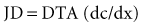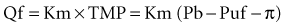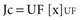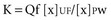115 Renal Replacement Therapy
 Principles of Renal Replacement Therapy
Principles of Renal Replacement Therapy
The principles of renal replacement therapy have been extensively studied and described.1–3 The two fundamental principles of renal replacement therapy particularly relevant to critical care physicians are summarized here.
Water Removal
Solute Removal
where J is solute flux, D is diffusion coefficient, T is temperature of the solution, A is membrane surface area, dc is concentration gradient between the two compartments, and dx is diffusion distance (thickness of the membrane). In dialysis, blood and dialysate are separated by a membrane. Bidirectional diffusive transport of molecules occurs in response to a concentration gradient.
where Qf is filtration, Km is coefficient of permeability of the membrane, TMP is transmembrane pressure, Pb is hydrostatic pressure of blood, Puf is hydrostatic pressure in the ultrafiltrate compartment, and π is oncotic pressure of blood. In convective treatments, the transport (Jc) of solute x is governed by the formula:
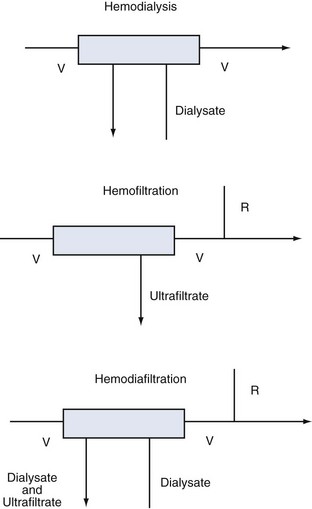
Despite these distinctions, diffusion and convection often act simultaneously, and it is almost impossible to divide these transport mechanisms physically. The term hemodialysis may not aptly describe the mode of treatment in the case of highly permeable membranes. A more suitable term would be hemodiafiltration (if replacement solution is needed) or high-flux dialysis (if a filtration-back filtration mechanism is present and no replacement fluid is required). The various modalities are described in Figure 115-1.
 Indications for Renal Replacement Therapy
Indications for Renal Replacement Therapy
The treatment of AKI requires a different style and philosophy from renal replacement therapy for chronic renal failure. In a critically ill patient, renal replacement therapy should be initiated early. It is physiologically irrational and clinically dangerous to wait for complications to appear before intervening. Fear of early dialysis stems from the well-known adverse effects of conventional intermittent hemodialysis with cuprophane membranes, especially hemodynamic instability, and from the risks and limitations of continuous or intermittent peritoneal dialysis.4–7 If extended dialysis techniques are used, they are minimized.8 Accordingly, the time-honored criteria for initiation of renal replacement therapy in patients with chronic renal failure may be inappropriate in critically ill patients.9 Modern criteria for initiation of renal replacement therapy in the intensive care unit (ICU) are presented in Table 115-1.
TABLE 115-1 Modern Criteria for Initiation of Renal Replacement Therapy in the ICU*
* If one criterion is present, renal replacement therapy should be considered. If two criteria are simultaneously present, renal replacement therapy is strongly recommended.
Once intermittent hemodialysis or continuous hemofiltration has been started, there are limited data on what is an “adequate” dose of dialysis. The concept of dialysis adequacy in AKI remains controversial and ill defined, and the current goal is maintenance of homeostasis at all levels.10 Emerging data suggest that better uremic control may translate into better survival.11–13 Patients at least should have urea levels maintained between 10 and 20 mmol/L throughout the treatment period. This level of uremic control should occur despite adequate nutrition support with a protein intake around 1.5 g/kg/d. If intermittent hemodialysis is used, daily treatment and/or extended treatment are more desirable,8 with the goal of ensuring at least some adequacy for small-solute removal. This means intermittent hemodialysis must guarantee at least a daily urea clearance in liters greater than or equal to the patient’s total body water. Total body water can be calculated from tables or simply as 60% of body weight. This relationship will be treated extensively in the following discussion.
 Continuous Renal Replacement Therapy
Continuous Renal Replacement Therapy
Continuous renal replacement therapy (CRRT) is now the most common form of renal replacement therapy in Australian and European ICUs. In the United States, however, CRRT reportedly is used in only 10% to 20% of ICU patients.14 CRRT has undergone several technical modifications since it was first described in 1977. Initially it was performed as an arteriovenous therapy (continuous arteriovenous hemofiltration) in which blood flow through the hemofilter was driven by the patient’s blood pressure. Clearances were low, however, and countercurrent dialysate flow soon was added to double or triple solute clearances (continuous arteriovenous hemodialysis/diafiltration) with or without spontaneous ultrafiltration. Double-lumen catheters and peristaltic blood pumps have come into use with or without control of ultrafiltration rate.
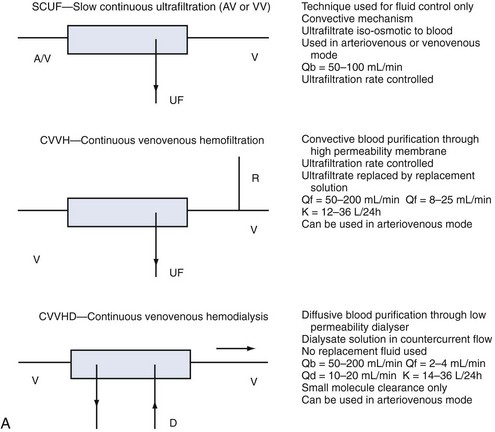
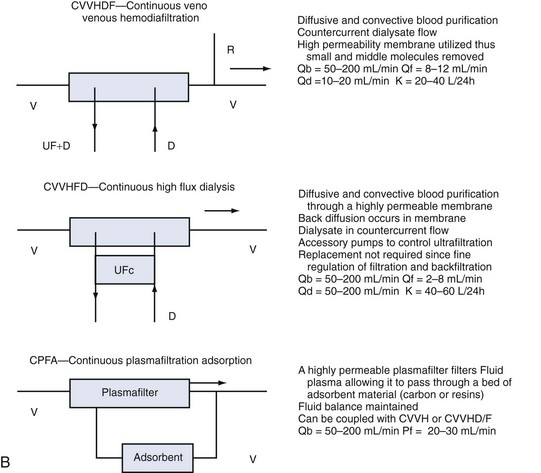
Whatever the technique of CRRT, the clearances achieved can be adjusted by adjusting ultrafiltration rate or dialysate flow rate or both, typically aiming to achieve a daily clearance at least equal to the patient’s total body water. A standardized nomenclature is now available for CRRT techniques.15 To make the reading easy and to make the reader familiar with the most accepted definitions and treatment schemes, we have summarized in Figure 115-2 the complete set of available techniques, including some hints on operational parameters. No matter what technique is used, the following outcomes are predictable, and the most important will be described:
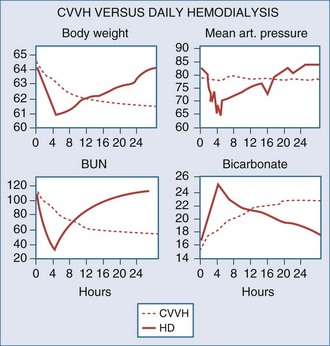
All critically ill patients need a high daily amount of volume infusions: blood and fresh frozen plasma, vasopressors and other continuous infusions, parenteral and enteral nutrition, which should be delivered without restriction or interruption. It is not uncommon for patients with AKI and associated septic shock to receive large amounts of fluid resuscitation, leading to fluid overload. The consequent positive fluid balance and tendency to interstitial edema causes the necessity for water removal and possibly the achievement of a negative daily fluid balance. Extracorporeal renal replacement therapies are typically utilized for ultrafiltration. Ultrafiltered water has a similar osmolarity to plasma water; for this reason, the process of “isolated ultrafiltration” substantially corresponds to blood dehydration, with possible increase of hematocrit values and smallest modification of solutes concentration.16 CRRT slowly and continuously removes a patient’s plasma water, mimicking urine output, whereas thrice-weekly intermittent hemodialysis must extract in few hours the equivalent of 2 days of administered fluids plus excess body water that may be present in the anuric patient. Intravascular volume depletion associated with excessive ultrafiltration rate is due to both the high rate of fluid removal required and the transcellular and interstitial fluid shifts caused by the rapid dialytic loss of solute. The major consequence of rapid fluid removal is hemodynamic instability. Consider the case of a septic patient with AKI who is receiving a high amount of vasopressors because of hemodynamic instability and needs appropriate fluid resuscitation, supplementation of nutrition, and blood product administration. The renal replacement modality of choice seems to be the one that warrants slow fluid removal, prolonged for many hours a day, to easily meet the highly variable required daily fluid balance. In particular, when volemic and uremic control is not a problem, an aggressive protein-rich nutritional policy (1.5–2.5 g/d) can be implemented in the care of AKI patients receiving CRRT, resulting in a marked improvement in daily nitrogen balance with possible favorable effects on immune function and overall outcome.17 Safe prescription of fluid loss during renal replacement therapy requires intimate knowledge of the patient’s underlying condition, understanding of the process of ultrafiltration, and close monitoring of the patient’s cardiovascular response to fluid removal. To preserve tissue perfusion in patients with AKI, it is important to optimize fluid balance by removing the patient’s excess water without compromising effective circulating fluid volume. It is still a matter of controversy which clinical parameter (actual patient weight/patient dry weight, mean arterial pressure, central venous pressure, wedge pressure, systemic saturation, mixed venous saturation, bioimpedance, etc.) or currently available monitoring (central venous catheter, Swan-Ganz catheter, transesophageal echocardiography, etc.) should be utilized to uniformly define the concept of “volume overload.” In patients who are clinically fluid overloaded, however, it is extremely important to accurately evaluate the amount of fluid to remove18; one of the main features of slow and constant ultrafiltration is the possibility for interstitial fluid to slowly and constantly refill the “dehydrated” bloodstream. This phenomenon is driven by hydrostatic and osmotic forces and allows for elimination of high plasma water volumes per day, with a reduced risk of hypovolemia and hypotension. In critically ill children, correction of water overload is considered a priority; it has been shown and recently confirmed that restoring adequate water content in small children is the main independent variable for outcome prediction.19 Similar results have been recently found in a large cohort of adult critically ill patients with AKI.20
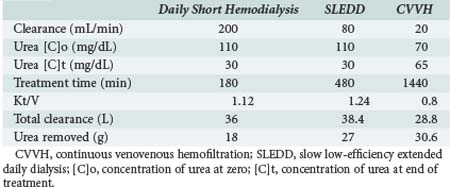
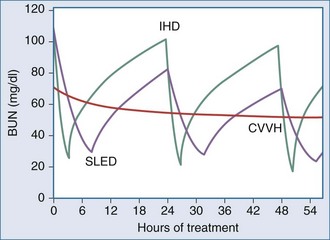
One of the measures utilized to quantify urea/creatinine removal is dialysis dose. One of the main aspects of dose to be understood is the concept of clearance (K): K is the volume of blood cleared from a given solute over a given time. K does not reflect the overall solute removal rate (mass transfer) but rather its value normalized by the serum concentration. Even when K remains stable over time, the removal rate will vary if the blood levels of the reference molecule change. K depends on solute molecular size, intercompartmental transmittance (Kc), transport modality (diffusion or convection), and circuit operational characteristics (blood flow rate, dialysate flow rate, ultrafiltration rate, hemodialyzer type, and size). As originally conceived, K is utilized to evaluate renal function among disparate individuals whose kidneys are operating 24 hours a day and urea/creatinine blood levels are at steady state. For this reason, the concept of K is easily applicable to continuous treatments, and its use to describe intermittent therapy efficiency is a sort of adaptation. Because K represents only the instantaneous efficiency of the system, during treatments with different time schedules, information about the time span during which K is delivered is fundamental to compare the different RRT doses. For example, K is typically higher in IHD than in CRRT and sustained low-efficiency daily dialysis. However, daily mass removal may be greater during CRRT or sustained low-efficiency daily dialysis because the K is applied for 12/24 hours (Table 115-2). In any case, from a physiologic point of view, even if a continuous and an intermittent therapy were prescribed in order to provide exactly the same marker solute removal, still they could not be comparable: during continuous treatments, where a relatively low K is applied, a slow but prolonged removal of solutes approaches a pseudo-steady state slope (Figure 115-3). In highly intermittent therapies, the intensive K, limited to 4 to 6 hours per day, thrice a week, causes the sawtooth slope in solute removal and eventual rebound during the time span without treatment. These peaks and valleys of solutes, bicarbonate, electrolytes, plasma osmolarity, and volemia are not physiologic and might have a detrimental impact on a patient’s hemodynamics and the balance of electrolytes, acid-base, and other “osmoles.” Furthermore, in the case of intermittent hemodialysis, the Kc (i.e., the variable tendency of different tissues to “release” a solute into the bloodstream) is much more relevant than during low-efficiency treatments. As a matter of fact, solute control is optimized during CRRT.
It has been calculated that if the solute target in a 70-kg patient was, for example, a mean blood urea nitrogen level of 60 mg/dL, this would be easily obtainable with a “standard” continuous venovenous hemofiltration dose, but it might be very difficult to be reached by even intensive intermittent hemodialysis regimens.16 Some authors have recently suggested expressing CRRT daily dose as K indexed to patient body weight. The current recommendation is to administer a CRRT dose between 25 and 35 mL/h/kg per 24 hours.21 Simplifying for low-molecular-weight solutes, K equals replacement solution and/or dialysate flow, and “standard” dose of a CRRT session may be expressed in a 70-kg patient as about 2500 mL/h (35 mL/h × 70 kg) per 24 hours or 60 L/day (2500 mL/h × 24 h) of replacement solution during continuous venovenous hemofiltration (CVVH) or of dialysate during continuous venovenous hemodialysis (CVVHD). It is expected this recommended dose will be modified in the next years, after the production of new evidence in this field (see later).
Oligoanuric patients often have mild acidemia secondary to increased unmeasured anions (strong ion gap [SIG] 12.3 mEq/L), hyperphosphatemia, and hyperlactatemia. This acidosis is attenuated by the alkalizing effect of hypoalbuminemia. Uchino and coworkers22 compared the effect on acid-base balance of intermittent hemodialysis and continuous venovenous hemodiafiltration. Before treatment, metabolic acidosis was common in both groups (63.2% for intermittent hemodialysis and 54.3% for continuous venovenous hemodiafiltration). Both intermittent hemodialysis and continuous venovenous hemodiafiltration corrected metabolic acidosis, but the rate and degree of correction differed significantly. Continuous venovenous hemodiafiltration normalized metabolic acidosis more rapidly and more effectively during the first 24 hours than did intermittent hemodialysis (P < 0.01). Intermittent hemodialysis was also associated with a higher incidence of metabolic acidosis than was continuous venovenous hemodiafiltration during the subsequent 2-week treatment period. Accordingly, continuous venovenous hemodiafiltration can be considered physiologically superior to intermittent hemodialysis in the correction of metabolic acidosis.
In a comparison between CVVH and peritoneal dialysis, all patients randomized to CVVH achieved correction of acidosis by 50 hours of treatment, compared with only 15% of those treated by peritoneal dialysis (P < 0.001).23
Rocktaschel showed that once CVVH is commenced, acidemia is corrected within 24 hours. This change is associated with a decreased SIG and decreased phosphate and chloride concentrations. After 3 days of CVVH, patients develop alkalemia secondary to metabolic alkalosis due to a further decrease in SIG and a decrease in serum phosphate concentration in the setting of persistent hypoalbuminemia.24
Anticoagulation During Continuous Renal Replacement Therapy
The flow of blood through an extracorporeal circuit causes activation of the coagulation cascade and promotes clotting of the filter and circuit itself. To delay such clotting and achieve acceptable operational life (≈24 hours) for the circuit, anticoagulation frequently is used.25 Circuit anticoagulation increases the statistical risk of bleeding for the patient, however. The clinician must weigh the risks and benefits of more or less intense anticoagulation. In this regard, the intensivist has several strategies available (Table 115-3).
TABLE 115-3 Strategies for Circuit Anticoagulation During Continuous Renal Replacement Therapy
|
Regional anticoagulation (prefilter heparin and postfilter protamine usually at a 100 units:1 mg ratio)
|
In most patients, low-dose heparin (<10 units/kg/h) is sufficient to achieve adequate filter life.26 Heparin is easy and inexpensive to administer, easy to reverse, and at these doses has almost no effect on the patient’s coagulation tests. In some patients, a higher dose is necessary. In others (pulmonary embolism, myocardial ischemia), full heparinization may be indicated concomitantly and should be pursued. Regional citrate anticoagulation is effective but requires that the hospital pharmacy or ICU use a special dialysate or replacement fluid. Citrate anticoagulation is expensive and more complex to organize. Nonetheless, it provides excellent and effective anticoagulation at minimal risk to the patient and has become a first choice of anticoagulation in many centers.25 Regional heparin/protamine anticoagulation also is complex but may be useful if frequent filter clotting occurs and further anticoagulation of the patient is considered dangerous. Low-molecular-weight heparin also is efficacious but more expensive and hard to reverse because it accumulates in renal failure. It has not been shown to provide any advantages over unfractionated heparin. Heparinoids and prostacyclin may be useful if the patient has developed heparin-induced thrombocytopenia and thrombosis and citrate is not available. Serine proteinase inhibitors have been used but are not available outside Japan. Finally, in perhaps 10% to 20% of patients, anticoagulation is best avoided because of endogenous coagulopathy or recent surgery. In such patients, mean filter life of greater than 24 hours can be achieved provided that blood flow is kept at 200 mL/min and vascular access is reliable.27
Continuous Renal Replacement Therapy Technology
The increasing use of venovenous CRRT has led to the development of a series of CRRT technologies that offer different kinds of machines to facilitate its performance.28 Some understanding of these devices is important for the successful implementation of CRRT in any ICU. The simplest technical approach is to allow ultrafiltration to occur spontaneously, measure it, and replace it as indicated. In such a system, hourly measurement of effluent is necessary, and the only requirement is that of a blood pump to deliver blood to the filter and a volumetric pump to administer replacement fluid at the appropriate rate. This approach is clearly inadequate in a modern ICU; such a system is inherently unsafe and labor intensive. A volumetric pump can regulate effluent flow easily, however. One can have a simple blood pump with safety features (air bubble trap and pressure alarms) and use widely available volumetric pumps to control replacement or dialysate flow and effluent flow. Such adaptive technology is inexpensive (approximately $10,000 U.S. dollars) but is not user-friendly. Also, volumetric pumps have an inherent inaccuracy of about 5%, which in a system exchanging 50 L/d can cause problems.28 Various manufacturers have produced custom-made machines for hemofiltration. For a detailed discussion of such machines, the reader is referred to specialist textbooks.28 These machines are safer and have much more sophisticated pump-control systems, alarms, and graphic displays. They are much more user-friendly, especially with the setup procedure. Most if not all ICUs in developed countries now conduct CRRT with third-generation devices characterized by advanced built-in technology and a high degree of automation.
 Intermittent Hemodialysis
Intermittent Hemodialysis
Intermittent hemodialysis remains dominant in the United States. Vascular access is typically by double-lumen catheter as in continuous hemofiltration. Intermittent hemodialysis machines use high dialysate flows (300–400 mL/min), however, and generate dialysate by using purified water and concentrate. Conventionally, intermittent hemodialysis is applied for short periods (3–4 hours), usually every second day. These features are summarized in Figure 115-4. The same applies to acid-base control. Limited fluid and uremic control imposes unnecessary limitations on nutritional support. Rapid solute shifts increase brain water content and intracranial pressure.29 Finally, much controversy has surrounded the issue of membrane bioincompatibility. Standard low-flux dialyzing membranes made of cuprophane are known to trigger the activation of several inflammatory pathways, much more so than high-flux synthetic membranes (also used for continuous hemofiltration). It is possible that such a proinflammatory effect contributes to further renal damage and delays recovery or even affects mortality.30,31
The serious limitations of applying “conventional” intermittent hemodialysis (3–4 h/d every second day) to the treatment of AKI have been highlighted,8 and new approaches to intermittent therapies (so-called hybrid techniques) such as slow extended dialysis, slow low-efficiency daily dialysis, and intermittent extended hemofiltration are emerging. These techniques seek to adapt intermittent hemodialysis to the clinical circumstance and increase its tolerance and clearances. In our opinion, such hybrid approaches represent a welcome improvement in dialysis support and a clear recognition that AKI patients should not receive the dialysis offered to patients with end-stage renal failure.
 Peritoneal Dialysis
Peritoneal Dialysis
Peritoneal dialysis is not commonly used in the treatment of adult AKI, either in the United States or elsewhere.32 Typically, access is by the surgical insertion of an intraperitoneal catheter. Glucose-rich dialysate is inserted into the peritoneal cavity and acts as the “dialysate.” After a given “dwell time,” it is removed and discarded with the extra fluid and toxins that have moved from the blood vessels of the peritoneum to the dialysate fluid. Machines also are available that deliver and remove dialysate at higher flows through a double-lumen peritoneal catheter, providing intermittent treatment and higher solute clearances. Several major shortcomings make peritoneal dialysis relatively unsuited to the treatment of adult AKI:
There have been no reports since the 1980s of the sole use of peritoneal dialysis for treatment of adult ICU patients with AKI. Despite this, the new technique called continuous flow peritoneal dialysis might offer something new in this field. No studies have been conducted so far. Peritoneal dialysis is a frequently used option in the case of pediatric AKI, and the typical patient is the post–cardiac surgery neonate; in these patients, peritoneal dialysis is a fundamental contributor in optimization of fluid balance.33
 Drug Prescription During Dialysis Therapy
Drug Prescription During Dialysis Therapy
AKI and the need for renal replacement therapy profoundly affect drug clearance. A comprehensive description of changes in drug dosage according to the technique of renal replacement therapy, residual creatinine clearance, and other determinants of pharmacodynamics is beyond the scope of this chapter and can be found in specialist textbooks.34 Table 115-4 provides general guidelines for the prescription of drugs that are commonly used in the ICU.
| Drug | CRRT | IHD |
|---|---|---|
| Aminoglycosides | Normal dose q 36 h | Half normal dose q 48 h; two-thirds redose after IHD |
| Cefotaxime or ceftazidime | 1 g q 8-12 h | 1 g q 12-24 h after IHD |
| Imipenem | 500 mg q 8 h | 250 mg q 8 h and after IHD |
| Meropenem | 500 mg q 8 h | 250 mg q 8 h and after IHD |
| Metronidazole | 500 mg q 8 h | 250 mg q 8 h and after IHD |
| Co-trimoxazole | Normal dose q 18 h | Normal dose q 24 h after IHD |
| Amoxicillin | 500 mg q 8 h | 500 mg daily and after IHD |
| Vancomycin | 1 g q 24 h | 1 g q 96-120 h |
| Piperacillin | 3-4 g q 6 h | 3-4 g q 8 h and after IHD |
| Ticarcillin | 1-2 g q 8 h | 1-2 g q 12 h and after IHD |
| Ciprofloxacin | 200 mg q 12 h | 200 mg q 24 h and after IHD |
| Fluconazole | 200 mg q 24 h | 200 mg q 48 h and after IHD |
| Acyclovir | 3.5 mg/kg q 24 h | 2.5 mg/kg/d and after IHD |
| Ganciclovir | 5 mg/kg/d | 5 mg/kg/48 h and after IHD |
| Amphotericin B | Normal dose | Normal dose |
| Liposomal amphotericin B | Normal dose | Normal dose |
| Ceftriaxone | Normal dose | Normal dose |
| Erythromycin | Normal dose | Normal dose |
| Milrinone | Titrate to effect | Titrate to effect |
| Amrinone | Titrate to effect | Titrate to effect |
| Catecholamines | Titrate to effect | Titrate to effect |
| Ampicillin | 500 mg q 8 h | 500 mg daily and after IHD |
CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis.
* These values represent approximations and should be used as a general guide only. Critically ill patients have markedly abnormal volumes of distribution for these agents, which affects dosage. CRRT is conducted at variable levels of intensity in different units, also requiring adjustment. The values reported here relate to continuous venovenous hemofiltration at 2 L/h of ultrafiltration. Vancomycin is variably removed during continuous venovenous therapies, and constant evaluation of serum levels is recommended. IHD also may differ from unit to unit. The values reported here relate to standard IHD with low-flux membranes for 3 to 4 hours every second day.
 Controversies in Renal Replacement Therapy
Controversies in Renal Replacement Therapy
A single-center randomized controlled trial showed that increasing the ultrafiltration rate from 20 to 35 mL/kg/h significantly increased survival.11 After this landmark study, up to 2007, the best evidence supported use of at least 35 mL/kg/h for CRRT. Lower doses of renal replacement therapy (RRT) were not recommended. More evidence finally came from two very recent trials. A small randomized controlled trial on 200 critically ill patients with AKI concluded that patient survival or renal recovery was not different between patients receiving high-dose (35 mL/kg/h) or standard-dose (20 mL/kg/h) continuous venovenous hemodiafiltration.35 A second trial under the sponsorship of the Veterans Affairs/National Institutes of Health (VA/NIH) Acute Renal Failure Trial Network, randomly assigned 1124 critically ill patients with AKI and failure of at least one nonrenal organ or sepsis to receive intensive or less intensive RRT.36 In both groups, only hemodynamically stable patients underwent intermittent hemodialysis, whereas hemodynamically unstable patients underwent continuous venovenous hemodiafiltration or sustained low-efficiency dialysis. Patients receiving the intensive treatment strategy underwent intermittent hemodialysis (Kt/V 1.2) and sustained low-efficiency dialysis 6 times per week and continuous venovenous hemodiafiltration at 35 mL/h/kg of body weight. For patients receiving the less intensive treatment strategy, corresponding treatments were provided thrice weekly and at 20 mL/h/kg. Sixty-day mortality was 53.6% with intensive therapy and 51.5% with less intensive therapy. There was no significant difference between the two groups in duration of renal replacement therapy or rate of recovery of kidney function or nonrenal organ failure. After this trial, operators might reasonably change their standard RRT dose prescriptions to a lower level than previously recommended; nonetheless, many concerns about the study have risen. On a time-averaged basis, greater urea removal occurred in patients receiving less intensive continuous renal replacement on a given day than in those receiving intensive intermittent hemodialysis. This uncertain separation of the dose during periods of unknown duration makes failure to observe a treatment effect unsurprising in the study. Of note, despite being judged clinically hemodynamically stable, the relatively high rate of severe hypotensive events in patients treated with intermittent hemodialysis may argue the ATN approach to modality assignment and suggests that from a hemodynamic point of view, a greater number of patients may have benefited from more liberal use of continuous renal replacement than that chosen for the study. In any case, it is possible that strategies other than only increasing dialysis dose might help AKI patients. Current approaches to dialysis are probably inadequate to fully replace critical functions such as regulation of fluid balance, electrolyte and acid-base homeostasis, and efficient down-regulation of the inflammatory response, which might play a major role in the pathophysiology of AKI.
The Randomized Evaluation of Normal versus Augmented Level Replacement Therapy (RENAL) Trial was planned to test the hypothesis that higher-dose continuous venovenous hemodiafiltration at an effluent rate of 40 mL/kg/h would increase survival compared to continuous veno-venous hemodiafiltration at 25 mL/kg/h of effluent dose.37 This trial randomized 1508 critically ill patients in 35 ICUs in Australia and New Zealand: 747 were randomly assigned to higher-intensity therapy and 761 to lower-intensity therapy. The two study groups received treatment for an average of 6.3 and 5.9 days, respectively. At 90 days after randomization, 322 deaths had occurred in the higher-intensity group and 332 deaths in the lower-intensity group, for a mortality of 44.7% in each group. Overall, the mortality rates were significantly lower, and recovery of kidney function in surviving patients was more common in the RENAL study than in the ATN study. It is possible that these differences are related to alternative strategies for the timing of initiation of RRT and to greater use of continuous therapy, as compared with intermittent therapy, as the initial mode of renal replacement in the RENAL Study. However, they may also be due to differences between the two study populations. Results of the ATN and RENAL studies imply that if a threshold dose of therapy must be achieved to optimize clinical outcomes, increasing the intensity of therapy beyond this dose seems not to provide further clinical benefit. Unfortunately, as recently shown by the DoReMi study group, such minimal dosing threshold is often not achieved.38
Key Points
Bellomo R. Continuous hemofiltration as blood purification in sepsis. New Horiz. 1995;3:732-737.
Bellomo R, Ronco C. Adequacy of dialysis in the acute renal failure of the critically ill: the case for continuous therapies. Int J Artif Organs. 1996;19:129-142.
Kellum JA, Mehta RL, Angus DC, et al. The first international consensus conference of CRRT. Kidney Int. 2002;62:1855-1863.
Ronco C, Bellomo R. Acute renal failure and multiple organ dysfunction in the ICU: from renal replacement therapy (RRT) to multiple organ support therapy (MOST). Int J Artif Organs. 2002;25:733-747.
Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet. 2000;355:26-30.
RENAL Replacement Therapy Study InvestigatorsBellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627-1638.
VA/NIH Acute Renal Failure Trial NetworkPalevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7-20.
Kellum JA, Ronco C. Dialysis: results of RENAL—what is the optimal CRRT target dose? Nat Rev Nephrol. 2010;6:191-192.
1 Sargent J, Gotch F. Principles and biophysics of dialysis. In: Maher J, editor. Replacement of Renal Function by Dialysis. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1989:87-102.
2 Henderson L. Biophysics of ultrafiltration and hemofiltration. In: Maher J, editor. Replacement of Renal Function by Dialysis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1989:300-332.
3 Nolph KD. Peritoneal dialysis. In: Brenner BM, Rector FC, editors. The Kidney. Philadelphia: WB Saunders; 1986:1791-1845.
4 Conger JD. Does hemodialysis delay recovery from acute renal failure? Semin Dial. 1990;3:146-154.
5 Howdieshell TR, Blalock WE, Bowen PA, et al. Management of post-traumatic acute renal failure with peritoneal dialysis. Am Surg. 1992;58:378-382.
6 Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med. 1999;25:805-813.
7 Bellomo R, Boyce N. Continuous venovenous hemodiafiltration compared with conventional dialysis in critically ill patients with acute renal failure. ASAIO J. 1993;39:M794-M797.
8 Marshall MR, Golper TA, Shaver MJ, Chatoth DK. Hybrid renal replacement modalities for the critically ill. Contrib Nephrol. 2001;132:252-257.
9 Paganini EP. Dialysis is not dialysis! Acute dialysis is different and needs help!. Am J Kidney Dis. 1998;32:832-833.
10 Bellomo R, Ronco C. Adequacy of dialysis in the acute renal failure of the critically ill: The case for continuous therapies. Int J Artif Organs. 1996;19:129-142.
11 Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous venovenous haemofiltration on outcomes of acute renal failure: A prospective randomized trial. Lancet. 2000;355:26-30.
12 Kellum J, Angus DC, Johnson JP, et al. Continuous versus intermittent renal replacement therapy: A meta-analysis. Intensive Care Med. 2002;28:29-33.
13 Cole L, Bellomo R, Silvester W, Reeves JH. A prospective, multicenter study of the epidemiology, management and outcome of severe acute renal failure in a “closed” ICU system. Am J Respir Crit Care Med. 2000;162:191-196.
14 Mehta R, Letteri JM. Current status of renal replacement therapy for acute renal failure. Am J Nephrol. 1999;19:377-382.
15 Bellomo R, Ronco C, Mehta R. Nomenclature for continuous renal replacement therapies. Am J Kidney Dis. 1996;28(Suppl 3):S2-S7.
16 Ronco C, Ricci Z. Renal replacement therapies: physiological review. Intensive Care Med. 2008;34:2139-2146.
17 Bellomo R, Tan K, Bhonagiri S, Gopal I, Seacombe J, Daskalakis M, et al. High protein intake during continuous hemodiafiltration: impact on amino acids and nitrogen balance. Int J Artif Organs. 2002;25:261-268.
18 Gibney N, Cerda J, Davenport A, Ramirez J, Singbartl K, Leblanc M, et al. Volume management by renal replacement therapy in acute kidney injury. Int J Artif Organs. 2008;31:145-155.
19 Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316-325.
20 Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74.
21 Kellum JA, Ronco C. Dialysis: Results of RENAL–what is the optimal CRRT target dose? Nat Rev Nephrol. 2010;6:191-192.
22 Uchino S, Bellomo R, Ronco C. Intermittent versus continuous renal replacement therapy in the ICU: impact on electrolyte and acid–base balance. Intensive Care Med. 2001;27:1037-1043.
23 Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895-902.
24 Rocktaschel J, Morimatsu H, Uchino S, Ronco C, Bellomo R. Impact of continuous veno-venous hemofiltration on acid base balance. Int J Artif Organs. 2003;26:19-25.
25 Mehta R, Dobos GJ, Ward DM. Anticoagulation procedures in continuous renal replacement. Semin Dial. 1992;5:61-68.
26 Bellomo R, Teede H, Boyce N. Anticoagulant regimens in acute continuous hemodiafiltration: A comparative study. Intensive Care Med. 1993;19:329-332.
27 Tan HK, Baldwin I, Bellomo R. Hemofiltration without anticoagulation in high-risk patients. Intensive Care Med. 2000;26:1652-1657.
28 Ronco C, Brendolan A, Bellomo R. Current technology for continuous renal replacement therapies. In: Ronco C, Bellomo R, editors. Critical Care Nephrology Dordrecht. The Netherlands: Kluwer Academic Publishers, 1998.
29 Davenport A. The management of renal failure in patients at risk of cerebral edema/hypoxia. New Horiz. 1995;3:717-724.
30 Hakim RM, Wingard R, Parker RA. Effect of dialysis membrane in the treatment of patients with acute renal failure. N Engl J Med. 1994;331:1338-1342.
31 Schiffl H, Lang SM, Konig A, et al. Biocompatible membranes in acute renal failure: Prospective case controlled study. Lancet. 1994;344:570-572.
32 Phu NH, Hien TT, Mai NTH, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895-902.
33 Morelli S, Ricci Z, Di Chiara L, Stazi GV, Polito A, Vitale V, et al. Renal replacement therapy in neonates with congenital heart disease. Contrib Nephrol. 2007;156:428-433.
34 Buckmaster J, Davies AR. Guidelines for drug dosing during continuous renal replacement therapies. In: Ronco C, Bellomo R, editors. Critical Care Nephrology. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1998.
35 Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008;19:1233-1238.
36 VA/NIH Acute Renal Failure Trial NetworkPalevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7-20.
37 RENAL Replacement Therapy Study InvestigatorsBellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627-1638.
38 Vesconi S, Cruz DN, Fumagalli R, Kindgen-Milles D, Monti G, Marinho A, et al. DOse REsponse Multicentre International collaborative Initiative (DO-RE-MI Study Group). Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care. 2009;13:R57.