Chapter 12 Rehabilitation and Prosthetic Restoration in Upper Limb Amputation
Limb loss and limb deficiency occur in significant numbers worldwide. Amputations are performed to remove limbs that are no longer functional because of injury or disease. The more common causes are related to diabetes, peripheral vascular disease, trauma, and malignancy. Genetic variation and mutation are the typical causes for congenital deficiencies. Upper limb loss is more commonly due to trauma than lower limb loss. Before 1900, war-related injury was the major reason for limb loss in the United States. Surgical amputation has evolved significantly since the days of severing a limb from an unanesthetized patient and dipping the residual limb in boiling oil to achieve hemostasis. As America became industrialized, there was a rise in civilian trauma causing upper limb loss as a result of crush injury, laceration, and avulsion. We owe a great deal to our wounded military and manual laborers, then and now, for pushing us to develop the technologies and options for those with limb loss today. The evolution of the upper limb prosthesis is founded on the principles of Salisbury and Newton.33 Prosthetic scientists stood on the shoulders of these giants while building functional tools that assist with performing daily tasks. As technologies advance, we are even more dependent on the training and the technical skill of the upper limb prosthetist. Even though we have entered the bionic age, the cable-and-hook systems remain the staple of upper limb prostheses because of their relative versatility and simplicity.
Demographics, Incidence, and Prevalence
In the United States an estimated 185,000 persons undergo an amputation of the upper or lower limb each year.31 In 2008, it was estimated that 1.9 million persons were living with limb loss in the United States (Johns Hopkins Bloomberg School of Public Health, unpublished data). Of this estimate, 500,000 persons were living with minor (fingers, hands) upper limb loss, and 41,000 persons were living with major upper limb amputations.8 Because of the aging of the population and higher rates of dysvascular disease related to diabetes and obesity, it is projected that the number of people living with lower limb loss in the United States will double by the year 2050.8
Trauma accounts for 90% of all upper limb amputations. During the next 50 years, the incidence of amputations secondary to trauma is estimated to remain flat if not decrease.32 The incidence of upper limb trauma is hypothesized to decrease because of more successful occupational safety standards.17 The future is also likely to bring even more aggressive and successful limb reconstruction and reimplantation. Other causes of upper limb loss include burns, peripheral vascular disease, neurologic disorders, infections, malignancies, contracture, and congenital deformities.3
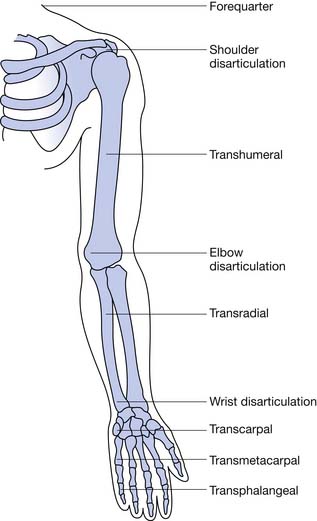
Finger amputation represents the highest percentage (78%) of upper limb amputations reported on hospital discharges.16 Most amputations involve single digits, with the index, ring, and long fingers accounting for 75% and the thumb 16%.3 Excluding finger amputation, the most common upper limb amputations are through the forearm (transradial) and humerus (transhumeral), respectively (Table 12-1, Figure 12-1).42 Most civilian limb injuries that result in amputation occur at work and involve saws or blades (e.g., lawnmowers and snowblowers). Blast-related injuries are rare in the civilian population (8.5%). In the active military, however, amputee injuries are from mortars, gunfire, improvised explosive devices, and rocket-propelled grenades. Because of the extreme forces involved, concomitant injuries such as traumatic brain injury, visual and hearing impairment, soft tissue loss, and burns are common. A fifth of all combat-related major amputations involve the upper limb.14,52 Two thirds of amputations resulting from trauma occur among adolescents and adult younger than 45 years.8 Males account for greater than 75% of those with upper limb loss, and the more severe the injury, the more likely the victim is male.3
| Procedure | Percentage of Total Upper Limb Amputation Procedures Performed |
|---|---|
| Amputation through the hand | 15 |
| Disarticulation through the wrist | 10 |
| Amputation through the forearm (transradial) | 31 |
| Disarticulation of the elbow | 7 |
| Amputation through the humerus (transhumeral) | 28 |
| Shoulder disarticulation | 7 |
| Forequarter amputation | 2 |
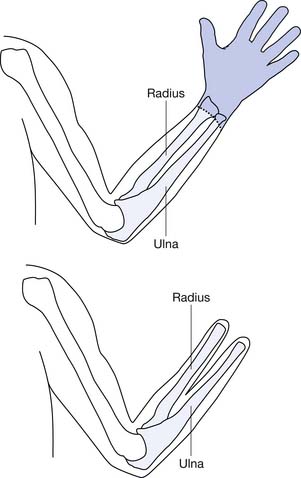
An estimated 4.1 per 10,000 babies are born each year with all or part of a limb missing, ranging from a missing part of a finger to the absence of both arms and both legs. Congenital deficiencies in the upper limb are more common (58%), and they occur slightly more often in boys. The most common congenital amputation is at the left short transradial level. Most cases of congenital upper limb deficiency have no hereditary implications. Congenital limb deficiencies occur because of the failure of part or all of a limb bud to form. The first trimester is the critical time for limb formation, with the bud occurring at 26 days’ gestation and differentiation through the eighth week of gestation. The etiology often is unclear, but teratogenic agents (e.g., medications and radiation exposure) and amniotic band syndrome are two common causes. Maternal ultrasound examination often identifies the limb deficiency before delivery. There have been many descriptions of congenital limb deficiencies (Box 12-1), with the development of the current and preferred system by the International Society for Prosthetic and Orthotics (ISPO; Box 12-2). The ISPO terminology divides the limb amputations into transverse or longitudinal. By definition, a child who has a transverse deficiency has no distal remaining parts. For example, a child with a transverse radial deficiency has a normal upper arm and a portion of the radius, but is missing the hand and fingers. Longitudinal deficiencies have distal portions present with a partial or total absence of a specific bone. The most common congenital limb deficiency in the upper limb is a longitudinal partial or complete lack of the radius. Longitudinal hand reductions represent half of all congenital upper limb reductions, and multiple limb reductions are found in less than 20% of live births.19,46
BOX 12-2 ISPO Classification System for Congenital Upper Limb Reductions
Nomenclature and Functional Levels of Amputations
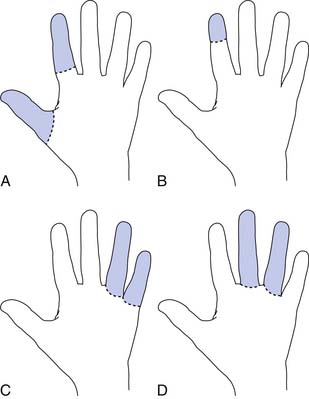
Radial amputations (Figure 12-2, A) involve the thumb and index finger and compromise grasp. Fingertip amputation (Figure 12-2, B) is the most common type of amputation. The thumb is the most functionally critical digit. Thumb amputation, partial or complete, results in loss of palmer grip, side-to-side pinch, and tip-to-tip pinch. Amputation of one of the other digits causes lesser functional loss. Transverse digit amputations occur at one or more digits and can be fit with functional finger prostheses. Ulnar amputations (Figure 12-2, C) involve digits IV and V, and hook grasp is lost. The loss of digit V is functionally under estimated because of this powerful grasp. Central amputation (Figure 12-2, D) involves digits III and IV, and reconstruction is usually not attempted, and a cosmetic substitute is used. The residual limb refers to the remaining part of the amputated limb. The sound limb refers to the nonamputated limb. Wrist disarticulations are rare, but are preferred over more proximal amputations because maximal pronation and supination are preserved.12
Proximal to the hand, amputations are divided into the following categories: transradial, transhumeral, shoulder disarticulation, and forequarter amputation. Depending on the percentage of the limb remaining compared with the sound side, further categorizations can be made such as “short” and “long” to define the residual limb. These categorizations have functional implications. For the transradial residual limb, the longer the length, the more pronation (normal, 120 degrees) and supination (normal, 180 degrees) is preserved. Of the pronation and supination preserved, 50% can be transmitted to the prosthesis.12
Transradial amputations are based on measurements made from the longest residual bone (ulna or radius) to the medial epicondyle. This is then compared with the measurement of the sound side from the ulnar styloid to the medial epicondyle. The remaining length impacts the ability to pronate and supinate the forearm with the prosthetic device. A long transradial amputation preserves 55% to 90% length, allows up to 60 degrees of supination and pronation with a prosthesis, and maintains strong elbow flexion.47 A medium transradial amputation preserves 35% to 55% length, and pronation and supination with a prosthesis are lost. Elbow flexion is reduced because of the inhibiting prosthesis. A short transradial amputation is defined as 0% to 35% preservation, which results in difficult prosthetic suspension and the additional loss of full range of motion (ROM) at the elbow.
The elbow disarticulation creates functional and prosthetic fit difficulties related to suspension and elbow joint placement. This level of amputation preserves humeral rotation to the prosthesis and can be accommodated by modern socket fabrication techniques and cosmesis. It is most suitable for the growing child to preserve the epiphysis for growth.38 The elbow disarticulation is recommended instead of bilateral transhumeral because of functional prosthetic control.
The transhumeral amputation can also be classified into three levels. The more humeral length preserved, the more optimal the prosthetic restoration. The long transhumeral is defined as preservation of 50% to 90% of length relative to the sound side. Glenohumeral motions are preserved and uninhibited by the prosthetic socket. The short transhumeral is defined as preservation of 30% to 50% of length, which results in loss of glenohumeral motion because of the inhibition of the prosthetic socket that encompasses the acromion.47 The glenohumeral motions of flexion, extension, and abduction are lost with humeral neck level amputation, shoulder disarticulation, and forequarter amputation. They are usually amputations related to malignancy and severe trauma in which no distal level amputation was possible. These levels of amputation present challenges to achieving adequate suspension and functional use of the prosthesis. Newer myoelectric techniques are gaining ground in achieving the multijoint control that is needed in optimal prosthetic restoration for these very proximal upper limb amputations.
Principles of Limb Salvage and Amputation Surgery
Limb Salvage
Injury scores were developed for severe trauma-related limb injuries, to help determine which vascular injury patients would benefit from primary amputation versus an attempt at limb salvage. Their validity has been questioned. The mangled extremity syndrome is defined as significant injury to at least three of the four tissue groups (skin/soft tissue, nerve, vessel and bone).24 The mangled extremity scoring systems have been shown to be poor predictors of amputation or salvage with regard to functional outcome.18,39,48 Ly et al.37 concluded that the available injury severity scores are not predictive of functional recovery of patients who undergo reconstruction surgery. Bosse et al.,6 using the Sickness Impact Profile, presented evidence that the functional outcomes from limb salvage and reconstruction after severe trauma were the same at 2 years for those who underwent amputation. Finally, in this salvage-versus-amputation equation, no significant long-term psychological outcome advantage has been reported for limb salvage surgery compared with amputation.45 Consequently, objective measures have not functionally supported the natural desires of the patient and the tendency of the trauma team to make all attempts at salvaging the limb.
In severe limb trauma that includes defects from burns and tumor resection, the appropriate soft tissue restoration is an essential component of the overall treatment. This is common both to limb salvage and amputation, especially when critical lengths are being preserved. It requires a vascularized flap that can protect the neurovascular and musculotendonous structures (Box 12-3). The pedicle flap is one where a local muscle inclusive of the overlying skin is moved over with it own blood supply to fill a large defect. A microvascular free flap is one in which the donor tissue is taken from a different site and the microvasculature of the donor tissue is anastomosed to the available vessels in the site of the defect. The feasibility of limb salvage is determined partly by the ability to reconstruct the soft tissue defect. In the upper limb, few pedicle flap options are available to repair significant defects. The recent advancement of microvascular reconstruction techniques and free flaps from sites like the rectus abdominus have promoted the option of limb salvage and preserved limb length.
BOX 12-3 Skin Flaps
For those with malignant tumors, 70% to 85% are treated by limb salvage without compromising the oncologic result.50 The goal of this type of surgery is to preserve function, prevent tumor recurrence, and enable the rapid administration of chemotherapy or radiation therapy. In the tumors of the hand, ray resection is done. In the wrist, multiple options are available such as an endoprosthesis implant or an allograft or vascularized bone transplant (e.g., fibula). For the elbow an endoprosthetic reconstruction is the best possible option. The humerus is similar to the wrist in that an endoprosthesis, or an allograft, or a vascularized bone transplant can be used. For tumors of the scapula or proximal humerus, a forequarter amputation or flail arm is prevented by reconstruction with a combination of an endoprosthesis and allograft. These types of reconstruction would not be possible without major improvements in radiography, chemotherapy, radiotherapy, and staging.50
The complication rate is much higher after limb salvage than after amputation in the oncology population. These complications can be divided into early and late. The earliest complications include infection, wound necrosis, and neurapraxia. The late complications include aseptic loosening, prosthetic fracture and dislocation, and graft nonunion.4 Consequently additional surgery is often necessary. Advancements in resection techniques, radiation, and chemotherapy have improved both functional limb survival and life expectancy. Serletti et al.,45 using the Enneking Outcome Measurement Scale, reported the functional outcome as “excellent” or “good” in greater than 70% of the patients who had reconstruction after resection of limb sarcomas. The Enneking Outcome Measurement Scale is an outcome tool that assesses seven characteristics of upper limb use: ROM, stability, deformity, pain level, strength, functional activity, and emotional acceptance. Limb salvage has cosmetic advantages, but whether the quality of life of these patients is superior to that of those who undergo amputation is unclear.
Reimplantation of traumatically amputated limbs is now possible, especially in children, because of the potential for successful neurologic recovery.30 Effective treatment of the patient and the ischemic, detached body part requires appropriate early cooling and prompt reimplantation within the initial 12-hour window. Predictors of successful reimplantation include adequate preservation, contraction of the muscle in the amputated limb after stimulation, the level of injury, and no tobacco use. The best predictor of success is the serum potassium level in the amputated segment. If the serum potassium level is higher than 6.5 mmol/L, reimplantation should be avoided.51
Reimplantation is indicated in levels from the distal forearm to the fingers. The more proximal to the wrist, the greater the amount of ischemic muscle mass and the more complex the metabolic and surgical demands. Approximately 85% of replanted parts remain viable. Sensory recovery with two-point discrimination occurs in 50% of adults.35 The functional results are more promising in children, but the viability rate is lower because of the technically demanding microvascular surgery. Major limb reimplantation entails significant metabolic disturbance and risk. It requires scrupulous medical management. Reimplantation is contraindicated in those with crushed and mangled limbs and those with atherosclerosis. Because nerves transected in the proximal arm must regenerate over a considerable length, only limited motor return is typically seen in the forearm and hand. Useful function of the wrist and hand is unusual and limited at best. Function can often be improved by converting these patients to transradial prosthetic wearers. Unfortunately it means performing a transradial amputation after successful transhumeral reimplantation. This is known as segmental reimplantation, in which portions of compromised limbs are salvaged that would otherwise have been discarded.
During the past 10 years, collaboration between hand surgeons and immunologists has led to successes in hand transplantation. Recent advances learned from clinical organ transplant immunosuppression known as composite tissue allograft (CTA) have permitted hand transplantation to progress beyond the first one done in the United States in 1997. CTA is the term used to describe transplantation of multiple tissues (skin, muscle, bone, cartilage, nerve, tendon, blood vessels) as a functional unit. In addition to the usual problems of identifying an organ donor, selecting a donor for a hand transplant must involve additional and careful emphasis on matching skin color, skin tone, gender, ethnicity and race, and the size of the hand. After a hand has been lost, much time can pass before a donor is found. Representation of the hand in the individual’s brain is lost because of cortical reorganization during this time. Researchers have learned through functional magnetic resonance imaging that after transplantation, amputation-induced cortical reorganization is reversed to reestablish the hand “image.”5 During transplantation the surgeon repairs the tissues in the following order: bone fixation, tendon repair, artery repair, nerve repair, and vein repair. The surgery can last from 12 to 16 hours, almost double that of heart and liver transplants. Immunosuppression after CTA is composed of two elements: treating the patient with monoclonal antibodies on the day of transplant, followed by a donor bone marrow infusion several days later. Typical postoperative complications include vessel thrombosis, infections, and rejection. Rejection can appear as a spotty, patchy, or blotchy rash. It could appear anywhere on the transplant and is usually painless. As rejection appears first in the skin, the clinical team and patients are encouraged to carefully watch for the signs. Unlike internal organ transplants, where rejection is difficult to detect early, it is relatively easy to monitor in the hand, allowing for early biopsy and treatment. Recovery is relatively slow, requiring an extensive program of occupational therapy. As of this printing the longest reported patient follow-up has been 43 months. This patient, a paramedic instructor, is now reportedly able to start intravenous lines and perform endotracheal intubation.
Amputation
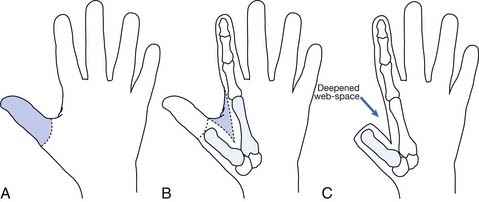
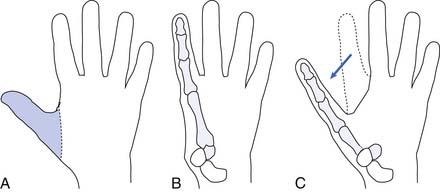
Hand function is vital in our competitive and industrialized society. There are many techniques for reconstruction of the hand. It is much better to have a painless hand with some grasp function and sensation preserved than to have a prosthesis. The most important part of the hand is the opposable thumb. The goal is to preserve as much of the sensate thumb as possible. Phalangization (Figure 12-3) of the metacarpals is a reconstructive technique in which the web space is deepened between the digits to provide more mobile digits. This works well for the thumb especially if the first metacarpal is adjusted to create opposition to the thumb. Pollicization (Figure 12-4) is the process of moving a finger with its nerve and blood supply to the site of the amputated thumb. This allows fine and gross grasp through opposition. A prosthesis for a hand amputation is inferior to the functional outcomes achieved with reconstructed hands.9 In reconstructing the hand, three issues should be considered: (1) preservation of sensitivity to the grasping surface; (2) the consequences of scarring; and (3) cosmetic acceptability.
Wrist disarticulation involves removal of the radius and ulna to the styloid processes, because there is no benefit to retaining the carpal bones. It retains the distal radial-ulnar joint, preserving more forearm rotation. The prosthetic attachment to the bulbous end is enhanced if the distal radial flare is retained for suspension. Burkhalter et al.10 indicate that it is important that the radial and ulnar styloids be resected slightly to minimize the discomfort the amputee will experience in active supination and pronation within the prosthetic socket. Tenodesis of the major forearm muscles stabilizes these groups and improves functional outcome, including myoelectric performance. Pronation and supination, as well as full elbow motion, are preserved with wrist disarticulation. Some will argue that (1) the wrist disarticulation creates a complicated prosthetic situation with difficult socket fabrication; (2) conventional wrist units are too long and cannot be used; and (3) it is harder to fit with a myoelectric prosthesis because there is no room to conceal the electronics and power supply.
Transradial amputation involves the myodesis of the forearm muscles and equal volar and dorsal skin flaps for closure. It is extremely functional, with forearm rotation and strength that is proportional to the length retained. The shorter the transradial amputation, the more the elbow and humerus are needed for suspension. Preserving the elbow joint is paramount because of the functional outcome possible with prosthetic enhancement. If the amputation must be very proximal, then an ulna 1.5 to 2 inches long is still adequate to preserve the elbow joint. To fit this very short residual limb with a prosthesis, it might be helpful to detach the biceps and reattach it to the ulna.36
A couple of special situations arise with transradial amputations. One is when the forearm bone is considerably longer than the other and the longer bone can be covered with an adequate soft tissue envelope. Rather than decrease prosthetic function by shortening the longer bone, it may be preferable to create a one-bone forearm. Another is the Krukenberg amputation (Figure 12-5), which transforms the residual ulna and radius into digits that have significant forceful prehension and retained ability to manipulate because of preserved sensation. This is an option for patients who have at least 4 inches of residual limb, those with bilateral amputation, and those with limited prosthetic facilities. It can be fitted with conventional as well as myoelectric prostheses.
Elbow disarticulation allows the transfer of humeral rotation to the prosthesis through the myodesis of the biceps and triceps, and it preserves a stronger lever. Although the skin flaps are approximately equal, the posterior muscle flap remains longer than the anterior muscle flap, to wrap around and cushion the end of the humerus. The ultimate position of the scar is not critical with modern total-contact sockets, but beware of the vulnerable skin over the medial epicondyle. The full humeral length precludes the use of a myoelectric elbow. Elbow disarticulation causes some prosthetic fitting challenges because the outside “elbow” hinge creates a bulky limb that is longer and asymmetric compared with the opposite limb. Disarticulation is the level of choice for juvenile amputees. The high incidence of residual limb revision because of bony overgrowth is avoided, and humeral growth is preserved. It remains controversial who is a good candidate for elbow disarticulation, but modern prosthetic fabrication techniques can overcome the socket and cosmetic difficulties.12,14
Transhumeral amputations are performed at or proximal to the supracondylar level. The humerus is sectioned at least 3 cm from the joint to allow for fit of the prosthetic elbow mechanism. Transhumeral amputations should be performed with minimal periosteal stripping to prevent the occurrence of bony spurs. Rough edges should be removed, but beveling of the bone is unnecessary. All possible length should be preserved to transmit glenohumeral motions through the prosthesis. To help preserve humeral length, at times it should be considered whether free-flap coverage and skin graft coverage are possible alternatives to allow primary closure. The anterior and posterior fascias over the flexor and extensor muscle groups are sutured together to cover the end of the humerus. Biceps and triceps myoplasty preserves strength for prosthetic control and myoelectric signals. Myodesis is rarely needed.38 Performing a more proximal amputation at the level of the surgical neck, which is the site of insertion of the pectoralis major, results in the same function as if a shoulder disarticulation had been done. This is because independent motion of the humerus is no longer possible. However, because the terminal device is controlled by active shoulder girdle motion, the humeral head should be preserved when amputation has to be done proximally.
Acute Management: Preamputation Through Early Rehabilitation
Acute Postamputation
This phase begins with an understanding that the decision to amputate is emotionally powerful for the patient, family, and clinical team. Amputation is not a failure but rather reconstructive surgery that creates improved functional possibilities and resumption of one’s life. The focus of the immediate postamputation period is to control pain and edema, promote wound healing, prevent contractures, initiate remobilization, and continue the supportive counseling and education (Box 12-4). This must be individualized to meet the needs of each patient. Surgical site infection needs to be seriously considered when pain, drainage, and edema are increasing despite the reasonable control measures instituted. The earlier an infection is eradicated, the earlier the time-sensitive prosthetic phase can begin. The goal for rehabilitation is for patients to acquire the skills and equipment needed to achieve prosthetic acceptance and holistic reintegration back into their own lives. It is imperative that the prosthesis be introduced at the earliest possible time after amputation.
Pain control requires an early, aggressive approach that considers the multiple potential pain generators in the postsurgical period. The patient-controlled analgesia systems are often the first-line treatment by the surgical team. This is transitioned to regularly scheduled long- and short-acting oral narcotic medications. It is imperative to maintain consistent pain control. Loss of adequate pain control is painful for the patient and disrupts the timely pursuit of the rehabilitation program. The escalation of the doses of opiates needs to be avoided, if possible, by addressing other pain generators. Understanding the characteristics of postsurgical residual limb pain and phantom pain allows the clinical team to choose pain interventions wisely. Residual limb pain is located in the remaining limb and generated from the soft tissue and musculoskeletal components. Phantom limb pain is pain in the absent limb and is considered neuropathic.17 The nonsteroidal antiinflammatory drugs and nonopiate pain relievers are helpful, and these can diminish the need for higher doses of opiates. Opiates administered at safe doses are often ineffective against phantom pain. Careful attention should be given to the description, timing, and quality of the pain complaint to tease out the central neuropathic pain component inclusive of painful phantom sensations versus peripheral nerve–generated pain. Peripheral nerve pain is more intense at night, and it is characterized as burning, stabbing, and buzzing. Phantom sensations occur in greater than 70% of amputees and do not have to be treated unless painful and disruptive. The use of medications known for controlling neuropathic pain and sensations, such as some anticonvulsants and antidepressants, can also diminish the need for opiates.
The new amputee should be taught how to change the dressings and self-administer the desensitization techniques. Desensitization techniques help to eliminate the hypersensitivity to touch. They include compression, tapping, massage, and application of different textures. These techniques are performed for 20 to 30 minutes three times per day as tolerated by the skin and scar.22 The use of modalities such as transcutaneous nerve stimulation, heat, and cold are also useful adjuvants for pain and diminish opiate need. Ramachandran and Rogers-Ramachandran43 have reduced phantom pain using mirrors to visually trick the brain. Because the loss of a limb is emotionally “painful,” the team should address and acknowledge this. It should be kept in mind that from the individual’s psychological standpoint, it might be more socially acceptable to express the psychological pain in terms of generalized pain complaints. It is important to address the psychological pain early, through grief counseling, peer visitation, and education.
Edema control begins once the last suture or staple is placed by the surgeon. If there is no contraindication and the surgeon has the appropriate training, an immediate postoperative rigid dressing (IPORD) can be placed in the operating room. This is a special cast placed on the residual limb by the surgeon or certified professional. The control of edema leads to earlier wound healing and improved pain control through the reduction of pain mediators in the accumulated “third-spaced fluid.” Typically additional shrinkage of the residual limb occurs after the initial IPORD placement, necessitating its early replacement. The rigid dressing can be removed in 5 to 7 days and replaced with a fresh cast. The attachment of joints and a terminal device to this rigid dressing creates an immediate postoperative prosthesis that can allow early functional use of the residual limb. The IPORD is the preferred treatment approach for the transradial amputation, and if healing progresses without issue, the second cast can be replaced with the first prosthesis.19
The formation of heterotopic bone impairing joint function and ROM should be considered in these complex trauma cases and can be diagnosed with the help of laboratory testing and triple-phase bone scan. The treatment of heterotopic ossification beyond trying to maintain ROM is limited, and surgical intervention is not feasible until the heterotopic bone matures at approximately 12 to 18 months after injury.23 Proper limb positioning and frequent monitoring of joint mobility are necessary. Any loss of ROM in a joint of the residual limb can have significant effects on functional use of the prosthesis. The loss of ROM needs to be investigated and aggressively managed to maximize range.
Preprosthetic training begins with the early postsurgical therapy visit and continues until prosthetic fitting is completed. Prosthetic fabrication and fitting ideally should be completed within 4 to 8 weeks after surgery. Early prosthetic fitting is important, because prosthetic acceptance declines if fitting is delayed beyond the third postoperative month.20 Preprosthetic training is critical to maintain motivation and create an easier transition to prosthetic use. Amputation results in a loss of body symmetry. This imbalance results in shoulder elevation and scapula rotation on the affected side, as well as loss of neutral positioning of the residual limb. Close attention must be paid to the individual’s awkward or compensatory body motions when approaching an object. The rebalancing begins with observing and correcting static postures in the mirror. The mirror remains an important tool in conscious recognition and correction of the abnormal positioning. The amputee is encouraged to use muscle memory to relearn correct postural and limb positioning control.22 As remobilization progresses, emphasis is placed on recognizing the abnormal postures and positioning that occur with basic activities of daily living (ADLs).
ADLs are mastered with one hand and, when appropriate, with the use of adaptive equipment. The amputee progresses from independence with basic hygiene to the advanced homemaking tasks. Hand dominance is retrained when necessary, especially with handwriting and keyboarding. Repetitive tasks can be used for strengthening. These tasks include fine motor exercises with nuts and bolts or tweezers, as well as gross motor exercises with equipment and mirrors. Proprioceptive neuromuscular facilitation is a particularly effective approach that enables the therapist to work in diagonal planes, vary the amount of resistance, and concentrate on specific areas of weakness. Isometric exercises are effective in creating muscle bulk for stabilization of the arm in the socket of the prosthesis. The stability of the prosthesis depends on both the bulk of the stabilizing musculature and the amputee’s ability to voluntarily vary residual limb configuration. Because balance is often disrupted in a new amputee, the goals should include strengthening of the trunk, core, and lower limbs using isometric exercise and aerobic training. Depending on level of loss, the upper limb amputee should begin to practice several motions that will be needed to control the prosthesis (Box 12-5).
BOX 12-5 Specific Movements Necessary to Control a Prosthesis and Maintenance of Range of Motion
Specific Movements Necessary to Control a Prosthesis
Maintenance of Range of Motion
Upper Limb Prostheses
Introduction to Upper Limb Prosthetic Systems
Each prosthesis is unique and customized to the individual. While two prostheses might be alike or very similar, there is no such thing as a “normal” or “standard” prosthesis (especially in the world of upper limb prosthetics). There are four categories of upper limb prosthetic systems: the passive system, the body-powered system, the externally powered system, and the hybrid system. Selecting among these can be difficult. Each patient’s functional and vocational goals, geographic location, anticipated environmental exposures, access to prosthetists for maintenance, and financial resources all need to be considered (Box 12-6).
The field of prosthetics has a unique vocabulary that is not part of the everyday practice of medicine (Box 12-7).
BOX 12-7 Prosthetic Terminology
Socket and Suspension Choices
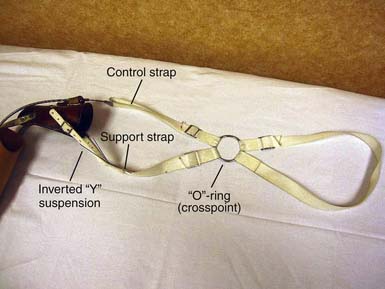
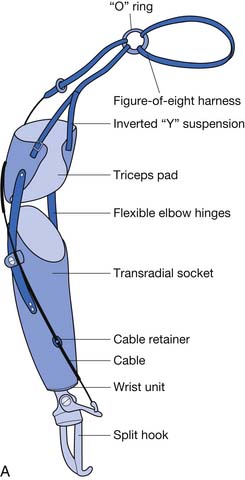
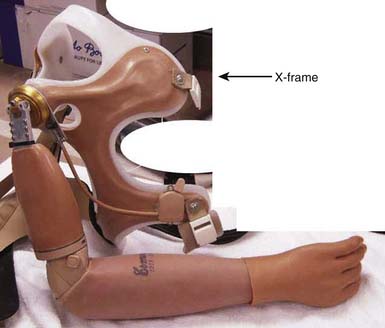
With a figure-of-eight harness for control and suspension, the harness not only operates the terminal device but also functions to keep the socket correctly positioned. There are four main components to the harness; the axilla loop, the anterior support strap, the control attachment strap, and the crosspoint (Figure 12-6). While wearing a prosthesis with this harness the amputee is able to open and control the terminal device with shoulder forward flexion.
Power
If amputees are given training on both the myoelectric and the body-powered prostheses, they will self-select their primary choice. It is not uncommon for the amputee to prefer myoelectric for one activity and body powered for another, which then drives what TD is selected.24 Body-powered prostheses use forces generated by body movements transmitted through cables to operate joints and terminal devices. An example is forward flexing the shoulder to provide tension on the control cable of the prosthesis, resulting in opening the terminal device. Relaxing the shoulder forward flexion results in return of the terminal device to the static closed position. An alternative movement for opening the terminal device is biscapular abduction, which is commonly used when operating the terminal device close to the body. Body-powered prostheses are more durable, give higher sensory feedback, and are less expensive and lighter than myoelectric prostheses.
When limited control sites (muscles) in a residual limb are available to control all the desired features of the prosthesis, a one-site/two-function (single-site) system can be used. This device uses a single electrode to control both functions of a paired activity such as flexion and extension. The patient uses muscle contractions of different strengths to differentiate between flexion and extension. For example, a strong contraction opens the device, and a weak contraction closes it. When multiple powered components on a single prosthesis must be controlled, sequential or multistate controllers can be used, allowing the same electrode pair to control several by a brief cocontraction of the muscle or by a switch used to cycle between control-mode functions.27
Switch-controlled, externally powered prostheses use small switches to operate the electric motors. These switches typically are enclosed inside the socket or incorporated into the suspension harness of the prosthesis, such as the “nudge,” which is operated by the chin depressing the switch on the anterior chest strap. A switch can be activated by the movement of a remnant digit or part of a bony prominence against the switch or by a pull on a suspension harness. This can be a suitable option when myoelectric control is otherwise not feasible.40 A hybrid system is one that incorporates both power options.
Level-Specific Upper Limb Amputation Prostheses
Partial hand prostheses are not commonly used. Amputation distal to the wrist is one of the most common upper limb deficiencies, but is difficult to treat successfully with a prosthesis. Poor results are due to functional limitations of prosthetic technology, discomfort at the prosthetic interface, unsatisfactory appearance, and absence of tactile sensation.11 With the advancement of new technologies, the availability of new prostheses has created additional challenges for the prosthetist. Many patients with limb deficiencies distal to the wrist have declined prosthetic intervention in the past, and most limbs makers have limited experience with partial hand amputations. Partial hand amputation can involve various levels of longitudinal and transverse loss that dictate different treatment options. The person with a partial hand deficiency has four prosthetic options: (1) no prosthetic intervention, (2) a passive prosthesis, (3) a body-powered prosthesis, and (4) multiple task-specific prostheses. Individuals with passive prostheses actively use their prostheses as frequently as do individuals with functional prostheses.21 Even though passive prostheses do not offer active grasp and release, they can be used to stabilize objects, to push against items, and to perform other functional tasks. This type of prosthesis usually incorporates a secure socket that is stabilized about the residual limb by means of a total contact suction fit. Body-powered prostheses for partial hand deficiencies can be divided into two categories: cable-driven and wrist- or finger-driven devices. Adequate functional grasp from both system types is limited. Task-specific prostheses are available for both vocational and avocational activities. These prostheses are usually highly customized to effectively meet the functional needs of the individual.
Wrist disarticulation prostheses are suspended using the patient’s remaining anatomy, specifically the radial and ulnar styloid processes. The benefit of a wrist disarticulation is that it preserves a longer and more powerful lever arm, as well as maximal preservation of forearm pronation and supination. When fitting someone with a wrist disarticulation prosthesis, preserving symmetric limb length becomes an issue. This can be a difficult problem, and wrist units are often not used with wrist disarticulation, to conserve length and preserve symmetry. When not using the wrist unit, compensation is gained through maximizing preserved forearm supination and pronation through the socket. Wrist disarticulation is harder to fit with a myoelectric prosthesis because less space is available in which to conceal the electronics and power supply.
There are a number of traditional transradial socket options (Figure 12-7). Three traditional styles use anatomic suction suspension so that a harness is not needed. This is known as a “self-suspending” system. These three are each designed to be used with a different length of residual limbs and are named the Muenster, the Northwestern, and the TRAC (Transradial Anatomically Contoured) designs. The Muenster-type socket was introduced in the 1960s, and it introduced a fitting technique for a short transradial level amputation that provided more intimate encapsulation of the residual limb. The elbow is set in a preflexed position (usually 35 degrees), and a channel is provided at the antecubital space for the biceps tendon. This allows for unobstructed flexion, and the suspension is achieved through anterior-posterior compression around the olecranon. It is not an optimal design for bilateral amputees because it is donned with a pull sock. This led to additional innovations that included the popular Northwestern socket design. Unlike the Muenster socket, the Northwestern uses medial-lateral compression of the arm above the epicondyles and less restrictive anterior-posterior compression, and is used primarily in those with long residual limbs. The reduced anterior-posterior compression creates a less snug suspension and can lead to problems with electrode contact and increased forces on the distal residual bone.34 The socket is known for its ease of donning and is a popular choice for bilateral amputees. The trim-lines of the transradial socket are dependent on the length of the residual limb; the shorter the limb, the higher the trim-line. The longer the limb, the lower the trim-line, and there is more allowance for pronation-supination. The patient’s ROM will be limited by a transradial prosthesis to approximately 70% of the motion possible without a prosthesis. It might be necessary for the prosthetist to add flexion to the socket so that the end range allows for easy contact with the person’s mouth and face. The TRAC socket incorporates design elements from both the Muenster and Northwestern sockets, but with more aggressive contouring of the limb to maximize load-tolerant areas of the residual limb. Similar to the Muenster, the TRAC retains the encapsulation of the olecranon posteriorly and the generous relief of the biceps anteriorly. The TRAC uses both anterior-posterior and medial-lateral compression for enhanced stability and comfort. The TRAC socket, through detailed anatomic contouring, transfers the load from the distal end of the radius to the more load-tolerant proximal musculature.
An elbow disarticulation socket or a long transhumeral socket (Figure 12-8) includes the residual limb and excludes the acromion, the deltopectoral groove, and the lateral border of the scapula. At this level of amputation, humeral rotation is captured by the intimate fitting at and above the epicondyles, which creates a well-suspended socket. Elbow disarticulation prostheses require the use of outside locking joints located on either side of the humeral epicondyles and external to the socket. This level might add active rotary control but at the expense of additional bulk to the medial-lateral dimension of the socket. When stabilizing the elbow joint with these hinges, the result is excellent weight-bearing and force dispersion. The elbow disarticulation amputation is least desirable because of the cosmetic asymmetry produced when using the prosthesis, including problems with clothing. Few prosthetic elbows are compatible, and amputees typically dislike the appearance. In the bilateral upper limb amputee in whom the transhumeral level is an option, the elbow disarticulation is more desirable in spite of the poor cosmetic appearance of the externally placed elbow. The functional advantages of disarticulation for the bilateral upper limb amputee are in the use of the residual limb for self-care. It is also preferred over the transhumeral level in children because the epiphysis is preserved and bony overgrowth is prevented.
Amputation at the level of the proximal third of the humerus (proximal to the deltoid insertion) is prosthetically challenging. Control is by scapular motion with assistance from the humerus. At this level there is a reduction in strength and leverage, and cable-powered prosthetic control is severely limited. Body-powered systems require up to 5 inches of total excursion of scapular motion to open the terminal device with the elbow in the fully flexed position.7 A transhumeral prosthesis uses two control cables, compared with that of the transradial system where only one cable is used. One of these cables flexes the elbow and operates the terminal device, while the remaining cable is used to lock and unlock the elbow. To fully operate the system, a cycle of movements must take place (Box 12-8). The body motions that typically operate these two cables are shoulder flexion and the simultaneous movement of abduction and slight extension of the shoulder joint. Shoulder flexion is used to apply tension to the cable, causing flexion of the elbow and operating the terminal device. To lock the elbow in flexion, the simultaneous movement of abduction and extension of the shoulder joint (similar to the motion of “elbowing” someone standing behind you) is used. The “figure-of-eight” body harness can be worn with a transhumeral prosthesis. Often additional straps and modifications are made to capture as much excursion as possible, especially with higher level amputations.
BOX 12-8 Cycle of Movements
Forequarter-level amputations present even more of a challenge to fit with a functional prosthesis. Most control options have been removed with the residual limb. The “nudge” control, which is a force-sensitive resistor operated by the chin, can operate the elbow and hand. For those who choose, a lightweight passive prosthesis anchored from the contralateral limb can be fabricated (Figure 12-9). It functions by creating a cosmetically natural appearance. If no prosthetic is used, a cosmetically sculpted insert should be offered for the shoulder symmetry needed for clothing fit.
Advantages and disadvantages of myoelectric and body-powered prostheses are summarized in Box 12-9.
Terminal Devices and Wrist Units
Multitudes of terminal devices are available for upper limb amputees, although the functionality of these terminal devices is limited. Terminal devices generally are broken down into two categories: passive and active (Box 12-10). Passive terminal devices fall into two classes: those designed primarily for function and those that provide cosmesis. Examples of the functional passive terminal devices include the child mitt frequently used on an infant’s first prosthesis to facilitate crawling, or the ball-handling terminal devices used by older children and adults for ball sports.
BOX 12-10 Types of Terminal Devices
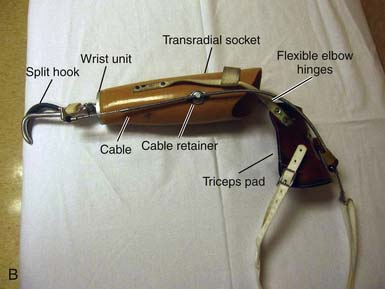
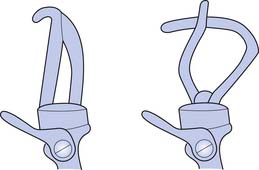
Hooks (Figure 12-10) have many advantages; they are simple in design, lightweight, enable efficient grasp, are durable, have low maintenance, and permit visual feedback that is unavailable with a mechanical hand (Boxes 12-11 and 12-12). In general, hook-style terminal devices provide the equivalent of active lateral pinch grip, whereas active hands provide a three-point chuck action. Many different options are available for terminal devices that address occupations, hobbies, and sports.
BOX 12-11 Typical Advantages of Split Hooks
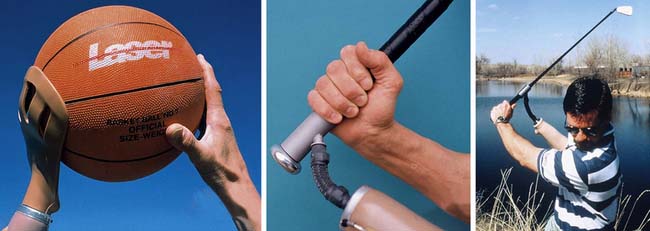
The major function of the hand that the prosthesis tries to replicate is grip (Boxes 12-13 and 12-14). Although artificial hands are generally less functional than hooks and prehensors, people often choose them because they look more natural. A prosthetic hand usually is bulkier and heavier than a hook. It can be powered by a cable or use external power. With a myoelectrically controlled device, it is possible to initiate palmar–finger tip grasp by contracting residual forearm flexors, and to release by contracting residual extensors.26 Many specialized terminal device designs are available or are custom fabricated for individual amputees. Most of the commercially available specialized terminal devices are designed for various vocational and recreational activities. Terminal devices are available for specific activities, such as playing musical instruments, golfing, bowling, swimming, tennis, weightlifting, fishing, skiing, shooting pool, rock climbing, baseball, hunting (bow and rifle), and photography (Figure 12-11).
BOX 12-14 The Five Types of Grip
Various types of electronic hands and terminal devices are available. Some of the current hands on the market have unique grasping characteristics; these include a feature used to eliminate slipping of an item being grasped. A sensor in the second digit senses an item slipping from grasp and tells the hand to grip harder.41 Just as a real hand would squeeze a cup a little harder when it gets heavier as water is poured into it, this hand automatically monitors grip force and grabs harder when objects get heavier so that they do not fall out of the user’s grasp. As a result, users do not have to be as precise with their grip force. Most electronic hands only have motors in the first three digits. This means that the fourth and fifth digit close passively as they are attached to the second and third digits. Not all electronic hands look like natural hands. Hands made for heavy-duty industrial purposes have other, hook-like shapes.
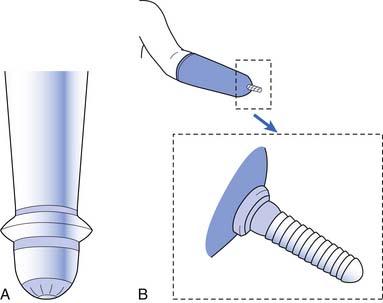
The wrist unit provides orientation of the terminal device in space. It can be positioned manually, by cable operation, or with external power (whether myoelectrically or by switch). Once positioned, the wrist unit is held in place by a friction lock or mechanical lock. Several different designs are available, including a quick-disconnect unit, a locking unit, and a flexion unit (Box 12-15). Friction-control wrist units are easy to position but can slip easily when carrying heavier loads. Wrists and elbows can also be controlled electronically. The electronic wrists can allow for full 360-degree rotation. The elbows flex and extend when sent signals from electrodes, touch pads, or pressure (force) sensors. These elbows contain microprocessor computer technology that allows for fine-tuned adjustments.49
BOX 12-15 Wrist Unit Designs
The Bilateral Upper Limb Amputee
The conceptual framework used for prosthetic fitting and training of the unilateral amputee changes significantly when fitting and training the bilateral amputee. The unilateral upper limb amputee uses the prosthesis as an assist, and the sound limb for sensory feedback and fine manipulator activities. The bilateral amputee has no “sound limb” for the prosthetic limb to assist. All activities must be performed with the prostheses. Wear and tear on joints and cables typically is far greater than for the unilateral amputee. The ability to handle complex sensory feedback and fine manipulation is lost. The goal remains, however, to master independent basic ADLs and vocational and avocational tasks. The sockets need to be easily donned and doffed for independence to occur. Bilateral wrist flexion units are mandatory to obtain the positioning necessary to master basic hygiene. The most functional terminal device is the hook. The new amputee rarely appreciates the functional advantages of a prosthetic hook over a prosthetic hand. It must be explained that a prosthetic hook is not an attempt to duplicate the human hand, because it obviously does not look or function like a hand. The prosthetic hook represents an efficient tool that is used for several functions. A major problem unique to the bilateral upper-limb amputee is the inability to use sensory feedback once the residual limb skin is covered by the socket. For this reason the prosthesis should be constructed so it can be partially removed for sensory feedback through the residual limb and then easily reapplied. For example, the socket could have a window or be open ended to expose the distal portion of the residual limb for such maximal sensory purposes (Figure 12-12) (e.g., the Krukenberg prosthesis). With a bilateral upper limb amputee the Caryle formula (Box 12-16) is used to determine proper limb length.
BOX 12-16 The Caryle Formula
For wrist disarticulation and the long and medium-length transradial amputation, a conventional socket is indicated with a sufficiently low anterior trim-line to permit a full range of elbow flexion. For the more proximal transradial amputee, flexible elbow hinges that are attached to the triceps pad are required for transradial socket stabilization and to permit pronation and supination.13 The shorter the residual limb, the greater is the indication for a polycentric elbow hinge so that prosthetic and anatomic joint congruity can be approached as closely as possible. Polycentric hinges require more maintenance than single pivot hinges. The socket is aligned in such a way that it brings the terminal device closer to the center of the body. The conventional socket design is unchanged in the bilateral transhumeral amputation. The anterior and posterior wings of the socket should extend sufficiently to stabilize the prosthesis against axial rotation. The shorter the amputation level, the higher the socket trim-line must extend, particularly the posterior and anterior wings. This is necessary to provide adequate control against longitudinal rotation as well as to provide suspension.13 Elbow joints with alternating locks and a friction-controlled turntable for internal-external rotation are standard components. It is best to use externally powered prostheses for control of the elbow or terminal device. The alignment needs to be adjusted for wheelchair users. A synergistic, interconnecting harnessing system is needed that interfaces with sockets. Some amputees prefer that each arm be harnessed independently so that they have the option of wearing only one prosthesis. Donning and doffing are accomplished by an over-the-head maneuver. Removal is done in a way that places the prostheses in position for redonning.
Advances in Prosthetic Technology
Bionic Hands
A newer type of electronic hand has motors and sensors in every digit (Figure 12-13). The hand has two unique features. First, a separate motor is in each finger, which means that each finger is independently driven and can articulate. Second, like the human thumb, the electronic thumb can rotate 90 degrees. Two electrodes sit on the skin and record myoelectric signals. They are used by the computer (which sits in the back of the hand) to do two things: interpret those signals and control the hand. This translates into the wearer being able to generate or use myoelectric signals in the arm to control the grabbing function of the hand. The digits do not move separately, although they appear to do so. When gripping, each finger senses contact with the item being gripped. The motors in the first, second, and third digits stall because of contact with the item being held, and the fourth and fifth digits continue to move until they reach a contact point. This creates the illusion of independent, “natural” movement between the digits. In addition, the first digit of this electronic hand has the capability of being manually positioned to create multiple grip patterns.29
Neuroprostheses
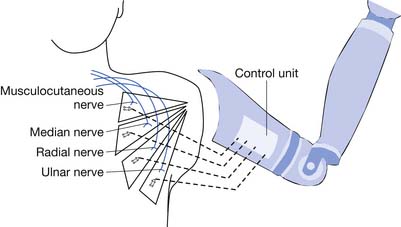
In 2005 the United States began funding over $70 million for the advancement of prosthetic technology through the U.S. Defense Advanced Research Projects Agency. The program has spanned worldwide, with engagement of multiple universities, and engineering and science laboratories to create the most advanced prosthetic arm that simulates the human arm. A human arm is capable of more than 25 degrees of freedom (independent motions)14 as well as sensory feedback. No prosthetic arm has even remotely achieved this simulation to date. The task is to create a biologically controlled intelligent prosthesis with capacity for sensory feedback. Surgical techniques reroute unused nerves to functional muscles. The technique is called targeted muscle reinnervation (Figure 12-14) and rewires the nerves that no longer have innervation points to the pectoral muscle. Myoelectrodes create the interface with the prosthetic arm. The new prosthetic system has built-in feedback loops that allow the user some sensory feedback. Wireless electrodes have been developed that can be implanted into muscle, and brain electrodes have been developed that can sense nerve impulses. Signal processing algorithms are used. A virtual reality physical and occupation therapy training has been created. The implanted electrodes (both muscle and brain) make the prosthesis easy to wear, by eliminating weight and bulk. To date the implantable electrodes have been successful and are awaiting clinical trials from the Food and Drug Administration. This newer prosthetic technology has been sufficiently successful that manufacturing companies have already been contacted for mass production upon Food and Drug Administration approval. At the time of this writing, at least two amputees are currently using the technology, and by reports, able to play Guitar Hero (a video game that uses an electronic guitar), something no other prosthetic device can control. One of the more interesting features of this research project is the amount of technology that has been deemed “open source,” or free for anyone to access and expand upon. This allows the world to expand upon this work, potentially increasing engineering advancements at much faster paces.28
Prosthetic Training
The prosthetic training phase begins with the delivery of the prosthesis. Focus is on donning and doffing and wearing the prosthesis for short periods. The goal is integration of the prosthesis into daily activity. Numerous issues arise during this period of rapid change, including maladaptive habits that occur quickly. After the introduction to the prosthesis is completed (Box 12-17), training progresses toward mastering basic ADLs. After ADLs are completed, the amputee is then moved to higher level homemaking skills and community reentry activities such as driving, work, and recreation. Protocols for controls training for the body-powered prosthesis and the myoelectric prosthesis, have been reported by Ganz et al.22 A list of activities and a rating guide (Table 12-2), designed by Northwestern University, is a helpful tool for the therapist to use to guide activities and assess progress for the unilateral upper limb amputee.2 In the case of the bilateral upper limb amputee the controls training is a more complex and coordinated motor process. The therapist needs to maintain and progress the strength and coordination gained in the preprosthetic training and facilitate the independence of the amputee with this as a daily routine. Proprioceptive neuromuscular facilitation enables the therapist to key into specific areas of muscle weakness. Isotonic exercises are effective in maintaining muscle bulk for stabilization of the arm in the socket of the prosthesis. The stability of the prosthesis depends on both the bulk of the stabilizing musculature and the amputee’s ability to voluntarily vary residual limb configuration. For the transhumeral level, this would be the external rotators and the biceps that stabilize the socket; for the transradial it would be the supinators and pronators that stabilize. There is a natural linear flow of these three rehabilitation phases, and as the amputee users attempt to integrate back into their life prosthetic training advances to mastering more specific and unique tasks (Box 12-18). The prosthetic training phase ends with the proficient use of the prosthesis.
BOX 12-17 Introduction to the Prosthesis
| Criteria | Examples | Grade∗ |
|---|---|---|
| Personal needs | Don/doff pullover shirt Manage zippers and snaps |
|
| Eating procedures | Cut meat Open milk carton |
|
| Desk procedures | Use phone and take notes Sharpen pencil |
|
| General procedures | Operate door knob Set time on watch |
|
| Housekeeping procedure | Hand wash dishes Dry dishes with a towel |
|
| Use of tools | Hammer Tape measure |
|
| Car procedures | Open and close doors, trunk, and hood Perform steps required to operate vehicles |
∗ Rating guide grading: 0, not possible; 1, mostly clumsy but accomplished; 2, minimal clumsiness; 3, smooth. (Grading system developed for the individualized goals.)
Follow-up
The need for regular lifelong follow-up by the rehabilitation team inclusive of the physiatrist cannot be overemphasized. After discharge from the therapy program, the amputee should be regularly monitored in an outpatient clinic by the rehabilitation team (Box 12-19). Follow-up should be considered the most important aspect of prosthetic rehabilitation and yet might be the most often neglected. Without this consistent communication, the many barriers to successful prosthetic use cannot be addressed, and functional use is sacrificed. Issues such as pain, depression, skin irritation, limb size change, and activity change are all more easily addressed early and thoroughly by “the team” before new behavior patterns start and abandonment of the prosthesis occurs. Many aspects of upper limb prosthetic rehabilitation cannot be addressed until the patient has had reasonable time to become acclimated to the rapid life and functional changes being experienced. The physiatrist is often asked to state the patient’s level of disability (Table 12-3).
| Disability | Rating (%) |
|---|---|
| Loss of one upper limb | 50 |
| Loss of one hand | 45 |
| Thumb amputation | 23 (50% of one hand) |
The team is available to address questions and nurture the newly mastered skills. The fit, comfort, and function of the prosthesis must be maintained and optimized over time as amputees alter and refine their initial goals and aspirations. The successful long-term use of an upper limb prosthesis depends primarily on its comfort and its perceived value to the amputee. Innovative design and careful custom adaptation of socket and harness, careful attention to follow-up adjustments, and prescription revisions based on the amputee’s changing needs are the essential factors for successful prosthetic rehabilitation. Atkins devised a rating scale to quantify success of prosthetic functional adaption (Box 12-20).2
BOX 12-20 Atkins1 Prosthetic Functional Adaption Rating Scale
Box 12-21 lists tips for maintenance and use of the prosthesis.
BOX 12-21 Tips for Prosthetic Maintenance and Use
1. Atkins D.J. Adult upper limb prosthetic training. In Bowker J.H., Michael J.W., editors: Atlas of limb prosthetics: surgical, prosthetic, and rehabilitation principles, ed 2, St Louis: Mosby, 1992.
2. Atkins D.J., Meier R.H., editors. Comprehensive management of the upper limb amputee. New York: Springer-Verlag, 1989.
3. Atroshi I., Rosberg H.E. Epidemiology of amputation and severe injuries of the hand. Hand Clin. 2001;17:343-350.
4. Benevenia J., Blackskin M.F., Patterson F.R. Complications after limb salvage surgery. Curr Probl Diagn Radiol. 2004;33:1-15.
5. Biemer E., Iribacher K., Machertanz J., et al. Reorganization of human motor cortex after hand replantation. Ann Neurol. 2001;50:240-249.
6. Bosse M.J., MacKenzie E.J., Kellam J.F., et al. An analysis of outcomes of reconstruction or amputation after leg threatening injuries. N Engl J Med. 2002;347:1924-1931.
7. Bray J.J. Prosthetic principles: upper extremity amputations (fabrication and fitting principles), ed 3. Los Angeles: Prosthetics Orthotics Education Program, University of California Press; 1989.
8. Brookmeyer R., Ephraim P.L., MacKenzie E.J., et al. Estimating the prevalence of limb loss in the United States 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
9. Bunnell S. Management of the nonfunctional hand: reconstruction vs. prosthesis. In Callahan A.D., Hunter J.M., Mackin E.J., editors: Rehabilitation of the hand, ed 2, St Louis: Mosby, 1984.
10. Burkhalter W.E., Hampton F.L., Smeltzer J.S. Wrist disarticulation and below-elbow amputation. In: John H., Bowker, Michael J.W. Atlas of limb prosthetics: surgical and prosthetic principles. St Louis: Mosby, 1981.
11. Caldwell R.R., Sanderson E.R., Wedderburn A., et al. A wrist powered prosthesis for the partial hand. J Assoc Child Prosthet Clin. 1986;21:42-45.
12. Cook T.M., Shurr D.G. Upper extremity prosthetics. In: Cook T.M., Shurr D.G., editors. Prosthetics and orthotics. East Norwalk, CT: Appleton and Lange, 1990.
13. Dickey R., Lehneis H.R. Special considerations: fitting and training the bilateral upper-limb amputee. In Bowker J.H., Michael J.W., editors: Atlas of limb prosthetics: surgical, prosthetic, and rehabilitation principles, ed 2, St Louis: Mosby, 1992.
14. Dillingham T.R. Rehabilitation of upper limb amputee. In: Dillingham T.R., Belandres P.V., editors. Textbook of military medicine. Part IV. Surgical combat casualty care: rehabilitation of the injured combatant, vol. I. Washington, DC: Office of the Surgeon General at TMM Publications, 1998.
15. Dillingham T.R., Esquenazi A., Huston C. Rehabilitation of the lower extremity amputee. In: Dillingham T.R., Belandres P.V., editors. Textbook of military medicine. Part IV. Surgical combat casualty care: rehabilitation of the injured combatant, Vol. 1. Washington, DC: Office of the Surgeon General at TMM Publications, 1998.
16. Dillingham T.R., MacKenzie E.J., Pezzin L.E. Incidence, acute care length of stay, and discharge to rehabilitation of traumatic amputee patients: an epidemiologic study. Arch Phys Med Rehabil. 1998;79:279-287.
17. Dillingham T.R., MacKenzie E.J., Pezzin L.E. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95:875-883.
18. Durham R.M., Mazuski J.E., Mistry B.M., et al. Outcome and utility of scoring systems in the management of the mangled extremity. Am J Surg. 1996;172:569-573.
19. Esquenazi A. Upper limb amputee rehabilitation and prosthetic restoration. In Braddom R.L., editor: Physical medicine and rehabilitation, ed 3, Philadelphia: Saunders, 2007.
20. Fleming L.L., Malone J.M., Robertson J. Immediate, early and late post surgical management of upper limb amputation. Prosthet Orthot Int. 1999;23:55-58.
21. Fraser C.M. An evaluation of the use made of cosmetic and functional prosthesis by unilateral upper limb amputees. Prosthet Orthot Int. 1998;22:216-223.
22. Ganz O., Gulick K., Smurr L.M., et al. Managing the upper extremity amputee: a protocol for success. J Hand Ther. 2008;21:160-176.
23. Garrison S.J., Subbar J.V. Heterotopic ossification: diagnosis and management, current concepts and controversies. J Spina Cord Med. 1990;22:273-283.
24. Gould R.J., Gregory R.T., Peclet M., et al. The mangled extremity syndrome (M.E.S.): a severity grading system for multisystem injury of the extremity. J Trauma. 1985;25:1147.
25. Lane D., Lu J., Peters C.: Degrees of freedom. Available at: http://onlinestatbook.com/chapter8/df.html. Accessed January 9, 2009.
26. Lawrence M., Gross G.-P., Lang M., et al. Assessment of finger forces and wrist torques for functional grasp using new multichannel textile neuroprostheses. Artif Organs. 2008;32:634-638.
27. Heckathorne C.W. Components for electric-powered systems. In Bowker J.H., Michael J.W., Smith D.G., editors: Atlas of amputation and limb deficiencies, ed 3, Rosemont, IL,: American Academy Orthopedic Surgeons, 2004.
28. IEEE Spectrum. Available at: http://spectrum.ieee.org/consumer-electronic/gaming/for-those-without-hands-Theses-guitar-hero. Accessed November 10, 2008.
29. I-Limb brochure http://www.touchbionics.com/i-LIMB. Accessed August 10, 2010.
30. Jaeger S.H., Kleinert H.E., Tsai T. Upper extremity replantation in children. Orthop Clin North Am. 1981;12:897.
31. Kozak L., Owings M. Ambulatory and inpatient procedures in the United States, 1996. National Center for Health Statistics. Vital Health Stat. 1998;13(139):1-119.
32. Lake C. The evoluation of upper limb prosthetic socket design, JPO 20:85-92, 2008.
33. Lake C. The evolution of upper limb prosthetic socket design. J Prosthet Orthot. 2008;20(3):83-92.
34. Lake C., Miguelez J.M. The transradial anatomically contoured (TRAC) interface: design principles methodology. J Prosthet Orthot. 2003;15:148-157.
35. Lee W.P., Wilhelmi B.J., et al. Replantation in the mutilated hand. Hand Clin. 2003;19:89.
36. Amputations Louis D. In: Green D.L., editor. Operative hand surgery, ed 2, New York: Churchill Livingstone, 1988.
37. Ly T.V., Travison T.G., Castillo R., et al. The ability of lower-extremity severity scores to predict functional outcome after limb salvage. J Bone Joint Surg Am. 2008;90:1738-1743.
38. McAuliffe J.A. Elbow disarticulation and transhumeral amputation/shoulder disarticulation and forequarter amputations. In Bowker J.H., Michael J.W., editors: Atlas of limb prosthetics: surgical, prosthetic, and rehabilitation principles, ed 2, St Louis: Mosby, 1992.
39. Mohamed A.E. Arterial reconstruction after mangled extremity: injury severity scoring systems are not predictive of limb salvage. Vascular. 2005;13:114-119.
40. Muzumdar A. Powered upper limb prosthesis. New York: Springer; 2004.
41. Myoelectric upper extremity prosthesis. OTTO BOCK 2009. Available at: http://www.ottobockus.com/cps/rde/xchg/ob_us_en/hs.xsl/6874.html. Accessed January 9, 2009.
42. National estimates from Healthcare Utilization Project (HCUP) Nationwide Inpatient Sample (NIS), Agency for Healthcare Research and Quality (AHRQ), based on data collected by individual states and provided to AHRQ by the states.
43. Ramachandran V.S., Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263(1369):377-386.
44. Segraves K.B., Simon M.A., Waddington W.W.Jr. Psychological outcome of extremity sarcoma survivors undergoing amputation or limb salvage. J Clin Oncol. 1985;3:1393-1399.
45. Serletti M., Carras A.J., O’Keefe R.J., et al. Functional outcome for soft tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg. 1998;102:1576-1583.
46. Smith D.: Limb loss in children congenital limb deficiencies and acquired amputations, In Motion. Available at: www.amputee-coalition.org/immotion/may./congenital.part3.html. January 9, 2009
47. Taylor C.L. The biomechanics of control in upper-extremity prosthesis. Artif Limbs. 2(4-25), 1955.
48. Togawa S., Yamami N., Nakayama H., et al. The validity of the mangled extremity severity score in the assessment of upper limb injuries. J Bone Joint Surg. 2005;87:1516-1519.
49. Utah Arm 3. Motion Control, Inc, 2009. Available at: http://www.utaharm.com/ua3.php. Accessed January 9, 2009.
50. Van Hoesel R., Veth R., Bökkerink JP., et al. Limb salvage in musculoskeletal oncology. Lancet Oncol. 2003;4:343-350.
51. Vanadurongwan V., Waikakul S., Sakkarnkosol S., et al. Prognostic factors for major limb re-implantation at both immediate and long term follow-up. J Bone Jnt Surg Br. 1998;80:1024-1030.
52. Walter Reed Amputee Care Program Database. Military Amputee Care Program, Washington, DC. Updated January 18, 2007.