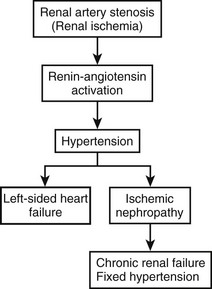
Renovascular Hypertension
In 1934, Goldblatt and coworkers described the association between coarctation of the aorta and renal artery stenosis with hypertension.1 However, it was not until the 1960s that activation of the renin–angiotensin system, which leads to the release of renin and the production of angiotensin II, was found to be the mechanism for the hypertension (Fig. 63-1). Subsequently, it has been shown that diminished renal perfusion pressure has direct effects on sodium excretion, sympathetic nerve activity, nitric oxide production, and intrarenal prostaglandin concentrations, resulting in renovascular hypertension.2 Other studies have demonstrated different fractional angiotensin elevation patterns in children with renovascular hypertension as compared with essential hypertension.3
The incidence of hypertension in children in all age groups is 2–10%, with the higher figure representative of adolescence. However, because blood pressure is not routinely measured in infants and younger children, the diagnosis of hypertension is often delayed. Table 63-1 lists the common causes of hypertension in children according to the organ system involved. This chapter will focus on renovascular causes of hypertension, most of which are correctable.
TABLE 63-1
Causes of Hypertension in Children
| Essential Hypertension | Causes |
| Renal | Glomerulonephritis, pyelonephritis, renal hypoplasia, polycystic kidney, Wilms tumor, neuroblastoma, arteritis, aneurysms, trauma |
| Cardiovascular | Aortic coarctation, Takayasu arteritis, neurofibromatosis, tuberous sclerosis, renovascular stenosis, collagen vascular disease |
| Central nervous system | Encephalitis, intracranial mass with increased pressure, dysautonomia |
| Endocrine | Pheochromocytoma, aldosteronoma, adrenogenital syndrome, Cushing syndrome |
| Secondary hypertension | Lead and mercury poisoning, glucocorticoid drugs, oral contraceptives |
Essential hypertension is the most common cause (60%) of hypertension between birth and 20 years of age. However, the incidence of correctable hypertension is very high in patients younger than 15 years. In previous studies by our group, the incidence of correctable hypertension in the birth to 5-year age group was around 80%; in the 6- to 10-year age group, 45%; and in the 11- to 15-year and 16- to 20-year age groups, 20%.4 Although a variety of causes were identified, the vast majority were renovascular. Renovascular disease comprises 8–10% of all forms of hypertension in children, while it is 1% of all forms in adults. Additionally, long-term follow-up of patients with renovascular hypertension clearly indicates that patients who have had successful repair of their lesions have sustained results and longer survival than those who do not. Moreover, the younger the patient, the better the result.5 Such studies support an aggressive approach to correction of identified renovascular lesions in young patients.
Etiology
Renovascular hypertension may be congenital or acquired. Congenital causes include arterial hypoplasia or aplasia; neurofibromatosis and tuberous sclerosis tumors involving the renal artery; and Williams syndrome, which includes manifestations of supravalvular aortic stenosis, peripheral vascular stenosis particularly in the subclavian and renal arteries, mid-aortic narrowing, hypercalcemia, and elfin facies.6 The most common acquired forms of renovascular hypertension are fibromuscular dysplasia (FMD), which involves one or both renal arteries, or a more generalized type of disease, such as Takayasu arteritis and subisthmic abdominal coarctation, now referred to as the mid-aortic syndrome.7,8 Stenoses of visceral arteries such as the superior mesenteric and celiac arteries are also common in mid-aortic syndrome.7 Other less common forms of acquired renovascular hypertension are renal artery trauma or thrombosis, thrombosis secondary to antithrombin deficiency, Kawasaki disease, and an anastomotic stenosis in renal transplants.9
Overall, the vast majority of children with renovascular hypertension have FMD. Presentation of newborns with aortic and arterial aplasia and hypoplasia suggest a congenital origin. In young adults, FMD appears to have an acquired pattern and may have a genetic basis in the syndromes mentioned above.6 The majority of patients initially present at several years of age, usually with an active inflammatory phase followed by a quiescent phase of arteritis.4,7 Although it was initially thought that FMD might be an autoimmune disease, relatively recent evidence suggests that T-cell-based immune mechanisms, macrophages, and antigen-presenting cells are mainly responsible for renovascular arteritis, and a variety of other arteritides as well.10 The histology of the vascular lesion seen in the renal artery and aorta reveals medial and perimedial fibroplasia, which has inherent implications about approaches to treatment, particularly angioplasty. Although FMD is a systemic, occlusive arteriopathy that may involve the entire abdominal aorta and its branches, the renal arteries are the predominant vessels involved.11
Clinical Presentation
Children with renovascular hypertension generally come to attention in one of two ways. Approximately 70% of patients are asymptomatic and are identified when they have their blood pressure taken during a routine evaluation. It is usually not known how long the hypertension has been present. Repeated measurements verify the chronic nature of this problem, which leads to diagnostic evaluation. Approximately one-half of these asymptomatic patients will be found to have correctable hypertension. The remaining 30% of children are symptomatic with headaches, vision problems, encephalopathy, congestive heart failure, oliguric renal failure, and, occasionally, leg claudication. Physical findings may indicate the presence of heart failure with enlargement of the liver and heart, as well as retinopathy and retinal hemorrhage. In cases of mid-aortic syndrome, peripheral pulses and blood pressure may be diminished in the lower extremities. Although an abdominal bruit could probably be heard in the majority of instances of renal artery stenosis, it is not commonly found until a diagnosis is suspected.
In contrast to a young female predominance in adults with FMD, gender incidence is equal in children. Both renal arteries are involved in approximately 70% of patients. In occasional patients with unilateral lesions, FMD may develop in the opposite renal artery years later. Renovascular causes of hypertension are more common in children younger than 10 years, as evidenced by an average age of 7 years in one of our studies.5 In infants and toddlers, malignant forms of hypertension with encephalopathy and retinopathy are more likely to develop than in older children.
Diagnosis
Imaging
Duplex color-coded Doppler ultrasonography (US) is capable of demonstrating the renal arteries, as well as measuring flow velocity as an index of the degree of stenosis. However, Doppler ultrasound studies, whether performed preoperatively or postoperatively for follow-up, have limitations in terms of demonstrating precise anatomic detail, particularly in small vessels. The same is true of captopril scintigraphy and contrast-enhanced ultrasound. Magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) are capable of demonstrating the renal arteries and the aorta and its branches better than Doppler ultrasound, but there are resolution issues in small subjects.12,13 MRA is less accurate than CTA, and the volume of contrast material for CTA is often the same as with aortography with selective angiography, so no advantage accrues from the standpoint of nephrotoxicity. If one takes the point of view that the prime purpose of an imaging study is to select the most suitable therapeutic method, aortography with selective arteriography is the most definitive study and the one most capable of demonstrating precise anatomic detail (Fig. 63-2). Doppler ultrasound, MRA, and CTA are now considered best as screening or follow-up studies in children.
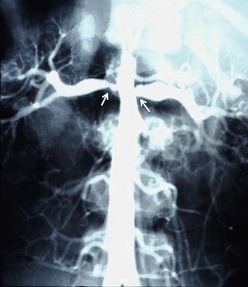
FIGURE 63-2 The aortogram performed on a 6-year-old boy with severe hypertension demonstrates bilateral orificial narrowing of the renal arteries (arrows) with post-stenotic dilatation and some degree of associated aortic narrowing (mid-aortic syndrome). He was treated with aorto-aortic bypass and bilateral renal artery bypasses, and is normotensive 19 years later.
The diagnostic approach to children, who usually have bilateral renal artery stenosis associated with FMD, is simpler than the workup in adults who primarily have atherosclerotic lesions. Consequently, many of the noninvasive tests designed for adults with renovascular hypertension are not useful in children. For example, in an adult study, it was found that the preoperative calculation of a high renal resistance-index value derived by Doppler ultrasound is a predictor of the lack of success of operative correction of renal artery stenosis.13 However, because successful revascularization in children routinely results in alleviation of hypertension, such studies are not relevant. The same is true for renal vein renin studies because renal artery stenosis in children with hypertension is always significant. Conversely, renal vein renin studies may be useful in determining the significance of distal stenoses or aneurysms of renal artery branches, or postoperative anastomotic stenoses.
As FMD is a systemic, occlusive arteriopathy, it is necessary to survey the entire lower thoracic and abdominal aorta to delineate those patients who not only have renal artery stenosis but also the mid-aortic syndrome, visceral artery stenosis, or other forms of renovascular hypertension.7,11,14 Thus, full angiography is needed to delineate all diseased vessels so that an appropriate single-stage operation can be performed. It is interesting to note that hypertensive children who have renal artery stenosis, without co-morbid conditions, usually have a single, focal branch artery lesion.12
Treatment
Medical Treatment
Antihypertensive medications are needed to control blood pressure before undertaking any invasive procedure, including arteriography. Drug therapy is not an alternative to operative correction, but an integral part of patient management, preoperatively, intraoperatively, and postoperatively. Most patients referred for surgical or interventional treatment have severe hypertension with symptoms that are most effectively managed with intravenous drugs. Once the hypertension is controlled, the patient can gradually be transitioned to oral medications including diuretics, calcium-channel blockers, beta and alpha blockers, converting enzyme inhibitors, and angiotension II receptor antagonists. In severe cases, nitroprusside may be required as the initial treatment. Either concurrently or sequentially, intravenous labetalol or nifedipine, or both, are useful drugs. The same three medications are utilized in postoperative management if hypertension is exacerbated as a consequence of temporary ischemia associated with revascularization procedures. For long-term and outpatient treatment, propranolol, atenolol, minoxidil, and oral diuretics in various combinations are often used. Angiotensin-converting enzyme (ACE) inhibitors such as captopril and ramipril are very effective antihypertensive drugs, but they must be used with great caution in patients with renovascular hypertension because of the potential for drug-induced renal ischemia and possible oliguric renal failure.5,15 Thus, if a decision is made to use ACE inhibitor medication, renal function must be monitored closely.
Interventional Procedures
Balloon angioplasty with and without stenting has been the subject of many reports with atherosclerotic disease as well as with FMD.16,17 The results in patients with FMD have been more favorable in instances in which the orifice of the renal artery is not involved. Balloon dilation for orifice lesions has generally resulted in short-term improvement only. Experience reported in children with balloon angioplasty indicates that balloon dilation of orifice lesions is as ineffective as it is in adults. However, it is very successful in providing long-term relief from stenosis of the main renal artery or its branches (Fig 63-3).18–20

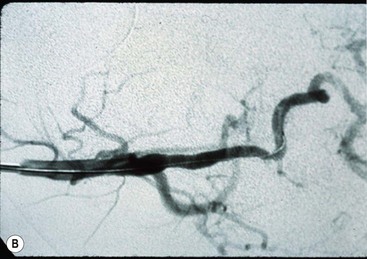
FIGURE 63-3 (A) This selective left renal arteriogram shows typical findings (arrows) of FMD distal to the aortic orifice. (B) The artery is normal-sized following balloon angioplasty of the distal stenoses. Note the catheter in the renal artery.
Nonetheless, there are a number of reports in the interventional radiology literature that promote percutaneous transluminal angioplasty (PTA) for children with all forms of renal artery stenosis, and even describe the placement of stents in very small children. Unfortunately, very few of these reports describe long-term outcomes. Tobin and colleagues have recently proposed cutting balloon angioplasty in children with resistant renal artery stenosis, although that technique may carry a higher complication rate than surgical repair.21 In one long-term follow-up report by Shroff and coworkers, PTA and stenting in a series of 33 children with a mean age of 10 years was associated with a high rate of re-stenosis, one procedure-related death, and a number of complications.17 McTaggart and colleagues reported that PTA was ineffective for patients with neurofibromatosis lesions of the renal artery because of the fibrotic nature of the disease.19 Lacombe and Ricco emphasize that when patients sustain complications of PTA such as dissection, rupture, or thrombosis, operative repair may then be difficult.22 Due to the relatively greater degree of fibroplasia seen in children with FMD, it is not surprising that PTA is associated with a much greater incidence of intimal dissection and thrombosis in children who most often have lesions at the ostia. Due to ongoing growth and young age, there is a reluctance to insert stents into these lesions after balloon dilation as stents may induce intimal hyperplasia or create obstruction as the child grows. However, dissolvable stents may prove useful in the future in some children.
Surgical Treatment
Effective operative management for renovascular hypertension in children is preferable to nonsurgical treatment. The method selected must be based on the etiology and distribution of the lesions causing the stenosis.23,24
Although children with FMD have a predominance of ostial lesions, sufficient involvement of the wall of the artery may exist such that the extent of the lesion is greater than what might be apparent on the arteriogram. Thus, patch angioplasty is only rarely effective. Reimplantation of the renal artery into another site on the aorta is the most effective approach, but its success depends on the aortic wall being normal where the new orifice is to be created and on sufficient renal artery length to reach without tension. Although autotransplantation is an alternative under these circumstances, it is more complicated because both arterial and venous anastomoses are needed. Reimplantation is contraindicated in patients with mid-aortic syndrome in which the aortic wall is involved with FMD. Also, direct anastomosis to the aorta is not an option for patients with branch vessel lesions, which must be treated with balloon dilatation, bypass, or partial nephrectomy for distally located lesions. It is unfortunate that reimplantation is usually not possible in this group of patients because it provides the best and most durable results.5
Due to the complicating factors mentioned previously, aortorenal bypass is usually the best option for revascularization. Bypass from the aorta to the side of the renal artery distal to the stenosis was often used in the past. More recently, division of the diseased artery from the aorta and an end-to-end anastomosis between the bypass graft and the distal renal artery has been preferred. Depending on the size of the anastomosis, either a continuous suture technique which is interrupted several times, or an interrupted suture technique for the anastomosis is appropriate. The anastomosis of the bypass graft to the aorta can usually be performed with a continuous suture that is interrupted at least three times. The opening in the aortic wall is made with an appropriate-sized punch instrument rather than a simple incision. A 6-0 polypropylene suture is commonly used for the aorta-to-graft anastomosis, and 7-0 polypropylene can be used for the distal graft-to-renal artery anastomosis. In instances of mid-aortic syndrome in which such severe coarctation exists that an aorto-aortic bypass is needed, the renal bypass grafts may be taken off the aortic bypass graft. The patient should be heparinized in the standard fashion during aortic clamping.
Some debate exists regarding the best choice of aortorenal bypass grafts. In adults, Gore-Tex (W.L. Gore & Associates, Elkton, MD) grafts are frequently used. However, thrombosis occurs more commonly in prosthetic grafts than with autogenous material. There is little debate that the best choice for bypass in children is autogenous hypogastric artery, provided that it is not involved with FMD and that a sufficient length of artery can be harvested for the bypass. Additionally, because 70% of patients have simultaneous bilateral renal artery stenosis, this would require both hypogastric arteries to be harvested. Certainly some concern exists about taking both hypogastric arteries in children, although the exact risks of impotence and incontinence are not known. Due to these potential complications, the hypogastric artery is not an option for many patients. Therefore, the next best option, and the one most frequently used today for aortorenal bypass in small patients, is the saphenous vein. Because of the risk of aneurysmal dilatation in as many as 40% of patients who have such grafts placed in the visceral location, a procedure to cover the saphenous vein bypass grafts with a loose mandrill of Dacron mesh has been effective over the long term.18,20,25
Another debated issue relates to a subset of patients with mid-aortic syndrome who have varying degrees of narrowing of the superior mesenteric and celiac arteries.7,8,25,26 A few scattered reports of patients with visceral artery stenosis who were initially seen with severe intermittent abdominal pain or intestinal infarction are available. However, in contrast to adults with lesions of this nature, most children are not symptomatic. In children with signs or symptoms related to superior mesenteric or celiac narrowing, a revascularization procedure by either direct reimplantation or bypass grafting can be performed. There is no consensus about whether asymptomatic children with marked visceral artery stenosis should have preemptive repair. Certainly, concomitant visceral artery bypass carries a high risk in a patient who is already having a bilateral renal artery bypass procedure, and possibly a simultaneous aorto-aortic bypass. Generally selective treatment of symptomatic patients with splanchnic artery occlusive disease is favored.7,24,25 As the overwhelming majority of patients with lesions of this nature are asymptomatic and have a remarkable amount of collateral circulation demonstrable on aortography, visceral artery revascularization in asymptomatic patients is not routinely performed (Fig. 63-4). Observation for as long as 30 years has found that these patients have remained well, supporting a conservative approach.5
Complications and Outcomes
A number of long-term follow-up studies of children who have had surgical repair for renovascular hypertension and its variants report a high degree of success and durable results, with cure rates ranging from 66–80%, improvement rates of 18–22%, and rare failures, with no mortality.5,11,20,25 Additionally, no patients developed renal failure, and those who had preoperative oliguric renal failure invariably returned to normal after revascularization. Intraoperative renal thrombosis, embolization during the procedure, and intraoperative and postoperative hemorrhage were not reported. Postoperative graft thrombosis occurs in fewer than 5% of renal repairs. In cases where graft thrombosis is encountered, an immediate reoperation with thrombectomy and anastomotic revision, if necessary, is preferred over the use of thrombolytic agents. Late thrombosis can occur, but the incidence is less in children than in adults because of the absence of atherosclerosis. In patients who have late thrombosis, repeat bypass grafting is preferable, but often partial or total nephrectomy is required. It is important to monitor the patient’s blood pressure indefinitely in follow-up because recurrence of hypertension usually indicates a problem with the vascular reconstruction or recurrent disease. Iliorenal bypass has been used in selected high-risk patients as a remedial operation with good results.27
Postoperative Imaging
Recurrent hypertension after revascularization procedures is due to either recurrent FMD or an anastomotic stenosis. As with renal transplant arterial stenosis, balloon dilatation has been an effective treatment approach. For follow-up, it is best to have the family monitor the child’s blood pressure frequently at home with at least 6-month follow-up visits initially. The patient’s blood pressure should remain normal, even with exercise. Some patients may require medication to keep their blood pressure within the normal range. In the asymptomatic patient, noninvasive imaging such as CTA or MRA is suggested every 5 years, with definitive selective angiography performed if these studies suggest abnormal findings.
Even when complicated, complete revascularization can be performed at a single operation and results are excellent. Whether patients have aortorenal bypass to either one or both kidneys, or a simultaneous aorto-aortic bypass procedure, 80% of children have been cured of their hypertension without the need for medications and 18% are markedly improved, needing minimal antihypertensive medications.5,7 Only about 2% of patients are unchanged after operation. In the absence of a demonstrated vascular lesion, these patients probably have sustained hypertension because of ischemic nephrosclerosis.
References
1. Goldblatt, H, Lynch, J, Hanzel, RF, et al. Studies on experimental hypertension: Production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934; 59:347–349.
2. Safian, RD, Textor, SC. Renal artery stenosis. N Engl J Med. 2001; 334:431–442.
3. Simoes, E, Silva, AC, Diniz, JS, et al. The renin-angiotensin system in childhood hypertension: Selective increase in angiotensin (1–7) in essential hypertension. J Pediatr. 2004; 145:93–98.
4. Foster, JH, Pettinger, WA, Oates, JA, et al. Malignant hypertension secondary to renal artery stenosis in children. Ann Surg. 1966; 164:700–713.
5. O’Neill, JA. Long-term outcome with surgical treatment of renovascular hypertension. J Pediatr Surg. 1998; 33:106–111.
6. Morris, CA. Williams Syndrome. In: Pagon RA, Bird TD, Colan CR, et al, eds. Gene Reviews, (internet). Seattle: University of Washington, 2006.
7. O’Neill, JA, Berkowitz, H, Fellows, KJ, et al. Mid-aortic syndrome and hypertension in childhood. J Pediatr Surg. 1995; 30:164–172.
8. Connolly, JE, Wilson, SE, Lawrence, PL, et al. Middle aortic syndrome: Distal thoracic and abdominal coarctation, a disorder with multiple etiologies. J Am Coll Surg. 2002; 194:774–781.
9. Miura, K, Takahashi, T, Takahashi, I, et al. Renovascular hypertension due to antithrombin deficiency in childhood. Pediatr Nephrol. 2004; 19:1294–1296.
10. Johnson, SL, Lock, RJ, Gompels, MM. Takayasu arteritis: A review. J Clin Pathol. 2002; 55:481–486.
11. Piercy, KT, Hundley, JC, Stafford, JM, et al. Renovascular disease in children and adolescents. J Vasc Surg. 2005; 41:973–982.
12. Vo, NJ, Hammelman, BD, Rocadio, JM, et al. Anatomic distribution of renal artery stenosis in children: Implications for imaging. Pediatr Radiol. 2006; 36:1032–1036.
13. Radermacher, J, Chavan, A, Bleck, J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal artery stenosis. N Engl J Med. 2001; 344:410–417.
14. Upchurch, GR, Henke, PK, Eagleton, MJ, et al. Pediatric splanchnic arterial occlusive disease: Clinical relevance and operative treatment. J Vasc Surg. 2002; 35:860–867.
15. Hricick, DE, Bronning, PJ, Kopelman, R, et al. Captopril-induced functional renal insufficiency in patients with bilateral renal stenosis or renal artery stenosis in a solitary kidney. N Engl J Med. 1983; 308:373–376.
16. Marshalleck, F. Pediatric arterial interventions. Tech Vasc Interventional Rad. 2010; 13:238–243.
17. Shroff, R, Roebuck, DJ, Gordon, I, et al. Angioplasty for renovascular hypertension in children: 20-year experience. Pediatrics. 2006; 118:268–275.
18. Berkowitz, HD, O’Neill, JA. Renovascular hypertension in children. J Vasc Surg. 1989; 9:46–55.
19. McTaggart, SJ, Gulati, S, Walker, RG, et al. Evaluation and long-term outcome of pediatric renovascular hypertension. Pediatr Nephrol. 2000; 14:1022–1029.
20. Lacombe, M. Role of surgery in the treatment of renovascular hypertension in the child. Bull Acad Natl Med. 2003; 187:1081–1093.
21. Tobin, RB, Pelchovitz, OJ, Baskin, KM, et al. Cutting balloon angioplasty in children with resistant renal artery stenosis. J Vasc Interv Radiol. 2007; 18:663–669.
22. Lacombe, M, Ricco, JB. Surgical revascularization after complicated or failed percutaneous transluminal renal angioplasty. J Vasc Surg. 2006; 44:537–544.
23. Barral, X, de Latour, B, Vola, M, et al. Surgery of the abdominal aorta and its branches in children: Late follow-up. J Vasc Surg. 2006; 43:1138–1144.
24. O’Neill, JA. Anomalies of the aorta and branches. In: Mulliken JB, Burrows PE, Fishman SJ, eds. Vascular Anomalies: Hemangiomas and Malformations. NY: Oxford Univ Press, 2012.
25. Stanley, JC, Criado, E, Upchurch, GR, et al. Pediatric renovascular hypertension: 132 primary and 30 secondary operations in 97 children. J Vasc Surg. 2006; 44:1219–1228.
26. Delis, KT, Gloviczki, P. Middle aortic syndrome: From presentation to contemporary open surgical and endovascular treatment. Perspect Vasc Surg Endovasc Ther. 2005; 17:187–203.
27. Grigoryants, V, Henke, PK, Watson, NC, et al. Iliorenal bypass: Indications and outcomes following 41 reconstructions. Ann Vasc Surg. 2007; 21:1–9.