39 Reducing Errors in Cardiac Anesthesiology
Although the safety of anesthesiology has improved over recent years, with an attributable mortality on the order of 1 in 53,500 anesthetics,* errors still occur. Like other medical practitioners, anesthesiologists are prone to human error. The complex arena of cardiac anesthesia provides an opportunity for making just about any of the errors that can possibly be made by an anesthesiologist. It is just because of the complexity and the high stakes encountered in the cardiac anesthesia environment that cardiac anesthesiologists should be most interested in methods for reducing errors. This is particularly relevant now as regulatory agencies and the public in general have become acutely aware of medical errors since the publication of the Institute of Medicine’s landmark report on the subject.1 In response, initiatives have been developed by a wide range of organizations with the intention of reducing errors and improving safety for surgical patients. These have been aimed at the entire surgical patient population (e.g., the World Health Organization’s safe surgery saves lives checklist)2,3 and, more specifically, at cardiac surgical patients. The Society of Cardiovascular Anesthesiologists (SCA) Foundation has undertaken an initiative called Flawless Operative Cardiovascular Unified Systems (FOCUS). They intend to take a multidisciplinary approach to identifying and mitigating hazards. One of the interventions they envision developing is peer-to-peer assessment. An overview of the project and an outline of their plan have been recently published.4
In each of these categories of errors, anesthesiologists will see that there are methods that should enable them to reduce error. The reader also may find previous descriptions of mechanisms of error in anesthesia useful.5–7
Errors involving the placement of central catheters
Central catheters have long been regarded as dangerous by practitioners, manufacturers, and the U.S. Food and Drug Administration. (FDA). Complications from central catheters have been reviewed recently.8–11 Hall and Russell12 have authored an editorial that provides, in three pages, a concise but complete description of safe practices for placing central catheters; if the reader has time to read only a single article on this topic, it is highly recommended. This section concentrates on errors related to the internal jugular vein (IJV) route of central catheter insertion because this is the most common insertion route used by cardiac anesthesiologists (Box 39-1).
Agency for Healthcare Research and Quality
The Agency for Healthcare Research and Quality (AHRQ) is a part of the Public Health Service of the federal Department of Health and Human Services. The mission of the AHRQ is “to support research designed to improve the quality, safety, efficiency, and effectiveness of healthcare for all Americans.” In 2001, AHRQ published a document entitled, “Making Health Care Safer: A Critical Analysis of Patient Safety Practices,” an evidence-based review of practices intended to improve patient safety.13 There were 11 practices that were most highly rated of 79 practices that were reviewed in detail, based on the strength of evidence supporting their widespread implementation. These included three practices related to the management of central venous catheters (CVCs):
Central Venous Catheter Complications and the American Society of Anesthesiologists Closed Claims Project Database
The authors’ review of the Closed Claims Project database confirmed the hazards previously associated with central catheters.14 Among the 6449 claims reported through December 2002, there were 110 claims for injuries related to CVCs (1.7%). Claims related to CVCs had a high severity of patient injury with an increased proportion of death (47%) compared with other claims in the database (29%; P < 0.01).
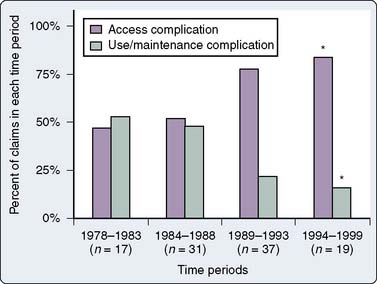
The main results of the review are shown in Table 39-1. Inspection of this table reveals that the most important injuries, both in terms of numbers and death rate, are cardiac tamponade and injuries to major arteries and veins (combining “carotid artery puncture/cannulation,” “hemothorax,” and “miscellaneous other vessel injury”), representing 16 of 110 and 39 of 110 cases, respectively, not including pulmonary artery (PA) injuries. This impression is reinforced when the injuries reported after 1990 are compared with those reported before 1990 (Figure 39-1). Since 1990, most injuries have been accounted for by vascular injuries.
TABLE 39-1 Central Catheter-Related Injuries from the American Society of Anesthesiologists Closed Claims Project Database
| Type of Complication | n | Death, n (%) |
|---|---|---|
| Wire/catheter embolus | 20 | 1 (5)* |
| Cardiac tamponade | 15 | 12 (80)* |
| Carotid artery injury | 14 | 5 (36) |
| Hemothorax | 14 | 12 (92)* |
| Pneumothorax | 12 | 2 (15)* |
| Miscellaneous vessel injury | 7 | 2 (29) |
| Pulmonary artery rupture | 6 | 6 (100) |
| Hydrothorax | 5 | 2 (40) |
| Air embolism | 4 | 3 (75) |
| Fluid extravasation in neck | 4 | 2 (50) |
| Cardiac arrhythmia | 1 | 0 (0) |
* P < 0.05 compared with other complications.
Data from Domino KB, Bowdle TA, Posner KL, et al: Injuries and liability related to central vascular catheters. Anesthesiology 100:1411–1418, 2004.
Although a great deal of attention has been paid to PA injuries caused by pulmonary artery catheters (PACs), it is interesting that they make up only 7 of 110 central catheter-related cases in the Closed Claims Project database.14 In addition, the anecdotal literature suggests that injuries to pulmonary arteries by PACs are sporadic and probably not related to specific errors or problems with technique. Although highly lethal15 and certainly to be feared, probably the main thing the practitioner can do to reduce the liklihood of PA injury is to avoid using a PAC in the first place. The appropriate use of PACs has been highly contentious,16–29 and because the literature is not clear regarding the safety of PACs, they will not be considered further in this chapter (see Chapter 14).
Preventing Cardiac Tamponade
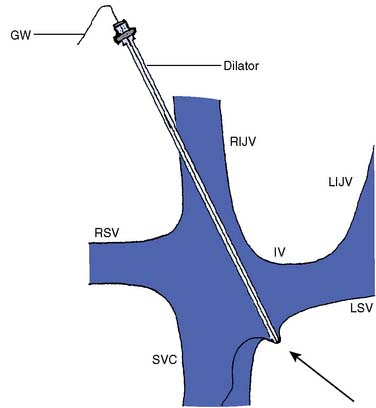
Cardiac tamponade from a central catheter occurs when the tip of a CVC is allowed to remain in the right atrium, or up against the wall of the vena cava at an acute angle, resulting in perforation of the atrium or cava. Perforation can occur immediately after placement or, more commonly, after hours to days. This problem has been documented by numerous case reports30–36 and accounted for 16 of 110 cases of central catheter-related claims in the ASA Closed Claims Project database; 13 of these 16 cases resulted in death.14 Perforation can occur immediately after placement, or more commonly after hours to days. Package inserts in CVC kits contain vigorous warnings against placing a CVC into the right atrium or with the CVC tip at an acute angle to the superior vena cava (SVC; Figure 39-2). Despite some controversy, most authors have concluded that the catheter tip should be outside of the pericardial sac and parallel to the walls of the vena cava.37 It is a widely accepted practice to obtain a chest radiograph after CVC placement, usually in the recovery room or intensive care unit (ICU) after surgery. This allows the position of the catheter tip to be assessed and adjusted if necessary (Figures 39-3 and 39-4).38,39 The carina is a useful structure for assessing the position of the catheter tip on the chest radiograph because the vena cava is outside the pericardium at the level of the carina.40,41 Locating the catheter tip at or above the level of the carina should ensure that the catheter tip lies outside of the pericardial sac. The carina may be a better landmark than the radiographic cardiac sillouette because several centimeters of the SVC may lie inside the pericardium but outside of the radiographic cardiac sillouette.40,41 Kwon et al42 made measurements of the SVC in vivo in 61 cardiac surgery patients and found that approximately half of the length of the SVC is within the pericardium. Particular caution should be exercised when catheters are placed from the left side (left internal jugular or subclavian veins) that the catheter tip either remains within the innominate vein37 or makes the turn into the SVC, ending with the tip parallel to the cava and not abutting the wall of the cava at an acute or right angle. Many catheters can be inserted to a depth sufficient to reach the right atrium (Figure 39-5), and this should be avoided by placing the CVC only to a depth of 10 to 12 cm from the right IJV approach in the typical adult. Because of anatomic variation, the placement should be confirmed by chest radiograph. Alternative approaches to verifying the position of the catheter tip include intravascular electrocardiography43 and TEE.44 An interesting editorial regarding tip location of CVCs is available.37
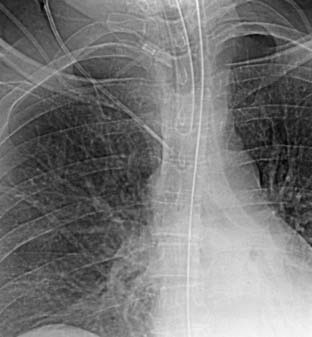
Figure 39-3 Routine chest radiograph taken in the recovery room showed the central venous catheter pointing toward the wall of the superior vena cava at an acute angle. Such positioning may increase the risk for perforation of the vena cava by the tip of the catheter. This catheter was repositioned as shown in Figure 39-4.
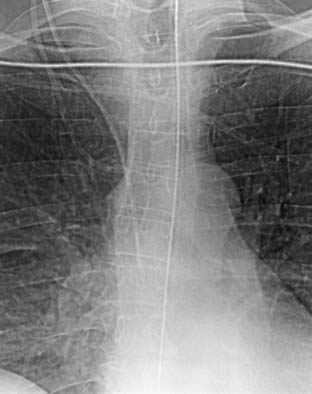
Figure 39-4 The central venous catheter shown in Figure 39-3 was repositioned so that the catheter was parallel to the walls of the superior vena cava. This position is thought to be safer than the original position shown in Figure 39-3 in which the catheter was pointed toward the wall of the vena cava at an acute angle.
Preventing Vascular Injury
Pressure Waveform Measurement
In 1983, Jobes et al45 reported on a retrospective study of 1021 attempts at IJV access in which there were 43 arterial punctures; 5 of 43 arterial punctures were unrecognized, resulting in the placement of 8-French introducer sheaths into an artery and resulting in one fatality from hemothorax. Subsequently, these investigators performed a prospective trial of 1284 attempts at IJV access in which they measured a pressure waveform from the vessel before inserting the guidewire. Before measuring the pressure waveform, a clinical assessment was made as to whether the needle was in an artery or vein, based on the usual criteria of color and pulsatility. There were 51 arterial punctures, 10 of which were incorrectly identified as being venous based on color and pulsatility but were determined to be arterial from the pressure waveform. Thus, 10 inadvertant cannulations of the carotid artery were avoided by pressure waveform monitoring.
Ezaru et al46 recently published a retrospective analysis of 9348 CVC placements requiring mandatory use of manometry to verify venous access. In a single institution, over a 15-year period, there were no cases of arterial injury. During the final year of the study, 511 catheters were placed. Arterial puncture (defined as placement of an 18-gauge finder needle or catheter into an artery) occurred in 28 patients (5%). Arterial puncture was recognized from color and pulsatility in 24 cases, without manometry; but in four cases, the arterial placement was only recognized with manometry. Despite these findings, and the anecdotal experiences of many anesthesiologists who have placed catheters and sheaths into carotid arteries, many anesthesiologists continue to rely on color and pulsatility of blood in the hub of a needle to differentiate arterial from venous puncture.
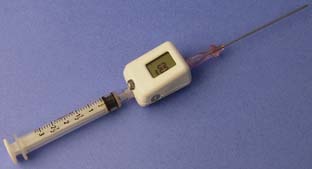
The pressure waveform can be most conveniently measured using the setup shown in Figure 39-6.47 This setup has the advantage of giving the pressure waveform without the need to disconnect the syringe and connect monitoring tubing to the needle, with the risk for dislodging the needle from the vein. An alternative method used by many anesthesiologists uses manometry. The syringe is disconnected from the needle and a length of tubing is connected, allowed to fill with blood, and then held vertically to identify the pressure from the height of the blood column. There are no data comparing these alternative methods of pressure measurement; however, the manometry technique requires an additional step to connect the manometry tubing. A novel approach to measuring a pressure waveform with a miniature, single-use in-line pressure transducer is illustrated in Figure 39-7.
Ultrasound Guidance
The availability of relatively inexpensive, portable ultrasound equipment led to the application of two-dimensional ultrasound imaging to guide CVC placement. Ultrasound imaging allows the presence of the IJV to be confirmed, its patency can be demonstrated, and its anatomic relation to the carotid artery can be defined. Real-time use can guide needle placement into the vein and confirm the presence of a wire in the vein. Troianos et al48 first reported the use of ultrasound-guided central vascular access in the anesthesia literature in 1991. Their prospective, randomized study of ultrasound guidance versus the traditional landmark method found a greater overall success rate, a greater success rate on the first attempt, and reduced rate of arterial puncture with ultrasound guidance. Numerous studies of ultrasound guidance and two major meta-analyses have appeared subsequently. As noted earlier, a review commissioned by the AHRQ strongly advocated the use of ultrasound guidance.10 In the United Kingdom, the National Institute of Clinical Excellence (NICE) recommends routine use of ultrasound for central venous catheterization.*
The two major meta-analyses of ultrasound guidance concluded that ultrasound guidance was superior to landmarks for overall success rate, a greater success rate on the first attempt, and reduced complications from arterial puncture for the IJV approach.49,50 The advantage of ultrasound guidance for the subclavian approach was less clear.
The authors examined CVC complications from the ASA Closed Claims Project database in an attempt to determine whether the use of pressure waveform monitoring or ultrasound guidance would have prevented the complications. This is clearly inferential; nevetheless, it is interesting that nearly half (48/110) of the complications were judged to be possibly preventable by the use of either pressure waveform monitoring or ultrasound guidance, only by ultrasound guidance, only by pressure waveform monitoring, or by chest radiograph (Table 39-2).14
TABLE 39-2 Potential Effect of Ultrasound Guidance or Pressure Waveform Monitoring on Central Venous Catheter–Related Injuries from the American Society of Anesthesiologists Closed Claims Project Database
| Possibly preventable by either ultrasound guidance or pressure waveform monitoring (n =19) | |
| Carotid artery puncture/cannulation | 16 |
| Hemothorax | 1 |
| Wire/catheter embolus | 1 |
| Miscellaneous other vessel injury | 1 |
| Possible preventable by pressure waveform monitoring only (n = 6) | |
| Miscellaneous other artery injury | 5 |
| Hemothorax | 1 |
| Possibly preventable by ultrasound guidance only (n = 9) | |
| Hemothorax | 4 |
| Pneumothorax | 4 |
| Miscellaneous other vessel injury | 1 |
| Possibly preventable by chest radiograph (n = 14) | |
| No chest radiograph taken (n = 7) | |
| Carotid tamponade | 2 |
| Wire/catheter embolus | 1 |
| Pneumothorax | 4 |
| Misread, not read, or inappropriate action taken (n = 7) | |
| Cardiac tamponade | 4 |
| Wire/catheter embolus | 3 |
The cases related to central venous catheters from the ASA Closed Claims database were examined to determine whether the use of ultrasound guidance, pressure waveform monitoring, or chest radiograph (after placement) might have prevented the injuries. A total of 48 of 110 injuries were thought to be potentially preventable by these means.
Data from Domino KB, Bowdle TA, Posner KL et al: Injuries and liability related to central vascular catheters. Anesthesiology 100:1411–1418, 2004.
Wigmore et al51 reported their experience in a single tertiary referral center in Britain after implementation of the National Institute of Clinical Excellence guideline. This is a particularly interesting study because it illustrates the effect in a single center of attempting to implement a national guideline. During the study, adoption of ultrasound guidance increased from less than 10% to more than 80%. There were 19 of 152 complications during a preguideline audit, 18 of which involved the landmark technique without ultrasound guidance. After introduction of the guideline, there were 13 of 286 complications, 10 from cases in which only the landmark technique was used (8.7%) and 3 from cases in which ultrasound guidance was used (1.7%), an absolute risk reduction of 6.9% with ultrasound. Complications consisted of arterial punctures, neck hematomas, and a pneumothorax. There was also a significant reduction in failed insertions with ultrasound guidance (7/115 for the landmark group and 1/169 for the ultrasound group; P < 0.01).
Recently, Hosokawa et al53 have demonstrated the utility of ultrasound guidance in neonates and infants less than 7.5 kg. They compared ultrasound guidance with the traditional anatomic landmark method, and later they compared ultrasound guidance for skin marking (without live ultrasound during needle puncture) with live ultrasound guidance. There was a 97% success rate for ultrasound guidance compared with a 62% success rate for the anatomic landmark method.52 Live ultrasound compared with ultrasound for skin marking (without live ultrasound during needle puncture) resulted in significantly faster cannulation and fewer needle passes.53 Fewer than three attempts at puncture were made in 100% of patients in the live ultrasound group compared with 74% of patients in the ultrasound for skin marking group.
An important caveat for the use of ultrasound guidance is that the needle and/or wire may not always be visualized in the vein, depending on the type of ultrasound equipment used and the skill of the operator. Although it may be possible to visualize the tip of the needle with ultrasound,54 because of the tomographic nature of an ultrasound beam, it may be difficult to distinguish the shaft of the needle from the tip. Needles with a special echogenic surface that may enhance ultrasound visualization are available (see Chapter 14).
Given the abundance of data in favor of the use of ultrasound guidance, it is reasonable to consider the use of ultrasound guidance to be the “preferred method of insertion.”55 Interestingly, several surveys have found relatively low rates of using ultrasound guidance. A survey of pediatric anesthesiologists in the United Kingdom found that only 39% used ultrasound routinely.56 A more recent survey of pediatric anesthesiologists in the United Kingdom found that only 26% always used ultrasound.57 Another recent survey in the United Kingdom of senior members of the Association of Anaesthetists of Great Britain and Ireland found that only 27% used ultrasound guidance as their first choice.58 A survey of members of the SCA found that only 15% always used ultrasound.59 A shortage of suitable ultrasound equipment is sometimes a reason for not using ultrasound guidance. A study in the United Kingdom found that 86% of anesthetic departments had ultrasound equipment for CVC placement60; however, Bailey et al59 found that 33% of anesthesiologists in their survey of members of the SCA never or almost never had ultrasound equipment available.
Preventing Injuries to Intrathoracic Arteries or Veins
Guidewires can take a circuitous route so that when devices are advanced over them, the device may trap the wire up against the wall of the vein, and if the device is stiff enough, perforation of the vein may occur (Figure 39-8).61 The inner dilators of PAC introducer sheaths are very stiff and may be particularly likely to cause this kind of perforation. The dilator is intended to create a passage through the skin and fascia but has no useful role once the device has entered the vein. Therefore, it is prudent to advance the dilator the minimum distance necessary to reach the vein and no farther. The introducer sheath may then be advanced over the dilator into the vein.
Kulvatunyou et al62 reviewed a collection of cases of injury to the right subclavian artery during attempted right IJV cannulation. The right subclavian artery is in close proximity when the right IJV is approached low in the neck. Because of interference from the clavicle, puncture of the subclavian artery may not be seen with ultrasound but will be detected by pressure waveform monitoring. The authors have seen several instances of presumed subclavian artery puncture during attempted IJV cannulation low in the neck, in which ultrasound guidance was used, but the needle tip was not visualized in the vein and arterial puncture was detected with pressure waveform monitoring.
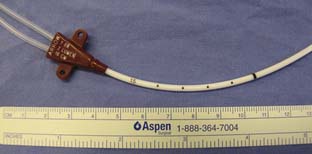
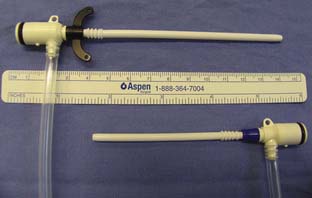
The left IJV approach may be particularly hazardous because the innominate (brachiocephalic) vein crosses the IJV at a right angle. A device inserted into the left IJV could perforate the innominate vein, and anecdotal reports verify that this is indeed the case. The right IJV should be preferred to the left IJV primarily for this reason. If the left IJV is used, special care should be exercised not to advance the dilator of a PAC introducer beyond the IJV. The authors prefer to use a shorter-than-normal introducer sheath in this situation (Figure 39-9). Another potential problem with the left-sided approach is injury to the thoracic duct, which terminates variably in the left IJV, the left subclavian vein, or the left innominate vein. The duct may be perforated in the process of placing a CVC, or rarely, the duct may actually be cannulated. Chylothorax and chylopericardium may result, and infusion of fluids into the thoracic duct may produce cardiac tamponade or constrictive pericarditis by retrograde flow into the pericardial lymphatics.63
Treatment of Inadvertent Cannulation of Arteries
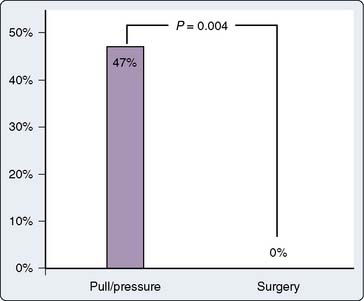
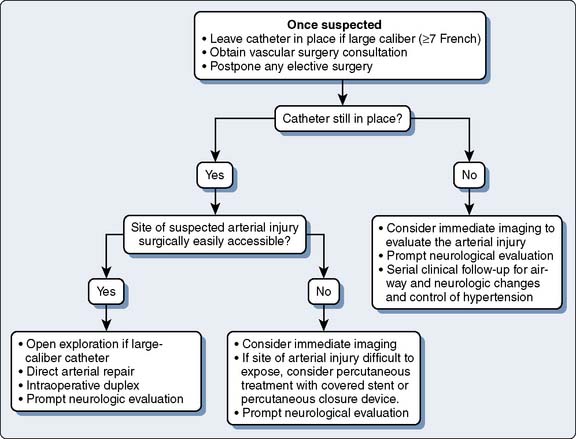
Although prevention of inadvertent arterial cannulation with large-bore CVCs is paramount, an approach to treating inadvertent arterial cannulation may be needed in rare circumstances. There have been no guidelines in the literature for the treatment of accidental cannulation of arteries with large-bore catheters, but two recently published case series documented better outcomes with surgical or endovascular intervention when compared with removal and compression (“pull/pressure”).64,65 Guilbert et al65 published a proposed algorithm for dealing with inadvertent arterial cannulation based on cases from their institutions and review of the literature. They found that the “pull/pressure” method was associated with a large incidence of serious complications (47%), including death, whereas the surgical or endovascular approach was not (Figure 39-10). Based on this, they suggested the algorithm shown in Figure 39-11. Interestingly, a survey of vascular surgeons presented with a hypothetical case of an 8.5-French catheter in a carotid artery found that the respondents saw this complication one to five times per year, and two thirds would simply pull the catheter and apply pressure. However, when vascular surgeons were shown the data from the study by Guilbert et al65 at a meeting, most of them changed their management to the surgical or endovascular approach as judged by pretest and post-test questions. Several of the specific findings of Guilbert et al’s65 study are worth noting:
Preventing Infections Related to Central Catheters
Catheter-related bloodstream infections occur 0.5 to 4.8 times per 1000 catheter days, have an attributable mortality rate of 0% to 11%, cause an excess hospital stay of 9 to 12 days per episode, and are expensive for the health care system.66–68 Infections from central catheters and PACs are related to the time that the catheter is in place and increase significantly after 3 days.69,70 Because many CVCs placed by anesthesiologists for intraoperative monitoring are removed soon after surgery, the risk for infection is reduced. Nevertheless, anesthesiologists should use the evidence-based methods for preventing CVC infections.71 Implementation of a CVC insertion “care bundle” can significantly reduce infection rates. The bundle of interventions used in Pronovost et al’s72 study included appropriate hand hygiene, use of chlorhexidine for skin antisepsis, use of maximal sterile barrier precautions (mask, sterile gown, sterile gloves, and large sterile drapes) during catheter insertion, avoidance of the femoral vein, if possible, and prompt removal of unnecessary catheters. There is substantial evidence that chlorhexidine is a more effective skin cleaning solution than povidone-iodine. Antibiotic ointments applied to the skin puncture site do not affect the risk for bloodstream infection and may actually increase the rate of colonization by fungi and promote antibiotic-resistant bacteria. Recent data suggest that the use of a chlorhexidine-impregnated sponge dressing at the line insertion site may reduce the rate of catheter-related infections.73 The use of antimicrobial-impregnated catheters reduces the incidence of bloodstream infections related to CVCs, and their use should be considered.74–76 As noted earlier, antimicrobial-impregnated catheters are recommended in the AHRQ report on evidence-based safety measures. However, these catheters are more expensive and may not be cost-effective when the catheters are going to be removed soon after surgery. For catheters that are intended to remain in place or for high-risk patients (e.g., immunosuppression), antibiotic-impregnated catheters may be worthwhile.76
Preventing intraoperative awareness
Three large, prospective, multicenter studies of intraoperative awareness have reported similar overall rates of intraoperative awareness of around 0.1% to 0.2% (Box 39-2).77–79 A recent report from China found a larger incidence rate of about 0.4% in that country.80 An analysis of cases of awareness during anesthesia from the ASA Closed Claims Project was reported in 1999.81 Undergoing cardiac surgery increases the risk for intraoperative awareness from the overall level of around 0.1% to 0.2%78,79 to around 0.4% to 1%,79,82–86 an increased risk of up to 10-fold. Ghoneim et al87 recently conducted a review of awareness cases in the literature and confirmed that cardiac surgery is associated with an increased risk. Intraoperative awareness may be a minor or a major complication depending on the severity of the episode of awareness and the response of the individual patient. Intraoperative awareness may result in a significant psychiatric condition such as post-traumatic stress disorder.88 The explanation for increased risk for intraoperative awareness during cardiac surgery is unknown. Possible causes include deliberately light anesthesia during periods of hemodynamic instability and gaps in anesthetic delivery during separation from cardiopulmonary bypass (CPB) when the volatile anesthetic being administered into the CPB circuit is discontinued. Although vasoactive drugs used during cardiac surgery may mask hemodynamic responses to light anesthesia, it is clear that intraoperative awareness often occurs in the absence of hemodynamic clues, rendering that explanation less likely. Whatever the cause may be, cardiac anesthesiologists should be concerned about the high incidence of intraoperative awareness during cardiac surgery.
BOX 39-2. Avoiding Intraoperative Awareness
Some anesthesiologists are skeptical about an incidence rate of intraoperative awareness of 0.1% to 1%. This skepticism is usually based on personal experience such that individual anesthesiologists believe that the rate of intraoperative awareness in their personal practice is much less than this. However, caution should be used in applying personal experience because of observations in the literature showing that anesthesiologists frequently may miss cases of intraoperative awareness for a variety of reasons, not the least of which is that patients do not always have the opportunity to relate their experience to the anesthesiologist.89 Several studies have shown that the patient may not report their awareness experience immediately after surgery.78,79
Electroencephalography (EEG) has been used extensively by neuropharmacologists since the 1960s to study the effects of anesthetic drugs. As the power of desktop computers increased, it became possible to process raw EEG using fast Fourier transforms and other mathematical methods to make the information contained in the raw EEG immediately accessible in the operating room to those without formal training in its interpretation. The original use of processed EEG in the operating room was for the detection of cerebral ischemia during carotid endarterectomy, and it has been only in recent years that attention has turned once again to the use of EEG to measure the effects of anesthetic drugs. The technologic obstacles to reliably measuring the effects of anesthetic drugs in the operating room with EEG and providing clinically useful information to the anesthesiologist were daunting. Many individuals who were familiar with EEG technology believed that it would never be possible to measure “anesthetic depth” in real time in the typical operating room setting. Nevertheless, the major obstacles have been overcome, and now several commercial products are available for this purpose. The processed EEG monitor known as BIS (bispectral index) is by far the most prevalent, and by far the best documented. Therefore, most of the information here will be concerned with BIS. However, it should be clearly understood that there are other commercial products that purport to provide similar data for clinical monitoring (see Chapter 16). BIS monitoring recently has been reviewed.90,91
The result of EEG processing by the BIS monitor is a number ranging from 0 to 100, where 0 is an isoelectric (flat) EEG and 100 is “awake.” Assuming that artifacts, interference, signal quality, and other idiosyncrasies of EEG processing have been appropriately evaluated by the user (see later), a BIS value less than 60 should be associated with an extremely low probability of awareness during anesthesia.92,93 It is possible that if BIS monitoring were used and BIS values kept to less than 60, the incidence of intraoperative awareness could be reduced to a very low level. However, an alternative notion has been proposed94 that the use of BIS monitoring might actually increase the incidence of intraoperative awareness because of the well-documented tendency of anesthesiologists to administer lower doses of anesthetic drugs when BIS monitoring is used as a guide to anesthetic drug administration.95,96
Three studies have been published that support the hypothesis that the use of BIS monitoring can result in a lower incidence of intraoperative awareness. In a retrospective case-comparison study, 5057 consecutive BIS-monitored patients were compared with 7826 non-BIS monitored cases from the same institutions.97 There were two cases of intraoperative awareness (during intubation; both had BIS values > 60) in the BIS-monitored series compared with 14 in the non-BIS–monitored series (P < 0.039). In a prospective, randomized multicenter trial, 2503 “high-risk” (e.g., cardiac anesthesia, trauma, obstetrics) patients were randomized to BIS or non-BIS monitoring.86 There were 2 cases of intraoperative awareness in the BIS-monitored group (in 1 case, the BIS was <60), compared with 11 in the non-BIS monitoring group (odds ratio, 0.18; 95% adjusted confidence interval, 0.02–0.84; P = 0.022). A single-center randomized trial compared BIS monitoring with “targeted end-tidal anesthetic gas analysis” (target range, 0.7–1.3 minimum alveolar concentration) in 2000 patients at “high risk” for intraoperative awareness; 25% of the patients underwent cardiac surgery.98 The authors predicted an incidence rate of awareness of 1% but found that either BIS monitoring or “targeted end-tidal gas analysis” reduced the incidence rate to 0.2%. These studies suggest that BIS monitoring may be a useful tool for helping to reduce the incidence of intraoperative awareness.
Kunisawa et al103 and Kakinohana et al104 have pointed out that during thoracic aortic surgery with partial left-heart bypass when the descending thoracic aorta is cross-clamped, BIS values can be altered substantially depending on whether the drugs are administered in the circulation above or below the cross-clamp. If the drugs are administered into a vein in the lower body, cross-clamping the aorta may result in a reduction in drug concentrations in the circulation and the brain above the cross-clamp,103 whereas if the drugs are administered into a vein in the upper body, the concentration in the circulation and the brain above the cross-clamp may increase,104 presumably because of pharmacokinetic changes relating to the altered circulation with partial left-heart bypass. The change in drug concentrations will be reflected in the BIS, which may increase if the drugs are administered below the cross-clamp, or decrease if the drugs are administered above the cross-clamp.
Drug errors
Adverse drug events are a major cause of morbidity and mortality in hospitalized patients, affecting more than 770,000 patients per year in the United States. Hospital costs for treating patients who suffer adverse drug events are enormous, estimated at between $1.6 and $5.6 billion annually. Most adverse drug events in hospitalized patients are caused by errors of various kinds,105 as brought to wide public attention in the Institute of Medicine’s report, “To Err Is Human: Building a Safer Health System.”1 This is an international problem, as shown by a recent investigation that used “disguised observation” to discover that one or more errors occurred in the preparation and administration of 58 of 122 intravenous drug doses on the nursing wards of a German hospital. Many of the errors were minor, but there were three cases of wrong dose, one omitted dose, and two “unauthorized” doses.106 A review of medication errors in the ICU setting is available.107
A great deal of attention has been directed at errors that occur in the process of writing and transcribing physician’s orders for drugs. There is evidence that computerization of physician ordering (computerized physician order entry [CPOE]), together with computerized advice (clinical decision support), can significantly reduce these types of errors.108 The AHRQ review “Making Health Care Safer: A Critical Analysis of Patient Safety Practices” rated CPOE and clinical decision support as patient safety practices with medium strength of evidence.13 The Leapfrog Group (“a coalition of more than 150 public and private organizations…created to help save lives and reduce preventable medical mistakes by mobilizing employer purchasing power to initiate breakthrough improvements in the safety of health care and by giving consumers information to make more informed hospital choices”), founded by the Business Roundtable, a national association of Fortune 500 CEOs, has made CPOE one of its “safety standards” (http://www.leapfroggroup.org). On balance, CPOE appears to be useful and probably can reduce drug errors109,110; however, there is the possibility of harm from unintended consequences,111,112 as with any technology. In a particularly dramatic example of unintended consequences, introduction of CPOE at the University of Pittsburgh apparently resulted in an increase in mortality rate from 2.8% to 6.3% of children transported to the university for special care, because of unintended delays in delivery of critically important medications.112 Clearly, the details of implementing technologies intended to reduce drug errors are extremely important, as illustrated for CPOE.113 This theme is repeated for bar coding and smart pumps later in this chapter.
Because most anesthesiologists seldom write orders for drugs (critical care settings such as the ICU being a notable exception), CPOE is of less interest than the administration of drugs by the anesthesiologist at the “point of care.” There is substantial evidence that drug administration errors are common in anesthetic practice (Box 39-3). In 1993, the Australian Incident Monitoring Study identified 144 instances of a wrong drug being given or nearly given to a patient among the first 2000 incidents reported to the study.114 There was the potential for serious harm in 74% of reports, but no deaths occurred. In 1995, Merry and Peck115 reported on a survey of 75 anesthesiologists in New Zealand, 89% of whom indicated they had made at least one error of drug administration, with 12.5% indicating that they had harmed a patient by a drug-related error. Subsequently, Merry’s group performed a survey of 10,806 anesthetics in two hospitals in New Zealand. Anesthesiologists volunteered information about drug errors using a standardized reporting form.116 The overall rate of drug error was 0.75%, or 1 in 133 anesthetics. Drug errors resulted in 1 case of intraoperative awareness, 2 cases of prolonged neuromuscular blockade, and 47 cases of transient physiologic effects, 5 of which required intervention. A similar survey of 687 anesthesiologists in Canada found that 85% of the respondents had made at least one drug error or “near miss.”117 Four deaths resulted from a total of 1038 reported errors. A report based on annual surveys conducted by the Japanese Society of Anesthesiologists between 1999 and 2002, reflecting 4,201,925 anesthetics,118 found an incidence of “critical events” caused by drug administration error rate of 0.02%. The incidence rate of death resulting from drug error was 0.00044%. Additional recent reports from Australia,119 Japan,120,121 and South Africa122 suggest that anesthetic drug error continues to be a significant problem around the world.
BOX 39-3. Reducing Errors Related to Drug Administration
The incidence of drug administration errors in an academic practice in the United States appears to be similar to that reported from elsewhere in the world. In a study modeled after the New Zealand study cited earlier, anesthesiologists and nurse anesthetists at the University of Washington completed survey forms; 6066 forms were returned for 6709 anesthetics, a response rate of 90%123 (Table 39-3). There were 41 reports of errors (0.68%) and 23 reports of pre-errors (near misses; 0.38%). Drug administration errors resulted in transient unintended drug effects (<5 minutes) in 17 cases and prolonged unintended drug effects (>5 minutes) in 12 cases. Of these 29 cases of unintended drug effect, 14 were associated with drug infusions administered by a pump. One patient had possible intraoperative awareness associated with an empty vaporizer, and one patient had a longer than expected hospital stay because of inadvertent administration of neuraxial morphine. There were no cases of drug-related permanent physical injury.
| Anesthetics | 6705 |
| Forms completed | 6066 |
| Total errors | 41 (0.68%) |
| Incorrect dose | 18 |
| Substitution | 7 |
| Insertion | 6 |
| Repetition | 2 |
| Incorrect route | 2 |
| Omission | 3 |
| Incorrect label | 1 |
| Unintended effects | 31 |
| < 5 minutes | 18 |
| > 5 minutes | 13 |
A study of self-reported drug-related errors by anesthesiology attendings, residents, and certified registered nurse anesthetists (CRNAs) in a university medical center found the errors shown in the table. The overall rate of drug-related error was 0.68% of anesthetics. There were no permanent injuries; however, in 31of 41 cases unintended drug effects occurred. One patient had possible intraoperative awareness associated with an empty vaporizer, and one patient had a longer than expected hospital stay because of inadvertent administration of neuraxial morphine.
Data from Bowdle A, Kruger C, Grieve R, et al: Anesthesia drug administration errors in a university hospital. Anesthesiology 99:A1358, 2003.
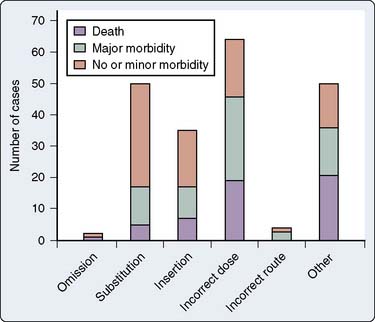
Analysis of the ASA Closed Claims Project database also reveals interesting information about anesthetic drug administration errors in the United States.124 As of 2003 (a more current update is pending), there were 205 drug errors, 4% of the database of 5803 cases. Drug errors were categorized into the following categories (after Webster et al116):
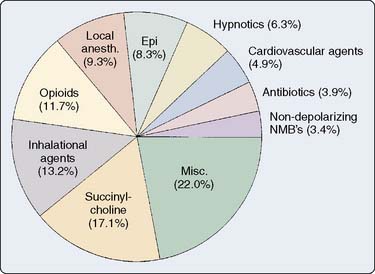
The numbers of cases in each category and the types of drugs involved are shown in Figures 39-12 and 39-13. The most common distinct mechanisms of drug administration error were substitution, insertion, and incorrect dose. Drug errors were also a significant factor in claims that involved multiple problems in patient management (“other”). Two drugs in particular were most involved.
Succinylcholine was involved in 35 cases (17%), and epinephrine was involved in 17 cases (8%). Twelve of the 35 cases involving succinylcholine resulted in patients being awake while paralyzed because of succinylcholine boluses given before induction agents or succinylcholine infusions that were started inadvertently in awake patients. Succinylcholine was administered to five patients with a previous history of definite or probable pseudocholinesterase deficiency, resulting in prolonged neuromuscular blockade. Hyperkalemic cardiac arrest occurred in two paraplegic patients and a patient with Guillain-Barré syndrome who received succinylcholine. Succinylcholine infusions were involved in 14 of the 35 succinylcholine-related cases. Drug administration errors involving epinephrine were particularly dangerous, with death or major morbidity resulting in 11 of the 17 epinephrine-related cases. Six of the 17 cases involving epinephrine were caused by ampoule swaps. Drugs interchanged with epinephrine were ephedrine (two cases), oxytocin (three cases), and hydralazine (one case). An informative case report describing the nearly fatal results of inadvertent epinephrine administration because of an ampoule swap has been published.126
An analysis of litigation against the National Health Service in England related to anesthetic drug errors found a spectrum of errors that were similar to those found by the ASA Closed Claims Project.127
Drug Errors by Perfusionists
Cardiac surgery requiring CPB presents the relatively unusual situation in which the anesthesiologist may not be the only person in the operating room administering drugs intravenously to the patient. Perfusionists frequently administer anesthetic drugs during CPB and may administer a variety of other drugs as well. Rarely, the perfusionist also may be an anesthesiologist (particularly in Australia). Whether the anesthesiologist supervises the perfusionist in the administration of drugs varies depending on the particular practice setting. In some practice settings, the anesthesiologist may leave the operating room during some portion of the time on CPB. Although the suggestion has been made that the anesthesiologist should not leave the operating room during CPB (http://www.asawebapps.org/Newsletters/2004/04_04/gravlee04_04.html), the practice of doing so probably is widespread, particularly outside of academic institutions. Apparently, there are no studies documenting drug errors made by perfusionists, despite several general reports on error and incidents during CPB.128–131 Any comprehensive effort to reduce drug errors during cardiac surgery should include the perfusionist. Common sense suggests that the perfusionist and anesthesiologist should work together as much as possible to ensure that the patient receives drugs appropriately during CPB.
Prevention of Drug Administration Errors—Bar Coding
Although drug administration errors are clearly important, relatively little is known about preventing these errors. A recent review evaluated the evidence for various measures for reducing drug administration errors in anesthetic practice and made recommendations; however, most of the evidence available for review was anecdotal.132 Recommendations included carefully reading the label on any ampoule or syringe, optimizing legibility and contents of labels, labeling syringes, formal organization of drugs drawers and workspaces, and double-checking drugs with another person or a device.
A bar-code reader represents just such a device, and bar-coding drugs at the point of care to verify the correctness of the drug and the dose is widely regarded as a technologic solution that might improve the accuracy of drug administration. The Healthcare Information and Management Systems Society has a position statement in support of bar coding in the healthcare environment for a variety of purposes (http://www.himss.org/content/files/BarCoding StatementFINAL20020603_19028.pdf). ECRI, the nonprofit health services research agency, also has published a review concerning the bar coding of medications.133,134 The proceedings of the Healthcare Information and Management Systems Society from 2000 (“Veterans Affairs: Eliminating Medication Errors Through Point-of-Care Devices”; available online at: http://www.himss.org/content/files/proceedings/2000/sessions/ses073.pdf) contains interesting anecdotal information about the experience of the Department of Veterans Affairs with bar coding. According to this report, wireless, point-of-care bar coding of patient wristbands and medications was used at the Comery-O’Neil Veterans Affairs Medical Center to deliver 5.7 million doses and in the process prevented 378,000 errors. The report claims that “no medication errors occurred when the technology was used as designed,” although errors continued to occur when the technology was not properly used. Application of bar-coding technology reduced wrong medication errors by 74%, wrong dose errors by 57%, wrong patient errors by 91%, wrong time errors by 92%, and omission errors by 70%. Clearly, these are promising results that should cause clinicians to look carefully at the prospects for bar coding in the anesthesia environment. Interestingly, the Veterans Affairs medical centers experienced some significant difficulties with point-of-care bar coding, which is described in an article by Mills et al.135 Some of the most common problems were simple but critically important, such as nonreadable bar codes on patients wrist bands and drug containers. According to Mills et al,135 “Several anecdotal reports have indicated that bar-coding systems successfully lower medication administrations errors…Nevertheless, within the VA, there have been significant negative effects since introducing the bar-coded medication administration (BCMA) system. More generally, the VA discovered that introducing new technology to complex medical systems, while beneficial, also presents new challenges.”
A study of point-of-care bar-code medication administration in a network of six community hospitals examined error logs and determined the likely severity of outcomes associated with errors that were prevented by the bar-code system.136 Only 1% of prevented errors were judged to be likely to produce severe adverse effects, and 8% moderate adverse effects. However, given that 18 million doses were administered in the hospital network since the bar-code system was implemented, 17,000 errors expected to produce severe or moderate adverse effects may have been prevented.
The FDA issued a rule in February 2004 that requires bar codes on most prescription drugs, certain over-the-counter drugs, and blood products (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFR Search.cfm?fr=201.25). The FDA believes that bar codes would be used in the following manner:
To the best of the authors’ knowledge, there are two commercially available systems designed specifically for bar-coding drugs in the anesthesia clinical environment. One was designed by an anesthesiologist and marketed by DocuSys (Hartland, WI); another was designed by an anesthesiologist and marketed by Safer Sleep (Cambridge, United Kingdom).137 Unfortunately, there are no prospective, randomized data showing that the use of these devices affects outcome, although a recent study of user ratings of the Safer Sleep system gave results in favor of the bar-coding system in comparison with the traditional system of drug administration.138 The Safer Sleep system was reported to result in a 19% increase in revenue from drug charges in a cardiac anesthesiology practice by improving documentation for billing.139 Hypothetically, automated anesthesia record-keeping systems provided by other vendors could be adapted to bar coding of drugs because bar-code readers can be operated by most desktop computer systems.
Prevention of Drug Errors—Infusion Pumps
As noted earlier, the study of drug errors at the University of Washington found that among 29 cases of unintended drug effect, 14 were associated with drug infusions administered by a pump.123 Although there is no comprehensive assessment of the incidence and nature of errors related to infusion pumps, it is clear from anecdotal reports that programming errors are a significant source of error. Obviously, infusion pumps play a major role in drug administration in cardiac surgery patients, so errors related to infusion pumps are of significant concern to cardiac anesthesiologists.
ECRI, the nonprofit health services research agency, recently made recommendations concerning improvements in the safety of infusion pumps. Based on reports of pump-related problems and accident investigations, ECRI has found that pump incidents typically involve either unintentional free-flow of a drug or the delivery of an incorrect dose because of a practitioner error in programming the pump.140
Pump sets that prevent free-flow when the set is removed from the pump have been available for many years, but not all pumps in current use require the use of sets that prevent free-flow, and unprotected sets continue to be available. ECRI recommends the use of pumps with tubing sets that prevent free-flow when the set is removed from the pump, and that only pumps that require the use of sets with free-flow prevention be purchased. They rate pumps that do not require set-based free-flow protection as “unacceptable.”140
Some infusion pumps have advisory systems known as “error reduction systems” that use predefined dosing limits and warn the practitioner if the dosing parameters that are entered at the point of care will result in a dose that is out of the predefined dosing limits. Such systems also can keep a computerized log that yields data regarding the incidence of “reprogramming” in response to an alert message; this is essentially the incidence of potential errors because of programming errors. Anecdotal information suggests that error reduction systems may be valuable for helping practitioners to recognize and avoid administering incorrect doses because of programming errors. A recent report of experience with 135 pumps with an error reduction system installed in a community hospital found that 40,644 infusion starts generated 693 alert messages (1.7%), resulting in 158 programming changes.141 The 1400-bed hospital projected that if all of their pumps had error reduction systems, that in a year more than 1 million infusion starts would result in 18,500 alert messages and 4000 programming changes. All pumps rated “preferred” or “acceptable” by ECRI include dose error reduction systems, whereas pumps without such systems are rated “not recommended” for new purchase.142 A prospective, randomized trial of “smart” infusion pumps conducted in 2002 in a cardiac surgery ICU found numerous serious medication errors related to pumps (approximately 2 serious errors per 100 patient pump-days).143 There was no significant improvement with the use of smart pumps, although the investigators observed that the pumps were frequently not used as intended. For example, the drug library that is intended to prevent manual programming errors was bypassed 25% of the time. This study demonstrates that technology alone may not solve a problem, and that close attention to the details of implementing the technology and making it work properly are essential (as noted for bar coding at the point-of-care; see earlier). Nuckols et al144 also found that the smart pumps they tested were capable of preventing only 4% of the adverse drug events in the ICU, with many of the events having to do with bolus dosing and failure to adequately monitor for and respond to drug-related problems. However, they did not conclude that smart pumps should be abandoned; to the contrary, they advocated smarter and more capable pumps.
An error programming the concentration of morphine in a patient-controlled analgesia pump caused the death of a patient who received a fivefold overdose of morphine because of the programming error. The authors of the case report made an estimate of the likely death rate from similar programming errors of the particular patient-controlled analgesia pump involved in this incident, based on an FDA database and other reports, using a denominator supplied by the pump manufacturer. The estimate was adjusted to take the likely under-reporting of incidents into account. Their estimate was a range of 1 death in 33,000 pump uses to 1 in 338,800, amounting to 65 to 667 deaths for 22,000,000 pump uses in the period 1988 to 2000.145
Transfusion Safety
Although bar coding of drugs has received greater publicity, bar coding of blood units and the use of bar coding to ensure accurate matching of blood component to recipient is another promising avenue for application of bar coding technology to prevent error. ABO mismatched transfusion because of administering the wrong blood is a classic example of a preventable medical error and is a significant cause for concern in the practice of cardiac anesthesia. A study of transfusion errors in New York State estimated the incidence of ABO mismatched transfusion at 1:12,000 to 1:33,000.146 A recent report from a hospital in Japan specializing in cardiovascular disease described a computer-assisted transfusion management system with bar coding at the bedside; 60,000 blood components were transfused without error, and one human error was prevented. The system also improved the efficiency of blood component management, reducing the outdate rate on red blood cells from 3.9% to 0.32%.147 Bar coding blood components in the operating room could contribute to both efficiency and accuracy, especially if the data from the bar coding operation were automatically entered in a computerized anesthesia record. As noted earlier, the FDA has mandated bar codes on blood products, as well as drugs.
Fatigue and error in cardiac anesthesia
The Institute of Medicine Committee on Optimizing Graduate Medical Trainee Hours and Work Schedules to Improve Patient Safety conducted a comprehensive review of the medical and scientific literature and concluded that working for more than 16 consecutive hours is unsafe for both trainees, because of a marked increase risk for a motor vehicle accident while driving home, and for their patients, because of an increase in attentional failure, serious errors, and diagnostic mistakes.3 After being awake for 24 hours, impairment of reaction time is comparable with that produced by a blood alcohol concentration of 0.10 g/dL (the equivalent of 4 drinks for a 70-kg individual, and over the legal limit for driving while intoxicated in most countries).148,149 A survey of resident physicians suggested that fatigue-related errors resulting in death or injury of a patient are not uncommon (Box 39-4).150
BOX 39-4. Reducing Errors Related to Fatigue and Distraction
Interestingly, there is significant variability between individuals in susceptibility to the effects of sleep deprivation. The effect of a single night of acute sleep deprivation on neurobehavioral functions is greater in younger people than in people older than 55 years.151 This may explain the fact that 55% of sleep-related motor vehicle accidents occur in drivers 25 or younger.152 However, older people are more vulnerable to the effects of a sequence of night shifts because of greater difficulty obtaining recovery sleep in an adverse portion of the circadian cycle.153 There also are inter-individual differences in the effects of sleep deprivation on otherwise healthy young people, and these differences may be specific for particular tasks. These differences have been linked to particular genetic polymorphisms.154,155 Czeisler156 has speculated that, in the future, it may be possible to identify from a cheek swab the genetic subset of individuals who tolerate sleep deprivation relatively well from those who are very sensitive.
Sleep-deprived individuals develop the subjective experience of sleepiness early, and if sleep deprivation persists, they have a poor capacity to recognize their fatigue, rendering them with a reduced capacity to work safely.157 Recognition that sleep deprivation adversely affects performance has led to policies that limit work hours. Limitations on work hours for pilots date from the 1950s. In the United States, the Accreditation Council for Graduate Medical Education limits the hours of trainees in approved programs. In other countries, a variety of regulations may apply. For example, in New Zealand, a labor agreement limits consecutive work hours for trainees to 16. There are no work-hour limitations in the United States for nontrainee physicians. In the European Union, all occupations are limited to 13 consecutive work hours and 48-hour work weeks. Whether limiting trainee work hours lessens the effectiveness of training programs has been the subject of much debate, which recently was reviewed.158 There has been widespread noncompliance with work hour limits in Accreditation Council for Graduate Medical Education–accredited training programs.159 However, given the available evidence connecting sleep deprivation with impairment in performance, it seems likely that work hour limitations will become more stringent rather than less. Moreover, it seems likely that consideration will be given to extending the limitations applied currently to trainees to physicians in practice.
Effects of Fatigue on Error in Anesthesia
Fatigue has been implicated as a contributor to impaired performance, critical incidents, and errors in anesthesia.160,161 The work hours and schedules of anesthesiologists expose them to circadian disruption, and both acute and chronic sleep deprivation cause fatigue. The performance of anesthesiologists may be more susceptible to the effects of even mild sleep deprivation compared with other medical specialties because of the vigilance required to provide safe anesthesia care. However, study of the effects of fatigue on the clinical performance of anesthesiologists is difficult, and to date, no clinical studies have shown a definite link between fatigued anesthesiologists and errors.
In a well-designed, realistic, simulation-based trial, Howard et al162 studied 12 residents who anesthetized a simulated patient for 4 hours in both a sleep-deprived state and a rested state. In the sleep-deprived group, there was a trend to poorer vigilance with slower response to vigilance probes during the simulation. In clinical tasks, the sleep-deprived group took longer to detect and correct abnormal clinical events, but this was not statistically significant. During the simulated anesthetic, nearly one third of the sleep-deprived group fell asleep at some stage.
In an analysis of the first 5700 critical incidents reported to the Australian Incident Monitoring Study database, 2.7% of incidents listed fatigue as a factor contributing to the incident.161 Drug errors (syringe swaps/wrong drug, underdose and overdose) were more frequent, and anesthesiologists reported that haste, distraction, inattention, failure to check equipment, fault of technique, and pressure to proceed with surgery were all more common in fatigue-related than non–fatigue-related incidents. Experience and training did not render an anesthesiologist any less likely to make a fatigue-related error. A healthy patient and relief anesthesiologist/staff change were factors identified that tended to minimize the critical incident. In a survey of 301 anesthesiologists and trainees in New Zealand, 86% responded that they had at some time made an error in patient care related to fatigue.163 Fifty percent of trainees and 27% of anesthesiologists believed that their average working week exceeded what they believed they could do on an ongoing basis while maintaining patient safety. In addition, the anesthesiologists who exceeded their self-defined safety limits for continuous anesthesia administration (with breaks) or their weekly safe working hours were more likely to report a fatigue-related error in the 6 months before the survey when compared with those who had not exceeded their limits. The conclusions from these two studies are limited because they are based on retrospective, self-reported data, but the majority of respondents consistently indicate that quality of care is compromised and attribute some errors to working while fatigued.
A Scottish study raised different issues. Flin et al164 surveyed 222 anesthesiologists. When questioned about stress and fatigue, 83% agreed they were less effective when stressed or tired, but 37% thought they performed effectively during the critical phases of surgery even when tired. Eighty-four percent had made errors during patient care, but they did not attribute any causation to fatigue and did not list being rested or changing work schedules/reducing work hours as methods for reducing errors. The authors conclude, “With regard to attitudes suggesting invulnerability to the effects of stress and fatigue, a significant number of anesthetists were found to show these beliefs” and this “suggests that anesthetists (like pilots) may benefit from additional training in human performance limitations, both in postgraduate education or for consultants through continuing professional development programs.”
Gander et al165 studied work patterns, sleep, and performance on a psychomotor vigilance task of 28 anesthesia trainees and 20 specialists (practicing physicians) during a 2-week work cycle in 2 urban public hospitals in New Zealand. This study is significant because it included specialists and trainees. Reaction times of trainees were slower after night shifts than after day shifts, and after night shifts, poorer performance was associated with longer shift length, longer time since waking, greater acute sleep loss, and more total work over 24 hours. Reaction times of specialists slowed in a progressive, linear fashion over 12 consecutive days of duty (without any days off), and poorer performance was associated with greater acute sleep loss and longer time since waking. Work hours of trainees, but not specialists, are limited in New Zealand by a labor agreement.
Sleep quality and quantity become impaired in those around age 50 and older.166,167 There have been no formal studies assessing how these changes might affect the performance of older anesthesiologists. However, there is reduced tolerance of late-night and shift work with aging.167 Thus, many of the fatigue-related performance impairments identified in residents are potentially worse in older anesthesiologists.
Although working at night may contribute to error, the nature of anesthetic work makes it difficult to avoid at least some work during night-time hours. Therefore, work patterns need to be designed to minimize the possibility of fatigue-related error. These designs should not only involve consideration of total individual work hours, but the importance of the effects of shift work on sleep and circadian rhythm. Useful overviews for individual practitioners on the impact of fatigue on learning and good sleep hygiene to mitigate the impact of fatigue and for departments on the impacts of different rostering systems are available.168–172 Some areas to address to limit fatigue and fatigue-related error in anesthesia providers are listed in Table 39-4.
TABLE 39-4 Some Areas to Address to Limit Fatigue and Fatigue-Related Error in Anesthesia Providers
| Individual |
| Length of time without break |
| Length of time for shifts |
| Length of break between shifts |
| Handover170,175 |
| Sleep hygiene education |
| Education on effects of fatigue on performance and increased likelihood of errors |
| Naps |
| Pharmacologic aids (e.g., modafinil176–179) |
| Tighten monitor alarm limits after hours |
| Departmental |
| Roster design to limit circadian rhythm disruption |
| Staffing levels |
| Stop after hours or night work for older anesthesiologists |
| Sleep hygiene education |
| Use of written protocols/checklists, especially for complex cases after hours |
| Hospital |
| Lighting levels after hours |
| Nap rooms |
| Food and drink provision |
| Limit after-hours work to emergency cases |
These topics are intended as a starting point for consideration of possible ways in which errors related to fatigue might be reduced.
Effects of the use of transesophageal echocardiography on vigilance
The introduction and subsequent widespread use of TEE has been a major advance in cardiac surgery and anesthesia for both diagnosis and monitoring intraoperatively. Anecdotally, the authors have noticed times when all attention in the operating room is focused on the TEE machine rather than the patient. This seems to be more noticeable during the phase of learning TEE than with those who are more experienced. The surgical team also may comment that the anesthesia providers are all looking at the TEE rather than the patient! The authors are aware of only one study that has addressed this issue. During a study on task distribution, workload, and vigilance during cardiac anesthesia, Weinger et al173 found that there was a significant increase in response time to vigilance probes (a light was illuminated on the monitor screen) when the TEE was being manipulated and analyzed compared with times when it was not. The authors concluded, “The use of the TEE may decrease vigilance to changes in other clinical data.” Several factors limit the applicability of this study—it involved anesthesia residents with limited cardiac anesthesia experience and limited TEE experience. The operating room setup had the TEE machine placed opposite to the anesthesia machine and monitor display (where the vigilance light was located) and the study involved only 20 cases. The findings of the study were questioned in a subsequent letter to the editor.174
American society of anesthesiologists closed claims project results for cardiac anesthesia
The ASA Closed Claims database contains 327 claims related to cardiac anesthesia. When compared with 4986 other claims for other surgical procedures under general anesthesia, cardiac surgery claims had an increased proportion of claims for brain damage, stroke, and awareness during anesthesia and a decreased proportion of claims for airway injury (Table 39-5). The primary damaging event, for example, the specific incident that led to the injury, was more often related to equipment problems, particularly with central or peripheral catheters (Table 39-6). In addition, a greater proportion of cardiac claims were related to surgical technique or patient condition than other surgical claims in the database (see Table 39-6). Approaches for avoiding problems with intraoperative awareness and central venous access have been reviewed in this chapter. Brain damage and stroke during cardiac anesthesia is a vast topic unto itself that is addressed in Chapter 36.
TABLE 39-5 Influence of Type of Surgery on Severity of Injury
| Injury | Cardiac Surgery, n (%) | Other Surgery, n (%) |
|---|---|---|
| Major Category | ||
| Death | 103 (31%) | 1746 (35%) |
| Brain damage | 60 (18%)* | 548 (11%)* |
| Nerve damage | 54 (17%) | 770 (15%) |
| Specific Complications | ||
| Stroke | 30 (9%)* | 156 (3%)* |
| Airway injury | 21 (6%)† | 501 (10%)† |
| Awareness | 20 (6%)* | 99 (2%)* |
| Myocardial infarction | 5(2%) | 157 (3%) |
Cases related to cardiac surgery from the American Society of Anesthesiologists Closed Clams Project database (N = 8954) were compared with noncardiac surgery cases (only cases of general anesthesia were considered).
* P < 0.01 cardiac surgery vs. other surgery by Fisher’s exact test.
† P < 0.05 cardiac surgery vs. other surgery by Fisher’s exact test.
TABLE 39-6 Influence of Type of Surgery on Primary Damaging Event
| Damaging Event | Cardiac Surgery, n (%) | Other Surgery, n (%) |
|---|---|---|
| Respiratory | 33 (10%)* | 1507 (30%)* |
| Cardiovascular | 53 (16%) | 788 (16%) |
| Wrong blood administered | 4 (1%) | 19 (< 1%) |
| Equipment | 104 (32%)* | 579 (12%)* |
| Peripheral catheter | 38 (12%)* | 99 (2%)* |
| Central catheter | 28 (9%)* | 112 (2%)* |
| Adverse drug reaction/drug error | 30 (9%) | 336 (7%) |
| Surgical/patient condition | 35 (11%)† | 315 (6%)† |
Cases related to cardiac surgery from the American Society of Anesthesiologists Closed Clams Project database (N = 8954) were compared with noncardiac surgery cases (only cases of general anesthesia were considered).
1 Institute of Medicine. To Err Is Human. Building a Safer Health Care System. Washington, DC: National Academy Press, 2000.
2 Haynes A.B., Weiser T.G., Berry W.R., et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
3 Resident Duty Hours. Enhancing Sleep, Supervision and Safety. Washington, DC: The National Academies Press, 2008.
4 Martinez E.A., Marsteller J.A., Thompson D.A., et al. The Society of Cardiovascular Anesthesiologists’ FOCUS initiative: Locating Errors through Networked Surveillance (LENS) project vision. Anesth Analg. 2010;110:307-311.
5 Arnstein F. Catalogue of human error. Br J Anaesth. 1997;79:645-656.
6 Gaba D.M., Fish K.J., Howard S.K. Crisis Management in Anesthesiology. New York: Churchill Livingstone, 1994,;5-53.
7 Reason J. Human error: Models and management. BMJ. 2000;320:768-770.
8 McGee D.C., Gould M.K. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123-1133.
9 Bowdle T. Complications of invasive monitoring. Anesthesiol Clin North America. 2002;20:333-350.
10 Polderman K.H., Girbes A.R.J. Central venous catheter use. Part 1: Mechanical complications. Intensive Care Med. 2002;28:1-17.
11 Polderman K.H., Girbes A.R.J. Central venous catheter use. Part 2: Infectious complications. Intensive Care Med. 2002;28:18-28.
12 Hall A.P., Russell W.C. Toward safer central venous access: Ultrasound guidance and sound advice. Anaesthesia. 2005;60:1-4.
13 Making health care safer, A critical analysis of patient safety practices; 2001; Evidence report/technology assessment: Number 43. AHRQ Publication No. 01-E058
14 Domino K.B., Bowdle T.A., Posner K.L., et al. Injuries and liability related to central vascular catheters. Anesthesiology. 2004;100:1411-1418.
15 Urschel J.D., Myerowitz D. Catheter-induced pulmonary artery rupture in the setting of cardiopulmonary bypass. Ann Thorac Surg. 1993;56:585-589.
16 Harvey S., Harrison D.A., Singer M., et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): A randomised controlled trial. Lancet. 2005;366:472-477.
17 Shah M.R., Hasselblad V., Stevenson L.W., et al. Impact of the pulmonary artery catheter in critically ill patients: Meta-analysis of randomized clinical trials. JAMA. 2005;294:1664-1670.
18 Connors A.F., Speroff T., Dawson N.V., et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA. 1996;276:889-897.
19 Dalen J.E., Bone R.C. Is it time to pull the pulmonary catheter? JAMA. 1996;276:916-918.
20 Isaacson I.J., Lowdon J.D., Berry A.J., et al. The value of pulmonary artery and central venous monitoring in patients undergoing abdominal aortic reconstructive surgery: A comparative study of two selected, randomized groups. J Vasc Surg. 1990;12:754-760.
21 Mimoz O., Rauss A., Rekik N., et al. Pulmonary artery catheterization in critically ill patients: A prospective analysis of outcome changes associated with catheter-prompted changes in therapy. Crit Care Med. 1994;22:573-579.
22 Ramsey S.D., Saint S., Sullivan S.D., et al. Clinical and economic effects of pulmonary artery catheterization in non-emergent coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2000;14:113-118.
23 Tuman K.J., McCarthy R.J., Spiess B.D., et al. Effect of pulmonary artery catheterization on outcome in patients undergoing coronary artery surgery. Anesthesiology. 1989;70:199-206.
24 Bashein G., Johnson P.W., Davis K.B., et al. Elective coronary bypass surgery without pulmonary artery catheter monitoring. Anesthesiology. 1985;63:451-454.
25 Bender J.S., Smith-Meek M.A., Jones C.E. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: Results of a prospective, randomized trial. Ann Surg. 1997;226:229-236.
26 Coles N.A., Hibberd M., Russell M., et al. Potential impact of pulmonary artery catheter placement on short-term management decisions in the medical intensive care unit. Am Heart J. 1993;126:815-819.
27 Mueller H.S., Chatterjee K., Davis K.B., et al. Present use of bedside right heart catheterization in patients with cardiac disease. JACC J Am Coll Cardiol. 1998;32:840-864.
28 Richard C., Warszawski J., Anguel N., et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: A randomized controlled trial. Jama. 2003;290:2713-2720.
29 Sandham J.D., Hull R.D., Brant R.F., et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5-14.
30 Bar-Joseph G., Galvis A.G. Perforation of the heart by central venous catheters in infants: Guidelines to diagnosis and management. J Pediatr Surg. 1983;18:284-287.
31 Brandt R.L., Foley W.J., Fink G.H., et al. Mechanism of perforation of the heart with production of hydropericardium by a venous catheter and its prevention. Am J Surg. 1970;119:311-316.
32 Collier P.E., Ryan J.J., Diamond D.L. Cardiac tamponade from central venous catheters—a report of a case and review of english literature. Angiology. 1984;35:595-600.
33 Jiha J.G., Weinberg G.L., Laurito C.E. Intraoperative cardiac tamponade after central venous cannulation. Anesth Analg. 1996;82:664-665.
34 Maschke S.P., Rogove H.J. Cardiac tamponade associated with a multilumen central venous catheter. Crit Care Med. 1984;12:611-612.
35 Scott W.L. Complications associated with central venous catheters. Chest. 1988;94:1221-1224.
36 Sheep R.E., Guiney W.B. Fatal cardiac tamponade: Occurrence with other complications after left internal jugular vein catheterization. JAMA. 1982;248:1632-1635.
37 Fletcher S.J., Bodenham A.R. Safe placement of central venous catheters: Where should the tip of the catheter lie? Br J Anaesth. 2000;85:188-191.
38 Tocino I.M., Watanabe A. Impending catheter perforation of superior vena cava: Radiographic recognition. Am J Roentgenol. 1986;146:487-490.
39 Trigaux J.P., Goncette L., Van Beers B., et al. Radiologic findings of normal and compromised thoracic venous catheters. J Thorac Imaging. 1994;9:246-254.
40 Albrecht K., Nave H., Breitmeir D., et al. Applied anatomy of the superior vena cava—the carina as a landmark to guide central venous catheter placement. Br J Anaesth. 2004;92:75-77.
41 Schuster M., Nave H., Piepenbrock S., et al. The carina as a landmark in central venous catheter placement. Br J Anaesth. 2000;85:192-194.
42 Kwon T.D., Kim K.H., Ryu H.G., et al. Intra- and extra-pericardial lengths of the superior vena cava in vivo: Implication for the positioning of central venous catheters. Anaesth Intensive Care. 2005;33:384-387.
43 Gebhard R.E., Szmuk P., Pivalizza E.G., et al. The accuracy of electrocardiogram-controlled central line placement. Anesth Analg. 2007;104:65-70.
44 Jeon Y., Ryu H.G., Yoon S.Z., et al. Transesophageal echocardiographic evaluation of ECG-guided central venous catheter placement. Can J Anaesth. 2006;53:978-983.
45 Jobes D.R., Schwartz A.J., Greenhow D.E., et al. Safer jugular vein cannulation: Recognition of arterial puncture and preferential use of the external jugular route. Anesthesiology. 1983;59:353-355.
46 Ezaru C.S., Mangione M.P., Oravitz T.M., et al. Eliminating arterial injury during central venous catheterization using manometry. Anesth Analg. 2009;109:130-134.
47 Bowdle A., Kharasch E., Schwid H. Pressure waveform monitoring during central venous catheterization. Anesth Analg. 2009;109:2030-2031. author reply 2031
48 Troianos C.A., Jobes D.R., Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72:823-826.
49 Randolph A.G., Cook D.J., Gonzales C.A., et al. Ultrasound guidance for placement of central venous catheters: A meta-analysis of the literature. Crit Care Med. 1996;24:2053-2058.
50 Hind D., Calvert N., McWilliams R., et al. Ultrasonic locating devices for central venous cannulation: Meta-analysis. BMJ. 2003;327:361-368.
51 Wigmore T.J., Smythe J.F., Hacking M.B., et al. Effect of the implementation of NICE guidelines for ultrasound guidance on the complication rates associated with central venous catheter placement in patients presenting for routine surgery in a tertiary referral centre. Br J Anaesth. 2007;99:662-665.
52 Shime N., Nomura M., Matsuyama H., et al. Internal jugular vein cannulation in infants and children using a new portable ultrasound designed for vascular access. J Jpn Soc Intensive Care Med. 2005;12:407-411.
53 Hosokawa K., Shime N., Kato Y., et al. A randomized trial of ultrasound image-based skin surface marking versus real-time ultrasound-guided internal jugular vein catheterization in infants. Anesthesiology. 2007;107:720-724.
54 Chapman G.A., Johnson D., Bodenham A.R. Visualisation of needle position using ultrasonography. Anaesthesia. 2006;61:148-158.
55 Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; vol 2005; 2002; National Institute for Clinical Excellence; London
56 Bosman M., Kavanagh R.J. Two dimensional ultrasound guidance in central venous catheter placement; a postal survey of the practice and opinions of consultant pediatric anesthetists in the United Kingdom. Paediatr Anaesth. 2006;16:530-537.
57 Tovey G., Stokes M. A survey of the use of 2D ultrasound guidance for insertion of central venous catheters by UK consultant paediatric anaesthetists. Eur J Anaesthesiol. 2007;24:71-75.
58 McGrattan T., Duffty J., Green J.S., et al. A survey of the use of ultrasound guidance in internal jugular venous cannulation. Anaesthesia. 2008;63:1222-1225.
59 Bailey P.L., Glance L.G., Eaton M.P., et al. A survey of the use of ultrasound during central venous catheterization. Anesth Analg. 2007;104:491-497.
60 Harris N., Hodzovic I., Latto P. A national survey of the use of ultrasound during central venous cahteterization. Anaesthesia. 2007;62:306-307.
61 Oropello J.M., Leibowitz A.B., Manasia A., et al. Dilator-associated complications of central vein catheter insertion: Possible mechanisms of injury and suggestions for prevention. J Cardiothorac Vasc Anesth. 1996;10:634-637.
62 Kulvatunyou N., Heard S.O., Bankey P.E. A subclavian artery injury, secondary to internal jugular vein cannulation, is a predictable right-sided phenomenon. Anesth Analg. 2002;95:564-566. table of contents
63 Welliver M.D., Masoud P.J. Thoracic duct cannulation during central venous catheterization: A case report. Am J Anesthesiol. 2001;28:199-202.
64 Shah P.M., Babu S.C., Goyal A., et al. Arterial misplacement of large-caliber cannulas during jugular vein catheterization: Case for surgical management. J Am Coll Surg. 2004;198:939-944.
65 Guilbert M.C., Elkouri S., Bracco D., et al. Arterial trauma during central venous catheter insertion: Case series, review and proposed algorithm. J Vasc Surg. 2008;48:918-925. discussion 925
66 Soufir L., Timsit J.F., Mahe C., et al. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: A matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol. 1999;20:396-401.
67 Renaud B., Brun-Buisson C. Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am J Respir Crit Care Med. 2001;163:1584-1590.
68 Maki D.G., Kluger D.M., Crnich C.J. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159-1171.
69 Raad I.I., Bodey G.P. Infectious complications of indwelling cascular catheters. Clin Infect Dis. 1992;15:197-208.
70 Mermel L.A., Maki D.G. Infectious complications of Swan-Ganz pulmonary artery catheters. Pathogenesis, epidemiology, prevention and management. Am J Respir Crit Care Med. 1994;149:1020-1036.
71 Saint S., Matthay M.A. Risk reduction in the intensive care unit. Am J Med. 1998;105:515-523.
72 Pronovost P., Needham D., Berenholtz S., et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
73 Timsit J.F., Schwebel C., Bouadma L., et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: A randomized controlled trial. JAMA. 2009;301:1231-1241.
74 Hockenhull J.C., Dwan K.M., Smith G.W., et al. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: A systematic review. Crit Care Med. 2009;37:702-712.
75 Casey A.L., Mermel L.A., Nightingale P., et al. Antimicrobial central venous catheters in adults: A systematic review and meta-analysis. Lancet Infect Dis. 2008;8:763-776.
76 Gilbert R.E., Harden M. Effectiveness of impregnated central venous catheters for catheter related blood stream infection: A systematic review. Curr Opin Infect Dis. 2008;21:235-245.
77 Myles P.S., Williams D.L., Hendrata M., et al. Patient satisfaction after anaesthesia and surgery: Results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6-10.
78 Sandin R.H., Enlund G., Samuelsson P., et al. Awareness during anaesthesia: A prospective case study. Lancet. 2000;355:707-711.
79 Sebel P.S., Bowdle T.A., Ghoneim M.M., et al. The incidence of awareness during anesthesia: A multicenter United States study. Anesth Analg. 2004;99:833-839.
80 Xu L., Wu A.S., Yue Y. The incidence of intra-operative awareness during general anesthesia in China: A multi-center observational study. Acta Anaesthesiol Scand. 2009;53:873-882.
81 Domino K.B., Posner K.L., Caplan R.A., et al. Awareness during anesthesia: A closed claims analysis. Anesthesiology. 1999;90:1053-1061.
82 Ranta SO-V, Hernanen P., Hynynen M. Patient’s conscious recollections from cardiac anesthesia. J Cardiothorac Vasc Anesth. 2002;16:426-430.
83 Ranta S., Jussila J., Hynynen M. Recall of awareness during cardiac anesthesia: Influence of feedback information to the anesthesiologist. Acta Anaesthesiol Scand. 1996;40:554-560.
84 Dowd N.P., Cheng D.C.H., Karski J.M., et al. Intraoperative awareness in fast-track cardiac anesthesia. Anesthesiology. 1998;89:1068-1073.
85 Phillips A.A., McLean R.F., Devitt J.H., et al. Recall of intraoperative events after general anaesthesia and cardiopulmonary bypass. Can J Anaesth. 1993;40:922-966.
86 Myles P.S., Leslie K., McNeil J., et al. Bispectral index monitoring to prevent awareness during anaesthesia: The B-Aware randomized controlled trial. Lancet. 2004;363:1757-1763.
87 Ghoneim M.M., Block R.I., Haffarnan M., et al. Awareness during anesthesia: Risk factors, causes and sequelae: A review of reported cases in the literature. Anesth Analg. 2009;108:527-535.
88 Osterman J.E., Hopper J., Heran W.J., et al. Awareness under anesthesia and the development of posttraumatic stress disorder. Gen Hosp Psychiatry. 2001;23:198-204.
89 Moerman N., Bonke B., Oosting J. Awareness and recall during general anesthesia. Facts and feelings. Anesthesiology. 1993;79:454-464.
90 Bowdle T.A. Can we prevent recall during anesthesia? In Fleisher L., editor: Evidence-Based Practice of Anesthesiology, ed 2, Philadelphia: Saunders, 2009.
91 Bowdle T.A. The Bispectral Index (BIS): An update. Curr Rev Clin Anesth. 2004;25:17-28.
92 Glass P.S., Bloom M., Kearse L., et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836-847.
93 Islein-Chaves I.A., Flaishon R., Sebel P.S., et al. The effect of the interaction of propofol and alfentanil on recall, loss of consciousness, and the bispectral index. Anesth Analg. 1998;87:949-955.
94 Kalkman C.J., Drummond J. Monitors of depth of anesthesia, quo vadis? Anesthesiology. 2002;96:784-787.
95 Gan T., Glass P., Windsor A., et al. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil and nitrous oxide anesthesia. Anesthesiology. 1997;87:808-815.
96 Liu S.S. Effects of bispectral index monitoring on ambulatory anesthesia. A meta-analysis of randomized controlled trials and a cost analysis. Anesthesiology. 2004;101:311-315.
97 Ekman A., Lindholm M-L, Lennmarken C., et al. Reduction in the incidence of awareness using BIS monitoring. Acta Anaesthesiol Scand. 2004;48:20-26.
98 Avidan M.S., Zhang L., Burnside B.A., et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097-1108.
99 Rampil I.J., Kim J.S., Lenhardt R., et al. Bispectral EEG index during nitrous oxide administration. Anesthesiology. 1998;89:671-677.
100 Nieuwenhuijs D., Coleman E.L., Douglas N.J., et al. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94:125-129.
101 Sebel P.S., Lang E., Rampil I.J., et al. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect. Anesth Analg. 1997;84:891-899.
102 Bouillon T.W., Bruhn J., Radulescu L., et al. Pharmacodynamic interaction between propofol and remifentanil regarding hypnosis, tolerance of laryngoscopy, bispectral index, and electroencephalographic approximate entropy. Anesthesiology. 2004;100:1353-1372.
103 Kunisawa T., Ueno M., Suzuki A., et al. Bispectral index monitor prevented intraoperative awareness during partial cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2010;24:740.
104 Kakinohana M., Miyata Y., Kawabata T., et al. Bispectral index decreased to “0” in propofol anesthesia after a cross-clamping of descending thoracic aorta. Anesthesiology. 2003;99:1223-1225.
105 Bates D.W., Cullen D.J., Laird N., et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA. 1995;274:29-34.
106 Taxis K., Barber N. Incidence and severity of intravenous drug errors in a German hospital. Eur J Clin Pharmacol. 2004;59:815-817.
107 Camire E., Moyen E., Stelfox H.T. Medication errors in critical care: Risk factors, prevention and disclosure. CMAJ. 2009;180:936-943.
108 , Reducing and preventing adverse drug events to decrease hospital costs; March 2001; [AHRQ Publication No. 01-0020] Research in Action
109 Eslami S., de Keizer N.F., Abu-Hanna A. The impact of computerized physician medication order entry in hospitalized patients—a systematic review. Int J Med Inform. 2008;77:365-376.
110 Kaushal R., Shojania K.G., Bates D.W. Effects of computerized physician order entry and clinical decision support systems on medication safety: A systematic review. Arch Intern Med. 2003;163:1409-1416.
111 Koppel R., Metlay J.P., Cohen A., et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293:1197-1203.
112 Han Y.Y., Carcillo J.A., Venkataraman S.T., et al. Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2005;116:1506-1512.
113 Khajouei R., Jaspers M.W. The impact of CPOE medication systems’ design aspects on usability, workflow and medication orders: A systematic review. Methods Inf Med. 2010;49:3-19.
114 Currie M., Mackay P., Morgan C., et al. The “wrong drug” problem in anaesthesia: An analysis of 2000 incident reports. Anaesth Intens Care. 1993;21:596-601.
115 Merry A.F., Peck D.J. Anaesthetists, errors in drug administration and the law. N Z Med J. 1995;108:185-187.
116 Webster C.S., Merry A.F., Larsson L., et al. The frequency and nature of drug administration error during anaesthesia. Anaesth Intensive Care. 2001;29:494-500.
117 Orser B.A., Chen R.J.B., Yee D.A. Medication errors in anesthetic practice: A survey of 687 practitioners. Can J Anesth. 2001;48:139-146.
118 Irita K., Kawashima Y., Iwao Y., et al. [Annual mortality and morbidity in operating rooms during 2002 and summary of morbidity and mortality between 1999 and 2002 in Japan: A brief review]. Masui. 2004;53:320-335.
119 Abeysekera A., Bergman I.J., Kluger M.T., et al. Drug error in anaesthetic practice: A review of 896 reports from the Australian Incident Monitoring Study database. Anaesthesia. 2005;60:220-227.
120 Yamamoto M., Ishikawa S., Makita K. Medication errors in anesthesia: An 8-year retrospective analysis at an urban university hospital. J Anesth. 2008;22:248-252.
121 Sakaguchi Y., Tokuda K., Yamaguchi K., et al. Incidence of anesthesia-related medication errors over a 15-year period in a university hospital. Fukuoka Igaku Zasshi. 2008;99:58-66.
122 Gordon P.C., Llewellyn R.L., James M.F. Drug administration errors by South African anaesthetists— a survey. S Afr Med J. 2006;96:630-632.
123 Bowdle A., Kruger C., Grieve R., et al. Anesthesia drug administration errors in a university hospital. Anesthesiology. 2003;99:A1358.
124 Bowdle T.A. Drug administration errors from the ASA closed claims project. ASA Newsl. 2003;67:11-13.
125 Bell D. Recurrent wrong-route drug error—a professional shame. Anaesthesia. 2007;62:541-545.
126 Orser B.A., Oxorn D.C. An anaesthetic drug error: Minimizing the risk. Can J Anaesth. 1994;41:120-124.
127 Cranshaw J., Gupta K.J., Cook T.M. Litigation related to drug errors in anaesthesia: An analysis of claims against the NHS in England 1995-2007. Anaesthesia. 2009;64:1317-1323.
128 Svenmarker S., Soren H., Jansson E., et al. Quality assurance in clinical perfusion. Eur J Cardiothorac Surg. 1998;14:409-414.
129 Jenkins O.F., Morris R., Simpson J.M. Australasian perfusion incident survey. Perfusion. 1997;12:279-288.
130 Mejak B.L., Stammers A., Rauch E., et al. A retrospective study on perfusion incidents and safety devices. Perfusion. 2000;15:51-61.
131 Stammers A., Mejak B.L. An update on perfusion safety: Does the type of perfusion practice affect the rate of incidents related to cardiopulmonary bypass. Perfusion. 2001;16:189-198.
132 Jensen L.S., Merry A.F., Webster C.S., et al. Evidence-based strategies for preventing drug administration errors during anaesthesia. Anaesthesia. 2004;59:493-504.
133 ECRI. Bar-coded medication administration systems (BCMA) systems: Future promise, present challenges. Health Devices. 2003;32:373-381.
134 Bruhn J., Bouillon T.W., Radulescu L., et al. Correlation of approximate entropy, bispectral index, and spectral edge frequency 95 (SEF95) with clinical signs of “anesthetic depth” during coadministration of propofol and remifentanil. Anesthesiology. 2003;98:621-627.
135 Mills P.D., Neily J., Mims E., et al. Improving the bar-coded medication administration system at the Department of Veterans Affairs. Am J Health Syst Pharm. 2006;63:1442-1447.
136 Sakowski J., Newman J.M., Dozier K. Severity of medication administration errors detected by a bar-code medication administration system. Am J Health Syst Pharm. 2008;65:1661-1666.
137 Merry A.F., Webster C.S., Mathew D.J. A new, safety-oriented, integrated drug administration and automated anesthesia record system. Anesth Analg. 2001;93:385-390.
138 Webster C.S., Merry A.F., Gander P.H., et al. A prospective, randomized clinical evaluation of a new safety-oriented injectable drug administration system in comparison with conventional methods. Anaesthesia. 2004;59:80-87.
139 Nolen A.L., Rodes W.D.2nd. Bar-code medication administration system for anesthetics: Effects on documentation and billing. Am J Health Syst Pharm. 2008;65:655-659.
140 New perspectives on general-purpose infusion pumps. Advances in the technology, changes in our ratings. Health Devices. 2002;31:354-359.
141 Eskew J.A., Jacobi J., Buss W.F., et al. Using innovative technologies to set new saety standards for the infusion of intravenous medications. Hosp Pharm. 2002:1179-1189.
142 Eye on medical errors: Dose error reduction systems—A valuable tool for reducing iv medication errors. Health Devices. 2002;31:356-358.
143 Rothschild J.M., Keohane C.A., Cook E.F., et al. A controlled trial of smart infusion pumps to improve medication safety in critically ill patients. Crit Care Med. 2005;33:533-540.
144 Nuckols T.K., Bower A.G., Paddock S.M., et al. Programmable infusion pumps in ICUs: An analysis of corresponding adverse drug events. J Gen Intern Med. 2008;23(Suppl 1):41-45.
145 Vicente K.J., Kada-Bekhaled K., Hillel G., et al. Programming errors contribute to death from patient-controlled analgesia: Case report and estimate of probability. Can J Anesth. 2003;50:328-332.
146 Linden J.V., Paul B., Dressler K.P. A report of 104 transfusion errors in New York state. Transfusion. 1992;32:601-606.
147 Miyata S., Kawai T., Yamamoto S., et al. Network computer-assisted transfusion-management system for accurate blood component-recipient identification at the bedside. Transfusion. 2004;44:364-372.
148 Cajochen C., Khalsa S.B., Wyatt J.K., et al. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640-R649.
149 Dawson D., Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235.
150 Barger L.K., Ayas N.T., Cade B.E., et al. Impact of extended-duration shifts on medical errors, adverse events, and attentional failures. PLoS Med. 2006;3:e487.
151 Duffy J.F., Willson H.J., Wang W., et al. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57:1245-1251.
152 Pack A.I., Pack A.M., Rodgman E., et al. Characteristics of crashes attributed to the driver having fallen asleep. Accid Anal Prev. 1995;27:769-775.
153 Folkard S. Shift work, safety, and aging. Chronobiol Int. 2008;25:183-198.
154 Viola A.U., Archer S.N., James L.M., et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613-618.
155 Groeger J.A., Viola A.U., Lo J.C., et al. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159-1167.
156 Czeisler C.A. Medical and genetic differences in the adverse impact of sleep loss on performance: Ethical considerations for the medical profession. Trans Am Clin Climatol Assoc. 2009;120:249-285.
157 Van Dongen H.P., Maislin G., Mullington J.M., et al. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117-126.
158 Olson E.J., Drage L.A., Auger R.R. Sleep deprivation, physician performance, and patient safety. Chest. 2009;136:1389-1396.
159 Landrigan C.P., Barger L.K., Cade B.E., et al. Interns’ compliance with accreditation council for graduate medical education work-hour limits. JAMA. 2006;296:1063-1070.
160 Gravenstein J.S., Cooper J.B., Orkin F.K. Work and rest cycles in anesthesia practice. Anesthesiology. 1990;72:737-742.
161 Morris G.P., Morris R.W. Anaesthesia and fatigue: An analysis of the first 10 years of the Australian Incident Monitoring Study 1987-1997. Anaesth Intensive Care. 2000;28:300-304.
162 Howard S.K., Gaba D.M., Smith B.E., et al. Simulation study of rested versus sleep-deprived anesthesiologists. Anesthesiology. 2003;98:1345-1355. discussion 1345A
163 Gander P.H., Merry A., Millar M.M., et al. Hours of work and fatigue-related error: A survey of New Zealand anaesthetists. Anaesth Intensive Care. 2000;28:178-183.
164 Flin R., Fletcher G., McGeorge P., et al. Anaesthetists’ attitudes to teamwork and safety. Anaesthesia. 2003;58:233-242.
165 Gander P., Millar M., Webster C., et al. Sleep loss and performance of anaesthesia trainees and specialists. Chronobiol Int. 2008;25:1077-1091.
166 Kryger M., Monjan A., Bliwise D., et al. Sleep, health, and aging. Bridging the gap between science and clinical practice. Geriatrics. 2004;59:24-26. 29–30
167 Reilly T., Waterhouse J., Atkinson G. Aging, rhythms of physical performance, and adjustment to changes in the sleep-activity cycle. Occup Environ Med. 1997;54:812-816.
168 Anonymous: Shift work, the anaesthetist and Santayana’s warning. Anaesthesia. 2004;59:735-737.
169 Howard S.K., Rosekind M.R., Katz J.D., et al. Fatigue in anesthesia: Implications and strategies for patient and provider safety. Anesthesiology. 2002;97:1281-1294.
170 Fatigue and Anaesthetists. London: Association of Anaesthetists of Great Britain and Ireland, 2004.
171 Best Practice Rostering: Training and Resource Kit. Practical Tools for Rostering Doctors. Kingston: Australian Capital Territory: Australian Medical Association, 2003.
172 Jha A., Duncan B., Bates D. Fatigue, Sleepiness and Medical Errors. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality, 2001. Evidence Report/Technology Assessment: Number 43 [AHRQ Publication No. 01-E058]
173 Weinger M.B., Herndon O.W., Gaba D.M. The effect of electronic record keeping and transesophageal echocardiography on task distribution, workload, and vigilance during cardiac anesthesia. Anesthesiology. 1997;87:144-155. discussion 129A–130A
174 Aronson S., Cook R. Vigilance—a main component of clinical quality. Anesthesiology. 1998;88:1122-1124.
175 Guidelines on the Handover of Responsibility during an Anaesthetic. Melbourne, Australia: Australian and New Zealand College of Anaesthetists, 2004.
176 Batejat D.M., Lagarde D.P. Naps and modafinil as countermeasures for the effects of sleep deprivation on cognitive performance. Aviat Space Environ Med. 1999;70:493-498.
177 Buguet A., Montmayeur A., Pigeau R., et al. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. II. Effects on two nights of recovery sleep. J Sleep Res. 1995;4:229-241.
178 Caldwell J.A., Caldwell J.L., Smith J.K., et al. Modafinil’s effects on simulator performance and mood in pilots during 37 h without sleep. Aviat Space Environ Med. 2004;75:777-784.
179 Caldwell J.A.Jr, Caldwell J.L., Smythe N.K.3rd, et al. A double-blind, placebo-controlled investigation of the efficacy of modafinil for sustaining the alertness and performance of aviators: A helicopter simulator study. Psychopharmacology (Berl). 2000;150:272-282.
* http://www.anzca.edu.au/resources/books-and-publications/reports/mortality/Safety%20of%20Anaesthesia%20in%20Australia.pdf
* http://guidance.nice.org.uk/TA49
* Inadvertent injection of intravenous medications into epidural catheters has prompted efforts to design unique neuraxial injection ports that would prevent such errors.125