Chapter 18 Renal Tumors
Introduction*
Cancer of the kidneys constitutes nearly 2% of the total human cancer burden occurring in all world regions, with a slight increased incidence in developed countries. Renal neoplasms represent several histologic subtypes that show a characteristic pattern of somatic mutations, which along with histopathology, constitutes the major criteria for their classification. The 2004 World Health Organization (WHO) classification of adult renal neoplasms1 is based on pathology and genetic abnormalities and recognizes several newer subtypes. In addition, renal tumors are a feature in several inherited cancer syndromes including von Hippel–Landau (vHL) disease and Birt-Hogg-Dubé syndrome.
Epidemiology and Risk Factors
RCC constitutes over 90% of all malignancies occurring in the kidney in adults. Overall, it is the 12th most common site in men and 17th in women and represents 2% of all solid cancers in adults worldwide.2 In males living in industrialized countries including Japan, it is more common, ranking 6th along with non-Hodgkin’s lymphoma, whereas in less developed countries, it is 16th. In the United States, it is the 7th most common site in males and the 8th most common site of cancer in females with 13,120 deaths and 60,920 new cases expected in the United States in 2011.3
The incidence of RCCs has been gradually increasing each year in both the United States and Europe over the past few decades. This is not entirely accounted for by increased detection of incidental carcinomas owing to frequent use of imaging studies because the incidence of advanced RCC has also increased over time.4 RCC is nearly twice as common in men as in women and in whites as in blacks. However, over the past few decades, there has been a rapid increase in incidence in women and also in blacks, thereby tilting these ratios. Age-adjusted incidence rates of RCC among white men, white women, black men, and black women in the United States during the period 1992 to 2002 were 13.8, 6.6, 16.8, and 8.0 per 100,000 person years, respectively.3,4
Rates of RCC vary considerably internationally, sometimes by up to a factor of 10, with increased incidence in several Western countries and Australia/New Zealand and the lowest rates in Asia and Africa. These demographic trends highlight the significant role of exogenous risk factors.2,5
Several exogenous and endogenous factors appear to contribute to the development of RCC (Table 18-1). Cigarette smoking is an established causal risk factor for developing RCC and is estimated to account for nearly 20% to 30% of cancers in men and 10% to 20% cancers in women. A meta-analysis including more than 20,000 patients estimated the relative risks of 2.0 and 1.6 among heavy smokers for males and females, respectively, with a slow decline in risk with several years of cessation.6
Table 18-1 Risk Factors for Developing Sporadic Renal Cell Carcinoma
| Cigarette smoking |
| Obesity |
| Dietary factors |
| Hypertension and antihypertensive medications |
| Analgesics |
| Hormonal and reproductive factors |
| Kidney transplantation and dialysis |
| Radiation |
Several studies have now established compelling evidence that links obesity to an excess risk of RCC. It is estimated that the relative risk of RCC is increased by 1.07 per unit increase in body mass index.7 Obesity is considered to be a causal factor in nearly 30% to 40% of all RCCs in the Western world.4 The mechanism underlying the pathways of association between obesity and RCC is not clear, but several metabolic and hormonal pathways such as increased levels of peptide and steroid hormones, estrogen, or elevated levels of insulin-like growth factor-1 (IGF-1) and interleukin-6 (IL-6) pathway have been hypothesized as possible associations.
The differences in incidence of RCC between Asia and the West suggest that diet may have an important role in the etiology of RCCs. In general, diets low in fat and rich in fruits and vegetables have a protective effect.8 There is an increased incidence of RCC in patients with hypertension and the odds ratio is calculated by many authors to be 1.3 to 2.0. It is now thought that hypertension-induced renal injury is the risk factor, although the exact mechanism is unknown.4,8 Although historically, analgesic use, especially phenacetin, was linked to the risk of RCC, this is not proved. Ionizing radiation is weakly linked to the development of RCC and this increased incidence has been recorded in patients who received treatment for ankylosing spondylitis and cervical cancer.4
Anatomy
The kidneys are paired structures situated in the retroperitoneum on either side of the vertebral bodies. Whereas most individuals have single renal arteries, multiple renal arteries are the most common anatomic variant, seen in up to 30% of normal individuals.9,10
Bilateral multiple renal arteries occur in approximately 10% to 15% of the population. Accessory renal arteries most commonly arise from the abdominal aorta and are classified as polar (supplying one of the poles) and hilar (running along with the main renal artery). The polar accessory renal arteries are usually slender, but the hilar renal artery is not always smaller than the main renal artery. Rarely, the accessory renal arteries arise from the celiac, mesenteric, lumbar, middle colic, or middle sacral artery.11
The left renal vein receives the gonadal and lumbar veins before draining into the inferior vena cava (IVC) anterior to the aorta and posterior to the superior mesenteric artery. The right renal vein, usually shorter than the left, directly drains into the IVC. Renal veins usually lie anterior to the arteries at the hilum. Multiple renal veins are the most common anatomic variant, seen in 15% to 30% of the population and are more common on the right side. The most common anomaly of the left renal vein is the circumaortic renal vein, seen in 2% to 17% of the population, in which the left renal vein divides into ventral and dorsal limbs that encircle the aorta. A rarer anatomic variant, the retroaortic renal vein, is seen in 2% to 3% of the population.11
Pathology
It is now well understood that RCC is actually a family of related tumors with different histologies, prognoses, and therapies. The 2004 WHO classification describes various categories and entities based on pathologic and genetic analyses and builds upon the previous classifications including the previous Heidelberg classification. Histologic subtypes include clear cell (“conventional”) adenocarcinoma (80%), papillary (15%), chromophobe (5%), collecting duct (1%), and unclassified (4%).1,12,13 Major changes in the 2004 WHO classification include emphasis placed on defining familial RCC, carcinoma associated with Xp11 translocations, carcinoma associated with neuroblastoma, multilocular cystic RCC, tubular, mucinous and spindle cell carcinoma, and mixed epithelial and stromal tumor (Table 18-2).
Table 18-2 World Health Organization Classification of Renal Tumors
| Clear cell carcinoma |
| Multilocular clear cell RCC |
| Papillary RCC |
| Chromophobe RCC |
| Renal medullary carcinoma |
| Xp11 translocation carcinoma |
| Carcinoma associated with neuroblastoma |
| Mucinous tubular and spindle cell carcinoma |
| RCC unclassified |
RCC, renal cell carcinoma.
From Eble JM, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumors of the urinary system and male genital organs. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2004.
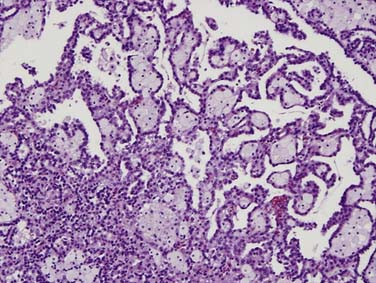
Clear cell carcinoma is the most common subtype, accounting for nearly 80% of all RCCs. Papillary RCC (15%) and chromophobe (5%) are the second and third most common subtypes. Each of these subtypes has differing cytogenetic and immunohistochemical profiles and each is thought to arise from a different part of the nephron. Histopathologic grading of the nuclei of the tumor is made by dividing them into the four-tier Fuhrman nuclear classification14 with grade I being the best-differentiated and grade IV the most anaplastic. There is a separate category of spindle-shaped cells with ominous prognostic significance seen in all histologic subtypes, referred to as a sarcomatoid variant. Clear cell carcinoma displays large, uniform cells with abundant clear cytoplasm rich in glycogen and lipid (Figure 18-1). All kidney tumors of clear cell type of any size are considered malignant. Most clear cell carcinoma are solitary cortical neoplasms. They may be multicentric in up to 4% and bilateral in 0.5% to 3%. The term granular cell carcinoma, indicating acidophilic cytoplasm, was an entity in the 1998 WHO classification and is now considered to be clear cell carcinoma based on clinical and pathologic similarities. Clear cell carcinoma is typically highly vascular and has the worst overall prognosis. Defects in the vHL suppressor gene are reported in over 60% of sporadic cases of clear cell carcinoma.15 The vHL gene is found on the short arm of chromosome 3, at 3p25. vHL protein, the product of this gene, is a potent tumor suppressor. Its loss, mutation, or inactivation in turn leads to higher levels of a transcription factor named hypoxia-inducible factor (HIF), which is produced constitutively but bound to and degraded by functional vHL in normoxic conditions. In hypoxic conditions, or in absence of functional vHL, high levels of HIF will result in the transcription and translation of several proteins involved in tumor angiogenesis, including vascular endothelial growth factor (VGEF).
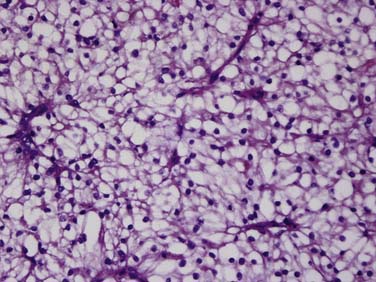
Figure 18-1 Clear cell carcinoma. Histology image (×100 magnification) shows abundant clear cells filled with glycogen.
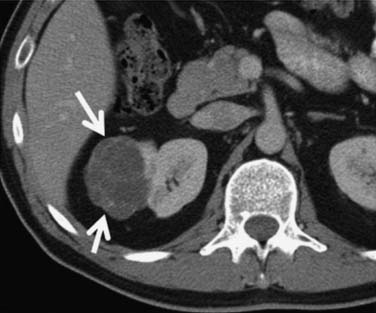
Multilocular cystic RCC is a variant of conventional clear cell tumors characterized by a cystic mass with less than 25% solid components and entirely composed of cysts of variable size separated from the kidney by a fibrous capsule (Figures 18-2 and 18-3).16,17 These tumors tend to be indolent and have a very low metastatic potential.
Papillary tumors are characterized by abnormalities of chromosomes 7 and 17 and loss of chromosome Y. They typically have a papillary growth pattern and exhibit a frondlike appearance on histologic examination (Figure 18-4). There is a 5:1 male predominance. Hereditary papillary RCC is an autosomal dominant form of the disease associated with multifocal papillary RCC.18 Papillary RCCs may have a less aggressive course than the clear cell RCC and are subdivided into cellular type 1 and type II. These tumors have cells arranged over a fibrovascular core and a papillary growth pattern. These terminologies are preferred over the terms “basophilic” (type 1 papillary) and “eosinophilic” (type 2 papillary) RCC used in previous classifications. Type 2 papillary RCC is typically of a higher grade and more aggressive than type 1. Once metastatic, patients with papillary RCC fare more poorly than patients with clear cell histologies, owing to the absence of effective systemic therapies.
Chromophobe RCCs (Figure 18-5) are a less aggressive subtype with 86% of tumors being T1 or T2 at diagnosis. The relationship between oncocytoma and chromophobe RCC is still under investigation and both are considered to be derived from the intercalated cells of the collecting duct. The Birt-Hogg-Dubé syndrome is a form of inherited RCC also characterized by hair follicle hamartomas. Patients with this syndrome often have chromophobe tumors or mixed chromophobe tumors and oncocytomas.19
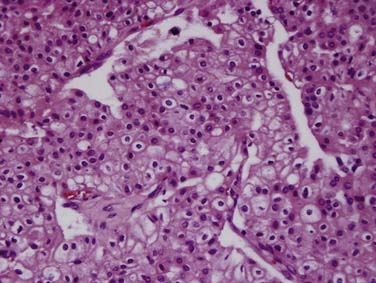
Figure 18-5 Chromophobe RCC. Histology specimen shows prominent pale cytoplasm with perinuclear halos.
Sporadic oncocytomas are relatively common and are considered benign but indistinguishable from RCCs on imaging (Figure 18-6).
Collecting duct tumors, which arise from the medullary collecting duct, often occur in younger patients and frequently present with advanced disease at the time of diagnosis. Renal medullary carcinoma is a rare subtype, closely related to collecting duct carcinoma, with a very poor prognosis, that occurs in young patients with sickle cell trait.20,21
RCC has a propensity for extending, as tumor thrombus, into the tributaries of the renal veins and subsequently to the main renal vein, IVC, hepatic veins, and potentially the right atrium. Rarely, the tumor may extend directly into the ipsilateral adrenal gland, or adjacent musculature, liver, spleen, pancreas, and colon. Hematogenous metastases are more common and occur earlier than lymphatic dissemination, the former most commonly to the lungs and bone, but essentially to any organ, including the subcutaneous tissues and skeletal muscle. Lymphatic dissemination, however, can involve regional nodes and spread to the chest via the cisterna chylii with mediastinal nodal involvement.
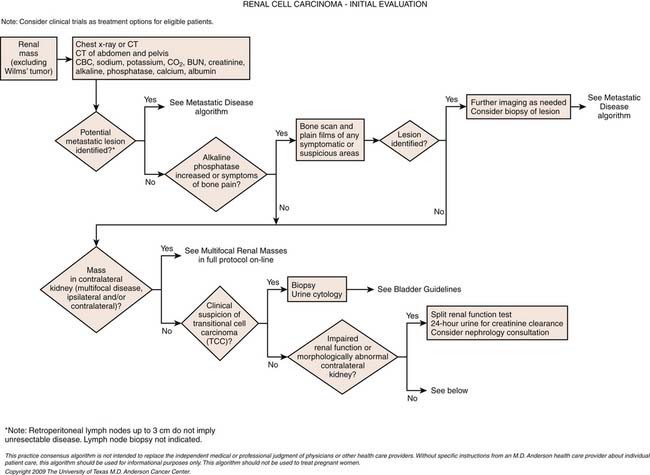
Staging
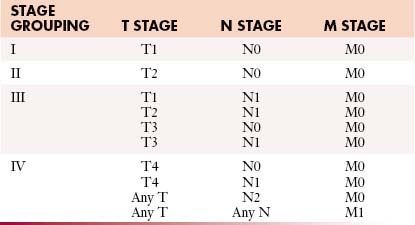
Staging systems are designed to reflect the modes of spread (Figure 18-7) and are used to stratify treatment options and prognoses and assess survival characteristics. The current version, the 2009 tumor-node-metastasis (TNM) staging of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) is presented in Tables 18-3 and 18-4 and Figure 18-7.3,22
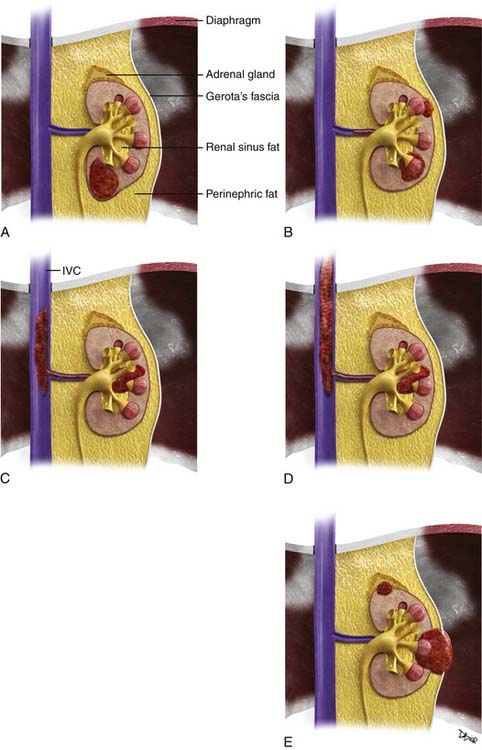
Figure 18-7 A-E, T staging according to the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system for RCC. Tumors confined to the kidney are staged as T1 when smaller than 7 cm and as T2 when larger than 7 cm. Tumors invading the renal sinus fat or perinephric fat are staged as T3a, and tumors involving Gerota’s fascia and adrenal gland are staged T4. IVC, inferior vena cava. See Table 18-3.
Table 18-3 Tumor-Node-Metastasis Staging for Renal Cell Carcinoma
| STAGE | CHARACTERISTICS |
|---|---|
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor confined to the kidney < 7 cm in greatest dimension |
| T1a | Tumor confined to kidney < 4 cm in greatest dimension |
| T1b | Tumor confined to kidney > 4 cm but < 7 cm in greatest dimension |
| T2 | Tumor confined to kidney and > 7 cm in greatest dimension |
| T2a | Tumor confined to kidney > 7 cm but < 10 cm in greatest dimension |
| T2b | Tumor confined to kidney > 10 cm |
| T3 | Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota’s fascia |
| T3a | Tumor grossly extends into the renal vein or its segmental (muscle containing) branches, or invades perirenal and/or renal sinus fat but not beyond Gerota’s fascia |
| T3b | Tumor grossly extends into the vena cava below the diaphragm |
| T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava |
| T4 | Tumor invades beyond Gerota’s fascia (including contiguous extension into the ipsilateral adrenal gland) |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in regional lymph node(s) |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
From Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010.
Prognosis is generally reflected in staging severity (Table 18-5) with lower stage disease associated with longer survival rates. For example, a patient with a T1a N0 M0 lesion has a 90% to 100% 5-year survival, whereas the survival for a patient with a T3b-c N0 M0 lesion decreases to 40% to 65%. Systemic metastases at the time of presentation are associated with only a 0% to 20% 5-year survival. The histologic type of tumor is an important consideration not included in the TNM system. For example, the time from nephrectomy to metastasis is twice as long for patients with chromophobe tumors than for those with clear cell or papillary subtypes.23
Table 18-5 Survival by Tumor-Node-Metastasis Stage
| DISEASE EXTENT | TNM STAGE | 5-YEAR SURVIVAL (%) |
|---|---|---|
| All organs confined | T1-T2 N0 M0 | 70-90 |
| ≤4 cm | T1a N0 M0 | 90-100 |
| 4-7 cm | T1b N0 M0 | 80-90 |
| ≥7 cm | T2 N0 M0 | 70-80 |
| Invasion of perinephric fat | T3a N0 M0 | 60-80 |
| Adrenal involvement | T3a N0 M0 | 0-30 |
| Venous involvement | T3b-c N0 M0 | 40-65 |
| Locally advanced | T4 N0 M0 | 0-20 |
| Lymphatic involvement | Any T, N+, M0 | 0-20 |
| Systemic metastases | Any T, Any N, M1 | 0-10 |
Imaging Evaluation
It is generally considered that a risk-adapted approach is used for workup of possible sites of metastases. Chest CT should be performed if the primary tumor is large or locally aggressive, because metastases are more common in these patients.24 Plain chest radiography without CT should be reserved for patients with a very low risk of metastatic disease or for those in long-term follow-up. Brain MRIs and nuclear medicine bone scans are generally only justified if there are symptoms and signs to suggest disease at these sites or if the tumor is large and locally aggressive (although even the latter is debated).25 Technetium-99m methylene diphosphate (99mTc-MDP) bone scans, however, may have limitations in detecting the typical osteolytic bone metastases from RCC. The value of pelvic CT in staging is limited.26
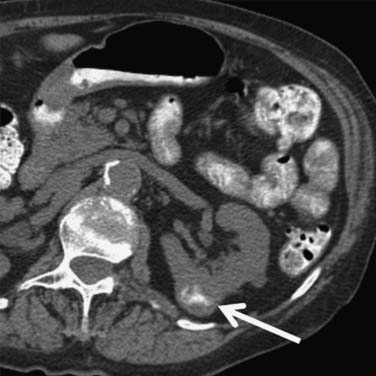
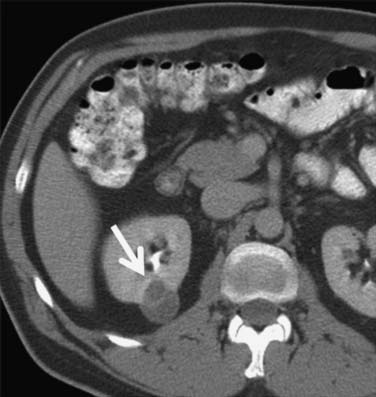
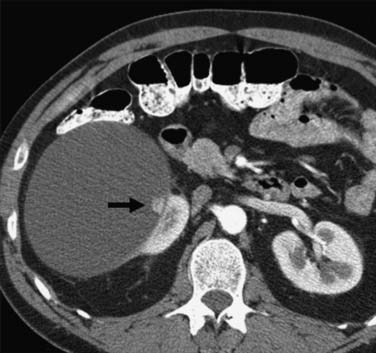
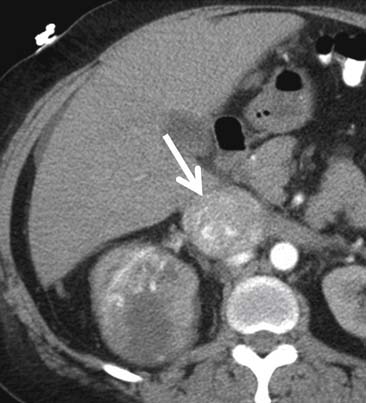
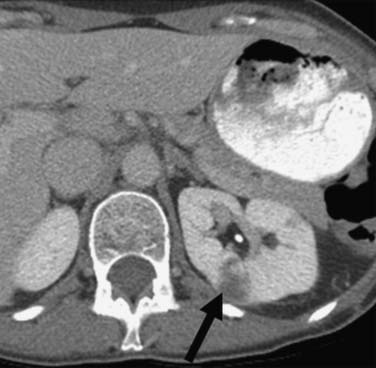
Calcification, which can be detected on an abdominal radiograph in as many as 20% of renal adenocarcinomas, is even easier to detect on an unenhanced CT examination (Figure 18-8). However, calcification also may be seen in other renal masses, including common lesions such as benign cysts. In all but very large tumors, renal function is preserved. In fact, diminished renal function suggests tumor thrombus in the renal vein. The tumor is identified as a mass with mottled density that causes a focal bulge in the renal contour. Such tumors are termed exophytic (Figure 18-9). Tumors that do not deform the renal contour are termed intrarenal or central (Figure 18-10). Tumors that have both an exophytic and a central component are referred to as mixed. Involvement of the collecting system is seen as irregularity of the urothelial surface or complete occlusion of a calyx or infundibulum. A filling defect within the collecting system may represent a blood clot or tumor invasion but is relatively uncommon with renal parenchymal tumors. In fact, if a mass is present in the collecting system, the lesion is more likely to represent a transitional cell carcinoma that has invaded the renal parenchyma rather than an RCC that invades the collecting system. Some tumors, particularly those with sarcomatoid features, have an infiltrative appearance in which the reniform shape of the kidney is preserved (Figure 18-11)
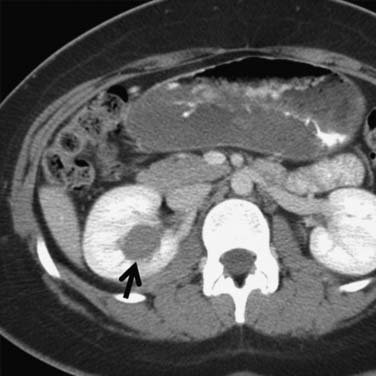
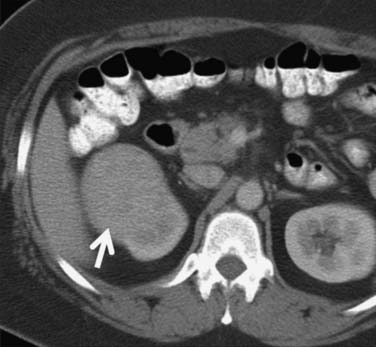
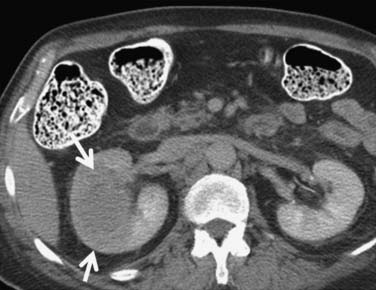
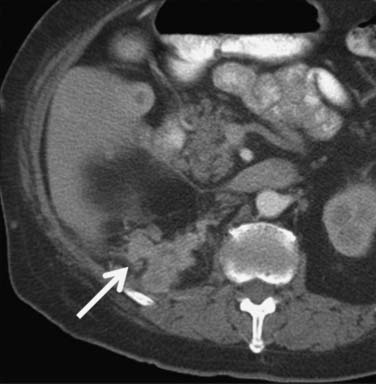
Figure 18-10 Axial CT scan of the abdomen with IV contrast shows a central tumor (arrow) in the right kidney.
Occasionally, the tumor can be found arising in the wall of a simple renal cyst (Figure 18-12). In some cases, the tumor itself has a cystic appearance and may be difficult to distinguish from a complex but benign renal cyst. Hartman27 estimates that 15% of cases of RCC are cystic on radiologic and pathologic examination. The clinical features of cystic RCC are similar to those that are completely solid. The majority will have clear cell histology, but some may also be papillary.
Computed Tomography Evaluation
Suggested protocols for preoperative evaluation and follow-up, using multislice computed tomography (MSCT) are discussed. In addition to assessing tumor staging, preoperative evaluation focuses on delineating the tumor with particular attention to the relationship of the tumor to adjacent structures, including vascular relationships. In comparison, follow-up evaluation is directed toward surveillance for residual or recurrent disease. In general, 100 to 150 mL of iodinated intravenous (IV) contrast medium is used, with a flow rate of 2 to 3 mL/sec.
Preoperative Computed Tomography Evaluation
During the nephrographic phase, the contrast, filtered through the glomeruli, enters the loop of Henle and the collecting ducts. It is to be noted that the nephrographic phase can occur earlier in patients injected with contrast at a rapid rate. During the nephrographic phase, renal medullary enhancement is similar to that of the cortex. The excretory phase begins when the contrast is excreted into the calyces at approximately 3 to 5 minutes. Although the nephrograms remain homogeneous in the excretory phase, the attenuation decreases uniformly owing to a decrease in the plasma concentration of contrast and continued excretion from the kidneys.28 The corticomedullary phase and all the subsequent phases can last longer in patients with renal dysfunction and in those with diminished cardiac output.
Multiplanar re-formatted and three-dimensional (3D) volume-rendered presentations of the renal phase images are helpful in allowing appreciation of the relationships of structures, particularly for surgeons (Figure 18-13). Such re-formations are best obtained with the thinnest possible collimation and some degree of reconstruction interval overlap (typically, <1.5 mm interval and 10-50% overlap), and transferred to a workstation for the purpose of creating multiplanar re-formatted images, 3D volume rendering, and maximum intensity projection (MIP) images. The late-arterial phase provides a useful angiographic image of arterial and venous supply to the kidney, but it has limited additional utility for lesion detection and characterization when a nephrographic phase is employed. The combination of excretory and nephrographic phases have been shown to improve lesion detection and staging.29,30
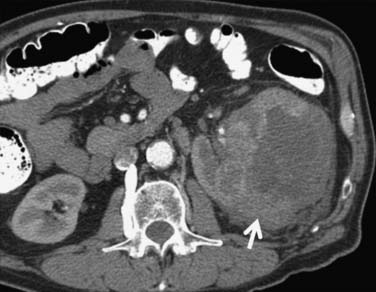
It has been shown by several investigators that clear cell RCC enhances to a greater extent and is more heterogeneous in appearance than other histologic subtypes of RCC (Figure 18-14). Kim and coworkers31 showed that an increase in attenuation of 84 Hounsfield units in the corticomedullary phase differentiated clear cell RCC from non–clear cell tumors with a sensitivity of 74% and a specificity of 100%. Herts and colleagues32 showed that papillary tumors were more homogeneous and had a much lower tumor-to-parenchyma enhancement ratio than was present in nonpapillary subtypes, particularly with tumors less than 3 cm in diameter (Figure 18-15). Chromophobe tumors also are less hypervascular than clear cell tumors and tend to have a more peripheral pattern of enhancement; their appearance, however, is not sufficiently characteristic to allow them to be reliably differentiated from papillary lesions (Figure 18-16). Oncocytomas cannot be reliably differentiated from RCCs by imaging and, thus, are also considered to be surgical lesions (see Figure 18-15). The well-known prominent central scar that can be used to suggest the diagnosis may also be present in necrotic clear cell tumors. Collecting duct and medullary RCC tumors are located centrally within the kidney and show variable, typically limited, postcontrast enhancement (Figures 18-17 and 18-18). They cannot be reliably distinguished from other RCCs or urothelial tumors, but a medullary tumor can be suggested when an aggressive tumor in a young patient with sickle cell disease or trait is encountered.
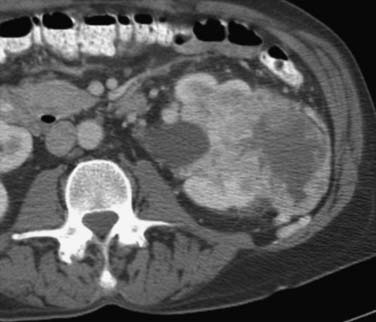
Figure 18-14 Large heterogeneous-enhancing mass in the left kidney revealed clear cell carcinoma on excision.
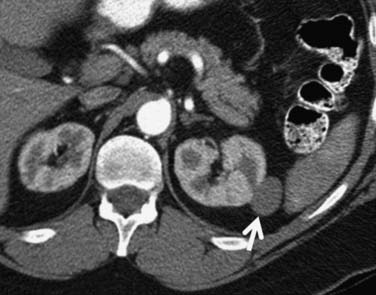
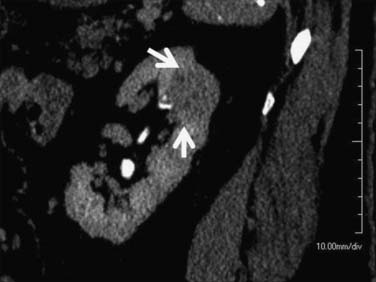
Figure 18-15 Papillary RCC. A small, poorly enhancing mass (arrow) arises from the lateral aspect of the left kidney.
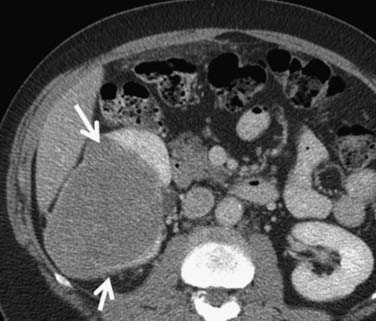
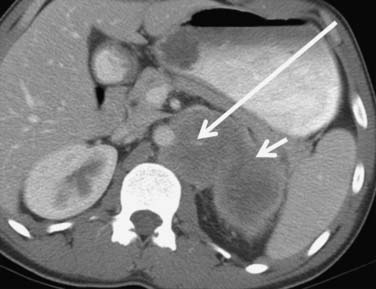
Figure 18-16 Chromophobe RCC. Relatively hypovascular, homogeneous expansile mass (arrows) arises from the right kidney.
Extension of tumor into the renal veins has been reported to occur in 20% to 35% of patients and into the IVC in 4% to 10%, infrahepatic (50%), intrahepatic (40%), and intra-atrial (10%).33–35 IVC thrombus is more common from right-sided tumors because of the shorter renal vein on the right.36,37
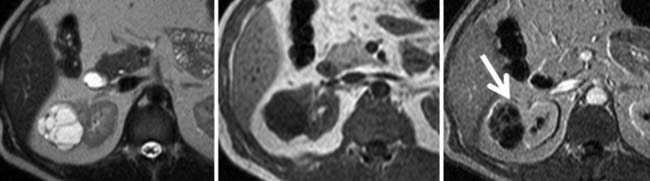
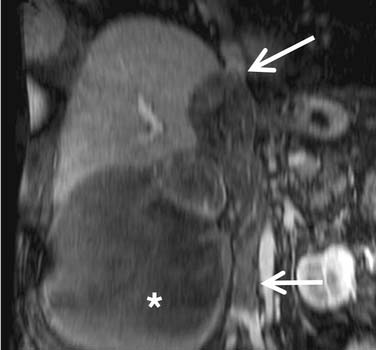
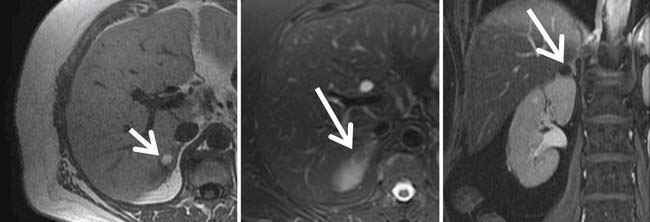
Identification of thrombus within the venous system, especially the IVC, is particularly important because it affects surgical management, typically necessitating an anterior abdominal approach. Furthermore, if thrombus extends into the heart, a combined thoracic and intracardiac approach with cardiac bypass may be required. Current CT techniques have reported sensitivities and specificities for detecting renal vein thrombus of 85% and 98%, respectively.38 Color Doppler ultrasonography has reported sensitivities and specificities for detecting thrombus in the renal veins of 75% and 96%, respectively, with 100% accuracy for detection of thrombus in the IVC.39 MRI has similar reported sensitivity and specificity for detecting thrombus in the renal vein of 86% to 94% and 75% to 100%, respectively, also with 100% accuracy for detecting IVC thrombus.40,41 MRI, with its multiplanar capability, is probably the best modality for identifying the superior extent of IVC involvement (Figure 18-19).42,43
Fortunately, prognosis is not adversely affected by tumor within the venous system, nor its level within the IVC, provided the tumor is free-floating and can be successfully removed (5-yr survival rates of 50-69%, in the absence of metastatic disease). It is, however, severely impaired if the tumor invades into the wall of the IVC (5-yr survival 25%), although this can improve if the involved IVC can be resected completely (5-yr survival rate 57%).44–46 Unfortunately, current imaging modalities have some limitations in distinguishing bland from tumor thrombus and in distinguishing invasive from noninvasive tumor with respect to the vessel wall.46 Intravascular tissue that enhances after IV contrast administration or that contains neovascularization (“thread-and-streak” sign) is good evidence of tumor, rather than bland, thrombus (Figure 18-20).
Magnetic Resonance Imaging Evaluation
MRI is generally used when optimal CT cannot be performed as in the case of severe allergy to iodinated contrast medium or pregnancy. MRI also finds application in instances in which there is equivocal contrast enhancement on CT or in instances of hemorrhagic lesions. MRI has similar reported overall staging accuracies compared with CT. Its multiplanar capability, however, is particularly useful for delineating the superior extent of tumor within the IVC.46–48
Coronal and axial conventional T1-weighted and axial fast spin echo T2-weighted fat-suppressed images of the abdomen are generally used. Images are supplemented by dynamic contrast-enhanced 3D fast spoiled gradient echo sequences (FSPGRs) to further delineate the primary tumor and/or liver lesions and to evaluate any vascular thrombus identified; in particular tumor (rather than bland) thrombus is indicated by the presence of enhancing vessels within the thrombus. Multiple dynamic acquisitions can be employed to obtain arterial, nephrogenic, and pyelographic-like images.43,49–51
Multidetector row CT with 3D re-formations and MRI have similar overall staging accuracies for RCC.52
Ultrasound Evaluation
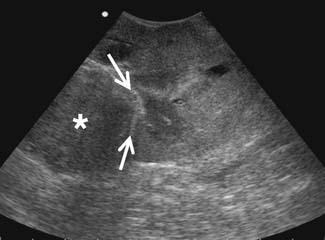
Ultrasound can be useful to assessing the presence and extent of venous thrombus. It can also be helpful in distinguishing cysts from hypovascular solid tumors seen on CT (e.g., papillary RCC). Ultrasound is able to see the septations better owing to complex interfaces to the ultrasound beam. Ultrasound has reported accuracies for T staging of 77% to 85% and detection of venous thrombus of 87%.37,39,53 However, it has limitations in visualizing the retroperitoneum and perinephric tissues, although some proponents argue otherwise.24,37,39,54
Partial nephrectomy has seen an increased use of image guidance during the operative procedure. Of the potential intraoperative modalities that can be used, ultrasound is the best based on its portability, real-time capabilities, and access. Use of ultrasound at the time of operation allows high-resolution imaging of the tumor and the kidney because it allows for use of a high-frequency probe that can be applied directly on the surface of the kidney. Intraoperative ultrasound guidance offers multiple advantages to the surgeon (Figure 18-21).55
Small tumors that are completely below the surface of the kidney can be identified by intraoperative ultrasound guidance, thereby allowing the surgeon to “see” before placing an incision on the surface of the kidney.56 Moreover, the accurate depiction of the relationship of the tumor to the vascular structures and the calyces allows the surgeon to avoid injuries to these structures and still achieve negative margins. Intraoperative ultrasound frequently identifies occult lesions that are not seen on conventional imaging modalities such as contrast-enhanced CT or MRI. Such lesions can also be characterized. Purely cystic lesions are benign and can be ignored. Partially cystic and solid lesions are suspicious and can be excised. This modality can be used for both open and laparoscopic approaches. Intraoperative ultrasound can also be used to monitor radiofrequency ablation (RFA) or cryotherapy of a tumor during laparoscopy.57
Percutaneous Biopsy
Attitudes toward biopsy have undergone some refinement in the past ten years. In the past, biopsy was discouraged or even thought to be potentially dangerous, but this is no longer the case and biopsy is now recognized as a valuable adjunct to management in selected patients. Preoperative percutaneous biopsy of the renal lesion is still not routinely undertaken in the nonmetastatic setting or prior to cytoreductive nephrectomy in the metastatic setting because the results usually do not affect what therapy will be recommended, except in patients with multiple tumors or occasionally in patients with an underlying predisposing condition. Percutaneous biopsies may be considered in selected cases, for example, when abscesses or metastatic disease from a known primary is suspected, especially from lymphoma or melanoma.47,58 It is also generally performed in patients who will undergo ablative or thermal therapy in order to determine the underlying histologic subtype of the tumor or the need for retreatment in patients who have undergone ablative therapy in whom imaging studies are equivocal. Biopsy is also mandated in the presence of central lesions that could arise from the renal collecting system to differentiate between a transitional cell carcinoma and an RCC.
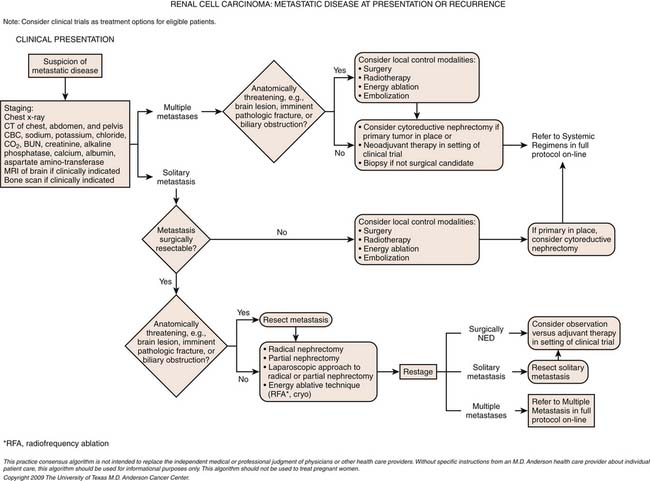
Patterns of Metastasis
RCC metastasizes both hematogenously and by means of lymphatic spread. Hematogenous spread occurs via the renal vein and into the lungs; when tumor cells invade the systemic circulation, blood-borne metastases ensue. Metastasis to regional lymph nodes in the perinephric space is the initial manifestation of lymphatic spread. Tumor cells may then travel via the thoracic duct to involve nodal groups elsewhere in the body. In addition, RCC may recur in the surgical bed (Figure 18-22). In a comprehensive review of patterns of tumor recurrence and spread, Chae and associates59 concluded that 83% of recurrences occurred within the first 2 years after surgery; nonetheless, late recurrences, sometimes as long as 10 years after initial surgery, have been reported. Lung and bone metastases most commonly manifest within 1 to 2 years after initial surgery. Recurrence in the nephrectomy site, however, occurred at varying times between 1 and 3 years after surgery. As expected, there was a significant correlation between tumor size and stage and the occurrence of metastatic disease and the nuclear (Fuhrman) grade of the tumor. The most frequent sites of distant metastasis are, in decreasing order of frequency, lung (38-71%), bone (17-37%), lymph nodes (12-63%), and liver (15-23%) followed by adrenal gland, contralateral kidney, retroperitoneum, and brain, with approximately 5% each.60
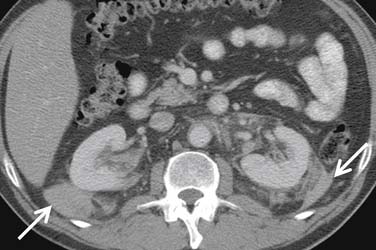
Metastasis to almost any organ and muscle (Figure 18-23), however, can occur.
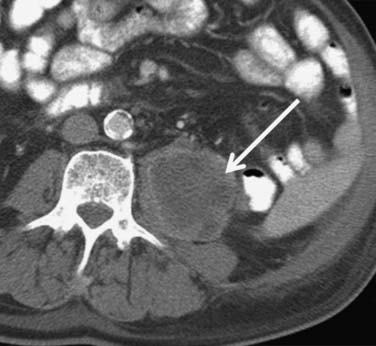
The pancreas (Figure 18-24) is an uncommon, but well-recognized, site for metastasis from RCC. They tend to occur with a long lead time after initial diagnosis; Ghavamian and coworkers61 reported a mean of 10 years after initial presentation. Typically, pancreatic metastases are well-defined single or multiple hypervascular masses found anywhere in the pancreas. Surgical resection of the metastases are feasible, and in one report, mean survival after resection was more than 4 years. Because the majority of such lesions are hypervascular, Ng and colleagues emphasized the value of early-phase images for their detection.62
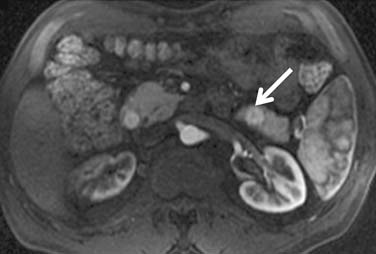
Figure 18-24 Axial T1-weighted postcontrast MRI shows an enhancing metastatic RCC (arrow) in the tail of the pancreas.
Therapy
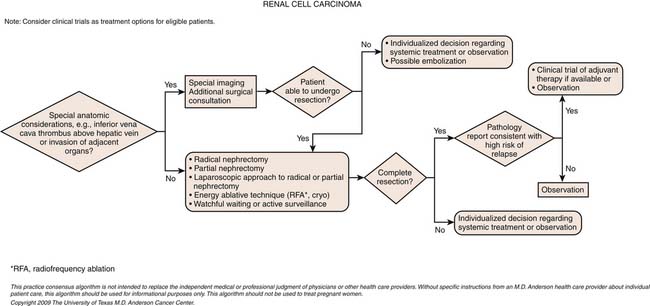
Surgical Therapy
Radical Nephrectomy
Survival after the surgical treatment of RCC markedly improved after the 1960s when Robson and associates63 showed that radical nephrectomy had a superior survival advantage over other forms of surgery performed at the time. Other studies soon followed and corroborated Robson and associates’ initial reports.64,65 Radical nephrectomy, as initially defined by Robson and associates, included early vascular ligation, extrafascial (outside Gerota’s fascia) dissection of the kidney, en bloc resection of the adrenal gland, and extensive lymphadenectomy from the crus of the diaphragm to the aortic bifurcation. Since then, these associated procedures have been shown to be unnecessary in most cases. For example, the overall incidence of adrenal metastasis is quite low, generally less than 5%. The possibility of adrenal involvement seems to increase with left-sided, large, upper pole, and high-stage tumors, as well as when multiple metastatic sites are present.66 Contemporary radiographic imaging has been found to be a very sensitive tool for detection of adrenal metastasis, but it may not be specific enough in all cases because benign lesions may be confused for malignancy.67 A 99.4% negative predictive value of preoperative CT scans has been reported.68 The stage is dependent, with T1-T2 disease associated with a 0.6% rate of adrenal involvement, T3 with 7.8%, and T4 disease with 40%. Therefore, resection of the adrenal gland during radical nephrectomy is not always necessary, particularly if it appears normal on axial imaging, and especially for clinical T1 tumor that does not abut or involve the adrenal gland.
As another example of the shifting trend toward “less radical” radical nephrectomy, lymphadenectomy is not frequently performed for the typical case of RCC because of the lack of a meaningful survival benefit. It is primarily used for staging in select cases only.69–71 The incidence of lymph node metastases can range from 3% to 15%, varying based on the number of lymph nodes removed, as well as tumor stage, the presence of a sarcomatoid component, necrosis, and tumor grade.71–75 In addition, lymph node enlargement measuring less than 2 cm on axial imaging is not always due to malignant causes.76 Thus, in these cases, although resection may be reasonable to perform for diagnostic purposes, the therapeutic benefit for most patients is unclear.77 Interestingly, during the cytokine era, it was shown that patients with metastatic disease but without lymph node involvement (N0 M1) were more likely to respond to systemic IL-2 therapy than patients having N+ M1 disease.70,78 It should be noted also that these data exclusively apply to the conventional, or clear cell, form of RCC. For papillary RCC, lymphadenectomy can actually provide a survival advantage for those with N+ M0 disease, because this variant represents a distinctly different biology.79 Such patients may derive a significant recurrence-free survival with complete surgical resection.
As a further example of the shifting trends, the gold standard of radical nephrectomy is being supplanted by nephron-sparing surgery (NSS) for nearly all tumors less than 4 cm in size and for many 4 to 7 cm in size. Several long-term studies have shown equivalent survival of patients undergoing NSS versus radical nephrectomy for a unilateral renal tumor less than 4 cm in size, with equivalent recurrence-free survival, lower overall survival (OS) for those undergoing radical nephrectomy (likely as a result of less induction of renal failure and its associated risks),83 as well as improved quality of life.80–83 The increasing call to perform NSS more frequently was highlighted in the recent publication of the American Urological Association Guideline for the Treatment of the Clinical Stage 1 Renal Mass.84–87
Laparoscopic Radical Nephrectomy
Although the first laparoscopic radical nephrectomy (LRN) was reported in 1991, laparoscopic surgery for kidney cancer was not fully accepted until over 10 years later, owing to various oncologic concerns including dissemination of cancer cells, port site seeding, and other issues.88 These concerns have been assuaged with several long-term follow-up studies showing equivalent survival of patients undergoing LRN versus open radical nephrectomy.89 The laparoscopic approach is associated with reduced blood loss, less pain, less narcotic usage, a shorter hospitalization, and generally a quicker recovery. General contraindications to LRN include tumor size larger than 13 cm, prior partial nephrectomy, presence of IVC tumor thrombus, extensive locoregional disease extension (clinical T4 disease), and the presence of bulky adenopathy. There are no differences in complication rates, and some would argue that there is a better adverse event profile with laparoscopic surgery, but no prospective, randomized data exist. Retrospective comparative studies indicate an overall average of approximately 12% to 15% rate of adverse events, as well as operative time, which is equivalent to open surgery in experienced hands, and with lower cost.90–92 In cases of cytoreduction for metastatic disease, LRN can also be done with the same tumor guidelines as those without metastatic disease.93,94 There are some who propose to morcellate the specimen during LRN in order to prevent having to make a small extraction incision and, thus, improve the cosmetic results, even though this has never been shown to cause less pain. Specimen morcellation is not performed at our center, because of the desire for a good pathologic evaluation of the intact specimen. Upward of 26% of patients with clinical T1-T2 tumors have been found to harbor high-risk disease on pathologic evaluation, warranting a more intensive follow-up regimen or enrollment into an adjuvant trial.95
Management of Metastatic Renal Cell Carcinoma: Role of Cytoreductive Nephrectomy
In the era of immunotherapy, cytoreductive nephrectomy prior to the administration of systemic therapy was an important part of a multidisciplinary approach in properly selected patients. Initially, several retrospective studies demonstrated the safety and potential efficacy of this approach, citing that cytoreductive surgery, prior to the administration of interferon or IL-2, may improve responses to systemic therapy and survival in appropriately selected patients.96–99 What was clear from these retrospective studies was that optimal patient selection was paramount to success, defined as minimal perioperative morbidity and the timely administration of systemic immunotherapy postoperatively. Proper patient selection included those with clear cell histology; good performance status; the absence of brain, liver, or extensive bone metastases; few if any comorbid conditions that could preclude surgery; the absence of sarcomatoid dedifferentiation; and the ability to resect greater than 75% of overall tumor burden.97 Subsequently, the Southwest Oncology Group (SWOG) and the European Organization for Research and Treatment of Cancer (EORTC) independently conducted phase III randomized trials that demonstrated an improved survival for patients who underwent cytoreductive nephrectomy prior to systemic interferon therapy. These studies solidified the role of cytoreductive surgery as part of a multidisciplinary approach to patient management.
Despite the lack of a consistent and significant primary tumor response to systemic targeted therapy, some investigators believe that the concept of neoadjuvant or presurgical therapy for patients with metastatic RCC requires serious consideration. In this treatment paradigm, patients are given a defined course of systemic therapy, and those who demonstrate stable disease or evidence of a response would be offered surgery, whereas those who progress through therapy would be spared from a potentially morbid surgery that they are unlikely to benefit from. Several phase II studies have investigated this treatment paradigm and shown it to be safe.100–106 Further study will be required to determine whether this approach provides a better survival advantage than upfront cytoreductive surgery.
Nephron-Sparing Surgery: Open, Laparoscopic, and Robotic Partial Nephrectomy
Imperative indications for NSS include the presence of bilateral renal tumors, a systemic condition threatening renal function (such as severe hypertension or diabetes mellitus), a local condition threatening the contralateral kidney (such as stone disease or chronic pyelonephritis), chronic kidney disease, and a solitary functioning kidney. The basic principles of open partial nephrectomy include a flank approach, allowing the surgeon to operate on the kidney at skin level. The salient steps include hilar clamping, allowing resection in a bloodless, well-visualized field to allow sharp dissection of the renal tumor with a negative margin, followed by suture ligation of any collecting system entry, transected renal vessels, and completed by renorrhaphy. Although resection with a negative margin is performed, and as long as an actual margin is present, it is not necessary to take wide margins so that maximal renal preservation can be achieved.107–109 It should be noted that nearly all data on surgical treatments are not based on randomized, controlled studies, although studies from multiple international centers have independently corroborated the advantages of NSS.
Publications on minimally invasive technologies likewise are based on retrospective data, which are still evolving and growing. Laparoscopic partial nephrectomy (LPN) was developed as a way to perform NSS without the flank incision and to reduce the morbidity of open surgery. It has since been shown to be an effective approach for select small renal tumors, adhering to the principles of open surgery detailed previously.110 Nevertheless, LPN is one of the most complex laparoscopic procedures performed by any surgical specialty, given the requirement for expedient, and flawless, tumor resection and renal reconstruction under the duress of renal ischemia, requiring extensive laparoscopic experience and careful patient selection. With the availability of a robotic surgery platform (daVinci, Intuitive Corp., Sunnyvale, CA), the laparoscopic approach to NSS has been facilitated. Robot-assisted partial nephrectomy (RAPN) has the potential to improve the results of LPN and allow a larger cadre of urologists to offer minimally invasive NSS. The most recent data, which are still early and growing, suggest that RAPN is associated with shorter warm ischemia time and a shorter operation than LPN, advantages that are seen even in surgeons experienced with LPN.111
Cryoablation and Radiofrequency Ablation
Laparoscopic or percutaneous energy-ablative therapies such as cryoablation and RFA, are generally reserved for small (<3.5 cm) tumors and patients at higher operative risk. Percutaneous renal cryoablation or RFA may be employed under MRI or CT guidance, respectively, for tumors that are safely accessible percutaneously. Laparoscopic approaches can be used for lesions that are anterior or in close approximation to the ureter, bowel, or other critical structures. Alternatively, several adjunct percutaneous procedures such as hydrodissection and balloon displacement may be used to allow a percutaneous route. Small exophytic tumors are probably treated just as well with either approach, whereas larger (>3.5 cm) and more central lesions have a higher rate of failure with either therapy. Both cryoablation and RFA have a favorable adverse outcome profile, and oncologic efficacy ranges in the 90% range, which although not unfavorable, is less than surgical excision, which has an efficacy of over 98%.87 Whether cryoablation or RFA is used, two other important factors must be considered. A biopsy to obtain a tissue diagnosis is imperative, and intensive postoperative follow-up imaging is needed to document effective treatment, given the lack of any confirmation of pathologic cure and sparse long-term data. Current recommendations include obtaining a renal mass protocol CT or MRI within the first 3 months after therapy, then at 6, 12, 18, and 24 months and semiannually or annually thereafter.112 Several centers, including ours, have additionally started to biopsy the zones of ablation 6 months or later after treatment if there has not been satisfactory involution in order to confirm adequate treatment because data exist showing that absence of enhancement by itself may not be a fully reliable finding, as well as the possibility of other causes of false-positive and false-negative imaging findings.113,114
Medical Therapy
Systemic therapy for RCC has changed substantially in the past several years. Up until the early 1980s, no consistently useful therapy existed for this disease. With the cloning of the genes for interferon-alpha (IFN-α) and IL-2 and recognition that an immune reaction may be mounted against some individuals with RCC, IL-2 and IFN agents were assessed in clinical trials, and found to have some utility. In 1992, IL-2 was U.S. Food and Drug Administration (FDA)–approved for mRCC on the basis of outcomes in 255 patients.115
The discovery of the vHL gene in 1993 expanded our understanding of the molecular biology of RCC and led to the assessment of antiangiogenic agents in this disease.116,117
Four anti-VEGF agents have been approved for use in advanced RCC by the FDA. These include sorafenib, sunitinib, bevacizumab plus IFN-α, and pazopanib.118–122 Sorafenib (Nexavar, Bayer AG), a small molecule inhibitor of vascular endothelial growth factor receptor (VEGFR), was tested in a 903-patient study that randomized previously treated individuals with clear cell mRCC between sorafenib and placebo and showed a doubling of progression-free survival (PFS) from 2.8 to 5.5 months in the sorafenib arm (p < .01).118 OS, the primary endpoint, was numerically higher in the sorafenib arm but did not reach statistical significance. Sunitinib (Sutent, Pfizer Inc.), a small molecule VEGFR inhibitor, was evaluated in a 750-patient study that randomized individuals with clear cell mRCC between study drug and IFN-α.123 A doubling of PFS from 5.0 to 11.0 months was seen in the sunitinib arm (P < .001). A borderline improvement in OS was observed; 21.8 months in the IFN-α arm versus 26.4 months in the sunitinib arm (P = .051).124 Bevacizumab (Avastin, F. Hoffmann–La Roche Ltd.), an antibody against VEGF, was tested in combination with IFN-α and compared with IFN-α monotherapy in two large randomized studies that enrolled previously untreated patients with clear cell RCC. In an industry-sponsored trial, 649 patients were enrolled, and PFS in the combination arm was 10.2 months versus 5.4 months in the IFN-α arm (P = .0001).120 In the Cancer and Leukemia Group B (CALGB)–led study, 732 patients were enrolled and the PFS was 8.5 months versus 5.2 months in the IFN-α arm (P < .0001).121 Neither study showed a statistically significant OS advantage for the combination arm. Most recently, pazopanib (Votrient, Glaxo Smith Kline plc), a small-molecule VEGFR inhibitor, was assessed in a study that randomized 435 patients with clear cell mRCC between pazopanib and placebo, with crossover permitted in the placebo arm at the time of progression. Roughly half the patients were treatment naïve, and the balance had received prior immunotherapy. The PFS in the pazopanib arm was 9.2 months versus 4.2 months in the placebo arm (P < .0001).122
The mammalian target of rapamycin (mTOR) was shown in phase II studies to be a potential target in RCC. The biologic rationale for this sensitivity is unclear but may be due in part to mTOR’s regulation of HIF. Two mTOR-inhibiting agents, both rapamycin analogues, were FDA approved for use in RCC.125,126 In a 626-patient study enrolling patients with poor-prognosis RCC, patients who received temsirolimus (Torisel, Pfizer Inc.), an IV sirolimus ester, achieved an OS of 10.9 months versus 7.3 months for patients who received IFN-α monotherapy (P = .008).125 This trial was also unique in that 20% of patients had non–clear cell histology, and only two thirds had undergone prior nephrectomy. A 410-patient study assessed the efficacy of everolimus (Afinitor, Novartis AG), an orally bioavailable treatment, in patients who had previously received sorafenib, sunitinib, or both. Patients were randomized 2:1 between everolimus and placebo. Patients who received everolimus showed a PFS of 4.0 months versus 1.9 months in the placebo arm.126
Mesenchymal Tumors
Angiomyolipoma
A strong association exists between angiomyolipomas and tuberous sclerosis.127 However, sporadic angiomyolipomas are being recognized with increasing frequency on cross-sectional imaging examinations. Although 80% of patients with tuberous sclerosis have an angiomyolipoma, less than 40% of patients with an angiomyolipoma have an element of the tuberous sclerosis complex.
Tuberous sclerosis, or Bourneville’s disease (Figure 18-25), is caused by an autosomal dominant gene with variable expressivity. Only approximately 50% of patients with tuberous sclerosis have family members with one or more manifestations of the disease. The tuberous sclerosis syndrome includes epilepsy, mental retardation, and various hamartomas. In addition to renal angiomyolipomas and multiple renal cysts, patients often develop retinal phakomas and cerebral hamartomas. Adenoma sebaceum may be seen in the malar areas of the face. In some patients, the clinical syndrome is incompletely manifest. Among patients with tuberous sclerosis, the angiomyolipomas are usually multiple and bilateral. In patients without tuberous sclerosis, the tumors are usually solitary. The female predilection seen in sporadic angiomyolipomas does not occur in patients with tuberous sclerosis. The angiomyolipomas associated with tuberous sclerosis occur at a younger age and tend to be larger in size than the sporadic lesions. As the spatial resolution of CT and ultrasound continues to improve, tiny angiomyolipomas are being detected in asymptomatic patients as incidental findings.
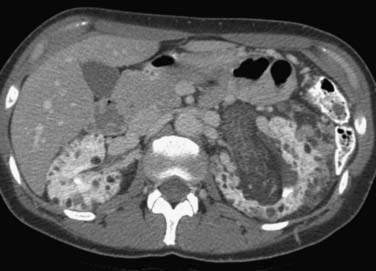
Figure 18-25 Axial CT scan in a patient with tuberous sclerosis shows multiple bilateral fat-containing angiomyolipomas.
The typical ultrasonographic finding of a highly echogenic renal mass depends on the fat content of the tumor.128 If there is relatively little fat, the ultrasonographic pattern will be indistinguishable from other renal masses. Even the very echogenic appearance can occasionally be mimicked by renal adenocarcinoma. If there has been hemorrhage, sonolucent areas may be seen.
The most reliable imaging modality for angiomyolipoma is CT.129 The detection of a renal mass of fat density makes the diagnosis of angiomyolipoma highly likely. Although Wilms’ tumor, oncocytoma, metastasis, and renal adenocarcinoma have all been reported with macroscopic fat demonstrable in the tumor mass, these are rare occurrences. When fat is seen with renal carcinoma, it is due either to a large tumor that engulfs perinephric fat or to osseous metaplasia in which calcifications are present. Because angiomyolipomas do not calcify, the presence of calcification should suggest RCC, even when fat is identified. Both lipomas and liposarcomas also may be fat-dense and, thus, are indistinguishable from an angiomyolipoma by imaging techniques; however, they are very rare renal tumors. Because some angiomyolipomas contain little or no fat, the absence of fat does not exclude the diagnosis of angiomyolipoma. These tumors are sometimes referred to as “lipid-poor.” Angiomyolipomas without detectable fat are homogeneous tumors with attenuation higher than normal renal parenchyma. They demonstrate homogeneous enhancement after the IV administration of contrast material.
MRI also can detect fat within an angiomyolipoma (Figure 18-26). A high signal intensity is seen on both T1- and T2-weighted images. When clearly imaged, MRI should have the same accuracy as CT in diagnosing an angiomyolipoma. However, MRI is probably not as sensitive as CT in detecting fat in small tumors.130,131
Lymphoma
Most patients with renal lymphoma have disease in other locations that dominates the clinical presentation. Fever, weight loss, and palpable adenopathy are common complaints. Occasionally, diffuse involvement of both kidneys or ureteral obstruction by adenopathy may compromise renal function. Renal lymphoma is usually clinically silent and occurs late in the course of the disease.
CT is routinely used to stage and monitor patients with malignant lymphoma and is an excellent method of detecting renal involvement. Lymphomatous masses are homogeneous and rounded. Renal lymphoma is difficult to detect on unenhanced examinations unless the masses are large. After IV contrast administration, lymphoma is usually well-demarcated from the normal parenchyma by its diminished enhancement. The CT pattern reflects the pathologic involvement as a solitary mass, multiple nodules, or diffuse involvement. Adjacent lymphadenopathy and direct tumor extension to the kidney also are well depicted with CT. Renal or perirenal involvement (Figure 18-27) without evidence on CT of other retroperitoneal disease is common. Positron-emission tomography (PET)/CT is also very useful in detecting and characterizing renal involvement.
Solid Tumor Metastases
Although renal metastases are common in autopsy series, where they outnumber primary renal malignancy 4:1, they are not commonly seen clinically. Most patients with renal metastases have widespread metastatic disease, and imaging studies are not needed to demonstrate renal involvement as well. In the series reported by Choyke and coworkers,49 more than 50% of the patients died within 3 months of the demonstration of renal metastases.
Renal metastases are most commonly detected by CT because this modality is most frequently used to stage and monitor oncology patients (Figure 18-28). Unenhanced scans are seldom useful in detecting metastases but may be helpful in excluding renal calculi. Metastases often are small and multiple, but certain primary tumors such as colonic carcinomas may produce a solitary large renal metastasis. These may be indistinguishable from primary renal adenocarcinomas. Renal biopsy may be required to make this distinction. Tumor invasion of the renal vein and extension into the IVC is commonly seen in renal adenocarcinoma but is rare in renal metastases. Although vascular lesions may enhance as much as the normal renal parenchyma during the early vascular phase, they later become hypodense. Small lesions may resemble cysts but can be differentiated by their higher density.
1. Lopez-Beltran A., et al. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798-805.
2. Parkin D.M., Whelan S.L., Ferlay J., et al. Cancer Incidence in Five Continents. Lyon, France: IARC Press, 2002.
3. American Cancer Society. Cancer Facts and Figures. Atlanta: ACS; 2011.
4. McLaughlin J.K., Lipworth L., Tarone R.E. Epidemiologic aspects of renal cell carcinoma. Semin Oncol. 2006;33:527-533.
5. Eble J.M., Sauter G., Epstein J.I., Sesterhenn I.A. Pathology and genetics of tumors of the urinary system and male genital organs. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2004.
6. Hunt J.D., et al. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101-108.
7. Bergstrom A., et al. Obesity and renal cell cancer—a quantitative review. Br J Cancer. 2001;85:984-990.
8. Murai M., Oya M. Renal cell carcinoma: etiology, incidence and epidemiology. Curr Opin Urol. 2004;14:229-233.
9. Geyer J.R., Poutasse E.F. Incidence of multiple renal arteries on aortography. Report of a series of 400 patients, 381 of whom had arterial hypertension. JAMA. 1962;182:120-125.
10. Williams P.L., Warwick R., Dyson M., Bannister L.H., et al. The urinary organs. Gray’s Anatomy. 37th ed.. New York: Churchill Livingstone; 1989. :1397-1416
11. Turkvatan A., et al. Multidetector CT angiography of renal vasculature: normal anatomy and variants. Eur Radiol. 2009;19:236-244.
12. Shanks J.H. Pathology of renal cell carcinoma: recent developments. Clin Oncol (R Coll Radiol). 1999;11:263-268.
13. Kovacs G., et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131-133.
14. Fuhrman S.A., Lasky L.C., Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655-663.
15. Cohen H.T., McGovern F.J. Renal-cell carcinoma. N Engl J Med. 2005;353:2477-2490.
16. Suzigan S., et al. Multilocular cystic renal cell carcinoma: a report of 45 cases of a kidney tumor of low malignant potential. Am J Clin Pathol. 2006;125:217-222.
17. Nassir A., et al. Multilocular cystic renal cell carcinoma: a series of 12 cases and review of the literature. Urology. 2002;60:421-427.
18. Zbar B., Linehan W.M. Hereditary papillary renal cell carcinoma: clinical studies in 10 families. J Urol. 1996;156:1781.
19. Fahmy W., et al. Multiple/bilateral renal tumors in patients with Birt-Hogg-Dubé syndrome. Int Urol Nephrol. 2007;39:995-999.
20. Yoon S.K., et al. Collecting duct carcinoma of the kidney: CT and pathologic correlation. Eur J Radiol. 2006;57:453-460.
21. Blitman N.M., et al. Renal medullary carcinoma: CT and MRI features. AJR Am J Roentgenol. 2005;185:268-272.
22. Choyke P.L., et al. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194:629-642.
23. Crotty T.B., Farrow G.M., Lieber M.M. Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol. 1995;154:964-967.
24. Choyke P.L., et al. Renal cell carcinoma staging. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215(Suppl):721-725.
25. Staudenherz A., et al. Is there a diagnostic role for bone scanning of patients with a high pretest probability for metastatic renal cell carcinoma? Cancer. 1999;85:153-155.
26. Fielding J.R., et al. Staging of 119 patients with renal cell carcinoma: the yield and cost-effectiveness of pelvic CT. AJR Am J Roentgenol. 1999;172:23-25.
27. Hartman D.S. Cysts and cystic neoplasms. Urol Radiol. 1990;12:7-10.
28. Yuh B.I., Cohan R.H. Different phases of renal enhancement: role in detecting and characterizing renal masses during helical CT. AJR Am J Roentgenol. 1999;173:747-755.
29. Cohan R.H., et al. Renal masses: assessment of corticomedullary-phase and nephrographic-phase CT scans. Radiology. 1995;196:445-451.
30. Kopka L., et al. Dual-phase helical CT of the kidney: value of the corticomedullary and nephrographic phase for evaluation of renal lesions and preoperative staging of renal cell carcinoma. AJR Am J Roentgenol. 1997;169:1573-1578.
31. Kim J.K., et al. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol. 2002;178:1499-1506.
32. Herts B.R., et al. Enhancement characteristics of papillary renal neoplasms revealed on triphasic helical CT of the kidneys. AJR Am J Roentgenol. 2002;178:367-372.
33. Kearney G.P., et al. Results of inferior vena cava resection for renal cell carcinoma. J Urol. 1981;125:769-773.
34. Davits R.J., Blom J.H., Schroder F.H. Surgical management of renal carcinoma with extensive involvement of the vena cava and right atrium. Br J Urol. 1992;70:591-593.
35. Kallman D.A., et al. Renal vein and inferior vena cava tumor thrombus in renal cell carcinoma: CT, US, MRI and venacavography. J Comput Assist Tomogr. 1992;16:240-247.
36. Oto A., et al. Inferior vena cava tumor thrombus in renal cell carcinoma: staging by MR imaging and impact on surgical treatment. AJR Am J Roentgenol. 1998;171:1619-1624.
37. Bos S.D., Mensink H.J. Can duplex Doppler ultrasound replace computerized tomography in staging patients with renal cell carcinoma? Scand J Urol Nephrol. 1998;32:87-91.
38. Welch T.J., LeRoy A.J. Helical and electron beam CT scanning in the evaluation of renal vein involvement in patients with renal cell carcinoma. J Comput Assist Tomogr. 1997;21:467-471.
39. Habboub H.K., et al. Accuracy of color Doppler sonography in assessing venous thrombus extension in renal cell carcinoma. AJR Am J Roentgenol. 1997;168:267-271.
40. Hricak H., et al. Detection and staging of renal neoplasms: a reassessment of MR imaging. Radiology. 1988;166:643-649.
41. Roubidoux M.A., et al. Renal carcinoma: detection of venous extension with gradient-echo MR imaging. Radiology. 1992;182:269-272.
42. Goldfarb D.A., et al. Magnetic resonance imaging for assessment of vena caval tumor thrombi: a comparative study with venacavography and computerized tomography scanning. J Urol. 1990;144:1100-1103. discussion 1103–1104
43. Hallscheidt P.J., et al. Preoperative staging of renal cell carcinoma with inferior vena cava thrombus using multidetector CT and MRI: prospective study with histopathological correlation. J Comput Assist Tomogr. 2005;29:64-68.
44. Hatcher P.A., et al. Surgical management and prognosis of renal cell carcinoma invading the vena cava. J Urol. 1991;145:20-23. discussion 23–24
45. Giberti C., et al. Radical nephrectomy for renal cell carcinoma: long-term results and prognostic factors on a series of 328 cases. Eur Urol. 1997;31:40-48.
46. Choyke P., et al. ACR appropriateness criteria: renal cell carcinoma staging. 2002. Available at www.acr.org, 2002 [cited]
47. Hilton S. Imaging of renal cell carcinoma. Semin Oncol. 2000;27:150-159.
48. Pretorius E.S., Wickstrom M.L., Siegelman E.S. MR imaging of renal neoplasms. Magn Reson Imaging Clin North Am. 2000;8:813-836.
49. Choyke P.L. Detection and staging of renal cancer. Magn Reson Imaging Clin North Am. 1997;5:29-47.
50. Zhang J., et al. Masses and pseudomasses of the kidney: imaging spectrum on MR. J Comput Assist Tomogr. 2004;28:588-595.
51. Tuite D.J., et al. Three-dimensional gadolinium-enhanced magnetic resonance breath-hold FLASH imaging in the diagnosis and staging of renal cell carcinoma. Clin Radiol. 2006;61:23-30.
52. Hallscheidt P.J., et al. Diagnostic accuracy of staging renal cell carcinomas using multidetector-row computed tomography and magnetic resonance imaging: a prospective study with histopathologic correlation. J Comput Assist Tomogr. 2004;28:333-339.
53. Frohmuller H.G., Grups J.W., Heller V. Comparative value of ultrasonography, computerized tomography, angiography and excretory urography in the staging of renal cell carcinoma. J Urol. 1987;138:482-484.
54. Fritzsche P.J., Millar C. Multimodality approach to staging renal cell carcinoma. Urol Radiol. 1992;14:3-7.
55. Choyke P.L., Daryanani K. Intraoperative ultrasound of the kidney. Ultrasound Q. 2001;17:245-253.
56. Campbell S.C., et al. Intraoperative evaluation of renal cell carcinoma: a prospective study of the role of ultrasonography and histopathological frozen sections. J Urol. 1996;155:1191-1195.
57. Gill I.S., et al. Laparoscopic renal cryoablation in 32 patients. Urology. 2000;56:748-753.
58. Russo P. Renal cell carcinoma: presentation, staging, and surgical treatment. Semin Oncol. 2000;27:160-176.
59. Chae E.J., et al. Renal cell carcinoma: analysis of postoperative recurrence patterns. Radiology. 2005;234:189-196.
60. Griffin N., Gore M.E., Sohaib S.A. Imaging in metastatic renal cell carcinoma. AJR Am J Roentgenol. 2007;189:360-370.
61. Ghavamian R., et al. Renal cell carcinoma metastatic to the pancreas: clinical and radiological features. Mayo Clin Proc. 2000;75:581-585.
62. Ng C.S., et al. Metastases to the pancreas from renal cell carcinoma: findings on three-phase contrast-enhanced helical CT. AJR Am J Roentgenol. 1999;172:1555-1559.
63. Robson C.J., Churchill B.M., Anderson W. The results of radical nephrectomy for renal cell carcinoma. Trans Am Assoc Genitourin Surg. 1968;60:122-129.
64. Skinner D.G., Vermillion C.D., Colvin R.B. The surgical management of renal cell carcinoma. J Urol. 1972;107:705-710.
65. Robson C.J., Churchill B.M., Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101:297-301.
66. Sagalowsky A.I., et al. Factors influencing adrenal metastasis in renal cell carcinoma. J Urol. 1994;151:1181-1184.
67. Kletscher B.A., et al. Prospective analysis of the incidence of ipsilateral adrenal metastasis in localized renal cell carcinoma. J Urol. 1996;155:1844-1846.
68. Tsui K.H., et al. Is adrenalectomy a necessary component of radical nephrectomy? UCLA experience with 511 radical nephrectomies. J Urol. 2000;163:437-441.
69. Blom J.H., et al. Radical nephrectomy with and without lymph node dissection: preliminary results of the EORTC randomized phase III protocol 30881. EORTC Genitourinary Group. Eur Urol. 1999;36:570-575.
70. Vasselli J.R., et al. Lack of retroperitoneal lymphadenopathy predicts survival of patients with metastatic renal cell carcinoma. J Urol. 2001;166:68-72.
71. Pizzocaro G., Piva L., Salvioni R. Lymph node dissection in radical nephrectomy for renal cell carcinoma: is it necessary? Eur Urol. 1983;9:10-12.
72. Tsukamoto T., et al. Regional lymph node metastasis in renal cell carcinoma: incidence, distribution and its relation to other pathological findings. Eur Urol. 1990;18:88-93.
73. Terrone C., et al. The number of lymph nodes examined and staging accuracy in renal cell carcinoma. BJU Int. 2003;91:37-40.
74. Ditonno P., et al. Role of lymphadenectomy in renal cell carcinoma. Prog Clin Biol Res. 1992;378:169-174.
75. Blute M.L., et al. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol. 2004;172:465-469.
76. Studer U.E., et al. Enlargement of regional lymph nodes in renal cell carcinoma is often not due to metastases. J Urol. 1990;144:243-245.
77. Minervini A., et al. Regional lymph node dissection in the treatment of renal cell carcinoma: is it useful in patients with no suspected adenopathy before or during surgery? BJU Int. 2001;88:169-172.
78. Pantuck A.J., et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol. 2003;169:2076-2083.
79. Margulis V., et al. Analysis of clinicopathologic predictors of oncologic outcome provides insight into the natural history of surgically managed papillary renal cell carcinoma. Cancer. 2008;112:1480-1488.
80. Novick A.C., et al. Long-term follow-up after partial removal of a solitary kidney. N Engl J Med. 1991;325:1058-1062.
81. Belldegrun A., et al. Efficacy of nephron-sparing surgery for renal cell carcinoma: analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol. 1999;17:2868-2875.
82. Herr H.W. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year follow-up. J Urol. 1999;161:33-34. discussion 34–35
83. Lau W., Zincke H. Matched comparison of radical nephrectomy versus elective nephron-sparing surgery for renal cell carcinoma (RCC): evidence for increased renal failure rate on long term follow-up (>10 years). J Urol. 2000;163:S153.
84. Clark P.E., et al. Quality of life and psychological adaptation after surgical treatment for localized renal cell carcinoma: impact of the amount of remaining renal tissue. Urology. 2001;57:252-256.
85. Ficarra V., et al. Psycho-social well-being and general health status after surgical treatment for localized renal cell carcinoma. Int Urol Nephrol. 2002;34:441-446.
86. Shinohara N., et al. Impact of nephron-sparing surgery on quality of life in patients with localized renal cell carcinoma. Eur Urol. 2001;39:114-119.
87. American Urological Association. Guideline for Management of the Clinical Stage 1 Renal Mass [cited]. Available at http://www.auanet.org
88. Clayman R.V., et al. Laparoscopic nephroureterectomy: initial clinical case report. J Laparoendosc Surg. 1991;1:343-349.
89. Permpongkosol S., et al. Long-term survival analysis after laparoscopic radical nephrectomy. J Urol. 2005;174:1222-1225.
90. Matin S.F., et al. Evaluation of age and comorbidity as risk factors after laparoscopic urological surgery. J Urol. 2003;170:1115-1120.
91. Fazeli-Matin S., et al. Laparoscopic renal and adrenal surgery in obese patients: comparison to open surgery. J Urol. 1999;162:665-669.
92. Meraney A.M., Gill I.S. Financial analysis of open versus laparoscopic radical nephrectomy and nephroureterectomy. J Urol. 2002;167:1757-1762.
93. Matin S.F., Madsen L.T., Wood C.G. Laparoscopic cytoreductive nephrectomy: the M. D. Anderson Cancer Center experience. Urology. 2006;68:528-532.
94. Walther M.M., et al. Laparoscopic cytoreductive nephrectomy as preparation for administration of systemic interleukin-2 in the treatment of metastatic renal cell carcinoma: a pilot study. Urology. 1999;53:496-501.
95. Cohen D.D., et al. Evaluation of the intact specimen after laparoscopic radical nephrectomy for clinically localized renal cell carcinoma identifies a subset of patients at increased risk for recurrence. J Urol. 2005;173:1487-1490. discussion 1490–1491
96. Levy D.A., et al. Timely delivery of biological therapy after cytoreductive nephrectomy in carefully selected patients with metastatic renal cell carcinoma. J Urol. 1998;159:1168-1173.
97. Fallick M.L., et al. Nephrectomy before interleukin-2 therapy for patients with metastatic renal cell carcinoma. J Urol. 1997;158:1691-1695.
98. Rackley R., et al. The impact of adjuvant nephrectomy on multimodality treatment of metastatic renal cell carcinoma. J Urol. 1994;152:1399-1403.
99. Wood C.G. The role of cytoreductive nephrectomy in the management of metastatic renal cell carcinoma. Urol Clin North Am. 2003;30:581-588.
100. Thomas A.A., et al. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol. 2009;181:518-523. discussion 523
101. Jonasch E., et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4076-4081.
102. Thomas A.A., et al. Surgical resection of renal cell carcinoma after targeted therapy. J Urol. 2009;182:881-886.
103. Amin C., et al. Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology. 2008;72:864-868.
104. Margulis V., et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008;180:94-98.
105. Bex A., et al. Neoadjuvant sunitinib for surgically complex advanced renal cell cancer of doubtful resectability: initial experience with downsizing to reconsider cytoreductive surgery. World J Urol. 2009;27:533-539.
106. Wood C.G., Margulis V. Neoadjuvant (presurgical) therapy for renal cell carcinoma: a new treatment paradigm for locally advanced and metastatic disease. Cancer. 2009;115(Suppl 10):2355-2360.
107. Castilla E.A., et al. Prognostic importance of resection margin width after nephron-sparing surgery for renal cell carcinoma. Urology. 2002;60:993-997.
108. Sutherland S.E., et al. Does the size of the surgical margin in partial nephrectomy for renal cell cancer really matter? J Urol. 2002;167:61-64.
109. Piper N.Y., et al. Is a 1-cm margin necessary during nephron-sparing surgery for renal cell carcinoma? Urology. 2001;58:849-852.
110. Gill I.S., et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol. 2003;170:64-68.
111. Benway B.M., et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866-872.
112. Matin S.F., et al. Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol. 2006;176:1973-1977.
113. Weight C.J., et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008;179:1277-1281. discussion 1281–1283
114. Matin S. Determining failure after renal ablative therapy for renal cell carcinoma: false-negative and false-positive imaging findings. Urology. 2010;75:1254-1257.
115. Fyfe G., et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688-696.
116. Latif F., et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317-1320.
117. Maxwell P.H., et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271-275.
118. Escudier B., et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125-134.
119. Motzer R.J., et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115-124.
120. Escudier B., et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103-2111.
121. Rini B.I., et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422-5428.
122. Sternberg C.N., et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
123. Motzer R.J., Figlin R.A., Hutson T.E., et al. Sunitinib versus interferon alfa (IFN-α) as first-line treatment of metastatic renal carcinoma (mRCC): updated results and analysis of prognostic factors. J Clin Oncol. 2007;18S:5024.
124. Motzer R.J., et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
125. Hudes G., et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271-2281.
126. Motzer R.J., et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456.
127. Bissler J.J. Kingswood JC. Renal angiomyolipomata. Kidney Int. 2004;66:924-934.
128. Lemaitre L., et al. Imaging of angiomyolipomas. Semin Ultrasound CT MR. 1997;18:100-114.
129. Bosniak M.A., et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentgenol. 1988;151:497-501.
130. Kim J.Y., et al. CT histogram analysis: differentiation of angiomyolipoma without visible fat from renal cell carcinoma at CT imaging. Radiology. 2008;246:472-479.
131. O.A. Catalano. et al. Pixel distribution analysis: can it be used to distinguish clear cell carcinomas from angiomyolipomas with minimal fat?. Radiology.. 2008;247:738-746.