Chapter 47 Rehabilitation of Lower Cranial Nerve Deficits after Neurotologic Skull Base Surgery
The lower cranial nerves function in concert to facilitate speech, swallowing, and airway protection (Fig. 47-1). Interruption of the complex interactions of these nerves results in articulation deficits, inanition, and aspiration. With speech and swallowing therapy, most patients are able to compensate for the loss of a single lower cranial nerve. The loss of multiple nerves, particularly in an elderly patient, may result in permanent inability to swallow despite intensive therapy. Paradoxically, preoperative loss of cranial nerve function from tumor compression allows most patients to compensate slowly over time, and may predict better postoperative speech and swallowing rehabilitation. Patient age and preoperative cranial nerve function are important factors in the decision to proceed with complete surgical resection, partial resection, or radiation therapy.
PATIENT EVALUATION
When lateral skull base surgery is elected, CN V3 to XII and the sympathetic trunk are at risk. A careful head and neck examination including cranial nerve evaluation and flexible fiberoptic examination of the hypopharynx and larynx should be performed at the bedside on the 1st postoperative day to confirm known deficits and to determine the extent of any additional cranial nerve deficits. The state of the airway, vocal fold function and cord position, pooling of secretions, and the ability to clear secretions by coughing should be noted. A modified barium swallow is the “gold standard” for evaluating aspiration, and should be performed as soon as the patient is awake and alert enough to cooperate with the study. The level of oropharyngeal dysphagia may be identified and addressed by the speech and language pathologist at the time of this study. Flexible videoendoscopic evaluation has been described to evaluate swallowing at the bedside, but is not the study of choice because it cannot identify aspiration during the pharyngeal phase of swallowing, which is the most significant component of swallowing dysfunction in lateral skull base surgery.1
After thorough evaluation has been completed, efforts are directed at rehabilitation. The remainder of this chapter describes the pertinent regional cranial nerve anatomy and function,2,3 associated postoperative deficits, and methods for rehabilitation.
LOWER CRANIAL NERVE DEFICITS AND REHABILITATION
Trigeminal Nerve: Mandibular Division (Cranial Nerve V3)
Atrophy of the temporalis muscle is an inevitable sequela of CN V3 sacrifice. For this reason, attempts at masseter or temporalis transfer for facial nerve rehabilitation are doomed to failure and should not be attempted. By 1 year, noticeable temporalis wasting occurs, and results in a significant cosmetic defect. Most patients desire placement of a silicone or methylmethacrylate implant beneath the residual temporalis muscle (Fig. 47-2). Care must be taken to contour the implant where it abuts the lateral orbital rim, or else a residual crease results in this region.
Abducens Nerve (Cranial Nerve VI)
During lateral transtemporal approaches to the cranial base, the abducens nerve is often encountered in its course along the clivus and medial aspect of the petrous apex. Its sole function is to supply somatic motor innervation to the lateral rectus muscle. After exiting the brainstem, the abducens nerve lies along the clivus and enters Dorello’s canal inferomedial to the root of the trigeminal nerve. It passes beneath (rarely above) the superior sphenopetrous ligament in a sulcus on the petrous apex. It courses through the cavernous sinus lateral to the carotid into the superior orbital fissure. Injury may occur anywhere along this course and result in lateral rectus muscle dysfunction with limitation of lateral gaze with associated diplopia. CN VI is exquisitely sensitive to pressure injury in the cavernous sinus, and great care must be taken not to pack the cavernous sinus vigorously to control bleeding because this may result in permanent lateral rectus palsy despite anatomic integrity of the nerve. If the nerve is known to be intact, treatment is conservative; prism glasses allow compensation until function returns, usually within 3 to 4 months. Botulinum toxin injection into the ipsilateral medial rectus has been used successfully to relieve diplopia by weakening the antagonist action of the medial rectus muscle.4 When it seems that permanent palsy has occurred, superior and inferior rectus muscle transposition may be performed to improve lateral gaze function.4
Glossopharyngeal Nerve (Cranial Nerve IX)
The final function of CN IX is visceral sensory from the carotid body and carotid sinus by way of the nerve of Hering. Disruption of CN IX at the skull base causes loss of the carotid sinus reflex on the ipsilateral side and was originally described for the treatment of carotid sinus syndrome.5 Unilateral loss does not interfere with the control of blood pressure and pulse, presumably because of the presence of an intact reflex on the contralateral side. When there is bilateral alteration of this system, acute elevation of blood pressure to greater than 220 mm Hg may be seen within a 60 second period. Preoperatively, one should consider the potential for a bilateral deficit in any patient who has had surgery on the contralateral neck where CN IX fibers may have been disrupted, such as in carotid endarterectomy. Appropriate intraoperative management of this scenario includes administration of a pure α-blocker, such as phenoxybenzamine hydrochloride. Sodium nitroprusside may be added for further control. The patient is weaned to clonidine hydrochloride in the postoperative period. Permanent maintenance of blood pressure may be required. Consultation with an intensivist in the preoperative period is strongly recommended if a bilateral loss is anticipated.
Vagus Nerve (Cranial Nerve X)
Nonsurgical approaches to VPI have included speech and swallowing therapy, palatal lift prostheses, and palatal obturators. Various surgical procedures have been described for the treatment of VPI secondary to cleft palate, including pharyngeal augmentation and pharyngoplasty. The most popular of the pharyngoplasty techniques is the superiorly based pharyngeal flap as described by Jackson.6 Vagal injury at or above the jugular foramen causes paralysis of not only the palate, but also the pharyngeal constrictors, resulting in loss of lateral wall motion on the affected side. Superiorly based flaps and pharyngeal augmentation are used to address VPI in cleft palate patients and are designed to leave an open lateral port. Lateral pharyngoplasty techniques7 are adynamic, and do not recreate lateral pharyngeal wall movement. A simple alternative technique that addresses the palatal and pharyngeal component of VPI in skull base patients is to close the lateral port by adhering the palate on the affected side to the posterior pharyngeal wall. The chief advantage of this procedure in patients who may already have multiple cranial nerve deficits and abnormal swallowing is that it does not alter pharyngeal anatomy with long mucosal flaps, and does not carry the risk of pharyngeal stenosis. Unilateral palatal adhesion successfully eliminates hypernasality and nasal regurgitation, and has become the procedure of choice for the correction of velopharyngeal incompetence in neurotologic skull base patients.8,9
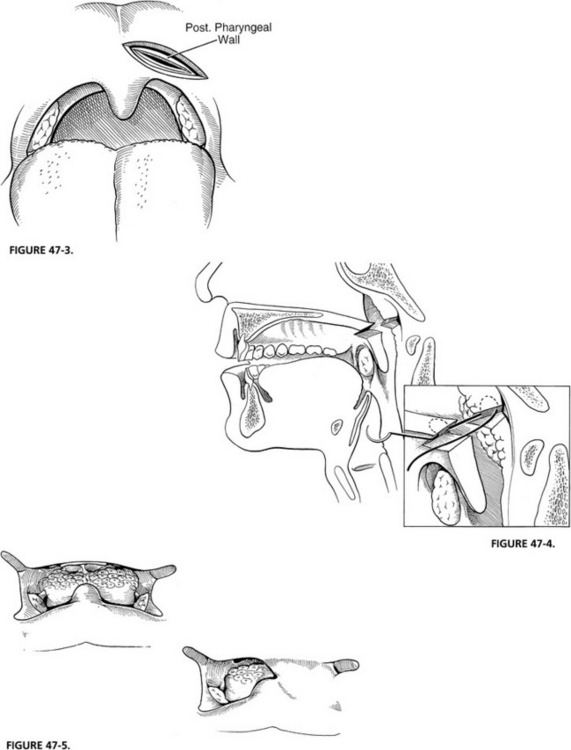
Palatal adhesion is usually performed several months after resection when swallowing function has stabilized, and when it is certain that an injured but intact vagus nerve has not recovered. Preoperative assessment by nasopharyngoscopy with either a rigid or a flexible scope shows unilateral closure of the nasopharynx. Under general anesthesia, the palate is exposed with a Dingman mouth gag. The paralyzed hemipalate and posterior pharyngeal wall are injected with epinephrine. Care is taken to inject just below any residual adenoid tissue. A transpalatal incision is performed in the area of the palatal crease that forms with normal palatal elevation, and the posterior pharyngeal wall is viewed through this incision (Fig. 47-3). A similar incision is created into the posterior pharyngeal wall down to the prevertebral fascia. The pharyngeal mucosa is elevated for just a few millimeters all around to create an edge to which the nasopharyngeal mucosa of the palate is sewn. Multiple deep mattress sutures are used to suture the nasopharyngeal surface of the palate to the posterior pharyngeal wall (Fig. 47-4). The oral surface of the palate is closed on itself, creating a unilateral palatal adhesion (Figs. 47-5 to 47-7).
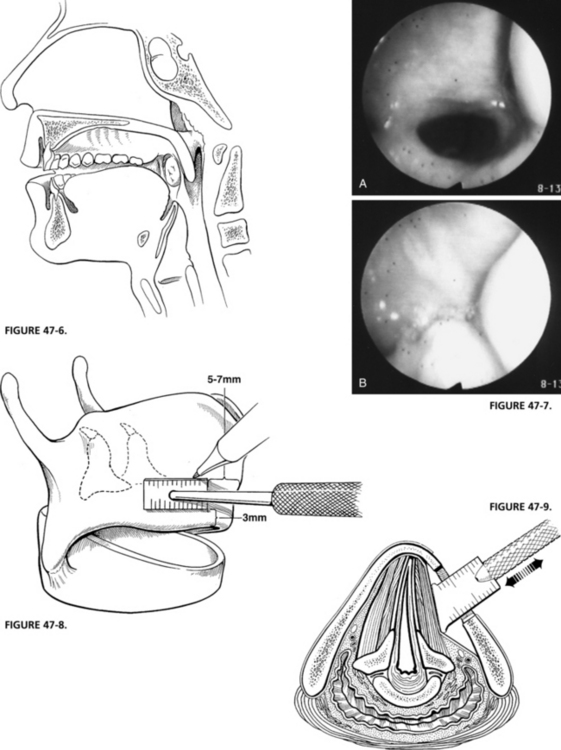
FIGURE 47-6 Sagittal view of completed palatal adhesion.
FIGURE 47-8. Alar window is positioned as shown, taking care to preserve inferior alar strut.
FIGURE 47-9. Depth gauge is used to medialize true vocal fold based on auditory and visual feedback.
Postoperatively, the patient is immediately allowed to take a liquid diet and is discharged from the hospital as soon as pain control is adequate. The only complication that has occurred from this procedure is a wound dehiscence that granulates over several months, producing a secondary adhesion with minimal VPI. Sleep apnea has not been seen as a complication of this procedure. Improvement of nasal regurgitation and reduction in hypernasal speech occur in all patients.8,9
The third motor branch of the vagus is the recurrent laryngeal nerve, which provides innervation to the intrinsic laryngeal musculature and the cricopharyngeus muscle. True vocal fold paralysis and lack of cricopharyngeal relaxation result from resection or neural injury at the time of surgery. The resultant glottal incompetence leads to a weak, breathy voice and an inefficient cough. This deficit combined with lack of cricopharyngeal relaxation results in aspiration and poor pulmonary hygiene. Tracheotomy has been universally performed to protect the airway until early swallowing rehabilitation can be accomplished. From an airway perspective, most patients with high vagal injury tolerate a unilateral vocal fold paralysis, making tracheotomy generally unnecessary; for the reasons discussed earlier, tracheotomy prolongs swallowing rehabilitation and adds morbidity to the postoperative course. Silastic medialization with arytenoid adduction has been the procedure of choice for addressing vocal fold paralysis in these patients.10 Although cricopharyngeal myotomy is not routinely necessary in these patients, it has been shown to decrease aspiration and promote early swallowing.11
The primary goal for vocal fold medialization in skull base patients is (1) to provide glottal competence, (2) to provide an efficient mechanism for coughing, and (3) to give support and volume to the voice. Generally, most patients with a unilateral vocal fold paralysis undergo a absorbable gelatin sponge (Gelfoam) injection postoperatively regardless of whether the nerve was taken. This injection lasts approximately 6 to 8 weeks, and greatly facilitates speech and swallowing rehabilitation while the nerve regains function or until Silastic medialization and arytenoid adduction may be performed. It is prudent to wait 2 to 3 months before proceeding with thyroplasty because denervation vocal fold atrophy occurs over several weeks, which alters the size of the Silastic implant and the degree of arytenoid adduction necessary to achieve good voice and airway results. Several weeks postoperatively, the patient should return for airway evaluation, videostroboscopy, and airflow measurements. A Silastic medialization under local anesthesia is performed. The technique is outlined in the following paragraphs and has been described elsewhere.10,12–14
Under local anesthesia, a midline horizontal incision or an extension of the cervical portion of the skull base incision is made overlying the midpoint of the thyroid cartilage. The sternohyoid muscle is divided at its medial attachments to the hyoid bone superiorly. A perichondrial flap is created from the midline back to the posterior edge of the thyroid cartilage, elevating the remaining attachments of the sternohyoid muscle and the thyrohyoid muscle with the flap, and exposing the inferior edge of the thyroid ala. A rectangular window is outlined so that the anterior extent lies 5 mm back from the anterior commissure in women and 7 mm back in men. The window is placed as low as possible, leaving an inferior 3 mm thyroid cartilage strut that is wide enough not to fracture when the implant is placed (Fig. 47-8).
Next, the long and short phonosurgery intralaryngeal elevators (Xomed, Jacksonville, MS) are used to elevate the inner perichondrium in all directions except anteriorly. Medialization is attempted, but it is rarely possible to achieve the required medialization with the inner perichondrium intact. The superior, posterior, and inferior margins of the perichondrium are incised discretely without injuring the lateral fascia of the thyroarytenoid muscle just deep to this. Branches of the superior laryngeal artery and vein can usually be seen lying just under this fascia. Although some surgeons fear that dividing the perichondrium leads to implant extrusion, this has not occurred in several hundred medialization procedures using this technique.13
The depth gauge is used to medialize the cord, and voice quality and cord position are assessed (Fig. 47-9). Based on these visual and auditory data, an implant is carved to appropriate dimensions from a preformed Silastic block (Fig. 47-10). Its inferior border is thicker than the superior border, and the posterior border is thicker than the anterior border. This creates an even medialization of the vocal fold with the point of maximum medialization at the lower edge of the window. Good alignment of the true vocal cord in the midline is usually obtained with little medialization of the false cord. All edges of the Silastic block are beveled to allow ease of insertion through the window. The average size of the block that is used when performing this under general anesthesia is 2 to 3 mm thick anteriorly and 5 to 7 mm at the point of maximal medialization, with an overall length of 13 to 18 mm. The block is inserted in the window by placing the lower flange behind the lower window strut while compressing the upper flange until it expands under the cartilage. Some 4-0 polypropylene (Prolene) sutures are used to stabilize the implant to the inferior strut of thyroid cartilage (Fig. 47-11).
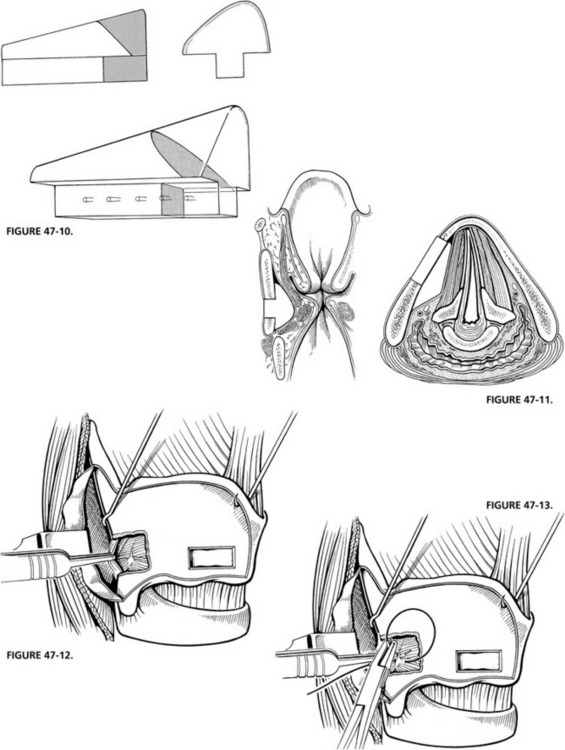
FIGURE 47-10 Preformed Silastic block is trimmed to fit auditory and visual feedback.
FIGURE 47-11. Final position of vocal cord after placement of Silastic implant.
High vagal injury with loss of the superior laryngeal nerve is usually best addressed with medialization and arytenoid adduction. Arytenoid adduction has been described elsewhere,15,16 and is performed at the time of Silastic medialization. When the cartilage window has been created, the voice is assessed using the depth gauge as described earlier, and the decision to proceed with arytenoid adduction is made.
Exposure of the arytenoid cartilage is achieved by first placing a hook beneath the posterior border of the thyroid cartilage and rotating the larynx forward. The perichondrium at the posterior border is divided, and the piriform sinus mucosa is elevated away from the inner surface of the thyroid cartilage. A 5 mm Kerrison rongeur is used to remove the posterior border of the thyroid cartilage, exposing the paraglottic space lateral to the muscular process of the arytenoid. Patients are then asked to purse their lips and blow out to confirm the location of the piriform mucosa so that dissection may be carried out anteriorly to expose the posterior cricoarytenoid muscle. The muscular process is palpated and moved in its plane of abduction and adduction while observing the monitor (Fig. 47-12).
A double-armed 4-0 Prolene suture is passed through the lateral edge of the muscular process in a figure-eight fashion to secure the arytenoid (Fig. 47-13). The goal of this stitch is to mimic the vector of force that rotates the vocal process of the arytenoid down (inferior) and in (medial) during phonation. To accomplish this, one end of the suture is brought through the paraglottic space and out through the cartilage just anterior to the window. The other end of the suture is passed from the window below the lower cartilage strut and then through the cricothyroid membrane soft tissue in the midline. Gentle traction is applied to the arytenoid, and its motion is observed on the monitor. The implant is carved and placed. Both arytenoid sutures pass deep to the implant. Final adjustments are made to the arytenoid stitch, and it is tied down. The perichondrium and strap muscles are laid back into position, and the wound is closed with absorbable suture.
Spinal Accessory Nerve (Cranial Nerve XI)
The spinal accessory nerve has only a branchial motor component. Its lower motor neuron cell bodies arise in the spinal cord, and its motor fibers ascend into the cranium and then descend through the pars nervosa of the jugular foramen, passing superficial to (70%), posterior to (27%), or through (3%) the jugular vein to innervate the sternocleidomastoid and trapezius muscles.17 Loss of sternocleidomastoid function results in weakness when turning the head away from the operated side, although this is rarely noticed by most patients. Loss of trapezius function results in downward and lateral rotation of the scapula with a shoulder droop. This causes severe shoulder disability secondary to weakness and pain. Whether the nerve may be grafted successfully or not, a shoulder exercise program that emphasizes strengthening of the levator, scalene, and rhomboid muscles should be administered by a qualified physical therapist. This program should continue indefinitely to maintain the strength and support of the shoulder girdle.
Hypoglossal Nerve (Cranial Nerve XII)
The hypoglossal nerve provides somatic motor control to all the intrinsic and extrinsic muscles of the tongue except the palatoglossus. The nerve exits the skull base through the hypoglossal canal medial to the jugular foramen. As it passes laterally, it shares fibers with the vagus nerve near the inferior (nodose) ganglion. Separation of these fibers leads to vocal fold paresis or paralysis and should be avoided.18 In time, most patients can compensate for unilateral tongue paralysis. The deficit is characterized by difficulty in positioning the bolus during the oral phase of swallowing. Often the bolus becomes lodged beneath the tongue on the paralyzed side. With swallowing therapy and lingual exercise, patients learn to position the bolus on the nonparalyzed side.
Cervical Sympathetic Chain
The sympathetic innervation to the head and neck structures originates in the upper thoracic segment of the spinal column. The preganglionic fibers ascend in the sympathetic chain to exit through one of its four ganglia (superior, middle, vertebral, and stellate). The superior cervical ganglion lies on the surface of the longus capitus muscle at the level of the second or third cervical vertebra, deep to the internal carotid artery at the most distal or superior point of the cervical sympathetic chain. From this ganglion, postganglionic fibers typically reach the first three or four cervical rootlets. They also communicate with CN IX, X, XI, and XII as part of the pharyngeal plexus. Finally, they form the sympathetic plexus that ascends along the internal carotid artery.2,19
Damage to the superior cervical ganglion or the internal carotid plexus results in Horner’s syndrome, consisting of ptosis, miosis, and anhidrosis. For most patients, there is little functional deficit. Rarely, visual fields may be partially obstructed because of the ptosis. More commonly, it is a cosmetic nuisance. Elevation of the eyelid to its normal open position can be accomplished by either levator shortening or resection of Müller’s muscle.20
The other significant sequela from injury to the cervical sympathetics at the skull base is loss of sympathetic innervation to the parotid gland. This complication is frequently seen with resection of high vagal paragangliomas when the sympathetic chain is either resected or damaged. In this setting, a prolonged course of cramping pain is associated with the first bite of each meal and has been named “firstbite syndrome.”18 Patients usually describe this pain as a spasm over the parotid region that begins with the first bite and subsides within the next several bites. The intensity of the pain is increased with strong sialogogues, such as vinegar or lemon. In the early postoperative period, the pain can be so severe as to limit oral intake. Early management consists of dietary modification with bland foods and oral carbamazepine (100 to 200 mg twice daily). Slowly, over time, the symptoms improve.
1. Bastian R.W. Videoendoscopic evaluation of patients with dysphagia: An adjunct to the modified barium swallow. Otolaryngol Head Neck Surg. 1991;104:339.
2. Hollinshead W.H. Anatomy for Surgeons: The Head and Neck, 3rd ed. Philadelphia: Lippincott; 1982.
3. Wilson-Pouwels L., Akesson E.J., Stewart P.A. Cranial Nerves: Anatomy and Clinical Comments. Philadelphia: Decker; 1988.
4. Rosenbaum A.L., Kushyner B. Vertical rectus muscle transposition and botulinum toxin after abducens nerve palsy. Arch Ophthalmol. 1989;107:820.
5. Ray B.S., Stewart H.J. Role of the glossopharyngeal nerve in the carotid sinus reflex in man: Relief of carotid sinus syndrome by intracranial section of the glossopharyngeal nerve. Surgery. 1948;23:411.
6. Jackson I. Discussion: A review of 236 cleft palate patients treated with dynamic muscle sphincter. Plast Reconstr Surg. 1983;71:187.
7. Orticochea M. A review of 236 cleft palate patients treated with dynamic muscle sphincter. Plast Reconstr Surg. 1983;71:180.
8. Netterville J.L., Vrabec J.T. Unilateral palatal adhesion for paralysis after high vagal injury. Arch Otolaryngol Head Neck Surg. 1994;120:218.
9. Netterville J.L., Civantos F.J. Rehabilitation of cranial nerve deficits after neurotologic skull base surgery. Laryngoscope. 1993;103:45.
10. Netterville J.L., Jackson C.G., Civantos F.J. Thyroplasty in the functional rehabilitation of neurotologic skull base surgery patients. Am J Otol. 1993;14:460.
11. Montgomery W.W., Hillman R.E., Varvares M.A. Combined thyroplasty type I and inferior constrictor myotomy. Ann Otol Rhinol Laryngol. 1994;103:858.
12. Isshiki N. Recent advances in phonosurgery. Folia Phoniatr Logop. 1984;32:119.
13. Netterville J.L., Stone R.E., Lukens L.S., et al. Silastic medialization and arytenoid adduction: The Vanderbilt experience—a review of 116 phonosurgical procedures. Ann Otol Rhinol Laryngol. 1993;102:413.
14. Wanamaker J.R., Netterville J.L., Ossoff R.H. Phonosurgery: Silastic medialization for unilateral vocal fold paralysis. Operative Tech Otolaryngol Head Neck Surg. 1993;4:207.
15. Isshiki N., Tanabe M., Sawada M. Arytenoid adduction for unilateral vocal fold paralysis. Arch Otolaryngol. 1978;104:555.
16. Miller F.R., Bryant G.L., Netterville J.L. Arytenoid adduction in vocal fold paralysis. Operative Tech Otolaryngol Head Neck Surg. 1999;10:36.
17. Parsons F.G., Keith A. Seventh report of the Committee of Collective Investigation of the Anatomical Society of Great Britain and Ireland, for the year 1896-97: Question III: The position of the spinal accessory nerve. Whether it passes outward between the jugular vein and internal carotid artery, or between the jugular vein and the atlas? Whether it perforates the sterno-mastoid or not: If so does the whole nerve perforate or only part? Which division of the sterno-mastoid does it perforate? J Anat Physiol. 1897;32:177.
18. Netterville J.L., Reilly K.M., Robertson D., et al. Carotid body tumors: A review of 30 patients with 46 tumors. Laryngoscope. 1995;105:115.
19. Collins S.L. Cervical sympathetic nerves in the surgery of the neck. Otolaryngol Head Neck Surg. 1992;105:544.
20. Dortzbach R.K. Superior tarsal muscle resection to correct blepharoptosis. Ophthalmology. 1979;86:1883.