Red cells
Prior to discharge from marrow sinuses into the peripheral blood, red cells shed their nuclei. This gives the advantages of reduced weight and transformation into a biconcave disc with increased deformability compared with the more rigid spheroidal nucleated precursor (Fig 2.1).
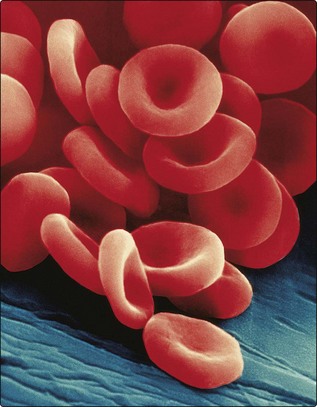
Fig 2.1 Scanning electron microscope picture of mature red cells showing clearly the characteristic biconcave shape. (Copyright Dennis Kunkel Microscopy Inc.)
Erythropoietin
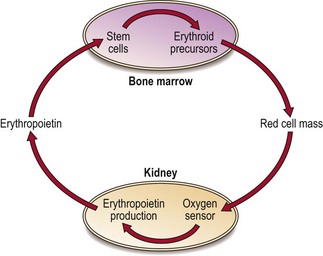
Unlike other growth factors, erythropoietin is mainly synthesised by the peritubular endothelial cells of the kidney. Production is triggered by tissue hypoxia (lack of oxygen). Cells can sense hypoxia via mediators such as the transcription factor HIF (hypoxia-inducible factor). HIF activates genes vital in the adaptive response to hypoxia including the erythropoietin gene. Erythropoietin molecules bind to specific membrane receptors on primitive erythroid cells in the bone marrow and induce maturation. The increase in red cells released into the blood stops when normal oxygen transport is restored – this feedback circuit is illustrated in Figure 2.2.
Structure
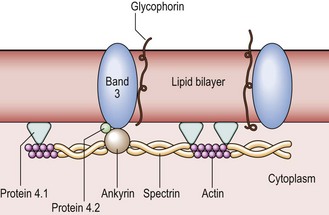
The red cell membrane is composed of a collapsible lattice of specialised proteins (the ‘cytoskeleton’) and an outer lipid bilayer (Fig 2.3). The protein skeleton is responsible for maintaining red cell shape while the lipid bilayer provides a hydrophobic skin. The main skeletal proteins are spectrin, actin, proteins 4.1 and 4.2, and ankyrin. Spectrin is the most abundant and consists of alpha and beta chains wound around each other. Spectrin heterodimers can align at the ends to form tetramers (i.e. four chains). Spectrin tetramers are joined together by actin in association with protein 4.1. This flexible skeleton is attached to the rest of the membrane by ankyrin, which interacts with protein 4.2 to link the spectrin beta chain to the cytoplasmic end of the transmembrane protein band 3. The lipid bilayer consists mainly of a mixture of phospholipids and cholesterol. Cholesterol molecules are inserted between phospholipid molecules in such a way that they stiffen the membrane while still allowing a degree of fluidity between the bilayers.
Haemoglobin and oxygen transport
The key function of red cells, to carry oxygen to the tissues and return CO2 from the tissues to the lungs, depends on the specialised protein haemoglobin which is present in large amounts in mature cells. The normal adult haemoglobin molecule (HbA) contains four polypeptide chains (‘globin’ chains): the two alpha chains and two beta chains are often notated as α2β2. Combined with each of the polypeptide chains is a ‘haem’ molecule which contains ferrous iron (Fe2+) and protoporphyrin (Fig 2.4). The iron combines reversibly with oxygen and thus haem forms the oxygen-carrying part of the molecule. Other globin chains are formed by the fetus and the change from fetal to adult haemoglobin occurs in the first 3–6 months of life. However, the subunits designated γ and δ persist into later life and small amounts of fetal haemoglobin (HbF; α2γ2) and HbA2 (α2δ2) are found in adults.
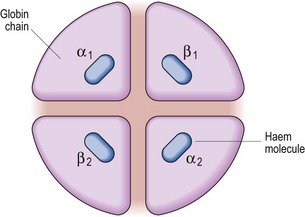
Fig 2.4 The essential elements of the haemoglobin molecule.
In reality each globin chain has a complex helical structure. The α chain has 141 amino acids and the β chain 146. The haem molecule consists of four pyrrole rings arranged around a ferrous ion.
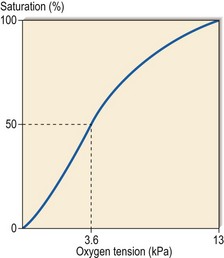
Haemoglobin is more than an inert carrier molecule. The individual globin chains interact with each other to facilitate the offloading of oxygen at lower oxygen saturations. The metabolite 2,3-diphosphoglyceride (2,3-DPG) generated in a side-arm of the Embden–Meyerhof pathway has an important role in the process, which results in a sigmoid-shaped oxygen dissociation curve (Fig 2.5). In anatomical terms haemoglobin has a high affinity for oxygen in the lungs and a much lower affinity in the tissues. The oxygen dissociation curve moves to the left when oxygen affinity increases; this occurs when H+ ion concentration is reduced or haemoglobin F (which cannot bind 2,3-DPG) raised. The curve moves to the right when oxygen affinity decreases; for instance when 2,3-DPG concentration rises or the abnormal sickle haemoglobin (HbS) is present. The P50 level is defined as the partial pressure of oxygen at which haemoglobin is half saturated.