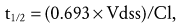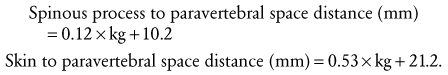41 Regional Anesthesia
THE USE OF REGIONAL anesthesia techniques in children has increased dramatically in the past two decades.1–9 Regional anesthesia is most commonly used in conjunction with general anesthesia, although in certain circumstances regional anesthesia may be the sole technique. In addition to central neuraxial blocks, peripheral nerve blocks are now employed with increasing frequency, in part because of the introduction of high-resolution portable ultrasound imaging. Ultrasound-guided visualization of anatomic structures permits both greater precision of needle or catheter placement and confirmation that the drug has been deposited at the site of choice (see Chapter 42). The advent of ultrasound guidance has also facilitated the performance of several blocks, including truncal blocks, which could not be otherwise performed safely using landmark techniques, especially in children. Evidence from recent large-scale collaborative studies of regional blockade in children supports the contention that peripheral nerve blockade is assuming greater prominence in pediatric anesthesia, and data from the Pediatric Regional Anesthesia Network (PRAN) suggests that increased use of ultrasound guidance may be, at least in part, driving this trend.10 Supplementing a general anesthetic with a nerve block can result in a pain-free awakening and postoperative analgesia without the potentially deleterious side effects associated with parenteral opioids (see Chapter 43).11 This benefit may be of particular importance to neonates, former preterm infants, and in children with cystic fibrosis and other conditions.4 There is also evidence that suggests that regional anesthesia may improve pulmonary function in children who have undergone thoracic or upper abdominal surgery.12–15 Lastly, the greatly increased number of “same day surgery” cases in recent years has made the advantages of regional anesthesia, such as the rapid awakening, enhanced postoperative analgesia with no sedation or altered sensorium, and lack of opioid-induced nausea or vomiting, even more apparent. The safe and effective use of these techniques in children, however, requires an understanding of both the developmental anatomy of the region in which the block is placed and the developmental pharmacology of local anesthetics. This chapter will focus on landmark-guided techniques for those who do not have the advantage of ultrasound imaging; ultrasound techniques are described in Chapter 42.
Pharmacology and Pharmacokinetics of Local Anesthetics
There are two classes of clinically useful local anesthetics, the amino amides (amides) and the amino esters (esters) (Table 41-1). The amides are degraded in the liver by cytochrome P450 enzymes, whereas the esters are hydrolyzed primarily by plasma cholinesterases.16–20 These degradation pathways account for some of the differences in distribution and metabolism of local anesthetics, particularly in neonates when compared with adults.
| Esters | Amides |
|---|---|
| Procaine | Lidocaine |
| Tetracaine | Mepivacaine |
| 2-Chloroprocaine | Bupivacaine |
| Levobupivacaine | |
| Ropivacaine | |
| Etidocaine |
Amides
Amide local anesthetics commonly used in children include lidocaine, bupivacaine (and its isomer levobupivacaine), and ropivacaine. The choice of agent most often depends on the desired speed of onset and duration of action of the block, but in small infants and children issues related to potential toxicity are also important. Compared with an adult, the neonatal liver has limited enzymatic activity to metabolize and biotransform drugs (E-Fig. 41-1; see also Chapter 6). The ability to oxidize and to reduce drugs, in particular, is immature.19–25 Clearance is immature in preterm neonates and matures rapidly through infancy. Neonates do not metabolize mepivacaine, with most of it excreted unchanged in the urine.26–32 Conjugation reactions are limited at birth and do not reach adult rates until approximately 3 to 6 months of age.21–2428
Older children also differ from adults with respect to the pharmacokinetics of local anesthetics. Children achieve peak plasma concentrations of amide local anesthetics more rapidly than adults after intercostal nerve blocks, but at similar times (approximately 30 minutes with lidocaine and bupivacaine) after caudal epidural administration.33–35 Ilioinguinal nerve blocks in children weighing less than 15 kg may produce plasma concentrations of bupivacaine in the toxic range if more than 1.25 mg/kg is administered.36
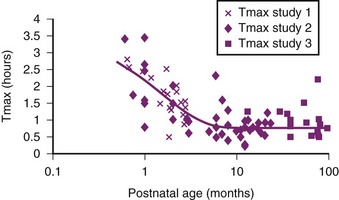
The nature of the epidural space in infants differs from that in the adult with increased vascularity, less fat, and a smaller absorptive surface for local anesthetics. Anatomical studies have shown that the epidural fat is spongy and gelatinous in appearance, with distinct spaces between individual fat globules.37 With increasing age, fat becomes more tightly packed and fibrous. The absorption half-time of epidural levobupivacaine decreases from 0.36 hours at 1 month postnatal age (PNA) to 0.14 hours at 6 months PNA. This, combined with reduced clearance (by the cytochrome P450, CYP3A4), causes a time to maximum plasma concentration (Tmax) to decrease from 2.2 hours at 1 month PNA to reach 80% of the mature value (0.75 hours) by 6 months PNA.38 The steady-state volume of distribution (Vdss) for amides in children is greater than that in adults, whereas their clearances (Cl) are similar.35,39,40 Because the elimination half-life (t1/2) is related to the volume of distribution and clearance,
a larger steady-state volume of distribution directly prolongs the elimination half-life. However it is clearance that determines steady-state concentrations with continuous amide infusion; reduced clearance in neonates implies that repeated doses and continuous infusions will cause an accumulation of local anesthetic (see E-Fig. 41-1).41–43 Thus infusion rates and local anesthetic concentrations must be reduced in this vulnerable age group when prolonged administration of amides are employed for postoperative analgesia.
Bupivacaine
Bupivacaine is highly bound to plasma proteins, particularly to α1-acid glycoprotein. It is a racemic mixture of the levorotary and dextrorotary enantiomers; the l-isoform is the bioactive one with regard to clinical effect, and the d-isoform contributes more to toxicity. Levobupivacaine, the l-enantiomer of bupivacaine, retains similar efficacy and duration of blockade as the racemic formulation (demonstrated in both an ovine model and in adult volunteers), yet carries up to a 30% reduced risk of cardiac and central nervous system (CNS) toxicities.44,45 Although levobupivacaine’s beneficial toxicity profile has resulted in its widespread use, it is currently unavailable in the United States.46
Several new experimental preparations of bupivacaine (for example, the recently approved liposome extended-release formulation) have the prospect to dramatically prolong analgesia with a reduced potential for toxicity.47–49 Bioerodible encapsulated microspheres of bupivacaine administered for peripheral neural blockade50 release local anesthetic over many hours to several days, depending on the formulation of the microsphere, thus producing very prolonged analgesia.51 The addition of dexamethasone to the microspheres prolongs the block up to 13-fold, and plasma bupivacaine concentrations in animal studies were far below the toxic threshhold.52 No adverse local reactions were noted. Several different preparations have been developed and studied, including synthetic bioerodible microspheres, protein-lipid-sugar spheres, or lipospheres.53–55 The first such preparation (Exparel, Pacira Pharmaceutical, Inc., Parsippany, N.J.) has recently been approved for use in patients 18 years of age or older by the FDA for surgical incision pain; toxicology studies suggest a low risk because of the slow rate of drug release.47,56–58 If this and other formulations become available for use in children, they may be particularly useful for those who cannot have an indwelling catheter for a regional anesthetic, but still require prolonged neural blockade for analgesia. Potential applications include intercostal blockade for children with rib fractures, postoperative analgesia for ambulatory surgery, and children in whom an indwelling epidural catheter poses an excessive risk of infection.59
Ropivacaine
Like levobupivacaine, ropivacaine is an l-enantiomer that has reduced risks of cardiac and neurologic toxicities compared with bupivacaine.60 The lethal dose in 50% of animals (LD50) appears to be greater than that of bupivacaine. Rats of different maturity exhibit a threefold greater tolerance to equipotent doses of ropivacaine than of bupivacaine, when administered for a femoral nerve block.61 Ropivacaine is also reputed to produce a less dense motor block at equianalgesic potency to other local anesthetics, although the data are conflicting in this regard. Some studies report a greater sparing of motor function compared with bupivacaine, whereas others have report no difference in motor and sensory block. Ropivacaine produces a denser blockade of the Aδ and C fibers than bupivacaine when low concentrations are used, lending mechanistic credence to the idea of differential blockade.62 Much of the infant animal data, however, do not support the existence of a greater sensorimotor differential block than that after bupivacaine. The few clinical studies in infants and children currently available do not report a detectable motor-sensory differential, in contrast to the data in adults.61,63 Several clinical studies in infants and children report a prolonged duration of analgesia with ropivacaine, despite using a solution of reduced potency.63–65 Although there are still only limited data available in children, the decreased potential for toxicity makes ropivacaine an attractive agent in this age group. Most clinical studies have used a 0.2% solution (2 mg/mL) with a volume of drug that was similar to that of bupivacaine but that depended on the type of block and size of the child. We use concentrations of 0.1% for infusions with opioid for continuous postoperative analgesia.
Lidocaine
Because lidocaine has a relatively short duration of action compared with bupivacaine and ropivacaine, it is not commonly used for single injection blocks in pediatric regional anesthesia, where a prolonged effect for postoperative analgesia is usually a priority. However, it can be used effectively in continuous blocks where the drug is continuously infused via a catheter, although here too, ropivacaine, levobupivacaine and bupivacaine are far more commonly employed. In vitro laboratory experiments have suggested that lidocaine might have greater potential for neurotoxicity in the developing nervous system than other local anesthetics, although the clinical implications of these findings remain unclear and unproved.66
Esters
The pharmacokinetics of the ester local anesthetics are also affected by the quantitative and qualitative difference in plasma proteins. Plasma pseudocholinesterase activity in neonatal umbilical blood is decreased compared with adults.67 The impact of this reduced activity on clearance is less certain. Other drugs that depend on plasma cholinesterase have mature clearance at birth (e.g., remifentanil). Limited data suggest that 2,3-chloroprocaine may be safe for neonatal regional techniques, and that potentially toxic accumulation does not occur after several hours of use with a 1.5% concentration.68,69
Another enzymatic system with decreased activity in neonates is methemoglobin reductase, which is responsible for maintaining hemoglobin in a reduced valence state where it is capable of binding and transporting oxygen. Hepatic metabolism of prilocaine yields o-toluidine, which can produce methemoglobinemia, thereby rendering red blood cells less capable of carrying oxygen.70 The decreased activity of methemoglobin reductase and the increased susceptibility of fetal hemoglobin to oxidization make prilocaine an unsuitable local anesthetic for use in neonates. Although prilocaine is no longer available for use in the United States as an injected local anesthetic, it is one of the components of EMLA cream (Eutectic Mixture of Local Anesthetics, AstraZeneca, Wilmington, Del.), a commonly employed transdermal local anesthetic. The total dose and surface area for EMLA application is therefore limited in neonates because methemoglobinemia has been reported; however, the dose must be carefully chosen even in infants and toddlers.71 Other local anesthetics, particularly topical agents, such as benzocaine, are potentially dangerous in infants because of the risk of methemoglobinemia by this same mechanism.72 EMLA should only be applied to normal intact skin in appropriate doses73 (less than 5 kg, 1 g applied to a maximum of approximately 10 cm2 surface area; 10 to 20 kg, 10 g applied to a maximum of approximately 100 cm2 surface area; greater than 20 kg, 20 g applied to a maximum of approximately 200 cm2 surface area. The dose must be reduced if EMLA is applied to mucosal surfaces (such as the glans of the penis) compared with intact skin. The duration of action is 1 to 2 hours after the cream is removed. Adverse reactions include skin blanching, erythema, itching, rash, and methemoglobinemia. EMLA should be used with caution and with strict attention to the amount of cream and surface area of application in children younger than 1 month of age. Infants receiving drugs that may induce methemoglobinemia, such as phenytoin, phenobarbital, and sulfonamides may be at increased risk, and caution is warranted. It is contraindicated in children with congenital or idiopathic methemoglobinemia. Topical amethocaine (Ametop) has a more rapid onset of analgesia and increased depth of penetration through the skin and produces minimal vasoconstriction of the skin compared with EMLA.74 Iontophoresis of lidocaine can also produce excellent transdermal skin analgesia with much faster onset of action (10 minutes) and greater depth of skin penetration than EMLA.74
Toxicity of Local Anesthetics
With the exception of uncommon effects, such as producing methemoglobinemia, the major toxic effects of local anesthetics are on the cardiovascular system and the CNS. Local anesthetics readily cross the blood-brain barrier to cause alterations in CNS function. A consistent sequence of symptoms can be observed as plasma local-anesthetic concentrations progressively increase, although this may not be readily apparent in infants and small children. Because of the smaller threshold for cardiac toxicity with bupivacaine, cardiac and CNS toxicity may occur virtually simultaneously in infants and children, or cardiac toxicity may even precede CNS toxicity. During the intraoperative use of bupivacaine, the risk of cardiac toxicity may be increased by the concomitant use of inhalational anesthetics and the CNS effects of the general anesthetic may obscure the signs of CNS toxicity until devastating cardiovascular effects are apparent.75
In awake adults, the earliest symptom of local-anesthetic toxicity is circumoral paresthesia, which is because of the high tissue concentrations of local anesthetic rather than CNS effects. The development of circumoral paresthesia is followed by the prodromal CNS symptoms of lightheadedness and dizziness, which progress to both visual and auditory disturbances, such as difficulty in focusing and tinnitus. Objective signs of CNS toxicity during this time are shivering, slurred speech, and muscle twitching. As the plasma concentration of local anesthetic continues to increase, CNS excitation occurs, resulting in generalized seizures. Further increases in the local anesthetic concentration depresses the CNS, with respiratory depression leading to a respiratory arrest. In adults, cardiovascular toxicity usually follows CNS toxicity. In this case, the systemic blood pressure decreases because the peripheral vasculature dilates, and because of direct myocardial depression, leading to a progressive bradycardia. These effects culminate in a cardiac arrest. In large doses, bupivacaine produces ventricular dysrhythmias, including ventricular tachycardia, peaked T waves, and ST segment changes suggestive of myocardial ischemia, especially when epinephrine-containing solutions are used. Bupivacaine has a particularly strong affinity for the fast sodium channels, as well as the calcium and slow potassium channels in the myocardium. These effects explain why it is so difficult to resuscitate children from a toxic dose of bupivacaine.76–78 Stereoselectivity of the sodium channel in the open state, however, has not been demonstrated. There is also evidence that the slow or “flicker” potassium channels may play a significant role in bupivacaine toxicity.79
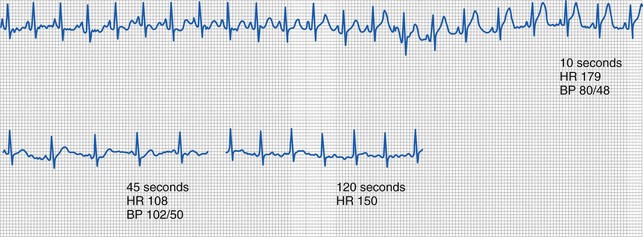
With an intravascular injection of bupivacaine with epinephrine, characteristic changes on the electrocardiogram (ECG) may be seen without any observable symptoms of CNS toxicity. Figure 41-1 shows an ECG tracing obtained during an IV injection of bupivacaine with and without epinephrine. Even a small IV dose of 1 to 2 µg/kg of epinephrine in a 1 : 200,000 solution with 0.25% bupivacaine produces peaked T waves with elevated ST segments, particularly in the lateral chest leads.80–82 As opposed to the serious risk of ischemia and dysrhythmias with larger unintended doses of bupivacaine with epinephrine, small test doses result in only brief, transient changes in the ECG and may therefore be useful in the detection of an intravascular catheter or needle placement. Tachycardia is not a reliable indicator of an intravascular injection of bupivacaine, occurring in only 73% of intravascular injections during general anesthesia. When the ECG effects of bupivacaine, with and without epinephrine, and epinephrine alone were compared in children, the most reliable ECG changes (peaked T waves at 1 minute), and an increase in arterial pressure and heart rate, required the presence of epinephrine.83 It should be noted, however, that these changes in the T waves depended on age, diminishing in responsiveness beyond 8 years of age. These data suggest that careful observation of the ECG during test-dose administration may be a sensitive indicator of unintended intravascular injection of bupivacaine in the child anesthetized with an inhalational anesthetic.84
Plasma protein binding is the most important pharmacologic factor that determines the toxicity of local anesthetics, particularly for amides, because it is the free (unbound) fraction of the drug that produces toxicity. Reduced plasma protein concentrations cause more drug to remain in the unbound active form with greater potential for toxicity (see Chapter 6). Plasma concentrations of α1-acid glycoprotein are less in neonates than in older children, and adults, producing a clinically crucial greater free fraction of amide local anesthetics.41,85–89 Current data suggest that the plasma concentration of free drug may be 30% greater in infants younger than 6 months of age and even greater in preterm infants than in adolescents90 as a result of reduced clearance and reduced α1-acid glycoprotein concentrations. α1-Acid glycoprotein is an acute phase reactant whose concentration increases after surgery. Concentrations of α1-acid glycoprotein in infants are less in those undergoing elective rather than emergency surgery.91 This increased concentration of α1-acid glycoprotein increases the total plasma concentrations for low to intermediate extraction drugs, such as bupivacaine. The unbound concentration, however, will not change because the clearance of the unbound drug is affected only by the intrinsic metabolizing capacity of the liver (i.e., clearance).92,93
Plasma concentrations of lidocaine that depress the cardiovascular and respiratory systems in human neonates are about half those that cause similar toxicity in adults.94 In contrast, 2-day-old guinea pigs were less susceptible to the toxic effects of bupivacaine than 2-week or 2-month-old guinea pigs, even though the blood concentrations in the 2-day-olds were greater.75 Data regarding toxicity in infant versus adolescent versus adult rats for both bupivacaine and ropivacaine are similar.61 Young dogs, however, have a decreased threshold to both seizure and cardiac toxicity caused by excessive doses of bupivacaine.95 Because the responses to toxic concentrations of local anesthetics vary among species, it is difficult to know which animal model and thus which study results are most applicable to human neonates.96 No data exist in humans regarding age-dependent differences in the toxic threshold of bupivacaine at a given blood concentration. Seizures and cardiovascular collapse have been reported in human infants at the same blood concentrations of bupivacaine as in adults. Whereas data from some animal studies suggest that the greater volume of distribution of amides in younger children may protect against bupivacaine toxicity, retrospective analyses of large databases of infants who have received epidural infusions indicate that these findings may not be applicable to the human infant, particularly during continuous infusions or with repeated dosing. Current data on the pharmacokinetic and pharmacodynamic differences associated with early infancy suggest that caution should be exercised when using local anesthetics in infants. Several reports document that infants and children may develop systemic toxicity, including dysrhythmias, seizures, and cardiovascular depression from the accumulation of bupivacaine administered via epidural infusions.41,97–100 Meticulous attention must be paid to the total dose of local anesthetic administered, the rate of administration, the site of injection, and the use of vasoconstrictors to diminish the rate of uptake of the local anesthetic. This is particularly important when a continuous regional anesthetic technique is used postoperatively or when repeated doses of local anesthetic are administered during prolonged surgery.
We recommend that both the bolus and infusion doses of bupivacaine and lidocaine be reduced by approximately 30% for infants younger than 6 months of age to decrease the risk for toxicity. This would mean that the maximum rate of bupivacaine administration should not exceed 0.2 to 0.3 mg/kg/hr.42 Whereas these recommendations are particularly applicable to continuous infusions of bupivacaine for postoperative analgesia, the same caveats apply to large single injections, repeated injections, and continuous infusions of local anesthetics during prolonged surgeries.
The l-stereoisomer (or enantiomer) of racemic mixtures of local anesthetics is associated with less cardiac and CNS toxicity compared with the d-enantiomer in the mixture.78 Both ropivacaine and levobupivacaine, which are l-enantiomers, the latter the l-enantiomer of bupivacaine, induce less cardiac toxicity than the racemic mixture of bupivacaine in adults and experimental animals.79 This may be due in part, to the reduced affinity of the l-enantiomers for cardiac and CNS binding sites.60 The inclusion of levobupivacaine and ropivacaine into the clinical armamentarium may decrease the toxicity of local anesthetics.
Prevention of Toxicity
There are few data that correlate the anesthetic block, blood concentration of local anesthetic, and dose in infants and children. Most dosing guidelines have been extrapolated from studies in adults. Table 41-2 lists the maximum recommended doses of local anesthetics, as well as their approximate durations of action. To avoid overdose and the possibility of toxic effects, it is prudent to remain within these guidelines until studies in children clarify the pharmacokinetics and pharmacodynamics of local anesthetics for specific nerve blocks. As discussed earlier, all of these doses should be reduced by approximately 30% in infants younger than 6 months of age.
TABLE 41-2 Maximum Recommended Doses and Duration of Action of Commonly Used Local Anesthetics
| Local Anesthetic | Maximum Dose (mg/kg)* | Duration of Action (minutes)† |
|---|---|---|
| Procaine | 10 | 60-90 |
| 2-Chloroprocaine | 20 | 30-60 |
| Tetracaine | 1.5 | 180-600 |
| Lidocaine | 7 | 90-200 |
| Mepivacaine | 7 | 120-240 |
| Bupivacaine | 2.5 | 180-600 |
| Ropivacaine | 3 | 120-240 |
*These are maximum doses of local anesthetics. Doses of amides should be decreased by 30% in infants younger than 6 months of age. When lidocaine is being administered intravascularly (e.g., during intravenous regional anesthesia), the dose should be decreased to 3 to 5 mg/kg; there is no need to administer long-acting local anesthetic agents for intravenous regional anesthesia, and such a practice is potentially dangerous.
†Duration of action is dependent on concentration, total dose, site of administration, and the child’s age.
Toxic reactions from local anesthetics are a function of (1) the total dose administered, (2) the site of administration, (3) the rate of uptake, (4) pharmacologic alterations in toxic threshold, (5) the technique of administration, (6) the rate of degradation, metabolism, and excretion of local anesthetic, and (7) the acid-base status of the child.101–106 Thus recommendations (including those of the authors) of a specific dose limit for a given drug are both overly simplistic and potentially misleading.107 Because of the multiplicity of factors that drive the final concentration of unbound local anesthetic, and in the absence of definitive and comprehensive data, conservative dosing remains the most prudent course.
Site of Injection
Injection of local anesthetics into very vascular areas leads to greater blood concentrations than the same dose injected into less vascular areas. The order of uptake (i.e., maximum blood concentration) of local anesthetics (in order from greatest to least) with regional blocks in adults is (1) intercostal nerve blocks, (2) caudal blocks, (3) epidural blocks, and (4) brachial plexus and femoral-sciatic nerve blocks.108 An easy way to remember this is by the mnemonic ICE Block:
Studies are required to determine whether this order holds true for children. Blood concentrations of bupivacaine twice those measured in older children have been reported in children weighing less than 15 kg after ilioinguinal nerve block for herniorrhaphy using only 1.25 mg/kg of 0.5% bupivacaine without epinephrine, which is half the usual recommended maximal dose.36 However, the fascia iliaca block in older children produced blood concentrations of bupivacaine that were within the acceptable safe range.109 Local infiltration of the wound in herniorrhaphy has not been associated with increased blood concentrations of local anesthetics,110 but scalp infiltration during neurosurgery may produce relatively greater blood concentrations.111 As would be expected, spinal anesthesia results in very small blood concentrations, even in neonates.89
Rate of Uptake
The rate of uptake of a local anesthetic depends on the vascularity of the site of injection. Increased perfusion increases uptake, whereas decreased perfusion decreases uptake.112 The rate of uptake in children is usually more rapid than in adults. In general, the addition of a vasoconstrictor to the local anesthetic reduces the rate of uptake and prolongs the duration of the block. In adults, the dose of epinephrine is usually limited when used in conjunction with potent anesthetic agents because of the risk of inducing cardiac arrhythmias. If epinephrine is combined with the ester anesthetics, there is no increased risk of arrhythmias. For example, in adults anesthetized with halothane, the maximum recommended dose of epinephrine is 1.0 to 1.5 µg/kg. In children, however, larger doses of epinephrine may be safe.113–115 We have used as much as 10 µg/kg of epinephrine in children, with a maximum dose of 250 µg, during halothane anesthesia without evidence of ventricular irritability, and these doses are likely to be even more safe with currently used inhalational anesthetics, which do not sensitize the myocardium to the arrhythmogenic effects of epinephrine. An epinephrine concentration of 1 : 100,000 should not be exceeded, and 1 : 200,000 or less is generally used. A quick reference for converting local anesthetic concentrations and the amount of epinephrine in various dilutions is presented in Tables 41-3 and 41-4. Epinephrine is contraindicated in blocks in which vasoconstriction of an end-artery could lead to tissue necrosis, such as for digital and penile blocks, although this historical practice has recently been challenged, setting the stage for prospective studies.116–118
| Epinephrine Dilution | µg/mL |
|---|---|
| 1 : 100,000 | 10 |
| 1 : 200,000 | 5 |
| 1 : 400,000 | 2.5 |
| 1 : 800,000 | 1.25 |
TABLE 41-4 Local Anesthetic Concentration and Its Conversion to mg/mL
| Concentration (percent) | mg/mL |
|---|---|
| 3 | 30 |
| 2.5 | 25 |
| 2 | 20 |
| 1 | 10 |
| 0.5 | 5 |
| 0.25 | 2.5 |
| 0.125 | 1.25 |
Alteration in Toxic Threshold
Medications, such as diazepam or midazolam, that increase the seizure threshold (i.e., the threshold for CNS toxicity) can be valuable adjuncts to regional anesthesia. Premedication with diazepam (0.15 to 0.3 mg/kg) decreases a child’s anxiety but also offers some protection from the toxic CNS effects of a local anesthetic overdose.119 Although diazepam is no longer in common clinical use in children in the perioperative period, evidence in animal models and considerable clinical experience in humans suggests that midazolam is also effective in terminating seizure activity.120 Animal data, however, suggest that the concomitant use of diazepam and bupivacaine decreases the elimination of bupivacaine from serum and cardiac tissue in mice.121 This effect is not a result of changes in protein binding.122 It is not known if this is true for all benzodiazepines or if this is also the case in humans. Although premedication with a benzodiazepine prevents manifestations of CNS toxicity, the threshold for cardiovascular toxicity is unchanged.123 Thus after premedication with a benzodiazepine, cardiovascular collapse can occur without warning because the symptoms of CNS toxicity may be blunted. Because most regional anesthesia administrations that are performed in children are placed after induction of general anesthesia (with the exception of some blocks in former preterm infants), this may be a moot point in most circumstances.
Technique of Administration
Whenever regional anesthesia is performed, the operator must be prepared for an adverse reaction and resuscitation supplies, including drugs, suction, and airway equipment, must be immediately available. The needle or catheter must always be inspected for blood as soon as it is positioned, but before injecting the local anesthetic, to determine if the tip is within an artery or vein. It is preferable to observe the needle or catheter for passive blood flow rather than to actively aspirate for blood because the blood vessels, such as the epidural venous plexus, are thin walled and collapse readily when negative pressure is applied. As a result, the inability to aspirate blood is not absolute proof that the needle or catheter is not in a blood vessel. For this reason, a small volume of local anesthetic with a marker for intravascular injection, such as epinephrine in a concentration of 1 : 200,000, is administered first, while the ECG is observed for 30 to 60 seconds. Data from awake adults indicate that the heart rate will increase within 1 minute of intravascular administration.124 When the drugs are administered during general anesthesia, however, the efficacy of the test dose to detect an intravascular injection may be greatly reduced. Heart rate increases in only 73% of children after an IV injection of 0.5 µg/kg of epinephrine during halothane anesthesia, suggesting that this marker of an intravascular injection is not completely reliable.125 Administration of atropine several minutes before the test dose increased the rate of positive responders to 92%, suggesting that vagal tone and the anesthetic’s blunting of the sympathetic reflexes are responsible for the reduced sensitivity to the test dose. Test doses during isoflurane anesthesia appear to have the same limitations.126 With sevoflurane, positive results were obtained in 100% of children, if the threshold for a positive response was an increase in heart rate of 10 beats per minute and a dose in excess of 0.5 µg/kg of epinephrine was used; positive results were obtained in 85% if 0.25 µg/kg of epinephrine was used.127 In all children, a change in the T-wave amplitude was a reliable indicator of intravascular injection with both doses of epinephrine (see Fig. 41-1). All children in that study were pretreated with atropine. It is not known if increasing the dose of epinephrine to 1.0 µg/kg or increasing the concentration of epinephrine in the test dose solution to 1 : 100,000 during general anesthesia would increase the sensitivity of the heart rate response test without atropine. Systolic blood pressure increased by more than 10% within 60 seconds of the test dose injection, suggesting that an increase in blood pressure may be a more sensitive indicator of intravascular injection than heart rate during inhalation anesthesia. ST-segment and T-wave changes also appear to be sensitive indicators of intravascular injection of local anesthetic. Observation of the ECG yields a highly sensitive indicator of an intravascular injection of bupivacaine with epinephrine. These ECG changes were present in 97% of infants and children who received an IV dose of bupivacaine and epinephrine.81 These investigators did not confirm the efficacy of pretreatment with atropine on the heart rate. Isoproterenol (added to the local anesthetic rather than epinephrine), 0.075 to 0.1 µg/kg, increases the heart rate in 90% to 100% of children during halothane anesthesia.128,129 It also increases the heart rate in adults during isoflurane and sevoflurane anesthesia, but this has not been extensively studied in children.130–132 During total intravenous anesthesia (TIVA) with propofol and remifentanil, however, quite different results have been reported. A prospective study of children who received an IV injection of bupivacaine with epinephrine as a simulated positive test dose during TIVA, found that T-wave alterations were inconsistent and could not be relied on as an indicator of intravascular injection.107 Only blood pressure (particularly diastolic) was a consistent and reliable marker of intravascular injection, increasing more than 10% in all subjects during TIVA.
Even though no test dose regimen is completely infallible, it appears most prudent to use an epinephrine-containing test dose before administering the therapeutic dose of local anesthetic, particularly if the block is administered in an anatomic location near a blood vessel.127 The test dose should be repeated before subsequent bolus injections through a catheter. If the drug injection is visualized in real time using ultrasound, test dosing may be less critical, as the actual drug deposition can be visualized during the injection, although this, too, may not be infallible. If the child is receiving a general inhalation anesthetic, blood pressure and the ST-segment configuration, in addition to the heart rate, should be carefully and frequently observed after injection of the test dose.84 Pretreatment with atropine may increase the rate of detecting an unintended intravascular injection. In addition, the rate of injection may also be a factor in the development of toxicity. If injection is partially or completely intravascular, a slow injection may not exceed the toxic threshold, whereas a rapid injection could. Thus slow, incremental injection of the therapeutic blocking dose of local anesthetic (over several minutes), even though repeated injections within a brief period might also result in toxic reactions, may further increase the safety of regional blockade.
Treatment of Toxic Reactions
Treatment of toxic reactions to local anesthetic overdose requires knowledge of the signs and symptoms previously described. The signs of local-anesthetic toxicity, with the exception of the catastrophic cardiovascular events, are all masked by general anesthesia. Indeed, inhaled anesthetics may actually raise the threshold for seizures and thereby delay the detection of toxicity until cardiovascular collapse occurs. Even in the nonanesthetized child, the progression from prodromal signs to cardiovascular collapse may be very rapid and the initial resuscitative therapy in some cases may need to be directed at reestablishing circulation and normal cardiac rhythm, including the timely institution of chest compressions, while definitive treatments are being readied. As always, initial management should consist of establishing and maintaining a patent airway and providing supplemental oxygen. The timely administration of a CNS depressant that alters the seizure threshold may prevent seizures. Midazolam (0.05 to 0.2 mg/kg IV), thiopental (2 to 3 mg/kg IV), or propofol (1 to 3 mg/kg IV) effectively prevent or terminate seizure activity, however the latter two agents are also potent myocardial depressants and should be used with caution. If seizure activity is present and the airway is not secured, the use of succinylcholine or other relaxant may facilitate tracheal intubation but does not prevent seizure activity. It should be remembered, however, that the acute morbidity from seizures is the result of airway complications (hypoxia and aspiration) and that securing the airway takes precedence over the actual control of the electrical activity of the seizure. CNS excitability is exacerbated in the presence of hypercarbia; it is, therefore, important to mildly hyperventilate children who have seizures. None of these interventions should in any way supplant or delay the administration of lipid emulsion to directly treat the toxic levels of local anesthetic, and help should be sought to carry out these treatments simultaneously. Indeed, two case reports suggest that lipid emulsion may reverse the CNS symptoms of local-anesthetic toxicity in the absence of cardiovascular collapse, and might be a preferable first line therapy.133,134
Advances in the treatment of local-anesthetic toxicity have dramatically altered the therapeutic interventions that should be initiated in the event of cardiovascular collapse after a large intravascular injection of an amide local anesthetic. IV lipid emulsion has been shown to be effective for resuscitation of cardiac arrest caused by both bupivacaine and ropivacaine toxicity. In dogs, successful resuscitation after cardiac arrest and 10 minutes of external cardiac massage was demonstrated after lipid administration.135 Although 100% of the dogs that received an infusion of 20% lipid emulsion were successfully resuscitated, none of the controls that received a saline infusion survived. A clear relationship between the tissue concentration and the response has also been established.136 These animal studies have been corroborated by several anecdotal reports of rescue from cardiac arrest in humans that followed intravascular injections of all of the amide local anesthetics in common use.137–140 The mechanism of action of lipid emulsions in bupivacaine toxicity is not entirely understood, but studies in isolated rat heart preparations concluded that lipid treatment promotes the elution of bupivacaine from the myocardium and accelerates the recovery from bupivacaine-induced asystole.141 This “lipid sink” hypothesis suggests a novel mechanism of action as compared with more conventional antidysrhythmic drugs, and the treatment appears to be more effective as well. An inverse relationship between the concentration of lipid emulsion and the myocardial bupivacaine concentration (greater lipid concentrations were more effective at decreasing the bupivacaine concentration in the myocardium) suggests that this lipid sink hypothesis is correct.136 In support of this theory, in vitro evidence indicates that the lipid solubility of the local anesthetic determines, in part, its responsiveness to lipid resuscitation.142 Although long-chain lipids are recommended for resuscitation after the pH is normalized, recent evidence suggests that a lipid formulation that contains a mixture of medium- and long-chain lipids is superior to long-chain lipid to extract local anesthetic, and that the binding efficiency of the former is independent of blood pH.143
In a rat study of cardiac arrest, excessive epinephrine during the initial arrest phase may actually impair resuscitation with lipid emulsion and prevent a sustained response to treatment, and whereas the initial return to cardiac function may be quicker after epinephrine, sustained recovery was better after the lipid emulsion when compared with the high-dose (greater than 10 µg/kg) epinephrine group. This contrasts with a pig model of severe bupivacaine toxicity (50% reduction in arterial pressure) in which initial treatment with epinephrine more rapidly restored vital signs than did Intralipid (lipid emulsion) administration.144 The same investigators also demonstrated that during bupivacaine-induced cardiac arrest in a pig model, survival after epinephrine alone or the combination of epinephrine and Intralipid was greater than after Intralipid alone or in combination with vasopressin.144
The anecdotal human reports and experimental literature suggests that 1.5 mL/kg of 20% lipid emulsion should be administered over 1 minute and repeated every 3 to 5 minutes, up to a maximum of 3 mL/kg, followed by a maintenance infusion rate of 0.25 mL/kg/min until the circulation is restored.145 Several pediatric events report success with this intervention, with a range of doses similar to those reported in adults.138,146,147 Therefore, although the dose of lipids in children remains speculative and has not been subject to controlled investigation, the adult guidelines appear to be effective in children. There is a growing consensus that 20% lipid emulsions should be immediately available in any location where regional anesthesia is performed, to permit rapid treatment of cardiac toxicity. Although propofol is compounded in a lipid emulsion, it is not recommended as a substitute for Intralipid for resuscitation from local-anesthetic toxicity.
Because the initial stage of cardiovascular toxicity consists of peripheral vasodilation, supportive treatment should include IV fluid loading (10 to 20 mL/kg of isotonic crystalloid) and, if necessary, titration of a peripheral vasoconstrictor, such as phenylephrine (initial rate of 0.1 µg/kg/min), to maintain vascular tone and systemic blood pressure at acceptable limits. As toxicity progresses to cardiovascular collapse, profound decreases in myocardial contractility occur, followed by dysrhythmias. In dogs, echocardiography showed that decreased systolic function always preceded the development of dysrhythmias. Before lipid emulsion treatment was described, norepinephrine was shown to be more effective than epinephrine, dopamine, isoproterenol, or amrinone to treat bupivacaine cardiac toxicity in rats.148,149 In infants, a report suggested that phenytoin (5 mg/kg administered by a slow IV infusion) treated bupivacaine cardiac toxicity.150 Many toxic reactions are self-limited because the local anesthetic redistributes throughout the body and plasma concentrations rapidly decrease. Excretion of local anesthetic is hastened by hydration and alkalization of the urine by IV administration of sodium bicarbonate.151,152 Cardiopulmonary bypass has also been used to successfully resuscitate an adult with bupivacaine-induced cardiac toxicity.153 All current data, however, strongly suggest that, in addition to the standard CPR algorithm, lipid infusion is the most successful treatment, and immediate administration of this agent should be the next first line of therapy.
Hypersensitivity to Local Anesthetics
Hypersensitivity reactions to local anesthetics are rare.154–156 Ester local anesthetics are metabolized to p-aminobenzoic acid, which is usually responsible for allergic reactions in this group. However, these agents may cause allergic phenomena in children who are sensitive to sulfonamides, sulfites, or thiazide diuretics.157,158 Among the amide local anesthetics, only one case of a true allergic reaction has been documented. These drugs may contain the preservative, methylparaben, which can produce allergic reactions in those sensitive to p-aminobenzoic acid.158,159 When in doubt, local anesthetic allergy must be ruled out. Detailed protocols are described elsewhere.160
Equipment
Use of Ultrasound
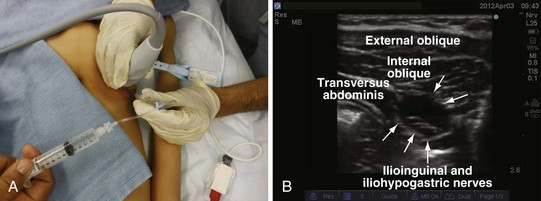
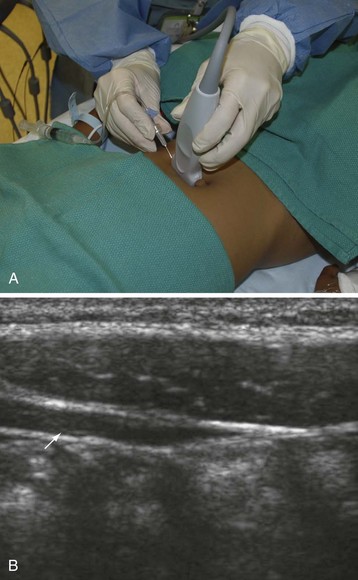
Recently there has been great interest in the use of ultrasound-guided peripheral nerve blocks in children.161 The availability of high-resolution portable ultrasound machines has become increasingly commonplace and ultrasound-guided treatment will likely soon become the new standard of care for many peripheral nerve blocks. Although this requires sophisticated expensive equipment and the acquisition of new skills, it is likely to have a larger role in pediatric regional blockade because most blocks are performed while the child is anesthetized. Several manufacturers make ultrasound machines that are about the size of a laptop computer and have been designed for ease of use by the anesthesiologist. The cost-effectiveness of acquiring these devices is justified because they serve a dual purpose for placing invasive central lines. Direct visualization of the nerve may facilitate correct placement of the local anesthetic and may also help reduce the total dose of local anesthetic needed for successful blockade. It is imperative to use an ultrasound machine that is capable of scanning superficially because most of the nerves in children are usually less than a few millimeters from the skin. A more in-depth discussion of ultrasound guidance for peripheral nerve blocks can be found in Chapter 42. Because ultrasound might still not be available to every practitioner, a complete discussion of landmark and nerve-stimulator guided peripheral blockade is included in this chapter.
Use of a Nerve Stimulator
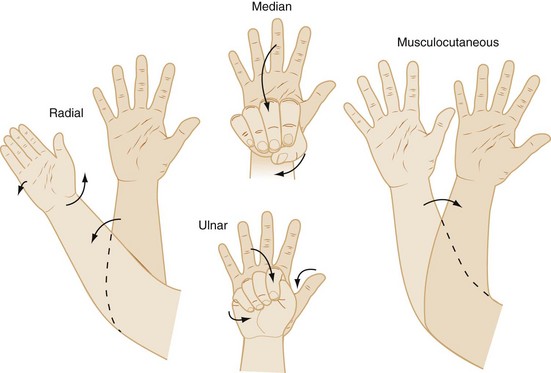
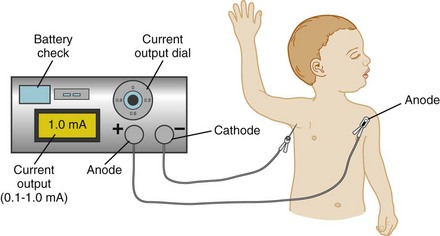
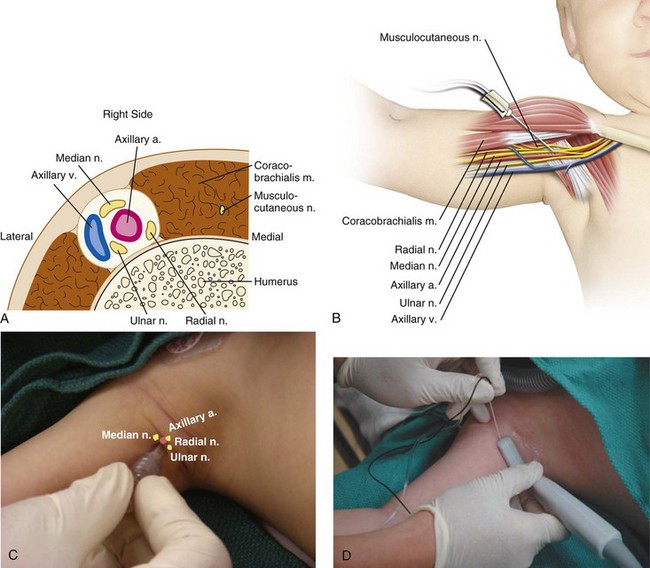
The use of a peripheral nerve stimulator is an alternative safe and effective method to locate the nerve to be blocked. Despite the increasing use of ultrasound guidance for placement of peripheral nerve blocks, some anesthesiologists still use nerve stimulation to verify the position of the tip of the needle when ultrasound is not available, or in combination with ultrasound, particularly when imaging is suboptimal. A nerve stimulator is not a substitute for anatomic knowledge, but is a useful adjunct that allows the performance of the block in an unconscious or uncooperative heavily sedated child. It avoids the need to seek sensory paresthesias or to rely purely on anatomic landmarks. The tiny amount of current flowing from the uninsulated needle tip stimulates the nerve and produces a motor response when the needle is in close proximity to the nerve. The nerve stimulator is attached to the child as shown in E-Figure 41-2. The cathode (negative pole) cable is attached to the low output terminal of the nerve stimulator at one end and to the proximal (uninsulated) shaft of a Teflon-insulated needle via a sterile alligator clip, or to the plug-in lead of a specially designed block needle at the other end (the child). The anode (positive lead) cable is attached to the high-output terminal of the stimulator at the one end and to the child, distant to the block site, via an ECG electrode at the other end.162,163 The needle is advanced in the appropriate anatomic direction, and when it appears to be in the correct position, the nerve stimulator is adjusted to approximately 0.5 mA with repetitive single pulse output at 1-second intervals. Local muscle contraction should be minimal at this setting, although direct muscle stimulation can occur and must be distinguished from neural stimulation. The area innervated by the nerves to be blocked is observed for the appropriate muscle contractions. As the uninsulated needle tip approaches the nerve, the muscle contractions will increase in intensity and become less strong as the needle tip moves away from the nerve. One should be able to decrease the current to approximately 0.2 mA with continued elicitation of easily perceptible muscle contraction to be sure that the needle tip is correctly positioned. It should be noted that the injection of even a very small volume of local anesthetic will ablate or dramatically attenuate responses produced by the low current of the nerve stimulator, so the needle position should be optimized before injection (![]() Video: Poppliteal Fossa Block of Sciatic Nerve with Nerve Stimulator). The responses to stimulation of the radial, median, ulnar, and musculocutaneous nerves are shown in Figure 41-2.
Video: Poppliteal Fossa Block of Sciatic Nerve with Nerve Stimulator). The responses to stimulation of the radial, median, ulnar, and musculocutaneous nerves are shown in Figure 41-2.
Nerve stimulation has been used to verify the dermatomal level to which the tip of an insulated epidural catheter has been threaded. A low-dose current applied to a fine wire within the lumen of the epidural catheter stimulates motor paresthesias in the muscles of the abdomen and thorax and the most cephalad responses represent the dermatomal level corresponding to the tip of the catheter.164,165
Specific Procedures
Central Neuraxial Blockade
Anatomic and Physiologic Considerations
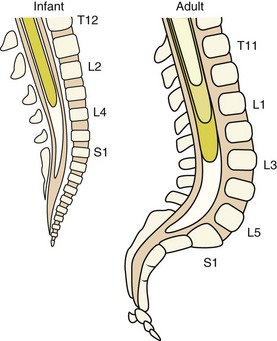
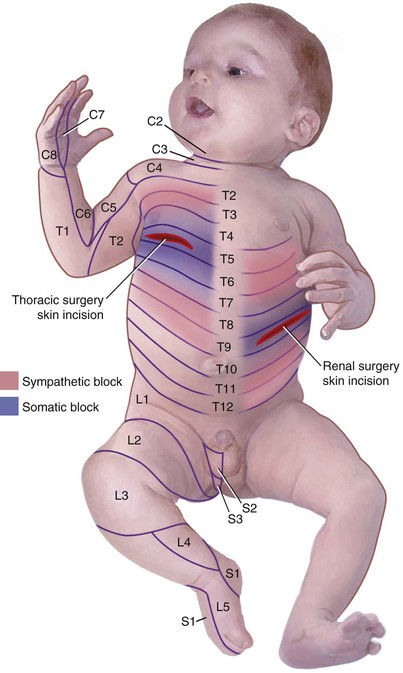
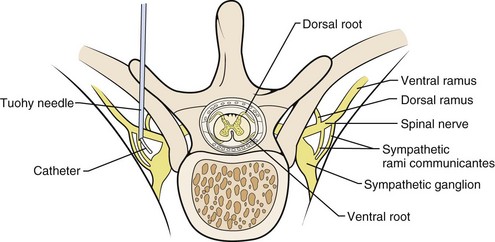
Several anatomic and physiologic differences between adults and children affect the performance of regional anesthetic techniques. The conus medullaris (the terminus of the spinal cord) in neonates and infants is located at the L3 vertebral level, which is more caudal than in adults. It does not reach the adult level at L1 until approximately 1 year of age (E-Fig. 41-3) owing to the difference in the rates of growth between the spinal cord and the bony vertebral column. Thus lumbar puncture for subarachnoid block in neonates and infants should be performed at the L4-5 or L5-S1 interspace to avoid needle injury to the spinal cord. The vertebral laminae are poorly calcified at this age, so a midline approach is preferable to a paramedian one in which the needle is “walked off” the laminae. Another anatomic difference is noted in the sacrum. In neonates, the sacrum is narrower and flatter than in adults (see E-Fig. 41-3). The approach to the subarachnoid space from the caudal canal is much more direct in neonates than in adults, making dural puncture more likely, so the needle must not be advanced deeply in neonates.166 The presence of a deep sacral dimple may be associated with spina bifida occulta, greatly increasing the probability of dural puncture. Thus a caudal block may be contraindicated in these children.
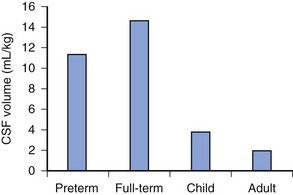
The distance from the skin to the subarachnoid space, which is very small in neonates (approximately 1.4 cm) and increases progressively with age (Fig. 41-3).167 The ligamenta flava are much thinner and less dense in infants and children than in adults, which makes the engagement of the epidural needle more difficult to detect and unintended dural puncture during epidural catheter placement a greater risk for the infrequent operator. Cerebrospinal fluid (CSF) volume as a percentage of body weight is greater in infants and young children than in adults (E-Fig. 41-4), although these studies are limited.168–172 This finding may account in part for the comparatively larger doses of local anesthetics required for surgical anesthesia with subarachnoid block in infants and young children. The CSF turnover rate is also greater in infants and children, accounting in part for the much briefer duration of subarachnoid block with any given agent compared with adults. These anatomic differences necessitate meticulous attention to detail to achieve successful and uncomplicated spinal or epidural anesthesia.
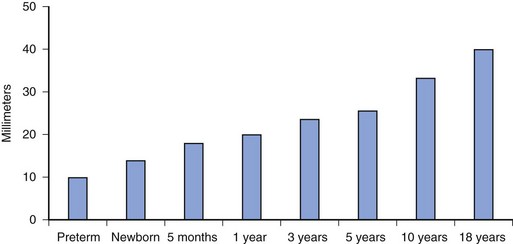
FIGURE 41-3 Distance from the skin to the subarachnoid space as a function of age.
(Data from Bonadio WA, Smith DS, Metrou M, et al. Estimating lumbar puncture depth in children. N Engl J Med 1988;319:952-3; Kosaka Y, Sato K, Kawaguchi R. [Distance from the skin to the epidural space in children.] Masui 1974;23:874-5; Lau HP. [The distance from the skin to the epidural space in a Chinese patient population.] Ma Tsui Hsueh Tsa Chi 1989;27:261-4.)
In contrast to older children and adults, subarachnoid and epidural blockade in infants and small children is characterized by hemodynamic stability, even when the level of the block reaches the upper thoracic dermatomes.173,174 Although heart rate variability, as determined by spectral analysis, is less, the heart rate is preserved, because the parasympathetic activity modulating heart rate appears to be attenuated in infants who receive spinal anesthesia.175 This attenuated vagal tone allows the heart rate to compensate for any changes in peripheral vascular tone, an effect that may be the most important factor in preserving hemodynamic stability compared with any other factor, such as the relatively small venous capacitance in the lower extremities in infants, and the relative lack of resting sympathetic peripheral vascular tone.176 Very high levels of spinal anesthesia can cause significant bradycardia that may require an anticholinergic.177 Nonetheless, data suggest that alterations in vascular resistance and blood flow to some vascular beds may occur, at least under certain conditions, in infants. In former preterm infants who received isobaric bupivacaine for subarachnoid block, cerebral blood flow decreased concomitant with changes in systemic blood pressure, although the conditions under which the baseline pressures were measured were not clear.178 In a study in which changes in regional temperature were used as a surrogate sign of sympathetic activity, extremity but not truncal temperature increased during subarachnoid block, together with small insignificant changes in blood pressure.179 In our clinical experience, and that of others, including the University of Vermont neonatal spinal anesthesia database, clinically significant systemic blood pressure changes do not occur in young infants after a subarachnoid block.177
Central neuraxial blockade can affect the respiratory mechanics of the chest wall and diaphragm by diminution in intercostal muscle activity. This may be particularly relevant in infants and young children, whose chest walls are very compliant because of limited ossification of the ribs.180 Infants rely on the diaphragm for the maintenance of tidal volume to a greater extent than older children and adults. One may also observe some chest wall paradox, that is, inward displacement of the rostral edge of the rib cage in infants during deep sleep, even in the absence of airway obstruction. Studies of infants during sleep have demonstrated that during rapid eye movement (REM) and deep sleep, paradoxical chest wall motion occurs commonly and increases as the force of diaphragmatic excursion increases.181 In a similar fashion, suppression of intercostal muscle activity, leading to decreased rib cage contribution to ventilation, has also been measured during spontaneous breathing in infants undergoing halothane anesthesia.182 When respiratory inductance plethysmography was used to study rib cage and diaphragmatic contributions to breathing in seven former preterm infants who underwent spinal anesthesia for herniorrhaphy,180 high thoracic (T2-4) levels of motor blockade were achieved, outward motion of the lower rib cage decreased, and paradoxical motion of the lower rib cage was noted in more than half of the infants. The diaphragmatic contribution to respiration, as estimated by abdominal displacement, was increased in all infants. This suggests a shift of respiratory workload from rib cage to diaphragm in compensation for the loss of the intercostal muscle contribution to breathing. Other factors, such as the alteration of the conformation of the diaphragm relative to the chest wall, with concomitant changes in the size of the zone of apposition (the portion of the anterior diaphragm that lies against the lower rib cage), may also contribute. These measurements were compared with each infant’s measurements before administration of the spinal anesthetic, but it is not known if these findings would be different if measured in unanesthetized infants during deep sleep. It is likely that these effects are well tolerated and that the ability of the diaphragm to compensate for loss of the contribution of the rib cage to breathing is adequate in the vast majority of infants.
Upper abdominal and thoracic surgery causes changes in respiration in the postoperative period by neurally inhibiting diaphragmatic function.183–185 The afferent pathways that induce this inhibition are presumed to arise from the chest and abdominal walls and perhaps the diaphragm itself, although they have not been conclusively identified.186 Contrary to popular belief, pain itself is not a major contributor to postoperative respiratory dysfunction. Numerous studies have demonstrated that opioids administered either parenterally or in the central neuraxis exert little effect on postoperative respiratory function.187–189 Regional blockade, on the other hand, has been shown to improve several measures of postoperative respiratory function.15,184,190 These data suggest that regional anesthesia has an important role to play in the attenuation of postoperative diaphragmatic dysfunction. Although the mechanism for this improvement has been attributed to blockade of the putative inhibitory neural pathways, other data suggest that alterations in respiratory mechanics, in particular an increase in the resting length of the diaphragm to its control value and the shift of the workload from rib cage to diaphragm, may play a greater role.14 These data also suggest that the beneficial effects of regional anesthesia on postoperative respiratory function may, in part, be related to the degree of motor blockade. It is not known if the preoperative administration of regional blockade enhances respiratory function compared with postoperative application of the blockade, as has been postulated in “preemptive analgesia.”
Spinal Anesthesia
Spinal anesthesia has been successfully performed in children since the first published reports in 1900 to 1910.191–194 The observation of postanesthetic apnea in former preterm infants led to a resurgence in the use of spinal anesthesia in infants, particularly for herniorrhaphy in the latter 20th century.195,196 Spinal anesthesia has even been administered for myelomeningocele repair197 by direct injection of tetracaine into the sac and supplemented as needed by direct application of tetracaine by the surgeon. It has also been used with success for a variety of other surgical procedures performed on infants.197–200 It has been recognized since the early 1980s that preterm and former preterm infants are at significant risk for developing perioperative apnea after undergoing general anesthesia.201–203 The reason for these postanesthetic apneas is not well understood. Spinal anesthesia has been proposed as an alternative to general anesthesia to reduce the incidence of perioperative apnea. However, when the spinal anesthetic was supplemented with ketamine, the incidence of postoperative apnea was even greater than with general anesthesia.204 Both retrospective and prospective studies of infants undergoing spinal anesthesia demonstrated fewer or no episodes of postanesthetic apnea compared with those undergoing general anesthesia.196,204 Although most of these studies were confounded by the absence of preoperative and postoperative pneumograms to control for baseline apnea and bradycardia, a prospective investigation confirmed that neonates who received a subarachnoid block exhibited no changes in heart rate and oxygen saturation when preoperative and postoperative pneumograms were compared.205 The infants in the general anesthesia group, however, exhibited both reduced oxygen saturation and slower heart rates in the postoperative period and these episodes were more severe than those seen preoperatively. However, these events were not consistently associated with central apnea, which was defined as a cessation of respirations lasting more than 10 seconds with no demonstrable chest wall movement. These data suggest that the several case reports of apnea after subarachnoid block may have occurred even in the absence of the anesthesia and surgery; however, one cannot entirely discount that they may have indeed been caused by the anesthetic or, perhaps, by the stress of surgery itself. Although the data from this study are persuasive, we remain cautious and have not modified the use of routine postoperative monitoring in these infants. Until studies with greater numbers of infants can distinguish which criteria would define a group with low apnea risk, all former preterm infants less than approximately 60 weeks postconceptional age should be treated similarly, regardless of the anesthetic technique.206–208 However, the best data available at this time suggest that for surgeries amenable to neuraxial blocks, spinal anesthesia (or caudal epidural analgesia) is preferable to general anesthesia for former preterm infants. It should be noted that the use of epidural anesthesia has also been reported for lower extremity and abdominal surgery in former preterm infants. Continuous spinal and epidural anesthesia for surgical procedures that outlast the duration of a “single shot” subarachnoid block have also been reported and are alternative strategies that can achieve the same goals as single injection subarachnoid block. The risk of local-anesthetic toxicity with a continuous caudal epidural blockade must be recognized; some have suggested that 2,3-chloroprocaine may be the prudent choice for local anesthetic in this situation.209 For cardiac surgery, high spinal anesthesia with tetracaine (2.4 mg/kg) has been used for ligation of the patent ductus arteriosus. Total spinal anesthesia has been used in conjunction with isoflurane general anesthesia for cardiac surgery in infants and children.210,211 Those infants whose tracheas were not intubated before surgery were successfully extubated immediately after the operation. Hemodynamic stability was maintained without the use of inotropic agents in most children. A series of infants with gastroschisis closures, as well as open pyloromyotomies, have been reported using subarachnoid block.200,211 Because spinal anesthesia in infants is associated with hemodynamic stability, it has been advocated as an ideal anesthetic for cardiac catheterization in infants with congenital heart defects and has been used, in conjunction with a light general anesthetic, for cardiac surgery.212–214
Recent concerns regarding the potential for adverse neurodevelopmental sequelae of general anesthetics administered to infants has led to additional interest in spinal anesthesia even in term infants. Although animal and retrospective human data are worrisome, a recent neonatal rat study demonstrated that memory was not impaired after sevoflurane anesthesia if the rats were exercised after the anesthetic.215 There are no prospective data yet from which to make definitive conclusions, and several longitudinal prospective studies that will evaluate long-term neurocognitive development are in progress, including one in which infants undergoing herniorrhaphy are randomized to receive general or spinal anesthesia (see Chapter 23).216–219
When a solid block37,208,220–223 is achieved, the majority of neonates fall asleep. This has been attributed to deafferentation, that is, a reduced level of consciousness because of diminished sensory input to the reticular activating system from the periphery, a mechanism confirmed in an animal model using spectral edge frequency analysis.224 Sedation levels have been measured using bispectral index and spectral edge frequency analysis in infants undergoing spinal anesthesia without the use of adjunctive agents.225 The investigators found a significant decrease in bispectral index, from 97 to 66.5 after 30 minutes, and in spectral edge frequency, from 26.1 to 9.9. These data indicate that sedation with dense regional blockade is a real physiologic phenomenon and can be used to the anesthesiologist’s advantage during spinal anesthesia in infants. They also suggest that if sedation is administered to these infants, smaller doses than usual should be considered to avoid oversedation.
Technique
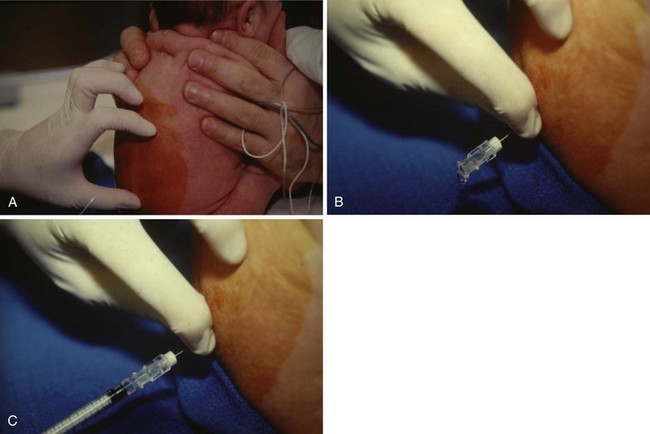
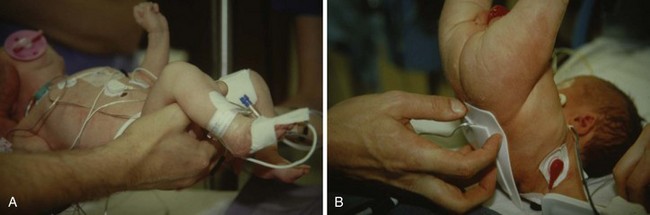
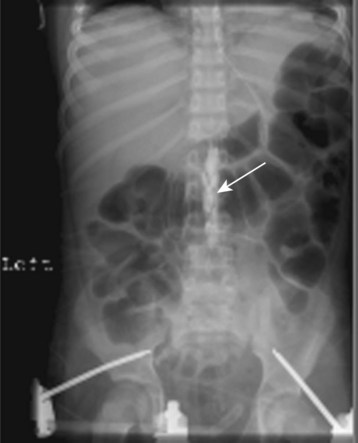
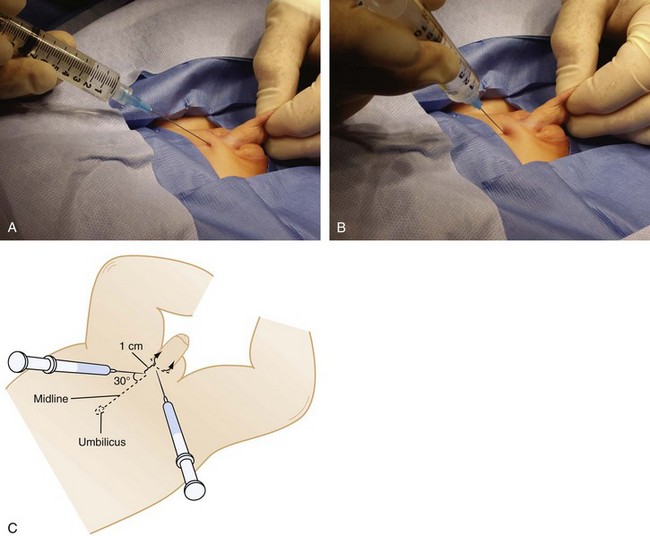
After routine monitors (ECG, blood pressure cuff, pulse oximeter, and precordial stethoscope) are affixed, the child is placed in a sitting or lateral decubitus position. For neonates and infants, care must be taken to avoid excessive flexion of the neck (as in adults) because this position may obstruct the airway (Fig. 41-4, A).226,227 The sitting position may aid in recognizing successful dural puncture by the increased CSF hydrostatic pressure and greater flow through the spinal needle. The skin is infiltrated with a minute quantity of 1% lidocaine (less than 0.25 mL should be sufficient; the authors use a 30-gauge needle on an insulin syringe), or a small amount of EMLA or other transcutaneous local anesthetic cream is applied to the infant’s lumbar area at least 1 hour before spinal placement. The lumbar puncture is performed using a midline approach with a 22-gauge or smaller, 1.5-inch styletted spinal needle (see Fig. 41-4, B and C). We do not routinely use a 25-gauge spinal needle because of the time delay between entering the subarachnoid space and the appearance of CSF in the needle hub. This delay may make it difficult to recognize that the subarachnoid space has been entered. Whitaker, Sprotte, Marx, and other “pencil point” needles are available in pediatric sizes.228,229 Lumbar puncture is performed only at the L4-5 or L5-S1 interspaces, for reasons previously described. The subarachnoid space in infants less than 60 weeks postconceptional age is approximately 1.5 cm from the skin (see Fig. 41-3); care must be taken not to pass the needle too deeply, passing beyond the subarachnoid space.167 When the subarachnoid space is located, the local anesthetic is slowly administered and the child is immediately placed in the supine position. After subarachnoid administration of local anesthetic, the child’s body should remain completely horizontal to preclude cephalad spread of the local anesthetic. If the legs are raised, a “total” spinal anesthetic results (Fig. 41-5, A and B).230 The grounding pad can be safely placed by lifting the entire infant, while maintaining the body in the horizontal plane or log-rolling the infant, or the pad can be affixed to the anterior thigh if sufficient space and muscle mass are available. The legs should never be raised above the torso as this may produce a high spinal block.
Spinal anesthesia maintains remarkable hemodynamic stability in infants, even when a high spinal block occurs. Some pediatric anesthesiologists advocate starting the IV line after the onset of the block to avoid pain associated with inserting an IV, although if the airway had to be instrumented or resuscitation drugs administered, not having IV access would be very problematic. In addition, should IV access prove difficult, valuable operating time and spinal block time would be lost while searching for a suitable vein. We apply the pulse oximeter to a toe of one leg and the blood pressure cuff to the thigh of the other which avoid disturbing the neonate during the surgical procedure (e.g., inguinal herniorrhaphy) (E-Fig. 41-5).
Because the addition of sedatives has been associated with postanesthetic apnea with an incidence at least as great as that of general anesthesia, we try to avoid all sedatives, especially ketamine.204 Most neonates will fall asleep once the block has set. A pacifier dipped in 50% dextrose will also help the infant to remain quiet and still. Gentle restraint is necessary in many cases, particularly during the most stimulating phase of the operation, when there is traction on the hernia sac, and thus on the peritoneum. It is particularly important for the infant to be still and not bear down when the hernia sac is dissected to avoid both tearing of the sac and of the extrusion of abdominal contents through the open hernia.
Selection of Drug
Neonates and Infants.
The proportional dose of local anesthetic required for subarachnoid block in neonates is much greater than that required for adults. When calculated on a per kilogram basis, there is a 5- to 10-fold greater drug requirement in neonates as for adults to reach a similar dermatomal distribution. In addition, the duration of the relatively larger dose lasts only about one-third to one-half as long as in the adult. As discussed previously, this appears to be due in part to the greater volume of CSF per kilogram and to the more rapid turnover of CSF in this age group. The drugs that have commonly been used for spinal anesthesia in neonates and infants include tetracaine, bupivacaine, and lidocaine.168,231–235 Reported doses of tetracaine range from 0.22 to 1.0 mg/kg, with larger doses used more commonly to achieve an adequate height and duration of blockade. We use hyperbaric tetracaine (0.75 to 1.0 mg/kg [equal volumes of tetracaine 1.0% and 10% dextrose]) with 0.01 mL/kg of epinephrine (1 : 100,000) or hyperbaric bupivacaine (0.75 mg/kg of 0.75% bupivacaine in 8.25% dextrose). Epinephrine prolongs the duration of tetracaine blockade by more than 30%.236 We draw a 1 : 1,000 epinephrine solution into a tuberculin or glass syringe and expel the contents in the manner of heparinizing a blood gas syringe. This leaves only a residual amount of epinephrine “wash” in the hub of the needle. The tetracaine dose and dextrose, if they are packaged separately, are combined. This dose usually provides adequate analgesia for inguinal hernia repair with a duration of motor block of 90 to 120 minutes and a dermatome height in the mid to upper thoracic region. For surgeries of limited duration that involve a lower extremity, smaller doses (0.5 to 0.6 mg/kg) may be used. The duration of bupivacaine is similar. Both isobaric and hyperbaric bupivacaine (0.5 to 1.0 mg/kg of a 0.5% solution) have been used in neonates and infants, with a reported duration similar to that of tetracaine, although the duration of action of the isobaric solution is slightly greater than for the hyperbaric solution.234,235,237 A dose-ranging study reported that the addition of clonidine (1 µg/kg) prolonged the duration of blockade from a mean of 67 minutes (plain bupivacaine) to 111 minutes.238 The use of larger doses of clonidine (2 µg/kg), however, caused transient hypotension and apnea, which required treatment with caffeine. Although lidocaine (2 mg/kg) is useful for a block of brief duration, such as for a muscle biopsy of the lower extremity, the duration of useful block is only approximately 30 minutes. In light of concerns regarding lidocaine in the subarachnoid space, we no longer recommend it in infants.239–241 A summary of doses for commonly used local anesthetics for subarachnoid block in neonates and infants is provided in Table 41-5.
TABLE 41-5 Local Anesthetics for Spinal Anesthesia in Neonates and Infants
| Anesthetic Drug | Usual Dose (mg/kg) | Range (mg/kg) |
|---|---|---|
| 1% Tetracaine in 5% dextrose | 0.75 | 0.6-1 |
| 0.5% Bupivacaine (isobaric) | 0.8 | 0.5-1 |
| 0.75% Bupivacaine in 8.25% dextrose | 0.75 | 0.5-1 |
Children.
There is little information on the doses of local anesthetics for spinal anesthesia in children, as subarachnoid block is much less commonly used beyond infancy. When a regional technique is desirable in children, an epidural or caudal block together with a “light” general anesthetic is often preferable. For spinal anesthesia, 0.3 to 0.5 mg/kg of bupivacaine (5 mg/mL concentration) may be used in children 2 months to 12 years of age.229 Doses of 0.3 to 0.4 mg/kg hyperbaric tetracaine have been used for subarachnoid block in children between 12 weeks and 2 years of age, and 0.2 to 0.3 mg/kg in children older than 2 years of age.242–244 Based on these limited data, the dose requirement for spinal anesthesia decreases with increasing age. Because there are few data available on drug doses and the height of anesthetic block produced in this age-group, it is prudent to use these values as an appropriate reference point and to revise the dose as determined by clinical experience.
Complications
Complications after spinal anesthesia include total spinal anesthesia, post–dural puncture headache, backache, neurologic sequelae, and the risk of lumbar epidermoid tumors if nonstyletted needles are used for subarachnoid puncture.228–230,245–251
Total spinal anesthesia has been reported in neonates. It is most commonly manifested by apnea with no change in systemic blood pressure or heart rate, although should pronounced bradycardia occur, a reduction in cardiac output is likely and should be treated aggressively.177,230,252 It can occur after a dose of as little as 0.6 mg/kg of tetracaine.230 Alteration in position, particularly by raising the lower body above the level of the head or thorax, may be the most common cause of a high spinal block. Although the rate of administration of the local anesthetic does not appear to affect the level of spinal anesthesia in adults, similar studies have not been conducted in neonates or infants.253 It is possible that factors, such as the use of a relatively large-bore needle (22 gauge) and a tuberculin syringe providing the means for injecting with high pressure, along with the small distance between vertebrae, combine to make the rate of injection an important consideration in neonates and infants by producing unintended barbotage. We have also observed this complication with rapid drug administration. Management consists of assisted or controlled ventilation until the return of spontaneous respiratory function.
The incidence of post–dural puncture headache appears to be infrequent in infants and children, although the incidence in preverbal children is unknown. An early study reported an incidence of spinal headache of approximately 2% using 20- to 22-gauge needles in children 2 to 17 years of age.244 However, no details were provided about the distribution of headache with respect to age. Other studies reported a 5% incidence of headaches in children ranging from 2 months to nearly 10 years of age, but, again, no age distribution was cited.228,229 A prospective study of pediatric oncology patients undergoing diagnostic or therapeutic lumbar puncture with a 20-gauge needle reported that post–dural puncture headache was relatively rare in children younger than 13 years of age.246 In most instances, the headaches were mild and resolved spontaneously. It is not entirely clear why young children should have a very low incidence of post–dural puncture headache. Several possible reasons include reduced CSF pressure,247 the increased rate of CSF production, and hormonal changes with age.246 As more pediatric regional anesthesia equipment becomes readily available, we expect that the use of “pencil point” (e.g., Whitaker, Marx, or Sprotte) needles will become more commonplace and will further reduce this already low incidence of headache.
Backache is a frequent postoperative complaint after both general and regional anesthesia in adults. It is thought to occur because of flattening of the normal lordotic lumbar curve secondary to muscle and ligament relaxation that occurs with spinal anesthesia. The incidence in children is unknown. Neurologic sequelae after spinal anesthesia are exceedingly rare. There are no reports in the literature of permanent neurologic injury caused by subarachnoid block, but good data in children are lacking. There have been no cases detected in over 1700 consecutive spinal anesthesias at the University of Vermont Medical Center, in another large series from Schneider Children’s Medical Center (Petah Tiqva, Israel), or in the PRAN database.10,177,252
Epidural Anesthesia
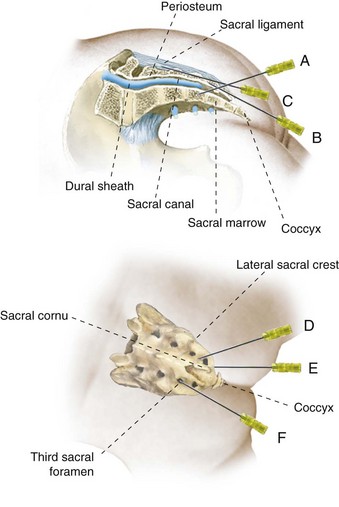
Caudal Epidural Anesthesia
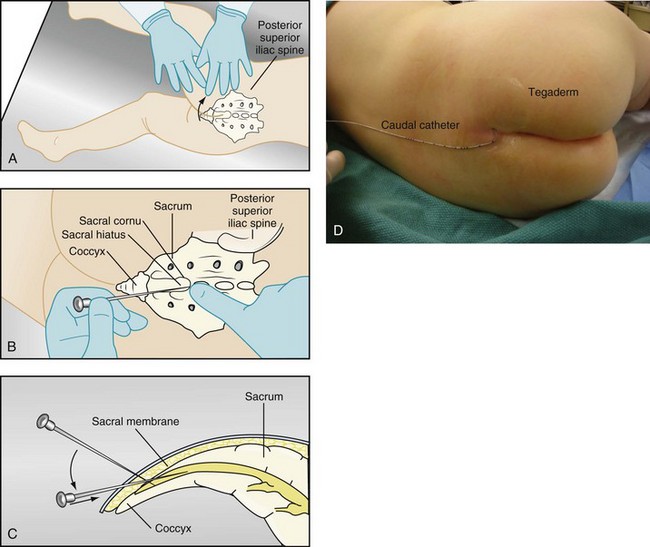
Caudal epidural anesthesia is the regional technique that is used with the greatest frequency in children, although this may decline as familiarity with lumbar and thoracic epidural techniques and peripheral nerve blocks grows. Although its use was first described in 1933,254 it was not until the early 1960s that caudal anesthesia gained any degree of popularity.255–270 Improvements in catheter material and the availability of pediatric-sized needles and catheters, and the growing recognition of the benefits of regional analgesia in general, have increased the interest in this technique for children.
Technique.
The child is placed either in the lateral decubitus or prone position with a small roll beneath the anterior iliac crests. The cornua of the sacral hiatus are most easily palpated as two bony ridges, about 0.5 to 1.0 cm apart, when the examiner moves his or her finger in a medial to lateral direction (Fig. 41-6, A). When the sacral cornua are not prominent or easily appreciated, it may prove easier to locate the space by palpating the L4-5 intervertebral space in the midline and then palpate in a caudal direction until the sacral hiatus is reached. Because the space between the sacrum and coccyx may be mistaken for the sacral hiatus, the latter technique may make identification of the landmarks easier. The proper location is often, but not always, located just at the beginning of the crease of the buttocks. A short-bevel styletted needle, 22-gauge, should be used because a long-bevel needle may increase the risk of intravascular injection.271 Some practitioners think that a styletted needle avoids the possibility of introducing a dermal plug into the caudal space whereas others think that if the skin and subcutaneous tissues are punctured with an 18-gauge needle, an IV catheter can be inserted without entraining a dermal plug and transferring it to the subarachnoid space. Still others suggest that if using an IV catheter, it should be inserted with the bevel facing down because once in place, easy advancement of the IV catheter off the needle suggests that the caudal canal has been entered and may reduce the risk of intravascular placement. Although smooth and easy advancement of the cannula off the stylet is not a completely reliable sign that placement is correct, difficulty in doing so is virtually always a sign that the needle has penetrated bone or another extra-spinal structure. The needle is initially directed cephalad at a 45- to 75-degree angle to the skin until it “pops” through the sacrococcygeal ligament (see Fig. 41-6, B) into the caudal canal, which is contiguous with the epidural space. If bone is encountered before the sacrococcygeal ligament, the needle should be withdrawn 1 to 2 mm, the angle with the skin decreased to approximately 30 degrees, and the needle again should be advanced in a cephalad direction until the sacrococcygeal ligament is pierced (see Fig. 41-6, C ). As the needle is advanced slightly farther, bone (the anterior table of the sacrum) is encountered, and the needle should be leveled in orientation before further advancement, so that it is nearly parallel to the child’s back. Once the caudal-epidural space has been entered, the needle should be advanced only a few millimeters. If an IV catheter was used, the catheter should be advanced off the needle several millimeters in distance, while transfixing the latter. Advancing the needle any further should not be attempted because the dural sac lies relatively caudad in infants and may be entered a very short distance from the ligament (Video: Single Shot Caudal Epidural)![]() .166,272
.166,272
After the caudal epidural location has been confirmed, a test dose of local anesthetic is administered. If neither ECG changes during inhalational anesthesia nor blood pressure changes during TIVA are evident after the test dose, the remainder of the dose of local anesthetic should be slowly injected in an incremental fashion over at least 1 to 2 minutes. Some view the entire volume of local anesthetic as simply an aggregate of multiple (1 to 2 mL) test doses and administer the entire in increments while observing the ECG for peaked T waves and changes in heart rate and/or blood pressure. Data from the PRAN showed that positive test doses occurred in 20 of 5,958 single injection neuraxial blocks, a rate of 0.34%, when there was no aspiration of blood [unpublished data, PRAN].273 We strongly recommend the use of a test dose, even with “single shot” caudal anesthesia.
The block may be placed before the onset of surgery without a significant decrement in duration of postoperative analgesia for short surgical procedures.274 This has the advantage of reducing the amount of general anesthesia needed, resulting in a more rapid recovery. In addition, there is adequate time for the block to “set up,” improving the chances of a pain-free awakening.
Inserting a catheter for a continuous caudal block follows a similar procedure (Video: Caudal Catheter Placement)![]() . First, one should determine the length of catheter that should be inserted into the caudal space by measuring the distance from the sacral hiatus to the desired site where the catheter tip will be positioned. Instead of a small-gauge needle or catheter, an 18-gauge IV catheter275 or an 18-gauge Crawford needle is used to enter the epidural space (see Fig. 41-6, D). Because the internal diameters of different IV cannulae vary, it is advisable to test that the epidural catheter easily passes through the IV cannula before puncturing the sacrococcygeal membrane. Once the epidural space has been accessed, the IV catheter and needle are advanced several millimeters. The catheter is then advanced off the needle several millimeters. Localization of the IV catheter tip in the epidural space is confirmed by lack of resistance to the injection of a small volume of saline and the absence of CSF or blood. If the needle had perforated the sacrum, the cannula will not easily advance off the needle. During injection, the area of the back overlying the IV catheter tip should be palpated; swelling or a fullness on injection of local anesthetic indicates a subcutaneous (SC) rather than an epidural catheter placement. The epidural catheter is advanced through the IV catheter and the IV catheter is withdrawn. After confirming the presence of neither blood nor CSF, a test dose of local anesthetic containing 1 : 200,000 epinephrine may be administered (see earlier). Test doses should be repeated each time a catheter is reinjected with a bolus dose of local anesthetic.
. First, one should determine the length of catheter that should be inserted into the caudal space by measuring the distance from the sacral hiatus to the desired site where the catheter tip will be positioned. Instead of a small-gauge needle or catheter, an 18-gauge IV catheter275 or an 18-gauge Crawford needle is used to enter the epidural space (see Fig. 41-6, D). Because the internal diameters of different IV cannulae vary, it is advisable to test that the epidural catheter easily passes through the IV cannula before puncturing the sacrococcygeal membrane. Once the epidural space has been accessed, the IV catheter and needle are advanced several millimeters. The catheter is then advanced off the needle several millimeters. Localization of the IV catheter tip in the epidural space is confirmed by lack of resistance to the injection of a small volume of saline and the absence of CSF or blood. If the needle had perforated the sacrum, the cannula will not easily advance off the needle. During injection, the area of the back overlying the IV catheter tip should be palpated; swelling or a fullness on injection of local anesthetic indicates a subcutaneous (SC) rather than an epidural catheter placement. The epidural catheter is advanced through the IV catheter and the IV catheter is withdrawn. After confirming the presence of neither blood nor CSF, a test dose of local anesthetic containing 1 : 200,000 epinephrine may be administered (see earlier). Test doses should be repeated each time a catheter is reinjected with a bolus dose of local anesthetic.
For those younger than 5 years of age, the catheter can often be advanced to any level desired without exiting a dural sleeve or becoming tangled or knotted, although one study found a surprisingly high rate of catheter misplacements as detected using epidurograms, that were not suspected clinically.37,276 Epidurograms are easily performed in the operating room when the catheter is placed. A small (0.5 to 2 mL max) volume of iopamidol is injected and imaged using fluoroscopy. A characteristic “bubbly” pattern in the midline is diagnostic of proper placement (Fig. 41-7). In addition to confirming placement in the epidural space and the location of the catheter tip, spread of the contrast may give a sense of how much volume is needed to cover the desired dermatomes. Besides epidurography, there are two other useful localizing techniques. The Tsui stimulating catheter, described earlier, produces motor twitches in the myotome at the catheter’s tip. One can mark the catheter’s advance by watching the motor paresthesias move up the legs, abdomen and thorax. Ultrasound has also been used to visualize the catheter in the epidural space, although this technique becomes more difficult with increasing age because of ossification of the posterior spinal column, and is frequently not possible in children older than 6 months of age.277,278 For infants less than 5 kg, successful catheter advancement may be less reliable than in older children, and an epidurogram, ultrasound, or use of a stimulating catheter may be needed to confirm proper placement of the catheter tip if the catheter is not radiopaque.279 An epidurogram can be useful in any situation in which the catheter is threaded or when there is concern for proper catheter position, although the routine use of an epidurogram is not standard practice. We usually strive to place the catheter tip at a level near or at the midpoint of the dermatomes encompassing the surgical incision. This position allows a more specific site of administration for both intraoperative anesthesia and continuous infusions for postoperative pain management, with the attendant advantage of being able to use reduced doses of medication. If the catheter does not pass easily to the desired level, it should be withdrawn several millimeters and a localizing technique employed to ascertain its location. The catheter should never be forced or advanced against resistance. It is thus prudent to use some method of localization when a catheter is threaded cephalad more than several centimeters.
Lumbar and Thoracic Epidural Anesthesia
It may be preferable to place epidural catheters at a lumbar or thoracic interspace when the area of operation is innervated by higher dermatomes. Advantages include less risk of contamination by stool and urine, closer proximity to the desired tip location and smaller volume of drug required for a more cephalad dermatomal level (if the caudal catheter is not threaded cephalad). Both lumbar and thoracic epidural catheters may be safely placed in anesthetized infants and children by experienced anesthesiologists.280 Although the adult literature has raised concerns about the risks of neural injury in the unconscious patient, no longitudinal studies have shown this to be the case in practice. An expert panel has recommended that, although caution must be used in all cases, there are few contraindications to the performance of regional blocks in anesthetized or heavily sedated children, and little evidence to support the notion that blocks placed while the patients are awake have fewer complications.281,282 All large-scale prospective studies of regional anesthetics in children have found that the risk of permanent neural injury related to regional anesthetics in children, the vast majority of which are performed under general anesthesia, is exceedingly small when performed by experienced pediatric anesthesiologists.11,66,283,284 Indeed, many of the arguments against regional anesthesia in the unconscious child are speculative, especially when one considers as the alternative a moving and uncooperative child.280
The technique for both lumbar and thoracic epidural catheter placement is similar to that in adults, with certain important exceptions (see Chapter 42 for ultrasound techniques). The midline approach is most commonly used, for the same reasons cited earlier regarding subarachnoid block. The ligamenta flava are considerably thinner and less dense in infants than in older children and adults. This makes recognition of engagement in a ligament more difficult and requires both extra care and slower, more deliberate passage of the needle to avoid subarachnoid puncture. It takes experience to perceive the more subtle differences in “feel” that are characteristic of the tissue planes in small children. The angle of approach to the epidural space is slightly more perpendicular to the plane of the back than in older children and adults, owing to the orientation of the spinous processes in infants and small children. The loss of resistance technique should be used with saline, not air. There are several reports of venous air embolism in infants and children when air was used to test for loss of resistance.285–287 Another method used to identify the epidural space is to attach an IV infusion chamber with a mini-drip or other free-flowing fluid delivery device to the epidural needle. When the epidural space is entered, the IV set begins to drip (Video: Hanging Drop Technique)![]() .288–290 We use a short (5 cm) 18-gauge Tuohy needle and a 20- or 21-gauge catheter in infants and children. The shorter length offers much better control than an adult-length (9 to 10 cm) needle. These catheters have fewer problems than the 24-gauge catheters, which in our experience are prone to kinking under the skin and have very high resistance to flow. Epidural kits specifically for infants and children are available, but aside from the substitution of a shorter needle they are identical to the adult sets.
.288–290 We use a short (5 cm) 18-gauge Tuohy needle and a 20- or 21-gauge catheter in infants and children. The shorter length offers much better control than an adult-length (9 to 10 cm) needle. These catheters have fewer problems than the 24-gauge catheters, which in our experience are prone to kinking under the skin and have very high resistance to flow. Epidural kits specifically for infants and children are available, but aside from the substitution of a shorter needle they are identical to the adult sets.
Selection of Drug.
The drug dose required to place an epidural block at a given dermatomal level depends on the volume (not concentration) of the local anesthetic and the volume and capacitance of the epidural space, which changes with age. There is a general impression that it takes greater volume on a per kilogram basis to achieve equal block heights in smaller children as compared with their older counterparts, but this has not been confirmed. Numerous studies have discussed the doses of local anesthetic drugs used for caudal anesthesia in children.255,257–259,261,262,264–270 The volumes of local anesthetic that block from a T4 to a T10 dermatome level span a fivefold range. In our experience, the formula of Takasaki and colleagues262 has best approximated good clinical results: Volume (mL) = 0.05 mL/kg/dermatome to be blocked.
A third simple regimen for a caudal block used in UK and Australasia is that of Armitage269: 0.5 mL/kg for lumbosacral, 1 mL/kg thoracolumbar, and 1.25 mL/kg mid thoracic. If the total volume is less than 20 mL then use bupivacaine 0.25%. If the volume exceeds 20 mL then use bupivacaine 0.19%.
Continuous Epidural Infusions
Although intermittent doses of local anesthetic are often used to maintain epidural anesthesia during a prolonged surgical procedure, it is also common practice to initiate continuous infusions of local anesthetics during surgery. Continuous infusions maintain the block at a constant level, assuming that the infusion rate is appropriate. This obviates the need for repetitive test dosing. Theoretically, fewer entries into the epidural catheter may reduce the risk of infection and the risk of accidental administration of the wrong drug. Strict attention to the total drug administered per hour (i.e., the drug concentration and infusion rate) is required to preclude potentially toxic drug doses. We recommend that the same dosing guidelines for postoperative infusion rates be followed intraoperatively: a maximum of 0.4 mg/kg/hr of bupivacaine after the initial block is established, with this dose reduced by approximately 30% for infants younger than 6 months of age.42 The concentration of local anesthetic solution depends on the age of the child, the surgical procedure, and the extent of area that needs to be blocked. When a more dense block is required in a small infant, it may be beneficial to use 2,3-chloroprocaine because its action is terminated by ester hydrolysis and has a minimal risk of accumulation compared with amide local anesthetics. A denser block with a more concentrated solution may then be achieved. The amides ropivacaine and levobupivacaine, because they are levorotary enantiomers and carry reduced risks of toxicity, may also successfully address these issues and allow the administration of more concentrated agents to produce denser blockade with less potential for adverse effects (see Chapters 42 and 43). In a study of children 1 to 9 years of age, infusion rates of up to 0.4 mg/kg/hr of ropivacaine that followed a 2 mg/kg bolus were found to result in stable levels of unbound ropivacaine in plasma, all well below the toxic threshold; clearance did not differ with age.291
Epidural Opioids
Epidural opioids can be safely used to augment intraoperative anesthesia in children, as well as to provide postoperative analgesia. Their use is discussed in detail in Chapter 43. If extubation of the trachea is expected at the end of the surgical procedure, one must take into account both the systemic and the central neuraxial opioid doses to avoid excessive respiratory depression.
Adjunctive Drugs
Numerous agents have been injected into the epidural space in attempts to prolong analgesia, to improve the quality of analgesia while reducing the dose of opioid and local anesthetic, or to replace the local anesthetic or opioid with a drug thought to possibly have fewer side effects. It is concerning, however, that several of these agents have not undergone exhaustive neurotoxicity testing and are not prepared or labeled for neuraxial use.292 In the United States, the only adjunctive drug accepted for epidural administration is clonidine, an α2-adrenoceptor agonist. There is some controversy in the literature regarding the beneficial effect of clonidine on the duration of epidural analgesia. Several studies, including a meta-analysis, found a significant prolongation of analgesia (2 hours), as measured by the time to first supplemental analgesic requirement.292–296 Other investigators found no significant increase in the duration of analgesia, including one double blind, randomized trial.297,298 In neonates, neuraxial clonidine, particularly at doses of 2 µg/kg or greater, has been associated with apnea. Increased sedation is often reported in older infants and children at those doses as well.
Complications
Complications after epidural anesthesia or analgesia include cardiac arrest from an intravascular or intraosseous injection, hematoma, neural injury, and infection. E-Figure 41-6 illustrates sites of unintended needle placement during the performance of a caudal epidural block. Injection of local anesthetic into an epidural blood vessel or intraosseous injection into the marrow cavity may result in a rapid increase in the blood concentration of the local anesthetic and a toxic reaction as discussed previously. It is also possible to pass the needle through the sacrum and perforate bowel or the pelvic organs, particularly in infants in whom ossification of the sacrum is incomplete.
There are now several large-scale prospective audits that have examined both the incidence and the nature of complications in regional anesthetics in children. The prospective audit from the UK and Ireland is the largest and most carefully described study on complications of epidural anesthesia in pediatrics to date. 10,633 cases were accrued over a period of 5 years, and all complications were reviewed and categorized by severity and type. Only five complications were graded as serious, and of these only one, the result of a drug error, had lasting sequelae. The French-Language Society of Pediatric Anesthesiologists (ADARPEF) published a follow-up prospective study on regional anesthetics in children in which they reported on 10,098 epidural blocks.66 There were no permanent sequelae seen in any child. The PRAN consortium in the United States, reported data from their first prospective cohort in which 9087 epidural and caudal blocks were accrued, 6210 single injection (mostly caudal) and 2946 continuous caudal or epidural anesthesias.10 In this study there were no complications of any kind lasting more than 3 months. The most common complication was catheter displacement or malfunction in the postoperative period in continuous blocks.
Infection is of grave concern when it occurs in either the subarachnoid or the epidural space.299 A study of 1620 children over a 6-year period found no incidence of epidural abscess.300 Catheters remained in situ for a mean of 2 days (maximum 8 days). The adult literature also suggests that infection is an uncommon complication.301,302 However, both superficial and deep abscesses may rarely occur, particularly in those children with immunodeficiency syndromes and cancer who are receiving long-term infusions.303 Epidural abscess and meningitis are the most potentially serious complications.299,304 The development of an epidural abscess is a surgical emergency, because failure to treat it can lead to a permanent neurologic injury. The signs and symptoms (Table 41-6) are the same as for epidural hematomas, although fever, increased erythrocyte sedimentation rate, and increased leukocyte count with a leftward shift are also often present. Surgical drainage may be necessary. In the British audit, three serious infections (two epidural abscesses and one case of meningitis) were noted. These infections were all related to insertion site infections. All cultures grew Staphylococcus aureus. Twenty-five local infections were reported, mostly S. aureus, and 80% were associated with catheters left in place more than 48 hours. Of note is that some localized infections that developed at the catheter insertion site only became apparent several days after the catheter had been removed (see Fig. 43-11). Similar findings were reported in the PRAN data. In the British study one case progressed to an epidural abscess. Whether these infections developed while the catheter was in place leaving bacteria to track through the open site in the skin after the catheter was removed, or by hematologic spread is unknown, although the former etiology is most frequently cited. Infants and toddlers who are in diapers require meticulous management of these catheters and their insertion site. A mild erythema occasionally occurs at the site of catheter insertion when children have indwelling catheters in place for several days, and this must be distinguished from a cellulitis (see Fig. 43-11). In most cases these superficial infections resolve with removal of the catheter and local care. On occasion these superficial infections may require treatment with a systemic antibiotic. If there is any question that the site is infected, the catheter should be removed. Although no serious systemic infection occurred in a prospective study of 210 children with 170 caudal catheters (age 3 ± 1 years) and 40 lumbar epidural catheters (age 11 ± 3 years) that were in place for 3 ± 1 days, 35% were colonized with bacteria.305 This rate of colonization was similar with both caudal (25%) and lumbar epidural (23%) approaches. These results suggest that colonization is not synonymous with infection and that caudal catheters are not necessarily associated with greater infectious risk than lumbar epidural catheters. The factors that transform colonization into infection remain unknown.
TABLE 41-6 Signs and Symptoms of Epidural Hematoma and Abscess
| Abscess | Hematoma |
|---|---|
| Fever | Afebrile |
| ± Increased WBC | WBC normal |
| ± Increased sedimentation rate | Sedimentation rate normal or slightly increased |
| ± Left WBC shift | |
| Localized back pain | Localized back pain |
| Radicular pain | Radicular pain |
| Paraplegia | Paraplegia |
| Sensory loss | Sensory loss |
| Urinary and fecal retention | Urinary and fecal retention |
| Incontinence | Incontinence |
| Local tenderness | Local tenderness |
| Defect on myelography | Defect on myelography |
| Localized lesion on magnetic resonance imaging | Localized lesion on magnetic resonance imaging |
WBC, White blood cell count.
It is common to have epidural fluid leak from the insertion site in caudal epidural catheters, especially in the presence of presacral edema. If an indwelling caudal epidural catheter is in place when a child develops a fever of unknown origin, the catheter should be removed because it may be causing the infection or become seeded by the infection (see Chapter 43).
Epidural hematoma is also a rare complication after epidural blockade. Optimal outcome depends on rapid diagnosis and prompt treatment and decompression. Signs and symptoms are presented in Table 41-6. The presence of clinically important coagulopathy or thrombocytopenia is an unacceptable risk for developing an epidural hematoma and is a contraindication to central neuraxial blockade. Guidelines for the conduct of neural blockade in the anticoagulated patient have been published by the American Society for Regional Anesthesia.306 Of particular note is that there is a difference in the management of the patient who is receiving conventional (unfractionated) heparin and low molecular weight heparins, such as enoxaparin. Guidelines for the management of patients who are anticoagulated are shown in Table 41-7; however, each case must be decided on an individual basis.306 Postoperatively, urinary retention has been associated with the presence of both epidural and spinal anesthesia. In this regard, it is important to distinguish between the effects of local anesthetics and central neuraxial opioids in the blocks. There is no evidence to support the notion that regional anesthesia with local anesthesia causes urinary retention, and, indeed, there are data to the contrary. In a prospective study of infants and children undergoing inguinal herniorrhaphy or orchiopexy, caudal blockade, ilioinguinal-iliohypogastric nerve block by the surgeon, or a control consisting of caudal injection of 1 : 200,000 epinephrine (no local anesthetic) yielded similar times to voiding postoperatively.307 In a retrospective study of 326 children undergoing inguinal herniorrhaphy and urologic surgery, 237 received a caudal block and 66 received local anesthesia by the surgeon. The incidence of urinary retention was similar for the two groups, with the type of surgery being the primary determinant of urinary retention.308
The epidural and subarachnoid use of opioids, however, is associated with an increased incidence of urinary retention. Epidural morphine in a dose of 70 µg/kg (a dose that would now be considered excessive) was associated with a 50% incidence of urinary retention309; 70% of those with urinary retention required treatment. Another study reported an incidence of urinary retention of 27% after caudally administered morphine, 33 to 100 µg/kg, although most of the children had urinary catheters.310 Finally, 50 µg/kg diacetylmorphine was associated with an 11% incidence of urinary retention.311 A dose of 33 µg/kg epidural morphine is the most common recommended in current practice.
Data from the large prospective databases indicate that the incidence of neural injury after epidural blockade is very small and that long-term neurologic sequelae of neuraxial blockade is rare. One must slightly temper these conclusions, however, based on the limited follow up of children in these studies who did not have problems reported within the immediate time frame of the block. A prospective study of more than 2,500 infants and children who received epidural blocks demonstrated no evidence of neurologic complications, although a retrospective review of the first ADARPEF data determined that 1 in 5000 infants younger than 3 months of age had neurologic complications with MRI evidence of spinal cord ischemia.99,312 In four of the five cases reported in that study the epidural space was located using loss of resistance to air (in the fifth the technique was not specified) and the authors thought that air embolus was the etiology of the neurologic injury. Based on these data, the use of air for loss of resistance in infants and children has been strongly discouraged, and saline should be used instead. In the follow up ADARPEF study there were no cases of neurologic injury.66 The British epidural audit found six cases of neural injury in 10,633 children in this prospective study. Of particular note was the delay in recognition of the injury, as no cases were discovered before 2 days had elapsed from the time the block was placed, and some diagnoses were not made for 10 days after the block. All children had complete resolution of their symptoms within 1 year. Two children were referred to a chronic pain service and treated with gabapentin, and one child developed a common peroneal nerve injury that was attributed to malpositioning of the leg during surgery. We have had one child who developed symptoms of complex regional pain syndrome after common peroneal nerve injury from positioning in the postoperative period. This child had persistent motor block, which emphasizes (1) the importance of early recognition of motor blockade as a potential for injury after surgery and (2) the critical importance of positioning and nursing care in preventing pressure injuries. There were no cases of persistent neurologic injury in the initial PRAN data cohort. An in vivo study in young rabbits, using colored microspheres to assess spinal cord and organ blood flow, found that a decrease in blood pressure, when accompanied by epidural anesthesia with lidocaine, decreased spinal cord blood flow.313 The addition of epinephrine to the local anesthetic solution did not increase the incidence of ischemia. These studies suggest that it may be particularly important to maintain adequate systemic blood flow during “combined technique” anesthesia in infants and children, and to treat hypotension promptly. Because blood pressure changes caused by neuraxial blockade are uncommon in infants and small children, hypotension in these patients is likely to be due to other causes and should prompt an assessment of intravascular filling pressures, inotropic state, and the depth of general anesthesia.
Peripheral Nerve Blocks
• A targeted area is anesthetized.
• Side effects, such as weakness of extremities, are minimal.
• The dose of local anesthetic is reduced.
• There is no risk of an unintended spinal anesthetic.
• There is no risk of urinary retention.
• They can be used in areas where a central neuraxial block is not possible (e.g., face and scalp).
There are many peripheral nerve blocks that can be used in the practice of pediatric anesthesia and each is described below (Table 41-8; see also Chapter 42).
TABLE 41-8 Peripheral Nerve Blocks
Selection of a Local Anesthetic
Local anesthetics commonly used for peripheral blocks in children include lidocaine, mepivacaine, bupivacaine, and, more recently, levobupivacaine and ropivacaine.63,65,314 Longer-acting agents have a greater role in peripheral blocks than shorter-acting agents because of the increased duration of postoperative analgesia. Lidocaine can be combined with bupivacaine to provide both a rapid onset and a long duration of action, a practice that we advocate if the child is having the procedure performed with a local block under sedation. If this is done, one must be careful to calculate the doses of the two drugs properly to avoid toxicity. Alternatively, the addition of sodium bicarbonate (1 mEq of bicarbonate/10 mL of local anesthetic [lidocaine]) can speed the onset and reduce the pain of injection of the block by increasing the pH of the solution.315–318 This alters the pKa of the solution, increasing the active cationic form of the local anesthetic in the solution.319 Bicarbonate should be added to the local anesthetic solution immediately before administration because precipitation of the local anesthetic and therefore loss of bioavailability increases over time (it should be administered within 10 minutes after alkalinization).320,321 This is particularly a problem with mepivacaine, bupivacaine, and ropivacaine in which the addition of 0.1 mL of 8.4% bicarbonate results in precipitation within 10 minutes.319,320 The total drug dose administered should not exceed the maximum milligram per kilogram dose permitted for the local anesthetic (see Table 41-2). The addition of epinephrine (1 : 200,000) may decrease both the vascular absorption and the potential for toxicity; for some local anesthetics the addition of epinephrine will also extend the duration of the block. The exact dose of local anesthetic in terms of volume or concentration needed for most peripheral blocks in children has not been adequately studied. Most blocks performed in children are based on adult experience. Suggested dosing for common peripheral blocks based on volume per kilogram and our experience is presented in Table 41-9.
TABLE 41-9 Suggested Dosing for Local Anesthetic Volumes for Common Peripheral Nerve Blocks
| Technique | Dose (mL/kg) | Usual Volume (mL) |
|---|---|---|
| Head and neck blocks | 0.05 | 3 |
| Brachial plexus blocks | 0.2-0.3 | 10 |
| Ilioinguinal nerve block | 0.075 | 4 |
| Rectus sheath block | 0.1 | 4 |
| Femoral nerve block | 0.2-0.3 | 10 |
| Sciatic nerve | 0.2-0.3 | 15 |
| Digital nerves | 0.05 | 2 |
Head and Neck Blocks
Peripheral nerve blocks for postoperative pain relief for the head and neck can be performed with the child under general anesthesia.322 These blocks can also be used for the provision of pain relief in children with chronic painful problems, such as headaches. Anatomically, two major nerves, the ophthalmic division (V1) of the trigeminal nerve and the branches of the cervical root C2, supply the sensory innervations of the face and scalp (Fig. 41-8).
Supraorbital and Supratrochlear Nerve Block
Anatomy.
The supraorbital and supratrochlear are the end branches of the ophthalmic division (V1) of the trigeminal nerve. The supraorbital nerve, the terminal branch of V1, exits the supraorbital foramen to supply the scalp anterior to the coronal suture. The supratrochlear nerve leaves the orbit between the trochlea and the supraorbital foramen and innervates the lower part of the forehead (Fig. 41-9, A). We use this combined block to provide pain relief in children undergoing frontal craniotomies and in children undergoing frontal ventriculoperitoneal shunt revisions. The technique can be used as the sole anesthetic in very sick neonates,323 and also to control postoperative pain in children undergoing excision of scalp lesions.324 The major advantage is the avoidance of opioids, thereby facilitating an early hospital discharge.
Technique.
With the child supine and the head in the neutral position, the supraorbital notch is palpated by running a finger from the midline laterally along the eyebrow (the supraorbital notch is usually located in line with the pupil with the eye in midline position). The skin is prepared with povidone-iodine or chlorhexidine and care is taken to avoid spilling the solution into the eye. A 27-gauge needle is inserted in the proximity of the supraorbital notch; 0.5 to 1.0 mL of bupivacaine (0.25% with epinephrine 1 : 200,000) is injected into the space after careful aspiration to prevent intravascular placement (see Fig. 41-9, B). To block the supratrochlear nerve, the needle is withdrawn to the skin level and then directed and advanced several millimeters medially toward the apex of the nose; 0.5 to 1.0 mL of bupivacaine (0.25% with epinephrine 1 : 200,000) is injected (Video: Supraorbital Nerve Block)![]() .
.
Greater Occipital Nerve Block
The greater occipital nerve block is used to diagnose and treat occipital pain. If this technique is used for the diagnosis of occipital neuralgia, a careful history and physical examination is performed to rule out other pathologic causes of headaches, including posterior fossa tumors and Arnold-Chiari malformation.325 It can also be used to treat postoperative pain in the posterior fossa after posterior fossa surgery, and in children undergoing posterior ventriculoperitoneal shunt revisions.326
Anatomy.
The cervical spinal nerves innervate the posterior head and neck. The dorsal rami of C2 end in the greater occipital nerve, which provides the cutaneous innervation to the major portion of the posterior scalp (Fig. 41-10, A). The nerve becomes subcutaneous slightly inferior to the superior nuchal line by passing above the aponeurotic sling; here it is in close proximity and medial to the occipital artery.
Technique.
With the child supine and the head laterally rotated or with the child prone, the occipital artery is palpated at the level of the superior nuchal line. The occipital artery is usually located at approximately one-third of the distance from the external occipital protuberance to the mastoid process on the superior nuchal line (see Fig. 41-10, B). A total volume of 2 mL of bupivacaine (0.25% with 1 : 200,000 epinephrine) is injected SC (Video: Greater Occipital Nerve Block)![]() . A recent technique using ultrasound guidance has been used in children for performing occipital nerve blocks.327
. A recent technique using ultrasound guidance has been used in children for performing occipital nerve blocks.327
Infraorbital Nerve Block
Anatomy.
The infraorbital nerve is the termination of the second division of the trigeminal nerve, the maxillary nerve (Fig. 41-11, A). This nerve is entirely sensory in function. It leaves the skull through the foramen rotundum and enters into the pterygopalatine fossa. It then enters the infraorbital groove and passes through the infraorbital canal. The nerve emerges in front of the maxilla through the infraorbital foramen and then divides into four branches: the inferior palpebral, the external nasal, the internal nasal, and the superior labial. The anatomic location of the infraorbital foramen has been studied using CT scans: the average distance from the midline (in millimeters) is 21.3 + 0.5 × age (years).328,329 The branches of the infraorbital nerve innervate the lower eyelid, the lateral inferior portion of the nose and its vestibule, the upper lip, the mucosa along the upper lip, and the vermilion. This block is effective for surgery of the upper lip and the vermilion after a cleft lip repair,330 for reconstructive procedures on the nose (including septal reconstruction and rhinoplasty),331 and for endoscopic sinus surgery.332 There are two approaches to the infraorbital nerve: intraoral and extraoral.
Intraoral Approach.
This is our preferred method for this block. The infraorbital foramen is located by palpation of the infraorbital notch. After folding back the upper lip, a 27-gauge needle is inserted through the buccal mucosa approximately parallel to the maxillary second molar and passed SC with the tip of the needle directed toward the infraorbital foramen. It is important to place a finger over the infraorbital foramen so as to palpate the progress of the needle beneath the skin and prevent unintended passage of the needle into the orbit. With the tip of the needle at the level of the infraorbital foramen and after careful aspiration, 0.5 to 1.0 mL of local anesthetic is injected (see Fig. 41-11, B, and Video: Infraorbital Nerve Block)![]() . Bupivacaine (0.25% with epinephrine 1 : 200,000) provides prolonged postoperative analgesia with this block.
. Bupivacaine (0.25% with epinephrine 1 : 200,000) provides prolonged postoperative analgesia with this block.
Extraoral Approach.
The infraorbital ridge of the maxillary bone should be identified and the infraorbital foramen palpated. A 27-gauge needle is advanced toward the foramen at a 45-degree angle to the maxilla (see Fig. 41-11, C ). After careful aspiration, 0.5 to 1.0 mL of bupivacaine (0.25% with epinephrine 1 : 200,000) is injected.
Great Auricular Nerve Block
The great auricular nerve supplies the sensory innervation to the mastoid area and the external ear. It is a branch of the superficial cervical plexus. Cervical plexus blocks were first performed by Halstead in 1884. This block has been used to provide postoperative analgesia in children undergoing otoplasty repair,333 as well as in tympanomastoid surgery.334 We found that the great auricular nerve block decreases the incidence of nausea and vomiting, which is a major morbidity associated with tympanomastoid surgery.334 It provides surface analgesia but not muscle relaxation and hence can be used for intraoperative analgesia despite the need for facial nerve monitoring in children undergoing tympanomastoid procedures.
Anatomy.
The cervical plexus is formed by the anterior primary division of the anterior and posterior roots of cervical nerves C2-4. The great auricular nerve is derived from C3, which was described by McKinney. The anatomic location of the nerve for blockade has been described as the McKinney point.335 The great auricular nerve wraps around the belly of the sternocleidomastoid muscle at the level of the cricoid cartilage and emerges to supply the area of the mastoid and external ear (Fig. 41-12, A).
Technique.
With the child under general anesthesia, the cricoid cartilage is identified. A line is drawn from the superior margin of the cricoid cartilage laterally to the posterior border of the sternocleidomastoid muscle (McKinney point). Bupivacaine (2 to 3 mL, 0.25% with epinephrine 1 : 200,000) is injected superficially at this point (see Fig. 41-12, B, and Video: Great Auricular Nerve Block)![]() .
.
Nerve of Arnold (Auricular Branch of Vagus)
The auricular branch of the vagus supplies the sensory nerve that supplies the innervation to the auditory canal, as well as the inferior portion of the tympanic membrane. This is useful for providing analgesia to the tympanic membrane following myringotomy and tube placement, as well as for tympanoplasty surgery. In a randomized controlled trial, intranasal fentanyl and blockade of the auricular branch of the vagus provided equivalent analgesia without adverse effects.336
Indications.
This block is used to provide analgesia for myringotomy and tube placement, and for tympanoplasty.
Truncal Blocks
Truncal blocks are performed in children for a variety of different surgical procedures. The most common blocks include intercostal blocks,33 ilioinguinal blocks, penile blocks, rectus sheath blocks, and paravertebral blocks.
Intercostal Nerve Block
Intercostal blocks after thoracotomy are useful in reducing opioid requirements, optimizing respiratory mechanics, and encouraging early ambulation.33,337,338 Their major disadvantage is the limited duration of analgesia. Currently, we more commonly employ epidural blockade for this purpose. The development of degradable bupivacaine microspheres and the recent approval of liposomal encapsulated bupivacaine, which produce analgesia of dramatically greater duration, may change this practice in the future.47,56–59,61 There are, however, still situations in which intercostal blocks are useful, particularly in children who cannot have a neuraxial catheter placed.
The uptake of local anesthetic after intercostal blocks is the most rapid of all sites of regional anesthesia, yielding the greatest plasma concentrations of local anesthetics following any other regional block. Furthermore, plasma concentrations in children increase more rapidly than after identical blocks in adults.339 For this reason, epinephrine (1 : 200,000) should be added to reduce the absorption of local anesthetic. We commonly use 0.25% bupivacaine with epinephrine in a dose of 1 to 5 mL for each nerve being blocked, depending on the size of the child and the number of ribs to be blocked. A maximum of 2 mg/kg of bupivacaine is used, although this amount should be reduced by about 30% for infants younger than 6 months of age. The concentration of bupivacaine should be decreased to provide adequate volume for the desired number of intercostal blocks while avoiding the risk of systemic toxicity.
Technique.
The site of injection may be either paravertebral or in the midaxillary line. The lower rib margin is located, and the skin is retracted cephalad (Fig. 41-13, A). The needle is inserted perpendicular to the skin over the rib and advanced until the rib is encountered (see Fig. 41-13, B). The skin through which the needle is passed is allowed to retract caudally, and the needle is then walked off the lower edge of the rib a distance of 2 to 3 mm (see Fig. 41-13, C ). This method may reduce the potential for pneumothorax, because the needle strikes the rib and is not advanced more than half of the thickness of the rib. A distinct pop may be felt as the needle enters the neurovascular sheath. After negative aspiration for blood, an appropriate volume of anesthetic is injected. Recently, with the use of ultrasound guidance, we can visualize the pleura, thereby avoiding puncturing the pleura because it can be adequately visualized while performing the block (E-Fig. 41-7; see also Fig. 42-11).
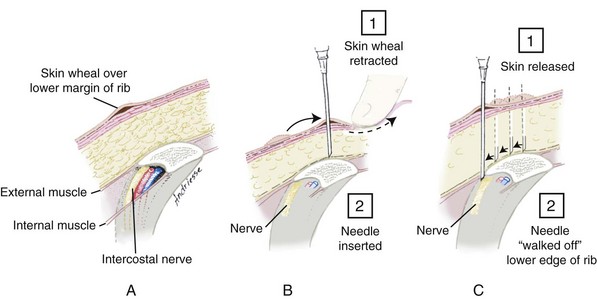
FIGURE 41-13 Intercostal block. A skin wheal is inserted on the lower rib margin (A). The skin wheal is retracted over the body of the rib, and a needle is inserted until contact is made with the rib (B). The skin is released, and the needle is carefully “walked” off the edge of the rib margin (C). After negative aspiration for blood, the appropriate volume of drug is injected. This block is used for postoperative analgesia for thoracotomy or chest tube insertion (see also E-Fig 41-7).
Complications.
Pneumothorax has been reported after intercostal blockade, with an incidence of approximately 0.07% in adults.340 However, the majority of the blocks in that study were performed by residents in training. If a small pneumothorax occurs, reabsorption is facilitated with the use of oxygen. Placement of a chest tube is only indicated if respirations are compromised. A more significant complication is the toxic effect of absorbed local anesthetic drugs. Using smaller volumes of more dilute local anesthetic may reduce the risk of achieving toxic plasma concentrations. The risk of intravascular injection may be reduced with incremental injection and frequent aspiration. A third complication is a high subarachnoid block, usually associated with the posterior paravertebral approach.
Inguinal Block (Ilioinguinal and Iliohypogastric Nerves)
Inguinal block, supplemented by wound infiltration, is sometimes used in adult patients undergoing inguinal hernia repair. However, in children, it is used almost exclusively as an adjunct to general anesthesia and for postoperative pain management. This block is as effective as caudal anesthesia for inguinal repairs.341,342 Blockade of the ilioinguinal and iliohypogastric nerves is very successful for this purpose and has few associated complications, although injection into the femoral vessels and potential femoral nerve block is a possibility.343,344 The risks of toxicity from excessive drug doses may be greater than previously recognized, as discussed earlier, but ultrasound guidance has demonstrated adequate analgesia with reduced doses of local anesthetic solution because of the optimal drug placement.345 This block may be (and commonly is) performed in conjunction with infiltration of the wound. The use of ultrasound may also avoid the risk of bowel perforation (see also Chapter 42, and Figs. 42-35 and 42-36).346
Anatomy.
The inguinal area is innervated by the subcostal nerve (T12), iliohypogastric and ilioinguinal nerves (derived from L1). These nerves lie in close proximity to each other medial and superior to the anterior superior iliac spine (Fig. 41-14, A). After piercing the internal oblique 2 to 3 cm medial to the anterior superior iliac spine, the nerve then lies between the internal oblique and the external oblique aponeurosis. Here it accompanies the spermatic cord (in males) to the genital area.
FIGURE 41-14 Ilioinguinal and iliohypogastric nerve blocks. A and B, The anterior superior iliac spine (ASIS) is palpated, and a point 1.0 to 1.5 cm cephalad and toward the midline is located (dashed line). A 22-gauge needle is passed through the external and internal oblique muscles, and 1.0 to 5.0 mL of local anesthetic is deposited in a fan-like fashion cephalad toward the umbilicus, medially, and caudad toward the groin (solid arrows). Just before removal from the skin, another 0.5 to 1.0 mL of local anesthetic is injected subcutaneously to block the iliohypogastric nerve. Blockade of these nerves provide postoperative analgesia for inguinal hernia and orchiopexy procedures (see also E-Fig. 41-8).
Technique.
The block may be performed either at the beginning of surgery or before the end of general anesthesia. If bupivacaine is used, a minimum of 15 minutes is usually required from the completion of the block until maximal analgesia is obtained. Thus blocks placed at the beginning of the surgical procedure (our preference) are usually more effective than those performed at the end of surgery. Blocks performed before skin incision may also provide “preemptive analgesia,” although the evidence for this is still under debate and somewhat confusing.347–350 The duration of postoperative analgesia is unaffected by the timing of placing the block: at the beginning of the procedure, assuming that the surgical procedure is not of more than 1.5 hours in duration or at the end. A short-bevel 27-gauge needle is inserted at a 45-degree angle at a point one-fourth of the way toward the midline along a line drawn from the anterior superior iliac spine to the umbilicus (1.0 to 1.5 cm cephalad and toward the midline from the anterior superior iliac spine in a 10- to 15-kg child). As the needle is advanced through the external and internal oblique muscles (see Fig. 41-14, B), two pops are elicited and provide useful guides of proper needle placement. Negative aspiration should be confirmed several times during the incremental injection of local anesthetic. A volume of 0.3 mL/kg of local anesthetic solution is injected in a fan-like fashion, cephalad toward the umbilicus, caudad toward the groin, and medially. Before removal of the needle from the skin, an additional 0.5 to 1.0 mL of local anesthetic is injected SC to block the iliohypogastric nerve (Video: Ilioinguinal Nerve Block with Ultrasound)![]() . Care must be taken to avoid entering the peritoneum, which has been reported after the blind injection approach.346 For inguinal herniorrhaphy, orchiopexy, or other inguinal procedures, local anesthetic deposited directly into the wound before it is closed has also proved effective for postoperative analgesia.342 The volume of drug used by this approach, like the volume of drug used for wound infiltration, must be accounted for when calculating the maximal dose of local anesthetic that can be used. It is important to note that this block will not provide pain relief for scrotal procedures because this is supplied by the genitofemoral nerve and hence it is important to have the surgeon infiltrate the scrotum for complete pain relief following orchiopexy or any other scrotal procedures. As mentioned earlier, an ultrasound-guided technique may be easier and leads to fewer complications while performing the block (E-Fig. 41-8).345
. Care must be taken to avoid entering the peritoneum, which has been reported after the blind injection approach.346 For inguinal herniorrhaphy, orchiopexy, or other inguinal procedures, local anesthetic deposited directly into the wound before it is closed has also proved effective for postoperative analgesia.342 The volume of drug used by this approach, like the volume of drug used for wound infiltration, must be accounted for when calculating the maximal dose of local anesthetic that can be used. It is important to note that this block will not provide pain relief for scrotal procedures because this is supplied by the genitofemoral nerve and hence it is important to have the surgeon infiltrate the scrotum for complete pain relief following orchiopexy or any other scrotal procedures. As mentioned earlier, an ultrasound-guided technique may be easier and leads to fewer complications while performing the block (E-Fig. 41-8).345
Penile Block
A penile block is used for anesthesia and postoperative analgesia for circumcision, urethral dilatation, and hypospadias repair. Caudal anesthesia is superior for proximal shaft or penoscrotal hypospadias repair because a penile block provides analgesia only for the distal two-thirds of the penis.266,351,352 The block is easily performed and has a high success rate. Bupivacaine, levobupivacaine, and ropivacaine are the most useful agents because of their prolonged duration of action. Epinephrine must never be used for this block because the dorsal artery of the penis is an end artery and vasospasm caused by epinephrine could cause necrosis.
Anatomy.
The nerve supply of the penis is from the pudendal nerve and the pelvic plexus (Fig. 41-15, A). Along the dorsal artery to the penis are two dorsal nerves that separate at the level of the symphysis pubis; they supply the sensory innervation to the penis.
Technique.
There are two commonly used techniques for penile block: (1) a ring block and (2) blockade of the dorsal nerve. One investigation compared the efficacy of these techniques and concluded that the ring block provided analgesia of prolonged duration, although both techniques provided analgesia superior to EMLA cream.353 The ring block is performed by inserting a 27-gauge needle at the base of the penis and, after negative aspiration, injecting the local anesthetic without epinephrine in a ring-shaped pattern around the base of the penile shaft. The needle may be inserted once in the midline and then redirected to each side (see Fig. 41-15, B). The alternate dorsal nerve block is performed with a 27-gauge needle inserted 1 cm above the symphysis pubis, in the midline, at a 30-degree angle and directed caudally (see Fig. 41-15, C ). The needle is advanced 1 cm after it pierces the penile fascia. After negative aspiration for blood, 1 to 4 mL of local anesthetic without epinephrine is injected slowly. There is a small risk of injury to the adjacent neurovascular structures.
Rectus Sheath Block
Although reported almost 20 years ago,354 this block has recently become popular for children undergoing repair of an umbilical hernia.355–357 The rectus sheath contains the thoracic intercostal nerves (T-10) that can be blocked at the paraumbilical area using a small volume of local anesthetic solution.
Technique.
On either side of the umbilicus, about 1 cm from midline a needle is inserted into the rectus sheath (Fig. 41-16, A). A pop can be felt as the needle advances beyond the anterior rectus sheath, through the rectus abdominis muscle and then just anterior to the posterior rectus sheath. After aspiration, a volume of 0.1 mL/kg of local anesthetic solution is injected (Video: Rectus Sheath Block with Ultrasound)![]() . This provides excellent analgesia for most umbilical area surgery, including laparoscopic surgery. Use of ultrasound may facilitate performing this block358 (see Fig. 41-16, A and B; see also Chapter 42, and E-Figs. 42-2 and 42-3).
. This provides excellent analgesia for most umbilical area surgery, including laparoscopic surgery. Use of ultrasound may facilitate performing this block358 (see Fig. 41-16, A and B; see also Chapter 42, and E-Figs. 42-2 and 42-3).
Paravertebral Block
This block is also gaining some popularity for use in children. The main advantage is that deposition of local anesthetics in the paravertebral space will lead to strict unilateral anesthesia of one or more adjacent dermatomes (Fig. 41-17); the main indications for a paravertebral nerve block are unilateral thoracic or abdominal surgical procedures.
Anatomy.
The paravertebral space is a triangular wedge-shaped area situated in the angle between the lateral border of the vertebral body and the anterior surface of the transverse process (Fig. 41-18). The paravertebral space exists only between T1 and T12. Below T12 the space is sealed off by the origin of the psoas muscle from the vertebral body and the transverse process.359 Cranially the space appears to communicate with fascial planes in the neck, because an upper thoracic paravertebral block may cause Horner syndrome. The communication of different thoracic levels of the paravertebral space is the foundation for spread of local anesthetic to multiple segments (see Fig. 41-17). The medial boundary of the paravertebral space is the lateral part of the vertebral body and disc, the dorsal limitation is the transverse process and costotransverse ligament, and the anterolateral boundary is the parietal pleura. Structures that pass through the paravertebral space include the spinal nerve root–intercostal nerve, the sympathetic chain, and the intercostal vessels. The paravertebral space is not like the epidural space, because the pleura are very adhesive to the other structures but should instead be viewed as a “potential space.” This accounts for the slight difficulty in introducing a percutaneous catheter into the paravertebral space. In the lumbar region a paravertebral block is still possible but each individual space must be blocked separately because there are no communications between adjacent lumbar levels.
Technique.
Three approaches to perform a paravertebral block in children have been described:
Loss-of-Resistance Technique359: The skin is punctured laterally to the spinous process and the needle is advanced in a perpendicular manner until contact is made with the transverse process. A Tuohy needle (19 to 20 gauge if younger than 1 year of age, 18 gauge if older than 1 year of age) is then “walked” below (underneath) the transverse process and by means of a loss-of-resistance technique the costotransverse ligament is pierced and the paravertebral space located. Alternatively the needle can be “walked” above (over the top of) the transverse process, but by using this approach there is the risk of striking the neck of the rib before entering the paravertebral space. Occasionally this will redirect the needle, making it virtually impossible to obtain access to the paravertebral space. The approach from below the transverse process is clearly advantageous.
An estimate of the distance from the spinous process to the skin puncture site (spinous process to paravertebral space distance) and the distance from the skin to the paravertebral space can be approximated by the following equations360,361:
Nerve-Stimulator–Guided Technique.362 The intervertebral lines corresponding to the specific dermatomes are determined by manual palpation. The site of injection is marked 1 to 2 cm laterally from the midline on the intervertebral line according to the child’s weight. A 21-gauge insulated needle of appropriate length, attached to a nerve stimulator (initial stimulating current: 2.5 to 5 mA, 1 Hz), is introduced perpendicularly to the skin in all planes. A contraction of the paraspinal muscles is initially observed, and the needle is advanced until the costotransverse ligament is reached. At this point the contraction of the paraspinal muscles will disappear. After piercing the costotransverse ligament, muscle contractions of the corresponding level are sought and the needle tip is manipulated into a position allowing continued muscular contractions while reducing the stimulating current to 0.4 to 0.6 mA; the desired local anesthetic dose and volume is injected. Manipulation of the needle tip within the paravertebral space is not an “in and out” movement but is rather an angular manipulation and circumferential rotation around the axis of the needle to reach an optimal position of the needle tip with regard to the nerve within the paravertebral space.
Selection of Drug.
After a negative aspiration test and administration of a test dose, 0.5 mL/kg of the local anesthetic (levobupivacaine 0.25% with epinephrine 1 : 200,000, bupivacaine 0.25% with epinephrine 1 : 200,000, or lidocaine 1% with epinephrine 1 : 200,000) is injected in toddlers and older children. This dose will usually spread to cover at least five dermatomes. A typical distribution of the block will be unilateral analgesia of the trunk ranging from T4 to T12 (see Fig. 41-17). In neonates and infants, slightly modified dosage regimens are recommended363–365; these dosages have been found to be both effective and associated with acceptable plasma concentrations of bupivacaine.363
Complications.
The use of a percutaneous loss-of-resistance technique in a mixed adult and pediatric population was found to be associated with an overall failure rate of approximately 10% and the complications experienced were hypotension (5%; only adults), vascular puncture (4%), pleural puncture (1%), and pneumothorax (0.5%).366 The risk for block failure is reduced to less than 5% when a nerve-stimulator–guided technique is used, and this technique also appears to be associated with a reduced risk for complications.362,367 Use of ultrasound may further improve success while reducing complications.
Upper Extremity Blocks
Brachial Plexus Block
Of the four techniques used to block the brachial plexus (axillary, infraclavicular, supraclavicular, and interscalene), the axillary approach is most commonly used in children when using nerve stimulation or landmark technques.368 Advantages include ease of insertion, a high rate of success in experienced hands, and low morbidity. The block is also well suited for orthopedic or plastic surgical repairs on the hand or forearm in a child with a full stomach.369,370 In this situation, deeper levels of sedation, intravascular injection, or drug overdose places the child at risk for aspiration of gastric contents. Because it is unnecessary to elicit a sensory paresthesia, the block can also be performed in an anesthetized child for postoperative pain management. Toxicity is avoided if the dose of bupivacaine is less than 2.5 mg/kg.
Infraclavicular, supraclavicular, and interscalene blocks are not as frequently used as the axillary block in children. The infraclavicular block is our preferred technique for the placement of a continuous catheter in the postoperative period. Unintentional block of the phrenic and recurrent laryngeal nerves is much more common in young children because these nerves are close to the site of injection, especially with an interscalene block. Data suggest that some degree of phrenic nerve blockade is present in all children receiving interscalene blocks.371,372 Phrenic nerve blockade may cause respiratory failure in very young children whose breathing is almost totally dependent on the diaphragm, whereas block of the recurrent laryngeal nerve may cause increased airway resistance because of vocal cord paralysis. The risk of pneumothorax is greater because the apex of the lung is situated more rostral in infants and small children. Total spinal anesthesia is also more likely with the interscalene approach to axillary plexus blockade.373
Anatomy.
The brachial plexus arises in the neck from spinal nerves C5, C6, C7, C8, and T1, passes between the clavicle and first rib, and extends into the axilla. At that point, the axillary artery is surrounded by a narrow fascial sheath that contains the median nerve anteriorly, the ulnar nerve posteriorly, and the radial nerve on the posterolateral aspect (Fig. 41-19, A). In children, the axillary artery and, at times, the axillary sheath itself may be palpable.
Technique.
Several techniques can be used to establish that the needle is within the axillary sheath. The first is by eliciting a sensory paresthesia with the needle, but this has little application in pediatric practice, particularly in young children and in those who are anesthetized. The use of a nerve stimulator allows precise placement of the needle in the neurovascular sheath without either the cooperation of the child or the need for painful sensory paresthesias (see Fig. 41-2). In thin children, the sheath can often be palpated as a cord-like structure inferior to the coracobrachialis muscle, allowing the placement of the needle in the sheath by “feel.” A transarterial approach can also be used.374 With all techniques, it is useful to attach a short piece of extension tubing between the needle and syringe to facilitate precise handling during needle placement, aspiration, and drug injection.
The use of a nerve stimulator for the axillary approach to the brachial plexus is best accomplished by abducting the arm to 90 degrees (see E-Fig. 41-2). Care should be taken not to hyperabduct the arm, obscuring the axillary pulse. The artery is palpated in the axilla, and a short beveled needle is advanced toward it (see Fig. 41-19, B). When using a nerve stimulator, a distal motor response is elicited in the distribution of the radial, ulnar, or median nerves at a threshold of less than 0.2 mA (see E-Fig. 41-2). If one is not using a nerve stimulator, the needle is advanced until a distinct pop is felt as the needle pierces the axillary sheath. The axillary sheath may be divided into fascial compartments for each nerve, and these may limit the spread of local anesthetic within the axillary sheath. Although distinct paresthesias to the distribution of all three nerves may be elicited with the nerve stimulator, and divided doses of anesthetic may be administered to each of those locations, in practice it becomes extremely difficult to find the second and third motor paresthesia after the administration of even a very small amount of local anesthetic with the first injection (Video: Axillary Block with Ultrasound Guidance)![]() . Alternatively, the transarterial technique, which involves direct puncture of the axillary artery, allows deposition of local anesthetic in two sites within the sheath. The needle is aimed directly toward the axillary pulse. As soon as blood is aspirated, the needle is advanced through the posterior wall of the artery. When blood can no longer be aspirated, half of the dose of local anesthetic is deposited posterior to the artery. The needle is withdrawn through the anterior wall of the artery, and the remainder of the dose is deposited anterior to the artery after reconfirming a negative aspiration for blood. Regardless of technique, the local anesthetic is administered in incremental quantities with intermittent aspiration to confirm that the needle is still outside the blood vessel. Some practitioners advocate applying a tourniquet distal to the site where the block is to be performed. It is sometimes difficult to block the musculocutaneous nerve, which carries sensory fibers to the radial aspect of the forearm, because it exits the brachial plexus proximal in the axillary fossa. Applying a tourniquet may promote proximal spread of local anesthetic and enhance the chances of a successful block of this nerve. Alternatively, the musculocutaneous nerve may be blocked by infiltrating 1 to 3 mL (proportional to the size of the child) of local anesthetic into the body of the coracobrachialis muscle. Regardless of the technique chosen, an additional 1 to 3 mL of local anesthetic is deposited as a subcutaneous cuff to block the intercostobrachial nerve and its communications with the musculocutaneous nerve. These additional quantities of local anesthetic must be accounted for when calculating the total drug dose. An ultrasound may also be used in conjunction with a nerve stimulator to further improve the localization of each nerve bundle (see Figs. 42-16 and 42-21).370
. Alternatively, the transarterial technique, which involves direct puncture of the axillary artery, allows deposition of local anesthetic in two sites within the sheath. The needle is aimed directly toward the axillary pulse. As soon as blood is aspirated, the needle is advanced through the posterior wall of the artery. When blood can no longer be aspirated, half of the dose of local anesthetic is deposited posterior to the artery. The needle is withdrawn through the anterior wall of the artery, and the remainder of the dose is deposited anterior to the artery after reconfirming a negative aspiration for blood. Regardless of technique, the local anesthetic is administered in incremental quantities with intermittent aspiration to confirm that the needle is still outside the blood vessel. Some practitioners advocate applying a tourniquet distal to the site where the block is to be performed. It is sometimes difficult to block the musculocutaneous nerve, which carries sensory fibers to the radial aspect of the forearm, because it exits the brachial plexus proximal in the axillary fossa. Applying a tourniquet may promote proximal spread of local anesthetic and enhance the chances of a successful block of this nerve. Alternatively, the musculocutaneous nerve may be blocked by infiltrating 1 to 3 mL (proportional to the size of the child) of local anesthetic into the body of the coracobrachialis muscle. Regardless of the technique chosen, an additional 1 to 3 mL of local anesthetic is deposited as a subcutaneous cuff to block the intercostobrachial nerve and its communications with the musculocutaneous nerve. These additional quantities of local anesthetic must be accounted for when calculating the total drug dose. An ultrasound may also be used in conjunction with a nerve stimulator to further improve the localization of each nerve bundle (see Figs. 42-16 and 42-21).370
Selection of Drug.
Local anesthetics commonly used in our practice include lidocaine and bupivacaine (see Table 41-2). As with other regional techniques that involve larger volumes of local anesthetic, the addition of both levobupivacaine and ropivacaine to the armamentarium is likely to prove beneficial in reducing the risk of toxicity from local anesthetics. Because it is desirable to have a prolonged duration of postoperative analgesia, longer-acting agents are usually used in place of lidocaine. To help ensure block of the musculocutaneous nerve, we use large volumes (0.5 mL/kg), diluting the concentration of local anesthetic with normal saline as needed to avoid toxicity. Care must always be taken not to exceed the maximal allowable doses of bupivacaine on a milligram per kilogram basis (2.5 mg/kg).375 Adding epinephrine (1 : 200,000) may decrease vascular absorption and the potential for toxicity. Sodium bicarbonate (1 mEq/10 mL of local anesthetic) added to the local anesthetic will speed the onset of blockade by increasing the pH of the solution; this is particularly the case with the premixed anesthetic-epinephrine formulations that have a reduced pH.
Complications.
All of the nerves of the brachial plexus occupy a neurovascular bundle and hence are prone to unintended injection into a blood vessel. A hematoma may form at the site of injection. If it is large enough, the hematoma may compress the neurovascular bundle, rendering the limb ischemic. Hence it is important to know the child’s coagulation status before attempting the block. Intravascular injection may be avoided with incremental injection and frequent aspiration. Intraneural injection may be minimized by use of a nerve stimulator. Surgeons may feel the importance to check the viability of the radial, median, and ulnar nerves before injecting local anesthetic solution. This block can be carried out in the recovery room after the function of the nerves are determined. A simple rule of thumb is to check the radial nerve (extension of the thumb), median nerve (flexion of the proximal interphalangeal joint of the thumb), and ulnar nerve (scissoring of the fingers) (Video: Finger Examination)![]() . This can functionally check the nerves before injection of local anesthetic solution.376
. This can functionally check the nerves before injection of local anesthetic solution.376
Infraclavicular Approach.
This approach to the brachial plexus is very helpful, particularly in children who may have fractures making it painful to abduct the arm. A vertical approach to the infraclavicular brachial plexus is performed using the coracoid process as a landmark to access the nerve.377 We routinely use this technique in children who require continuous infusions of local anesthetic solution in the postoperative period.
Technique.
With the arm in abduction or adduction, the acromial process is palpated. A line drawn 2 cm below and medial to the coracoid process is usually where the needle is introduced (Fig. 41-20, A). At this level, the pleura is not usually affected. A sheathed needle with a nerve stimulator is introduced, and the nerve is stimulated at about 1 mA. Any stimulation other than forearm flexion is taken as a positive stimulation of the brachial plexus. Forearm flexion denotes stimulation of the musculocutaneous nerve. The needle should then be directed medial to provide a blockade of the cords of the brachial plexus (see Fig. 41-20, B). An ultrasound-guided technique may also be used (see also Chapter 42 and Figs. 42-17 to 42-19).