CHAPTER 6 Renal failure
Genitourinary assessment: general
Goal of system assessment
Evaluate for decreased renal function and assess the severity of renal dysfunction.
Detailed health history
• Chronic symptoms of fatigue, weight loss, anorexia, nocturia, and pruritus
• Renal-related symptoms including dysuria, edema, frequency, hematuria, flank pain, pyuria, frothy urine, bloody urine, and renal colic
• Presence of comorbidities: hypertension, congestive heart failure, diabetes, multiple myeloma, chronic infection, and myeloproliferative disorder
• Current medications including over-the-counter medications
Observation
Evidence of chronic versus acute process
• Skin: petechiae, purpura, ecchymosis, livedo reticularis, dryness, pallor, yellowness, decreased turgor
• Eyes: uveitis, ocular palsy, findings suggestive of hypertension, atheroembolic disease
• Inspection of the flank area in a standing and supine position for raised masses or unusual pulsations
Vital sign assessment
Evaluate for changes indicative of fluid volume excess or depletion and infection.
Palpation
Abdominal assessment to identify renal pathology
• Costovertebral angle (CVA) tenderness, which may occur with pyelonephritis
• Enlarged liver, which may occur with congestive heart failure
• Kidneys are difficult to palpate because of location. If they are enlarged and palpable, this could represent polycystic kidney disease or hydronephrosis.
• Ascites may occur with liver failure or acute renal failure.
Labwork
Acute renal failure/acute kidney injury
Pathophysiology
The product of ADQI was a graded definition of ARF designated as the RIFLE criteria. This led to the development of the Acute Kidney Injury network. The workings of these groups resulted in the adoption of the term acute kidney injury (AKI), which represents the entire spectrum of ARF. The RIFLE criteria are based on three graded levels of injury which reflect serum creatinine or urine output and two outcome measures (Table 6-1). Formation of urine is a three-step process consisting of (1) ultrafiltration of delivered blood by the glomeruli (renal cortex), (2) internal processing of the ultrafiltrate via tubular secretion and reabsorption (renal parenchyma), and (3) excretion of waste products from the kidneys through the ureters, bladder, and urethra. Corresponding to those steps, ARF/AKI is categorized as prerenal, intrarenal, and postrenal (Table 6-2).
Table 6-1 RIFLE CLASSIFICATION FOR ACUTE RENAL FAILURE (ARF)/ACUTE KIDNEY INJURY (AKI)
| Classification | GFR Criteria | Urine Output Criteria |
|---|---|---|
| Risk | Serum creatinine increased 1.5 times | Less than 0.5 ml/kg/hr for 6 hours |
| Injury | Serum creatinine increased 2 times | Less than 0.5 ml/kg/hr for 12 hours |
| Failure | Serum creatinine increased 3 times or greater than 355 μmol/L or mg/dl when there was an acute rise of greater than 44 μmol/L or mg/dl | Less than 0.3 ml/kg/hr for 24 hours or anuria for 12 hours |
| Loss | Persistent acute renal failure: complete loss of kidney function for longer than 4 weeks | |
| End-stage renal disease | End-stage renal disease for longer than 3 months |
Table 6-2 CAUSES OF ACUTE RENAL FAILURE
| Prerenal (Decreased Renal Perfusion) | Intrarenal (Parenchymal Damage; Acute Tubular Necrosis) | Postrenal (Obstruction) |
|---|---|---|
| Hypovolemia
Hepatorenal syndromeEdema-forming conditions Renal vascular disorders |
• Antibiotics (aminoglycosides, sulfonamides, methicillin)
• Diuretics (e.g., furosemide)
Organic solvents (e.g., carbon tetrachloride, ethylene glycol)Infection (gram-negative sepsis), pancreatitis, peritonitis transfusion reaction (hemolysis)
• Rhabdomyolysis with myoglobinuria (severe muscle injury)
• Drug-related: heroin, barbiturates, IV amphetamines, succinylcholine
Glomerular diseases
GI, gastrointestinal; IgA, immunoglobulin A; IV, intravenous.
Prerenal failure, or azotemia, is the result of decreased blood flow to the kidneys. The events leading to prerenal insults cause decreased renal vascular perfusion and may be associated with systemic hypoperfusion. If treated promptly, this form of ARF/AKI is readily reversible. Chronic heart failure, drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) and angiotensin-converting enzyme (ACE) inhibitors, volume loss, or sequestration and shock states (especially septic shock) all may lead to reduced renal perfusion. If not managed aggressively, parenchymal (intrarenal) involvement, or acute tubular necrosis (ATN), can result. Intrarenal damage may result from a mean arterial pressure less than75 mm Hg. Autoregulation fails; the sympathetic response increases and, with the action of the renin-angiotensin system, results in severe constriction of the afferent arteriole. Glomerular blood flow and hydrostatic pressure are reduced, and the GFR decreases. The amount of cellular damage depends on the duration of ischemia: mild damage (less than 25 minutes), moderate/severe damage (40 to 60 minutes), and irreversible damage (may occur within 60 to 90 minutes).
6-1 RESEARCH BRIEF
From Kelly AM. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med 148:284–294, 2008.
ATN is characterized by tubular cell necrosis, cast formation, and tubular obstruction caused by casts and cellular debris. Therapy is focused on maintenance of renal perfusion pressure, administering renal vasodilators to restore blood flow, and promoting diuresis to “wash out” the intratubular debris. Sometimes ATN is nonoliguric. Oliguria may occur with both toxic ATN and ischemic ATN. Common nephrotoxic agents are found in Table 6-3.
Table 6-3 COMMON NEPHROTOXIC AGENTS
| Drugs | X-ray Contrast Media |
|---|---|
| Antineoplastics Methotrexate Cisplatin Antibiotics Cephalosporins Aminoglycosides Tetracycline Nonsteroidal anti-inflammatory drugs Ibuprofen Ketorolac |
Biologic substances Myoglobin Tumor products Chemicals Ethylene glycol Pesticides Organic solvents Heavy metals Lead Mercury Gold |
Fluid, electrolyte, and acid-base disorders that occur with ARF include hypervolemia, hyperkalemia, hyperphosphatemia, hypocalcemia, hypermagnesemia, and metabolic acidosis (Table 6-4). Phosphate levels rise because of impaired excretion of phosphorus by the renal tubules with continued gastrointestinal (GI) absorption. Hypocalcemia results from the lack of active vitamin D, which is activated by the kidney, which would otherwise stimulate absorption of calcium from the GI tract, or high phosphate levels, which inhibit absorption of calcium. Hypocalcemia triggers the parathyroid glands to secrete parathyroid hormone (PTH), which mobilizes calcium from the bone into the blood. Hypermagnesemia is generally moderate (2 to 4 mg/dl) and is rarely symptomatic unless the patient receives magnesium-containing antacids (e.g., Maalox, Milk of Magnesia).
Table 6-4 ALTERED ELECTROLYTE BALANCE IN ACUTE RENAL FAILURE (ARF)
ARF, acute renal failure; Ca2+, calcium; DTR, deep tendon reflex; ECG, electrocardiogram; HCO3−, bicarbonate; HR, heart rate; I&O, intake and output; IV, intravenous; K+, potassium; LOC, level of consciousness; Mg2+, magnesium; Na+, sodium; OTC, over-the-counter; SOB, shortness of breath; VS, vital signs.
There are three identifiable stages/phases of ARF:
1. Oliguric phase: A drop in the 24-hour urinary output to less than 400 ml lasting approximately 7 to 14 days. About 30% of patients have nonoliguric renal failure.
2. Diuretic phase: A doubling of the urinary output from the previous 24-hour total. During this phase the patient may produce as much as 3 to 5 L of urine in 24 hours.
3. Recovery phase: A return to a normal 24-hour volume (1500 to 1800 ml). Usually, renal function continues to improve and may take 6 months to 1 year from the initial insult to return to baseline functional status.
Assessment
Goal of assessment
Evaluate fluid, electrolyte, and acid-base balances to prevent the development of metabolic encephalopathy (see Genitourinary Assessment: General, p. 583).
Vital signs
• BP may be elevated in states of fluid volume excess or decreased in states of fluid volume deficit.
• Heart rate (HR) may be increased or decreased with abnormal rhythms based on fluid and electrolyte abnormalities.
• Weight may be increased or decreased based on fluid volume status.
• Temperature: May be hyperthermic or hypothermic if patient is septic
Observation
Uremic manifestations
Screening labwork
• BUN and creatinine: Elevations indicative of renal impairment
• GFR: Most reliable estimation of renal function using 24-hour creatinine clearance or laboratory estimation, which is part of renal panel in most laboratories
• Electrolyte levels: Elevated or decreased potassium, phosphorus, magnesium, sodium
• Urinalysis: Presence of sediment including tubular epithelial cells, debris, casts, protein, RBC casts, or myoglobin
• Urinary sodium: Prerenal disease results in urinary sodium levels less than 10 mEq/L
• CBC and coagulation studies (PT, PTT): Evaluate for hematologic complications.
• Arterial blood gas (ABG) values: Evaluate for metabolic acidosis associated with ARF/AKI.
Diagnostic Tests For Acute Renal Failure (ARF)/Acute Kidney Injury (AKI)
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Ultrasonography | Provides general appearance, size and scarring | Small scarred kidneys Renal mass Kidney stones Hydronephrosis |
| Radionuclide renal scan | Evaluate renal perfusion | Renal thromboemboli Tumors or cysts Asymmetry of blood flow |
| Magnetic resonance imaging | More specific in detecting renal masses and vessel malformations | Tumors or cysts Vessel malformation |
| Retrograde or antegrade pyelography | Diagnose partial or complete obstruction | Ureteric or ureteral stenosis or obstruction |
| Renal angiography | Evaluate renal vessels | Thrombotic, stenotic lesions in the main renal vessels |
| Renal biopsy | Determine intrarenal pathology | Acute glomerulonephritis, vasculitis, or interstitial nephritis |
| Blood Studies | ||
| Complete blood count (CBC) Hemoglobin (Hgb) Hematocrit (Hct) RBC count (RBCs) WBC count (WBCs) |
Assess for anemia, inflammation, and infection; assists with differential diagnosis of septic cause of ARF/AKI | Decreased RBCs, Hgb, or Hct reflects anemia or recent blood loss. |
| Coagulation profile Prothrombin time (PT) with international normalized ratio (INR) Partial thromboplastin time (PTT) |
Assess for the presence of bleeding or clotting and disseminated intravascular coagulation (DIC) | Decreased PT with low INR promotes clotting; elevation promotes bleeding. |
| Blood urea nitrogen (BUN) Creatinine Estimated glomerular filtration rate (eGFR) |
Assess for the severity of renal dysfunction | Elevation indicates renal dysfunction. Creatinine may be markedly elevated in the presence of massive skeletal muscle injury (e.g., multiple trauma, crush injuries). BUN is influenced by hydration, catabolism, GI bleeding, infection fever, and corticosteroid therapy. The eGFR in ARF/AKI is usually less than 50 ml/min. |
| Electrolytes Potassium (K+) Sodium (Na+) Calcium (Ca2+) Magnesium (Mg2+) |
Assess for abnormalities associated with ARF/AKI | Increase or decrease in K+ may cause arrhythmias. Elevated Na+ may indicate dehydration. Decreased Na+ may indicate fluid retention. Low Mg2+ or Ca2+ may cause dysrhythmias. |
| Arterial blood gases (ABGs) | Assess for the presence of metabolic acidosis | Low PaCO2 and plasma pH values reflect metabolic acidosis. |
| Urinalysis | Assess for the presence of sediment | Presence of sediment containing tubular epithelial cells, cellular debris, and tubular casts supports diagnosis of ARF/AKI. Increased protein and many RBC casts are common in intrarenal disease. Sediment is normal in prerenal causes. Large amounts of myoglobin may be present in severe skeletal muscle injury or rhabdomyolysis |
| Urinary sodium | Differentiate prerenal from intrarenal cause | Urinary Na+ is less than 20 mEq/L in prerenal causes. Urinary Na+ is more than 20 mEq/L in intrarenal causes. |
Collaborative management
Care priorities for all arf/aki pathologies
The major priorities for all patients with ARF/AKI regardless of etiology are the assessment of the contributing causes of the initial injury, identification of the clinical course with an emphasis on comorbidities, assessment of volume status, and prevention of further deterioration in renal function (Table 6-5).
Table 6-5 MANAGEMENT CONSIDERATIONS: ACUTE RENAL FAILURE (ARF)/ACUTE KIDNEY INJURY (AKI)
| Treatment | Rationale |
|---|---|
| Volume replacement for dehydration | Replacement solutions include free water plus electrolytes lost through urine, wounds, drainage tubes, diarrhea, and vomiting. Usually losses are replaced on a volume-for-volume basis. Maintenance fluid approximately 1500 ml/24 hr. With moderate fluid deficit (5% weight loss), at least 2400 ml/24 hr. Severe deficit (more than 5% weight loss) requires replacement of at least 3000 ml/24 hr. |
| Forced alkaline diuresis | Use of mannitol or sodium bicarbonate solution to manage pigmenturia (myoglobinuria, hemoglobinuria) due to rhabdomyolysis or severe crush or skeletal muscle injury. In addition, aggressive volume replacement to maintain renal perfusion pressure and reduce cast formation leading to renal tubular obstruction. |
| Diuretics (furosemide, bumetanide, torsemide, ethacrynic acid) | Decrease filtrate reabsorption and enhance water excretion. Use only after adequate hydration to increase urine output or in an attempt to prevent onset of oliguria. If volume overload is present, they are used to prevent pulmonary edema. Osmotic diuretics such as mannitol may be used to increase intravascular volume, promote renal blood flow, increase glomerular filtration rate, and stimulate urinary output. See Table 6-7. |
| Dopamine | Controversial treatment: Low doses usually less than 2 mcg/kg/min used to stimulate dopaminergic receptors, encourage renal vasodilatation, and promote renal blood flow. Studies have shown that this approach is ineffective if the patient remains oliguric. Doses of 3-10 mcg/kg/min are used to stimulate beta1 receptors resulting in improved BP, cardiac output, and urine output. Doses greater than 10 mcg/kg/min may cause damaging renal vasoconstriction. May increase urine output in critically ill patients, but it neither prevents nor improves ARF. Increased diuresis may actually increase the risk of ARF in normovolemic and hypovolemic patients. Potentially detrimental effect of dopamine on splanchnic oxygen uptake Decreased GI motility Diminished respiratory drive |
| Nesiritide | Synthetic BNP (brain natruretic peptide), which results in vasodilatation, natriuresis, diuresis, and decreased renin-angiotensin activity, resulting in lower pulmonary artery occlusive pressure, decreased systemic vascular resistance, and increased cardiac output and cardiac index. Used to manage heart failure associated with prerenal azotemia. Increased cardiac output augments renal perfusion. Meta-analyses have revealed there is increased mortality and increased renal dysfunction with use of nesiritide compared to other medications. |
| Management of hyperkalemia | Intravenous calcium gluconate 10% or calcium chloride 10% (1 g of calcium chloride does not provide an equivalent dose to 1 g gluconate) (immediate onset) infusion of glucose, insulin, bicarbonate (20- to 60-minute onset) Inhaled albuterol (30- to 60-minute onset), sodium polystyrene sulfonate (Kayexalate) with sorbitol enema (1- to 4-hour onset). Hemodialysis (1- to 3-hour onset) may be used for control of elevated potassium. |
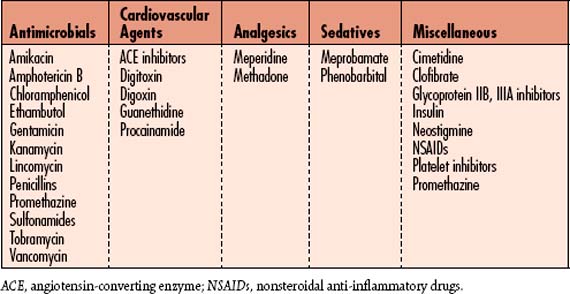
| Removal or discontinuation of toxin | Agents such as aminoglycoside antibiotics or angiotensin-converting enzyme (ACE) inhibitors used for blood pressure control and heart failure prevention; and nonsteroidal anti-inflammatory drugs (NSAIDs) used for pain management, must be discontinued or removed. |
| Prevention of contrast-induced nephropathy | Hydration, oral or IV Mucomyst (N-acetylcysteine) may be used before sending borderline or patients with renal insufficiency for radiologic procedures requiring contrast media. Aggressive hydration and possible IV mannitol after the procedure may also assist in clearing contrast from the patient. Intravenous fenoldopam (e.g., Corlopam) is no longer recommended in this setting. |
| Renal replacement therapy | Maintain homeostasis (see Continuous Renal Replacement Therapies, p. 603). |
| Nutrition therapy | Diet high in carbohydrates and with catabolic patients, essential and nonessential amino acids to prevent endogenous protein catabolism and muscle breakdown; low in sodium for individuals who retain sodium and water, high in sodium for those who have lost large volumes of sodium and water as a result of diuresis or other body drainage; low in potassium if the patient is retaining potassium; and if not catabolic, low in protein to maintain daily requirements while minimizing increases in azotemia. Nutrition is delivered via oral, enteral, or total parenteral nutrition (TPN). (See Box 1-4 for a list of foods high in sodium and Box 1-5 for a list of foods high in potassiums.) |
| Hematologic problems | Packed RBCs are given to maintain Hct. Anemia caused by decreased erythropoietin, low-grade GI bleeding from mucosal ulceration, blood drawing, and shortened life of the RBCs. Erythropoietin is used for primary prevention and treatment of anemia. Prolonged bleeding time is caused by decreased platelet adhesiveness. |
| Pharmacotherapy | Antihypertensives (see Table 5-22): phosphate binders (calcium carbonate antacids, calcium acetate) to bind phosphorus and control hyperphosphatemia and hypermagnesemia are given with meals. Sodium bicarbonate is given to control metabolic acidosis and promote the shift of potassium back into the cells. Water-soluble vitamin supplements are given to patients on dialytic therapy. |
| Relief of obstruction | Achieved via catheterization with indwelling urinary catheter or nephrostomy tube, or ureteral stent to relieve obstruction prior to surgical intervention or lithotripsy to disintegrate stones. |
a. Use of pulmonary artery catheters to measure filling pressures, cardiac output, and systemic vascular resistance to determine volume status
b. In preoperative patients, to prevent kidney hypoperfusion and ischemia
c. Adjust dose of nephrotoxic agents based on patient’s GFR and serum levels.
d. Hydration prior to radiographic studies and the use of acetylcysteine (Mucomyst) before and after the administration of contrast. Acetylcysteine is an antioxidant with vasodilatory properties and may minimize vasoconstriction and oxygen free radical generation from radiocontrast materials.
e. Normal saline administration in patient with rhabdomyolysis to maintain urine output of 200 to 300 ml/hr
a. Vasoactive agents such as low-dose dopamine are no longer shown to improve kidney function; consider nesiritide (synthetic BNP) infusion.
b. Debate continues on the effectiveness of crystalloids versus colloids.
c. Correction of fluid and electrolyte and acid-base balance (judiciously monitor for hyperkalemia and hyperphosphatemia)
d. Consider forced alkaline diuresis for patients with crush injuries or rhabdomyolysis.
6. Initiation of renal replacement therapy:
Renal replacement therapies include hemodialysis and continuous renal replacement therapies.
![]()
![]() See Table 6-6 for a list of common medications that require dosage modification for patients with ARF/AKI. Drugs that require dosage modification in renal failure are those that are excreted primarily by the kidneys. Dosage must be governed by clinical responses, as well as serum levels, if available. Nephrotoxic drugs should be avoided (Box 6-1). Also avoid drugs that are toxic to other organs if they accumulate—those that aggravate uremic symptoms and those that contribute to metabolic derangements of renal injury. Diuretics must be used judiciously in patients with ARF/AKI. See Table 6-7 for a detailed explanation of the use of diuretics.
See Table 6-6 for a list of common medications that require dosage modification for patients with ARF/AKI. Drugs that require dosage modification in renal failure are those that are excreted primarily by the kidneys. Dosage must be governed by clinical responses, as well as serum levels, if available. Nephrotoxic drugs should be avoided (Box 6-1). Also avoid drugs that are toxic to other organs if they accumulate—those that aggravate uremic symptoms and those that contribute to metabolic derangements of renal injury. Diuretics must be used judiciously in patients with ARF/AKI. See Table 6-7 for a detailed explanation of the use of diuretics.
| Types | Mechanisms of Action | Potential Fluid and Electrolyte Abnormalities |
|---|---|---|
| Osmotic Diuretics | ||
| Mannitol Urea |
Increase osmotic pressure of the filtrate, which attracts water and electrolytes and prevents their reabsorption | Hyponatremia Hypokalemia Rebound volume expansion |
| Loop Diuretics | ||
| Furosemide Ethacrynic acid Bumetanide Torsemide |
Inhibit reabsorption of Na+ and Cl− at the ascending loop of Henle in the medulla; they produce a vasodilatory effect on the renal vasculature | Hypokalemia Hyperuricemia Hypocalcemia Hyperglycemia and impairment of glucose tolerance Dilutional hyponatremia Hypochloremic acidosis |
| Thiazides | ||
| Bendroflumethiazide Benzthiazide Chlorothiazide sodium Hydrochlorothiazide Hydroflumethiazide Polythiazide Trichlormethiazide |
Inhibit Na+ in the ascending loop of Henle at the beginning of the distal loop | Hypokalemia Dilutional hyponatremia Hypercalcemia Metabolic alkalosis Hypochloremia Hyperuricemia Hyperglycemia and impaired glucose tolerance |
| Thiazide-like Diuretics | ||
| Chlorthalidone Indapamide Metolazone Quinethazone |
Action same as thiazides | Same as thiazides |
| Potassium-sparing Diuretics* | ||
| Amiloride HCl Spironolactone Triamterene |
Inhibit aldosterone effect on the distal tubule, causing Na+ excretion and K+ reabsorption | Hyperkalemia Hyponatremia Dehydration Acidosis Transient increase in BUN |
| Carbonic Anhydrase-inhibitors | ||
| Acetazolamide sodium Dichlorphenamide Methazolamide |
Block the action of the enzyme carbonic anhydrase, producing excretion of Na+, K+, HCO3−, and water | Hyperchloremic acidosis Hypokalemia Hyperuricemia |
Note: Loop or osmotic diuretics (or a combination of both) are used in patients with acute renal failure to prevent hypervolemia and to stimulate urinary output.
BUN, blood urea nitrogen; Cl−, chloride; HCO3−, bicarbonate; K+, potassium; Na+, sodium.
CARE PLANS FOR ACUTE RENAL FAILURE/ACUTE KIDNEY INJURY
related to inability of kidney to normally excrete urine
Fluid Overload Severity; Fluid Balance
1. Document I&O hourly. Consult physician or midlevel practitioner if urinary output falls to less than 0.5 ml/kg/hr.
2. Weigh patient daily; consult physician or midlevel practitioner regarding significant weight gain (e.g., 0.5 to 1.5 kg/24 hr.
3. Assess for and document the presence of basilar crackles, jugular vein distention, tachycardia, pericardial friction rub, gallop, increased BP, increased CVP, or shortness of breath (SOB), any of which are indicative of fluid volume overload. Chronic heart failure may require additional support measures to help resolve ARF.
4. Assess for and document the presence of peripheral, sacral, or periorbital edema.
5. Restrict patient’s total fluid intake to 1200 to 1500 ml/24 hr or as prescribed. Measure all output accurately, and replace milliliter for milliliter at intervals of 4 to 8 hours or as prescribed.
6. Provide ice chips, chewing gum, or hard candy to help patient quench thirst and moisten mouth.
7. Monitor serum osmolality and serum sodium values. These values may be decreased because of the dilutional effect of fluid overload.
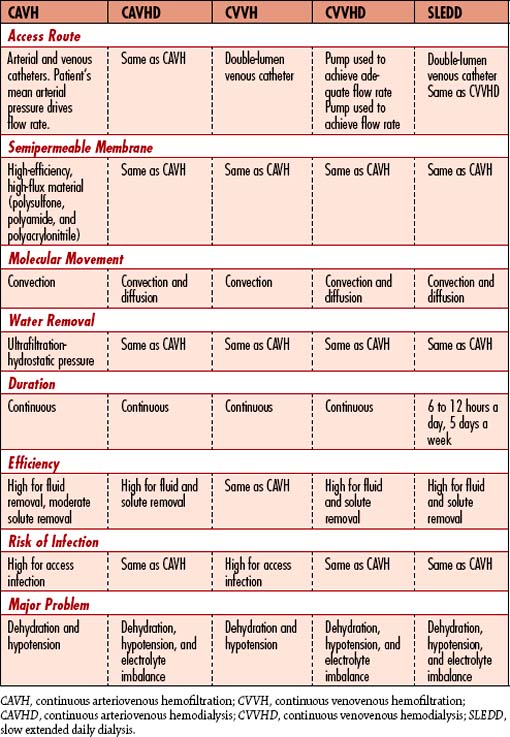
8. Recognize that if it is delivered, total parenteral nutrition (TPN) will provide the largest volume of fluid intake for the patient. If total fluid intake is greater than 2000 ml/day, ultrafiltration (UF) with hemodialysis or continuous renal replacement therapy (CRRT) (continuous venovenous hemofiltration [CVVH], continuous venovenous hemodialysis [CVVHD], continuous venovenous hemodiafiltration [CVVHDF], slow continuous ultrafiltration [SCUF], or continuous arteriovenous hemofiltration [CAVH]) may be necessary to maintain fluid and electrolyte balance (see Continuous Renal Replacement Therapies, p. 603).
9. If patient is retaining sodium, restrict sodium-containing foods (see Table 6-8, p. 604) and avoid diluting IV medications with high-sodium diluents. Also avoid sodium-containing medications such as sodium penicillin.
![]() related to overdiuresis and/or dehydration resulting in acute kidney injury
related to overdiuresis and/or dehydration resulting in acute kidney injury
1. Weigh patient daily. Consult physician or midlevel practitioner for weight loss of 1 to 1.5 kg/24 hr.
2. Monitor and document I&O hourly. Consult physician if patient’s output is less than 0.5 ml/kg/hr. With a deficit, intake should exceed output by 0.5 to 1 L (depending on severity of dehydration).
3. Consult physician or midlevel practitioner for increase in losses from vomiting, diarrhea, or wound drainage or sudden onset of diuresis.
4. Observe for and document indicators of dehydration and hypovolemia (e.g., poor skin turgor, dry and sticky mucous membranes, thirst, hypotension, tachycardia, decreasing CVP, increasing BUN and creatinine).
5. Encourage oral fluids if they are allowed. Ensure that IV fluid rates are maintained as prescribed.
6. Approximately 20% of patients with ARF have GI bleeding. Monitor hemoglobin (Hgb), hematocrit (Hct), and BUN levels. In the presence of bleeding with ARF, Hgb and Hct values will fall steadily, and they will fall rapidly if there is massive bleeding.
7. Test all stools, emesis, and peritoneal dialysate drainage for occult blood. Check urine and dialysate drainage at least every 8 hours.
8. To minimize the risk of bleeding, keep side rails up, minimize invasive procedures, use small-gauge needles for injections, minimize blood drawing, and promote the use of electric razors and soft-bristle toothbrushes. If possible, avoid intramuscular (IM) or subcutaneous injections for 1 hour after hemodialysis. Apply gentle pressure to injection sites for at least 2 to 3 minutes.
9. Inspect gums, mouth, nose, skin, and perianal and vaginal areas every 8 hours for bleeding. Also inspect hemodialysis insertion and/or peritoneal access sites for bleeding every 8 hours. Apply a soft, occlusive, sterile dressing to access sites to protect the skin from irritation and bleeding caused by catheter movement.
Imbalanced nutrition, less than body requirements
![]() related to the adverse effects of acute kidney injury on digestion and absorption of nutrients
related to the adverse effects of acute kidney injury on digestion and absorption of nutrients
1. Infuse enteral feedings and TPN as prescribed.
2. Assess and document patient’s intake of nutrients every shift.
3. Weigh patient daily. Consult physician or midlevel practitioner for significant findings (e.g., loss of greater than 1.5 kg/24 hr).
4. Control nausea and anorexia using small, frequent meals. Present appetizing food in a pleasant atmosphere; eliminate any noxious odors. As indicated, administer medication with prescribed antiemetic 30 minutes before meals.
![]() related to immunocompromised state associated with renal failure
related to immunocompromised state associated with renal failure
1. Monitor and record patient’s temperature every 8 hours. If it is elevated (i.e., greater than 37°C [98.6°F]), monitor temperature every 4 hours. Because ARF may be accompanied by hypothermia, even a slight rise in temperature of 1° to 2° may be significant especially if on CRRT as it is an extracorporeal therapy.
2. Inspect and record the color, odor, and appearance of all body secretions. Be alert to cloudy or blood-tinged peritoneal dialysate return, cloudy and foul-smelling urine, foul-smelling wound exudate, purulent drainage from any catheter site, foul-smelling and watery stools, foul-smelling vaginal discharge, or purulent sputum.
3. Be aware that uremia retards wound healing; therefore, it is important that all wounds (including scratches resulting from pruritus) be assessed for indicators of infection. Send samples of any suspicious fluid or drainage for culture and sensitivity (C&S) tests as indicated.
4. Monitor WBC count for elevations, and obtain specimens for C&S as prescribed.
5. Use aseptic technique when manipulating central lines, peripheral IV lines, and indwelling catheters. Avoid use of indwelling urinary catheter in patients with oliguria and anuria. The presence of a catheter in these patients further increases the risk of infection.
6. Be aware that catabolism of protein, which occurs with infection, causes potassium to be released from the tissues.
7. Provide oral hygiene every 2 hours to help maintain the integrity of the oral mucous membranes.
8. Reposition patient every 2 to 4 hours to help maintain the barrier of an intact integumentary system. Provide skin care at least every 8 hours.
9. Encourage good pulmonary hygiene by having patient practice deep-breathing exercises (and coughing, if indicated) every 2 to 4 hours.
related to disease process of acute renal failure
1. Determine patient’s and significant others’ knowledge about patient’s disease process and the biochemical alterations (hyperkalemia, hypokalemia, hypernatremia, hyponatremia, hypocalcemia, hyperphosphatemia, hypermagnesemia, metabolic acidosis, and uremia) that can occur.
2. Teach patient and significant others the signs and symptoms of the biochemical alterations (see Table 6-4 and care plan for Infection Protection, p. 600).
3. Provide lists of foods high in potassium (see Box 1-5), sodium (see Box 1-4), phosphorus (see Box 1-6), and magnesium (see Box 1-7), which patient should add or avoid when planning meals. In addition, provide a list of medications that contain magnesium (see Table 1-9), which should not be taken without physician or midlevel practitioner approval.
4. Explain the importance of consuming only the amount of protein prescribed by physician and avoiding exposure to persons with infection or a febrile illness to minimize catabolism of protein, which causes potassium to be released from the tissues. Reinforce that patient should consume the prescribed diet and limit strenuous activity as prescribed, both of which will spare protein and thus minimize potassium release.
5. Teach patient to report to physician or midlevel practitioner an increase in temperature or other signs of infection.
6. Reinforce the importance of taking vitamin D and calcium supplements as prescribed.
7. Teach the relationship between calcium and phosphate levels. Emphasize that maintaining good phosphate control and calcium balance may help control itching and prevent future problems with bone disease.
8. Stress the importance of taking phosphate binders (e.g., Amphojel, Alternagel, PhosLo) as prescribed and to avoid antacids containing magnesium (e.g., Maalox, Milk of Magnesia).
9. Teach patient not to take over-the-counter (OTC) medications without first consulting physician. Aspirin, for example, exacerbates the bleeding tendency caused by uremia.
10. Instruct patient about the importance of maintaining the prescribed dialysis schedule, inasmuch as dialysis will help correct acidosis, uremia, and many of the metabolic abnormalities that occur.
11. Teach patient to use lotions and oils to lubricate skin and relieve drying and cracking.
12. Stress the importance of follow-up monitoring of serum electrolyte levels.
1. Monitor patient for the following mentation and motor dysfunctions associated with ARF:
2. Explain to significant others that patient’s decreasing attention level necessitates simple and direct communication efforts.
3. To alleviate unpleasant metallic taste caused by uremia, provide frequent oral hygiene. Because patient with uremia is at increased risk for bleeding, ensure use of soft-bristle brushes.
4. If appropriate, provide chewing gum or hard candy, which may help alleviate the unpleasant metallic taste.
5. Encourage isometric exercises and short walks, if patient is able, to help maintain muscle strength and tone, especially in the legs.
6. Decrease environmental stimuli, and use a calm, reassuring manner in caring for patient.
7. Encourage establishment of sleep/rest patterns by scheduling daytime activities appropriately and promoting relaxation method (see Appendix 7).
8. Assess for decreased tactile sensations in the feet and legs, which may occur with peripheral neuropathy. Be alert to the potential for pressure sores and friction burns, which may occur with peripheral neuropathy.
9. Use splints and braces to aid in mobility for patients with severe neuropathic effects.
![]() related to fluid and electrolyte imbalance and reduced activity level
related to fluid and electrolyte imbalance and reduced activity level
Within 48 hours of onset, patient has bowel movements of soft consistency.
Constipation/impaction management
1. Monitor and record the number and quality of patient’s bowel movements.
2. Administer prescribed stool softeners and bulking agents, such as psyllium husks.
3. If these measures fail, administer oil retention or tap water enemas as prescribed. Because excess fluid can be absorbed from the gut, avoid using large-volume water enemas.
4. Encourage moderate exercise on a routine basis.
5. Establish a regular schedule for fluid intake within patient’s prescribed limits.
6. Administer metoclopramide as prescribed to increase motility in the presence of autonomic neuropathy.
Patient’s skin remains intact.
Tissue Integrity: Skin and Mucous Membranes
1. Monitor patient for presence of pruritus with resulting frequent and intense scratching. Pruritus decreases with reduced BUN level and control of hyperphosphatemia. Monitor laboratory values of BUN and phosphorus, and report levels outside the optimal range (BUN greater than 20 mg/dl and phosphorus greater than 4.5 mg/dl or less than 2.6 mEq/L). Pruritus increases in the presence of secondary hyperparathyroidism. Monitor serum calcium and PTH levels, and report elevations (Ca2+ greater than 10.5 mg/dL and PTH greater than 30% above the upper limit of the test used).
2. Administer phosphate binders (e.g., Alternagel) as prescribed, and, if possible, reduce patient’s dietary intake of phosphorus (see Box 1-6).
3. Ensure that patient’s fingernails are cut short and that the nail tips are smooth.
4. Because uremia retards wound healing, monitor scratches for indicators of infection.
5. Because of reduced oil gland activity associated with uremia, the patient’s skin may be quite dry. Use skin emollients liberally, and avoid harsh soaps and excessive bathing.
6. Advise patient of the potential for bruising because of clotting abnormality and capillary fragility.
7. Administer oral antihistamine, such as diphenhydramine, to relieve itching as prescribed.
Additional nursing diagnoses
For patients undergoing dialytic therapy (conventional hemodialysis), see nursing diagnoses and interventions in Continuous Renal Replacement Therapy. Also see the following as appropriate: Nutritional Support (p. 117), Prolonged Immobility (p. 149), and Emotional and Spiritual Support of the Patient and Significant Others (p. 200).
Continuous renal replacement therapies
1. Prevention of uremic complications
3. Electrolyte and volume homeostasis
The most common indications for CRRT include:
1. Hyperkalemia and other electrolyte disturbances refractory to medical management
2. Metabolic acidosis unresponsive to medical therapy
3. Intravascular volume excess refractory to diuretics
5. Need for removal of dialyzable substances (metabolites, drugs, toxins)
Other indications for CRRT include:
1. Massive fluid overload: CHF, overaggressive fluid resuscitation in multiple trauma
2. Fluid overload in the presence of hemodynamic instability
3. Cardiogenic shock with pulmonary edema
4. Oliguric patient unresponsive to diuretics
5. Patient with anuria who requires large volumes of parenteral fluid: acts as a supplement to hemodialysis to maintain fluid balance
Indications for early initiation of CRRT include:
1. Possible impending electrolyte or acid-base disturbances
2. Presence of oliguria and the need for infusion of large volumes of fluid for medications or nutrition
3. Presence of AKI with a poor prognosis for immediate recovery
4. AKI in the presence of sepsis or systemic inflammatory response syndrome (SIRS)
Pathophysiology
Historically, CRRT has evolved from an arteriovenous to a venovenous extracorporeal process to achieve solute and fluid removal in the critically ill patient. CRRT has been traditionally limited to the ICU setting based on the requirement for close hourly monitoring and fluid adjustments. The multiple acronyms and description of therapies are available in Table 6-8.
Diffusion: Movement of solutes, including high concentrations of waste products and excess electrolytes, from an area of greater concentration to an area of lesser concentration. Diffusion requires a concentration gradient. During CRRT, high concentrations of these excess particles diffuse into the dialysate/effluent, which contains much lower concentrations of these solutes.
Ultrafiltration: Removal of water from the blood compartment of the hemofilter by generating a lower pressure in the effluent compartment. In hemodialysis, lower or negative pressure in the dialysate facilitates the rapid removal of excess water from the blood compartment in which positive or higher pressure exists.
Convection: Removal of a substance with fluid across a semipermeable membrane over time. This occurs in response to the pressure gradient across the membrane. Small molecules freely pass into the ultrafiltrate. Large molecule removal is dependent on the pore size of the membrane. However, larger molecules tend to move across these membranes better by convection than diffusion. Some elements in plasma water (e.g., urea) are conveyed across the membrane as a result of the differences in hydrostatic pressure. The removal of large amounts of plasma water results in the removal of large amounts of filterable solutes.
The availability of a wide variety of CRRT therapies, however, has not led to standards for initiation of therapy, dosage, choice of modality, or the intensity and duration of therapy. The use of a highly permeable membrane, the infusion of various types of replacement solution, and the continuous nature of each of the techniques make them highly effective and versatile in managing control of fluids. CRRT techniques can serve as a regulatory system for fluids without compromising metabolic balance. More recent literature suggests use of daily hemodialysis may provide equally effective therapy in AKI patients or for those with more complex disease processes who are unable to receive consistent therapy due to machine problems, including clotting of the hemofilter. Multiple interruptions in therapy can undermine the efficacy of CRRT, as the patient does not receive continuous therapy if the system is frequently alarming, which interrupts diffusion, ultrafiltration, and convection.
Assessment: pre-crrt
Vital signs
• BP may be elevated in states of fluid volume excess or decreased in states of fluid volume deficit.
• HR rate may be increased or decreased with abnormal rhythms based on fluid and electrolyte abnormalities.
• Baseline electrocardiogram (ECG) and rhythm
• Hemodynamic parameters of cardiac output (CO), cardiac index (CI), ejection fraction, CVP, and pulmonary artery wedge pressure (PAWP)
• Weight may be increased or decreased based on fluid volume status.
• Temperature: may be hyperthermic or hypothermic if patient is septic
Observation
• Peripheral edema and periorbital edema
• Poor skin turgor, flushed skin, and dry mucous membranes
• Altered mental status and disorientation
• Signs of central nervous system depression
• Mechanical ventilation parameters
• Presence of any assist devices such as extracorporeal membrane oxygenator (ECMO)
Uremic manifestations
Screening labwork
Blood and urine studies will determine the level of renal dysfunction and can provide clues to the cause of ARF/AKI.
• BUN, creatinine: elevations indicative of renal impairment
• GFR: most reliable estimation of renal function using 24-hour creatinine clearance or laboratory estimation, which is part of renal panel in most laboratories
• Electrolyte levels: elevated or decreased potassium, phosphorus, magnesium, sodium
• Urinalysis: presence of sediment including tubular epithelial cells, debris, casts, protein, RBC casts, or myoglobin
• Urinary sodium: prerenal disease results in urinary sodium less than 10 mEq/L
• CBC and coagulation studies (PT, PTT): evaluate for hematologic complications
• ABG values: evaluate for metabolic acidosis associated with ARF/AKI
Diagnostic Tests Used in Association with Continuous Renal Replacement Therapy Blood Studies
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Complete blood count (CBC) Hemoglobin (Hgb) Hematocrit (Hct) RBC count (RBCs) WBC count (WBCs) |
Assess for anemia, inflammation, and infection. Assists with differential diagnosis of septic cause of acute renal failure (ARF)/acute kidney injury (AKI). | Decreased RBCs, Hgb, or Hct reflects anemia or recent blood loss. |
| Coagulation profile Prothrombin time (PT) with international normalized ratio (INR) Partial thromboplastin time (PTT) |
Assess for the presence of bleeding or clotting and disseminated intravascular coagulation (DIC). | Decreased PT with low INR promotes clotting. Elevation promotes bleeding. |
| Blood urea nitrogen (BUN) Creatinine Estimated glomerular filtration rate (eGFR) |
Assess for the severity of renal dysfunction. | Elevation indicates renal dysfunction. Creatinine may be markedly elevated in the presence of massive skeletal muscle injury (e.g., multiple trauma, crush injuries). BUN is influenced by hydration, catabolism, GI bleeding, infection fever, and corticosteroid therapy. The eGFR in ARF/AKI is usually <50 ml/min. |
| Electrolytes Potassium (K+) Sodium (Na+) Calcium (Ca2+) Magnesium (Mg2+) |
Assess for abnormalities associated with ARF/AKI. | Increase or decrease in K+ may cause arrhythmias. Elevated Na+ may indicate dehydration. Decreased Na+ may indicate fluid retention. Low Mg2+ or Ca2+ may cause arrhythmias. |
| Arterial blood gases (ABGs) | Assess for the presence of metabolic acidosis. | Low PaCO2 and plasma pH values reflect metabolic acidosis. |
| Urinalysis | Assess for the presence of sediment. | Presence of sediment containing tubular epithelial cells, cellular debris, and tubular casts supports diagnosis of ARF/AKI. Increased protein and many RBC casts are common in intrarenal disease. Sediment is normal in prerenal causes. Large amounts of myoglobin may be present in severe skeletal muscle injury or rhabdomyolysis. |
| Urinary sodium | Differentiate prerenal from intrarenal cause. | Urinary Na+ <20 mEq/L in prerenal causes. Urinary Na+ >20 mEq/L in intrarenal causes. |
Determining type and modality of crrt used
Advantages and disadvantages of CRRT methods are found in Table 6-9. Complications of CRRT are found in Table 6-10.
Table 6-9 ADVANTAGES AND DISADVANTAGES OF METHODS OF RENAL REPLACEMENT THERAPY
| Advantages | Disadvantages |
|---|---|
| SLEDD | |
| Very efficient; Modification of intermittent hemodialysis therapy by extending the time and slowing the rate of solute and fluid removal. | Special equipment and trained staff required Heparinization usually required Possible difficulty in maintaining vascular access Risk of blood loss necessitating transfusion |
| Continuous Arteriovenous Therapies | |
| Physiologic process Ideal for hemodynamically unstable patient Allows for administration of large volumes of fluid (e.g., TPN) MAP must be greater than 60 mm Hg |
Must maintain MAP for effective process Must maintain an arterial and venous access, which becomes a problem in a restless patient Risk of blood loss if arterial line displaced Increased responsibilities for the ICU nurses |
| Continuous Venovenous Therapies | |
| Physiologic process ideal for the patient who is hemodynamically unstable Allows administration of large volumes (e.g., TPN) CVVH effective in patients with MAP less than 70 mm Hg |
Low-efficiency solute removal unless CVVHD or CVVHDF Large-volume fluid replacement Potential for electrolyte imbalance Increased responsibilities for ICU nurses |
Table 6-10 COMPLICATIONS OF RENAL REPLACEMENT THERAPIES
| SLEDD | Hemofiltration |
|---|---|
| Hypotension | Bleeding |
| Air embolus | Infection |
| Volume depletion | |
| Blood loss (dialyzer rupture) | Blood leakage |
| Infection | Decreased ultrafiltration |
| Hemolysis | Filter clotting |
| Hemorrhage | Electrolyte disturbances |
| Septicemia | Air embolus with CVVH |
| Clotting | Poor functioning vascular access |
| High-output heart failure | Hypothermia |
CVVH, continuous venovenous hemofiltration.
CVVH, CVVHD, CVVHDF, SCUF, SLEDD, and CAVH are types of renal replacement therapy performed to manage fluid and solute overload in critically ill patients. Their advantage over conventional dialytic therapies is that ultrafiltration occurs more gradually, thus avoiding drastic volume changes and rapid fluid shifts. Treatment duration may be 6 to 24 hours or several days, depending on the total amount of fluid to be removed. The type best suited for each situation is chosen based on clinical status, including the ability to safely anticoagulate the patient and what type of vascular access is available. Catabolism, for example, causes rapid rises in BUN, creatinine, and potassium values. The patient needs rapid removal of metabolic wastes. These patients may require hemodialysis with supplemental CRRT (see Table 6-8). The use of CAVH requires that the patient have an average mean arterial blood pressure (MAP) of 70 mm Hg. Hypotensive patients will not benefit from CAVH but can benefit from CVVH (or any other venovenous therapy).
Principles applied to specific therapies
Ultrafiltration: For ultrafiltration to occur, there must be a pressure gradient across the membrane that favors filtration. The pressure in the blood compartment must exceed the pressure in the filtrate compartment of the hollow filter. In CAVH or CVVH/other venovenous hemofiltration therapies, this is called transmembrane pressure (TMP). Its major determinants are hydrostatic pressure and oncotic pressure. The higher pressure in the blood compartment is a function of the individual’s blood pressure when using CAVH. Pressure in the blood compartment is adequate when the MAP is 50 to 70 mm Hg. Higher pressures enhance ultrafiltration.
Venovenous therapies do not rely on the patient’s blood pressure for ultrafiltration. Negative pressure for ultrafiltration can be achieved by lowering the collection container 20 to 40 cm below the hemofilter. The differences in hydrostatic pressure also cause the crossing of some elements, such as glucose and some vitamins. The longer it takes for blood to clear the filter, the more likely it is that intermediate molecules (vitamins, glucose) will be filtered out of the patient’s system. Opposing the hydrostatic pressure is oncotic pressure, which is maintained by plasma proteins that do not pass through the membrane. When hydrostatic pressure exceeds oncotic pressure, filtration of water and solutes occurs.
Sieving coefficient (SC): Clearance of medication during CRRT is impacted by the SC of the drug as it passes through the membrane. The SC is equal to the ultrafiltrate concentration of the drug divided by the plasma concentration. Drugs that are more protein-bound have a lower clearance during CRRT. However, due to the long duration of CRRT, more of these drugs may be removed.
Procedure
If the objective is the removal of large amounts of fluid and solute (i.e., urea, potassium, creatinine), it is necessary to infuse large volumes of filtration replacement fluid (FRF) or replacement fluid to maintain electrolyte balance. Nursing responsibilities include initiating treatment, monitoring the patient and the system, and discontinuing treatment. Tables 6-9 and 6-10 discuss the advantages, disadvantages, and complications of CRRTs. Table 6-11 discusses troubleshooting major problems with CRRT.
Table 6-11 TROUBLESHOOTING MAJOR PROBLEMS IN CONTINUOUS RENAL REPLACEMENT THERAPY
| Problem | Cause | Intervention |
|---|---|---|
| Hypotension | Cardiac dysfunction Excessive intravascular volume removal |
Cardiotonic and pressor support Fluid replacement Recalculate UF rate |
| Poor ultrafiltration | High Hct Decreased MAP Clotted filter |
Predilution fluid replacement Pressor support to increase MAP Flush filter; replace if necessary |
| Clotted hemofilter | Inadequate anticoagulation Poor blood flow rates Kinks in blood tubing |
Check ACT or aPTT hourly, and adjust heparin infusion Pressors or fluid replacement to increase MAP Check tubing hourly to guard against kinks Change filter and restart therapy |
ACT, activated clotting time; aPTT, activated partial thromboplastin time; Hct, hematocrit; MAP, mean arterial pressure; UF, ultrafiltration.
1. The Fresenius 2008K with a CRRT option can be used for intermittent hemodialysis, SLEDD, or CRRT (Fresenius Medical Care, Bad Homburg, Germany).
2. The Prisma, and Prismaflex, are automated integrated systems for CRRT and continuous fluid management (Gambro, Stockholm, Sweden).
3. Other integrated systems available in the United States are:
Anticoagulation
Factors that may contribute to coagulation
Heparin
Systemic heparinization: Heparin can be infused in a separate IV line for systemic heparinization or into the arterial line of the CRRT device. In addition to the complication of bleeding, heparin-induced thrombocytopenia can occur. This complication has limited the use of heparin in recent years.
Regional heparinization: A relatively uncommon procedure that produces anticoagulation in the circuit but not systemically to the patient. There is little research available on regional heparinization. When done, it requires two infusion devices: one to infuse heparin pre filter (before the hollow fiber filter) and another for protamine, a heparin antagonist that is run post filter into the return line to neutralize the heparin. This process requires determining the aPTT systemically from the patient and post-filter preprotamine infusion. The goal is to heparinize the circuit without systemically heparinizing the patient. This process is labor intensive and requires meticulous monitoring and frequent dose adjustment of both the heparin and protamine. Use of protamine in this fashion is quite uncommon, so few centers engage in this method of anticoagulation.
Filtration replacement fluid (replacement fluid)
Approaches to filtration fluid replacement and calculation of FRF rate can be found in Table 6-12 and Box 6-2.
Table 6-12 APPROACHES TO CONTINUOUS RENAL REPLACEMENT THERAPY FILTRATION FLUID REPLACEMENT
| Predilution: Replacement Fluid Infused Proximal to the Filter | Postdilution: Replacement Fluid Infused Distal to the Filter |
|---|---|
| Patient population: Those with poor blood flow and elevated BUN and Hct levels | Patient population: All types |
| Replacement fluid infused into arterial line | Replacement fluid infused into venous line |
| Used to enhance urea clearance to ≥18%; decreases oncotic pressure, increasing net TMP; moves urea from erythrocytes into plasma | Used to maintain fluid and electrolyte balance |
| Increases net fluid removal | Less replacement fluid required |
| Potentially increases filter life | Simplified clearance determination |
| *Urea clearance 12.5 ml/min | Urea clearance 10.6 ml/min |
BUN, blood urea nitrogen; Hct, hematocrit; TMP, transmembrane pressure.
* If increased urea clearance is desired, predilution mode of fluid replacement is used.
Assessment: during continuous renal replacement therapy
Vital signs
![]() • BP may increase or decrease based on fluid volume status
• BP may increase or decrease based on fluid volume status
• HR may increase or decrease in response to fluid and electrolyte changes
• Hyperthermia or hypothermia in the presence of sepsis
• Hypothermia is a common complication of CRRT
• Cardiac arrhythmias may occur with hypothermia
• Cold patients are prone to use more energy and increase CO
• PAWP and CVP will change with volume status
• Oxygen saturation to assess respiratory status
• Body weight to assess fluid balance
• Urine output and other fluid losses (blood loss drainage fluid)
Observation
Collaborative management
| Key Considerations | Goals |
| Total parenteral nutrition | Maintain nutritional requirements. |
| Predilution fluid replacement | Used if increased solute removal is required |
| Filtration replacement fluid | Used to maintain fluid and electrolyte balance |
| Anticoagulation | To prevent clotting in the circuit |
| Vasopressors | Used with CAVH only to maintain arterial pressure |
| Vascular access | Double-lumen catheter in the subclavian or internal jugular vein for venovenous procedures |
| Arterial access needed for CAVH |
Care priorities
Prevention of hemodynamic instability and maintenance of homeostasis are the goals of CRRT. This includes fluid removal and electrolyte replacement. Continuous monitoring and frequent prescription changes based on patient condition and needs are required to meet the goal of therapy. Fluid removal, electrolyte balance, and maintaining nutrition in these critically ill catabolic patients present a major care challenge for the treatment team.
5. Maintain patency of the crrt machine circuit.
CARE PLANS FOR CONTINUOUS RENAL REPLACEMENT THERAPY
For patients undergoing continuous renal replacement therapies: venovenous or arteriovenous
related to fluid overload creating heart failure
1. Assess and document BP, HR, and RR hourly for the first 4 hours of hemofiltration, and then every 2 hours. Be alert to indicators of fluid volume deficit, manifested by a drop in systolic BP to less than 100 mm Hg, tachycardia, and tachypnea.
2. Assess and document peripheral pulses and color, temperature, and capillary refill in the extremities every 2 hours. Be alert to decreased amplitude of peripheral pulses and to coolness, pallor, and delayed capillary refill in the extremities as indicators of decreased perfusion.
3. Measure and record I&O hourly. Consult physician or midlevel practitioner for a loss of greater than 200 ml/hr over desired loss.
4. Monitor cardiac rhythm continuously; notify physician of decrease in BP greater than 20 mm Hg from baseline, tachycardia, depressed T waves and ST segments, and dysrhythmias, which can occur with hypovolemia, potassium changes, or calcium changes.
5. Ensure prescribed rates of ultrafiltration and replacement fluid infusion (see Box 6-2), and adjust if ultrafiltration rate changes. Use an infusion pump for replacement fluids to ensure precise rate of infusion. Also maintain TPN and IV rates, as well as oral intake, within 50 ml of the values used to calculate the filtration fluid replacement rate. If any parameters change greater than 50 ml, recalculate filtration fluid replacement rate and adjust accordingly.
6. Monitor serum electrolyte values, being alert to changes in potassium, calcium, phosphorus, and bicarbonate. Compare patient’s values with the following normal ranges: potassium 3.5 to 5 mEq/L, calcium 8.5 to 10.5 mg/dl, phosphorus 2.5 to 4.5 mg/dl, and bicarbonate 22 to 26 mEq/L (see Fluid and Electrolyte disturbances, p. 37, and Acid-Base Imbalances, p. 1).
Risk for deficient fluid volume
![]() related to ultrafiltration during CRRT
related to ultrafiltration during CRRT
1. Measure and record I&O every 30 minutes for the first 2 hours and then hourly. Ensure that it is within desired limits.
2. Weigh patient every 8 hours. Be alert to a daily loss of greater than 2.5 kg.
3. Record cumulative ultrafiltrate loss hourly. Measure amount in the ultrafiltrate container. The difference between this value and total hourly intake is the cumulative loss per hour.
4. Check replacement fluid rate hourly to ensure that it is within prescribed limits: usually 25 ml of the calculated rate.
5. Consult physician or midlevel practitioner for unanticipated fluid loss from vomiting, diarrhea, fever, and wound drainage.
6. Consult physician or midlevel practitioner for increased filtration rate, which may occur because of increased BP, or for increased negative pressure, which may be caused by lowering the ultrafiltration collection device.
7. Monitor vital signs hourly; consult physician or midlevel practitioner for increased arterial pressure (greater than 10 mm Hg above baseline), which would increase flow through the hemofilter, thereby increasing the rate of ultrafiltration.
8. Adjust the filtration replacement fluid rate as prescribed when ultrafiltration rate increases.
9. Maintain intake (oral, enteral, IV, TPN) within 25 to 50 ml of the value used to calculate fluid replacement rate. If not possible, fluid replacement rate must be recalculated.
![]() related to renal insufficiency
related to renal insufficiency
1. Monitor BP every 30 minutes for the first 2 hours, and then hourly. Consult physician for a 10 mm Hg drop in BP, which would decrease the rate of ultrafiltration significantly.
2. If ultrafiltration rate is decreased to 50% of the baseline, consult physician or midlevel practitioner and decrease FRF rate as prescribed.
3. Check tubes hourly for kinks.
4. Maintain constant heparin infusion per infusion pump to maintain ACT at two to three times that of the baseline value. System must be functional for patient to get the full benefit of CRRT.
5. Monitor clotting time every 2 hours. Use of an ACT device is advisable.
6. Inspect vascular access filter and lines for patency hourly. If clotting or clogging with protein is suspected, flush the system with 50 ml normal saline to check patency.
7. If clots are present, consult physician or midlevel practitioner. As prescribed, change the filter and recheck ACT/PTT to ensure necessary adjustment in heparin infusion rate.
8. On an hourly basis, assess for and document the presence of physical indicators of hypervolemia: CVP greater than 6 mm Hg, BP elevated greater than 20 mm Hg over baseline, tachycardia, jugular venous distention, basilar crackles, increasing edema (peripheral, sacral, periorbital), and tachypnea.
![]() Electrolyte Management: Hypokalemia; Electrolyte Management: Hyponatremia; Fluid/Electrolyte Management; Fluid Management; Fluid Monitoring. Additional, optional interventions include Dysrhythmia Management; Feeding; Gastrointestinal Intubation; Hemodialysis Therapy; Hemodynamic Regulation; Invasive Hemodynamic Monitoring; Medication Management; Phlebotomy: Arterial and Venous Blood Samples; Positioning; Skin Surveillance; and Weight Management.
Electrolyte Management: Hypokalemia; Electrolyte Management: Hyponatremia; Fluid/Electrolyte Management; Fluid Management; Fluid Monitoring. Additional, optional interventions include Dysrhythmia Management; Feeding; Gastrointestinal Intubation; Hemodialysis Therapy; Hemodynamic Regulation; Invasive Hemodynamic Monitoring; Medication Management; Phlebotomy: Arterial and Venous Blood Samples; Positioning; Skin Surveillance; and Weight Management.
Deficient knowledge of crrt procedure/treatment
Knowledge: Treatment/Procedure
1. Assess patient’s knowledge of the procedure, and intervene accordingly.
2. Explain necessity of vascular access and the sensations that can be anticipated during cannula insertion.
3. Explain importance of and rationale for limited movement of the involved extremity after cannula placement.
4. Describe equipment that will be used for the procedure (e.g., CRRT machine, filter, lines, infusion pumps).
5. Explain that vital signs will be assessed and blood tests will be performed at frequent intervals to monitor patient’s status during the procedure.
6. Explain to patient that his or her blood will be visible in the filter and lines.
7. Reinforce that a staff member will be close to patient at all times during the procedure and will explain each step as it occurs.
8. Explain that the procedure may require 24 hours or longer to attain fluid balance.
9. Teach patient that the typical access sites are the femoral artery and the femoral vein, or the internal jugular, or the subclavian vein.
![]() related to weakness ensuing with critical illness
related to weakness ensuing with critical illness
1. Secure access catheters with gauze wraps (elastic wrap may compress access site and cause clotting) and tape to ensure safe movement of the involved limb without disruption of access cannula.
2. Explain to patient the need for care and assistance when moving the involved limb.
3. Use soft restraints if movement must be restrained markedly.
4. Turn and reposition patient at least every 2 hours, maintaining good body alignment.
5. Massage bony prominences during every position change to promote comfort and circulation.
6. Support involved extremities with pillows.
7. Teach patient-assisted range-of-motion (ROM) exercises on uninvolved extremities. Encourage isometric, isotonic, and quadriceps-setting exercises on uninvolved extremities, especially for patients whose CAVH or CVVH lasts longer than 24 hours.
1. Tape and secure all connections within the system.
2. Check connections hourly to ensure that they are secure.
3. Avoid concealing lines, filter, or connections with linen.
4. Position filter and lines close to the access extremity; secure them with gauze wraps and tape to prevent traction on the connections.
5. Inspect ultrafiltrate hourly for any signs of blood. If unsure whether ultrafiltrate contains blood, check the solution for occult blood.
6. If the test is positive for blood, clamp the ultrafiltrate port and consult physician or midlevel practitioner for further interventions.
American Nephrology Nurses Association. Core curriculum for nephrology nursing. Pitman, NJ: Anthony J. Janetti; 2008.
American Nephrology Nurses Association. Nephrology nursing standards of practice and guidelines for care. Pitman, NJ: Anthony J. Janetti; 2005.
Bagshaw SM, Berthiaume LR, et al. Continuous versus intermittent renal replacement therapy for acute kidney injury: a meta-analysis. Crit Care Med. 2008;35(2):610–617.
Barrett BJ, Pharfrey PS. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379–386.
Bouchard J, Mehta RL. Acid-base disturbances in the intensive care unit: current issues and the use of renal replacement therapy as a customized treatment tool. Int J Artif Organs. 2008;31(1):6–14.
Cerda J, Lameire N, Eggers P, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881–886.
Chrysochoou G, Marcus RJ, et al. Renal replacement therapy in the critical care unit. Crit Care Nurse Q. 2008;31(4):282–290.
Molzhan AE, Butera E. Contemporary nephrology nursing: principles and practice, ed 2. Anthony J. Janetti: Pitman, NJ, 2006.
Davenport A, Bouman C, et al. Delivery of renal replacement therapy in acute kidney injury, what are the key issues. Clin J Am Soc Nephrol. 2008;3:869–875.
Dirkes S, Hodge K. Continuous renal replacement therapy in the adult intensive care unit. History and current trends. Crit Care Nurse. 2007;27(2):61–80.
Eknoyan G. Emergence of the concept of acute kidney injury. Adv Chronic Kidney Dis. 2008;15(3):308–313.
Ghossein C, Grouper S, Soong W. Renal replacement therapy in the intensive care unit. Int Anesthesiol Clin. 2009;47(1):15–34.
Gibney N, Hoste E, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI, unanswered key questions. Clin J Am Soc Nephrol. 2008;3:876–880.
Himmelfarb J. Continuous renal replacement therapy in the treatment of acute renal failure, critical assessment is required. Clin J Am Soc Nephrol. 2007;2:385–389.
Himmelfarb J, Joannidis M, Molitoris B, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:962–967.
Hoste E, Schurgers M. Epidemiology of acute kidney injury: how big is the problem. Crit Care Med. 2008;36(4, Suppl):S146-S151.
Kellum J. Acute kidney injury. Crit Care Med. 2008;36(4, Suppl):S141-S145.
Kielstein J, Kretschmer U, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. Am J Kidney Dis. 2004;43(2):342–349.
Kyung S, Rosner M, Okusa M. Pharmacologic treatment of acute kidney injury, why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365.
Mehta R. Continuous renal replacement therapy in the critically ill patient. Kidney Int. 2005;67:781–795.
Mehta R, Kellum J, Shah S, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. .
Morgera S, Slowirski T, et al. Renal replacement therapy with high-cutoff hemofilters: impact of convection and diffusion on cytokine clearances and protein status. Am J Kidney Dis. 2004;43(3):444–453.
Oudenmans-van Straaten HM, Wester JPJ, et al. Anticoagulation strategies in continuous renal replacement therapy, can the choice be evidence based. Intens Care Med. 2006;32:188–202.
Palevsky P. Definition of acute kidney injury (acute renal failure). www.uptodate.com, 2008.
Palevsky PM, Baldwin I, et al. Renal replacement therapy and the kidney, minimizing the impact of renal replacement therapy on recovery of acute renal failure. Curr Opin Crit Care. 2005;11:548–554.
Palsson R, Laliberte KA, Niles JL. Choice of replacement solution and anticoagulant in continuous venovenous hemofiltration. Clin Nephrol. 2006;65(1):34–42.
Pannu N, Klarenbach S, et al. Renal replacement therapy in patients with acute renal failure. A systematic review. JAMA. 2008;299(7):793–805.
Price Rabetoy C. Acute renal failure. In Molzahn A, editor: Contemporary nephrology nursing: principles and practice, ed 2, Pitman, NJ: Anthony J. Janetti, 2006.
Schrier RW, Wang W. Acute renal factors and sepsis. N Engl J Med. 2004;351:159–169.
The VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20.
Vanbiesen W, Vanholder R, Lameire N. Defining acute renal failure, RIFLE and beyond. Clin J Am Soc Nephrol. 2006;1:1314–1319.
Venkataraman R. Can we prevent acute kidney injury. Crit Care Med. 2008;36(4, Suppl):S166-S171.
Waikar S, Liu K, Chertow G. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;13:844–861.
Zappitelli M, Parikh C, Akcan-Ariken A, et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Soc Nephrol. 2008;3:948–954.