Chapter 62A Resectional techniques
Pancreaticoduodenectomy, distal pancreatectomy, segmental pancreatectomy, total pancreatectomy, and transduodenal resection of the papilla of Vater
Overview
The modern surgeon can certainly appreciate the trepidations felt by early surgical pioneers faced with such a clinical situation. Early series on pancreatic operations for cancer published in the late 1960s reported postoperative morbidity rates of 60% and mortality rates approaching 25%, with dismal long-term outcomes. Consequently, Crile (1970) opined that patients would be better served by a bypass procedure rather than putting them through a futile and risky resection. Such a nihilistic view was the prevailing attitude prior to the 1980s, to the point that surgeons asked themselves whether pancreatoduodenectomy (PD) should be abandoned as treatment for pancreatic cancer (van Heerden et al, 1981).
In the ensuing decades, however, a dramatic decline was seen in surgical mortality and morbidity rates. High-volume pancreatic surgical centers are consistently reporting mortality rates of less than 2% and morbidity rates of 36% (Büchler et al, 2003; Cameron et al, 2006; Wagner et al, 2004). Certainly, continual improvements in surgical technique have played a role, but credit cannot be claimed solely by the surgical profession; significant advances were achieved in tandem in other fields that included better understanding of pancreatic diseases, advances in diagnostics, better patient selection, and improvements in perioperative care. Perhaps one of the main contributors to this phenomenon was the establishment of high-volume centers (Birkmeyer et al, 2003; Fong et al, 2005). Such centers tend to boast larger facilities and therefore have a broader range of specialist- and technology-based services, with better staffed intensive care units. This also implies that complications are better recognized and managed.
Although the question of the safety of pancreatic resection has been effectively addressed, the long-term outcome is more controversial as far as pancreatic adenocarcinoma is concerned. The concept of cure following “curative” resection has recently been challenged (Gudjonsson, 1995), but surgical resection is the only therapy that offers significantly increased survival. A randomized multicenter trial affirmed the superior results of resection on survival compared with various forms of nonsurgical therapy (Imamura et al, 2004). The median survival following resection was 14.3 months, whereas patients who did not undergo surgery died at 4.9 months (Conlon et al, 1996); 10% to 30% of patients will be true 5-year survivors (Cameron et al, 2006; Wagner et al, 2004). Furthermore, although pancreatic cancer is the most common of the periampullary tumors, cancers of the ampulla, duodenum, and distal bile duct, as well as intraductal papillary mucinous neoplasm–associated adenocarcinoma, have a better long-term survival following curative PD (Poultsides et al, 2010). In addition, more and more cystic pancreatic and endocrine tumors are diagnosed today, and simple enucleation or segmental resection of the tumors at the appropriate stage can cure the disease. Even with advances in multimodal treatment, surgery remains the centerpiece of the treatment algorithm for pancreatic cancer because no truly effective chemotherapeutic agents for treating nonresectable disease have been developed (Gillen et al, 2010; Postier, 2003).
Technical refinements have led to a variety of surgical techniques that have allowed a more individualized, disease-directed approach. These modifications were partly responsible for the decline in surgical morbidity. This chapter focuses on some of these surgical techniques, as practiced in our institution, as well as some of the more pertinent perioperative issues for patients subjected to pancreatic resection. Surgical techniques specific for the treatment of acute pancreatitis (open and minimally invasive necrosectomy) and chronic pancreatitis (duodenum-preserving pancreatic head resection) are described in detail in Chapters 54 and 55B.
Preoperative Workup and Management
Diagnosis and Staging of Disease
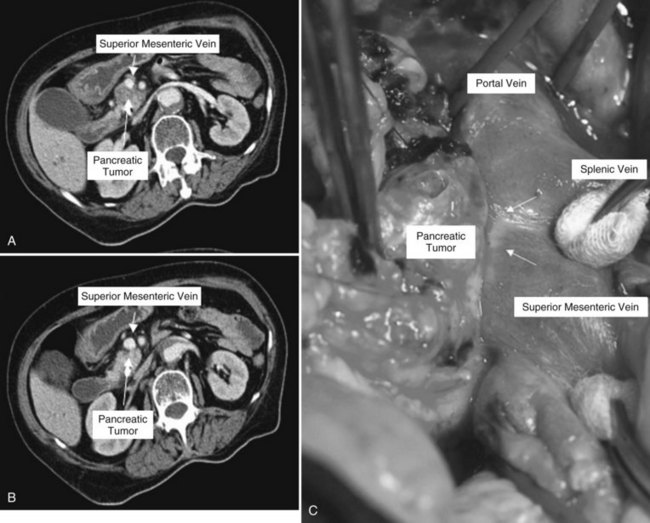
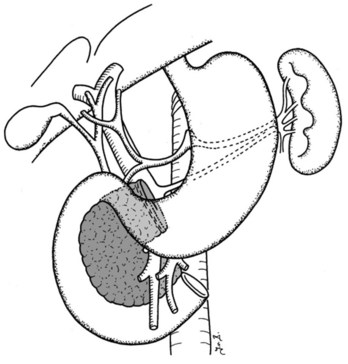
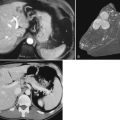
Clinical staging should reliably define the extent of disease, thereby avoiding unnecessary intervention and the accompanying morbidity, mortality, and diminished quality of life in patients with advanced disease (Spanknebel & Conlon, 2001). Although the tumor-node-metastasis (TNM) staging system is most often used in clinical trials, physicians in practice typically classify patients as having resectable, locally unresectable, or metastatic disease (Haller, 2002). Resectable pancreatic cancer is universally defined as a pancreatic tumor without evidence of involvement of the superior mesenteric artery (SMA) or the celiac axis and no evidence of distant metastasis. Tumors with infiltration of one of these central arteries are defined as locally irresectable, but those with infiltration of the superior mesenteric vein or abutment of visceral arteries are defined as borderline resectable (Adler et al, 2007; Katz et al, 2008). Portal vein (PV) involvement, although somewhat controversial, should not be a contraindication for surgery; today, this is still center dependent. In general, PV involvement does not preclude an R0 resection (Fig. 62A.1).
Imaging
Helical computed tomography (CT) scanning has been established as the single most effective initial staging study (Freeny, 2001; Klauss et al, 2008) and is often used as the entry point to a management algorithm. The findings then dictate the subsequent management, including additional diagnostics (see Chapter 16).
The previous selling point of magnetic resonance imaging (MRI) (see Chapter 17) was its being a one-stop modality that combines pancreatography, cholangiography, and angiography and also assists in the evaluation of tumors. However, MRI is being progressively overshadowed with the advent of the multidetector CT, which has nearly the same offerings. As such, MRI offers no added benefit over CT for evaluating pancreatic cancer, except perhaps in patients in whom a contrast-enhanced CT cannot be performed (White et al, 2003), and MRI seems to be superior for the differential diagnosis of pancreatic cystic lesions (see Chapter 57).
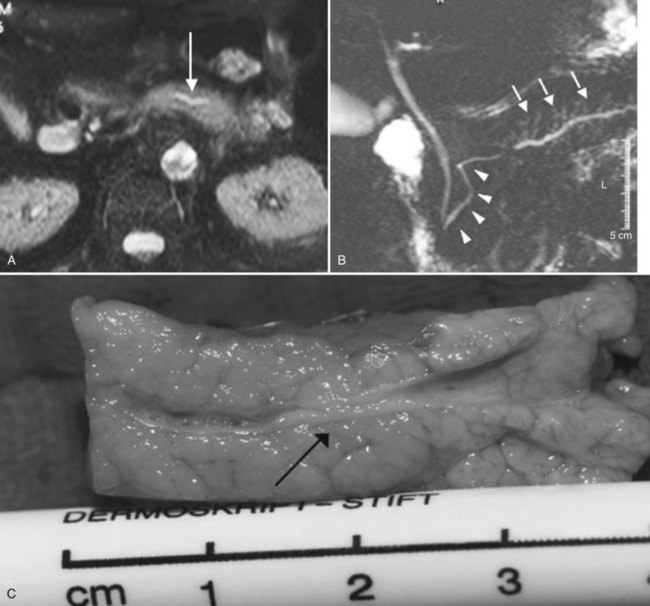
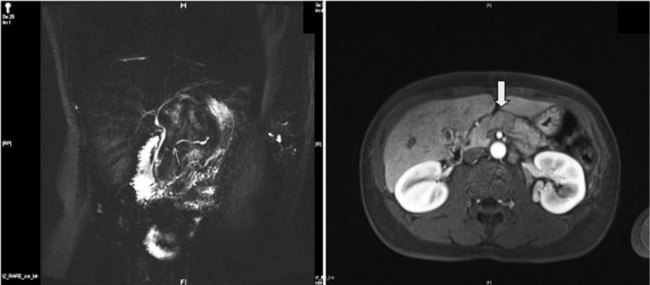
MR cholangiopancreatography (MRCP) offers a noninvasive delineation of the pancreatic and biliary ducts. It detects pancreatic or ampullary carcinoma by demonstrating the effect of a space-occupying lesion on the ducts, namely obstruction or displacement (Fig. 62A.2). The classic feature is the “double-duct” sign. However, even a strictly defined double-duct sign is only 80% to 85% specific for malignancy (Menges et al, 2000). More recent applications include secretin-enhanced MRCP, which can improve pancreatic duct and side-branch delineation. Furthermore, such pharmacologic stimulation of pancreatic juice secretion can potentially allow the evaluation of pancreatic flow dynamics and assessment of pancreatic exocrine function (Kalra et al, 2003).
Endoscopic ultrasonograpy (EUS) allows a sonographic transducer to be placed in close proximity to the pancreas (see Chapter 14). In doing so, interference from overlying bowel gas is eliminated, thus allowing higher frequencies to be used and resulting in markedly improved resolution of the imagery. Consequently, EUS is more sensitive in detecting smaller lesions (<20 mm), with a sensitivity in the range of 93% to 100% (Tamm et al, 2003). In a meta-analysis of studies comparing staging by EUS to results with other modalities, EUS without fine needle aspiration (FNA) more accurately predicted T stage, N stage, and PV involvement than did CT scanning. EUS also allows image-guided FNA of the primary tumor and the regional lymph nodes without the possible risk of tumor seeding along the needle tract, unlike FNA via the percutaneous route (Gress et al, 2001).
EUS-guided FNA biopsy has been reported to have a high sensitivity (93%) and specificity (100%) (Wiersema, 2002); however, EUS with FNA is only of diagnostic value if histology confirms a pancreatic tumor. The major limitations of this technology are operator dependence and a limited field of visualization for the detection of distant metastasis. In fact, in resectable pancreatic cancer, but also in cystic or endocrine tumors, preoperative biopsies are of no value (Hartwig et al, 2009a). Therefore, we use this modality on a highly selective basis, such as in obtaining tissue biopsy for patients scheduled for neoadjuvant therapy with locally advanced pancreatic cancer, or before palliative chemotherapy is applied in metastatic disease.
With the advent of MRCP, EUS, and multidedector CT with multiplanar three-dimensional (3D) reconstruction, the role of endoscopic retrograde cholangiopancreatography (ERCP) as a diagnostic tool has become increasingly limited. Furthermore, it has potentially high complication rates compared with other procedures (Ciocirlan & Ponchon, 2004). Such concerns have been summarized in a recent report by the American Society of Gastrointestinal Endoscopy (ASGE, 2003). In light of these data, the current view is that purely diagnostic uses of ERCP should be limited, a view affirmed by the National Institutes of Health (NIH) State of the Science Conference Statement (Cohen et al, 2002), which recommended that for patients with pancreatic or biliary cancer, the principal advantage of ERCP is palliation of biliary obstruction when surgery is not elected.
General Preoperative Patient Assessment
Pancreatic resections exert a significant physiologic stress on patients, many of whom are elderly; the peak incidence of pancreatic cancer falls in the 65- to 75-year age group (Lankisch et al, 2002). With the improvement of the safety profile of pancreatic operations, many centers have offered surgery to octogenarians, although in such patients the incidence of comorbidities is higher.
In our center, we found that systemic complications, chiefly cardiopulmonary complications, have become the main cause of operative death (Büchler et al, 2003). As such, we routinely subject our patients to a rigorous preoperative assessment of their cardiac, pulmonary, and renal functions. Cardiopulmonary exercise testing has been shown to more accurately evaluate the ability of the cardiorespiratory system to deliver oxygen under stress (Davies & Wilson, 2004). We have also included a pulmonary function test in our algorithm. Optimization of afterload and myocardial contractility take equal importance, and pulmonary artery catheters are occasionally inserted to facilitate this. The operation will only proceed when we can achieve an optimal hemodynamic profile.
Perioperative Management (See Chapters 22, 23, and 24)
Perioperative Anticoagulation
Meta-analysis of the role of low-molecular-weight heparin (LMWH) in the prevention of venous thromboembolic events in general surgery has shown that LWMH can significantly reduce the incidence of asymptomatic deep vein thrombosis (DVT), clinical venous thromboembolism, and pulmonary embolism, with a trend toward a reduction in overall mortality rate (Mismetti et al, 2001). Consequently, we routinely administer LMWH in a prophylactic dose to our patients, starting the evening before the day of surgery, until the patient is ambulant postoperatively. In addition, our patients are prescribed compression stockings to wear intraoperatively and for their entire inpatient stay because such stockings reduce pooling of blood in deep veins by mechanically preventing venous distension (Morris & Woodcock, 2004) and are thus a simple and cost-effective method of DVT prophylaxis.
Antibacterial Prophylaxis
Antibacterial prophylaxis is instrumental in the reduction of infection-related morbidity with clean-contaminated procedures (de Lalla, 2002); as such, it is recommended for all patients who undergo hepatobiliary or pancreatic surgery (Sganga, 2002). Drugs with antianaerobic activity are added if anaerobe encounter is anticipated during the procedure, in particular procedures involving the gastrointestinal (GI) tract. The general guideline is to use the highest licensed dosage of the chosen antimicrobial agent, administered at induction of anesthesia so as to achieve high peak tissue concentration at the site of the wound before the first incision, which should be maintained until the time of closure (Polk et al, 2000). For our pancreatic operations, in addition to 500 mg metronidazole, we routinely give 4 g mezlocillin, an ureidopenicillin with activity against both gram-positive and gram-negative organisms, including enteric bacilli. We generally do not give postsurgical doses unless the patient has had a recent bout of cholangitis or if the bile was found to be turbid or purulent intraoperatively. In any event, in all procedures that require entry into the biliary tract, bile is sent for microbiologic examination to guide postoperative antimicrobial treatment if the need arises.
Octreotide Analogues
The pancreatoenteric anastomosis is considered the Achilles heel of PD, a testimonial to the potentially disastrous sequelae of life-threatening intraabdominal sepsis and hemorrhage (Stojadinovic et al, 2003) in the event of a pancreatic leak. The secretion capacity of the remnant gland has been found to be a determining factor for the development of a leak because continuous pancreatic secretion has been hypothesized to hinder healing of the pancreatic stump. This led to the hypothesis that by reducing exocrine secretion, perhaps the incidence of pancreatic fistula could be reduced accordingly.
Octreotide, the octapeptide analogue of somatostatin, is a powerful inhibitor of pancreatic exocrine secretion. A number of randomized prospective trials have examined the role of prophylactic, perioperative octreotide and its impact on outcome after pancreatic surgery. The results seemed to display an intercontinental difference: level 1 studies from Europe generally showed that perioperative somatostatin has a protective effect, whereas all the North American studies failed to demonstrate any benefit. A recent systematic review of this topic came to the conclusion that the prophylactic administration of somatostatin or its analogue does not uniformly reduce the incidence of pancreatic anastomotic leak, overall morbidity, or mortality after pancreatic resection (Li-Ling & Irving, 2001). Based on the findings of the trials conducted by our center (Büchler et al, 1992; Friess et al, 1995), we believe that some benefits may still be reaped from the use of prophylactic somatostatin in high-risk patients (soft pancreas, small duct), in whom a PD is performed. If the pancreas is deemed to be high risk by the surgeon, because of a soft consistency and a pancreatic duct size of less than 2 mm in diameter, somatostatin is applied intraoperatively (200 µg subcutaneously), followed by three daily doses of 200 µg octreotide for 5 days. Other indications for applying somatostatin are when segmental resections or enucleations must be performed in patients with a soft pancreas, as is frequently observed with cystic or endocrine lesions.
Preoperative Biliary Drainage
Patients with pancreatic cancer who have jaundice are also at risk for associated coagulopathy, malabsorption, malnutrition, and immune dysfunction. Using a multiple-variant regression analysis, we had previously determined that jaundice (bilirubin level >5.8 mg/dL) is a significant risk factor for postoperative hemorrhage (Martignoni et al, 2001). This calls into question the role of preoperative biliary drainage (PBD) in reducing surgical morbidity through the normalization of liver function and the correction of coagulopathy. Sewnath and colleagues (2002) found in a meta-analysis no difference in the overall death rate among patients who had PBD and those who had surgery without PBD. Instead, the overall complication rate was adversely and significantly affected by PBD, and the hospital stay was also prolonged, so they concluded that PBD carries no benefit. Recent studies also demonstrate that routine PBD should not be performed because of the high rate of procedure-associated complications (Mezhir et al, 2009; van der Gaag et al, 2010). We concur with the opinion that ERCP should only be undertaken with therapeutic intent and believe that PBD as a routine practice should be halted. We would refer a patient for PBD in the presence of cholangitis or other severe complication of jaundice that would preclude a safe, early resection. An absolute indication for PBD is jaundice that requires induction therapy prior to surgical extirpation.
Fast-Track Surgery
Studies on so-called fast-track GI surgery have shown that epidural analgesia, combined with an intensive and standardized regimen of early feeding and mobilization, can reduce hospital stay (Basse et al, 2002). Epidural analgesia has been found to have many attributes, including a shorter duration of postoperative ileus, attenuation of the stress response, fewer pulmonary complications, improved postoperative pain and mobility, and patient perception of a quicker recovery (Fotiadis et al, 2004). Thoracic epidural analgesia is of particular benefit to patients with a high risk of cardiac or pulmonary morbidity, and it can reduce hospital stay and costs in this subgroup of patients.
Resectional Techniques
Pancreatoduodenectomy
Ductal adenocarcinoma is by far the most prevalent tumor of the pancreas, with a predominant localization within the pancreatic head (78%) (see Chapter 58A, Chapter 58B, Chapter 59 ; Schäfer et al, 2002). Furthermore, adenocarcinoma of the body or tail is seldom resectable on presentation. It is thus not surprising that PD is the best-studied pancreatic surgical procedure.
Technique
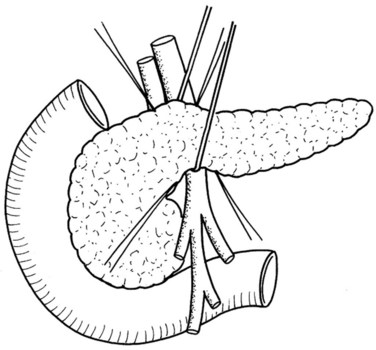
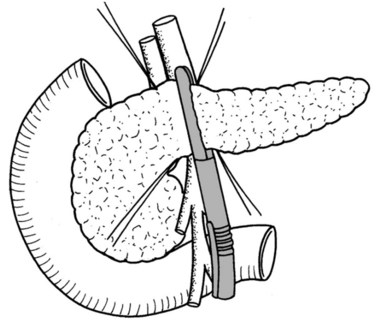
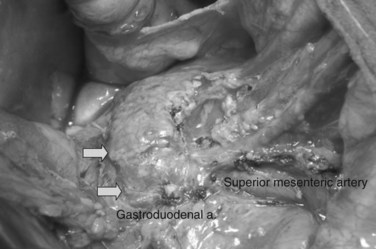
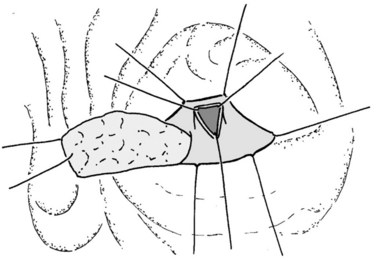
In thin patients, we prefer a midline incision beginning from the xiphoid process, but we perform a rooftop incision in patients with a short torso and a wide abdominal girth. After we have ruled out liver metastasis, the peritoneal surfaces are carefully inspected for metastatic deposits. Particular attention is paid to the pelvis for drop metastasis and to the root of the mesentery for evidence of tumor extension or matted nodes. A wide Kocher maneuver is then performed to assess the retroperitoneum and appraise the tumor and its relationship to the SMA (Fig. 62A.3). The mesenteric artery is inspected from the ligament of Treitz and dissected on its right hemicircumference to exclude any tumor infiltration of the artery. This technical approach, the so-called artery-first approach (Fig. 62A.4; Weitz et al, 2010), helps avoid R2 resections because any infiltration of the central arteries can be diagnosed early during exploration. We proceed with the resection only if we find no evidence of infiltration of the celiac trunk and SMA.
Cholecystectomy is performed in a typical fashion. The cystic duct is traced to its origin from the common bile duct (CBD), which is transected just cephalic to this point. Extreme care must be taken to avoid any iatrogenic injury to the right hepatic artery, which usually runs posterior to the hepatic duct (see Chapter 1B; Skandalakis et al, 2004). In addition, it is important to assess for an aberrant right hepatic artery, present in 20% of patients, by palpation of the posterior aspect of the hepatoduodenal ligament. The cystic artery is isolated and closed with ligation.
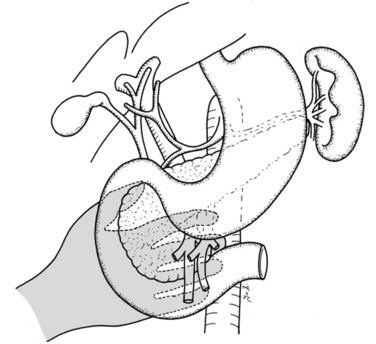
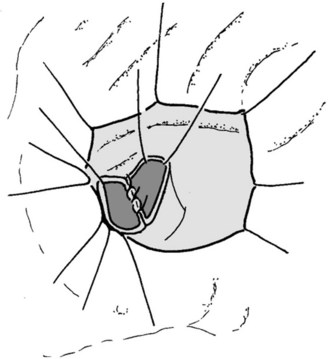
If preservation of the pylorus is planned, which is our usual practice, the right gastric and right gastroepiploic arteries are divided and the duodenum is skeletonized 2 cm beyond the pylorus. The suprapancreatic portion of the PV will now be widely exposed, and two stay sutures are similarly placed on the superior border of the pancreas; these sutures at the superior and inferior pancreatic borders also serve to ligate the superior and inferior pancreatic vessels respectively, running longitudinally in the parenchyma. Hence bleeding from the cut edges is reduced after transection. A tunnel is cautiously created between the SMV-PV trunk posteriorly and the pancreatic neck anteriorly. A silicon drain is then insinuated into this tunnel to loop up the neck (Fig. 62A.5).
Because the medial margin is the most crucial with regard to tumor infiltration and the R status of the resection (Esposito et al, 2008), we now perform the uncinate process–first approach (Hackert et al, 2010). After having exposed and evaluated the SMA, the ligament of Treitz is mobilized, and the mesenteric branches to the fourth part of the duodenum are divided to allow it to be delivered into the inframesocolic compartment under the SMA. The mesenteric window is extended to the proximal jejunum, which is transected at a point that allows a tension-free loop of jejunum to be brought into the supramesocolic compartment in a retrocolic fashion through a window created on the right side of the middle colic pedicle. The dissection is performed along the SMA and SMV with bipolar cautery and clips.
Standard Pancreatoduodenectomy Versus Pylorus-Preserving Pancreatoduodenectomy
In addition to the known postoperative complications associated with PD, the performance of a distal gastrectomy in the standard procedure also brings with it the possible long-term morbidities of gastric dumping, marginal ulceration, and bile-reflux gastritis (Poon & Fan, 2001). First introduced by Kenneth Watson (1944), it was not popular until 1978, when Traverso and Longmire (1978) reintroduced it. They hypothesized that the preservation of the stomach and pyloric function would improve GI function and hence reduce the morbidities associated with a gastroenterostomy. Indeed, better long-term digestive function and quality of life have been found after pylorus-preserving pancreatoduodenectomy (PPPD) in some retrospective studies. To retain a functioning pylorus, the entire stomach, 2 cm of the first part of the duodenum, and the neurovascular supply to both are preserved. Following the division of the right gastric and right gastroepiploic arteries at their origin, the duodenum is skeletonized 2 cm distal to the pylorus (Farnell et al, 2001), taking care to preserve the nerve of Latarjet. The duodenal bulb is then transected.
A recent meta-analysis demonstrated that the classical Kausch-Whipple procedure and the pylorus-preserving Whipple procedure are comparable with regard to perioperative morbidity and mortality and long-term outcome (Diener et al, 2008b). Hence, in the light of the available evidence, PPPD should be designated the “standard” procedure for pancreatic and periampullary neoplasm because no study to date has demonstrated any advantage to the removal of the stomach and the proximal duodenum (Bell, 2004). Hemigastrectomy should be performed only when gastric or proximal duodenal invasion or grossly abnormal peripyloric nodes are evident.
Vascular Resection
Over 30 years ago, Fortner (1973) reasoned that a more radical resection should improve survival by improving tumor clearance, specifically that tumor adherence to the PV or SMV, often regarded as a criterion of unresectability, could be overcome by en bloc resection of the involved vessels. Randomized evaluation of portal and superior mesenteric vein resection in patients with tumors adherent to these vessels is difficult because considerable intersurgeon variation in the interpretation of adherence is likely. Thus no randomized trial evaluates this topic.
A recently published systematic review (Siriwardana & Siriwardena, 2006) evaluated 52 manuscripts with 6333 patients in whom pancreatic resection was performed for pancreatic cancer; 1646 of these patients (26%) underwent synchronous portal and superior mesenteric vein resection. The median number of resections per publication evaluated was 82; of those, 23 patients underwent portal and superior mesenteric vein resection. The proportion of portal and superior mesenteric vein resection per publication varies widely, ranging from 2% to 77%, which mirrors the various treatment approaches of pancreatic cancer in different institutions today.
The assessment of pooled data is critically influenced by the quality of the single reports. The reports included have been published over the last 25 years, and the standards of perioperative care, surgical technique, and adjuvant therapy have developed dramatically. Thus the wide variations of outcome parameters—perioperative death, operative time, blood loss—mirror the nonhomogenous collective included in this review. However, the long-term survival rate demonstrates that resection of the portal or superior mesenteric vein is a potentially curative operation. It seems that patients who need a portal or superior mesenteric vein resection for cure include a high percentage of patients with a positive nodal stage. Because adjuvant treatment has been proven to increase survival but has only recently been introduced as standard care for patients with pancreatic cancer, the survival rates of patients with portal or superior mesenteric vein resection today should be even better than those reported in this review (Neoptolemos et al, 2004; Oettle et al, 2007).
In addition, surgical expertise has improved over the past few decades, and the quality of high-volume centers—especially for technically demanding surgeries, such as pancreatic resection—has been proven (Birkmeyer et al, 2003; Büchler et al, 2003). Thus it is not surprising that perioperative morbidity and mortality rates reported for pancreatic resections with portal or superior mesenteric vein resection are identical to those without it (Bachellier et al, 2001; Carrere et al, 2006; Leach et al, 1998; Nakao et al, 2006; Riediger et al, 2006; Wagner et al, 2004).
Tumor infiltration of the SMA, celiac trunk, or hepatic arteries are well-accepted criteria for unresectability of pancreatic adenocarcinoma. However, because adherence of tumors to the arteries does not automatically mean infiltration—in fact, the arterial wall is resected much longer than the venous wall—several published series of pancreatic resections included resection and reconstruction of visceral arteries (Hartwig et al, 2009a; Li et al, 2004; Sasson et al, 2002; Varadhachary et al, 2006). A recent review states that in 15% of pancreatic operations in which portal or mesenteric vein resection had to be performed, arteries were also resected. These included the common hepatic artery (50%), SMA (20%), celiac axis (10%), and other arteries (Siriwardena & Siriwardena, 2006).
Multivisceral Resections
The benefit of radical surgical resection of contiguously involved structures for locally advanced pancreatic cancer has been addressed in several reports (Hartwig et al, 2009; Sasson et al, 2002). In fact, about 35% of patients are seen initially with locally advanced pancreatic cancers with involvement of surrounding structures and organs. Although several reports in the past showed that morbidity of extended resection was increased and survival benefit limited, more recent publications demonstrate that en bloc resection of contiguously involved organs can be performed safely (Sasson et al, 2002). No difference was reported with regard to perioperative morbidity (35%) and mortality (3%) compared with standard resection. Although operation time is clearly longer because of the extended resection—which includes mesocolon, colon, adrenal glands, liver, and stomach—blood loss and hospital stay are not different from what is observed after the standard procedure. The main aim of the extended procedure must be an R0 resection because this is the most important predictor of long-term survival (Wagner et al, 2004). The survival rate after resection of mesocolon and colon, as well as stomach, is not significantly decreased compared with the standard procedure (Sasson et al, 2002). The 5-year survival rate was 16%, and the median survival of 26 months is much better than the 6 to 9 months reported for patients who are not resected at all.
Similarly, another study demonstrated that patients with pancreatic adenocarcinoma of the body or tail need extended resection for contiguous organ involvement more frequently because these tumors are generally detected later and have therefore reached a greater size than cancers of the pancreatic head by the time of diagnosis. The median survival in these patients was 15.9 months, and the survival at 5 and 10 years was 22% and 18%, respectively. In our series of more than 100 patients with multivisceral resections, we showed that perioperative mortality rate and long-term outcome are similar to those of standard resections (median survival, 20 months), but that the morbidity is increased. Thus these patients need careful postoperative observation in an experienced pancreatic center (Hartwig et al, 2009).
Lymphadenectomy
The rationale for extended lymphadenectomy is that lymph node studies have confirmed positive lymph nodes may be found outside the confines of the standard dissection (Cubilla et al, 1978). Even for small cancers, lymph nodes of the paraaortic region, between the celiac trunk and the origin of the inferior mesenteric artery, frequently harbor metastases; it has been suggested that these should be dissected en bloc during radical resections (Nagakawa et al, 1993). These revelations initiated a movement of extended lymphadenectomy among Japanese surgeons. A few studies reported similar surgical morbidity and mortality rates, but improved survival results were seen with extended surgery compared with the standard PDs (Ishikawa et al, 1993). However, these were all retrospective, nonrandomized studies.
What constitutes extended lymphadenectomy is still widely debated; this is epitomized by the multitude of terminologies used in the literature. In an attempt to harmonize this subject, a consensus conference on the surgical treatment of pancreatic cancer took place in Castelfranco Veneto, Italy (Pedrazzoli et al, 1999). This consensus provided a standardized definition of the different extent of lymphadenectomy that used the Japanese Pancreas Society rules for the study of pancreatic cancer to define the lymph node stations to be removed for the various procedures (Japan Pancreas Society, 1993). The three stages of radicality for PD have been named standard, radical, and extended radical, depending on the nodal stations removed. For cancers of the body or tail, according to the extent of lymphadenectomy, two different procedures are identified: standard and radical. It is hoped that this standardization of surgical procedures will facilitate comparison of results between different centers.
Between 1988 and 1999, eight retrospective studies were published on the extent of lymphadenectomy for PD. The data on survival are largely heterogeneous, but neither morbidity nor mortality seems to be altered. Altogether, two studies reported on morbidity rates, whereas five studies reported perioperative mortality rates. Especially in Japan, extended lymphadenectomy was widely used in the late 1980s and 1990s because of the initial reports of Ishikawa and colleagues (1988) and Manabe and colleagues (1989), who advocated an extension of the PD. In these studies, survival rates were significantly increased in the extended lymphadenectomy groups. Interestingly, some long-term survivors were seen in the extended lymphadenectomy groups, but none (Manabe et al, 1989) or very few (Ishikawa et al, 1988) of the patients in the standard lymphadenectomy arm survived for more than 5 years.
In terms of operative techniques, total pancreatectomies were used in many patients, which may have also altered the outcome. Interestingly, the largest retrospective studies (Hirata et al, 1997; Mukaiya et al, 1998), which together included 1330 patients, did not reproduce these results. The 1- and 3-year survival rates were comparable in the standard lymphadenectomy and extended lymphadenectomy groups, but data on morbidity and mortality were not provided. A study by Kawarada and colleagues was the only one to reproduce the results of Manabe and Ishikawa. However, patients included in the standard lymphadenectomy arm had been operated on between 1976 and 1981, whereas extended lymphadenectomy was performed between 1981 and 1993. Because the perioperative outcomes were significantly improved in the late 1980s, the survival benefit shown for extended lymphadenectomy may be due solely to generally advanced perioperative management. Hence, it cannot be concluded from the retrospective studies that extended lymphadenectomy provides a survival benefit.
Six prospective, nonrandomized studies have been published on this topic. A prospectively conducted study from Henne-Bruns and colleagues (1998), in which the decision to perform a standard or extended lymphadenectomy was made based on the clinical status of the patient, revealed no significant differences in mortality rate and long-term survival but did show a trend toward a better outcome in the standard lymphadenectomy group. This may be due to a higher percentage of patients with advanced-stage tumors in the extended lymphadenectomy group. Furthermore, extended lymphadenectomy was associated with an increased rate of severe diarrhea in the early postoperative phase. Gazzaniga and colleagues (2001) showed that morbidity, mortality, and long-term survival were unaltered between standard and extended lymphadenectomy. Interestingly, a third group of patients received extended lymphadenectomy and additional adjuvant chemoradiation and fared significantly better, with a 5-year survival rate of 17.6%. However, this promising concept of multimodal therapy was not confirmed by randomized trials. Another retrospective study from Italy showed a tendency toward increased survival rates with an extended lymphadenectomy, but morbidity was comparable (Iacono et al, 2002). Studies from both Popiela and colleagues (2002) and Capussotti and colleagues (2003) confirmed the results of the previous reports with unchanged morbidity, mortality, and survival rates. However, for node-negative cancer, extended lymphadenectomy significantly improved the 5-year survival rates (Popiela et al, 2002), and a slight improvement in short-term survival was also seen for patients in the extended lymphadenectomy group (Capussotti et al, 2003). Overall, the number of resected lymph nodes was significantly higher in the extended lymphadenectomy groups.
Four randomized controlled trials have been conducted so far and were summarized in a meta-analysis (Farnell et al, 2005; Michalski et al, 2007; Nimura et al, 2004; Pedrazzoli et al, 1998; Yeo et al, 2002). Interestingly, these four level I studies are from centers on three different continents. The first study by Pedrazzoli and colleagues did not reveal any differences with regard to morbidity, mortality, and survival between the standard and extended lymphadenectomy groups. Standard lymphadenectomy included the dissection of lymph nodes at the anterior and posterior pancreatoduodenal side, at the pylorus, bile duct, and superior and inferior part of the pancreatic head and body. For the extended procedure, additional lymph nodes were dissected within the complete circumference at the aorta, the inferior and superior mesenteric arteries, and the celiac trunk. Interestingly, a retrospective subgroup analysis revealed a significantly prolonged survival for node-positive patients who received an extended lymphadenectomy. This statement has since been intensively discussed because of the small difference in the number of resected lymph nodes between the standard and extended lymphadenectomy groups (13.3 vs. 19.8).
Considering the large number of patients and the detailed analysis of many variables, the conclusion that extended lymphadenectomy does not improve survival but may increase morbidity is to be supported. These data and conclusions are underlined by their follow-up publication and a study on quality of life (QOL) of the same patient group (Riall et al, 2005). Additional studies from the Mayo Clinic by Farnell and colleagues (2005) and from Japan by Nimura and colleagues (2004) supported those results. The Mayo Clinic trial included 79 patients randomized in two groups (lymphadenectomy: standard [n = 40] vs. extended [n = 39]). When performing an extended lymphadenectomy, the number of lymph nodes resected was greater (36 vs. 15), but morbidity and mortality rates were comparable. No significant differences were observed for the 1-, 3-, and 5-year survival rates. However, at 4 months postoperatively, diarrhea was significantly increased, and bowel control and body appearance were worse in the extended lymphadenectomy group. The multicenter trial from Nimura and colleagues revealed similar results with comparable mortality and survival rates but increased morbidity, which was due to severe diarrhea in 48% of the patients (Nimura et al, 2004). Of interest, the number of lymph nodes resected varies considerably between the studies. For examples, Nimura and colleagues resected about twice as many lymph nodes in the extended lymphadenectomy group as did Pedrazzoli and colleagues, which may reflect different operative techniques as well as differences in pathologic analysis.
In summary, none of the randomized, controlled trials (RCTs), except for a retrospective subgroup analysis within the study of Pedrazzoli and colleagues, showed any survival benefit for extended lymphadenectomy, whereas overall morbidity with diarrhea and delayed gastric emptying as particular complications tended to occur more frequently. Interestingly, both Yeo and colleagues and Farnell and colleagues particularly report a reduction in QOL in the extended lymphadenectomy group, primarily because of high rates of postoperative diarrhea with subsequent development of malnutrition (Michalski et al, 2007). Thus mortality and morbidity may be at best unaltered for the extended radical operation compared with the standard procedure. The randomized studies are altogether underpowered and lack standardization with regard to the actual extent of the performed lymphadenectomy. As for the potential survival benefit, further adequately powered randomized studies will need to be impractically large, thus precluding their realization. Therefore, the current standard of surgery for pancreatic head tumors remains PPPD without extended lymphadenectomy.
Reconstruction After Pancreatic Resection and Management of the Pancreatic Remnant
A pancreatic leak is the major cause of procedure-related death. Simply occluding the duct has been shown to result in higher fistula rates in addition to increasing the risk of pancreatic exocrine and endocrine insufficiency (Tran et al, 2002). Hence, drainage of the pancreatic remnant to the GI tract remains a crucial step, but it runs the risk of anastomotic breakdown. It is thus not surprising that the pancreatoenteric anastomosis has fascinated surgeons, motivating them to search for a more reliable technique to avoid this dreaded complication. Many techniques have been described, and the literature will continue to report novel techniques that promise to be even safer. However, more so than the choice of the variant used, successful management of a pancreatic anastomosis is most dependent on the surgeon’s concentration on meticulous execution of the chosen technique (Trede et al, 2001). For as long as the basic tenets of a safe anastomosis are met—namely, careful handling of the pancreatic tissues, a tension-free adaptation, good perfusion, and no distal obstruction—any pancreatoenteric anastomotic technique can have a good outcome.
One of the most commonly used techniques is a pancreatojejunal anastomosis, which can be performed by invaginating the transected pancreas into the end of the jejunum, the so-called dunking procedure. Another variant is to anastomose the pancreatic duct directly to a proper opening in the jejunum, the so-called duct-to-mucosa technique. The technique of pancreatojejunal anastomosis—whether end-to-side or end-to-end, duct-to-mucosa or dunking—does not seem to significantly influence the anastomotic leak rate. Another strategy is to anastomose the pancreatic stump to the stomach, and proponents of pancreatogastrostomy (PG) have cited various reasons to choose this method (Zenilman, 2000); it is easier to perform given the close proximity of the stomach to the pancreas, and the anastomosis is less prone to ischemia because of the rich gastric perfusion. Because the exocrine enzymes enter an acidic environment, it was once thought that the leak rate would be lower because the enzymes do not get activated, but this idea has since been debunked. In a prospective randomized trial comparing pancreatojejunostomy (PJ) to PG, the leak rates were not significantly different (PJ, 11%; PG, 12%) (Yeo et al, 1995).
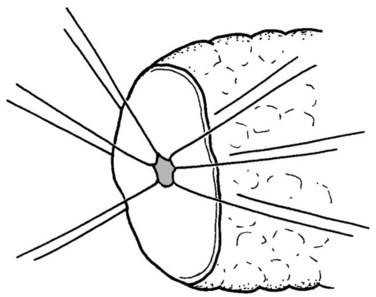
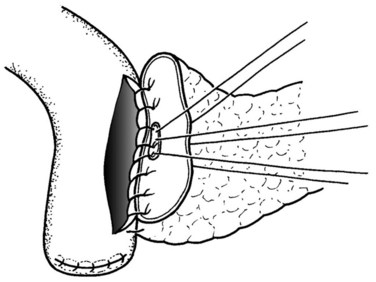
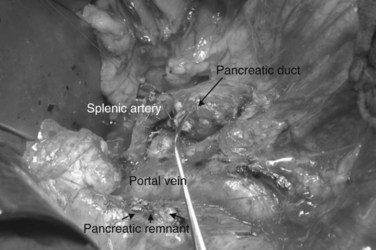
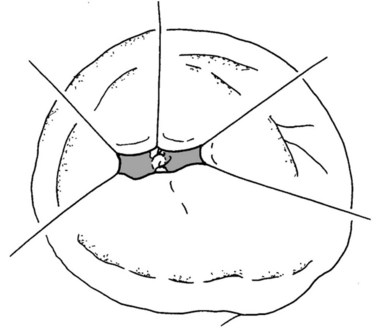
The authors’ institution uses a standardized technique of PJ performed in an end-to-side fashion with a retrocolic jejunal limb (Z’graggen et al, 2002). The anastomosis is performed in two layers with duct-to-mucosa adaptation using monofilament absorbable polydioxanon sutures (PDS 5/0) with an atraumatic JRB-1 (5/8) needle. The first step is the placement of the ductal sutures, first anteriorly from the outside in, then posteriorly from the inside out (Fig. 62A.6). We endeavor to place at least three ductal sutures on each side. This is generally no problem, because the duct is dilated in cases of pancreatic head tumors. Only in patients with very small ducts that cannot be cannulated with a small ductal probe, the duct sutures should be omitted to avoid obstruction of the duct.
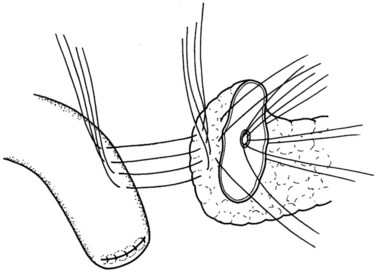
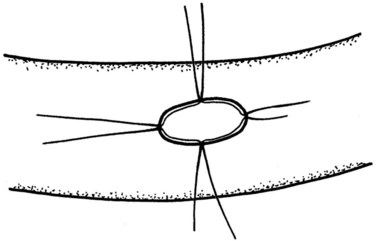
Because the pancreatic stump is dissected over 2 cm toward the left, good visualization of the posterior capsule can be achieved. A row of interrupted sutures is then placed through the dorsal capsule, with the needle entering the pancreatic tissues about 1 cm from the cut end of the stump and taking an adequate bite of the parenchyma. The needle is then driven through the seromuscular layer of the jejunal limb about 1 to 2 mm from the mesenteric border. The intersuture distance is about 4 to 6 mm (Fig. 62A.7). The jejunum is carefully adapted to the pancreas following meticulous and gentle tying of the knots. A longitudinal enterotomy is then performed about 5 mm from the posterior row of sutures (Fig. 62A.8) with the width of the opening tailored to the size of the pancreatic stump.
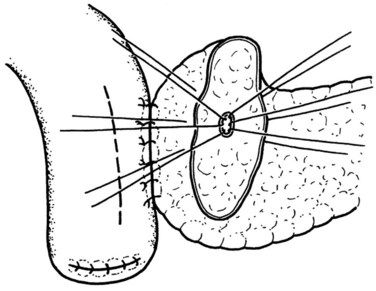
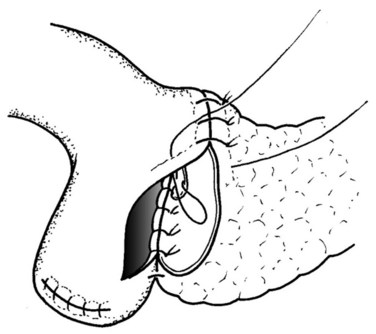
The posterior inner row of sutures is then constructed, first catching the pancreas in an inside-out fashion, ensuring an adequate bite of the parenchyma, and exiting just at the cut edge of the remnant pancreas. The suture is then driven through the posterior edge of the enterotomy in an outside-in fashion, ensuring a full-thickness bite. The previously placed ductal sutures are integrated in order, and each suture is clipped with an artery clamp (Fig. 62A.9). All knots are tied after completion of the suture row.
This is followed by the anterior inner row of sutures, which is driven through the pancreas in an outside-in manner, entering just at the anterior cut edge of the stump and ensuring an adequate bite of the pancreatic parenchyma. The suture is then placed through the anterior edge of the enterotomy in an inside-out fashion with a full-thickness bite (Fig. 62A.10). All knots are, again, only tied following completion of the suture row.
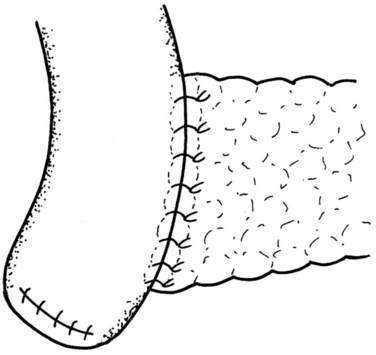
The final step is the second row of anterior sutures, the aim of which is to cover the inner suture line by imbricating the jejunal serosa over it (Fig. 62A.11). The suture first catches the anterior capsule of the pancreas close to the anterior inner row. A seromuscular bite is then taken of the jejunum at a point that will allow a fold of the jejunal wall to cover the inner row of sutures in a tension-free manner (Fig. 62A.12).
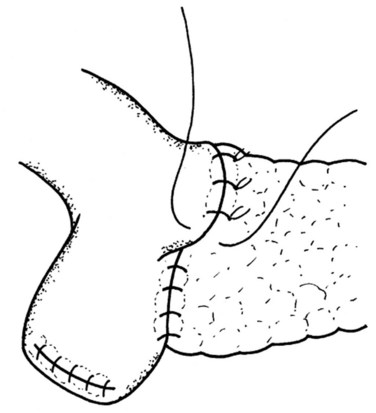
FIGURE 62A.11 The second layer of the anterior suture row, which serves to imbricate the first layer.
The end-to side technique allows the adaptation of the jejunal opening to the specific requirements of the pancreatic remnant. Separate duct-to-mucosa adaptation also keeps the duct orifice open, thereby ensuring the unobstructed flow of pancreatic secretions through the anastomosis. Consequently we see no need for the placement of any pancreatic stents. This technique was used in 93% of patients undergoing pancreatic head resection, with an overall leak rate of 3.2% (Büchler et al, 2003).
Bilioenteric Anastomosis
We position this anastomosis about 10 to 15 cm downstream from the PJ. The cut edge of the bile duct is freshened to obtain well-perfused healthy tissues, after which a small enterotomy is made at the antimesenteric border of the jejunal loop, with removal of a small cuff of mucosa. The protruding mucosa is then fixed at four quadrants of the enterotomy using 6/0 PDS sutures (Fig. 62A.13). These mucosa-fixation sutures ensure a full-thickness bite in the subsequent suture placement and thus guarantee a secured duct-to-mucosa coadaptation. The posterior suture layer is first constructed, with each individual suture held in clamps, until the entire suture row is completed and before knotting begins (Fig. 62A.14). This is then followed by the anterior suture row (Fig. 62A.15), and the final step is closure of the mesocolic opening around the jejunal limb.
Reconstitution of Gastrointestinal Continuity
Depending on whether a distal gastrectomy or PPPD is performed, the reconstruction ends either with a gastrojejunostomy or a duodenojejunostomy, respectively. Some concern has surrounded the question of whether an intact pylorus will lead to higher delayed gastric emptying (DGE) rates postoperatively following a PPPD; however, this controversy seems to have been finally laid to rest following the results of several controlled trials that to which we have previously alluded. Therefore, based on evidence available in 2004, it is apparent that pylorus preservation does not seem to increase the frequency of DGE (Horstmann et al, 2004).
The technique used to reconstitute GI continuity may have an impact on the incidence of DGE, however. One group speculated that a retrocolic reconstruction predisposes the jejunal limb to venous congestion and bowel edema, which can consequently retard recovery of jejunal peristalsis at the duodenojejunostomy (Park et al, 2003). Postoperative gastroparesis may also lead to temporary gastric distension, which can lead to angulation of the anastomosis because it lies relatively fixed through its retrocolic position (Horstmann et al, 2004). Additionally, the close proximity of the duodenojejunostomy to the PJ also predisposes the occurrence of DGE in the event of a small PJ leak or transient postoperative remnant pancreatitis. Opponents to this theory have suggested that the real culprit is an antecolic reconstruction (Riediger et al, 2003) that predisposes the relatively fixed stomach to angulation or torsion. The risk of DGE caused by local inflammation is reduced by placing the duodenojejunstomy in the infracolic compartment through a separate mesenteric window, away from the pancreatic and biliary anastomoses, which lie in the supracolic compartment.
Our unit’s philosophy is more in line with the first group, substantiated by the results of a retrospective review conducted in our center, in which we compared the outcomes following retrocolic placement of the duodenojejunostomy with those following an antecolic placement of the anastomosis. We found that the incidence of DGE was lower after using the second surgical strategy. After PPPD, we now routinely perform an antecolic end-to-side duodenojejunostomy about 50 cm downstream from the hepaticojejunostomy, using the transverse colon to splint the duodenojejunostomy away from the PJ. The anastomosis is constructed in two layers using monofilament absorbable sutures in a continuous fashion (PDS 4/0 or 5/0) (Fig. 62A.16).
Abdominal Drains and Nasogastric Tubes
Intraperitoneal drains have been placed in relation to the biliary and pancreatic anastomoses with the intention of controlling leakage of blood and biliary, lymphatic, or pancreatic secretions. Such a practice has been prophylactic in nature, and the reason for it stems more from habit than evidence. But this has been challenged by a randomized trial that addressed the value of drains following pancreatic resection and found that placement of drains did not translate into a reduction in surgical morbidity (Conlon et al, 2001). Rather, a significantly higher proportion of patients randomized to the drain group developed intraperitoneal sepsis, fluid collection, or fistula, although this trial was not sufficiently powered to determine whether the drain was the cause of these intraabdominal complications. Consequently, based on this trial alone, our unit has not found sufficient evidence for us to abandon this practice.
Our hesitancy to embrace a no-drain policy is further vindicated by the findings of a recently published meta-analysis of prophylactic drainage after GI surgery (Petrowsky et al, 2004). This evidence-based review found that drains do not reduce complications following hepatic, colorectal, or rectal resection with primary anastomosis, and drains should not be used following such procedures. With regard to many other GI procedures, including pancreatic resections, the jury is still out, and we have concluded that more well-designed trials are needed to clarify the role of prophylactic drainage. For our participation in a multicenter randomized (PANDRA) trial evaluating this issue, we routinely place two passive drainage tubes (Easy-flow; Dahlhausen & Company, Cologne, Germany): one below the diaphragm and the other in close proximity to the hepaticojejunostomy and the PJ.
We also routinely place nasogastric (NG) suction tubes for our patients during the operation and anesthesia, which are immediately removed at extubation. A meta-analysis on the need for NG decompression has quashed the long-held belief that routine NG decompression decreases the risk of postoperative nausea, vomiting, aspiration, wound dehiscence, and anastomotic leak (Cheatham et al, 1995). Instead, it was found that fever, atelectasis, and pneumonia were significantly less common, and days to first oral intake were significantly fewer, in patients managed without NG tubes. Although patients may develop abdominal distension or vomiting without an NG tube, this does not necessarily translate into an increase in complications or length of stay.
Distal Pancreatectomy
Management of the Pancreatic Remnant
Pancreatic fistula remains the most common surgery-related complication after distal pancreatectomy (Benoist et al, 1999; Ferrone et al, 2008). Many techniques have been described in the literature with regard to the management of the residual transected pancreatic parenchyma and the divided pancreatic duct to lower the risk of leak. These include direct duct ligation, enteric drainage, prolamine injection, sealing with fibrin glue, and transection with linear stapling devices. The plethora of surgical strategies described in the medical literature simply underlies the fact that no one technique has been convincingly shown to reduce the incidence of pancreatic leak consistently.
When the pancreas has a soft texture and is not too thick, we transect it with a linear stapling device (Fig. 62A.17). This device gives a staple line consisting of a triple row of closely placed staples. In all other cases, the pancreas is dissected by a knife, and the resection margin is closed with single PDS stitches; the pancreatic duct should be identified and closed by separate sutures. A meta-analysis comparing the safety of hand-sewn sutures versus staple closure of the pancreatic remnant after pancreatic left resection did not show a significant difference with regard to morbidity, including postoperative fistula (Knaebel et al, 2005). Results of a European multicenter randomized trial comparing the two techniques over the last several years will be available shortly (Diener et al, 2008a).
Splenic Preservation
Conventionally, the spleen has been removed en bloc with distal pancreatectomy. This was believed to be necessary because of the close relationship of the splenic artery and vein to the body of the pancreas; splenic preservation might compromise the oncologic resection (Shoup et al, 2002). Furthermore, this was thought to be technically simpler. Splenic preservation can be performed either with the division of the splenic artery and vein or with preservation of the entire length of both vessels. The paramount prerequisite in the former surgical approach is preservation of the gastrosplenic vessels to ensure splenic perfusion and allow venous drainage. In the latter technique, the splenic artery and vein must be uninvolved by tumor. Because of the important immune function of the spleen, it is not surprising that the incidence of infectious complications that require intervention is significantly higher in the splenectomy group (Shoup et al, 2002). More importantly, Schwarz and colleagues (1999) found that in their patients undergoing resection of pancreatic adenocarcinoma with curative intent, the median actuarial survival was 12.2 months with splenectomy versus 17.8 months without splenectomy. Such an adverse effect of splenectomy on survival was also evident when it accompanied surgical resection for gastric and colon cancer. Based on the available evidence, we concur with Conlon and colleagues (2001) in concluding that splenic preservation should be attempted in patients with low-grade malignancies or other more indolent tumors of the pancreatic body/tail, but we feel that splenic preservation may compromise oncologic clearance if performed for pancreatic adenocarcinoma.
Our preference is to attempt to preserve the splenic artery and splenic vein to reduce the risk of splenic necrosis and abscess formation; because splenic perfusion based on the short gastric vessels alone has been shown to be potentially inadequate (Gurleyik et al, 2000). Following division of the pancreas, the distal stump is elevated and rotated gently to the left. Multiple small, short branches of the splenic artery and vein are then identified and clipped serially with hemostatic clips and cut. The dissection proceeds from the proximal to distal pancreas, until the tail of the pancreas can be separated from the splenic hilum (Fig. 62A.18). This separation of the splenic vein from the pancreas can only proceed in the direction of the spleen because the vein has already divided into small vessels that can easily be injured at the level of the hilum. On the other hand, isolation of the pancreas from the splenic artery is described to be easier when performed from the hilum toward the pancreatic head (Jovine et al, 2004). This is also a less tedious task because there are fewer branches from the splenic artery, and they are all on one side. Any vessel injuries can be repaired by suturing with monofilament 5/0 vascular sutures. If attempts at hemostasis of such vascular injuries are unsuccessful, we divide the splenic pedicle. We avoid mobilizing the spleen medially into the operative field, as this step is unnecessary and carries the risk of iatrogenic splenic injury.
Distal Pancreatectomy with Splenectomy
We routinely perform lymphadenectomy when the indication for the operation is a malignant tumor of the body or tail. This involves excision of the nodal tissues along the common hepatic artery, left gastric artery, celiac axis, and along the SMV, as well as the peripancreatic and retroperitoneal lymph nodes and the left adrenal gland (Kleeff et al, 2007). A similar approach called the radical antegrade modular pancreatosplenectomy (RAMPS) allows a more comprehensive lymphadenectomy of the N1 nodes and reduces the risk of positive posterior resection margins (Strasberg et al, 2003).
Segmental Pancreatectomy
Letton and Wilson (1959) were the first to describe the technique of segmental pancreatectomy for two cases of traumatic transection of the pancreatic isthmus. Following a central segmental resection, the cephalic stump was oversewn, and a Roux-en-Y jejunal limb was constructed and anastomosed to the distal stump. Pancreatic surgeons were quick to recognize the versatility of this procedure and have since adapted it to the treatment of benign or borderline lesions of the pancreas situated in the neck. Benign or low-malignancy tumors arising from this region present a unique challenge. Conventionally, if the lesion is small, enucleation would be the optimal strategy. However, if the lesion measures more than 2 cm, or when it is situated deep within the parenchyma such that damage to the pancreatic duct becomes a real risk, the surgeon may have to resort to either an extended distal pancreatectomy or PD simply to remove the lesion. Such operative strategies for a small lesion come at the cost of a significant waste of normal tissue, not to mention the inherent risk of morbidity and mortality that accompanies such extended pancreatectomies.
Segmental pancreatectomy represents an organ-preserving extirpation technique that offers the advantages of avoiding the morbidity and mortality associated with PD; preserving splenic function by avoiding splenectomy, which often accompanies distal pancreatectomy; and preserving maximal pancreatic endocrine and exocrine function (Efron et al, 2004). Perhaps the most beneficial attraction seems to be the excellent preservation of endocrine function. This is not surprising because the risk of diabetes is proportional to the extent of resection and length of follow-up observation (Warshaw et al, 1998). On the other hand, the prime concern was whether pancreatic fistula rates would be significantly higher with two possible sources of pancreatic leakage. In the literature, the frequency of pancreatic leak in segmental pancreatectomy ranged from 14% to 30%, whereas in distal pancreatectomy, it ranged from 5% to 15%. These findings notwithstanding, in almost all reports patients do well with conservative management.
Iacono and colleagues (1998) succinctly summarized the prerequisites that allow segmental pancreatectomy to be considered as a reasonable approach: small lesions (<5 cm in diameter) (1) that are benign or low-grade malignant tumors (2) located in the neck or its contiguous portion (3), and a distal pancreas stump of at least 5 cm in length (4). A critical adjunct is the availability of frozen-section examination to confirm that the lesion is benign and to verify a free resection margin (Müller et al, 2006).
Technique
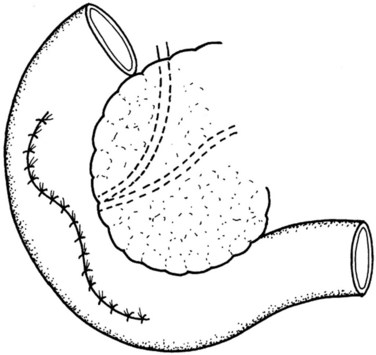
The lesser sac is entered, and the anterior aspect of the pancreas is widely exposed by dividing the adhesions between the posterior surface of the stomach and the pancreas. This is followed by intraoperative pancreatic ultrasonography to better delineate the lesion and to exclude concomitant lesions that may necessitate a change of plan. The pancreas is then elevated off the SMV-PV trunk. Stay sutures are placed in the superior and inferior pancreatic margins to indicate the proximal and distal limits of division and to aid in the subsequent dissection of the pancreas from the splenic vein. The pancreas is then divided proximally with a vascular stapler at least 1 cm from the lesion. The distal stump is then gently retracted toward the left to allow the cautious and tedious process of freeing the splenic vein from the posterior surface, clipping and cutting all the fine venous tributaries lying between the splenic vein and the pancreas. The pancreas is then divided distal to the lesion with a blade (Fig. 62A.19), and a specimen is sent for analysis and margin assessment.
Our usual technique of reconstruction follows that described in most other series and consists of the closure of the proximal stump and a Roux-en-Y PJ for the distal stump as described. The duct in the proximal pancreatic stump is closed by PDS single stiches, and the resection margin is covered by a serosal patch by apposing the serosa of the jejunal limb to the stump and taking seromuscular bites of the jejunum and the pancreatic capsule (Figs. 62A.20 to 62A.24).
A jejunal Roux limb is created by dividing the proximal jejunum 15 to 20 cm past the ligament of Treitz. This is then brought to the supracolic compartment in a retrocolic fashion, through a mesenteric window created on the left side of the middle colic vessels. The distal pancreatojejunal anastomosis is constructed first in the same two-layer fashion as previously described (see Management of the Pancreatic Remnant). This is then followed by the proximal anastomosis using the same jejunal Roux limb. The reconstruction is completed by an end-to-side jejunojejunostomy, and drains are placed.
Total Pancreatectomy
In the 1960s and 1970s, dissatisfaction with the early results of PD for cancer of the head of the pancreas led surgeons to look for an alternative operation. Total pancreatectomy (TP) was proposed by some to be a rational choice for several reasons: It can eliminate multifocal disease, achieve wider resection margins, and achieve a more extensive lymphadenectomy. It also avoids complications emanating from the pancreatic remnant. However, recent evidence has shown that multicentric disease is actually uncommon, seen only in perhaps 9% of cases (Karpoff et al, 2001). Furthermore, randomized trials on extended lymphadenectomy have shown that no tangible benefits could be reaped from such procedures, especially because the lymph nodes around the body and tail of the gland are rarely involved with metastatic disease from a cancer of the pancreatic head. Conversely, removal of the entire pancreas is associated with severe metabolic consequences as a result of the loss of pancreatic endocrine and exocrine functions. One of the more serious metabolic aftermaths is brittle diabetes and severe recurrent hypoglycemia. Indeed, Yeo and associates (1995) have shown that the median survival for patients who had TP performed for pancreatic head cancer was 10 months, as opposed to 16 months in the PD group. Likewise, researchers at Memorial Sloan-Kettering Cancer Center (MSKCC) have shown that patients with TP had a significantly lower overall survival compared with a contemporary cohort of patients who underwent PD and distal pancreatectomy (Karpoff et al, 2001). The length of stay was markedly prolonged, emphasizing the greater morbidity of this procedure. No apparent effect of positive margins on outcome was shown, and hence it was concluded that a positive margin of resection at the time of PD should not compel the extension of the resection to a TP.
We are of a different opinion, as we had previously shown that a curative resection (R0) is the single most important factor in determining outcome in patients with pancreatic adenocarcinoma (Wagner et al, 2004). Hence, positive resection margins on frozen-section surgical margin analysis remains one of the few indications for us to proceed with a TP. Similarly, longitudinal involvement of the pancreatic duct in cases of intraductal papillary mucinous neoplasm (IPMN) is another reason to cut back until lesion-free margins are obtained; on some occasions, TP becomes necessary (see Chapter 57). Recent evidence has also shown that the presence of atypia or carcinoma in situ at the ductal resection margin was not associated with a poor outcome (D’Angelica et al, 2004), suggesting abandonment of this obsession with clear margins in cases of IPMN. However, our center and others (Gigot et al, 2001) have held that surgical resection should be tailored to the longitudinal spreading into the pancreatic duct as established by routine frozen section of the surgical margins. It will be interesting to follow this debate as more evidence emerges. Another indication for TP is the presence of an atrophic, soft, friable pancreatic remnant that does not hold sutures. Most recent reports demonstrate that morbidity and mortality of TP have decreased dramatically and that the perioperative and oncologic long-term outcome are comparable to standard pancreatic resections (Müller et al, 2007; Reddy et al, 2009).
Besides rendering a patient diabetic, TP also has a nutritional toll as a consequence of the loss of exocrine function, leading to persistent diarrhea and steatorrhea. If TP is accompanied by gastric resection by a standard Whipple procedure, further nutritional compromise will ensue from decreased dietary intake as a result of the loss of gastric reservoir function. It is our center’s policy to preserve the pylorus whenever we perform a TP, unless tumor infiltrates into the proximal duodenum or suspicious peripyloric nodes are present that preclude an R0 resection if left in situ. This philosophy is the result of a study comparing patients who had a pylorus-preserving TP with those who had a classic TP in which a distal gastrectomy was performed. Patients in the first group showed a significantly faster rate of recovery of body weight compared with those in the second group, and no difference in survival was reported between these two groups (Wagner et al, 2001). Sugiyama and Atomi (2000) also demonstrated improved recovery of body weight and serum albumin at 6 months after surgery following pylorus-preserving TP, with a low incidence of persistent diarrhea and lower hyperalimentation requirements. Not surprisingly, the postoperative QOL was somewhat better in the pylorus-preserving group than in those who had a standard TP (Sugiyama & Atomi, 2000).
Technique
In cases of main duct IPMN, an en bloc total pancreatectomy without transaction of the pancreas may be necessary. In this case, the mobilization of the pancreatic head and the transection of the jejunum and duodenum are performed first. The pancreatic tail is mobilized together with the spleen, and the dissection of the pancreatic mesentery along the PV and the SMA is the last step of resection (Werner & Büchler, 2010).
For more low-grade tumors, such as IPMNs, we would attempt to preserve the spleen. In this case, we would preserve all the short gastric vessels in the event that the splenic vessels need to be divided. This is followed by the tedious process of ligating or clipping and cutting the multiple branches that connect the splenic vein and the splenic artery to the pancreas in an attempt to preserve both splenic artery and vein. Reconstruction after TP consists of an interrupted end-to-side, single-layer hepaticojejunal anastomosis with a retrocolic jejunal limb. An antecolic end-to-side duodenojejunostomy is then constructed about 50 to 60 cm downstream from the bilioenteric anastomosis (Kulu et al, 2009).
Transduodenal Resection of the Papilla of Vater
All ampullary tumors should be resected. Such a sweeping statement governing the strategy of managing such neoplasia stems from several observations. The most common benign tumors of the ampulla of Vater are villous and tubulovillous adenomas, and although they are classified as benign, they are truly premalignant neoplasms whose malignant transformation can only be confidently prevented by their complete removal. These tumors are also highly deceptive; consequently, distinguishing adenoma from carcinoma becomes an exceedingly difficult problem. Adenomas are known to harbor occult foci of carcinoma, with a reported incidence of coexistent carcinoma in duodenal or ampullary tumors ranging from 35% to 60% (Martin & Haber, 2003). Furthermore, preoperative endoscopic diagnosis is notoriously unreliable. False-negative results from endoscopic biopsies have been described in 25% to 60% of patients with carcinoma (Posner et al, 2000); thus complete excision is the only sure way of establishing whether an ampullary adenoma harbors undetected foci of carcinoma. Finally, among the various types of periampullary cancers, overall 5-year survival is greatest for ampullary cancers and duodenal cancers (Sarmiento et al, 2001). A recent series from de Castro and associates (2004) reported that overall 5-year actuarial survival of patients with adenocarcinoma after PD was 37%, which is considerably superior to that of patients with pancreatic adenocarcinoma. Another recent series echoed this finding; the authors found that survival after resection of ampullary cancer was far better than that after resection of other periampullary cancers, with a 5-year survival rate of 36% as opposed to 14% in the other group (Bettschart et al, 2004). Furthermore, the resectability rate of ampullary carcinoma is higher than the other periampullary tumors, with a 92% resection rate reported in this series. Adopting an aggressive approach toward ampullary tumors therefore appears justifiably warranted.
Although it is generally agreed that PD is the operation of choice for ampullary adenocarcinoma, the ideal treatment for benign neoplasms, with or without dysplasia, and lesions with microinvasion remains controversial. Some have argued that a radical resection should also be applied to benign lesions, citing a high incidence of malignancy in ampullary tumors and an increased tendency for these lesions to recur after local excision. Recurrence of villous tumors after transduodenal excision (TDE) is reportedly 32% at 5 years and 43% at 10 years; more importantly, it may recur as invasive cancer (Farnell et al, 2001). An added bonus is that PD obviates the need for endoscopic surveillance during the postoperative follow-up in cases of sporadic adenomas. Detractors, however, believe that TDE is an acceptable and adequate form of treatment with markedly decreased morbidity and mortality rates compared with radical resection. Posner and colleagues (2000) have shown that TDE of benign ampullary tumors, even those with varying degrees of dysplasia, can have low recurrence rates. Beger and colleagues (1999) also observed that TDE of lesions with only submucosal invasion does not compromise long-term survival. The transduodenal approach is also associated with a significantly shorter operation time, a significantly lower incidence of surgery-related morbidity and, consequently, a significantly shorter postoperative hospital stay (de Castro et al, 2004). Even after recurrence, most benign lesions do not lead to the patient’s demise, and many can be treated endoscopically or with reexcision.
We have established certain indications for which TDE would be a reasonable option (Paramythiotis et al, 2004), such as large (≥2 cm) villous or tubulovillous adenomas of the papilla, adenomas of the papilla with high-grade dysplasia, and adenomas with carcinoma in situ. Beger and colleagues (1999) have also offered TDE for pT1N0M0/G1 and G2 cancer of the mucosa with the caveat of additional lymphadenectomy of the anterior and posterior nodal groups of the pancreatic head and the supraduodenal lymph nodes. However, even in pT1 tumors, lymph node involvement has been observed in 6% to 10% of patients (Klein et al, 1996). Hence, we would recommend PD even for superficially invasive cancer of the ampulla, especially with the good safety profile of this procedure in experienced hands. However, in patients who are unfit for PD, we concede that TDE offers a chance for good palliation and even cure.
As with military operations, good planning prior to surgical operations requires good intelligence. We consider the ERCP critical in the preoperative workup. Besides being able to obtain tissues for histologic diagnosis, the endoscopic visualization may give some clues as to the biologic behavior of the lesion. Reliable endoscopic signs that indicate the likelihood of malignant transformation have been described such as induration and rigidity of the papilla on probing, ulceration, and a submucosal mass effect suggestive of tumor extension into the duodenal wall (Martin & Haber, 2003). Although not conclusive, the presence of such signs will certainly raise the index of suspicion. With its ability to clearly delineate the layered anatomy of the duodenal wall, EUS can identify the depth of tumor invasion with an accuracy of 75% to 85% (see Chapter 14; Posner et al, 2000). If a T2 tumor (invasion of duodenal wall) or involvement of contiguous structures is clearly demonstrated on EUS despite a negative biopsy, we would proceed with a radical procedure.
Technique
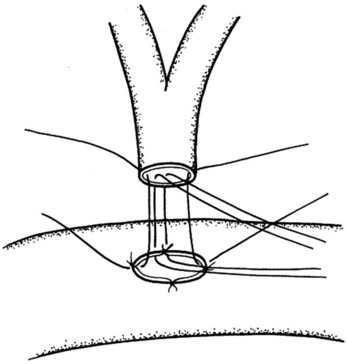
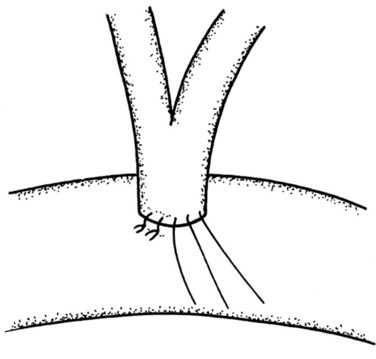
Because the ampulla is absent in nearly two thirds of patients (Sarmiento et al, 2001), it would be more often correct anatomically to use the term TDE of the papilla of Vater. The abdomen is entered through a midline incision in case a conversion to a more radical procedure is required. A generous Kocher maneuver is then performed to completely mobilize the second part of the duodenum, and three to four stay sutures are placed alongside the antimesenteric border on its anterior aspect to help elevate the duodenum toward the abdominal wound to facilitate the duodenotomy. A 6- to 8-cm long longitudinal duodenotomy is then made in a lazy-S fashion along the antimesenteric border opposite the pancreas; the incision is kept open with stay sutures (Fig. 62A.25). Circumferential stay sutures, using monofilament absorbable PDS (5/0), are placed about 1 cm beyond the gross border of the lesion and are kept long. Excision of the lesion is undertaken with electrocautery to minimize bleeding, along a resection margin just inside the circle formed by the circumferential stay sutures. A heavy stay suture is placed through the tumor mass to allow the tumor and the papillary region to be drawn upward. The dissection is carried around the tumor area in a stepwise fashion, and the incision must allow a full-thickness excision of the tumor that includes the biliopancreatic ductal junction. Ductal resection can be safely performed up to 1 cm (Nikfarjam et al, 2001); this is to safeguard against any possible tumor infiltrations of the bile and pancreatic ducts. As each duct is identified, stay sutures are placed through the ductal wall following complete transection (Fig. 62A.26). Following complete excision, the specimen is carefully oriented for the pathologist to facilitate frozen-section examination. If clear margins are reported, we would proceed with the reconstruction, which begins with the suturing together of the pancreatic and common bile ducts in a common septum (Fig. 62A.27). This is followed by the reimplantation of this new common channel back into the duodenal wall with 5/0 monofilament absorbable sutures to approximate the duodenal wall to the joined pancreatic and bile duct openings (Fig. 62A.28). Patency of the ducts is ensured before closure of the duodenotomy. The duodenal opening is then closed in two layers (Fig. 62A.29), and drains are routinely placed in the vicinity of the duodenotomy.
Postoperative Management
Following operation, we routinely monitor our patients in the intensive care unit or the high-dependency unit for the first 24 hours. The NG tube is usually removed at extubation or, at the latest, within the first 12 hours after extubation, when the patient has regained a reasonable level of consciousness and has an intact cough reflex. Intravenous antiemetics are given prophylactically. The abdominal drains are usually removed within the first 3 days after operation, based on the volume and the amylase/lipase content (Bassi et al, 2005; Molinari et al, 2007). However, if the volume is excessive (>700 mL/day), the drains should be kept in place. If the fluid amylase level is three times the serum amylase level or higher, the drains are kept in and the patient is treated as for a pancreatic leak.
On the question of the ideal route of administration, investigators at MSKCC conducted a prospective randomized trial of early enteral feeding (EEN) with an immune-enhancing formula in patients with upper GI malignancies. They found that EEN via a jejunostomy tube did not provide any benefit over postoperative crystalloid support in well-nourished patients (Heslin et al, 1997). A more recent publication echoed similar findings. Braga and colleagues (2001) observed that EEN did not improve outcome compared with total parenteral nutrition (TPN) in the overall population; however, they did find that in a subgroup of malnourished patients, EEN was associated with a significantly shorter length of stay. This was probably secondary to a lower overall complication rate, particularly the rate of infectious complications—noted in the EEN group compared with the TPN group. The prevalence of glucose and electrolyte disturbances was also lower during EEN than during TPN. In addition, researchers were able to demonstrate better gut oxygenation with EEN, not to mention significant cost savings compared with TPN. This makes EEN the ideal method for nutritional support, albeit in a selective manner.
EEN is not without risks, however. We previously demonstrated a significantly higher incidence of DGE in the enteral nutrition (EN) group compared to the no-EN group (Welsch et al, 2010). Furthermore, the use of a feeding jejunostomy tube is itself subject to the risk of adverse events, including tube dislocation, blockage, and leakage. Looking at the risk/benefit ratio, we agree with Braga and colleagues that the use of EEN should be targeted selectively at the group of patients with malnourishment, defined as the involuntary weight loss of more than 10% of the patient’s usual body weight in the preceding 6 months. In general, 25 to 30 kcal/kg/day and 1.2 to 1.5 g/kg/day of protein are reasonable goals in these patients (Sampliner, 2001). TPN is used when nutritional goals cannot be met with enteral feedings because of GI dysfunction.
On the other hand, positive effects have been demonstrated by mere institution of early oral intake. In the group randomized to clear liquids ad lib after surgery, followed by a soft diet for patients who can tolerate 500 mL of liquid. Steed and colleagues (2002) showed that length of stay was significantly reduced, but the rates of ileus, emesis, and morbidity remained identical with the control group.
Following TP, pancreatic enzyme substitutes are also be prescribed to this group of patients to treat their expected exocrine insufficiency. Although many patients complain of some form of diarrhea, this symptom resolves rapidly with high-dose enzyme substitution (Müller et al, 2007; Wagner et al, 2001).
Adler G, et al. S3 guidelines: exocrine pancreatic cancer. Z Gastroenterol. 2007;45(6):487-523.
American Society for Gastrointestinal Endoscopy. Complications of ERCP. Gastrointest Endosc. 2003;57:633-638.
Bachellier P, et al. Is pancreaticoduodenectomy with mesentericoportal venous resection safe and worthwhile? Am J Surg. 2001;182:120-129.
Basse L, et al. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg. 2002;89:446-453.
Bassi C, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8-13.
Beger HG, et al. Tumour of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526-532.
Bell RHJr. Save the pylori. Ann Surg. 2004;240:746-747.
Benoist S, et al. Is there a role of preservation of the spleen in distal pancreatectomy? J Am Coll Surg. 1999;188:255-260.
Bettschart V, et al. Presentation, treatment and outcome in patients with ampullary tumours. Br J Surg. 2004;91(12):1600-1607.
Birkmeyer JD, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117-2127.
Braga M, et al. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001;29:242-248.
Büchler MW, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992;163:125-130.
Büchler MW, et al. Changes in mortality after pancreatic resection: towards the end of completion pancreatectomy. Arch Surg. 2003;138:1310-1314.
Cameron JL, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10-15.
Capussotti L, et al. Extended lymphadenectomy and vein resection for pancreatic head cancer: outcomes and implications for therapy. Arch Surg. 2003;138:1316-1322.
Carrere N, et al. Pancreaticoduodenectomy with mesentericoportal vein resection for adenocarcinoma of the pancreatic head. World J Surg. 2006;30:1526-1535.
Cheatham ML, et al. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995;221:469-478.
Ciocirlan M, Ponchon T. Diagnostic ERCP. Endoscopy. 2004;36:137-146.
Cohen S, et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy. Gastrointest Endosc. 2002;56:803-809.
Conlon KC, Klimstra DS, Brennan MF. Long term survival after curative resection for pancreatic ductal adenocarcinoma: cliniopathologic analysis of five year survivals. Ann Surg. 1996;223:273-279.
Conlon KC, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487-494.
Crile GJ. The advantages of bypass operations over radical pancreaticoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130:1049-1053.
Cubilla AL, Fortner J, Fitzgerald PJ. Lymph node involvement in carcinoma of the pancreas area. Cancer. 1978;41:880.
D’Angelica M, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinipathologic features and outcome. Ann Surg. 2004;239:400-408.
Davies SJ, Wilson RJT. Preoperative optimization of the high-risk surgical patient. Br J Anaesth. 2004;93:121-128.
de Castro SMM, et al. Surgical management of neoplasm of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004;136:994-1002.
de Lalla F. Surgical prophylaxis in practice. J Hosp Infect. 2002;50(Suppl A):S9-S12.
Diener MK, et al. DISPACT trial: a randomized controlled trial to compare two different surgical techniques of DIStal PAnCreaTectomy—study rationale and design. Clin Trials. 2008;5(5):534-545.
Diener MK, et al. Pancreaticoduodenectomy (classic Whipple) versus pylorus-preserving pancreaticoduodenectomy (PP Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2, 2008. CD006053
Efron DT, et al. Central pancreatectomy with pancreaticogastrostomy for benign pancreatic pathology. J Gastrointest Surg. 2004;8:532-538.
Esposito I, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15(6):1651-1660.
Farnell MB, Nagorney DM, Sarr MG. The Mayo clinic approach to the surgical treatment of adenocarcinoma of the pancreas. Surg Clin North Am. 2001;81(3):611-623.
Farnell MB, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618-628.
Ferrone CR, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg. 2008;12(10):1691-1697.
Fong Y, et al. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242(4):540-544.
Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery. 1973;73:307-320.
Fotiadis RJ, et al. Epidural analgesia in gastrointestinal surgery. Br J Surg. 2004;91:828-841.
Freeny PC. Pancreatic carcinoma: what is the best imaging test? Pancreatology. 2001;1:604-609.
Friess H, et al. Randomised controlled multicentre study of the prevention of complications by octreotide for patients undergoing surgery for chronic pancreatitis. Br J Surg. 1995;82:1270-1273.
Gazzaniga GM, et al. D1 versus D2 pancreatoduodenectomy in surgical therapy of pancreatic head cancer. Hepatogastroenterology. 2001;48:1471-1478.
Gigot JF, et al. Surgical management of intraductal papillary mucinous tumours of the pancreas. Arch Surg. 2001;136:1256-1262.
Gillen S, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 7(4), 2010. e1000267
Gress F, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459-464.
Gudjonsson B. Carcinoma of the pancreas: critical analysis of costs, results of resections, and the need for standardized reporting. J Am Coll Surg. 1995;181:483-503.
Gurleyik E, et al. Perfusion and functional anatomy of the splenic remnant supplied by short gastric vessels. Am J Surg. 2000;179:490-493.
Hackert T, et al. Uncinate process first: a novel approach for pancreatic head resection. Langenbecks Arch Surg. 2010;395(8):1161-1164.
Haller DG. Future directions in the treatment of pancreatic cancer. Semin Oncol. 2002;29(Suppl 20):31-39.
Hartwig W, et al. Multivisceral resection for pancreatic malignancies: risk-analysis and long-term outcome. Ann Surg. 2009;250(1):81-87.
Hartwig W, et al. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96(1):5-20.
Henne-Bruns D, et al. Ductal adenocarcinoma of the pancreas head: survival after regional versus extended lymphadenectomy. Hepatogastroenterology. 1998;45:855-866.
Heslin MJ, et al. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg. 1997;226:567-580.
Hirata K, et al. Results of 1001 pancreatic resections for invasive ductal adenocarcinoma of the pancreas. Arch Surg. 1997;132:771-776.
Horstmann O, et al. Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas. 2004;28:69-74.
Iacono C, et al. Results of pancreaticoduodenectomy for pancreatic cancer: extended versus standard procedure. World J Surg. 2002;26(11):1309-1314.
Iacono C, Bortolasi L, Serio G. Is there a place for central pancreatectomy in pancreatic surgery? J Gastroinest Surg. 1998;2:509-517.
Imamura M, et al. A randomized multicenter trial comparing resection and radiochemotherapy for resectable locally invasive pancreatic cancer. Surgery. 2004;136:1003-1011.
Ishikawa O. What constitutes curative pancreatectomy for adenocarcinoma of the pancreas? Hepatogastroenterology. 1993;40(5):414-417.
Ishikawa O, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg. 1988;208:215-220.
Jovine E, et al. Spleen-preserving total pancreatectomy with conservation of the spleen vessels: operative technique and possible indications. Pancreas. 2004;28:207-210.
Kalra MK, et al. Correlation of positron emission tomography and CT in evaluating pancreatic tumours: technical and clinical implications. AJR Am J Roentgenol. 2003;181:387-393.
Karpoff HM, et al. Results of total pancreatectomy for adenocarcinoma of the pancreas. Arch Surg. 2001;136:44-47.
Katz MH, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833-846.
Kawarada Y, Isaji S. Stage classifications of pancreatic cancer: comparison of the Japanese and UICC classifications and proposal for a new staging system. Union Internationale Contre le Cancer. Pancreas. 1998;16(3):255-264.
Kawarada Y, et al. Modified standard pancreaticoduodenectomy for the treatment of pancreatic head cancer. Digestion. 1990;60(Suppl 1):120-125.
Klauss M, et al. A new invasion score for determining the resectability of pancreatic carcinomas with contrast-enhanced multidetector computed tomography. Pancreatology. 2008;8(2):204-210.
Kleeff J, et al. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245(4):573-582.
Klein P, et al. Is local excison of pT1 ampullary carcinomas justified? Eur J Surg Oncol. 1996;22:366-371.
Knaebel HP, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92(5):539.
Kulu Y, et al. Total pancreatectomy for pancreatic cancer: indications and operative technique. HPB (Oxford). 2009;11(6):469-475.
Lankisch PG, et al. Epidemiology of pancreatic diseases in Luneburg County: a study in a defined German population. Pancreatology. 2002;2:469-477.
Leach S, et al. Survival following pancreaticoduodenectomy with resection of the superior mesenteric–portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg. 1998;85:611-617.
Letton AH, Wilson JP. Traumatic severance of pancreas treated by Roux-Y anastomosis. Surg Gynecol Obstet. 1959;109:473-478.
Lewis SJ, et al. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ. 2001;323(7316):773-776.
Li B, et al. Pancreaticoduodenectomy with vascular reconstruction in treating carcinoma of the pancreatic head. Hepatobiliary Pancreat Dis Int. 2004;3:612-615.
Li-Ling J, Irving M. Somatostatin and octreotide in the prevention of postoperative pancreatic complications and the treatment of enterocutaneous pancreatic fistulas: a systematic review of randomized controlled trials. Br J Surg. 2001;88:190-199.
Manabe T, et al. Radical pancreatectomy for ductal cell carcinoma of the head of pancreas. Cancer. 1989;64:1132-1137.
Martignoni ME, et al. Effect of preoperative biliary drainage on surgical outcome after pancreaticoduodenectomy. Am J Surg. 2001;181:52-59.
Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endoscopy Clin North Am. 2003;13:649-669.
Menges M, Lerch MM, Zeitz M. The double duct sign in patients with malignant and benign pancreatic lesions. Gastrointest Endosc. 2000;52:74-77.
Mezhir JJ, et al. A matched case-control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J Gastrointest Surg. 2009;13:2163-2169.
Michalski CW, et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94(3):265-273.
Mismetti P, et al. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913-930.
Molinari E, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007;246(2):281.
Morris RJ, Woodcock JP. Evidence-based compression: prevention of stasis and deep vein thrombosis. Ann Surg. 2004;239:162-171.
Müller MW, et al. Middle segmental pancreatic resection: an option to treat benign pancreatic body lesions. Ann Surg. 2006;244(6):909-918.
Müller MW, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246(6):966-974.
Mukaiya M, et al. Lack of survival benefit of extended lymph node dissection for ductal adenocarcinoma of the head of the pancreas: retrospective multi-institutional analysis in Japan. World J Surg. 1998;22:248-252.
Nagakawa T, et al. The pattern of lymph node involvement in carcinoma of the head of the pancreas: histologic study of the surgical findings in patients undergoing extensive nodal dissections. Int J Pancreatol. 1993;13:15-22.
Nakao A, et al. Indications and techniques of extended resection for pancreatic cancer. World J Surg. 2006;30:976-982.
Neoptolemos JP, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210.
Nikfarjam M, et al. Local resection of ampullary adenocarcinomas of the duodenum. ANZ J Surg. 2001;71:529-537.
Nimura Y, et al. Standard versus extended lymphadenectomy in pancreatoduodenectomy for pancreatic cancer: a multicenter randomized controlled trial. Pancreatology. 2004;4:274.
Oettle H, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: randomized controlled trial. JAMA. 2007;297:267-277.
Paramythiotis D, et al. Still any role for transduodenal local excision in tumours of the papilla of Vater? J Hepatobiliary Pancreat Surg. 2004;11:239-244.
Park YC, et al. Factors influencing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Am Coll Surg. 2003;196:859-865.
Pedrazzoli S, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multi-centre prospective randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508-517.
Pedrazzoli S, et al. A surgical and pathological based classification of resective treatment of pancreatic cancer. Dig Surg. 1999;16:337-345.
Petrowsky H, et al. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240:1074-1085.
Polk HCJr, Christmas AB. Prophylactic antibiotics in surgery and surgical wound infections. Am Surg. 2000;66(2):105-111.
Poon TP, Fan ST. Opinions and commentary on treating pancreatic cancer. Surg Clin North Am. 2001;81:625-636.
Popiela T, Kedra B, Sierzega M. Does extended lymphadenectomy improve survival of pancreatic cancer patients? Acta Chir Belg. 2002;102:78-82.
Posner S, et al. Safety and long-tem efficacy of transduodenal excision for tumours of the ampulla of Vater. Surgery. 2000;128:694-701.
Postier RG. The challenge of pancreatic cancer. Am J Surg. 2003;186:579-582.
Poultsides GA, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470-476.
Reddy S, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term. survival. Ann Surg. 2009;250(2):282-287.
Riall TS, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: update on 5-year survival. J Gastrointest Surg. 2005;9(9):1191-1204.
Riediger H, et al. Delayed gastric emptying after pylorus-preserving pancreatoduodenectomy is strongly related to other postoperative complications. J Gastrointest Surg. 2003;7(6):758-765.
Riediger H, et al. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106-1115.
Sampliner JE. Postoperative care of the pancreatic surgical patient: the role of the intensivist. Surg Clin North Am. 2001;81:637-645.
Sarmiento JM, et al. Periampullary cancers: are there differences? Surg Clin North Am. 2001;81:543-555.
Sasson A, et al. En bloc resection for locally advanced cancer of the pancreas: is it worthwhile? J Gastrointest Surg. 2002;6:147-158.
Schäfer M, Müllhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137-148.
Schwarz RE, et al. The impact of splenectomy on outcomes after resection of pancreatic adenocarcinoma. J Am Coll Surg. 1999;188:516-521.
Sewnath ME, et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumours causing obstructive jaundice. Ann Surg. 2002;236:17-27.
Sganga G. New perspectives in antibiotic prophylaxis for intra-abdominal surgery. J Hosp Infect. 2002;50(Suppl A):S17-S21.
Shoup M, et al. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164-168.
Siriwardena H, Siriwardena A. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93:662-673.
Skandalakis JE, et al. Hepatic surgical anatomy. Surg Clin North Am. 2004;84:413-435.
Spanknebel K, Conlon KC. Advances in the surgical management of pancreatic cancer. Cancer J. 2001;7:312-323.
Steed HL, et al. A randomized controlled trial of early versus “traditional” postoperative oral intake after major abdominal gynecologic surgery. Am J Obstet Gynecol. 2002;186:861-865.
Stojadinovic A, et al. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. J Am Coll Surg. 2003;196:954-964.
Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003;133:521-527.
Sugiyama M, Atomi Y. Pylorus-preserving total pancreatectomy for pancreatic cancer. World J Surg. 2000;24:66-71.
Tamm EP, et al. Diagnosis, staging, and surveillance of pancreatic cancer. AJR Am J Roentgenol. 2003;180:1311-1323.
Tran K, et al. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg. 2002;236:422-428.
Traverso LW, Longmire WP. Preservation of the pylorus in pancreatico-duodenectomy. Surg Gynecol Obstet. 1978;146:959.
Trede M, Richter A, Wendl K. Personal observations, opinions, and approaches to cancer of the pancreas and periampullary area. Surg Clin North Am. 2001;81:595-610.
van der Gaag NA, et al. Preoperative biliary drtainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137.
van Heerden JA, et al. Radical pancreatoduodenectomy: a procedure to be abandoned? Mayo Clin Proc. 1981;56:601.
Varadhachary G, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Arch Surg Oncol. 2006;13:1035-1046.
Wagner M, et al. Pylorus-preserving total pancreatectomy: early and late results. Dig Surg. 2001;18:188-195.
Wagner M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586-594.
Warshaw AL, et al. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg. 1998;133:327-333.
Watson K. Carcinoma of the ampulla of Vater: successful radical excision. Br J Surg. 1944;31:368.
Weitz J, et al. The “artery first” approach for resection of pancreatic head cancer. J Am Coll Surg. 2010;210(2):e1-e4.
Welsch T, et al. Evaluation of the International Study Group of Pancreatic Surgery definition of delayed gastric emptying after pancreatoduodenectomy in a high-volume. centre. Br J Surg. 2010;97(7):1043-1050.
Werner J, Büchler MW, 2010: Total pancreatectomy. In Audisio RA: Atlas of Procedures in Surgical Oncology with Critical, Evidence-Based Commentary Notes. Singapore, World Scientific Publishing, pp 125-131.
White RR, Shah AS, Tyler DS. Pancreatic cancer since Halsted: how far have we come and where are we going? Ann Surg. 2003;238(6S):S123-S144.
Wiersema MJ. Identifying contraindications to resection in patients with pancreatic carcinoma: the role of endoscopic ultrasound. Can J Gastroenterol. 2002;16:109-111.
Yeo CJ, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221:721-731.
Yeo CJ, et al. Pancreaticoduodenectomy with or without extended distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2. Ann Surg. 2002;236:355-368.
Zenilman ME. Use of pancreaticogastrostomy for pancreatic reconstruction after pancreaticoduodenectomy. J Clin Gastroenterol. 2000;31:11-18.
Z’graggen K, et al. How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg. 2002;9:733-737.