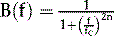Chapter 13 Regional and Global Ventricular Function and Volumes from SPECT Perfusion Imaging
INTRODUCTION
Electrocardiographic (ECG)-gated myocardial perfusion single-photon emission computed tomography (SPECT) is one of the most frequently performed techniques in nuclear cardiology, and in 2007 it accounted for over 90% of all myocardial perfusion SPECT imaging in the U.S.A. Key to its diffusion has been the relative ease with which gated SPECT acquisitions can be accomplished, especially in conjunction with technetium-99m 99mTc-based radioisotopes, multidetector cameras, and fast processing computers. Gated myocardial perfusion SPECT (gated SPECT) permits the assessment and quantification of various parameters of global and regional function for the left ventricle (LV), which in turn translates into improved diagnostic capabilities and better prognostic power. This chapter will start by identifying and discussing a number of issues connected with the acquisition and processing of gated SPECT data sets, then quantification of global and regional LV function will be addressed in terms of different algorithmic approaches, validation, and practical limitations to quantitative accuracy. Finally, the incremental diagnostic value of gated SPECT parameters over myocardial perfusion parameters will be described and evaluated. Their prognostic value will be addressed later in Chapter 16.
ACQUISITION
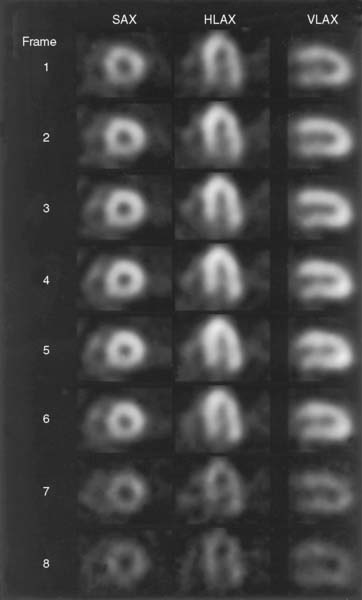
As Figure 13-1 shows, a gated SPECT acquisition is essentially equivalent to a standard SPECT acquisition, the only difference being that 8 to 16 projection images instead of one are acquired at each camera angle. Each of those 8 to 16 images is representative of a specific phase of the cardiac cycle, since count accumulation is triggered by the QRS complex of the patient’s ECG wave. It is therefore possible to reconstruct three-dimensional (3D) tomographic representations of the LV’s location and count distribution throughout the cardiac cycle and display them in rapid cinematic succession to achieve a realistic rendition of the four-dimensional (4D) LV motion (three dimensions plus time). Ideally it would be desirable that the number of gating intervals or “frames” employed be as high as possible so as to better follow changes in myocardial position and volumes; however, this consideration is balanced by the need for each projection image to contain adequate counts. Count statistics are influenced by numerous factors, including radioisotope used, injected dose, acquisition time, patient size, camera configuration and sensitivity, collimation, number of frames, and count acceptance criteria. For assessment of systolic ventricular function, gated myocardial perfusion SPECT has traditionally been performed using either 8-frame or 16-frame gating. We prefer the latter, since it can more reliably than the 8-frame approach contain a frame that will correspond closely to true end-systole, important for assessment of systolic function, and because it has a greater number of diastolic frames, important for assessment of diastolic function.
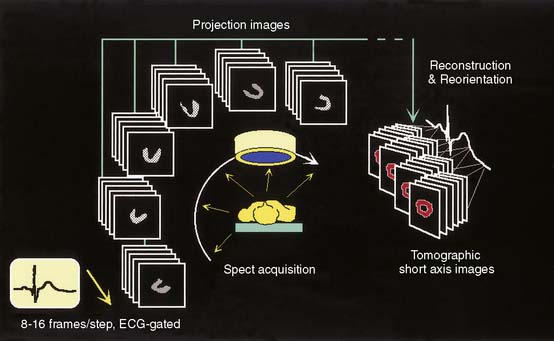
Figure 13-1 Schematic representation of ECG-gated perfusion SPECT acquisition and processing.
(Reproduced with permission from Germano G, Berman D: Acquisition and processing for gated perfusion SPECT: Technical aspects. In Germano G, Berman D [eds]: Clinical Gated Cardiac SPECT. Armonk, NY: Futura Publishing, 1999, pp 93-113.)
Importance of “Bad Beat” Rejection
Cardiac Beat Length Acceptance Window as a Tool for “Bad Beat” Rejection
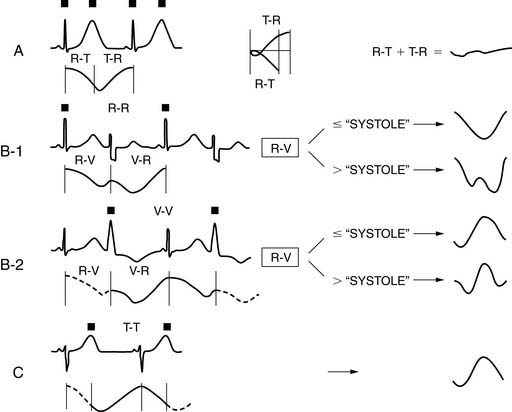
As explained earlier, the peak or R point of each QRS complex in the ECG triggers the binning of counts into projection images. If a “fixed temporal resolution framing” approach is employed,1 usually based on a sampling of the beats at the beginning of a gated SPECT acquisition, all gating intervals will be set to the same temporal length. For example, in 8-frame gating, the 8 intervals will span 125 milliseconds each if the expected heartbeat duration (R-R computer) is 1 second (Fig. 13-2). Because the actual time between successive R points (R-R patient) typically varies during the course of the acquisition, and particularly because of the impact of PVCs on systolic function and the systolic and diastolic timing noted, if there is no “bad beat” rejection, an error in the function measurements will be embedded in the data, with the magnitude of the error being proportional to the frequency of the premature beats. To circumvent this problem, most computers used in the acquisition process have beat length tolerances built into the count collection process. This is done by defining a beat length acceptance window, which essentially specifies how much shorter (or longer) than its 1-second expected duration a cardiac beat can be to not have its counts discarded for purposes of assessing ventricular function. As Figure 13-2 shows, a beat length acceptance window of 20% allows accumulation of data from cardiac beats of 900 to 1100 milliseconds’ duration. An acceptance window of 100%, on the other hand, allows accumulation of data from beats with duration in the range of 500 to 1500 milliseconds. Note that this is not equivalent to accepting 100% of the beats, which is instead consistent with having a window of infinite width.
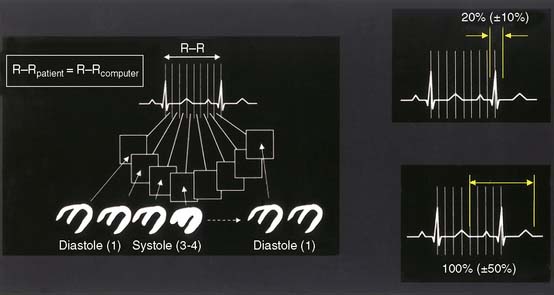
Figure 13-2 The cardiac beat length acceptance window.
(Adapted and reproduced with permission from Cullom SJ, et al: Electrocardiographically gated myocardial perfusion SPECT: Technical principles and quality control considerations. J Nucl Cardiol 5:418-425, 1998.)
Figure 13-3 shows that a gated SPECT acquisition produces both gated SPECT images (through reconstruction and reorientation of the projection data sets corresponding to the individual gating intervals) and standard SPECT images (through reconstruction and reorientation of the sum of the projection sets across all intervals). Whereas cardiac function is assessed from the former, myocardial perfusion is derived from the latter. Since the primary purpose of gated SPECT is usually to assess myocardial perfusion, it is optimal to include all of the data in the perfusion assessment, not just data from the accepted beats. To this end, a useful option is an “extra frame” in which all counts “rejected” by being outside the acceptance window for purposes of gated ventricular function assessment are still accumulated and used for perfusion assessment, leading to optimal count statistics for perfusion assessment purposes and optimal beats for function assessment. Cameras with this feature allow the use of a narrow acceptance window, typically 20% to 30%. If, on the other hand, no “extra frame” exists, it may be advisable to open the acceptance window to 100% or infinity so as to maximize the quality of the images from which perfusion will be assessed. The downside of the wide acceptance window is that counts from arrhythmic beats are allowed into the gated SPECT images, decreasing the accuracy of cardiac function assessment. Most camera manufacturers today do provide the “extra frame” feature in conjunction with gated SPECT imaging, as currently recommended.2 Another approach to the same end is to have simultaneous, independent acquisition of the gated and ungated data sets, a potentially optimal approach because it allows the use of a narrow window on the gated portion and no window at all on the ungated portion of the study.3
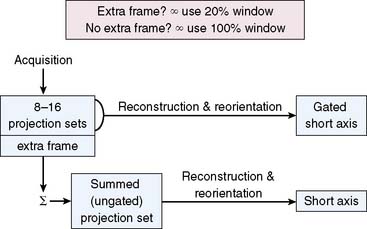
Figure 13-3 Setting the cardiac beat length acceptance window.
(Adapted and reproduced with permission from Germano G, Berman D: Gated single-photon emission computed tomography. In Iskandrian AS, Verani MS [eds]: Nuclear Cardiac Imaging: Principles and Applications, 3rd ed. New York: Oxford University Press, 2003, pp 121-136.)
Gating Errors
If a wide cardiac beat length acceptance window is used in the presence of arrhythmias (e.g., the 100% window mentioned), some arrhythmic beats will not be rejected. A typical example of this problem is the “count dropoff” phenomenon, in which shorter cardiac beats, but not short enough to be rejected, do not contribute counts to the later gating intervals of a “fixed temporal resolution” acquisition; this in turn causes “flickering” of gated SPECT images displayed in cinematic mode (Fig. 13-4).
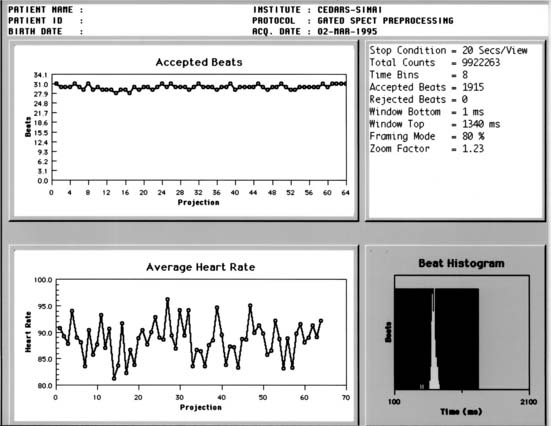
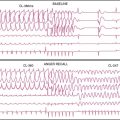
Excessive “bad beat” rejection can result in inadequate counting statistics for accurate function assessment from the remaining beats. In these circumstances, it is recommended that the ventricular function assessments not be considered valid. Examples of such arrhythmias are bigeminy, trigeminy, and frequent ventricular PVC couplets. In many laboratories, patients with more than 20% PVCs present are considered poor candidates for ventricular function measurements with gated SPECT.4 To detect cases in which arrhythmias develop during a gated acquisition employing a wide window, however, additional quality-control strategies can be useful, including (1) the generation, from an independent heart monitor, of paper printouts of the patient’s heart rate throughout the acquisition, (2) the use of software that aims at detecting gating errors from postacquisition analysis of the relative counts and count patterns in the various gated frames,5 and (3) when provided by the camera manufacturer, the review of graphs of accepted counts or heart rate as a function of the projection angle, as well as beat length histograms (Fig. 13-5).
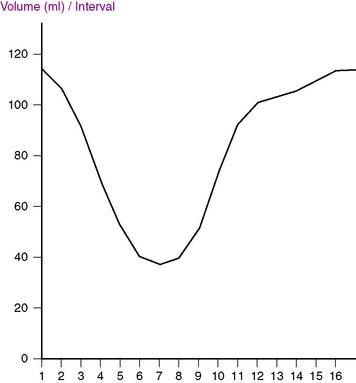
A simple yet effective quality-control tool that can easily be used postprocessing to identify gating errors is visual assessment of the time-volume curve generated by the quantification software available with most systems. A “canonical” curve would be expected to be approximately U-shaped, with the maximal volume (end-diastolic volume [EDV]) corresponding to the first gating interval, and the minimal volume (end-systolic volume [ESV]) slightly before the midpoint of the R-R interval (Fig. 13-6). If major deviations from this expected shape occur, the ECG gate has most likely sensed the R-R interval incorrectly or there have been excessive arrhythmic beats. As Figure 13-7 exemplifies, both arrhythmias and various gating errors are capable of causing severe deformations of the time-volume curve from which EDV, ESV, and left ventricular ejection fraction (LVEF) are calculated, naturally resulting in quantitative errors.
PROCESSING
As is the case with ungated acquisitions, projection images corresponding to the individual frames of a gated acquisition are tomographically reconstructed into transaxial images, which are in turn reoriented into short-axis images perpendicular to the long axis of the LV.6 Projection images are typically smoothed before reconstruction to reduce statistical noise; this is most frequently accomplished using a Butterworth filter, which in the frequency (f) domain is described by the following equation:
The degree of smoothing applied is proportional to fc, the critical or “cutoff” frequency, and depends to a lesser degree on n, the order of the filter. The cutoff frequency can be expressed as a value either in the 0 to 1 range or in the 0 to 0.5 range, depending on the particular convention adopted6—in any event, it is generally agreed that gated images must be smoothed more heavily than ungated or summed images because they contain fewer counts. A summary of the filters settings used at the authors’ institution can be found in Table 13-1.
Gated SPECT images are reconstructed using either the classic filtered backprojection approach, first described by Bracewell and Riddle for astronomical applications in 19677 and later extended to medical imaging by Shepp and Logan,8 or iterative reconstruction techniques. The latter have the potential advantages of reducing reconstruction artifacts caused by hepatic or other extracardiac activity,9 as well as the ease of incorporation of attenuation correction and other compensations into the reconstruction process. Iterative techniques are intrinsically slower than filtered backprojection; however, the speed of modern computers makes it both practical and advisable to reconstruct gated and ungated data sets using the same iterative reconstruction approach.
Automated algorithms to filter, reconstruct, and reorient gated SPECT images are widely available in modern nuclear cardiology10–13 and can greatly improve the accuracy and reproducibility of processing. One particularly useful feature of these approaches is that even though different filter cutoffs are used (see Table 13-1 and Fig. 13-8), the same reconstruction limits (see Fig. 13-8) and reorientation angles (Fig. 13-9) can be applied in lockstep to the gated and ungated image data sets, improving consistency between the perfusion and the function assessment.
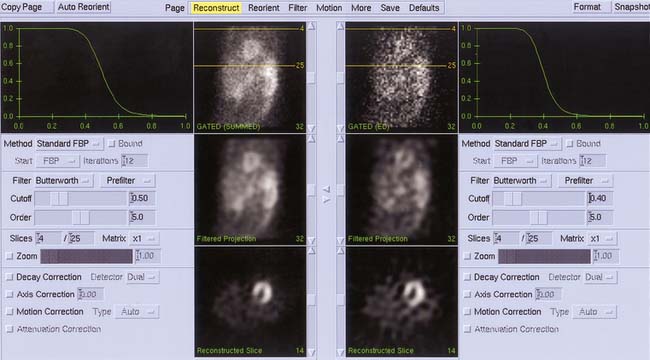
Figure 13-8 Simultaneous automated filtering and reconstruction of gated end-diastolic (right) and summed gated (left) projection images from a thallium-201 gated SPECT acquisition. Note that despite the difference in count statistics, the same reconstruction limits (yellow lines) are calculated for and applied to the two data sets. Cutoff frequencies are based on Table 13-1 but expressed using the 0 to 1 scale.
QUANTIFICATION
Gated perfusion SPECT images can be quantitatively analyzed with respect to a remarkable number of parameters of cardiac function, both global and regional, both systolic and diastolic. As Table 13-2 shows, global systolic function is typically characterized by measurement of the LVEF, EDV and ESV, whereas global diastolic function is similarly defined by assessment of the peak filling rate, time to PFR, peak ejection rate, time to PER, and mean filling fraction MFR/3. Regional parameters of LV function include myocardial wall motion and wall thickening. When these are semiquantitatively scored using a segmental model, the individual segment scores can be added to generate a global or summed wall motion score and a summed wall thickening score. Quantitative phase analysis can also be performed both in a global manner (synchrony of contraction of the LV as a whole) or regionally as the difference between the onset of contraction in different myocardial walls.
Table 13-2 Global and Regional Parameters of Systolic and Diastolic Left Ventricular Function Measured from Gated Myocardial Perfusion SPECT Images
| Parameter | Systolic | Diastolic |
|---|---|---|
| Global |
Global and regional parameters of systolic and diastolic LV function that can be effectively measured from gated myocardial perfusion SPECT images. EDV, end-diastolic volume; ESV, end-systolic volume; MFR/3, mean filling fraction; LVEF, left ventricular ejection fraction; PER, peak ejection rate; PFR, peak filling rate; SWM, summed wall motion score; SWT; summed wall thickening score; TPER, time to PER; TPFR, time to PFR; WM, myocardial wall motion; WT, myocardial wall thickening.
Gated SPECT imaging also offers interesting potential advantages, such as the ability to “freeze” LV motion so as to better identify mild perfusion defects,14–16 as well as the ability to measure gated transient ischemic dilation of the LV,17 which in its ungated form has proven a highly specific marker of severe and extensive coronary artery disease (CAD).18
Recent years have seen the development and validation of numerous algorithms aimed at quantifying parameters of cardiac function from gated SPECT images, and it is indeed estimated that the overwhelming majority of institutions and laboratories performing gated SPECT imaging today also employ quantification to some degree. Although many of those algorithms are important for historical reasons,19–27 only the four commercially available algorithms will be discussed in detail within this chapter. The algorithms are usually referred to as: (1) Cedars-Sinai’s software, or “Quantitative Gated SPECT” (QGS), also a component of AutoQUANT; (2) Emory University’s software, a component of the Emory Cardiac Toolbox (ECT); (3) the University of Michigan’s software, or 4D-MSPECT; and (4) Yale University’s CQ software.
The Cedars-Sinai Approach: Quantitative Gated SPECT
This approach is entirely automated and based in the 3D space. It starts with the segmentation of the LV myocardium using heuristic image thresholding, binarization, and clusterification, followed by iterative cluster refinement using pixel erosion and pixel growing. When a mask consistent with the expected size, shape, and location of the LV is obtained, ellipsoidal fitting is used to define a new sampling coordinate system, along which rays are drawn and count profiles measured normally to the myocardium. These count profiles are fitted to asymmetric Gaussian curves. The Gaussians’ maxima represent the midmyocardial surface, while the endocardial and epicardial surfaces are determined based on a phantom-determined fraction of the Gaussians’ standard deviations, and the valve plane is determined by fitting a plane to the most basal myocardial points. Surfaces can usually be accurately determined even in the apparent absence of perfusion, thanks to count gradient continuity constraints. LV cavity and myocardial volumes are calculated for each gating interval as the number of voxels bound by myocardial surfaces and the valve plane, and cavity volumes are displayed in the time-volume curve, from which diastolic function can be assessed, while the LVEF is derived from the EDV and ESV.28
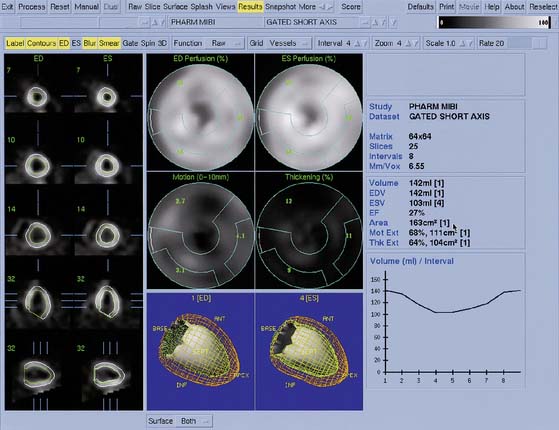
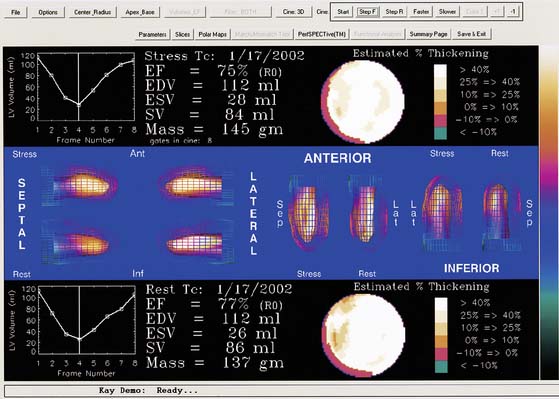
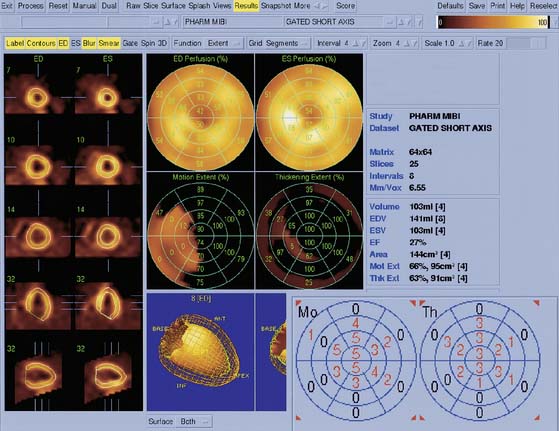
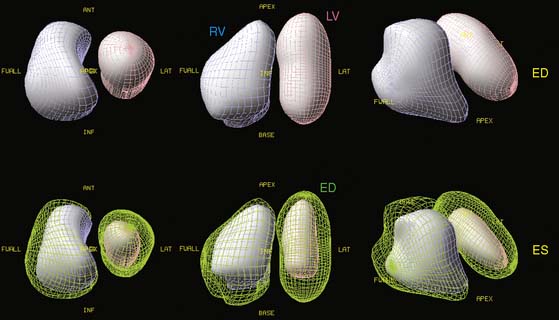
Myocardial motion is computed as the excursion of the endocardial surface from end-diastole to end-systole, and myocardial thickening is based on both geometric and count considerations.29 Synchrony of contraction can be measured for the LV as a whole, or as differences in the time of contraction of individual portions of the LV quantified.30 Both myocardial motion and thickening can be visualized through various 2D and 3D cinematic displays, and motion and thickening scores are calculated automatically using normal databases.31 A sample QGS display is presented in Figure 13-10, and a comprehensive description of the Cedars-Sinai approach can be found in Ref. 32.
The Emory University Approach: Emory Cardiac Toolbox
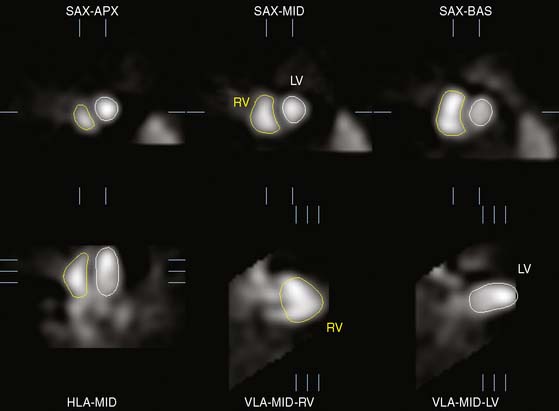
In this approach, maximal myocardial count samples are extracted at many locations about the LV during perfusion analysis for each gated frame of the study. Because of the SPECT point spread function, it is assumed that the maximum count value occurs in the middle of the myocardium at each sample. Therefore, these sample points define a midmyocardial surface at each frame. The frame where the volume of this midmyocardial surface is maximum is considered the probable end-diastolic frame, and the myocardium is assumed to be uniformly 1-cm thick at each sample of that frame.33 Wall thickening through the cardiac cycle at each myocardial sample is determined by fitting the change in counts to a cosine function and computing its amplitude. The ED wall thickness value of 1 cm is modified according to the percent thickening curve to compute wall thickness for each sample in each additional frame. Finally, the endocardial or epicardial surfaces are estimated at each frame by subtracting or adding, respectively, half of the thickness to the midmyocardial surface at each sample. Using these surfaces, a final calculation of volume is made for the endocardial surface to determine end-diastole, end-systole, LV volumes, and LVEF. A phase histogram from which global dyssynchrony measures are derived is also generated from the phase angles of the fitted cosine curves for each sample point.34 A sample ECT display is presented in Figure 13-11, and a comprehensive description of the Emory approach can be found in Ref. 35.
The University of Michigan Approach: 4D-MSPECT
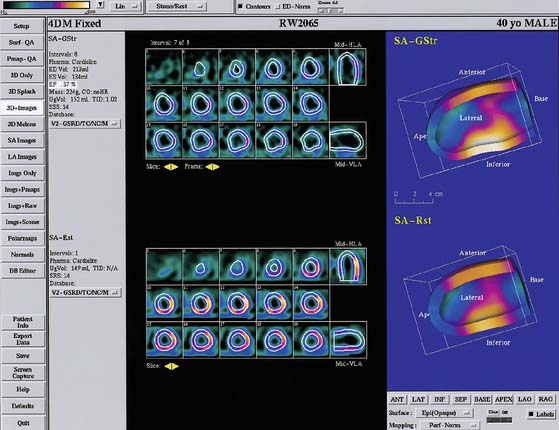
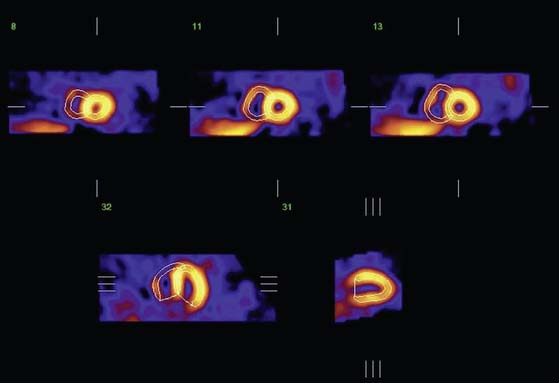
The surface estimation algorithm for 4D-MSPECT (4DM) utilizes a 2D gradient operator in conjunction with a segmented image and a contiguity constraint to provide initial surface estimates for the endocardial and epicardial surfaces. Weights are assigned based on measured intensity profiles within these initial surface boundaries. Using these weights and the estimated curvature of the heart, 2D and 1D weighted splines are utilized to “fill in” missing data (i.e., perfusion defects) while minimizing the inclusion of extracardiac activity.36 Using the intensity profiles bounded by these new surfaces, a Gaussian fit is used to find the peak activity and an estimate of the myocardial thickness. To compensate for the limited resolution of emission tomography, the myocardial thickness at end-diastole (max endocardial volume) is scaled to provide an average thickness of 10 mm. This scale factor in conjunction with a myocardial mass constraint is used to adjust the myocardial thickness throughout the cardiac cycle. Using the endocardial surfaces, a volume curve is generated that spans the cardiac cycle. From the volume curve data, systolic (LVEF, volumes, cardiac output, myocardial mass) and diastolic (peak and time to peak filling, and ejection rates) parameters are calculated. For diastolic function, a minimum of 16 frames of data are required to provide adequate sampling or the harmonic fitting to compute to the diastolic parameters. Use of 4DM also provides 2D and 3D regional wall motion and wall thickening maps with database comparisons, wall motion and wall thickening segmented scoring maps,37 and 2D and 3D cinematic displays for the visual interpretation of wall motion and wall thickening. To complement the functional estimates, 2D and 3D analysis of the myocardial intensity information within the detected LV surfaces is compared with normal databases to quantify myocardial perfusion, thereby providing correlative information of perfusion with function for the improved diagnosis of CAD. A sample 4DM display is presented in Figure 13-12, and a comprehensive description of the University of Michigan approach can be found in Ref. 38.
The Yale University Approach
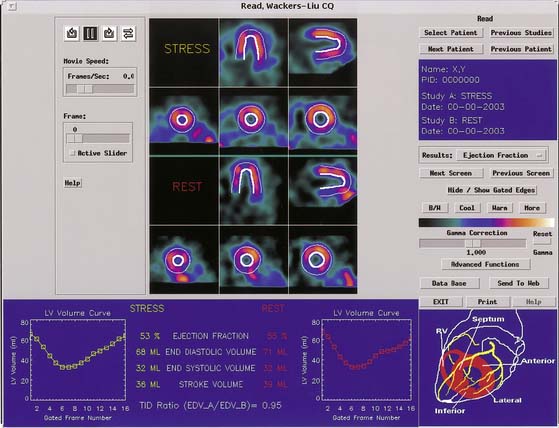
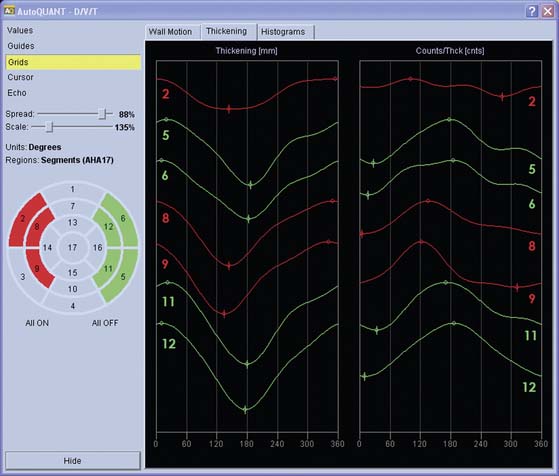
Quantification of regional myocardial wall thickening is based on the generation of circumferential maximal count profiles for each gating frame, and measurement of count changes in each sector from end-diastole to end-systole.39 End-diastole is defined as the first frame that corresponds to the R wave, and end-systole as the frame with maximal sectorial count density. The normalized maximal thickening profile is displayed in conjunction with the lower limit of normal wall thickening (mean thickening in normal subjects is 2SD). A sample Yale University display is presented in Figure 13-13, and a comprehensive description of the Yale approach can be found in Ref. 40.
EJECTION FRACTION
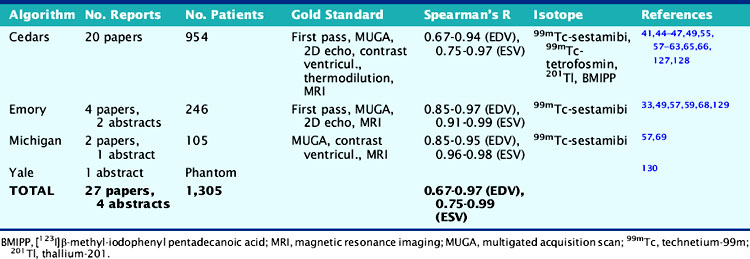
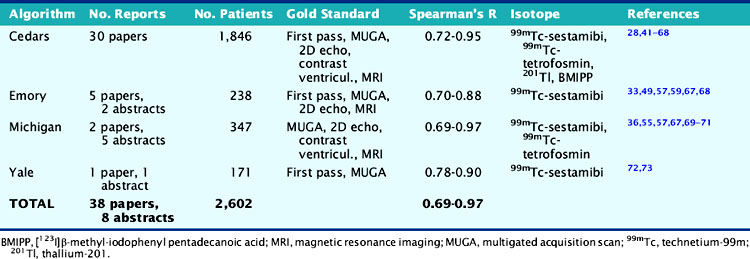
As Table 13-3 shows, the agreement between gated SPECT and reference standard measurements of quantitative LVEF is in the very good to excellent range and relatively independent of the isotope, protocol, standard, and algorithm used. Reproducibility (defined as the agreement between independent measurements of LVEF resulting from multiple applications of the same quantitative algorithm to the same gated SPECT study) is also excellent, being directly related to the degree of automation of the algorithm.22,57,66,67
Table 13-3 Clinical Validation of Quantitative Measurements of Left Ventricular Ejection Fraction Volume from Gated Perfusion SPECT
Repeatability (defined as the agreement between independent measurements of LVEF resulting from separate applications of the same quantitative algorithm to different, but presumably equivalent, gated SPECT studies) is also extremely good, both for sequential studies74–76 and for acquisition involving different isotopes,50,52,58,76–79 injected dose and acquisition time,80–82 acquisition orbit,83 cameras,84,85 number of gating intervals,53 and patient position.86,87 Moreover, the use of attenuation correction or resolution recovery in conjunction with gated SPECT imaging does not appear to greatly affect quantitative measurements of LVEF.88,89
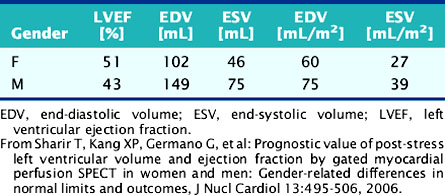
Cross-algorithm reproducibility (defined as the agreement between LVEF values measured by different quantitative algorithms applied to the same gated SPECT study) has been reported to be in the very good to excellent range. However, it is also well established that systematic differences among algorithms do exist and effectively prevent the direct comparison (or pooling) of differently analyzed data.55,59,90–95 Normal limits for LVEF are similarly algorithm dependent55,73,96–100 and also gender specific,98,100–108 as shown in Table 13-4 for the QGS software.
Table 13-4 Normal Limits for Quantitative Measurements of Global Left Ventricular Function from 8-Frame Gated Perfusion SPECT Images, Using the QGS Algorithm
There are some limitations common to most algorithms connected with the quantification of LVEF from gated perfusion SPECT. For example, it is well known that the relatively low resolution of nuclear cardiology images may make it difficult to visualize small objects, such as the LV cavity of patients with small ventricles, particularly at end-systole (Fig. 13-14). This phenomenon, also referred to as partial volume effect,26 will lead to the underestimation of LV cavity volumes (particularly the ESV), with consequent overestimation of the LVEF.109–111 The problem can be alleviated through magnification of the LV either in acquisition (by employing a larger acquisition zoom) and/or in reconstruction (by employing zoomed centered or zoomed off-axis reconstruction),111–114 or by applying numerical modeling and compensation of blurring.115,116 However, a simpler solution is to discard the overestimated value and report the LVEF as being “in the normal range.”
Another limitation related to the partial volume effect is that nuclear cardiology techniques are incapable of measuring myocardial thickness with high accuracy. Most quantitative gated SPECT algorithms are either calibrated for the range of thicknesses most typically encountered in clinical practice28 or assume a fixed myocardial thickness in the normal range33; consequently, gated SPECT LVEFs measured in patients with left ventricular hypertrophy are likely to be underestimated.117
Gated SPECT imaging traditionally has been performed with 8-frame gating, which undersamples the time-volume curve compared to 16-frame gating, and thus leads to mild underestimation of the LVEF. However, Figure 13-15 shows that the degree of underestimation is small (3 to 4 LVEF percentage points) and remarkably uniform over a wide range of ejection fractions, as also confirmed by other published reports.28,53,54,56,118–120
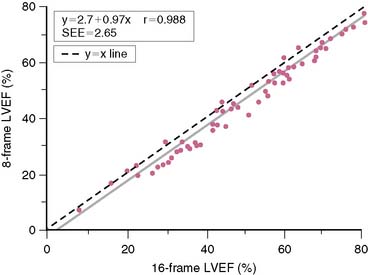
Figure 13-15 Left ventricular ejection fractions (LVEFs) measured by QGS on 65 patients, using 16-frame and 8-frame gated 99mTc-sestamibi SPECT, showing that the latter underestimates LVEF.28 The correlation between the two measurements is excellent, and the standard error of the estimate (SEE) low, making their relationship quite predictable.
(Reproduced with permission from Germano G, Berman D: Quantitative gated perfusion SPECT: Technical aspects. In Germano G, Berman D [eds]: Clinical Gated Cardiac SPECT. Armonk, NY: Futura Publishing, 1999, pp 115-146.)
Finally, most quantitative gated SPECT algorithms tend to assume a regular or “smooth” LV shape in areas with severely reduced or absent perfusion,28 and this would be expected to result in the “cutting off” of aneurysms, with consequent overestimation of the measured LVEF. Although a large number of published reports deny the presence of major discrepancies between true and quantitatively measured LVEF in patients with large perfusion defects and/or low LVEF,42,43,45,46,48,50,56,121–125 the possibility of aneurysms should always be considered in the presence of perfusion defects.69,126
END-SYSTOLIC AND END-DIASTOLIC VOLUME
As Table 13-5 shows, the agreement between gated SPECT and reference standard measurements of quantitative end-diastolic and end-systolic volumes is in the very good to excellent range and relatively independent of the isotope, protocol, standard, and algorithm used. These published findings are similar to those for LVEF (see Table 13-3), even though the LVEF can be considered relatively less vulnerable to errors in the EDV and ESV volume estimates (errors in the determination of EDV and ESV would be expected to occur in the same general direction and therefore would at least partially cancel out when the volumes are ratioed). As with LVEF, reproducibility is excellent,28,46,66,67,74 and repeatability has also been reported to be extremely high, both for sequential studies74 and for acquisition involving different isotopes,58,76,79 injected dose and acquisition time,80,81 number of gating intervals,53 and patient position.86,87 Cross-algorithm reproducibility of LV volume measurements at ES and ED has also been reported to be very high,57,94,95,99,129 although, as for LVEF, systematic differences among algorithms do exist and can be quite large.59,90,92,94,95
REGIONAL SYSTOLIC FUNCTION
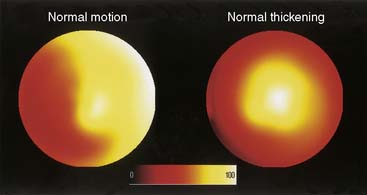
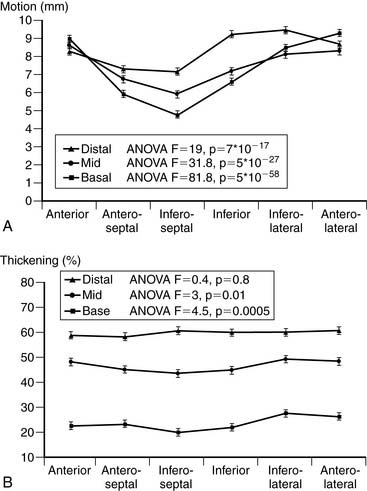
The quantitative measurement of regional LV myocardial wall motion and wall thickening from gated SPECT images poses a unique problem. While it is certainly possible to measure (at many sampling locations) the absolute motion of the endocardial surface of the LV or the “brightening” of the LV myocardium from ED to ES,29 these measurements are not as directly interpretable as regional measurements of myocardial perfusion. For example, neither myocardial wall motion nor thickening are uniform across the myocardium of a normal patient,131 as shown in Figure 13-16, so dark areas in the motion or thickening polar maps cannot be considered a visual clue that myocardial function is abnormal in those areas. Indeed, it has been demonstrated that normal regional myocardial contraction by gated myocardial perfusion SPECT is characterized by a substantial apex-to-base decline in thickening and circumferential heterogeneity in endocardial motion (Fig. 13-17).31
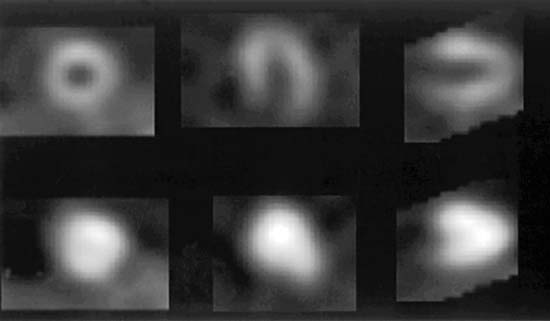
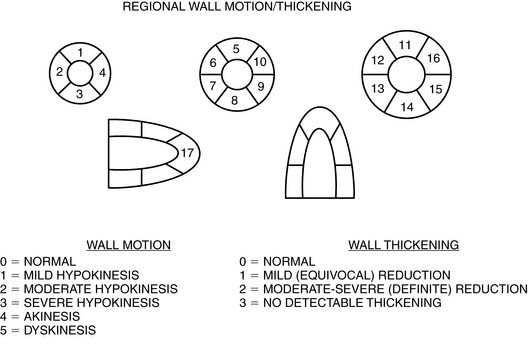
Validation of absolute quantitative measurements of regional myocardial function can be performed by comparing gated SPECT results to results from a high-resolution reference standard like MRI.132 However, absolute measurements have greater clinical value if translated into an assessment of function abnormality through the development of region-specific normal limits133–137 and criteria for abnormality.31 Normal limits are usually developed based on an expert observer’s classification of segmental motion and thickening using a semiquantitative or categorical scale and a multisegment model of the myocardium. The standardized 17-segment model recommended by the American Heart Association138 uses three short-axis slices (distal [apical], mid, and basal), with the apex represented by one segment visualized in a midvertical long-axis image,139 and each segment is scored with a 6-point (motion) or 4-point (thickening) scale, as exemplified in Figure 13-18.
Semiquantitative scoring of myocardial wall motion and thickening can now be generated automatically by computer algorithms based on normal limits and has been validated against reference semiquantitative visual assessment (Fig. 13-19).31,37,139
DIASTOLIC FUNCTION
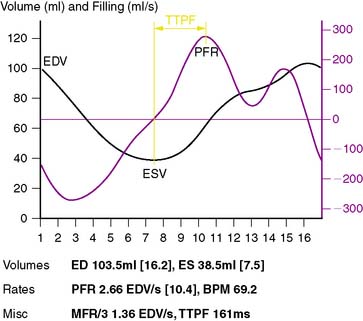
While it is generally agreed that 8-frame gated SPECT imaging does not allow for meaningful measurement of LV diastolic function,140 it has also been reported that 12-frame imaging may be somewhat adequate,141 16-frame imaging quite effective,142 and 32-frame imaging feasible and resulting in excellent agreement with a MUGA standard.51,53,54,143 As shown in Figure 13-20, parameters of LV diastolic function that can be quantitatively measured from gated myocardial perfusion SPECT images include the peak filling rate (PFR), the time at which the PFR occurs (TTPF), the peak ejection rate (PER), the time at which the PER occurs (TPER), and MFR/3. Normal limits for PFR and TTPF have been quantitatively determined by QGS from 16-frame gated SPECT images and proved very similar to those reported with gated blood pool studies.144
PHASE ANALYSIS
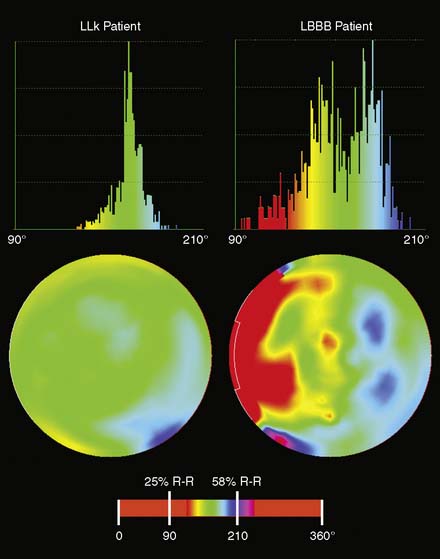
Phase analysis, initially described with nuclear cardiology methods in the 1970s, has reemerged as being of clinical interest in assessing the likelihood of benefit from and evaluation of the benefit from cardiac resynchronization therapy (CRT),145,146 as well as for potentially evaluating and guiding internal cardiac defibrillators (ICDs). Phase analysis can be applied to gated myocardial perfusion SPECT or gated blood pool SPECT. In the former, when sampling the LV myocardium for the purpose of quantitative perfusion and function assessment, it is also straightforward to create two one-dimensional arrays for each LV myocardium sampling point, the first one containing the distance (amount of wall motion) between the mid-myocardial surface and a reference position for each gating interval, and the second one containing the myocardial thickness (distance between the endo- and epicardial surfaces normally to the mid-myocardial surface), also for each gating interval. Each array represents a time-varying, periodic function that can be reduced to its first fourier harmonic (FFH), in turn defined by its phase and amplitude.147 Motion and thickening phase and amplitude for all the sampling points representative of the LV can be displayed in polar maps or global histograms, as shown in Figure 13-21, where thickening phase is compared between a low-likelihood patient and a patient with left bundle branch block. In addition, motion and thickening curves showing onset and peak of contraction can be derived and compared across different portions of the LV (segments, walls, coronary territories), which makes it easier to evaluate dyssynchrony between the lateral wall of the LV and the septum (Fig. 13-22). As previously mentioned, phase analysis calculations have been implemented in the ECT and the Cedars-Sinai software.
RIGHT VENTRICULAR FUNCTION
Nevertheless, it is conceptually possible to apply to the RV algorithms similar to those used for LV function quantitation, determine RV myocardial contours even in non hypertrophic RVs (Fig. 13-23), and derive estimates of both RVEF and RV volumes. It is anticipated that RV quantitation will become an integral part of gated perfusion SPECT quantification, even though it is not currently widely available or fully validated.
BLOOD POOL SPECT
If tomographic assessment of both LV and RV function is required, the most widespread nuclear medicine technique available today for that purpose is gated blood pool SPECT using 99mTc-labeled red blood cells148–151; unlike with traditional gated blood pool planar imaging, no background subtraction is needed with the SPECT approach, since the LV and RV are well separated in the 3D space.
Most of the technical issues examined in this chapter with respect to the acquisition and processing of gated perfusion SPECT studies also apply to gated blood pool SPECT imaging. However, in blood pool imaging, no perfusion information is acquired, and therefore it is advisable to always set the cardiac beat length acceptance window to 20% to 30% to minimize the effects of arrhythmia. With the use of 10% to 20% acceptance windows and 16-frame studies, assessment of diastolic function could be both feasible and accurate. Several quantitative algorithms have been recently introduced that aim at identifying the endocardial surfaces of the LV and (in a minority of cases) of the RV throughout the cardiac cycle (Fig. 13-24), so as to determine ventricular volumes and ejection fractions in a manner similar to gated perfusion SPECT. Although reported validations of these algorithm measurements are generally satisfactory,132,152–179 the gold standards used are very often planar, and RV parameters are rarely validated. Still, the increase in computer speed, the greater diffusion of multidetector cameras, and the general acceptance of state-of-the-art 3D analysis and display techniques (Fig. 13-25) have already considerably strengthened the case for gated blood pool SPECT imaging. The clinical circumstances in which this procedure is likely to become effective are the same as those in which resting blood pool scintigraphy is currently applied. Chief among these is the assessment of Adriamycin cardiotoxicity. Blood pool scintigraphy is also commonly employed in serial assessment of patients with aortic insufficiency, congestive heart failure, and patients who have undergone cardiac transplantation.
DIAGNOSTIC VALUE OF GATED SPECT
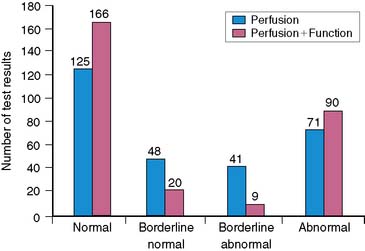
An important clinical role of myocardial perfusion SPECT stems from its ability to reduce the number of diagnoses deemed equivocal when based on perfusion assessment alone. The principal way in which this occurs is by defining the motion characteristics of segments with apparent nonreversible perfusion defects that are considered to possibly represent soft-tissue attenuation. The assumption is that regions with true perfusion defects that are nonreversible would contract abnormally, while those associated with attenuation artifacts would demonstrate normal motion and thickening. The incremental value of gated SPECT in this area was demonstrated by Smanio et al.,180 who interpreted myocardial perfusion SPECT studies using (1) perfusion information and (2) both perfusion and regional function information in 285 consecutive patients undergoing same-day rest and gated post-stress 99mTc sestamibi SPECT. Based on a 4-point classification of perfusion (normal, borderline normal, borderline abnormal, and abnormal), 31% of the patients had a “borderline” interpretation using the perfusion images alone; when wall motion from the gated SPECT images was also considered, the number of borderline interpretations fell to 10% (Fig. 13-26). Of note, the increase in normal interpretations occurred mostly in the subsample of patients with a low (<10%) pretest likelihood of disease, whereas the increase in abnormal interpretations was associated with the subsample of patients with documented CAD, suggesting that the addition of regional myocardial function assessment to perfusion assessment was improving the diagnostic accuracy of SPECT imaging.
The differentiation of true perfusion defects from attenuation artifacts with gated SPECT has been shown to increase the specificity of myocardial perfusion assessment in a population of 115 women with known or suspected CAD (n = 85) or with a less than 5% likelihood of CAD (n = 30) undergoing nongated stress/rest 99mTc sestamibi plus post stress gated 99mTc sestamibi SPECT,181 as well as in a mixed population of 91 patients (71 males) with equivocal fixed perfusion defects on stress/rest 99mTc tetrofosmin SPECT.182 In the latter study, two independent observers classified the defects as true or artifactual in three separate steps, by visually assessing (1) the stress/rest perfusion images, (2) the stress/rest perfusion images plus the stress/rest rotating projection images, and (3) all of the above plus myocardial wall motion from the rest gated SPECT images. The main finding was that the diagnostic accuracy for each observer (measured by ROC curve analysis) increased at each step. Moreover, the κ coefficient of agreement between the two observers improved with each step, signifying an increase in interobserver reproducibility.
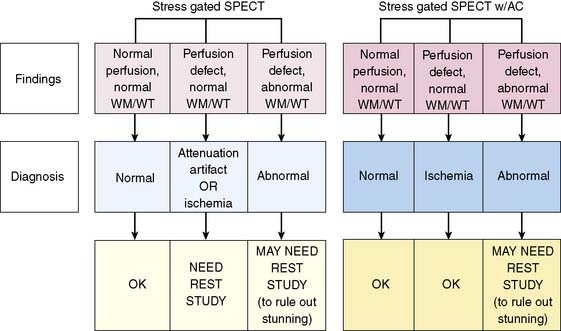
Since attenuation artifacts are relatively frequent in both women (mainly breast attenuation) and men (diaphragmatic attenuation), combining gated SPECT with attenuation correction might further improve the specificity of myocardial perfusion SPECT imaging. This has been suggested by preliminary studies,183 although considerable concerns still exist concerning the cost, standardization, and validation of currently available attenuation-correction protocols.184,185 If accurate and standardized attenuation correction could be achieved, however, its utilization in conjunction with gating could facilitate the diffusion of “stress-only” SPECT protocols in some types of patients.186–190 In essence, if a perfusion defect is present and can be assumed to be real because of attenuation correction, and if the myocardial region corresponding to the defect contracts or thickens on gated SPECT,191 the defect can be considered to be reversible and indicative of ischemia. As Figure 13-27 shows, in the gated SPECT with attenuation correction scenario, only patients with stress perfusion defects that do not contract or thicken would need to return for a resting perfusion study. This single-injection, single-acquisition protocol would offer advantages in regard to cost reduction and maximization of patient throughput, although it also would have the disadvantage of requiring a 2-day study in some patients, and possibly of decreasing the sensitivity associated with observing mild reversible perfusion defects, which might be interpreted as normal in stress-only protocols.
Myocardial wall motion abnormalities detected by postexercise gated SPECT may be a sign of prolonged postischemic stunning. Since exercise-induced stunning is considered to be a marker of severe ischemia and is usually related to a high-grade coronary stenosis, this finding can be of value in the attempts to use gated SPECT for risk stratification. Sharir et al. studied 98 patients who underwent nongated rest thallium-201/gated postexercise 99mTc sestamibi SPECT, with the latter acquisition commencing 15 to 30 minutes after exercise, and semiquantitatively scored both myocardial perfusion and function using a 20-segment model and a 5-point (perfusion) or 6-point (motion) scale.192 These investigators found, and others193 later confirmed, that in patients with normal resting myocardial perfusion, postexercise wall motion abnormalities are strongly associated with critical coronary stenoses. As a rule of thumb, approximately 90% of segments displaying this pattern are associated with a greater than or equal to 90% stenosis in the vessels supplying the segment. Sharir et al. also demonstrated that this “post stress stunning” had significantly higher sensitivity for the detection of severe CAD compared to severe perfusion defects. A mechanism by which to explain this finding is that perfusion SPECT images are normalized to the area of highest uptake within the myocardium, so that in the setting of multivessel ischemia, only the most severely abnormal region shows a perfusion defect, because myocardial perfusion SPECT depends on a regional decrease in perfusion compared to the “normal zone” for perfusion defect detection. This phenomenon can lead to an interpretation of single-vessel rather than multivessel disease, even when the patient actually has multivessel ischemia. An extreme (albeit rare) generalization of this scenario pertains to patients with balanced reduction of flow due to triple-vessel disease (where image normalization might lead to apparent normal perfusion across the myocardium), with the clue to its presence being the finding of diffuse post stress stunning. Table 13-6 summarizes the ways in which gated SPECT improves diagnostic assessment.
Table 13-6 Role of Gated SPECT Imaging in the Assessment of Cardiac Disease
Several studies have confirmed and added to these observations. Lima et al. demonstrated that the combination of perfusion and function (i.e., wall motion abnormalities) resulted in a significant increase in correct identification of multivessel CAD in patients with angiographically documented triple-vessel disease. The addition of functional data from gated SPECT to clinical, stress, and perfusion information also yielded a significant increase in the prediction of triple-vessel disease.194 Cullom et al. furthered this concept by showing added benefit of using gated acquisition during rest as well as post stress studies, demonstrating that a fall in ejection fraction on post stress is associated with higher risk. Following this work, it has become standard in our laboratories and many laboratories to use gated acquisitions as a routine for all gated SPECT acquisitions, even when the tracer employed is thallium-201 (rest or redistribution imaging), with its associated lower count rates.4,195,196 Emmett et al. subsequently also demonstrated that combined rest and post stress regional wall motion abnormality added incremental value to perfusion defect analysis with gated SPECT for the assessment of the severity of angiographic CAD.193 We have shown that gated SPECT wall motion assessment and EF assessment at the time of reporting adds to perfusion defect assessment in the correct classification of patients with left main CAD as being at high risk.197
PROGNOSTIC VALUE OF GATED SPECT
Increasingly, the most useful application of myocardial perfusion SPECT is that of risk stratification and guiding management decisions, where the focus is not on predicting who has CAD but on identifying patients at risk for specific adverse events (cardiac death or nonfatal myocardial infarction) and guiding subsequent management to reducing the risk of those outcomes. Although there are fewer reports of the incremental value of function measurements from gated SPECT over perfusion in these assessments, several recent studies have documented this added value. As a background regarding the importance of LVEF, as well as rest and peak-stress end-systolic volumes measured by nuclear cardiology methods, a series of reports from the Duke databank using rest and exercise first-pass radionuclide angiography demonstrated that patients with suspected cardiac disease could be risk-stratified according to their risk of subsequent cardiac death using a diagnostic threshold of resting LVEF equal to 50%.198–203
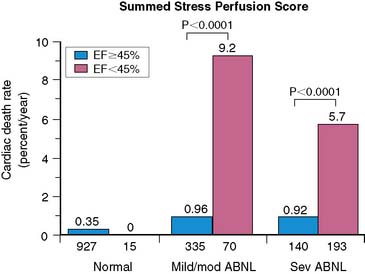
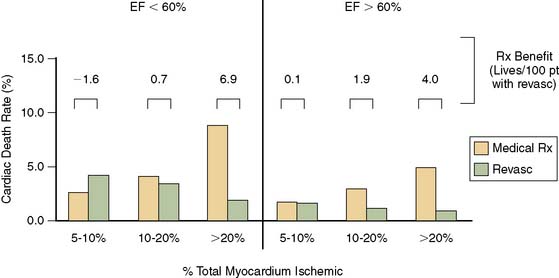
Using the Cedars-Sinai database, Sharir et al.204 demonstrated in 1680 patients that post stress LVEF, as measured by gated SPECT, provided significant information over the extent and severity of perfusion defect as measured by the summed stress score in the prediction of cardiac death (Fig. 13-28). These authors further demonstrated that LV end-systolic volume provided added information over post stress LVEF in prediction of cardiac death (Fig. 13-29). A relatively low cardiac death rate in patients with abnormal perfusion and normal LV function reported in this study is probably explained by a referral bias in which patients with greatest ischemia by summed stress score were preferentially sent for early revascularization and thus censored from assessment of the prognostic value of the test, whereas the referral bias was likely less prominent based on ventricular function. In a subsequent report in more than 2600 patients, Sharir et al. noted that while post stress EF provides incremental information over prescan and perfusion variables in prediction of cardiac death, the extent of ischemia was a stronger predictor of nonfatal myocardial infarction. Once prescan and perfusion variables were known, post stress LVEF did not provide incremental information with respect to the risk of nonfatal MI.205
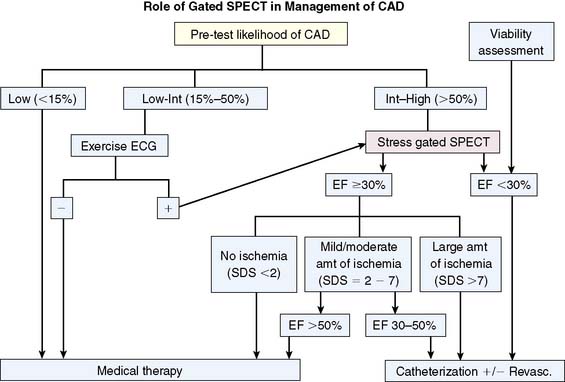
Sharir et al. further reported that the combination of the ejection fraction and reversible ischemia can be used in the prediction of cardiac events.205 If post stress EF is less than 30%. Cardiac death or nonfatal MI rates appear to be high regardless of the amount of ischemia as assessed by the summed difference score. In patients with post stress EF of 30% to 50%, mild amounts of ischemia were associated with relatively high cardiac event rates. In patients with ejection fractions of greater than 50%, only patients with moderately extensive ischemia were at high risk of cardiac events. The work of Sharir et al. was based on the summed difference score as a measure of ischemia, but we have recently advocated the use of a ratio between this score and the maximal score as a more useful clinical measurement.206 An algorithm incorporating myocardial perfusion scintigraphy and gated SPECT LVEF based on these findings is shown in Figure 13-30.
Similar findings were reported by Liao et al.,208 who studied 997 patients (75% male; median age = 60 years) who underwent exercise treadmill testing with first-pass ejection fraction (RNA-EF)-measured and myocardial perfusion SPECT imaging as a single test. During a median follow-up of 4.1 years, 175 patients experienced outcome events. In clinically risk-adjusted models, RNA-EF was the most powerful predictor of cardiovascular death compared with the Duke Treadmill Score (DTS) and myocardial perfusion SPECT (X2 = 40.5, 27.6, and 19.8, respectively). Conversely, exercise myocardial perfusion SPECT was a stronger predictor of nonfatal MI than the DTS or RNA-EF (X2= 26.7, 15.7, and 16.7, respectively).
A recent report by Thomas et al. illustrated the added value of the post stress EF from gated SPECT over myocardial perfusion measurements from a community-based nuclear cardiology laboratory that followed 1612 consecutive patients undergoing stress gated SPECT over a follow-up period of 24 ± 7 months.209 These authors found that post stress EF added incremental prognostic value over pre-SPECT and perfusion data. Even after adjustment for these variables, each 1% change in LVEF was associated with a 3% increase in risk of adverse events. In this study, perfusion data also added incremental value over EF data in both patients with EF lower than 40% and those with EF of 40% or greater. Travin et al. subsequently reported a series of 3207 patients who underwent stress SPECT and were followed up for adverse events.210 They found that both abnormal wall motion and abnormal EF were associated with increased risk; furthermore, they demonstrated that an abnormal gated SPECT wall motion score was also predictive of outcome. Similar to previous studies, myocardial infarction was predicted by the number of territories with a perfusion defect but not by EF. These authors also demonstrated that the results of gated SPECT added incremental value over both normal and abnormal SPECT perfusion. Similarly, Petix et al. studied 333 patients undergoing gated myocardial perfusion SPECT with both rest and post stress gating, demonstrating that addition of function data from gated SPECT provided a significant increase in the prediction of hard cardiac events.211
Most of the previously noted studies documenting the added prognostic value of LV function measurements over perfusion assessment using gated SPECT employed exercise as the form of stress. Although vasodilator stress is considered to be as likely as exercise stress to produce an abnormal perfusion study in patients with CAD, it less commonly produces true ischemia compared to exercise stress. Thus, vasodilator stress is expected to less commonly produce abnormalities of ventricular function. Little has been written regarding the added value of function measurements from gated SPECT for prognosis in patients undergoing vasodilator stress. Mast et al. reported findings in 240 patients undergoing pharmacologic gated SPECT, demonstrating that a significant minority of patients with CAD undergoing pharmacologic stress develop stress-induced wall motion abnormalities.212
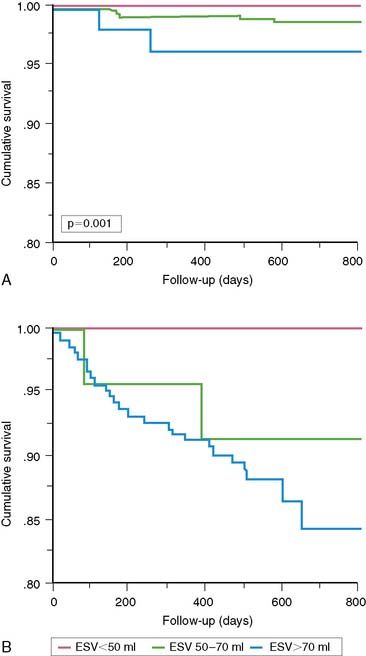
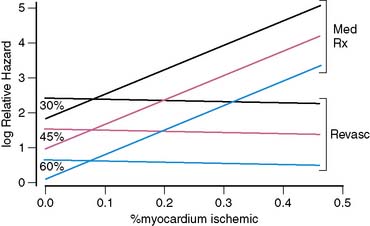
A problem of particular interest with regard to outcome research dealing with the use of nuclear cardiology procedures in guiding patient management is the impact of including both patients treated medically and those treated with early revascularization on the results of survival analyses. In a landmark manuscript dealing with myocardial perfusion variables without gating, Hachamovitch et al. demonstrated that the amount of ischemia by myocardial perfusion SPECT was predictive of benefit from revascularization.213 More recently, these investigators included the gated SPECT findings to examine the hypothesis that although EF predicts the risk of cardiac death, only measures of ischemia will identify which patients will accrue a survival benefit from revascularization compared with medical therapy after stress SPECT. In this study, 5366 consecutive patients without prior revascularization were followed up for 2.8 ± 1.2 years, during which 146 cardiac deaths occurred (2.7%, or 1% per year). After adjustment for pre-SPECT data and the use of a propensity score to adjust for nonrandomized treatment assignment, the authors found several interesting findings. First, as previously shown by Sharir et al., LVEF was the most powerful predictor of cardiac death. In addition, as shown before, stress perfusion results added incremental value over EF for prediction of cardiac death.214 Most important, only the percent ischemic myocardium was able to predict which patients would accrue a survival benefit with revascularization over medical therapy. On the other hand, LVEF played a crucial role in identifying the absolute benefit (number of lives saved per 100 treated, number of years of life gained with treatment) for a given patient (Figs. 13-31 and 13-32).
As previously described, prediction of absolute benefit after gated SPECT is also a function of clinical risk factors such as patient age, sex, diabetes mellitus, and type of stress performed.214 Optimal implementation of the results of gated SPECT for purposes of guiding patient management will require complex algorithms that incorporate all of the information from the perfusion and function variables derived from gated SPECT as well as the clinical response to stress and comprehensive assessment of variables that define the baseline risk of the patient.
ASSESSING MYOCARDIAL VIABILITY
An underappreciated potential use of gated SPECT is in assessment of myocardial viability by examining the recruitment of contractile function in asynergic segments in response to inotropic stimulation. Low-dose dobutamine echocardiography has become a commonly employed, established method in this regard.215 Gated SPECT can also be applied for this purpose. In this regard, Leoncini et al. documented the usefulness of dobutamine 99mTc sestamibi gated SPECT for prediction of improvement (or lack of improvement) of LVEF after coronary revascularization.216 In 37 patients undergoing this procedure as well as revascularization with follow-up gated SPECT, an increase in LVEF of ≥ 5 units during dobutamine was the optimal cutoff value for predicting a significant post revascularization improvement in LVEF, with sensitivity and specificity of 79% and 78%, respectively.
1. Bacharach S.L., Bonow R.O., Green M.V. Comparison of fixed and variable temporal resolution methods for creating gated cardiac blood-pool image sequences. J Nucl Med. 1990;31:38-42.
2. Bar Harbor Invitation Meeting 2000. J Nucl Cardiol. 2001;8:224-316.
3. Philips Medical Systems: Personal communication
4. Cullom S.J., Case J.A., Bateman T.M. Electrocardiographically gated myocardial perfusion SPECT: technical principles and quality control considerations. J Nucl Cardiol. 1998;5:418-425.
5. Nichols K., Dorbala S., DePuey E.G., et al. Influence of arrhythmias on gated SPECT myocardial perfusion and function quantification. J Nucl Med. 1999;40:924-934.
6. Germano G. Technical aspects of myocardial SPECT imaging. J Nucl Med. 2001;42:1499-1507.
7. Bracewell R., Riddle A. Inversion of fan-beam scans in radioastronomy. Astrophys J. 1967;150:427-434.
8. Shepp L.A., Logan B.F. The Fourier reconstruction of a head section. IEEE Trans Nucl Sci. 1974;NS-21:21-43.
9. Germano G., Chua T., Kiat H., et al. A quantitative phantom analysis of artifacts due to hepatic activity in technetium-99m myocardial perfusion SPECT studies. J Nucl Med. 1994;35:356-359.
10. Cauvin J.C., Boire J.Y., Maublant J.C., et al. Automatic detection of the left ventricular myocardium long axis and center in thallium-201 single photon emission computed tomography. Eur J Nucl Med. 1992;19:1032-1037.
11. Cooke C., Folks R., Jones M., et al. Automatic program for determining the long axis of the left ventricular myocardium used for thallium-201 tomographic reconstruction. J Nucl Med. 1989;30:806. (abstract)
12. Germano G., Kavanagh P.B., Chen J., et al. Operator-less processing of myocardial perfusion SPECT studies. J Nucl Med. 1995;36:2127-2132.
13. Germano G., Kavanagh P.B., Su H.T., et al. Automatic reorientation of three-dimensional, transaxial myocardial perfusion SPECT images [commentary]. J Nucl Med. 1995;36:1107-1114.
14. Slomka P.J., Nishina H., Berman D.S., et al. “Motion-Frozen” Display and quantification of myocardial perfusion. J Nucl Med. 2004;45:1128-1134.
15. Suzuki Y., Slomka P., Wolak A., et al. Motion-frozen myocardial perfusion SPECT improves detection of coronary artery disease in obese patients. J Nucl Med. 2008;49:1075-1079.
16. Taillefer R., DePuey E.G., Udelson J.E., et al. Comparison between the end-diastolic images and the summed images of gated 99mTc-sestamibi SPECT perfusion study in detection of coronary artery disease in women. J Nucl Cardiol. 1999;6:169-176.
17. Bestetti A., Di Leo C., Alessi A., et al. Transient left ventricular dilation during myocardial perfusion gated-SPECT in hypertensive patients. Eur J Nucl Med. 2001;28:OS239. (abstract)
18. Mazzanti M., Germano G., Kiat H., et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilation of the left ventricle in dual-isotope myocardial perfusion SPECT. J Am Coll Cardiol. 1996;27:1612-1620.
19. Buvat I., Bartlett M.L., Kitsiou A.N., et al. A “hybrid” method for measuring myocardial wall thickening from gated PET/SPECT images. J Nucl Med. 1997;38:324-329.
20. DePuey E.G., Nichols K., Dobrinsky C. Left ventricular ejection fraction assessed from gated technetium-99m-sestamibi SPECT. J Nucl Med. 1993;34:1871-1876.
21. Everaert H., Franken P.R., Flamen P., et al. Left ventricular ejection fraction from gated SPET myocardial perfusion studies: a method based on the radial distribution of count rate density across the myocardial wall. Eur J Nucl Med. 1996;23:1628-1633.
22. Goris M.L., Thompson C., Malone L.J., Franken P.R. Modelling the integration of myocardial regional perfusion and function. Nucl Med Commun. 1994;15:9-20.
23. Marcassa C., Marzullo P., Parodi O., et al. A new method for noninvasive quantitation of segmental myocardial wall thickening using technetium-99m 2-methoxy-isobutyl-isonitrile scintigraphy: Results in normal subjects. J Nucl Med. 1990;31:173-177.
24. Mochizuki T., Murase K., Fujiwara Y., et al. Assessment of systolic thickening with thallium-201 ECG-gated single-photon emission computed tomography: a parameter for local left ventricular function. J Nucl Med. 1991;32:1496-1500.
25. Nichols K., DePuey E.G., Rozanski A. Automation of gated tomographic left ventricular ejection fraction. J Nucl Cardiol. 1996;3:475-482.
26. Smith W.H., Kastner R.J., Calnon D.A., et al. Quantitative gated single photon emission computed tomography imaging: a counts-based method for display and measurement of regional and global ventricular systolic function. J Nucl Cardiol. 1997;4:451-463.
27. Williams K.A., Taillon L.A. Left ventricular function in patients with coronary artery disease assessed by gated tomographic myocardial perfusion images. Comparison with assessment by contrast ventriculography and first-pass radionuclide angiography. J Am Coll Cardiol. 1996;27:173-181.
28. Germano G., Kiat H., Kavanagh P.B., et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138-2147.
29. Germano G., Erel J., Lewin H., et al. Automatic quantitation of regional myocardial wall motion and thickening from gated technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1997;30:1360-1367.
30. Van Kriekinge S.D., Nishina H., Ohba M., et al. Automatic global and regional phase analysis from gated myocardial perfusion SPECT imaging: application to the characterization of ventricular contraction in patients with left bundle branch block. J Nucl Med. 2008;49:1790-1797.
31. Sharir T., Berman D.S., Waechter P.B., et al. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: Normal heterogeneity and criteria for abnormality. J Nucl Med. 2001;42:1630-1638.
32. Germano G., Kavanagh P.B., Slomka P.J., et al. Quantitation in gated perfusion SPECT imaging: The Cedars-Sinai approach. J Nucl Cardiol. 2007;14:433-454.
33. Faber T.L., Cooke C.D., Folks R.D., et al. Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med. 1999;40:650-659.
34. Chen J., Garcia E.V., Folks R.D., et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687-695.
35. Garcia E.V., Faber T.L., Cooke C.D., et al. The increasing role of quantification in clinical nuclear cardiology: The Emory approach. J Nucl Cardiol. 2007;14:420-432.
36. Chugh A., Ficaro E.P., Moscucci M., et al. Quantification of left ventricular function by gated perfusion tomography: Testing of a new fully automatic algorithm. J Am Coll Cardiol. 2001;37:394A. (abstract)
37. Ficaro E.P., Kritzman J.N., Corbett J.R. Automatic segmental scoring of myocardial wall thickening and motion: validation of a new semi-quantitative algorithm. J Nucl Med. 2001;42:171P. (abstract)
38. Ficaro E.P., Lee B.C., Kritzman J.N., Corbett J.R. Corridor4DM: The Michigan method for quantitative nuclear cardiology. J Nucl Cardiol. 2007;14:455-465.
39. Shen M.Y., Liu Y.H., Sinusas A.J., et al. Quantification of regional myocardial wall thickening on electrocardiogram-gated SPECT imaging. J Nucl Cardiol. 1999;6:583-595.
40. Liu Y.H. Quantification of nuclear cardiac images: The Yale approach. J Nucl Cardiol. 2007;14:483-491.
41. Abe M., Kazatani Y., Fukuda H., et al. Left ventricular volumes, ejection fraction, and regional wall motion calculated with gated technetium-99m tetrofosmin SPECT in reperfused acute myocardial infarction at super-acute phase: comparison with left ventriculography. J Nucl Cardiol. 2000;7:569-574.
42. Atsma D.E., Bavelaar-Croon C.D.L., Germano G., et al. Good correlation between gated single photon emission computed myocardial tomography and contrast ventriculography in the assessment of global and regional left ventricular function. Int J Card Imaging. 2000;16:447-453.
43. Bacher-Stier C., Müller S., Pachinger O., et al. Thallium-201 gated single-photon emission tomography for the assessment of left ventricular ejection fraction and regional wall motion abnormalities in comparison with two-dimensional echocardiography. Eur J Nucl Med. 1999;26:1533-1540.
44. Bavelaar-Croon C.D., Kayser H.W., van der Wall E.E., et al. Left ventricular function: correlation of quantitative gated SPECT and MR imaging over a wide range of values. Radiology. 2000;217:572-575.
45. Bax J.J., Lamb H., Dibbets P., et al. Comparison of gated single-photon emission computed tomography with magnetic resonance imaging for evaluation of left ventricular function in ischemic cardiomyopathy. Am J Cardiol. 2000;86:1299-1305.
46. Chua T., Yin L.C., Thiang T.H., et al. Accuracy of the automated assessment of left ventricular function with gated perfusion SPECT in the presence of perfusion defects and left ventricular dysfunction: correlation with equilibrium radionuclide ventriculography and echocardiography. J Nucl Cardiol. 2000;7:301-311.
47. Cwajg E., Cwajg J., He Z.X., et al. Gated myocardial perfusion tomography for the assessment of left ventricular function and volumes: comparison with echocardiography. J Nucl Med. 1999;40:1857-1865.
48. Everaert H., Bossuyt A., Franken P.R. Left ventricular ejection fraction and volumes from gated single photon emission tomographic myocardial perfusion images: comparison between two algorithms working in three-dimensional space. J Nucl Cardiol. 1997;4:472-476.
49. Faber T.L., Vansant J.P., Pettigrew R.I., et al. Evaluation of left ventricular endocardial volumes and ejection fractions computed from gated perfusion SPECT with magnetic resonance imaging: Comparison of two methods. J Nucl Cardiol. 2001;8:645-651.
50. He Z.X., Cwajg E., Preslar J.S., et al. Accuracy of left ventricular ejection fraction determined by gated myocardial perfusion SPECT with Tl-201 and Tc-99m sestamibi: comparison with first-pass radionuclide angiography. J Nucl Cardiol. 1999;6:412-417.
51. Higuchi T., Nakajima K., Taki J., et al. Assessment of left ventricular systolic and diastolic function based on the edge detection method with myocardial ECG-gated SPET. Eur J Nucl Med. 2001;28:1512-1516.
52. Inubushi M., Tadamura E., Kudoh T., et al. Simultaneous assessment of myocardial free fatty acid utilization and left ventricular function using 123I-BMIPP-gated SPECT. J Nucl Med. 1999;40:1840-1847.
53. Kikkawa M., Nakamura T., Sakamoto K., et al. Assessment of left ventricular diastolic function from quantitative electrocardiographic-gated (99)mTc-tetrofosmin myocardial SPET (ERRATA in vol 28, pg 1579, 2001). Eur J Nucl Med. 2001;28:593-601.
54. Kumita S., Cho K., Nakajo H., et al. Assessment of left ventricular diastolic function with electrocardiography-gated myocardial perfusion SPECT: Comparison with multigated equilibrium radionuclide angiography. J Nucl Cardiol. 2001;8:568-574.
55. Lipke C.S.A., Kuhl H.P., Nowak B., et al. Validation of 4D-MSPECT and QGS for quantification of left ventricular volumes and ejection fraction from gated Tc-99m-MIBI SPET: comparison with cardiac magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2004;31:482-490.
56. Manrique A., Koning R., Cribier A., Véra P. Effect of temporal sampling on evaluation of left ventricular ejection fraction by means of thallium-201 gated SPET: comparison of 16- and 8-interval gating, with reference to equilibrium radionuclide angiography. Eur J Nucl Med. 2000;27:694-699.
57. Nakajima K., Higuchi T., Taki J., et al. Accuracy of ventricular volume and ejection fraction measured by gated myocardial SPECT: Comparison of 4 software programs. J Nucl Med. 2001;42:1571-1578.
58. Nanasato M., Ando A., Isobe S., et al. Evaluation of left ventricular function using electrocardiographically gated myocardial SPECT with I-123- labeled fatty acid analog. J Nucl Med. 2001;42:1747-1756.
59. Nichols K., Lefkowitz D., Faber T., et al. Echocardiographic validation of gated SPECT ventricular function measurements. J Nucl Med. 2000;41:1308-1314.
60. Tadamura E., Kudoh T., Motooka M., et al. Use of technetium-99m sestamibi ECG-gated single-photon emission tomography for the evaluation of left ventricular function following coronary artery bypass graft: comparison with three-dimensional magnetic resonance imaging. Eur J Nucl Med. 1999;26:705-712.
61. Tadamura E., Kudoh T., Motooka M., et al. Assessment of regional and global left ventricular function by reinjection T1-201 and rest Tc-99m sestamibi ECG-gated SPECT: comparison with three-dimensional magnetic resonance imaging. J Am Coll Cardiol. 1999;33:991-997.
62. Thorley P.J., Plein S., Bloomer T.N., et al. Comparison of Tc-99m tetrofosmin gated SPECT measurements of left ventricular volumes and ejection fraction with MRI over a wide range of values. Nucl Med Commun. 2003;24:763-769.
63. Vaduganathan P., He Z.X., Vick G.W.3rd, et al. Evaluation of left ventricular wall motion, volumes, and ejection fraction by gated myocardial tomography with technetium 99m-labeled tetrofosmin: a comparison with cine magnetic resonance imaging. J Nucl Cardiol. 1999;6:3-10.
64. Vallejo E., Dione D.P., Sinusas A.J., Wackers F.J. Assessment of left ventricular ejection fraction with quantitative gated SPECT: accuracy and correlation with first-pass radionuclide angiography. J Nucl Cardiol. 2000;7:461-470.
65. Vourvouri E.C., Poldermans D., Bax J.J., et al. Evaluation of left ventricular function and volumes in patients with ischaemic cardiomyopathy: gated single-photon emission computed tomography versus two-dimensional echocardiography. Eur J Nucl Med. 2001;28:1610-1615.
66. Yoshioka J., Hasegawa S., Yamaguchi H., et al. Left ventricular volumes and ejection fraction calculated from quantitative electrocardiographic-gated 99mTc-tetrofosmin myocardial SPECT. J Nucl Med. 1999;40:1693-1698.
67. Higuchi T., Nakajima K., Taki J., et al. Accuracy and reproducibility of four softwares for the left-ventricular function with ECG-gated myocardial perfusion SPECT. J Nucl Cardiol. 2001;8:S64. (abstract)
68. Vansant J., Pettigrew R., Faber T., et al. Comparison and accuracy of two gated-SPECT techniques for assessing left ventricular function defined by cardiac MRI. J Nucl Med. 1999;40:166P. (abstract)
69. Cahill J., Chen M., Corbett J., Quaife R. Validation of three-dimensional analysis method for calculation of the LV mass and ejection fraction using Tc-99m sestamibi gated-SPECT perfusion imaging: Comparison between 4D-MSPECT and magnetic resonance imaging. J Nucl Med. 2003;44:197P. (abstract)
70. Ficaro E., Quaife R., Kritzman J., Corbett J. Accuracy and reproducibility of 3D-MSPECT for estimating left ventricular ejection fraction in patients with severe perfusion abnormalities. Circulation. 1999;100:I-26. (abstract)
71. Gayed I.W., Cid E., Boccalandro F. Correlation of left ventricular ejection fraction using Gated SPECT automated programs with echocardiography. J Nucl Med. 2001;42:177P-178P. (abstract)
72. Lam P.T., Wackers F.J.T., Liu Y.H. Validation of a new method for quantification of left ventricular function from ECG-gated SPECT. J Nucl Med. 2001;42:93P-94P. (abstract)
73. Liu Y.H., Sinusas A.J., Khaimov D., et al. New hybrid count- and geometry-based method for quantification of left ventricular volumes and ejection fraction from ECG-gated SPECT: Methodology and validation. J Nucl Cardiol. 2005;12:55-65.
74. Hyun I.Y., Kwan J., Park K.S., Lee W.H. Reproducibility of Tl-201 and Tc-99m sestamibi gated myocardial perfusion SPECT measurement of myocardial function. J Nucl Cardiol. 2001;8:182-187.
75. Johnson L.L., Verdesca S.A., Aude W.Y., et al. Postischemic stunning can affect left ventricular ejection fraction and regional wall motion on post-stress gated sestamibi tomograms [commentary]. J Am Coll Cardiol. 1997;30:1641-1648.
76. Lee D.S., Ahn J.Y., Kim S.K., et al. Limited performance of quantitative assessment of myocardial function by thallium-201 gated myocardial single-photon emission tomography. Eur J Nucl Med. 2000;27:185-191.
77. Agostini D., Filmont J., Darlas Y., et al. LVEF and LV volumes determinations with gated 123I-MIBG SPECT in dystrophic myotony. J Nucl Med. 2000;41:49P. (abstract)
78. Germano G., Erel J., Kiat H., et al. Quantitative LVEF and qualitative regional function from gated thallium-201 perfusion SPECT. J Nucl Med. 1997;38:749-754.
79. Maunoury C., Chen C.C., Chua K.B., Thompson C.J. Quantification of left ventricular function with thallium-201 and technetium-99m-sestamibi myocardial gated SPECT. J Nucl Med. 1997;38:958-961.
80. Everaert H., Vanhove C., Franken P.R. Gated SPET myocardial perfusion acquisition within 5 minutes using focusing collimators and a three-head gamma camera. Eur J Nucl Med. 1998;25:587-593.
81. Franken P.R., Everaert H., Momen A., Vanhove C. Automatic left ventricular cavity volume and ejection fraction measurements from one-day rest/stress perfusion gated tomograms. Eur J Nucl Med. 1999;26:1079. (abstract)
82. Mazzanti M., Germano G., Kiat H., et al. Fast technetium 99m-labeled sestamibi gated single-photon emission computed tomography for evaluation of myocardial function. J Nucl Cardiol. 1996;3:143-149.
83. Vanhove C., Franken P.R., Defrise M., Bossuyt A. Comparison of 180 degrees and 360 degrees data acquisition for determination of left ventricular function from gated myocardial perfusion tomography and gated blood pool tomography. Eur J Nucl Med Mol Imaging. 2003;30:1498-1504.
84. Kritzman J., Ficaro E., Corbett J. Reproducibility of 3-D MSPECT for quantitative gated SPECT sestamibi perfusion analysis. J Nucl Med. 2000;41:166P. (abstract)
85. Nakajima K., Nishimura T. Inter-institution preference-based variability of ejection fraction and volumes using quantitative gated SPECT with Tc-99m-tetrofosmin: a multicentre study involving 106 hospitals. Eur J Nucl Med Mol Imaging. 2006;33:127-133.
86. Berman D., Germano G., Lewin H., et al. Comparison of post-stress ejection fraction and relative left ventricular volumes by automatic analysis of gated myocardial perfusion single-photon emission computed tomography acquired in the supine and prone positions. J Nucl Cardiol. 1998;5:40-47.
87. Kubo N., Mabuchi M., Katoh C., et al. Validation of left ventricular function using gated SPECT with a scintillation crystal and semiconductor detectors camera system: A study of dynamic myocardial phantom. J Nucl Med. 2002;43:199P-200P. (abstract)
88. Daou D., Pointurier I., Coaguila C., et al. Performance of OSEM and depth-dependent resolution recovery algorithms for the evaluation of global left ventricular function in Tl-201 gated myocardial perfusion SPECT. J Nucl Med. 2003;44:155-162.
89. Ficaro E., Kritzman J., Hamilton T., et al. Effect of attenuaton corrected myocardial perfusion SPECT on left ventricular ejection fraction estimates. J Nucl Med. 2000;41:166P-167P. (abstract)
90. Boussaha M.R., Storto G., Antonescu C., Delaloye A.B. Ejection fraction evaluation by gated myocardial perfusion SPECT: Comparison between gated SPECT quantification (GSQ) and Emory Cardiac Tool Box (ECTB). Eur J Nucl Med. 2001;28:OS240. (abstract)
91. Dede F., Narin Y. Can different software programs give the same functional measurements in ECG gated SPECT: Comparison of two software programs. Eur J Nucl Med. 2002;29:S209. (abstract)
92. Franken P.R., Everaert H., Momen A., Vanhove C. Comparison of three automatic software to measure left ventricular cavity volume and ejection fraction from perfusion gated tomograms. Eur J Nucl Med. 1999;26:1076. (abstract)
93. Hambye A.S., Vervaet A., Dobbeleir A. Variability of left ventricular ejection fraction and volumes with quantitative gated SPECT: influence of algorithm, pixel size and reconstruction parameters in small and normal-sized hearts. Eur J Nucl Med Mol Imaging. 2004;31:1606-1613.
94. Lewis T., Grewal K., Calnon D. Discrepancies in estimating left-ventricular volumes and ejection fraction by two commercially available gated SPECT algorithms: comparison to echocardiography. J Nucl Cardiol. 2001;8:S18. (abstract)
95. Nichols K., Folks R., Cooke D., et al. Comparisons between “ECTb” and “QGS” software to compute left ventricular function from myocardial perfusion gated SPECT data. J Nucl Cardiol. 2000;7:S20. (abstract)
96. Kang D., Kim M., Kim Y. Functional data of gated myocardial perfusion SPECT by QGS and 4D-MSPECT program cannot exchanged each other (sic). J Nucl Med. 2004;45:225P. (abstract)
97. Krasnow J., Trask I., Dahlberg S., et al. Automatic determination of left ventricular function (LVEF) by gated SPECT; comparison of four quantitative software programs. J Nucl Cardiol. 2001;8:S138. (abstract)
98. Rozanski A., Nichols K., Yao S.S., et al. Development and application of normal limits for left ventricular ejection fraction and volume measurements from 99mTc-sestamibi myocardial perfusion gates SPECT. J Nucl Med. 2000;41:1445-1450.
99. Santana C.A., Garcia E.V., Folks R., et al. Comparison of normal values of left ventricular function between two programs: QGS and emory cardiac toolbox (ECTB). J Nucl Med. 2001;42:166P. (abstract)
100. Sharir T., Germano G., Friedman J., et al. Prognostic value of gated myocardial perfusion single photon emission computed tomography in women versus men. Circulation. 2000;102:II-544. (abstract)
101. Ababneh A., Sciacca R., Bergmann S. Normal limits for left ventricular ejection fraction, end-diastolic, and end-systolic volume as estimated with gated perfusion imaging. Circulation. 2000;102:II-724. (abstract)
102. Ababneh A.A., Sciacca R.R., Kim B., Bergmann S.R. Normal limits for left ventricular ejection fraction and volumes estimated with gated myocardial perfusion imaging in patients with normal exercise test results: influence of tracer, gender, and acquisition camera. J Nucl Cardiol. 2000;7:661-668.
103. De Bondt P., De Sutter J., De Winter F., et al. Normal values of left ventricular ejection fraction (LVEF) and left ventricular volumes (LVV) measured by gated myocardial SPECT in women and men at rest and after bicycle or dipyridamole (DIP) stress testing. J Nucl Med. 2000;41:159P. (abstract)
104. De Bondt P., Van de Wiele C., De Sutter J., et al. Age- and gender-specific differences in left ventricular cardiac function and volumes determined by gated SPET. Eur J Nucl Med. 2001;28:620-624.
105. Ficaro F., Kritzman J., Stephens G., Corbett J. Gender specific differences in normal ranges of cardiac functional parameters from gated SPECT. J Nucl Med. 2003;44:205P-206P. (abstract)
106. Hyun I., Kim D., Seo J., et al. Normal parameters of left ventricular volume and ejection fraction measured by gated myocardial perfusion SPECT: comparison of Tc99m MIBI and TI-201. Eur J Nucl Med. 2002;29:S205. (abstract)
107. Kim J., Kim N. Normal LVEF measurements are significantly higher in females assessed by 99m Tc-MIBI and Tetrofosmin quantitative gated myocardial perfusion SPECT: age and gender matched statistical analysis. J Nucl Med. 2003;44:106P. (abstract)
108. Sharir T., Germano G., Kang X.P., et al. Prognostic value of post-stress left ventricular volume and ejection fraction by gated myocardial perfusion single photon emission computed tomography in women: Gender related differences in normal limits and outcome. Circulation. 2002;106:II-523. (abstract)
109. Case J., Cullom S., Bateman T., et al. Overestimation of LVEF by gated MIBI myocardial perfusion SPECT in patients with small hearts. J Am Coll Cardiol. 1998;31:43A. (abstract)
110. Ford P.V., Chatziioannou S.N., Moore W.H., Dhekne R.D. Overestimation of the LVEF by quantitative gated SPECT in simulated left ventricles. J Nucl Med. 2001;42:454-459.
111. Nakajima K., Taki J., Higuchi T., et al. Gated SPET quantification of small hearts: mathematical simulation and clinical application. Eur J Nucl Med. 2000;27:1372-1379.
112. Ezuddin S., Sfakianakis G., Pay L., Sanchez P. Comparative study to determine the effect of different zoom factors on the calculation of LVEF from gated myocardial perfusion SPECT with Tl-201 and Tc-99m-sestamibi in patients with small hearts. J Nucl Med. 1999;40:169P. (abstract)
113. Manrique A., Gardin I., Brasse D., et al. Effect of reconstruction parameters on LV volume measurements from Tl-201 SPECT: a phantom study. J Nucl Cardiol. 2001;8:S71. (abstract)
114. Schwartz R., Mixon L., Germano G., et al. Gated SPECT reconstruction with zoom and depth dependent filter improves accuracy of volume and LVEF in small hearts. J Nucl Cardiol. 1999;6:S17. (abstract)
115. Case J., Bateman T., Cullom S., et al. Improved accuracy of SPECT LVEF using numerical modeling of ventricular image blurring for patients with small hearts. J Am Coll Cardiol. 1999;33:436A. (abstract)
116. Faber T., Cooke C., Folks R., et al. Correction of artifactually high EF from gated perfusion SPECT in small ventricles. J Nucl Cardiol. 2000;7:S20. (abstract)
117. Santos M., Lewin H., Hayes S., et al. A potential cause for underestimation of LVEF by QGS. J Nucl Cardiol. 2001;8:S130. (abstract)
118. Cohade C., Taillefer R., Gagnon A., et al. Effect of the number of frames per cardiac cycle and the amount of injected dose of radionuclide on the determination of left ventricular ejection fraction (LVEF) with gated SPECT myocardial perfusion imaging (GS). J Nucl Med. 2000;41:154P. (abstract)
119. Imai K., Azuma Y., Nakajima S., et al. Frames a cardiac cycle in quantitative gated SPECT (QGS) for clinical use: 8 versus 16. J Nucl Cardiol. 1999;6:S17. (abstract)
120. Manrique A., Vera P., Hitzel A., et al. 16-interval gating improves thallium-201 gated SPECT LVEF measurement in patients with large myocardial infarction. J Am Coll Cardiol. 1999;33:436A-437A. (abstract)
121. Everaert H., Vanhove C., Franken P.R. Low-dose dobutamine gated single-photon emission tomography: comparison with stress echocardiography. Eur J Nucl Med. 2000;27:413-418.
122. Itti E., Rosso J., Damien P., et al. Assessment of ejection fraction with Tl-201 gated SPECT in myocardial infarction: Precision in a rest-redistribution study and accuracy versus planar angiography. J Nucl Cardiol. 2001;8:31-39.
123. Nichols K., Tamis J., DePuey E.G., et al. Relationship of gated SPECT ventricular function parameters to angiographic measurements. J Nucl Cardiol. 1998;5:295-303.
124. Stollfuss J.C., Haas F., Matsunari I., et al. Regional myocardial wall thickening and global ejection fraction in patients with low angiographic left ventricular ejection fraction assessed by visual and quantitative resting ECG-gated 99mTc-tetrofosmin single-photon emission tomography and magnetic resonance imaging. Eur J Nucl Med. 1998;25:522-530.
125. Stollfuss J.C., Haas F., Matsunari I., et al. 99mTc-tetrofosmin SPECT for prediction of functional recovery defined by MRI in patients with severe left ventricular dysfunction: additional value of gated SPECT. J Nucl Med. 1999;40:1824-1831.
126. Canbaz F., Basoglu1 T., Durna K., et al. Left ventricular aneurysm in the scope of gated perfusion SPECT: accuracy of detection and ejection fraction calculation. Int J Cardiovasc Imaging. 2008;24:585-596.
127. Iskandrian A.E., Germano G., VanDecker W., et al. Validation of left ventricular volume measurements by gated SPECT 99mTc-labeled sestamibi imaging. J Nucl Cardiol. 1998;5:574-578.
128. Zuber E., Rosfors S. Effect of reversible hypoperfusion on left ventricular volumes measured with gated SPECT at rest and after adenosine infusion. J Nucl Cardiol. 2000;7:655-660.
129. Nichols K., Lefkovitz D., Faber T., et al. Ventricular volumes compared among three gated SPECT methods and echocardiography. J Am Coll Cardiol. 1999;33:409A. (abstract)
130. Liu Y. A new approach of quantification of left-ventricular volume for gated SPECT imaging: preliminary validation using a phantom. J Nucl Cardiol. 2001;8:S61. (abstract)
131. Adachi I., Morita K., Imran M.B., et al. Heterogeneity of myocardial wall motion and thickening in the left ventricle evaluated with quantitative gated SPECT. J Nucl Cardiol. 2000;7:296-300.
132. Faber T.L., Stokely E.M., Peshock R.M., Corbett J.R. A model-based four-dimensional left ventricular surface detector. IEEE Trans Med Imaging. 1991;10:321-329.
133. Cooke C., Garcia E., Folks R., Ziffer J. Myocardial thickening and phase analysis from Tc-99m sestamibi multiple gated SPECT: development of normal limits. J Nucl Med. 1992;33:926-927. (abstract)
134. Everaert H., Vanhove C., Franken P.R. Effects of low-dose dobutamine on left ventricular function in normal subjects as assessed by gated single-photon emission tomography myocardial perfusion studies. Eur J Nucl Med. 1999;26:1298-1303.
135. Fujino S., Masuyama K., Kanayama S., et al. Early and delayed technetium-99m labeled sestamibi myocardial ECG-gated SPECT by QGS program in normal volunteers. J Nucl Med. 1999;40:180P. (abstract)
136. Itoh Y., Adachi I., Kohya T., et al. Heterogeneity in myocardial perfusion, wall motion and wall thickening with Tc-99m-sestamibi quantitative gated SPECT in normal subjects. J Nucl Med. 1999;40:165P. (abstract)
137. Shirakawa S., Hattori N., Tamaki N., et al. [Assessment of left ventricular wall thickening with gated 99mTc-MIBI SPECT—value of normal file]. Kaku Igaku. 1995;32:643-650.
138. Cerqueira M.D., Weissman N.J., Dilsizian V., et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol. 2002;9:240-245.
139. Berman D., Germano G. An approach to the interpretation and reporting of gated myocardial perfusion SPECT. In: Germano G., Berman D., editors. Clinical Gated Cardiac SPECT. Armonk, NY: Futura Publishing Company; 1999:147-182.
140. Damrongpipatkij Y., Mohammed F., Brown E., et al. Quantitative cardiac SPECT: measuring diastolic function. J Nucl Med. 2000;41:154P. (abstract)
141. Higuchi T., Taki J., Yoneyama T., et al. Diastolic and systolic parameters obtained by myocardial ECG-gated perfusion study. J Nucl Med. 2000;41:160P. (abstract)
142. Nakajima K., Taki J., Kawano M., et al. Diastolic dysfunction in patients with systemic sclerosis detected by gated myocardial perfusion SPECT: an early sign of cardiac involvement. J Nucl Med. 2001;42:183-188.
143. Higuchi T., Nakajima K., Taki J., et al. The accuracy of left-ventricular time volume curve derived from ECG-gated myocardial perfusion SPECT. J Nucl Cardiol. 2001;8:S18. (abstract)
144. Akincioglu C., Berman D.S., Nishina H., et al. Assessment of diastolic function using 16-frame Tc-99m-sestamibi gated myocardial perfusion SPECT: Normal values. J Nucl Med. 2005;46:1102-1108.
145. Boogers M.M., Van Kriekinge S.D., Henneman M.M., et al. QGS derived phase analysis on gated myocardial perfusion SPECT detects LV dyssynchrony and predicts response to CRT. J Nucl Med. 2009. (in press)
146. Henneman M.M., Chen J., Dibbets-Schneider P., et al. Can LV Dyssynchrony as Assessed with Phase Analysis on Gated Myocardial Perfusion SPECT Predict Response to CRT. J Nucl Med. 2007;48:1104-1111.
147. Chen J., Henneman M.M., Trimble M.A., et al. Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2008;15:127-136.
148. Berman D.S., Salel A.F., DeNardo G.L., et al. Clinical assessment of left ventricular regional contraction patterns and ejection fraction by high-resolution gated scintigraphy. J Nucl Med. 1975;16:865-874.
149. Maublant J., Bailly P., Mestas D., et al. Feasibility of gated single-photon emission transaxial tomography of the cardiac blood pool. Radiology. 1983;146:837-839.
150. Moore M.L., Murphy P.H., Burdine J.A. ECG-gated emission computed tomography of the cardiac blood pool. Radiology. 1980;134:233-235.
151. Tamaki N., Mukai T., Ishii Y., et al. Multiaxial tomography of heart chambers by gated blood-pool emission computed tomography using a rotating gamma camera. Radiology. 1983;147:547-554.
152. Alexanderson E., Espinola N., Meavel A., Victoria D. Assessment of ventricular perfusion and function with nuclear scan and echocardiography in patients with corrected transposition of great arteries. J Nucl Cardiol. 2003;10:S66. (abstract)
153. Barat J.L., Brendel A.J., Colle J.P., et al. Quantitative analysis of left-ventricular function using gated single photon emission tomography. J Nucl Med. 1984;25:1167-1174.
154. Bartlett M.L., Srinivasan G., Barker W.C., et al. Left ventricular ejection fraction: comparison of results from planar and SPECT gated blood-pool studies. J Nucl Med. 1996;37:1795-1799.
155. Bunker S.R., Hartshorne M.F., Schmidt W.P., et al. Left ventricular volume determination from single-photon emission computed tomography. Am J Roentgenol. 1985;144:295-298.
156. Chin B.B., Bloomgarden D.C., Xia W., et al. Right and left ventricular volume and ejection fraction by tomographic gated blood-pool scintigraphy. J Nucl Med. 1997;38:942-948.
157. Corbett J.R., Jansen D.E., Lewis S.E., et al. Tomographic gated blood pool radionuclide ventriculography: analysis of wall motion and left ventricular volumes in patients with coronary artery disease. J Am Coll Cardiol. 1985;6:349-358.
158. Daou D., Harel F., Mariano-Goulart D., et al. Left ventricular function estimated with ECG-gated blood pool SPECT: Comparison of two different processing softwares. J Nucl Med. 2001;42:137P. (abstract)
159. Daou D., Van Kriekinge S.D., Coaguila C., et al: Automatic quantification of right ventricular function with gated blood pool SPECT, 11: 293-304, 2004.
>
160. Druz R.S., Akinboboye O.A., Grimson R., et al. Postischemic stunning after adenosine vasodilator stress. J Nucl Cardiol. 2004;11:534-541.
161. Ficaro E.P., Kritzman’ J.N., Corbett J.R. Normal LV ejection fraction limits using 4D MSPECT: Comparisons of gated perfusion and gated blood pool SPECT data with planar blood pool imaging. J Nucl Cardiol. 2003;10:S9. (abstract)
162. Ficaro E.P., Quaife R.F., Kritzman J.N., Corbett J.R. Validation of a new fully automatic algorithm for quantification of gated blood pool SPECT: Correlations with planar gated blood pool and perfusion SPECT. J Nucl Med. 2002;43:97P. (abstract)
163. Gill J.B., Moore R.H., Tamaki N., et al. Multigated blood-pool tomography: new method for the assessment of left ventricular function. J Nucl Med. 1986;27:1916-1924.
164. Groch M., Belzberg A., DePuey E., et al. Evaluation of ventricular performance using gated blood pool SPECT: a multicenter study. J Nucl Med. 2000;41:5P. (abstract)
165. Groch M., Marshall R., Erwin W., et al. Left ventricular ejection fraction computed from gated SPECT blood pool imaging correlates with conventional planar imaging. J Nucl Med. 2000;41:98P. (abstract)
166. Groch M.W., Erwin W.D., DePuey E.G., et al. Multicenter clinical evaluation of gated blood pool SPECT for the assessment of ventricular function. Eur J Nucl Med. 2001;28:PS85. (abstract)
167. Keng F., Chua T., Koh T. Quantitative blood pool single-photon emission computed tomography (QBS) program: comparison with conventional planar equilibrium blood pool imaging (MUGA). J Nucl Cardiol. 2003;10:S25. (abstract)
168. Keng F., Tan H., Chua T. Gated SPECT blood pool imaging: a comparison with equilibrium blood pool imaging. J Nucl Cardiol. 2000;7:S3. (abstract)
169. Nakajima K., Higuchi T., Taki J., et al. Quantitative gated SPECT with myocardial perfusion and blood-pool studies to determine ventricular volumes and stroke volume ratio in congenital heart diseases. J Nucl Cardiol. 2003;10:S11. (abstract)
170. Nichols K., Saouaf R., Ababneh A.A., et al. Validation of SPECT equilibrium radionuclide angiographic right ventricular parameters by cardiac magnetic resonance imaging. J Nucl Cardiol. 2002;9:153-160.
171. Rouzet F., Ederhy S., Daou D., et al. Respective role of inter and intraventricular delays in patients candidates to biventricular pacing evaluated by multiharmonic analysis of radionuclide angiography. J Nucl Med. 2004;45:242P. (abstract)
172. Schwartz R., Le Guludec D., Holder L., et al. Blood pool gated SPECT: validation of left ventricular volumes and ejection fraction with planar radionuclide angiography. J Nucl Cardiol. 2000;7:S2. (abstract)
173. Stadius M.L., Williams D.L., Harp G., et al. Left ventricular volume determination using single-photon emission computed tomography. Am J Cardiol. 1985;55:1185-1191.
174. Underwood S.R., Walton S., Ell P.J., et al. Gated blood-pool emission tomography: a new technique for the investigation of cardiac structure and function. Eur J Nucl Med. 1985;10:332-337.
175. Van Kriekinge S., Berman D., Germano G. Automatic quantification of left and right ventricular ejection fractions from gated blood pool SPECT. Circulation. 1999;100:I-26. (abstract)
176. Van Kriekinge S., Paul A.K., Hasegawa S., et al. Validation of quantitative left-ventricular end-diastolic and end-systolic volumes from gated blood pool SPECT. J Am Coll Cardiol. 2001;37:500A. (abstract)
177. Van Kriekinge S.D., Berman D.S., Germano G. Automatic quantification of left ventricular ejection fraction from gated blood pool SPECT. J Nucl Cardiol. 1999;6:498-506.
178. Vanhove C., Everaert H., Bossuyt A., Franken P. Automatic determination of LV ejection fraction and volumes by gated blood pool SPECT. J Nucl Med. 2000;41:187P. (abstract)
179. Vanhove C., Franken P.R. Left ventricular ejection fraction and volumes from gated blood pool tomography: Comparison between two automatic algorithms that work in three-dimensional space. J Nucl Cardiol. 2001;8:466-471.
180. Smanio P.E., Watson D.D., Segalla D.L., et al. Value of gating of technetium-99m sestamibi single-photon emission computed tomographic imaging. J Am Coll Cardiol. 1997;30:1687-1692.
181. Taillefer R., DePuey E.G., Udelson J.E., et al. Comparative diagnostic accuracy of Tl-201 and Tc-99m sestamibi SPECT imaging (perfusion and ECG-gated SPECT) in detecting coronary artery disease in women. J Am Coll Cardiol. 1997;29:69-77.
182. Choi J.Y., Lee K.H., Kim S.J., et al. Gating provides improved accuracy for differentiating artifacts from true lesions in equivocal fixed defects on technetium 99m tetrofosmin perfusion SPECT. J Nucl Cardiol. 1998;5:395-401.
183. Links J.M., DePuey E.G., Taillefer R., Becker L.C. Attenuation correction and gating synergistically improve the diagnostic accuracy of myocardial perfusion SPECT. J Nucl Cardiol. 2002;9:183-187.
184. Hendel R.C., Corbett J.R., Cullom S.J., et al. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: A joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Med. 2002;43:273-280.
185. O’Connor M.K., Kemp B., Anstett F., et al. A multicenter evaluation of commercial attenuation compensation techniques in cardiac SPECT using phantom models. J Nucl Cardiol. 2002;9:361-376.
186. Bateman T.M., Kolobrodov V.V., Vasin A.P., O’Keefe J.H.Jr. Extended acquisition for minimizing attenuation artifact in SPECT cardiac perfusion imaging. J Nucl Med. 1994;35:625-627.
187. Gibson P.B., Demus D., Noto R., et al. Low event rate for stress-only perfusion imaging in patients evaluated for chest pain. J Am Coll Cardiol. 2002;39:999-1004.
188. Heller G.V., Bateman T.M., Botvinick E.H., et al. Value of attenuation correction in interpretation of stress only exercise Tc-99m sestamibi SPECT imaging: Results of a multicenter trial. J Am Coll Cardiol. 2002;39:343A. (abstract)
189. Santana C.A., Garcia E.V., Vansant J.P., et al. Gated stress-only Tc-99m myocardial perfusion SPECT imaging accurately assesses coronary artery disease. Nucl Med Commun. 2003;24:241-249.
190. Thompson R.C., Heller G.V., Johnson L.L., et al. Value of attenuation correction on ECG-gated SPECT myocardial perfusion imaging related to body mass index. J Nucl Cardiol. 2005;12:195-202.
191. Chua T., Kiat H., Germano G., et al. Gated technetium-99m sestamibi for simultaneous assessment of stress myocardial perfusion, postexercise regional ventricular function and myocardial viability. Correlation with echocardiography and rest thallium-201 scintigraphy. J Am Coll Cardiol. 1994;23:1107-1114.
192. Sharir T., Bacher-Stier C., Dhar S., et al. Identification of severe and extensive coronary artery disease by postexercise regional wall motion abnormalities in Tc-99m sestamibi gated single-photon emission computed tomography. Am J Cardiol. 2000;86:1171-1175.
193. Emmett L., Iwanochko R.M., Freeman M.R., et al. Reversible regional wall motion abnormalities on exercise technetium-99m-gated cardiac single photon emission computed tomography predict high-grade angiographic stenoses. J Am Coll Cardiol. 2002;39:991-998.
194. Lima R.S.L., Watson D.D., Goode A.R., et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42:64-70.
195. Abidov A., Bax J.J., Hayes S.W., et al. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J Am Coll Cardiol. 2003;42:1818-1825.
196. Hachamovitch R., Rozanski A., Hayes S.W., et al. Predicting therapeutic benefit from myocardial revascularization procedures: Are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary. J Nucl Cardiol. 2006;13:768-778.
197. Berman D.S., Kang X., Slomka P.J., et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14:521-528.
198. Johnson S.H., Bigelow C., Lee K.L., et al. Prediction of death and myocardial infarction by radionuclide angiocardiography in patients with suspected coronary artery disease. Am J Cardiol. 1991;67:919-926.
199. Jones R.H., Johnson S.H., Bigelow C., et al. Exercise radionuclide angiocardiography predicts cardiac death in patients with coronary artery disease. Circulation. 1991;84:I52-I58.
200. Lee K.L., Pryor D.B., Pieper K.S., et al. Prognostic value of radionuclide angiography in medically treated patients with coronary artery disease. A comparison with clinical and catheterization variables. Circulation. 1990;82:1705-1717.
201. Morris K.G., Palmeri S.T., Califf R.M., et al. Value of radionuclide angiography for predicting specific cardiac events after acute myocardial infarction. Am J Cardiol. 1985;55:318-324.
202. Pryor D.B., Harrell F.E.Jr, Lee K.L., et al. Prognostic indicators from radionuclide angiography in medically treated patients with coronary artery disease. Am J Cardiol. 1984;53:18-22.
203. Upton M.T., Palmeri S.T., Jones R.H., et al. Assessment of left ventricular function by resting and exercise radionuclide angiocardiography following acute myocardial infarction. Am Heart J. 1982;104:1232-1243.
204. Sharir T., Germano G., Kavanagh P.B., et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999;100:1035-1042.
205. Sharir T., Germano G., Kang X.P., et al. Prediction of myocardial infarction versus cardiac death by gated myocardial perfusion SPECT: Risk stratification by the amount of stress-induced ischemia and the poststress ejection fraction. J Nucl Med. 2001;42:831-837.
206. Hachamovitch R.: Personal communication, 2006.
207. Klocke F.J., Baird M.G., Lorell B.H., et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—Executive summary—A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to revise the 1995 guidelines for the clinical use of cardiac radionuclide imaging). J Am Coll Cardiol. 2003;42:1318-1333.
208. Liao L., Smith W.T., Tuttle R.H., et al. Prediction of death and nonfatal myocardial infarction in high-risk patients: A comparison between the Duke treadmill score, peak exercise radionuclide angiography, and SPECT perfusion imaging. J Nucl Med. 2005;46:5-11.
209. Thomas G.S., Miyamoto M.I., Morello A.P., et al. Technetium(99m) sestamibi myocardial perfusion imaging predicts clinical outcome in the community outpatient setting—The Nuclear Utility in the Community (NUC) Study. J Am Coll Cardiol. 2004;43:213-223.
210. Travin M.I., Heller G.V., Johnson L.L., et al. The prognostic value of ECG-gated SPECT imaging in patients undergoing stress Tc-99m sestamibi myocardial perfusion imaging. J Nucl Cardiol. 2004;11:253-262.
211. Petix N.R., Sestini S., Coppola A., et al. Prognostic value of combined perfusion and function by stress technetium-99m Sestamibi gated SPECT myocardial perfusion imaging in patients with suspected or known coronary artery disease. Am J Cardiol. 2005;95:1351-1357.
212. Mast S.T., Shaw L.K., Ravizzini G.C., et al. Incremental prognostic value of RNA ejection fraction measurements during pharmacologic stress testing: A comparison with clinical and perfusion variables. J Nucl Med. 2001;42:871-877.
213. Hachamovitch R., Hayes S.W., Friedman J.D., et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900-2907.
214. Abidov A., Germano G., Hachamovitch R., Berman D.S. Gated SPECT in assessment of regional and global left ventricular function: Major tool of modern nuclear imaging. J Nucl Cardiol. 2006;13:261-279.
215. Cheitlin M.D., Armstrong W.F., Aurigemma G.P., et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to update the 1997 guidelines for the clinical application of echocardiography). Circulation. 2003;108:1146-1162.
216. Leoncini M., Marcucci G., Sciagrà R., et al. Nitrate-enhanced gated technetium 99m sestamibi SPECT for evaluating regional wall motion at baseline and during low-dose dobutamine infusion in patients with chronic coronary artery disease and left ventricular dysfunction: comparison with two-dimensional echocardiography. J Nucl Cardiol. 2000;7:426-431.