CHAPTER 1 RENAL PATHOLOGY
INTRODUCTION
PEDIATRIC RENAL TUMORS
OVERVIEW
WILMS’ TUMOR / NEPHROBLASTOMA
| Wilms’ aniridia genital anomaly retardation (WAGR) syndrome |
| Beckwith–Wiedemann syndrome |
| Hemihypertrophy |
| Denys–Drash syndrome |
| Familial nephroblastoma |
| Frasier syndrome |
| Simpson–Golabi–Behmel syndrome |
| Neurofibromatosis |
| Perlman syndrome |
Epidemiology
Genetics
Clinical features
Specific issues of specimen handling
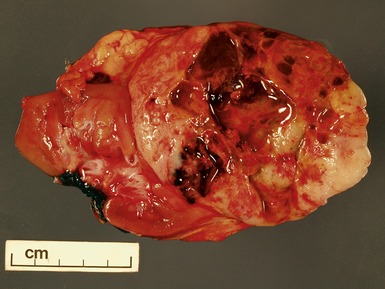
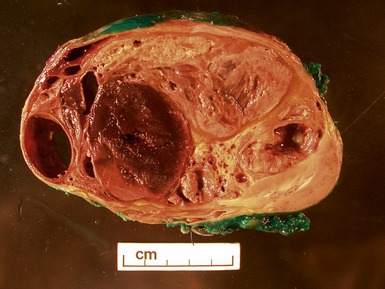
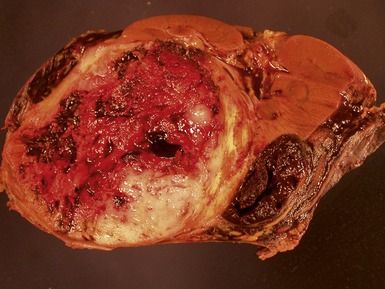
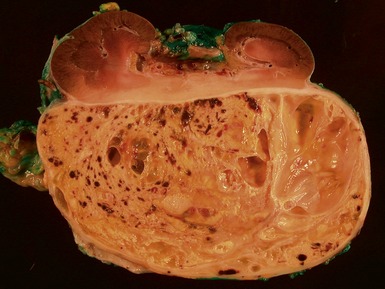
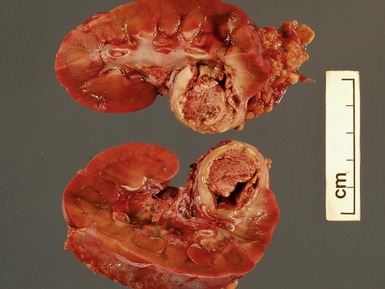
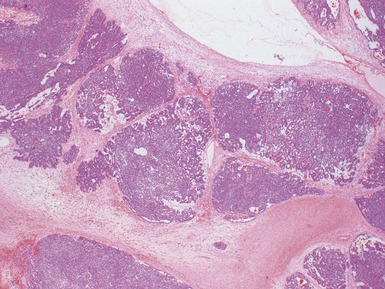
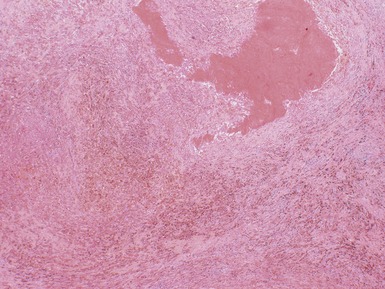
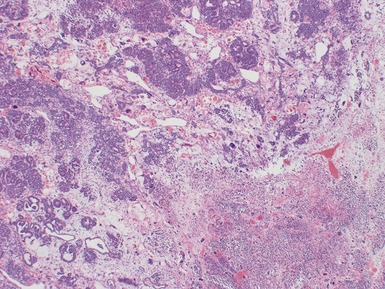
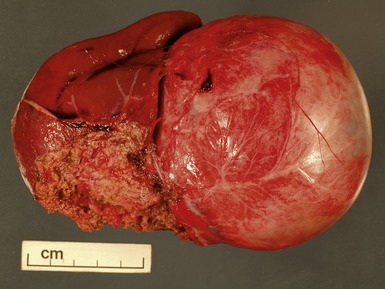
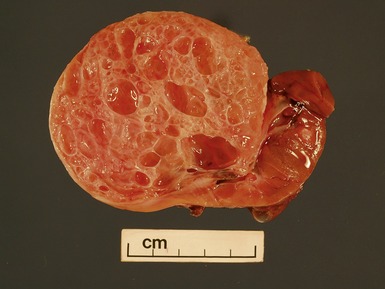
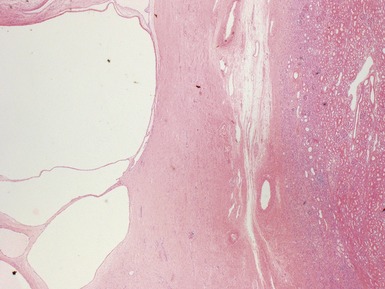
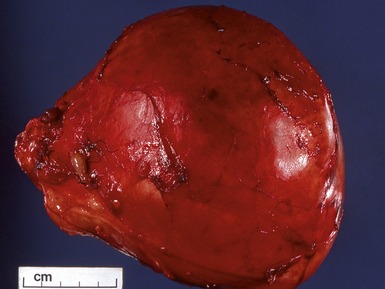
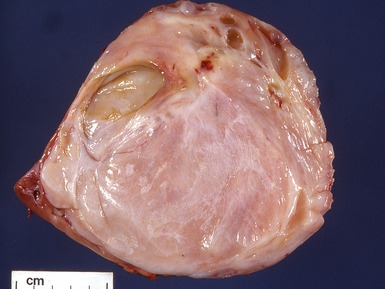
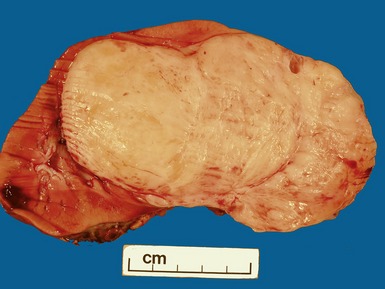
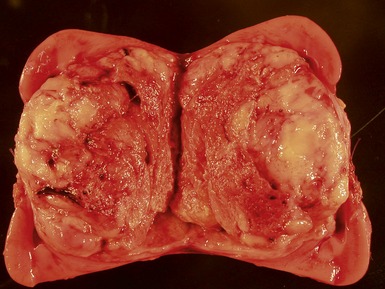
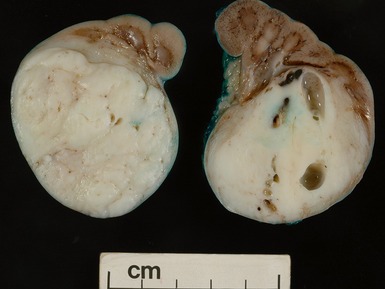
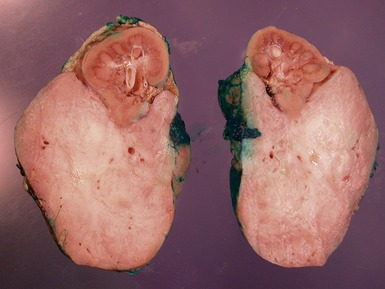
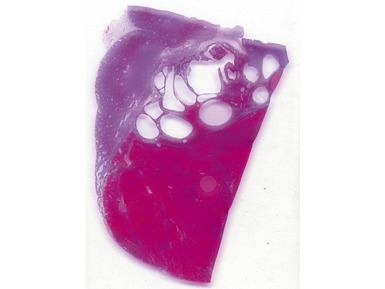
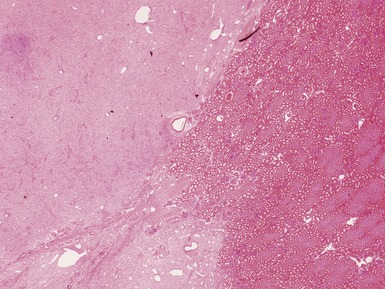
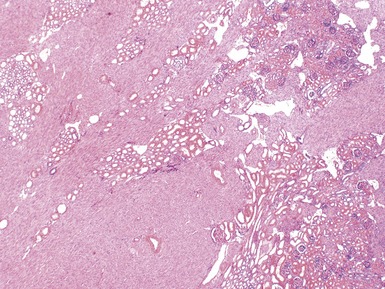
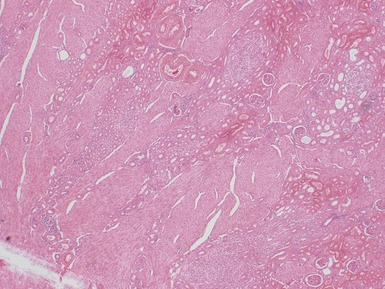
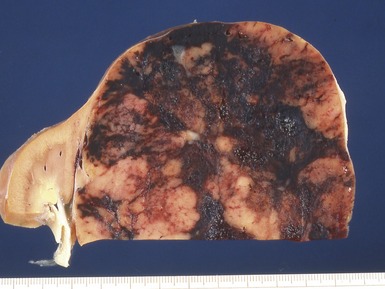
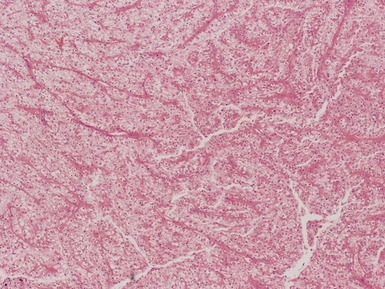
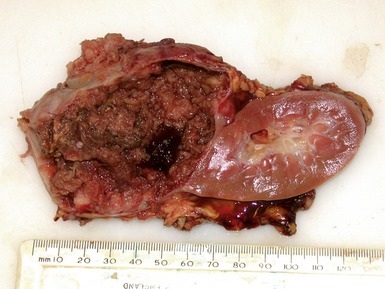
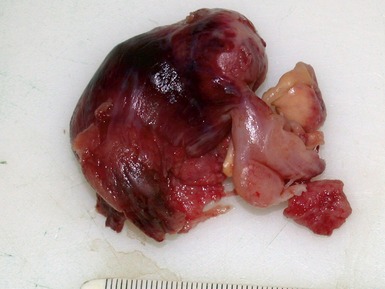
Macroscopic features (Figs 1.1–1.7)
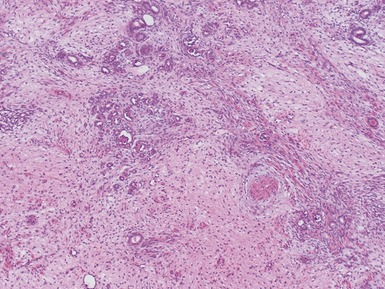
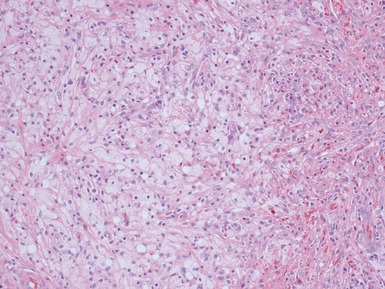
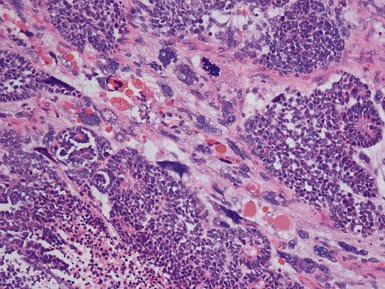
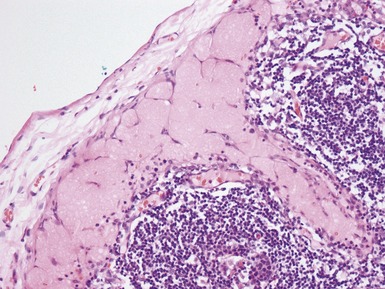
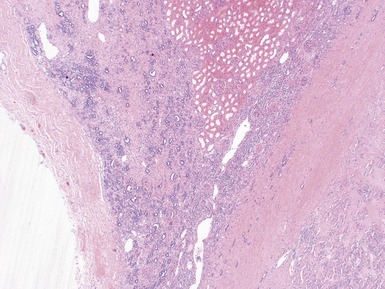
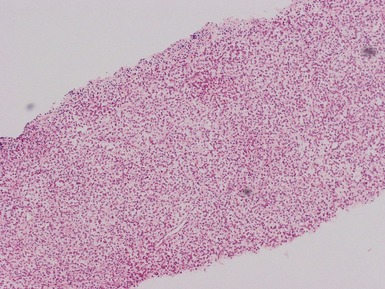
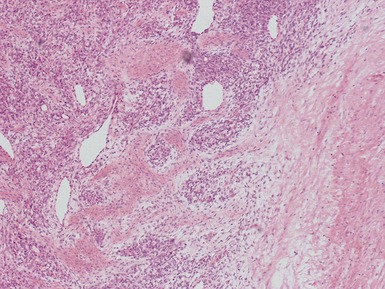
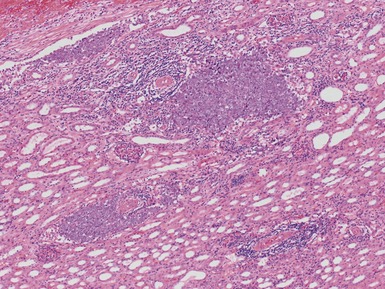
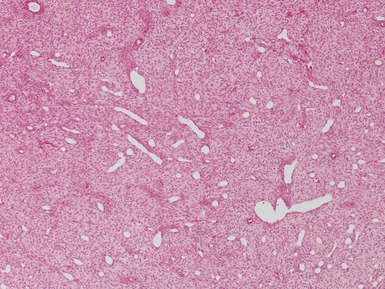
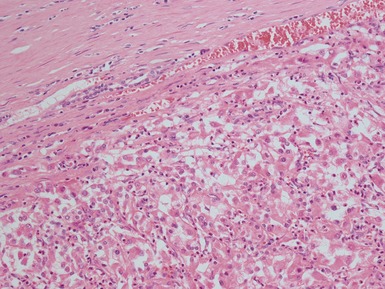
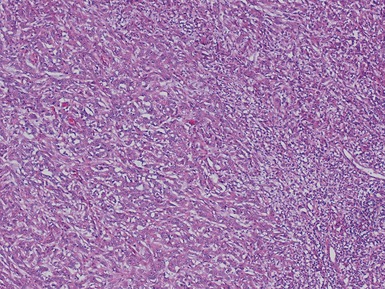
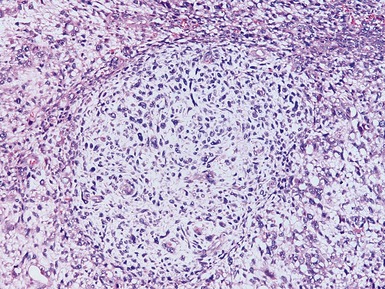
Histopathological features
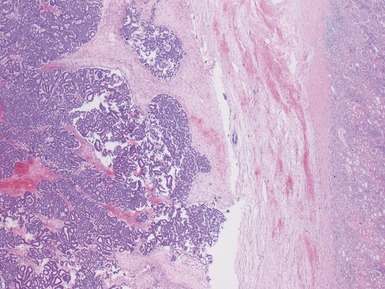
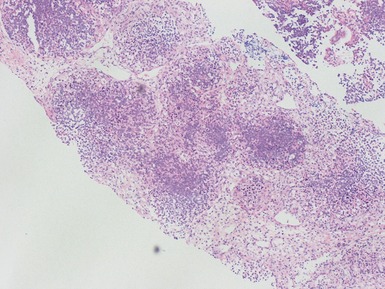
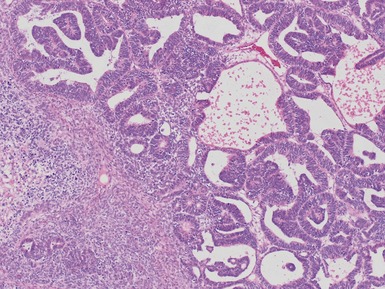
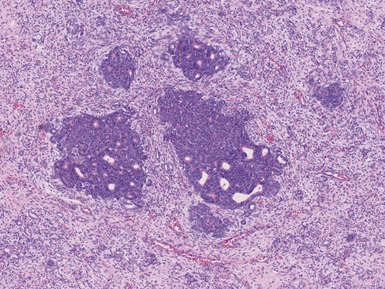
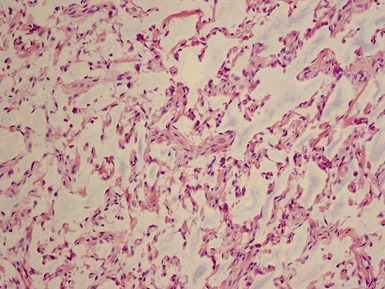
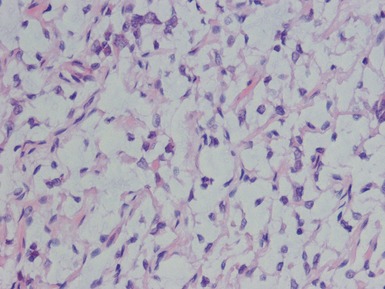
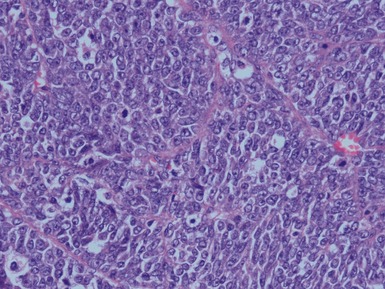
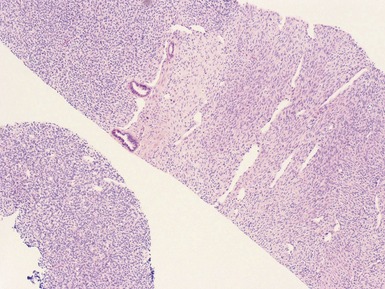
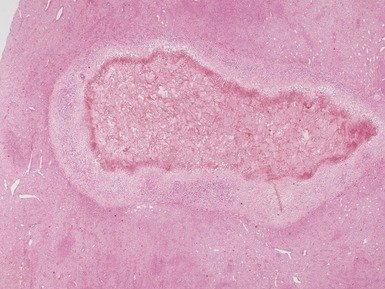
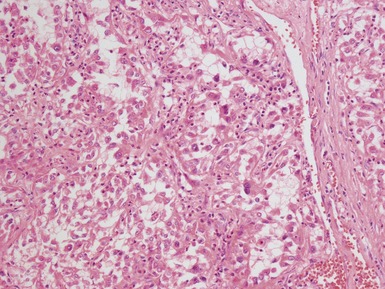
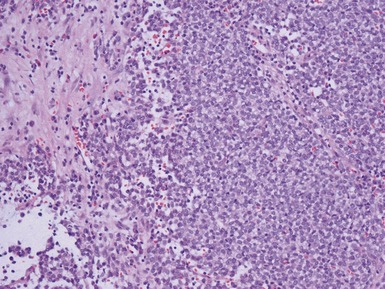
Figs 1.13–1.14 Photomicrographs of nephroblastomas showing the classical architectural and morphological mixture of elements.
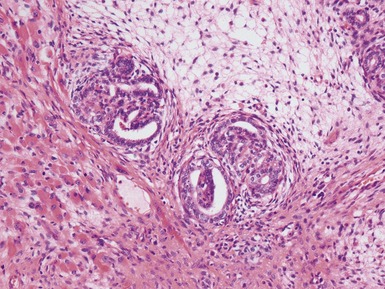
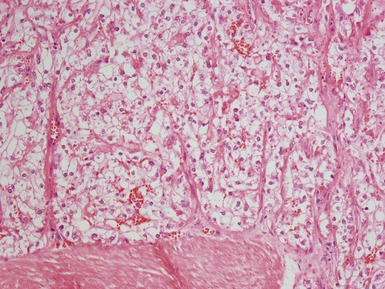
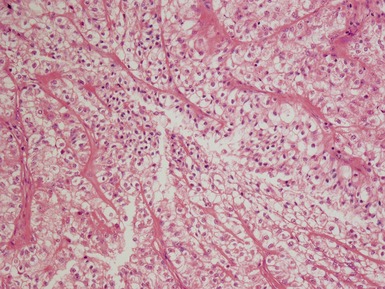
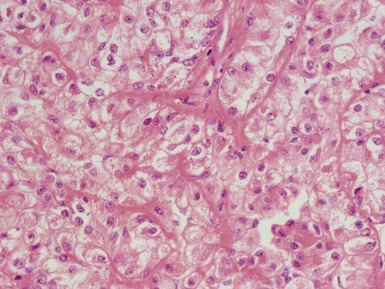
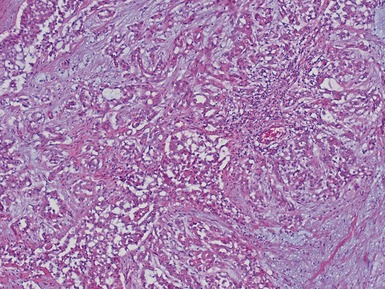
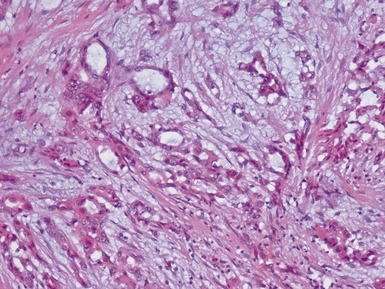
Epithelial component (Figs 1.15–1.18)
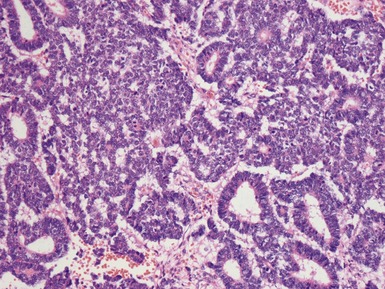
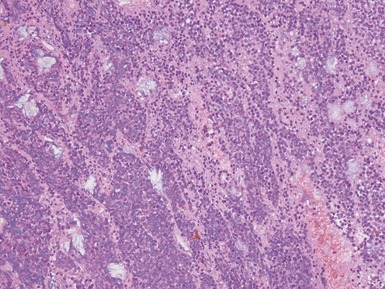
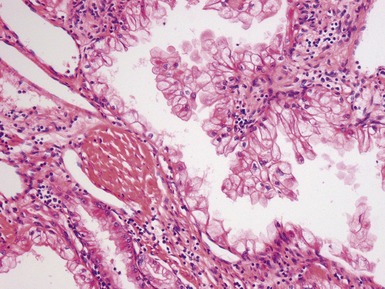
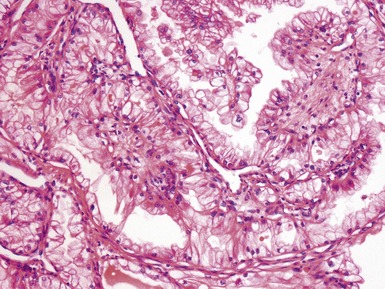
Figs 1.15–1.16 Photomicrographs of nephroblastomas showing epithelial elements manifest as well-formed tubular structures.
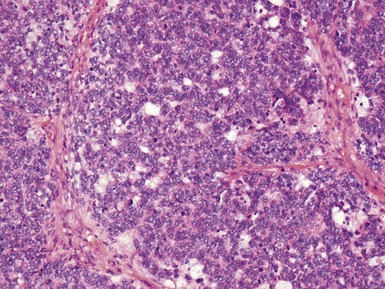
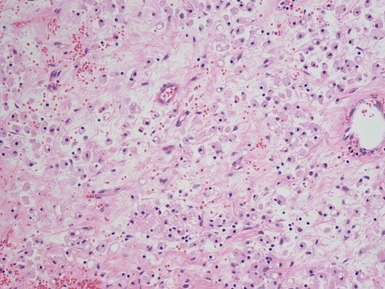
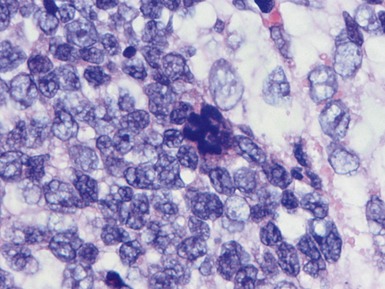
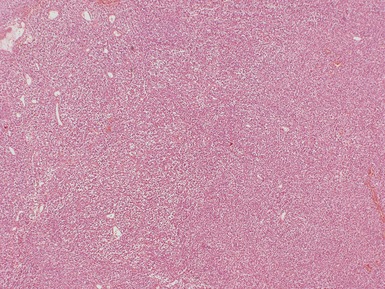
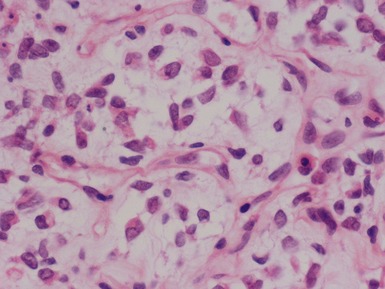
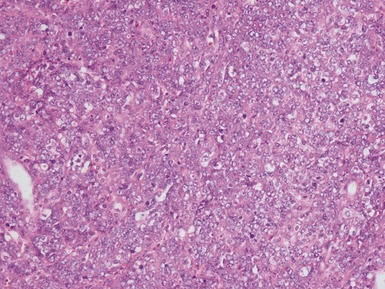
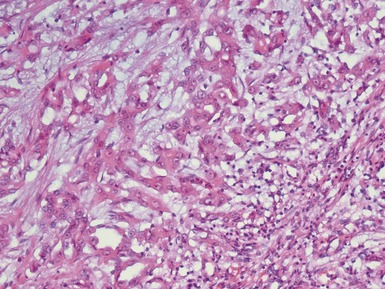
Blastemal component (Figs 1.19–1.21)
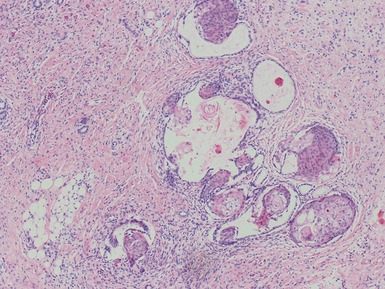
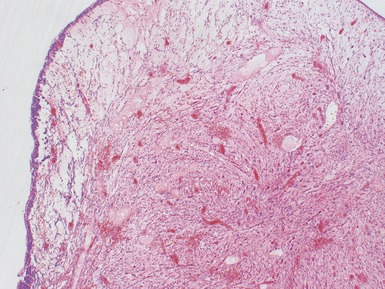
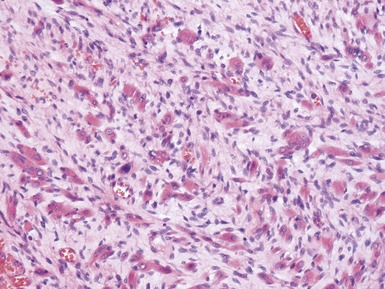
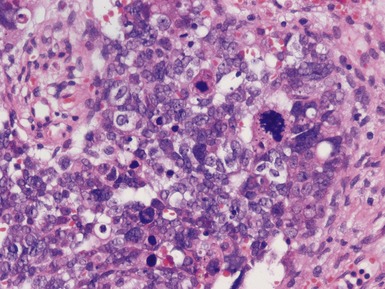
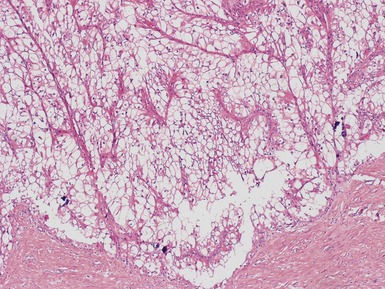
Stromal component (Figs 1.22–1.25)
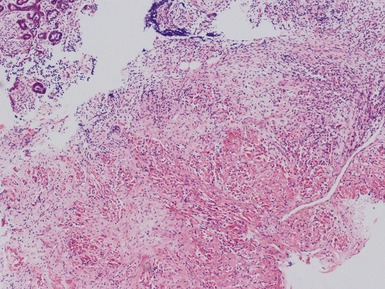
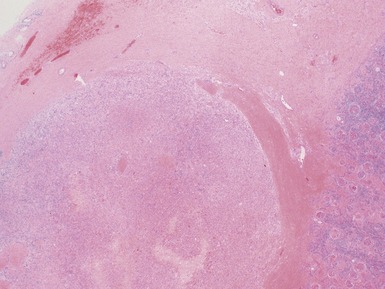
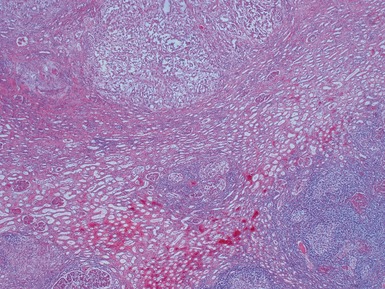
Post-chemotherapy features (Figs 1.26–1.30)
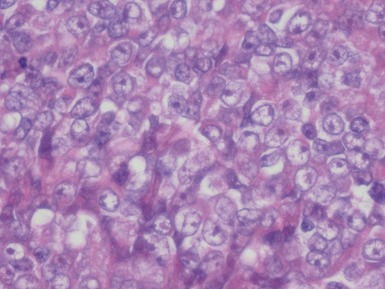
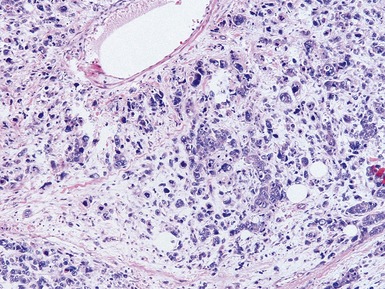
Unfavorable histology Wilms’ tumor (anaplasia) (Figs 1.31–1.34)
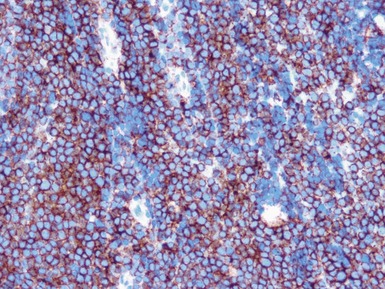
Immunohistochemical staining
Classification and staging
| A. Pre-treated cases | Low risk | Mesoblastic nephroma |
| Cystic partially differentiated nephroblastoma | ||
| Completely necrotic nephroblastoma | ||
| Intermediate risk | Nephroblastoma – epithelial type | |
| Nephroblastoma – stromal type | ||
| Nephroblastoma – mixed type | ||
| Nephroblastoma – regressive type | ||
| Nephroblastoma – focal anaplasia type | ||
| High risk | Nephroblastoma – blastemal type | |
| Nephroblastoma – diffuse anaplasia | ||
| Clear cell sarcoma of the kidney | ||
| Rhabdoid tumor of the kidney | ||
| B. Primary nephrectomy cases | Low risk | Mesoblastic nephromaCystic partially differentiated nephroblastoma |
| Intermediate risk | Non-anaplastic nephroblastoma and its variants | |
| Nephroblastoma – focal anaplasia | ||
| High risk | Nephroblastoma – diffuse anaplasia | |
| Clear cell sarcoma of the kidney | ||
| Rhabdoid tumor of the kidney |
If necrosis and regressive change comprises more than two-thirds of the tumor mass it is a regressive type.
If regressive change / necrosis comprises less than two-thirds of the tumor mass a predominant histological component should be looked for and the tumor subclassified accordingly.
For a component to be regarded as dominant, more than two-thirds of the viable tumor must be composed of the subtype.
If >10% and <66% of the viable tumor is blastema, mixed type regardless of ‘dominant’ component.
To fulfill the diagnosis for completely necrotic subtype there must be no viable tumor present on gross and microscopic examination of multiple blocks from different areas of the tumor sampled at least one block per centimeter of tumor largest diameter, in combination with the presence of regressive and necrotic changes caused by chemotherapy.
Table 1.3 Staging system: SIOP
| Stage I | a.The tumor is limited to kidney or surrounded with a fibrous pseudocapsule if outside of the normal contours of the kidney. The renal capsule or pseudocapsule may be infiltrated with tumor but it does not reach the outer surface, and is completely resected (resection margins clear) |
| b.The tumor may be bulging into the pelvic system and dipping into the ureter but not infiltrating their walls | |
| c.The vessels of the renal sinus are not involved | |
| d.Intrarenal vessel involvement may be present | |
| Fine needle aspiration or percutaneous cord needle biopsy does not upstage the tumor | |
| The presence of necrotic tumor or chemotherapy-induced change in the renal sinus and/or within the perirenal fat should not be regarded as a reason for upstaging a tumor providing it is completely excised and does not reach the resection margins | |
| Stage II | a.The tumor extends beyond the kidney or penetrates through the renal capsule and/or fibrous pseudocapsule into perirenal fat but is completely resected (resection margins clear) |
| b.Tumor infiltrates the renal sinus and/or blood and/or lymphatic vessels outside the renal parenchyma but is completely resected | |
| c.Tumor infiltrates adjacent organs or vena cava but is completely resected | |
| Stage III | a.Incomplete excision of the tumor which extends beyond resection margins (gross or microscopical tumor remains postoperatively) |
| b.Any abdominal lymph nodes are involved | |
| c.Tumor rupture before or intraoperatively | |
| d.The tumor has penetrated through the peritoneal surface | |
| e.Tumor implants are found on the peritoneal surface | |
| f.Tumor thrombi present at resection margins of vessels or ureter, transected or removed piecemeal by surgeon | |
| g.The tumor has been surgically biopsied (wedge biopsy) prior to preoperative chemotherapy or surgery | |
| The presence of necrotic tumor or chemotherapy-induced changes in a lymph node or at the resection margins is regarded as proof of previous tumor with microscopic residue and therefore the tumor is assigned stage III because of the possibility that some viable tumor is left behind in the adjacent lymph node or beyond resection margins | |
| Stage IV | Hematogenous metastasis or lymph node metastasis outside the abdominal and pelvic region |
| Stage V | Bilateral renal tumors at diagnosis. Each side should be sub staged according to the above classification |
| Stage I | Limited to kidney and completely resected, renal capsule intact |
| Fine needle aspiration or percutaneous core needle biopsy does not upstage the tumor | |
| The presence of necrotic tumor or chemotherapy-induced change in the renal sinus and/or within perirenal fat should not be regarded as a reason for upstaging a tumor providing it is completely excised and does not reach the resection margins | |
| Stage II | Tumor infiltrates beyond kidney but is completely resected |
| a.The tumor extends beyond the kidney or penetrates through the renal capsule and/or fibrous pseudocapsule into perirenal fat but is completely resected (resection margins clear) | |
| b.Tumor infiltrates the renal sinus and/or blood and/or lymphatic vessels outside the renal parenchyma but is completely resected | |
| c.Tumor infiltrates adjacent organs or ureter but is completely resected | |
| Stage III | Gross or microscopic residual tumor confined to abdomen |
| a.Incomplete excision of the tumor which extends beyond resection margins (gross or microscopical tumor remains postoperatively) | |
| b.Any abdominal lymph nodes are involved | |
| c.Tumor rupture before or intraoperatively | |
| d.The tumor has penetrated through the peritoneal surface | |
| e.Tumor implants are found on the peritoneal surface | |
| f.Tumor thrombi present at resection margins of vessels or ureter, transected or removed piecemeal by surgeon | |
| g.The tumor has been surgically biopsied (wedge biopsy) prior to preoperative chemotherapy or surgery | |
| Stage IV | Hematogenous metastasis or lymph node metastasis outside the abdominal and pelvic region |
| Stage V | Bilateral renal tumors at diagnosis. Each side should be sub staged according to the above classification |
Special diagnostic investigations
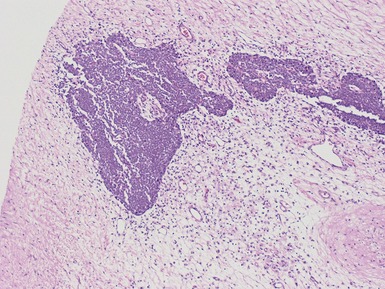
Differential diagnoses and pitfalls
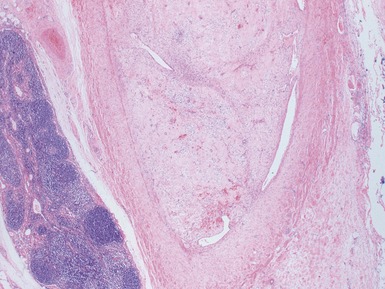
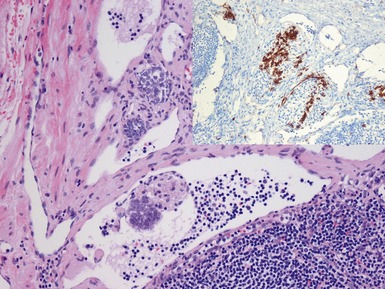
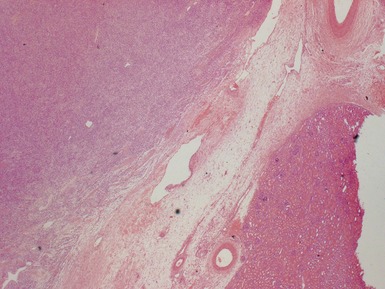
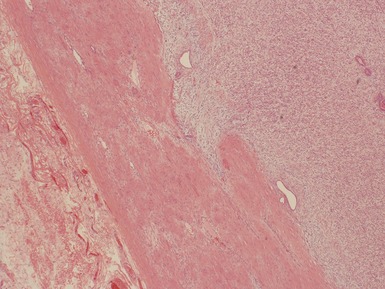
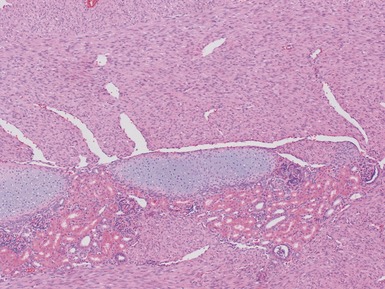
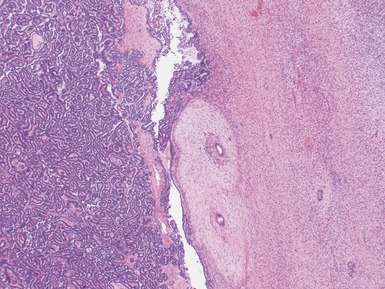
Fig 1.37 Photomicrograph showing involvement of hilar renal vein branch by postchemotherapy nephroblastoma.
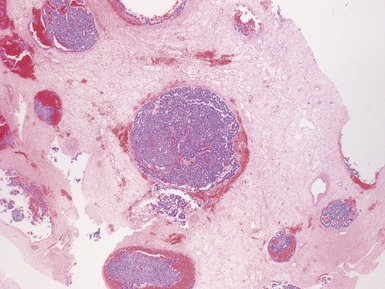
NEPHROGENIC RESTS AND NEPHROBLASTOMATOSIS
CYSTIC NEPHROMA AND CYSTIC PARTIALLY DIFFERENTIATED NEPHROBLASTOMA (CN/CPDN)
CLEAR CELL SARCOMA OF THE KIDNEY (CCSK)
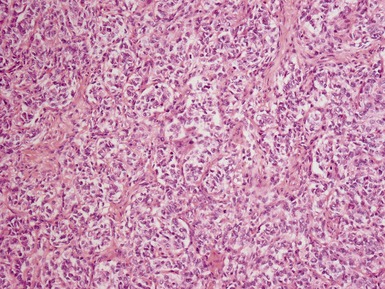
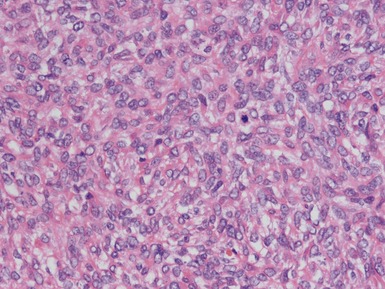
Histopathological features (Figs 1.49–1.58)
RENAL RHABDOID TUMOR
Genetics
Clinical features
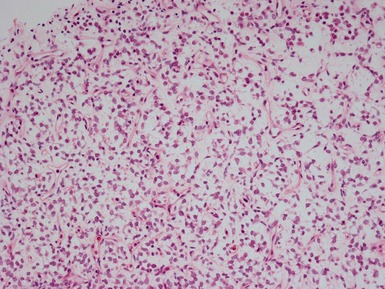
Histopathological features (Figs 1.60–1.64)
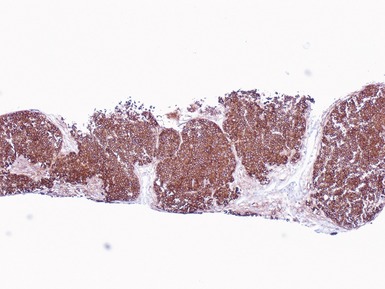
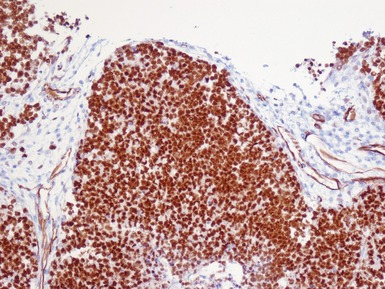
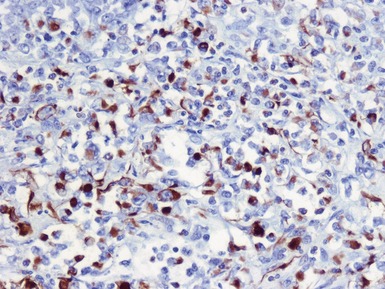
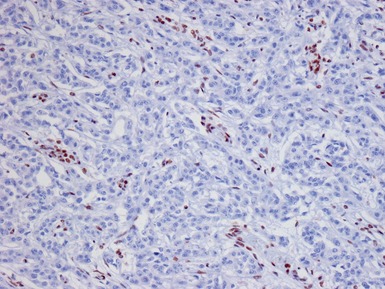
Immunohistochemical staining (Figs 1.65, 1.66)
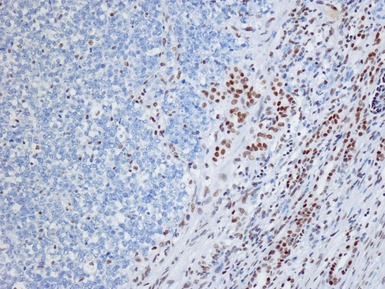
Figs 1.65–1.66 Photomicrographs of nephrectomy specimens of malignant renal rhabdoid tumor demonstrating characteristic ‘dot-like’ cytoplasmic expression of vimentin (Fig 1.65) and essentially diagnostic lack of nuclear expression of INI1 in the presence of appropriate positive normal control tissue (Fig 1.66).
CONGENITAL MESOBLASTIC NEPHROMA
Genetics
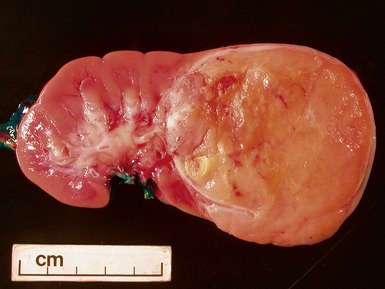
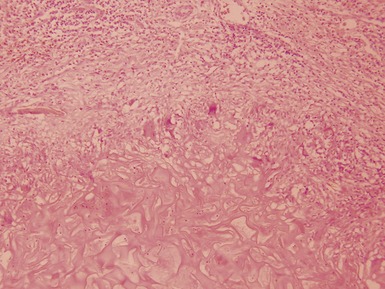
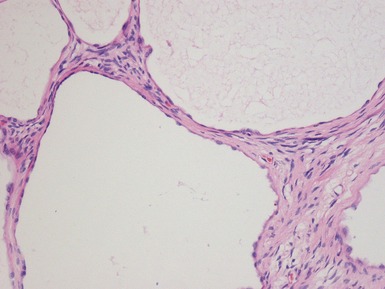
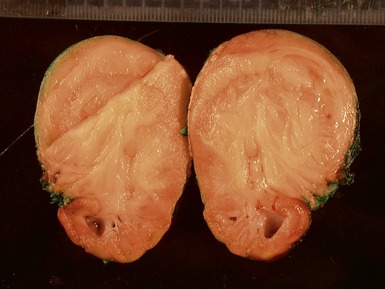
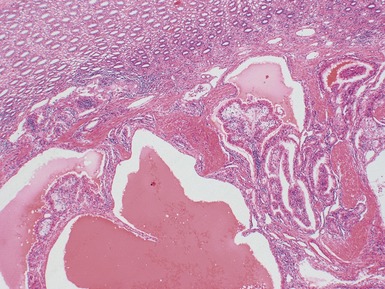
Macroscopic features (Figs 1.67–1.70)
Histopathological features (Figs 1.71–1.82)
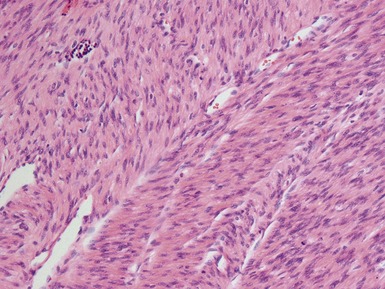
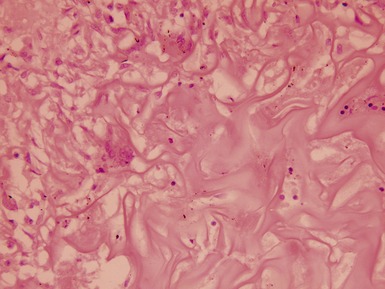
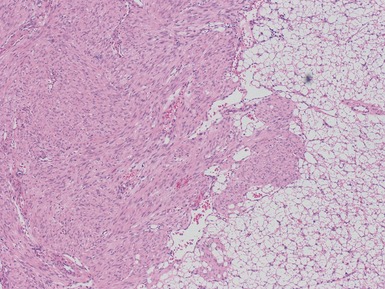
Figs 1.73–1.80 Photomicrographs of nephrectomy specimens of classical mesoblastic nephromas, demonstrating tumor composed of interlacing fascicles of bland spindle cells. Note the presence of cartilage focally and the infiltrating margin with normal kidney and the surrounding fat.
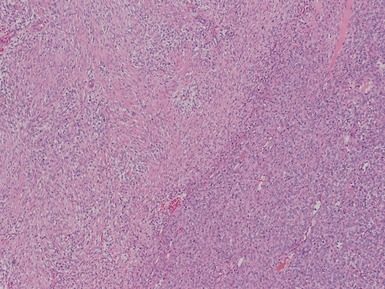
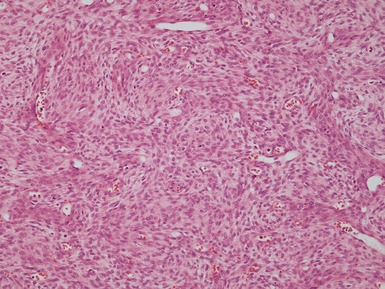
Figs 1.81–1.82 Photomicrographs of nephrectomy specimens of cellular (Fig 1.81) and mixed type (Fig 1.82) mesoblastic nephromas. The cellular subtype is composed of closely-packed plump ovoid cells, superficially resembling other blastematous tumors. In mixed variants, both morphological subtypes are present.
RENAL CELL CARCINOMA IN CHILDHOOD
Clinical features
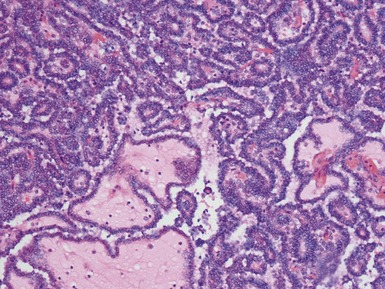
Histopathological features (Figs 1.83–1.87)
Xp11.2 ASSOCIATED RENAL CELL CARCINOMA
Genetics
Histopathological features (Figs 1.88–1.90)
NEUROBLASTOMA ASSOCIATED RENAL CELL CARCINOMA
Epidemiology
COLLECTING DUCT CARCINOMA
RENAL MEDULLARY CARCINOMA
Histopathological features (Figs 1.94–1.98)
RENAL ONCOCYTOMA
METANEPHRIC ADENOMA / METANEPHRIC ADENOFIBROMA
Clinical features
Histopathological features (Figs 1.100–1.103)
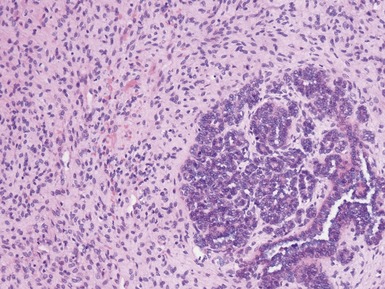
Figs 1.100–1.101 Photomicrographs of metanephric adenofibroma showing presence of both well-differentiated epithelial and stromal areas.
METANEPHRIC STROMAL TUMOR
OSSIFYING RENAL TUMOR OF INFANCY
RENAL ANGIOMYOLIPOMA
Clinical features
PRIMARY INTRARENAL PRIMITIVE NEUROECTODERMAL TUMOR
(See soft tissue tumor section also)
Clinical features
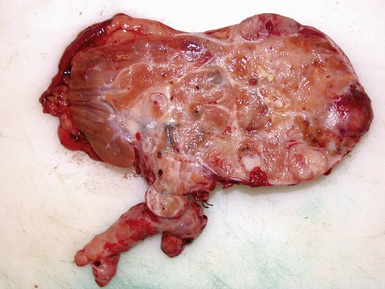
Macroscopic features (Figs 1.104, 1.105)
Histopathological features (Fig 1.106)
SYNOVIAL SARCOMA OF THE KIDNEY
(See soft tissue tumor section also)
Histopathological features
PRIMARY INTRARENAL NEUROBLASTOMA
(See soft tissue tumor section also)
ANAPLASTIC SARCOMA OF KIDNEY
RENAL BIOPSY AND NON-TUMOR PATHOLOGY
PEDIATRIC RENAL BIOPSY PATHOLOGY
Overview
General approach
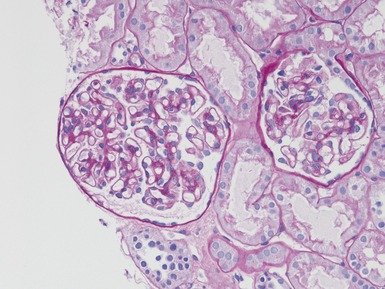
MINIMAL CHANGE DISEASE
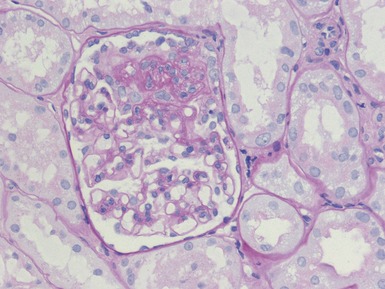
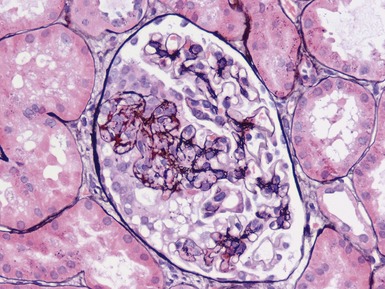
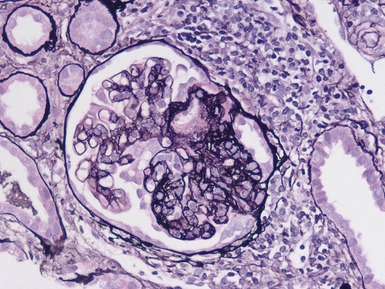
Histopathological features (Figs 1.110, 1.111)
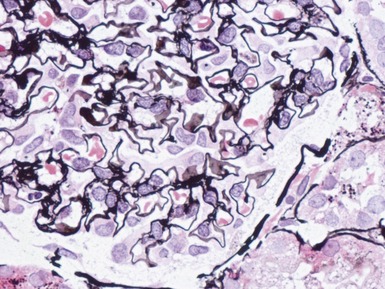
FOCAL SEGMENTAL GLOMERULOSCLEROSIS (FSGS)
Clinical features
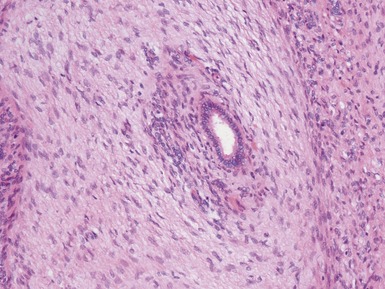
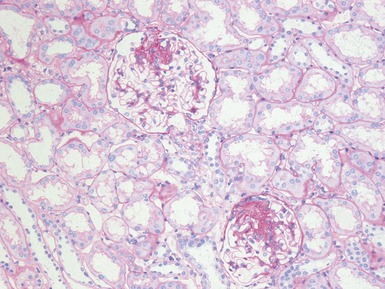
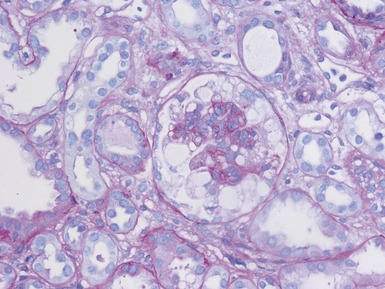
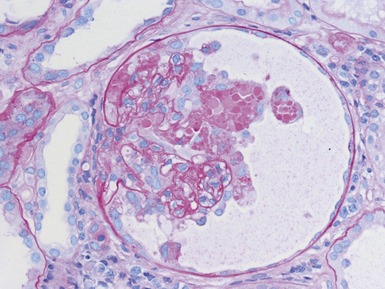
Histopathological features (Figs 1.112–1.116)
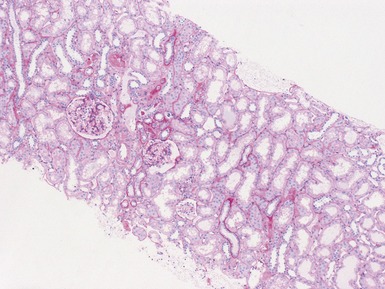
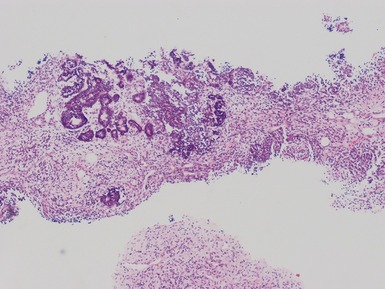
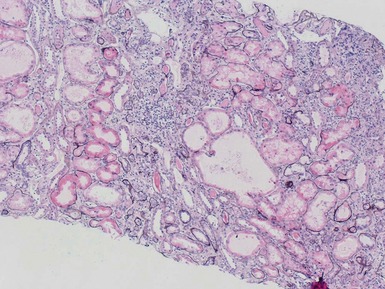
Fig 1.112 Photomicrograph of a renal biopsy with primary idiopathic FSGS, demonstrating a patchy area of tubular atrophy and interstitial fibrosis. In this clinical setting in a pediatric renal biopsy, such changes should prompt a thorough search for sclerotic glomerular lesions.