CHAPTER 172 Rehabilitation of Facial Paralysis
The facial nerve, once damaged, rarely attains full recovery of function. Given the challenges to the patient, counsultations regarding facial nerve paralysis require a clinician’s fullest thought and compassion. A realistic approach yields the rewards of patient compliance, understanding, satisfaction, and acceptance of reality. A recent review of all state and federal civil trials alleging malpractice and facial nerve paralysis demonstrates the importance of careful explanation and documentation, as well as the importance of good patient rapport and bedside manner in preventing lawsuits.1
Patient Assessment
An outline of assessment of facial nerve paralysis is presented in Box 172-1.
Assessment of the Deformity
Physical examination includes complete head and neck examination with attention to cranial nerve function and the presence of functional masseter and temporalis muscles. The degree of facial nerve function is recorded using the House-Brackmann Facial Grading System2 (Table 172-1). A number of facial nerve grading scales have been developed, but the House-Brackmann scale was adopted by the Facial Nerve Disorders Committee of the American Academy of Otolaryngology–Head and Neck Surgery in 1985 because of its reproducibility and ease of use.2 This scale is useful for evaluation of overall function, but it is insufficient for precise assessment of defects affecting one or more branches of the facial nerve, and it does not allow precise measurement of effectiveness of treatments isolated to one region of the face. Therefore, the examination should assess deformity of the upper, middle, and lower thirds of the face independently. This approach allows more precise characterization of defects, aids the decision-making process for rehabilitation, and allows more precise assessment of treatment results. Facial tone also is noted, as is the presence of any reinnervation. Thorough assessment of the eye also is performed. Visual acuity, corneal integrity, eyelid closure, tearing, Bell’s phenomenon, lagophthalmos, lower lid laxity, position of the lacrimal puncta, and eyebrow position are noted. Nasal examination focuses on the position of the ala and nasal septum and the presence or absence of nasal obstruction. Oral competence and height and position of the lower lip are carefully reviewed. In long-term paralysis (of more than 1 year’s duration), electromyography (EMG) of the facial muscles is performed before reinnervation procedures. Occasionally, muscle biopsy provides additional information about the presence of viable muscle for innervation. If nerve fibrosis is suspected, nerve biopsy occasionally is indicated.
| Grade | Description | Characteristics |
|---|---|---|
| I | Normal | Normal facial function in all areas |
| II | Slight |
From House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146.
Another important component of the assessment is evaluation of the patient’s smile pattern. The smile is created by the muscles of the lips, and smile patterns can be classified into one of three types.3 The “Mona Lisa” smile is the most common smile pattern (67%). It is dominated by action of the zygomaticus major muscle: The corners of the mouth move laterally and superiorly, with subtle elevation of the upper lip. The canine smile (31%) is dominated by levator labii superioris action, appearing as vertical elevation of the upper lip, followed by lateral elevation of the corner of the mouth. The least common smile is the full denture smile (2%), or “toothy smile,” produced by simultaneous contraction of the elevators and depressors of the lips and angles of the mouth. Knowledge of facial muscle anatomy and the smile pattern exhibited by the patient is important in considering rehabilitation techniques other than nerve grafting to recreate a balanced facial appearance at rest and the simulation of a symmetric smile.
Considerations in Facial Nerve Rehabilitation
Generally, however, the order of preference for rehabilitation procedures is as follows:
Time since Transection
A chronic, long-standing paralysis with complete muscle degeneration poses several problems with regard to eventual reinnervation surgery. The facial muscles may undergo denervation atrophy. Severe atrophy renders the reasonably normal muscles incapable of reinnervation and contraction. Such severe atrophy may occur after 18 months of complete denervation, although in some clinical situations, muscles have been known to persist inexplicably for many years without incurring such atrophy.4 EMG is the most helpful method for assessing facial muscle atrophy and is therefore a preoperative prerequisite for all reanimation candidates if the paralysis is of more than 12 months’ duration. The presence of nascent, polyphasic, or normal voluntary action potentials in a patient with facial paralysis indicates the occurrence of reinnervation. If more than 12 months have passed since the facial nerve injury, the situation can be assumed to be stable, and an attempt at surgical reanimation may be warranted. Within the first 12 months, however, the presence of potentials may mean that reinnervation is occurring and that facial movements may return in the next few months. Reanimation surgery should therefore be postponed. Fibrillation or denervation potentials mean that the EMG electrode is positioned in denervated muscle. This is an optimal situation for cable nerve grafting or, when no viable proximal facial nerve is available, for hypoglossal-facial anastomosis.
Status of the Proximal and Distal Facial Nerve
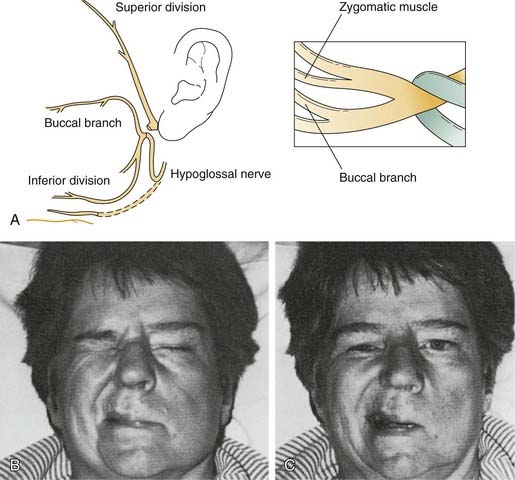
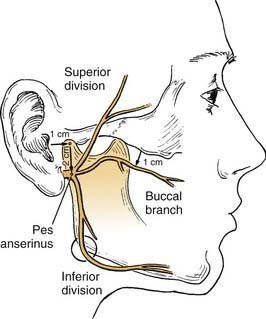
The facial nerve distal to the injury site serves as a conduit for neural regeneration to the facial muscles after neurorrhaphy, grafting, or hypoglossal-facial anastomosis. With acute injuries (incurred less than 72 hours previous), the electrical stimulator may be used to identify the distal nerve and the muscular innervation of distal branches. After this “golden period,” however, the surgeon must rely on visual identification of the divisions and branches of the distal nerve, because the capability of being stimulated electrically generally is lost after approximately 72 hours. For this reason, transected nerve branches in trauma or tumor cases should be tagged for identification by placing a small colored suture around or adjacent to each nerve branch. Any anatomic or surgical landmarks should be precisely dictated in the operative note. If no suture markers are available, and the golden period has elapsed, careful surgical searching (preferably with use of loupes or an operating microscope) may reveal each of the divisions or branches of the facial nerve. A topographic map is essential in guiding the dissection. A review by Bernstein and Nelson5 describes the variability with which these branches are placed. The following landmarks are helpful (Fig. 172-1).
(From House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146.)
As an example of selective routing, when a parotid tumor operation results in excision of the pes anserinus and the proximal facial branches, a branched nerve graft may be placed to reinnervate the zygomatic and the buccal branches, excluding unimportant branches. Fisch6 advises clipping branches of cervical branches in order to route innervation to the more important portions of the face. Data confirming the efficacy of this technique, however, are very limited.
If no nerve branches are found, and EMG shows that denervated facial muscles are present, the nerve graft may be sutured directly to the muscles targeted for reinnervation (muscular neurotization). In these instances, the most important muscles are those of the midface (zygomaticus major and minor, levator labii superioris) and orbicularis oculi muscles. Reinnervation will not be as complete as in routine nerve grafting because the regenerating axons must form new connections to the old motor endplates, or they must create their own.7
Viability of Facial Muscles
Four types of EMG responses are observed8:
Status of Donor Nerves
The hypoglossal nerve is the most frequently used nerve source for transfer. Reflex and physiologic similarities between the hypoglossal and facial nerves have been described.9 The integrity of the hypoglossal nerve must be determined before it is transferred for reinnervation. Irradiation of the brainstem, lesions of the skull base and hypoglossal canal, and surgical procedures of the upper neck may affect the integrity and function of this nerve.
The cross-face nerve graft procedure (faciofacial anastomosis) initially was thought to be the most appropriate and ingenious facial reanimation procedure.10,11 The procedure is unique in that it borrows appropriate neural input from the contralateral normal side and routes it to the paralyzed side. Such a procedure requires a fully intact (contralateral) facial nerve.
Age
The proximal neuron’s ability to regenerate declines with time because of denervation and aging-related changes. The etiologic mechanism probably involves diminishing regenerative vitality of the perikaryon (cell body), although peripheral scarring may play a role. The clinical implication is that facial reanimation surgery should always be performed as soon as possible, provided that the operative procedure does not interfere with or injure existing innervation or ongoing reinnervation.12
Prior Radiotherapy
Radiotherapy, a necessary component of treatment for certain salivary gland malignancies, appears to have a deleterious effect on reinnervation through facial nerve grafts. McCabe13 demonstrated satisfactory muscle reinnervation from grafting despite irradiation in animals. These investigators subsequently have documented return of facial function in nine patients who received post-grafting radiotherapy. McGuirt and McCabe13 and Conley and Miehlke14 have published reports indicating that facial nerve grafts function well even though irradiated, and that nerves are among the most radioresistant tissues of the human body. Pillsbury and Fisch15 found that radiotherapy reduced the average outcome from 75% to 25% of nerve function recovery in a review of 42 grafted patients. Irradiation probably affects the neovascularization of the nerve graft by decreasing vascularity of the tissue bed and probably injures the proximal and distal segments of the nerve as well. The most radiosensitive portion of the nerve—the pontine nucleus—should be assessed to determine whether it was present in the field of irradiation.
Congenital Paralysis
In a series of 95 infants with neonatal paralysis, Smith and associates8 found 74 of the cases to be secondary to intrauterine injury or birth trauma, whereas 21 were thought to be congenital. These infants should be studied with nerve excitability and EMG testing early in life to ascertain the status of nerves and muscles. Most patients with injury-related neonatal paralysis recover rapidly, whereas the paralysis associated with other congenital anomalies (such as Möbius syndrome) is permanent. Nerve exploration or transfer generally is futile in the latter cases.
Early Care of Facial Nerve Injury
Eye Protection: Evaluation and Treatment of Eyelid Paralysis
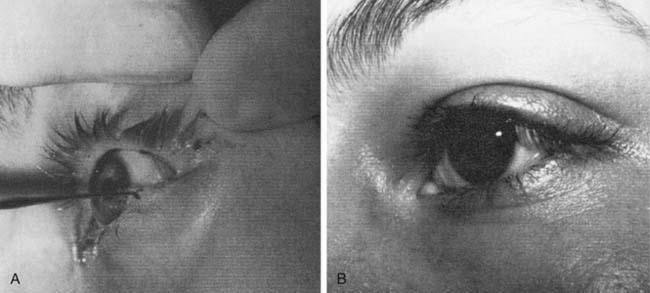
Lacriserts, contact lenses, and occlusive bubbles commonly are used, although patient compliance may be problematic16 (Fig. 172-2). The eyelids frequently are patched or taped, but if incorrectly used, these methods may result in corneal injuries. Tape should not be placed vertically across the eyelashes, but applied horizontally above the eyelashes on the upper eyelid, or supporting the lateral canthal portions of the lower eyelid.17 When an eye patch is used, care must be taken to ensure that the eye cannot open, because this would allow contact between the patch and the cornea.
Procedures to Treat Paralysis of the Lower Lid (Ectropion)
Tarsorrhaphy
For longer-lasting protection, the lid adhesion tarsorrhaphy is preferred. The lid margin (gray line) of each lid is denuded 4 to 6 mm from the lateral canthus, and a similar suture technique is used to approximate the denuded mucocutaneous junctions of upper and lower lids (Fig. 172-3). Like the temporary lateral tarsorrhaphy, this procedure can be reversed if function returns. The cosmetic deformity of tarsorrhaphy and the availability of improved, alternative techniques to rehabilitate the eyelids have reduced the use of tarsorrhaphies.
Wedge Resection and Canthoplasty for Paralytic Ectropion
The tarsal strip procedure described by Anderson and Gordy18 modifies the previously described technique by denuding the conjunctiva over the lateral tendon and separating the posterior lamella and the tendon from the anterior lamella. The isolated tarsal strip is then suspended to the lateral orbital rim. In cases of severe ectropion, the lower lid punctum may be everted and displaced laterally after lateral canthoplasty. In these instances, a medial canthoplasty is used to restore the physiologic relationship of the punctum to the globe.19,20 The lower lid also may be augmented with auricular cartilage to address inadequate support of the medial tarsal plate in cases not amenable to lateral tendon suspension alone.21
Procedures to Treat Paralysis of the Upper Lid (Lagophthalmos)
Weights, Springs, and Slings for Lagophthalmos
Under local anesthesia, an incision is made extending equally between the medial and middle thirds of the supratarsal crease, and the skin is elevated to the superior border of the tarsus. A pocket is formed immediately superficial to the tarsus to accommodate the dimensions of the weight. The weight is placed so that its inferior border is parallel to and just above the eyelash line. It is important to create a pocket directly on the tarsal plate, with care taken to preserve a thin cuff of tissue at the lid margin to prevent inferior extrusion of the implant. The implant is secured with clear nylon sutures superior and inferior to the tarsal plate, the orbicularis-levator complex is reapproximated, and the skin is closed.16
Palpebral springs and silicone (Silastic) slings as described by Morel-Fatio and Lalardrie22 and Arion,23 respectively, also have been used for lagopththalmos. Silastic slings are used less frequently and are complicated by lateral ectropion.24 Both types of implants share the disadvantage of extrusion, and they are more difficult to place than eyelid weights. Currently, titanium chain link implants are being used with greater frequency and with excellent results. These implants may be camouflaged to obtain a better cosmetic result, because they conform to the shape of the eyelid. Additionally, the titanium implants have been shown to result in less corneal astigmatism or cornea-altering effect on the globe itself than that typical with gold weights.25
Facial Nerve Grafting
Facial nerve grafting for acute causes such as parotid malignancy requires the surgeon to customarily identify the distal facial nerve trunk divisions or branches for the distal anastomosis. Nerve restitution should be performed at this time unless extenuating circumstances (e.g., anesthetic complications, intraoperative emergencies) rule out immediate grafting.26 If grafting is not undertaken at the time of nerve sacrifice, it should be completed within 72 hours thereafter, so that the facial nerve stimulator may be used to identify the distal branches. Conversely, the nerve ends can be tagged for identification at a later time. When grafting is not done, the distal branch becomes nonstimulatable and thus much more difficult to locate and identify.
Surgical Planning
Distally, several situations may be encountered that require some ingenuity in effecting reanimation. When the distal anastomotic site is at or proximal to the pes anserinus, a simple nerve-to-nerve suture will suffice. More frequently, however, after resection for parotid malignancy, several branches or divisions may require anastomosis. In such cases, priority must be given to the zygomatic and buccal branches, sometimes to the exclusion of other, less important facial nerve branches. Frequently, a two-branch graft can be prepared from the greater auricular nerve or the sural nerve. This situation favors suturing of one branch of the nerve graft to the buccal branch and the other graft branch to the zygomatic branch or superior division. This technique will direct innervation to the important orbicularis oculi muscle and the muscles of the buccal-smile complex (Fig. 172-4).
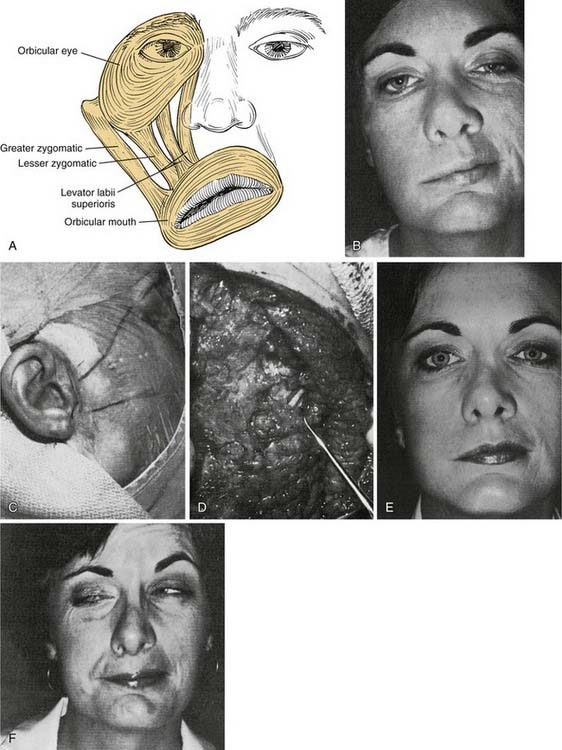
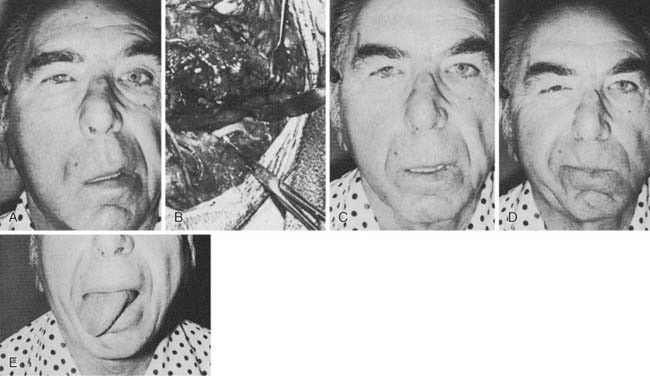
Figure 172-4. Facial muscles. A, The most important muscles for reanimation: The levator labii superioris, along with the lesser zygomatic muscle, probably is the most significant muscle for elevation of the upper lip. The greater zygomatic muscle also is critical as the strongest elevator of the oral commissura. B to F, Example of a delayed nerve graft in a patient in whom a parotid malignancy was removed without immediate reconstruction (because of intraoperative anesthesia considerations). B, The patient was referred for nerve grafting after 9 months elapsed. Note elongation of the buccal-smile complex of muscles (greater and lesser zygomatics, levator labii superioris) and paralysis of orbicular eye muscle. C, Preoperative surface markings for nerve branches. Nerve stimulator cannot be used to locate distal branches after 9-month time lapse. Markings are for the superior division and buccal branch, based on landmarks described in Figure 172-1 and the previous operative report. D, Superior division is found; it is ready for transection and anastomosis to sural graft (from midmastoid nerve segment). E, Result at 1 year after operation—typical for a delayed nerve graft. Orbicular eye and buccal branch muscle function is improved, but the muscles are not normally or completely reinnervated. F, Voluntary motion in both zygomatic and buccal branches, with synkinesia. This result also is typical for most nerve grafts in that the temporal branch (to occipitofrontal muscle) shows no reinnervation. Note the hairstyle designed to camouflage occipitofrontal muscle paralysis.
Choosing a Donor Nerve
The medial antebrachial cutaneous nerve has been described in the orthopedic literature for peripheral nerve repairs and is used in situ with forearm microvascular flaps for sensory innervation in head and neck cancer reconstruction. This nerve has several properties that warrant consideration for use in facial nerve reconstruction27: It has a consistent anatomy, traveling in the bicipital groove immediately adjacent to the basilic vein. Nerve diameter and branching pattern are similar to those of the facial nerve, and donor site morbidity is minimal with nerve harvest. Cheney27 has described the relevant anatomy in detail.
Surgical Technique
For the surgical technique of neurorrhaphy, interrupted sutures using 9-0 or 10-0 monofilament nylon are preferred. A straight and a curved pair of jeweler’s forceps as well as a Castroviejo needle holder are satisfactory instruments for performing the anastomosis. Both ends of the nerve graft and the proximal and distal stumps should be transected cleanly with a fresh sterile blade.13 For nerve trunk anastomosis, four simple epineural sutures will usually coapt the nerve ends accurately. However, obvious discrepancies in size or other epineural gaps should be closed with additional sutures. The needle should pass through epineurium only to avoid injury to the fascicular neural contents. The nerve graft should lie in the healthiest possible bed of supporting tissue, with approximately 8 to 10 mm of extra length for each anastomosis. Thus, the graft should lie in a somewhat “lazy S” configuration (see Fig. 172-4), which appears to minimize tension during healing. Suction drainage systems should be placed away from any portions of the nerve graft.
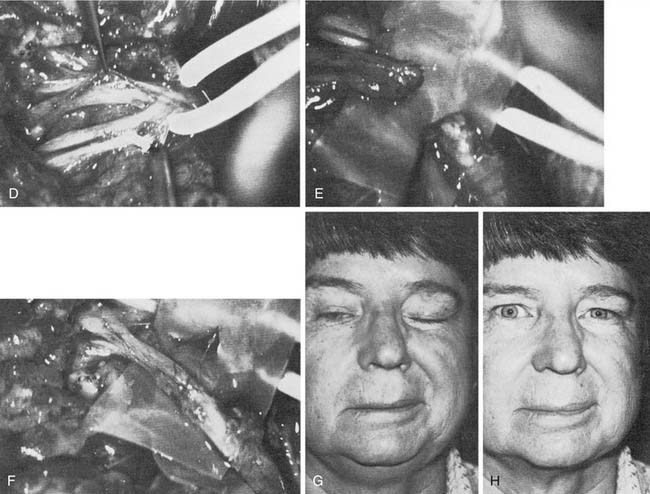
When one division is excised or injured and other portions of the nerve remain intact, it may be desirable to graft from a fascicle within the pes anserinus to a distal branch. To accomplish this, fascicular dissection is performed in parallel and along the plane of the nerve fascicles with curved jewler’s forceps into the pes anserinus (Fig. 172-5). The distal buccal branch often has several small filaments, so it may be necessary to select the larger of these for distal anastomosis.
Approximation of the nerve ends using an acrylic glue has been described (Histacryl or cyano-butyl-acrylate)28 with subsequent investigators revealing that neural anastomosis with tissue adhesive yields results similar to nerve suture. This technique is most helpful in the confined surgical considerations of the temporal bone rather than in distal facial anastomosis.29 Others have described using biodegradable nerve tubules.
Following temporal bone resection, the nerve may be routed from the tympanic or the labyrinthine portions directly to the face through a bony window near the posterior root of the zygomatic arch. This will shorten the necessary length of the nerve graft. However, when using this technique it is important to ensure the nerve graft’s protection from trauma at the temporomandibular joint if the joint is preserved. Conley and Baker30,31 have reported excellent results using similar techniques.
Millesi32 introduced interfascicular nerve repair, reasoning that the exact microsurgical approximation of nerve fascicles or fascicle groups may minimize synkinesis or mass movement. It is well known that this type of repair is preferred in nerve injuries in the extremities; however, such repairs have not been universally accepted for use in the facial nerve. Several reasons underlie this limited acceptance. The tympanic and, in many cases, the mastoid portions of the nerve have only one or two fascicles, and the intraneural topography is questionable. Few, if any, sensory fibers are present in the extratemporal portion of the facial nerve, so performing sensory-to-sensory fascicular repair is not of value.
Along with May and Miehlke, we have reported (independently) that discrete, spatially oriented fascicles are present in the nerve near the stylomastoid foramen.33–35 Other authors, notably Sir Sidney Sunderland36 and Tomander and colleagues,37 have reported conflicting data demonstrating that various portions of the face are represented in a random fashion in the proximal nerve. At present, it probably is best to perform fascicular repair when the injury obviously lends itself to the technique (e.g., clean lacerations through the pes anserinus and branch nerve grafts that require fascicular dissection in the pes). Basic research has yet to reveal the exact neural topography of the more proximal portions of the nerve.
Cross-Face Nerve Grafting
Overview
The creative and physiologic method of cross-face nerve grafting provides the possibility for facial nerve control of previously paralyzed facial muscles. It is the only procedure with the theoretic capability for specific divisional control of facial muscle groups (e.g., the buccal branch controlling the buccal branch distribution, the zygomatic branch innervating the orbicularis oculi). Originally described by Scaramella10 and Smith11 in independent reports in 1971, the technique has not proved to be as advantageous as was first thought. Anderl38 subsequently described his own results as good in 9 of 23 patients, whereas Samii39 reported that only 1 of 10 patients had good movement as a result of this technique. A more recent update by Ferreira40 indicates that those patients operated on within the first 6 months of the paralysis did better than those operated on at a later date. In some of these patients, however, partial spontaneous reinnervation may have occurred, because they appeared to have had lesions without total palsy, and the traditional waiting period of 1 year was not allowed to elapse.
Surgical Technique
The cross-face technique suffers from a lack of sufficient axon population and neural excitatory vitality. It is of marginal value when used alone, but when combined with microvascular transfer of muscle (see below), it can provide suitable innervation. Conley41 and Conley and Baker42 have discussed the shortcomings and unproven status of cross-face grafting. Cross-face grafting currently is used only in conjunction with free muscle transfers. Reinnervation of paralyzed facial muscles has not been proved to be sufficient to justify use of this procedure without muscle transfer.
Nerve Transposition
Hypoglossal Nerve Transfer
Of the various nerves available for anastomosis with the facial nerve, the hypoglossal nerve is preferred, because an anatomic and functional relationship exists between the facial and the hypoglossal nerves. They both arise from a similar collection of neurons in the brainstem, and they also share similar reflex responses to trigeminal nerve stimulation.9 In addition, the hypoglossal nerve is in close anatomic proximity and is readily available during other operations on the facial nerve. Hypoglossal nerve transection results in less donor disability than that typical with sacrifice of the spinal accessory, phrenic, or other regional nerves that have been used for facial reanimation. The most common criticism of hypoglossal nerve transfer is that it results in a lack of voluntary emotional control. Although this is true, ipsilateral facial nerve anastomosis often is associated with a similar drawback: Mass movements and spasms preclude any voluntary control of eye closure, smiling, or other emotional movements. In our practice, we have used hypoglossal nerve transfer for reanimation of the upper or lower division selectively by performing fascicular dissection in the pes anserinus to identify the specific fascicles that required reinnervation (see Fig. 172-5).
May and coworkers43 attempted to decrease morbidity from tongue atrophy by performing partial transection of the hypoglossal nerve with use of a jump graft from the partially transected nerve to the distal facial nerve. Decreased effectiveness of partial transection with jump graft has been reported by some investigators.44
Recently, “pure” end-to-side anastomosis of the facial nerve or a jump graft into the donor hypoglossal nerve has been rediscovered45 and reported in a small series of patients.46 The study investigators performed anastomosis of facial nerve either mobilized from the mastoid or bridged by interposition graft into the intact hypoglossal nerve, without removal of epineurium or perineurium. This technique relies on the presumed axon sprouting across intact epineurium of the hypoglossal nerve. Some evidence indicates lateral axonal growth after end-to-side nerve anastomosis occurs. Rat studies demonstrate axonal penetration of endoneurium, perineurium, and epineurium.47–49 Whether this technique will be applicable in humans who have decreased nerve regenerative capacity and much thicker perineurium and epineurium than in the rat remains to be seen. It may be more effective to remove an epineurial window and perform the anastomosis without disruption of nerve fibers yet still induce enough axonal regrowth to provide tone and function. Further animal and human studies will be necessary to determine the usefulness and role of end-to-side neurorrhaphy for facial rehabilitation.
Results
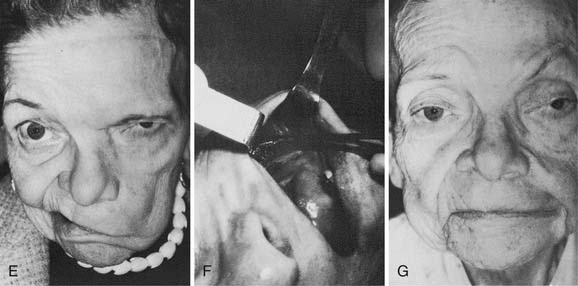
In the largest series to date, involving 137 patients, approximately 95% regained satisfactory tone in repose and regained some mass facial movement.50 Of these patients, 15% demonstrated hypertonia and excessive movement in the middle third of the face; however, none of these patients requested that the transferred nerve be reoperated. This excessive movement was found to decrease gradually over 10 to 20 years. However, Dressler and Schonle51 and Borodic and coworkers52 have had success treating facial hyperkinesia with selective injection of botulinum toxin. Seventy-eight percent of the patients had moderate to severe tongue atrophy, whereas 22% showed minimal atrophy; this wide variability in response of the tongue to hypoglossal nerve transection has been confirmed in other series53 (Fig. 172-6). Use of the interpositional jump graft with partial hypoglossal nerve preservation preserved tongue function in a majority of patients and provided satisfactory function. In a series of 20 patients, good facial tone and symmetry were observed at follow-up evaluation in all patients, and 13 had “excellent” restoration of facial movement, with development of 12th nerve deficits noted in only 3 patients.43
Other Nerve Transfers
The spinal accessory nerve was used before the hypoglossal nerve in nerve transposition techniques. Drobnik performed the first anastomosis of cranial nerves XI and VII in 1879.9 The phrenic nerve has been similarly used, but this technique causes paralysis of the diaphragm and induces undesirable involuntary inspiratory movements in the facial muscles.54 The technique is now obsolete.
The neuromuscular pedicle technique described by Tucker transfers a branch of the ansa hypoglossi nerve and a small muscle bloc directly to paralyzed facial muscles. According to Tucker,55 this procedure is valuable only for the perioral, depressor anguli oris, and zygomaticus muscles. The procedure is described as transferring innervated motor endplates to the denervated facial muscles without the usual delay period seen with free nerve grafts and nerve transfers. The technique allows limited reanimated strength because of the small number of axons present in the donor nerve. In addition, sound electrophysiologic confirmation that the technique produces reinnervation is somewhat lacking, despite a report by Anonsen and colleagues on this topic.56 Until physiologic data are presented and confirmation by other surgeons is obtained, the procedure has potential but remains unproved.
Neurotropic Factors
With several growth factors known to promote neuronal survival, application and delivery of the these factors constitute an attractive therapy for nerve injury and surgical repair. The trophic effects of nerve growth factor (NGF), glial cell–derived neurotropic factor (GDNF), brain-derived neurotropic factor (BDNF), and insulin growth factors I and II all are under current investigation.57 In ongoing rat experiments, embyronic stem cells are used to provide the trophic support factors to the host motor neurons in restitution of the neuromuscular junction.58 These preliminary findings provide a model for motor unit restoration and a potential therapeutic intervention in the treatment of paralysis. However, these studies are limited to the rat model and require further basic and clinical investigation.
Muscle Transfers
Masseter Transfer
Since the masseter was first used for facial reanimation in 1908, many modifications have been described.59 Many authors, notably Conley and Baker, prefer the masseter muscle for rehabilitation of the lower face and midface.30
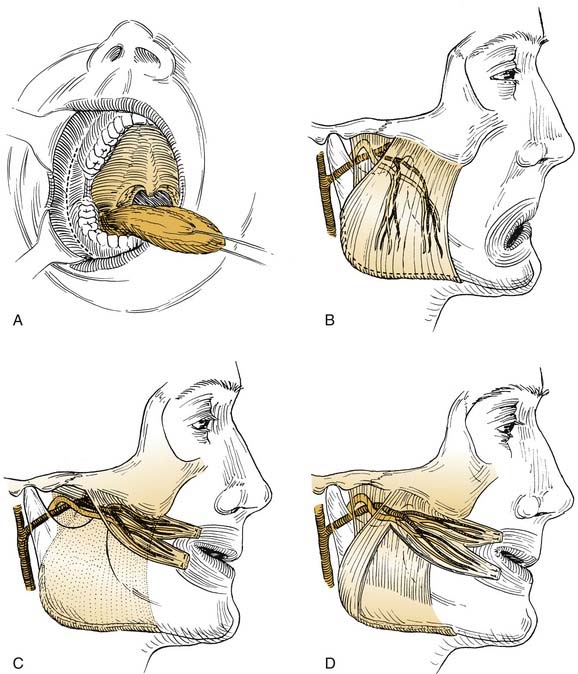
The masseter transfer procedure generally is performed for rehabilitation of the sagging paralyzed oral commissure and the buccal-smile complex of muscles. The masseter’s upper origin from the zygomatic arch allows a predominantly posterosuperior pull on the lower midface. Transfer of the muscle can be accomplished externally through a rhytidectomy-parotidectomy incision, or intraorally using a mucosal incision in the gingivobuccal sulcus lateral to the ascending ramus of the mandible (Fig. 172-7). The masseter’s blood supply is medial and deep, and its nerve supply passes through the sigmoid notch between the condylar and the coronoid processes of the mandible to reach the upper deep surface of the muscle. The nerve supply then ramifies and courses distally and inferiorly, terminating near the periosteal attachments on the lateral aspect of the mandibular angle and body. In general, the external approach is preferred, insofar as the intraoral approach is associated with somewhat limited access, poorer muscle mobilization, and less vascular control.
A generous parotidectomy incision is made and extended inferiorly below the mastoid tip. The parotid gland and masseteric fascia are exposed. The posterior border of the muscle is freed from the mandible’s ascending ramus and at the lower border of the mandible. The nerve supply courses along the deep surface approximately midway between the anterior and the posterior borders of the muscle (see Fig. 172-7). It is advisable to preserve the deep fascial layer in dissecting the muscle free from the mandible. Mobilization of the periosteal attachments along the inferior border will provide secure tissue for anchoring sutures and will provide greater length of the transposed muscle.
Temporalis Transfer
Although Gillies60 usually is given credit for introducing the temporalis procedure, Rubin61,62 deserves much credit for refining the goals of the procedure and the operative technique in the United States. Like the masseter transfer, the temporalis transfer procedure requires an intact ipsilateral V3 nerve. The nerve supply to the temporalis lies along the deep surface of the muscle. The blood supply comes from the deep temporal vessels from the external carotid artery. Thus, with total parotidectomy, extensive neck dissection, or infratemporal fossa dissection, the neurovascular supply may be tenuous at best. The upper origin of the temporalis muscle is fan-shaped and arises from the periosteum of the entire temporal fossa. The muscle belly converges on a short tendinous portion deep to the zygomatic arch and inserts on the coronoid process. The muscle is best exposed through an incision that passes above the ear, slightly posteriorly, and then in an anteromedial arc. This will expose the entire upper portion of the muscle (Fig. 172-8). A convenient aponeurotic dissection plane exists lateral to the temporalis fascia.
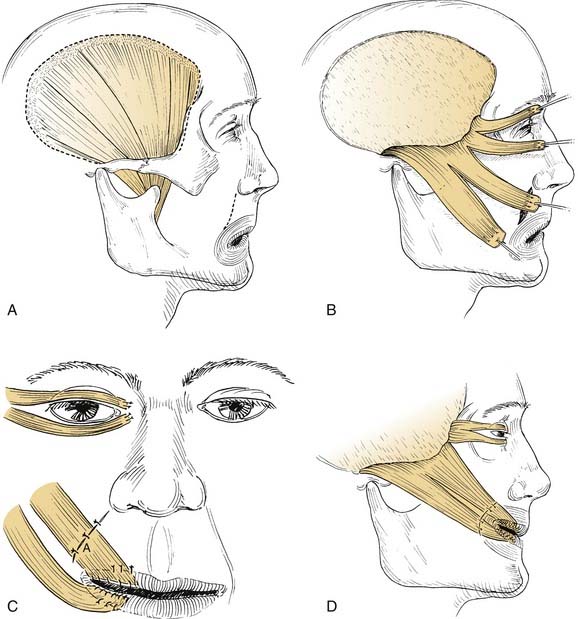
Figure 172-8. Temporalis muscle transposition. A, Dotted line illustrates the incision in the pericranium peripheral to the edge of the muscle. This results in strong periosteum to hold sutures in the transposed position. Nerve supply on deep side of muscle is not shown. B, Temporalis muscle divided into four slips. Note pericranium at the end of the slips sutured to the muscle to reinforce the suture site. Temporal fascia superficial to the muscle can be used in the same way. C, Transposed slips sutured to perioral muscles. Creativity and compulsivity are crucial during this portion of procedure. Overcorrection is mandatory. Sutures (A) must be placed in subdermis inferior to incision or in the submucosal portion of the wound deep to the orbicularis oris muscle. D, Completed procedure. Wide tunnel over the zygomatic arch precludes an unsightly bulge of muscle, which would otherwise be produced. Superior pull of temporalis muscle is somewhat preferred to posterolateral pull of masseter muscle (see Fig. 172-7).
In Rubin’s technique, the muscle is dissected free from the periosteum and attached to fascial strips, which are turned down inferiorly to reach the oral commissure and eyelid area. If these fascial strips are omitted, the transposed muscle’s length will be insufficient to reach the lateral oral commissure. More recently, Rubin62 refined his temporalis transfer technique by including a slip of masseter muscle that is sutured to the oral commissure and lower lip. The resulting masseteric pull improves results by providing more posterior and lateral vectors to the oral commissure.
We prefer to use the technique described by Baker and Conley,50 who describe retaining the integrity of the upper muscle and its overlying fascia. The latter is dissected free and then turned inferiorly for suturing to the oral commissure.
A tunnel at least 1 to 1.5 inches wide must be made over the zygomatic arch to allow the muscle to turn inferiorly and eliminate an unsightly bulge. The attachment of the strip should be just medial to the nasolabial fold so that the natural crease is reproduced by the muscle pull. As with the masseteric procedure, a marked overcorrection is necessary on the operating table. A soft silicone block or a temporoparietal fascia flap may be used to fill the temporal hollow. A modification of this technique by Sherris63 is performed by extending the transfer into the midregion of the upper and lower lips, to reduce stretching and thinning of the lips over time.
Several modifications of the temporalis transfer have been reported in attempts to avoid folding of the temporalis muscle over the zygomatic arch. Labbé and Huault64 describe partial inferior mobilization of the temporalis muscle from the skull by elevation of the posterior and superior attachments of the muscle. The coronoid attachments of the muscle are detached, and the muscle is inferiorly mobilized toward the upper lip. The coronoid insertion of the inferiorly displaced muscle is secured to the perioral musculature.
Although the gold weight–canthoplasty technique often is preferred, the temporalis muscle can be used for orbital rehabilitation. The anterior third of the temporalis muscle is turned laterally into the eyelids (see Fig. 172-8). Subcutaneous tunnel dissection between the paralyzed orbicularis oculi and the eyelid skin allows passage of the fascial strips medially through both eyelids to the medial canthus, where they are sutured. As with any reconstructive procedure, adjustments and suture revisions should be checked carefully on the operating table to ensure proper eyelid contour.
Microneurovascular Muscle Transfer
Microneurovascular muscle transfer was popularized in the 1970s and has been combined with cross-face nerve grafting to restore some facial movement.65 Because facial movements are highly complex and interrelated, enthusiasm for free muscle transfer was stimulated by the potential to use muscles that may provide isolated or independent segmental contractions, such as for superior elevation of the oral commissure. In patients with absence of facial musculature (such as those with congenital paralysis as in Möbius syndrome), microneurovascular muscle transfer seems to have strong potential.
A number of muscles and their respective nerve supply have been used for microneurovascular transfers. The most popular muscles include the gracilis, latissimus dorsi, and pectoralis minor muscle flaps.66 Ideally, the proximal stump of the ipsilateral facial nerve is used, but this often is not possible. Traditionally, reinnervation has been accomplished in two stages, with a preliminary cross-face nerve graft performed approximately 1 year before muscle transfer. Neural ingrowth within the grafted nerve is monitored by recording progression of Tinel’s sign along the path of the graft. When reinnervation of the graft has occurred, microvascular muscle transfer is then performed. One-stage procedures using long neurovascular pedicles connected directly to the contralateral facial nerve have been described.67–70 A recent comparison study found favorable outcomes with the one-stage reconstruction compared to the more traditional two-stage reconstruction.70 Muscle preservation using implantable intramuscular stimulators is under investigation and may allow preservation of facial muscles while reinnervation occurs.71 This technique shows promise in management of peripheral nerve injuries, and exploratory studies on the facial nerve have been initiated. When facial nerve input is not available, alternative nerves can be used for input, including the masseteric branch of V3, ansa hypoglossi, or the hypoglossal nerve.66,72
Composite tissue allograft procedures have gained considerable interest over the last 10 years. Devauchelle73 and Dubernard74 and their colleagues detailed the preliminary functional improvements of the first human partial face transplant, with the return of light touch, heat and cold perception, and mouth closure. These functional milestones were obtained by 10 months postoperatively; a normal smile was observed at 18 months. As with all tissue transplantation, however, immunosuppression is an essential adjunct and not without consequence. After transplantation, the patient suffered two episodes of acute rejection, two infectious complications (type 1 herpes simplex virus infection and molluscum contagiosum), renal failure, moderate thrombotic microanigiopathy, and hemolytic anemia. These preliminary findings reveal a new dimension of reconstructive surgery while also underscoring the inherent benefits of native tissues in facial reconstruction.
Static Procedures
Several materials have been used for static suspension. The most common have been fascia lata75 and acellular human dermis (Alloderm).76 In the past, expanded polytetrafluoroethylene (PTFE) (i.e., Gore-Tex) has been used more frequently.77 The acellular dermis preparation and PTFE have the advantage of avoiding donor site morbidity, but use of foreign materials carries a small risk of infection, which is of greater concern in persons undergoing radiation therapy.
Static Facial Sling
Technique
The implant can be placed through a preauricular or temporal incision. In patients with a well-developed contralateral nasolabial fold, a nasolabial incision may be used instead. Additional incisions are made at the vermilion border of the upper and lower lips, adjacent to the commissure. A subcutaneous dissection plane is created to connect the temporal region to the oral commissure. In selected patients with nasal alar collapse and nasal obstruction from soft tissue ptosis, the dissection is extended to include the midface immediately adjacent to the nasal ala. A single strip of implant material is adequate for the procedure. This is cut to appropriate size and can be split near the end to include slips for attachment to the upper and the lower lips. Permanent sutures are placed to secure the implant to the orbicularis oris muscle and deep dermis. Resorbable sutures are placed in the deep dermis to secure the material just medial to the proposed nasolabial fold. Similar fixation is performed for a strip to the ala if desired. The sling is then suspended and secured with permanent suture to the temporalis fascia, or to the periosteum of the zygoma or zygomatic arch. If fascia lata or acellular human dermis (AHD) is used, overcorrection of the smile is achieved before fixation. Current interest has been developed in the suspension of implant materials along the nasolabial fold with suture anchoring to the deep temporal fascia.78 In similar fashion, mimicking of the contralateral crease is achieved through suture tension along various points.
Adjunctive Procedures
Procedures for Upper Third of the Face
Paralysis of the upper third of the face produces significant functional and cosmetic deformities. Brow ptosis may cause superior visual field deficits as well significant facial asymmetry. This asymmetry may be further accentuated after procedures to address the lower face. Browlift techniques to manage paralytic ptosis are the same as for a cosmetic browlift, the only significant technical difference being exercise of restraint to avoid further compromising eyelid closure. Direct, mid-forehead, endoscopic, and indirect browlifts are all effective. When not overdone, a browlift in conjunction with lid loading or lower eyelid tightening, or both, generally produces satisfactory cosmetic and functional results. Brow ptosis associated with normal aging may be accentuated unnaturally by a unilateral lift, and in older patients, improved results are seen when bilateral browlifts are performed.79
Procedures for Middle Third of the Face
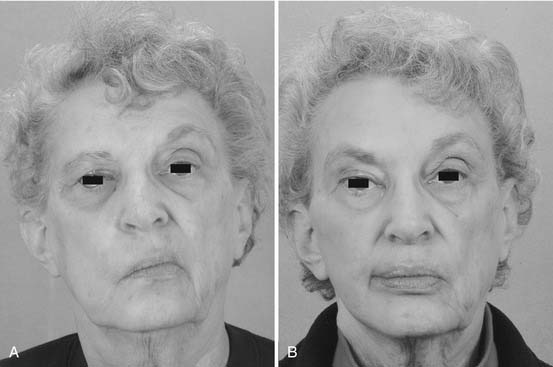
Midface soft tissue laxity and sagging, characteristic of the aging midface, is abnormally pronounced in the paralyzed face. In older patients with significant skin laxity, performing a facelift enhances the results of other treatments for midface deformity. Facelifts can be performed concurrently with other procedures, whether dynamic or static, and some patients will benefit from and prefer a bilateral facelift (Fig. 172-9).
Procedures for Lower Third of the Face
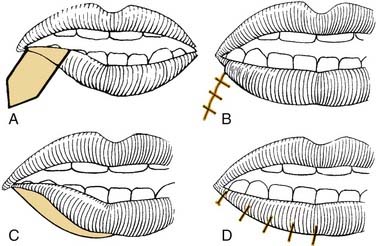
Reinnervation, free tissue transfer, dynamic slings, and to some degree even static slings assist in repositioning the oral commissure to re-create a more symmetric smile. The asymmetry of depressor function, the so-called “marginal mandibular lip,” is more troublesome and difficult to improve. The most commonly used procedures are wedge resection and transposition of the posterior belly of the digastric muscle. The wedge resection, with or without supplemental cheiloplasty technique to improve symmetry and oral competence, has been described by Glenn and Goode.80 A 2- to 2.5-cm full-thickness excision is performed 7 to 10 mm lateral to the commissure (Fig. 172-10).
The most common dynamic technique for depressor dysfunction is the digastric transposition, first described by Conley and colleagues.81 The digastric tendon is identified using a submandibular approach. It is released from the hyoid bone, transected near the mastoid tip, transposed superiorly, and attached to the orbicularis oris through a separate vermilion border incision. Care is taken to preserve the mylohyoid nerve innervating the anterior belly of the digastric muscle. The anterior belly of the digastric muscle may not be available after extirpative cancer surgery, however.82
Management of Synkinesis
Botulinum A Toxin
Botulinum A toxin is the most potent poison known to humankind, yet it has been used effectively for more than 2 decades to treat a variety of hyperfunctional disorders including torticollis,52 blepharospasm,83 spasmodic dysphonia,84 strabismus,85 hyperhidrosis,86 hyperdynamic skin creases,87 palatal myoclonus,88 hemifacial spasm,89 and facial synkinesis.90,91 Botulinum toxin causes paralysis by blocking the presynaptic release of acetylcholine at the neuromuscular junction. This typically results in paralysis of the treated muscle for approximately 3 to 6 months. Systemic weakness can occur with doses greater than 200 units. The lethal dose is approximately 40 units/kg.92
Adverse effects can occur, related to the diffusion of toxin into surrounding muscles. An example is the development of ptosis after periocular injection. This problem is uncommon, occurring in perhaps 5% of cases. It can be treated with apraclonidine 0.5% drops administered to the affected eye three or four times a day until the ptosis resolves.93 Apraclonidine causes contraction of Müller’s muscle to elevate the upper eyelid. Other complications from botulinum toxin treatment of the face include diplopia, further impairment of eyelid closure, lower eyelid ectropion, brow ptosis, and drooling.
In some patients, the effectiveness of botulinum toxin decreases over time. This diminishing effect may be the result of resprouting of motor endplates or the development of neutralizing antibodies and cannot be overcome with increased doses of the toxin.94 Patients who do not benefit from botulinum toxin treatments or demonstrate the development of resistance, or who desire a more permanent solution, may be candidates for selective myectomy.
Selective Myectomy
Neurolysis was for many years the procedure of choice for these patients. Although Fisch95 reported excellent results, others did not have the same success.96 Paralytic ectropion, lagophthalmos, lip paresis, oral incompetence, blunted facial expression, and other complications from neurolysis have been reported.97,98 Myectomy has been offered as a safer, more specific procedure for permanent resolution of synkinesis after facial paralysis.96 Myectomy results in a more predictable result because the removed muscle does not regrow, a phenomenon that can be seen with neurolysis. In patients desiring no further treatment with botulinum toxin, or for whom botulinum toxin was ineffective, a myectomy may be performed. Selective myectomies can be performed on synkinetic facial muscles.
Barrs DM. Facial nerve trauma: optimal timing for repair. Laryngoscope. 1991;101:835.
Bernstein L, Nelson RH. Surgical anatomy of the extraparotid distribution of the facial nerve. Arch Otolaryngol. 1984;110:177.
Borodic GE, Pearce LB, Cheney M, et al. Botulinum A toxin for treatment of aberrant facial nerve regeneration. Plast Reconstr Surg. 1993;91:1042.
Clark JM, Shockley W. Management of reanimation of the paralyzed face. In: Papel I, Larrabee W, Holt G, Park S, Sykes J, editors. Facial Plastic and Reconstructive Surgery. 2nd ed. New York: Thieme Medical Publishers; 2002:660.
Conley J, Baker DC. Myths and misconceptions in the rehabilitation of facial paralysis. Plast Reconstr Surg. 1983;71:538.
Conley J, Baker DC. The surgical treatment of extratemporal facial paralysis: an overview. Head Neck Surg. 1978;1:12.
Fisch U. Extracranial surgery for facial hyperkinesis. In: May M, editor. The Facial Nerve. New York: Thieme Medical Publishers; 1986:535.
Fisch U, editor. Facial Nerve Surgery. Birmingham, Ala: Aesculapius Publishing, 1977.
Fisher E, Frodel JL. Facial suspension with acellular human dermal allograft. Arch Facial Plast Surg. 1999;1:195.
Freeman MS, Thomas JR, Spector JG, et al. Surgical therapy of the eyelids in patients with facial paralysis. Laryngoscope. 1990;100:1086.
House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146.
Jelks GW, Smith B, Bosniak S. The evaluation and management of the eye in facial palsy. Clin Plast Surg. 1979;6:397.
Kazanjian VH, Converse JM. Facial palsy. In: Converse JM, editor. Surgical Treatment of Facial Injuries. Baltimore: Williams & Wilkins, 1974.
Kumar PA, Hassan KM. Cross-face nerve graft with free-muscle transfer for reanimation of the paralyzed face: a comparative study of the single-stage and two-stage procedures. Plast Reconstr Surg. 2002;109:451.
Levine R. Eyelid reanimation surgery. In: May M, editor. The Facial Nerve. New York: Thieme Medical Publishers, 1986.
May M, Sobol SM, Mester SJ. Hypoglossal-facial nerve interpositional-jump graft for facial reanimation without tongue atrophy. Otolaryngol Head Neck Surg. 1991;104:818.
Miehlke A, Stennert E, Chilla R. New aspects in facial nerve surgery. Clin Plast Surg. 1979;6:451.
Patel BCK, Anderson RL, May M. Selective myectomy. In: May M, Schaitkin BM, editors. The Facial Nerve. 2nd ed. New York: Thieme Medical Publishers; 2001:467.
Rubin LR. Reanimation of total unilateral facial paralysis by the contiguous facial muscle technique. In: Rubin LR, editor. The Paralyzed Face. St Louis: Mosby; 1991:156-177.
Rubin LR, Lee GW, Simpson RI. Reanimation of the long-standing partial facial paralysis. Plast Reconstr Surg. 1986;77:41.
Shindo M. Facial reanimation with microneurovascular free flaps. Facial Plast Surg. 2000;16:357.
Smith JD, Crumley RL, Harker LA. Facial paralysis in the newborn. Otolaryngol Head Neck Surg. 1981;89:1021.
Wesley RE, Jackson CG. Facial palsy. In: Hornblass A, editor. Oculoplastic, Orbital and Reconstructive Surgery. Vol I: Eyelids. Baltimore: Williams & Wilkins, 1988.
1. Lydiatt DD. Medical malpractice and facial nerve paralysis. Arch Otolaryngol Head Neck Surg. 2003;129:50.
2. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146.
3. Rubin LR. The anatomy of a smile: its importance in the treatment of facial paralysis. Plast Reconstr Surg. 1974;53:384.
4. Gutman E, Young J. The reinnervation of muscle after various periods of atrophy. J Anat. 1944;78:15.
5. Bernstein L, Nelson RH. Surgical anatomy of the extraparotid distribution of the facial nerve. Arch Otolaryngol. 1984;110:177.
6. Fisch U, editor. Facial Nerve Surgery. Birmingham, Ala: Aesculapius Publishing, 1977.
7. Aitken JT. Growth of nerve implants in voluntary muscle. J Anat. 1950;84:38.
8. Smith JD, Crumley RL, Harker LA. Facial paralysis in the newborn. Otolaryngol Head Neck Surg. 1981;89:1021.
9. Stennert EI. Hypoglossal facial anastomosis: its significance for modern facial surgery: II. Combined approach in extratemporal facial nerve reconstruction. Clin Plast Surg. 1979;6:471.
10. Scaramella LL. L’anastomosi tradue nervi facciali. Arch Otologia. 1971;82:208.
11. Smith JW. A new technique of facial reanimation. In: Transactions of Fifth International Congress of Plastic and Reconstructive Surgery. Melbourne, Australia: Butterworths; 1971:83-84.
12. Ducker TB, Kempe LG, Hayes GJ. The metabolic background for peripheral nerve surgery. J Neurosurg. 1969;30:270.
13. McGuirt WF, McCabe BF. Effect of radiation therapy on facial nerve autografts. Laryngoscope. 1977;87:415.
14. Conley J, Miehlke A. Factors influencing results in extratemporal facial nerve repair. In: Fisch U, editor. Facial Nerve Surgery. Birmingham, Ala: Aesculapius Publishing, 1977.
15. Pillsbury HC, Fisch U. Extratemporal facial nerve grafting and radiotherapy. Arch Otolaryngol. 1979;105:441.
16. Freeman MS, Thomas JR, Spector JG, et al. Surgical therapy of the eyelids in patients with facial paralysis. Laryngoscope. 1990;100:1086.
17. Wesley RE, Jackson CG. Facial palsy. In: Hornblass A, editor. Oculoplastic, Orbital and Reconstructive Surgery. Vol I. Eyelids. Baltimore: Williams & Wilkins, 1988.
18. Anderson RL, Gordy DD. The tarsal strip procedure. Arch Ophthalmol. 1979;97:2192.
19. Jelks GW, Smith B, Bosniak S. The evaluation and management of the eye in facial palsy. Clin Plast Surg. 1979;6:397.
20. Kazanjian VH, Converse JM. Facial palsy. In: Converse JM, editor. Surgical Treatment of Facial Injuries. Baltimore: Williams & Wilkins, 1974.
21. May M, Hoffmann DF, Buerger GFJr, et al. Management of the paralyzed lower eyelid by implanting auricular cartilage. Arch Otolaryngol Head Neck Surg. 1990;116:786.
22. Morel-Fatio D, Lalardrie JP. Palliative surgical treatment of facial paralysis: the palpebral spring. Plast Reconstr Surg. 1964;33:446.
23. Arion HG. Dynamic closure of the lids in paralysis of the orbicularis muscle. Int Surg. 1972;57:48.
24. Levine R. Eyelid reanimation surgery. In: May M, editor. The Facial Nerve. New York: Thieme Medical Publishers, 1986.
25. Mavrikakis I, Beckingsale P, Lee E, et al. Changes in corneal topography with upper eyelid gold weight implants. Ophthal Plast Reconstr Surg. 2006;22:331.
26. Barrs DM. Facial nerve trauma: optimal timing for repair. Laryngoscope. 1991;101:835.
27. Cheney ML. Medial antebrachial cutaneous nerve graft. In: Urken ML, editor. Atlas of Regional and Free Flaps for Head and Neck Reconstruction. New York: Raven Press, 1995.
28. Miehlke A. Probleme beider naht der feinstenperipheren neruen im bereich der otolaryngologie. Melsunger Med Mitteilungen. 1968;42:71.
29. Siedentop KH, Loewy A. Facial nerve repair with tissue adhesive. Arch Otolaryngol. 1979;105:423.
30. Conley J, Baker DC. The surgical treatment of extratemporal facial paralysis: an overview. Head Neck Surg. 1978;1:12.
31. Conley J, Baker DC. Hypoglossal-facial nerve anastomosis for reinnervation of the paralyzed face. Plast Reconstr Surg. 1979;63:63.
32. Millesi H. Facial nerve suture. In: Fisch U, editor. Facial Nerve Surgery. Birmingham, Ala: Aesculapius Publishing, 1977.
33. Crumley RL. Spatial anatomy of facial nerve fibers: a preliminary report. Laryngoscope. 1980;90:274.
34. May M. Anatomy of the facial nerve (spatial orientation of fibers in the temporal bone). Laryngoscope. 1973;83:1311.
35. Miehlke A. A topography of the course of fibers in the fascialis stem. Arch Klin Exp Ohren Nasen Kehlkopfkunde (Berlin). 171, 1958.
36. Sunderland S. Mass movements after facial nerve injury. In: Fisch U, editor. Facial Nerve Surgery. Birmingham, Ala: Aesculapius Publishing; 1977:285-289.
37. Tomander L, Aldshogius H, Grant G. Motor fiber organization of the facial nerve in the rat. In: House W, editor. Disorders of the Facial Nerve. New York: Raven Press; 1982:75-82.
38. Anderl H. Cross-face nerve transplant. Clin Plast Surg. 1973;6:433.
39. Samii M. Nerves of the head and neck: management of peripheral nerve problems. In: Omer G, Spinner M, editors. Management of Peripheral Nerve Problems. Philadelphia: WB Saunders, 1970.
40. Ferreira MC. Cross-facial nerve grafting. Clin Plast Surg. 1984;11:211.
41. Conley J. Facial rehabilitation: new potentials. In: Baker CD, editor. Clinical Plastic Surgery. Philadelphia: WB Saunders, 1979.
42. Conley J, Baker DC. Myths and misconceptions in the rehabilitation of facial paralysis. Plast Reconstr Surg. 1983;71:538.
43. May M, Sobol SM, Mester SJ. Hypoglossal-facial nerve interpositional-jump graft for facial reanimation without tongue atrophy. Otolaryngol Head Neck Surg. 1991;104:818.
44. Atlas MD, Lowinger DS. A new technique for hypoglossal-facial nerve repair. Laryngoscope. 1997;107:984.
45. Battal MN, Hata Y. A review on the history of end-to-side neurorrhaphy. Plast Reconstr Surg. 1997;99:2110.
46. Koh KS, Kim JK, Kim CJ, et al. Hypoglossal-facial crossover in facial-nerve palsy: pure end-to-side anastomosis technique. Br J Plast Surg. 2002;55:25.
47. Noah EM, Williams A, Fortes W, et al. A new animal model to investigate axonal sprouting after end-to-side neurorrhaphy. J Reconstr Microsurg. 1997;13:317.
48. Noah EM, Williams A, Jorgenson C, et al. End-to-side neurorrhaphy: a histologic and morphometric study of axonal sprouting into an end-to-side nerve graft. J Reconstr Microsurg. 1997;13:99.
49. Zhao JZ, Chen ZW, Chen TY. Nerve regeneration after terminolateral neurorrhaphy: experimental study in rats. J Reconstr Microsurg. 1997;13:31.
50. Baker DC, Conley J. Regional muscle transposition for rehabilitation of the paralyzed face. Clin Plast Surg. 1979;6:317.
51. Dressler D, Schonle PW. Botulinum toxin to suppress hyperkinesias after hypoglossal-facial nerve anastomosis. Eur Arch Otorhinolaryngol. 1990;247:391.
52. Borodic GE, Mills L, Joseph M. Botulinum A toxin for the treatment of adult-onset spasmodic torticollis. Plast Reconstr Surg. 1991;87:285.
53. Miehlke A, Stennert E, Chilla R. New aspects in facial nerve surgery. Clin Plast Surg. 1979;6:451-486.
54. Hardy R, Perret G, Myers RD. Phrenicofacial anastomosis for facial paralysis. J Neurosurg. 1957;14:400.
55. Tucker HM. The management of facial paralysis due to extracranial injuries. Laryngoscope. 1978;88:348.
56. Anonsen CK, Patterson HC, Trachy RE, et al. Reinnervation of skeletal muscle with a neuromuscular pedicle. Otolaryngol Head Neck Surg. 1985;93:48.
57. Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27(Jun):277.
58. Deshpande DM, Kim YS, Martinez T, et al. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006;60:32.
59. Lexer E, Eden R. Uber die chirurgische Behandlung der peripheren Facialislahmung. Beitr Klin. 1911;73:116.
60. Gillies H. Facial paralysis. Proc R Soc Med. 1934;27:1372.
61. Rubin LR. Reanimation of the Paralyzed Face: New Approaches. St Louis: Mosby; 1977.
62. Rubin LR. Reanimation of total unilateral facial paralysis by the contiguous facial muscle technique. In: Rubin LR, editor. The Paralyzed Face. St Louis: Mosby; 1991:156-177.
63. Sherris DA. Refinement in reanimation of the lower face. Arch Facial Plast Surg. 2004;6:49.
64. Labbé D, Huault M. Lengthening temporalis myoplasty and lip reanimation. Plast Reconstr Surg. 2000;105:1289.
65. Harii K, Ohmori K, Torii S. Free gracilis muscle transplantation, with microneurovascular anastomoses for the treatment of facial paralysis: a preliminary report. Plast Reconstr Surg. 1976;57:133.
66. Shindo M. Facial reanimation with microneurovascular free flaps. Facial Plast Surg. 2000;16:357.
67. Harii K, Harii K, Asato H, et al. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102:941.
68. Jiang H, Guo ET, Ji ZL, et al. One-stage microneurovascular free abductor hallucis muscle transplantation for reanimation of facial paralysis. Plast Reconstr Surg. 1995;96:78.
69. Jones BM. Cross-face reanimation of the paralysed face, with single stage microneurovascular gracilis transfer without nerve graft. Br J Plast Surg. 1995;48:519.
70. Kumar PA, Hassan KM. Cross-face nerve graft with free-muscle transfer for reanimation of the paralyzed face: a comparative study of the single-stage and two-stage procedures. Plast Reconstr Surg. 2002;109:451.
71. Nicolaidis SC, Williams HB. Muscle preservation using an implantable electrical system after nerve injury and repair. Microsurgery. 2001;21:241.
72. Ueda K, Harii K, Yamada A. Free neurovascular muscle transplantation for the treatment of facial paralysis using the hypoglossal nerve as a recipient motor source. Plast Reconstr Surg. 1994;94:808.
73. Devauchelle B, Badet L, Lengelé B, et al. First human face allograft: early report. Lancet. 2006;368:203.
74. Dubernard JM, Lengelé B, Morelon E, et al. Outcomes 18 months after the first human partial face transplantation. N Engl J Med. 2007;357:2451.
75. Brown JB. Support of the paralyzed face by fascia. JAMA. 1947;135:18.
76. Fisher E, Frodel JL. Facial suspension with acellular human dermal allograft. Arch Facial Plast Surg. 1999;1:195.
77. Okamura H, Yanagihara N. Multiple facial suspensions in protracted facial palsy. Auris Nasus Larynx. 1987;14:105.
78. Alam D. Rehabilitation of long-standing facial nerve paralysis with percutaneous suture-based slings. Arch Facial Plast Surg. 2007;9:205.
79. Clark JM, Shockley W. Management of reanimation of the paralyzed face. In: Papel I, Larrabee W, Holt G, Park S, Sykes J, editors. Facial Plastic and Reconstructive Surgery. 2nd ed. New York: Thieme Medical Publishers; 2002:660.
80. Glenn MG, Goode RL. Surgical treatment of the “marginal mandibular lip” deformity. Otolaryngol Head Neck Surg. 1987;97:462.
81. Conley J, Baker DC, Selfe RW. Paralysis of the mandibular branch of the facial nerve. Plast Reconstr Surg. 1982;70:569.
82. Tan ST. Anterior belly of digastric muscle transfer: a useful technique in head and neck surgery. Head Neck. 2002;24:947.
83. Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985;103:347.
84. Ford CN, Bless DM, Lowery JD. Indirect laryngoscopic approach for injection of botulinum toxin in spasmodic dysphonia. Otolaryngol Head Neck Surg. 1990;103:752.
85. Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044.
86. Lowe NJ, Yamauchi PS, Lask GP, et al. Efficacy and safety of botulinum toxin type a in the treatment of palmar hyperhidrosis: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2002;28:822.
87. Blitzer A, Brin MF, Keen MS, et al. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg. 1993;119:1018.
88. Varney SM, Demetroulakos JL, Fletcher MH, et al. Palatal myoclonus: treatment with Clostridium botulinum toxin injection. Otolaryngol Head Neck Surg. 1996;114:317.
89. Yoshimura DM, Aminoff MJ, Tami TA, et al. Treatment of hemifacial spasm with botulinum toxin. Muscle Nerve. 1992;15:1045.
90. Biglan AW, May M, Bowers RA. Management of facial spasm with Clostridium botulinum toxin, type A (Oculinum). Arch Otolaryngol Head Neck Surg. 1988;114:1407.
91. Borodic GE, Pearce LB, Cheney M, et al. Botulinum A toxin for treatment of aberrant facial nerve regeneration. Plast Reconstr Surg. 1993;91:1042.
92. Biglan AW, Patel BC, May M, Murdock TJ. Botulinum A toxin. In: May M, Schaitkin BM, editors. The Facial Nerve. 2nd ed. New York: Thieme Medical Publishers; 2001:441.
93. Carucci JA, Zweibel SM. Botulinum A exotoxin for rejuvenation of the upper third of the face. Facial Plast Surg. 2001;17:11.
94. Dressler D, Münchau A, Bhatia KP, et al. Antibody-induced botulinum toxin therapy failure: can it be overcome by increased botulinum toxin doses? Eur Neurol. 2002;47:118.
95. Fisch U. Extracranial surgery for facial hyperkinesis. In: May M, editor. The Facial Nerve, Vol 186. New York: Thieme Medical Publishers; 2000:535.
96. Patel BCK, Anderson RL, May M. Selective myectomy. In: May M, Schaitkin BM, editors. The Facial Nerve. 2nd ed. New York: Thieme Medical Publishers; 2001:467.
97. Bates AK, Halliday BL, Bailey CS, et al. Surgical management of essential blepharospasm. Br J Ophthalmol. 1991;75:487.
98. Frueh BR, Callahan A, Dortzbach RK, et al. The effects of differential section of the VIIth nerve on patients with intractable blepharospasm. Trans Am Acad Ophthalmol Otolaryngol. 1976;81:OP595.