Chapter 13 Rehabilitation and Prosthetic Restoration in Lower Limb Amputation
Despite advances in medicine, industry, and technology, amputation remains a leading source of disability. Approximately 159,000 lower limb amputations are performed each year,68 with peripheral vascular disease accounting for the vast majority of lower limb amputations. Amputations secondary to vascular conditions accounted for 82% of limb loss discharges, with the incidence increasing by 27% from 1988 to 1996.23 Trauma-related amputations accounted for 16% of amputations, whereas those resulting from malignancy and congenital deformity were responsible for 0.9% and 0.8% of amputations, respectively. Interestingly, the incidence of amputation secondary to a traumatic etiology has not gone up significantly within the United States Armed Services personnel despite recent conflicts.90 The incidence of amputations is not expected to subside anytime soon for a number of reasons, including the aging of the population and the increased incidence of diabetes in the United States. As the population ages, the number of amputations in persons older than 65 years is expected to double.36 Amputation prevalence is also expected to more than double from 2005 to 2050.106 Diabetes creates the greatest risk for amputation, surpassing the risks created by both smoking and hypertension. Diabetes is related to 67% of all amputations.81 The age-adjusted amputation rate for persons with diabetes is as high as 18 to 28 times more than that of persons without diabetes.94
In terms of level of amputation, Dillingham et al.23 reported that lower limb amputation accounted for 97% of all amputations between 1988 and 1996 with the following distributions: 31.5% toes, 10.5% midfoot, 0.8% ankle disarticulation, 27.6% transtibial, 0.4% knee disarticulation, 25.8% transfemoral, and 0.4% hip disarticulation. Other sources report that 64% to 73% of amputations were transtibial; 26% to 31%, transfemoral level; and 4.5%, knee disarticulation.4,35 Patients with diabetes are at greater risk for a second amputation, with rates as high as 18% at 2 years and 45% at 4 years.26 A second amputation is shown by more recent studies to be a conversion to a more proximal amputation level in 9%, with amputation of the contralateral limb in 11% to 21% of patients with amputation.4,35,49,91 In patients with vascular disease, 34% required more than one amputation procedure.80
Survival rates after amputation vary based on a variety of factors. Those who have amputations from trauma tend to have good long-term survival, but those who have amputations because of a vascular etiology face sobering survival statistics. After a vascular amputation, the 30-day mortality rate ranges from 9% to 21%,1,4,28,49,80 and long-term survival has recently been found to be 48% to 69% at 1 year, 42% at 3 years, and 35% to 45% at 5 years.4,15,28,79,80 More proximal levels of amputation have also been associated with decreased survival rates.4,15,36,79 Diabetes and end-stage renal disease have been shown to negatively affect survival, with 5-year survival rates as low as 30.9% and 14.4%, respectively.4
The chances of prosthetic fitting vary depending on the etiology of amputation, the level of amputation, and the age. In a survey of individuals registered with the Amputee Coalition of America, 95% of respondents had a prosthesis and used it extensively.70 MacKenzie et al.58 found that 97% of all traumatic amputees were ambulating with a prosthesis at 3 months. Success rates are lower for people with amputations resulting from vascular disease and diabetes. Overall success rates for prosthetic fitting in dysvascular patients have been reported to be greater than 80%.40,72 Fletcher et al.36 reported a 78% success rate in transtibial patients and a 57% success rate in transfemoral patients for functional prosthetic use among people older than 65 years who were referred to an amputee clinic. He noted that success of fitting diminished with age and level of amputation. Patients with transtibial amputation older than 85 years had less than a 2% chance of successful prosthetic fitting. Johannesson et al.49 reported that 43% of all patients received a prosthesis after primary amputation. Multiple limb amputees can be successful ambulators. Two studies have reported that 70% of bilateral transtibial amputees were able to use their prosthesis for ambulation.13,93
Regardless of its etiology, amputation remains a source of significant physical and psychological trauma in individuals facing limb loss. Although many patients and physicians alike might consider it to be a failure of medical and surgical management, amputation is a reconstructive surgery that maximizes the patient’s function and quality of life. Most patients have the potential for a successful outcome after amputation. Although elderly patients who have undergone vascular amputation might never run or participate in competitive sports, they can still have the potential for improved function with a prosthesis. For the young person with amputation, an active lifestyle with a prosthesis is expected. To illustrate the functional potential of a patient with amputation, the men’s record for the 100-m sprint for an athlete with an amputation is 10.91 seconds. This was set in April 2007 by Oscar Pistorious, a man with bilateral transtibial amputations, who later petitioned to qualify for the 2008 Olympics.57 Although he did not compete, that is not far behind the able-bodied record of 9.69 seconds set by Usain Bolt at the 2008 Olympics.107
Presurgical Management
Selection of Amputation Level
The earliest attempts to judge the appropriate level of amputation focused on the presence of palpable pulses, angiographic findings, skin color and temperature, character and location of pain, and most notably, the presence of incisional skin bleeding at the time of surgery. Various diagnostic methods exist to help determine the level at which healing will occur. These include Doppler pressure measurements, pulse volume recordings, photoplethysmographic pressures, laser Doppler blood flow studies, xenon-133 skin blood flow studies, arterial angiography, and transcutaneous oxygen determinations (see Chapter 57). However, these tests have not been more consistently reliable than clinical judgment in predicting wound healing at a given level.5,25 Most surgeons use a combination of objective data and assessment of the appearance of the tissues at the time of surgery, particularly bleeding, to decide on the site of amputation.
Pain Control
Good perioperative pain control is essential for the patient facing limb loss. Beyond patient comfort, adequate pain control minimizes the patient’s stress, and allows the patient to participate more fully in a rehabilitation program. Uncontrolled pain can result in the development of central nervous system–mediated pain and chronic pain,60 as well as impair postoperative healing and immune functions.8,22
The mainstay of perioperative pain control treatment is opioid therapy. Oral opiates are usually sufficient, but there should be no hesitation to use more aggressive pain control measures including transdermal, subcutaneous, intramuscular, or intravenous routes. A scheduled dose of a long-acting agent in conjunction with an immediate-release analgesic is recommended so that the patient has consistent pain relief. A sufficient dose of analgesia just before therapies often helps the patient fully participate. Caution, however, should be used in the geriatric population given the risk of delirium in these individuals, and opiate use should be streamlined in these instances.
Patient-controlled analgesia systems are useful for patients having severe pain in the perioperative period. They provide a continuous infusion of analgesic, with the ability of the patient to use a supplemental dose as needed. Patient-controlled analgesia provides excellent analgesia and can reduce some of the patient’s stress or fear related to pain. Continuous regional nerve blocks and epidural anesthesia can also be used for perioperative pain control,58 although typically these cannot be continued in the post–acute care setting.
Psychological Support
Psychological assessment and support for the patient facing limb loss should be a high priority. Assessment of the patient’s expectations and goals is important. Frequently, patients fear that they will never be able to walk again after amputation and might not expect to be able to accomplish more demanding occupational or recreational tasks. These perceptions are often unfounded because most amputees can do very well functionally. As noted above, several studies indicate that greater than 80% of amputees at all levels will be able to successfully walk with a prosthesis,40,63,72 and this is consistent with our experience. Each patient’s specific long-term goals should be identified before surgery, and a comprehensive rehabilitation program should be outlined to achieve these goals. Patients feel empowered and reassured when higher functions (such as sports) are recognized as legitimate goals, and the rehabilitation team helps them achieve these goals.
Describing the rehabilitation process in detail to patients and educating them about prostheses can help to allay their fear of the unknown. Providing educational literature and website addresses can be a valuable and calming service to patients and their families (Box 13-1). Some programs use a peer counseling program in which patients who are successful prosthesis users visit patients with new amputees on request. Finally, it is important to discuss phantom limb sensation and phantom limb pain with patients before surgery. Phantom limb sensation is the temporary nonpainful feeling that the amputated limb is still present. This feeling typically fades away over a period of weeks. Phantom pain is the usually temporary pain that can occur in a limb after its amputation, especially in a patient with a long history of preamputation limb pain. All patients need to be told to expect these sensations in the missing limb after surgery and to realize that they are normal.
BOX 13-1 Educational Resources Available for Amputees
Booklets
Postsurgical Management
Wound Care
In patients with vascular compromise, wound margin necrosis can develop. The limb should be monitored closely in these cases, kept clean, and protected from any trauma that could cause a dehiscence in such a fragile wound. Once the nonviable tissue is clearly demarcated, debridement can be considered. Adjuvant therapies such as negative pressure wound therapy and hyperbaric oxygen treatment are available options that have possible benefit but warrant further investigation to determine their efficacy with this population.43,91,98
Good nutrition must be encouraged and supported with supplements when necessary. Eneroth30 found that when supplementary nutrition was given to malnourished patients, twice as many adequately nourished patients healed their residual limb wound when compared with controls.
Edema Control
Several different edema control systems are available. The most commonly used treatment is elastic wraps on the residual limb (e.g., Ace bandages). Although elastic wraps can provide effective compression, they are high-maintenance items that must be properly applied and changed about every 4 to 6 hours to maintain consistent compression.61 This can be difficult and time-consuming for a patient, or even the health care team, to accomplish. Elastic wraps that are improperly applied or displaced in the normal course of regular movement can turn into tourniquets, causing pressure wounds and even limb ischemia. Consequently the use of elastic wrapping is not recommended.
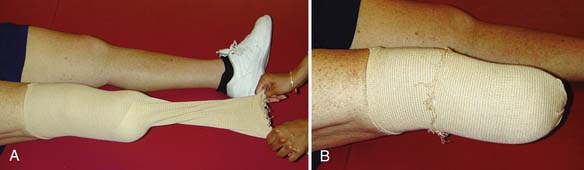
The use of elastic socks or elastic stockinette provides a better alternative than elastic wraps. Elastic stockinette (e.g., Compressigrip and Tubigrip) can be applied in multiple layers to give graded and increasing compression toward the end of the residual limb (Figure 13-1). It is inexpensive and easily applied. Premanufactured residual limb shrinkers can also be used. For transfemoral amputees, a residual limb shrinker with a waist belt must be used, because otherwise the dressing tends to slide off the conical-shaped residual limb. The waist belt should be attached to the elastic dressing at the side of the limb. If the attachment is worn in front, the shrinker will slide off during sitting.
Prosthetic elastomeric liners can also be used as compression socks for edema reduction in amputees.49,51 They can provide compression and also lend some degree of protection to the residual limb. Because of their suction fit, they can be used on the transfemoral amputee without a waist belt. When using any elastic dressing, bony prominences should be closely monitored because pressure can concentrate at protruding bony areas and lead to skin breakdown.
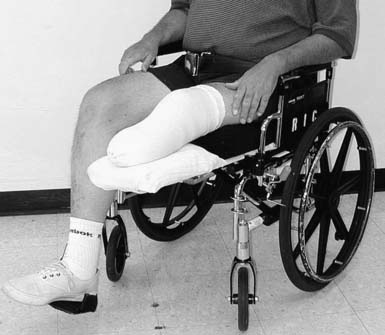
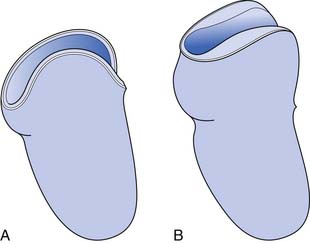
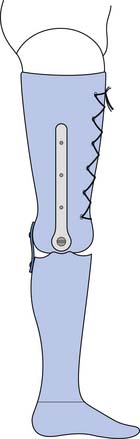
Rigid dressings provide additional benefits over soft dressings alone for transtibial amputees (Figure 13-2). Rigid dressings help protect the residual limb from any inadvertent trauma, such as a fall.31 They provide good compression to minimize edema, and the cast can be conformed to minimize pressure over bony prominences. Partial weight-bearing can also be started through the rigid dressing to help desensitize the limb and build tolerance to pressure.
FIGURE 13-2 Rigid dressings for transtibial amputee. (A) Rigid dressing. (B) Rigid removable dressing.
A nonremovable rigid dressing is a cast that is applied over the fully extended residual limb up to the midthigh (see Figure 13-2, A). This type of cast is helpful in preventing knee flexion contractures and can be used as an immediate postoperative prostheses (as discussed below). A nonremovable cast does not allow for wound inspection except at cast changes. It also does not allow patients to massage their residual limbs, an important part of the desensitization program.
A removable rigid dressing (RRD)104,105 is a custom-made cast that covers the residual limb up to the knee (see Figure 13-2, B). It is held in place with either elastic stockinette or a thigh cuff. As the limb shrinks, socks, shrinker socks, and elastic stockinette are added underneath the RRD to keep it snug. The RRD allows for frequent wound inspection and massage of the residual limb. It also helps to teach patients how to adjust sock ply, a necessary skill for using most types of prostheses. A suggested protocol is to start with a rigid nonremovable dressing applied immediately after surgery. After the first 3 to 6 days, the cast should be changed to an RRD, which should be used until most of the edema is resolved and the wound is well healed. If more than 12 to 18 ply of sock is required to keep the RRD snug, a new RRD is recommended.
Functional Rehabilitation
Patients should be educated in proper positioning. Prevention of hip and knee contractures is critical in this period. Patients should not have pillows placed under the knee because this can lead to knee flexion contracture. To prevent hip abduction contractures, pillows should not be placed between the legs. Dangling the residual limb over the side of the bed or wheelchair should be avoided. A knee extension board (Figure 13-3) can be fitted underneath the wheelchair or chair to promote knee extension and help prevent dependent edema. Wheelchair-elevating leg rests are generally less effective and more expensive than these boards. If knee flexion contractures are of great concern, a knee immobilizer while the patient is in bed can be used to maintain knee extension. Patients should be instructed to lie prone several times a day for 10 to 15 minutes at a time to prevent hip flexion contractures. Individuals who cannot tolerate prone positioning can lie supine on a mat while performing hip extension exercises of their affected limb.
ROM and strengthening exercises of the affected limb are important adjuncts to positioning. Muscles that oppose the common sites of contracture must be strengthened, especially knee and hip extensors. Other important muscles groups that should be strengthened include the hip adductors and abductors. Because patients are increasingly reliant on their arms to assist with mobility, arm strengthening and conditioning are needed. Specific exercises include strengthening of the wrist, elbow extensors, and scapular stabilizers. This training prepares patients to properly execute transfers and to correctly use crutches or a walker. Initiation of aerobic exercise is needed to increase endurance and cardiovascular fitness, but given the high incidence of concomitant cardiovascular disease in individuals with a vascular etiology of amputation, adherence to cardiac precautions is important.
Early partial weight-bearing can begin in the first few days if there are no wound complications. For transtibial amputees with rigid dressings, limited weight-bearing can be performed through their cast using a strap across their wheelchair (Figure 13-4). When the patient is up in the parallel bars, weight-bearing can be done via a tire jack or adjustable footstool. Immediate postoperative prostheses can also be used for early weight-bearing as described below. Inspection after weight-bearing is important in monitoring wound tolerance of pressure. This is especially important in individuals with amputation from vascular disease or patients with impaired sensation.
Pain Management
Identifying the etiology of postoperative limb pain is important for successful control of the pain. Because of nerve fiber damage and ongoing stimulation of the nerves in the residual limb, a generalized residual limb pain is initially expected secondary to the surgical incision and postoperative edema. Ectopic activity at the cut end of nerves is expected and can be due to unstable sodium channels or the uncovering of new pathologic receptors.6,7 Ephaptic transmission, which is the stimulation of afferent fibers (nociceptors) by efferent neurons (motor or sympathetic), can also contribute to limb pain.48,77 This acute pain responds well to intravenous or intramuscular opiates. It subsides fairly rapidly, and the parenteral opiates can usually be discontinued within 2 to 3 days. Scheduled doses of oral opiates with rescue medications as needed should be continued beyond this period and weaned slowly so that the patient continues to receive adequate pain control.
Desensitization techniques should be added to the treatment plan within a few days of surgery. Patients should be instructed to start massaging and tapping their residual limb, which can be performed through any soft dressing. This gentle stimulation can help to reduce residual limb pain by closing the pain gate,60 and gives patients a technique for controlling their pain independently. Self-massage also forces patients to attend to their amputation; this can help with their new body image and psychological adjustment to limb loss.
Even after the immediate postoperative period and residual limb healing, the incidence of pain is high in patients with amputation, with 68% reporting residual limb pain and 80% reporting phantom pain.32 The most common types of sensory problems reported include phantom pain, phantom sensation, and residual limb pain. The differential diagnoses for pain in an amputated lower limb are diverse, and treatment options differ significantly based on the etiology of pain. Consequently the source and mechanism of the pain must be investigated and identified so that an optimal treatment plan can be implemented.
Prosthesis use is a common cause of residual limb pain in individuals with amputation. In these instances, pain tends to be worse with prosthetic use and during ambulation. Usually there is skin irritation that correlates with the area of the prosthesis causing the pain. Multiple factors can contribute to prosthetic pain including socket fit, suspension, alignment, and gait pathology. Consideration must be given to all of these areas when there are complaints of pain in the residual limb during prosthetic use. As an example, Table 13-1 lists some of the common causes of distal tibial pain, a very common site for prosthesis-related pain in transtibial amputees. Addressing the prosthetic etiology typically results in pain reduction.
Table 13-1 Prosthetic Adjustments for Distal Tibial Pain in the Transtibial Amputee
| Contributing factors | Treatment |
|---|---|
| Excessive socket pressure | Socket relief. Remove sock ply. |
| Pistoning in the socket | Add socks to tighten fit. Build up the liner. Tighten socket. Tighten suspension system. |
| Excessive pressure from liner | Change suspension system. |
| Excessive early knee flexion | Move foot forward. Plantar flex foot. Decrease socket flexion. Soften heel of foot or shoe. Add anteroposterior ankle motion. Round heel of shoe or change shoe. Strengthen quadriceps/hamstring muscles. |
Phantom limb sensation and pain are likely maintained by afferent, central, and efferent (sympathetic) dysfunction.54 Phantom limb sensation and pain are neuropathic perceptions in a portion of the limb that was amputated. Most amputees experience some degree of phantom limb sensation and pain, but the natural history is for these feelings to diminish both in frequency and intensity over the first few weeks to months after the amputation.29 These sensations are highly variable in character. Phantom limb sensations are frequently described as numbness, tingling, pins and needles, or itching. Some amputees report the sensation of the phantom limb becoming shorter, known as telescoping. Patients can also complain that the missing limb feels like it is moving or is in a cramped or awkward position. It is important to explain that these are normal sensations that will likely diminish with time. Sometimes patients do not consider the sensation to be painful, but uncomfortable or “bothersome.” These sensations can be severe enough to interfere with sleep, impair patients’ functioning, or significantly reduce their quality of life. At this point the phantom limb sensation should be treated as neuropathic pain. The character of phantom limb pain is often described as sharp, burning, stabbing, tingling, shooting, electric, or cramping. Patients frequently perceive the same type of pain that they had before the amputation. For example, they might feel like they still have a painful foot ulcer.
Residual limb pain, previously referred to as stump pain, is another manifestation of central sensitization. Preliminary work in the area suggests that it is a form of allodynia (i.e., pain evoked by previously innocuous stimuli) or a spontaneous pain of peripheral neuropathic origin, central neuropathic origin, or both. Spontaneous residual limb pain is usually described as aching, burning, or throbbing, whereas evoked pain can be electrical or shooting (which can easily be confused with a clinically significant neuroma).95 The pain is localized in the residual limb and can be associated with phantom limb pain. Edhe et al.29 reported residual limb pain to be as common as phantom limb pain in lower limb amputees, and often more distressing.
The first line of treatment for bothersome phantom limb sensation, phantom limb pain, and residual limb pain is desensitization techniques. Massaging, tapping, slapping, wrapping, and friction rubbing of the residual limb often diminish such sensations. Patients frequently find that their phantom limb pain diminishes with the stimulation of using a prosthesis. Anecdotally, many patients find that for a phantom itch, scratching the remaining leg in the same spot is helpful. For cramped or malpositioned limb sensations, hypnosis can be helpful.27,65,88 Under hypnosis, the patient might be able to alleviate a cramped phantom hand or move an awkwardly phantom positioned limb to a more comfortable position.
If desensitization techniques are insufficient and these pains are significantly interfering with quality of life, pharmacologic treatment should be considered. Unfortunately, the literature is not robust with regards to demonstrating efficacy of any particular agent in treating phantom pain.69 However, the two primary categories of medicines generally used in clinical practice to treat chronic phantom limb phenomenon are antidepressants and anticonvulsants. Antidepressants have several advantages in addition to pain control. They can also treat depression, which is a common problem in new amputees. They generally have anxiolytic effects and, some being sedative, can improve sleep. They are convenient because they are usually taken just once per day, and they are generally less expensive than the newer antiseizure medications. Analgesic antidepressants apparently need to have both noradrenergic and serotonergic receptor activity to effectively treat neuropathic pain,33 which explains why the selective serotonin reuptake inhibitors do not help with the treatment of phantom limb pain. Tricyclic antidepressants have the most anecdotal and empirical support for treating pain, but they also have undesirable anticholinergic side effects.52,82 Some antidepressants have both serotonergic and noradrenergic activity without the anticholinergic activity (serotonin and norepinephrine reuptake inhibitors [SNRIs]), and might help in treating neuropathic pain. Mirtazapine is an SNRI that can be useful for the treatment of phantom limb pain because it has no anticholinergic side effects, and it enhances sleep (night is often when phantom limb pain is most problematic). It is an effective antidepressant and an anxiolytic, although weight gain can be a significant side effect.85 Venlafaxine and the newer agent duloxetine are also SNRI-type agents that might have some value for the treatment of neuropathic pain.
A number of anticonvulsants have been used to treat neuropathic pain syndromes.6 At present, gabapentin is probably the most widely prescribed neuropathic pain medicine in the United States. Despite its name, gabapentin’s mechanism of action is unknown. Nevertheless, gabapentin has demonstrated efficacy with neuropathic pain, has minimal side effects, and has been shown in a small study to have efficacy in phantom pain.10,45 However, it usually requires frequent dosing (generally three to four times a day). Traditional anticonvulsants such as carbamazepine and phenytoin are membrane stabilizing agents (sodium channel blockers) that have the widest historical use for the treatment of neuropathic pain syndromes.21,69 However, they have a high incidence of significant side effects. Other anticonvulsants that are being used to treat neuropathic pain include oxcarbazepine, topiramate, levetiracetam, and pregabalin, but few or no data exist yet concerning their efficacy. Anticonvulsants can also be used in combination with antidepressants or with each other to maximize relief from phantom limb pain. This must be done in a thoughtful fashion that uses complementary mechanisms.
Some success has been reported with topical anesthetic agents such as various analgesic balms, sprays, and patches. Capsaicin, a substance P inhibitor, has been anecdotally reported to be effective for phantom limb pain and residual limb pain.51 Lidocaine cream, ointment, and patches have also been used with some success.
Other nonpharmacologic modalities exist for the treatment of phantom limb pain such as stress-relaxation techniques and biofeedback.37,50,87,88 Transcutaneous electrical nerve stimulation has also been shown to give temporary pain relief.35,56 Mirror therapy, while primarily used with upper limb amputations, has been attempted in patients with lower limb loss,16,19 with some success.
Neuromas are bundles of nerve endings that form after a nerve is cut, as is done during an amputation. They can produce sharp, focal pain under pressure or upon palpation (i.e., Tinel’s sign).22 If a neuroma is superficial, then a small, tender mass can be palpated. It can cause significant pain and can preclude use of a prosthesis. Initially, prosthetic socket adjustments are used to relieve pressure over a neuroma. If this is unsuccessful, neuropathic pain medication, intralesional steroid and anesthetic injection, or neuroma ablation with phenol, alcohol or cryoablation can provide pain relief. However, literature demonstrating efficacy in patients with amputation remains severely limited. Ultrasound-guided phenol instillation has been shown in a small case series to provide some benefit in the treatment of neuroma pain.44 Radiofrequency ablation has also had some success in case reports for both neuroma and phantom limb–related pain.76,103
If these interventions are all ineffective, the neuroma can be surgically excised and the nerve endings buried deep in the soft tissue to protect them from mechanical pressures. Surgical resection has been found to be up to 80% successful.12,42
While the causal relationship between amputation and phantom limb pain or residual limb pain is obvious, other conditions can also cause pain in a phantom limb. In trauma, referred pain can be generated down the leg by a proximal nerve injury, plexopathy, radiculopathy, or occult fractures, even if the leg has been amputated. Referred leg pain from spinal stenosis or arthritis at the knee or hip can also be mistaken for phantom limb pain. These alternative diagnoses must be considered and clearly require different treatment plans.
Psychological Adjustment
The emotional impact of limb loss is devastating and is frequently underestimated by the rehabilitation team. Grieving over the loss of one’s limb is necessary, and a brief exogenous (reactive) depression is expected. Amputees are at high risk of developing more severe psychological problems. The incidence of persistent clinical depression is estimated to be 21% to 35% for people with limb loss.20,83 Risk factors for depression include low income, comorbid conditions, and the presence of phantom and back pain.20 Posttraumatic stress disorder is a recognized complication after traumatic amputation, but frequently goes untreated. Nontraumatic amputees can also develop anxiety disorders from the stress related to limb loss.
Peer counseling and amputee support groups are another important emotional support mechanism. The opportunity to talk to persons who have been through a similar amputation, and to see how well they are doing, can be very valuable to the patient. The Amputee Coalition of America (see Box 13-1) has a national peer network that provides peer counselor training sessions and lists amputee support groups by region.
The physician should regularly monitor the patient’s emotional adjustment to amputation by assessing mood, appetite, weight changes, quality of sleep, and the occurrence of nightmares. Any correlation between the patient’s perceived stress level and pain should be explored. Sometimes adjustment issues will not become problematic until shock and denial wear off and time passes. Because studies have shown that the incidence of depression in younger amputees increases with time,38 continued monitoring by the rehabilitation team is necessary. The whole team must be involved in monitoring the patient’s emotional state, and not just the physician. In most cases the therapists and prosthetists will spend much more time with the patient and might have better insight regarding a patient’s emotional status.
Skin Problems
Problems with skin are frequent in patients with amputation. It has been reported that 63% of patients experienced a skin problem in the past month in one questionnaire.61 Adequate treatment requires proper identification of etiology and type of skin issue.
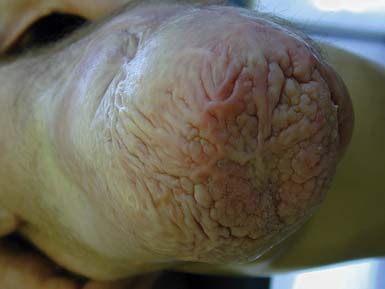
Verrucose Hyperplasia
Verrucose hyperplasia (also termed verrucous hyperplasia) is the development of a wart-like lesion on the end of the residual limb (Figure 13-5). Cracks in the skin and even infection can occur in severe cases. Verrucose hyperplasia is most common in the transtibial amputee, but can occur with other levels of amputation. This dysplastic skin condition is thought to be due to choking effect on the residual limb. It is hypothesized that if the prosthesis fits tightly around the limb (circumferentially) and there is a lack of distal pressure, then vascular congestion can occur that somehow leads to verrucose hyperplasia. The pathophysiology of the condition remains unknown. Adjusting the prosthesis to create adequate distal pressure usually resolves the verrucose hyperplasia within a few weeks or months. Adjustments such as simply adding an end pad to the socket can be sufficient; otherwise a new total contact socket is recommended.
Contact Dermatitis
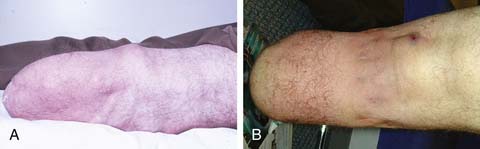
Contact dermatitis is inflammation of the skin manifested by erythema and sometimes mild edema. It can be caused by an irritant that causes scaling, or an allergic reaction that induces vesiculation. Contact dermatitis is a common problem with prostheses, especially with the increased use of elastomeric liners. It is treated by locating the causative agent and preventing it from contacting the residual limb. Hygiene of the residual limb and its prosthetic components must be considered first, because poor hygiene can allow the accumulation of allergens on the residual limb. Soaps can also be the causative agent, and the patient should wash the residual limb each night with a mild or hypoallergenic soap and then rinse the limb well. Similarly the skin interface system (e.g., socks, liners, or the socket itself) should be washed daily per the manufacturer’s recommendations, rinsed well, and allowed to dry before donning. If these simple measures fail, then a change in the skin interface system is needed. Wool socks can be changed to cotton or acrylic. Elastomeric liners made of different materials can be tried. If the contact dermatitis persists, then a change to a different interface system must be considered. For acute contact dermatitis, a short course of topical steroids can decrease inflammation and discomfort. Because the use of topical steroids cannot be considered a long-term plan, the causative agent must be identified and eliminated.
Hyperhidrosis
Hyperhidrosis has been reported to be one of the most common skin issues in patients with amputation, with 32% to 50% of patients reporting this as a symptom in one large study.59 It is usually associated with prosthetic use and can be seen with all types of suspension and liner materials, because heat dissipation is impaired with the use of a prosthetic device or material over the residual limb. Hyperhidrosis can be alleviated by using nylon sheaths as an interface with the liner material, and by using topical or spray antiperspirants. One case series showed promise with the use of botulinum toxin for alleviating this symptom.17
Infections
Once the residual limb is healed, other infections can arise. Aeration of the residual limb, good limb hygiene, good prosthetic hygiene, and proper prosthetic fitting can all prevent and treat such infections. Residual limbs tend to harbor more abundant bacterial flora than unaffected limbs.3 Folliculitis is a hair root infection that can occur in limbs with excessive perspiration and oily skin. This is aggravated by sweating and is worse in warmer, humid months. Skin maceration and moisture can allow bacterial invasion of the hair follicles. This can eventually lead to cellulitis.51 Treatment consists of warm compresses and topical antimicrobials. More severe cases warrant the use of oral or parenteral antibiotics, and incision and drainage of boils.
Epidermoid Cysts
Epidermoid cysts occur when sebaceous glands are plugged. These cysts are firm, round, mobile, subcutaneous nodules of variable size that are most commonly found in the popliteal fossa of transtibial amputees and the upper thigh of transfemoral amputees (Figure 13-6). They can be quite tender and can become inflamed by the pressure of the prosthesis. Sometimes bleeding into the cyst makes them appear dark. Ruptured epidermoid cysts have a purulent or serosanguineous discharge. Treatment with topical or oral antifungal and/or antibacterial agents has been recommended.52 Treatment should also include minimizing pressure over the cysts by adjusting the prosthesis and ensuring an optimal fit. Sometimes larger inflamed cysts require incision and drainage. However, recurrences are frequent because incision and draining does not remove the keratin-producing lining of the cyst. A more definitive treatment consists of surgical excision, but even this intervention cannot completely eliminate the possibility of recurrence.
Other Complications
Joint contractures are frequent complications during the rehabilitation of people with limb loss. Initial treatment consists of a stretching program both in therapy and at home. For knee contractures, extension devices like knee immobilizers can provide relief. For hip flexion contractures, prone lying on a daily basis is helpful. Ultrasound heating can also be an effective therapy when combined with aggressive stretching, provided the patient’s vasculature is adequate for vigorous heating. For transtibial patients who are expected to ambulate, the knee flexion contracture is initially accommodated in the prosthetic alignment. Once the patient is ambulating adequately, flexion is gradually taken out of the socket. This causes an extension stretch when the patient walks, and progressively reduces the contracture. In severe cases that do not respond to the above treatment, surgical release can be considered.
Bony growths at the end of the amputated bone are called bone spurs. They occur frequently and are usually asymptomatic. Similarly, heterotopic ossification (HO) is a spontaneous development of bone in soft tissue, usually occurring after traumatic amputations. The incidence of HO with traumatic amputation has been reported to be as high as 63% in recent armed conflicts.73 If a bone spur or HO protrudes distally and is not covered by adequate soft tissue, it can become painful and cause skin breakdown. Accommodating the spur or HO with a relief and/or padding in the socket might solve the problem. If this fails, surgical excision might be required.
Prostheses
Timing of Prosthetic Fitting
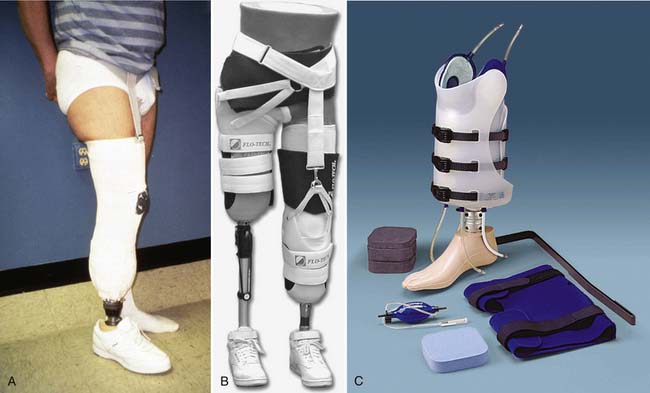
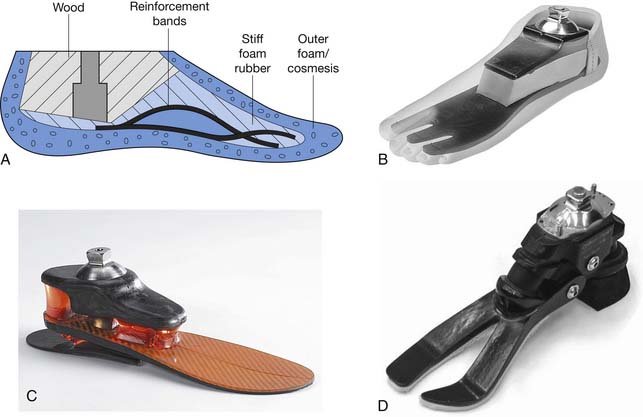
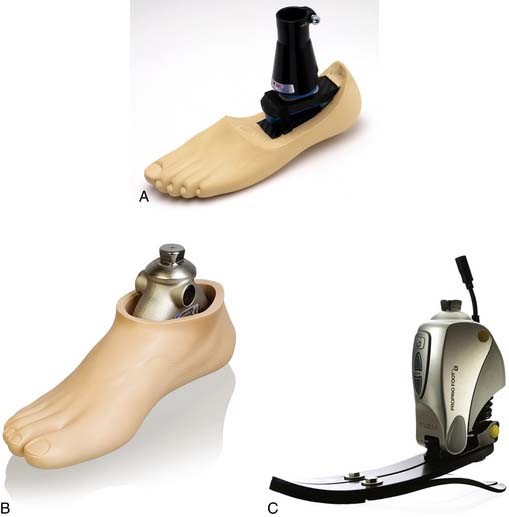
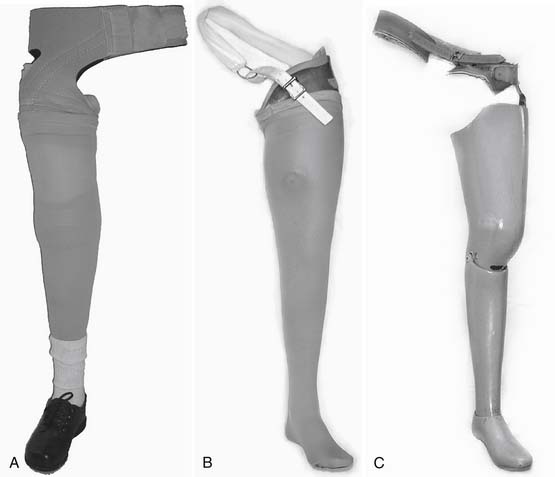
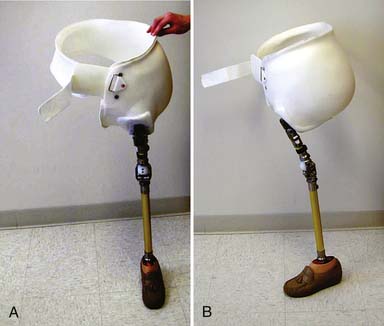
Determining when to fit the lower limb amputee with a prosthesis and what kind of prosthesis to use are issues open to considerable debate. One option for transtibial amputees is to use an immediate postoperative prosthesis (IPOP). IPOPs traditionally have been thigh-high casts with a pylon and foot attached (Figure 13-7, A). Prefabricated devices are also now available (Figure 13-7, B and C). These devices allow for earlier bipedal ambulation. Although many claims have been made for the advantages of IPOPs over soft dressing management (including improved psychological acceptance, reduction in pain, accelerated rehabilitation times, reduced revision rates, and time to healing), these claims have rarely been evaluated with controlled studies.89 A review of 10 controlled studies yielded only two proven claims for the IPOP: (1) that rigid plaster casts result in accelerated rehabilitation times and significantly less edema compared with soft dressings; and (2) that prefabricated pneumatic prostheses were found to have significantly fewer postsurgical complications and fewer higher level revisions compared with soft gauze dressings. Only limited weight-bearing can take place with an IPOP, and patient compliance is important for such success.89 Although weight-bearing is limited through IPOPs, patients clearly have greater stability in standing and walking with the bipedal support. Some studies have shown an increase in wound dehiscence and infection with these devices.18 Frequently the residual limb is too tender and painful to allow early weight-bearing to take full advantage of these devices. With the nonremovable IPOPs, patients are unable to inspect or massage their residual limbs, which can interfere with desensitization and emotional adjustment to their amputation.
The most conservative option is to wait until the wound on the residual limb is completely healed and surgical edema has resolved before the patient is fitted with a custom prosthesis. This generally takes 3 to 6 months with dysvascular amputees. Although this approach can minimize wound problems related to early weight-bearing, there is a higher risk of complications such as joint contracture, deconditioning, pressure ulcers, and an unnecessarily long delay in returning to functional ambulation.
Factors Affecting Prosthetic Prescription
In 1994 the federal government attempted to clarify which knee, ankle, and foot components should be used for patients with particular functional abilities or functional levels. The functional level is a measurement of the capacity and potential of the patient to accomplish his/her expected postrehabilitation, daily function. This functional classification was designed to assist the Durable Medical Equipment Regional Committees in determining appropriate reimbursement for prosthetic components.24 It limits patients with lower functional abilities to simpler prosthetic components, while allowing more active people to use more advanced (and expensive) devices. The determination of these levels is for individuals with unilateral lower limb amputations and is at the discretion of the prescribing physician. Items to take into consideration are the patient’s medical history, present medical condition, and functional status, as well as future ambulation goals and expectations. The prescribing physician should keep an open mind regarding the potential or expected functional level, focusing on the patient’s current condition and how it may affect the goals. Documentation of function should be maintained in the patient’s records.
The functional levels (often referred to as the K-modifiers) are listed in Box 13-2. These levels are based on the patient’s potential, not on the current level of function; even deconditioned patients can reach a higher level of function than anticipated with appropriate rehabilitation. Also, the K1 level includes the use of a prosthesis for assisting with transfers. Many low-functioning people with amputations below the knee or lower benefit from the use of a prosthesis. For higher levels of amputation, a prosthesis does not generally assist with transferring; instead, it often gets in the way.
BOX 13-2 Description of the K-Level Modifiers
| K0 | Does not have the ability or potential to ambulate or transfer safely with or without assistance, and a prosthesis does not enhance quality of life or mobility. |
| K1 | Has the ability or potential to use a prosthesis for transfers or ambulation on level surfaces at fixed cadence. Typical of the limited and unlimited household ambulator. |
| K2 | Has the ability or potential for ambulation with low-level environmental barriers such as curbs, stairs, and uneven surfaces. Typical of the limited community ambulator. |
| K3 | Has the ability or potential for ambulation with variable cadence. Typical of the community ambulator who can traverse most environmental barriers and has vocational, therapeutic, or exercise activity that demands prosthetic utilization beyond simple locomotion. |
| K4 | Has the ability or potential for prosthetic ambulation that exceeds basic ambulation skills, exhibiting high impact, stress, or energy levels. Typical of the prosthetic demands of the child, active adult, or athlete. |
Prosthesis Construction
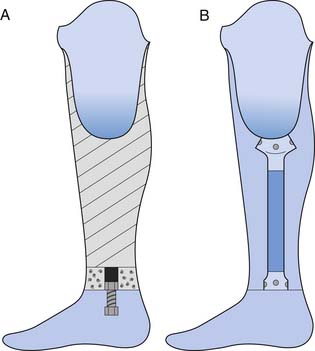
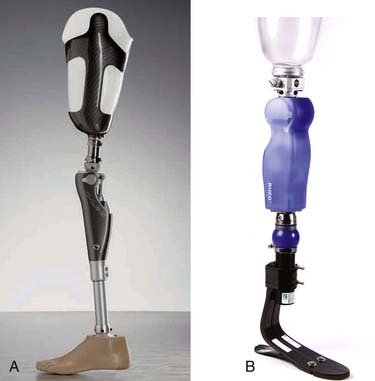
The prosthetic socket is connected to the remaining components in two ways: through exoskeletal or endoskeletal construction (Figure 13-8). In the more common endoskeletal construction (see Figure 13-8, B), the socket connects to the remaining components through pipes called pylons. The modularity of these components allows for angular and linear changes in both the sagittal and coronal planes, and makes it easy to adjust the height of the prosthesis if necessary. An important benefit of this modularity includes the ability to use many different components (e.g., adapters and feet). At higher levels (transfemoral and higher) the endoskeletal design is also lighter in weight. Many endoskeletal systems have carbon fiber or titanium pylons, which are even lighter in weight than standard steel components. Endoskeletal pylons also allow the ability to finish the prosthesis with softer, more realistic covers.
Exoskeletal construction (see Figure 13-8, A) uses a rigid exterior lamination from the socket down and has a lightweight filler inside. This rigid lamination gives the device strength. Since exoskeletal designs do not have a soft foam cosmesis, they are more durable and can be indicated for heavy-duty use and for children. Fewer components are designed for exoskeletal construction, so this construction can limit foot and knee options, as well as long-term adjustability.
Fitting Considerations
Shoe characteristics also affect gait. A tennis shoe with a rounded heel has less of a knee flexion moment at heel strike than a stiffer dress shoe. The tightness of the shoe also affects gait mechanics and should fit securely, but not so tight as to cause compression of the soft cover or heel of the foot within the shoe.75 The prosthetic foot size should allow for easy donning of shoes.
Partial Foot Amputations
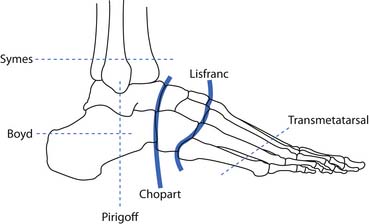
There are various levels of partial foot amputations including toe amputations, ray amputations, transmetatarsal amputations, the tarsometatarsal disarticulation (Lisfranc) amputation, and the transtarsal disarticulation (Chopart) amputations (Figure 13-9). For many partial foot amputations it is necessary to fit orthopedic shoes or shoes with a large toe box, to allow for the extra material of the prosthesis.
For toe and ray amputations, a custom foot orthosis with a toe filler is usually needed to load the remaining foot in an acceptable manner. The orthosis should distribute the pressure more evenly under the foot with relatively less pressure under vulnerable bony prominences (e.g., the end of the amputated toes, rays, or remaining prominent distal metatarsal heads), while relatively more pressure should be placed under the arch of the foot. The toe filler will also prevent movement of the remaining foot inside the shoe, reducing the possibility of frictional sores (blisters) from occurring. A carbon fiber footplate is often added to the insert, or a steel shank is built into the shoe to lengthen the foot’s lever arm. The longer lever arm prevents collapse of the shoe at the end of the amputation, and promotes a more even step length. Ray amputations are generally more successful with amputation of the fourth or fifth rays than with the first or second.
Syme’s Amputation
There are three conventional socket designs for the Syme’s level: posterior opening socket, medial opening socket, and a stovepipe construction (Figure 13-10). For the posterior opening socket, the posterior opening is cut down to the level of the malleoli and is constructed as a removable section that is held with Velcro. This is used for the more bulbous residual limbs. This is the weakest of the three designs because of the limited material in the sagittal plane. The medial opening design has a window cut out to allow the malleoli to pass. Because of the increased material in the sagittal plane, the bending resistance is increased, and this is a stronger design than the posterior opening. The strongest design is the stovepipe design. With the stovepipe design, no flaps or windows are cut in the socket. Instead, a soft insert is built up into a tapered cylinder to slide into a cylindrical socket, or an expandable wall is built into a cylindrical socket to allow passage of the malleoli. If the malleoli are not prominent, suspension is similar to transtibial prostheses.
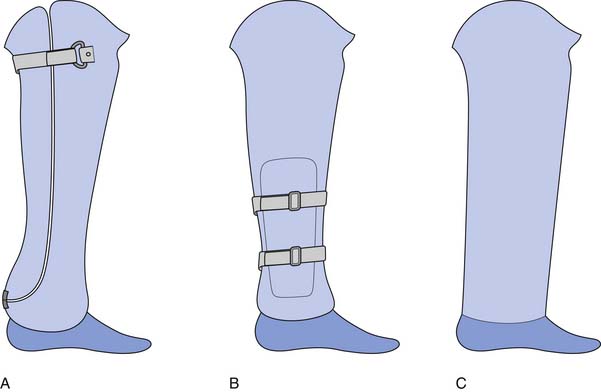
FIGURE 13-10 Three common Syme’s style prostheses: (A) posterior opening, (B) medial opening, and (C) stovepipe.
The Boyd and Pirogoff amputations are variations of the Syme’s where part of the calcaneous is fused to the distal tibia to lengthen the limb further and reduce limb length discrepancy. The Boyd uses the inferior half of the calcaneous, whereas the Pirogoff uses the posterior half. These amputations also allow distal weight-bearing, but the minimal limb shortening makes prosthetic fitting very challenging in adults. They can be useful in pediatric amputees when there is a desire to maintain length, or in situations where no prosthetic care would be available (e.g., developing countries).
Transtibial (Below-Knee) Amputation
A standard transtibial amputation, as advocated by Burgess and Romano,14 is performed one third of the way down the tibia, and a posterior myocutaneous flap is used to cover the residual tibia. At this length, the bulk of the posterior compartment muscles are available for a flap, they provide good soft tissue coverage over the distal tibia, and the primary vascular structures for the lower limb are preserved in the flap.
Long transtibial amputations are sometimes performed to give patients a longer lever arm and more surface area for load distribution.101 However, no functional muscle attachments are saved with a long transtibial amputation, and it is associated with multiple complications and poorer cosmesis.67,86 A standard-length transtibial amputation (from 35% to 50% of tibial length) is strongly recommended as the procedure of choice at this level. The fibula should be cut approximately 1 cm shorter than the tibia, and both bones should be beveled to eliminate sharp ends. Other suggested procedures, including skewed flaps and the Ertl procedure (a bone bridge between the tibia and fibula), have not gained widespread support.
Socket Designs
Patellar Tendon–Bearing
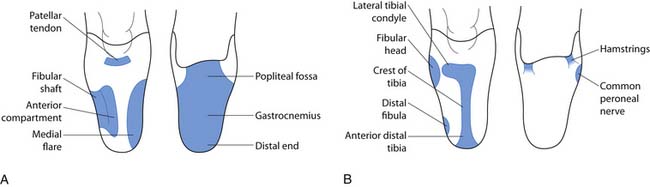
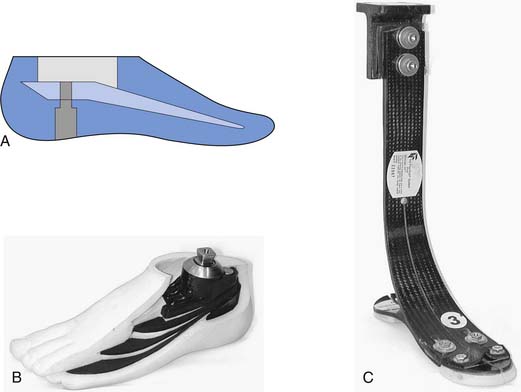
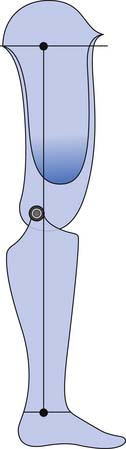
The PTB design focuses on specific weight-bearing areas. Although the socket has total contact with the residual limb, it concentrates force in pressure-tolerant areas and relieves force in pressure-sensitive areas (Figure 13-11). The PTB design gets its name from the amount of force that is borne on the patellar ligament. The socket is designed with an anteriorly directed force, from pressure in the popliteal area, pushing the residual limb onto a “bar” that is created between the distal pole of the patella and the tibial tubercle. Other regions of the residual limb that are used for distribution of forces (pressure-tolerant areas) include the pretibial and gastrocnemius musculature, the medial tibial flare, and the fibular shaft. Pressure-sensitive areas include the patella, hamstring tendons, fibular head, femoral epicondyles, tibial shaft, distal tibia, and distal fibula. An essential part of the PTB design is the alignment of the socket and prosthesis. The PTB design was created to take advantage of normal, or perpendicular, forces on the patellar ligament. This is done by adding initial flexion of the socket to that which is present on the individual’s residual limb. This increased socket flexion loads pressure-tolerant areas of the anterior surface by allowing the patellar ligament to be more parallel with the ground. This provides a surface that is more perpendicular to the ground reaction forces during stance phase. The foot is also aligned medially relative to the socket in order for the individual wearing the prosthesis to experience an external knee varus moment. This moment, in the coronal plane, simulates normal human locomotion and ensures that forces are distributed on the medial tibial flare and the fibular shaft, two of the pressure-tolerant areas on the transtibial limb.
Suspension
Fork Strap With Waist Belt
A fork strap combined with a waist belt is a very simple suspension technique (Figure 13-12). The fork strap, sometimes referred to as a Y-strap, is attached to the medial and lateral aspects of the socket with the junction proximal to the patella. The upper portion of the strap often has some elastic component inherent in its design to permit freedom of knee flexion and extension throughout ambulation and sitting. There is no medial-lateral or anterior-posterior support provided by the fork strap. Its sole purpose is to suspend the prosthesis securely to the waist belt attachment. Because of the bulk and cosmesis of this suspension, it is typically used only when maximum security is required and no other suspension technique is viable (e.g., in obese patients).
The fork strap can be combined with a PTB socket design, thigh-lacer, and joints and rigid sidebars called a joint and corset. This design has the maximum medial-lateral and anterior-posterior stability provided through the rigid sidebars and mechanical stop of the joints, respectively. The joints and corset are required for an unstable knee associated with a traumatic amputation. The joints and corset can also be cinched tightly to provide significant weight-bearing. This can be useful in difficult cases where off-loading of the end of the residual limb is needed to help heal a problematic wound that has failed other measures, or to relieve a hyperpathic residual limb. However, the joints and corset suspension system is very heavy and often not tolerated well by patients
Cuff
Cuff suspension (Figure 13-13) has been used successfully for centuries for individuals with transtibial amputations. Although it is sometimes referred to as a supracondylar cuff or strap, the suspension is provided on the proximal border of the patella and not over the femoral epicondyles. The cuff is attached to the medial and lateral aspects of the socket, over the proximal aspect of the patella, and encircles the distal thigh at a level just proximal to the femoral epicondyles. A prominent patella is required, making this design contraindicated for obese persons or those with excessive thigh musculature. When appropriately applied, the cuff provides an adequate means of suspension between 0 and 60 degrees of knee flexion, and loosens up after 60 degrees of knee flexion to permit comfortable sitting. Similar to the fork strap, the supracondylar cuff provides minimal, if any, medial-lateral or anterior-posterior stability to the knee and has considerable pistoning in the socket. The tightness and width of the circumferential strap can contraindicate the cuff for individuals with vascular compromise.
Sleeve
Suspension provided by a sleeve (Figure 13-14) is done with elastic and sometimes tacky material. The distal aspect of the sleeve is stretched over the proximal portion of the prosthesis and is held in place by the compression of the sleeve against the outer wall of the prosthesis. The proximal aspect of the sleeve is folded inside out and is reflected down beyond the trim-lines of the socket to facilitate donning. Once the individual has seated the residual limb appropriately inside the socket, the sleeve can be pulled up or rolled up onto the distal thigh to create a bond with the person’s skin and body. These sleeves come in a variety of materials and fabrics including neoprene, latex, silicone, and urethane. Some of these sleeves can provide a vacuum seal inside the socket to provide enhanced suspension via suction. Although most sleeves provide an excellent means of suspension, they are often difficult to don, retain heat, and provide minimal to no stability about the knee. They are relatively inexpensive but require frequent replacement.
Supracondylar Suspension
Supracondylar suspension (Figure 13-15, A) is designed to use the anatomy as a means of suspension. A compression of medial-lateral dimensions of the prosthesis above the femoral epicondyles differentiates this design from those previously mentioned. A supracondylar wedge, mostly on the medial aspect of the prosthesis, is used to create this suspension. The wedge can be incorporated into the prosthesis in three ways. First, it can be incorporated into a soft insert made from a material such as foam. The foam liner is donned on the limb first, and then the limb and foam liner are inserted into the hard socket. The hard socket keeps the wedge compressed against the medial distal thigh, thereby preventing the prosthesis from falling off.
Supracondylar-Suprapatellar Suspension
Supracondylar-suprapatellar suspension (Figure 13-15, B) is similar to the supracondylar design with one major addition. The suprapatellar socket addition provides added suspension over the proximal aspect of the patella and can provide a “stop” to help prevent hyperextension of the knee. This stop is accomplished by cupping in over the patella and creating a quadriceps-bar that comes into contact with the distal quadriceps during knee extension. Supracondylar-suprapatellar designs are a good choice for individuals with short residual limbs because the forces are distributed over as large a surface area as possible. This encapsulation of the limb can restrict some ROM and is designed to restrict excessive extension.
Gel or Elastomeric Liner Suspension
Suction sock suspension is a very popular design of recent times that provides both suspension and weight distribution through the use of a gel liner and pin-locking mechanism (Figure 13-16). The gel liners that are available, like the sleeves, are fabricated from various materials such as silicone, urethane and mineral oil gel. The liners come in different thicknesses, and some provide more padding in different areas than others. In addition to cushioning and suspension, these liners minimize shear on the limb. Custom-made liners are also available for use with residual limbs that have a complex shape (such as crevices from traumatic amputation) or other skin problems.
Appropriate donning of these gel liners is crucial to the comfort and maintenance of good skin integrity. Most of the liners need to be turned inside out so that the distal end of the inside of the liner is flat when it is applied to the distal residual limb. This is done so that no air becomes trapped between the distal limb and liner creating a negative pressure within the suction liner that can cause erythema and capillary rupture. A well-fitting, appropriately donned gel liner provides a barrier of comfort and creates a suction environment between the limb and liner.
Vacuum Suspension Systems
Lastly, and fairly new to the market, are systems that provide an active means of expelling air from the inner socket. These designs require the user to wear a gel liner and a sealing sleeve, and they have a valve at the distal end of the socket through which air is drawn. The removal of air to achieve a vacuum can be through a mechanical pump (a telescoping vertical compression unit built into the pylon section of the prosthesis) or a powered electric pump. This method has proven to be beneficial in the suspension of prostheses, while also maintaining limb volume throughout the course of daily use.9 The downside of this suspension is that if a hole forms in the outer sleeve, the vacuum seal is lost. These systems also increase the cost and weight of the device.
Foot-Ankle Assemblies
Solid-Ankle, Cushion-Heel Feet
The SACH feet, introduced in 1956 by Foort and Radcliffe, are one of the most basic and widely used feet in prosthetics (Figure 13-17). SACH feet have solid ankles in that there is no articulation within the foot. They attach to the distal aspect of the shank (endoskeletal pylon-ankle adapter or ankle block) in a way that permits no motion. It is important to understand that motion in all planes is arrested. No plantar flexion, dorsiflexion, inversion, eversion or transverse plane motion is allowed during gait. The cushion heel aspect of the foot allows for a simulated plantar flexion during initial contact-loading response by means of compression under loading. This compression lowers the forefoot to the ground and brings the ground reaction force anterior, simulating the function of true plantar flexion. Heel compression also acts to absorb shock during loading response. With correct heel stiffness, shock absorption benefits all of the lower limb, especially the reduction of external knee flexion moment that would be caused by a stiffer heeled foot. External knee flexion moments directly correlate to increased quadriceps activity and decreased stability in individuals with transtibial and transfemoral amputations, respectively. A drawback of SACH feet is that most are designed with keels (the main inner structure of the foot) that are not flexible or are nonresponsive to the typical loads that they will encounter. These keels are usually made of wood or hard plastic. However, these feet are very durable and inexpensive (as compared with other designs). They can vary in heel height and stiffness, and they come in Syme’s varieties. For these reasons, they are still widely used.
Single-Axis Feet
The usual method by which the ankle motion in a single-axis foot is permitted is by bumpers that are installed anteriorly and posteriorly to the ankle (Figure 13-18). These bumpers regulate the amount and speed of motion in which the foot can rotate about the mechanical axis. The plantar flexion bumper is posterior to the ankle axis. As the bumper is compressed, the foot is permitted to slowly or quickly rotate about the axis, depending on whether the bumper is firm or soft, respectively. If the bumper is too stiff, the external knee flexion moment will be great and vice versa. The dorsiflexion bumper is mounted anterior to the ankle. As the ground reaction force moves anterior to the ankle, the bumper begins to compress to permit graded ankle dorsiflexion. The speed and degree of this motion can be varied as well with the stiffness of these dorsiflexion bumpers.
Multiaxial Feet
Multiaxial feet are designed to replicate the actions of the anatomic foot. Multiaxial motion can be obtained either with a foot that has a flexible keel or one that has true mechanical joint axes. A flexible keel foot allows the motion to occur within the keel itself as the ground reaction forces cause deformation of the foot, especially on uneven terrain. This deformation is expected and is an inherent design of the foot. As the foot deforms, it maintains contact with the ground, thereby providing a stable base of support for the individual wearing the prosthesis.
Multiaxial feet have now been fabricated with the materials necessary to enhance their energy return as well (Figure 13-19). These multiaxial, dynamic response feet can give an individual the benefits of compensating for uneven ground, absorbing shock, and providing some responsiveness to reduce energy expenditure during ambulation.
Dynamic Response Feet
Dynamic response feet enhance the mobility of the user by using materials that are “energy storing.” Materials in the keel of these feet are required to deflect under load and return to their original shape. This return, while being unloaded, is what propels the foot and leg forward. In doing so, the foot is providing the response to users that lessens their energy expenditure.63 Single-speed, low-activity (often elderly) individuals do not have the ability to load the foot enough to warrant their use.
There are various types of dynamic response feet (Figure 13-20). Some low-profile feet simply have a dynamic keel inside a foot shell providing a smaller spring. Other feet incorporate the pylon and keel as one flexible larger spring. These feet are indicated for variable cadence ambulators and are much better for high-level activities such as running and jumping.
Ankles
As mentioned previously, the feet can be designed to accommodate for motions that have been lost by the absence of the anatomic ankle. Stand-alone ankles have also been designed to replicate these lost motions. Ankle units (Figure 13-21, A) can be added to most solid keel feet (SACH or low-profile dynamic response feet) that can provide the user with multiaxial motion in addition to the functions provided by the foot itself. This addition allows the user to more easily traverse inclines, declines, and uneven terrain. More recent ankle components with adjustable ankles (Figure 13-21, B) can accommodate for shoes with varying heel heights. Actively powered ankle components have also been marketed that provide active dorsiflexion and plantar flexion to accommodate for toe clearance in swing and transitional movements from sit to stand, and aid in stair and ramp ascent and descent (Figure 13-21, C).
Pylon Components
Other components are available for absorbing vertical shock or transverse plane motion, or to perform a combination of both (Figure 13-22). Torsion adapters are incorporated into pylons or are stand-alone components. These allow transverse plane rotation that simulates tibial rotation. The stiffness of the devices is adjustable based on the user’s size and activity level. They are particularly useful for people who do a lot of twisting motion (e.g., manual laborers), and for some sports, especially golf.
Gait Analysis
Many deviations occur secondary to muscle weakness and prosthetic alignment, and they vary greatly from person to person. Gait deviations based on sound mechanical principles are listed in Table 13-2. These gait deviations are primarily due to an incorrect placement of the foot with relationship to the socket, or vice versa. Other factors in this table include component selection. Although it can be easy to decipher these deviations, it might not be consistent in the gait pattern of the user. For example, an amputee might have had a wonderful gait pattern at one point of rehabilitation, only to present later with a dramatically changed gait pattern. Changes can be due to decreased or increased function by the user, a change in fit, or a change in shoe wear. The wearer might have had a contracture that has been reduced through use of the prosthesis and daily ambulation. This is an example where an increased function can lead to problems with the prosthesis. However, if the alignment of the prosthesis was rectified, the prosthesis would accommodate this improved ROM and greatly improve the gait and overall function of the user.
| Gait Deviation | Prosthetic Causes | Solutions |
|---|---|---|
| Delayed, abrupt, and limited knee flexion after heel strike | Heel wedge too soft Foot too far anterior Foot keel too stiff |
Stiffen heel wedge. Move foot posterior. Use more flexible keel foot. |
| Extended knee throughout stance phase | Too much plantar flexion | Dorsiflex foot. |
| Toe stays off floor after heel strike | Heel wedge too stiff Foot too anterior Too much dorsiflexion |
Soften heel wedge. Move foot posterior. Plantar flex foot. |
| “Hill-climbing” sensation toward end of stance phase | Heel wedge too soft Foot too far anterior Foot keel too stiff Too much plantar flexion |
Stiffen heel wedge. Move foot posterior. Use more flexible keel foot. Dorsiflex foot. |
| High pressure against patella throughout most of the stance phase; heel off floor when patient stands | Too much plantar flexion | Dorsiflex foot. |
| Knee too forcefully and rapidly flexes after heel strike; high pressure against anterior-distal tibia and heel strike and/or prolonged discomfort at this point | Heel wedge too stiff Foot too far posterior Foot too dorsiflexed Keel too soft |
Soften heel. Move foot anterior. Plantar flex foot. Use stiffer keel foot. |
| Hips level, but prosthesis seems short | Foot too far posterior Foot too dorsiflexed |
Move foot anterior. Plantar flex foot. |
| Drop off at end of stance phase | Keel too soft | Use stiffer keel foot. |
| Toe off of floor as patient stands, or knee flexed too much | Foot too dorsiflexed | Plantar flex foot. |
| Valgus moment at knee during stance; excessive pressure on distal-medial limb and/or proximal-lateral surface of knee | Foot too outset | Inset foot. |
| Excessive varus moment at knee during stance (a varus moment at the knee should occur in stance phase but should never be excessive); the distal-lateral and or proximal-medial residual limb is painful | Foot too inset Medial-lateral dimension of socket too large |
Reduce inset of foot. Fit of socket should be evaluated. |
Courtesy Northwestern University Prosthetic-Orthotic Center, Chicago, IL.
Knee Disarticulation and TranscondylarSupracondylar Amputation
The knee disarticulation amputation is a procedure that preserves the femoral condyles, either with or without the patella. This leaves a residual limb that serves as a long lever arm and provides for a stronger residual limb than a transfemoral amputation because the muscular attachments to the condyles are preserved as well. As a result of the long length and weight-bearing properties of a knee disarticulation amputation, the proximal socket trim lines are significantly lower than that of a traditional amputation, often resulting in more comfort and greater ROM in the hip. The bulbous nature of the limb has a poorer cosmesis but can be helpful in using supracondylar suspension of the prosthesis. Variations of knee disarticulation amputations have been described in which the condyles are partially resected to reduce the bulk of the residual limb, but this can interfere with supracondylar suspension. The primary problem with the knee disarticulation is the length at the knee. When a socket and prosthetic knee are added, the functional thigh length is too long. This mandates a poor appearance when sitting because the leg lengths are uneven. In addition, sitting in tight places such as church pews and public transportation is difficult, and kneeling is problematic. For these reasons the knee disarticulation amputation is rarely performed.
Socket Designs
The socket designs for the knee disarticulation limb are highly dependent on the musculature present and the size of the condyles. In designs similar to the transtibial supracondylar suspension, the knee disarticulation socket can be created with an insert to provide both cushioning and suspension. This insert has a thickness of padding, or wedge, incorporated proximal to the femoral epicondyles that provides suspension when donned into a hard socket. As is the case for a Syme’s prosthesis, a door can be cut out of the hard socket to permit donning, and then a strap or elastic webbing can close the door to provide suspension of the prosthesis. If no large discrepancy exists between the femoral epicondyles and the region proximal to them, hard sockets can be used with socks over the residual limbs to facilitate donning through the narrowing distal socket. Gel or foam liners have also been used to suspend the knee disarticulation prosthesis. When donning the hard socket, the liner displaces over the condyles to allow the residual limb to slip past the narrowing socket and then rebounds when the limb is fully seated into the socket.
Component Selection
Knee components are selected for the longer residual limb based on muscular control, limb length and cosmesis. Most individuals with long transfemoral limbs or knee disarticulation limbs have excellent control of their limbs in all planes, and when trained appropriately, can walk with a variety of component designs. The components for knee disarticulation prostheses are chosen to minimize hardware distally, enhancing its aesthetics. Outside (single pivot) hinges (Figure 13-23) are often used in the exoskeletal-style knee disarticulation prosthesis. This offers the best opportunity to equalize femoral segment lengths, but the joints offer no resistance to flexion and extension. Low-profile polycentric joints are very popular for this level of amputation because they provide for a more natural-looking femoral section during sitting. This is because the linkages allow the knee unit to fold under the femoral section. It must be noted, however, that these polycentric knee units also offer increased stability, which might not be necessary for the individual with this residual limb length. Care must be taken to appropriately align the prosthesis to readily permit knee flexion during the preswing and swing phases.
Transfemoral (Above-Knee) Amputation
The primary surgical goal of a transfemoral amputation is to stabilize the femur while retaining maximal femur length. During this procedure the adductor magnus is pulled over the end of the femur, with myodesis (suturing muscle to bone) to the lateral femur. Myoplasty (suturing of muscle to muscle) of the quadriceps and the hamstrings is recommended.41 This procedure provides optimal adductor magnus function and padding of the distal femur. Myoplasty alone is inadequate because it does not optimize muscle length. It also does not allow for adequate control of the femur. If the femur is too mobile within the residual limb, the lateral distal femur can be a focus of excessive pressure, causing pain, and the transfer of energy into the prosthesis is probably compromised.
Prosthetic Prescription
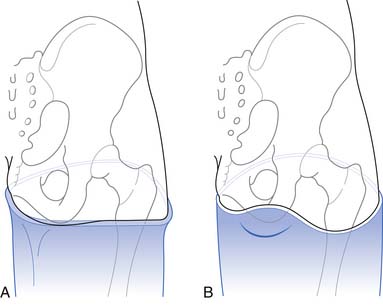
There are two standard socket designs for transfemoral prostheses: the quadrilateral design and the ischial containment design (Figure 13-24). The quadrilateral socket, described in 1955 by Charles Radcliff,74 is designed to allow for muscle function and to provide a seat for the ischium. The quadrilateral socket has four distinct walls with specific biomechanical objectives. The lateral wall supports the femur and provides a surface for the abductor muscles to fire against. The medial wall is in the line of progression and supports the adductor region. The posterior wall is angled away from the medial wall to allow for function of the gluteal tissue, which is critical for knee stability. The anterior wall is contoured to compress the Scarpa’s triangle bounded by the inguinal ligament, the adductor tendon, and the sartorius. There is also relief built into the shape for function of the rectus femoris. Compression of the anterior surface provides the counter force that maintains the ischium on the posterior shelf.
The ischial containment design was first described as the normal shape–normal alignment socket by Ivan Long in 1975. There are now multiple variations in the socket design for ischial containment sockets.46,66,84,96 However, the main biomechanical principle of this design is to provide for a bony lock of the ischium in the prosthetic socket. Preventing lateral movement of the socket through containment of the ischium increases medial lateral stability during the stance phase and allows better adduction. This is helpful for all amputees, but is even more important for individuals with shorter residual limbs and for those with mild hip abductor weakness. The ischial containment socket is now the most commonly used design.
Ischial containment sockets are often made with a flexible inner socket and external frame (Figure 13-25). The flexible socket can be made out of a variety of thermoplastic materials. It provides for increased comfort proximally, which is important with the more aggressive ischial containment designs. The frame can be made out of stiffer thermoplastic or laminated materials. Cutouts can be made in the frame posteriorly to provide increased sitting comfort, and along the anterior surface to provide freedom of movement for rectus femoris contraction.
Suspension
Suction Suspension
There are various methods for suspending a prosthesis, including suction, liners, and belts. Suction suspension is the most secure suspension method. A one-way valve is placed distally in the socket to allow air to exit the socket but not enter. To don a suction socket, a sock is placed on the limb and then the limb is slid into the socket. The sock is then pulled out of the valve opening, pulling the tissue into the socket. Variations on the sock-donning method include the use of a special nylon sock, which makes donning easier by reducing friction; the use of an elastic bandage spirally wrapped on the limb and then pulled out through the opening; and the “wet fit” method, in which lotion is placed on the limb before it is slid down into the socket. Although wet fit is an easier donning technique, if a large amount of soft tissue is present, it can be pushed up and out of the socket rather than being contained in the socket. This can result in a medial adductor roll, which can be a long-term fitting obstacle. The advantage of suction suspension is that it is the most secure suspension available and provides for the greatest control of the prosthesis. The disadvantage of suction is that it can be difficult to don and also that volume stability is critical for suspension. Volume loss can result in loss of suspension, and volume (weight) gain can result in an adductor roll, lack of distal contact, and/or erythema. A vacuum suspension system can also be fitted for those at this level of amputation.
Belt Suspension
Belts can be used to suspend a transfemoral prosthesis or provide increased medial-lateral stability. There are three main types of belts: a total elastic suspension belt, a Silesian belt, and a hip joint and pelvic band (Figure 13-26). Total elastic suspension belt belts, because they are elastic, provide less secure suspension and medial-lateral control. They are typically used more for auxiliary suspension and rotational control. The Silesian belt is made of Dacron webbing and can also be used for auxiliary suspension. When this belt is used alone for suspension, the socket is often worn with a sock ply fit. When better medial-lateral control is needed (for a short limb or for someone with mild hip abductor weakness), a Silesian belt should be used. A high lateral wall also increases the effectiveness of the belt in these cases. A hip joint and pelvic band should be worn for severe hip abductor weakness or by active individuals with a very short limb. The metal hip joint connects the socket to a metal pelvic band and leather belt. This provides the maximum medial-lateral stability; however, it also increases bulk and weight.
Prosthetic Knees
Some knee units also include an extension assist (Figure 13-27). An extension assist acts to help limit heel rise (similar to friction), and it also helps to initiate and ensure full extension. The extension assist can be built into the knee or in some cases can be an optional feature. Not all knee units can have an extension assist. They are more common on knees designed for individuals with less voluntary control.
Single-Axis Knees
Single-axis knees have a single axis of rotation similar to that of a basic door or hinge joint. Stability is achieved only through alignment and voluntary control. A single-axis knee is typically aligned with the weight line slightly in front of the knee axis. As the voluntary control increases, the knee can be moved further anterior to the zero mark, where the weight line passes through the knee axis, often referred to as trigger. The knee should not be moved ahead of this point or instability can occur when the user is momentarily distracted.
Polycentric Knees
Polycentric knees (see Figure 13-27) have more than one axis of rotation. Four-bar knees are a subclass of polycentric knees. Many polycentric knees have more than four axes. The advantages of polycentric knees are that they have a mobile center of rotation, provide an effective shortening of the prosthesis in swing phase, and fold under themselves for increased cosmesis in sitting. Polycentric knees can be designed for individuals with short residual limbs by having a center of rotation that is proximal and posterior to the mechanical joint axes. This allows for increased stability (because the center is posterior) and more voluntary control (because the center is proximal and closer to the residual limb). They can also be designed for long limbs or knee disarticulation amputees, where the center of rotation is close to the knee joint but the knee is designed to fold under the thigh, leaving a lower sitting profile and improving cosmesis.
Weight-Activated Stance-Control Knees
Weight-activated stance-control knees have a braking mechanism that prevents further flexion when weight is applied (Figure 13-28). In this figure the extension assist is external to the knee along the anterior. The braking mechanism is located internally and can be adjusted to be more or less sensitive to the weight required to engage the stance control. These knees have occasionally been referred to as “safety knees,” but because the possibility of knee flexion remains, this is an inappropriate term and should not be used. The braking mechanism of these knees function when weight is applied and the knee is flexed less than approximately 20 degrees. The sensitivity of this adjustment can be set for each individual user, allowing for momentary stability and stumble recovery for those with poor balance. The knee should not be aligned or used in a manner where every step relies on the breaking mechanism to prevent knee flexion, because the wear of the mechanism can cause premature failure and falls.
Manual Locking Knees
Manual locking knees (Figure 13-29) are the most stable of the knee designs. These knees have a switch release for sitting and snap into place at full extension. They cannot be flexed again until the switch release is engaged. Manual locking knees compromise gait mechanics because the patient must walk with a straight leg. The leg should be shorter than the contralateral side to allow clearance. This design is only used for those where maximal stability is the main goal: those with significant weakness or instability, some bilateral amputees, and/or those using the prosthesis primarily for transfers.
Microprocessor-Controlled Knees
New microprocessor-controlled knee systems are also available. One example, the C-leg by Otto Bock (Figure 13-30, A), measures the knee joint angle and the forces in the pylon to determine the phase of the gait cycle and the speed at which the user is ambulating. The microprocessor then adjusts valves to control fluid flow within the hydraulic knee unit. Another computerized knee, the Rheo Knee by Ossur (Figure 13-30, B), is filled with a magnetorheologic fluid that changes flow characteristics when a magnetic field is applied. This knee also uses measured force and position data to determine how to adjust friction for speed or stumbling. Instead of controlling valves, however, the microprocessor controls resistance by adjusting the magnetic field around the fluid. The advantage of computerized knee systems is that they allow knee friction to be varied based on knee angle, forces, speed, and type of activity. When the microprocessor determines that the user is walking faster, it increases friction in swing phase, eliminating excessive knee flexion. If the microprocessor determines that the user has stumbled or is not in a phase of the gait cycle where the knee should bend, it can lock the knee (or increase friction substantially) to allow for recovery and improved stability. These knees can also be set to allow an appropriate friction for easier descent of stairs. As microprocessor computing power evolves, computer-controlled components will continue to allow for a more dynamic responsiveness and make inadvertent flexion of the knee less likely. The primary disadvantages of these microprocessor-controlled knees is that they are very expensive, require robust maintenance, and are not well suited for wet or grimy environments.
Considerations for Other Component Selection
Transfemoral Prosthesis Alignment
Alignment of the transfemoral prosthesis depends on component selection and user strength and ROM. The foot and knee are usually aligned outset with respect to the ischium in the coronal plane. The foot should be further outset (up to approximately the middle of the socket) for those with shorter residual limbs or mild abductor weakness. For those with moderate abductor weakness or for those with a very short limb, however, a Silesian belt or hip joint and pelvic band must be used. In the sagittal plane the socket should be preflexed 5 degrees from vertical beyond the amputee’s hip flexion range. For example, if the amputee has a 5 degrees flexion contracture, the prosthesis should be aligned with 10 degrees of flexion in the socket. This accommodates the loss of knee flexion in late stance at opposite heel contact and allows the user to take a normal sound side step. If this alignment is not made, the prosthetic user might take a short step and, if a flexion contracture is not accommodated, can also result in an unstable knee. For knee alignment with the quadrilateral socket fitting, the trochanter-knee-ankle line is used (Figure 13-31). For this alignment a single-axis knee should fall 6 mm posterior to a line connecting the greater trochanter and ankle. For ischial containment sockets a weight line is typically used. The starting point for alignment is a plumb line from the bisection of the socket at ischial level. Each knee design has a manufacturer-suggested alignment, but generally the knee axis should be posterior to this line. Moving the knee axis further posterior increases the stability. A knee positioned closer to the weight line requires more voluntary control, but allows easier knee flexion in late stance phase and a more normal appearing gait. During dynamic alignment the goal is to have a knee that is stable but easy to control. The foot should progress from heel to toe smoothly, and in the coronal plane, the pelvis should be maintained close to level, and the foot should be flat on the floor. A list of common gait deviations is presented in Table 13-3.
Table 13-3 Common Gait Deviations for the Patient With a Transtibial Amputation
| Gait Deviation | Prosthetic Causes | Amputee Causes |
|---|---|---|
| Lateral trunk bending: excessive bending occurs laterally away from midline toward prosthetic side | Prosthesis too short Improperly shaped lateral wall fails to support femur Lack of ischial support (high medial wall cause amputee to hold prosthesis away to avoid ramus pressure) Prosthesis aligned in abduction, causing a wide-based gait |
Inadequate balance Abduction contracture Residual limb painful Short limb can fail to provide sufficient lever arm for the pelvis. Poor gait habit |
| Abducted gait: wide-based gait with prosthesis held away from body at all time (stance and swing) | Prosthesis too long Too much abduction built into prosthesis Lack of ischial support (high medial wall can cause amputee to hold prosthesis away to avoid ramus pressure) Improperly shaped lateral wall fails to support femur. Pelvic band can be positioned too far away from the body. |
Abduction contracture Poor gait habit |
| Circumducted gait: the swinging of the prosthesis laterally in a wide arc, returning to vertical for stance phase | Prosthesis too long Too much alignment stability or friction in the knee, making it difficult to bend in swing phase Extension aid too strong |
Abduction contracture Lack of confidence for flexing the knee because of muscle weakness or fear of stubbing the toe Poor gait habit |
| Vaulting | Prosthesis too long Inadequate suspension Excessive knee stability caused by alignment, excessive knee friction, or an excessive extension assist |
Poor gait habit Fear of stubbing the toe Residual limb pain |
| Rotation of the prosthetic foot at heel strike | Heel too firm Too much toe out Loose socket fit Posterior socket tightness |
User can extend limb too vigorously at heel strike. Poor muscle control of the limb |
| Uneven arm swing, with arm on prosthetic side held close to the body | Improperly fitting socket Poor suspension |
Poor balance Fear and insecurity accompanied by uneven timing Poor gait habit |
| Uneven timing, characterized by a short stance phase on the prosthetic side | Improperly fitting socket Weak extension aid or insufficient friction can cause excessive heel rise and result in a longer amount of time spent on the sound side. Knee instability |
Weak residual limb User cannot have developed good balance. Fear and insecurity Pain |
| Uneven heel rise | Knee joint can have insufficient friction. Inadequate extension aid |
User can be using more power than necessary to force the knee into flexion. |
| Terminal swing impact, characterized by rapid forward movement of the shin allowing the knee to reach maximum extension with too much force before heel strike | Insufficient knee friction Knee extension aid can be too strong. |
User can deliberately and forcibly extend residual limb to ensure full extension of knee. |
| Instability of the prosthetic knee, which creates a danger of falling | Knee joint can be aligned anterior to weight line or trochanter-knee-ankle line. Insufficient initial flexion can have been built into the socket. Heel can be too firm, causing the knee to buckle at heel strike. Foot keel can be too soft, allowing knee flexion to occur late in stance. |
User can have weak hip extensors. Severe flexion contracture can cause instability. |
| Medial or lateral whip: these are best observed as the user walks away. A medial whip is present when the heel travels medially on initial flexion at the beginning of swing phase, and a lateral whip exists when the heel moves laterally. | Lateral whips occur from excessive internal rotation of the knee with respect to the socket. Medial whips can result from excessive external rotation of the knee. Socket can fit too tightly, reflecting limb rotation. Excessive valgus in the prosthesis can contribute. Socket can have been donned improperly. |
Faulty gait habits can result in whips. |
| Foot slap: a rapid descent of the anterior of the foot at heel strike | Plantar flexion resistance is too soft (heel too soft). | User can be driving the prosthesis into the ground at heel strike to ensure knee stability. |
| Drop-off at the end of stance: downward movement of the trunk as the body moves forward over the prosthesis | Inadequate limitation of dorsiflexion of the foot Keel of the foot can be too short or too soft. Socket can have been placed too far anterior with respect to the foot. |
None |
| Long prosthetic step (or short sound side step): the user takes a longer step with the prosthesis than the sound leg. | Insufficient flexion included in the socket | A flexion contracture that cannot be accommodated prosthetically |
| Excessive trunk extension: during stance phase the user creates excessive, active lumbar lordosis. | Improperly shaped posterior wall causing forward rotation of the pelvis to avoid full weight-bearing on the ischium Insufficient initial flexion built into the socket |
Hip flexor tightness Weak hip extensors, substituted for by lumbar erector spinae Weak abdominal muscles Poor gait habit Poor balance |
| External rotation of the foot at heel off: viewed from posterior, the heel is seen to rotate internally before swing phase. | Insufficient flexion included in the socket | Hip flexor tightness |
Courtesy Northwestern University Prosthetic-Orthotic Center, Chicago, IL.
Hip Disarticulation and Transpelvic Amputation (Hemipelvectomy)
Hip disarticulation and hemipelvectomy amputations are usually performed on patients with femoral malignancy or severe trauma. The basic technique for hip disarticulation was described by Boyd.11 The wound is generally closed with the gluteus maximus being sutured to remnant of the adductor muscles to provide padding to the residual tissue.
For the hip disarticulation level of amputation, the pelvis should be intact. In some cases, those with short transfemoral amputation (when there is not enough femur length to control a transfemoral prosthesis) can be fitted with a hip disarticulation prosthesis. The hip disarticulation and hemipelvectomy socket must allow for weight-bearing during stance phase, provide coronal plane stability during stance phase, and provide suspension during swing phase. When the ischium is present (for hip disarticulation and some hemipelvectomy cases), it can provide a loading surface and increase medial-lateral stability. Some weight must also be applied along the lateral surface of the torso. Typically the socket wraps around the pelvis (Figure 13-32), with loading occurring above the iliac crest, and medial-lateral stability occurring through loading of the iliac fossa. For hemipelvectomy fittings where a significant portion of the pelvis has been removed, the trim-lines should continue to just below the costal margin and further include the abdominal tissue. The higher trim-lines ensure more area for weight-bearing.
Translumbar Amputation (Hemicorporectomy)
The translumbar amputation is essentially an ablation of the caudal 50% of the human body, including the legs, pelvis, and genitourinary and reproductive organs. It is also termed hemicorporectomy, although in this case the “hemi” refers to the lower half of the body as opposed to half of one side being amputated. The lower half of the body with pelvis is removed, with creation of a colostomy and a urinary diversion stoma for bowel and bladder elimination.99
Bilateral Amputee Considerations
The occurrence of bilateral transtibial amputations unfortunately is becoming increasingly common. Literature shows that between 28% and 51% of individuals with diabetic amputations lose their contralateral limb within 5 years of their first limb amputation.78 This population of patients requires that attention be paid to techniques that preserve the alternate limb (see Chapter 57). Bilateral amputees do not have a noninvolved side to compensate for the limitations of a prosthesis, especially the loss of ankle motion. A torsion pylon or foot with ankle motion in various planes is often recommended because it assists the patient in attempting to compensate for these lost motions. Multiaxial feet or ankles used to be thought of as being contraindicated for bilateral lower limb amputees, because it was thought that solid ankle systems were necessary for the amputee to be able to stand stably within a secure base of support. However, with proper fitting and training, they have proven to be a beneficial addition. Without the sensory feedback, however, some individuals feel unsteady and prefer solid ankle systems.
One way to facilitate a successful bilateral transfemoral prosthetic gait is to start with very short prostheses called “stubbies” (Figure 13-33). Stubbies are prostheses that are close to the ground, with the foot component attached directly to the distal end of the transfemoral socket (or close enough to make the height of the prosthesis even with the contralateral side). These training prostheses bring the center of mass closer to the ground, providing more stability for ambulation. The feet are initially mounted in reverse to keep the center of mass farther back in the user’s base of support and to minimize the risk of falling backward. There are no knees in stubbies. Thus the user does not have to concentrate on controlling the prosthetic knee and preventing the knee from flexing. Patients begin by focusing on socket fit and basic ambulation skills. As progress is made, the devices are raised to greater height, the feet are reversed to a forward-pointing direction, and knee units are added one at a time (usually on the longer residual limb first). Eventually the devices are raised to the desired final height, and the amputee can progress to using knees with less inherent stability, as well as using less restrictive gait aids. This is a long and time-consuming process that demands commitment from both the patient and the rehabilitation team.
FIGURE 13-33 Stubbies: bilateral transfemoral prosthesis with no knees and the feet mounted backward.
Energy Expenditure
The effect of amputation on the energy cost of ambulation is significant. Typically the cost of ambulation is measured in oxygen consumption. Although various measurement techniques exist, usually the volume of air inhaled and the amount of carbon dioxide exhaled is used to calculate energy expenditure and is subsequently scaled as a function of body mass. The results can be reported as a function of distance or as a rate of oxygen consumption.
With respect to rate of oxygen consumption, individuals with an amputation typically walk slower to keep their rate of oxygen consumption comparable to that of healthy controls. Individuals with or without amputation tend to self-select a comfortable walking speed that provides a comparable rate of oxygen consumption.101 In addition, the relative energy cost, defined as the rate of consumption divided by the maximum aerobic capacity, is comparable for amputations with a traumatic etiology and those with a vascular etiology. the only exception being that transfemoral amputations with a vascular etiology have a higher relative energy cost.101 Some studies have found that younger patients with amputations related to trauma can walk with a higher oxygen consumption rate but at a faster pace than those with older patients with amputation or patients with amputation secondary to vascular problems, but at a slower pace than healthy controls.100
While the rate of oxygen consumption tends to be similar across individuals, those with amputation incur a larger oxygen cost per distance than a nonamputee walking at the same speed. Individuals with vascular amputations have higher oxygen consumption per distance than those with traumatic amputations.101 Unilateral traumatic transtibial amputees use approximately 25% more energy to walk the same distance as nonamputees. This increases to 63% for traumatic transfemoral amputees. Vascular transtibial and transfemoral amputees use up to 40% and 120% more energy, respectively.100,102
In addition to oxygen consumption and rate, maximal aerobic capacity influences an individual’s ability to perform with a prosthesis. This capacity decreases with both age and vascular involvement.101 As such, older patients with higher level amputations have less aerobic capacity and are closer to their anaerobic threshold when ambulating.
Recreational activities
People with lower limb loss can enjoy most recreational activities with conventional prostheses, but some components are better suited than others for different activities. High-level activity functions require very secure suspension for safe participation. Suction-type suspension systems are generally preferred. Sports involving running and jumping are best served by high-profile dynamic response feet. For transfemoral amputees, running requires a high-performance variable cadence (hydraulic) knee. Competitive running is a sport where a dedicated prosthetic foot is seen. These feet are custom-made to provide the correct energy return for the individual’s weight and running style. Most designs lack a heel because during short runs, force is only applied to the toes (Figure 13-34). This makes stopping and standing difficult, especially for bilateral amputees. Insurance companies rarely fund these dedicated devices, and cost can make personal purchase prohibitive. Competitive athletes that require dedicated devices like a running foot are often sponsored by companies that supply the componentry.
Golf is a very popular sport for many amputees, including dysvascular amputees. The National Amputee Golf Association (see Box 13-1) exists to assist people with this sport and sponsors many events. While golfing can be done with most prostheses, the addition of a tibial rotation unit can be helpful because it allows the necessary tibial rotation to occur during the swing of the golf club.
Although most amputees enjoy swimming without the use of a prosthesis, a water leg is useful for the beach or being around water. This can be a prosthesis with water-resistant components or even just a pull-up covering that keeps the prosthesis reasonably dry. Prostheses for use with swimming are also made and include ankles that allow the foot to be plantar flexed for a kicking position. The Leisure Activity Ankle (Figure 13-35) has a design that can be incorporated into modular prostheses. The design permits the ankle to be locked in a neutral or plantar-flexed posture with the turn of a lever. The neutral position is used for limited ambulation, while the plantar-flexed position is designed to be used in water activities such as scuba diving. Because most prosthetic devices are not waterproof, dedicated sockets and attachments are required. Often hollow exoskeletal designs are used, which eliminate most of the metal components. It is also necessary to design a construction that can fill with water and drain easily to limit the effects of buoyancy of the limb. Some swimmers attach a fin directly to the end of their residual limb, eliminating the need for a dedicated prosthesis. All of these components are typically used by recreational swimmers and scuba divers, because competitive swimmers are not allowed to use assistive devices.
Individuals with a prosthesis sometimes struggle with bike riding because the prosthetic foot is difficult to keep on the pedal, especially for transfemoral amputees. Recreational bikers can either use cages to help keep the foot from sliding off the pedal, or biking shoes with cleats that lock onto the pedal. This creates a safety concern, however, because the lock can be difficult to disengage. Sometimes an old prosthesis can be dedicated to bike riding and aligned in a more advantageous position. Competitive cyclists are usually fitted with extremely rigid feet (to better transfer forces to the pedals) and shoe cleats that are built directly into the bottom of the foot.
Pediatric Amputation
Classifications of Limb Deficiencies
There have been many ways of describing limb deficiencies in the past, but currently there are two main headings for these classifications under the International Organization for Standardization and the International Society for Prosthetics and Orthotics classification of congenital limb deficiency: transverse and longitudinal.47 Transverse deficiencies are defined as normal development until the point of the deficiency, beyond which the normal anatomy does not exist. These transverse deficiencies present as guillotine-type amputations, in the fact that no segments distal to the amputation site exist. Most of the causes for these are unknown and are thought to have resulted from vascular disruptions in utero. Transverse deficiency can also result from amniotic band syndrome, where the limb bud is caught in a band that constricts it and prevents normal development. Other regions of the body can experience this banding as well.
Common Lower Limb Deficiencies
Longitudinal Deficiency of the Fibula
Longitudinal deficiency of the fibula is the most common long bone absence39 (Figure 13-36). It is often associated with other anomalies present including a deformed foot (usually with absent lateral ray[s]), valgus angulation at the knee, anterior (kyphoscoliotic) bowing of the tibia, a subcutaneous dimple over the apex of the tibia, ipsilateral femoral shortening, and limited hip internal rotation. The two most common treatments for this anomaly are some form of ankle disarticulation amputation, or lengthening with an external fixator and often subsequent ankle arthrodesis (fusion). There are conflicting opinions regarding the outcome of these two interventions. If the family elects to proceed with the ankle disarticulation, the child can be fitted with a prosthesis shortly thereafter. The more traditional Syme’s amputation for children with fibular deficiencies has proven to be very beneficial. Migration of the heel pad posterior-proximally has sometimes led surgeons to perform Boyd procedures to ensure the centralization of the heel pad. Once fitted with a prosthesis, the child is able to walk with a relatively normal gait. Although there are space limitations for prosthetic feet while the child is very young, the natural occurrence of ipsilateral femoral shortening or the use of an epiphysiodesis can lead to adequate room for most prosthetic foot designs as the child matures. Other factors that can compromise the prosthetic fittings are the lack of ability to end-bear as the child gets larger, and the increased genu valgum resulting from the absence of a lateral stabilizer. This valgus attitude, combined with a long residual limb and an appropriate prosthetic alignment, creates a large bump on the lateral aspect of the prosthesis, compromising cosmesis.
Longitudinal Deficiency of the Femur (Proximal Femoral Focal Deficiency)
Proximal femoral focal deficiency (PFFD), the second most common congenital deficiency of the lower limb, is a prosthetic, surgical, and rehabilitative challenge. PFFD is a congenital deficiency that can present in a large variety of ways, primarily with a clinical presentation of hip flexion, abduction, and external rotation. Several methods of classifications for femoral malformation or PFFD have been described. Aitken’s method is the most broadly accepted (Figure 13-37).2 Aitken classified the PFFD levels according to acetabular and femoral involvement. This classification of A through D is designed with class A being the least involved and class D being the most severely involved. In addition to the femoral and acetabular involvement, the aforementioned longitudinal deficiency of the fibula is present in a large percentage of individuals with PFFD. Because of this severe instability and proximal position of the foot, surgical intervention is often recommended to reshape the acetabulum, disarticulate the foot, fuse the knee, and/or rotate the foot posteriorly. These interventions vary based on clinical setting, geographic location, cultural issues related to body image, and parental wishes.
The third most common intervention for PFFD is to perform a rotationplasty of the foot 180 degrees (Figure 13-38). This procedure, popularized by Van Nes,97 allows children to use their ankle as a knee unit. To ensure success of a rotationplasty, the hip joint and ankle joint integrity must not be compromised. The ankle dorsiflexors will be used as knee flexors, while the ankle plantar flexors will be used as knee extensors. It is critically important for these individuals to maintain ankle strength and ROM for this procedure to be a success. Once again, individuals can be successfully fitted with a prosthesis that has outside hinges. They might also need an endoskeletal knee below the foot complex to provide increased knee flexion to enter tight spaces such as cars.
Training and Treatment Goals
Training for children is, in most cases, playing outside and participating in daily activities. The initial training for children requiring prostheses below the knee is minimal. They will begin with a very abducted gait pattern at first and then progress to a more normal gait as they mature. Children with amputations above the knee, at various PFFD levels or hip disarticulation, require more formalized gait training. All of these children are encouraged to use the prostheses for pull-to-stand activities, which occur anywhere from 8 to 18 months. The children’s progress will be based on their limb lengths, strength, and ROM in their affected limb.
1. Adunsky A., Wershawski M., Arad M., et al. Non-traumatic lower limb older amputees: a database survey from a geriatric centre. Disabil Rehabil. 2001;23(2):80-84.
2. Aitken G.: Proximal femoral focal deficiency: definition, classification, and management. In Aiken CT, editor: Proximal femoral focal deficiency: a congenital anomaly, Washington, DC, 1969, National Academy of Science
3. Allende M.F., Barnes G.H., Levy S.W., et al. The bacterial flora of the skin of amputation stumps. J Invest Dermatol. 1961;36:165-166.
4. Aulivola B., Hile C.N., Hamdan A.D., et al. Major lower limb amputation: outcome of a modern series. Arch Surg. 2004;139(4):395-399. discussion 399
5. Bacharach J.M., Rooke T.W., Osmundson P.J., et al. Predictive value of transcutaneous oxygen pressure and amputation success by use of supine and elevation measurements. J Vasc Surg. 1992;15(3):558-563.
6. Backonja M.M. Anticonvulsants (antineuropathics) for neuropathic pain syndromes. Clin J Pain. 2000;16(suppl 2):S67-S72.
7. Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16(suppl 2):S12-S20.
8. Bennett G.J. Neuropathic pain. In: Wall P.D., Melzack R., editors. Textbook of pain. Edinburgh: Churchill Livingstone, 1994.
9. Board W.J., Street G.M., Caspers C. A comparison of trans-tibial amputee suction and vacuum socket conditions. Prosthet Orthot Int. 2001;25(3):202-209.
10. Bone M., Critchley P., Buggy D.J. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Reg Anesth Pain Med. 2002;27(5):481-486.
11. Boyd H. Anatomic disarticulation of the hip. Surg Gynecol Obstet. 1947;84:346-349.
12. Bradley N., Miller W.A., Evans J.P. Plantar neuroma: analysis of results following surgical excision in 145 patients. South Med J. 1976;69(7):853-854.
13. Brodzka W.K., Thornhill H.L., Zarapkar S.E., et al. Long-term function of persons with atherosclerotic bilateral below-knee amputation living in the inner city. Arch Phys Med Rehabil. 1990;71(11):895-900.
14. Burgess E.M., Romano R.L. The management of lower limb amputees using immediate postsurgical prostheses. Clin Orthop. 1968;57:137-146.
15. Canfield J.A., Reiber G.E., Cannard C., et al. Survival following lower-limb amputation in a veteran population. J Rehabil Res Dev. 2001;38(3):341-345.
16. Chan B.L., Witt R., Charrow A.P., et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357(21):2206-2207.
17. Charrow A., DiFazio M., Foster L., et al. Intradermal botulinum toxin type A injection effectively reduces residual limb hyperhidrosis in amputees: a case series. Arch Phys Med Rehabil. 2008;89(7):1407-1409.
18. Cohen S.I., Goldman L.D., Salzman E.W., et al. The deleterious effect of immediate postoperative prosthesis in below-knee amputation for ischemic disease. Surgery. 1974;76(6):992-1001.
19. Darnall B.D. Self-delivered home-based mirror therapy for lower limb phantom pain. Am J Phys Med Rehabil. 2009;88(1):78-81.
20. Darnall B.D., Ephraim P., Wegener S.T., et al. Depressive symptoms and mental health service utilization among persons with limb loss: results of a national survey. Arch Phys Med Rehabil. 2005;86(4):650-658.
21. Davis R.W. Phantom sensation, phantom pain, and stump pain. Arch Phys Med Rehabil. 1993;74(1):79-91.
22. Devor M. The pathophysiology of damaged peripheral nerves. In: Wall P.D., Melzack R., editors. Textbook of pain. Edinburgh: ChurchillLivingstone, 1994.
23. Dillingham T.R., Pezzin L.E., MacKenzie E.J. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95(8):875-883.
24. Health Care Financing Administration: Healthcare common Procedure Coding System, Washington, DC, 2001, US Government Printing Office.
25. Dwars B.J., van den Broek T.A., Rauwerda J.A., et al. Criteria for reliable selection of the lowest level of amputation in peripheral vascular disease. J Vasc Surg. 1992;15(3):536-542.
26. Ebskov B., Josephsen P. Incidence of reamputation and death after gangrene of the lower limb. Prosthet Orthot Int. 1980;4(2):77-80.
27. Edelson J., Fitzpatrick J.L. A comparison of cognitive-behavioral and hypnotic treatments of chronic pain. J Clin Psychol. 1989;45(2):316-323.
28. Eggers P.W., Gohdes D., Pugh J. Nontraumatic lower limb amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56(4):1524-1533.
29. Ehde D.M., Czerniecki J.M., Smith D.G., et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81(8):1039-1044.
30. Eneroth M: Improved wound healing in transtibial amputees receiving supplementary nutrition. Presented at the IXth World Congress of the International Society of Prosthetics and Orthotics, Amsterdam, June 28–July 3, 1998.
31. English R. Removable rigid dressing for transtibial amputees: a randomized study. Paper presented at: X World Congress of the International Society of Prosthetics and Orthotics, 2001.
32. Ephraim P.L., Wegener S.T., MacKenzie E.J., et al. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86(10):1910-1919.
33. Fields H.L., Heinricher M.M., Mason P. Neurotransmitters in nociceptive modulatory circuits. Ann Rev Neurosci. 1991;14:219-245.
34. Finsen V., Persen L., Lovlien M., et al. Transcutaneous electrical nerve stimulation after major amputation. J Bone Joint Surg Br. 1988;70(1):109-112.
35. Fletcher D.D., Andrews K.L., Butters M.A., et al. Rehabilitation of the geriatric vascular amputee patient: a population-based study. Arch Phys Med Rehabil. 2001;82(6):776-779.
36. Fletcher D.D., Andrews K.L., Hallett J.W.J., et al. Trends in rehabilitation after amputation for geriatric patients with vascular disease: implications for future health resource allocation. Arch Phys Med Rehabil. 2002;83(10):1389-1393.
37. Flor H. Cortical reorganisation and chronic pain: Implications for rehabilitation. J Rehabil Med. 2003;41(suppl):66-72.
38. Frank R.G., Kashani J.H., Kashani S.R., et al. Psychological response to amputation as a function of age and time since amputation. Br J Psychiatry. 1984;144:493-497.
39. Froster U.G., Baird P.A. Congenital defects of lower limbs and associated malformations: a population based study. Am J Med Genet. 1993;45(1):60-64.
40. Gauthier-Gagnon C., Grise M.C., Potvin D. Enabling factors related to prosthetic use by people with transtibial and transfemoral amputation. Arch Phys Med Rehabil. 1999;80(6):706-713.
41. Gottschalk F. Transfemoral amputation. In Bowker J., Michael J., editors: The atlas of limb prosthetics: surgical, prosthetic, and rehabilitation principle, ed 2, St Louis: Mosby, 1992.
42. Greenfield J., Rea J.Jr., Ilfeld F. Morton’s interdigital neuroma. Indications for treatment by local injections versus surgery. Clin Orthop. 1984;(185):142-144.
43. Gregor S., Maegele M., Sauerland S., et al. Negative pressure wound therapy: a vacuum of evidence? Arch Surg. 2008;143(2):189-196.
44. Gruber H., Glodny B., Kopf H., et al. Practical experience with sonographically guided phenol instillation of stump neuroma: predictors of effects, success, and outcome. AJR Am J Roentgenol. 2008;190(5):1263-1269.
45. Harden R.N. Gabapentin: a new tool in the treatment of neuropathic pain. Acta Neurol Scand. 1999;173(suppl):43-47.
46. Hoyt C., Littig D., Lundt J., et al. The UCLA CAT-CAM above knee socket. Los Angeles: UCLA Prosthetics Education and Research Program; 1987.
47. International Organization for Standardization: ISO 8598-1: Prosthetics and orthotics—limb deficiencies. Part I. Method of describing limb deficiencies present at birth, Geneva, 1989, International Organization for Standardization.
48. Janig W. Pathophysiology of nerve following mechanical injury in man. In: Dubner R., Gebhart G., Bond M., editors. Pain research and clinical management. Amsterdam: Elsevier, 1988.
49. Johannesson A., Larsson G.U., Oberg T. From major amputation to prosthetic outcome: a prospective study of 190 patients in a defined population. Prosthet Orthot Int. 2004;28(1):9-21.
50. Kuiken T., Huang M., Harden N.: Peri-operative rehabilitation of the transtibial and transfemoral amputee, Phys Med Rehabil State Art Rev 16(3):521-537, 2002
51. Larsson G. Post operative treatment with silicone liner in 176 amputees. Presented at X World Congress of the International Society of Prosthetics and Orthotics, Glasgow; 2001.
52. Levy C.E., Bryant P.R., Spires M.C., et al. Acquired limb deficiencies. 4. Troubleshooting. Arch Phys Med Rehabil. 2001;82(3 suppl 1):S25-S30.
53. Levy S. Skin problems of the amputee. In: Bowker J., editor. Atlas of limb prosthetics: surgical, prosthetic, and rehabilitation principle. St Louis: Mosby, 1992.
54. Levy S.: Skin problems in the amputee. In Smith D., Michael J., Bowker J., editors: Atlas of amputations and limb deficiencies, 2004, American Academy of Orthopaedic Surgeons
55. Loeser J. Pain after amputation: phantom limb and stump pain. In: Bonica J., editor. The management of pain. London: Churchill Livingstone; 1994:244-256.
56. Loeser J.D., Black R.G., Christman A. Relief of pain by transcutaneous stimulation. J Neurosurg. 1975;42(3):308-314.
57. Longman J. Debate on an amputee sprinter: is he disabled or too-abled? The New York Times 2007 May 15; Sect. A.
58. MacKenzie E.J., Bosse M.J., Castillo R.C., et al. Functional outcomes following trauma-related lower-limb amputation. J Bone Joint Surg Am. 2004;86(8):1636-1645.
59. Melzack R. The tragedy of needless pain. Science. 1990;262:27-33.
60. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971-979.
61. Meulenbelt H.E., Geertzen J.H., Jonkman M.F., et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90(1):74-81.
62. Muilenberg A., Wilson A.: A manual for above knee amputees, Alexandria, VA, 1989, American Academy of Orthotists and Prosthetists
63. NgDavid B.E., Hunter G., Berbrayer D. Transtibial amputation: preoperative vascular assessment and functional outcome. J Prosthet Orthot. 1996;8(4):123-129.
64. Nielsen D., Shurr D., Golden J., et al. Comparison of energy cost and gait efficiency during ambulation in below-knee amputees using different prosthetic feet: a preliminary report. J Prosthet Orthot. 1988;1(1):24-31.
65. Oakley D.A., Whitman L.G., Halligan P.W. Hypnotic imagery as a treatment for phantom limb pain: two case reports and a review. Clin Rehabil. 2002;16(4):368-377.
66. Ortiz M. Medical ramus containment socket design for transfemoral prosthesis. Presented at American Academy of Orthotists and Prosthetists Annual Meeting and Scientific Symposium, New Orleans. 2004.
67. Ouriel K., Green R. Arterial disease. In Shires E., editor: Principles of surgery, ed 7, New York, McGraw Hill, 1999.
68. Owings M.F., Kozak L.J. Ambulatory and inpatient procedures in the United States, 1996, Vital Health Stat 13. 1998;139:1-119.
69. Pasquina P.F., Bryant P.R., Huang M.E., et al. Advances in amputee care. Arch Phys Med Rehabil. 2006;87((3 suppl 1):S34-S43.
70. Patterson J.F. Carbamazepine in the treatment of phantom limb pain. South Med J. 1988;81(9):1100-1102.
71. Pezzin L.E., Dillingham T.R., Mackenzie E.J., et al. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729.
72. Pinzur M.S., Littooy F., Daniels J. Multidisciplinary preoperative assessment and late function in dysvascular amputees. Clin Orthop. 1992;281:239-243.
73. Potter B.K., Burns T.C., Lacap A.P., et al. Heterotopic ossification following traumatic and combat-related amputation: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89(3):476-486.
74. Radcliffe C. Functional considerations in the fitting of above-knee prostheses. Artif Limbs. 1955;1:35-60.
75. Radcliffe C., Foort J. The patellar-tendon-bearing below-knee prosthesis. San Francisco–Berkeley: University of California Biomechanics Laboratory; 1961.
76. Ramanavarapu V., Simopoulos T.T. Pulsed radiofrequency of lumbar dorsal root ganglia for chronic post-amputation stump pain. Pain Physician. 2008;11(4):561-566.
77. Raymond S., Rocco A. Ephaptic coupling of large fibers as a clue to mechanisms in chronic neuropathic allodynia following damage to dorsal roots. Pain. 1990;5:S276.
78. Reiber G., Boyko E., Smith D.: Lower limb foot ulcers and amputations in diabetes. In Harris M., Cowie C., Sterm MP., editors: Diabetes in America, ed 2, Washington, DC, 1995, National Institutes of Health Publication
79. Reiber G.E., Smith D.G., Carter J., et al. A comparison of diabetic foot ulcer patients managed in VHA and non-VHA settings. J Rehabil Res Dev. 2001;38(3):309-317.
80. Remes L., Isoaho R., Vahlberg T., et al. Major lower extremity amputation in elderly patients with peripheral arterial disease: incidence and survival rates. Aging Clin Exp Res. 2008;20(5):385-393.
81. Resnick H.E., Valsania P., Phillips C.L. Diabetes mellitus and nontraumatic lower limb amputation in black and white Americans: the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study, 1971-1992. Arch Intern Med. 1999;159(20):2470-2475.
82. Robinson L.R., Czerniecki J.M., Ehde D.M., et al. Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Arch Phys Med Rehabil. 2004;85(1):1-6.
83. Rybarczyk B., Nicholas J. Nyenhuis. Coping with a leg amputation: integrating research and clinical practice. Rehabil Psychol. 1997;42(3):241-256.
84. Sabolich J. Contoured adducted trochanteric-controlled alignment method (CAT-CAM): introduction and basic principles. Clin Prosthet Orthot. 1985;9:15-26.
85. Schechtman L., Kuiken T., Harden N. Phantom limb pain treatment with mirtazapine: a case series. Arch Phys Med Rehabil. 2002;83:1668.
86. Scully S., Harrelson J. Amputation and limb substitution. In Sabiston D., editor: Textbook of surgery: the biological basis of modern surgical practice, ed 5, Philadelphia: WB Saunders, 1997.
87. Sherman R.A., Gall N., Gormly J. Treatment of phantom limb pain with muscular relaxation training to disrupt the pain–anxiety–tension cycle. Pain. 1979;6(1):47-55.
88. Siegel E.F. Control of phantom limb pain by hypnosis. Am J Clin Hypn. 1979;21(4):285-286.
89. Smith D.G., McFarland L.V., Sangeorzan B.J., et al. Postoperative dressing and management strategies for transtibial amputations: a critical review. J Rehabil Res Dev. 2003;40(3):213-224.
90. Stansbury L.G., Lalliss S.J., Branstetter J.G., et al. Amputations in U.S. military personnel in the current conflicts in Afghanistan and Iraq. J Orthop Trauma. 2008;22(1):43-46.
91. Stone P.A., Flaherty S.K., Hayes J.D., et al. Lower extremity amputation: a contemporary series. W V Med J. 2007;103(5):14-18.
92. Thackham J.A., McElwain D.L., Long R.J. The use of hyperbaric oxygen therapy to treat chronic wounds: a review. Wound Repair Regen. 2008;16(3):321-330.
93. Thornhill H.L., Jones G.D., Brodzka W., et al. Bilateral below-knee amputations: experience with 80 patients. Arch Phys Med Rehabil. 1986;67(3):159-163.
94. Trautner C., Haastert B., Giani G., et al. Amputations and diabetes: a case-control study. Diabet Med. 2002;19(1):35-40.
95. Tremont-Lukats I.W., Megeff C., Backonja M.M. Anticonvulsants for neuropathic pain syndromes: mechanisms of action and place in therapy. Drugs. 2000;60(5):1029-1052.
96. Uellendahl J., Edwards M., Uellendahl E. Northwestern University transfemoral fitting manual: ischial containment above knee prosthesis. Chicago: Northwestern University Prosthetic Certification Program; 1998.
97. Van Nes C. Rotation-plasty for congenital defects of the femur: making use of the ankle shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg Br. 1950;32:12-16.
98. Vikatmaa P., Juutilainen V., Kuukasjarvi P., et al. Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg. 2008;36(4):438-448.
99. Wagman L., Terz J. Translumbar amputation (hemicorpectomy). In: Bowker J., Michael J., editors. Atlas of limb prosthetics: surgical, prosthetic, and rehabilitation principles. St Louis: Mosby, 1992.
100. Waters R.L., Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. 1999;9(3):207-231.
101. Waters R.L., Perry J., Antonelli D., et al. Energy cost of walking of amputees: the influence of level of amputation. J Bone Joint Surg Am. 1976;58(1):42-46.
102. Waters R.L., Perry J., Chambers R. Energy expenditure of amputee gait. In: Moore W., Malone J., editors. Lower limb amputation. Philadelphia: W.B. Saunders, 1989.
103. Wilkes D., Ganceres N., Solanki D. Pulsed radiofrequency treatment of lower extremity phantom limb pain. Clin J Pain. 2008;24(8):736-739.
104. Wu Y., Brncick M., Krick M.J., et alPutnam T.D. Scotchcast P.V.C. interim prosthesis for below-knee amputees. Bull Prosthet Res. 1981;10-36:40-45.
105. Wu Y., Keagy R.D., Krick H.J., et al. An innovative removable rigid dressing technique for below-the-knee amputation. J Bone Joint Surg Am. 1979;61(5):724-729.
106. Ziegler-Graham K., MacKenzie E.J., Ephraim P.L., et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429.
107. Zinser L. Bolt blazes to gold medal and a world record. The New York Times. 2008 August 17. Sect. SP