Test for FMH not required.
Test for FMH not required.
Test for FMH not required.
Test for FMH not required.
cordocentesis
Test for FMH not required. After 20 weeks of gestation.
Test for FMH not required.
CVS: chorionic villus sampling; ECV: external cephalic version; FMH: fetomaternal hemorrhage; IU: international units; h: hours.
Preventable RhD alloimmunization occurs in susceptible RhD-negative women for the following reasons:
Safety, availability, dosage and administration of anti-D Ig
Anti-D Ig used in the UK is polyclonal and is prepared from pooled plasma from non-UK blood donors who have high levels of anti-D Ig, either due to previous sensitization or intentional immunization. In addition to screening for hepatitis B surface antigen, anti-human immunodeficiency virus infection and hepatitis C virus RNA, the plasma also undergoes viral inactivation steps in order to reduce risk of viral transmission. The theoretical transmission of Creutzfeldt–Jakob disease remains unquantifiable, though is likely to be extremely small. The incidence of possible adverse events from an injection of anti-D is estimated to be less than one event per 80,000 doses of anti-D Ig, the majority of which are not considered serious.
Although very rare, severe hypersensitivity, including anaphylaxis, may occur. Medications to treat anaphylaxis, such as adrenaline and steroids, should be readily available in case of such severe reactions. The probable mechanism by which anti-D works includes rapid clearance of anti-D coated D-positive red cells by macrophages and downregulation of antigen-specific B cells.
The minimum recommended dose of anti-D is mentioned above. The actual dose depends on the quantity of FMH and type of anti-D Ig preparations available in individual centers, and thus may be higher than is clinically necessary. The dose of anti-D is specified in IU and 1 mcg of anti-D Ig is equivalent to 5 IU. A dose of 500 IU, IM is considered sufficient to treat a FMH of up to 4 mL fetal red cells. Where it is necessary to give additional doses of anti-D Ig, as guided by tests for FMH, the dose calculation is traditionally based on 125 IU anti-D Ig/mL fetal or inadvertently transfused D-positive red cells for IM administration.
Management of red cell alloimmunization
In current clinical practice, obstetricians will encounter two clinical scenarios where knowledge of Rh disease becomes important:
(1) positive Rh antibody screening report, indicating presence of antibodies that may cause HDN
(2) raised Rh antibodies and affected fetus or newborn in a previous pregnancy.
As with most other areas of fetal medicine, the key elements of appropriate management strategy include careful history taking, maternal and fetal diagnostic tests, antenatal surveillance and treatment when necessary.
Obstetric history
An attempt should be made to establish the sensitizing event. Faced with a parous patient with no previous history of Rh sensitization, the following questions are helpful.
(1) Did you have any pregnancy losses, ectopic pregnancy or invasive tests in previous pregnancies? If yes, did you receive an (anti-D) injection?
(2) Did you receive antenatal/postnatal anti-D prophylaxis in previous pregnancies?
(3) Did you receive any blood transfusions in the past? (This is particularly relevant in the presence of non-D antibodies.)
The following questions are helpful when faced with a patient with history of Rh sensitization in previous pregnancy, particularly when full obstetric records are not available.
(1) Were you refered to a fetal medicine unit for monitoring in your last pregnancy?
(2) Did you have any invasive tests/fetal blood transfusions in your last pregnancy?
(3) Was your labor induced because of antibodies? If yes, at which gestation?
(4) Did any of your babies need a transfusion after birth?
(5) Did your baby need phototherapy (“under blue light”) after birth?
As a rule, the HDN tends to worsen in subsequent affected pregnancies. History of early fetal death or hydrops is particularly ominous, as hydrops recurs in 90% of affected pregnancies, often at an earlier gestation. Neonatal jaundice due to hemolysis is also likely to recur to the same degree of severity in subsequent affected pregnancies.
Paternal testing
Testing for paternal genotype should be considered routine in the presence of maternal Rh antibodies. If the father is homozygous then all his children will be at risk of Rh disease, whereas if he is heterozygous there is a 50:50 chance of his fathering a baby who will not be affected.
However, this counseling should not be trivialized because in certain scenarios this is effectively a test of paternity. For example, if presumed father is shown to be RhD homozygous, but the baby is found to be RhD negative, this may cause a considerable distress to the family in question. In the authors’ practice, a frank discussion is often held with a pregnant woman without the partner’s presence. If there is even a remote possibility that the current partner may not be the father of the child, then fetal genotyping should be considered (see below) without checking paternal blood group.
Prenatal diagnosis of fetal RhD status
One of the most important advances in recent years in the antenatal management of red cell alloimmunization has been the introduction of fetal blood grouping using cell-free fetal DNA (cffDNA) from maternal blood. Several studies have shown that detection of fetal RhD, RhC, RhE, Rhc and Kell antigens in maternal blood in alloimmunized pregnancies is highly accurate and can eliminate unnecessary fetal monitoring[27–30]. For antigens other than those mentioned above, invasive testing (CVS or amniocentesis) may be considered if fetal anemia is a concern or if invasive testing is performed for another reason, e.g., karyotyping.
In the UK, the Bristol International Blood Group Reference Laboratory (http://ibgrl.blood.co.uk/) perform prenatal diagnosis for the blood group antigen RhD, Kell, Rhc and RhE. When a test is negative, either the fetus is antigen negative or there was no fetal DNA in the maternal blood sample. In such circumstances, testing for the presence of the sex-determining region Y (SRY) gene in the fetal cells in maternal plasma can confirm the presence of a male fetus, and hence the presence of fetal DNA in the sample. The presence of a female fetus can potentially complicate the test result, but one useful option is to repeat the test after a few weeks. The last option would be to do the genetic testing of amniocytes obtained from amniocentesis, but this option is rarely needed. As diagnostic accuracy of cffDNA testing is not quite 100%, blood levels of antibody should be tested every 4 weeks to detect any false-negative results[31].
Routine testing of cffDNA for fetal RhD genotyping for all D-negative pregnant women can potentially limit the administration of prophylactic anti-D only to those women in whom it is needed. This could result in a significant saving of anti-D Ig, as women with D-negative fetuses (about one-third) will not require routine immunoprophylaxis or prenatal anti-D following sensitizing events (e.g., invasive procedures). It may also negate the need for testing the serologic status of the newborn at delivery, as DNA testing is more accurate than serology.
Maternal serology
The aims of antenatal serologic testing are to identify Rh-negative women who will benefit from anti-D Ig prophylaxis, to detect maternal alloimmunization and to ascertain risk to the fetus from alloimmune hemolytic disease. All pregnant women should be tested for ABO, RhD typing and for irregular serum antibodies at the initial antenatal visit, preferably in the first trimester and repeated at 28 weeks’ gestation (prior to administration of prophylactic anti-D).
Conventionally, an indirect antiglobulin test (IAT) is performed with untreated red cells in order to detect clinically important IgG antibodies, which may cross the placenta. Often an additional test is carried out using enzyme modified red cells, which facilitates early detection of low levels of antibodies, some of which may be clinically important.
When clinically significant alloantibodies are detected, quantification is usually performed by an autoanalyser technique (for anti-D and anti-c) or by IAT titration, usually reported as a titer score. Anti-D and anti-c values are expressed as IU/mL.
By convention, the quantification of other other antibodies is reported as the integer of the greatest tube dilution with a positive agglutination reaction (i.e., a titer of 16 is equivalent to a dilution of 1:16).
It is important to note that an increase in titer from 1 in 32 to 1 in 64 or from 1 in 64 and to 1 in 128 is not considered clinically significant. The increase should be more than double, i.e., from 1 in 32 to 1 in 128. This is the reason why old samples should be stored frozen and tested in parallel with the current sample using the same techniques.
The absolute level of antibody is not as important as the trend, with a rising level requiring more frequent monitoring, particularly if there is a history of previous HDFN. Significant HDFN due to anti-D is highly unlikely below a concentration of 4 IU/mL. Where quantification is not routine, antibody titration is performed using the IAT, with different laboratories establishing critical titers for RhD antibody, varying from 1:8 to 1:32. Close monitoring is usually indicated if the titre is >1/8[32–35] .
Ultrasound monitoring
A variety of ultrasonographic parameters have been used in an attempt to determine when fetal anemia is present. These have included placental thickness, umbilical vein diameter, hepatic length, splenic perimeter and polyhydramnios[36]. However, most of these features have not proven reliable in clinical practice. Late ultrasound findings include abdominal ascites, pleural and pericardial effusion and scalp edema.
The major breakthrough in the monitoring of Rh disease came with the introduction of the use of Doppler ultrasound to measure peak systolic velocity (PSV) of blood flow in the fetal middle cerebral artery (MCA) of the fetus[37].
The technique for obtaining fetal MCA Doppler waveforms is as follows[38].
-
An axial section of the brain, including the thalami and the sphenoid bone wings, should be obtained and magnified.
-
Color flow mapping should be used to identify the circle of Willis and the proximal MCA.
-
The pulsed-wave Doppler gate should then be placed at the proximal third of the MCA, close to its origin in the internal carotid artery (the systolic velocity decreases with distance from the point of origin of this vessel).
-
The angle between the ultrasound beam and the direction of blood flow should be kept as close as possible to zero degrees.
-
Care should be taken to avoid any unnecessary pressure on the fetal head.
-
At least three and fewer than 10 consecutive waveforms should be recorded. The highest point of the waveform is considered as the PSV (cm/s).
-
The PSV can be measured using manual calipers or autotrace. The latter yields significantly lower medians than does the former, but more closely approximates published medians used in clinical practice.
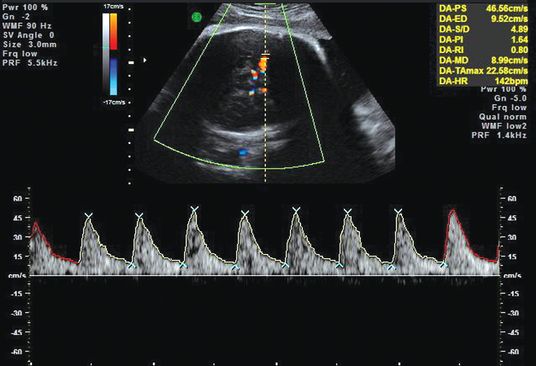
Doppler ultrasound examination of middle cerebral artery.
The threshold values for PSV in the MCA at different gestational ages are shown in Table 15.2. All of the fetuses with moderate or severe anemia had PSV values above 1.50 times the median.
| Week of gestation | Multiples of the median (MoM) | |||
|---|---|---|---|---|
| 1.00 MoM | 1.29 MoM | 1.50 MoM | 1.55 MoM | |
| 18 | 23.2 | 29.9 | 34.8 | 36.0 |
| 20 | 25.5 | 32.8 | 38.2 | 39.5 |
| 22 | 27.9 | 36.0 | 41.9 | 43.3 |
| 24 | 30.7 | 39.5 | 46.0 | 47.5 |
| 26 | 33.6 | 43.3 | 50.4 | 52.1 |
| 28 | 36.9 | 47.6 | 55.4 | 57.2 |
| 30 | 40.5 | 52.2 | 60.7 | 62.8 |
| 32 | 44.4 | 57.3 | 66.6 | 68.9 |
| 34 | 48.7 | 62.9 | 73.1 | 75.6 |
| 36 | 53.5 | 69.0 | 80.2 | 82.9 |
| 38 | 58.7 | 75.7 | 88.0 | 91.0 |
| 40 | 64.4 | 83.0 | 96.6 | 99.8 |
Amniotic fluid spectrophotometry
In most developed countries and many developing countries, amniotic fluid spectrophotometry (AFS) has now been superseded by antenatal fetal Doppler ultrasound examination for assessment and monitoring. Briefly, AFS was first introduced by Liley[39] in 1961 when he obtained amniotic fluid from 101 Rh-sensitized pregnant women between 27 and 41 weeks’ gestation, and spectrophotometrically analyzed it for bilirubin at an optical density of 450 nm. Liley used his data to delineate three zones related to gestational age. The optical density difference (ΔOD450) values in the lower zone (zone 1) indicated a fetus with mild or no hemolytic disease, whilst those in the upper zone (zone 3) indicated severe hemolytic disease with fetal death probable within 7–10 days.
In order to decide the optimum time of intervention (delivery or in utero transfusion), Whitfield et al.[40] introduced the concept of an action line based on the presence, severity and likely further trend of hemolysis in the fetus as indicated by the trend of amniotic fluid bilirubin concentration.
Fetal blood sampling and intrauterine transfusion
Fetal blood sampling (FBS) is ultrasound-directed, which allows for the direct measurement of fetal blood indices as early as 17–18 weeks’ gestation.
Traditionally, fetal blood is obtained from placental cord insertion or intrahepatic umbilical vein. In certain circumstances when access is a problem, it may be necessary to puncture a loop of cord that floats freely in the amniotic fluid. Specimens of fetal blood can be used for direct measurement of complete blood count, reticulocyte, and red cell antigen phenotyping. Full blood count is done in the local hematology laboratory and may take 5–10 min. Most fetal medicine units also use preliminary bedside testing (e.g., Hamecue) to decide whether to start fetal transfusion whilst waiting for the confirmatory result from the laboratory.
There is no universal agreement on a critical Hb level that indicates the need for transfusion. In modern practice, FBS is only performed in sensitized pregnancies with fetal hydrops or significantly raised PSVs in the fetal MCA. It is therefore extremely rare to perform FBS and find fetal Hb >10 g/L. When indicated, fetal transfusion is perfomed with O, Rh-negative, cytomegalovirus-negative, washed irradiated packed cells crossmatched against maternal blood. It is important to have the blood ready.
Transfusion volume depends on estimated fetoplacental blood volume for a particular gestation, fetal hematocrit and donor hematocrit.
In order to calculate exact transfusion, volume nomograms have been created by Nicolaides’s group from the Harris Birthright Fetal Medicine Unit at King’s College Hospital, London. Total fetoplacental blood volume increases from around 26 mL at 18 weeks to 241 mL at 36 weeks of gestation (Table 15.3)[41]. Based on pretransfusion fetal hematocrit and donor blood hematocrit, they derived a multiplication factor (F) in order to achieve a post-transfusion fetal hematocrit of around 40% (Table 15.4)[42]. The amount of blood to be transfused is calculated by multiplying the fetopalcental volume with this factor F. In current practice, these calculations are used to determine the initial volume of transfused blood. Post-transfusion fetal Hb is checked using a bedside test for full blood count. Additional volume is transfused, if necessary, aiming for a post-transfusion fetal Hb of around 130–150 g/L. In certain circumstances, particularly in a hydropic baby, the risk of volume overload is high, and it may be necessary to ‘under-transfuse’ in the first instance before a further top-up transfusion a week later. This will allow the fetal cardiovascular system to compensate for the acute change in viscosity. In the past, the follow-up transfusion was scheduled based on an anticipated decline in hematocrit of approximately 1% per day. Increasingly, MCA Doppler measurements are used to determine the timing of subsequent transfusions.
| Gestational age (weeks) | Estimated fetoplacental blood volume in fetuses with erythroblastosis fetalis (mL) |
|---|---|
| 18 | 26 |
| 20 | 34 |
| 22 | 50 |
| 24 | 69 |
| 26 | 86 |
| 28 | 112 |
| 30 | 136 |
| 32 | 166 |
| 34 | 203 |
| 36 | 241 |
| Donor hematocrit (%)►Fetal hematocrit (%)▼ | 50 | 60 | 70 | 80 |
|---|---|---|---|---|
| 10 | 3 | 1.5 | 1 | 0.75 |
| 20 | 2 | 1 | 0.7 | 0.5 |
| 30 | 1.125 | 0.55 | 0.35 | 0.3 |
Intraperitoneal transfusion
Intraperitoneal transfusion (IPT) is a technique in which donor red cells are placed in the fetal peritoneal cavity and are absorbed via subdiaphragmatic lymphatics and thoracic duct into the fetal circulation. This was superseded by intravascular intrauterine transfusion because of several disadvantages such as being less effective in hydropic fetus, a lack of information on pre- and post- transfusion blood indices, and possibly, increased intraperitoneal pressure compromising the venous return. However, IPT may be the only choice when treatment becomes essential at very early gestations (<18 weeks) because intravascular access is technically difficult[43]. Some have suggested that combined intravascular and intraperitoneal transfusion may be useful in prolonging the interval between the transfusion, but most centers use intravascular transfusion alone to avoid the need for two procedures and shorten the procedure time[44].
Management protocol
Stable antibodies <4 IU/mL or <1:64
Confirm that the partner is homozygous for the relevant antigen. If in doubt, organize fetal gentotyping. Serial antibody measurement is performed every 4 weeks before 28 weeks and 2 weeks after 28 weeks. Some minor antibodies can be repeated every 4 weeks. Multiple antibodies should be repeated every fortnight.
In such cases, timing, mode and place of delivery is dependent on standard obstetric grounds. Good quality evidence is not available to influence decision making. Usually, delivery is undertaken around 38–39 weeks’ gestation.
Antibodies >4 IU/mL or >1:64 and/or previously affected child
All such cases should be referred/discussed with tertiary fetal medicine units. Women with hydrops in previous pregnancy should be seen as early as 14–16 weeks. As a rule, MCA Doppler to estimate PSV should start around 18 weeks and usually continued fortnightly. If antibody levels rise, then Doppler studies may be needed weekly or even twice per week. Timing of repeat Doppler studies depends on the history, antibody levels and MCA values. Stable MCA below 1.29 multiples of the median (MoM) is reassuring.
When MCA-PSV exceeds 1.5 MoM before 32 weeks, most centers are likely to opt for FBS to detect fetal Hb levels. The authors’ view is that each FBS procedure has to be organized and planned meticulously, with blood for transfusion being readily available and an operating theatre on stand-by (after 24 weeks’ gestation).
As a rule, this group of women will be delivered between 36 and 38 weeks and the timing of invasive procedures often reflect that. In the past, the last in-utero transfusion used to be scheduled around 32 weeks, but with more experience and better results the upper cut-off point seems to be shifting towards later gestations, particularly when access is thought to be easier (e.g., anterior placenta).
Some units have attempted to modify the disease using high-dose IV gammaglobulins, plasmapheresis or steroids as an adjunct to intravascular transfusion in severe hemolytic disease where treatment is required prior to 20 weeks[44], or even before pregnancy. The mechanism of action could be downregulation of the maternal immune system or antagonistic action in the fetal reticular endothelial system[46].
Indications for referral to a fetal medicine specialist for a fetus at risk of HDFN[47]
-
Anti-D level of >4 IU/mL. At antibody levels >4 IU/mL but <15 IU/mL the fetus carries a moderate risk of HDFN and levels above 15 IU/mL can cause severe HDFN.
-
Anti-c level of >7.5 IU/mL. At antibody levels >7.5 IU/mL but < 20 IU/mL the fetus carries a moderate risk of HDFN, whereas antibody levels >20 IU/mL correlate with a high risk of HDFN.
-
Anti-K antibodies can lead to severe anemia even at low titres, and therefore referral should take place once anti-K antibody is detected.
-
Anti-E potentiates the severity of HDFN due to anti-c antibodies, and hence referral is indicated at lower titers or levels when both these antibodies are present simultaneously.