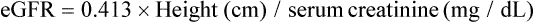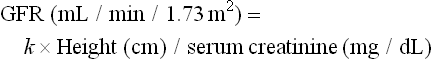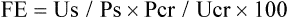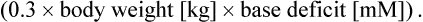Renal Impairment
Body Fluid and Electrolyte Regulation
Effective kidney function maintains the normal volume and composition of body fluids. Although there is a wide variation in dietary intake and nonrenal expenditures of water and solute, water and electrolyte balance is maintained by the excretion of urine, with the volume and composition defined by physiologic needs. Fluid balance is accomplished by glomerular ultrafiltration of plasma coupled with modification of the ultrafiltrate by tubular reabsorption and secretion.1,2 The excreted urine, the modified glomerular filtrate, is the small residuum of the large volume of nonselective ultrafiltrate modified by transport processes operating along the nephron. The glomerular capillaries permit free passage of water and solutes of low molecular weight while restraining formed elements and macromolecules. The glomerular capillary wall functions as a barrier to the filtration of macromolecules based on their size, shape, and charge characteristics. The glomerular filtrate is modified during passage through the tubules by the active and passive transport of certain solutes into and out of the lumenal fluid and the permeability characteristics of specific nephron segments. The transport systems in renal epithelial cells serve to maintain global water, salt, and acid–base homeostasis.
The rate of ultrafiltration across the glomerular capillaries is determined by the same forces that allow the transmural movement of fluid in other capillary networks.3 These forces are the transcapillary hydraulic and osmotic pressure gradients and the characteristics of capillary wall permeability. A renal autoregulatory mechanism enables the kidney to maintain relative constancy of blood flow in the presence of changing systemic arterial and renal perfusion pressures.1 This intrinsic renal autoregulatory mechanism appears to be mediated in individual nephrons by tubuloglomerular feedback involving the macula densa (a region in the early distal tubule that juxtaposes the glomerulus) and the magnitude of resistance in the afferent and efferent arterioles,
Renal Function Evaluation
The evaluation of kidney function begins with the history, physical examination, and laboratory studies. Persistent oliguria or significant impairment in renal concentrating capacity should be evident from the history. Examination of the urinary sediment may provide evidence of renal disease if proteinuria and/or cellular casts are present. Normal serum concentrations of sodium, potassium, chloride, total CO2, calcium, and phosphorus indicate appropriate renal regulation of the concentration of electrolytes in body fluids. The serum creatinine concentration is the usual parameter for GFR. Important limitations and caveats must be observed when using creatinine to estimate GFR. Urinary creatinine excretion reflects both filtered and secreted creatinine because creatinine is not only filtered by the glomerular capillaries, but is also secreted by renal tubular cells. As a consequence, creatinine clearance, which is calculated by using serum creatinine concentration and the urinary excretion of creatinine, overestimates true GFR measured by using inulin clearance by 10–40%.4 Serum creatinine concentration and the rate of urinary creatinine excretion are affected by diet. The ingestion of meat, fish, or fowl, which are substances containing preformed creatinine and creatinine precursors, causes an increase in serum creatinine concentration and in urinary creatinine excretion.5 The overestimation of GFR by creatinine clearance increases as kidney function deteriorates owing to the relative increase in the tubular component of urine creatinine. Another caveat should be applied in the case of the patient with an abnormal muscle mass. The smaller the muscle mass, the lower is the release of creatinine into the circulation resulting in lower blood levels and urine excretion rates. The opposite picture will be seen in a patient with a very large muscle mass.
During the past 15 years, the serum concentration of cystatin C, a nonglycosylated 13.3 kDa basic protein, has been shown to correlate with GFR as well as or better than serum creatinine.6–9 From about age 12 months and up until age 50 years, normal serum cystatin C concentrations are similar in children and adults (0.70–1.38 mg/L). Currently, the measurement of cystatin C has not been incorporated into routine clinical practice. However, in the near future it may become a new tool in the assessment of GFR. In contrast, a ‘bedside’ equation is used to estimate GFR:
has recently been developed in children with chronic kidney disease based on data generated from the measurement of GFR using the plasma disappearance of iohexol.10 The bedside formula is most applicable to those children whose GFR is in the range of 15–75 mL/min/1.73m2.
Urine Volume
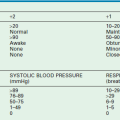
The appropriate urine volume depends on the status of body fluids, fluid intake, extrarenal losses, obligatory renal solute load, and renal concentrating and diluting capacity. Patients with impaired renal concentrating capacity require a larger urinary volume for excretion of the obligatory renal solute load. On the other hand, patients with elevated levels of antidiuretic hormone (ADH) retain water out of proportion to solute and are prone to hyponatremia. Increased levels of ADH may occur because of physiologic factors such as hypertonic body fluids or a decrease in the effective circulatory volume (as encountered with low levels of serum albumin or with generalized vasodilatation as with sepsis). Some researchers have expressed concern that ‘usual maintenance fluids’ (Table 4-1) providing 2–3 mEq/L of sodium, potassium, and chloride per 100 calories metabolized may contribute to the development of hyponatremia in children hospitalized with conditions likely to be associated with ADH excess.11 The children at risk are those with nonosmotic stimuli for ADH release, such as central nervous system disorders, the postoperative patient, pain, stress, nausea, and emesis. It has been proposed that in patients prone to develop the syndrome of inappropriate secretion of ADH, isotonic 0.9% normal saline might be a better choice for maintenance fluid therapy.12
TABLE 4-1
Usual Maintenance Water Requirements
| Weight Range (kg) | Maintenance Water |
| 2.5–10 | 100 mL/kg |
| 10–20 | 1000 mL + 50 mL/kg >10 kg |
| >20 | 1500 mL + 20 mL/kg >20 kg |
Approximately 30 mOsm of obligatory renal solute/100 mL of usual maintenance water is taken as the obligatory renal solute load in children aged 2 months and older.13 Urinary concentrating capacity increases rapidly during the first year of life and reaches the adult level of 1200–1400 mOsm/L at around year two.14 The maximum urinary concentrating capacity of the term infant from 1 week to 2 months of age is about 800 mOsm/L; from 2 months to 3 years, about 1000 mOsm/L; and beyond that age, about 1200 mOsm/L.
Noteworthy is the recent re-characterization of acute renal failure as acute kidney injury (AKI) to better describe renal dysfunction.l5,16 Nonoliguric AKI occurs about as frequently as oliguric AKI. It is diagnosed when the patient with normal urine output has elevated serum creatinine and urea nitrogen concentrations.
Glomerular Filtration Rate
GFR is the most useful index of renal function because it reflects the volume of plasma ultrafiltrate presented to the renal tubules. Decline in GFR is the principal functional abnormality in both acute and chronic renal failure. Assessment of GFR is important not only for evaluating the patient with respect to kidney function, but also for guiding the administration of antibiotics and other drugs. Inulin clearance, which is the accepted standard for measurement of GFR, is too time consuming and inconvenient for use in the clinical evaluation of most patients. Serum urea nitrogen concentration shows so much variation with dietary intake of nitrogen-containing foods that it is not a satisfactory index of GFR. Serum creatinine concentration and creatinine clearance have become the usual clinical measures for determining the GFR. However, precautions should be taken when creatinine alone is used for estimation of GFR because of the effect of diet as well as common medications on serum creatinine concentration and excretion rate. Ingestion of a meal containing a large quantity of animal protein increases serum creatinine levels about 0.25 mg/dL in two hours and increases the creatinine excretion rate about 75% over the next three- to four-hour period.5 Serum creatinine concentrations are also increased by ingestion of commonly used medications such as salicylate and trimethoprim.l7,18 These agents compete with creatinine for tubular secretion through a base-secreting pathway. They do not alter GFR but do elevate the serum creatinine concentration.
Because of the difficulties in timed urine collection, several equations have been developed to estimate GFR. Historically the most commonly used equation has been the one developed by Schwartz19–21 and is based on the serum creatinine value (as determined by the Jaffe kinetic method) and the child’s height:
where k for low birth weight infants is 0.33, full-term infants, 0.45; males 2–12 and females 2–21 years old, 0.55: and males 13–25 years old, 0.70.
Creatinine is formed by the nonenzymatic dehydration of muscle creatinine at a rate of 50 mg creatinine/kg muscle.4 The serum creatinine concentration in the neonate reflects the maternal level for the first three to four days of life and somewhat longer in the premature infant due to delayed maturation of kidney function. After this time, the serum creatinine concentration should decrease. From age 2 weeks to 2 years, the value averages about 0.4 ± 0.04 mg/dL (35 ± 3.5 µM).22 The serum creatinine concentration is relatively constant during this period of growth because the increase in endogenous creatinine production, which is directly correlated with muscle mass, is matched by the increase in GFR. During the first two years of life, GFR increases from 35–45 mL/min/1.73 m2 to the normal adult range of 90–170 mL/min/1.73 m2. The normal range for serum creatinine concentration increases from 2 years through puberty, although the GFR remains essentially constant when expressed per unit of surface area. This occurs because growth during childhood is associated with increased muscle mass and, therefore, increased creatinine production, which is greater than the increased GFR per unit of body weight.22 Table 4-2 shows the mean values and ranges for plasma or serum creatinine levels at different ages.23 Normative data of serum creatinine may differ from one laboratory to another, depending on the methodology used, although efforts are being made for standardization.20,24
TABLE 4-2
Plasma Creatinine Levels at Different Ages
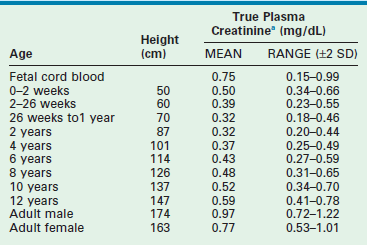
aConversion factor: mmol/L = mg/dL × 88.4
Adapted from Changler C, Barratt TM. Laboratory evaluation. In: Holiday MA, editor. Pediatric Nephrology. 2nd ed. Baltimore: Williams & Wilkins; 1987. p. 282–99.
Fractional Excretion of Substances
where Us is urine solute concentration, Ps is plasma solute concentration, Pcr is plasma creatinine concentration, and Ucr is urine creatinine concentration.
Fractional Excretion of Sodium
The fractional excretion of sodium (FE Na) is 2–3% in normal newborns and may be higher in premature infants. In older children it is usually less than 1%, but may be elevated with high salt intake, adaptation to chronic renal failure, and diuretic administration.25 When a decrease in renal perfusion occurs, which is common in intravascular volume depletion or congestive heart failure, the normal renal response results in a marked increase in the tubular reabsorption of sodium leading to a decrease in sodium excretion and consequently a FE Na of less than 1%. The FE Na, is usually greater than 2% in ischemic AKI (also known as acute tubular necrosis), reflecting the impaired ability of the tubules to reabsorb sodium.
Renal Tubular Acidosis
Renal tubular acidosis (RTA) describes a group of disorders in which metabolic acidosis occurs as a result of an impairment in the reclamation of filtered HCO3 in the proximal tubule or from a defect in the renal hydrogen ion excretion in the distal tubule, assuming an absence of a significant reduction in GFR.26 RTA is considered in the differential diagnosis of the patient with metabolic acidosis; a normal serum anion gap (hyperchloremic metabolic acidosis), and, other than a few exceptions, a urinary pH above 6.0. It is important to remember that an identical biochemical profile is seen in the child with diarrhea, which needs to be excluded before considering the diagnosis of RTA.
In addition to several genetic disorders such as cystinosis, proximal tubular damage is often seen in children receiving chemotherapy. The diagnosis of a defect in proximal tubular reabsorption of HCO3 is made by showing that the fraction excretion of bicarbonate (FE HCO3) is greater than 15% when the plasma HCO3 concentration is normalized with alkalization. Classic distal RTA is caused by a defect in the secretion of H+ by the cells of the distal nephron. It is characterized by hyperchloremic metabolic acidosis, urine pH greater than 6.0 at normal as well as at low serum HCO3 concentrations, and a FE HCO3 less than 5% when the serum HCO3 is normal.26,27
Type IV RTA, a form of distal RTA associated with low urinary pH (<6.0) and hyperkalemia, is a result of decreased H+ and K+ secretion in the distal tubule and is related to a failure to reabsorb sodium.26,27 Type IV RTA is probably the most commonly recognized type of RTA in both adults and children. The hyperkalemia inhibits ammonia synthesis, resulting in decreased available ammonia to serve as a urinary buffer. Therefore, a low urinary pH occurs despite decreased H+ secretion (NH3 + H+ = NH4+). Type IV RTA is physiologically equivalent to aldosterone deficiency, which is one cause of the disorder. In children, it may reflect true hypoaldosteronism but it is much more common as a consequence of renal parenchymal damage, especially that due to obstructive uropathy. In children, the physiologic impairment of type IV RTA resolves in a few weeks to months after relief of an obstructive disorder.28
Acute Kidney Injury
Pathophysiology
AKI is characterized by an abrupt decrease in kidney function. Because AKI is caused by a decrease in the GFR, the initial clinical manifestations are elevations in serum urea nitrogen and creatinine concentrations, and frequently a reduction in urine output. Among pediatric surgical patients, an impairment in kidney function is most common to those who are undergoing cardiopulmonary procedures.29,30 In recent years, research has focused on the identification of biomarkers that indicate imminent kidney failure, even before a rise in serum creatinine is noted.31 The idea is to identify urine and possibly blood proteins and enzymes released from the tubules very early in the development of AKI. A substantial amount of data has been collected in children undergoing elective heart surgery, using the biomarker neutrophil gelatinase-associated lipocalin (NGAL).31 However, at this point, such markers have not been incorporated into routine clinical practice.32
The most important factor in the pathogenesis of postoperative kidney failure is decreased renal perfusion. In the early phase, the reduction in renal blood flow results in a decline in GFR. Intact tubular function results in enhanced reabsorption of sodium and water. This clinical condition is recognized as prerenal azotemia. Analysis of the patient’s urine reveals a high urinary osmolality of greater than 350 mOsm/kg H2O and a urine sodium concentration less than 10 mEq/L (20 mEq/L in the neonate).33 The most useful index for the tubular response to renal hypoperfusion with intact tubular function is FE Na. The FE Na test is invalid if the patient received diuretics before giving the urine sample. With renal hypoperfusion and intact renal function, FE Na is less than 1% in term infants and children, and below 2.5% in premature infants.34 In most patients with prerenal azotemia, intravascular volume depletion is clinically evident. However, in patients with diminished cardiac output (pump failure), clinical appreciation of reduced renal perfusion can be obscured because body weight and central venous pressure may suggest fluid overload. Similarly, assessment of volume status is difficult in patients with burns, edema, ascites, anasarca, or hypoalbuminemia. The reduced effective intra-arterial volume might be evident from the reduced systemic blood pressure, tachycardia, and prolonged capillary refill time.
Prerenal azotemia can be alleviated by improving renal perfusion either by repleting the intravascular fluid volume or by improving the cardiac output. The improved kidney function is recognized by increased urine output and normalization of serum urea nitrogen and creatinine concentrations. However, if renal hypoperfusion persists for a significant period or if other nephrotoxic factors are present, parenchymal kidney failure can result. Factors that may predispose the patient to AKI include preexisting congenital urinary anomalies or impaired kidney function, septicemia, hypoxemia, hemolysis, rhabdomyolysis, hyperuricemia, drug toxicity, and the use of radiocontrast agents. Also, abdominal compartmental syndrome resulting from tense ascites may impair renal perfusion. In this setting, kidney failure may be alleviated by abdominal decompression.35
Medical Management
If urinary output is inadequate after the fluid challenge, an intravenous infusion of furosemide, 1 mg/kg, may be given. Patients with renal failure may require higher doses, up to 5 mg/kg. If no response occurs after the initial infusion of furosemide, a second, higher dose can be repeated after one hour. Some patients may require furosemide every four to eight hours to maintain satisfactory urinary volume. A protocol with constant furosemide infusion has been successfully used in oliguric children after cardiac surgery.36 Furosemide is infused at 0.1 mg/kg/h, with the dose increased by 0.1 mg after two hours if the urinary volume remains less than 1 mL/kg/h. The maximum dose is 0.4 mg/kg/h. At times, urine output can be increased by the use of vasoactive agents such as dopamine, However, their efficacy in otherwise altering the course of AKI is not well established.37,38 It is very important to maintain adequate blood pressure and effective renal plasma flow.
Careful monitoring of the patient’s fluid and electrolyte status is essential. Those children who fail to respond to furosemide are at risk for fluid overload. Overzealous fluid administration during anesthesia and surgery and for the management of persistent hypoperfusion, along with decreased urinary output, can result in hypervolemia, hypertension, heart failure, and pulmonary edema. In extreme cases, fluid administration must be decreased to the minimum necessary to deliver essential medications. In less severe instances, and in euvolemic patients with impaired kidney function, total fluid intake should equal insensible water loss, urine volume, and any significant extrarenal fluid losses. Urine output must be monitored hourly and fluid management should be re-evaluated every four to 12 hours, as clinically indicated. Valuable information about the patient’s overall fluid status can be obtained by carefully monitoring blood pressure, pulse, and body weight. The preoperative values of these parameters help serve as a baseline for postoperative evaluation. Ideally, the patient’s hemodynamic status should be assessed continuously by using central venous pressure monitoring. In patients with complicated cardiac problems, a Swan–Ganz catheter that monitors pulmonary wedge pressure should be used.
Fluid overload can lead to hyponatremia. In most cases, because total body sodium remains normal or high, the best way to normalize serum sodium concentration is by restriction of fluid intake and enhancement of urinary volume.39 In patients with acute symptomatic hyponatremia, careful infusion of NaCl 3% solution (512 mEq Na/L or 0.5 mEq/mL) may be given to correct hyponatremia. Rapid correction at a rate of 1–2 mEq/h over a two to three-hour period, with an increase of serum sodium level by 4–6 mEq/L, is usually well tolerated and adequate. Infusion of 6 mL/kg of 3% NaCl increases serum sodium concentration by about 5 mEq/L. Hyponatremia present for more than 24 to 48 hours should not be corrected at a rate more rapid than 0.5 mEq/L/h.
In children with AKI, hyperkalemia often develops. The early sign of potassium cardiotoxicity is peaked T waves on the electrocardiogram. Higher levels of serum potassium can cause ventricular fibrillation and cardiac asystole. The treatment of hyperkalemia is shown in Box 4-1. Emergency treatment of hyperkalemia is indicated when the serum potassium concentration reaches 7.0 mEq/L or when electrocardiographic changes are noted.
In children with AKI, metabolic acidosis rapidly develops. Owing to decreased kidney function, fewer hydrogen ions are excreted. Organic acids accumulate in the body, causing a reduction in the serum HCO3 concentration. Although a child with uncompromised ventilatory capacity is able to hyperventilate and achieve partial compensation, a child with compromised pulmonary function or a hypercatabolic state is at risk for profound acidosis. Metabolic acidosis is usually treated by administering NaHCO3. However, attention should be directed toward the excess sodium load associated with this mode of therapy. Because hypocalcemia develops in many patients with AKI, treatment with alkali should be done cautiously to protect them from hypocalcemic tetany due to a shift of ionized calcium from free to albumin-bound. It is not necessary to correct the metabolic acidosis completely to prevent the untoward effects of acidemia. Increasing the serum HCO3 concentration to 15 mEq/L is usually satisfactory.40
Dialysis
The most common indication for postoperative dialysis in a child is hypervolemia caused by repeated attempts at fluid resuscitation, administration of medications, and total parenteral nutrition.41 Repeated intravenous catheter flushes and endotracheal tube lavages can add a significant amount of water and solute to the total intake. Fluid overload in the postoperative patient can cause pulmonary edema and hypertension and may have a significant impact on patient recovery.42
Dialysis Methods.
The three modes of dialysis therapy include hemodialysis (HD), peritoneal dialysis (PD), and continuous renal replacement therapy (CRRT). Although PD has historically been used most often in children, more recent data have revealed an increased use of CRRT in those centers where the expertise and resources are available.38,41,43,44 Recognition of the needs of the patient, the resources of the treating facility, and the advantages and disadvantages of each dialytic technique dictate which modality is best (Table 4-3).41
TABLE 4-3
Characteristics of Dialysis Modalities
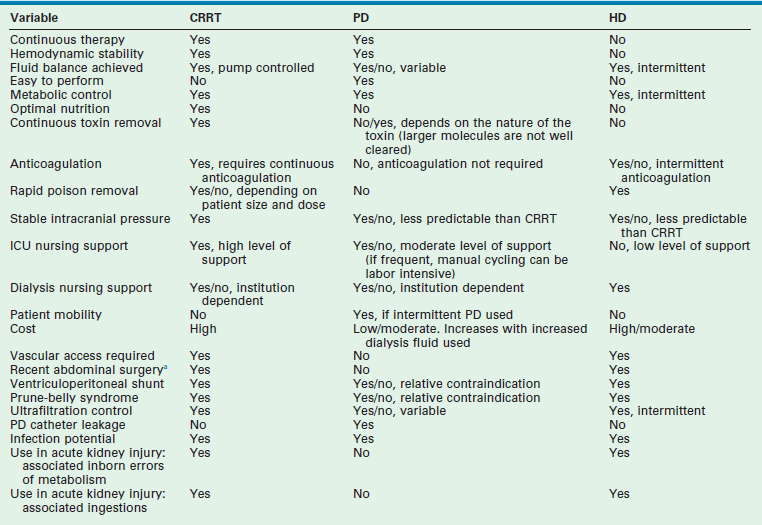
CRRT, continuous renal replacement therapy; HD, hemodialysis; PD, peritoneal dialysis.
aOmphalocele, gastroschisis, frequent or extensive abdominal surgery. Varies, depending on the location of the hemodialysis catheter.
Adapted from Changler C, Barratt TM. Laboratory evaluation. In Holiday MA (ed): Pediatric Nephrology, 2nd ed. Baltimore, Williams & Wilkins, 1987, pp. 282–99.
The intrinsic factors that affect the efficacy of PD include peritoneal blood flow, peritoneal vascular permeability, and peritoneal surface area. Although removal of up to 50% of the peritoneal surface area does not seem to interfere with dialysis efficacy, hypoperfusion of the peritoneal membrane vasculature renders PD ineffective.45 PD is feasible in the postoperative patient even in the presence of peritonitis or immediately after major abdominal operations.43,46–48 Increased intra-abdominal pressure caused by the dialysis fluid can cause respiratory embarrassment and can contribute to leakage from the incisions and the exit site of the PD catheter. If leakage persists, the smallest effective dialysis fluid volume (10–20 mL/kg) can be tried. Common complications associated with PD are peritonitis, exit site infection, dialysate leakage, catheter obstruction from omentum or fibrin, and abdominal wall hernia. Sclerosing peritonitis can also occur and is seen more commonly in females. It results in peritoneal scarring and can lead to bowel obstruction and perforation. Resection and stoma may be needed due to concerns about healing with a primary repair.
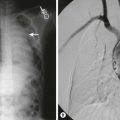
The provision of antibiotics at the time of catheter placement is recommended and may decrease the risk of peritonitis.49,50 Also, the use of fibrin glue at the site of catheter entry into the peritoneum has been associated with a decreased incidence of dialysate leakage during the immediate postoperative period and may be particularly beneficial when PD is initiated soon after catheter placement.51 A study in 2000 showed placement of a Tenckhoff catheter (Fig. 4-1) to be superior to the Cook catheter (Cook Medical, Bloomington, IN, USA) in terms of complication-free survival.52 More recently, there is evidence for equal outcomes with the Cook Multipurpose Drainage catheter, a flexible catheter that is placed at the bedside, in contrast to the Tenckhoff catheter, which typically requires operative insertion.53
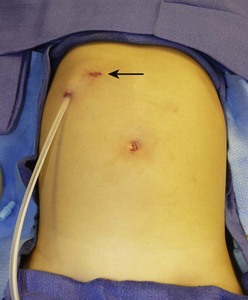
FIGURE 4-1 This 4-year-old child developed hemolytic uremic syndrome related to Escherichia coli colitis. A peritoneal dialysis catheter was inserted laparoscopically. Note the catheter is oriented caudally at the exit site which is felt to be the optimal way the peritoneal dialysis catheter should be oriented. A 5 mm incision in the right upper abdomen (arrow) is the site where the peritoneal dialysis catheter was introduced into the abdominal cavity. A 5 mm cannula and telescope were inserted in the umbilicus for visualization.
PD is performed with dialysis solutions that contain a 1.5%, 2.5%, or 4.25% glucose concentration. Dialysate with a 1.5% glucose concentration has an osmolality of 350 mOsm/kg H2O, which is moderately hypertonic to normal plasma (280–295 mOsm/kg H2O). Other factors being equal, the higher the tonicity of the dialysate and the greater the osmotic gradient between blood and dialysate, the greater the ultrafiltrate (fluid removed from the body). Owing to the rapid movement of water and glucose across the peritoneal membrane, the effect of PD on fluid removal is maximal when short dialysis cycles of 20 to 30 minutes are used. When solutions containing glucose concentrations higher than 1.5% are used, close monitoring of the patient’s serum glucose concentration is necessary. If hyperglycemia develops with a blood glucose concentration greater than 200 mg/dL, it can be controlled by the addition of insulin to the dialysate solution or by intravenous insulin drip. The volume of fluid removed by dialysis in a 24-hour period should generally be limited to 500 mL in the neonate, 500–1000 mL in infants and 1000–1500 mL in young children. The effect of dialysis on the removal of solutes depends mainly on the length of the dwell time of the dialysate within the peritoneal cavity and the molecular weight of the solute. The following are the relative rates of removal of common substances: urea> potassium> sodium> creatinine> phosphate> uric acid> calcium> magnesium. Standard dialysate solutions do not contain potassium. Therefore, hyperkalemia may be controlled with a few hours of effective PD.
Hemodialysis has the advantage of more rapid ultrafiltration and solute removal than either PD or CRRT. Adequate vascular access is the most important requirement, and a variety of temporary pediatric catheters are available.54 Ideally, insertion of the dialysis catheter in the right internal jugular vein is preferred, followed by the femoral vein and the left internal jugular vein. Placement in the subclavian vein should be discouraged because of the potential development of subclavian stenosis and the subsequent inability to create a dialysis fistula in the ipsilateral arm of those patients who go on to develop end-stage renal disease.38 Fluid removal can be problematic in the patient who is hypotensive and receiving HD because of poor patient tolerance, and is better accomplished by either PD or CRRT in this clinical setting.
The types of CRRT consist of continuous venovenous hemodialysis (CVVHD), continuous venovenous hemofiltration (CVVH), and continuous venovenous hemodiafiltration (CVVHDF). CRRT is now widely practiced in many tertiary pediatric centers because of the safety and efficacy of the technique in even the sickest patients. The choice of one method of CRRT over another depends on whether one chooses to make use of the diffusive (CVVHD) or convective (CVVH) method or a combination of the two (CVVHDF) properties of the technique. As in HD, a well-functioning vascular access catheter is crucial for CRRT. Data suggest that the optimal access is the one with the largest diameter preferably located within the internal jugular vein.55 Likewise, large extracorporeal blood volumes are necessary for the CRRT (and HD) circuit, and require blood products in the small patient in whom the circuit volume exceeds 10% of the patient’s blood volume. Particular attention must be paid to the possible development of hemofilter-related reactions that might occur with the initiation of therapy.56,57 The predictability and efficiency of ultrafiltration and solute removal make CRRT an ideal dialytic technique for hemodynamically unstable patients. In children at risk for hemorrhage, a protocol using citrate instead of heparin as the anticoagulant has been developed.58–60 Finally, new information has provided direction regarding the preferred timing of dialysis initiation. Fluid overload itself appears to be a significant risk factor for mortality, and its early and aggressive management with dialysis may prove particularly beneficial.61 A recent analysis has revealed 29.6% mortality with <10% fluid overload, 43.1% with 10–20% fluid overland, and 65.6% with >20% fluid overload.42
Acute Kidney Injury in the Neonate
AKI occurs in as many as 24% of all patients admitted to the neonatal intensive care unit (NICU).62,63 The definition of AKI in a term neonate has historically been considered to be a serum creatinine level above 1.5 mg/dL for more than 24 hours in the setting of normal maternal renal function.64 On occasion, it may be diagnosed in the term infant with a serum creatinine value less than 1.5 mg/dL when it fails to decrease in a normal manner over the initial days/weeks of life.65,66 More recently, it has been defined by an age independent increase in serum creatinine to ≥1.5 times baseline, which is known to have occurred within the past 7 days or a urine volume <0.5 mL/kg/h for 6 hours.38 A recent proposed neonatal specific classification has characterized stage 1 AKI as an increase in serum creatinine of 0.3 mg/dL or an increase in serum creatinine of 1.5–2 times the previous value.63 A pediatric modification (pRIFLE) of an adult AKI classification system has also been developed.67 The limited availability of cystatin C data from the neonatal population currently precludes its routine use to define AKI.68,69
AKI is of the oliguric variety when the elevated serum creatinine concentration is accompanied by a urine output below 1 mL/kg/hr after the initial 24 hours of life and when urine output fails to improve in response to a fluid challenge.70 In contrast, solute retention develops in some neonates, as evidenced by an elevated serum creatinine level, with a normal (>1.0 mL/kg/h) urine flow rate. These neonates are diagnosed as having nonoliguric AKI.71 The nonoliguric form is particularly common in neonates with AKI secondary to perinatal asphyxia and appears to be associated with a better prognosis than does the oliguric form.66,71 The diagnosis of nonoliguric AKI can be missed if patients at risk for developing renal insufficiency are monitored solely by the evaluation of urine output without repeated assessments of the serum creatinine concentration.
The causes of AKI in newborns traditionally have been divided into three categories: prerenal, intrinsic, and postrenal (Box 4-2). This division, based on the site of the problem, has important implications because the evaluation, treatment, and prognosis of the three groups can be quite different.
Prerenal Acute Kidney Injury
Impairment of renal perfusion is the cause of 70% of AKI during the neonatal period.62,63,65,66 Prerenal AKI may occur in any patient with hypoperfusion of an otherwise normal kidney. Although prompt correction of the low perfusion state usually reverses this impairment, delay in fluid resuscitation may result in renal parenchymal damage.
Intrinsic Acute Kidney Injury
Intrinsic AKI occurs in 6–8% of admissions to the NICU and implies the presence of renal cellular damage associated with impaired kidney function.65 Intrinsic AKI usually falls into one of the following categories: ischemic (acute tubular necrosis), nephrotoxic (aminoglycoside antibiotics, indomethacin), congenital renal anomalies (autosomal recessive polycystic kidney disease), and vascular lesions (renal artery or vein thrombosis), especially with a solitary kidney.72
Postrenal Acute Kidney Injury
Postrenal AKI results from obstruction of urine flow from both kidneys or from a solitary kidney. The most common causes of postrenal AKI in neonates are posterior urethral valves (PUV), bilateral ureteropelvic junction obstruction, and bilateral ureterovesical junction obstruction.73,74 Although these types of obstructions are characteristically reversible, neonates with long-standing intrauterine obstruction have varying degrees of permanent impairment of kidney function.75,76 This impairment may be due not only to the presence of renal dysplasia but also to cellular damage secondary to AKI.
Diagnostic Evaluation
Laboratory studies are an important component of this evaluation and include the following measures: complete blood cell count and determination of serum concentrations of urea nitrogen, creatinine, electrolytes, uric acid, calcium, glucose, and phosphorus. The serum creatinine value during the first several days of life is a reflection of the maternal value. In term infants, a value of 0.4–0.5 mg/dL is expected after the first week of life. In contrast, the expected value in preterm infants is related to their gestational age, with an initial increase followed by a gradual decrease.77,78 In all cases, a urinalysis should be obtained to check for the presence of red blood cells, protein, and casts suggestive of intrinsic renal disease.
Urine indices can help distinguish intrinsic renal failure from prerenal azotemia in the oliguric newborn.37,79 As mentioned previously, the index usually found to be most useful is the FE Na. This factor is based on the assumption that the renal tubules of the poorly perfused kidney reabsorb sodium avidly, whereas the kidney with intrinsic renal disease and tubular damage is unable to do so. Accordingly, in most cases of neonatal oliguric renal failure secondary to intrinsic disease, the FE Na is >2.5–3.0%, a value that is different from that of the older child.62,70 The FE Na should be measured before administering furosemide. In addition, the results should be interpreted with caution in the very premature infant who normally has an even higher (i.e., >5%) FE Na.64,80
Ultrasonography commonly is the initial imaging study.81 The urinary tract should be evaluated for the presence of one or two kidneys and for their size, shape, and location. A voiding cystourethrogram (VCUG) may also be necessary, specifically when the diagnosis of PUV or vesicoureteral reflux is entertained. In most cases, a VCUG is deemed preferable to radionuclide cystography in this setting because of its superior ability to provide reliable anatomic information about the grading of vesicoureteral reflux or the appearance of the urethra.82 Antegrade pyelography or diuretic renography with either 99mTc-dimercaptosuccinic acid (DMSA) or 99mTc-dimercaptoacetyltriglycine (MAG3) may be needed to evaluate for ureteral obstruction. Finally, assessment of the differential kidney function may be performed with radioisotope scanning as well.
Management
The treatment of neonatal AKI should proceed simultaneously with the diagnostic workup. Bladder catheter placement is a good immediate therapy for PUV, whereas high surgical drainage may be needed for other obstructive lesions in the neonate. The fluid challenge for the neonate should consist of 20 mL/kg of an isotonic solution containing 25 mEq/L of NaHCO3 infused over a one- to two-hour period. In the absence of a prompt diuresis of 2 mL or more of urine per kilogram over one to two hours, intravenous furosemide at 1–3 mg/kg may be helpful, As noted previously, the potential role of low-dose (0.5–3.0 µg/kg/min) dopamine continues to be debated, but recent guidelines recommend against its use to prevent or treat AKI.37,38 The failure to achieve increased urinary output after volume expansion in the neonate with an adequate cardiac output and an unobstructed urinary tract indicates the presence of intrinsic kidney disease and the need to manage oliguric or anuric kidney failure appropriately.
Maintenance of normal fluid balance is of primary concern in the management of the patient with AKI. Daily fluid intake should equal insensible water loss, urine output, and fluid losses from nonrenal sources. In term infants, insensible water losses amount to 30–40 mL/kg/day, whereas premature infants may require as much as 50–100 mL/kg/day.80,83 A frequent assessment of the neonate’s body weight is essential for fluid management. The electrolyte content of the fluids administered should be guided by frequent laboratory studies. Insensible water losses are electrolyte free and should be replaced by using 5% dextrose in water.
Important systemic disturbances that may arise secondary to AKI include hyperkalemia, hyponatremia, hypertension, hypocalcemia, hyperphosphatemia, and metabolic acidosis. All exogenous sources of potassium should be discontinued in patients with AKI. Despite this restriction, elevated serum potassium levels develop in many neonates and must be treated aggressively due to the potential for cardiac toxicity.34 Treatment should be initiated by correction of metabolic acidosis with NaHCO3. A dose of 1–2 mEq/kg should be given intravenously over a ten to 20-minute period, provided that salt and water balance is not problematic. The quantity of NaHCO3 to be prescribed also can be calculated in the following manner:
Associated hypocalcemia should be treated with the intravenous administration of 10% calcium gluconate at a dose of 0.5–1.0 mL/kg injected slowly over a five- to 15-minute period with continuous monitoring of the heart rate. If a progressive increase in the serum potassium concentration is noted, additional treatment measures may include the use of a sodium–potassium exchange resin (sodium polystyrene sulfonate in 20–30% sorbitol, 1 g/kg by enema), with recognition of its frequent ineffectiveness and/or associated complications when used in low birth weight infants.84 The use of glucose (0.5–1.0 g/kg) followed by insulin (0.1–0.2 unit regular insulin per gram glucose over a 1-hour period) may be the preferred approach. Either intravenous salbutamol or inhaled albuterol is an additional therapeutic option.85–87 Dialysis should be considered if these measures prove unsuccessful.48,87,88
When serum sodium levels are less than 120 mEq/L and are associated with symptoms (e.g., seizures), prompt treatment with hypertonic (3%) saline is indicated. The provision of 10–12 mL/kg of 3% saline is generally therapeutic.
The treatment of persistent hypertension may include parenterally administered hydralazine (0.15 to 0.6 mg/kg/dose), labetalol (0.20 to 1.0 mg/kg/dose or 0.25 to 3.0 mg/kg/hr infusion), or enalapril at (5.0 to 10 µg/kg/dose). Orally administered amlodipine (0.05 to 0.3 mg/kg/dose) can be prescribed for the patient who is without symptoms. Treatment of the patient with marked or refractory hypertension can include intravenous sodium nitroprusside (0.5 to 10 µg/kg/min infusion), nicardipine (1 to 4 µg/kg/min infusion), or labetalol.89 Caution should be exercised when initiating therapy with captopril (initial oral dose, 0.01 to 0.05 mg/kg/dose), owing to the profound hypotension that can occur in neonates in association with higher doses.90,91
In the infant in whom AKI does not fully resolve and becomes chronic renal failure (CKD), the development of hyperphosphatemia (serum phosphorus level > 7 mg/dL) necessitates a low phosphorus infant formula and possibly calcium carbonate (50–100 mg/kg/day) as a phosphate binder.89 The use of aluminum hydroxide as a binder is contraindicated, owing to its association with aluminum toxicity in infants and children with renal insufficiency.90 No experience has been published about the use of newer, noncalcium-containing phosphate-binding agents, such as sevelamer, in the neonatal population.91,92 Hypocalcemia, as ref1ected by a low total serum calcium level, often occurs in AKI in association with hypoalbuminemia. Less commonly, the ionized calcium level is low and the patient is symptomatic. In these cases, intravenous 10% calcium gluconate, 0.5–1.0 mL/kg, over a five-minute period with cardiac monitoring should be given until the ionized calcium level is restored to the normal range.
This dose may be given orally or added to parenteral fluids and infused during several hours.
Adequate nutrition should be provided, with the goal of 100–120 calories and 1–2 g of protein/kg/day, provided intravenously or orally. Additional protein may be needed to account for dialysis related losses in those patients receiving PD and CRRT.93,94 For neonates who can tolerate oral fluids, a formula containing low levels of phosphorus and aluminum, such as Similac PM 60/40 (Abbott Labs, Abbott Park, IL), is recommended. An aggressive approach to nutrition may well contribute to kidney recovery by providing necessary energy at the cellular level.34
Although most neonates with AKI can be managed conservatively, occasional patients require PD or CRRT for the treatment of the metabolic complications and fluid overload.95–97 The mortality rate in this group of patients can be exceedingly high in the setting of AKI post-cardiac surgery.47,98–100 Apart from the need for pressor support, the procedure was well tolerated in one report on the use of CRRT in 85 children weighing less than 10 kg, with survival rates of 25% and 41% for those weighing less than 3 kg and from 3–10 kg, respectively.97 A recent retrospective study of PD treatment of AKI post-cardiac surgery in 146 neonates and infants revealed that the mortality rate was decreased by more than 40% in those patients who received ‘early PD’ (day of surgery or postoperative day 1) versus ‘delayed PD’ (postoperative day 2 or later).101 Finally, when AKI occurred in neonates receiving extracorporeal membrane oxygenation, the mortality rate was 3.2 times higher than in those without AKI.102 Moreover, patients who required renal replacement therapy had 1.9 higher odds of death than those who did not receive this therapy.
Obstructive Uropathy
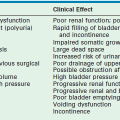
Obstructive uropathy in the neonate is the most common renal abnormality diagnosed prenatally and is most often the result of ureteropelvic junction obstruction, PUV, or ureterovesical junction obstruction.73 Obstruction also represents a significant cause of end-stage renal disease in children, accounting for 13% of all cases.103 Accordingly, early recognition and treatment of these lesions are desirable because of the adverse effects that obstruction can have on kidney function.39,40,75,76,104 Regardless, after surgical intervention and relief of obstruction, alterations of GFR, renal blood flow, and renal tubular function can still occur.76,104,105 Specifically, injury to the renal tubule can result in an impaired capacity to reabsorb sodium, to concentrate urine, and to secrete potassium and hydrogen, all of which can have profound clinical implications. The resorption of other solutes, such as magnesium, calcium and phosphorus, may also be affected.76,105
The ability of the renal tubule to reabsorb salt and water after relief of the obstruction typically depends on whether the obstruction is unilateral or bilateral. In unilateral obstruction, the proximal tubules of the juxtamedullary nephrons are unable to reabsorb salt and water maximally, whereas the fractional reabsorption of salt and water is increased in the superficial nephrons.105 However, the amount of sodium excreted by the previously obstructed kidney is not different from that of the contralateral kidney, because tubuloglomerular balance is maintained. In contrast, relief of bilateral obstruction or, on occasion, unilateral obstruction in neonates, results in a postobstructive diuresis characterized by a marked elevation in the absolute amount of sodium and water lost.106 In part, these changes are a result of an osmotic diuresis secondary to retained solutes, such as urea.106,107 Some contribution may also occur from atrial natriuretic factor, the plasma level of which is elevated during obstruction, as well as from enhanced synthesis of prostaglandins.105 Decreased renal medullary tonicity and decreased hydraulic water permeability of the collecting duct in response to ADH, the latter a result of reduced aquaporin channels, contribute to the impaired concentrating ability of the kidney.36,104
Ureteral obstruction also can result in the impairment of hydrogen and potassium secretion and the syndrome of hyperkalemia, hyperchloremic metabolic acidosis, or type IV RTA.108–110 This clinical situation appears to be the result of the impaired turnover of the sodium-potassium pump or a decreased responsiveness of the distal renal tubule to the actions of aldosterone. In a portion of the patients with this presentation, the FE Na is normal and the FE κ is inappropriately low, relative to the elevated serum level. Treatment is directed toward correcting the underlying obstructive abnormality as well as providing NaHCO3 to alleviate the metabolic acidosis and hyperkalemia.
Finally, the outcome of obstructive uropathy in the neonate in terms of preservation of GFR is, in part, related to how promptly relief of obstruction occurs. In these patients, the serum creatinine obtained at age 12 months has been shown to be predictive of long-term kidney function.39,40,76,104 Attempts to preserve renal function with fetal surgery in the patient with obstructive uropathy have not proven to be successful.111
References
1. Brenner, B, Dworkin, L, Kchikawa, L, Glomerular ultrafiltration. The Kidney. Brenner, B, Rector, F, eds. The Kidney; Vol.1. WB Saunders, Philadelphia, 1986:124–144.
2. Hogg, R, Stapleton, F. Renal tubular function. In: Holliday M, Barratt T, Vernier R, eds. Pediatric Nephrology. Baltimore: Williams & Wilkins; 1987:59–77.
3. Yared, A, Ichikawa, I. Renal blood flow and glomerular filtration rate. In: Holliday M, Barratt T, Vernier R, eds. Pediatric Nephrology. Baltimore: Williams & Wilkins; 1987:45–58.
4. Perrone, R, Madias, N, Levey, A. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem. 1992; 38:1933–1953.
5. Hellerstein, S, Hunter, J, Warady, B. Creatinine excretion rates for evaluation of kidney function in children. Pediatr Nephrol. 1988; 2:419–424.
6. Newman, D, Thakkar, H, Edwards, R, et al. Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995; 47:312–318.
7. Bokenkamp, A, Domanetzki, M, Zinck, R, et al. Cystatin C serum concentrations underestimate glomerular filtration rate in renal transplant recipients. Clin Chem. 1999; 45:1866–1868.
8. Finney, H, Newman, D, Price, C. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000; 31:49–59.
9. Fisehbach, M, Graff, V, Terzie, J, et al. Impact of age on reference values for serum concentration of cystatin C in children. Pediatric Nephrol. 2002; 17:104–106.
10. Schwartz, GJ, Munoz, A, Schneider, M, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009; 20:629–637.
11. Moritz, M, Ayus, J. Prevention of hospital-acquired hyponatremia: A case for using isotonic saline. Pediatrics. 2003; 111:227–230.
12. Moritz, M, Ayus, J. Hospital-acquired hyponatremia—why are hypotonic parenteral fluids still being used? Nat Clin Pract Nephrol. 2007; 3:374–382.
13. Holliday, M, Segar, W. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957; 19:823–832.
14. Polacek, B, Vocel, J, Neugebauerova, L, et al. The osmotic concentrating ability in healthy infants and children. Arch Dis Child. 1965; 40:291–295.
15. Zappitelli, M, Parikh, C, Akcan-Arikan, A, et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008; 3:948–954.
16. Hui-Stickle, S, Brewer, E, Goldstein, S. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005; 45:96–101.
17. Burry, H, Dieppe, P. Apparent reduction of endogenous creatinine clearance by salicylate treatment. Br Med. 1976; 2:16–17.
18. Berglund, F, Killander, J, Pompeius, R. Effect of trimethoprim-sulfamethoxazole on the renal excretion of creatinine in man. J Urol. 1975; 114:802–808.
19. Work, D, Schwartz, G. Estimating and measuring glomerular filtration rate in children. Curr Opin Nephrol Hypertens. 2008; 17:320–325.
20. Fadrowski, J, Neu, A, Schwartz, GJ, et al. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol. 2011; 6:1427–1435.
21. Schwartz, GJ, Schneider, M, Maier, P, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Inf. 2012; 82(4):445–453.
22. Hellerstein, S, Holliday, M, Grupe, W, et al. Nutritional management of children with chronic renal failure. Summary of the task force on nutritional management of children with chronic renal failure. Pediatr Nephrol. 1987; l:195–211.
23. Chantler, C, Barratt, T. Laboratory evaluation. In: Holliday M, Barratt T, Vernier R, eds. Pediatric Nephrology. Baltimore: Williams & Wilkins; 1987:282–299.
24. Srivastava, T, Garg, U, Alon, U. Impact of standardization of creatinine methodology on the assessment of glomerular filtration rate. Pediatr Res. 2008; 65:113–116.
25. Steiner, R. Interpreting the fractional excretion of sodium. Am J Med. 1984; 77:699–702.
26. Halperin, M, Goldstein, M, Stinebaugh, B, et al. Renal tubular acidosis. In: Maxwell M, Kleeman C, Narins R, eds. Clinical Disorders of Fluid and Electrolyte Metabolism. New York: McGraw-Hill; 1987:675–689.
27. Rodriguez-Soriano, J, Vallo, A. Renal tubular acidosis. Pediatr Nephrol. 1990; 4:268–275.
28. Alon, U, Chan, J. Inherited form of renal tubular acidosis. In: Fernandes J, Saudubray J, Tada K, eds. Inherited Metabolic Diagnosis and Treatment. New York: Springer-Verlag; 1990:585–595.
29. Wedekin, M, Ehrich, J, Offner, G, et al. Aetiology and outcome of acute and chronic renal failure in infants. Nephrol Dial Transplant. 2008; 23:1575–1580.
30. Goldstein, S. Pediatric acute renal failure: Demographics and treatment. In: Ronco C, Bellomo R, Brendolan A, eds. Sepsis, Kidney and Multiple Organ Dysfunction. Basel: Karger; 2004:284–290.
31. Fadel, F, Abdel Rahman, A, Mohamed, M, et al. Plasma neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury after cardiopulmonary bypass in pediatric cardiac surgery. Arch Med Sci. 2012; 8:250–255.
32. Devarajan, P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011; 23:194–200.
33. Cohen, M, Ritkind, D. The pediatric abacus. Boca Raton: The Parthenon Publishing Group; 2002.
34. Gaudio, K, Siegel, N. Pathogenesis and treatment of acute renal failure. Pediatr Clin North Am. 1987; 34:771–787.
35. Bailey, J, Shapiro, M. Abdominal compartment syndrome. Crit Care Med. 2000; 4:23–29.
36. Singh, N, Kissoon, N, Al-Mofada, S, et al. Furosemide infusion versus furosemide bolus in the postoperative pediatric cardiac patient. Pediatr Res. 1990; 27:35A.
37. Kellum, J, Decker, JM. Use of dopamine in acute renal failure: A meta-analysis. Crit Care Med. 2001; 29:1526–1531.
38. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group, KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012; 2:1–138.
39. Trachtman, H. Sodium and water homeostasis. Pediatr Clin N Am. 1995; 2:1343–1363.
40. Feld, L, Cachero, S, Springate, J. Fluid needs in acute renal failure. Pediatr Clin N Am. 1990; 37:337–350.
41. Walters, S, Porter, C, Brophy, P. Dialysis and pediatric acute kidney injury: Choice of renal support modality. Pediatr Nephrol. 2008; 24:37–48.
42. Sutherland, S, Zappitelli, M, Alexander, S, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: The prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010; 55:316–325.
43. Sebestyen, JF, Warady, BA. Advances in pediatric renal replacement therapy. Adv Chronic Kidney Dis. 2011; 18:376–383.
44. Warady, B, Bunchman, T. Dialysis therapy for children with acute renal failure: Survey results. Pediatr Nephrol. 2000; 15:11–13.
45. Alon, U, Bar-Maor, JA, Bar-Joseph, G. Effective peritoneal dialysis in an infant with extensive resection of the small intestine. Am J Nephrol. 1988; 8:65–67.
46. Bonifati, C, Pansini, F, Torres, D, et al. Antimicrobial agents and catheter-related interventions to prevent peritonitis in peritoneal dialysis using evidence in the context of clinical practice. Int J Artif Organs. 2006; 29:41–49.
47. Pedersen, K, Hjortdal, V, Christensen, C, et al. Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int. 2008; 108:S81–S86.
48. Zaritsky, J, Warady, B. Peritoneal Dialysis in the Newborn. In: Kiessling S, Chisthti A, Alam S, eds. Kidney and Urinary Tract Diseases in the Newborn. Springer Medical Publishing, 2012.
49. Warady, BA, Bakkaloglu, S, Newland, J, et al. Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int. 2012; 32:S29–S86.
50. Bonifati, C, Pansini, F, Torres, D, et al. Antimicrobial agents and catheter-related interventions to prevent peritonitis in peritoneal dialysis: Using evidence in the context of clinical practice. Int J Artif Organs. 2006; 29:41–49.
51. Sojo, E, Grosman, M, Monteverde, M, et al. Fibrin glue is useful in preventing early dialysate leakage in children on chronic peritoneal dialysis. Perit Dial Int. 2004; 24:186–190.
52. Chadha, V, Warady, B, Blowey, D, et al. Tenckhoff catheters prove superior to Cook catheters in pediatric acute peritoneal dialysis. Am J Kidney Dis. 2000; 35:1111–1116.
53. Auron, A, Warady, B, Simon, S, et al. Use of the multipurpose drainage catheter for the provision of acute peritoneal dialysis in infants and children. Am J Kidney Dis. 2007; 49:650–655.
54. Bunchman, T, Donckerwolcke, R. Continuous arterial-venous diahemofiltration and continuous veno-venous diahemofiltration in infants and children. Pediatr Nephrol. 1994; 8:96–102.
55. Hackbarth, R, Bunchman, T, Chua, A, et al. The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: A report from the PPCRRT registry. Int J Artif Organs. 2007; 30:1116–1121.
56. Strazdins, V, Watson, A, Harvey, B, European Pediatric Peritoneal Dialysis Working Group. Renal replacement therapy for acute renal failure in children: European guidelines. Pediatr Nephrol. 2004; 19:199–207.
57. Brophy, P, Mottes, T, Kudelka, T, et al. AN-69 membrane reactions are pH-dependent and preventable. Am J Kidney Dis. 2001; 38:173–178.
58. Brophy, P, Somers, M, Baum, M, et al. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant. 2005; 20:1416–1421.
59. Chadha, V, Garg, U, Warady, B, et al. Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr Nephrol. 2002; 17:819–824.
60. Symons, J, Chua, A, Somers, M, et al. Demographic characteristics of pediatric continuous renal replacement therapy: A report of the Prospective Pediatric Continuous Renal Replacement Therapy Registry. Clin J Am Soc Nephrol. 2007; 2:732–738.
61. Goldstein, S, Somers, M, Baum, M, et al. Pediatric patients with multi-organ system dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005; 67:653–658.
62. Chan, J, Williams, D, Roth, K. Kidney failure in infants and children. Pediatr Rev. 2002; 23:47–60.
63. Jetton, J, Askenazi, D. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012; 24:191–196.
64. Whyte, D, Fine, R. Acute renal failure in children. Pediatr Rev. 2008; 29:299–306.
65. Stapleton, F, Jones, D, Green, R. Acute renal failure in neonates: Incidence, etiology and outcome. Pediatr Nephrol. 1987; 1:314–320.
66. Drukker, A, Guignard, J. Renal aspects of the term and preterm infant: A selective update. Curr Opin Pediatr. 2002; 14:175–182.
67. Akcan-Arikan, A, Zappitelli, M, Loftis, L, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007; 71:1028–1035.
68. Finney, H, Newman, D, Thakkar, H, et al. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000; 82:71–75.
69. Harmoinen, A, Ylinen, E, Ala-Houhala, M, et al. Reference intervals for cystatin C in pre- and full-term infants and children. Pediatr Nephrol. 2000; 15:105–108.
70. Andreoli, S. Acute renal failure in the newborn. Semin Perinatol. 2004; 28:112–123.
71. Karlowicz, M, Adelman, R. Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol. 1995; 9:718–722.
72. Blowey, D, Ben, D, Koren, G. Interactions of drugs with the developing kidney. Pediatr Clin N Am. 1995; 42:1415–1431.
73. Elder, J, Duckett, J. Management of the fetus and neonate with hydronephrosis detected by prenatal ultrasonography. Pediatr Ann. 1988; 17:19–28.
74. Saphier, C, Gaddipati, S, Applewhite, L, et al. Prenatal diagnosis and management of abnormalities in the urologic system. Clin Perinatol. 2000; 27:921–945.
75. Chevalier, R. Obstructive uropathy: State of the art. Pediatr Med Chir. 2002; 24:95–97.
76. Kemper, M, Muller-Wiefel, D. Renal function in congenital anomalies of the kidney and urinary tract. Curr Opin Urol. 2001; 11:571–575.
77. Gallini, F, Maggio, L, Romagnoli, C, et al. Progression of renal function in preterm neonates with gestational age < 32 weeks. Pediatr Nephrol. 2000; 15:119–124.
78. Feldman, W, Guignard, J. Plasma creatinine in the first month of life. Arch Dis Child. 1982; 57:123–126.
79. Bellomo, R, Chapman, M, Finfer, S, et al. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000; 356:2139–2143.
80. Anand, S. Acute renal failure. In: Taeusch H, Ballard R, Avery M, eds. Diseases of the Newborn. Philadelphia: W B Saunders; 1991:894–895.
81. Mercado-Deane, M, Beeson, J, John, S. Ultrasound of renal insufficiency in neonates. Radiographics. 2002; 22:1429–1438.
82. Kraus, S. Genitourinary imaging in children. Pediatr Clin N Am. 2001; 48:1381–1424.
83. Roy, R. Hydration of the low birth-weight infant. Clin Perinatol. 1975; 2:393–417.
84. Ohlsson, A, Hosking, M. Complications following oral administration of exchange resins in extremely low-birth-weight infants. Eur J Pediatr. 1987; 146:571–574.
85. Singh, B, Sadiq, H, Noguchi, A, et al. Efficacy of albuterol inhalation in treatment of hyperkalemia in premature neonates. J Pediatr. 2002; 141:16–20.
86. Mildenberger, E, Versmold, H. Pathogenesis and therapy of non-oliguric hyperkalemia of the premature infant. Eur J Pediatr. 2002; 161:415–422.
87. Vemgal, P, Ohlsson, A. Interventions for non-oliguric hyperkalaemia in preterm neonates. Cochrane Database Syst Rev. 24(1), 2007.
88. Goldstein, S. Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial. 2011; 24:187–191.
89. Alon, U, Davidai, G, Bentur, L, et al. Oral calcium carbonate as phosphate binder in infants and children with chronic renal failure. Miner Electrolyte Metab. 1986; 12:320–325.
90. American Academy of Pediatrics. Aluminum toxicity in infants and children. Pediatrics. 1996; 97:412–416.
91. Slatopolsky, E, Burke, S, Dillon, M, et al. RenaGel, a nonabsorbed calcium- and aluminum-free phosphate binder, lowers serum phosphorus and parathyroid hormone. Kidney Int. 1999; 55:299–307.
92. Salusky, I. A new era in phosphate binder therapy: What are the options? Kidney Int. 2006; 105:S10–S15.
93. Zappitelli, M, Goldstein, S, Symons, J, et al. Protein and calorie prescription for children and young adults receiving continuous renal replacement therapy: A report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Crit Care Med. 2008; 36:3239–3245.
94. National Kidney Foundation. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD. Am J Kidney Dis. 2009; 53:S1–124.
95. Flynn, J. Choice of dialysis modality for management of pediatric acute renal failure. Pediatr Nephrol. 2002; 17:61–69.
96. Golej, J, Kitzmueller, E, Hermon, M, et al. Low-volume peritoneal dialysis in 116 neonatal and pediatric critical care patients. Eur J Pediatr. 2002; 161:385–389.
97. Symons, J, Brophy, P, Gregory, M, et al. Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis. 2003; 41:984–989.
98. Pedersen, K, Povlsen, J, Christensen, S, et al. Risk factors for acute rena1 failure requiring dialysis after surgery for congenital heart disease in children. Acta Anaesthesiol Scand. 2007; 51:1344–1349.
99. Blinder, JJ, Goldstein, SL, Lee, W, et al. Congenital heart surgery in infants: Effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012; 143:368–374.
100. Baskin, E, Saygili, A, Harmanci, K, et al. Acute renal failure and mortality after open-heart surgery in infants. Ren Fail. 2005; 27:557–560.
101. Bojan, M, Gioanni, S, Vouhe, P, et al. Early initiation of peritoneal dialysis in neonates and infants with acute kidney injury following cardiac surgery is associated with a significant decrease in mortality. Kidney Int. 2012.
102. Askenazi, D, Ambalavanan, N, Hamilton, K, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011; 12:1–6.
103. North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). 2011 Annual Dialysis Report 2011.
104. Chevalier, R, Kim, A, Thornhill, B, et al. Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int. 1999; 55:793–807.
105. Klahr, S, Harris, K, Purkerson, M. Effects of obstruction on renal functions. Pediatr Nephrol. 1992; 147:430–432.
106. Boone, T, Allen, T. Unilateral postobstructive diuresis in the neonate. J Urol. 1992; 147:43–432.
107. Harris, R, Yarger, W. The pathogenesis of post-obstructive diuresis: The role of circulating natriuretic and diuretic factors, including urea. J Clin Invest. 1975; 56:880–887.
108. Rodriguez-Soriano, J, Vallo, A, Oliveros, R, et al. Transient pseudo-hypoaldosteronism secondary to obstructive uropathy in infancy. J Pediatr. 1983; 103:375–380.
109. Yarger, W, Buerkert, J. Effect of urinary tract obstruction on renal tubular function. Semin Nephrol. 1982; 2:17–30.
110. Alon, U, Kordoff, M, Broecker, B, et al. Renal tubular acidosis type IV in neonatal unilateral kidney diseases. J Pediatr. 1984; 104:855–860.
111. Hodges, S, Patel, B, McLorie, G, et al. Posterior urethral valves. Scientific World Journal. 2009; 14:1119–1126.