of bed, mobilising (see Chapter 4) |
| Communication | Communication aids, speaking valves |
| Activities | Activity plan/routine, entertainment (TV/Films), visitors, outings |
| Sleep | Clustering cares, reducing stimuli to promote sleep (see Chapter 7) |
| Family support | Importance of providing physical, emotional and/or spiritual support to family members (see Chapter 8) |
| Tracheostomy follow-up | Outreach team: follow-up care by nurses experienced in tracheostomy care can prevent complications and improve outcomes |
| End-of-life decisions in ARF | see Chapter 5 |
Medications
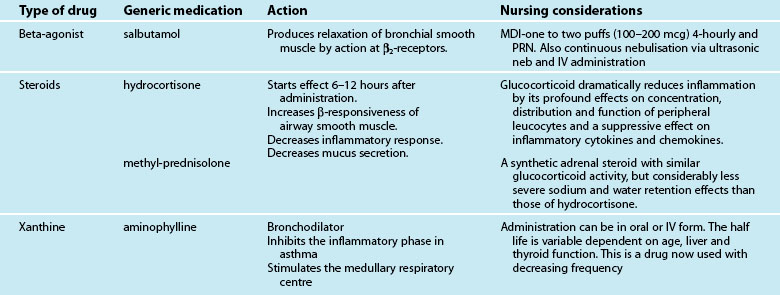
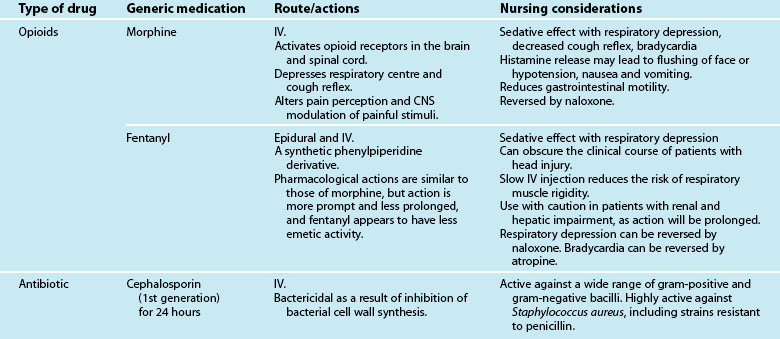
Medications commonly prescribed in respiratory failure include inhalation steroids and bronchodilators, intravenous steroids and bronchodilators, antibiotic therapy, analgesia and sedation to maintain patient–ventilator synchrony, but may also involve nitric oxide, glucocorticoid or surfactant administration. A patient’s condition, comorbidities and the above-mentioned pharmacological therapy may also be supported with inotropic and other resuscitation therapies (see Chapter 11). As the use of medications will vary depending on the underlying cause of respiratory failure, these are discussed in each section respectively.
Special Considerations
Respiratory failure in patients who are pregnant, elderly or have comorbidities require specific attention to avoid clinical deterioration. Respiratory physiology and the respiratory tract itself are altered during pregnancy; this may result in exacerbation of preexisting respiratory disease or increased susceptibility to disease (see Chapter 26). Upper airway mucosal oedema may increase the likelihood of upper respiratory tract infection. Lung function and lung volume are also altered, compensated by an increase in respiratory drive and minute ventilation. The impact of these alterations on chronic conditions such as asthma/COPD and acute illness are explored in the subsequent sections. The impact on the fetus of infection, hypoxia and drug therapy is an important consideration.6
The elderly have ageing organs and systems and other comorbidities that may exacerbate their respiratory dysfunction. Drug metabolism and excretion is slowed, complicating drug dosing and response.16 Metabolism of anaesthetic agents is slower due to the diminished physio-logy of ageing organs. Common comorbidities may also be present, including obesity, heart disease, diabetes, and renal impairment or muscle wasting. Pneumonia is a common presentation in the elderly and is often exacerbated by chronic lung conditions.6
Post-anaesthesia Respiratory Support
Short-term respiratory support may be required after major surgery, in cases of extended anaesthesia, preexisting comorbidities and/or diminished physical reserve (e.g. elderly, patients with obstructive sleep apnoea). Most patients requiring ventilation in the early postoperative period have had cardiothoracic surgery, and so much of the available research relates to this patient group (see also Chapter 12).
Preoperative assessment and management is a key factor in preventing respiratory complications. This involves optimising physical condition and nutritional status, planning the timing of surgery to reduce the likelihood of preexisting respiratory infection and patient education regarding the importance of respiratory support, including postoperative mobilisation and physiotherapy. Patients with suspected or confirmed chronic conditions require a thorough diagnostic work-up prior to surgery to determine the best management strategy in the postoperative period.17
The key focus in management of postoperative ventilation is to limit ventilation time, as prolonged ventilation time is associated with poor outcome. Once a patient has reached normothermia, is haemodynamically stable, responsive and has adequate analgesia, weaning of ventilation is commenced. Rapid and/or nurse-led weaning protocols are often implemented to minimise delays in the weaning process. Anaesthetic care in these patients includes use of short-acting or regional anaesthesia (e.g. epidural analgesia) to minimise respiratory depression.18
Pneumonia
Pathophysiology
The normal human lung is sterile, unlike the gastrointestinal tract and upper respiratory tract which have resident bacteria. A number of defence mechanisms exist to prevent microorganisms entering the lungs, such as particle filtration in the nostrils, sneezing and coughing to expel irritants and mucus production to trap dust and infectious organisms and move particles out of the respiratory system. Infection occurs when one or more of these defences are not functioning adequately or when an individual encounters a large amount of microorganisms at once and the defences are overwhelmed.6 An invading pathogen provokes an immune response in the lungs, resulting in the following pathophysiological processes:
• alteration in alveolar capillary permeability that leads to an increase in protein-rich fluid in the alveoli; this impacts on gas exchange and causes the patient to breathe faster in an effort to increase oxygen uptake and remove CO2
• mucous production increases and mucous plugs may develop which block off areas of the lung, further reducing capacity for gas exchange
• consolidation occurs in the alveoli, filling with fluid and debris; this occurs particularly with bacterial pneumonia where debris accumulates from the large number of white blood cells involved in the immune response.6
Aetiology
Pneumonia is caused by a variety of microorganisms, including bacteria, viruses, fungi and parasites. In many cases, the causative organism may not be known and current practice in many cases is to initiate antimicrobial treatment as soon as possible, based on symptoms and patient history, rather than waiting for microorganism culture results.19 The true incidence of pneumonia is not well known as many patients do not require hospitalisation. Different ages and characteristics of the patient are often associated with different causative organisms. Viral pneumonias, especially influenza, are most common in young children, while adults are more likely to have pneumonia caused by bacteria such as Streptococcus pneumoniae and Haemophilus influenzae. Pneumonia is a particular concern among elderly adults as they experience an increase in the frequency and severity of pneumonia.6
Table 14.47 outlines the principal diagnoses of patients hospitalised with pneumonia in Australia during 2007–2008. This information reflects the high proportion of viral pneumonia and the large number of cases where the causative organism may not be known.
TABLE 14.4 Principal diagnoses of patients hospitalised with pneumonia in Australia during 2007–2008
| Principal Diagnosis | Hospitalisations | |
|---|---|---|
| n | % | |
| Pneumonia due to identified influenza virus | 1668 | 2.4 |
| Influenza, virus not identified | 1429 | 2.0 |
| Viral pneumonia, not elsewhere classified | 1899 | 2.7 |
| Pneumonia due to Streptococcus pneumoniae | 1331 | 1.9 |
| Pneumonia due to Haemophilus influenzae | 1029 | 1.5 |
| Bacterial pneumonia, not elsewhere classified | 3184 | 4.5 |
| Pneumonia due to other infectious organisms, not elsewhere classified | 292 | 0.4 |
| Pneumonia, organism unspecified | 59,389 | 84.6 |
| Total | 70,232 | 100.0 |
Community-acquired Pneumonia
Clinical assessment, especially patient history, is important in distinguishing the aetiology and likely causative organism in patients with community-acquired pneumonia (CAP). Specific information regarding exposure to animals, travel history, nursing home residency and any occupational or unusual exposure may provide the key to diagnosis.9 Personal habits such as smoking and alcohol consumption increase the risk of developing pneumonia and should be explored. Many patients admitted to hospital or ICU with CAP have comorbidities, suggesting that those who are chronically ill have an increased risk of developing ARF. The most common chronic illnesses involved are respiratory disease (including smoking history, COPD/asthma), congestive cardiac failure and diabetes mellitus.6,20 Table 14.5 outlines aspects of the clinical history associated with particular causative organisms in CAP.6,9,21
TABLE 14.5 Clinical history/comorbidities associated with particular causative organisms in CAP
| Condition | Causative organisms |
|---|---|
| Individual factors | |
| Alcoholism | Streptococcus pneumoniae (including penicillin-resistant), anaerobes, gram-negative bacilli (possibly Klebsiella pneumoniae), tuberculosis |
| Poor dental hygiene | Anaerobes |
| Elderly | group B streptococci, Moraxella catarrhalis, H. influenzae, L. pneumophila, gram-negative bacilli, C. pneumoniae and polymicrobial infections |
| Smoking (past or present) | S. pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Aspergillus spp. |
| IV Drug use | S. aureus, anerobes, M. tuberculosis, S. pneumoniae |
| Comorbidities | |
| COPD | S. pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Aspergillus spp. |
| Post influenza pneumonia | S. pneumoniae, S. aureus, H. influenzae |
| Structural disease of lung (e.g., bronchiectasis, cystic fibrosis) | P. aeruginosa, P. cepacia or Staphylococcus aureus |
| Sickle cell disease, asplenia | Pneumococccus, H. influenzae |
| Previous antibiotic treatment and severe pulmonary comorbidity, (e.g. bronchiectasis, cystic fibrosis, and severe COPD) | P. aeruginosa |
| Malnutrition related diseases | Gram-negative bacilli |
| Environmental exposure | |
| Air conditioning | Legionella pneumophila |
| Residence in nursing home | S. pneumoniae, gram-negative bacilli, H. influenzae, S. aureus, Chlamydia pneumoniae; consider M. tuberculosis. Consider anaerobes, but less common. |
| Homeless population | S. pneumoniae, S. aureus, H. influenzae, Cryptococcus gattii: caused by inhalation of spores while sleeping, associated with red gum trees (Australia, Southeast Asia, South America) |
| Suspected bioterrorism | Anthrax, tularaemia, plague |
| Animal exposure | |
| Bat exposure | Histoplasma capsulatum |
| Bird exposure | Chlamydia psittaci, Cryptococcus neoformans, H. capsulatum |
| Rabbit exposure | Francisella tularensis |
| Exposure to farm animals or parturient cats | Coxiella burnetii (Q fever) |
| Travel history | |
| Travel to southwestern USA | Coccidioidomycosis; hantavirus in selected areas |
| Travel to southeast Asia | Severe acute respiratory syndrome (coronavirus), Mycobacterium tuberculosis, melioidosis |
| Residence or travel to rural tropics | Melioidosis (Burkholderia pseudomallei) |
| Travel to area of known epidemic | Avian influenza (H5N1), Swine influenza (H1N1) and SARS (coronavirus) |
The Australian CAP study collaboration20 examined episodes of CAP in which all patients underwent detailed assessment for bacterial and viral pathogens. Aetiology was identified in 46% of episodes, with the most frequent causes being Streptococcus pneumoniae (14%), Mycoplasma pneumoniae (9%) and respiratory viruses (15%). Mechanical ventilation or vasopressor support was required in 11% of cases.
Diagnosis of CAP
Routine screening of patients with suspected pneumonia continues to rely on microscopy and culture of lower respiratory tract specimens, blood cultures, detection of antigens in urine and serology. Methods for detection of antigens are now widely available for several pneumonia pathogens, particularly S. pneumoniae, Legionella and some respiratory viruses.22 Culture of respiratory secretions may be limited due to difficulty in obtaining sputum samples. For this reason, nasopharyngeal aspirates or swabs may be taken as part of the routine screening for CAP.23
Severity assessment scoring
International guidelines recommend a severity-based approach to management of CAP. CURB65, CRB65 and the Pneumonia Severity Index (PSI) are the most widely recommended systems that produce scores and assess severity based on patient demographics, risk factors, comorbidities, clinical presentation and laboratory results.6 Recent evaluation found no significant differences between these systems in their ability to predict mortality.24 The Australian CAP Collaboration team devised and validated the SMART-COP scoring system for predicting the need for intensive respiratory or vasopressor support in patients with CAP. The acronym relates to the factors: low Systolic blood pressure, Multilobar chest radiography involvement, low Albumin level, high Respiratory rate, Tachycardia, Confusion, poor Oxygenation and low arterial pH.25
Hospital-acquired and Ventilator-associated Pneumonia
Hospital-acquired or nosocomial pneumonia is defined as pneumonia occurring more than 48 hours after hospital admission.9 It is the second-most common nosocomial infection and the leading cause of death from infection acquired in-hospital. Ventilator-associated pneumonia (VAP) is a nosocomial pneumonia in patients who are mechanically ventilated. The incidence of VAP is reported at 10–30% among patients who require mechanical ventilation for greater than 48 hours.26
Critically ill ventilated patients commonly experience chest colonisation as a result of translocation of bacteria from the mouth to the lungs via the endotracheal tube (ETT). This may lead to clinical signs of infection, or the patient may remain colonised without an infective process. The patient’s severity of disease, physiological reserve and comorbidity influence the development of infection.6 Most cases (58%) of VAP are associated with infection involving gram-negative bacilli such as Pseudomonas aeruginosa and Acinetobacter spp. A high number of cases (20%) are associated with gram-positive Staphylococcus aureus. Many cases of VAP are associated with multiple organisms.6 As in CAP, the presence of comorbidities and other risk factors influence the causative organism.
Diagnosis and treatment of VAP
VAP can be difficult to diagnose, as clinical features can be non-specific and other conditions may cause infiltrates on chest X-ray (CXR). However, it is often suspected when there are new infiltrates observed on CXR or when clinical signs of infection begin to develop, e.g. new onset of pyrexia, raised white blood cell counts, purulent sputum and a difficulty in maintaining adequate oxygenation.6 Specific risk factors associated with increased mortality in VAP have been identified over the last decade. The most widely-recognised risk factor is the provision of appropriate antibiotic treatment, which has reduced mortality and the rate of complications. Timeliness of antibiotic admini-stration is an independent risk factor for mortality; mortality was increased where administration of antibiotics was delayed for more than 24 hours after diagnosis.26 When VAP is suspected there are two treatment strategies, although a systematic review did not demonstrate any differences in mortality, length of ICU stay or length of ventilation period:19
• Clinical Strategy: involves treatment of patients with new antibiotics, based on patient risk factors and the local microbiologic and resistance patterns. Therapy is adjusted based on culture results and the patient’s response to treatment.
• Invasive Strategy: involves collection and quantitative analysis of respiratory secretions from samples obtained by bronchoalveolar lavage (BAL) to confirm the diagnosis and causative organism. Antibiotic therapy is then guided by specific protocols.
Clinical Manifestations
Symptoms for pneumonia are both respiratory and systemic. Common characteristics include fever, sweats, rigours, cough, sputum production, pleuritic chest pain, dyspnoea, tachypnoea, pleural rub, inspiratory crackles on auscultation, plus radiological evidence of infiltrates or consolidation. Cough is the most common finding and is present in up to 80% of all patients with CAP.6,9
Collaborative Practice
Early recognition of pneumonia and timely administration of antibiotic therapy are key aspects for patient management. The most important aspect of management in VAP is prevention and this is a key emphasis in the care of all mechanically-ventilated patients. One approach in encouraging the implementation of VAP prevention was the combination of four aspects of patient management into one evidence based guideline, known as the Ventilator Care Bundle: elevating the head of bed to 30–45 degrees, daily sedation vacation and assessment of readiness to extubate, peptic ulcer disease (PUD) prophylaxis and deep vein thrombosis (DVT) prophylaxis.27 Effectiveness of this strategy and implementation issues have been further evaluated, with some additional perspectives offered. While it is apparent that daily spontaneous awakening and breathing trials are associated with early liberation from mechanical ventilation and VAP reduction, the strategies included for DVT and PUD prophylaxis do not directly affect VAP reduction. Semi-recumbent positioning has been associated with a significant reduction in VAP but is difficult to maintain in ventilated patients.14 It has been suggested that other methods to reduce VAP, such as oral care and hygiene, chlorhexidine in the posterior pharynx and specialised endotracheal tubes (continuous aspiration of sub-glottic secretions, silver-coated), should be considered for inclusion in a revised Ventilator Bundle more specifically aimed at VAP prevention.14
Development of VAP is attributed in part to aspiration of oral secretions that are colonised by a variety of bacteria. Maintenance of oral hygiene is therefore a key element in the care of mechanically-ventilated patients.6 Oral mucosa and dental plaque may also be colonised with bacteria and the use of an oral antiseptic solution (e.g. Chlorhexidine) may further reduce the risk of developing VAP.28
Supportive ventilation is a key focus for managing patients with pneumonia. In some instances this may include increased oxygen delivery and positive end expiratory pressure (PEEP) to maintain oxygenation and prevent alveolar collapse. Chest physiotherapy assists in the prevention of VAP29 and remains a key component of management of all ventilated patients. However, its contribution towards improving mortality in patients with pneumonia is unclear.30 Upright positioning and early mobilisation are important elements of both prevention and management of pneumonia. The effectiveness of additional strategies, such as use of beds with a continuous lateral rotation or a vibration function to assist in the removal of secretions is yet to be shown.31 See Chapter 15 for further discussion.
Medications
Antibiotic administration is fundamental to a patient’s clinical progress. As noted earlier, the importance of accurate and timely administration of antibiotics directly impacts on patient outcome. In particular, the first dose of antibiotics is required as soon as possible after the diagnosis of pneumonia has been made. Studies where the first dose of antibiotic therapy was delayed showed an increase in mortality.32 Antibiotic cover for pneumonia depends on the causative organism and sensitivity to drugs (see Table 14.66). Review of antibiotic prescribing practices in Australia and New Zealand has shown that prescription of antibiotics in pneumonia is consistent with current guidelines.33
| Type of infection | Preferred agent(s) |
|---|---|
| Community-acquired pneumonia | |
| Streptococcus pneumoniae | PCN-susceptible: Penicillin G, amoxicillin, clindamycin, doxycycline, telithromycinPCN-resistant: cefotaxime, ceftriaxone, vancomycin, and fluoroquinolone |
| Mycoplasma | Doxycycline, macrolide |
| Chlamydophila pneumoniae | Doxycycline, macrolide |
| Legionella | Azithromycin, fluoroquinolone (including ciprofloxacin), erythromycin (rifampicin) |
| Haemophilus influenzae | Second- or third-generation cephalosporin, clarithromycin, doxycycline, β-lactam/β-lactamase inhibitor, trimethoprim/sulfamethoxazole, azithromycin, telithromycin |
| Moraxella catarrhalis | Second- or third-generation cephalosporin, trimethoprim-sulfamethoxazole, macrolide doxycycline, β-lactam–β-lactamase inhibitor |
| Neisseria meningitidis | Penicillin |
| Streptococci (other than S. pneumoniae) | Penicillin, first-generation cephalosporin |
| Anaerobes | Clindamycin, β-lactam–β-lactamase inhibitor, β-lactam plus metronidazole |
| Staphylococcus aureus Methicillin-susceptible Methicillin-resistant |
Oxacillin, nafcillin, cefazolin; all rifampin or gentamicin Vancomycin, rifampicin or gentamicin |
| Klebsiella pneumoniae and other Enterobacteriaceae (excluding Enterobacter spp.) | Third-generation cephalosporin or cefepime (all aminoglycoside) carbapenem |
| Hospital-acquired infections | |
| Enterobacter spp. | Carbapenem, β-lactam–β-lactamase inhibitor, cefepime, fluoroquinolone; all + aminoglycoside in seriously ill patients |
| Pseudomonas aeruginosa | Anti-pseudomonal β-lactam + aminoglycoside, carbapenem + aminoglycoside |
| Acinetobacter | Aminoglycoside + piperacillin or a carbapenem |
Special Considerations
Pneumonia is a leading cause of maternal and fetal morbidity and mortality. It also increases the likelihood of the complications of pneumonia, including requirement for mechanical ventilation. Bacterial pneumonia is the most common type experienced in pregnancy although diagnosis is often delayed as a result of the reluctance to obtain a chest X-ray. Management is similar to a non-pregnant patient with antibiotic therapy adjusted to consider the impact on the fetus.6
CAP is a major cause of morbidity and mortality in elderly patients. Streptococcus pneumoniae is the most common causative organism, with an increase in drug-resistance being reported more widely in the over-65 age group. Treatment of elderly patients with pneumonia is similar to younger patients, with emphasis on supportive care, prevention of sepsis and management of preexisting chronic conditions. Immunisation with pneumococcal and influenza vaccines is beneficial in the prevention of pneumonia in elderly patients.34
Respiratory Pandemics
Serious outbreaks of respiratory infections that spread rapidly on a global scale are termed pandemics. Their spread is so rapid because the infection is usually associated with emergence of a new virus where the majority of the population has no immunity. These infections are characterised by extremely rapid ‘transmission with concurrent outbreaks throughout the globe; the occurrence of disease outside the usual seasonality, including during the summer months; high attack rates in all age groups, with high levels of mortality particularly in healthy young adults; and multiple waves of disease immediately before and after the main outbreak’.35 Several severe respiratory infections have progressed to become pandemics in recent years; these have been associated with the Coronavirus and Influenza viruses. Prediction of the interval between pandemics is difficult, but occurrence is likely to continue and therefore requires that the health care community be well prepared.
Severe Acute Respiratory Syndrome
In 2002–03 an outbreak of a novel Coronavirus occurred in China and rapidly spread throughout the world. The infection was highly virulent with over 8000 cases reported and a mortality rate of 11%. The infection was called Severe Acute Respiratory Syndrome (SARS) due to the severity of the disease, characterised by diffuse alveolar infiltrates, resulting in about 20% of patients requiring respiratory support. The SARS outbreak provoked a rapid and intense public health response coordinated by the World Health Organization (WHO), resulting in a cessation of disease transmission within ten months.35
Influenza Pandemics
A feature of the influenza virus that explains why it continues to be associated with epidemic and pandemic disease is its high frequency of antigenic variation. This occurs in two of the external glycoproteins and is referred to as antigenic drift or antigenic shift, depending on the extent of the variation. The result of this is that new viruses are introduced into the population, and due to the absence of immunity to the virus, a pandemic of influenza results.6
Pandemics of influenza were observed a number of times in the twentieth century, and were believed to have involved viruses circulating in humans that originated from influenza A viruses in birds. The ‘Spanish influenza’ pandemic of 1918–19 resulted in the death of over 50 million people worldwide and remains unprecedented in its severity.35
The first reported infection of humans with avian influenza viruses occurred in Hong Kong in 1997, with six recorded fatalities. The increased virulence of this disease was observed in the acuity of those affected by the outbreak of the highly pathogenic avian influenza virus (H5N1) in 2004–2005.35 Most patients presented with non-specific symptoms of fever, cough and shortness of breath. In many patients this progressed rapidly to ARF requiring ventilation and other supportive measures. The majority of people affected (90%) were less than 40 years of age with case fatality rates highest in the 10–19-year-old age group.36
The most recent influenza pandemic declared by WHO occurred in 2009 when a novel H1N1 influenza A virus emerged in Mexico and the USA. This virus contained genes from avian, human and swine influenza virus and affected millions of people worldwide.37 Patients typically presented with nonspecific flu-like symptoms, however in a quarter of patients this was accompanied by diarrhoea and vomiting. The disease spread globally with millions of cases reported and resulted in over 16,000 deaths by March 2010.38
Australia and New Zealand communities had a high proportion of cases of H1N1 influenza-A infection, with 856 patients being admitted to ICU; 15 times the incidence of influenza A in other recent years. Infants (aged 0–1 years) and adults aged 25–64 years were at particular risk; others at increased risk were pregnant women, adults with a BMI over 35 and indigenous Australian and New Zealand populations. Australian and New Zealand Intensive Care Society (ANZICS) investigators prepared a report based on the Australian and New Zealand experience to assist those in the northern hemisphere to better prepare for their winter influenza season.39
The emergence of novel swine-origin influenza A virus was not anticipated and it is unlikely, given the limitations of current knowledge, that future pandemics can be predicted. The threat of pandemic disease from avian influenza remains high with the rapid evolution of H5N1 viruses; however the direction this will take is unpredictable. Priorities for prevention and management of future influenza pandemics therefore involve development of an international surveillance and response network for early detection and containment of the disease, local preparation for controlling the spread of the infection and further development of vaccines and antiviral agents.38
Influenza Vaccinations
Influenza vaccines are formulated annually based on current and recent viral strains. Success in protecting an individual against influenza requires that the virus strains included in the vaccine are the same as those currently circulating in the community. Vaccines are commonly effective in 70–90% in preventing influenza in healthy adults younger than 65 years of age. Efficacy appears lower in elderly persons. Health care workers are a key target group for the influenza vaccine, at the very least to reduce absenteeism over what is often the busiest period for most hospitals and health services35 and to reduce the risk of nosocomial influenza in hospitals.40
Isolation Precautions and Personal Protective Equipment
Key aspects of infection control in an epidemic or pandemic situation focus on limiting opportunities for nosocomial spread and the protection of health care workers. Guidelines for institutional management of these infections involve designing and implementing appropriate isolation procedures and recommending appropriate personal protective equipment (PPE). The impor-tance of adequate PPE was highlighted particularly in the SARS epidemic where there was overrepresentation of health care workers who became patients infected with the virus.35
Specific infection control guidelines are usually developed for individual institutions, based on government recommendations for management of staff, appropriate PPE and isolation procedures. Table 14.7 summarises the recommendations from the Australian41 and New Zealand42 governments.
TABLE 14.7 Recommendations for personal protective measures in respiratory pandemics
| Section | Protective measure |
|---|---|
| Staff management | Assessment of staff at increased risk of complications from the specific infection should be redeployed if possibleMonitoring health care workers for signs of illness and management with antivirals as a priority |
| Personal protection: basic measures | Hand hygiene, social distancing, safe cough/sneeze etiquette, and good ventilation |
| Personal Protective Equipment |
In all settings, it is important to ensure that staff members are familiar with respiratory protection devices. In areas or situations where respirators (P2 or N-95 masks) are used, a fit-testing program ensures understanding of how the devices work and maximal safety. During the SARS epidemic, infection of staff members through inappropriate or ineffective use of these masks occurred, and infection due to failure to wear adequate eye protection was also reported.43
Acute Lung Injury
Acute lung injury (ALI) is a generic term that encompasses conditions causing physical injury to the lungs. Acute respiratory distress syndrome (ARDS) is a severe form of ALI as a result of bilateral and diffuse alveolar damage due to an acute insult, and is the predominant form of ALI observed in ICU.6
Aetiology
ARDS is a characteristic inflammatory response of the lung to a wide variety of insults. Approximately 200,000 patients are diagnosed annually in the USA with ARDS, accounting for 10–15% of ICU admissions.44 Commonly associated clinical disorders can be separated into those that directly or indirectly injure the lung9 (see Table 14.8).
| Direct lung injury | Indirect lung injury |
|---|---|
The most common cause of indirect injury resulting in ALI/ARDS is sepsis, followed by severe trauma and haemodynamic shock states. Transfusion-related ALI (TRALI) is not common but is observed in ICU. ARDS arising from direct injury to the lung is most commonly seen in patients with pneumonia. An individual’s risk of developing ARDS increases significantly when more than one predisposing factor is present.6
Pathophysiology
Inflammatory damage to alveoli from inflammatory mediators (released locally or systemically) causes a change in pulmonary capillary permeability, with resulting fluid and protein leakage into the alveolar space and pulmonary infiltrates. Dilution and loss of surfactant causes diffuse alveolar collapse and a reduction in pulmonary compliance and may also impair the defence mechanisms of the lungs.45 Intrapulmonary shunt is confirmed when hypoxaemia does not improve despite supplemental oxygen administration.6 The characteristic course of ARDS is described as having three phases:6,45
1. Oedematous phase: involves an early period of alveolar damage and pulmonary infiltrates resulting in hypoxaemia. This phase is characterised by migration of neutrophils into the alveolar compartment, releasing a variety of substances including proteases, gelatinases A and B, and reactive nitrogen and oxygen species that damage the alveoli. Further damage is caused by resident alveolar macrophages and release of proinflammatory cytokines that amplify the inflammatory response in the lung. Significant ventilation–perfusion (intrapulmonary shunt) mismatch evolves causing hypoxaemia.
2. Proliferative phase: begins after 1–2 weeks as pulmonary infiltrates resolve and fibrosis and remodelling occurs. This phase is characterised by reduced alveolar ventilation and pulmonary compliance and ventilation–perfusion mismatch. Reduced compliance (stiff lungs) causes further atelectasis in the mechanically ventilated patient as alveoli are damaged by increased volume and/or pressure on inspiration.
3. Fibrotic phase: the final phase where alveoli become fibrotic and the lung is left with emphysema-like alterations.
Diagnosis
A standardised definition of ARDS was first described in 1988, with three clinical findings; hypoxia, decreased pulmonary compliance and diffuse infiltrates observed on a chest X-ray. The Murray Lung Injury Score was developed as a method for clarifying and quantifying the existence and severity of the disease.46 The American-European Consensus Conference on ARDS provided the following definition:
• acute onset of arterial hypoxaemia (PaO2:FiO2 ratio < 200)
• bilateral infiltrates on radiography without evidence of left atrial hypertension or congestive cardiac failure.
The spectrum of disease was also acknowledged and the term ALI was introduced to describe patients with a less severe but clinically similar form of respiratory failure (PaO2:FiO2 ratio <300).47 It has been suggested that these definitions require review as they include such a broad, heterogenous group of patients that has limited investigation of appropriate management strategies. This may also be because the interventions studied were ineffective, but it is just as likely that the broadly inclusive definition of ARDS captures a heterogeneous group of patients that respond differently to current therapies.48
Clinical Manifestations
While no specific test exists to determine whether a patient has ARDS, it should be considered in any patient with a predisposing risk factor who develops severe hypoxaemia, reduced compliance and diffuse pulmonary infiltrates on a chest X-ray.44 ARDS usually occurs 1–2 days following onset of a presenting condition and is characterised by rapid clinical deterioration. Common symptoms include severe dyspnoea, dry cough, cyano-sis, hypoxaemia requiring rapidly-escalating amounts of supplemental oxygen and persistent coarse crackles on auscultation.6
Assessment
A patient with ARDS requires ongoing monitoring of oxygenation and ventilation through ABG analysis and pulse oximetry and monitoring of PaCO2 to assess permissive hypercapnia. Monitoring of ventilatory pressures and volumes ensures that additional lung injury is prevented. As many patients with ARDS require cardiovascular support, assessment of haemodynamics and peripheral perfusion is important to ensure oxygen delivery to cells is achieved.6
Collaborative Practice
The key principles of management are treatment of the precipitating cause and providing supportive care during the period of acute respiratory failure.6,45 Mortality rates from ARDS have decreased over time; this is not attributed solely to the use of low tidal volume ventilation promoted by the ARDS Network group, but to other advances in critical care.44 Specific strategies include cautious fluid management, adequate nutrition, prevention of ventilator-associated pneumonia, prophylaxis for deep venous thrombosis and gastric ulcers, weaning of sedation and mechanical ventilation as early as possible, and physiotherapy and rehabilitation (similar to ARF management). Management involves a coordinated collaborative approach including supportive ventilation, patient positioning and medication administration.
Ventilation Strategies
The key focus of ventilation in ARDS is the prevention of refractory hypoxaemia rather than reversing it after it develops. The use of small tidal volumes and adequate levels of PEEP, along with careful attention to fluid status and patient–ventilator synchrony, may be sufficient to maintain oxygenation at an appropriate level while minimising further damage from barotrauma and nosocomial pneumonia.6,47 The use of rescue therapies is controversial as none to date have reduced mortality when studied in large heterogeneous populations of patients with ARDS. Some therapies however demonstrated improved oxygenation, which may be an important goal in many patients who experience severe hypoxaemia. The key focus of rescue ventilatory strategies is alveolar recruitment, including higher levels of PEEP, use of airway pressure release ventilation (APRV), high-frequency oscillatory ventilation (HFOV) and high-frequency percussive ventilation (HFPC) (see Chapter 15). If hypoxaemia is severe, extracorporeal life support may also be considered. As there is no evidence to support the use of one strategy over another, the choice of therapy is often based on equipment availability and clinician expertise. An evidence based approach is likely to involve lung-protective ventilation (volume and pressure limitation with modest PEEP) requiring permissive hypercapnia and permissive hypoxaemia.49
Prone Positioning
Use of prone positioning in patients with ARDS was described almost 30 years ago as a means of improving oxygenation. This improvement is largely due to the effect that the prone position has on chest wall and lung compliance. The result is a more homogenous ventilation of the lungs and improved ventilation–perfusion matching.6 Investigation into the effectiveness of this as a therapy in ARDS has noted improvement in oxygenation, but no corresponding improvement in mortality. It is therefore recommended as a rescue therapy for the patient at risk of death from hypoxia, rather than as a routine treatment.50 See Chapter 15 for further discussion.
Medications
A number of non-ventilatory strategies may form part of the treatment of patients with ARDS. Neuromuscular blocking agents (NMBAs) are used to promote patient–ventilator synchrony, especially when non-conventional modes of ventilation are used. Improvements in oxygenation are usually observed and may be attributed to reduction in oxygen consumption and improved chest wall compliance. The use of NMBAs, however, is also associated with an increased risk of myopathy, so any benefits gained should be weighed against known risks.51
Inhaled nitric oxide (iNO) therapy may be used to improve oxygenation through selective vasodilation of the pulmonary blood vessels, promoting improvement in ventilation–perfusion matching. Despite the lack of evidence regarding its effectiveness in improving outcomes of patients with ARDS, its use is reasonably widespread. Improvement in oxygenation should be observed within the first hour of treatment to support its ongoing use.51 Some groups have reported the use of iNO to be harmful and recommend that it not be used, given the lack of evidence demonstrating reduction in mortality.52A similar effect, in terms of pulmonary vasodilation, has been achieved using inhaled prostacyclines and this remains under investigation as an alternative therapy.51
A number of medications are currently being investigated to treat ARDS in acute and subacute exudative phases. These include agents that target the disrupted surfactant system (exogenous surfactant therapy), oxidative stress and antioxidant activity (antioxidants), neutrophil recruitment and activation, expression and release of inflammatory mediators (corticosteroids), activation of the coagulation cascade (immunomodulating agents and statins), and microvascular injury and leak (beta2-agonists).53 The use of low-dose corticosteroids has been associated with improved outcomes for patients with ARDS,54 although its use remains controversial and further investigation is recommended.
Special Considerations
ALI and ARDS occur in pregnancy usually as a result of aspiration pneumonitis, sepsis or pneumonia. Management of ventilation is similar to the non-pregnant patient, although consideration of the impact on the fetus is important in medication usage and ventilatory management.6 Elderly patients who develop ARDS are likely to experience an increased severity of disease, yet have a mortality rate comparable to other patients. Development of other organ dysfunction depends on the presence of chronic conditions such as renal and cardiovascular diseases.55
Asthma and Chronic Obstructive Pulmonary Disease
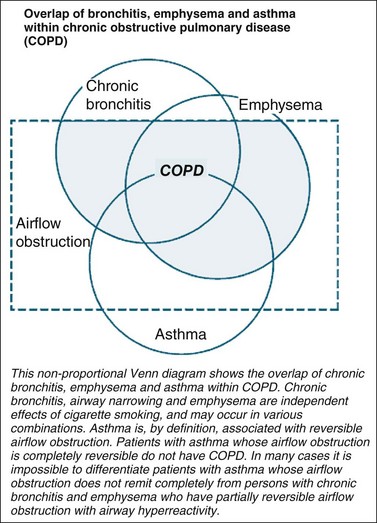
Asthma is defined as a respiratory condition where airflow limitation may be fully or partially reversible either spontaneously or with treatment.56–58 COPD is a respiratory condition defined by a largely fixed airflow limitation. The partial airway responsiveness to therapy in COPD results in a clinical overlap between COPD, asthma and chronic bronchitis. A non-proportional Venn diagram (see Figure 14.2), originally used by the American Thoracic Society59 and now in the Australian and New Zealand expert guidelines60,61 depict this overlap between conditions. It is not uncommon for people with an obstructive lung disease to share clinical characteristics for more than one respiratory condition, although the dominant clinical symptom is usually indicative of the underlying condition.62 It is however important to differentiate between COPD and asthma as they have different management and illness trajectories.56
Pathophysiology
Asthma is a complex syndrome influenced by genetic and environmental factors.63 Altered airway physiology and airway wall remodelling in asthma are consequences of inflammatory processes.64 While initial symptoms can occur at any age, most patients exhibit episodes of wheezing and obstruction before the age of six.65,66 The increasing incidence of disease burden in children may be attributable to a greater awareness and diagnosis of the condition, with the overall differences in global prevalence now becoming less.67
In contrast, COPD is a systemic, permanent and progressive condition with a number of mechanisms involved in its development. Smoking is the cardinal risk factor and continuation is the most significant determinant for disease progression.60,68 The concept of ‘pack years’ is used to quantify smoking, and is independent of whether an individual is a current or reformed smoker.69 A history of more than 20 pack years of smoking is a significant risk factor for the development of COPD.70 Continued smoking accelerates the decline of respiratory function in susceptible individuals.71,72 However, less than 15% of smokers actually develop clinically-significant COPD68,73,74 suggesting that other factors are also involved, including environmental and occupational pollutants, genetic predisposition, hyper-responsive airways and respiratory infections.68,75–79 Disease progression in susceptible individuals is most likely to be dependent on the synergistic effects of these factors.
Ventilation abnormalities in COPD result from airway inflammation, bronchoconstriction, increased mucus secretion and oedema. Perfusion abnormalities arise from hypoxic-induced vasoconstriction of the capillary beds. Pulmonary ventilation/perfusion (V/Q) abnormalities, and hyperinflation contribute to increased pulmonary vascular resistance (PVR), and respiratory muscle fatigue.80 Increased PVR and hypoxaemia require the heart’s right side heart to work harder, over time resulting in hypertrophy, remodelling and cor pulmonale.81,82 The incidence of right ventricular hypertrophy approximates 40% for patients with moderate levels of COPD (i.e. FEV1 <1000 mL).60 The left ventricle may also be compromised by hyperinflation, which generates an increased work of afterload.83 Heart disease is therefore a frequent concomitant condition with COPD84–86 (see Chapter 11 for further discussion). Impaired ventilation and perfusion leads to hypoxaemia and mechanical dysfunctions, with the primary cause of adverse lung mechanics being hyperinflation.
Hyperinflation has two components: static and dynamic.83 Loss of elastic recoil (static hyperinflation) and incomplete expiratory airflow (dynamic hyperinflation) leads to air trapping and a reduced inspiratory capacity.87,88 The effects of incomplete and prolonged expiration accounts for increased work of breathing, dyspnoea and reduced exercise tolerance experienced by people with COPD.89–95 Severity of COPD promotes hyperinflation of the lungs, and hyperinflation is a catalyst for hypoventilation.96
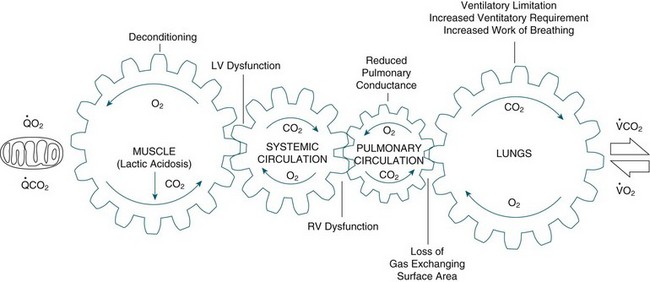
COPD is also a systemic condition that has an effect on the skeletal muscles, the intercostals and diaphragm.97–99 Bloodflow is diverted from lower limb muscles to meet the oxygen requirements of these respiratory muscles; a phenomenon referred to as circulatory steal.82 Use of supplemental oxygen to hypoxaemic patients with COPD has been found to reduce dynamic hyperinflation, dyspnoea and improve exercise tolerance;88,97 reduce PVR;76,86,100 reduce ventilatory requirements and circulating lactate levels.101 The systemic limitations that arise with COPD are therefore profound and complex.102 These inter-relationships are illustrated in Figure 14.3.
Clinical Manifestations
With asthma and COPD, a patient may present with wheeze, cough and/or dyspnoea. History and physical assessment are fundamental to determining the severity of presentation. Presence of diminished or silent breath sounds, central cyanosis, an inability to speak, an altered level of consciousness, an upright posture and diaphoresis indicate a life-threatening case.58 Chest pain or tightness may be present. Underestimation of severity is associated with higher mortality.58 Recent longitudinal datasets for Australia and New Zealand highlight a trend in reduced ICU admissions following an exacerbation of asthma and an improved health outcome.103 Conversely, studies in patients with COPD identified poorer 12 month health outcomes following an ICU admission for hypercapnoeic respiratory failure.104,105
Assessment and Diagnostics
Communication with patients that builds trust, through honesty and effective intervention, contributes considerably to the de-escalation of panic and fear in patients presenting with hypoxaemia. Creating a calm and trusting environment is paramount for those struggling for breath. Forward-planning for potential deterioration and constant assessment of respiratory, cardiovascular and neurological systems are fundamental in determining optimal clinical progress for these patients. Where possible, diagnostic tests and procedures involve peak flow monitoring, spirometry, radiology and ABGs.58
The ‘gold standard’ for diagnosing COPD is spirometry.60,75,106 While there is no gold standard in the diagnosis of asthma, spirometry is the lung function test of choice.104 In Australia, respiratory function tests are usually performed according to standard principles.107 Values obtained are expressed at body temperature, ambient pressure, saturated with water vapour (BTPS), in absolute units (L or L/sec) and as a percentage of predicted normal values. The carbon monoxide pulmonary diffusing capacity (TLCO), may be measured using the single breath technique modified by Krogh. Diffusing capacity indicates the available surface area for gas exchange, and is reduced with emphysema but can be normal with asthma.108 The TLCO can be a directly measured value or as a percentage of predicted normal for age, sex, height and weight. A number of reference tables of predicted normal values enable comparison with population norms.109 A continuing lack of consensus remains for differentiating asthma and COPD.71 The most commonly used criterion in Australia and New Zealand is airway reversibility in response to bronchodilator therapy: <15% reflects COPD; >15% reflects asthma.60,110
Collaborative Practice
Contemporary management of asthma follows an asthma management plan, to minimise the acute exacerbation and any subsequent respiratory arrest. Many presentations will be managed in the emergency department (see Chapter 22 for further discussion). For patients requiring ventilatory support, a case series noted that patients were better managed with noninvasive ventilation (NIV), as mechanical ventilation was associated with significant mortality and morbidity111 from hyperinflation and aggravation of bronchospasm.58 Contemporary management of COPD has advocated a care plan for patients in the community setting. This has an effect on prompting patients to recognise a change in their symptoms and seek appropriate care. However, improving symptom recognition does not reduce health care utilisation.112 Patients with COPD managed with NIV in a timely manner have a reduced length of hospital stay, reduced need for endotracheal intubation and reduced mortality rate.113 There are published guidelines on the prevention, identification and management of asthma56 and COPD.61
Pneumothorax
Pneumothorax describes air that has escaped from a defect in the pulmonary tree and is trapped in the potential space between the two pleura. A pneumothorax normally resolves with treatment. A pneumothorax is termed persistent if the air leak lasts for more than five days,114 while one reappearing on the same side after seven days is termed reoccurring.115 A pneumothorax can arise spontaneously, from disease or from traumatic injury and can be life-threatening.
A tension pneumothorax involves significant and progressive respiratory or haemodynamic compromise that is quickly offset by decompression.116 A patient with a tension pneumothorax can present with symptoms similar to asthma, i.e. ‘respiratory distress, wheeze, tachycardia, tachypnoea, desaturation, hyper-expansion, agitation and decreased air entry.’117, p. 525 Fortunately, tension pneumothorax is a far less common condition, and the patient is more likely to report additional chest pain. The actual incidence of a tension pneumothorax is relatively unexamined but it is more likely to occur in a ventilated patient where a pneumothorax has been missed on assessment.117
Pathophysiology
If the pleural defect functions as a one-way valve, air enters the pleural cavity on inspiration but is unable to exit on expiration, leading to increasing ipsilateral intrapleural pressure. This causes further lung collapse, diaphragmatic depression, and (dependent on mediastinal distensibility) contralateral lung compression.117
Clinical Manifestations
Severe presentations are identified by history and clinical examination (respiratory distress, cyanosis, tachycardia, tracheal shift and unilateral movement of the chest). They are also detected on CXR with a translucent appearance of the air and absence of lung markings118 (see Chapter 13 for interpretation of CXR).
Collaborative Practice
Insertion of a thoracic underwater seal drain allows the collapsed lung to re-expand. This is facilitated with mechanical ventilation if required. If a haemothorax is present, suction on the underwater seal drain (20–60 mmHg) will expedite drainage and re-expansion of the lung.118
No differences in short- and long-term health outcomes were reported between insertion of an underwater seal drainage system and simple aspiration of the air for patients with a spontaneous pneumothorax.119 Treatments for pneumothorax where there is concomitant lung disease, e.g. cystic fibrosis, identified a paucity of data to guide practice.120
Pain management and facilitation of respiratory care with oxygen therapy, non-invasive or invasive ventilation, positioning and deep-breathing and coughing, and the monitoring of the chest tube and drainage for presence of air-leak and serous drainage, are key to recovery without development of further complications.121 Drainage system connections need to be tight and supported to prevent drag on the patient. Evidence is available for the development of clinical practice guidelines on thoracostomy.121 Chapter 12 discusses chest tube management in more detail.
Pulmonary Embolism
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are two aspects of the disease process known as venous thromboembolism (VTE).122 Certain factors lead to higher incidence: immobilisation (due to long bone, pelvic and spinal fractures) and closed head injury in particular (see Table 14.11 for a list of risk factors).123 Most PE originate in the lower limbs, pelvic veins or inferior vena cava. Three predisposing risk factors for thrombosis are venous stasis, vein wall injury and hypercoagulability of blood. Clinical risk factors are immobility, surgery, trauma, malignancy, pregnancy or thrombophilia. PE may have no clinical consequence or it may be catastrophic, causing sudden death,123 and is responsible for 10% of in-hospital deaths.124 The morbidity and costs associated with VTE are also significant. An evidence-based clinical practice guideline has been published to address this significant health issue122 and addresses the risks and benefits of treatments for medical, surgical and oncology patients. Further, VTE guidelines for patients with heparin-induced thrombocytopenia; pregnancy and childbirth are outlined with a listing of the publications to support the level of evidence for the clinical management guidelines.
TABLE 14.11 Risk factors for venous thromboembolism (VTE)123
| Primary hypercoaguable states (thrombophilia) | Secondary hypercoagulable states |
|---|---|
Clinical Manifestations
Pulmonary artery obstruction causes release of vasoactive agents from accumulating platelets, with subsequent raised pulmonary vascular resistance and acute pulmonary hypertension. The arterial obstruction causes severe shunting and life-threatening hypoxaemia. Symptomatic patients present with dyspnoea (most common), pleuritic chest pain and haemoptysis. The physical signs of tachypnoea, fever, tachycardia and right ventricular dysfunction may also be present. If a massive PE has occurred, the patient exhibits hypotension with pale, mottled skin and peripheral and/or central cynanosis.124
Assessment and Diagnostics
Investigations to confirm VTE include compression ultrasonography for a suspected DVT, pathology test for elevated levels of D-dimer in plasma125 and a ventilation-perfusion (V/Q) isotope scan, computed tomographic (CT) and pulmonary angiography (helical CT) scan for PE.122
Collaborative Practice
Current and ongoing treatment modalities for PE are selected according to the patient’s individual circumstances. In general, options include medications and percutaneously inserted vena caval filters.126
To prevent VTE, prophylactic interventions include hydration and early mobilisation that, depending on the need for patient admission are not always possible in the critical care setting. Mechanical measures of prophylaxis aim to reduce venous stasis via external compression. Commonly employed measures include knee- or thigh- length graduated compression stockings and/or intermittent pneumatic compression and/or venous foot pumps. Clinical practice guidelines are published to support evidence-based care.124 Two Cochrane systematic reviews have established that combined modalities reduce the incidence of DVT but the effect on PE remains unknown.127,128
Medications
Table 14.12 outlines the key medications recommended and prescribed for patients with PE. Risk reductions for DVT postsurgery have been reported following the use of prophylactic medications.129 Studies continue to postulate the efficacy of novel versus standard medication administration for VTE with only preliminary conclusions available.130,131
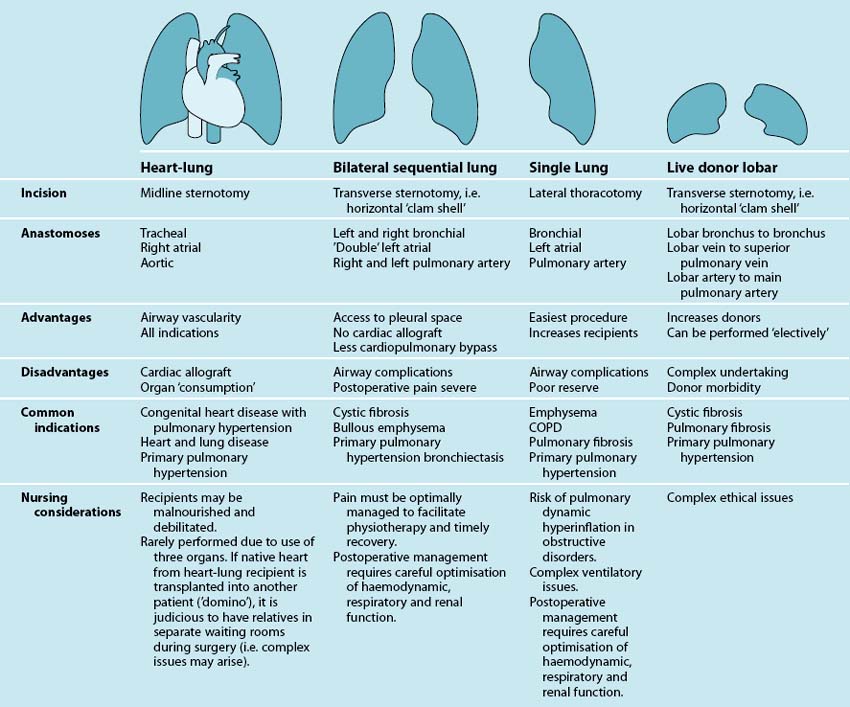
Lung Transplantation
Transplantation is a life-saving and cost-effective form of treatment that enhances the quality of life for people with chronic respiratory disease. Lung transplantation is facilitated by organ donation from patients with brain death or donation after cardiac death.132 Donation after cardiac death has the potential to significantly increase the number of organs available for lung transplantation.132 In 1985, 13 lung transplant procedures were reported worldwide.133 In subsequent years, the number of recipients worldwide has steadily increased to be in excess of 2700 annually.134 Patients have received lung transplants in Australasia since the early 1990s. Lung transplantation can be either single or double, depending on a patient’s underlying disease state. In the postoperative period, clinicians need to carefully balance fluid management to optimise respiratory function without causing haemodynamic compromise or renal dysfunction. As severe pain, particularly for transverse thoracotomy incisions, can compromise recovery significantly, effective analgesic regimens to facilitate physiotherapy are critical.
Indications
The two generally-accepted criteria for lung transplantation in patients with end-stage pulmonary or pulmonary vascular disease are a poor prognosis (less than 50% chance of surviving 2 years) and poor quality of life.135 In terms of quality of life, prospective lung transplant recipients usually struggle to perform activities of daily living, may be oxygen-dependent and have New York Health Authority functional class III or IV symptoms. As a result, most patients presenting for surgery are at risk of being debilitated and may be malnourished or overnourished, and therefore require specific interventions by health team members.
Description
The four possible forms of lung transplantation, indications for each form of surgery and salient nursing implications are outlined in Table 14.13. Currently, lung transplantation takes two main forms: bilateral sequential lung transplantation (BSLTx) and single-lung transplantation (SLTx). BSLTx is the most common form of lung transplantation and confers a survival advantage over and above SLTx. However the advantage of SLTx over BSLTx is that twice as many people receive life-saving surgery. For SLTx recipients with COPD, there is an increase in the complexity of postoperative respiratory management, and for this reason some centres may perform BSLTx for patients with COPD. SLTx is also utilised for patients with idiopathic pulmonary fibrosis (IPF) and other forms of interstitial lung disease (ILD) who have a high waiting list mortality.136
Clinical Manifestations
Postoperative nursing and medical management common to all forms of lung transplant recipients involves intensive clinical monitoring similar to that for heart transplant recipients, with a focus on the stabilisation and optimisation of haemodynamic, respiratory and renal status. Great skill by clinicians is required to manage this complex interplay. Respiratory dysfunction can develop due to severe allograft dysfunction secondary to ischaemia-reperfusion injury, pulmonary oedema, hyperacute rejection and pulmonary venous or artery anastomotic obstruction. Other major complications in the early postoperative period that affect respiratory management include severe pain, diaphragmatic dysfunction, acute rejection and infection. Patients who receive a SLTx for COPD are at risk of developing pulmonary dynamic hyperinflation, requiring independent lung ventilation. Haemodynamic function can be compromised in the early postoperative phase due to cardiac and respiratory problems; renal and gastrointestinal dysfunction is also prevalent. Long-term respiratory complications include airway anastomotic problems (stricture and dehiscence), suboptimal exercise performance, and chronic rejection manifesting as bronchiolitis obliterans syndrome. The most important aspects of these complications are discussed below in relation to nursing practice, and Table 14.14 provides a summary.
TABLE 14.14 Possible causes of low cardiac output in first week after lung transplantation
| Cardiovascular |
Respiratory Dysfunction
Respiratory dysfunction within the first 24–48 hours postoperatively is usually caused by primary graft dysfunction (PGD), a syndrome characterised by non-specific alveolar damage, lung oedema and hypoxaemia.137 Primary graft dysfunction may be aggravated by factors associated with the donor (e.g. trauma, mechanical ventilation, aspiration, pneumonia and hypotension), cold ischaemic storage,137 inadequate preservation and disruption of pulmonary lymphatics. Clinical signs of PGD range from mild hypoxaemia with infiltrates on chest X-rays to severe ARDS requiring high-level ventilatory support, pharmacological support and ECMO.138 Australian researchers have shown a decrease in the severity and incidence of PGD following the implementation of an evidence-based guideline for managing patients’ respiratory and haemodynamic status postoperatively.139 The guideline directs clinicians to minimise crystalloid fluids, use vasopressors as the first-line treatment to maintain blood pressure if cardiac output is adequate and use ARDSNet principles for ventilatory support.139,140 Respiratory dysfunction beyond 72 hours is likely to be due to infection or hemidiaphragm paralysis secondary to phrenic nerve damage. Although BSLTx is usually performed without cardiopulmonary bypass, for those patients who require cardiopulmonary bypass for surgery, it is recognised that there is a higher incidence of PGD but management principles are essentially the same.
Nursing practice
Severity of allograft dysfunction is assessed by ABG analysis, respiratory function and patient comfort, chest X-ray, bronchoscopy and haemodynamic parameters. A careful balance in the management of haemodynamic, respiratory and renal status is vital in the first 12 hours, and their optimisation should be achieved with inotropes (e.g. adrenaline, noradrenaline) and limited and judicious use of colloid fluids to ensure adequate end-organ perfusion without causing pulmonary overload. Fluid management should aim to keep filling pressures low to normal in light of a recent retrospective review that found a high CVP (>7 mmHg) was associated with prolonged mechanical ventilation and high mortality.141 Importantly, there was no evidence of renal complications associated with these low filling pressures.141 Fluid resuscitation should include products to correct anaemia and preoperative low plasma protein levels.142
For patients who have required intraoperative cardiopulmonary bypass, high doses of inotropes are often needed to overcome a transient relative hypovolaemia. Additionally, gentle rewarming measures are needed to re-establish normothermia in order to prevent haematological and peripheral perfusion impairments associated with hypothermia. Slow rather than rapid rewarming, and close monitoring of CI, CVP and PAWP should minimise the development of pulmonary oedema at this time. For patients with allograft dysfunction accompanied by high pulmonary pressures, inhaled NO is useful in decreasing high pulmonary pressures and intrapulmonary shunting.143,144 Ongoing nursing assessments of MAP, CI, PAP, PAWP, CVP and urine output guide and evaluate haemodynamic therapeutic interventions (see Chapter 9).
To assess the causes and progress of allograft dysfunction, chest X-rays provide vital information about line placement, ETT position, lung expansion, lung size, position of the diaphragm and mediastinum and the presence of pneumothorax, oedema and atelectasis.145 Allograft dysfunction due to ischaemia-reperfusion injury appears on chest X-rays as a rapidly-developing diffuse alveolar pattern of infiltration that is greater in the lower regions,142 most commonly seen on the first postoperative day but may occur up to 72 hours following surgery. The presence of rapidly worsening pulmonary infiltrates (especially if associated with low cardiac indices) should however prompt urgent echocardiography to assess cardiac function and pulmonary venous anastamosis patency. Beyond 72 hours, alveolar and interstitial infiltration may indicate either acute rejection or an infective process.146 This information is combined with other respiratory and haemodynamic data to inform appropriate collaborative interventions.
Commonly, ventilatory settings and respiratory weaning are guided by pH rather than CO2 levels. A modest degree of hypercarbia is anticipated postoperatively and resolves over time. Given that low-volume ventilation has a positive impact on lung recovery and long-term outcomes in patients with adult respiratory distress syndrome (ARDS),147 it has now been recommended that SLTx and BSLTx recipients receive similar settings to prevent barotrauma while providing adequate ventilation.142 In SLTx recipients, ventilation perfusion mismatches can also be improved by inhaled NO and by positioning patients regularly with the allograft uppermost.
Allograft dysfunction can develop in SLTx recipients with a remaining native COPD lung who are ventilated via a single-lumen ETT, due to gas trapping in the overdistensible native lung, a condition known as pulmonary dynamic hyperinflation (PDH) (see Figure 14.4). Any condition that lowers the compliance of the allograft can lead to PDH in these patients. Nurses need to be aware of the patients who can potentially develop PDH and to remain hypervigilant, as early signs and opportunities to stabilise patients’ haemodynamic and respiratory status quickly can be easily missed. Initial presentation of PDH is usually a set of ABGs showing inadequate ventilation (hypercarbia and hypoxaemia). However, this pattern of ABG values must not be responded to with increases in respiratory rate, tidal volume or PEEP, as these actions will exacerbate the degree of native lung hyperinflation; rather, minute ventilation must be reduced.148
Other common presenting cues of PDH include a haemodynamic profile of cardiac tamponade, tracheal deviation, obvious hyperinflation of the native lung with or without mediastinal shift on chest X-ray, decreased air entry to the allograft on auscultation and pneumothorax. The early stages of PDH in a patient with a left SLTx for COPD can be seen on the chest X-ray in Figure 14.5. Immediate management of the condition requires attempts to minimise hyperinflation with altered ventilatory settings and bronchodilators. If this fails, a skilled physician is required to administer an anaesthetic, insert a dual-lumen ETT, check the position of each lumen’s position and cuff with an intubating bronchoscope. Secure placement of the tube is paramount, to avoid slight movement of the position and consequent displacement of correct cuff placement (see Figure 14.6 for correct positioning of a dual-lumen ETT).
FIGURE 14.6 Correct positioning of double-lumen endotracheal tube for pulmonary dynamic hyperinflation
Independent lung ventilation is then established to ensure that the native lung receives no PEEP and a minimal tidal volume and rate (e.g. four breaths of 100 mL/min).148 The allograft may require high levels of PEEP to provide adequate ABGs. Ongoing assessment of respiratory function determines the timing of weaning from the dual-lumen ETT and independent lung ventilation to a single-lumen ETT and standard ventilatory practice. If PDH is not recognised until the patient has a cardiac arrest, the single-lumen ETT should be pushed into the bronchus of the transplanted lung in order to selectively ventilate the allograft until the patient’s condition is stable, when a dual-lumen ETT can be safely inserted.
Classic clinical signs of pulmonary infections include a low-grade fever, increasing dyspnoea and sputum production, cough and infiltrates on a chest X-ray. Hypotension, a reduced cardiac index and subtle changes in respiratory parameters during mechanical ventilation noted above may also be present. Pulmonary infections may be acquired through nosocomial, community or donor means, with recipient-colonised and opportunistic infections prevalent. Regardless of the means of acquisition, all infections are treated promptly with specific antibiotic, antifungal or antiviral therapies. The risk of developing CMV and Pneumocystis carinii in lung transplant recipients is somewhat higher than in heart transplant recipients, so prophylactic therapies for both infections are provided. Clinicians play an important role in preventing the transmission of infection between patients and cross-contamination within patients. Meticulous hand-washing between patients and between procedures, as well as minimising traffic into and out of patient care areas, are important measures in reducing infection rates.149
Pain
All recipients of lung transplantation can experience severe pain afterwards due to the incisions and chest drains. However, recipients of BSLTx in particular experience extremely severe postoperative pain secondary to the transverse sternotomy (clam-shell incision) and presence of four chest tubes. The recent use of a minimally invasive thoracotomy rather than transverse sternotomy for patients with obstructive respiratory illnesses may also reduce the postoperative pain experienced by recipients. Ideally, all lung transplant recipients should receive epidural analgesia; however, the insertion of an epidural catheter at the time of surgery may be contraindicated due to preoperative anticoagulation therapy. In these circumstances, epidural analgesia should be instituted as soon as appropriate after surgery. Higher failure rates of transition from epidural to oral analgesia have been reported in lung transplant recipients than in other thoracotomy patients,150 and in our experience it is not uncommon for BSLTx recipients to require opiate analgesia for a month after surgery in order to perform activities of daily living and physiotherapy.
Nursing practice
Consultation with pain services to ensure that patients receive optimal analgesic regimens should be an integral component of patients’ postoperative management (see Chapter 19). Paracetamol is beneficial in relieving mild to moderate pain, and may be used as an adjunct to centrally-acting analgesics for moderate to severe pain.151 The use of non-steroidal antiinflammatory drugs should be avoided, due to their detrimental effects on renal and gastrointestinal function.151
The nursing management of intercostal chest tubes is similar to that for cardiac surgical patients152 (see Chapter 12), with a few additional considerations. Recipients of SLTx have one apical and one basal chest tube, whereas BSLTx recipients have four chest tubes: two apical and two basal. Both BSLTx and HLTx recipients have one pleural space, so the amount and consistency of drainage from basal tubes will vary depending on patient positioning. Apical chest tubes are removed prior to basal tubes. Once lung expansion is optimal and any pneumothoraces have resolved, the apical tubes are removed. Basal chest tubes are removed once drainage is considered minimal in volume (approximately 250 mL/day) and serous in nature.
Haemodynamic Instability
As noted earlier, all lung transplant patients can experience haemodynamic compromise and renal impairment postoperatively as a result of managing respiratory function. Potential causes of a low cardiac output are outlined in Table 14.14. Patients with pulmonary hypertension must be carefully managed in the early postoperative period because of impaired cardiac output and changes in right ventricular dynamics. Prior to surgery, prolonged periods of a high right ventricular afterload lead to right ventricular thickening and stiffness, accompanied by limited wall motion of the left ventricle.153
Nursing practice
During arousal from anaesthesia and patient activity, fluctuations in oxygenation and systemic and pulmonary pressures exacerbate haemodynamic instability.154,155 When weaning from mechanical ventilation, as ventilation pressures fall, increases in preload may precipitate acute pulmonary oedema, even days after surgery.154 Conversely, if the patient is hypovolaemic at the time of weaning, right ventricular outflow obstruction may occur.142 These potential events confirm that careful titration of fluid and inotropic therapies, guided by frequent, accurate monitoring of invasive haemodynamic parameters, is required in patients with preoperative pulmonary hypertension.
Renal and Gut Dysfunction
Reasons for renal dysfunction in LTx recipients in the early postoperative phase are similar to those for heart recipients. The situation is, however, compounded in lung recipients due to aminoglycoside and NSAID use preoperatively, the high number of patients with diabetes and a requirement for ‘dry’ lungs postoperatively. Fortunately, the use of interleukin-2 receptor antibody drugs can assist in lowering the doses of calcineurin inhibitor agents to offer some early protection to the kidneys without inducing acute rejection.156
Nursing practice
Routine management of gut function is an important aspect of nursing practice, including the prevention of constipation (see Chapter 19). For patients receiving surgery for cystic fibrosis, pancreatic enzyme supplements are required postoperatively. As these patients are invariably debilitated preoperatively, enteral feeds that do not require pancreatic enzyme supplements should be commenced as soon as possible after surgery, as these supplements cannot be administered via enteral feeding tubes. Further specific information on managing patients with cystic fibrosis is available.157
Psychosocial Care
In the early postoperative period, corticosteroids, sedatives, sleep deprivation and persistent pain contribute to acute organic brain syndrome135 (see Chapter 7). Rejection episodes can be emotionally demanding, and the requirement for higher doses of corticosteroids can lead to irritability, insomnia, profound depression, mania or psychosis.135
Although LTx surgery offers recipients relief from shortness of breath and increased exercise tolerance, many patients have to continue managing other aspects of their underlying disease (e.g. cystic fibrosis). Thus, the burden of living with a chronic illness remains. Conversely, some recipients experience wellness for the first time in their life, and this can alter family and relationship dynamics. In circumstances where lung function deteriorates after initial success, patients and families experience feelings of devastation and hopelessness. Counselling services are essential in both the preoperative and the postoperative phase.135,158–166
Long-term Sequelae
Long-term sequelae for lung transplant recipients include renal impairment, hypertension and increased risk of malignancies, similar to those with heart trans-plantations. Further information about long-term complications specific to lung transplantation, such as bronchiolitis obliterans syndrome and other non-pulmonary complications, is available.135
Summary
Case study
• CNS: GCS 15, Temperature 38.6 °C
• CVS: HR 135/min, sinus tachycardia, BP 150/78 mmHg, brisk capillary refill
• RESP: RR 40/min, shallow rapid breathing, appears tired. SpO2 95% while receiving oxygen at 6 L/minute via Hudson Mask, improving to 98% with increased flow to 8 L/minute.
Research vignette
Critique
This clinical enquiry was a randomised controlled study. Each study participant had a stabilisation period and following this, proceeded to be randomised to either protocol A or B. The stabilisation period became the control period for each participant and increased the strength of the study design. The merit of this experimental design is that extraneous variables are controlled for. Extraneous variables may be antecedent or intervening. Examples of antecedent variables include age, gender, socioeconomic status and premorbid health status. These data provide a baseline to confirm similarity between groups prior to assessment of an effect of the intervention.167 Intervening variables may occur during the course of the study and are unrelated to the clinical trial but may influence the dependent variables. For example a media report on the merit of clinical research may influence the public’s attitude to participation in a clinical trial.
The study was well conducted. The researchers were transparent in their handling of data and reporting of all those initially recruited into this study via the CONSORT statement.168 The duration of ventilation prior to extubation report wide confidence intervals. This could suggest that a wide profile of patients were enrolled into this study. Due to this trial being a prospective evaluation undertaken in the local setting it is possible to generalise the applicability of findings across the population in Australia and New Zealand. Outcome measures were a combination of quantifiable data such as arterial blood gas analysis and vital signs in addition to subjective measures i.e. the nurse’s report of the patient’s comfort and tolerance for the flow delivery system using the visual analogue scale (VAS). The VAS is a ubiquitous, valid and sensitive measure in a range of age groups.169
Certain limitations were apparent within the study. The recruitment period of time for the study is unreported. It is not reported whether random number generation was responsible for the creation of the randomisation sequence (n = 50) or whether this was undertaken by the recruiters. The researchers had identified their study’s limitations. This could not be a double- or even single-blind study as the patient participating and their nurse were aware of which high-flow modality was being used. Further, it is thought,170 but remains unclear, what window of time with one high-flow set up before change over to the alternative strategy is a sufficient length of time to wash out one intervention before measuring the clinical, self report and bedside observer patient data.
There are a number of recognised factors that influence gas exchange such as skeletal muscle conditioning, haematological profile and diffusion capacity. Variability of these factors was offset by the trial design as each participant was their own control in this study’s design. However, it was unclear whether patient positioning was consistent within and between the study’s participants. Patient positioning could influence the level of alertness, airway clearance and gas exchange. It would need to be assumed when interpreting these data that the temperature/humidity of the inspired gas with each intervention was consistent across the sample. The function of airway mucosa and temperature of inspired gas has been long established.171
The sample size was small (n = 44) and the risk of drawing conclusions based on a small sample size risks a Type II error. These results support the researchers’ contention that a larger sample size is required. Power calculations to determine equivalence between interventions exist.172 It is unclear how many bedside nursing staff participated in this study and if any and/or regular staff in-service education occurred to achieve inter-rater reliability of their reports of the patients’ tolerance with these two high-flow modalities. Each high-flow set up was trialled in 30-minute episodes. How frequently the bedside nurse observed the patient’s tolerance of the high-flow set up may have been variable. Interestingly the utility of nasal-delivered high flow oxygen therapy in generating a positive airway pressure has been examined and reported in healthy subjects as proportional to rates of gas flow and reduced pressure in mouth breathers in Australia and New Zealand in healthy subjects173 and ICU patients174 albeit with small sample sizes. This study is important, as it is the first randomised trial that compares two popular high flow delivery systems and highlights that further generation of evidence is vital to support our clinical decision making in everyday practice.
Learning activities
1. A patient has severe ARDS following aspiration pneumonia. Their FiO2 is 1 and PaO2 60 mmHg. Core temperature is 40°C and the only medications are antibiotics. Any activity including suctioning causes profound desaturation. What additional measures could be implemented to minimise this effect?
2. What is your understanding of ARDS?
3. List the interventions required for a nurse to safely care for a patient with a provisional diagnosis of a novel infectious disease.
4. Describe and compare the differences between a simple, persistent and reoccurring pneumothorax.
5. At the commencement of your shift you undertake a respiratory assessment of your patient. List the parameters that should be examined.
American Association for Respiratory Care. http://www.aarc.org/.
ARDS Network. http://www.ardsnet.org.
Asian Pacific Society of Respirology. http://www.apsresp.org/.
Asthma Foundation. http://www.asthmaaustralia.org.au/.
Australian & New Zealand Society of Respiratory Science. http://www.anzsrs.org.au/.
Australian Department of Health and Ageing. http://www.health.gov.au/internet/wcms/Publishing.nsf/Content/health-sars-index.htm.
Australian Lung Foundation. http://www.lungnet.org.au/.
Become an expert in spirometry. http://www.spirxpert.com/.
Centers for Disease Control and Prevention. http://www.cdc.gov.
Critical Care Medicine Tutorials. http://www.ccmtutorials.com.
Lung Health Promotion Centre. The Alfred Hospital, Victoria – resources http://www.lunghealth.org
New Zealand Ministry of Health. http://www.moh.govt.nz/sars.
Respiratory Care online. http://www.rcjournal.com/.
Respiratory Research. http://respiratory-research.com/.
Thoracic Society of Australia and New Zealand. http://www.thoracic.org.au/index.html.
Crunden E, Boyce C, Woodman H, Bray B. An evaluation of the impact of the ventilator care bundle. Nurs Crit Care. 2005;10(5):242–246.
Gardner A, Hughes D, Cook R, Henson R, Osborne S, Gardner G. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Aust Crit Care. 2005;18(4):158. 160–5
Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intens Care Med. 2006;32(2):230–236.
Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Joint Commission J Qual Patient Safety. 2005;31(5):243–248.
Rose L, Nelson S. Issues in weaning from mechanical ventilation: literature review. J Adv Nurs. 2006;54(1):73–85.
1 Moran J, Bristow P, Solomon P, George C, Hart G. Mortality and length-of-stay outcomes, 1993–2003, in the binational Australian and New Zealand intensive care adult patient database. Crit Care Med. 2008;36(1):46–61.
2 Australian and New Zealand Intensive Care Society (ANZICS). Annual Report 2008. Centre for Outcome and Resource Evaluation (CORE). ANZICS, Melbourne. http://www.anzics.com.au/core, 2008. [Cited September 2010]. Available from
3 Australian Institute of Health and Welfare (AIHW). Australian Hospital Statistics 2007–08. AIHW, Australian Government, Canberra. http://www.aihw.gov.au/publications/hse/84/11173.pdf, 2010. [Cited September 2010]. Available from
4 Partridge M. Understanding respiratory medicine: a problem orientated approach. London: Manson Publishing; 2006.
5 Drennan K, Hart G, Hicks P. Intensive Care Resources and Activity: Australia and New Zealand 2006–07. Australian and New Zealand Intensive Care Society Centre for Outcome and Resource Evaluation, Melbourne. http://www.anzics.com.au/core/reports, 2009. [Cited September 2010]. Available from
6 Mason R, Broaddus V, Martin T, et al. Murray and Nadel’s textbook of respiratory medicine, 5th edn. Philadelphia: Saunders; 2010.
7 Australian Institute of Health and Welfare (AIHW). Australian Hospital Statistics 2008–09. AIHW, Australian Government, Canberra. http://www.aihw.gov.au/publications/hse/84/11173.pdf, 2010. [Cited September 2010]. Available from
8 West J. Respiratory physiology: the essentials, 8th edn. Baltimore: Lippincott: Williams & Wilkins; 2008.
9 Fink M, Abraham E, Vincent JL, Kochanek P. Textbook of critical care, 5th edn. London: Elsevier; 2005.
10 Nettina SM, ed. Lippincott manual of nursing practice, 8th edn, Philadelphia: Lippincott, Williams and Wilkins, 2006.
11 Tobin M, Laghi F, Jubran A. Narrative review: ventilator-induced respiratory muscle weakness. Annals Intern Med. 2010;153(4):240–245.
12 Yang M, Yuping Y, Yin X, Wang BY, Wu T et al. Chest physiotherapy for pneumonia in adults. Cochrane Database of Systematic Reviews 2010; (2): CD006338.
13 Gosselink R, Bott J, Johnson M, Dean E, Nava S, et al. Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intens Care Med. 2008;34(7):1188–1199.
14 Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. J Quality and Patient Safety. 2005;31(5):243–248.
15 Engels P, Bagshaw S, Meier M, Brindley P. Tracheostomy: from insertion to decannulation. Canadian J Surgery. 2009;52(5):427–433.
16 Bagshaw S, Webb S, Delaney A, George C, Pilcher D, Hart G, Bellomo R. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care. 2009;13(2):R45.
17 Serrano N, Garcia C, Villegas J, Huidobro S, Henry C, Santacreu R, Mora M. Prolonged intubation rates after coronary artery bypass surgery and ICU risk stratification score. Chest. 2005;128(2):595–601.
18 Myles PS, Daly DJ, Djaiani G, et al. A systematic review of the safety and effectiveness of fast-track cardiac anesthesia. Anesthesiol. 2003;99(4):982–987.
19 Berton D, Kalil A, Cavalcanti M, Teixeira P. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Systematic Review 2008; (4): CD006482.
20 Charles P, Whitby M, Fuller A, Stirling R, Wright A, et al. The aetiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46(10):1513–1521.
21 King J, DeWitt M. Cryptococcosis. eMedicine Specialties-Infectious Diseases-Fungal Infections. http://emedicine.medscape.com/article/215354-overview, 2009. Available from
22 Murdoch D, O’Brien K, Scott J, Karron R, Bhat N, et al. Breathing new life into pneumonia diagnostics. J Clin Micro. 2009;47(11):3405–3408.
23 Stralin K, Tornqvist E, Kaltoft M, Olcen P, Holmberg H. Etiologic diagnosis of adult bacterial pneumonia by culture and PCR applied to respiratory tract samples. J Clin Micro. 2006;44(2):643–645.
24 Chalmers J, Singanayagam A, Akram A, Mandal P, Short P, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia: Systematic review and meta-analysis. Thorax. 2010;65(10):878–883.
25 Charles P, Wolfe R, Whitby M, Fine J, Fuller A, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community acquired pneumonia. Clin Infect Dis. 2008;47(3):375–384.
26 Torres A, Ferrer M, Badia J. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010;51(Suppl1):S48–S53.
27 Wip C, Napolitano L. Bundles to prevent ventilator-associated pneumonia: how valuable are they? Current Opin Infect Dis. 2009;22(2):159–166.
28 Panchabhia T, Dangayach N, Krishnan A, Kothari V, Karnard D. Oropharyngeal cleansing with 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients: an open-label randomized trial with 0.01% potassium permanganate as control. Chest. 2009;135:1150–1156.
29 Ntoumenopoulos G, Presneill J, McElholum M, Cade J. Chest physiotherapy for the prevention of ventilator-associated pneumonia. Inten Care Med. 2002;28(7):850–856.
30 Yang M, Yuping Y, Yin X, Wang B, Wu T, Liu G, Dong B. Chest physiotherapy for pneumonia in adults. Cochrane Database Systematic Review 2010; CD006338.
31 Staudinger T, Bojic A, Holzinger U, Meyer B, Rohwer M, et al. Continuous lateral rotation therapy to prevent ventilator-associated pneumonia. Crit Care Med. 2010;38(2):486–490.
32 Iregui M, Ward S, Sherman G, Fraser V, Kollef M. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122(1):262–268.
33 Delhunty J, Webb S, Paterson D, Bellomo R, Myburgh J, et al. A survey of antibiotic prescribing practices in Australian and New Zealand intensive care units. Crit Care Resus. 2010;12(3):162–170.
34 Gutierrez F, Masia M. Improving outcomes of elderly patients with community-acquired pneumonia. Drugs and Aging. 2008;25(7):585–610.
35 Mandell G, Bennett J, Dolin R. Mandell. Douglas and Bennett’s principles and practice of infectious diseases, 7th edn. Philadelphia: Churchill Livingstone; 2009.
36 Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459(7249):931–939.
37 Shinde V, Bridges C, Uyeki T. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. New Engl J Med. 2009;360:2616–2625.
38 Taubenberger J, Morens D. Influenza: the once and future pandemic. Public Health Report. 2010;125(Suppl3):16–26.
39 ANZICS Influenza Investigators. Critical care services and 2009 influenza in Australia and New Zealand. New Eng J Med. 2009;361(20):1925–1934.
40 Salgado C, Gianetta E, Hayden F, Farr B. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Inf Con and Hosp Epid. 2004;25(11):923–928.
41 Department of Health and Ageing, Australian Government. Infection Control Recommendations 2009. Canberra: Department of Health and Ageing. [Cited August 2010]. Available from http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/clinical-infection
42 Ministry of Health. Infection prevention and control during an influenza pandemic. Wellington: NZ Ministry of Health; 2006. [Cited August 2010]. Available from http://www.moh.govt.nz/moh.nsf/0/4013C98B3E5B384DCC2571490017EC3D/$File/influena-pandemic.pdf
43 World Health Organization. Clarification: Use of Masks by Health Care Workers in Pandemic Settings. In: WHO global influenza preparedness plan: The role of WHO and recommendations for national measures before and during pandemics. Switzerland: World Health Organization; 2005.
44 Erikson S, Martin G, Davis J, Matthay M, Eisner M, for the ARDS Network. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37(5):1574–1579.
45 Chapman S, Robinson G, Stradling J, West S. Oxford handbook of respiratory medicine. New York: Oxford University Press; 2009.
46 Murray J, Matthay M, Luce J, Flick M. An expanded definition of the adult respiratory distress syndrome. Am Rev of Resp Dis. 1988;138(3):720–723.
47 Bernard G, Artigas A, Brigham K, Carlet J, Falke K, et al. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respiratory Crit Care Med. 1994;149(3Pt1):818–824.
48 Phua J, Stewart R, Ferguson N. Acute respiratory distress syndrome 40 years later: time to revisit its definition. Crit Care Med. 2008;36(10):2912–2921.
49 Esan A, Hess D, Raoof S, George L, Sessler C. Severe hypoxaemic respiratory failure: part 1: ventilatory strategies. Chest. 2010;137(5):1203–1216.
50 Taccone P, Pesenti A, Latini R, et al. Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984.
51 Raoof S, Goulet K, Esan A, Hess D, Sessler C. Severe hypoxaemic respiratory failure: part 2: nonventilatory strategies. Chest. 2010;137(6):1437–1448.
52 Afshari A, Brok J, Moller A, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Systematic Review 2010; (7): CD002787.
53 Bosma K, Taneja R, Lewis J. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: current and experimental approaches. Drugs. 2010;70(10):1255–1282.
54 Tang B, Craig J, Eslick G, Seppelt I, McLean A. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37(5):1594–1603.
55 Eachempati S, Hydo L, Shou J, Barie P. Outcomes of acute respiratory distress syndrome (ARDS) in elderly patients. Trauma. 2007;63(2):344–350.
56 National Asthma Council of Australia. National Asthma Handbook 06. http://www.nationalasthma.org.au/cms/images/stories/amh2006_web_5.pdf, 2006. [Cited November 2010]. Available from
57 Barnard A. Management of an acute asthma attack. Aust Fam Phys. 2005;34(7):531–534.
58 Tuxen D, Naughton M. Acute severe asthma. In: Bersten N, Soni N. Oh’s Intensive Care Manual. Oxford: Elsevier; 2009:399–413.
59 American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152(Supp):S77–120.
60 McKenzie DK, Frith PA, Burdon JG, Town GI. The COPDX Plan: Australian & New Zealand Guidelines for the management of chronic obstructive pulmonary disease 2003. Med J Aust. 2003;178(Supp):S7–39.
61 McKenzie DK, Abramson M, Crockett AJ, Glasgow N, Jenkins S, et al. The COPD-X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2010. [Cited November 2010]. Available from http://www.copdx.org.au/home
62 Leidy N. Functional performance in people with chronic obstructive pulmonary disease. Image J Nurs Sch. 1995;27(1):23–34.
63 Busse W, Lemanske R. Advances in immunology: asthma. New Eng J Med. 2001;344(5):350–362.
64 Bradding P, Walls A, Holgate S. The role of the mast cell in the pathophysiology of asthma. J All Clin Imm. 2006;117(6):1277–1284.
65 Gern J, Lemanske R, Busse W. Early life origins of asthma. J Clin Invest. 1999;104(7):837–843.
66 O’Sullivan S. Asthma death, CD+8 T cells & viruses. Proc ATS. 2005;2(2):162–165.
67 Pearce N, Ait-Khaled N, Beasley R, Mallol J, Keil U, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007;62(9):758–766.
68 Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(5Suppl):S121–S126.
69 Lowe A, Campbell D, Walker P, Farish S, Jackson B, Dharmage S. Clustering effects of chronic obstructive pulmonary disease specific quality of life in hospitalized patients. Respirol. 2003;8(3):339–343.
70 British Thoracic Society. Guidelines for the management of Chronic Obstructive Pulmonary Disease. Thorax. 1997;52(Suppl 5):S1–28.
71 Reid DW, Soltani A, Johns DP, Bish R, Williams TJ, Burns GP, Walters EH. Bronchodilator reversibility in Australian adults with chronic obstructive pulmonary disease. Intern Med J. 2003;33(12):572–577.
72 Lacasse Y, Maltais F, Goldstein R. Smoking cessation in pulmonary rehabilitation: goal or prerequisite? J Cardiopulm Rehabil. 2002;22(3):148–153.
73 Croxton TL, Weinmann GG, Senior RM, Wise RA, Crapo JD, Buist AS. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med. 2003;167(8):1142–1149.
74 Regional COPD working group. COPD prevalence in 12 Asia-Pacific countries and regions: Projections based on the COPD prevalence estimation model. Respirol. 2003;8:192–198.
75 Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46(8):798–825.
76 Bach PB, Brown C, Gland SE, McCrery DC. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001;134(7):600–620.
77 Chen JC, Mannino D. Worldwide epidemiology of chronic obstructive pulmonary disease. Curr Opin Pulmonary Med. 1999;5(2):93.
78 Chitkara RK, Sarinas P. Recent advances in diagnosis and management of chronic bronchitis and emphysema. Curr Opin Pulmonary Med. 2002;8(2):126–136.
79 Gerald LB, Bailey W. Global initiative for chronic obstructive lung disease. J Cardiopulmonary Rehab. 2002;22(4):234–244.
80 Gronkiewicz C, Borkgren-Okonek M. Acute exacerbations of COPD: nursing application of evidenced based guidelines. Crit Care Nurs Q. 2004;27(4):336–352.
81 Farquhar SL, Fantasia L. Pulmonary anatomy and physiology and the effects of COPD. Home Health Nurse. 2005;23(3):167–174.
82 Sietsema K. Cardiovascular limitations in chronic pulmonary disease. Med Sci Sports Exerc. 2001;33(7 Suppl):S656–S661.
83 Mergoni M, Rossi A. Physiopathology of acute respiratory failure in COPD and asthma. Minerva Anestesiol. 2001;67(4):198–205.
84 Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–2666.
85 Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158(12):1171–1181.
86 Sin DD, Man S. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519.
87 Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–597.
88 Diaz O, Villafranca C, Ghezzo H, Borzone G, Leiva A, et al. Breathing pattern and gas exchange at peak exercise in COPD patients with and without tidal flow limitation at rest. Euro Resp J. 2001;17(6):1120–1127.
89 Sutherland ER, Cherniack R. Management of chronic obstructive pulmonary disease. New Engl J Med. 2004;350(26):2689–2697.
90 Alvisi V, Mirkovic T, Nesme P, Guerin C, Milic-Emili J. Acute effects of hyperoxia on dyspnea in hypoxemia patients with chronic airway obstruction at rest. Chest. 2003;123(4):1038–1046.
91 Barbarito N, Ceriana P, Nava S. Respiratory muscles in chronic obstructive pulmonary disease and asthma. Minerva Anestesiol. 2001;67(9):653–658.
92 Decramer M. Respiratory muscles in COPD: regulation of trophical status. Verh K Acad Geneeskd Belg. 2001;63(6):577–602.
93 Haccoun C, Smountas AA, Gibbons WJ, Bourbeau J, Lands LC. Isokinetic muscle function in COPD. Chest. 2002;121(4):1079–1084.
94 ODonnell DE. Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001;33(7 Suppl):S647–S655.
95 Polkey MI. Muscle metabolism and exercise tolerance in COPD. Chest. 2002;121(5):131–135.
96 Haluszka J, Chartrand DA, Grassino AE, Milic-Emili J. Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary disease. American Rev Resp Dis. 1990;141(5Pt1):1194–1197.
97 Larson JL, Covey MK, Corbridge S. Inspiratory muscle strength in chronic obstructive pulmonary disease. AACN Clinical Issues. 2002;13(2):320–332.
98 Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. New Engl J Med. 1997;337(25):1799–1806.
99 Casaburi R. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001;33(7Supp):S662–S670.
100 Fujimoto K, Matsuzawa Y, Yamaguchi S, Koizumi T, Kubo K. Benefits of oxygen on exercise performance and pulmonary hemodynamics in patients with COPD with mild hypoxemia. Chest. 2002;122(2):457–463.
101 Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(1):19–38.
102 Cooper CB. Determining the role of exercise in patients with chronic pulmonary disease. Med Sci Sports Exerc. 1995;27(2):147–157.
103 Stow PJ, Pilcher D, Wilson J, George C, Bailey M, et al. Improved outcomes from acute severe asthma in Australian intensive care units (1996–2003). Thorax. 2007;62(10):842–847.
104 Connors AF, Jr., Dawson NV, Thomas C, Harrell FE, Jr., Desbiens N, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med. 1996;154(4Pt1):959–967.
105 Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274(23):1852–1857.
106 Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123(5):1684–1692.
107 American Thoracic Society. Standardization of Spirometry: 1994 Update. Am J Respir Crit Care Med. 1995;152(3):1107–1136.
108 Hughes J, Pride N. Lung function tests: physiological principles and clinical applications. London: Saunders; 2000.
109 Pierce RJ, Hillman D, Young IH, O’Donoghue F, Zimmermann PV, et al. Respiratory function tests and their application. Respirology. 2005;10(Suppl2):S1–19.
110 Reid DW, Soltani A, Johns DP, Bish R, Williams TJ, et al. Bronchodilator reversibility in Australian adults with chronic obstructive pulmonary disease. Intern Med J. 2003;33(12):572–577.
111 Williams T, Tuxen D, Scheinkestel C, Czarny D, Bowes G. Risk factors for morbidity in mechanically ventilated patients with acute severe asthma. Am Rev Respir Dis. 1992;146(3):607–615.
112 Walters JAE, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010; (5): CD005074.
113 Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. New Engl J Med. 1995;333(13):817–822.
114 Schidlow D, Taussig L, Knowles M. Cystic Fibrosis Foundation consensus conference report on pulmonary complications of cystic fibrosis. Pediatric Pulmonology. 1993;15:187–196.
115 Tschopp JM, Rami-Porta R, Noppen M, Astoul A. Management of spontaneous pneumothorax: state of the art. Euro Resp J. 2006;28(3):637–650.
116 Leigh-Smith S, Harris T. Tension pneumothorax in asthma time for a re-think? Emerg Med J. 2005;22(1):8–16.
117 Leigh-Smith S, Christey G. Tension pneumothorax in asthma. Resuscitation. 2006;69(3):525–527.
118 Padley S. Imaging the chest. In: Berstern N, Soni N. Oh’s Intensive Care Manual. 5th edn. Oxford: Elsevier; 2003:387–403.
119 Wakai A, O’Sullivan R, McCabe G. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database of Systematic Reviews 2007; CD004479.
120 Amin R, Noone PG, Ratjen F. Chemical pleurodesis versus surgical intervention for persistent and recurrent pneumothoraces in cystic fibrosis. Cochrane Database of Systematic Reviews 2009; CD007481.
121 Adrales G, Huynh T, Broering B, et al. A thoracostomy tube guideline improves management efficiency in trauma patients. J Trauma. 2002;52(2):210–216.
122 National Health and Medical Research Council (NHMRC). Clinical practice guideline for the prevention of venous thromboembolism in patients admitted to Australian Hospitals. Melbourne: NHMRC; 2009.
123 Schuerer D, Whinney E, Robb R, Freeman B, Nash J, et al. Evaluation of the applicability, efficacy and safety of a thromboembolic event prophylaxis guideline designed for quality improvement of the traumatically injured patient. Trauma. 2005;58(4):731–739.
124 Brown M, Vance S, Kline J. An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study. Acad Emerg Med. 2005;12(1):20–25.
125 Perrier A, Roy P, Aujesky D, Chagnon I, Howarth N, et al. Diagnosing pulmonary embolism in outpatients with clinical assessment, D-Dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study. Am J Med. 2004;116(5):291–299.
126 Young T, Tang H, Hughes R. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database of Systematic Reviews 2010; CD006212.
127 Kakkos S, Caprini JA, Geroulakos G, Nicolaides AN, Stansby GP, Reddy DJ. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism in high-risk patients. Cochrane Database of Systematic Reviews 2008; CD005258.
128 Sachdeva A, Dalton M, Amaragiri SV, Lees T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database of Systematic Reviews 2010; CD001484.
129 Ramos J, Perrotta C, Badariotti G, Berenstein G. Interventions for preventing venous thromboembolism in adults undergoing knee arthroscopy. Cochrane Database of Systematic Reviews 2008; CD005259.
130 Salazar CA, Malaga G, Malasquez G. Direct thrombin inhibitors versus vitamin K antagonists or low molecular weight heparins for prevention of venous thromboembolism following total hip or knee replacement. Cochrane Database of Systematic Reviews 2010; CD005981.
131 Watson L, Armon M. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2004; CD002783.
132 Snell GI, Levvey BJ, Oto T, McEgan R, Pilcher D, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transpl. 2008;8:1282–1289.
133 International Society of Heart Lung Transplantation (ISHLT). Lung transplantation. J Heart Lung Transpl. 2004;23(7):804–815.
134 Hertz MI, Aurora P, Christie JD, et al. Scientific registry of the International Society for Heart and Lung Transplantation: Introduction to the 2010 annual reports. J Heart Lung Transpl. 2010;29(10):1083–1141.
135 Williams TJ, Snell GI. Lung transplantation. In: Albert RK, Spiro SG, Jett JR. Clinical respiratory medicine. St. Louis: Mosby; 2004:831–845.
136 Keating D, Levvey B, Kotsimbos T, et al. Lung transplantation in pulmonary fibrosis: challenging early outcomes counterbalanced by surprisingly good outcomes beyond 15 years. Transplantation Proceedings. 2009;41(1):289–291.
137 de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Resp Crit Care Med. 2003;167(4):490–511.
138 King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann of Thor Surg. 2000;69(6):1681–1685.
139 Currey J, Pilcher DV, Davies A, Scheinkestel C, Botti M, et al. Implementation of a management guideline aimed at minimizing the severity of primary graft dysfunction following lung transplantation. J Thor and Card Surg. 2010;139(1):154–161.
140 The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory. New Eng J Med. 2000;342(18):1301–1308.
141 Pilcher DV, Scheinkestel CD, Snell GI, Davey-Quinn A, Bailey MJ, Williams TJ. High central venous pressure is associated with prolonged mechanical ventilation and increased mortality after lung transplantation. J Thor and Card Surg. 2005;129(4):918.
142 Snell GI, Klepetko W. Lung transplant perioperative management. ERS monograph on lung transplantation. 2003;26:130–143.
143 Ardehali A, Hughes K, Sadeghi A, Esmailian F, Marelli D, et al. Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation. 2001;72(4):638–641.
144 Thabut G, Brugiere O, Leseche G, Stern JB, Fradi K, et al. Preventive effect of inhaled nitric oxide and pentoxifylline on ischemia/reperfusion injury after lung transplantation. Transplantation. 2001;71(9):1295–1300.
145 Van Breuseghem I, De Wever W, Verschakelen J, Bogaert J. Role of radiology in lung transplantation. JBR-BTR. 1999;82(3):91–96.
146 Ward S, Muller NL. Pulmonary complications following lung transplantation. Clin Rad. 2000;55(5):332–339.
147 Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. New Eng J Med. 2000;342(18):1301–1308.
148 Weill D, Torres F, Hodges TN, Olmos JJ, Zamora MR. Acute native lung hyperinflation is not associated with poor outcomes after single lung transplant for emphysema. J Heart Lung Transpl. 1999;18(11):1080–1087.
149 Walsh TR, Guttendorf J, Dummer S, Hardesty RL, Armitage JM, et al. The value of protective isolation procedures in cardiac allograft recipients. Ann of Thor Surg. 1989;47(4):539–544.
150 Richard C, Girard F, Ferraro P, Chouinard P, Boudreault D, et al. Acute postoperative pain in lung transplant recipients. Ann of Thor Surg. 2004;77(6):1951–1955.
151 National Health and Medical Research Council (NHMRC). Acute pain management: scientific evidence. Canberra: NHMRC; 1998.
152 Charnock Y, Evans D. Nursing management of chest drains: A systematic review. Aust Crit Care. 2001;14(4):156–160.
153 Birsan T, Kranz A, Mares P, Artemiou O, Taghavi S, et al. Transient left ventricular failure following bilateral lung transplantation for pulmonary hypertension. J Heart Lung Transpl. 1999;18(1):4–9.
154 Mendeloff EN, Meyers BF, Sundt TM, Guthrie TJ, Sweet SC, et al. Lung transplantation for pulmonary vascular disease. Ann of Thor Surg. 2002;73(1):209–217.
155 Simpson KP, Garrity ER. Perioperative management in lung transplantation. Clin Chest Med. 1997;18(2):277–284.
156 Garrity ER, Jr., Villanueva J, Bhorade SM, Husain AN, Vigneswaran WT. Low rate of acute lung allograft rejection after the use of daclizumab, an interleukin 2 receptor antibody. Transplantation. 2001;71(6):773–777.
157 Egan JJ, Woodcock AA, Webb AK. Management of cystic fibrosis before and after lung transplantation. J Royal Soc of Med. 1997;90:47–58.
158 Burker EJ, Evon DM, Sedway JA, Egan T. Appraisal and coping as predictors of psychological distress and self-reported physical disability before lung transplantation. Prog Transpl. 2004;14(3):222–232.
159 Smolin TL, Aguiar LJ. Psychosocial and financial aspects of lung transplantation. Crit Care Nurs Clin N Am. 1996;8(3):293–303.
160 van Menen M. Researching lived experience: Human science for an action sensitive pedagogy. New York: State University of New York Press; 1990.
161 Collins TJ. Organ and tissue donation: A survey of nurses’ knowledge and educational needs in an adult ITU. Intens and Crit Care Nurs. 2005;21(4):226–233.
162 Kim JR, Elliott D, Hyde C. Korean health professionals’ attitudes and knowledge toward organ donation and transplantation. Int J Nurs Stud. 2004;41(3):299–307.
163 Albert PL. Grief and loss in the workplace. Prog in Transpl. 2001;11:169–173.
164 Chernenko SM, Jensen L, Newburn-Cook C, Bigam DL. Organ donation and transplantation: a survey of critical care health professionals in non-transplant hospitals. Prog Transpl. 2005;15(1):69–77.
165 Krasko A, Deshpande K, Bonvino S. Liver Failure, transplantation, and critical care. Crit Care Clin. 2003;19:155–183.
166 Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surgery, Gynecology & Obstetrics. 1963;117:659–676.
167 Elliott DT. Common quantitative methods. In: Schneider Z, Whitehead D, Elliott D, Lobiondo-Wood G, Haber J. Nursing and midwifery research. Sydney: Elsevier, 2007.
168 Moher D, Jones A, Lepage L. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;285(15):1992–1995.
169 Tiplady B, Jackson SH, Maskrey VM, Swift CG. Validity and sensitivity of visual analogue scales in young and older healthy subjects. (United Kingdom). Age Ageing. 1998;27(1):63.
170 Cakar N, Tuõrul M, Demirarslan A, Nahum A, Adams A, et al. Time required for partial pressure of arterial oxygen equilibration during mechanical ventilation after a step change in fractional inspired oxygen concentration. Intens Care Med. 2001;27(4):655–659.
171 Williams R, Rankin N, Smith T, Galler D, Seakins P. Rela-tionship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med. 1996;24(11):1920–1929.
172 Jones B, Jarvis P, Lewis J, Ebbutt A. Trials to assess equivalence: the importance of rigorous methods. BMJ. 1996;313(7048):36–39.
173 Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20(4):126–131.
174 Parke R, McGuinness S, Eccleston M. Nasal high flow therapy delivers low level positive airway pressure. Brit J Anaes. 2009;103(6):886–890.